Key Points
Question
Does aflibercept treatment of moderate to severe nonproliferative diabetic retinopathy prevent vision-threatening complications and benefit visual acuity compared with sham treatment?
Findings
In this randomized clinical trial of 328 adults (399 eyes) with nonproliferative diabetic retinopathy without center-involved diabetic macular edema, the 2-year rate of developing center-involved diabetic macular edema with vision loss or proliferative diabetic retinopathy was 16.3% with aflibercept vs 43.5% with sham. The difference between aflibercept and sham in 2-year mean visual acuity change was 0.5 letters.
Meaning
In this study, aflibercept injections reduced the development of vision-threating complications; however, through 2 years, preventive treatment did not confer visual acuity benefit compared with observation plus aflibercept if complications developed.
Abstract
Importance
The role of anti–vascular endothelial growth factor injections for the management of nonproliferative diabetic retinopathy (NPDR) without center-involved diabetic macular edema (CI-DME) has not been clearly established.
Objective
To determine the efficacy of intravitreous aflibercept injections compared with sham treatment in preventing potentially vision-threatening complications in eyes with moderate to severe NPDR.
Design, Setting, and Participants
Data for this study were collected between January 15, 2016, and May 28, 2020, from the ongoing DRCR Retina Network Protocol W randomized clinical trial, conducted at 64 US and Canadian sites among 328 adults (399 eyes) with moderate to severe NPDR (Early Treatment Diabetic Retinopathy Study severity level, 43-53), without CI-DME. Analyses followed the intent-to-treat principle.
Interventions
Eyes were randomly assigned to 2.0 mg of aflibercept injections (n = 200) or sham (n = 199) given at baseline; 1, 2, and 4 months; and every 4 months through 2 years. Between 2 and 4 years, treatment was deferred if the eye had mild NPDR or better. Aflibercept was administered in both groups if CI-DME with vision loss (≥10 letters at 1 visit or 5-9 letters at 2 consecutive visits) or high-risk proliferative diabetic retinopathy (PDR) developed.
Main Outcomes and Measures
Development of CI-DME with vision loss or PDR through May 2020, when the last 2-year visit was completed.
Results
Among the 328 participants (57.6% men [230 of 399 eyes]; mean [SD] age, 56 [11] years), the 2-year cumulative probability of developing CI-DME with vision loss or PDR was 16.3% with aflibercept vs 43.5% with sham. The overall hazard ratio for either outcome was 0.32 (97.5% CI, 0.21-0.50; P < .001), favoring aflibercept. The 2-year cumulative probability of developing PDR was 13.5% in the aflibercept group vs 33.2% in the sham group, and the 2-year cumulative probability of developing CI-DME with vision loss was 4.1% in the aflibercept group vs 14.8% in the sham group. The mean (SD) change in visual acuity from baseline to 2 years was −0.9 (5.8) letters with aflibercept and −2.0 (6.1) letters with sham (adjusted mean difference, 0.5 letters [97.5% CI, −1.0 to 1.9 letters]; P = .47).
Conclusions and Relevance
In this randomized clinical trial, among eyes with moderate to severe NPDR, the proportion of eyes that developed PDR or vision-reducing CI-DME was lower with periodic aflibercept compared with sham treatment. However, through 2 years, preventive treatment did not confer visual acuity benefit compared with observation plus treatment with aflibercept only after development of PDR or vision-reducing CI-DME. The 4-year results will be important to assess longer-term visual acuity outcomes.
Trial Registration
ClinicalTrials.gov Identifier: NCT02634333
This randomized clinical trial examines the efficacy of intravitreous aflibercept injections compared with sham treatment in preventing potentially vision-threatening complications in eyes with moderate to severe nonproliferative diabetic retinopathy.
Introduction
Intravitreal anti–vascular endothelial growth factor (VEGF) therapy is effective first-line treatment for vision-threatening complications of diabetes, including center-involved diabetic macular edema (CI-DME) and proliferative diabetic retinopathy (PDR).1,2,3,4 The role of anti-VEGF therapy for eyes with nonproliferative diabetic retinopathy (NPDR) in the absence of vision-threatening complications is less clear.
In the PANORAMA (Study of the Efficacy and Safety of Intravitreal [IVT] Aflibercept for the Improvement of Moderately Severe to Severe Nonproliferative Diabetic Retinopathy [NPDR]) study, eyes with moderately severe to severe NPDR (Diabetic Retinopathy Severity Scale [DRSS] level,5 47-53) without CI-DME were randomly assigned to receive intravitreal aflibercept every 8 weeks after an initial 5 monthly doses, intravitreal aflibercept every 16 weeks after an initial 3 monthly doses, or sham injections.6 At 2 years, 62% of the eyes that received aflibercept every 8 weeks and 50% of the eyes that received aflibercept every 16 weeks had an improvement of 2 or more steps in the DRSS vs 13% of the eyes in the sham group. Through 2 years, 19% of the eyes that received aflibercept every 8 weeks and 16% of the eyes that received aflibercept every 16 weeks developed vision-threatening complications vs 50% of the eyes in the sham group. No difference was identified between groups in mean visual acuity (VA) letter score change when the study ended at 2 years (aflibercept every 8 weeks, −0.8; aflibercept every 16 weeks, 0.5; and sham, 0).
The DRCR Retina Network Protocol W was designed as a long-term study to determine whether there is a benefit of aflibercept for a 2- and 4-year period for the prevention of PDR or CI-DME in eyes with moderate to severe NPDR, and if so, whether there is an associated visual benefit of aflibercept for the prevention of PDR or CI-DME with vision loss compared with observation and aflibercept treatment if vision-threatening complications develop.
Methods
Study data included in this report were collected from January 15, 2016, when the study was initiated, through May 28, 2020, when the last 2-year visit was completed. This study adhered to the tenets of the Declaration of Helsinki.7 The ethics boards associated with the following sites provided approval: Jaeb Center for Health Research; University of Wisconsin, Health Sciences Institutional review boards; Johns Hopkins Office of Human Subjects Research; Northwestern University Institutional Review Board; University of Pennsylvania, Office of Regulatory Affairs; University of North Carolina at Chapel Hill Office of Human Research Ethics; The New York Eye and Ear Infirmary; Oregon Health and Science University Institutional Review Board; Loma Linda University Health; Office for the Protection of Research Subjects, University of Illinois at Chicago; Baylor Institutional Review Board; University Health Network Research Ethics Board; University of Arizona Institutional Review Board; University of British Colombia Clinical Research Ethics Board; University of Miami Human Subjects Research Office; Nova Scotia Health Authority Research Ethics Board; WCG IRB, Canada; and University of California Davis Institutional Review Board Administration. Study participants provided written informed consent. Participants were reimbursed between $25 and $100 depending on the visit and the calendar year. An independent data and safety monitoring committee provided oversight. The study protocol and the statistical analysis plan are provided in Supplement 1. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines.
