Highlights
-
•
Adolescents with a family history of SUD are impulsive in the delay discounting task.
-
•
Adolescents with and without family history of SUD did not differ in brain activity.
-
•
The delay discounting task engaged the expected reward and control brain regions.
-
•
Despite behavioral differences we observed no clear functional brain signatures for SUD risk.
Keywords: Delay discounting, Family history, Substance use disorder, fMRI
Abstract
Adolescents with a family history (FH+) of substance use disorder (SUD) are at a greater risk for SUD, suggested to be partly due to the transmission of behavioral impulsivity. We used a delay discounting task to compare impulsivity in decision-making and its associated brain functioning among FH+ and FH - minority adolescents. Participants chose between Smaller Sooner (SS) and Larger Later (LL) rewards. The SS was available immediately (Now trials) or in the future (Not-Now trials), allowing for greater differentiation between impulsive decisions. The FH+ group showed greater impatience by responding SS more frequently than the FH - group, only on the Now trials, and even when the relative reward differences (RRD) increased. Surprisingly, there were no differences in brain activity between the groups. Combined, the groups showed greater reward activity during the Now vs. Not-Now trials in medial prefrontal/anterior cingulate, posterior cingulate, precuneus, and inferior frontal gyrus (i.e., an immediacy effect). As the RRD increased activation in the reward network decreased, including the striatum, possibly reflecting easy decision-making. These results indicate that risk for SUD, seen behaviorally among FH+ adolescents, may not yet be associated with discernable brain changes, suggesting that early intervention has the potential to reduce this risk.
1. Introduction
Adolescence is marked by impulsive and risk-taking behaviors (Chambers et al., 2003) and thus it is a period with increased susceptibility to substance use (SU) and to developing a substance use disorder (SUD). This is often explained by adolescents’ preference for experiences with a high probability of immediate rewards, and by a less developed capacity for forethought regarding potentially negative consequences (Gladwin et al., 2011). Typically, SU begins in early adolescence (Wu et al., 2008, 2007) and increases sharply between ages 12 and 21 (Masten et al., 2008; Volkow and Fowler, 2000). However, some adolescents have a greater than average risk of developing SUD, and this may be due to genetic (Yu and McClellan, 2016), familial (Hummel et al., 2013), social, or environmental factors (Feldstein Ewing et al., 2012; Hicks et al., 2014; Jackson et al., 2016; Respress et al., 2013; Rudolph et al., 2018; Tobler et al., 2012). Environmental influences on the development of SU/SUD include: socioeconomic status, neighborhood characteristics (Wilson et al., 2005), cultural factors (Alegria et al., 2012; Vega et al., 1993), and discrimination (Respress et al., 2013; Tobler et al., 2012). In addition, residential segregation patterns are also related to risk of SUD among adolescents in those communities. Importantly, a family history (FH+) of SUD puts adolescents at a significantly increased risk of developing SUD (Bohman, 1978; Goodwin et al., 1973; Hill, 2010; Hill et al., 2011; Kendler et al., 2008; Masten et al., 2008; Tessner and Hill, 2010). This increased risk for SUD in adolescents due to parental SUD may interact with other risk factors. Previous studies have shown that FH+ offspring are more impulsive than children from FH- parents (Habeych et al., 2006; Martin et al., 1994). In addition, longitudinal studies reported that low behavioral inhibition or self-control among FH+ children and adolescents is associated with earlier onset of SU and accelerated progression to SUD for multiple substances (Shoal and Giancola, 2001; Tarter et al., 2003; Wong et al., 2006). Given these patterns, we were interested in understanding the relationship between adolescent impulsivity in decision making and the additional risk for SUD conferred by FH+ status. We drew from an ongoing longitudinal study of the development of SU in which all adolescent participants (FH+ and FH-) resided in the South Bronx neighborhood of New York City, an environment in which multiple levels of disadvantage converge to engender high risk for SUD (Galea et al., 2003).
Impulsivity is a complex and multifaceted construct, whose different dimensions are captured by different measures. For example, adolescents’ engagement in risky behaviors such as fast driving, unprotected sex, and extreme sports, is often used as a proxy of impulsive behavior (Dalley et al., 2011). Particular aspects of impulsivity related to attention and planning can be assessed via questionnaires, like the BIS-11 (Patton et al., 1995). Motor components of impulsivity can be assessed by the Go/No-Go task, and is indicated by the inability to suppress prepotent motor responses leading to a higher false-alarm rate. In this study, we focused on measuring impulsivity by choice preferences on a Delay Discounting (DD) task. DD measures the capacity to postpone gratification and defines impulsivity as preferences for sooner smaller (SS) rewards over larger later (LL) rewards (Loewenstein, 1988). It is typical that a reward that is delayed in time has a lower subjective value (its value is discounted) compared to the same reward available sooner. However, when this discounting becomes extreme, as when someone prefers $20 now over $200 in two weeks, it indicates an inability to postpone gratification, thus the DD task captures this aspect of impulsivity. The rate of discounting varies among people based on several factors including socioeconomic status (Jachimowicz et al., 2017). Importantly, it is well-established that a steeper rate of discounting future rewards is associated with drug addiction (Coffey et al., 2003; Hoffman et al., 2006; Kirby and Petry, 2004; Lempert et al., 2018; Moeller and Dougherty, 2002; Monterosso et al., 2001). While the discounting rate may decrease with reduction in dependence (Bickel et al., 2014), cocaine users who had been abstinent for 30 days still showed steep rates of discounting. This suggests that the preference for immediate rewards is long lasting and may not only be the result of the drug use per se (Heil et al., 2006). Cross sectional studies of adolescents also demonstrate that elevated sensation seeking behavior and steeper delay discounting were associated with earlier onset of drug experimentation (Kollins, 2003; Martin, 2002; Martin et al., 2004; Reynolds et al., 2003). Therefore, it is possible that the DD task could be used as a marker of an adolescent’s risk status for SU initiation and progression, as was suggested by Dougherty (Dougherty et al., 2014).
Adolescents’ impulsivity has been attributed to the differential developmental trajectories of two brain systems. Steinberg (Steinberg, 2008) described the dominating role of the socio-emotional brain system in driving reward seeking behavior at a time when the self-regulatory brain system is, as yet, underdeveloped. Similarly, Casey (Casey et al., 2008; Casey and Jones, 2010) proposed that during adolescence there are pronounced hierarchical changes in the brain circuitry of the subcortical and cortical regions and in their interconnections. Thus, over-activation of the brain’s reward system and under-activation of the cognitive control brain mechanisms lead to the impulsive and sensation seeking behaviors seen among adolescents (Somerville et al., 2010). Interestingly, these patterns of maturational development map rather well onto the time course of typical SU initiation and progression. During the DD tasks, this same integration of processing in the reward and control systems, both of which are undergoing changes during adolescence, appears to determine choice behaviors (Christakou et al., 2011; Ripke et al., 2012). Indeed, typically developing adolescents show steeper DD than adults (de Water et al., 2014; Steinberg et al., 2009). In the current study, we use a DD task to assess whether FH+ adolescents have higher discount rates than FH-, which may in-turn reflect their increased risk for SUD initiation.
In addition to the behavioral aspect of decision-making tasks designed to distinguish between SUD and control participants, neuroimaging studies of decision making tasks reported differences in frontostriatal reward areas encoding the subjective value of both immediate and delayed rewards (Carter et al., 2010; Glimcher, 2009; Goldstein and Volkow, 2011; Liu et al., 2011; Luijten et al., 2017; McClure and Bickel, 2014; Tervo-Clemmens et al., 2020; Tomko et al., 2016; Wesley and Bickel, 2014). This network includes anterior cingulate (ACC), medial prefrontal cortex (mPFC), orbitofrontal cortex (OFC), inferior frontal gyrus (IFG), ventral striatum (VS), and posterior cingulate cortex (PCC). Differences were also found in the lateral prefrontal cortex (LPFC) (Figner et al., 2010) and inferior parietal lobule (Liu et al., 2011) which are involved in executive processes. Functional connectivity between OFC and dorsolateral prefrontal cortex (DLPFC) during DD tasks increases during development as seen in children (Steinbeis et al., 2016) and adolescents (Christakou et al., 2011). Furthermore, in typically developing adolescents, individual variations in choices were associated with differential activity of brain areas implicated in cognitive control (lateral PFC including IFG regions, and parietal cortex) and reward valuation (VS and mPFC) (de Water et al., 2017). In adolescents at risk, Wetherill noted that those who later transitioned to heavy drinking had lower BOLD responses during the Go/No-Go task in brain inhibitory control circuits (including, inferior, superior, and medial frontal gyri) compared to those who did not transition to heavy drinking (Wetherill et al., 2013). Overall, poor task performance on the Go/No-Go task indicated vulnerability to substance use initiation (Verdejo-García et al., 2008) and later SUD (Hester and Garavan, 2004; Ivanov et al., 2008; Kaufman et al., 2003). Using an Emotional Stroop task in a pilot study, we demonstrated that inner city FH+ adolescents had increased conflict-related neural activity in a brain region which is part of the cognitive control network (Qiao et al., 2015). Similarly, a study of 16−22-year-olds with and without FH of alcohol use, reported differential activation in fronto-striatal areas during a Go/No-Go task (Heitzeg et al., 2010). While the participants in the latter study were not all substance naïve, the demonstrated differences may well be indicative of actual deficits in everyday self-regulation, impulsivity, and decision making, which, in turn, may increase the risk for SU initiation and progression.
One of the challenges in understanding the behavioral and brain imaging findings in SUD is whether the behaviors and the brain imaging differences represent pre-existing risk factors for SU, or whether substance use has altered brain functions. The literature supports the idea that SU creates neurocognitive deficits that may then reinforce SU behaviors and lead to full blown SUD (Hardin and Ernst, 2009; Robinson and Berridge, 2008; Volkow et al., 2012). Yet, only a few studies have adequately examined the connection between behavioral and neurocognitive differences among substance naïve young adolescents and their subsequent SU. For example, in offspring of alcohol dependent parents as well as in naïve adolescents who later developed SUD, smaller OFC volume has been associated with higher impulsivity (Cheetham et al., 2017; Hill et al., 2009), suggesting that the small OFC volume measured before drug exposure may be indicative of SUD risk. Those findings are intriguing but inconclusive and, thus, our study is designed to further investigate the connection between the behavioral and neuroimaging aspects of DD task performance and risk for SUD based on history of FH+/FH- in adolescents. We, therefore, examined brain regions known to be associated with decision making, as they have been shown to be differentially activated in adults with SUD.
The present study describes the baseline phase of a longitudinal investigation of inner-city adolescents designed to explore the nature of transmitted familial SUD risk. Thus, this paper reports the behavioral and fMRI findings from those baseline assessments. The study compares task performance and brain functioning in adolescents from FH+ and FH- families at an age when SU initiation is expected. We assessed brain activity using fMRI during a DD task, in which adolescents make binary choices between two different amounts of money, each available at a specified sooner or later time (Van Den Bos et al., 2015; Weber et al., 2007). To investigate the role of immediacy in reward choices in these adolescents, the reward associated with the sooner option was available either immediately, so called Now trials, or in two weeks, so called Not-Now trials (McClure et al., 2004a, 2004b). We hypothesized that FH+ adolescents would show more impulsivity in task performance compared to age-matched FH- controls, and that this effect would be more pronounced in the Now trials. Moreover, this behavioral difference would be reflected in brain activation in regions that are associated with reward valuation and with cognitive inhibition, as had been found in adults with SUD. Thus, the goal of this study was to understand the effect of parental SUD on impulsivity during early adolescence in a high-risk cohort of low SES families with similarly disadvantaged environments. This would help to identify if, before SU initiation, there are neurocognitive differences in offspring based solely on FH status and not on other demographic characteristics.
