Abstract
Heat stress can decrease poultry performance indices, immune function, and intestinal development, which can reduce birds' innate protective mechanisms and may be more susceptible for pathogens. Ma chickens heat-stressed with 41°C for 12 h and recovered for 7 d had extremely low immunity. In this study, a susceptible chicken model induced by heat stress and then infected with Escherichia coli O157:H7 was established to explore the mechanisms of birds' intestinal immune function changes. Ma chickens in heat stress + E. coli (HS + E. coli) group were stressed at 41°C for 12 h and recovered for 7 d, then chickens in E. coli group and HS + E. coli group were orally administered with 1 mL E. coli O157:H7 (1 × 109 cfu/mL). Chickens were sacrificed at the fourth day after E. coli administration. Results showed that the HS + E. coli group had increased intestinal length and weight, had higher E. coli counts in cecum contents than the E. coli group. Heat stress also enhanced serum diamine oxidase and decreased IgA level in chickens infected by E. coli. Heat stress had protective effects in small intestinal morphology except for duodenum by using hematoxylin and eosin staining. Compared with the E. coli group birds, IL-1β, TNF-α, and caspase-1 protein levels in the duodenum and ileum were significantly increased. Heat stress also can significantly enhance the gene and protein expression of Hsp70, TLR4, and NF-κB in the duodenum and ileum, respectively. The gene expression of Hsp70, TLR4, and NF-κB in the jejunum was not influenced, but the protein expression of Hsp70 and NF-κB was inhibited by heat stress. The results indicated heat stress can amplify the effect of E. coli on intestinal inflammatory injury of Ma chickens through increasing TLR4-NF-κB signaling pathway.
Key words: heat stress, chicken, intestinal inflammation, Escherichia coli, NF-κB, TLR4
Introduction
Stresses can reduce animal welfare, performance indices, and immunological parameters in poultry production (Bartlett and Smith, 2003; Mashaly et al., 2004) and high ambient temperature is one of the most relevant stressors. It's reported that heat stress (HS) can depress the growth rate, decrease body weight gain, and induce a high mortality rate (Syafwan et al., 2011). Heat stress also was reported to negatively affect intestinal development, especially the integrity of the intestinal epithelium (Soderholm and Perdue, 2001; Quinteiro-Filho et al., 2010, 2012). Moreover, stress-induced perturbation of the integrity of the gut epithelium reduces the efficacy of the birds' innate protective mechanisms and may increase the potential for pathogens (such as Escherichia coli, Salmonella spp) to bind to and damage the intestinal epithelium (Burkholder et al., 2008). The loss of intestinal barrier integrity allows for the paracellular transport of endotoxins into the blood stream leading to the activation of the innate immune system and systemic inflammation (Hall et al., 2001).
Escherichia coli is an important inducer of endemic and epidemic diarrheal disease worldwide and can induce life-threatening illnesses that require hospitalization that can even result in death (Barton Behravesh et al., 2011). Escherichia coli O157:H7 is an enterohemorrhagic serotype of the bacterium E. coli and a cause of infection, which could result in poor growth, increased mortality, and a great economic loss in poultry industry (Alonso et al., 2011). Clinical data indicate that patients with E. coli disease had obvious intestinal inflammatory responses. The inflammatory responses were related with the host intestinal damage and the subsequent decreased of host innate immune responses. It's reported that chronic intestinal diseases, such as Crohn's disease and ulcerative colitis, are developed due to an aberrant immune response to intestinal microbes in susceptible hosts (Xavier and Podolsky, 2007).
The intestinal mucosa is continuously exposed to a heavy load of antigenic molecules from ingested food and microorganisms, such as resident and invading bacteria and viruses. If the intestinal barrier is disturbed, antigen and bacterial passage may therefore increase, thereby damaging the mucosa and leading to pathological conditions (Keita and Soderholm, 2010). Stressors have been reported to enhance the risk of infectious diseases and to influence their severity (Glaser and Kiecolt-Glaser, 2005). Chronic and acute HS can compromise intestinal function, with gut barrier integrity compromised; pathogenic microbes and other debris from the intestinal tract are more likely to cause systemic infection. This is primarily attributed to inflammation, oxidative stress, and the effects of microbial toxins and stress hormones (Zhang et al., 2015). Furthermore, HS disrupts the balance of the intestinal microbial ecology and induces proliferation of harmful pathogens including total aerobic bacteria, such as Salmonella and E. coli (Park et al., 2013), resulting in intestinal dysfunction and damage. Diamine oxidase (DAO) can reflect the injury of the intestinal mucosa. IgA exists in the mucosal tissues and can avoid pathogen invasion. Serum DAO and IgA levels in chickens are indicators to reflect whether the intestinal mucosal damaged or not.
Acute and chronic HS in broiler chickens can increase its serum corticosterone levels and induce intestinal inflammation (Quinteiro-Filho et al., 2010, 2012). Heat stress can affect the integrity of the intestinal barrier, and the damaged intestinal barrier integrity leads to increased intestinal permeability, inducing local intestinal inflammation (Chappell et al., 2009), which stimulates mRNA expressions of proinflammatory cytokines (Varasteh et al., 2015). Lots of cytokines have been used to measure intestinal health and inflammatory processes. It's reported that increased gut IL-1β, IL-6, and TNF-α concentrations could enhance intestinal cell permeability. In addition, the observed intestinal inflammation may induce the release of inflammatory cytokines (such as IL-6, IL-1β, TNF-α) that can induce sickness-related behaviors in birds (Dantzer, 2001). TLR4 plays a pivotal role in stress-induced inflammatory priming. Although lots of research studies report that HS can reduce birds' innate protective mechanisms to damage intestinal development, little is known about the relationship between HS and intestinal inflammatory response after E. coli challenge in birds. The objective of the present study was to explore the influence of HS on intestinal inflammatory against E. coli in Ma chicken. Our findings provide evidence that 12 h heat-stressed chicken had serious intestinal inflammatory injury after E. coli challenge by enhancing TLR4-NK-κB signaling pathway.
Materials and methods
Bacteriology E. coli O157:H7 Strain
The E. coli O157:H7 strain was originally obtained from Guangdong Microbial Culture Collection Center (Guangdong, China). The frozen strain was thawed and 100 μL was inoculated into sterile tubes containing 10 mL of sterile Luria-Bertani broth. The inoculated broth was incubated at 37°C with orbital shaking for 24 h (HZQ incubator; Harbin Donglian Electronic Technology Co. Ltd., Heilongjiang, China). The stock culture was prepared in sterile saline solution and adjusted to 1 × 109 cfu/mL of E. coli O157:H7 as the inoculum.
