Abstract
The adipocyte hormone leptin regulates glucose homeostasis both centrally and peripherally. A key peripheral target is the pancreatic β-cell, which secretes insulin upon glucose stimulation. Leptin is known to suppress glucose-stimulated insulin secretion by promoting trafficking of KATP channels to the β-cell surface, which increases K+ conductance and causes β-cell hyperpolarization. We have previously shown that leptin-induced KATP channel trafficking requires protein kinase A (PKA)-dependent actin remodeling. However, whether PKA is a downstream effector of leptin signaling or PKA plays a permissive role is unknown. Using FRET-based reporters of PKA activity, we show that leptin increases PKA activity at the cell membrane and that this effect is dependent on N-methyl-D-aspartate receptors, CaMKKβ, and AMPK, which are known to be involved in the leptin signaling pathway. Genetic knockdown and rescue experiments reveal that the increased PKA activity upon leptin stimulation requires the membrane-targeted PKA-anchoring protein AKAP79/150, indicating that PKA activated by leptin is anchored to AKAP79/150. Interestingly, disrupting protein phosphatase 2B (PP2B) anchoring to AKAP79/150, known to elevate basal PKA signaling, leads to increased surface KATP channels even in the absence of leptin stimulation. Our findings uncover a novel role of AKAP79/150 in coordinating leptin and PKA signaling to regulate KATP channel trafficking in β-cells, hence insulin secretion. The study further advances our knowledge of the downstream signaling events that may be targeted to restore insulin secretion regulation in β-cells defective in leptin signaling, such as those from obese individuals with type 2 diabetes.
Keywords: leptin, protein kinase A (PKA), A-kinase anchoring protein (AKAP), cyclic AMP (cAMP), fluorescence resonance energy transfer (FRET), ATP-sensitive potassium channel
Abbreviations: AC, adenylyl cyclase; AKAP, A-kinase anchoring protein; AMPK, AMP-activated protein kinase; CaMKKβ, calcium/calmodulin-dependent kinase kinase β; FRET, fluorescence resonance energy transfer; GSIS, glucose-stimulated insulin secretion; NMDAR, N-methyl-D-aspartate receptor; PKA, protein kinase A; PP2B, protein phosphatase 2B
Pancreatic β-cells produce and secrete insulin making them critical for maintaining glucose homeostasis. In order to secrete insulin in a timely and controlled manner, β-cells must interpret and respond to a myriad of physiological stimuli and hormones. One such hormone is the adipocyte-derived hormone leptin, which regulates serum insulin levels by suppressing glucose-stimulated insulin secretion (GSIS) from β-cells (1, 2, 3, 4, 5). Leptin does so by promoting trafficking of ATP-sensitive potassium (KATP) channels to the β-cell membrane (6, 7, 8, 9, 10). This increased surface expression of KATP channels increases the total KATP channel conductance, thereby causing membrane hyperpolarization and reducing β-cell electrical activity. Our studies to date have shown that leptin-induced KATP channel trafficking involves the following series of signaling events (7, 8, 9, 10). Leptin stimulates Src kinase to phosphorylate and potentiate N-methyl-D-aspartate (NMDA) receptor (NMDAR) activity, resulting in enhanced Ca2+ influx that activates calcium/calmodulin-dependent kinase β (CaMKKβ), which then phosphorylates and activates AMP-activated protein kinase (AMPK). Downstream of AMPK actin depolymerization occurs and results in increased trafficking of KATP channels to the plasma membrane (7, 8, 9, 10). We have shown previously that actin remodeling and subsequent KATP channel trafficking following AMPK activation requires protein kinase A (PKA). However, it remains elusive whether PKA functions as an active or permissive player in leptin signaling.
PKA is a serine/threonine kinase consisting of two regulatory subunits and two catalytic subunits. Binding of cAMP to the regulatory subunits causes a conformational change that releases the autoinhibition of the catalytic subunits to activate PKA (11, 12). Due to the ubiquity of PKA in mammalian cells and the promiscuity of its catalytic subunits, PKA is involved in a multitude of cellular processes. In pancreatic β-cells PKA has been identified by several studies as a positive regulator of insulin granule trafficking and exocytosis (13, 14, 15, 16, 17). However, evidence that PKA promotes KATP channel trafficking suggests that PKA is also involved in signaling events that lead to inhibition of insulin secretion (7, 18). These findings illuminate the complexity of β-cell signaling and raise the question of how cellular signaling networks are coordinated to fine-tune insulin secretion. It is well established that PKA interacts with a family of scaffolding proteins termed A-kinase anchoring proteins (AKAPs). Different AKAPs direct PKA activity toward specific cell signaling machinery by anchoring PKA and other signaling molecules to distinct subcellular locations (19). Several AKAPs have been identified as having roles in regulating insulin secretion, but the mechanisms by which they do so remain poorly understood (20, 21, 22, 23, 24, 25).
In this study we show that leptin increases PKA signaling at the β-cell membrane, but not in the cytoplasm, through our previously identified pathway involving NMDAR, CaMKKβ, and AMPK. Moreover, we find that anchoring of PKA by AKAP79/150 is necessary for leptin-mediated KATP channel trafficking and that overexpression of an AKAP79/150 mutant associated with increased basal PKA activity due to a loss of protein phosphatase 2B (PP2B) binding recapitulates the effects of leptin. Additionally, we present evidence that leptin locally increases concentrations of the PKA activator cAMP near AKAP79/150 expressed at the cell membrane. These findings reveal a novel function of the PKA-AKAP79/150 signaling complex for orchestrating leptin signaling to regulate KATP channel surface expression and thus β-cell excitability.
Results
Leptin increases PKA activity near the plasma membrane
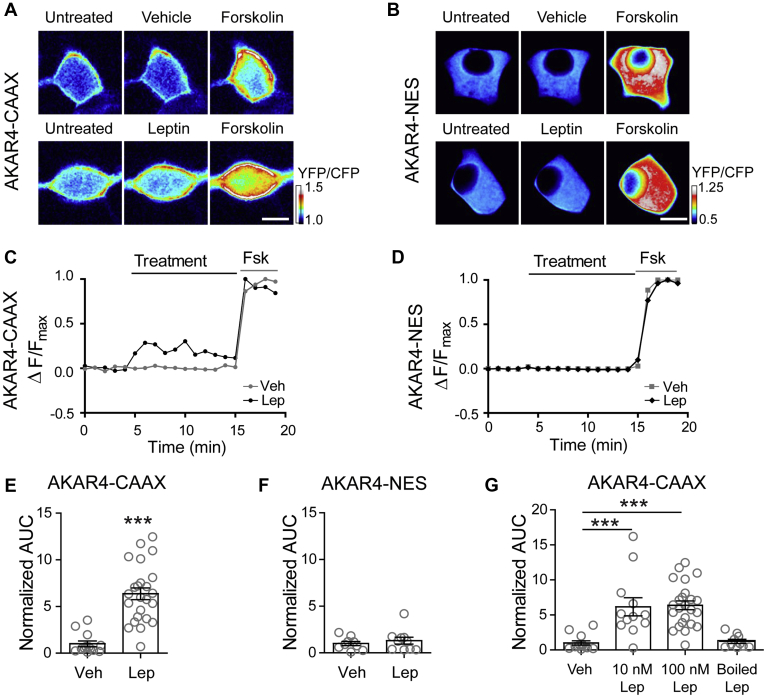
We have previously shown that leptin promotes trafficking of KATP channels to the plasma membrane, causing increased K+ conductance and cell hyperpolarization in rat insulinoma INS-1 clone 832/13 cells (referred to herein as INS-1 832/13) as well as primary mouse and human β-cells (7, 8, 9, 10). Our prior work revealed that a critical event for this process is actin remodeling (7), which presumably allows vesicles containing potassium channels to translocate and insert into the β-cell membrane (26, 27, 28, 29). Furthermore, we found that the ability of leptin to remodel the actin cytoskeleton and increase surface KATP channel density could be blocked by the protein kinase A (PKA)-specific inhibitor peptide (PKI) suggesting that PKA is essential to this signaling mechanism (7). However, whether leptin stimulation directly enhances PKA signaling remains unknown. To determine whether leptin signaling increases PKA signaling, we monitored PKA activity using the fluorescence resonance energy transfer (FRET)-based PKA activity reporter A-kinase activity reporter 4 (AKAR4) (30). AKAR4 contains a PKA substrate motif that is phosphorylated by PKA causing the sensor to undergo a conformational change to increase FRET detected as an increased YFP acceptor/CFP donor emission ratio in response to excitation of the CFP donor. AKAR4 targeted to the plasma membrane with a farnesylation motif (CAAX; Fig. 1A) and AKAR4 targeted to the cytoplasm by a nuclear export signal (NES; Fig. 1B) were both tested. INS-1 832/13 cells expressing either AKAR4-CAAX or AKAR4-NES were treated with leptin (100 nM) or vehicle. In order to control for the expression level of AKAR4 between cells the potent PKA activator forskolin was administered at the end of each experiment and cells that responded robustly to forskolin with a 10–15% increase in FRET were chosen for analysis. For quantification purposes, FRET traces were normalized to the maximal forskolin response (Fig. 1, C and D) and then analyzed for area under the curve (AUC) during the treatment period with vehicle or leptin. In AKAR4-CAAX expressing cells, leptin treatment led to a 6.37-fold increase in PKA activity (p < 0.0005, n = 24) compared with vehicle-treated cells (n = 13) (Fig. 1E). In contrast, AKAR4-NES expressing cells showed no change in PKA activity between vehicle (n = 9) and leptin (n = 10) treatments (Fig. 1F). To confirm that the increase in PKA activity in AKAR4-CAAX expressing cells was due to leptin and not an experimental artifact, we tested the effect of various leptin concentrations compared with inactive boiled leptin. While we found that leptin at concentrations of 10 nM and 100 nM increased PKA activity, boiled 100 nM leptin did not (Fig. 1G). This result indicates that leptin does indeed increase PKA activity, as reported by AKAR4-CAAX, and that this increase is spatially restricted to the plasma membrane.
