Abstract
On the commercial level, the poultry industry strives to find new techniques to combat bird's infection. During the first week, mortality rate increases in birds because of several bacterial infections of about ten bacterial species, especially colisepticemia. This affects the flock production, uniformity, and suitability for slaughter because of chronic infections. Escherichia coli (E. coli) causes various disease syndromes in poultry, including yolk sac infection (omphalitis), respiratory tract infection, and septicemia. The E. coli infections in the neonatal poultry are being characterized by septicemia. The acute septicemia may cause death, while the subacute form could be characterized through pericarditis, airsacculitis, and perihepatitis. Many E. coli isolates are commonly isolated from commercial broiler chickens as serogroups O1, O2, and O78. Although prophylactic antibiotics were used to control mortality associated with bacterial infections of neonatal poultry in the past, the commercial poultry industry is searching for alternatives. This is because of the consumer's demand for reduced antibiotic-resistant bacteria. Despite the vast and rapid development in vaccine technologies against common chicken infectious diseases, no antibiotic alternatives are commercially available to prevent bacterial infections of neonatal chicks. Recent research confirmed the utility of probiotics to improve the health of neonatal poultry. However, probiotics were not efficacious to minimize death and clinical signs associated with neonatal chicks' bacterial infections. This review focuses on the causes of the increased mortality in broiler chicks during the first week of age and the methods used to minimize death.
Key words: bacterial infection, broiler, pathogenic Escherichia coli, first week mortality
Introduction
The commercial poultry industry depends on raising birds in large quantities at high stocking densities, especially in broiler production systems. According to the European standards, Yassin et al. (2009) postulated that when the daily cumulative mortality rates are too high, the farmer should reduce the number of broiler chicks in the next cycle. However, owing to the highly intensive management systems with overcrowded barn environments, infectious diseases are inevitable. Hence, minimizing the mortality in a flock is crucial to gain profit in subsequent cycles. Notably, the first week of life is very important for the chick as its entire life is transforming during this period. Starting from a conditioned life in the hatchery, they move on to an independent life in a barn where they have to adjust to new feed, water, thermoregulation, and competition and fight infections at the same time (Vieira et al., 1999). Moreover, as their immune systems are not fully developed at hatch (Sedeik et al., 2019; El-Shall et al., 2020), the chick is susceptible to various infections, including bacterial, viral, and parasitic. Out of these infections, bacterial infections occur worldwide in a similar pattern causing massive economic losses to the poultry industry (Nhung et al., 2017).
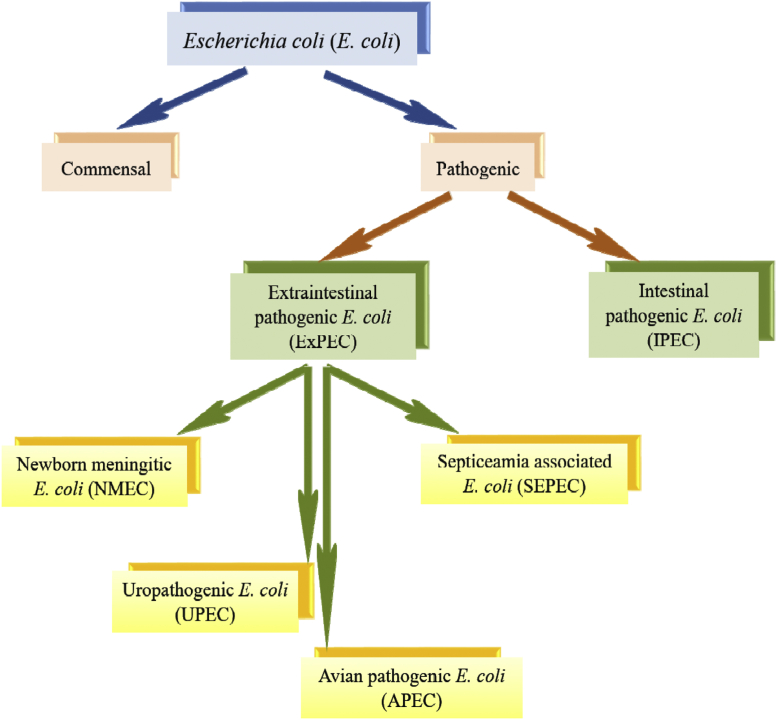
Infections caused by harmful bacteria, particularly during the first week of birds' lives, result in severe mortalities, poor weight gain, and poor flock uniformity. This leads to economic losses for producers. Using the prophylactic and in-feed growth-promoting antibiotics was the preventative strategy for a long time to face the on-going problems. However, various public health concerns, such as the emergence of antibiotic-resistant bacteria in the environment and antibiotic residues in food, have raised questions regarding this practice (Cervantes, 2015; Mehdi et al., 2018). The poultry industry worldwide is choosing antibiotic-free production because of rapidly increasing customer demand resulting from public health points of view (Cervantes, 2015). Several bacterial infections were proved to cause high mortalities in chicks (first-week mortality [FWM]). As a major cause of high FWM, Escherichia coli infections lead to various disease syndromes in baby chicks, including yolk sac infection (YSI) or omphalitis, enteritis, swollen head, respiratory tract infection, cellulitis, and septicemia (Nolan et al., 2020). The acute form of E. coli infection results in septicemic lesions and mortality. In the subacute form, pericarditis, airsacculitis, and perihepatitis are the prominent lesions (Lutful Kabir, 2017; Nolan et al., 2020). Many E. coli isolates commonly associated with commercial broiler chickens belonged to serogroups O1, O2, and O78 (Gomis et al., 2001; Ewers et al., 2004). Pathogenic E. coli isolates were categorized into intestinal pathogenic E. coli, or extraintestinal pathogenic E. coli (ExPEC), depending on the location of the infection. The intestinal E. coli includes enteropathogenic, enterotoxigenic, enteroinvasive, enterohemorrhagic, and enteroaggregative E. coli. In this respect, the study of Russo et al. (2000) identified several traits for distinguishing the 3 pathotypes of ExPEC, including avian pathogenic E. coli (APEC), neonatal meningitis E. coli, and uropathogenic E. coli. Ewers et al. (2003) reported that E. coli pathogenicity was generally enhanced or initiated by several influencing factors: environmental factors, viral infections, mycoplasma infections, and immune suppression. Generally, young birds are more susceptible to severe infections than adults (Rodriguez-Siek et al., 2005a). The horizontal infections with E. coli occur by the contact with other birds, in addition to fecal and oral routes. On the other hand, the E. coli vertical transmission was reported from breeders through eggshell contamination (Nolan et al., 2020). Similar to E. coli, paratyphoid Salmonella species cause YSI and septicemia in newly hatched chicks triggering increased FWM which causes economic losses to the poultry industry (Yassin et al., 2009; Kemmett et al., 2014). The present review article highlights the various bacterial infections that cause the FWM with special reference to E. coli as a major and main participant in FWM of commercial broiler chicks.
FWM in Broilers
The FWM is an essential indicator of the chick quality as well as welfare. The ideal FWM rate in a poultry flock is 0 to 1% (Heier et al., 2002). As FWM highly contributes to the total mortality of the operation, it is important to control it. Multiple factors contribute to increasing the rate of FWM, including breeder factors such as age, strain, genetic line, and egg weight (Lighter eggs cause more chick mortality) (Sedeik et al., 2019). Also, hatchery, feed, and environmental or housing climate (excess ammonia or dust) and season are included factors (Yassin et al., 2009). A study carried out in Taiwan reported that broiler chicks' cumulative mortality depends on the type of ventilation, flock size, distance of shipping the chicks, and delivery route (Chou et al., 2004). The infectious causes contributed to 50% of FWM, while dehydration and nephropathy associated with visceral gout contributed as the other causes in layer chicks (Olsen et al., 2012).
Bacterial Infections as a Cause of FWM
Bacterial infections are a leading cause of FWM (Karunarathna et al., 2017). Despite the scarcity of informative studies, Kemmett et al. (2014) reported their findings on the significant contribution of E. coli on chicks' mortality within the first 48-72 h of placement. In their study, approximately 70% of the dead chicks displayed colibacillosis signs, and thirty different virulence profiles were identified in the E. coli isolates. In another study on 48 layer flocks, the average FWM was about 1.4%. More than half of the flocks showed FWM due to infectious causes, YSI followed by septicemia being demonstrated in 94% of the flocks. Salmonella is the next frequently isolated genus from YSI with high FWM in broiler and layer chicks, followed by many other bacterial species as Pseudomonas, Staphylococcus, Streptococcus, Proteus, Klebsiella, Enterococcus, Corynebacterium, Citrobacter, Aeromonas, Bacillus, Clostridium, Micrococcus, Yersinia, Enterobacter, Aerobacter, Achromobacter, and Alcaligenes leading to several economic losses (Shivaprasad, 2000; Cortés et al., 2004; Khan et al., 2004; Olsen et al., 2012). E. coli and Enterococcus faecalis were the most frequently isolated organisms from those infections (Olsen et al., 2012). In a study in Ethiopia during 2010-2011, a prevalence of 33.1% YSI was recorded in the FWM predominantly among 3- to 5-day-old chicks. E. coli was isolated as the most prevalent bacteria from these cases, followed by Staphylococcus aureus (Amare et al., 2013). Moreover, Rai et al. (2005) reviewed the YSI prevalence data in the previous studies. They reported that YSI is the most common cause of FWM in chicks. The various reports concluded that the incidence of YSI varied between 5.1 and 20.7%, and mortality has been up to 31.5%. However, Karunarathna et al. (2017) reported the incidence of Enterococcus (29.71%) and E. coli (19.46%) isolated from dead chick embryos during the incubation.
E. coli Infections in Chickens
E. coli is the species of the genus Escherichia that belongs to the family Enterobacteriaceae. It is a gram-negative, non–acid-fast, uniform staining, non–spore-forming bacillus. Most of the E. coli strains have peritrichous flagella and therefore are motile. They are able to grow in both aerobic and anaerobic environments. They can grow on nutrient media at 18 to 44°C, fermenting glucose and producing gas. The E. coli produce diffused turbidity in both cultures, and on blood agar, colonies are usually 1 to 3 mm. The colony morphology may vary with rough colonies larger with irregular margins and smooth colonies raised, wet-looking with well-demarcated margins (Nolan et al., 2020). Generally, the recorded E. coli serotypes are more than 1,000 but fewer have been implicated in poultry diseases (Awad et al., 2020). E. coli is commonly present in the intestinal tract, mucosal surfaces, bird's skin, and feathers. Chickens' intestine contains about 106 colony-forming units of E. coli per gram feces. These strains always belong to both pathogenic and nonpathogenic types (Awad et al., 2020). Nevertheless, only these strains have specific virulence factors contributing to the ability to cause diseases in birds and are known as APEC. These infections are mostly extraintestinal, with a majority being respiratory and systemic infections. As a result, APEC has been categorized as ExPEC that share similar virulence to the E. coli strains that cause human urinary tract infections, sepsis, and newborn meningitis (Manges et al., 2012; Mellata, 2013) (Figure 1). Fecal contamination of eggs leads to YSI, which results in late embryonic mortality or early chick mortality up to 3 wks after hatching (Nolan et al., 2020). It was reported that APEC's egg transmission is common, leading to high FWM with consideration of vertically transmitted fluoroquinolone-resistant E. coli from clinically normal breeders (Rosario et al., 2004; Giovanardi et al., 2005; Petersen et al., 2006). Based on the infection's acquired time, embryonic, or at hatch, mortality could start as soon as hatching or as late as 20 h after hatch. Early studies on APEC strains showed that O1, O2, O15, O35, and O78 serotypes were mostly associated with colibacillosis outbreaks (Dho-Moulin et al., 1999). A later study by Nolan et al. (2020) confirmed the presence of O18, O81, O115, O116, and O132 serotypes, which were signals for the emergence of new pathogenic serotypes. In addition, Younis et al. (2017) and El-Sawah et al. (2018) showed wide antigenic diversity among APEC strains worldwide.
Figure 1.
Prevalence and possible transmission of extraintestinal pathogenic Escherichia coli (EXPEC).
Serogroups, Antigenic Structure, and Virulence Factors of APEC
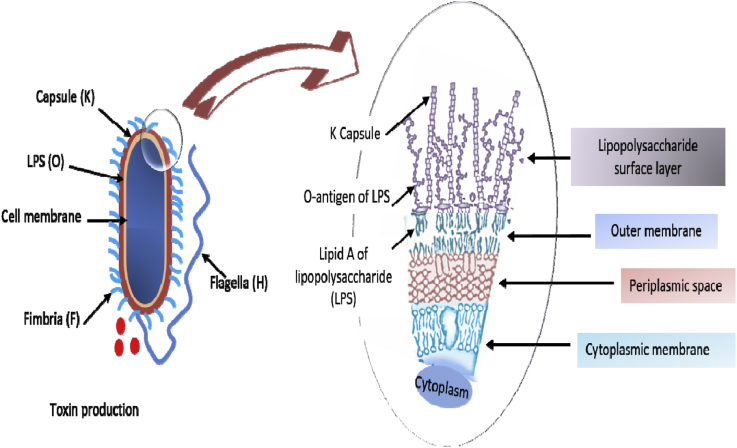
In E. coli, O antigen determines the serogroup and K (capsular) or H (flagellar) antigens determine the serotypes (Fratamico et al., 2016). O somatic antigen comprises the antigenic portion of the lipopolysaccharide (LPS) in the cell wall (Figure 2). Owing to the O antigens' high variability, it is used to differentiate serogroups as an epidemiological tool (Stenutz et al., 2006). There are 180 O, 60 H, 80 K, and 17 F (fimbrial or pillus-P) antigens (Stenutz et al., 2006). The immune response in poultry birds is mainly directed toward the O antigen of E. coli. According to serotyping, the most common serotypes that have been associated with poultry infections are O1, O2, O18, O35, O36, O78, and O111 (Nolan et al., 2020). Out of those, O1, O2, and O78 are the most commonly isolated from cases (Gomis et al., 2001; Ewers et al., 2004). K1 capsular antigen is frequently associated with APEC strains, and it is associated with resistance to serum bactericidal effects (La Ragione and Woodward, 2002; Nakazato et al., 2009). Flagellar (H) antigen, which projects out of the cell, is rotated to provide motility. In E. coli and several other species, the central region of the flagellum is variable and consists of H-serotype–specific epitopes (Wang et al., 2003).
Figure 2.
Antigenic structure of pathogenic Escherichia coli (note: K, capsule; O, antigen and lipid A of LPS; F, fimbriae; and H, flagella).