Study Population
Protocol W recruited adults (age, ≥18 years) with type 1 or 2 diabetes and severe NPDR, as determined by the investigator, at 64 clinical sites in the US and Canada. Study eyes had moderate to severe NPDR (reading center–assessed DRSS level, 43-53; 9 months after recruitment began, the lower cutoff was modified from 47), with no evidence of neovascularization (NV) within the 7 modified Early Treatment Diabetic Retinopathy Study (ETDRS) fields detected on fluorescein angiographic image. Eyes with CI-DME detected on results of clinical examination or a central subfield thickness (CST) greater than machine and sex optical coherence tomography thresholds were excluded.8 Study eyes had a best-corrected VA letter score of 79 or greater (Snellen equivalent, ≥20/25), no history of diabetic macular edema (DME) or diabetic retinopathy (DR) treatment within the prior 12 months, and no prior panretinal photocoagulation (PRP). We collected participant-reported race/ethnicity based on fixed categories per the National Institutes of Health policy9 and consistent with US Food and Drug Administration guidelines.10
Study Design
Eyes were assigned randomly (1:1) on the study website to receive 2 mg of intravitreal aflibercept (Eylea; Regeneron) or sham injections. Randomization for participants with 1 study eye was stratified by DRSS5 (moderate NPDR, level 43; moderately severe, level 47A, 47B-D; severe NPDR, level 53 with NV identified outside the 7 fields on fundus photograph11; or severe NPDR, level 53 without NV identified outside the 7 fields on fundus photographs). For participants with 2 study eyes, the participant was randomly assigned to receive sham in the eye with the worse DRSS and aflibercept in the other, or aflibercept in the eye with the worse DRSS and sham in the other.
Visits occurred at baseline and 1, 2, and 4 months, then every 4 months through 4 years. Additional visits occurred if DME or PDR treatment was initiated. Study participants, technicians for annual visits, and central reading center graders were masked to treatment assignment. Investigators were not masked.
Study Treatment
Prevention injections (either aflibercept or sham) were given at randomization and at each study visit through 2 years. Thereafter, injections were deferred if DR severity was mild NPDR or better (DRSS level ≤35) based on results of clinical examination. Aflibercept was initiated for CI-DME if CST increased by 10% or more from baseline with a 10-letter or more decrease in VA at 1 visit or a 5- to 9-letter decrease at 2 consecutive visits with vision loss presumed to be due to CI-DME. Aflibercept was also initiated if high-risk PDR developed, with PRP given if aflibercept treatment failed. Once treatment was initiated, the DRCR Retina Network algorithms for anti-VEGF retreatment of CI-DME or PDR were followed.12,13
Primary Anatomical Outcome
The primary outcome was development of CI-DME with vision loss or PDR. Development of PDR was defined as NV within the 7 modified ETDRS fields detected on fundus photography or fluorescein angiographic image (graded by the reading center), NV of the iris or angle, neovascular glaucoma, traction retinal detachment, vitreous hemorrhage, preretinal hemorrhage greater than half the disc area, or a procedure undertaken to treat PDR. Development of CI-DME was defined as CST increased by 10% or more from baseline with vision loss (as already defined), or treatment for CI-DME.
Statistical Analysis
The study planned to enroll 386 eyes, which provided 89% power to reject the null hypothesis of no treatment group difference (hazard ratio, 1) for the primary anatomical outcome, assuming exponentially distributed event times consistent with rates of 15% for aflibercept and 30% for sham, 10% loss to follow-up at 2 years, and type I error rate of 5%. To preserve the type I error rate for analysis at multiple time points, a 2.5% type I error was allocated to the current analysis, and a 2.5% type I error was allocated to the final analysis to be conducted when the 4-year follow-up is completed. To preserve the type I error rate at 2 years, a hierarchical approach was used in which mean change in VA was compared only if there was a significant difference (P ≤ .025) in the primary anatomical outcome. The sample size provided 89% power to detect a difference in mean VA if the true difference was at least 3 letters with an SD of 8.
The comparison of the time to PDR or DME development was performed using the marginal Cox proportional hazards regression model adjusted for baseline DRSS, study eye laterality, and correlation between eyes of participants with 2 study eyes. The proportional hazards assumption was verified using Martingale residuals.14 Data from eyes not meeting the outcome criteria were censored at the last completed visit. Two-year cumulative probabilities were calculated at the end of the 2-year visit window (815 days after randomization) using the Kaplan-Meier estimator.15
Analyses followed the intent-to-treat principle. Descriptive statistics are reported using observed data and include data from eyes that received treatment for DME or PDR. For treatment group comparisons, missing values for VA and CST were imputed using the Markov chain Monte Carlo method (100 imputations). Secondary outcomes were analyzed using linear mixed models, logistic regression with generalized estimating equations, or marginal Cox proportional hazards regression models as appropriate.
All P values are 2-sided, with P ≤ .025 considered significant. Analyses were completed using SAS software, version 9.4 (SAS Institute Inc). Because of the potential for type I error due to multiplicity, analyses of secondary outcomes and adverse events should be interpreted as exploratory.