Figure 1.
Leptin increases PKA activity.A, B, ratiometric images of INS-1 832/13 cells transfected with the FRET-based PKA activity reporter A-kinase activity reporter 4 (AKAR4), which has been targeted to the plasma membrane with a farnesylation motif (AKAR4-CAAX) or to the cytoplasm with a nuclear export signal (AKAR4-NES). Cells were treated with vehicle or leptin (100 nM) followed by the robust PKA activator forskolin (20 μM). Scale bar, 5 μm. C, D, FRET traces of the cells in (A, B) normalized to the maximal forskolin response. E, group analysis of AKAR4-CAAX cell traces. Graph shows the fold-change in area under the curve (AUC) normalized to vehicle treatment (n = 13). In total, 100 nM leptin (n = 24) was used for these experiments. ∗∗∗p < 0.0001 by unpaired student's t-test. F, group data of AKAR4-NES expressing cells treated with vehicle (n = 9) or 100 nM leptin (n = 10). G, group analysis for AKAR4-CAAX cells treated with vehicle (n = 13), 10 nM leptin (n = 12), 100 nM leptin (n = 24), or boiled 100 nM leptin (n = 10). ∗∗∗p < 0.001 by one-way ANOVA (F(3,55) = 15.05, p < 0.0001) followed by a post hoc Dunnett's multiple comparison test. In all figures, circles represent individual cells except as otherwise specified.
Leptin signals via the NMDAR-CaMKKβ-AMPK axis to enhance PKA signaling
Next, we sought to determine the signaling mechanism that underlies an increase in PKA activity. Our previous studies have shown that the leptin signaling pathway leading to increased KATP channel trafficking involves potentiation of NMDAR activity and Ca2+ influx to activate CaMKKβ; this results in phosphorylation and activation of AMPK, which is followed by PKA-dependent actin depolymerization (7, 8, 9, 10). Thus, a logical hypothesis is that leptin upregulates PKA activity via the NMDAR-CaMKKβ-AMPK signaling axis. To test this, we implemented the membrane-targeted PKA activity sensor AKAR4-CAAX in conjunction with pharmacological reagents. As shown in Figure 2A, inhibiting the initial potentiation of NMDARs with the competitive NMDAR antagonist D-APV (50 μM) or preventing the ensuing Ca2+ influx with the Ca2+ chelator BAPTA (10 mM) reduced leptin enhancement of PKA activity by 2.5-fold (n = 12) and 5-fold (n = 11), respectively, levels that were not significantly different from the vehicle control. Consistent with these findings, inhibiting CaMKKβ with STO-609 (1 μM) also prevented leptin from increasing PKA activity (n = 9). Finally, the role of AMPK for leptin activation of PKA was tested using Compound C (CC, also known as Dorsomorphin, 1 μM). Blocking AMPK activity with CC (n = 15) greatly diminished the effect of leptin such that PKA activity levels resembled those of vehicle-treated cells. These results support the notion that PKA is activated downstream of AMPK in the leptin signaling cascade.
Figure 2.
Leptin activates PKA via the NMDAR-CaMKKβ-AMPK signaling cascade. INS-1 832/13 cells were transfected with AKAR4-CAAX followed by various treatments. A, PKA activity in response to 100 nM leptin (n = 24) alone or in the presence of the NMDAR inhibitor D-APV (50 μM; n = 12), the Ca2+ chelator BAPTA (10 mM; n = 11), the CaMKKβ inhibitor STO-609 (1 μM; n = 9), or the AMPK inhibitor Compound C (CC, 1 μM; n = 15). Treatments were compared with vehicle control (n = 13). ∗∗∗p < 0.001 as determined by one-way ANOVA (F(4,66) = 12.13, p < 0.0001) followed by a post hoc Dunnett's multiple comparison test. B, effects of NMDAR coagonists NMDA/glycine (100 μM/100 μM; n = 11) and the AMPK activator AICAR (500 μM; n = 20) on PKA activity. ∗∗p < 0.01, ∗∗∗p < 0.001 by one-way ANOVA (F(3,64) = 15.77, p < 0.0001) followed by a post hoc Tukey's multiple comparison test. C, PKA activity in response to NMDAR activation by NMDA/glycine in the absence or presence of the AMPK inhibitor CC (1 μM; p = 0.002, n = 10) and to AMPK activation by AICAR in the absence or presence of the NMDAR inhibitor D-APV (50 μM; p = 0.99 n = 10). Statistical analysis by unpaired student's t-test.
To further corroborate the above findings, we tested whether pharmacological activation of NMDAR or AMPK could increase PKA activity. Treating AKAR4-CAAX expressing cells with the NMDAR coagonists NMDA (100 μM) and glycine (100 μM) or the AMPK agonist AICAR (500 μM) significantly increased FRET (Fig. 2B). While direct activation of AMPK caused a 6.25-fold increase (p < 0.001, n = 20) over vehicle that was equivalent to leptin treatment, NMDAR coagonists caused a 10.66-fold increase (p < 0.001, n = 11) compared with vehicle, which was 1.67 times (p < 0.01, n = 11) greater than that of leptin. This suggests that the direct activation of NMDARs may have caused a greater extent of NMDAR activity and consequently more PKA activity. To confirm that NMDARs signal through the leptin pathway involving AMPK, we treated cells with the NMDAR coagonists NMDA and glycine in the presence of the AMPK inhibitor CC (1 μM) and found that CC significantly reduced the effect of NMDAR activation on PKA activity (n = 10, p < 0.001) (Fig. 2C). On the contrary, the effects of the AMPK agonist AICAR were not blocked by the NMDAR inhibitor D-APV (50 μM) indicating that NMDAR lies upstream of AMPK. Collectively, these findings demonstrate that leptin signaling through the NMDAR-CaMKKβ-AMPK cascade increases PKA activity.
