Highlights
-
•
Skull Base Metastases need multidisciplinary treatment.
-
•
Surgical decompression has a decisive role in relieving neurological symptoms and improving Quality of Life.
-
•
The virtual planning step consists of a phantom-base procedure with the aid of Stealth Navigation.
-
•
New generations of custom-made PolyMethyl MethAcrylate (PMMA) cranioplasty allow a quick access to complementary therapies.
-
•
Stealth navigation during the surgical step allows a precise tumor resection and drives to an accurate cranial reconstruction.
Abbreviations: CT, computer tomography; MRI, magnetic resonance imaging; SBM, skull base metastasis; PMMA, PolyMethyl Methacrylate; ChT, chemotherapy; ChT, radiosurgery (RS)
Keywords: Skull brain metastasis, Custom-made implants, Cranio-orbital reconstruction, Single-step surgery, Case report
Abstract
Introduction and importance
Brain metastasis involving the skull base is a rare complication of malignant tumors. Besides radiotherapy, surgical treatment is a therapeutical option even though it may apply complex technical procedures that may delay complementary therapies. However, in recent days, the innovation of custom-made implants allows treating selected patients with fewer complications and better results.
Case presentation
We describe a single case of a complex fronto-orbital skull base metastasis requiring skull bone reconstruction that we treated with a single-step surgery and custom-made implant. Our procedure consists of two steps: in the first one, we perform a “virtual” craniotomy on a 3D phantom model previously built on a high-resolution bone CT scan. In the second step, the actual surgical procedure, the patient undergoes the resection and reconstruction of the cranial defect with an implant of PMMA custom-made cranioplasty. A three-month clinical and radiological follow-up is reported, which documented the extent of resection and good aesthetic results.
Clinical discussion
In our case, we performed a skull reconstruction of cranio-orbital region with macroscopic gross-total resection of the tumor. Complementary radiotherapy was obtained after one month. Three-month follow-up showed good esthetic results and progression-free disease. A recent review of the literature is provided to discuss different reconstruction techniques.
Conclusion
“Single-Step” resection and custom-made reconstruction is a relatively new technique that could be helpful not only for benign tumors, which remains its main application but also in selected cases of malignant tumors when immediate reconstruction and faster complementary treatments are needed.
1. Introduction
Skull base metastasis (SBM) are rare tumors that comprise about 4% of all malignant neoplasms [1]. Primary cancers comprise breast (20–30%), prostate (14–38,5%), lymphatic system (8%), lung (6%) and others (27%) [2]. A recent literature review shows that the main treatment options for brain metastasis are surgery, radiotherapy, stereotactic radiosurgery, and chemotherapy [3]. Instead, SBM treatment is relatively less known for its low incidence and scarcely published papers, mainly consisting of single case reports and series with a low number of cases [4,5]. The treatment of malignant lesions involving the cranio-orbital region is particularly complex due to the susceptible anatomical structures, which require an accurate bone reconstruction after surgical resection [6,7]. These techniques provide two methods: the direct implant of bone graft or plastic material in a single procedure or a two-step surgery (resection followed by a custom-made cranioplasty reconstruction). While the former may result in a poor cosmetic outcome, the latter involves a more significant amount of time due to the double procedure with an increased risk of infective and anesthetic complications. We propose an alternative technique to perform excision and optimal bone reconstruction in a single-step surgical procedure. Such an approach is already known for lesions involving the skull base and orbital region. Due to time-consuming and elevated costs [8], this technique was mainly used for benign lesions but recently, new technologies, cheaper materials, and shorter realization times have allowed an increasing utilization of this procedure [9,10]. It consists of two phases: in the first phase, we simulate a cranial resection on a 3D Phantom-model of the patient’s skull (build on a previous CT scan) with the Stealth Navigation guide’s aid. Afterward, the phantom undergoes a further CT scan to create a custom-made cranioplasty. In the “surgical” phase, we perform a stealth-guided craniotomy aiming at a "single-step" accurate resection and reconstruction with custom-made PolyMethyl MethAcrylate (PMMA) cranioplasty. This study was reported according to the 2020 SCARE Guidelines [11].