Results
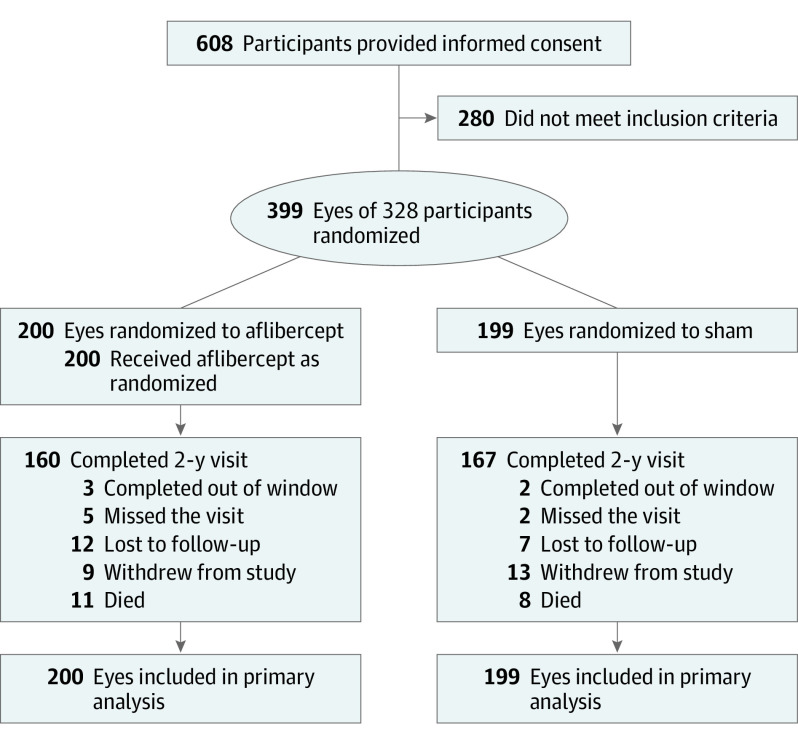
From January 2016 to March 2018, 399 eyes (328 participants) were randomly assigned to receive 2.0 mg of aflibercept (n = 200) or sham (n = 199) (Figure 1). The baseline characteristics of the participants and study eyes appeared balanced between treatment groups (Table 1). The median age was 57 years (interquartile range [IQR], 51-64 years), 57.6% were men (189 of 328), 42.4% were women (139 of 328), 46.7% were White (153 of 328), 30.5% were Hispanic or Latino (100 of 328), and 15.2% were Black or African American (50 of 328). Based on reading center assessment, 68 eyes (17.0%) had moderate NPDR (DRSS level 43), 126 eyes (31.6%) had moderately severe NPDR (DRSS level 47A), 109 eyes (27.3%) had moderately severe NPDR (DRSS level 47B-D), and 96 eyes (24.1%) had severe NPDR (DRSS level 53). The median baseline VA letter score was 88 (Snellen equivalent, 20/20) in both groups. Excluding deaths, 160 of 189 participants (84.7%) assigned to aflibercept and 167 of 191 participants (87.4%) assigned to sham completed the 2-year visit, with a mean (SD) number of visits through 2 years of 10 (3) for the aflibercept group and 11 (4) for the sham group. The median follow-up for the aflibercept group was 2.4 years (IQR, 2.0-3.0 years; maximum, 4.1 years), and the median follow-up for the sham group was 2.4 years (IQR, 2.0-3.0 years; maximum, 3.9 years). Excluding deaths, 59 of 67 participants (88.1%) with 2 study eyes completed the 2-year visit; the median follow-up duration was 2.3 years (IQR, 2.0-3.0 years).
Figure 1. Study Flow Diagram.
Protocol-specified visits occurred at baseline (randomization), 1 month (±2 weeks), 2 months (±1 week), and 4 months (±8 weeks), then every 4 months (±12 weeks for annual visits, ±8 weeks otherwise) through 4 years.
Table 1. Baseline Participant and Study Eye Characteristics.
| Baseline characteristica | No. (%) | |
|---|---|---|
| Aflibercept (n = 200 eyes) | Sham (n = 199 eyes) | |
| Participant characteristics | ||
| No. of study eyes | ||
| 1 | 129 (64.5) | 128 (64.3) |
| 2 | 71 (35.5) | 71 (35.7) |
| Sex | ||
| Female | 83 (41.5) | 86 (43.2) |
| Male | 117 (58.5) | 113 (56.8) |
| Age, median (IQR), y | 57 (51-63) | 56 (48-62) |
| Race/ethnicity | ||
| White | 92 (46.0) | 86 (43.2) |
| Hispanic or Latino | 62 (31.0) | 67 (33.7) |
| Black or African American | 29 (14.5) | 32 (16.1) |
| Asian | 10 (5.0) | 9 (4.5) |
| More than 1 race/ethnicity | 3 (1.5) | 1 (0.5) |
| Unknown or not reported | 2 (1.0) | 3 (1.5) |
| American Indian or Alaskan Native | 1 (0.5) | |
| Native Hawaiian or other Pacific Islander | 1 (0.5) | 1 (0.5) |
| Diabetes type | ||
| Type 1 | 12 (6.0) | 23 (11.6) |
| Type 2 | 188 (94.0) | 176 (88.4) |
| Duration of diabetes, median (IQR), y | 17 (10-22) | 16 (11-22) |
| Insulin used | 139 (69.5) | 139 (69.8) |
| Hemoglobin A1c, median (IQR), %b | 8.6 (7.1-10.0) | 8.3 (7.3-9.7) |
| Mean arterial pressure, median (IQR), mm Hg | 100 (92-107) | 98 (91-106) |
| BMI, median (IQR) | 31 (28-37) | 31 (27-36) |
| Prior myocardial infarction | 16 (8.0) | 19 (9.5) |
| Prior stroke | 13 (6.5) | 11 (5.5) |
| Preexisting renal disease | 6 (3.0) | 6 (3.0) |
| Study eye characteristics | ||
| Visual acuity, letter score, median (IQR) | 88 (84-90) | 88 (85-91) |
| Visual acuity, Snellen equivalent, median (IQR) | 20/20 (20/20-20/16) | 20/20 (20/20-20/16) |
| Visual acuity ≥20/20 (letter score ≥84) | 156 (78.0) | 161 (80.9) |
| OCT machine | ||
| Heidelberg Spectralis | 111 (55.5) | 112 (56.3) |
| Zeiss Cirrus | 89 (44.5) | 87 (43.7) |
| OCT CST, median (IQR), µmc | 283 (264-300) | 283 (265-299) |
| CST ≥ sex- and machine-specific DME thresholdd | 3 (1.5) | 2 (1.0) |
| OCT retinal volume, median (IQR), mm3b,e | 7.4 (7.0-7.9) | 7.4 (6.9-7.8) |
| Diabetic Retinopathy Severity Scale level at screening based on reading center assessment | ||
| Moderate NPDR (level 43) | 33 (16.5) | 35 (17.6) |
| Moderately severe NPDR (level 47A) | 65 (32.5) | 61 (30.7) |
| Moderately severe NPDR (level 47B-D) | 54 (27.0) | 55 (27.6) |
| Severe NPDR (level 53) | 48 (24.0) | 48 (24.1) |
| Randomization stratification factor based on reading center assessment of eligibility | ||
| Moderate NPDR (level 43) | 33 (16.5) | 33 (16.6) |
| Moderately severe NPDR (level 47A) | 65 (32.5) | 63 (31.7) |
| Moderately severe NPDR (level 47B-D) | 54 (27.0) | 55 (27.6) |
| Severe NPDR (level 53) with NV identified outside 7-field fundus photograph | 1 (0.5) | 1 (0.5) |
| Severe NPDR (level 53) without NV identified outside 7-field fundus photograph | 47 (23.5) | 47 (23.6) |
| Intraocular pressure, median (IQR), mm Hg | 15 (13-17) | 15 (13-17) |
| Lens status | ||
| PC IOL | 34 (17.0) | 32 (16.1) |
| Phakic | 166 (83.0) | 167 (83.9) |
| Noncentral DMEb,f | 75/188 (39.9) | 83/195 (42.6) |
| Prior treatment for DME | 19 (9.5) | 22 (11.1) |
| Prior anti-VEGF therapy for DME | 8 (4.0) | 5 (2.5) |
| Prior focal or grid laser for DME | 12 (6.0) | 19 (9.5) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CST, central subfield thickness; DME, diabetic macular edema; IQR, interquartile range; IOL, intraocular lens; NPDR, nonproliferative diabetic retinopathy; NV, neovascularization; OCT, optical coherence tomography; PC, posterior chamber; VEGF, vascular endothelial growth factor.