Virulence Factors of Pathogenic E. coli Isolated From Birds
Protections or Serum Resistance Genes
Serum resistance virulence genes (VGs) allow the bacteria to survive in the exterior of the gastrointestinal tract and overcome the host's defense mechanisms involving complement and antimicrobial peptides (Schouler et al., 2012). Mellata et al. (2003) reported that the capsular K1 and somatic O78 LPS increased serum resistance of the APEC, leading to bacteremia. Nilsson et al. (2014) showed a strong correlation between the APEC pathogenicity and 4 serum resistance VGs such as increased serum survival (iss), structural genes of colicin V operon (cvaC), surface exclusion protein, and outer membrane protein A. This was significantly associated with APEC compared with avian fecal E. coli. However, the individual existence of the iss gene or whether this iss gene was a marker gene for the presence of the plasmids correlating with APEC pathogenicity was not confirmed. The determination of APEC resistance to heterophils and macrophages is another important trait that promotes the successful infection. It may be related to complement resistance. Kottom et al. (1997) noticed that a complement-sensitive mutant E. coli (C3 subunits) bound and phagocytozed is more significant than the wild-type APEC strain from which it had been derived. The presence of type 1 and P fimbriae, O78 antigen, and the 0-min chromosomal region contributed to the protection of APEC against the bactericidal effect of phagocytes, in particular, heterophils (Mellata et al., 2003). Certain APEC strains can survive within macrophages and cause their destruction through apoptosis (Bastiani et al., 2005). Caspases, enzymes essential for apoptosis, were activated by a strain of APEC (APEC17), which resulted in cytotoxicity within 8 h of infection (Bastiani et al., 2005).
Adhesions
Bacterial adhesion is based on the recognition between bacterial surface components and specific receptors in the host tissues. Arp et al. (1980) concluded that piliated and motile E. coli were more virulent to turkey poults. This indicates the importance of structures that provide adhesive properties (adhesins), increasing virulence in ExPEC. ExPEC strains encode many adhesions that promote the attachment of the bacteria to cell receptors and are very important for developing septicemia (Monroy et al., 2005). Type 1 fimbriae (F1 fimbriae) have been involved with the initial stages of upper respiratory colonization.
In contrast, the P fimbriae were involved in the colonization of the internal organs such as air sacs, lungs, kidney, blood, and pericardial fluid, depicting their involvement in later stages of infection (Wooley et al., 1998). The F1 fimbriae were encoded by 9 fim genes which include a major protein named FimA and minor proteins named as FimF, FimG, and FimH adhesions. Nevertheless, an earlier study of Arne et al. (2000) showed that the APEC FimH mutant strain failed to adhere to the chicken trachea epithelial cells in vitro. The P fimbriae are hemagglutinating fimbriae with mannose-resistant properties; they were found in E. coli strains producing human urinary tract infections as well as some APEC (Arne et al., 2000). Moreover, they were linked to the colonization of internal organs, which led to septicemia and lethality in one-day-old chickens. This is a temperature-sensitive hemagglutinin (tsh) gene that causes hemagglutination activity at 26°C and repression at 42°C (Provence et al., 1994). The tsh gene is an important virulence marker of APEC, having a strong association with internal organs colonization, septicemia, and lethality in one-day-old chicks (Ngeleka et al., 2002). At the same time, this protein's mucinolytic ability suggests that it must be playing an important role in the colonization of the tracheal mucosa (Kobayashi et al., 2010). P fimbriae were encoded by pyelonephritis-associated pili gene clusters (pap). This pap gene cluster involved 11 genes (papI, papB, papA, papH, papC, papD, papJ, papK, papE, papF, papG), for the biogenesis and synthesis of the P fimbriae (Dozois et al., 2000). Another type of adhesin in APEC, other E. coli strains, and Salmonella enterica is the curli fimbriae (Nakazato et al., 2009). They were identified and reported as thin coiled surface structures that consist of a single type of subunit called curlin. It is associated with the bacterial binding to the extracellular matrix proteins such as fibronectin, laminin, fibrinogen, and H kininogen, enhancing the survival of such bacteria in the extracellular environment (Olsén et al., 1998). According to La Ragione and Woodward (2002), the genes accountable for curli fimbriae expression were encoded by 2 types of operons: csgBAC and csgDEFG. CsgA sequence was recognized in all APEC and recovered from chickens suffering from septicemia (Amabile de Campos et al., 2005). Additional adhesions recognized between APEC strains suggested to be involved in these strains' pathogenesis include type 1–like fimbriae, AC/1 fimbriae, Afa, Sfa, F17, and Eae fimbriae–related sequences (McPeake et al., 2005).
Toxins
Cytotoxic activity in APEC was first studied in the early 1990s (Fantinatti et al., 1994). Several toxins were described in APEC strains but with unclear roles in pathogenesis. These involve cytolethal distending toxin, enterohaemolysin, cytotoxic necrotizing factor 1, cytotoxin designated VT2y (Parreira et al., 1998), microcin ColV (cvaC), haemolysin, and secreted autotransporter toxin (Tóth et al., 2003). According to Ewers et al. (2005), the vacuolating autotransporter-type toxin coded on a pathogenicity island named VAT-PAI was found to have a role in APEC's virulence as it was identified with a high frequency between APEC compared with avian fecal E. coli. The existence of enterohaemolysin, secreted autotransporter toxin, and cytotoxic necrotizing factor 1 genes has also been described in APEC strains (da Silva et al., 2017). However, their function in pathogenesis was not fully clarified. In addition, some of the toxins' genes (haemolysin, cytolethal distending toxin, and cvaC) have been associated with large transmissible plasmids, indicating that these VGs might be easily transmitted to other strains (Mellata et al., 2012). A recent study by Murase et al. (2016) suggested that hlyF, one of the ColV plasmid genes, is a molecular indicator for APEC. Moreover, this gene was directly included in the outer membrane vesicle production. Shiga toxin gene had been detected in APEC by PCR, but the proof of its expression was little. Lately, a strong mediator for apoptosis (caspase 3/7-induced) and cytotoxic action was described, after a 6-h infection assay using macrophage cell line by an APEC strain (Bastiani et al., 2005). Other toxins described in APEC strains involved the heat-labile enterotoxin and the heat-stable enterotoxin 1 (AstA), homolog of enteroaggregative E. coli (Janben et al., 2001).
Iron acquisition mechanisms
In APEC, various iron-acquisition mechanisms or operons were found on large plasmids (siderophores, aerobactin or hydroxamate siderophore, yersiniabactin, sit, and iro systems) which are less common in commensal E. coli strains (Dozois et al., 2000; La Ragione and Woodward, 2002; Sabri et al., 2006; Johnson et al., 2006a, b; Caza et al., 2008; Tivendale et al., 2009; Caza et al., 2011). Similar to S. enterica serovar Typhimurium isolate (Zhou et al., 1999), this sit operon was described in APEC using genomic subtractive hybridization and signature-tagged mutagenesis (Li et al., 2005; Schouler et al., 2012). The sit operon encodes an ABC transport system involved in iron and manganese metabolism and resistance to hydrogen peroxide (Sabri et al., 2006).
Invasins
The ibeA gene is found in chromosomal pathogenicity islands (PAIs) (APEC O1) of APEC, promoting the invasion of brain microvascular endothelial cells (BMECs) by neonatal meningitis ExPEC. The inactivation of ibeA significantly reduced the abilities of APEC strain BEN2908 to invade human BMEC, adhere to BMEC, and cause avian colibacillosis. The ibeA occurs in 14–20% of APEC (Rodriguez-Siek et al., 2005b, Rodriguez-Siek et al., 2005a; Germon et al., 2005; Kariyawasam et al., 2006; Cortes et al., 2008). Also, ibeA of APEC BEN2908 dominates the cellular endocytic pathways to invade cultured avian hepatocytes and human pneumocytes (Chanteloup et al., 2011).
Virulence Gene Traits in APEC Isolated From Chicks Worldwide
The pathogenicity of APEC in relation to the presence of certain VG patterns was studied. Several patterns have been suggested as diagnostic tools for rapid detection of APEC. These patterns include pentaplex pattern containing hlyF, iutA, iroN, iss, and ompT genes (Johnson et al., 2008), the presence of 5 to 8 genes of the iss, tsh, papC, astA, irp2, vat, iucD, and cva/cvi genes (Kwon et al., 2008), and the presence of crl, fimH, and aer gene patterns (Ghanbarpour et al., 2011). Recently, the presence of one of 4 combination patterns of VGs—A [iutA+, P(F11)+], B [iutA+, P(F11)-, frzorf4+], C [iutA+, P(F11)-, frzorf4-, O78+], and D [iutA-, sitA + aec26+]—were reported (Schouler et al., 2012). Finally, at least 8 to 13 VGs were detected in chicken, whereas intermediate pathogenic isolates contained at least about 5 to 8 VGs (Wang et al., 2015).
In Egypt, 4 VGs (iucD, irp2, iss, and tsh) were detected using multiplex PCR in APEC serotype O27, while O78 and O86 had 2 VG (iucD and iss) and O115 had iss VGs gene only (Ammar et al., 2011). Helal (2012) found out iss and iucD VGs using multiplex PCR. Both genes were present in E. coli serotypes O1 and O146, while O114 and O119 serotypes only have iss gene. Regarding the VGs in APEC showing extended-spectrum beta-lactamase activity, AbdEl-Tawab et al. (2018) detected iss and outer membrane protein A VGs only in all examined isolates.
Pathobiology of Colibacillosis in Neonatal Chicks
In young chickens, E. coli mostly transmitted horizontally through the respiratory tract by the inhalation of contaminated fecal dust in hatchers or barn. Chicks also get infected in the hatcheries by APEC-contaminated or infected eggs (Nolan et al., 2020). These infections were suggested to happen by the entry of bacteria through the yolk sac. Sometimes, vertical transmission of E. coli is also possible from a hen with salpingitis caused by E. coli. During the incubation period, APEC causes YSI and embryo mortality. Posthatch infections happen 24 to 48 h after hatching, and mortality elevate up to 10 to 20% for 2 to 3 wks because of septicemia. In the initial part of the infection, lung congestion, edematous serous membranes, and splenitis were apparent. A few days later, acute, fibrinoheterophilic polyserositis appears involving the pericardium, pleura, air sacs, and perihepatic tissue. These chicks usually have retained yolk sacs and yolk abscesses, indicating the yolk sac entry of bacteria. Out of the survivors, up to 5% of the flock may be stunted, and the unaffected birds may grow naturally (Lutful Kabir, 2017; Nolan et al., 2020).
Bacterial Contamination of Poultry Feeds
Sanderson et al. (2005) reported that coliform bacteria, including Escherichia spp., Klebsiella spp., and Enterobacter spp., were the species contaminating animal feeds most. As such, they prompt zoonotic disease outbreaks and secondary infections in humans resulting in major hospitalization cases. Therefore, the ingredient quality control component of a poultry operation feed mill is an important first step in preventing birds' contamination on the farm. Magwedere et al. (2015) indicated that foodborne and waterborne illnesses associated with Salmonella spp. were more commonly due to increased fecal pollution of feed resources. The study also reiterated that animal waste as fertilizer for crops or raw materials destined for producing animal feed is a common practice by some farmers. The Salmonella serotypes found in feed ingredients are often not the same as those commonly found in processed poultry. It is plausible that such raw materials may lead to subsequent contamination of the feed mill environment. Poultry feed, which comprises 68% of total production costs, is typically composed of maize and soybean meal mixtures, including several vitamins and minerals, and generally contains 2 or 3 medications (Jones et al., 2016). The microbial contamination of different animal feeds depend on the water activity, oxygen concentration, pH, and nutrient composition of the feed ration (Kim et al., 2007). Feed materials are usually inoculated during growing, harvesting, processing, storage, and dispersal of the feed (Maciorowski et al., 2004; Jones et al., 2016). A soil mixed with animal feces can contaminate standing crops either by direct deposition or fertilizer. Houseflies and cockroaches feeding on the fecal matter can act as both vectors and reservoirs for pathogens in the environment (Maciorowski et al., 2004; Kim et al., 2007). Most plant-based feeds provided to small animals have a difficulty to be treated or disinfected. As a result, the risk of foodborne diseases on animals and consumers will be possible (Maciorowski et al., 2007). Heat treatment is the most effective control method used to inactivate feed pathogens (Abd El-Hack et al., 2017; Saeed et al., 2017). Reductions in bacterial contamination by heat depend on the temperature, treatment time, and moisture content of the feed (Maciorowski et al., 2004). Chemical treatments, such as organic acids alone or formaldehyde, are sometimes used (Meeker and Meisinger, 2015). Also, pelleting is a process of pressing conditioned material with specific dimensions of openings and thickness. One of the supplementary benefits of pelleting is the destruction of pathogens and reduced total microorganisms due to the increased temperature during processing (Zimonja, 2009). Applications of steam and water in animal feed manufacturing have long been recognized as a good way to achieve high-quality pellets (Sredanović et al., 2005). Because poultry feeds are a main source of foodborne pathogens, on-farm control efforts are needed to decrease and prevent feed contamination (Sanderson et al., 2005; Maciorowski et al., 2007). Improving biosecurity measures for feed storage at the feed mill or on the farm would likely be a more cost-effective risk management strategy to lower pathogen proliferation in animal feeds.
Bacterial Contamination in Water Resources
Water composition varies with geographical region and environmental conditions. Water contamination can occur if surface water drains into the well, especially if the water source is exposed. Several researchers have demonstrated a positive association between drinking water contaminations with E. coli O157:H7 and this organism's presence in poultry feces (Saha et al., 2009; Levantesi et al., 2012). Poor water quality may interfere with digestion and subsequent bird performance (Narita et al., 2004; Bain et al., 2014). The effectiveness of vaccines and medications administered through the water can be reduced when water quality is poor (Narita et al., 2004). Leaky water through nipples inside the poultry house will wet the litter and lead to increasing ammonia production and prompt microorganism's proliferation (Ashbolt, 2015). Therefore, control measures should be prioritized to prevent the occurrence of diseases that are spread through water and would certainly result in great economic losses (Kostyla et al., 2015). In this regard, water is an excellent transmission route for agents responsible for human and animal diseases, mainly those in which fecal-oral transmission occurs because contamination of water supplies still gradually increases because of urban and rural activities (Bain et al., 2014). In 2004, about 4 million people were still obtaining water from rivers, ponds, and springs, which were usually fecally contaminated and not treated. So, safe and sufficient water and sanitation would reduce animal mortalities and child deaths by 50% and prevent 25% of diarrhea (Momba et al., 2003a,b). Such waterborne outbreaks often lead to a considerable number of individuals being simultaneously affected, and in most cases, the outbreak subsides when the water supply is adequately treated (Pillsbury et al., 2010). Momba et al. (2006) reported the abundance of pathogenic E. coli, Salmonella typhimurium, and Vibrio cholerae in both surface and groundwater sources in South Africa. The presence of these pathogenic bacteria in drinking water sources poses a serious health risk to consumers. Eliminating E. coli O157:H7 from poultry drinking water may be a meritorious goal and an effective measure to reduce poultry drinking water contamination with this pathogenic bacterium (Bucher et al., 2007).