Animals and Experimental Design
All animals' work was conducted in accordance with the guidelines for the care and use of experimental animals established by the Ministry of Science and Technology of the People's Republic of China (approval number: 2006-398), and was approved by the Laboratory Animal Management Committee of Foshan University.
A total of 36 two-week-old Ma chickens are a pure line of local Qing Yuan Ma Chickens from a commercial hatchery (Foshan Nanhai Poultry Corporation, Foshan, China). The chickens were randomly divided into 3 groups with 12 replicates per group. The treatments were as follows: control group, E. coli group, and heat stress + E. coli (HS + E. coli) group. Chickens in HS + E. coli group were treated with 41°C HS for 12 h and then recovered for 7 d (at 21-day-old). At the seventh day, the E. coli and HS + E.coli group chickens were orally administrated with 1 mL E. coli O157:H7 inoculum (109 cfu/mL) using a polyethylene tube attached to a syringe. The chickens in the control group were given the same amount of saline solution. Chickens had free access to experimental diets and drinking water. A combination of daylight and artificial light was used, with a 12-h light/dark cycle.
Birds were selected for sample collection at the fourth day after E. coli challenge. Blood samples were taken from the jugular vein and used for the measurement of DAO and IgA level. The birds were then killed by CO2 inhalation and small intestines were collected, weighed, and the length measured. Then the duodenum, jejunum, ileum, and cecum were weighed and their lengths were measured. Some duodenum, jejunum, and ileum tissues were retained for qPCR to detect cytokine production, and for Western blot to detect protein production. The duodenum, jejunum, ileum, and cecum were fixed in 4% (v/v) paraform aldehyde for evaluation of the tissue pathology changes by using hematoxylin and eosin staining. The cecum contents were removed and put in sterilized tubes, then stored at −20°C for determination of E.coli cDNA expression.
Serum Diamine Oxidase and IgA Analysis
Serum IgA level was analyzed using immunoglobulin A assay kit (H108) and DAO level was measured by the DAO assay kit (A088-1-1). The two kits were provided by Jiancheng Bioengineering Institute (Nanjing, China).
Intestinal Morphology
At necropsy, a 3 cm long segment of the duodenum, jejunum, ileum, and cecum were longitudinally cut in each chicken. The tissues were rinsed with 0.9% NaCl solution and then fixed immediately in 4% (v/v) paraformaldehyde solution in PBS until processing. The paraffin-embedded intestinal samples were sectioned (5 μm) and stained with hematoxylin and eosin for light microscopy. The villus height (VH) and corresponding crypt depth (CD) were randomly chosen from different well-orientated parts of the sections. The following histological parameters were measured with computer-assisted microscopy (Nikon ECLIPSE E200, Tokyo, Japan): the VH, CD, and the VH/CD ratio (V/C).
Western Blot Analysis
Standard Western blot analysis was performed on the duodenum, jejunum, and ileum tissues of birds. Briefly, tissues were homogenized with protein lysis buffer. Protein concentration was determined using the BCA protein assay kit (Thermo Fisher Scientific) as per the manufacturer's instructions and using a multimode microplate reader for spectroscopic measurements. About 30 μg proteins were mixed with 3 μL loading buffer and subjected to 10% SDS-PAGE electrophoresis followed by blotting onto a polyvinylidene difluoride membrane. Membranes were immersed in blocking solution (3% BSA in tris buffered saline tween) for 2 h and then immunoblotted with primary antibodies (Hsp70, TLR4, NF-κBp65, caspase-1, pro-IL-1β, IL-1β, IL-6, TNF-α, and β-actin; Cell Signaling Technology, Danvers, MA) diluted with 3% BSA for 2 h at room temperature. The membranes were washed with tris buffered saline tween buffer 3 times followed by 1 h incubation with HRP-conjugated secondary anti-mouse IgG from goat (or anti-rabbit IgG). And the gray level of the protein expression was analyzed by using the software, ImageJ.
Gene Expression Analysis
A 5 cm segment of the duodenum, jejunum, and ileum tissues was snap-frozen in liquid nitrogen and stored at −80°C until mRNA extraction, gene expression performed. Total RNA was extracted from the tissue samples using the TRIzol Reagent (Hlingene Corporation, Shanghai, China) as per the manufacturer's guidelines, and 10 ug of RNA was used for cDNA synthesis using UEIris Ⅱ RT-PCR System for First-Strand cDNA Synthesis (Jiangsu, China) to examine expression of Hsp70, TLR4, and NF-κB. RNA was spectrophotometrically quantified (A260) and its integrity was verified by agarose gel electrophoresis.
To investigate the presence of E. coli in the cecum of the E. coli–challenged birds, cecum samples were taken from 8 birds per group. The cecum content DNA was extracted with the EZNA bacterial DNA kit (Omega, Doraville, GA). The E. coli DNA was spectrophotometrically quantified (A260) and its integrity was verified by agarose gel electrophoresis.
All-in-one TM qPCR mix kit (GeneCopoeia) in accordance with the manufacturer's protocol for the LightCycler 480 Real-Time PCR system (Applied Roche, Basel, Switzerland). The PCR efficiency and melting curves were checked to ensure consistent amplification of a single PCR product. Gene expression was normalized to β-actin (internal reference) or rpsl (bacterial internal reference) and presented as relative fold change compared with the control. All samples were tested in triplicate.
PCR was performed using specific primers for Hsp70, TLR4, NF-κB, and E. coli. The primers used in qPCR are given in Table 1.
Table 1.
The primers used in qPCR.
| Primers | Gene | Sequence (5′ to 3′) |
|---|---|---|
| Hsp70 for | 120 bp | CGGGCAAGTTTGACCTAA |
| Hsp70 rev | TTGGCTCCCACCCTA TCTCT | |
| TLR4 for | 132 bp | TCTTTCAAGGTGCCACATCCA |
| TLR4 rev | AGCGACGTTAAGCCATGGAA | |
| NF-κB for | 118 bp | TGGGAGTGTCAGATCCCCAA |
| NF-κB rev | TACGGTCCATCTGCTGTTCG | |
| β-actin for | 298 bp | ACGTCTCACTGGATTTCGAGCAGG |
| β-actin rev | TGCATCCTGTCAGCAATGCCAG | |
| E. coli for | 82 bp | GTGTGATATCTACCCGCTTCGC |
| E. coli rev | AGAACGCTTTGTGGTTAATCAGGA | |
| rpsl for | 75 bp | TACGTGGTGCGCTTGACTGC |
| rpsl rev | TAGGACGCTTCACGCCATAC |
Statistical Analysis
All data were analyzed using SPSS 17.0 for one-way ANOVA. The data are presented as the mean ± standard deviation. Differences were considered statistically significant at P < 0.05.