Leptin signaling via PKA requires AKAPs
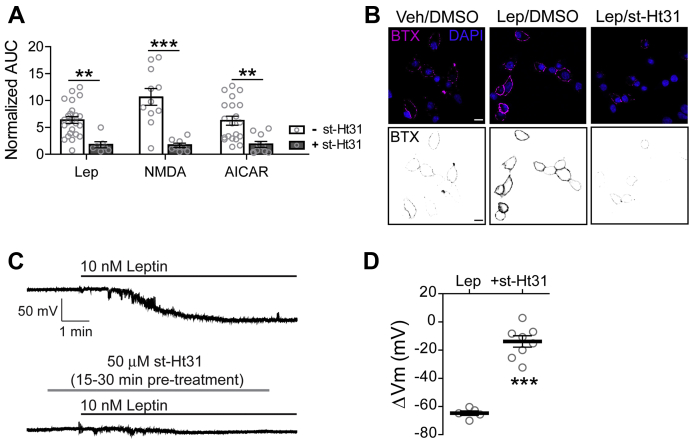
During the live cell PKA activity imaging experiments, it was apparent that leptin increased PKA activity at the cell membrane but not throughout the cytoplasm. This suggests that leptin acts on a subset of cellular PKA that is localized near the plasma membrane. It is widely documented that a high level of regulation and specificity of PKA signaling is maintained by a family of scaffolding proteins known as A-kinase anchoring proteins (AKAPs), which target PKA and its signaling partners to distinct subcellular regions, thereby creating PKA signaling microdomains and nanodomains (19, 31, 32). To test if AKAPs are involved in targeting PKA to the cell membrane for leptin signaling, we used the PKA–AKAP interaction disruptor peptide st-Ht31 (50 μM) (33). This peptide binds the regulatory subunits of PKA and prevents PKA from binding AKAPs. We first introduced st-Ht31 to INS-1 832/13 cells expressing AKAR4-CAAX and monitored PKA activity in response to various stimuli (Fig. 3A). Disrupting the PKA–AKAP interaction with st-Ht31 led to a significant decrease in PKA activity by leptin (3.6-fold decrease, p < 0.01), NMDAR agonists NMDA and glycine (6.4-fold decrease, p < 0.001, n = 8), and AMPK activator AICAR (3.4-fold decrease, p < 0.01, n = 10). These experiments showed that increased PKA activity at the cell membrane by leptin signaling requires AKAPs.
Figure 3.
Leptin signaling through PKA requires an A-kinase anchoring protein (AKAP).A, effects of the PKA–AKAP interaction disruptor peptide st-Ht31 on PKA activity. Group FRET data of cells expressing AKAR4-CAAX in response to various stimuli: 100 nM leptin (n = 7), NMDAR coagonists NMDA/glycine (100 μM/100 μM) (n = 8), and AMPK activator AICAR (500 μM) (n = 10) in the presence of st-Ht31 (50 μM). Results are compared with those shown in Figure 2B of cells treated with leptin (n = 24), NMDA/glycine (n = 11) and AICAR (n = 20) in the absence of st-Ht31 ∗∗p < 0.01, ∗∗∗p < 0.001. Data was analyzed by two-way ANOVA followed by a post hoc Bonferroni's test. Analysis revealed significant main effects of st-Ht31 (F1,74 = 54.17, p < 0.0001), but no significant main effects of stimuli (F2,74 = 2.76, p = 0.07) nor a significant interaction between these variables (F2,74 = 3.11, p = 0.05). B, INS-1 832/13 cells transduced with bungarotoxin binding motif-tagged SUR1 (BTX-SUR1) and Kir6.2 subunits of KATP channels and treated with leptin (10 nM) for 30 min in the presence of 0.01% DMSO or 50 μM st-Ht31. Surface KATP channels were then labeled with Alexa 555-conjugated bungarotoxin (BTX) and nuclei were stained with DAPI. Top panels show representative confocal microscopy images. Inverse gray scale representations of BTX-labeled surface KATP channels are shown in bottom panels. Scale bar, 10 μm. C, representative INS-1 832/13 cell-attached membrane recordings in response to leptin (10 nM) in the absence or presence of st-Ht31 (50 μM) preincubation (15–30 min). D, group data showing the extent of membrane hyperpolarization in response to leptin without st-Ht31 preincubation (−64.76 ± 1.61 mV, n = 5) or with st-Ht31 preincubation (−14.53 ± 3.96 mV, n = 8). ∗∗∗p < 0.001 by unpaired student's t-test. For graph analysis of membrane potential recordings, the mean is represented by a thick line with error bars depicting the standard error of the mean.
Having established that PKA–AKAP interactions are necessary for leptin-mediated PKA activity, we wanted to determine if such an interaction is essential for downstream KATP channel trafficking. To visualize KATP channels at the plasma membrane, INS-1 832/13 cells were transduced with recombinant adenoviruses containing the KATP channel subunits Kir6.2 and SUR1 tagged with an N-terminus extracellular bungarotoxin-binding motif (BTX-SUR1) (7); surface KATP channels were then labeled with Alexa 555-conjugated BTX (BTX) following a 30 min treatment period. Leptin (10 nM) treated cells showed a marked increase in surface BTX staining of BTX-SUR1 compared with vehicle-treated cells as expected (Fig. 3B) (7). However, this effect of leptin was greatly attenuated by the presence of st-Ht31 indicating that blocking PKA from binding AKAPs prohibits leptin from increasing KATP channel surface density. Previously we have shown that the increased abundance of KATP channels in the membrane enhances total K+ conductance and causes β-cells to hyperpolarize (7, 8, 9, 10). To further verify the importance of PKA-AKAP interactions for KATP channel translocation, we monitored the effects of st-Ht31 on cell membrane potential following leptin treatment using cell-attached current clamp recording, which is a noninvasive approach for detecting changes in membrane potential without disturbing cellular integrity or compromising intracellular soluble factors important for signaling (34, 35). Cell-attached membrane recordings of INS-1 832/13 cells showed that leptin (10 nM) elicits a mean hyperpolarization extent of –64.76 ± 1.61 mV (n = 5) (Fig. 3, C and D). However, in the presence of st-Ht31 (50 μM), leptin-induced hyperpolarization was significantly reduced to –14.53 ± 3.96 mV (p < 0.001, n = 8). This data strongly implicates that an AKAP is required to anchor PKA for leptin-mediated KATP channel trafficking.