Fecal contamination of water bodies can lead to waterborne illnesses and is detrimental to human health, with microbial contamination being a major cause. Concentrated animal feeding operations (CAFOs) are a potential culprit for microbial water contamination. Human waste from these operations can end up in streams, rivers, and lakes, especially from rain events (Hribar, 2010). Fecal indicator bacteria such as E. coli have been used to detect and determine the level of fecal contamination in environmental waters to protect the general population from water-related pathogens (USEPA, 2009). However, owing to antibiotic use to protect animals from infection, CAFOs have consequently grown antibiotic-resistant bacteria, including E. coli, which also can end up in the environment from animal waste (Hribar, 2010). American agriculture has transitioned from family-owned small farming to large-scale corporate farming in the last century, with a few companies producing most of the food animals (MacDonald et al., 2009). Today, these animals' production occurs in CAFOs, which are essentially large-scale industrialized agricultural factory farms. To qualify as a CAFO, a farming operation must first be considered as an animal feeding operation, which is defined as “a lot or facility where animals are kept confined and fed or maintained for 45 or more d per y, and crops, vegetation, or forage growth are not sustained over a normal growing period” (USEPA, 2009; Hribar, 2010).
CAFOs are classified by type and number of animals and how they discharge their animal waste into the nearest body of water. There are size thresholds in considering a CAFO to be small, medium, or large. For poultry, especially laying hens or broilers, the CAFO (which has a liquid manure handling system) is considered large with 30,000 or more chickens, medium with 9,000 – 29,999, and small with 9,000 or fewer chickens (USEPA, 2009). A large number of animals means a large amount of waste, where most environmental health issues arise. Poultry CAFO waste can have several contaminants, such as nutrients, pathogens, and antibiotics (Hribar, 2010). Previous studies have shown that poultry CAFOs have contaminated surrounding watersheds by runoff containing poultry litter (bedding contaminated with feces) (Campagnolo et al., 2002; Mallin and Cahoon, 2003). Poultry litter can lead to degradation of water quality through chemical and microbial pollution. A study conducted by Harden (2015) found that watersheds that had swine and poultry CAFOs exhibited significantly greater nutrient contamination, including ammonia, nitrate, and total N, than watersheds that lacked these operations. Previously, Stone et al. (1995) also found a stream that had both swine and poultry CAFOs and elevated nutrient concentrations during both dry and wet weather events than a nearby background stream that lacked these operations. Nutrients in poultry waste, such as nitrogen and phosphorus, can contribute to eutrophication, which is when there are excessive amounts of nutrients in a body of water, which leads to algal bloom growth which can be detrimental to local ecosystems (USEPA, 2009; Slonczewski, 2017).
A study conducted by Hurby (2015) in the United States found that some of these pathogens from poultry manure can survive weeks in soil, which means heavy precipitation events could also cause water contamination by collecting and discharging the slurry (soil and water mixture) into the nearest body of water. Antibiotics are found in residual levels in waste as they were used to ensure the animals can stay healthy in fighting off a potential infection and, until recently, to promote growth (Marshall et al., 2011). However, overuse of antibiotics has led to antibiotic resistance in pathogens such as E. coli because of selective pressure, causing some treatments for infection to be ineffective (Kaufman, 2000; Martínez-Antonio et al., 2008). In 2013, the Food & Drug Administration announced a plan to phase out certain medical antibiotics used in livestock to curb antibiotic resistance (USDA, 2013).
Tyson, the leading poultry producer in the United States, has curtailed antibiotic usage, and the company notably claimed it would eliminate antibiotics important to human medicine for raising its poultry by 2017 (Meyer, 2017). Monitoring E. coli and its potential for antibiotic resistance helps maintain water quality and subsequently, public health.
E. coli and Its Role in Water Quality
E. coli is a fecal coliform bacterium that is found in the intestines of humans and animals. Most strains are considered harmless to humans, but some strains produce shiga-toxin, causing hemorrhagic diarrhea, and the serotype O157:H7 is the one most related to foodborne illnesses (WHO, 2018). E. coli is commonly used as a fecal indicator organism (FIO) of human and/or animal fecal contamination in freshwater (Pitout et al., 2017; CDC, 2018). E. coli is monitored in water as its presence is indicative of other potential pathogenic microbes that could also be present (Edberg et al., 2000). For E. coli to qualify as an FIO, it should ideally meet criteria according to the Routledge Handbook of Water and Health (Bartram, 2015). While no one FIO currently satisfies all the criteria under all circumstances, many regulatory agencies and scientists still consider E. coli and members of the fecal coliform group as the best for microbial water quality testing (USEPA, 2009).
Owing to high volumes of antibiotics used in animal agriculture, monitoring antibiotic-resistant E. coli is also of interest. Some poultry CAFOs use antibiotics to prevent or treat diseases and, until recently, promote growth, but this can create potential antibiotic resistance in bacteria such as E. coli because of selective pressure (Martínez-Antonio et al., 2008). Antibiotic resistance occurs when the antibiotic kills most of the intended susceptible bacteria. Still, a small number naturally immune to this antibiotic can survive and reproduce, creating a population of predominantly antibiotic-resistant bacteria (CDC, 2018).
Antibiotic resistance has been an increasing public health issue in relation to CAFOs, as the use and overuse of antibiotics in animal feed have led to microbes becoming antibiotic-resistant (Kaufman et al., 2000). Animals such as poultry do not completely metabolize antibiotics and still exist in their waste (Hribar, 2010). One study tested antibiotic resistance of E. coli in fecal samples of turkeys and broilers, in farms and slaughterers, and found that there was significantly higher (P < 0.005) antibiotic resistance to ciprofloxacin, flumequine, and neomycin than laying hens that did not have high antibiotic usage (Bogaard et al., 2001). A related study found a high prevalence of antibiotic resistance but of S. aureus in turkey (79%; 22/28) and chicken (26%; 6/23) isolates. Antibiotic resistance is not confined to poultry CAFOs but is a concern across industrial food animal production facilities. In 2007, Sapkota et al. (2007) analyzed surface water samples downstream of a swine CAFO and found significant antibiotic resistance of erythromycin (P = 0.02) and clindamycin (P < 0.001) in enterococci. Christenson and Stewart (2018) also found higher antibiotic resistance in E. coli isolated from surface water downstream of CAFOs than from background watersheds (19% vs. 6%). Some studies have also noted higher levels of contamination after precipitation events.
Prevention of Bacterial Infections in the Poultry Folks
Prevention of various infections that could be detrimental to poultry health is a significant responsibility in managing a poultry operation. Poultry producers, veterinarians, and other stakeholders have worked together in developing protocols applying strict biosecurity measures to prevent the introduction of infections at various stages of the production cycle.
Antibiotics use
For years, prophylactic antibiotics have been used in the commercial poultry industry to prevent bacterial infections such as APEC, salmonellosis, and necrotic enteritis. Antibiotic growth promoters were introduced in the 1940s when feeding dried mycelia of Streptomyces aureofaciens containing chlortetracycline residues was found to improve growth in animals (Castanon, 2007). The earliest report of the use of growth-promoting antibiotics ran to 1946 when streptothricin, sulfasuxidin, and streptomycin were used in the feed (Moore et al., 1946). Most commonly, ceftiofur (no longer in use), gentamicin, and lincomycin-spectinomycin were given in ovo at commercial hatcheries. Once they reached the barns, enrofloxacin (no longer in use), amoxicillin, penicillin-streptomycin, oxytetracycline, sulfamethazine, sulfaquinoxaline, tetracycline, and tetracycline-neomycin were used in water to prevent or treat neonatal diseases and bacterial septicemias. As for the in-feed antibiotics, the report indicates that penicillin, sulfatrimethoprim, tylosin, virginiamycin, bacitracin, and oxytetracycline were used mainly to prevent necrotic enteritis. Ionophorous and chemical anticoccidial drugs such as lasalocid, maduramicin, monensin, narasin, salinomycin, and nicarbazin were used in feed to prevent coccidiosis (Agunos et al., 2017).
Antibiotic Resistance and the Need for Alternatives
Antibiotic resistance in APEC strains isolated from several regions, including Asia, Africa, the United States, Brazil, and Spain reported >70% resistance to ampicillin, amoxicillin, and tetracycline. Resistance against ciprofloxacin, neomycin, and chloramphenicol was 50-70% (Nhung et al., 2017). As the public health concerns of antibiotic resistance and the resulting withdrawal of antibiotics from prophylactic uses started happening in recent years, a dire need for alternative strategies has sparked in the production animal industry. Many researchers seek the potential of various natural and synthetic molecules in fighting bacterial infections, weighing their efficacy and cost-effectiveness on the scale. Probiotics, prebiotics, synbiotics, postbiotics, organic acids, vaccinations, innate immune stimulation, and improving biosecurity are at the forefront of these alternatives. Although these strategies may not have shown consistent efficiency throughout research, combinations of 2 or more approaches have exhibited proficiency (Caly et al., 2015; Postma et al., 2015; Suresh et al., 2017).
Antimicrobial resistance has drastically increased because of the global misuse and overuse of antibiotics (Fletcher, 2015). This is prompted by the high prevalence of foodborne zoonotic pathogens such as E. coli, Enterococcus spp., S. aureus, and nontyphoidal Salmonella and Campylobacter spp. (Landoni and Albarellos, 2015; Abd El-Hack et al., 2021). Global monitoring epidemiology reported the resistance of E. coli and Salmonella strains to ampicillin, chloramphenicol, and trimethoprim-sulfamethoxazole (Hendriksen et al., 2011). They also confirmed that the application of antimicrobials in food animals might lead to the development of resistant strains of bacteria, which propagates to infect both animals and humans (Hendriksen et al., 2011).
Oguttu et al. (2008), on antimicrobial drug resistance of E. coli isolated from poultry abattoir workers at risk and broilers, reported antimicrobial usage in food animals to be increasing and resulting in the development of antimicrobial drug–resistant bacteria. It has been suggested that this resistant bacterial strain can be transferred to people working with such animals, for example, abattoir workers (Oguttu et al., 2008).
Antimicrobial drug resistance was investigated for E. coli from broilers raised on feed supplemented with antimicrobials and the people who carry out evisceration, washing, and packing of intestines in a high-throughput poultry abattoir. In addition to their specific effects, edible tissues of poultry might contain veterinary drug residues, which would cause hazardous health effects in humans, such as toxicological effects, hypersensitivity, allergic reactions, change of gut microflora, and increased bacterial resistance to antimicrobials (Beyene, 2016). Serious concerns are raised on the antimicrobial resistance in zoonotic enteropathogens (Salmonella spp., Campylobacter spp.), commensal bacteria (E. coli, enterococci), and bacterial pathogens of animals (Pasteurella, Actinobacillus spp.).
Alternative Strategies to Antibiotics
Since antibiotic use is diminishing because of concerns such as the emergence of antibiotic-resistant bacteria, alternative strategies have become valuable in the control of infections (El-Saadony et al., 2019, Abdelnour et al., 2020a, Abdelnour et al., 2020b; Abd El-Hack et al., 2020a,b; Akl et al., 2020; Ashour et al., 2020; El-Saadony et al., 2020a,b; Reda et al., 2020; Sheiha et al., 2020).
Probiotics, Prebiotics, Synbiotics, and Postbiotics
The Food and Agriculture Organization and the World Health Organization collectively defined probiotics as “live microorganisms that grant a health benefit to the host” (Bajagai et al., 2016). Several microorganisms are being used as probiotics for poultry species, such as bacterial and nonbacterial organisms. Some of the bacterial probiotics are several Lactobacillus species (Mookiah et al., 2014; Forte et al., 2018), Bifidobacterium (Khaksar et al., 2012; Pedroso et al., 2013), Bacillus (Abdelqader et al., 2013), and Enterococcus (Mountzouris et al., 2010). Yeast or fungal probiotics are added to feed such as Saccharomyces cervisiae (Aluwong et al., 2013; Bai et al., 2013), Aspergillus oryzae (Daşkıran et al., 2012; Shim et al., 2012), Candida pintolopsii (Daşkıran et al., 2012), and Saccharomyces boulardii (Rajput et al., 2013) that caused positive effects on the performance and gut health.
There are also certain adverse effects reported contradictory to the positive effects (Philpot, 2015). However, multiple reports depict the positive enteric health effects of probiotics in improving intestinal integrity (Dalloul et al., 2003; Ritzi et al., 2014) and mentioning that they could be a great alternative to antibiotics in the poultry industry (Nava et al., 2005; Li et al., 2014).
According to Food and Agriculture Organization, prebiotics is defined as nondigestible food ingredients that benefit the host. They selectively stimulate the growth and activity of bacteria in the colon and thereby improve host health (Pineiro et al., 2008). They are supplying a substrate for beneficial microorganisms in the gastrointestinal tract. Although the previous definition has focused only on few carbohydrates, researchers have redefined prebiotics, including various oligosaccharides containing varying carbon lengths, and collectively designated them as nondigestible oligosaccharides. Different molecules such as fructooligosaccharides, galactooligosaccharides, mannan oligosaccharides, inulin, and isomaltooligosaccharide are among the nondigestible oligosaccharides that have beneficial properties as prebiotics (Ferreira et al., 2011; Ricke, 2018; Alyileili et al., 2020a,b). Reports suggested that certain nondigestible oligosaccharides such as fructose oligosaccharides, inulin-type fructans, and mannanoligosaccharides can modulate gastrointestinal microbiota by increasing the Lactobacillus population while reducing harmful pathogens such as E. coli and Clostridium perfringens (Kim et al., 2011; Pourabedin and Zhao, 2015). Alyileili et al. (2020c) studied the application of nondegraded date pits and degraded date pits using the cellulolytic fungus Trichoderma reesei as prebiotics in broiler's diets on the gut bacterial growth and growth performance. They demonstrated that the supplementation of 10% of degraded date pits in broilers' diet, partly replaced with dietary maize, served as a gut health enhancer and thus a growth promoter in the diet for broilers without the use of antibiotics (Alyileili et al., 2020c).
Synbiotics are made by synergistically combining probiotics and prebiotics to provide beneficial effects to the host by promoting microbial feed supplements in the gastrointestinal tract (Andersson et al., 2001). Studies have shown the beneficial effects of synbiotics on reducing harmful bacteria such as Campylobacter jejuni (Baffoni et al., 2012) and Salmonella typhimurium (Revolledo et al., 2009). Markazi et al. (2018) recently reported that drinking water with synbiotic supplementation helps to influence a healthy microbiota and improved immune response in the intestines of laying hens during a Salmonella challenge. The most recent review by Micciche et al. also compiled study reports of synbiotics effect on improving gut health and weight gain other than reducing the harmful effects of Salmonella (Micciche et al., 2018). Markowiak and Slizewska (2018) published a review in 2018, compiling most of the studies that showed beneficial effects of synbiotic use in poultry birds and other production animals rather than using probiotics or prebiotics alone. Depending on the probiotic and prebiotic combination, the host's immunomodulatory and microbiome modification effects can vary, as observed via in ovo delivery (Dunislawska et al., 2017). An interesting study revealed that synbiotic treatment during heat stress could act as an effective management tool to minimize detrimental effects, particularly in hot regions of the world (Mohammed et al., 2018).
A new term called postbiotics is recently introduced into the poultry industry, referring to the soluble factors (products or metabolic byproducts) secreted by live bacteria or released after bacterial lysis, such as enzymes, peptides, teichoic acids, peptidoglycan-derived muropeptides, polysaccharides, cell surface proteins, and organic acids. It is characterized by their clear chemical structure, long shelf-life, safe usage, and dosage. Their properties including anti-inflammatory, immunomodulatory, antiobesogenic, antihypertensive, hypocholesterolemic, antiproliferative, hepatoprotective, and antioxidant led to the improvement of host health through enhancing several physiological functions (Aguilar-Toalá et al., 2018). Postbiotics are mainly derived from Lactobacillus, Bifidobacterium, Streptococcus, and Faecalibacterium species (Konstantinov et al., 2013; Tsilingiri and Rescigno, 2013).