SI conversion factor: To convert hemoglobin A1c to proportion of total hemoglobin, multiply by 0.01.
Unless otherwise specified, baseline values reflect values at the randomization visit.
Hemoglobin A1c values were missing for 11 participants in the aflibercept group and 9 participants in the sham group. OCT retinal volume and noncentral DME status were missing for 12 eyes in the aflibercept group and 4 eyes in the sham group.
Heidelberg Spectralis machine equivalent. Spectralis = 40.78 + 0.95 × cirrus.
DME thresholds are female CST less than 290 µm and male CST less than 305 µm on the Zeiss Cirrus machine or female CST less than 305 µm and male CST less than 320 µm on the Heidelberg Spectralis machine.
Zeiss Stratus machine equivalent.
At least 2 noncentral macular subfields with OCT thickness above threshold (average normal + 2 SD) or at least 1 noncentral macular subfield with OCT thickness at least 15 µm above threshold (average normal + 2 SD).
Treatment Effect on DR and DME
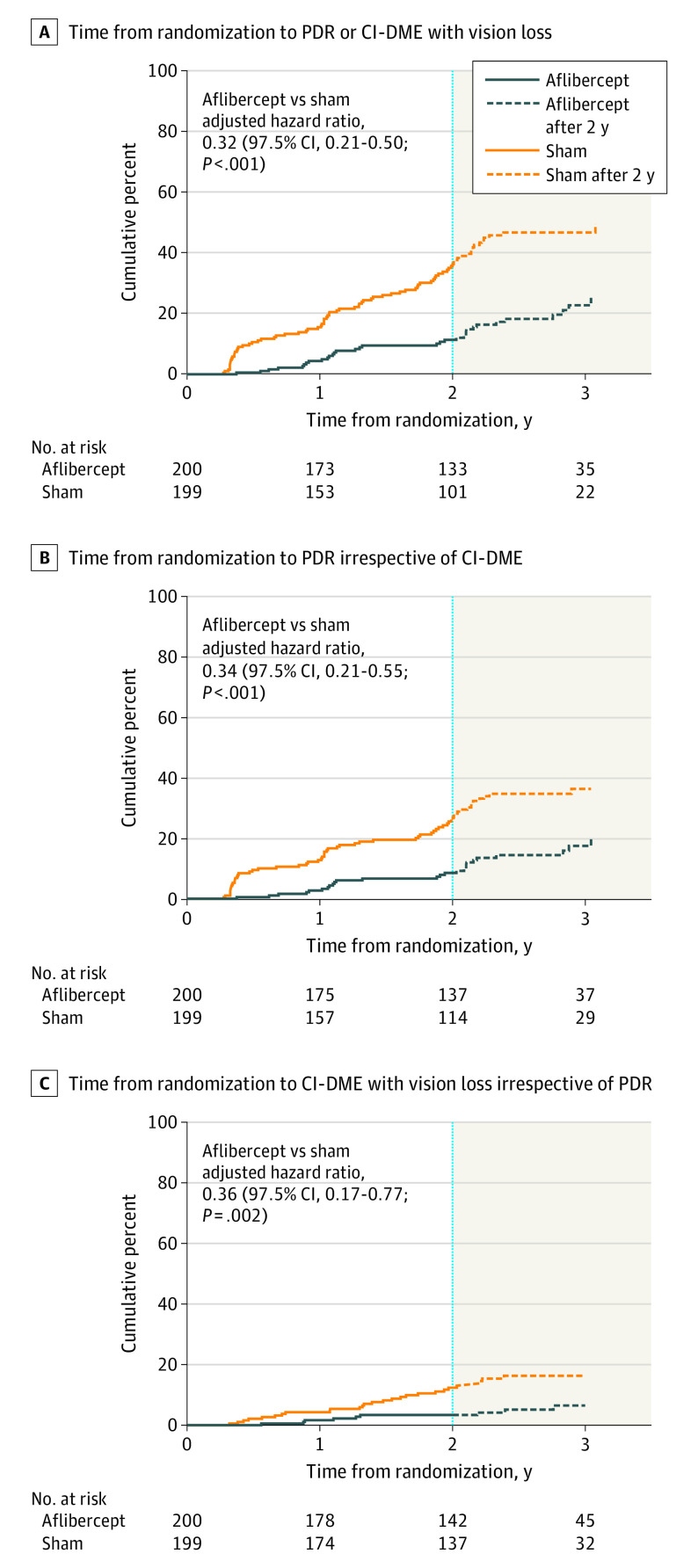
For the development of CI-DME with vision loss or PDR (primary outcome), the overall adjusted hazard ratio comparing aflibercept with sham was 0.32 (97.5% CI, 0.21-0.50; P < .001) (Figure 2A). The cumulative probability of developing CI-DME with vision loss or PDR within 2 years was 16.3% with aflibercept vs 43.5% with sham. For the development of PDR, the adjusted hazard ratio was 0.34 (97.5% CI, 0.21-0.55; P < .001) (Figure 2B), and for the development of CI-DME with vision loss, the adjusted hazard ratio was 0.36 (97.5% CI, 0.17-0.77; P = .002) (Figure 2C). The cumulative probability of developing PDR within 2 years from randomization was 13.5% with aflibercept vs 33.2% with sham. The cumulative probability of developing CI-DME with vision loss within 2 years from randomization was 4.1% with aflibercept vs 14.8% with sham. The cumulative probability of developing high-risk PDR (DRSS levels of 71, 75, 81, and 85) any time within 2 years was 2.4% with aflibercept and 8.9% with sham. None of the preplanned subgroup analyses (baseline DRSS, presence of non–center-involved DME, race/ethnicity, and sex) indicated a significant subgroup effect (eTable 1 in Supplement 2).
Figure 2. Time From Randomization to Development of Proliferative Diabetic Retinopathy (PDR) or Center-Involved Diabetic Macular Edema (CI-DME).
A, Time from randomization to development of PDR or CI-DME, whichever came first. B, Time from randomization to development of PDR irrespective of CI-DME. C, Time from randomization to development of CI-DME irrespective of PDR. Hazard ratios include all available data through 4 years and were adjusted for diabetic retinopathy severity at the screening visit, study eye laterality, and correlation between eyes of participants with 2 study eyes. The figure was truncated at the time point at which data from fewer than 20 eyes in each treatment group were available.