PKA and AKAPs are also required for leptin signaling in human β-cells
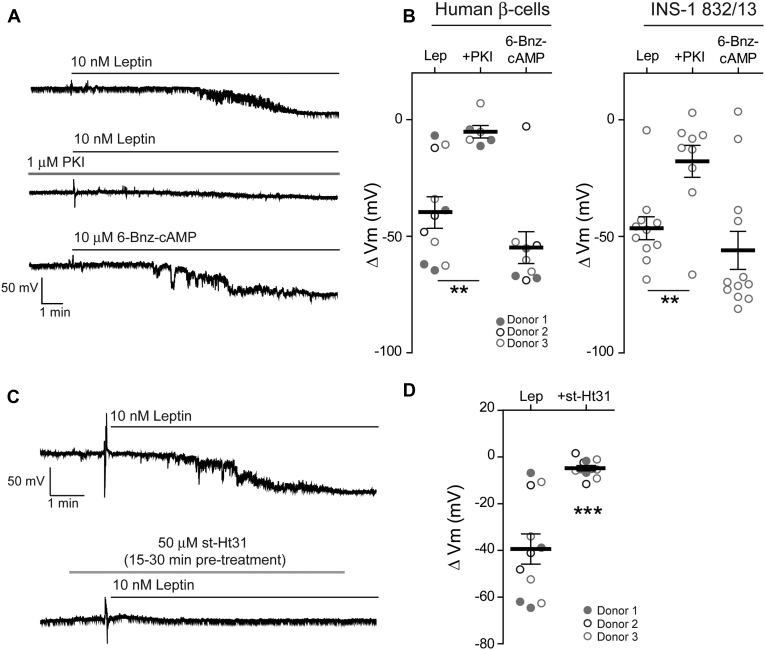
Our previous studies have shown that leptin regulation of KATP channel trafficking is conserved in human β-cells. To test whether PKA and AKAPs are also involved in leptin signaling in human β-cells, we conducted cell-attached current-clamp recording experiments to monitor cell membrane potential response while pharmacologically manipulating PKA activity and the PKA–AKAP interaction as described above for INS-1 832/13 cells. Individual human β-cells dissociated from islets from three different donors (Table 1) were tested and the results were compared with those using INS-1 832/13 cells (Fig. 4). Bath application of 10 nM leptin induced membrane hyperpolarization in human β-cells to a similar extent (-39.40 ± 6.46 mV, n = 11) as in INS-1 832/13 cells (-46.49 ± 4.92 mV, n = 11) (Fig. 4, A and B). Consistent with PKA being required for KATP channel trafficking, the myristoylated form of the PKA inhibitor PKI (1 μM) significantly reduced leptin-induced hyperpolarization to –5.18 ± 2.65 mV (p < 0.01, n = 6) and –17.82 ± 6.88 mV (p < 0.01, n = 9) in human β-cells and INS-1 832/13 cells, respectively (Fig. 4, A and B). Conversely, treatment with the PKA-specific agonist 6-Bnz-cAMP (10 μM) recapitulated the effects of leptin and caused a mean hyperpolarization of –54.84 ± 6.83 mV (n = 9) in human β-cells and –56.00 ± 8.15 mV (n = 12) in INS-1 832/13 cells. Interestingly, at the concentration used, 6-Bnz-cAMP tended to induce a greater membrane hyperpolarization compared with leptin, which may be due to variation in the degree of PKA activation. The findings in human β-cells agree with those in INS-1 832/13 cells indicating that PKA is both necessary and sufficient to promote KATP channel trafficking and subsequent membrane hyperpolarization in human β-cells. We then examined whether disrupting the PKA–AKAP interaction would negatively impact leptin-induced hyperpolarization in human β-cells. Preincubating human β-cells with st-Ht31 (50 μM) to disrupt PKA-AKAP interactions occluded the effects of leptin on β-cell membrane potential (–4.81 ± 1.13 mV, p < 0.0001, n = 11). These studies demonstrate the importance of our findings to human biology and support that a PKA–AKAP complex plays an essential role for leptin signaling in human β-cells. In the studies that follow, we will further elucidate the mechanism of PKA–AKAP interaction in leptin signaling using INS-1 832/13 cells as they are more amenable to biochemical and molecular genetic manipulations.
Table 1.
Human islets donor information
| Donor (date received) | T2D | Age | Gender | BMI | Cause of death | Islet viability (%) | Islet purity (%) |
|---|---|---|---|---|---|---|---|
| 10/09/181 | N | 47 | F | 24.1 | Stroke | 90 | 90 |
| 10/19/182 | N | 55 | F | 35.7 | Stroke | 98 | 94 |
| 11/07/183 | N | 48 | M | 24.4 | Head trauma | 90 | 90 |
The superscript numbers denotes the donor (Donor 1, 2, 3) whose β-cells were used to collect data as shown in Figure 4, B and D.
Figure 4.
AKAP anchoring of PKA is necessary for leptin-induced hyperpolarization in human β-cells.A, representative cell-attached membrane recordings of individual human β-cells treated with 10 nM leptin (top), leptin in the presence of the PKA inhibitor PKI (middle; PKI, 1 μM), or the PKA-specific activator 6-Bnz-cAMP (bottom; 6-Bnz-cAMP, 10 μM). B, group analysis of the extent of membrane hyperpolarization of human β-cells (left) or INS-1 832/13 cells (right) treated with leptin (human β-cells: –39.40 ± 6.46 mV, n = 11; INS-1 832/13 cells: –46.49 ± 4.92 mV, n = 11), leptin with PKI (human β-cells: –5.18 ± 2.65 mV, n = 6; INS-1 832/13 cells: –17.82 ± 6.88 mV, n = 9), or 6-Bnz-cAMP (human β-cells: –54.84 ± 6.83 mV, n = 9; INS-1 832/13 cells: –56.00 ± 8.15 mV, n = 12). ∗∗p < 0.01 by one-way ANOVA followed by a post hoc Dunnett's multiple comparison test. C, representative membrane potential recordings of human β-cells in response to leptin (10 nM) following preincubation without (top) or with 50 μM st-Ht31 (bottom) for 15–30 min. D, group data of human β-cells showing the degree of membrane hyperpolarization in response to leptin without st-Ht31 preincubation (–39.40 ± 6.46 mV, n = 11) or with st-Ht31 preincubation (–4.81 ± 1.13 mV, n = 11). ∗∗∗p < 0.0001 by unpaired student's t-test. Donors are indicated by the circle color and fill.
AKAP79/150 coordinates leptin signaling to regulate KATP channel trafficking
The above results that PKA–AKAP interactions are required for leptin to exert its signaling effect raise the question of which AKAP(s) is involved in localizing PKA. There are more than 50 known AKAPs (19). We chose to focus on AKAPs that have been shown to be expressed in β-cells and localize at the cell membrane based on the observation that increased PKA activity occurs near the plasma membrane upon leptin stimulation (Fig. 1). Among the possible candidates, AKAP79/150 (human/murine proteins, AKAP5 gene) stood out as a most interesting candidate (20, 36, 37, 38), because in neurons AKAP79/150 has been shown to co-immunoprecipitate with NMDARs (39, 40), a known player in the leptin signaling pathway being studied here. To test the idea that AKAP79/150 could serve as a scaffold to coordinate a complex of leptin signaling molecules in β-cells and regulate KATP channel trafficking, we genetically knocked down AKAP150 expression and monitored surface KATP channels using surface staining, electrophysiology, and surface biotinylation experiments.
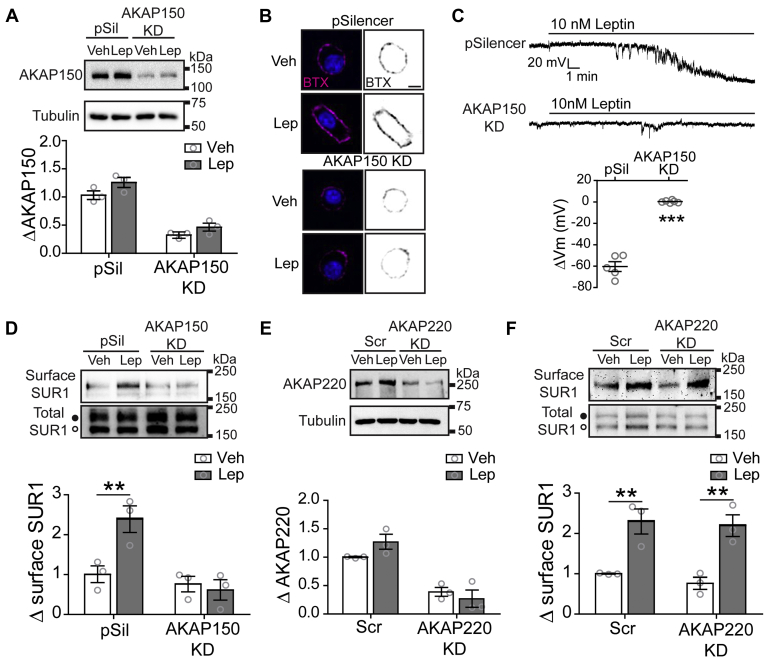
INS-1 832/13 cells were transiently transfected with AKAP150 shRNAi to knock down AKAP150 (AKAP150 KD) or with the empty pSilencer vector as a control (41, 42). AKAP150 KD cells showed a significant decrease in AKAP150 expression compared with control cells regardless of whether they received vehicle or leptin (10 nM) treatment for 30 min (Fig. 5A). Surface staining of exogenously expressed BTX-SUR1/Kir6.2 KATP channels was again used to visualize KATP channels in the membrane. Similar to untransfected INS-1 832/13 cells (Fig. 3B), pSilencer-transfected control cells also showed increased surface KATP channel expression when treated with leptin, but this effect appeared to be reduced in AKAP150 KD cells (Fig. 5B). In agreement with this observation, cell-attached current-clamp recordings showed that leptin-induced hyperpolarization was drastically reduced from –60.40 ± 4.60 mV (n = 5) in pSilencer control cells to 0.30 ± 0.49 mV (p < 0.0001, n = 6) in AKAP150 KD cells (Fig. 5C). To further substantiate the role of AKAP150 in leptin signaling, we carried out surface biotinylation experiments to examine the expression of endogenous KATP channels at the membrane. Control and AKAP150 KD cells were treated with vehicle or leptin (10 nM) for 30 min followed by surface biotinylation and western blot analysis. In pSilencer control cells, leptin caused a significant 2.39-fold increase (p < 0.01) of surface SUR1 compared with vehicle treatment (Fig. 5D). In contrast, AKAP150 KD cells failed to show an increase of surface KATP channels upon leptin treatment. Taken together these findings identify a novel relationship between AKAP79/150 and leptin signaling in β-cells.