2. Methods and materials
2.1. Participants
One hundred and twenty-five healthy adolescents (60 female) participated in this fMRI study. Sixty-five adolescents (mean age 14.98±1.29) had family history of SUD (based on DSM-IV) and were categorized as FH+, and 60 (mean age 15.11±1.36) served as controls (FH-). Ninety-seven percent of the adolescents were drug naïve, defined as having consumed less than 6 alcoholic drinks, smoked marijuana less than 6 times, or used any other combination of drugs less than 3 times in their lifetime.
Since high trait anxiety (general feelings of anxiety) scores have previously been associated with adolescent substance use (Ste-Marie et al., 2006), we assessed the participants with the State and Trait Anxiety Inventory (STAI) (Spielberger et al., 1983). Similarly, as previous research with adolescents has indicated that higher Barratt Impulsiveness Scale (BIS-11) scores are associated with earlier onset of substance use and a higher likelihood of abuse/dependency issues later on in life (Von Diemen et al., 2008), we included the BIS-11 (Patton et al., 1995). The BIS-11 examines impulsiveness using three subscales: attentional, motor, and non-planning impulsiveness, with higher scores indicating a higher likelihood of impulsive behaviors.
FH+ adolescents had parents with life-time history of drug use disorder (22.5%), alcohol use disorder (42.5%), or both (35%), or both (35%). The FH+ and FH- groups were similar in their demographic characteristics as well as in their anxiety and impulsivity measures (Tables 1 & 2 and sample details in SM). One adolescent refused to perform the DD task in the scanner so behavioral data includes 124 subjects. Due to image artifacts (N = 1), diffuse brain intensities (N=2), signal or motion outliers (N=18), or missing too many trials of the task (N=1), fMRI analysis was performed on 102 participants, 53 were FH+ and 49 were FH- (see SM for the demographic and psychological characteristics of this N=102 subsample). The study was reviewed and approved by the New York State Psychiatric Institute IRB. Informed consent was obtained from parents, and adolescents signed assent forms. All participants received an $80 gift card for their participation in the MRI study, as well as the reward amount they chose in one randomly selected trial of the task (see details below).
Table 1.
Demographic Information and Comparison of the FH+ and FH- Groups.
| FH+ (N=65) |
FH- (N=60) |
Chi-square | df | P | N missing | |
|---|---|---|---|---|---|---|
| N (%) | N (%) | |||||
| Female | 30 (46.15) | 30 (50.00) | 0.18 | 1 | 0.67 | 0 |
| SES (household income) | ||||||
| <$15K | 20 (31.25) | 22(37.29) | 0.56 | 2 | 0.76 | 2 |
| $15-50K | 31 (48.44) | 27(45.76) | ||||
| >$50K | 13 (20.31) | 10 (16.95) | ||||
| Ethnicity | ||||||
| Hispanic | 37 (56.92) | 39 (65.00) | 0.88 | 2 | 0.64 | 0 |
| Black | 18 (27.69) | 14 (23.33) | ||||
| Other | 10 (15.38) | 7 (11.67) | ||||
Table 2.
Impulsivity and Anxiety Measures Comparison of the FH+ and FH- Groups.
| FH+ (N=65) |
FH- (N=60) |
T -test | df | P | N missing | |
|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | |||||
| BIS-111 | ||||||
| Attentional impulsiveness | 16.52 (3.21) | 15.68 (2.84) | −1.53 | 121 | 0.13 | 2 |
| Motor impulsiveness | 21.69 (4.29) | 21.07 (3.69) | −0.85 | 120 | 0.4 | 3 |
| Non-planning impulsiveness | 27.97 (5.17) | 27.07 (4.59) | −1.02 | 122 | 0.31 | 1 |
| STAI2 | ||||||
| State | 34.89 (8.52) | 32.01 (8.50) | −1.89 | 122 | 0.06 | 1 |
| Trait | 36.98 (9.18) | 35.02 (8.94) | −1.2 | 121 | 0.23 | 2 |
Note: 1For details about BIS 11 see (Patton et al., 1995). 2 For details about STAI see (Spielberger et al., 1983).
2.2. Delay discounting task (DD)
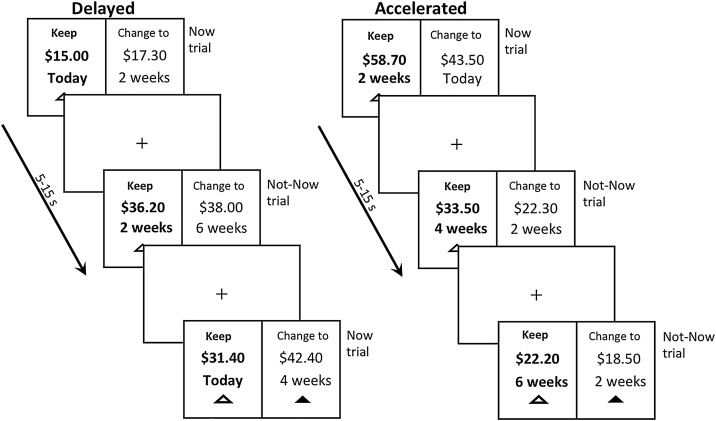
Participants performed the DD task (Decker et al., 2015) in the scanner after a short practice. Fig. 1 presents a schematic of the task design. Participants made binary choices between different amounts of money available with various delays, thus, the choice was always between a smaller-sooner (SS) and larger-later (LL) reward in all trial types. The Time Differences between SS and LL were either 2 or 4 weeks (split evenly). The Relative Reward Differences (RRD) in dollar amount between SS and LL (i.e., (LL-SS)/SS) was either 1%, 3%, 5%, 10 %, 15%, 20%, 25%, 35%, or 50%. The dollar amounts were drawn from a distribution with a mean of $45 (SD=$20) and truncated so that it was between $15 and $85. However, unlike other versions of DD tasks, our DD task included two trial types (Now/Not-Now), and two framing conditions (Delay/Acceleration). In the Now/Not-Now trials, half of the SS options were available “today” (Now), and in half, even the sooner rewards were not available for two weeks (Not-Now), while the LL were further delayed. In the Delay/Acceleration framing, half the trials were presented with a so called “Delay Frame” in which the SS was presented as the default (i.e., presented as the first option by a green triangle under the choice), and half with an “Acceleration Frame” in which the LL was presented as the default.
Fig. 1.
Trial procedure of the delay discounting task in the delay and the acceleration conditions. In both conditions, participants were presented with a choice between a smaller amount of money available sooner (SS) and a larger amount available later (LL). Time of delivery for the SS was either today (Now trial) or in 2 weeks (Not-Now trials), and the time of delivery for the LL was either 2 or 4 weeks after the SS. In the delayed condition, the default option presented to the subject was the SS amount, whereas in the accelerated condition it was the LL amount. This was indicated by the green triangle under the choice option. Amounts offered ranged from $15 to $85.
In each trial, the SS and LL choices were shown on the screen until subjects responded or when 9 s passed. Then, the participants received feedback for 1 s, indicating that their choice had been recorded. The feedback was a change in the colour of the triangle below the chosen option which turned green, while the triangle below the alternative option disappeared. The feedback was followed by an interval that ranged between 4 and 6 s (randomized based on uniform distribution) with a fixation cross. Participants performed each type of frame twice (Delay and Acceleration), in 4 runs each lasting about 5 min (due to time constraints, one participant had only 3 runs), with counterbalanced ordering of the frames between subjects, for an average of 147 valid trials (range 85–180; with 149.74±17.65 trials for the FH+ and 146.53±12.63, P > 0.2). Trials with no responses or with reaction times (RTs) < 200 ms were excluded, but both were rare. Participants were told that there were no correct answers and were instructed to choose the option they truly preferred, because one trial would be selected randomly, and the participant would be paid according to their preference on that trial. That is, if the randomly selected trial was a Now trial, and the participant chose SS, they received the amount of the SS choice at the end of the session in cash. If it was a Not-Now trial, and the participant chose SS, they received the amount of the SS choice as a check in 2 weeks. In both trial types, if they chose LL, a check for the LL amount was sent to them at the time indicated by the trial. Stimuli were projected on a screen using Presentation® software (Version 18.0, Neurobehavioral Systems, Inc., Berkeley, CA, www.neurobs.com).
2.3. Image acquisition
Imaging was performed on a GE Discovery MR750 3.0 T scanner using a 32-channel head coil. Functional images were acquired using standard gradient-echo EPI pulse sequence (TE = 25 ms, flip angle = 77˚, TR = 2 s, field of view = 192 mm, matrix size = 64 × 64, 3 mm isotropic voxels, 45 contiguous axial slices), with ascending interleaved slice order. Four fMRI runs of 150 images each were acquired. High-resolution T1-weighted anatomical images were acquired in the same axial plane as the functional images using a 3D BRAVO sequence (TE = 2.7, flip angle = 12˚, TR = 7.2, field of view = 256 × 256, 176 slices with a spatial resolution of 1.0 × 1.0 × 1.0 mm3).
2.4. Data analyses
2.4.1. Behavioral
Data for the 124 adolescents were analyzed at the trial level without aggregation. Missing response trials were excluded. The main model, a Bayesian generalized linear mixed-effects model, was performed on SS vs. LL choices as a function of Group (FH- and FH+), the immediacy of the SS option (available Now versus Not-Now), framing, the time difference between the SS and LL (2 or 4 weeks), the reward magnitude of the SS, and the RRD between the SS and LL. We also included an interaction term for each of these factors with Group. In addition to these fixed effects, we included a random intercept varying over participants, random slopes varying over participants for each of the fixed effects, as well as all possible pairwise covariances between the random effects. Thus, this model constitutes a "maximal model" with respect to the random effects (Barr et al., 2013) to safeguard against inflated Type 1 errors. All categorical predictors were sum-to-zero coded. All the other, continuous predictors were standardized. The models were implemented using the package brms (Bürkner, 2017) in R (R Core Team, 2019), which provides an interface to Stan (Carpenter et al., 2017). The Bayesian model was checked for convergence. We deemed a regression coefficient statistically significant if its 95 % posterior credible interval (CI) did not include 0. See SM for more details and for the same analyses performed on the N=102 participants for which we had usable fMRI data.