Results
Effects of Heat Stress on Changes of Intestinal Length and Weight in E. coli–Infected Chicken
Changes of intestinal length in Ma chicken are shown in Table 2. The results showed the intestinal length in the E. coli group birds was longer than that of the control group birds (P < 0.05). Compared with the E. coli group, the changes of intestinal length were significantly decreased in the HS + E. coli group Ma chicken with 7 d of recovery from HS (P < 0.05). The length of the duodenum, ileum, and cecum in the E. coli group birds significantly increased compared with the control group birds (P < 0.05, P < 0.01, P < 0.01, respectively), and HS obviously inhibited the increase in length of the duodenum, ileum, and cecum in the HS + E. coli group compared with the E. coli group birds except the jejunum (P < 0.05, P < 0.01, P < 0.01, respectively).
Table 2.
Changes of intestinal length in Escherichia coli–challenged Ma chickens treated with heat stress.
| Item | Control (cm) | E. coli group (cm) | HS + E. coli group (cm) |
|---|---|---|---|
| Small intestine | 69.25 ± 8.17 | 86.17 ± 10.35# | 62.50 ± 4.19∗ |
| Duodenum | 17.25 ± 2.49 | 22.50 ± 2.69# | 18.00 ± 1.73∗ |
| Jejunum | 32.75 ± 4.26 | 27.00 ± 4.13 | 29.67 ± 3.86 |
| Ileum | 19.25 ± 4.26 | 36.67 ± 4.33## | 14.83 ± 1.86∗∗ |
| Cecum | 9.50 ± 1.12 | 16.17 ± 2.03## | 7.81 ± 1.92∗∗ |
#P < 0.05, ##P < 0.01 vs. control group; ∗P < 0.05, ∗∗P < 0.01 vs. E. coli group, n = 12.
Abbreviation: HS, heat stress.
Results showed the intestinal weight between the E. coli group and control group birds had no change (Table 3). Compared with the E. coli group, HS significantly decreased intestinal weight in the HS + E. coli group Ma chicken (P < 0.05). The weight changes of the ileum had the same trend with the length change. However, the weight of the jejunum was decreased in the E. coli group compared with the control group (P < 0.05), and HS significantly reversed the decrease of the jejunum weight compared with E.coli alone–treated birds (P < 0.05).
Table 3.
Changes of intestinal weight in Escherichia coli–challenged Ma chickens treated with heat stress.
| Item | Control (g) | E. coli group (g) | HS + E. coli group (g) |
|---|---|---|---|
| Small intestine | 12.83 ± 2.54 | 12.58 ± 1.52 | 10.22 ± 1.83∗ |
| Duodenum | 3.78 ± 0.64 | 3.63 ± 0.47 | 3.00 ± 0.59 |
| Jejunum | 5.84 ± 1.48 | 3.75 ± 0.54# | 4.58 ± 0.50∗ |
| Ileum | 3.21 ± 0.61 | 5.20 ± 0.89## | 2.64 ± 0.99∗∗ |
| Cecum | 2.34 ± 0.94 | 3.59 ± 1.62 | 2.19 ± 0.99 |
#P < 0.05, ##P < 0.01 vs. control group; ∗P < 0.05, ∗∗P < 0.01 vs. E. coli group, n = 12.
Abbreviation: HS, heat stress.
The Effects of Heat Stress on E. coli RNA Levels in the Chicken Cecum
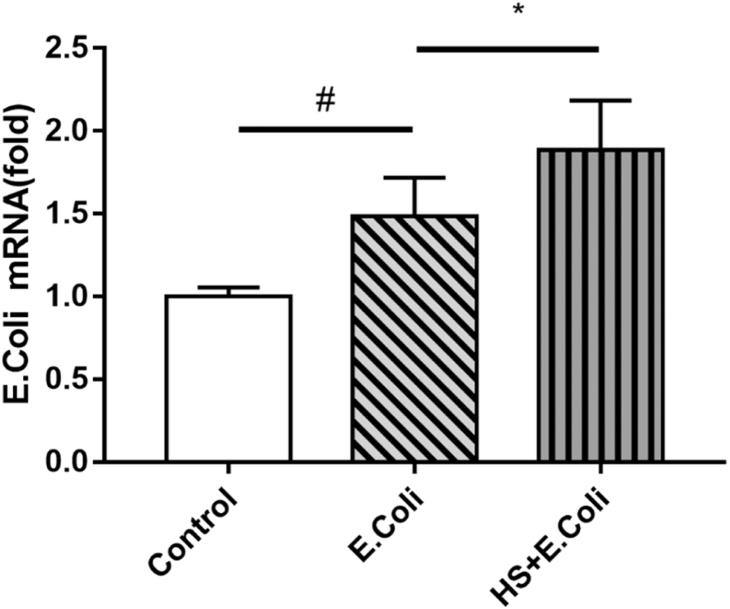
The data in Figure 1 demonstrated that the cecum contents of E. coli RNA level was significantly increased in the E. coli group birds, compared with the control group birds (P < 0.05). However, HS increased the cecum contents of E. coli RNA level (P < 0.05) in the HS + E. coli group birds, compared with the E. coli group birds. Results showed that HS promoted E. coli replication in E. coli–infected Ma chicken.
Figure 1.
Cecum contents of Escherichia coli RNA level in E. coli–infected Ma chickens loaded with heat stress. Escherichia coli replication in cecum contents of Ma chickens were measured on day 4 after E. coli infection. #P < 0.05 vs. control group; ∗P < 0.05, vs. E. coli group, (n = 8).