2. Case presentation
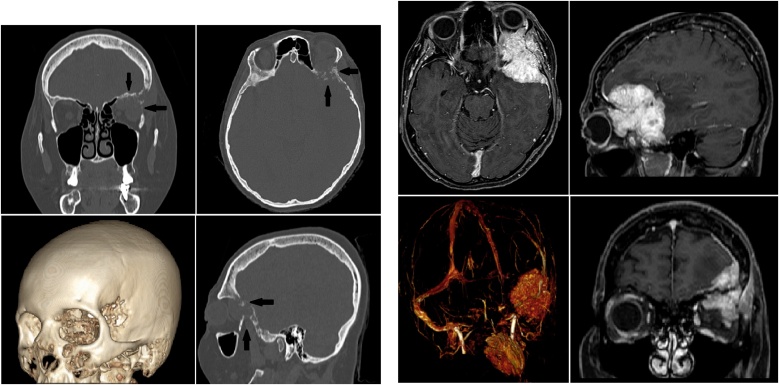
A 48-year-old woman presented with frontal headache, exophthalmos, diplopia, and left ptosis. CT and MRI scans (Figs. 1 and 2) showed a unique frontal skull base lesion infiltrating the left orbital region. The patient was recently operated for breast cancer (T3N1M0) with negative human epidermal growth factor receptor type 2 (HER2) and was on Radiotherapy, and Chemiotherapy treatment cycles (Doxorubicin 60 mg/m2 IV). No adverse reactions or allergies were reported. Family history was negative for oncological diseases, and BRCA1/BRCA2 were negatives. Total-Boby CT scan did not show further metastatic localizations. After a multidisciplinary briefing with consideration of the staging disease, Karnofski Performance Grade (KPS), and survival prognosis (more than 12 months based on oncological consultation board), we decided to submit the patient to a single-step craniotomy resection and reconstruction with PMMA custom-made cranioplasty. The surgical procedure consisted of a single-step procedure with resection of the malignant lesion and reconstruction of the bone defect with custom-made PMMA cranioplasty (APTIVA-BIOCAD Factory). This procedure's program was organized in two different steps: "simulation plan" and "surgical phase."
Figs. 1 and 2.
Pre-operative CT and MRI scans of skull base lesion infiltrating the left fronto-orbital region. In Fig. 1, black arrows show the sign of bone erosion.
2.1. Simulation plan
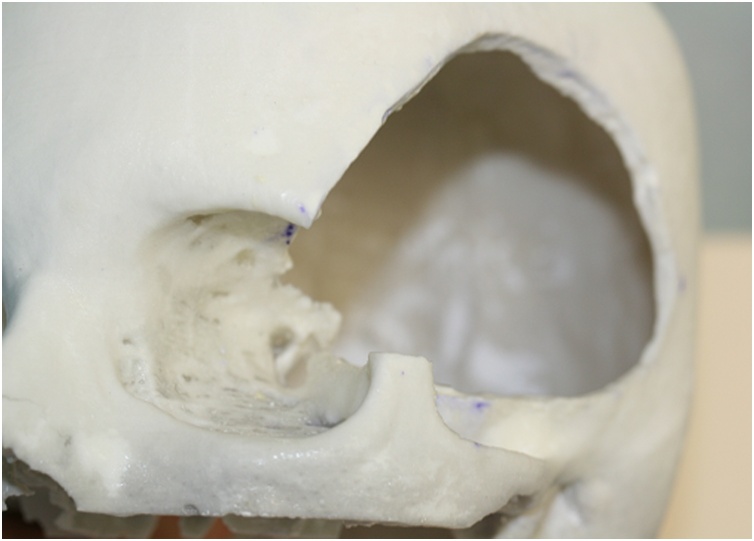
Based on DICOM CT High Resolution (HR) scan, a 3D Phantom model in plaster material was build according to factory guidelines. A simulation craniotomy with stealth-neuronavigation (S7 Medtronic) guide merging CT with MRI scans was performed on the phantom (Fig. 4). Afterward, a phantom CT scan comprising fronto-orbital craniotomy was obtained. Therefore, phantom CT scans in DICOM format were sent to the factory to build a PMMA cranioplasty based on the simulation model. Before obtaining the final model, we modified the digital phantom model according to the suggestion and collaboration with the engineering team to refine and optimize the implant precision. Within ten days, the gamma rays sterilized PMMA cranioplasty was built and sent in double copy to our department.