2.4.2. Neuroimaging
We used a standard preprocessing and analysis approach (detailed in SM). Briefly, preprocessing of the fMRI images included slice timing correction, motion correction, normalization, and smoothing. Individual-level analysis was modeled according to the factors that showed significant interactions with group in the behavioral analysis (i.e., Immediacy; RRD). Two general linear models (GLM) were used for the whole brain analysis of the individual subject data (Friston et al., 1995). First, GLM1 was option-based and included a regressor for each trial type (i.e., Now and Not-Now trials) to investigate the immediacy effect, and a regressor for the RRD as a parametric modulator. Two 1st level contrasts were performed: Now vs. Not-Now trials to investigate immediacy effect, and all trials modulated by RRD to investigate the effect of reward value differences between choices. Using this option-based GLM model, there were equal numbers of Now and Not-Now trials entering the analysis, as that did not depend on the participants choices. Second, GLM2 was based on participants choices (i.e., SS or LL) using three regressors; SS choices in Now trials, SS choices in Not-Now trials, and LL choices in both Now and Not-Now trials. We investigated the immediacy effect with Now vs Not-Now trials 1st level contrast. Therefore, GLM2 contrast analysis, unlike GLM1, excluded the contributions of LL choices in the Now and Not-Now trials. This GLM2 analysis included fewer participants (N=89) because some participants responded more often SS or mostly LL and did not have a minimum 10 trials for at least one of the comparison conditions. This reduction in the number of participants did not change the behavioral outcomes (see SM for N=89), which were similar to those reported here (N=124), and for the N=102 reported in the SM. In both GLM analyses, we modeled the decision time by including RT as an event duration, and regressors were convolved with a canonical HRF. We also included regressors of no interest reflecting the mean whole-brain activity on an acquisition-by-acquisition basis, null covariates for bad volumes (obtained with ArtRepair) and 6 motion parameters. Results from individual-level analysis (1st level contrast) were submitted to random-effects whole-brain group analysis. One-sample t-tests were used to identify activation in the combined groups, and for each group separately. Two-sample t-tests were used to compare activation between the FH+ and the FH- groups. Multiple comparison corrections were implemented with AFNI program 3d ClusSim (Cox, 1996; Cox et al., 2016), using Monte Carlo simulations, on p < 0.001 uncorrected maps to estimate the minimum cluster size that would be significant at p (corrected) < 0.05. The cluster extent thresholds for each contrast are noted in each Figure. An uncorrected p-value of 0.005 with extent threshold of 50 voxels was used to explore the presence of subthreshold activations for the combined groups (See SM). MNI coordinates for the peak voxel in each significant cluster were calculated based on SPM output and corresponding anatomical labels were obtained using xjView toolbox (http://www.alivelearn.net/xjview). To interpret the contribution of each regressor of the imaging results of GLM1, we obtained the peak beta value for active regions using SPM 12. In addition, we investigated differential activity modulated by RRD as included in GLM1 for all trials (both Now and Not-Now) between groups, and for the combined groups (uncorrected p = 0.005 at the voxel level and extended cluster threshold of 50 voxels).
3. Results
3.1. Behavioral
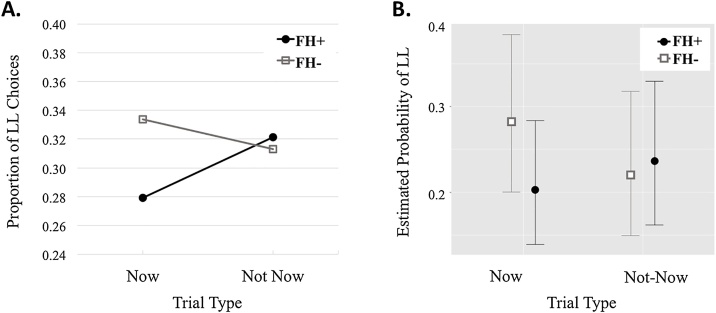
Table 3 presents the regression coefficients and the confidence intervals for the behavioral performance in the DD task using the Bayesian mixed-effects model. Participants’ choices were affected by SS reward amount, time difference, immediacy, and RRD. Importantly, as seen in Fig. 2, the Now/Not-Now factor interacts with Group, suggesting that the groups differ in their choices between SS and LL depending whether they were in the Now or Not-now trials. In the Now trials, this group difference was driven by the FH+ participant choosing LL less often (SS more often) than the FH- participants. Interestingly, it is the FH- group that showed fewer LL choices in the Not-Now compared with the Now trials (seen more clearly in the SM with the subgroup of 102 participants included in the fMRI analysis).
Table 3.
The Bayesian Model Results for the Behavioral Performance in the DD (N = 124).
| Variables | Beta Est. | Est. Error | L-95 % CI | U-95 % CI |
|---|---|---|---|---|
| SS Rewarda | 0.718 | 0.064 | 0.594 | 0.844 |
| Now/Not-Nowa | −0.334 | 0.134 | −0.595 | −0.070 |
| Relative Reward Differences (RRD)a | 1.063 | 0.096 | 0.878 | 1.251 |
| Time Differencea | −0.648 | 0.107 | −0.858 | −0.437 |
| Frame | 0.170 | 0.120 | −0.067 | 0.408 |
| Group | −0.441 | 0.317 | −1.074 | 0.190 |
| Now/Not-Now by Groupa | 0.529 | 0.180 | 0.177 | 0.883 |
| RRD by Groupa | −0.300 | 0.104 | −0.504 | −0.099 |
| Time Difference by Group | 0.079 | 0.143 | −0.206 | 0.361 |
| Frame by Group | −0.105 | 0.168 | −0.435 | 0.224 |
The Bayesian model included predictors that represented main effects of Group, Now/Not-Now trials, Delay/Acceleration Frame, Time Difference, SS Reward, Relative Reward Differences (RRD), and interactions with Group for all variables but SS Reward. All continuous predictors (SS Reward, RRD) were mean-centered and standardized, all categorical predictors (Group, Now/Not-now, Time Differences, Frames) were coded using sum-to-zero contrasts. All primary results are derived from this model. Coefficients were deemed statistically significant if the associated 95 % posterior credible intervals were non-overlapping with zero.
< 0.05.
Fig. 2.
Proportion of LL choices for the Now and Not-Now trials for the FH+ and FH- groups. A. represents the choice proportions computed from the raw data. B. represents the estimated marginal mean probabilities based on the Bayesian model (with 95 % CIs).
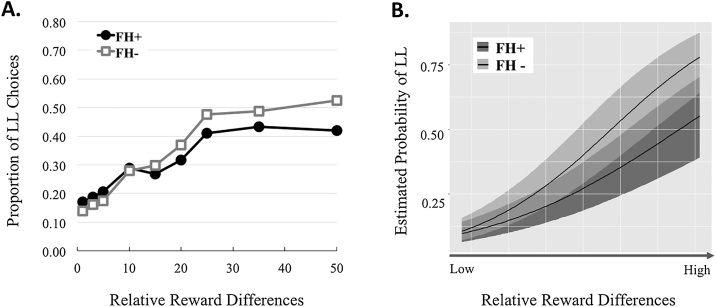
As expected, and shown in Fig. 3, participants were more likely to choose the LL as RRD increased. The RRD also significantly interacted with Group indicating that the groups differ in their choice of SS and LL as a function of how much larger the LL was compared to the SS. However, the increase in LL choices in FH+ is smaller than the increase in LL choices seen in the FH-.
Fig. 3.
The proportion of LL choices as a function of the Relative Reward Differences (RRD) for the FH + and FH- groups. A. represents the choice proportions computed from the raw data. B. represents the estimated marginal mean probabilities based on the Bayesian model (with 95 % CIs).
Table 4 presents the RTs for the SS and LL choices for each of the trial types for the FH+ and FH- groups. Comparisons between groups using t-tests revealed that FH+ responded significantly faster than FH- when making SS choices. Both groups, however, were choosing SS faster in the Now trials than in the Not-Now trials (p < 0.001 for both FH+ and FH-). In addition, LL responses did not show a difference in RT across groups or trial types.
Table 4.
Reaction Time Comparisons between the FH + and FH- Groups.
| FH+ (N = 64) |
FH- (N = 60) |
t -test | df | P | ||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) ms | Mean (SD) ms | |||||||
| SS | Now | 1542 | (1104) | 1808 | (1083) | −9.69 | 6269 | <0.001 |
| Not-Now | 1691 | (1161) | 1947 | (1091) | −8.96 | 6250 | <0.001 | |
| LL | Now | 1936 | (1284) | 1977 | (1135) | −0.87 | 2686 | 0.38 |
| Not-Now | 1915 | (1277) | 1996 | (1209) | −1.78 | 2894 | 0.08 | |
3.2. Neuroimaging
GLM models were based on the behavioral results and thus included the comparison of Now vs. Not-Now, and an examination of the effect of RRD on the modulation of the BOLD responses for all trials.
3.2.1. Brain regions involved in immediacy based on option presentation (GLM1)
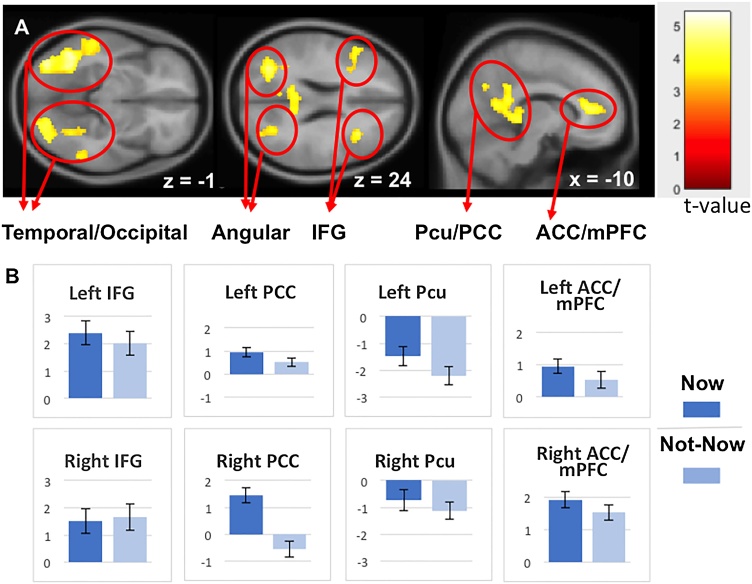
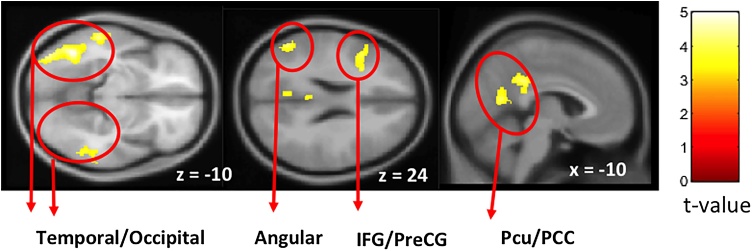
The comparison of Now vs Not-Now contrast across groups showed no significant activation after multiple comparisons correction. Therefore, we investigated the effect of immediacy (Now vs. Not-Now) of the combined groups data. Fig. 4A and Table 5 show significantly greater activation for Now compared to Not-Now trials in frontal regions, including the ACC/mPFC, left inferior and middle frontal gyrus, and in a large bilateral tempo-occipital cluster including medial temporal gyrus, hippocampal gyrus, middle and inferior occipital gyrus and extending to the parietal precuneus (Pcu), PCC and angular regions. No deactivations were observed, indicating greater involvement of brain regions for the immediate reward (Now trials) than for the Not-Now reward trials. Notably, striatum regions that have been associated with reward processing were observed only at uncorrected threshold with small cluster size (see SM).
Fig. 4.
Group random effects for the Now vs. Not-Now trials for FH- and FH+ groups combined (N=102) in the option-based analysis (GLM1). A. Maps are threshold at p < 0.05 FPR corrected determined by a p-unc<0.001 at the voxel level and cluster size threshold of 218. B. The beta coefficients from the group random-effect analysis done on the combined groups shown in A. Data are shown for the peak value of the (sub) cluster comparing the Now and the Not- Now trials. IFG, inferior frontal gyrus; Pcu, precuneus; PCC, posterior cingulate cortex; ACC, anterior cingulate cortex; mPFC, medial prefrontal cortex.
Table 5.