Vaccines against E. coli
Vaccination is a common strategy used in the control of most viral infections. However, several vaccines have been developed to prevent diseases caused by bacterial pathogens such as Salmonella, E. coli, Campylobacter, Pasteurella multocida (fowl cholera), Avibacterium paragallinarum (infectious coryza), Ornithobacterium rhinotracheale, and Bordetella avium in poultry birds (Desin et al., 2013; Filho et al., 2013). Although several vaccine candidates such as whole organism and subunit vaccines have been experimentally tested, the protective efficacies of these commercial vaccines against salmonellosis and colibacillosis have been minimally studied.
Poultry researchers use different strategies from generating autogenous vaccines to modified live, inactivated, and subunit vaccines to help producers protect against E. coli infections (Ghunaim et al., 2014). There are currently 3 commercially available vaccines against colibacillosis in the market; Nobilis E. coli (MSD Animal Health, Summit, NJ) is an inactivated subunit vaccine consisting of fimbrial antigen F11 and a flagellar toxin (Gregersen et al., 2010; Mombarg et al., 2014).
Gregersen et al. (2010) conducted a study to test breeder vaccination's effect on broiler mortality and found no significant impact or benefit of this vaccine against colibacillosis in the progeny. However, they found a significant effect of this vaccine on the breeders against E. coli infection. The introduction of the live attenuated commercial vaccine Poulvac E. coli, (Zoetis, Florham Park, NJ) has tempted poultry producers to begin using it because of the reports of its protective effect (Galal et al., 2018). The current vaccine against colibacillosis in the market comprises live attenuated E. coli EC34195 serotype O78 with deleted aroA gene as the active substance (La Ragione et al., 2013). Researchers reported that the Enterobacteriaceae family members require aroA genes for the biosynthesis of aromatic amino acids to acquire the full expression of virulence (Galal et al., 2018).
Thus, the deletion of the gene and adding the aromatic amino acids would result in sufficient bacterial growth on minimal media to produce safe live-vaccine candidates (Hoiseth and Stocker, 1981). Studies revealed that coarse spray vaccination of one-day-old chicks with this vaccine could protect birds against homologous and some heterologous E. coli serotypes challenge (Filho et al., 2013; La Ragione et al., 2013; Sadeyen et al., 2015). At the same time, it was identified as safe (Mombarg et al., 2014). Sadeghi et al. (2018) reported that this vaccine was efficient against E. coli O78 and that the birds did not develop any lesions (Sadeghi et al., 2018).
Furthermore, it has been elucidated that APEC dominates the immune response induced by the vaccine in the early stage, diminishing by the end of 7 d. Cell-mediated immunity managed by CD 4+TCRVβ1+ on the mucosal surfaces producing immunoglobulin (IgA) and CD8 cells are hypothesized as the protective mechanisms of the vaccine-induced immune response (Filho et al., 2013). In Japan, a group of researchers reported that only the deletion of the aroA gene was insufficient to attenuate full virulence. They reported the mutation of the crp gene of O78 in APEC as the development of a live vaccine candidate (Nagano et al., 2012). They mentioned a vaccine containing this bacterial strain had been marketed in Japan since 2012. Their studies indicated that with this vaccine, survival rate, and egg-laying rate improved in egg layers (Uotani et al., 2017). The third commercially available vaccine is a live mutant called Nisseiken Avian Colibacillosis Vaccine CBL (Nisseiken Co., Ltd., Tokyo, Japan). This vaccine comprises a 107–109 colony-forming units/dose of AESN1331 O78 APEC strain, which has a deleted crp gene. A study by Abd El-Mawgoud et al. (2020) recorded the efficacy of this vaccine against homologous (O78) but not heterologous (O125) APEC challenge.
A recombinant multiantigen vaccine comprising a combination of common EXPEC surface proteins has been recently reported to produce significant levels of IgY against specific antigens and impose immune responses favorable for killing the bacteria. It has reduced bacterial growth of multiple APEC serotypes, reduced bacterial load in organs, and reduced lesions (Van Goor et al., 2017). They demonstrated increased IgA and IgY and reduced air sac lesions against homologous challenge using this vaccine (Ebrahimi-Nik et al., 2018).
Meanwhile, the careless use of antimicrobials in the developing countries, and the difficulty of discovering new antimicrobial therapies for resistant E. coli, led to the suggestion of using vaccines as the best choice to control E. coli infections in poultry farms. Multiple trials have been conducted to evaluate the efficacy of using vaccination against E. coli infecting poultry. However, several difficulties hindered such efforts, including the vaccine's capability to induce cross-protection against various APEC serogroups, vaccine mass delivery methods, and vaccination (Ghunaim et al., 2014). Generally, studies revealed that the inactivated vaccines protected homologous challenges only (Roland et al., 2004). Meanwhile, research on live attenuated E. coli vaccines resulted in the production of 2 commercial vaccines. Both vaccines are currently used in Egypt; however, their field efficacy against homologous and heterologous E. coli needs to be further evaluated (Galal et al., 2018). Although subunit vaccines demonstrated better immune response and better protection against homologous and heterologous challenges, large scale experiments are yet not conducted.
Other Strategies
In addition to the aforementioned methods, poultry producers use various strategies to prevent bacterial infections in farms. Biosecurity remains a vital measure that is typically underrated in the field. Keeping strict biosecurity in segregation, traffic control, cleaning, and disinfection, helps prevent a large proportion of harmful bacteria and viruses from entering poultry barns (Segal, 2013). Apart from good management practices, there are many alternative approaches proposed and explored by researchers worldwide to overcome bacterial infections in birds. While immuno-modulatory agents will be discussed separately in this article, it is worth brushing over the other strategies here.
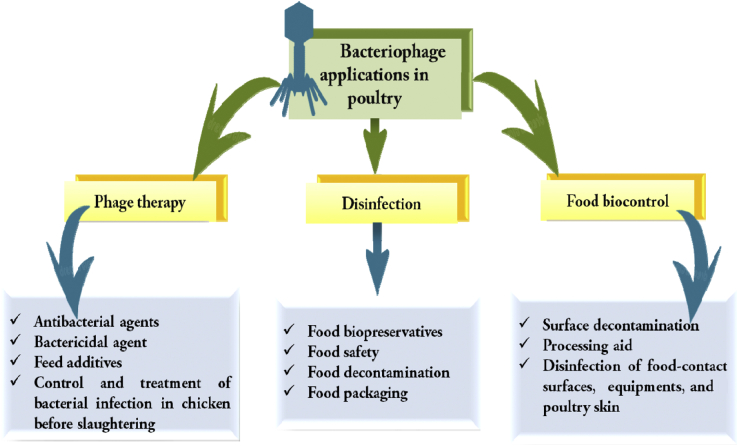
Bacteriophages and their lysins have been studies for a long time for their antibacterial effects as potential solutions (Figure 3). These viruses that specifically attack bacteria are currently being recognized for their ability to prevent infections by pathogens such as E. coli, Salmonella, and Campylobacter (Cheng et al., 2014). Huff et al. (2005) demonstrated that the administration of 2 bacteriophages as an aerosol spray and an intramuscular injection reduced mortality significantly against E. coli infection. However, owing to these phages' obligatory parasitic nature and their strict dependence on specific bacterial species, many precautions have to be taken during the phage preparation process. Despite the challenges, phage therapy (Figure 3) has lots of potential because of its natural presence, minimal harm, and the ability to adjust to its host bacterium's mutations (Rios et al., 2016).
Figure 3.
Applications of bacteriophage in the poultry.
Several plant extracts have been identified with antimicrobial properties and applied in poultry studies as alternatives to antibiotics (Cheng et al., 2014). Phytobiotics or phytogenic compounds are divided into several categories, such as herbs, spices, phenols, essential oils, alkaloids, and lectins. Adding these to animal diets has been identified to improve the quality of the animals' lives and the food derived from them (Windisch et al., 2008). Essential oils such as oregano, rosemary, and lavender oil used in broiler trials have shown efficient antimicrobial activity (Mathlouthi et al., 2012; Yang et al., 2015; Adaszyńska-Skwirzyńska and Szczerbińska, 2018).
Immune Modulation as an Alternative to Antibiotics
Various immuno-modulatory strategies are hot topics as alternatives to antibiotics in food animal production. Antibacterial vaccines are part of this category, although they are not able to control all bacterial infections because of their specificity to different bacterial agents. Nevertheless, the essence of immune-modulation is about modulating the host immune system or immune functions using the action of immuno-modulatory molecules such as toll-like receptor (TLR), ligands and agonists, hyperimmune antibodies, bacteriophages (Figure 3), probiotics, herbs, and essential oils (Lillehoj and Lee, 2012). Out of the agents, TLR ligands and agonists have been widely studied as strategies to control infections owing to their potent immunogenicity as stand-alone immune stimulants as well as vaccine adjuvants.
As opposed to vaccines, the other benefit of these TLR agonists is that they target the host rather than the pathogen. It is improbable that their repeated use would revert to any virulence or give rise to resistance (Mifsud et al., 2014). TLRs are a cluster of pattern recognition receptors that recognize pathogen-associated molecular patterns that comprise the invading pathogenic organisms such as cell wall components such as LPS, peptidoglycans, bacterial deoxyribonucleic acid, and double-stranded viral ribonucleic acid (Kannaki et al., 2010). Synthetic counterparts of CpG-oligodeoxynucleotides, polyinosinic:polycytidylic acid, and other molecules are promising analogs that are mostly being used in research as TLR agonists (Zhang et al., 2017).
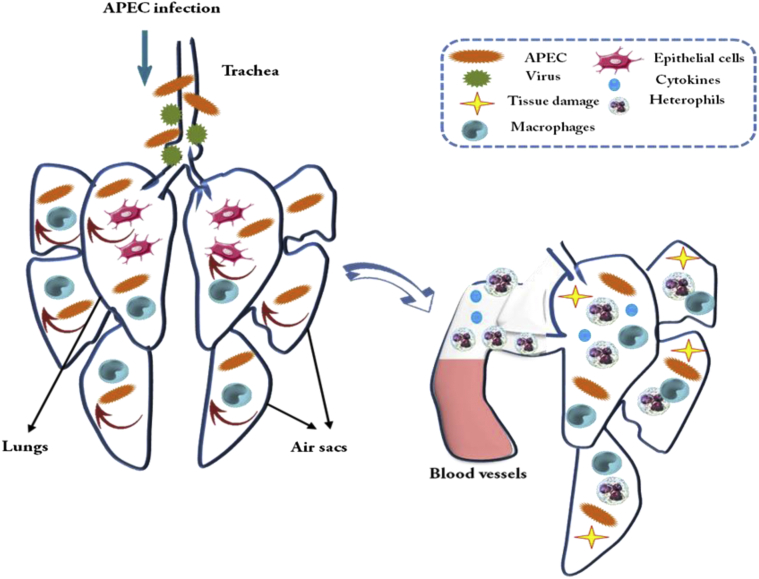
Recognition of specific pathogen-associated molecular patterns by the TLRs results in the activation of signaling cascades leading to the expression of various innate immune responses (Figure 4) such as proinflammatory cytokine genes, reactive oxygen intermediates, and nitrogen intermediates as reported by Takeda and Akira (2005). Such immunomodulatory properties (Figure 4) have been acknowledged to use in vaccine production as antigens, adjuvants, and direct immune stimulators to prevent or fight pathogenic infections (Ahmad-Nejad et al., 2002; Barjesteh et al., 2014). As we discussed using immunomodulatory molecules as alternatives to antibiotics, it would be useful to review the organization of the avian immune system to understand their mechanisms of action.
Figure 4.
Inflammatory response against avian pathogenic Escherichia coli (APEC) in chicken respiratory tract. After inhalation of contaminated aerosol particles, APEC interacts with tracheal epithelial cells, where previous viral infection can facilitate APEC colonization.
Conclusions
In conclusion, minimizing bacterial infections, especially E. coli, during the first week of age in chicks is very critical. This will help to obtain high performance of broiler chickens (body weight, weight gain, homogeneity of the bird's weight, immune response to vaccination as well as meat quality) and consequently increasing the profitability. Different strategies should be used for decreasing mortality during the first week of life. The biosecurity, selection of good quality chicks, and immune modulation remain as the main factors to achieve the best results.
Acknowledgments
The authors extend their appreciation to the Deputyship for Research & Innovation, “Ministry of Education” in Saudi Arabia for funding this research work through the project number IFKSURP-327.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.psj.2021.101039.
Disclosures
Authors declare no conflict of interests.