Figure 5.
AKAP150 is necessary for leptin-induced KATPchannel trafficking.A, INS-1 832/13 cells transfected with the control pSilencer vector (pSil) or AKAP150 shRNAi (AKAP150 KD) and analyzed for AKAP150 expression via western blot (top). Graph shows quantification of AKAP150 relative to tubulin (bottom). Analysis determined significant main effects of cells (F(1,8) = 113.37, p < 0.0001), treatment (F(1,8) = 6.68, p = 0.03), but no significant interaction between these variables (F(1,8) = 0.38, p = 0.55). B, confocal images of BTX labeled surface KATP channels following vehicle or 10 nM leptin treatment in pSil and AKAP150 KD cells (top panels). Bottom panels show inverse gray scale representations. Scale bar, 5 μm. C, representative membrane potential recordings from pSil (top; –60.40 ± 4.60 mV, n = 5) and AKAP150 KD (bottom; 0.30 ± 0.49 mV, n = 6) cells treated with 10 nM leptin. Below the traces is the group analysis of the extent of membrane hyperpolarization. ∗∗∗p < 0.0001 by unpaired student's t-test. D, surface biotinylation experiments. Western blots show surface expression of the KATP channel subunit SUR1 and total SUR1 in pSil and AKAP150 KD cells treated with vehicle or 10 nM leptin (top). Note, the upper band in the total SUR1 corresponds to the complex-glycosylated SUR1 (filled circles) that traffics to the surface and the lower band corresponds to the ER-core glycosylated SUR1 (open circle). Normalized quantification of surface SUR1 relative to total upper SUR1 band, which represents mature KATP channels that may be trafficked to the cell membrane (bottom). Data analysis revealed significant main effects of cells (F(1,8) = 15.79, p = 0.004), treatment (F(1,8) = 5.95, p = 0.04), and a significant interaction between these variables (F(1,8) = 9.18, p = 0.016). E, western blot analysis (top) and quantification (bottom) of AKAP220 expression in control scramble siRNA (Scr) or AKAP220 siRNA (AKAP220 KD) cells. Significant main effects of cells (F(1,8) = 56.41, p < 0.0001), no effect of treatment (F(1,8) = 0.43, p = 0.53), and no interaction (F(1,8) = 3.16, p = 0.11) by two-way ANOVA. F, western blots showing the effects of AKAP220 siRNA on surface SUR1 relative to total SUR1. Analysis determined no effect of cells (F(1,8) = 0.61, p = 0.48), a significant effect of treatment (F(1,8) = 38.89, p = 0.0002), and no significant interaction between these variables (F(1,8), = 0.10, p = 0.76). Biochemical experiments (A, D, E, F) were repeated three times (n = 3; represented as circles) and normalized to vehicle-treated controls. ∗∗p < 0.01, ∗∗∗p < 0.001 by two-way ANOVA followed by a post hoc Bonferroni's test unless stated otherwise.
To determine whether the effect observed with AKAP150 KD is specific, we examined the effect of knockdown of another membrane-associated AKAP, AKAP220 (43, 44). INS-1 832/13 cells were transfected with scramble control siRNA or AKAP220 siRNA (AKAP220 KD) and knockdown of AKAP220 expression was confirmed by western blot analysis (Fig. 5E). Surface biotinylation experiments were then carried out to assess KATP channel trafficking in response to leptin. As expected, scramble control cells treated with leptin showed a significant 2.30-fold increase (p < 0.01) in surface SUR1 expression compared with vehicle control (Fig. 5F). AKAP220 KD cells stimulated with leptin also showed a significant 2.19-fold increase (p < 0.01) in surface SUR1 expression compared with vehicle-treated cells. The amount of surface SUR1 in leptin-treated AKAP220 KD cells was comparable with scramble control cells treated with leptin and in stark contrast to AKAP150 KD cells. From these observations we concluded that AKAP220 is not involved and that AKAP79/150 plays a unique role for leptin signaling.
AKAP79/150 mediates leptin signaling via its interaction with PKA
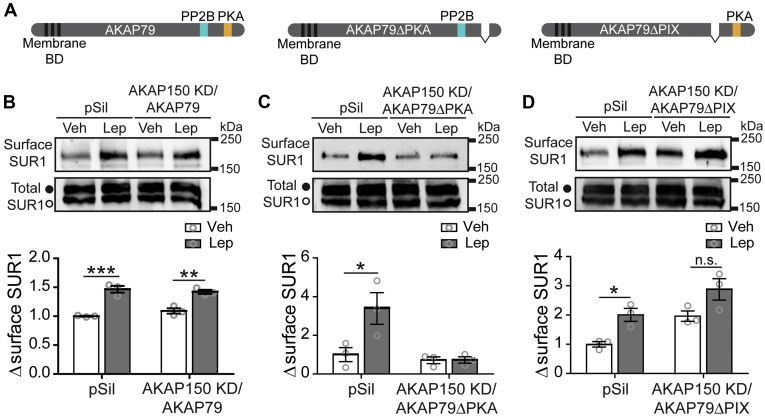
As a PKA anchoring protein, AKAP79/150 presumably participates in leptin signaling by binding to PKA and bringing PKA in proximity to the other signaling molecules. However, in addition to PKA, AKAP79/150 has also been found to anchor protein phosphatase 2B (PP2B; also known as calcineurin; see Fig. 6A) (45, 46), which opposes PKA activity by dephosphorylating PKA substrates. This dual specificity of AKAP79/150 coordinates PKA and PP2B signaling and allows anchored-PKA actions on downstream substrates to be tightly regulated. Indeed, AKAP79/150 mutants that lack the PP2B binding PxIxIT-like motif (AKAP79ΔPIX; Fig. 6A) (45, 46, 47) display increased localized PKA activity (42, 45, 47, 48). To address whether the role of AKAP79/150 in leptin signaling involves AKAP79/150 interaction with PKA, PP2B, or both, we performed AKAP150 KD and rescue experiments using full-length WT AKAP79 (human ortholog of mouse AKAP150) or AKAP79 mutants deficient in PKA or PP2B binding.
Figure 6.
AKAP79 rescues leptin-induced KATPchannel trafficking in AKAP150 KD cells.A, schematic of AKAP79 showing key membrane binding domains (BD) as well as PP2B and PKA binding regions. B, INS-1 832/13 cells were transfected with control pSilencer vector (pSil) or cotransfected with AKAP150 shRNAi and WT AKAP79 (AKAP150 KD/AKAP79). Transfected cells were treated with vehicle or 10 nM leptin for 30 min followed by surface biotinylation. Western blots show surface SUR1 and total SUR1 (top). Quantification of surface SUR1 relative to total upper SUR1 band (bottom). There was a significant effect of treatment (F(1,8) = 91.33, p < 0.0001), no significant effect of cells (F(1,8) = 0.33, p = 0.58), and no interaction between these variables (F(1,8) = 2.624, p = 0.14). C, same as (B) except AKAP150 KD cells were cotransfected with AKAP79 mutants that cannot bind PKA (AKAP150 KD/AKAP79ΔPKA). Both cells (F(1,8) = 10.18, p = 0.0128) and treatment (F(1,8) = 6.73, p = 0.0319) had significant effects in these experiments, and there was a significant interaction between these variables (F(1,8) = 6.573, p = 0.033). D, same as (B) except AKAP150 KD cells were cotransfected with AKAP79 mutants that cannot bind PP2B (AKAP150 KD/AKAP79ΔPIX). Analysis of these data revealed that both cells (F(1,8) = 15.08, p = 0.0047) and treatment (F(1,8) = 16.61, p = 0.0036) had significant effects, but there was no interaction between the two (F(1,8) = 0.039, p = 0.8485). Each experiment was performed three independent times (n = 3; shown as circles) and results were normalized to vehicle-treated control pSil cells. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 by two-way ANOVA followed by a post hoc Bonferroni's test.