Brain regions active for the contrast of Now > Not-Now trials for the combined groups in the option-based analysis (GLM1).
| Brain Regions | Brodmann Area | Peak t-value | Cluster size | MNI Coordinates |
|||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| L | Temporal (MTG, ITG), Occipital (MOG, IOG, FUS), Parietal (SPL, ANG, Pcu) | 37, 20, 22, 18, 19, 39, 7 | 5.4 | 3439 | −44 | −56 | −14 |
| R | Temporal (MTG, ITG), Occipital (MOG, IOG, Fus), Parietal (SPL, ANG, Pcu) | 37, 20, 18, 19, 39, 7 | 5.13 | 2561 | 54 | −44 | −16 |
| L/R | Parietal (PCC, Pcu) , Occipital (CU, LG) , Temporal (Hipp), Thalamus | 30, 31, 23, 27 | 4.78 | 1334 | −6 | −58 | 14 |
| L | Frontal (IFG, MFG, PreCG) | 9, 46, 45, 13 | 4.53 | 427 | −48 | 26 | 14 |
| L/R | Frontal (ACC/mPFC, SFG) | 32, 24, 9, 10 | 4.4 | 758 | −10 | 40 | 10 |
| R | Frontal (IFG, MFG), Insula | 46, 13 | 4.37 | 353 | 34 | 22 | 24 |
P < 0.05 FPR cluster-level corrected (voxel level P < 0.001 and a cluster size > 218 voxels).
Abbreviations: L, left; R, right; MTG, middle temporal gyrus; ITG, inferior temporal gyrus, MOG, middle occipital gyrus; IOG, inferior occipital gyrus; FUS, fusiform gyrus; SPL, superior patietal lobe, ANG, angular gyrus, Pcu, precuneus; PCC, posterior cingulate gyrus; CU, cuneus, LG, lingual gyrus; Hipp, hippoampus; IFG, inferior frontal gyrus; MFG, middle frontal gyrus; PreCG, precentral gyrus; ACC, anterior cingulate gyrus; MPFC, medial prefrontal cortex; SFG, superior frontal gyrus.
To further understand the underlying Now vs. Not-Now BOLD signal, we examined the beta coefficients of peak voxel for each active region resulting from the combined group analysis. As can be seen in Fig. 4B, Now trials had larger activation than Not-Now trials in the right ACC and bilateral PCC. In contrast, Not-Now trials had larger deactivation in left Pcu compared to Now trials.
3.2.2. Brain regions involved in immediacy based on participants’ choices (GLM2)
Comparing brain activity associated directly with the behavioral choices, we found no difference between the groups in SS choices in the Now and Not-Now trials. Comparing brain activity associated with SS choices during Now and Not-Now trials for the combined groups (Fig. 5 and Table 6) revealed significantly greater activation in the following clusters: left frontal region, including left inferior and middle frontal gyrus, a large tempo-occipital-parietal region including left middle, inferior and superior temporal gyri, and bilateral Pcu, PCC and angular regions of the parietal lobe. As in the GLM1, no deactivations were observed, indicating greater involvement of brain regions for the immediate reward (Now trials) than for the Not-Now reward trials. Striatum regions were not observed even at uncorrected thresholds using small cluster size (50 voxels) (see SM).
Fig. 5.
Group random effects for SS choices only, during the Now vs. Not-Now trials, for FH- and FH + groups (N=89) combined in the choice-based analysis (GLM2). Maps are threshold at p < 0.05 FPR corrected determined by a p-unc<0.001 at cluster size threshold of 181. IFG, inferior frontal gyrus; PreCG, precentral gyrus; Pcu, precuneus; PCC, posteriro cingulate cortex.
Table 6.
Brain regions active for the contrast of SS Now choices > SS Not-Now choices for the combined groups for the choice-based analysis (GLM2).
| Brain Regions | BA | Peak t-value | Cluster size | MNI Coordinates |
|||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| L | Temporal (MTG, ITG, FUS), Occipital (IOG) | 37, 20, 18, 19 | 5.02 | 923 | −42 | −60 | −8 |
| L | Temporal (MTG, STG, ITG), Parietal (ANG, SMG) | 21, 22, 39, 40 | 4.51 | 850 | −60 | −32 | −8 |
| L/R | Parieteal (Pcu, PCC), Occipital (CU, LG) | 23, 30, 31 | 4.42 | 208 | −6 | −62 | 14 |
| L | Frontal (MFG, IFG) | 9, 44, 45, 46 | 4.38 | 435 | −48 | 22 | 16 |
| R | Frontal (MFG, SFG) | 8, 9 | 4.35 | 303 | 26 | 30 | 54 |
| L/R | Parietal (PCC, Pcu, MCC) | 23, 31 | 4.22 | 392 | −2 | −38 | 32 |
| R | Temporal (MTG, ITG, FUS), Cerebellum | 37, 20, 21 | 4.21 | 344 | 38 | −44 | −28 |
P < 0.05 FPR cluster-level corrected (voxel level P < 0.001 and a cluster size > 181 voxels).
Abbreviations: L, left; R, right; MTG, middle temporal gyrus; ITG, inferior temporal gyrus; FUS, fusiform gyrus; IOG, inferior occipital gyrus; STG, superior temporal gyrus; ANG, angular gyrus; SMG, supramarginal gyrus; Pcu, precuneus; PCC, posterior cingulate gyrus; CU, cuneuus; LG, lingual gyrus; MFG, middle frontal gyrus; IFG, inferior frontal gyrus; SFG, superior frontal gyrus; MCC, middle cingulate gyrus.
Overall, the brain activity seen in this choice-based analysis was similar to the one reported above for the option-based analysis (see Fig. 4A and B). The main difference between the two analyses was the lack of activation in the choice-based analysis in the ACC/mPFC, regions that are part of the valuation network.
3.2.3. Brain regions modulated by RRD (GLM1)
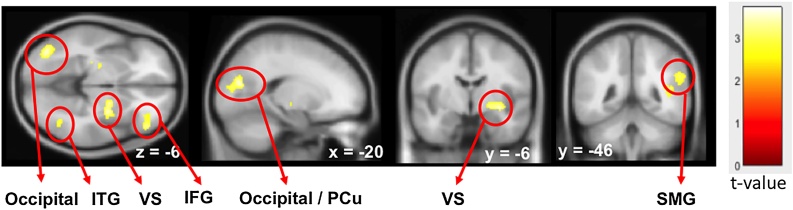
Comparison of parametric modulation of the BOLD signal for the Now and Not-Now trials by the RRD factor did not yield a significant difference across groups. For the combined groups, there was decreased activity in the VS, MFG and IFG, middle and superior occipital gyrus, superior and middle temporal gyrus and angular and supramarginal gyrus in the parietal lobe with larger RRD (Fig. 6 and Table 7). Only the occipital and temporal clusters overlap with immediacy related activity as shown in Fig. 4A, suggesting that most of the RRD related processes occurred in brain regions not signifying valuation processes.
Fig. 6.
Increasing negative modulation of BOLD activity by the Relative Reward Differences (RRD) of the group random-effects for all trials for combined (N=102) FH- and FH + groups (GLM1). Maps are threshold at p-unc<0.005 at the voxel level and cluster size threshold of 50. ITG, inferior temporal gyrus; VS, ventral striatum; IFG, inferior frontal gyrus; Pcu, precuneus; SMG, supramarginal gyrus.
Table 7.
Brain regions demonstrating negative parametric modulation of RRD for all trials for the combined groups (GLM1).
| Brain Regions | BA | Peak t-value | Cluster size | MNI Coordinates |
|||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| L | Occipital (IOG) Temporal (MTG, ITG) | 19, 37 | −3.71 | 239 | −38 | −78 | −4 |
| L | Occipital (SOG, MOG) Parietal (Pcu) | 17, 18, 31 | −3.62 | 277 | −24 | −74 | 16 |
| R | Temporal (STG, MTG), Parietal (SMG, Ang) | 22, 40 | −3.61 | 399 | 42 | −50 | 14 |
| R | VS (Put, Gl. Pallidus), Thalamus | −3.43 | 235 | 30 | −6 | −6 | |
| L | VS (Put, Gl. Pallidus), Thalamus | −3.39 | 133 | −24 | −20 | 4 | |
| R | Frontal (MFG, IFG) | 47, 11 | −3.2 | 155 | 48 | 38 | −6 |
| R | Temporal (ITG, MTG) | 37 | −3.1 | 61 | 38 | −60 | −2 |
| R | Insula, Putamen | 13 | −3 | 58 | −40 | 4 | 6 |
Punc < 0.005 and a cluster size > 50 voxels.
Abbreviations: L, left, R, right; ITG, inferior temporal gyrus; SOG, superior occipital gyrus; MOG, middle occipital gyrus; SMG, supramarginal gyrus; Ang, angular gyrus; VS, ventral striatum; Gl. Pallidus, globus pallidus; Put, putamen.
4. Discussion
This study investigated the susceptibility of adolescents with a family history of SUD to exhibit impulsive choices on a DD task. Our initial hypothesis was that the well-documented increased risk for developing SUD in adolescents with FH+ would be manifested as a behavioral preference for immediate rewards over LL rewards, and this would be reflected in their brain activity. The present findings support this behavioral hypothesis, as we found statistically significant differences in DD performance (both in % choice of SS and in RTs) between FH+ and FH- groups. However, the imaging hypothesis were not supported as there were no statistically significant differences in brain activity across FH+ and FH-.
4.1. Behavioral findings
In the DD task, FH+ adolescents (compared to FH-) were more sensitive to manipulation of immediacy, i.e., Now/Not-Now, resulting in FH+ subjects choosing significantly fewer LL (more SS) rewards in the Now trials compared to FH- subjects. Figner et al. (Figner et al., 2010) argued that choosing LL in the Now trials requires more self-control than choosing the LL in Not-Now trials, because Now trials offer more salient immediate rewards (McClure, Laibson, et al., 2004). This would suggest that the FH+ adolescents may have exerted less self-control than the FH- adolescents in the Now trials (Figner et al., 2010). Moreover, the FH+ adolescents showed increased LL choices for the Not-Now trials whereas the FH- adolescents showed the opposite, fewer LL choices for the Not-Now trials. A possible explanation is related to the impulsive nature of the FH+ group. The FH+ participants are more impulsive in the sense that they are present oriented, such that an immediate reward dominates their decisions. Once they cannot receive the reward immediately, as in the Now trials, all delays are more or less equal. Thus, in the Not-Now trials where all rewards are in the future, the SS option is not as attractive as SS option in the Now trials, and we see it as an increase in proportions of LL choices. On the other hand, one might expect that the FH- would make a similar proportion of LL choices in both the Now and Not-Now trials, but that was not the case. This may be related to the patient nature of the FH- group. The FH- participants are future oriented and were willing to wait for later rewards. As the delay options are salient for them, they are sensitive to the additional delay in the Not-Now trials. The Not-Now trials in practice have longer delay from today, maximum 6 weeks from today compared to 4 weeks from today in the Now trials. Therefore, the FH- participants may become less patient in the Not-Now trials and preferred an earlier reward. For the FH- group, the two trial types are not equivalent as the participants are more sensitive to variability in time delay than the FH + participants which are more sensitive to immediacy.
Fast motor response is one aspect of impulsive behavior (Verdejo-García et al., 2008), and in our data it is seen only for SS choices. Both groups showed longer RT for LL than for SS. However, FH+ adolescents were faster than FH- adolescents in choosing SS, suggesting that the FH- adolescents took time to assess their options, whereas the FH+ were more impulsive in making immediate choices. However, both groups showed the fastest responses in making SS choices in the Now-trials compared with the Not-Now trials. This suggests that overall Now trials generate impulsive responses even in the less impulsive adolescents.