Supplementary Data
References
- Abd El-Hack M.E., Alagawany M., El-Sayed S.A.A., Fowler J. Influence of dietary inclusion of untreated or heat-treated Jatropha meal on productive and reproductive performances and biochemical blood parameters of laying Japanese quail. Poult. Sci. 2017;96:2761–2767. doi: 10.3382/ps/pex089. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shehata A.M., Arif M., Paswan V.K., Batiha G.E. Campylobacter spp. colonization in broiler chickens: a review. Environ. Sci. Pollut. Res. 2021;28:4989–5004. doi: 10.1007/s11356-020-11747-3. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shafi M.E., Zabermawi N.M., Arif M., Batiha G.E., Khafaga A.F., Abd El-Hakim Y.M., Al-Sagheer A.A. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: a review. Int. J. Biol. Macromol. 2020;164:2726–2744. doi: 10.1016/j.ijbiomac.2020.08.153. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shafi M.E., Qattan S.Y.A., Batiha G.E., Khafaga A.F., Abdel-Moneim A.M.E., Alagawany M. Probiotics in poultry feed: a comprehensive review. J. Anim. Physiol. Anim. Nutr. 2020;104:1835–1850. doi: 10.1111/jpn.13454. [DOI] [PubMed] [Google Scholar]
- Abd El-Mawgoud A.I., El-Nahass E.E., Shany S.A.S., EL-Sawah A.A., Dahshan A.M., Nasef S.A., Ali A. Efficacy of live attenuated vaccine and commercially available lectin against avian pathogenic E. coli infection in broiler chickens. Vet. Sci. 2020;7:65. doi: 10.3390/vetsci7020065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelnour S.A., Swelum A.A., Salama A., Al-Ghadi M.Q., Qattan S.Y.A., Abd El-Hack M.E., Khafaga A.F., Alhimaidi A.R., Almutairi B.O., Ammari A.A., El-Saadony M.T. The beneficial impacts of dietary phycocyanin supplementation on growing rabbits under high ambient temperature. Ital. J. Anim. Sci. 2020;19:1046–1056. [Google Scholar]
- Abdelnour S.A., El-Saadony M.T., Saghir S.A.M., Abd El-Hack M.E., Al-Shargi O.Y.A., Al-Gabri N., Salama A. Mitigating negative impacts of heat stress in growing rabbits via dietary prodigiosin supplementation. Livest. Sci. 2020;240:104220. [Google Scholar]
- Abdelqader A., Al-Fataftah A., Das G. Effects of dietary Bacillus subtilis and inulin supplementation on performance, eggshell quality, intestinal morphology and microflora composition of laying hens in the late phase of production. Anim. Feed Sci. Technol. 2013;179:103–111. [Google Scholar]
- AbdEl-Tawab A.A., El-Hofy F.I., El-Gamal A.M., Ibrahim H.O. Phenotypic and genotypic characterization of E. coli isolated from fish and human. Benha Vet. Med. J. 2018;34:41–50. [Google Scholar]
- Adaszyńska-Skwirzyńska M., Szczerbińska D. The antimicrobial activity of lavender essential oil (Lavandula angustifolia) and its influence on the production performance of broiler chickens. J. Anim. Physiol. Anim. Nutr. 2018;102:1020–1025. doi: 10.1111/jpn.12907. [DOI] [PubMed] [Google Scholar]
- Aguilar-Toalá J.E., García-Varela R., García H.S., Mata-Haro V., González-Córdova A.F., Vallejo-Cordoba B., Hernández-Mendoza A. Postbiotics: an evolving term within the functional foods field. Trends Food Sci. Technol. 2018;75:105–114. [Google Scholar]
- Agunos A., Léger D.F., Carson C.A., Gow S.P., Bosman A., Irwin R.J., ReidSmith R.J. Antimicrobial use surveillance in broiler chicken flocks in Canada, 2013-2015. PLoS One. 2017;12:e0179384. doi: 10.1371/journal.pone.0179384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad-Nejad P., Häcker H., Rutz M., Bauer S., Vabulas R.M., Wagner H. Bacterial CpG-DNA and lipopolysaccharides activate Toll-like receptors at distinct cellular compartments. Eur. J. Immunol. 2002;32:1958–1968. doi: 10.1002/1521-4141(200207)32:7<1958::AID-IMMU1958>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Akl B.A., Nader M.M., El-Saadony M.T. Biosynthesis of silver nanoparticles by Serratia marcescens ssp sakuensis and its antibacterial application against some pathogenic bacteria. J. Agric. Chem. Biotech. Mansoura Univ. 2020;11:1–8. [Google Scholar]
- Aluwong T., Kawu M., Raji M., Dzenda T., Govwang F., Sinkalu V., Ayo J. Effect of yeast probiotic on growth, antioxidant enzyme activities and malondialdehyde concentration of broiler chickens. Antioxidants. 2013;2:326–339. doi: 10.3390/antiox2040326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alyileili S.R., Belal I.E.H., Hussein A.S., El-Tarabily K.A. Effect of inclusion of degraded and non-degraded date pits in broilers’ diet on their intestinal microbiota and growth performance. Animals. 2020;10:2041. doi: 10.3390/ani10112041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alyileili S.R., El-Tarabily K.A., Belal I.E.H., Ibrahim W.H., Sulaiman M., Hussein A.S. Effect of Trichoderma reesei degraded date pits on antioxidant enzyme activities and biochemical responses of broiler chickens. Front. Vet. Sci. 2020;7:338. doi: 10.3389/fvets.2020.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alyileili S.R., El-Tarabily K.A., H Belal I.E., Ibrahim W.H., Sulaiman M., Hussein A.S. Intestinal development and histomorphometry of broiler chickens fed Trichoderma reesei degraded date seed diets. Front. Vet. Sci. 2020;7:349. doi: 10.3389/fvets.2020.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amabile de Campos T., Stehling E.G., Ferreira A., Pestana de Castro A.F., Brocchi M., Dias da Silveira W. Adhesion properties, fimbrial expression and PCR detection of adhesin-related genes of avian Escherichia coli strains. Vet. Microbiol. 2005;106:275–285. doi: 10.1016/j.vetmic.2004.12.025. [DOI] [PubMed] [Google Scholar]
- Amare A., Amin A.M., Shiferaw A., Nazir S., Negussie H. Yolk sac infection (omphalitis) in kombolcha poultry farm, Ethiopia. Am-euras. J. Sci. Res. 2013;8:10–14. [Google Scholar]
- Ammar A.M., Norhan K.A., Yousreya H.M., AbdEl-Aziz E.E. Advanced studies on diagnosis of single M. gallisepticum infection and combined with E. coli in chickens. Zag. Vet. J. 2011;39:110–114. [Google Scholar]
- Andersson H., Asp N.-G., Bruce A., Roos S., Wadström T., Wold A.E. Health effects of probiotics and prebiotics A literature review on human studies. Näringsforskning. 2001;45:58–75. [Google Scholar]
- Arne P., Marc D., Bree A., Schouler C., DhoMoulin M. Increased tracheal colonization in chickens without impairing pathogenic properties of avian pathogenic Escherichia coli MT78 with a fimH deletion. Avian Dis. 2000;44:343–355. [PubMed] [Google Scholar]
- Arp L. H. Jensen, Piliation A.E. Hemagglutination, motility, and generation time of Escherichia coli that are virulent or avirulent for turkeys. Avian Dis. 1980;24:153–161. [Google Scholar]
- Ashbolt N.J. Microbial contamination of drinking water and human health from community water systems. Curr. Environ. Health Rep. 2015;2:95–106. doi: 10.1007/s40572-014-0037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashour E.A., Abd El-Hack M.E., Shafi M.E., Alghamdi W.Y., Taha A.E., Swelum A.A., Tufarelli V., Mulla Z.S., El-Ghareeb W.R., El-Saadony M.T. Impacts of green coffee powder supplementation on growth performance, carcass characteristics, blood indices, meat quality and gut microbial load in broilers. Agriculture. 2020;10:457. [Google Scholar]
- Awad A., El-Shall N.A., Khalil D., Abd El-Hack M.E., Swelum A.A., Mahmoud A.H., Ebaid H., Komany A., Sammour R.H., Sedeik M.E. Incidence, pathotyping, and antibiotic susceptibility of avian pathogenic Escherichia coli among diseased broiler chicks. Pathogens. 2020;9:114. doi: 10.3390/pathogens9020114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baffoni L., Gaggìa F., Di Gioia D., Santini C., Mogna L., Biavati B.A. Bifidobacterium-based synbiotic product to reduce the transmission of C. jejuni along the poultry food chain. Int. J. Food Microbiol. 2012;157:156–161. doi: 10.1016/j.ijfoodmicro.2012.04.024. [DOI] [PubMed] [Google Scholar]
- Bai S.P., Wu A.M., Ding X.M., Lei Y., Bai J., Zhang K.Y., Chio J.S. Effects of probiotic-supplemented diets on growth performance and intestinal immune characteristics of broiler chickens. Poult. Sci. 2013;92:663–670. doi: 10.3382/ps.2012-02813. [DOI] [PubMed] [Google Scholar]
- Bain R., Cronk R., Wright J., Yang H., Slaymaker T., Bartram J. Fecal contamination of drinking-water in low- and middle-income countries: a systematic review and meta-analysis. Plos Med. 2014;11:e1001644. doi: 10.1371/journal.pmed.1001644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajagai A., Klieve V., Dart P.J., Bryden W.L. Vol. 179. FAO Animal Production and Health; Rome: 2016. (Probiotics in Animal Nutrition–Production, Impact and Regulation by Yadav S). [Google Scholar]
- Barjesteh N., Behboudi S., Brisbin J.T., Villanueva A.I., Nagy E., Sharif S. TLR ligands induce antiviral responses in chicken macrophages. PLoS One. 2014;9:e105713. doi: 10.1371/journal.pone.0105713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartram J. Taylor and Francis; Florence: 2015. Routledge Handbook of Water and Health. [Google Scholar]
- Bastiani M., Vidotto M.C., Horn F. An avian pathogenic Escherichia coli isolate induces caspase 3/7 activation in J774 macrophages. FEMS Microbiol. Lett. 2005;253:133–140. doi: 10.1016/j.femsle.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Beyene T. Veterinary drug residues in food-animal products: its risk factors and potential effects on public health. J. Veterinar Sci. Technol. 2016;7:85–298. [Google Scholar]
- Bogaard A.E., London N., Driessen C., Stobberingh E. Antibiotic resistance of faecal Escherichia coli in poultry, poultry farmers and poultry slaughterers. J. Antimicrob. Chemotherap. 2001;47:763–771. doi: 10.1093/jac/47.6.763. [DOI] [PubMed] [Google Scholar]
- Bucher O., Holley R.A., Ahmed R., Tabor H., Nadon C., Ng L.K., D'Aoust J.Y. Occurrence and characterization of Salmonella from chicken nuggets, strips, and pelleted broiler feed. J. Food Prot. 2007;70:2251–2258. doi: 10.4315/0362-028x-70.10.2251. [DOI] [PubMed] [Google Scholar]
- Caly D.L., D’Inca R., Auclair E., Drider D. Alternatives to antibiotics to prevent necrotic enteritis in broiler chickens: a microbiologist’s perspective. Front. Microbiol. 2015;6:1336. doi: 10.3389/fmicb.2015.01336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagnolo E.R., Johnson K.R., Karpati A., Rubin C.S., Kolpin D.W., Meyer M.T., Mcgeehin M. Antimicrobial residues in animal waste and water resources proximal to large-scale swine and poultry feeding operations. Sci. Total Environ. 2002;299:89–95. doi: 10.1016/s0048-9697(02)00233-4. [DOI] [PubMed] [Google Scholar]
- Castanon J.I.R. History of the use of antibiotic as growth promoters in European poultry feeds. Poult. Sci. 2007;86:2466–2471. doi: 10.3382/ps.2007-00249. [DOI] [PubMed] [Google Scholar]
- Caza M., Lepine F., Dozois C.M. Secretion, but not overall synthesis, of catecholate siderophores contributes to virulence of extraintestinal pathogenic Escherichia coli. Mol. Microbiol. 2011;80:266–282. doi: 10.1111/j.1365-2958.2011.07570.x. [DOI] [PubMed] [Google Scholar]
- Caza M., Lepine F., Milot S., Dozois C.M. Specific roles of the iroBCDEN genes in virulence of an avian pathogenic Escherichia coli O78 strain and in production of salmochelins. Infect. Immun. 2008;76:3539–3549. doi: 10.1128/IAI.00455-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Antibiotic/Antimicrobial Resistance. 2018. https://www.cdc.gov/drugresistance/about.html Accessed May 2020.
- Cervantes H.M. Antibiotic-free poultry production: is it sustainable? J. Appl. Poult. Res. 2015;24:91–97. [Google Scholar]
- Chanteloup N.K., Porcheron G., Delaleu B., Germon P., Schouler C., Moulin-Schouleur M., Gilot P. The extra-intestinal avian pathogenic Escherichia coli strain BEN2908 invades avian and human epithelial cells and survives intracellularly. Vet. Microbiol. 2011;147:435–439. doi: 10.1016/j.vetmic.2010.07.013. [DOI] [PubMed] [Google Scholar]
- Cheng G., Hao H., Xie S., Wang X., Dai M., Huang L., Yuan Z. Antibiotic alternatives: the substitution of antibiotics in animal husbandry? Front. Microbiol. 2014;5:1–15. doi: 10.3389/fmicb.2014.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou C.C., Jiang D.D., Hung Y.P. Risk factors for cumulative mortality in broiler chicken flocks in the first week of life in Taiwan. Br. Poult. Sci. 2004;45:573–577. doi: 10.1080/000716604000006248. [DOI] [PubMed] [Google Scholar]
- Christenson E., Stewart J. Water Resources Research Institute of the University of North Carolina, Chapel Hill; NC: 2018. Understanding How Land Use Characteristics Affect the Prevalence of Antibiotic Resistant, Virulent E. coli and Host-specific Markers in Watersheds with and without Swine Cafos. Department of Environmental Sciences and Engineering Report No. 471. [Google Scholar]
- Cortés C.R., Téllez Isaías G., Ló pez Cuello C., Villaseca-Flores J.M., Anderson R.C., Eslava Campos C. Bacterial isolation rate from fertile eggs, hatching eggs, and neonatal broilers with yolk sac infection. Rev. Latinoam. Microbiol. 2004;46:12–16. [PubMed] [Google Scholar]