The details of the event of first meeting the PDR or DME development criteria are described in Table 2, with the cumulative probability of each component shown in eTable 2 in Supplement 2. The most common event, NV of the disc or elsewhere as determined by a central reading center, occurred in 20 eyes (12.4%) in the aflibercept group and 53 eyes (30.9%) in the sham group within 2 years.
Table 2. Components of PDR and DME Composite Outcome Met at First Occurrence of Outcome.
| Primary outcomea | No. | |||
|---|---|---|---|---|
| Within 2 y | Within total time in study | |||
| Aflibercept (n = 200) | Sham (n = 199) | Aflibercept (n = 200) | Sham (n = 199) | |
| Development of PDR and/or DME (whichever came first)b | 27 | 75 | 35 | 81 |
| First PDR and/or DME criteria met | ||||
| NVA | 0 | 1 | 0 | 1 |
| NVD or NVE | 14 | 38 | 17 | 39 |
| NVD or NVE and preretinal hemorrhage (>half disc area) | 1 | 5 | 1 | 5 |
| NVD or NVE and VH due to PDR | 1 | 3 | 2 | 3 |
| NVD or NVE and VH due to PDR and preretinal hemorrhage (>half disc area) | 0 | 3 | 0 | 4 |
| NVI (≥2 cumulative clock hours) | 0 | 1 | 0 | 1 |
| VH due to PDR | 4 | 4 | 4 | 4 |
| VH due to PDR and preretinal hemorrhage (>half disc area) | 0 | 0 | 0 | 1 |
| CI-DME with ≥10% increase in CST and 5- to 9-letter decrease in VA at 2 consecutive visits (≥21 d apart)c | 0 | 3 | 0 | 4 |
| CI-DME with ≥10% increase in CST and ≥10-letter decrease in VAc | 4 | 11 | 6 | 12 |
| NVD or NVE and CI-DME with ≥10% increase in CST and ≥10-letter decrease in VAc | 2 | 1 | 2 | 2 |
| PRP for PDR | 0 | 1 | 0 | 1 |
| Anti-VEGF therapy for DME | 1 | 3 | 3 | 3 |
| Focal or grid laser for DME | 0 | 1 | 0 | 1 |
| Development of PDRb | 22 | 57 | 26 | 62 |
| First PDR criteria met | ||||
| NVA | 0 | 1 | 0 | 1 |
| NVD or NVE | 16 | 39 | 19 | 42 |
| NVD or NVE and preretinal hemorrhage (>half disc area) | 1 | 5 | 1 | 5 |
| NVD or NVE and VH due to PDR | 1 | 3 | 2 | 3 |
| NVD or NVE and VH due to PDR and preretinal hemorrhage (>half disc area) | 0 | 3 | 0 | 4 |
| NVI (≥2 cumulative clock hours) | 0 | 1 | 0 | 1 |
| VH due to PDR | 4 | 4 | 4 | 4 |
| VH due to PDR and preretinal hemorrhage (>half disc area) | 0 | 0 | 0 | 1 |
| PRP for PDR | 0 | 1 | 0 | 1 |
| Development of DMEb | 7 | 25 | 11 | 28 |
| First DME criteria met | ||||
| CI-DME with ≥10% increase in CST and 5- to 9-letter decrease in VA at 2 consecutive visits (≥21 d apart)c | 0 | 3 | 0 | 4 |
| CI-DME with ≥10% increase in CST and ≥10-letter decrease in VAc | 6 | 15 | 8 | 17 |
| Anti-VEGF therapy for DME | 1 | 5 | 3 | 5 |
| Focal or grid laser for DME | 0 | 2 | 0 | 2 |
Abbreviations: CI-DME, center-involved diabetic macular edema; CST, center subfield thickness; DME, diabetic macular edema; NVA, neovascularization of the angle; NVD, neovascularization of the disc; NVE, neovascularization elsewhere; NVG, neovascular glaucoma; NVI, neovascularization of the iris; PDR, proliferative diabetic retinopathy; PRP, panretinal photocoagulation; TRD, traction retinal detachment; VA, visual acuity; VEGF, vascular endothelial growth factor; VH, vitreous hemorrhage.
Outcomes based on reading center assessment only: NVD or NVE, CI-DME, and change in CST. Outcomes based on reading center assessment or clinical examination: TRD, VH, and preretinal hemorrhage. Outcomes based on clinical examination only: NVA, NVG, NVI, change in VA, and any treatment for PDR or DME.
First development of criteria meeting end point. Eyes that met any criteria are then censored from contributing to the next criteria. Eyes that did not meet the outcome were censored at the time of the last completed visit. Each outcome appears only once under “First PDR and/or DME criteria met.” Outcomes appear under “Development of PDR” if PDR developed at any time in the study (regardless of if or when DME developed) and outcomes appear under “Development of DME” if DME developed at any time in the study (regardless of if or when PDR developed).
Vision loss presumed to be from DME.
Among participants who completed the 2-year visit, DR severity improved 2 steps or more from baseline to 2 years in 69 of 154 eyes (44.8%) receiving aflibercept vs 22 of 161 eyes (13.7%) receiving sham (adjusted odds ratio, 5.91 [97.5% CI, 3.19-10.95]; P < .001); DR severity worsened 2 or more steps in 8 of 154 eyes (5.2%) receiving aflibercept vs 20 of 161 eyes (12.4%) receiving sham (adjusted odds ratio, 0.37 [97.5% CI, 0.13-1.01]; P = .03) (eTable 3 and eTable 4 in Supplement 2). Compared with baseline, the mean (SD) CST at 2 years decreased by 6 (27) μm with aflibercept vs 1 (28) μm with sham (adjusted mean difference, −4 µm [97.5% CI, −12 to 4 µm]; P = .26). Data on additional CST and optical coherence tomography retinal volume outcomes are provided in eFigure 1, eFigure 2, eTable 5, and eTable 6 in Supplement 2.