Our data also demonstrate that as RRD increases all participants choose LL more often. As expected, and consistent with previous studies, participants were more likely to wait for the LL when the payoff for doing so was larger (Decker et al., 2015). However, the rate of increase of the LL choice as RRD increases is significantly less for the FH + than the FH- group, indicating an attenuated response to future rewards. Furthermore, for both groups, the rates of LL choices as a function of RRD are much smaller than those previously reported in adults. At the largest RRD our adolescents choose LL on 50% of the trials in comparison to adults who choose LL in 75%–80% of those trials (Decker et al., 2015; McClure, Laibson, et al., 2004). This could reflect steeper discounting in adolescents compared to adults, as observed by de Water and colleagues (de Water et al., 2014).
Together, these data suggest that the FH+ group is biased towards immediate rewards, even as the payoffs that come with waiting are significantly increased. In other words, the FH+ groups showed greater sensitivity to timing of immediately available rewards and less sensitivity to magnitude (and timing) of later rewards. It is tempting to speculate that these biases toward immediate reward represent the specific component of impulsivity that is, at least partially, responsible for the increased risk for SU initiation and progression towards SUD found in FH+ adolescents. Whereas in the DD task the FH+ group was more impulsive than the FH- group, the groups did not differ on the BIS-11 measures of impulsivity (attention, motor, and planning). Because the BIS-11 captures different aspects of impulsive behavior our data suggest that the DD task maybe more indicative of SUD risk than the BIS-11, at least in our sample groups, which were well matched in most variables other than family history of SUD. Our behavioral findings are in agreement with previous cross-sectional studies that reported increased impulsivity in FH+ offspring (Dougherty et al., 2014), for review see (Ivanov et al., 2008; Verdejo-García et al., 2008), as well as in adult FH+ offspring (VanderBroek et al., 2016).
4.2. Imaging findings
In this study, comparison of brain activity of the Now vs. Not-Now trials between FH+ and FH- groups, for either analysis, did not yield a cluster that survived statistical correction for multiple comparisons suggesting that there are no detectable differences between groups in brain processes during the DD task. Similar findings were reported in adolescents of alcohol use disorder parents (Bjork et al., 2008). As expected, examining the immediacy effect in the combined groups resulted in increased activation across a distributed network. Specifically, based on option presentation analysis (GLM1), stronger BOLD signal for the Now trials, compared to the Not-Now, was observed in the ACC/mPFC, Pcu, posterior cingulate, inferior and middle frontal gyri, and superior-frontal gyrus, temporal gyrus and occipital gyrus. Subthreshold activations were also observed in the VS and dorsal striatum (caudate, putamen) (SM). The mPFC, OFC, VS, and the PCC have been proposed as regions that code the subjective value of a reward (Carter, 2010; Hare, O'Doherty, Camerer, Schultz, & Rangel, 2008; Juechems et al., 2017; Kable and Glimcher, 2007; McClure et al., 2004a, 2004b; Tanaka et al., 2004), and the VS as associated with valuation of delayed rewards (Hare et al., 2014; Prévost et al., 2010). Activity in the PCC and neighboring Pcu have been implicated in encoding time intervals and valuation of delayed or probabilistic rewards (Clithero et al., 2009; Glimcher, 2010; Harrington et al., 2004; Kable and Glimcher, 2007; McClure, Laibson, et al., 2004). In addition, there was activation in the angular gyrus in the choice-based analysis (GLM2), probably representing the process of choice per se. Thus, our results are in agreement with previous studies of DD showing that these brain regions are engaged in reward processing and thus, more broadly, in decision making (Carter, 2010; Carter et al., 2010; Figner et al., 2010; Fröhner et al., 2019; Frost and McNaughton, 2017; Hare et al., 2009, 2008; Kable and Glimcher, 2007; McClure, York, et al., 2004). Furthermore, studies that compare the Now and Not-Now trials yielded activation in the mPFC, VS and PCC similar to our findings in the combined group analysis (Kable and Glimcher, 2010; Ludwig et al., 2015; McClure, Laibson, et al., 2004; Weber and Huettel, 2008). These regions showed greater activation in the Now compared with Not-Now trials. In contrast, the Pcu show greater deactivation for Not-Now compared to Now trials (Fig. 4B), similar to that reported by Ikink (Ikink et al., 2019).
The choice-based analysis (GLM2, Fig. 5) shows a similar activation to that of the option-based analysis (GLM1, Fig. 4) with the exception of frontal ACC/mPFC, and striatal regions, with no significant differences between groups. The question is then, why we do not see greater activation for the FH+ group, since behaviorally they made more SS choices during the Now trials compared with the FH- group? While there are no significant differences in brain activity between groups, in either the option-based or the choice-based analysis, some characteristics of the brain activation may reflect the small behavioral differences found between the groups. In particular, the unexpected increased brain activity during the Now trials (compared to the Not-Now) for the FH- group may point to their greater valuation of LL choices (See SM Figure S5). A closer look at the ACC activity we described for the combined groups indicated that it is mostly due to activity seen in the FH- group, as frontal activity in the FH+ alone did not survive the statistical correction (See SM Figure S5). Whereas the lack of differentiation in brain activity of the Now vs. Not-Now trials in the FH+ group may stem from the fact that they made fewer LL choices in the Now trials, or due to factors other than valuation affecting their choices, such as focus on timing of the reward. Further support of greater valuation of LL choices can be discerned from comparison of brain activity reported for GLM1 and GLM2. These two analyses differ in that the option-based analysis contrast included trials with LL choices, whereas in the choice-based analysis contrast, trials with LL choices were not included (as we compared SS choices between Now and Not-Now trials). Therefore, we can assume that any difference between these two analyses stemmed from the exclusion of trials with LL choices. Thus, the regions seen only in the option-based analysis probably reflect greater valuation processes during trials when LL were chosen. Because FH- participants were more likely to choose LL during the Now trials it resulted in more pronounced brain activity in the option-based analysis (GLM1) for the FH- compared to the FH+ group as shown in SM (Figure S5).
We also observed that the BOLD activity in Pcu, supramarginal gyrus, inferior frontal gyrus and VS for all trial types decreased with increases in RRD. Most of these areas can be viewed as part of the frontoparietal circuitry which has been proposed to signify a choice network. Indeed, these regions did not show BOLD activation during the valuation process as was demonstrated in the Now/Not-Now comparison. Our findings agree with a previous proposal separating two computational networks, a frontoparietal choice network that computes the relative subjective value in preparation for choice, and the frontostriatal valuation network (Kable and Glimcher, 2010). Change in BOLD activity in the choice network was inversely correlated with RRD, similar to that reported by Hare et al. (Hare et al., 2014), suggesting that this network does not compute choice difficulty based on monetary values alone but maybe based on other parameters. Lateral fronto-parietal regions have been proposed to carry out decision computations that involve contextual parameters of rewards, such as their temporal distribution, frequency, and level of abstraction (Christakou et al., 2009; McClure, Laibson, et al., 2004). Specifically, in this study, the monetary stimuli included decimal values (e,g, $22.30 vs. $33.50) which could have presented greater challenges in stimuli discrimination especially when the amounts were relatively close. This difficulty was seen as greater BOLD activation with decrease in RRD. Another possibility is that the larger BOLD signal with lower RRD is an artifact of a larger number of trials in the lower end (6 sampling intervals between 1%–20%) of the RRD, compared with the upper end (3 sampling intervals between 25%–50%).
Few neuroimaging studies investigating delay-discounting have been performed in adolescents. For example, mPFC and striatum were observed in healthy adolescents who performed a DD task (Schneider et al., 2014; Van Den Bos et al., 2015). Ripke and colleagues (Ripke et al., 2012) reported similar brain activation patterns in the reward and cognitive control areas in adolescents and adults. However, adolescent discounting was steeper, and they were less consistent in their choices than the adults. Moreover, the choice inconsistencies were associated with reduced parietal activation. Our FH- participants demonstrated brain activity that is in line with those reported in healthy adolescents.
To the best of our knowledge, only very few studies have included high-risk FH+ children prior to regular substance use (Ivanov et al., 2019; Qiao et al., 2015). For example, Ivanov and colleagues (Ivanov et al., 2018) used a flanker task to study 8−13-year-old children with attention-deficit hyperactivity disorder (ADHD) with and without FH+ and non-ADHD controls, and reported no performance differences between groups. However, a whole-brain analysis revealed significant differences in widely distributed networks related to cognitive control and reward processing. In our study, using a DD task, there were performance differences between the groups which were not associated with quantitative differences in brain activation.
Although behavioral responses were significantly different between the groups, the actual percent difference in choosing SS is small, reflecting subtle differences between groups that could not be detected in the BOLD signal. The fact that, at this developmental stage, the effect of FH+ is not prominent in the brain may suggest that early intervention could prevent neurobehavioral changes that could lead to SUD. We will have the opportunity to test whether there is a differentiation in brain signal between the groups in the follow up phase of the study (3 years after baseline). Critically, our study strength lies in the fact that the only major difference between the groups is the FH status. Many studies assessing risk of SUD among FH+ compared this group to a group that is FH- but from a very different demographic and environmental characteristics (income level, parental education, parental marital status, neighborhood, etc.) (Ryan et al., 2016; Tarter et al., 2003). Each of these variables, either alone or in combination, could contribute substantially to impulsive decisions in the DD task, as well as influence brain activity. The fact that we have isolated this one major variable to differentiate the groups allows us to more clearly attribute possible findings to this variable per se, a strength of this study. We can argue the FH status alone may not increase risk for SUD (both groups in our study may have similar increase risk because of their disadvantaged environment). Importantly, it is possible that FH status alone does not induce clear brain changes and that is the reason that we could not find differences in brain activity. Furthermore, our studies (under review) of diffusion tensor imaging (DTI) as well as anatomical MRI and resting state connectivity of this sample did not reveal major differences between the FH- and the FH+ groups either. To understand behavioral outcomes and potential brain changes associated with FH status, future studies should consider ways to reduce variability in the sample to further validate whether there is a significant effect of FH status alone on brain function.
4.2.1. Study limitations
The DD task we used was designed to compare Now vs. Not-Now trials while keeping participants engaged by using cognitively demanding reward and time options. This may have increased variability in performance strategy and in the associated brain functions. It is possible that greater variability in the fMRI signal reduces the power to detect between-subject effects (Elliott et al., 2020). However, this is probably not due to sample size because other studies with adults, using the exact same task, did find both behavioral and neural differences across groups using a much smaller sample (Decker et al., 2015; Pinto et al., 2014). However, it is possible that at this stage of development or in circumstances where the adolescents are not using or abusing substances, the neuronal differences, if they exist, are still so small that they are not as easily detectable as in the other studies where the participants psychopathology is well-defined. There are other sources of variation that could have made it hard to detect small changes in fMRI signal. In particular, the age range of 12–17 in the present study may be too wide and includes a developmental period that is known to demonstrate large brain changes that were similar across groups. It is possible that the brain activities associated with SUD risk among FH+ adolescents could not be detected due to the developmental variability intrinsic during this time period. Other variabilities stemmed from the fact that FH+ adolescents have varied parental SUD characteristics, such as SUD duration, onset, childhood exposure, one vs. two parents with SUD, and a variety of substances of abuse (Biederman et al., 2000). While some authors have suggested that the specific substance of abuse by parents is less important than the common risk factor among those who are FH+ (Tsuang et al., 1998), others have argued that familial risk for SUD is substance specific (Merikangas et al., 2009). Moreover, heterogeneity of susceptible brain systems among FH+ offspring would reduce the power in detecting differences between the FH groups. Critically, among the FH+ adolescents, not all will develop SUD. Indeed, Martz et al. (Martz et al., 2019) showed that FH+ offspring with reduced connectivity between the DLPFC and the PCC were more likely to initiate SU, as they have less cognitive control. In contrast, FH+/resilient offspring, who did not experiment with substances, showed stronger synchrony between brain regions associated with cognitive control.