Fig. 4.
“Virtual Step”: Stealth-neuronavigation guided simulated craniotomy performed on the phantom.
2.2. Surgical procedure
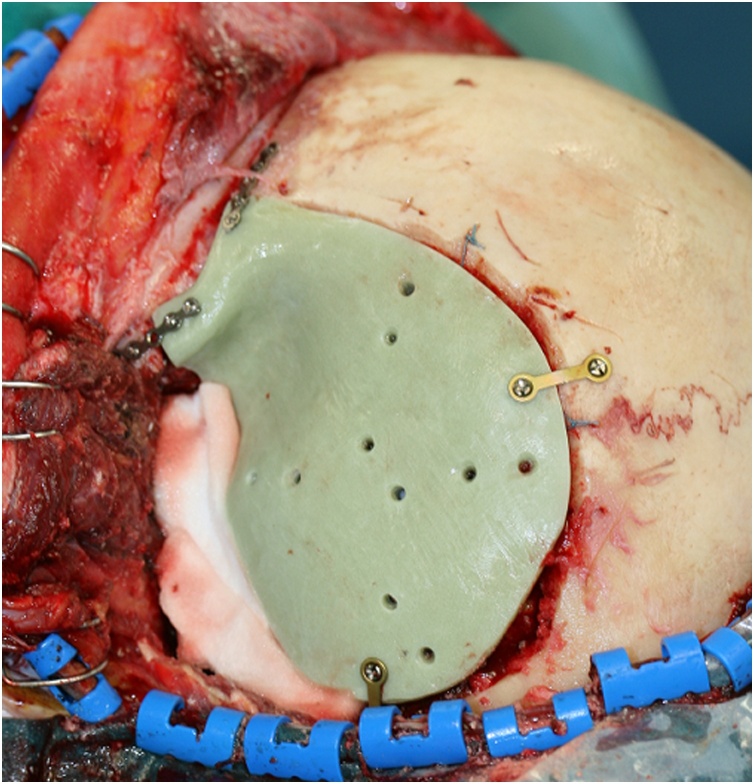
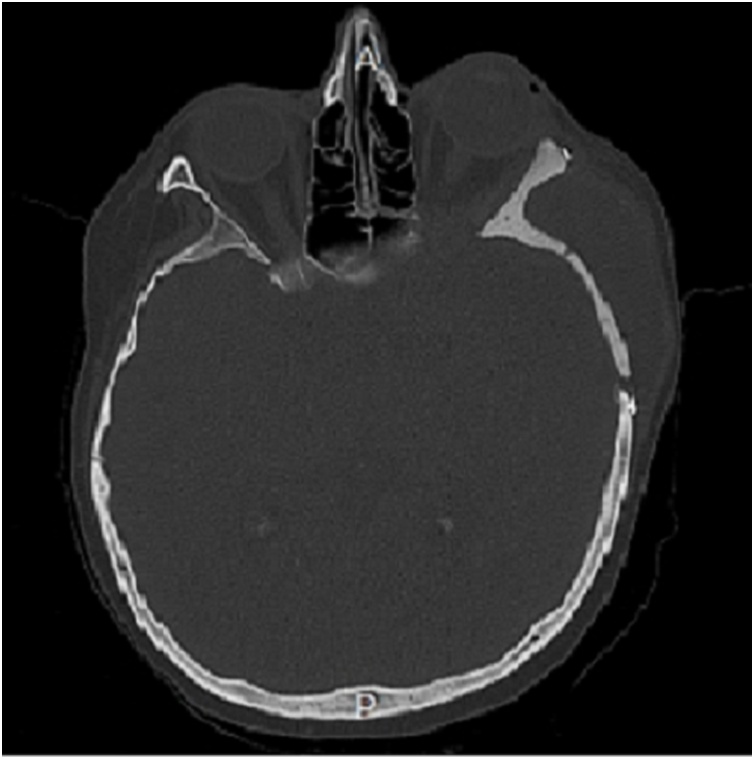
The surgical intervention was performed by a multidisciplinary team composed by the senior neurosurgeon and maxillofacial surgeons. In general anesthesia with the head fixed in Mayfield headboard, a left fronto-temporal skin incision with temporal interfascial dissection was made. A fronto-orbital craniotomy was then performed under Stealth guide using CT and MRI merged scans. The intradural lesion was operated with microsurgical technique (OPMI Pentero 900 Zeiss). Dural reconstruction was performed with a dural substitute fixed with watertight suture and fibrin glue. Therefore, custom-made cranioplasty was implanted and fixed with titanium low profile plates and screws (CIzeta System) (Fig. 5). A post-operative CT scan (Fig. 3) showed a gross total tumor resection with orbital decompression and accurate bone reconstruction. Complementary radiotherapy was obtained after one month, and three-month clinical follow-up showed excellent functional and aesthetic results.