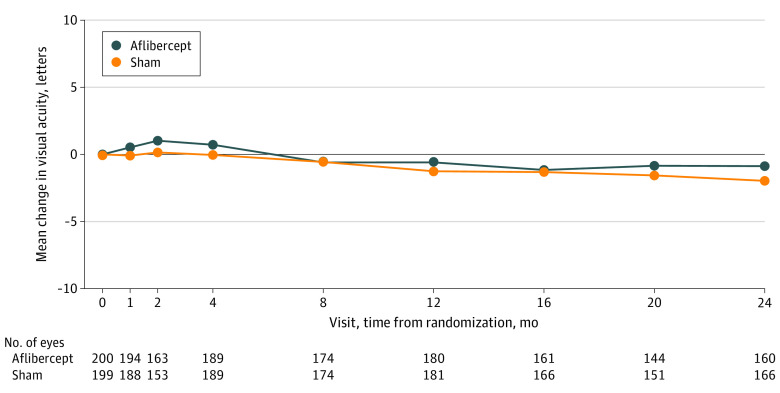
Visual Acuity
The mean (SD) change in VA from randomization to 2 years was −0.9 (5.8) letters with aflibercept and −2.0 (6.1) letters with sham (adjusted mean difference, 0.5 letters [97.5% CI, −1.0 to 1.9 letters]; P = .47) (Table 3 and Figure 3). At 2 years, 120 of 160 eyes receiving aflibercept (75.0%) and 119 of 166 eyes receiving sham (71.7%) had VA of 20/20 or better (≥84 letters); no eyes in either group had VA of 20/200 or worse (≤38 letters). Visual acuity loss of 10 or more letters at 2 years occurred in 11 of 160 eyes receiving aflibercept (6.9%) and in 14 of 166 eyes receiving sham (8.4%). Additional VA outcomes are summarized in Table 3 and eTable 7 in Supplement 2. None of the preplanned subgroup analyses (baseline DRSS, non–center-involved DME, race/ethnicity, and sex) indicated a significant subgroup effect (eTable 8 in Supplement 2).
Table 3. Visual Acuity Outcomes at 2 Yearsa.
| Characteristic | Observed data | Aflibercept vs sham adjusted mean difference or odds ratio (97.5% CI) | |||
|---|---|---|---|---|---|
| Primary analysisb | Sensitivity analyses | ||||
| Aflibercept (n = 160) | Sham (n = 166) | Complete case | Per-protocolc | ||
| Visual acuity at randomization, letter score, mean (SD) | 87.3 (4.5) | 87.8 (4.6) | NA | NA | NA |
| Visual acuity at 2 y, letter score, mean (SD) | 86.3 (7.8) | 85.7 (7.9) | NA | NA | NA |
| Visual acuity change from randomization to 2 y, letters | |||||
| Mean (SD) | −0.9 (5.8) | −2.0 (6.1) | 0.5 (−1.0 to 1.9) | 0.9 (−0.5 to 2.3) | 0.5 (−0.9 to 1.8) |
| P value | NA | NA | .47 | .14 | .40 |
| Mean visual acuity change over 2 y (AUC), letters | |||||
| Mean (SD) | −0.3 (4.2) | −1.0 (3.5) | 0.1 (−0.7 to 1.0) | NA | NA |
| P value | NA | NA | .73 | NA | NA |
| Visual acuity loss of ≥10 letters at 2 y | |||||
| No. (%) | 11 (6.9) | 14 (8.4) | 1.05 (0.43 to 2.56) | NA | NA |
| P value | NA | NA | .90 | NA | NA |
| Visual acuity loss of ≥15 letters at 2 y, No. (%) | 5 (3.1) | 10 (6.0) | NA | NA | NA |
| Visual acuity loss of ≥5 letters at 2 y and the previous study visit, No. (%) | 22 (13.8) | 21 (12.7) | NA | NA | NA |
| Visual acuity gain of ≥5 letters at 2 y and the previous study visit, No. (%) | 12 (7.5) | 9 (5.4) | NA | NA | NA |
| Visual acuity of ≥20/20 (≥84 letters) at 2 y, No. (%) | 120 (75.0) | 119 (71.7) | NA | NA | NA |
| Visual acuity of ≥20/40 (≥69 letters) at 2 y, No. (%) | 155 (96.9) | 158 (95.2) | NA | NA | NA |
| Visual acuity of ≤20/200 (≤38 letters) at 2 y, No. (%) | 0 | 0 | NA | NA | NA |
Abbreviations: AUC, area under the curve; DME, diabetic macular edema; NA, not applicable; PDR, proliferative diabetic retinopathy.
Mean differences between treatment groups were estimated using linear mixed models with a random intercept for the correlation between eyes of participants having 2 study eyes and fixed effects for treatment group, study eye laterality, retinopathy severity at screening, and visual acuity at randomization. Odds ratios between treatment groups were estimated using generalized estimating equations with an exchangeable correlation structure for the correlation between eyes of participants having 2 study eyes and regression terms for treatment group, study eye laterality, retinopathy severity at screening, and visual acuity at randomization. Visual acuity change was truncated to the mean ±3 SD (−1.6 ± 3 × 6.7) for 3 eyes in the aflibercept group and 3 eyes in the sham group. Trunction limits were calculated from 2-year data with treatment groups combined.
The primary analysis followed the intent-to-treat principle with multiple imputation (m = 100) for missing data in the analysis of visual acuity change, visual acuity AUC, and visual acuity loss of 10 or more letters. The imputation model included laterality, retinopathy severity, visual acuity at randomization, and change in visual acuity from baseline at each protocol assessment visit up to and including the analysis time point.
The per-protocol analysis included 136 eyes in the aflibercept group and 142 eyes in the sham group that completed the 2-year visit and received at least 80% of the study injections and had no other treatment for PDR or DME before the 2-year visit.
Figure 3. Mean Change in Visual Acuity Through 2 Years.
Treatment of DR and DME
Among participants completing the 2-year visit, the mean (SD) number of aflibercept injections (including those given for prevention and treatment) was 8.0 (1.2) in the aflibercept group and 7.7 (0.7) for prevention alone (eTable 9 in Supplement 2); the mean (SD) number of aflibercept injections in the sham group was 1.1 (2.7), and the mean (SD) number of sham injections was 7.4 (1.1). Seven of 160 eyes (4.4%) in the aflibercept group and 32 of 167 eyes (19.2%) in the sham group received at least 1 aflibercept injection by 2 years for CI-DME or PDR. Among eyes in the sham group that received at least 1 aflibercept injection, the mean (SD) number of injections through 2 years was 5.7 (3.2).
Safety
There were 3 cases of endophthalmitis, all in eyes randomly assigned to aflibercept and from injections administered for prevention, among 3406 injections in study and nonstudy eyes (eTable 10 in Supplement 2). The rate of any cardiovascular or cerebrovascular adverse event was not different among the treatment groups (6 of 71 bilateral [8.5%], 11 of 129 unilateral aflibercept [8.5%], and 11 of 128 unilateral sham [8.6%]; P > .99). eTables 11, 12, 13, and 14 in Supplement 2 provide additional information on ocular and systemic adverse events.
Discussion
Through 2 years, the proportion of eyes with moderate to severe NPDR that developed PDR or CI-DME with vision loss was lower in the aflibercept group than in the sham group. Despite these differences in anatomical outcomes, the mean VA change from baseline to 2 years was similar between the 2 groups. Additional follow-up is needed to determine whether early treatment leads to visual benefit long term. This study will continue through 4 years.