There were additional limitations that may have contributed to the lack of significant differences in brain activation between the FH+ and FH- groups. First, the greater modulation of brain activation by the RRD in FH+ in comparison to FH-, as suggested by behavioral results, could be hard to detect due to lack of power resulting from the distribution of the RRD intervals. Specifically, based on the behavioral results, the brain activity modulation across groups was expected to increase with increased RRD. However, the behavioral results indicated that the greatest difference was in the large RRD (25%, 35% and 50%) which constituted only about 30 % of the trials. Second, although monetary delay discounting tasks have been extensively used (de Water et al., 2014; Figner et al., 2010; McClure, Laibson, et al., 2004; Solway et al., 2017), to the best of our knowledge, they have not been widely used with low SES minority adolescents, who might discount more steeply, either due to an immediate financial need or because of a lack of trust in obtaining later rewards (Lempert et al., 2018). We are, however, confident that the participants trusted that they would receive the payment from our research team since they have been followed as part of a longitudinal epidemiological study. Although, we cannot be sure about their immediate financial needs, this should not be a major concern as we expect it to affect adolescents in both groups equally. As we continue to follow our cohort, we will be able to separate the FH+ group into those who are more at risk (as SU initiation progresses) and those who have greater resilience.
5. Conclusion
The present study provides evidence that in a cohort of young adolescents from an inner-city minority community, family history of SUD predicts a tendency to choose immediate SS rewards over LL rewards in the DD task compared to FH-. This behavior is stronger when the SS rewards are available immediately. Moreover, FH+ participants were less likely to switch to LL rewards as the RRD increases. Brain activity supports the notion that subjects’ decision-making represents higher subjective value during the Now trials compared to the Not-Now trials. Overall, the brain processes involved in performing the DD task by this unique sample of adolescents is similar to those reported for typical adolescents and for adults. At this stage in adolescents’ development, our study could not identify brain regions as markers for the FH+ status or future SUD risk. A longitudinal follow up of this cohort is already underway and may be able to identify at what point in brain development, if any, the risk conveyed by FH+ status manifests in measurable functional brain differences.
Data statement
The raw data support the findings of this study will be available as per the data sharing agreement with National Institute of Drug Abuse.
Declaration of Competing Interest
The authors report no financial disclosures or conflict of interest.
Acknowledgments
This work was supported by NIDA grant R01-DA038154 to Christina Hoven, and Gender Supplement to Diana V. Rodriguez Moreno and Re-entry supplement to Yael M. Cycowicz.
Partial data was presented at the Annual Meeting of the Cognitive Neuroscience Society (CNS) 2018. Cycowicz, Y.M., Rodriguez Moreno, D.V., Amsel, L.V., Hill, C.A., Wang, Z., He X., Hoven, C. Family History of Substance Abuse Affects Adolescents’ Choices.
We are grateful to Kelsang C. Bista, Chase Hill, Nicole Garcia, Drucilla Lefevre, Michael Wang and Sharon Cuchacovich for data acquisition and analysis. Special thanks to Dr. George Musa for his support throughout this project. We would also thank all our family participants.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2021.100942.
Contributor Information
Diana V. Rodriguez-Moreno, Email: Diana.Moreno@nyspi.columbia.edu.
Yael M. Cycowicz, Email: yc60@cumc.columbia.edu.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Alegria M., Strathdee S.A., Pantin H. Substance risk, prevention treatments and the role of the environmental and cultural context in addressing latinos and other ethnic/racial populations. Drug Alcohol Depend. 2012;125S:S2–S3. doi: 10.1016/j.drugalcdep.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Barr D.J., Levy R., Scheepers C., Tily H.J. Random effects structure for confirmatory hypothesis testing: keep it maximal. J. Mem. Lang. 2013;68(3):255–278. doi: 10.1016/j.jml.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel W.K., Koffarnus M.N., Moody L., Wilson A.G. The behavioral-and neuro-economic process of temporal discounting: a candidate behavioral marker of addiction. Neuropharmacology. 2014;76:518–527. doi: 10.1016/j.neuropharm.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J., Faraone S.V., Monuteaux M.C., Feighner J.A. Patterns of alcohol and drug use in adolescents can be predicted by parental substance use disorders. Pediatrics. 2000;106(4):792–797. doi: 10.1542/peds.106.4.792. [DOI] [PubMed] [Google Scholar]
- Bjork J.M., Knutson B., Hommer D.W. Incentive-elicited striatal activation in adolescent children of alcoholics. Addiction. 2008;103(8):1308–1319. doi: 10.1111/j.1360-0443.2008.02250.x. [DOI] [PubMed] [Google Scholar]
- Bohman M. Some genetic aspects of alcoholism and criminality. A population of adoptees. Arch. Gen. Psychiatry. 1978;35(3):269–276. doi: 10.1001/archpsyc.1978.01770270019001. [DOI] [PubMed] [Google Scholar]
- Bürkner P.-C. Advanced Bayesian multilevel modeling with the R package brms. arXiv preprint arXiv. 2017;1705:11123. [Google Scholar]
- Carpenter B., Gelman A., Hoffman M., D, Lee D., Goodrich B., Betancourt M., Riddell A. Stan: A probabilistic programming language. J. Stat. Softw. 2017;76(1) doi: 10.18637/jss.v076.i01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter B.J. Evidence-based decision-making: practical issues in the appraisal of evidence to inform policy and practice. Aust. Health Rev. 2010;34(4):435–440. doi: 10.1071/AH09778. [DOI] [PubMed] [Google Scholar]
- Carter R.M., Meyer J.R., Huettel S.A. Functional neuroimaging of intertemporal choice models: a review. J. Neurosci. Psychol. Econ. 2010;3(1):27. [Google Scholar]
- Casey B.J., Jones R.M. Neurobiology of the adolescent brain and behavior: implications for substance use disorders. J. Am. Acad. Child Adolesc. Psychiatry. 2010;49(12):1189–1201. doi: 10.1016/j.jaac.2010.08.017. quiz 1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Getz S., Galvan A. The adolescent brain. Dev. Rev. 2008;28(1):62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers R.A., Taylor J.R., Potenza M.N. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am. J. Psychiatry. 2003;160(6):1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham A., Allen N.B., Whittle S., Simmons J., Yücel M., Lubman D.I. Orbitofrontal cortex volume and effortful control as prospective risk factors for substance use disorder in adolescence. Eur. Addict. Res. 2017;23(1):37–44. doi: 10.1159/000452159. [DOI] [PubMed] [Google Scholar]
- Christakou A., Brammer M., Giampietro V., Rubia K. Right ventromedial and dorsolateral prefrontal cortices mediate adaptive decisions under ambiguity by integrating choice utility and outcome evaluation. J. Neurosci. 2009;29(35):11020–11028. doi: 10.1523/JNEUROSCI.1279-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakou A., Brammer M., Rubia K. Maturation of limbic corticostriatal activation and connectivity associated with developmental changes in temporal discounting. Neuroimage. 2011;54(2):1344–1354. doi: 10.1016/j.neuroimage.2010.08.067. [DOI] [PubMed] [Google Scholar]
- Clithero J.A., Carter R.M., Huettel S.A. Local pattern classification differentiates processes of economic valuation. Neuroimage. 2009;45(4):1329–1338. doi: 10.1016/j.neuroimage.2008.12.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey S.F., Gudleski G.D., Saladin M.E., Brady K.T. Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Exp. Clin. Psychopharmacol. 2003;11(1):18. doi: 10.1037//1064-1297.11.1.18. [DOI] [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cox R.W., Reynolds R.C., Taylor P.A. AFNI and clustering: false positive rates redux. BioRxiv. 2016 doi: 10.1089/brain.2016.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley J.W., Everitt B.J., Robbins T.W. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69(4):680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- de Water E., Cillessen A.H.N., Scheres A. Distinct age‐related differences in temporal discounting and risk taking in adolescents and young adults. Child Dev. 2014;85(5):1881–1897. doi: 10.1111/cdev.12245. [DOI] [PubMed] [Google Scholar]
- de Water E., Mies G.W., Figner B., Yoncheva Y., van den Bos W., Castellanos F.X., Scheres A. Neural mechanisms of individual differences in temporal discounting of monetary and primary rewards in adolescents. Neuroimage. 2017;153:198–210. doi: 10.1016/j.neuroimage.2017.04.013. [DOI] [PubMed] [Google Scholar]
- Decker J.H., Figner B., Steinglass J.E. On weight and waiting: delay discounting in anorexia nervosa pretreatment and posttreatment. Biol. Psychiatry. 2015;78(9):606–614. doi: 10.1016/j.biopsych.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty D.M., Charles N.E., Mathias C.W., Ryan S.R., Olvera R.L., Liang Y., Acheson A. Delay discounting differentiates pre-adolescents at high and low risk for substance use disorders based on family history. Drug Alcohol Depend. 2014;143:105–111. doi: 10.1016/j.drugalcdep.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott M.L., Knodt A.R., Ireland D., Morris M.L., Poulton R., Ramrakha S., Hariri A.R. What Is the Test-Retest Reliability of Common Task-Functional MRI Measures? New Empirical Evidence and a Meta-Analysis. Psychol. Sci. 2020;31(7):792–806. doi: 10.1177/0956797620916786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein Ewing S.W., Wray A.M., Mead H.K., Adams S.K. Two approaches to tailoring treatment for cultural minority adolescents. J. Subst. Abuse Treat. 2012;43(2):190–203. doi: 10.1016/j.jsat.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figner B., Knoch D., Johnson E.J., Krosch A.R., Lisanby S.H., Fehr E., Weber E.U. Lateral prefrontal cortex and self-control in intertemporal choice. Nat. Neurosci. 2010;13(5):538–539. doi: 10.1038/nn.2516. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Holmes A.P., Worsley K.J., Poline J.B., Frith C.D., Frackowiak R.S. Statistical parametric maps in functional imaging: a general linear approach. Hum. Brain Mapp. 1995;2:189–210. [Google Scholar]
- Fröhner J.H., Teckentrup V., Smolka M.N., Kroemer N.B. Addressing the reliability fallacy in fMRI: similar group effects may arise from unreliable individual effects. Neuroimage. 2019;195:174–189. doi: 10.1016/j.neuroimage.2019.03.053. [DOI] [PubMed] [Google Scholar]
- Frost R., McNaughton N. The neural basis of delay discounting: a review and preliminary model. Neurosci. Biobehav. Rev. 2017;79:48–65. doi: 10.1016/j.neubiorev.2017.04.022. [DOI] [PubMed] [Google Scholar]
- Galea S., Ahern J., Vlahov D., Coffin P.O., Fuller C., Leon A.C., Tardiff K. Income distribution and risk of fatal drug overdose in New York City neighborhoods. Drug Alcohol Depend. 2003;70(2):139–148. doi: 10.1016/s0376-8716(02)00342-3. [DOI] [PubMed] [Google Scholar]