First, we performed surface biotinylation experiments in AKAP150 KD cells transfected with WT AKAP79 (AKAP150 KD/AKAP79) to test whether expression of AKAP79 would rescue leptin-induced KATP channel trafficking in AKAP150 KD cells. In this set of experiments, leptin treatment caused a significant 1.47-fold increase (p < 0.001) of surface SUR1 in pSilencer-transfected control cells and a similar 1.42-fold increase (p < 0.01) was observed in AKAP150 KD/AKAP79 cells demonstrating that AKAP79 successfully rescues a loss of AKAP150 (Fig. 6, A and B). This result also confirms that the lack of leptin response in AKAP150 KD cells (Fig. 5D) was not due to off-target effects of the shRNAi. Next, we performed the same experiment using an AKAP79 mutant that lacks the PKA binding motif (AKAP79ΔPKA; Fig. 6A) (46, 47) to test the importance of AKAP79/150-anchored PKA for leptin signaling. AKAP150 KD/AKAP79ΔPKA cells treated with leptin failed to show a significant change in surface KATP channel expression compared with vehicle-treated cells (Fig. 6C) indicating that PKA anchored by AKAP79/150 serves a critical function in the leptin signaling cascade. Lastly, surface biotinylation experiments were performed in AKAP150 KD cells cotransfected with an AKAP79 mutant lacking the PP2B-binding motif (AKAP150 KD/AKA79ΔPIX). Interestingly, compared with vehicle pSilencer controls, vehicle-treated AKAP150 KD/AKA79ΔPIX cells exhibited a 1.96-fold increase in surface SUR1 expression levels akin to that seen in leptin-treated controls (Fig. 6D). Stimulating AKAP150 KD/AKA79ΔPIX cells with leptin led to an even greater 2.87-fold increase in surface KATP channels, although this increase was not statistically significant when compared with vehicle-treated AKAP150 KD/AKA79ΔPIX cells (p > 0.05). The higher than normal KATP channel surface expression in unstimulated AKAP150-KD/AKAP79ΔPIX cells implies that PP2B constitutively bound to AKAP79 likely limits anchored-PKA signaling such that disrupting PP2B anchoring mimics the effect of leptin to increase basal PKA signaling and KATP channel trafficking. The combined results from these experiments provide compelling evidence that leptin increases AKAP79/150-anchored PKA activity and suggest that these actions of PKA are basally opposed by AKAP79/150-anchored PP2B.
Leptin increases cAMP concentrations near AKAP79
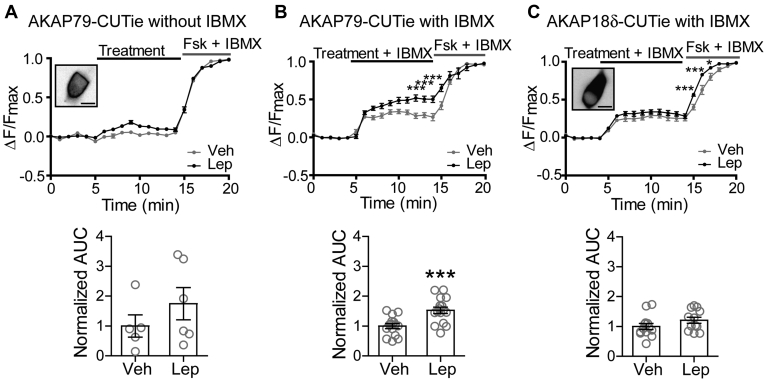
The results thus far indicate that leptin increases the activity of AKAP79/150 anchored-PKA to regulate KATP channel trafficking, but how leptin activates PKA remains to be addressed. PKA is typically activated by cAMP, which binds to PKA regulatory subunits and relieves autoinhibition to unleash the catalytic subunits. Alternatively, inhibiting the opposing activity of PP2B could also enhance net PKA signaling (11, 31, 47, 48, 49). Our results above implicating that AKAP150 KD/AKAP79ΔPIX cells have increased basal PKA signaling are consistent with an inhibitory role of PP2B on steady-state PKA signaling. However, the observation that AKAP150 KD/AKAP79ΔPIX cells still responded to leptin suggests a PKA activation mechanism independent of PP2B inhibition. This prompted us to ask whether leptin increases cAMP levels near AKAP79/150. To monitor changes in cAMP near AKAP79/150, we employed the FRET-based cAMP CUTie sensor targeted to AKAP79 (AKAP79-CUTie) (50). AKAP79-CUTie was found to express at the cell membrane where we had also observed increases in PKA activity. Similar to FRET-based PKA activity experiments, we treated the cells with vehicle or leptin (100 nM), normalized the FRET traces to a maximal response, and then analyzed the traces for AUC. To generate a maximal response, we applied forskolin (20 μM) and IBMX (10 μM), which increases cAMP production by adenylyl cyclases (ACs) and prevents cAMP degradation by phosphodiesterases (PDEs), respectively. During our first set of experiments, we did not see a significant difference in cAMP levels between treatments, although there appeared to be a trend toward increased cAMP in leptin-treated cells (Fig. 7A). Since AKAPs have also been shown to anchor PDEs to tightly regulate PKA activity via cAMP degradation (19), we repeated the experiments in the presence of IBMX to prevent potential cAMP degradation that could obscure cAMP signals. In the presence of IBMX, leptin-treated cells showed a significant 1.53-fold increase (p < 0.0005, n = 16) in AKAP79-CUTie cAMP sensor activity compared with vehicle (n = 15) (Fig. 7B). This effect of leptin on cAMP levels appears to be specific to AKAP79 as leptin did not significantly increase cAMP levels monitored by a cytoplasmic AKAP18δ-CUTie sensor (Fig. 7C). Curiously, in the presence of forskolin, leptin accelerated cAMP accumulation as detected by AKAP18δ-CUTie suggesting that leptin may augment cAMP production by forskolin. These results lead us to conclude that leptin signaling activates PKA at least in part by increasing local cAMP levels near AKAP79/150.
Figure 7.
Leptin increases cAMP levels near AKAP79.A, cAMP CUTie sensor targeted to AKAP79 (human orthologue of AKAP150) was utilized to detect changes in cAMP levels in response to vehicle or 100 nM leptin. The average FRET traces (top) and the group data of FRET traces analyzed for area under the curve (bottom) are shown. Inset shows INS-1 832/13 cell expressing AKAP79-CUTie sensor. Scale bar, 10 μm. B, experiments in (A) were repeated in the presence of the phosphodiesterase inhibitor IBMX (50 μM) to prevent rapid degradation of cAMP. Two-way ANOVA analysis of average traces determined a significant effect of treatment (F(1,629) = 40.08, p < 0.0001) and time (F(20,629) = 212.5, p < 0.0001) and there was a significant interaction between these variables (F(20,629) = 3.96, p < 0.0001). This was followed by a post hoc pairwise comparison of time points by Bonferroni's test ∗∗∗p < 0.0001 (top). Below, normalized AUC was analyzed by unpaired student's t-test (∗∗∗p < 0.0005). C, same as (B) except cAMP levels were monitored using the AKAP18δ-CUTie sensor, which is largely expressed in the cytosol as shown in the inset. Average traces were analyzed by two-way ANOVA, which revealed a significant effect of treatment (F(1,629) = 46.10, p < 0.0001) and time (F(20,629) = 339.8, p < 0.0001) and there was a significant interaction between these variables (F(20,629) = 2.27, p = 0.0014). ∗p < 0.05, ∗∗∗p < 0.001 by post hoc Bonferroni's test (traces, top). Scale bar, 10 μm.