Fig. 5.
PMMA custom-made cranioplasty implant.
Fig. 3.
Post-operative CT scan.
3. Discussion
SBM is a relatively rare entity, more frequently seen during autopsy [4]. Its incidence in oncology patients is about 4% [1], and in one-third of cases, it represents the clinical onset [1]. A recent analysis of the literature describes an overall median survival of 31 months, which showed a substantial improvement compared to 12 months of previous reports [11]. The main clinical syndrome depends on the involved region. In a recent review, Laigle-Donadey [1] described six different types of syndromes: Orbit (125%), Parasellar and sellar (29%), Gasserin ganglion (6%), Jugular foramen (3,5%), Occipital condyle (16%), and others (33%), as shown in Table 1. Diagnosis is essentially obtained from neurological examinations (Table 2) and radiological exams. According to a recent review published by Roukotz [2], an accurate diagnosis of the primary disease and a multidisciplinary approach are mandatory to obtain the best treatment in terms of functional outcome and tumor progression control. SBM usually show a hypointensity on T1-weighted MRI sequences and a variable appearance on T2-weighted sequences and after gadolinium contrast. MRI is also useful to evaluate cavernous sinus infiltration and orbital and optic nerve involvement [11]. On the other hand, CT scans allow a better indication of cranial base invasion, hyperostosis, and osteolysis [12]. Radionuclide, PET, and SPECT scans could be helpful in the differential diagnosis and disease staging [13]. In selected cases, a biopsy could be mandatory to obtain a diagnosis. Rarely, Cerebro Spinal Fluid (CSF) examination is obtained to exclude Central Nervous System (CNS) diffusion. The most important neoplasms for SBM’s differential diagnosis comprise Meningiomas, Chordomas, Chondroblastoma, and Solitary Fibrous Tumor. Conversely, SBM diagnosis could be complicated especially when the primary tumor is unknown or occult. Among non-surgical procedures, radiotherapy is the most common treatment of SBM [14]. Greenberg et al. [5] documented an overall improvement of clinical symptomatology in 86% of cases. The efficacy of radiotherapy appears strongly correlated with the treatment's timing, whose delay may dramatically compromise the results. Generally, the authors recommend 32 Gy in 14 fractioned doses within three weeks. These doses are usually well tolerated. In select patients with long life expectancy, the radiant dose could be increased to 50 Gy [2]. Chemotherapy (ChT) is less effective than radiotherapy, and its utilization may change for the histopathological diagnosis of the primary tumor [2]. ChT associated with radiotherapy improves overall survival in some subtypes of the tumor (breast and prostate cancer) [15]. Recently, immunotherapy and Target therapy seem to find an encouraging role in stopping disease progression [1]. Radiosurgery (RS) is another therapeutic option in SBM. RS is often used as a secondary treatment after surgery (residual tumor). Alternately, it is the first choice when the tumor size is relatively small [1,2]. RS can obtain control of tumor growth and functional status in about 61–67% of the cases [16]. Cranial neuropathy (optic nerve, facial, trigeminal, and abducent) is the most common complication [17]. On the other hand, surgical resection of SBM remains an important therapeutic option for selected patients. Compared with past literature, modern and accurate surgery have allowed obtaining excellent functional results and better tumor growth control [2]. KPS represents an important prognostic factor in SBM surgery and should be accurately evaluated [1,2]. All authors agree that surgical decompression has a decisive role in relieving neurological symptoms such as visual loss or ophthalmoplegia and improving Quality of Life (QoL) [2,18]. In an International Collaborative Study, Patel et al. [19] analyzed 1307 patients affected with SBM treated with surgical resection. Surgical complications were reported in 33% of the cases. The most frequent complication was wound healing (18%). The mortality rate was of 4%. The median follow-up was 25 months. Surgical reconstruction was obtained in 78% of cases. Locoregional flaps were the most common reconstruction methods. Free flap and autologous non vascularized tissue was used in 21% and 16% of the cases. Only in 2% of patients, mesh or autologous bone were used for cranial reconstruction. Single step resection/reconstruction is a well-described surgery procedure in literature especially for benign lesion (Meningiomas, osteomas, dysplasia fibrosa) [8,10,20]. From an accurate review of the literature, the single-step procedure is rarely used for malignant skull base tumors. On the other hand, concerning autologous reconstruction, modern biomaterials involve minor reabsorption and infective risks, quicker access to complementary therapies, and a better aesthetic and functional result. Moreover, new technologies involve cheaper materials, lesser realization time, and increased biocompatibility [8,9,10,20], allowing greater utilization even in selected metastatic lesions.