The Effects of Heat Stress on Intestinal Histopathological Changes in Chicken
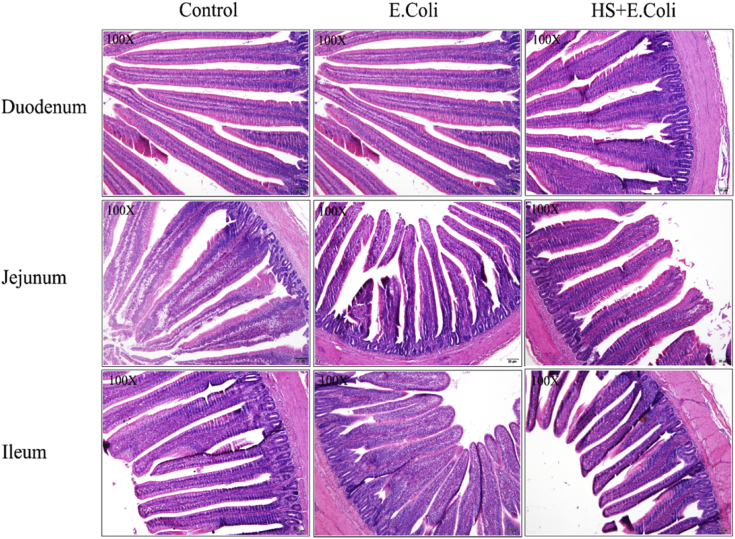
Intestinal histological changes were observed in Figure 2 and Table 4. There were no alterations in the morphology (VH and CD) of the duodenum, jejunum, or ileum in the control group chicken. However, compared with the control group chickens, a mild decrease of VH and increase of CD were observed in the jejunum and ileum of the E. coli group birds (Table 4). Compared with the E. coli group chickens, HS decreased the jejunum and ileum CD levels (P < 0.01, P < 0.01) and enhanced ileum VH level (P < 0.01). But the change of VH in the duodenum was in contrast (Table 4). These results indicated that HS may have protective effects in small intestinal absorption capacity except for the duodenum when chicken are infected with E. coli.
Figure 2.
Intestinal histopathological changes on day 4 after Escherichia coli infection in Ma chickens loaded with heat stress. Representative histologic sections of intestine from experimental Ma chickens were stained by hematoxylin and eosin (H&E, 100 × ). The duodenum, jejunum, and ileum histopathological changes were examined.
Table 4.
Changes of intestinal histopathological in Escherichia coli–challenged Ma chickens treated with heat stress.
| Small intestine | Index | Control group | E. coli group | HS + E. coli group |
|---|---|---|---|---|
| Duodenum | Villus height (VH) | 118.28 ± 7.00 | 126.85 ± 7.13 | 109.05 ± 4.93∗ |
| Crypt depth (CD) | 13.60 ± 1.44 | 12.93 ± 0.61 | 13.73 ± 0.47 | |
| V/C | 8.82 ± 1.27 | 9.83 ± 0.53 | 7.95 ± 0.34∗∗ | |
| Jejunum | Villus height (VH) | 97.34 ± 3.64 | 79.13 ± 3.15## | 81.53 ± 0.96 |
| Crypt depth (CD) | 12.73 ± 0.66 | 21.06 ± 2.42## | 10.78 ± 0.71∗∗ | |
| V/C | 7.68 ± 0.60 | 3.796 ± 0.31## | 7.596 ± 0.45∗∗ | |
| Ileum | Villus height (VH) | 80.78 ± 4.92 | 48.60 ± 5.43## | 62.40 ± 1.10∗∗ |
| Crypt depth (CD) | 14.00 ± 0.55 | 15.53 ± 0.98# | 11.33 ± 1.09∗∗ | |
| V/C | 5.78 ± 0.45 | 3.15 ± 0.45## | 5.57 ± 0.61∗∗ |
#P < 0.05, ##P < 0.01 vs. control group; ∗P < 0.05, ∗∗P < 0.05 vs. E. coli group.
Abbreviations: HS, heat stress.
The Effects of Heat Stress on Serum Diamine Oxidase and IgA Levels in Chicken
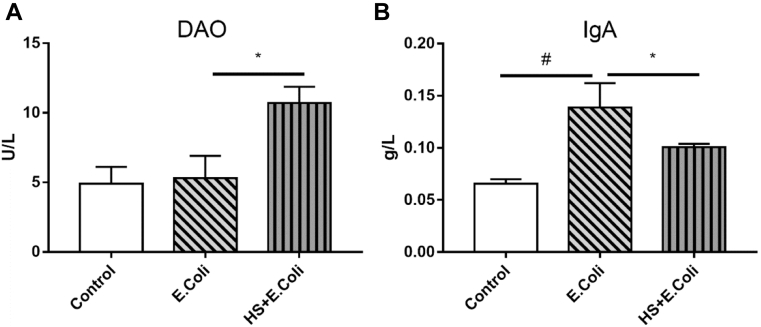
To explore the influence of HS on intestinal mucosal injury, serum DAO and IgA level in Ma chicken were measured (Figure 3).
Figure 3.
Serum diamine oxidase (DAO) and IgA levels in Escherichia coli–infected Ma chickens loaded with heat stress. (A) Measurement of serum diamine oxidase (DAO) level in Ma chickens. (B) Measurement of serum IgA level in Ma chickens. #P < 0.05 vs. control group; ∗P < 0.05 vs. E. coli group, (n = 8).
Results shown that the E. coli group birds had higher IgA levels than that of the control group birds (P < 0.05), but had no change in DAO level (Figure 3A). However, HS significantly increased DAO levels and decreased IgA contents in the HS + E. coli group birds, compared with those in the E. coli group (P < 0.05, P < 0.05). These results suggested that HS induced gut barrier dysfunction and resulted in weakened intestinal defenses in E. coli–challenged Ma chicken.