In this study, preventive treatment with aflibercept resulted in a more than 3-fold reduction (from 14.8% with sham to 4.1% with aflibercept) in eyes that developed CI-DME with decreased VA and a more than 2-fold reduction (from 33.2% with sham to 13.5% with aflibercept) in new-onset PDR. Eyes receiving treatment for PDR and CI-DME often have suboptimal visual outcomes; therefore, preventing these conditions may reduce vision loss over time. For example, in the DRCR Retina Network Protocol I, of eyes with CI-DME with decreased VA receiving anti-VEGF therapy, only 40% returned to VA of 20/25 or better by 5 years.16 Similarly, in Protocol S, of eyes with PDR randomized to receive anti-VEGF therapy, approximately 70% had VA of 20/25 or better by 5 years.12
Despite the potential benefits of disease prevention, the findings from prior studies suggest that earlier treatment does not always confer long-term visual benefit. The ETDRS recommended that early PRP be deferred for eyes with mild or moderate NPDR because treatment risks outweighed functional benefits.17 In eyes with CI-DME and good VA (≥20/25), Protocol V demonstrated that initial observation, with anti-VEGF treatment given only if eyes lost VA, led to VA outcomes that were similar to those that received immediate anti-VEGF therapy.18 In this study, no statistically significant difference between aflibercept and sham was identified in mean VA through 2 years, with aflibercept treatment given for disease progression. In addition, approximately three-quarters of each group had a VA letter score of 20/20 or better and less than 10% of each group lost 2 or more lines of VA at 2 years. These data may suggest that when patients are monitored closely, with follow-up examinations performed at least every 16 weeks as was done in this protocol, treatment of vision-threatening complications after they develop may be adequate to recover lost vision or prevent VA loss, on average. Conversely, it is also possible that average VA loss stemming from higher rates of PDR and CI-DME and their complications in the sham group will increase with continued follow-up. The 4-year results from this study will provide greater clarity as to whether there is a long-term functional benefit of using anti-VEGF therapy as a preventive strategy in eyes with moderate or severe NPDR.
Despite all eyes in the aflibercept group receiving at least 1 injection per year and undergoing a mean of 8 anti-VEGF injections through 2 years, 16.3% of aflibercept group eyes developed PDR or CI-DME with VA loss by 2 years. This finding demonstrates that anti-VEGF treatment as provided in this study does not guarantee prevention of vision-threatening complications in this high-risk cohort. Continued ophthalmic follow-up and routine examinations are needed to diagnose and treat PDR and CI-DME irrespective of whether intravitreal anti-VEGF therapy is given for prevention of these conditions.
Eyes in the Protocol W and PANORAMA aflibercept groups experienced a higher rate of DR improvement, as measured by the DRSS, compared with sham. Nonetheless, recent studies have suggested that although anti-VEGF therapy improves DR severity level, it may not substantially modify associated pathologic conditions such as retinal nonperfusion.19 Couturier et al20 imaged eyes undergoing 3 monthly anti-VEGF injections for CI-DME and did not demonstrate reperfusion of vessels or the capillary network in areas that were nonperfused at baseline. Similarly, eyes that received aflibercept vs PRP in the CLARITY (Clinical Efficacy of Intravitreal Aflibercept Versus Panretinal Photocoagulation for Best Corrected Visual Acuity in Patients With Proliferative Diabetic Retinopathy at 52 Weeks) study did not have significant differences in the retinal nonperfusion area at 52 weeks.21 Thus, the question remains whether anti-VEGF treatment delivered to specifically improve DR severity will be beneficial in the long term, especially after cessation of therapy.
All eyes in this study were judged to have severe NPDR by the enrolling investigator. However, the reading center graded 75.9% (303 of 399) as moderate or moderately severe NPDR. This study capped enrollment of eyes at less severe levels of baseline DRSS owing to concerns that progression rates in these eyes would not be sufficient to observe a treatment effect. The 2-year progression rates of eyes receiving sham with moderate NPDR at baseline were lower (24%) than in eyes with severe NPDR (68%). However, aflibercept treatment appeared beneficial at preventing PDR or CI-DME outcomes across all DR severity levels in this study.
Limitations
This study has some limitations. First, not excluding deaths, retention through 2 years was 80.0% in the aflibercept group (160 of 200) and 83.9% in the sham group (167 of 199). Thus, although treatment adherence was excellent for participants who continued follow-up, and baseline characteristics were similar between those who completed and those who did not complete the 2-year visit, results may be biased by the loss to follow-up. Second, the primary outcome included components determined by an unmasked investigator. However, only 3.5% of the outcomes were based on investigator assessment, and if only outcomes not based on unmasked investigator assessment are evaluated, the results of the study are not substantially different. Third, although it is possible that some of the primary outcome components (eg, vitreous hemorrhage) might not be due to diabetic pathologic conditions, it was infrequent that diabetic retinopathy progression was not verified on retinal images. Fourth, this study used aflibercept as the intervention, and the treatment algorithm used to determine need for aflibercept injection was developed based on DRCR Retina Network investigator consensus. Results using another anti-VEGF agent or a different treatment approach may differ.
Conclusions
The proportion of eyes with moderate to severe NPDR that developed vision-threatening complications of CI-DME with vision loss or PDR was lower with aflibercept treatment compared with sham through at least 2 years. However, through 2 years, preventive treatment with aflibercept did not confer visual benefit, on average, compared with initial observation and intravitreal anti-VEGF therapy given only after PDR or DME development. The 4-year results will be critical to assess whether PDR and DME prevention with aflibercept results in long-term VA benefit.