Table 1.
SBM main clinical syndromes according to the involved region (Laigle-Donadey et al., [1]).
| Main Clinical Syndrome | Orbit | 12.5% |
| Parasellar and sellar | 29% | |
| Gasserian ganglion | 6% | |
| Jugular foramen | 3,5% | |
| Occipital condyle | 16% | |
| Others | 33% |
Table 2.
Main neurological symptoms according to Greenberg et al [5].
| Region | Symptom | Sign |
|---|---|---|
| Orbit | Supraorbital headache | Proptosis |
| Diplopia | Ophtalmoplegia | |
| +/- facial numbness (V1) | ||
| +/- decreased vision | ||
| +/-periorbital swelling | ||
| Parasellar (sella turcica, petrous apex) |
Frontal headache | Ophtalmoplegia |
| Diplopia | facial numbness (V1) periorbital swelling |
|
| Gasserian ganglion (petrous ridge) |
Facial numbness, paresthesia | facial numbness (V2,V3) |
| Atypical facial pain | Abducens palsy (anterior ridge) | |
| Facial palsy (posterior ridge) | ||
| Jugular foramen | Occipital pain | Cranial nerve palsies IX,X, XI |
| Hoarseness | ||
| Dysphagia | ||
| Occipital condyle | Occipital pain | Cranial nerve palsy XII |
| dysarthria |
4. Conclusion
Despite newer oncology therapies, SBM remains a challenger entity due to its multiple feeble involved structures requiring prompt and aggressive treatment. According to us, a single-step procedure, successfully used for skull base benign lesions, can also be performed for selected patients with malignant tumors involving cranial bone. We believe that immediate cranial reconstruction is essential to guarantee rapid access to complementary therapies, obtain better tumor progression control and achieve excellent functional and esthetic results.
Declaration of Competing Interest
None.
Sources of funding
Not applicable.
Ethical approval
Not applicable.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contribution
Vincenzo Antonelli and Giuseppe Maimone wrote this paper. All authors read and approved the final manuscript.
Registration of research studies
Not applicable.
Guarantor
Giuseppe Maimone and Vincenzo Antonelli.
Provenance and peer review
Not commissioned, externally peer-reviewed.