- Gladwin T.E., Figner B., Crone E.A., Wiers R.W. Addiction, adolescence, and the integration of control and motivation. Dev. Cogn. Neurosci. 2011;1(4):364–376. doi: 10.1016/j.dcn.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glimcher P.W. Neuroeconomics. Elsevier; 2009. Choice: towards a standard back-pocket model; pp. 503–521. [Google Scholar]
- Glimcher P. Oxford University Press; 2010. Foundations of Neuroeconomic Analysis. [Google Scholar]
- Goldstein R.Z., Volkow N.D. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat. Rev. Neurosci. 2011;12(11):652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin D.W., Schulsinger F., Hermansen L., Guze S.B., Winokur G. Alcohol problems in adoptees raised apart from alcoholic biological parents. Arch. Gen. Psychiatry. 1973;28(2):238–243. doi: 10.1001/archpsyc.1973.01750320068011. [DOI] [PubMed] [Google Scholar]
- Habeych M.E., Folan M.M., Luna B., Tarter R.E. Impaired oculomotor response inhibition in children of alcoholics: the role of attention deficit hyperactivity disorder. Drug Alcohol Depend. 2006;82(1):11–17. doi: 10.1016/j.drugalcdep.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Hardin M.G., Ernst M. Functional brain imaging of development-related risk and vulnerability for substance use in adolescents. J. Addict. Med. 2009;3(2):47. doi: 10.1097/ADM.0b013e31819ca788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare T.A., O’Doherty J., Camerer C.F., Schultz W., Rangel A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. J. Neurosci. 2008;28(22):5623–5630. doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare T.A., Camerer C.F., Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324(5927):646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Hare T.A., Hakimi S., Rangel A. Activity in dlPFC and its effective connectivity to vmPFC are associated with temporal discounting. Front. Neurosci. 2014;8:50. doi: 10.3389/fnins.2014.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington D.L., Boyd L.A., Mayer A.R., Sheltraw D.M., Lee R.R., Huang M., Rao S.M. Neural representation of interval encoding and decision making. Cogn. Brain Res. 2004;21(2):193–205. doi: 10.1016/j.cogbrainres.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Heil S.H., Johnson M.W., Higgins S.T., Bickel W.K. Delay discounting in currently using and currently abstinent cocaine-dependent outpatients and non-drug-using matched controls. Addict. Behav. 2006;31(7):1290–1294. doi: 10.1016/j.addbeh.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Heitzeg M.M., Nigg J.T., Yau W.Y.W., Zucker R.A., Zubieta J.K. Striatal dysfunction marks preexisting risk and medial prefrontal dysfunction is related to problem drinking in children of alcoholics. Biol. Psychiatry. 2010;68(3):287–295. doi: 10.1016/j.biopsych.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R., Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J. Neurosci. 2004;24(49):11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks B.M., Johnson W., Durbin C.E., Blonigen D.M., Iacomo W.G., McGue M. Delineating selection and mediation effects among childhood personality and environmental risk factors in the development of adolescent substance abuse. J. Abnormal Child Psychol. 2014;42:845–859. doi: 10.1007/s10802-013-9831-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill S.Y. Neural plasticity, human genetics, and risk for alcohol dependence. Funct. Plast. Genet. Var. Insights Neurobiol. Alcohol. 2010;91:53–94. doi: 10.1016/S0074-7742(10)91003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill S., Y, Wang S., Kostelnik B., Carter H., Holmes B., McDermott M., Keshavan M.S. Disruption of orbitofrontal cortex laterality in offspring from multiplex alcohol dependence families. Biol. Psychiatry. 2009;65(2):129–136. doi: 10.1016/j.biopsych.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill S.Y., Wang S.H., Carter H., Tessner K., Holmes B., McDermott M., Stiffler S. Cerebellum volume in high-risk offspring from multiplex alcohol dependence families: Association with allelic variation in GABRA2 and BDNF. Psychiatry Res.Neuroimaging. 2011;194(3):304–313. doi: 10.1016/j.pscychresns.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman W.F., Moore M., Templin R., McFarland B., Hitzemann R.J., Mitchell S.H. Neuropsychological function and delay discounting in methamphetamine-dependent individuals. Psychopharmacology. 2006;188(2):162–170. doi: 10.1007/s00213-006-0494-0. [DOI] [PubMed] [Google Scholar]
- Hummel A., Shelton K.H., Heron J., Moore L., van den Bree M.B.M. A systematic review of the relationships between family functioning, pubertal timing and adolescent substance use. Addiction. 2013;108(3):487–496. doi: 10.1111/add.12055. [DOI] [PubMed] [Google Scholar]
- Ikink I., Engelmann J.B., van den Bos W., Roelofs K., Figner B. Time ambiguity during intertemporal decision-making is aversive, impacting choice and neural value coding. Neuroimage. 2019;185:236–244. doi: 10.1016/j.neuroimage.2018.10.008. [DOI] [PubMed] [Google Scholar]
- Ivanov I., Schulz K.P., London E.D., Newcorn J.H. Inhibitory control deficits in childhood and risk for substance use disorders: a review. Am. J. Drug Alcohol Abuse. 2008;34(3):239–258. doi: 10.1080/00952990802013334. [DOI] [PubMed] [Google Scholar]
- Ivanov I., Flory J., Newcorn J.H., Halperin J.M. Childhood serotonergic function and early adult outcomes in youth with ADHD: a 15-year follow-up study. Eur. Neuropsychopharmacol. 2018;28(12):1429–1438. doi: 10.1016/j.euroneuro.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov I., Schulz K., Li X., Newcorn J. Reward processing in drug-naive youth with various levels of risk for substance use disorders: a pilot study. J. Child Adolesc. Psychopharmacol. 2019;29(7):516–525. doi: 10.1089/cap.2018.0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jachimowicz J.M., Chafik S., Munrat S., Prabhu J.C., Weber E.U. Community trust reduces myopic decisions of low-income individuals. Proc. Natl. Acad. Sci. 2017;114(21):5401–5406. doi: 10.1073/pnas.1617395114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson P., Yule A., Wilens T. The MassGeneral Hospital for Children Adolescent Medicine Handbook. Springer; 2016. Adolescent substance use and prevention; pp. 259–281. [Google Scholar]
- Juechems K., Balaguer J., Ruz M., Summerfield C. Ventromedial prefrontal cortex encodes a latent estimate of cumulative reward. Neuron. 2017;93(3):705–714. doi: 10.1016/j.neuron.2016.12.038. [DOI] [PubMed] [Google Scholar]
- Kable J.W., Glimcher P.W. The neural correlates of subjective value during intertemporal choice. Nat. Neurosci. 2007;10(12):1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable J.W., Glimcher P.W. An “as soon as possible” effect in human intertemporal decision making: behavioral evidence and neural mechanisms. J. Neurophysiol. 2010;103(5):2513–2531. doi: 10.1152/jn.00177.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J.N., Ross T.J., Stein E.A., Garavan H. Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. J. Neurosci. 2003;23(21):7839–7843. doi: 10.1523/JNEUROSCI.23-21-07839.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler K.S., Schmitt E., Aggen S.H., Prescott C.A. Genetic and environmental influences on alcohol, caffeine, cannabis, and nicotine use from early adolescence to middle adulthood. Arch. Gen. Psychiatry. 2008;65(6):674–682. doi: 10.1001/archpsyc.65.6.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby K.N., Petry N.M. Heroin and cocaine abusers have higher discount rates for delayed rewards than alcoholics or non‐drug‐using controls. Addiction. 2004;99(4):461–471. doi: 10.1111/j.1360-0443.2003.00669.x. [DOI] [PubMed] [Google Scholar]
- Kollins S.H. Delay discounting is associated with substance use in college students. Addict. Behav. 2003;28(6):1167–1173. doi: 10.1016/s0306-4603(02)00220-4. [DOI] [PubMed] [Google Scholar]
- Lempert K.M., Steinglass J.E., Pinto A., Kable J.W., Simpson H.B. Can delay discounting deliver on the promise of RDoC? Psychol. Med. 2018;49(2):190–199. doi: 10.1017/S0033291718001770. [DOI] [PubMed] [Google Scholar]
- Liu X., Hairston J., Schrier M., Fan J. Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neurosci. Biobehav. Rev. 2011;35(5):1219–1236. doi: 10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein G.F. Frames of mind in intertemporal choice. Manage. Sci. 1988;34(2):200–214. [Google Scholar]
- Ludwig V.U., Nüsser C., Goschke T., Wittfoth-Schardt D., Wiers C.E., Erk S., Walter H. Delay discounting without decision-making: medial prefrontal cortex and amygdala activations reflect immediacy processing and correlate with impulsivity and anxious-depressive traits. Front. Behav. Neurosci. 2015;9:280. doi: 10.3389/fnbeh.2015.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luijten M., Schellekens A.F., Kühn S., Machielse M.W.J., Sescousse G. Disruption of reward processing in addiction: an image-based meta-analysis of functional magnetic resonance imaging studies. JAMA Psychiatry. 2017;74(4):387–398. doi: 10.1001/jamapsychiatry.2016.3084. [DOI] [PubMed] [Google Scholar]
- Martin E. Contributions of cognitive neuroscience to clinical neuropsychology: the role of reaction time-based studies. Neuropsychology, development, and cognition. Section A. J. Clin. Exp. Neuropsychol. 2002;24(7) [Google Scholar]
- Martin C.S., Earleywine M., Blackson T.C., Vanyukov M.M., Moss H.B., Tarter R.E. Aggressivity, inattention, hyperactivity, and impulsivity in boys at high and low risk for substance abuse. J. Abnorm. Child Psychol. 1994;22(2):177–203. doi: 10.1007/BF02167899. [DOI] [PubMed] [Google Scholar]
- Martin E.M., Pitrak D.L., Weddington W., Rains N.A., Nunnally G., Nixon H., Bechara A. Cognitive impulsivity and HIV serostatus in substance dependent males. J. Int. Neuropsychol. Soc. 2004;10(7):931–938. doi: 10.1017/s1355617704107054. [DOI] [PubMed] [Google Scholar]
- Martz M.E., Cope L.M., Hardee J.E., Brislin S.J., Weigard A., Zucker R.A., Heitzeg M.M. Frontostriatal resting state functional connectivity in resilient and non-resilient adolescents with a family history of alcohol use disorder. J. Child Adolesc. Psychopharmacol. 2019;29(7):508–515. doi: 10.1089/cap.2018.0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten A.S., Faden V.B., Zucker R.A., Spear L.P. Underage drinking: a developmental framework. Pediatrics. 2008;121(Suppl 4):S235–251. doi: 10.1542/peds.2007-2243A. [DOI] [PubMed] [Google Scholar]
- McClure S.M., Bickel W.K. A dual-systems perspective on addiction: contributions from neuroimaging and cognitive training. Ann. N. Y. Acad. Sci. 2014;1327:62. doi: 10.1111/nyas.12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure S.M., Laibson D.I., Loewenstein G., Cohen J.D. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306(5695):503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- McClure S.M., York M.K., Montague P.R. The neural substrates of reward processing in humans: the modern role of FMRI. Neuroscientist. 2004;10(3):260–268. doi: 10.1177/1073858404263526. [DOI] [PubMed] [Google Scholar]
- Merikangas K.R., Li J.J., Stipelman B., Yu K., Fucito L., Swendsen J., Zhang H. The familial aggregation of cannabis use disorders. Addiction. 2009;104(4):622–629. doi: 10.1111/j.1360-0443.2008.02468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller F.G., Dougherty D.M. Impulsivity and substance abuse: what is the connection? Addict. Disord. Their Treat. 2002;1(1):3–10. [Google Scholar]