Discussion
The study we present here reveals a novel relationship between leptin and PKA for KATP channel trafficking in β-cells that is mediated by AKAP79/150. From this work and our previous studies, we propose a model in which the PKA-AKAP79/150 complex orchestrates leptin-induced KATP channel trafficking to suppress GSIS from pancreatic β-cells (Fig. 8). In this model, leptin stimulation increases PKA activity anchored by AKAP79/150 through a signaling cascade wherein leptin activates Src kinase, which phosphorylates and potentiates NMDARs, resulting in an enhanced Ca2+ influx that activates CaMKKβ to phosphorylate and activate AMPK. AMPK then increases PKA activity by elevating cAMP concentrations localized near AKAP79/150, possibly by activating an AC, culminating in actin remodeling and the translocation of KATP channels. A greater abundance of KATP channels in the membrane increases the total K+ conductance causing β-cell membrane hyperpolarization and inhibition of Ca2+ influx through voltage-dependent Ca2+ channels to prevent insulin exocytosis. Importantly, we found that in human β-cells, the ability of leptin to reduce electrical activity was also dependent on PKA–AKAP interactions suggesting that this signaling mechanism serves an important function in the regulation of insulin secretion in humans.
Figure 8.
Proposed model depicting AKAP79/150 mediates leptin signaling to regulate KATPchannel trafficking. AKAP79/150 is a scaffolding protein that creates PKA signaling microdomains localized at cell membranes. AKAP79/150 also anchors PP2B, which opposes PKA activity by dephosphorylating PKA substrates and ACs, which enhance PKA activity by producing the PKA activator cAMP. Signaling complexes coordinated by AKAP79/150 allow for PKA signaling to be tightly regulated. In pancreatic β-cells AKAP79/150 anchoring of PKA renders a localized increase of PKA activity following leptin activation of Src kinase to initiate the NMDAR–CaMKKβ–AMPK signaling cascade. This enhancement of PKA activity may at least in part be due to the leptin signaling axis increasing cAMP levels near AKAP79/150. Actin remodeling downstream of PKA allows for increased KATP channel trafficking and a subsequent increase in K+ conductance, which causes cell hyperpolarization and suppresses GSIS.
Mechanism of leptin-induced PKA activity
Although our previous studies suggested that PKA-dependent actin remodeling was necessary for leptin to promote KATP channel trafficking (7), there was very little evidence outside of our own findings for such a signaling relationship between leptin and PKA (51). By implementing FRET-based live cell imaging to monitor PKA activity in combination with pharmacology, we demonstrate here that leptin significantly increases PKA activity at the cell membrane via the NMDAR–CaMKKβ–AMPK signaling axis. The finding that AMPK activation can increase PKA activity in β-cells was notable as two prior studies, one in vascular smooth muscle cells and the other in cardiomyocytes, had only tentatively implicated AMPK upstream of PKA (52, 53). One potential mechanism for AMPK to increase PKA signaling is by inhibiting protein phosphatase activity to prevent the dephosphorylation of PKA effectors. While there are some studies to suggest that AMPK may counteract protein phosphatases (54), others show that AMPK is inactivated by protein phosphatases (55, 56) or that AMPK increases protein phosphatase activity (57, 58). Our experiments using the AKAP79ΔPIX mutant that cannot bind PP2B showed that even vehicle-treated cells displayed increased surface KATP channel expression, which could be the result of a loss of AKAP79-anchored PP2B opposing basal AKAP79-anchored PKA activity. Thus, it is possible that leptin signals via AMPK to inhibit AKAP79-anchored PP2B activity resulting in increased KATP channel surface density. However, the fact that leptin still increases surface KATP channels in AKAP150 KD/AKAP79ΔPIX cells, albeit not statistically significant compared with vehicle-treated control due to already elevated basal KATP channel surface expression, suggests that leptin increases PKA activity independent of PP2B. In this regard, our finding that leptin stimulation raises cAMP levels near AKAP79-CUTie suggests an alternative mechanism by which AMPK increases PKA activity. It is unlikely that AMPK increases cAMP by inhibiting PDEs as we were only able to detect significantly elevated cAMP levels in the presence of the PDE inhibitor IBMX, and in other contexts AMPK has been shown to increase PDE activity (59). Rather, AMPK may act via ACs either directly or indirectly to cause a rise in cAMP. Indeed, several ACs have been reported to interact with AKAP79/150 (21, 60, 61, 62, 63, 64). Of note, we observed a small but significant increase in cAMP detected by the cytosolic AKAP18CUTie in response to forskolin stimulation in leptin-treated cells compared with vehicle-treated cells; this raises the possibility that leptin may also enhance forskolin-induced AC activation in the cytosol, although the significance of this observation awaits further investigation. Other possibilities that AMPK phosphorylates a yet-to-be identified intermediary or directly phosphorylates PKA to induce catalytic activity also need to be considered (65). It is clear that more work is required to determine the precise mechanism by which AMPK activates PKA.
Spatiotemporal regulation of PKA activity in β-cells
A key observation made during these studies is that leptin causes an increase in PKA activity that is restricted near the cell membrane. While our studies support that leptin signals via PKA to increase KATP channel surface density, K+ conductance, and subsequent β-cell hyperpolarization known to inhibit GSIS (7, 8), others have found that the incretin hormone glucagon-like peptide 1 (GLP-1) signals via PKA to promote insulin granule trafficking (14) and Ca2+-induced exocytosis to augment GSIS (15, 16, 17, 23). All of these studies have used pharmacological or genetic manipulations of total PKA activity to implicate PKA in either inhibiting or enhancing GSIS. Although some of these discrepancies may be explained in part by differences in experimental design, it is also likely that they are the result of the indiscriminate activation and/or inhibition of total cellular PKA. In contrast, endogenous hormonal and physiological stimuli such as leptin and GLP-1 are likely to act in a more nuanced manner due to high spatial and temporal regulation. This is supported by our data demonstrating that leptin has a much more limited effect on PKA activity compared with the global PKA activator forskolin. Specificity of PKA signaling within cells is attributed primarily to AKAPs, such as AKAP79/150, organizing PKA signaling microdomains. Interestingly, recent work in vascular smooth muscle cells also illustrates how local cAMP-PKA signaling in an AKAP79/150-organized complex can dictate a very different downstream cellular response from that regulated by more global engagement of cAMP and PKA (66). Of note, some AKAPs expressed in β-cells, including AKAP79/150, have already been implicated in either inhibiting or promoting insulin secretion (20, 21, 22, 23, 24, 25). This highlights that a high level of structural cellular organization is essential for β-cells to incorporate multiple complex signaling networks in order to function properly, including the opposing effects of the hormones leptin and GLP-1.