The Effects of Heat Stress on Intestinal Inflammatory Cytokines Expressions in Chicken
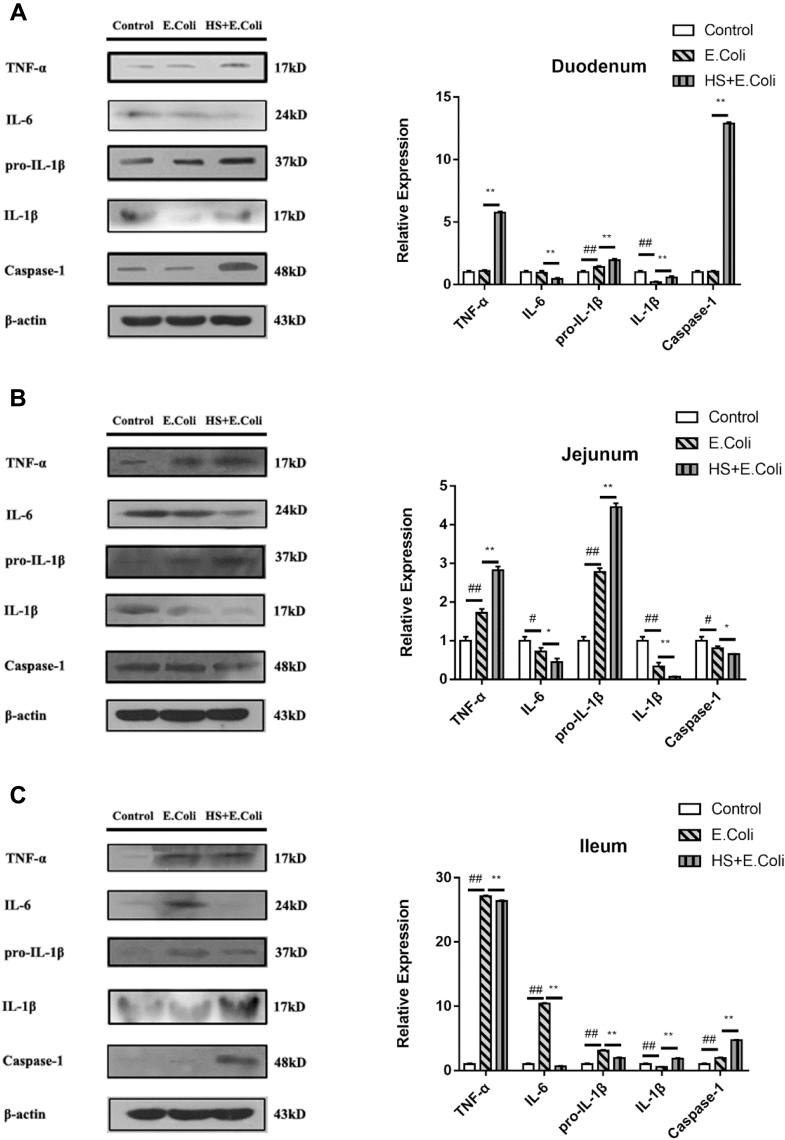
Compared with the control group chicken, E. coli significantly enhanced the protein expression of TNF-α and IL-6 in the ileum of chicken, and HS significantly inhibited that of inflammatory cytokines expressions (Figure 4). However, in the jejunum, E. coli increased TNF-α and decreased IL-6 expressions, and the HS + E. coli birds had higher TNF-α and lower IL-6 expressions. We got the same trend in the duodenum in the HS + E. coli birds compared with the E. coli group birds. Our results observed that E. coli administration did not have influence on duodenum inflammatory cytokines (IL-6, TNF-α) but have obvious effects on the jejunum and ileum. Heat stress can increase the inflammatory cytokines' expressions in the duodenum and jejunum. It also can induce caspase-1 so as to activate pro-IL-1β into mature IL-1β in the duodenum and ileum.
Figure 4.
Intestinal changes of inflammatory cytokines expressions in Escherichia coli–infected Ma chickens loaded with heat stress. (A) Duodenum, (B) jejunum, and (C) ileum inflammatory cytokines expression changes from experimental Ma chickens were measured by Western blot. #P < 0.05, ##P < 0.01 vs. control group; ∗P < 0.05, ∗∗P < 0.01 vs. E.coli group.
The Effects of Heat Stress on Gene Expressions of TLR4-NF-κB Signaling Pathway in the Chicken Intestine
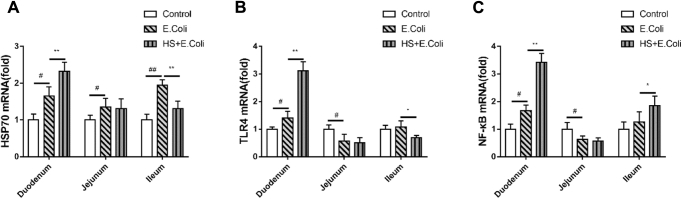
Inflammatory responses during pathogens infection were triggered by the activation of NF-κB. To confirm the dependence of inflammation responses on these key factors, the relative gene expressions of TLR4, NF-κB, and Hsp70 in the intestine of E. coli–infected Ma chicken were examined.
The results in Figure 5 express that compared with the control group, Hsp70 gene expressions were enhanced in the duodenum, jejunum and ileum of the E. coli group birds (P < 0.05, P < 0.05, P < 0.01, respectively). Heat stress significantly increased Hsp70 gene expressions in the duodenum and decreased Hsp70 gene expressions in the ileum compared with those in the E. coli alone–treated group birds (P < 0.05, P < 0.05).
Figure 5.
Intestinal changes of HSP70, TLR4, and NF-κB gene expressions in Escherichia coli–infected Ma chickens loaded with heat stress. A quantitative RT-PCR analysis of HSP70, TLR4, and NF-κB mRNA in Ma chickens were measured. (A) HSP70, (B) TLR4, and (C) NF-κB gene expression changes in the small intestine. #P < 0.05, ##P < 0.01 vs. control group; ∗P < 0.05, ∗∗P < 0.01 vs. E. coli group.
Compared with the control group, E. coli challenge enhanced TLR4 and NF-κB genes expressions in the duodenum, suppressed its expressions in the jejunum, and had no influence on the ileum of birds (Figures 5B and 5C). Heat stress significantly increased TLR4 and NF-κB gene expressions in the duodenum, decreased TLR4 gene expressions in the ileum, and promoted NF-κB gene expressions in the ileum, compared with those in the E. coli group birds. However, there was no obvious difference that was observed in the jejunum between the E. coli and HS + E. coli group birds on the HSP70, TLR4, and NF-κB genes expressions.