References
- 1.Laigle-Donadey F., Taillibert S., Martin-Duverneuil N. Skull-base metastases. J. Neurooncol. 2005;75(1):63e9. doi: 10.1007/s11060-004-8099-0. [DOI] [PubMed] [Google Scholar]
- 2.Roukoz B., Chamoun M., De Monte F. Management of skull base metastases. Neurosurg. Clin. N. Am. 2011;22(1):61–66. doi: 10.1016/j.nec.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Kalkanis S., Kondziolka D., Gaspar L. The role of surgical resection in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J. Neurooncol. 2010;96(1) doi: 10.1007/s11060-009-0061-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gloria-Cruz T., Schachern P., Paparella M. Metastases to temporal bones from primary non systemic malignant neoplasms. Arch. Otolaryngol. Head Neck Surg. 2000;126(2) doi: 10.1001/archotol.126.2.209. [DOI] [PubMed] [Google Scholar]
- 5.Greenberg H., Deck M., Vikram B. Metastasis to the base of the skull: clinical findings in 43 patients. Neurology. 1981;5(2):530e7. doi: 10.1212/wnl.31.5.530. [DOI] [PubMed] [Google Scholar]
- 6.Gaillard S., Pellerin P., Dhellemmes P., Pertuzon B., Christiaens J. Strategy of craniofacial reconstruction after resection of spheno-orbital“ en plaque” meningiomas. Plast. Reconstr. Surg. 1997;100:1113–1120. doi: 10.1097/00006534-199710000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Kraus D., Shah J., Galicich J., Strong E. Complications of craniofacial resection for tumors involving the anterior skull base. Head Neck. 1994;16:307–312. doi: 10.1002/hed.2880160403. [DOI] [PubMed] [Google Scholar]
- 8.Vougioukas V., Hubbe U., Van Velthoven V., Freiman T., Schramm A., Spetzger U. Neuronavigation-assisted cranial reconstruction. Neurosurgery. 2004;55:162–167. doi: 10.1227/01.neu.0000126940.20441.e7. [DOI] [PubMed] [Google Scholar]
- 9.Tel A., Costa F., Sembronio S., Lazzarotto A., Robiony M. All-in-one surgical guide: a new method for cranial vault resection and reconstruction. J. Craniomaxillofac. Surg. 2018;46:967–973. doi: 10.1016/j.jcms.2018.03.020. [DOI] [PubMed] [Google Scholar]
- 10.Guerrini F., Dallolio V., Grimod G., Cesana C., Vismara D., Franzin A. It is time to reduce free-hand manipulation: case report of our proposal for an innovative 1-Step cranioplasty. World Neurosurg. 2017;107 doi: 10.1016/j.wneu.2017.08.111. 1052-e7. [DOI] [PubMed] [Google Scholar]
- 11.Agha R., Franchi T., Sohrabi C., Mathew G., For the SCARE Group The SCARE 2020 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 12.Harrison R., Nam J., DeMonte F. Intracranial dural, calvarial, and skull base metastases. Handb. Clin. Neurol. 2018:205–225. doi: 10.1016/B978-0-12-811161-1.00014-1. [DOI] [PubMed] [Google Scholar]
- 13.Loevner L., Yousem D. Overlooked metastatic lesions of the occipital condyle: a missed case treasure trove. Radiographics. 1997;17:1111–1121. doi: 10.1148/radiographics.17.5.9308105. [DOI] [PubMed] [Google Scholar]
- 14.Fukumoto M., Osaki Y., Yoshida D., Ogawa Y., Fujiwara M. Dual-isotope SPECT diagnosis of a skull-base metastasis causing isolated unilateral hypoglossal nerve palsy. Ann. Nucl. Med. 1998;12:213–216. doi: 10.1007/BF03164848. [DOI] [PubMed] [Google Scholar]
- 15.Vikram B., Chu F. Radiation therapy for metastases to the base of the skull. Radiology. 1979;130(2):465e8. doi: 10.1148/130.2.465. [DOI] [PubMed] [Google Scholar]
- 16.Ripa Saldias S., Ayuso Blanco T., Delpon Perez E. Involvement of cranial pairs as manifestation, 42 of prostatic cancer. Actas Urol. Esp. 1994;18:911–914. [PubMed] [Google Scholar]
- 17.Francel P., Bhattacharjee S., Tompkins P. Skull base approaches and gamma knife radiosurgery for multimodality treatment of skull base tumors. J. Neurosurg. 2002;97:674–676. doi: 10.3171/jns.2002.97.supplement. [DOI] [PubMed] [Google Scholar]
- 18.Ripa Saldias S., Ayuso Blanco T., Delpon Perez E. Involvement of cranial pairs as manifestation, 42 of prostatic cancer. Actas Urol. Esp. 1994;18:911–914. [PubMed] [Google Scholar]
- 19.Mukoyama N., Nobuaki N. Prospective evaluation of health-related quality of life in patients undergoing anterolateral craniofacial resection with orbital exenteration. J. Neurol. Surg. Part B. 2020;81(5):585–593. doi: 10.1055/s-0039-1694010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel S., Singh B., Polluri A. Craniofacial surgery for malignant skull base tumors: report of an international collaborative study. Cancer. 2003;98:1179–1187. doi: 10.1002/cncr.11630. [DOI] [PubMed] [Google Scholar]