- Monterosso J., Ehrman R., Napier K.L., O’Brien C.P., Childress A.R. Three decision‐making tasks in cocaine‐dependent patients: Do they measure the same construct? Addiction. 2001;96(12):1825–1837. doi: 10.1046/j.1360-0443.2001.9612182512.x. [DOI] [PubMed] [Google Scholar]
- Patton J.H., Stanford M.S., Barratt E.S. Factor structure of the Barratt impulsiveness scale. J. Clin. Psychol. 1995;51(6):768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Pinto A., Steinglass J.E., Greene A.L., Weber E.U., Simpson H.B. Capacity to delay reward differentiates obsessive-compulsive disorder and obsessive-compulsive personality disorder. Biol. Psychiatry. 2014;75(8):653–659. doi: 10.1016/j.biopsych.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prévost C., Pessiglione M., Météreau E., Cléry-Melin M.-L., Dreher J.-C. Separate valuation subsystems for delay and effort decision costs. J. Neurosci. 2010;30(42):14080–14090. doi: 10.1523/JNEUROSCI.2752-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao J., Wang Z., Geronazzo-Alman L., Amsel L., Duarte C., Lee S., Doan T. Brain activity classifies adolescents with and without a familial history of substance use disorders. Front. Hum. Neurosci. 2015;9:219. doi: 10.3389/fnhum.2015.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . R Foundation for Statistical Computing.; Vienna, Austria: 2019. R: A Language and Environment for Statistical Computing (Version 3.5. 2, R Foundation for Statistical Computing, Vienna, Austria, 2018) [Google Scholar]
- Respress B.N., Small E., Francis S.A., Cordova D. The role of perceived peer prejudice and teacher discrimination on adolescent substance use: a social determinants approach. J. Ethn. Subst. Abuse. 2013;12(4):279–299. doi: 10.1080/15332640.2013.836728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B., Karraker K., Horn K., Richards J.B. Delay and probability discounting as related to different stages of adolescent smoking and non-smoking. Behav. Processes. 2003;64(3):333–344. doi: 10.1016/s0376-6357(03)00168-2. [DOI] [PubMed] [Google Scholar]
- Ripke S., Hübner T., Mennigen E., Müller K.U., Rodehacke S., Schmidt D., Smolka M.N. Reward processing and intertemporal decision making in adults and adolescents: the role of impulsivity and decision consistency. Brain Res. 2012;1478:36–47. doi: 10.1016/j.brainres.2012.08.034. [DOI] [PubMed] [Google Scholar]
- obinson T.E., Berridge K.C. Review. The incentive sensitization theory of addiction: some current issues. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 2008;363(1507):3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph A., Song M., Brook M.N., Milne R.L., Mavaddat N., Michailidou K., Wilcox A.N. Joint associations of a polygenic risk score and environmental risk factors for breast cancer in the Breast Cancer Association Consortium. Int. J. Epidemiol. 2018;47(2):526–536. doi: 10.1093/ije/dyx242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan S.R., Acheson A., Charles N.E., Lake S.L., Hernandez D.L., Mathias C.W., Dougherty D.M. Clinical and social/environmental characteristics in a community sample of children with and without family histories of substance use disorder in the San Antonio area: a descriptive study. J. Child Adolesc. Subst. Abuse. 2016;25(4):327–339. doi: 10.1080/1067828X.2014.999202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S., Peters J., Peth J.M., Büchel C. Parental inconsistency, impulsive choice and neural value representations in healthy adolescents. Transl. Psychiatry. 2014;4(4):e382. doi: 10.1038/tp.2014.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoal G.D., Giancola P.R. Cognition, negative affectivity and substance use in adolescent boys with and without a family history of a substance use disorder. J. Stud. Alcohol. 2001;62(5):675–686. doi: 10.15288/jsa.2001.62.675. [DOI] [PubMed] [Google Scholar]
- Solway A., Lohrenz T., Montague P.R. Simulating future value in intertemporal choice. Sci. Rep. 2017;7:43119. doi: 10.1038/srep43119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L.H., Hare T., Casey B.J. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. J. Cogn. Neurosci. 2010 doi: 10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger C.D., Gorsuch R.L., Lushene R., Vagg P.R., Jacobs G.A. Consulting Psychologists Press; Palo Alto, CA: 1983. Manual for the State-Trait Anxiety Inventory. [Google Scholar]
- Steinbeis N., Haushofer J., Fehr E., Singer T. Development of behavioral control and associated vmPFC–DLPFC connectivity explains children’s increased resistance to temptation in intertemporal choice. Cereb. Cortex. 2016;26(1):32–42. doi: 10.1093/cercor/bhu167. [DOI] [PubMed] [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Dev. Rev. 2008;28(1):78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L., Graham S., O’brien L., Woolard J., Cauffman E., Banich M. Age differences in future orientation and delay discounting. Child Dev. 2009;80(1):28–44. doi: 10.1111/j.1467-8624.2008.01244.x. [DOI] [PubMed] [Google Scholar]
- Ste-Marie C., Gupta R., Derevensky J.L. Anxiety and social stress related to adolescent gambling behavior and substance use. J. Child Adolesc. Subst. Abuse. 2006;15(4):55–74. [Google Scholar]
- Tanaka S.C., Doya K., Okada G., Ueda K., Okamoto Y., Yamawaki S. Behavioral Economics of Preferences, Choices, and Happiness. Springer; 2004. Prediction of immediate and future rewards differentially recruits cortico-basal ganglia loops; pp. 593–616. [DOI] [PubMed] [Google Scholar]
- Tarter R.E., Kirisci L., Mezzich A., Cornelius J.R., Pajer K., Vanyukov M., Clark D. Neurobehavioral disinhibition in childhood predicts early age at onset of substance use disorder. Am. J. Psychiatry. 2003;160(6):1078–1085. doi: 10.1176/appi.ajp.160.6.1078. [DOI] [PubMed] [Google Scholar]
- Tervo-Clemmens B., Quach A., Calabro F.J., Foran W., Luna B. Meta-analysis and review of functional neuroimaging differences underlying adolescent vulnerability to substance use. Neuroimage. 2020;209 doi: 10.1016/j.neuroimage.2019.116476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessner K.D., Hill S.Y. Neural circuitry associated with risk for alcohol use disorders. Neuropsychol. Rev. 2010;20(1):1–20. doi: 10.1007/s11065-009-9111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler A.L., Maldonado-Molina M.M., Staras S.A.S., O’Mara R.J., Livingston M.D., Komro K.A. Perceived racial/ethnic discrimination, problem behaviors, and mental health among minority urban youth. Ethn. Health. 2012;18(4):337–349. doi: 10.1080/13557858.2012.730609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomko R.L., Bountress K.E., Gray K.M. Personalizing substance use treatment based on pre-treatment impulsivity and sensation seeking: a review. Drug Alcohol Depend. 2016;167:1–7. doi: 10.1016/j.drugalcdep.2016.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuang M.T., Lyons M.J., Meyer J.M., Doyle T., Eisen S.A., Goldberg J., Eaves L. Co-occurrence of abuse of different drugs in men: the role of drug-specific and shared vulnerabilities. Arch. Gen. Psychiatry. 1998;55(11):967–972. doi: 10.1001/archpsyc.55.11.967. [DOI] [PubMed] [Google Scholar]
- Van Den Bos W., Rodriguez C.A., Schweitzer J.B., McClure S.M. Adolescent impatience decreases with increased frontostriatal connectivity. Proc. Natl. Acad. Sci. 2015;112(29):E3765–E3774. doi: 10.1073/pnas.1423095112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderBroek L., Acker J., Palmer A.A., de Wit H., MacKillop J. Interrelationships among parental family history of substance misuse, delay discounting, and personal substance use. Psychopharmacology. 2016;233(1):39–48. doi: 10.1007/s00213-015-4074-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega W.A., Zimmerman R.S., Warheit G.J., Apospori E., Gil A.G. Risk factors for early adolescent drug use in four ethnic and racial groups. Am. J. Public Health. 1993;83(2):185–189. doi: 10.2105/ajph.83.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-García A., Lawrence A.J., Clark L. Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci. Biobehav. Rev. 2008;32(4):777–810. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Volkow N.D., Fowler J.S. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb. Cortex. 2000;10(3):318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Volkow N.D., Wang G.-J., Fowler J.S., Tomasi D. Addiction circuitry in the human brain. Annu. Rev. Pharmacol. Toxicol. 2012;52:321–336. doi: 10.1146/annurev-pharmtox-010611-134625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Diemen L., Bassani D.G., Fuchs S.C., Szobot C.M., Pechansky F. Impulsivity, age of first alcohol use and substance use disorders among male adolescents: a population based case–control study. Addiction. 2008;103(7):1198–1205. doi: 10.1111/j.1360-0443.2008.02223.x. [DOI] [PubMed] [Google Scholar]
- Weber B.J., Huettel S.A. The neural substrates of probabilistic and intertemporal decision making. Brain Res. 2008;1234:104–115. doi: 10.1016/j.brainres.2008.07.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber E.U., Johnson E.J., Milch K.F., Chang H., Brodscholl J.C., Goldstein D.G. Asymmetric discounting in intertemporal choice: a query-theory account. Psychol. Sci. 2007;18(6):516–523. doi: 10.1111/j.1467-9280.2007.01932.x. [DOI] [PubMed] [Google Scholar]
- Wesley M.J., Bickel W.K. Remember the future II: meta-analyses and functional overlap of working memory and delay discounting. Biol. Psychiatry. 2014;75(6):435–448. doi: 10.1016/j.biopsych.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill R.R., Squeglia L.M., Yang T.T., Tapert S.F. A longitudinal examination of adolescent response inhibition: neural differences before and after the initiation of heavy drinking. Psychopharmacology (Berl.) 2013 doi: 10.1007/s00213-013-3198-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson N., Syme S.L., Boyce W.T., Battistich V.A., Selvin S. Adolescent alcohol, tobacco, and marijuana use: the influence of neighborhood disorder and hope. Am. J. Health Promot. 2005;20(1):11–19. doi: 10.4278/0890-1171-20.1.11. [DOI] [PubMed] [Google Scholar]
- Wong M.M., Nigg J.T., Zucker R.A., Puttler L.I., Fitzgerald H.E., Jester J.M., Adams K. Behavioral control and resiliency in the onset of alcohol and illicit drug use: a prospective study from preschool to adolescence. Child Dev. 2006;77(4):1016–1033. doi: 10.1111/j.1467-8624.2006.00916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P., Hoven C.W., Okezie N., Fuller C.J., Cohen P. Alcohol abuse and depression in children and adolescents. J. Child Adolesc. Subst. Abuse. 2007;17(2):51–69. [Google Scholar]
- Wu P., Hoven C.W., Liu X.H., Fuller C.J., Fan B., Musa G., Cook J.A. The relationship between depressive symptom levels and subsequent increases in substance use among youth with severe emotional disturbance. J. Stud. Alcohol Drugs. 2008;69(4):520–527. doi: 10.15288/jsad.2008.69.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C., McClellan J. Genetics of substance use disorders. Child Adolesc. Psychiatr. Clin. N. Am. 2016;25(3):377–385. doi: 10.1016/j.chc.2016.02.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.