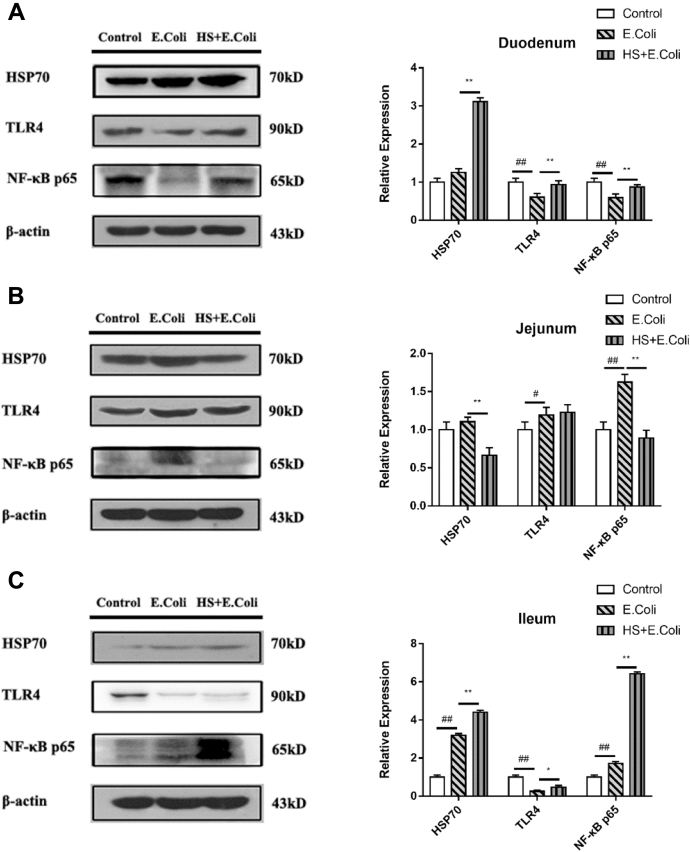
The Effects of Heat Stress on Protein Expressions of TLR4-NF-κB Signaling Pathway in the Chicken Intestine
Heat stress significantly enhanced Hsp70 protein expressions compared with the E. coli group birds of the duodenum and ileum (P < 0.01, P < 0.01, respectively). Compared with the control group, E. coli challenge inhibited TLR4 and NF-κB p65 protein expressions in the duodenum, increased its expressions in the jejunum of birds (Figure 6). Heat stress significantly increased TLR4 and NF-κB p65 protein expressions in the duodenum and ileum, compared with those in the E. coli group birds.
Figure 6.
Intestinal changes of HSP70, TLR4, and NF-κB p65 protein expressions in Escherichia coli–infected Ma chickens loaded with heat stress. HSP70, TLR4, and NF-κB p65 protein expressions changes in the (A) duodenum, (B) jejunum, and (C) ileum. #P < 0.05, ##P < 0.01 vs. control group; ∗P < 0.05, ∗∗P < 0.01 vs. E. coli group.
Discussion
Heat stress is known to decrease growth rate, body weight gain, and induce enteritis in broiler chickens (Shini et al., 2009). Stress-induced decrease in performance of broiler chickens may be related to alterations in HPA axis function by released corticosterone (Quinteiro-Filho et al., 2010). It's reported in pigs that E. coli can reduce the total percentage of intestinal mucosa, which therefore changes the host metabolic pattern (Sugiharto et al., 2012). The intestinal mucosa has been noted as the first line of defense against ingested pathogens, such as Salmonella and E. coli (Fagarasan and Honjo, 2003). Some studies found that the intestinal tract is one of the primary organs by heatstroke (Yi et al., 2017). Heat stress induces a subsequent reduction of the intestinal absorption and gastrointestinal injuries (Quinteiro-Filho et al., 2010). Our results demonstrated that HS enhanced jejunum weight, increased cecum E. coli counts, increased serum DAO, and reduced serum IgA level. These results suggest that HS can decrease host health status through inducing gut barrier dysfunction, with cecum E. coli counts increased, serum DAO increased, and serum IgA level reduced in E. coli–challenged broilers.
Absence of inflammation also allow for normal gut functions. Villus height and CD are important indicators of gut function and animal health (Uni et al., 1995). Increasing of villi height and decreased CD may result in higher nutrient absorption, reduced secretion in the gastrointestinal tract, and improvement of growth performance (Xu et al., 2003). Our results found that HS could improve intestinal morphology in broilers, as evidenced by increased VH and V/C ratios in the jejunum and ileum as compared with the E. coli group. This finding provides further information to the previous studies showing positive effects of HS on mucosal architecture with regard to VH (Thanh et al., 2009). However, HS decreased VH and V/C ratios in the duodenum, which increased the risk of villi damage caused by higher pathogen populations in the gut, as found in the present study.
Researchers reported that gut integrity damage occurs because of HS-associated ischemia of the gut epithelia (Leon and Helwig, 2010). TNF-α is an eficient proinfammatory factor. Various HS can make injured cells to produce IL-6, which is necessary for tissue homeostasis. IL-1β has been categorized as a master regulator of mediating inflammation (Basu et al., 2004). Pro-IL-1β must be cleaved by caspase-1 to form the biologically active, mature form of IL-1β. In our study, E. coli administration did not have influence on the duodenum inflammatory cytokines (IL-6, TNF-α, and IL-1β), but have obvious effects on the jejunum and ileum. Compared with the control group chicken, E. coli significantly enhanced the protein expression of TNF-α and IL-1β in the ileum of chicken, and HS significantly inhibited inflammatory cytokines expressions. However, in the jejunum, E. coli increased TNF-α and IL-1β, and decreased IL-6 expressions, and the HS + E. coli birds had higher TNF-α and IL-1β but lower IL-6 expressions. A similar trend was observed in the duodenum in the HS + E. coli birds compared with the E. coli group birds. Heat stress can increase the inflammatory cytokines expressions especially in the duodenum and jejunum.
Indeed, HS induced enteritis in the jejunum of broiler chickens with increased production of proinflammatory cytokines (Quinteiro-Filho et al., 2010), which can increase the permeability of the mucosa to the pathogenic bacteria (Al-Sadi et al., 2008). We demonstrated mild enteritis in heat-stressed Ma chickens with E. coli infection. This inflammation was characterized by increased inflammatory cytokines infiltrates in all regions of the small intestine (duodenum, jejunum, and ileum) in the E. coli group chickens. We also observed increased, moderate enteritis throughout the small intestine in the chickens that were both heat-stressed and infected with E. coli. Another important finding was the presence of an increased area of inflammation in the duodenum of the birds that were both stressed and infected with E. coli. This inflammation was characterized by a larger number of villi and crypts that exhibited prominent inflammatory infiltrates. TNF-α and IL-1β expressions were significantly increased in the duodenum of heat-stressed chicken infected with E. coli. Results suggested that the duodenum is a critical part of the small intestine, which is more sensitive to inflammation caused by HS than other parts.