- Cortes M.A.M., Gibon J., Chanteloup N.K., Moulin-Schouleur M., Gilot P., Germon P. Inactivation of ibeA and ibeT results in decreased expression of type 1 fimbriae in extraintestinal pathogenic Escherichia coli strain BEN2908. Infect Immun. 2008;76:4129–4136. doi: 10.1128/IAI.00334-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva G.C., Rossi C.C., Santana M.F., Langford P.R., Bossé J.T., Bazzolli D.M.S. p518, a small floR plasmid from a South American isolate of Actinobacillus pleuropneumoniae. Vet. Microbiol. 2017;204:129–132. doi: 10.1016/j.vetmic.2017.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalloul R.A., Lillehoj H.S., Shellem T.A., Doerr J.A. Enhanced mucosal immunity against Eimeria acervulina in broilers fed a Lactobacillus-based probiotic. Poult. Sci. 2003;82:62–66. doi: 10.1093/ps/82.1.62. [DOI] [PubMed] [Google Scholar]
- Daşkıran M., Önol A.G., Cengiz Ö., Ünsal H., Türkyılmaz S., Tatlı O., Sevim Ö. Influence of dietary probiotic inclusion on growth performance, blood parameters, and intestinal microflora of male broiler chickens exposed to posthatch holding time. J. Appl. Poult. Res. 2012;21:612–622. [Google Scholar]
- Desin T.S., Köster W., Potter A.A. Salmonella vaccines in poultry: past, present and future. Expert Rev. Vaccin. 2013;12:87–96. doi: 10.1586/erv.12.138. [DOI] [PubMed] [Google Scholar]
- Dho-Moulin M., Fairbrother J.M. Avian pathogenic Escherichia coli (APEC) Vet. Res. 1999;30:299–316. [PubMed] [Google Scholar]
- Dozois C.M., Dho-Moulin M., Bree A., Fairbrother J.M., Desautels C., Curtiss R. Relationship between the Tsh autotransporter and pathogenicity of avian Escherichia coli and localization and analysis of the Tsh genetic region. Infect Immun. 2000;68:4145–4154. doi: 10.1128/iai.68.7.4145-4154.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunislawska A., Slawinska A., Stadnicka K., Bednarczyk M., Gulewicz P., Jozefiak D., Siwek M. Synbiotics for broiler chickens—in vitro design and evaluation of the influence on host and selected microbiota populations following in ovo delivery. Plos One. 2017;12:e0168587. doi: 10.1371/journal.pone.0168587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimi-Nik H., Bassami M.R., Mohri M., Rad M., Khan M.I. Bacterial ghost of avian pathogenic E. coli (APEC) serotype O78:K80 as a homologous vaccine against avian colibacillosis. PLoS One. 2018;13:e0194888. doi: 10.1371/journal.pone.0194888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edberg S.C., Rice E.W., Karlin R.J., Allen M.J. Escherichia coli: the best biological drinking water indicator for public health protection. Symp. Ser. Soc. Appl. Microbiol. 2000;88:106S–116S. doi: 10.1111/j.1365-2672.2000.tb05338.x. [DOI] [PubMed] [Google Scholar]
- El-Saadony M.T., Abd El-Hack M.E., Taha A.E., Fouda M.M.G., Ajarem J.S., Maodaa S.N., Allam A.A., Elshaer N. Ecofriendly synthesis and insecticidal application of copper nanoparticles against the storage pest Tribolium castaneum. Nanomaterials. 2020;10:587. doi: 10.3390/nano10030587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Elsadek M.F., Mohamed A.S., Taha A.E., Ahmed B.M., Saad A.M. Effects of chemical and natural additives on cucumber juice’s quality, shelf life, and safety. Foods. 2020;9:639. doi: 10.3390/foods9050639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., El-Wafai N.A., El-Fattah H.I.A., Mahgoub S.A. Biosynthesis, optimization and characterization of silver nanoparticles using a soil isolate of Bacillus pseudomycoides MT32 and their antifungal activity against some pathogenic fungi. Adv. Anim. Vet. Sci. 2019;7:238–249. [Google Scholar]
- El-Sawah A.A., Dahshan A.L.H.M., El-Nahass E.-S., El-Mawgoud A.I.A. Pathogenicity of Escherichia coli O157 in commercial broiler chickens. Beni-suef Univ. J. Basic Appl. Sci. 2018;7:620–625. [Google Scholar]
- El-Shall N.A., Shewita R.S., Abd El-Hack M.E., AlKahtane A., Alarifi S., Alkahtani S., Abdel-Daim M.M., Sedeik M.E. Effect of essential oils on the immune response to some viral vaccines in broiler chickens, with special reference to Newcastle disease virus. Poult. Sci. 2020;99:2944–2954. doi: 10.1016/j.psj.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewers C., Janssen T., Wieler L.H. Avian pathogenic Escherichia coli (APEC). Berl. Munch. Tierarztl. Wochenschr. 2003;116:381–395. [PubMed] [Google Scholar]
- Ewers C., Janssen T., Kiessling S., Philipp H.C., Wieler L.H. Rapid detection of virulence associated genes in avian pathogenic Escherichia coli by multiplex polymerase chain reaction. Avian Dis. 2005;49:269–273. doi: 10.1637/7293-102604R. [DOI] [PubMed] [Google Scholar]
- Ewers C., Janssen T., Kiessling S., Philipp H.-C., Wieler L.H. Molecular epidemiology of avian pathogenic Escherichia coli (APEC) isolated from colisepticemia in poultry. Vet. Microbiol. 2004;104:91–101. doi: 10.1016/j.vetmic.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Fantinatti F., Silveira W.D., Castro A.F.P. Characteristics associated with pathogenicity of avian septicaemic Escherichia coli strains. Vet. Microbiol. 1994;41:75–86. doi: 10.1016/0378-1135(94)90137-6. [DOI] [PubMed] [Google Scholar]
- Ferreira C.L., Salminen S., Grzeskowiak L., Brizuela M.A., Sanchez L., Carneiro H., Bonnet M. Terminology concepts of probiotic and prebiotic and their role in human and animal health. Rev. Salud Anim. 2011;33:137–146. [Google Scholar]
- Filho T.F., Fávaro C., Ingberman M., Beirão B.C.B., Inoue A., Gomes L., Caron L.F. Effect of spray Escherichia coli vaccine on the immunity of poultry. Avian Dis. 2013;57:671–676. doi: 10.1637/10456-112612-ResNote.1. [DOI] [PubMed] [Google Scholar]
- Fletcher S. Understanding the contribution of environmental factors in the spread of antimicrobial resistance. Environ. Health Prev. Med. 2015;20:243–252. doi: 10.1007/s12199-015-0468-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forte C., Manuali E., Abbate Y., Papa P., Vieceli L., Tentellini M., Trabalza-Marinucci M., Moscati L. Dietary Lactobacillus acidophilus positively influences growth performance, gut morphology, and gut microbiology in rurally reared chickens. Poult. Sci. 2018;97:930–936. doi: 10.3382/ps/pex396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratamico P.M., DebRoy C., Liu Y., Needleman D.S., Baranzoni G.M., Feng P. Advances in molecular serotyping and subtyping of Escherichia coli. Front. Microbiol. 2016;7:1–8. doi: 10.3389/fmicb.2016.00644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galal H.M., Tawfek A.M., Abdrabou M.I., Hessain A.M., Alhaaji J.H., Kabli S.A., Elbehiry A., Alwarhi W.K., Moussa I.M. Recent approaches for control of E. coli and respiratory complex in Middle East. Saudi J. Biol. Sci. 2018;25:1302–1307. doi: 10.1016/j.sjbs.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germon P., Chen Y.H., He L., Blanco J.E., Bree A., Schouler C., Huang S.H., Moulin-Schouleur M. ibeA, a virulence factor of avian pathogenic Escherichia coli. Microbiology. 2005;151:1179–1186. doi: 10.1099/mic.0.27809-0. [DOI] [PubMed] [Google Scholar]
- Ghanbarpour R., Sami M., Salehi M., Ouromiei M. Phylogenetic background and virulence genes of Escherichia coli isolates from colisepticemic and healthy broiler chickens in Iran. Trop. Anim. Health Pro. 2011;43:153–157. doi: 10.1007/s11250-010-9667-2. [DOI] [PubMed] [Google Scholar]
- Ghunaim H., Abu-Madi M.A., Kariyawasam S. Advances in vaccination against avian pathogenic Escherichia coli respiratory disease: potentials and limitations. Vet. Microbiol. 2014;172:13–22. doi: 10.1016/j.vetmic.2014.04.019. [DOI] [PubMed] [Google Scholar]
- Giovanardi D., Campagnari E., Ruffoni L.S., Pesente P., Ortali G., Furlattini V. Avian pathogenic Escherichia coli transmission from broiler breeders to their progeny in an integrated poultry production chain. Avian Pathol. 2005;34:313–318. doi: 10.1080/03079450500179046. [DOI] [PubMed] [Google Scholar]
- Gomis S.M., Riddell C., Potter A.A., Allan B.J. Phenotypic and genotypic characterization of virulence factors of Escherichia coli isolated from broiler chickens with simultaneous occurrence of cellulitis and other colibacillosis lesions. Can. J. Vet. Res. 2001;65:1–6. [PMC free article] [PubMed] [Google Scholar]
- Gregersen R.H., Christensen H., Ewers C., Bisgaard M. Impact of Escherichia coli vaccine on parent stock mortality, first week mortality of broilers and population diversity of E. coli in vaccinated flocks. Avian Pathol. 2010;39:287–295. doi: 10.1080/03079457.2010.495744. [DOI] [PubMed] [Google Scholar]
- Harden S.L. Surface-water quality in agricultural watersheds of the North Carolina coastal plain associated with concentrated animal feeding operations. U.S. Geol. Surv. Scientific Invest. Rep. 2015:5080. [Google Scholar]
- Heier B.T., Høgåsen H.R., Jarp J. Factors associated with mortality in Norwegian broiler flocks. Prev. Vet. Med. 2002;53:147–158. doi: 10.1016/s0167-5877(01)00266-5. [DOI] [PubMed] [Google Scholar]
- Helal W.M.E.A. Zagazig Univ; 2012. Comparison between pathogenicity of E. coli serotypes isolated from intestinal and respiratory infections in chickens. M. V. Sc. Thesis, Fac. Vet. Med. [Google Scholar]
- Hendriksen R.S., Vieira A.R., Karlsmose S., Lo Fo Wong D.M., Jensen A.B., Wegener H.C., Aarestrup F.M. Global monitoring of Salmonella serovar distribution, world health organization foodborne infections network country data bank: results of quality assured laboratories from 2001 to 2007. Foodborne Pathog. Dis. 2011;8:887–900. doi: 10.1089/fpd.2010.0787. [DOI] [PubMed] [Google Scholar]
- Hoiseth S.K., Stocker B.A.D. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- Hribar C. National Association of Local Boards of Health; Bowling Green, OH: 2010. Understanding Concentrated Animal Feeding Operations and Their Impact on Communities. [Google Scholar]
- Huff W.E., Huff G.R., Rath N.C., Balog J.M., Donoghue A.M. Alternatives to antibiotics: utilization of bacteriophage to treat colibacillosis and prevent foodborne pathogens. Poult. Sci. 2005;84:655–659. doi: 10.1093/ps/84.4.655. [DOI] [PubMed] [Google Scholar]
- Hurby C.E.K. Vol. 14408. Iowa State University; IA: 2015. Impacts of poultry manure application on bacteria and phosphorus in soils and drainage tile-waters. (Graduate Theses and Dissertations). [Google Scholar]
- Janben T., Schwarz C., Preikschat P., Voss M., Philipp H.C., Wieler L.H. Virulence associated genes in avian pathogenic Escherichia coli (APEC) isolated from internal organs of poultry having died from colibacillosis. Int. J. Med. Microbiol. 2001;291:371–378. doi: 10.1078/1438-4221-00143. [DOI] [PubMed] [Google Scholar]
- Johnson T.J., Johnson S.J., Nolan L.K. Complete DNA sequence of a ColBM plasmid from avian pathogenic Escherichia coli suggests that it evolved from closely related ColV virulence plasmids. J. Bacteriol. 2006;188:5975–5983. doi: 10.1128/JB.00204-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson T.J., Skyberg S.J., Nolan L.K. Multiple antimicrobial resistance region of a putative virulence plasmid from an Escherichia coli isolate incriminated in avian colibacillosis. Avian Dis. 2006;48:351–360. doi: 10.1637/7121. [DOI] [PubMed] [Google Scholar]
- Johnson T.J., Wannemuehler Y., Doetkott C., Johnson S.J., Rosenberger S.C., Nolan L.K. Identification of minimal predictors of avian pathogenic Escherichia coli virulence for use as a rapid diagnostic tool. J. Clin. Microbiol. 2008;46:3987–3996. doi: 10.1128/JCM.00816-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D.R., Guard J., Gast R.K., Buhr R.J., Fedorka-Cray P.J., Abdo Z., Plumblee J.R., Bourassa D.V., Cox N.A., Rigsby L.L., Robison C.I., Regmi P.D., Karcher M. Influence of commercial laying hen housing systems on the incidence and identification of Salmonella and Campylobacter. Poult. Sci. 2016;95:1116–1124. doi: 10.3382/ps/pew036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannaki T.R., Reddy M.R., Shanmugam M., Verma P.C., Sharma R.P. Chicken toll-like receptors and their role in immunity. Worlds Poult. Sci. J. 2010;66:727–738. [Google Scholar]
- Kariyawasam S., Johnson T.J., DebRoy C., Nolan L.K. Occurrence of pathogenicity island IAPEC-O1 genes among Escherichia coli implicated in avian colibacillosis. Avian Dis. 2006;50:405–410. doi: 10.1637/7462-102705R.1. [DOI] [PubMed] [Google Scholar]
- Karunarathna R., Popowich S., Wawryk M., Chow-Lockerbie B., Ahmed K.A., Yu C., Liu M., Goonewardene K., Gunawardana T., Kurukulasuriya S., Gupta A., Willson P., Ambrose N., Ngeleka M., Gomis S. Increased incidence of enterococcal infection in nonviable broiler chicken embryos in western Canadian hatcheries as detected by matrix assisted laser desorption/ionization-time-of-flight (MALDI-TOF) mass spectrometry. Avian Dis. 2017;61:472–480. doi: 10.1637/11678-052317-Reg.1. [DOI] [PubMed] [Google Scholar]
- Kaufman M. Worries Rise over Effect of Antibiotics in Animal Feed; Humans Seen Vulnerable to Drug-Resistant Germs. Washington Post. A01. 2000. http://www.upc-online.org/000317wpost_animal_feed.html Accessed May 2020.