Study Protocol and Statistical Analysis Plan
eFigure 1. Mean Change in Central Subfield Thickness Over 2 Years
eFigure 2. Mean Change in Optical Coherence Tomography Retinal Volume Over 2 Years
eTable 1. Subgroup Analysis for the Development of PDR and CI-DME
eTable 2. Number of Eyes That Met Each Specific PDR/DME Outcome Criterion at Any Time Within 2 Years
eTable 3. Change in Diabetic Retinopathy Severity at 2 Years
eTable 4. Change in Diabetic Retinopathy Severity at 1 Year
eTable 5. Change in OCT Central Subfield Thickness and Retinal Volume at 2 Years
eTable 6. Change in OCT Central Subfield Thickness and Retinal Volume at 1 Year
eTable 7. Change in Visual Acuity at 1 Year
eTable 8. Subgroup Analysis for Change in Visual Acuity at 2 Years
eTable 9. Annual Treatments for PDR and DME
eTable 10. Endophthalmitis Cases
eTable 11. Ocular Adverse Events of Interest in Study Eyes
eTable 12. Systemic Adverse Events of Interest
eTable 13. All Systemic Adverse Events
eTable 14. All Ocular Adverse Events
Data Sharing Statement
References
- 1.Diabetic Retinopathy Clinical Research Network . Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117(6):1064-1077. doi: 10.1016/j.ophtha.2010.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wells JA, Glassman AR, Ayala AR, et al. ; Diabetic Retinopathy Clinical Research Network . Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372(13):1193-1203. doi: 10.1056/NEJMoa1414264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gross JG, Glassman AR, Jampol LM, et al. ; Writing Committee for the Diabetic Retinopathy Clinical Research Network . Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA. 2015;314(20):2137-2146. doi: 10.1001/jama.2015.15217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sivaprasad S, Prevost AT, Vasconcelos JC, et al. ; CLARITY Study Group . Clinical efficacy of intravitreal aflibercept versus panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): a multicentre, single-blinded, randomised, controlled, phase 2b, non-inferiority trial. Lancet. 2017;389(10085):2193-2203. doi: 10.1016/S0140-6736(17)31193-5 [DOI] [PubMed] [Google Scholar]
- 5.Wilkinson CP, Ferris FL 3rd, Klein RE, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110(9):1677-1682. [DOI] [PubMed] [Google Scholar]
- 6.Regeneron Pharmaceuticals Inc . Eylea (aflibercept) injection reduced risk of developing vision-threatening events by 75% after two years in patients with diabetic retinopathy. Published February 8, 2020. Accessed January 19, 2021. https://newsroom.regeneron.com/index.php/news-releases/news-release-details/eylear-aflibercept-injection-reduced-risk-developing-vision
- 7.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 8.Bressler SB, Edwards AR, Chalam KV, et al. ; Diabetic Retinopathy Clinical Research Network Writing Committee . Reproducibility of spectral-domain optical coherence tomography retinal thickness measurements and conversion to equivalent time-domain metrics in diabetic macular edema. JAMA Ophthalmol. 2014;132(9):1113-1122. doi: 10.1001/jamaophthalmol.2014.1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Institutes of Health. NIH policy and guidelines on the inclusion of women and minorities as subjects in clinical research. Accessed April 11, 2019. https://grants.nih.gov/grants/funding/women_min/guidelines.htm
- 10.US Food and Drug Administration. Collection of race and ethnicity data in clinical trials: guidance for industry and Food and Drug Administration staff. Published October 26, 2016. Accessed April 11, 2019. https://www.fda.gov/downloads/RegulatoryInformation/Guidances/UCM126396.pdf?source=govdelivery&utm_medium=email&utm_source=govdelivery
- 11.Aiello LP, Odia I, Glassman AR, et al. Comparison of Early Treatment Diabetic Retinopathy Study standard 7-field imaging with ultrawide-field imaging for determining severity of diabetic retinopathy. JAMA Ophthalmol. 2019;137(1):65-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gross JG, Glassman AR, Liu D, et al. ; Diabetic Retinopathy Clinical Research Network . Five-year outcomes of panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA Ophthalmol. 2018;136(10):1138-1148. doi: 10.1001/jamaophthalmol.2018.3255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wells JA, Glassman AR, Ayala AR, et al. ; Diabetic Retinopathy Clinical Research Network . Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology. 2016;123(6):1351-1359. doi: 10.1016/j.ophtha.2016.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin DY, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of Martingale-based residuals. Biometrika. 1993;80(3):557-572. doi: 10.1093/biomet/80.3.557 [DOI] [Google Scholar]
- 15.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457-481. doi: 10.1080/01621459.1958.10501452 [DOI] [Google Scholar]
- 16.Elman MJ, Ayala A, Bressler NM, et al. ; Diabetic Retinopathy Clinical Research Network . Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: 5-year randomized trial results. Ophthalmology. 2015;122(2):375-381. doi: 10.1016/j.ophtha.2014.08.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Early Treatment Diabetic Retinopathy Study Research Group . Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Ophthalmology. 1991;98(5)(suppl):766-785. doi: 10.1016/S0161-6420(13)38011-7 [DOI] [PubMed] [Google Scholar]
- 18.Baker CW, Glassman AR, Beaulieu WT, et al. ; DRCR Retina Network . Effect of initial management with aflibercept vs laser photocoagulation vs observation on vision loss among patients with diabetic macular edema involving the center of the macula and good visual acuity: a randomized clinical trial. JAMA. 2019;321(19):1880-1894. doi: 10.1001/jama.2019.5790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wykoff CC, Nittala MG, Zhou B, et al. ; Intravitreal Aflibercept for Retinal Nonperfusion in Proliferative Diabetic Retinopathy Study Group . Intravitreal aflibercept for retinal nonperfusion in proliferative diabetic retinopathy: outcomes from the randomized RECOVERY trial. Ophthalmol Retina. 2019;3(12):1076-1086. doi: 10.1016/j.oret.2019.07.011 [DOI] [PubMed] [Google Scholar]
- 20.Couturier A, Rey PA, Erginay A, et al. Widefield OCT-angiography and fluorescein angiography assessments of nonperfusion in diabetic retinopathy and edema treated with anti-vascular endothelial growth factor. Ophthalmology. 2019;126(12):1685-1694. doi: 10.1016/j.ophtha.2019.06.022 [DOI] [PubMed] [Google Scholar]
- 21.Nicholson L, Crosby-Nwaobi R, Vasconcelos JC, et al. Mechanistic evaluation of panretinal photocoagulation versus aflibercept in proliferative diabetic retinopathy: CLARITY substudy. Invest Ophthalmol Vis Sci. 2018;59(10):4277-4284. doi: 10.1167/iovs.17-23509 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study Protocol and Statistical Analysis Plan
eFigure 1. Mean Change in Central Subfield Thickness Over 2 Years
eFigure 2. Mean Change in Optical Coherence Tomography Retinal Volume Over 2 Years
eTable 1. Subgroup Analysis for the Development of PDR and CI-DME
eTable 2. Number of Eyes That Met Each Specific PDR/DME Outcome Criterion at Any Time Within 2 Years
eTable 3. Change in Diabetic Retinopathy Severity at 2 Years
eTable 4. Change in Diabetic Retinopathy Severity at 1 Year
eTable 5. Change in OCT Central Subfield Thickness and Retinal Volume at 2 Years
eTable 6. Change in OCT Central Subfield Thickness and Retinal Volume at 1 Year
eTable 7. Change in Visual Acuity at 1 Year
eTable 8. Subgroup Analysis for Change in Visual Acuity at 2 Years
eTable 9. Annual Treatments for PDR and DME
eTable 10. Endophthalmitis Cases
eTable 11. Ocular Adverse Events of Interest in Study Eyes
eTable 12. Systemic Adverse Events of Interest
eTable 13. All Systemic Adverse Events
eTable 14. All Ocular Adverse Events
Data Sharing Statement