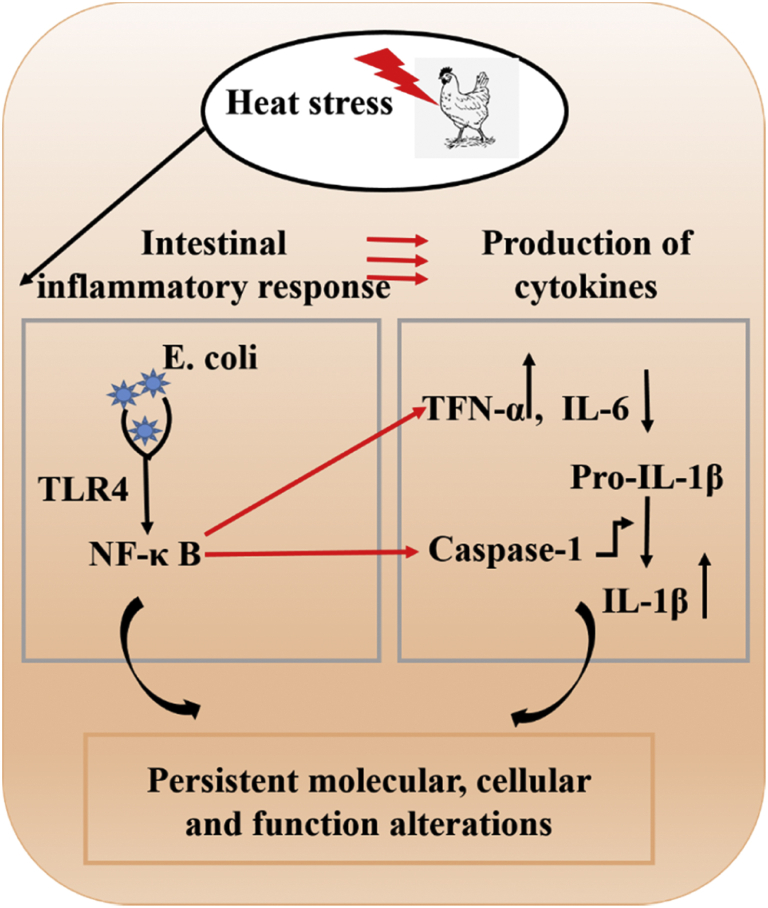
It's reported that HS can disturb the microecological balance of the intestinal microbiota, which caused bacterial translocation so as to induce intestinal endotoxins (LPS) production. These endotoxins can activate the TLR4-mediated response, including the initiation of NF-κB and MAPK pathways. Signaling at TLR4 initiates an intracellular signaling cascade to activate the transcription factor NF-κB (Kawai and Akira, 2010), which is a critical step to promote the overproduction of the inflammatory cytokine (Kagan and Medzhitov, 2006). Therefore, it is necessary to reduce the inflammatory status by restraining the activation of TLR4-mediated NF-κB pathway. In the present investigation, TLR4 and NF-κB mRNA expression were examined by qPCR analysis in small intestine (duodenum, jejunum, and ileum). Escherichia coli infection significantly increased TLR4 and NF-κB mRNA expression in the duodenum and decreased its expressions as compared with the control group chickens. TLR4 and NF-κB mRNA were higher in the HS + E. coli group chicken than in the E. coli alone–treated group. But, the effects of HS on expression of TLR4 and NF-κB in the jejunum were not obvious. These results suggested that TLR4 expression may activate NF-κB to regulate the inflammatory cytokines responses. NF-κB signaling pathway has been detected in various tissues after exposure to stressful environmental conditions. To explore the effects of HS on NF-κB pathway, we examined the TLR4 and NF-κB protein expression and NF-κB-mediated inflammatory cytokines in the small intestine. Thus, the results concluded that HS can significantly enhance TLR4, NF-κB protein expressions, and its downstream molecules in the duodenum and ileum. Moreover, the effects of HS on protein expressions of TLR4 and NF-κB in the jejunum were not obvious. These results suggested that TLR4 expression may activate NF-κB pathways and its downstream molecules which regulate the inflammatory cytokines responses in the duodenum and ileum. Consequently, the results of current study indicated that HS has excellent promoting inflammatory ability by activating TLR4-NF-κB pathways, which induced the inflammatory cytokines responses, in the duodenum and ileum in chickens with E. coli infection (Figure 7).
Figure 7.
Priming and activation of the TLR4-NF-κB signaling pathway. Pattern recognition receptor TLR4 recognizes Escherichia coli and subsequent activates NF-κB, which then drives transcription of TNF-α and caspase-1.
Conclusions
In the present study, HS in Ma chickens induced more severe inflammatory status than in the control and E. coli alone–treated group chickens, which is reflected by upregulating TLR4–NF-κB pathway and enhancing its downstream inflammatory cytokines protein expressions. The available findings also indicated that HS improves the inflammatory status of birds and should have proper control in temperature range in the poultry industry so as to improve economic efficiency.
Acknowledgments
This work was supported by the Natural Science Foundation of Guangdong Province, China [grant numbers 2017A030310607]; Guangdong Science and Technology Department, China [grant numbers 2015A040404048].
Disclosures
No potential conflict of interest was reported by the authors.