- Kemmett K., Williams N.J., Chaloner G., Humphrey S., Wigley P., Humphrey T. The contribution of systemic Escherichia coli infection to the early mortalities of commercial broiler chickens. Avian Pathol. 2014;43:37–42. doi: 10.1080/03079457.2013.866213. [DOI] [PubMed] [Google Scholar]
- Khaksar V., Golian A., Kermanshahi H. Immune response and ileal microflora in broilers fed wheat-based diet with or without enzyme Endo feed and supplementation of thyme essential oil or probiotic PrimaLac®. Afr. J. Biotechnol. 2012;11:14716–14723. [Google Scholar]
- Khan K.A., Khan S.A., Aslam A., Rabbani M., Tipu M.Y. Factors contributing to yolk retention in poultry: a review. Pakistan Vet. J. 2004;24:46–51. [Google Scholar]
- Kim G.B., Seo Y.M., Kim C.H., Paik I.K. Effect of dietary prebiotic supplementation on the performance, intestinal microflora, and immune response of broilers. Poult. Sci. 2011;90:75–82. doi: 10.3382/ps.2010-00732. [DOI] [PubMed] [Google Scholar]
- Kim M., Kim S., Yun S.J., Kwon J.W., Son S.W. Evaluation of PCDD/Fs characterization in animal feed and feed additives. Chemosphere. 2007;69:381–386. doi: 10.1016/j.chemosphere.2007.05.025. [DOI] [PubMed] [Google Scholar]
- Kobayashi R.K., Gaziri L.C., Vidotto M.C. Functional activities of the Tsh protein from avian pathogenic Escherichia coli (APEC) strains. J. Vet. Sci. 2010;11:315–319. doi: 10.4142/jvs.2010.11.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostantinov S.R., Kuipers E.J., Peppelenbosch M.P. Functional genomic analyses of the gut microbiota for CRC screening. Nat. Rev. Gastroenterol. Hepatol. 2013;10:741–745. doi: 10.1038/nrgastro.2013.178. [DOI] [PubMed] [Google Scholar]
- Kostyla C., Bain R., Cronk R., Bartram J. Seasonal variation of fecal contamination in drinking water sources in developing countries: a systematic review. Sci. Total Environ. 2015;514:333–343. doi: 10.1016/j.scitotenv.2015.01.018. [DOI] [PubMed] [Google Scholar]
- Kottom T.J., Nolan L.K., Robinson M., Brown J., Gustad T., Horne S.M., Giddings C.W. Further characterization of a complement sensitive mutant of a virulent avian Escherichia coli isolate. Avian Dis. 1997;41:817–823. [PubMed] [Google Scholar]
- Kwon S.-G., Cha S.-Y., Choi E.-J., Kim B., Song H.-J., Jang H.-K. Epidemiological prevalence of avian pathogenic Escherichia coli differentiated by multiplex PCR from commercial chickens and hatchery in Korea. J. Bacteriol. Virol. 2008;38:179–188. [Google Scholar]
- La Ragione R.M., Woodward M.J. Virulence factors of Escherichia coli serotypes associated with avian colisepticaemia. Res. Vet. Sci. 2002;73:27–35. doi: 10.1016/s0034-5288(02)00075-9. [DOI] [PubMed] [Google Scholar]
- La Ragione R.M., Woodward M.J., Kumar M., Rodenberg J., Fan H., Wales A.D., Karaca K. Efficacy of a live attenuated Escherichia coli O78∶K80 vaccine in chickens and turkeys. Avian Dis. 2013;57:273–279. doi: 10.1637/10326-081512-Reg.1. [DOI] [PubMed] [Google Scholar]
- Landoni M.F., Albarellos G. The use of antimicrobial agents in broiler chickens. Vet. J. 2015;205:21–27. doi: 10.1016/j.tvjl.2015.04.016. [DOI] [PubMed] [Google Scholar]
- Levantesi C., Bonadonna L., Briancesco R., Grohmann E., Toze S., Tandoi V. Salmonella in surface and drinking water: occurrence and water-mediated transmission. Food Res. Int. 2012;45:587–602. [Google Scholar]
- Li G., Laturnus C., Ewers C., Wieler L.H. Identification of genes required for avian Escherichia coli septicemia by signature-tagged mutagenesis. Infect. Immun. 2005;73:2818–2827. doi: 10.1128/IAI.73.5.2818-2827.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Xu Q., Yang C., Yang X., Lv L., Yin C., Liu X., Yan H. Effects of probiotics on the growth performance and intestinal micro flora of broiler chickens. Pak. J. Pharm. Sci. 2014;27:713–717. [PubMed] [Google Scholar]
- Lillehoj H.S., Lee K.W. Immune modulation of innate immunity as alternatives-toantibiotics strategies to mitigate the use of drugs in poultry production. Poult. Sci. 2012;91:1286–1291. doi: 10.3382/ps.2012-02374. [DOI] [PubMed] [Google Scholar]
- Lutful Kabir S.M. Avian colibacillosis and salmonellosis: a closer look at epidemiology, pathogenesis, diagnosis, control and public health concerns. Int. J. Environ. Res. Public Health. 2017;7:89–114. doi: 10.3390/ijerph7010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald J.M., McBride W.D. Econ. Inf. Bull. No. 43, CreateSpace Independent Publishing Platform Scotts Valley, CA; 2009. The transformation of U.S. livestock agriculture: scale, efficiency, and risks. [Google Scholar]
- Maciorowski K.G., Jones F.T., Pillai S.D., Ricke S.C. Incidence,sources, and control of food-borne Salmonella spp. in poultry feed. World’s Poult. Sci. J. 2004;60:446–457. [Google Scholar]
- Maciorowski K.G., Herrera P., Jones F.T., Pillai S.D., Ricke S.C. Effects on poultry and livestock of feed contamination with bacteria and fungi. Anim. Feed Sci. Technol. 2007;133:109–136. [Google Scholar]
- Magwedere K., Rauff D., De Klerk G., Keddy K.H., Dziva F. Incidence of nontyphoidal Salmonella in food-producing animals, animal feed, and the associated environment in South Africa, 2012-2014. Clin. Infect. Dis. 2015;61:283–289. doi: 10.1093/cid/civ663. [DOI] [PubMed] [Google Scholar]
- Mallin M., Cahoon L. Industrialized animal production—a major source of nutrient and microbial pollution to aquatic ecosystems. Popul. Environ. 2003;24:369–385. [Google Scholar]
- Manges A.R., Johnson J.R. Food-borne origins of Escherichia coli causing extraintestinal infections. Clin. Infect. Dis. 2012;55:712–719. doi: 10.1093/cid/cis502. [DOI] [PubMed] [Google Scholar]
- Markazi A., Luoma A., Shanmugasundaram R., Mohnl M., Raj Murugesan G., Selvaraj R. Effects of drinking water synbiotic supplementation in laying hens challenged with Salmonella. Poult. Sci. 2018;97:3510–3518. doi: 10.3382/ps/pey234. [DOI] [PubMed] [Google Scholar]
- Markowiak P., Śliżewska K. The role of probiotics, prebiotics and synbiotics in animal nutrition. Gut Pathog. 2018;10:21. doi: 10.1186/s13099-018-0250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall B.M., Levy S.B. Food animals and antimicrobials: impacts on human health. Clin. Microbiol. Rev. 2011;24:718–733. doi: 10.1128/CMR.00002-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Antonio A., Janga S.C., Thieffry D. Functional organization of Escherichia coli transcriptional regulatory network. J. Mol. Biol. 2008;381:238–247. doi: 10.1016/j.jmb.2008.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathlouthi N., Bouzaienne T., Oueslati I., Recoquillay F., Hamdi M., Urdaci M., Bergaoui R. Use of rosemary, oregano, and a commercial blend of essential oils in broiler chickens: in vitro antimicrobial activities and effects on growth performance. J. Anim. Sci. 2012;90:813–823. doi: 10.2527/jas.2010-3646. [DOI] [PubMed] [Google Scholar]
- McPeake S.J.W., Smyth J.A., Ball H.J. Characterization of avian pathogenic Escherichia coli (APEC) associated with colisepticaemia compared to faecal isolates from healthy birds. Vet. Microbiol. 2005;110:245–253. doi: 10.1016/j.vetmic.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Meeker D.L., Meisinger J.L. Companion animals symposium: Rendered ingredients significantly influence sustainability, quality, and safety of pet food. J. Anim. Sci. 2015;93:835–847. doi: 10.2527/jas.2014-8524. [DOI] [PubMed] [Google Scholar]
- Mehdi Y., Letourneau-Montminy M.P., Gaucher M.L., Chorfi Y., Suresh G., Rouissi T., Brar S.K., Caroline Cote C., Ramirez A.A., Godbout S. Use of antibiotics in broiler production: global impacts and alternatives. Anim. Nutr. 2018;4:170–178. doi: 10.1016/j.aninu.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellata M. Human and avian extraintestinal pathogenic Escherichia coli: infections, zoonotic risks, and antibiotic resistance trends. Foodborne Pathog. Dis. 2013;10:916–932. doi: 10.1089/fpd.2013.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellata M., Maddux J.T., Nam T., Thomson N., Hauser H., Stevens M.P., Mukhopadhyay S., Sarker S., Crabbé A., Nickerson C.A. New insights into the bacterial fitness associated mechanisms revealed by the characterization of large plasmids of an avian pathogenic E. coli. PLoS One. 2012;7:e29481. doi: 10.1371/journal.pone.0029481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellata M., Dho-Moulin M., Dozois C.M., Curtiss R., Brown P.K., Arné P., Brée A., Desautels C., Fairbrother J.M. Role of virulence factors in resistance of avian pathogenic Escherichia coli to serum and in pathogenicity. Infect Immun. 2003;71:536–540. doi: 10.1128/IAI.71.1.536-540.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer Z. Tyson Foods eliminate antibiotics in chicken. Report. 2017. https://www.usatoday.com/story/money/business/2017/05/01/poultry-giant-tyson-bootantibiotics-chicken/100970854/ Accessed May 2020.
- Micciche A.C., Foley S.L., Pavlidis H.O., McIntyre D.R., Ricke S.C. A Review of prebiotics against Salmonella in Poultry: current and future potential for microbiome research applications. Front. Vet. Sci. 2018;5:191. doi: 10.3389/fvets.2018.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mifsud E.J., Tan A.C.L., Jackson D.C. TLR agonists as modulators of the innate immune response and Their potential as agents against infectious disease. Front. Immunol. 2014;5:79. doi: 10.3389/fimmu.2014.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed A.A., Jacobs J.A., Murugesan G.R., Cheng H.W. Effect of dietary synbiotic supplement on behavioral patterns and growth performance of broiler chickens reared under heat stress. Poult. Sci. 2018;97:1101–1108. doi: 10.3382/ps/pex421. [DOI] [PubMed] [Google Scholar]
- Momba M.N.B., Kaleni P. Re-growth and survival of indicator microorganisms on the surfaces of household containers used for the storage of drinking water in rural communities of South Africa. Water Res. 2003;36:3023–3028. doi: 10.1016/s0043-1354(02)00011-8. [DOI] [PubMed] [Google Scholar]
- Momba M.N.B., Notshe T.L. The microbiological quality of groundwater-derived drinking water after long storage in household containers in a rural community of South Africa. Aqua. 2003;52:67–77. [Google Scholar]
- Momba M.N.B., Malakate V.K., Theron J. Abundance of pathogenic Escherichia coli, Salmonella typhimurium and Vibrio cholerae in Nkonkobe drinking water sources. J. Water Health. 2006;4:289–296. doi: 10.2166/wh.2006.011. [DOI] [PubMed] [Google Scholar]
- Mombarg M., Bouzoubaa K., Andrews S., Vanimisetti H.B., Rodenberg J., Karaca K. Safety and efficacy of an aroA-deleted live vaccine against avian colibacillosis in a multicentre field trial in broilers in Morocco. Avian Pathol. J. WVPA. 2014;43:276–281. doi: 10.1080/03079457.2014.917760. [DOI] [PubMed] [Google Scholar]
- Monroy M.A.R., Knöbl T., Bottino J.A., Ferreira C.S.A., Ferreira A.J.P. Virulence characteristics of Escherichia coli isolates obtained from broiler breeders with salpingitis. Comp. Immunol. Microbiol. Infect. Dis. 2005;28:1–15. doi: 10.1016/j.cimid.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Mookiah S., Sieo C.C., Ramasamy K., Abdullah N., Ho Y.W. Effects of dietary prebiotics, probiotic and synbiotics on performance, caecal bacterial populations and caecal fermentation concentrations of broiler chickens. J. Sci. Food Agric. 2014;94:341–348. doi: 10.1002/jsfa.6365. [DOI] [PubMed] [Google Scholar]
- Moore P.R., Evenson A., Luckey T.D., McCoy E., Elvehjem C.A., Hart E.B. Use of sulfasuxidine, streptothricin, and streptomycin in nutritional studies with the chick. J. Biol. Chem. 1946;165:437–441. [PubMed] [Google Scholar]
- Mountzouris K.C., Tsitrsikos P., Palamidi I., Arvaniti A., Mohnl M., Schatzmayr G., Fegeros K. Effects of probiotic inclusion levels in broiler nutrition on growth performance, nutrient digestibility, plasma immunoglobulins, and cecal microflora composition. Poult. Sci. 2010;89:58–67. doi: 10.3382/ps.2009-00308. [DOI] [PubMed] [Google Scholar]
- Murase K., Martin P., Porcheron G., Houle S., Helloin E., Penary M., Nougayrede J.P., Dozois C.M., Hayashi T., Oswald E. HlyF Produced by extraintestinal pathogenic Escherichia coli is a virulence factor that regulates outer membrane vesicle biogenesis. J. Infect. Dis. 2016;213:856–865. doi: 10.1093/infdis/jiv506. [DOI] [PubMed] [Google Scholar]
- Nagano T., Kitahara R., Nagai S. An attenuated mutant of avian pathogenic Escherichia coli serovar O78: a possible live vaccine strain for prevention of avian colibacillosis. Microbiol. Immunol. 2012;56:605–612. doi: 10.1111/j.1348-0421.2012.00482.x. [DOI] [PubMed] [Google Scholar]
- Nakazato G., de Campos T.A., Stehling E.G., Brocchi M., da Silveira W.D. Virulence factors of avian pathogenic Escherichia coli (APEC) Pesqui. Veterinária Bras. 2009;29:479–486. [Google Scholar]
- Narita S., Matsuyama S., Tokuda H. Lipoprotein trafficking in Escherichia coli. Arch. Microbiol. 2004;182:1–6. doi: 10.1007/s00203-004-0682-4. [DOI] [PubMed] [Google Scholar]
- Nava G.M., Bielke L.R., Callaway T.R., Castañeda M.P. Probiotic alternatives to reduce gastrointestinal infections: the poultry experience. Anim. Health Res. Rev. 2005;6:105–118. doi: 10.1079/ahr2005103. [DOI] [PubMed] [Google Scholar]
- Ngeleka M., Brereton L., Brown G., Fairbrother J.M. Pathotypes of avian Escherichia coli as related to tsh-, pap-, pil-, and iuc-DNA sequences, and antibiotic sensitivity of isolates from internal tissues and the cloacae of broilers. Avian Dis. 2002;46:143–152. doi: 10.1637/0005-2086(2002)046[0143:POAECA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Nhung N.T., Chansiripornchai N., Carrique-Mas J.J. Antimicrobial resistance in bacterial poultry pathogens: a Review. Front. Vet. Sci. 2017;4:126. doi: 10.3389/fvets.2017.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson O., Börjesson S., Landén A., Bengtsson B. Vertical transmission of Escherichia coli carrying plasmid-mediated AmpC (pAmpC) through the broiler production pyramid. J. Antimicrob. Chemother. 2014;69:1497–1500. doi: 10.1093/jac/dku030. [DOI] [PubMed] [Google Scholar]
- Nolan L.K., Vaillancourt J.-P., Barbieri N.L., Logue C.M. Colibacillosis. In: Swayne D.E., editor. Diseases of Poultry. Wiley Online Library; Ames, IA: 2020. pp. 770–830. [Google Scholar]
- Oguttu J.W., Veary C.M., Picard J.A. Antimicrobial drug resistance of Escherichia coli isolated from poultry abattoir workers at risk and broilers on antimicrobials. J. S. Afr. Vet. Assoc. 2008;79:161–166. doi: 10.4102/jsava.v79i4.266. [DOI] [PubMed] [Google Scholar]
- Olsén A., Wick M.J., Mörgelin M., Björck M., Curli L. Fibrous surface proteins of Escherichia coli, interact with major histocompatibility complex class I molecules. Infect. Immun. 1998;66:944–949. doi: 10.1128/iai.66.3.944-949.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen R.H., Frantzen C., Christensen H., Bisgaard M. An investigation on first week mortality in layers. Avian Dis. 2012;56:51–57. doi: 10.1637/9777-051011-Reg.1. [DOI] [PubMed] [Google Scholar]
- Parreira V.R., Arns C.W., Yano T. Virulence factors of avian Escherichia coli associated with swollen head syndrome. Avian Pathol. 1998;27:148–154. doi: 10.1080/03079459808419316. [DOI] [PubMed] [Google Scholar]
- Pedroso A.A., Hurley-Bacon A.L., Zedek A.S., Kwan T.W., Jordan A.P., Avellaneda G., Hofacre C.L., Oakley B.B., Collett S.R., Maurer J.J., Lee M.D. Can probiotics improve the environmental microbiome and resistome of commercial poultry production? Int. J. Environ. Res. Public Health. 2013;10:4534–4559. doi: 10.3390/ijerph10104534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen A., Christensen J.P., Kuhnert P., Bisgaard M., Olsen J.E. Vertical transmission of a fluoroquinolone-resistant Escherichia coli within an integrated broiler operation. Vet. Microbiol. 2006;116:120–128. doi: 10.1016/j.vetmic.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Philpot C.S. Poult. Sci. Dept. Univ. Arkansas; United States: 2015. Saccharomyces boulardii as an enteric health promoter in broiler chickens. Undergraduate Honors Thesis. [Google Scholar]
- Pillsbury L., Miller E.A., Boon C., Pray L. National Academies Press; Washington DC: 2010. Providing Healthy and Safe Foods as We Age. Workshop Summary. [PubMed] [Google Scholar]
- Pineiro M., Asp N.G., Reid G., Macfarlane S., Morelli L., Brunser O., Tuohy K. FAO technical meeting on prebiotics. J. Clin. Gastroenterol. 2008;42:S156. doi: 10.1097/MCG.0b013e31817f184e. [DOI] [PubMed] [Google Scholar]
- Pitout J., Cheng A., Thielman N., Guerrant R. Escherichia Coli. 2017. http://www.antimicrobe.org/b104.asp Accessed June 2020.
- Postma M., Stärk K.D., Sjölund M., Backhans A., Beilage E.G., Lösken S., Belloc C., Collineau L., Iten D., Visschers V. Alternatives to the use of antimicrobial agents in pig production: a multi-country expert-ranking of perceived effectiveness, feasibility and return on investment. Prev. Vet. Med. 2015;118:457–466. doi: 10.1016/j.prevetmed.2015.01.010. [DOI] [PubMed] [Google Scholar]
- Pourabedin M., Zhao X. Prebiotics and gut microbiota in chickens. FEMS Microbiol. Lett. 2015;362:fnv122. doi: 10.1093/femsle/fnv122. [DOI] [PubMed] [Google Scholar]
- Provence D.L., Curtiss R. Isolation and characterization of a gene involved in hemagglutination by an avian pathogenic Escherichia coli strain. Infect. Immun. 1994;62:1369–1380. doi: 10.1128/iai.62.4.1369-1380.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai M.F., Khan S.A., Aslam A., Saeed K. Effects of yolk sac infection in chicken. Avian Poult. Biol. Rev. 2005;16:87–93. [Google Scholar]
- Rajput I.R., Li L.Y., Xin X., Wu B.B., Juan Z.L., Cui Z.W., Yu D.Y., Li W.F. Effect of Saccharomyces boulardii and Bacillus subtilis B10 on intestinal ultrastructure modulation and mucosal immunity development mechanism in broiler chickens. Poult. Sci. 2013;92:956–965. doi: 10.3382/ps.2012-02845. [DOI] [PubMed] [Google Scholar]
- Reda F.M., El-Saadony M.T., Elnesr S.S., Alagawany M., Tufarelli V. Effect of dietary supplementation of biological curcumin nanoparticles on growth and carcass traits, antioxidant status, immunity and caecal microbiota of Japanese quails. Animals. 2020;10:754. doi: 10.3390/ani10050754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revolledo L., Ferreira C.S.A., Ferreira A.J.P. Prevention of Salmonella typhimurium colonization and organ invasion by combination treatment in broiler chicks. Poult. Sci. 2009;88:734–743. doi: 10.3382/ps.2008-00410. [DOI] [PubMed] [Google Scholar]
- Ricke S.C. Impact of prebiotics on poultry production and food safety. Yale J. Biol. Med. 2018;91:151–159. [PMC free article] [PubMed] [Google Scholar]
- Rios A.C., Moutinho C.G., Pinto F.C., Del Fiol F.S., Jozala A., Chaud M.V., Vila M.M., Teixeira J.A., Balcão V.M. Alternatives to overcoming bacterial resistances: State-of-the-art. Microbiol. Res. 2016;191:51–80. doi: 10.1016/j.micres.2016.04.008. [DOI] [PubMed] [Google Scholar]
- Ritzi M.M., Abdelrahman W., Mohnl M., Dalloul R.A. Effects of probiotics and application methods on performance and response of broiler chickens to an Eimeria challenge. Poult. Sci. 2014;93:2772–2778. doi: 10.3382/ps.2014-04207. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Siek K.E., Giddings C.W., Doetkott C., Johnson T.J., Nolan L.K. Characterizing the APEC pathotype. Vet. Res. 2005;36:241–256. doi: 10.1051/vetres:2004057. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Siek K.E., Giddings C.W., Doetkott C., Johnson T.J., Fakhr M.K., Nolan L.K. Comparison of Escherichia coli isolates implicated in human urinary tract infection and avian colibacillosis. Microbiol. 2005;151:2097–2110. doi: 10.1099/mic.0.27499-0. [DOI] [PubMed] [Google Scholar]
- Roland K., Karaca K., Sizemore D. Expression of Escherichia coli antigens in Salmonella typhimurium as a vaccine to prevent airsacculitis in chickens. Avian Dis. 2004;48:595–605. doi: 10.1637/7178-031004R1. [DOI] [PubMed] [Google Scholar]
- Rosario C.C., Lopez A.C., Tellez I.G., Navarro O.A., Anderson R.C., Eslava C.C. Serotyping and virulence genes detection in Escherichia coli isolated from fertile and infertile eggs, dead-in-shell embryos, and chickens with yolk sac infection. Avian Dis. 2004;48:791–802. doi: 10.1637/7195-041304R. [DOI] [PubMed] [Google Scholar]
- Russo T.A., Johnson J.R. Proposal for a new inclusive designation for extraintestinal pathogenic isolates of Escherichia coli: ExPEC. J. Infect. Dis. 2000;181:1753–1754. doi: 10.1086/315418. [DOI] [PubMed] [Google Scholar]
- Sabri M., Leveille S., Dozois C.M. A SitABCD homologue from an avian pathogenic Escherichia coli strain mediates transport of iron and manganese and resistance to hydrogen peroxide. Microbiology. 2006;152:745–758. doi: 10.1099/mic.0.28682-0. [DOI] [PubMed] [Google Scholar]
- Sadeghi M., Tavakkoli H., Golchin M., Ghanbarpour R., Amanollahi S. Efficacy and safety of Poulvac E. coli vaccine in broiler chickens challenged with E. coli serotype O78 and an acute field isolate. Comp. Clin. Pathol. 2018;27:1629–1636. [Google Scholar]
- Sadeyen J.R., Wu Z., Davies H., van Diemen P.M., Milicic A., La Ragione R.M., Kaiser P., Stevens M.P., Dziva F. Immune responses associated with homologous protection conferred by commercial vaccines for control of avian pathogenic Escherichia coli in turkeys. Vet. Res. 2015;46:1–14. doi: 10.1186/s13567-014-0132-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed M., Arain M.A., Arif M., Alagawany M., Abd El-Hack M.E., Kakar M.U., Manzoor R., Erdenee S., Chao S. Jatropha (Jatropha curcas) meal is an alternative protein source in poultry nutrition, World's Poult. Sci. J. 2017;73:783–790. [Google Scholar]
- Saha T., Murhekar M., Hutin Y.J. Ramamurthy, T. An urban, waterborne outbreak of diarrhoea and shigellosis in a district town in eastern India. Natl. Med. J. India. 2009;22:237–239. [PubMed] [Google Scholar]
- Sanderson M.W., Sargeant J.M., Renter D.G., Griffin D.D., Smith R.A. Factors associated with the presence of coliforms in the feed and water of Farm animals. Appl. Environ. Microbiol. 2005;71:6026–6032. doi: 10.1128/AEM.71.10.6026-6032.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapkota A.R., Curriero F.C., Gibson K.E., Schwab K.J. Antibiotic-resistant enterococci and fecal indicators in surface water and groundwater impacted by a concentrated swine feeding operation. Environ. Health Persp. 2007;15:1040–1045. doi: 10.1289/ehp.9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouler C., Schaeffer B., Brée A., Mora A., Dahbi G., Biet F., Oswald E., Mainil J., Blanco J., Moulin-Schouleur M. Diagnostic strategy for identifying avian pathogenic Escherichia coli based on four patterns of virulence genes. J. Clin. Microbiol. 2012;50:1673–1678. doi: 10.1128/JCM.05057-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedeik M.E., El-shall N.A., Awad A.M., Abd El-Hack M.E., Alowaimer A.N., Swelum A.A. Comparative evaluation of HVT-IBD vector, immune complex, and live IBD vaccines against vvIBDV in commercial broiler chickens with high maternally derived antibodies. Animals. 2019;9:72. doi: 10.3390/ani9030072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal Y. Vol. 64. FAO; 2013. (Prevention and Control of Poultry Diseases for Better Farm Profitability). [Google Scholar]
- Sheiha A.M., Abdelnour S.A., Abd El-Hack M.E., Khafaga A.F., Metwally K.A., Ajarem J.S., Maodaa S.N., Allam A.A., El-Saadony M.T. Effects of dietary biological or chemical-synthesized nano-selenium supplementation on growing rabbits exposed to thermal stress. Animals. 2020;10:430. doi: 10.3390/ani10030430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim Y.H., Ingale S.L., Kim J.S., Kim K.H., Seo D.K., Lee S.C., Chae B.J., Kwon I.K. A multi-microbe probiotic formulation processed at low and high drying temperatures: effects on growth performance, nutrient retention and caecal microbiology of broilers. Br. Poult. Sci. 2012;53:482–490. doi: 10.1080/00071668.2012.690508. [DOI] [PubMed] [Google Scholar]
- Shivaprasad H.L. Fowl typhoid and pullorum disease. Rev. Sci. Tech. 2000;19:405–424. doi: 10.20506/rst.19.2.1222. [DOI] [PubMed] [Google Scholar]
- Slonczewski J. Microcystis Aeruginosa and the Effects of Microcystin-LR on Ecosystems and Human Health. 2017. https://microbewiki.kenyon.edu/index.php/Microcystis_aeruginosa_and_the_Effects_of_Micro cystin-LR_on_Ecosystems_and_Human_Health Accessed June 2020.
- Sredanović S., Lević J., Đuragić O. Identification of feed raw material hazard properties. J. Process. Energy Agric. 2005;9:120–123. [Google Scholar]
- Stenutz R., Weintraub A., Widmalm G. The structures of Escherichia coli O polysaccharide antigens. FEMS Microbiol. Rev. 2006;30:382–403. doi: 10.1111/j.1574-6976.2006.00016.x. [DOI] [PubMed] [Google Scholar]
- Stone K.C., Hunt P.G., Coffey S.W., Matheny T.A. Water quality status of a USDA water quality demonstration project in the eastern coastal Plain. J. Soil Water Conserv. 1995;50:567–571. [Google Scholar]
- Suresh G., Das R.K., Brar S.K., Rouissi T., Ramirez A.A., Chorfi Y., Godbout S. Alternatives to antibiotics in poultry feed: molecular perspectives. Crit. Rev. Microbiol. 2017;44:318–335. doi: 10.1080/1040841X.2017.1373062. [DOI] [PubMed] [Google Scholar]
- Takeda K., Akira S. Toll-like receptors in innate immunity. Int. Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- Tivendale K.A., Noormohammadi A.H., Allen J.L., Browning G.F. The conserved portion of the putative virulence region contributes to virulence of avian pathogenic Escherichia coli. Microbiology. 2009;155:450–460. doi: 10.1099/mic.0.023143-0. [DOI] [PubMed] [Google Scholar]
- Tóth I., Hérault F., Beutin L., Oswald E. Production of cytolethal distending toxins by pathogenic Escherichia coli strains isolated from human and animal sources: establishment of the existence of a new cdt variant (Type IV) J. Clin. Microbiol. 2003;41:4285–4291. doi: 10.1128/JCM.41.9.4285-4291.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsilingiri K., Rescigno M. Postbiotics: what else? Benef. Microbes. 2013;969:101–107. doi: 10.3920/BM2012.0046. [DOI] [PubMed] [Google Scholar]
- U.S. Food & Drug Administration (USDA) Press Announcements - FDA Takes Significant Steps to Address Antimicrobial Resistance. 2013. http://wayback.archiveit.org/7993/20161022070002/http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncemen ts/ucm378193.htm Accessed June 2020.
- United States Environmental Protection Agency, (USEPA) Animal Feeding Operations. 2009. http://cfpub.epa.gov/npdes/home.cfm?program_id=7 Accessed June 2020.
- Uotani Y., Kitahara R., Imai T., Tsutsumi N., Sasakawa C., Nagai S., Nagano T. Efficacy of an avian colibacillosis live vaccine for layer breeder in Japan. J. Vet. Med. Sci. 2017;79:1215–1219. doi: 10.1292/jvms.17-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Goor A., Stromberg Z.R., Mellata M. A recombinant multi-antigen vaccine with broad protection potential against avian pathogenic Escherichia coli. PLoS One. 2017;12:e0183929. doi: 10.1371/journal.pone.0183929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira S.L., Moran E.T. Effects of egg of origin and chick post-hatch nutrition on broiler live performance and meat yields. World Poult. Sci. J. 1999;55:125–142. [Google Scholar]
- Wang J., Tang P., Tan D., Wang L., Zhang S., Qiu Y., Dong R., Liu W., Huang J., Chen T. The pathogenicity of chicken pathogenic Escherichia coli is associated with the numbers and combination patterns of virulence-associated genes. Open J. Vet. Med. 2015;5:243–254. [Google Scholar]
- Wang L., Rothemund D., Curd H., Reeves P.R. Species-wide variation in the Escherichia coli Flagellin (H-antigen) gene. J. Bacteriol. 2003;185:2936–2943. doi: 10.1128/JB.185.9.2936-2943.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windisch W., Schedle K., Plitzner C., Kroismayr A. Use of phytogenic products as feed additives for swine and poultry. J. Anim. Sci. 2008;86:E140–E148. doi: 10.2527/jas.2007-0459. [DOI] [PubMed] [Google Scholar]
- Wooley R.E., Gibbs P.S., Brown T.P., Glisson J.R., Steffens W.L., Maurer J.J. Colonization of the chicken trachea by an avirulent avian Escherichia coli transformed with plasmid pHK11. Avian Dis. 1998;42:194–198. [PubMed] [Google Scholar]
- World Health Organization (WHO) E. coli. 2018. http://www.who.int/news-room/fact-sheets/detail/e-coli Accessed June 2020.
- Yang C., Chowdhury M.A.K., Hou Y., Gong J. Phytogenic compounds as alternatives to in-Feed antibiotics: potentials and challenges in application. Pathogens. 2015;4:137–156. doi: 10.3390/pathogens4010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassin H., Velthuis A.G.J., Boerjan M., van Riel J. Field study on broilers’ first week mortality. Poult. Sci. 2009;88:798–804. doi: 10.3382/ps.2008-00292. [DOI] [PubMed] [Google Scholar]
- Younis G., Awad A., Mohamed N. Phenotypic and genotypic characterization of antimicrobial susceptibility of avian pathogenic Escherichia coli isolated from broiler chickens. Vet. World. 2017;10:1167–1172. doi: 10.14202/vetworld.2017.1167-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A., Lai H., Xu J., Huang W., Liu Y., Zhao D., Chen R. Evaluation of the protective efficacy of Poly I:C as an adjuvant for H9N2 Subtype avian influenza inactivated vaccine and its mechanism of action in ducks. PLoS One. 2017;12:e0170681. doi: 10.1371/journal.pone.0170681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D., Hardt W.D., Galan J.E. Salmonella typhimurium encodes a putative iron transport system within the centisome 63 pathogenicity island. Infect. Immun. 1999;67:1974–1981. doi: 10.1128/iai.67.4.1974-1981.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimonja O. Pages 45-50 in Current Issues in Pelleting in Respect to Physical Pellet Analyses. 1st Workshop Feed-To-Food FP7 REGPOT-3. XIII Symposium Feed Technology. In: Lević J., Ðuragić O., Sredanović S., editors. 2009. Proceedings. Novi Sad, Serbia, 29 September-1 October. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.