References
- Alonso M.Z., Padola N.L., Parma A.E., Lucchesi A. Enteropathogenic E. Coli contamination at different stages of the chicken slaughtering process. Poult. Sci. 2011;90:2638–2641. doi: 10.3382/ps.2011-01621. [DOI] [PubMed] [Google Scholar]
- Al-Sadi R., Ye D., Dokladny K., Ma T.Y. Mechanism of IL-1b-induced increase in intestinal epithelial tight junction permeability. J. Immunol. 2008;180:5653–5661. doi: 10.4049/jimmunol.180.8.5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett J.R., Smith M.O. Effects of different levels of zinc on the performance and immunocompetence of broilers under heat stress. Poult. Sci. 2003;82:1580–1588. doi: 10.1093/ps/82.10.1580. [DOI] [PubMed] [Google Scholar]
- Barton Behravesh C., Jones T.F., Vugia D.J., Long C., Marcus R., Smith K. Deaths associated with bacterial pathogens transmitted commonly through food: foodborne diseases active surveillance network (Food Net), 1996-2005. J. Infect. Dis. 2011;204:263–267. doi: 10.1093/infdis/jir263. [DOI] [PubMed] [Google Scholar]
- Basu A., Krady J.,K., Levison S.,W. Interleukin-1: a master regulator of neuroinflammation. J. Leukoc. Biol. 2004;81:1–5. doi: 10.1002/jnr.20266. [DOI] [PubMed] [Google Scholar]
- Burkholder K.M., Thompson K.L., Einstein M.E., Applegate T.J., Patterson J.A. Influence of stressors on normal intestinal microbiota, intestinal morphology, and susceptibility to Salmonella Enteritidis colonization in broilers. Poult. Sci. 2008;87:1734–1741. doi: 10.3382/ps.2008-00107. [DOI] [PubMed] [Google Scholar]
- Chappell L., Kaiser P., Barrow P., Jones M.A., Johnston C., Wigley P. The immunobiology of avian systemic salmonellosis. Vet. Immunol. Immunopathol. 2009;128:53–59. doi: 10.1016/j.vetimm.2008.10.295. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behavior: mechanisms and implications. Ann. New York Acad. Sci. 2001;933:222–234. doi: 10.1111/j.1749-6632.2001.tb05827.x. [DOI] [PubMed] [Google Scholar]
- Fagarasan S., Honjo T. Intestinal IgA synthesis: regulation of front-line body defences. Nat. Rev. Immunol. 2003;3:63–72. doi: 10.1038/nri982. [DOI] [PubMed] [Google Scholar]
- Glaser R., Kiecolt-Glaser J.K. Stress-induced immune dysfunction: implications for health. Nat. Rev. Immunol. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- Hall D.M., Buettner G.R., Oberley L.W., Xu L.J., Matthes R.D., Gisolfi C.V. Mechanisms of circulatory and intestinal barrier dysfunction during whole body hyperthermia. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H509–H521. doi: 10.1152/ajpheart.2001.280.2.H509. [DOI] [PubMed] [Google Scholar]
- Kagan J.C., Medzhitov R. Phosphoinositide-mediated adaptor recruitment controls toll-like receptor signaling. Cell. 2006;125:943–955. doi: 10.1016/j.cell.2006.03.047. [DOI] [PubMed] [Google Scholar]
- Kawai T., Akira S. The role of pattern-recognition receptors in innate immunity: update on toll-like receptors. Nat. Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- Keita A.V., Soderholm J.D. The intestinal barrier and its regulation by neuroimmune factors. Neurogastroenterol. Motil. 2010;22:718–733. doi: 10.1111/j.1365-2982.2010.01498.x. [DOI] [PubMed] [Google Scholar]
- Leon L.R., Helwig B.G. Heat stroke: role of the systemic inflammatory response. J. Appl. Physiol. 2010;109:1980–1988. doi: 10.1152/japplphysiol.00301.2010. [DOI] [PubMed] [Google Scholar]
- Mashaly M.M., Hendricks G.L., Kalama M.A., Gehad A.E., Abbas A.O., Patterson P.H. Effect of heat stress on production parameters and immune responses of commercial laying hens. Poult. Sci. 2004;83:889–894. doi: 10.1093/ps/83.6.889. [DOI] [PubMed] [Google Scholar]
- Park S., Hwangbo J., C, Ryu B., Park H., Chae H., Choi H., Kang O., Seo, Choi Y. Effects of extreme heat stress on growth performance, lymphoid organ, IgG and cecum microflora of broiler chickens. Int. J. Agric. Biol. 2013;15:1204–1208. [Google Scholar]
- Quinteiro-Filho W.M., Gomes A.V.S., Pinheiro M.L., Ribeiro A., Ferraz-de-Paula V., Astolfi-Ferreira, Ferreira A.J.P., Palermo-Neto J. Acute heat stress impairs performance parameters and induces mild intestinal enteritis in broiler chickens: role of acute HPA axis activation. J. Anim. Sci. 2012;90:1986–1994. doi: 10.2527/jas.2011-3949. [DOI] [PubMed] [Google Scholar]
- Quinteiro-Filho W.M., Ribeiro A., Ferraz-de-Paula V., Pinheiro M.L., Sakai M., Sa L.R., Ferreira A.J., Palermo-Neto J. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult. Sci. 2010;89:1905–1914. doi: 10.3382/ps.2010-00812. [DOI] [PubMed] [Google Scholar]
- Shini S., Shini A., Huff G.R. Effects of chronic and repeated corticosterone administration in rearing chickens on physiology, the onset of lay and egg production of hens. Physiol. Behav. 2009;98:73–77. doi: 10.1016/j.physbeh.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Soderholm J.D., Perdue M.H. Stress and gastrointestinal tract. II. Stress and intestinal barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;280:7–13. doi: 10.1152/ajpgi.2001.280.1.G7. [DOI] [PubMed] [Google Scholar]
- Sugiharto S., Hedemann M.S., Jensen B.B., Lauridsen C. Diarrhea-like condition and intestinal mucosal responses in susceptible homozygous and heterozygous f4r+ pigs challenged with enterotoxigenic Escherichia coli. J. Anim. Sci. 2012;90:281–283. doi: 10.2527/jas.53840. [DOI] [PubMed] [Google Scholar]
- Syafwan S.K., Kwakkel R.P., Verstegen M.W.A. Heat stress and feeding strategies in meat-type chickens. World’s Poult. Sci. J. 2011;67:653–673. [Google Scholar]
- Thanh N.T., Loh T.C., Foo H.L., Hair-Bejo M., Azhar B.K. Effects of feeding metabolite combinations produced by Lactobacillus plantarum on growth performance, faecal microbial population, small intestine villus height and faecal volatile fatty acids in broilers. Br. Poult. Sci. 2009;50:298–306. doi: 10.1080/00071660902873947. [DOI] [PubMed] [Google Scholar]
- Uni Z., Noy Y., Sklan D. Posthatch changes in morphology and function of the small intestines in heavy and light-strain chicks. Poult. Sci. 1995;74:1622–1629. doi: 10.3382/ps.0741622. [DOI] [PubMed] [Google Scholar]
- Varasteh S., Braber S., Akbari P., Garssen J., FinkGremmels J. Differences in susceptibility to heat stress along the chicken intestine and the protective effects of galactooligosaccharides. PLoS One. 2015;10:e0138975. doi: 10.1371/journal.pone.0138975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier R.J., Podolsky D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- Xu Z., Hu C., Xia M., Zhan X., Wang M. Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of male broilers. Poult. Sci. 2003;82:1030–1036. doi: 10.1093/ps/82.6.1030. [DOI] [PubMed] [Google Scholar]
- Yi G., Li L., Luo M., He X., Zou Z., Gu Z., Su L. Heat stress induces intestinal injury through lysosome and mitochondrial-dependent pathway in vivo and vitro. Oncotarget. 2017;8:40741–40755. doi: 10.18632/oncotarget.16580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Hornef M.W., Dupont A. The intestinal epithelium as guardian of gut barrier integrity. Cell Microbiol. 2015;17:1561–1569. doi: 10.1111/cmi.12501. [DOI] [PubMed] [Google Scholar]