Implications for the role of AKAP79/150 in insulin secretion
Our results that AKAP150 knockdown or expression of AKAP79ΔPKA prevents leptin-induced KATP channel trafficking indicate that AKAP79/150 coordinates PKA and leptin signaling to impact insulin secretion. Interestingly, neither AKAP150 knockdown nor AKAP79ΔPKA expression affected basal KATP channel surface expression, suggesting that while AKAP79/150 anchored-PKA is critical for leptin signaling, it is unlikely to be involved in constitutive KATP channel trafficking. On the other hand, our findings that expression of AKAP79ΔPIX, a mutant that cannot bind PP2B, leads to a greater abundance of KATP channels in the β-cell membrane in the absence of leptin stimulation suggests that increasing AKAP79/150 anchored-PKA activity is sufficient to promote KATP channel translocation. Note, a previous study has reported that mouse islets lacking AKAP150 exhibit reduced GSIS (20), which was attributed to the effects of AKAP150 on L-type Ca2+ channels. Curiously, the same study found that while expressing AKAP150ΔPKA in AKAP150 knockout islets did not affect GSIS, expressing AKAP150ΔPIX significantly reduced GSIS (20). These observations are congruent with our findings in INS-1 832/13 cells that disrupting PKA tethering did not affect basal surface KATP channel density but disrupting PP2B tethering increases basal surface KATP channel density. This raises the possibility that the reduced GSIS observed in AKAP150ΔPIX-expressing islets is in part due to increased KATP channel surface expression. Noteworthy, the activity of PP2B is stimulated by Ca2+ raising the question of whether Ca2+ influx through NMDARs following leptin stimulation also increases PP2B activity to oppose the effect of leptin on PKA and KATP channel trafficking. Such a dual mode of Ca2+ effect on KATP channel surface expression may play a role in setting the range of KATP channel surface density and will be an interesting topic to pursue in the future. It is also worth mentioning that PKA and PP2B have been reported to modulate KATP channel phosphorylation and subsequently KATP channel gating in some contexts (67, 68, 69, 70, 71). However, our published (7) data showed that there is little difference in the gating properties of KATP channels between vehicle-treated and leptin-treated cells, suggesting that the effect of altered PKA and/or PP2B signaling following leptin stimulation affects primarily KATP channel trafficking rather than gating. Also important, while our current study focuses on the role of AKAP79/150 in mediating PKA signaling by leptin, AKAP79/150 is multifaceted and can coordinate multiple signaling complexes, as has been shown in neurons (39, 42, 72) and other cell types (66, 73). Thus, AKAP79/150 in β-cells may be involved in organizing multiple signaling networks with distinct functions. Finally, increased KATP channel trafficking has been observed following glucose starvation via activation of AMPK (74) and following high-glucose stimulation in a PKA-dependent manner (18). It will be interesting to determine in the future whether these regulations are similarly mediated by AKAP79/150.
In summary, we have identified a novel role of AKAP79/150 in coordinating leptin and PKA signaling to regulate KATP channel trafficking in β-cells, hence GSIS. Importantly, our recent studies showed that leptin failed to promote KATP channel trafficking and membrane hyperpolarization in human β-cells from obese type II diabetic donors and β-cells from obese diabetic db/db mice lacking functional leptin receptors; however, activation of NMDARs downstream of leptin reenacted the effect of leptin (10). Thus further exploration into the leptin signaling pathway coordinated by AKAP79/150 will likely provide valuable insight into how the β-cell regulates KATP channel trafficking to tune its function and may even identify potential therapeutic targets to combat type II diabetes.
Experimental procedures
Chemicals
Leptin and glutamate were from Sigma-Aldrich. PKI, 6-Bnz-cAMP, NMDA, Compound C (Dorsomorphin), D-APV, and STO-609 were from Tocris Bioscience. AICAR was from Selleck Chemicals. st-Ht31 inhibitor peptide was from Promega.
INS-1 832/13 cell culture
INS-1 cells (clone 832/13, referred to herein as INS-1 832/13) were cultured in RPMI 1640 medium with 11.1 mM D-glucose (Invitrogen) supplemented with 10% fetal bovine serum (FBS), 100 units/ml penicillin, 100 μg/ml streptomycin, 10 mM HEPES, 2 mM glutamine, 1 mM sodium pyruvate, and 50 μM β-mercaptoethanol. Only cells within passage numbers 55–75 were used for experiments.
Dissociation of human pancreatic β-cells
Human β-cells were dissociated from human islets obtained through the Integrated Islets Distribution Program (IIDP) as described previously (7, 8, 9, 10). Human islets were cultured in RPMI 1640 medium with 10% FBS and 1% L-glutamine. Islets were dissociated into single cells by trituration in a solution containing 116 mM NaCl, 5.5 mM D-glucose, 3 mM EGTA, and 0.1% bovine serum albumin (BSA), pH 7.4. Dissociated cells were then plated on 0.1% gelatin-coated coverslips and allowed to recover overnight in culture media. For electrophysiological experiments, β-cells were identified by their high level of autofluorescence at 488 nm excitation due to β-cells having high concentrations of unbound flavin adenine dinucleotide (75, 76). Dithizone (Sigma-Aldrich) staining confirmed β-cell identity at the end of each experiment (77). Donor information is provided in Table 1.
Plasmids, viruses, and siRNA
pcDNA3-EGFP (Addgene plasmid #13031) was a gift from Doug Golenbock (UMass, MA). pcDNA3-AKAR4-CAAX (Addgene plasmid #61621) and pcDNA3-AKAR4-NES (Addgene plasmid #64727) were gifts from Jin Zhang (UCSD, CA). AKAP79-CUTie and AKAP18δ-CUTie were gifts from Manuela Zaccolo (Oxford, UK). AKAP150 shRNAi and pSilencer vector were gifts from John Scott (UW, Washington) (41), AKAP79-GFP, AKAP79ΔPIX, and AKAP79ΔPKA were described previously (36, 42, 45, 47). Recombinant rat Kir6.2 and BTX-tag SUR1 adenoviruses were generated in our lab and described previously (7). AKAP220 siRNA (5′-CCAAUGUAAGCA GUAGUCCUCUAA A-3′) and scramble siRNA (5′-UUUAGAGGACUACUGCUUACA UUGG-3′) were from Millipore.
Electrophysiology
Cell-attached recordings were performed using an Axon 200B amplifier (Molecular Devices) and pClamp software. Signals were acquired at 20 kHz and filtered at 2 kHz. Micropipettes were pulled from nonheparinized Kimble glass (Thermo Fisher Scientific) on a horizontal puller (Sutter Instruments) and filled with 140 mM NaCl. The bath solution (Tyrode's solution) contained (in mM): 137 NaCl, 5.4 KCl, 1.8 CaCl2, 0.5 MgCl2, 5 Na-HEPES, 3 NaHCO3, and 0.16 NaH2PO4, 11 glucose, pH 7.2. Pipette resistance was typically between 2 and 6 MΩ. The seal resistance between the recording pipette and the cell ranged between 2 and 8 GΩ. Membrane potentials were recorded in current clamp mode (I = 0) and signals were analyzed using Clampfit (pClamp). After correcting for the measured liquid junction potential (−10 mV), the average baseline Vm was around –4 mV. In this configuration, the estimated Vm is dependent on the ratio of the seal resistance and the combined patch and cell resistance where the ratio of recorded Vm and true membrane potential = (Rseal/Rpatch + Rcell)/[1+(Rseal/Rpatch + Rcell)] (35). Note, as the recorded Vm is an underestimate of the actual membrane potential, we only used it to track changes in membrane potential (34, 35). Seal resistance was monitored before and after the recording. Only cells that showed stable baseline membrane potential prior to leptin/drug application and which maintained good seal resistance were included for analysis.
Live cell FRET imaging
INS-1 832/13 cells were grown in 35-mm dishes and transfected at 50–60% confluency with FRET-based biosensors (2.5 μg plasmid DNA) using Lipofectamine 2000 (6 μl) (Invitrogen). Twenty-four hours posttransfection, cells were plated in μ-slide eight-well glass bottom chamber (Ibidi) and allowed to recover overnight. Cells were then imaged at 37 °C in Tyrode's solution (described above). Images were acquired every minute using an Olympus IX71 inverted microscope with a 40×, 1.35 NA, oil immersion, UApo objective (Olympus). The microscope was equipped with a Nikon Coolsnap ES2 HQ camera. Image processing was performed using Fiji software (National Institutes of Health) (78). FRET was calculated as the ratio of acceptor fluorophore emission (545 nm) to donor emission (480 nm) in response to donor excitation (435 nm). F/Fmax was obtained for each time point by subtracting the average baseline FRET ratio and normalizing to the maximal forskolin response. Only cells that showed a 10–15% increase in FRET in response to forskolin were used for analysis. AUC was calculated using GraphPad Prism for each experimental treatment time course.
Surface staining
INS-1 832/13 cells at ∼70% confluency were washed once with phosphate-buffered saline (PBS) and incubated for 2 h at 37 °C in Opti-MEM (Thermo Fisher Scientific) and a mixture of viruses: tetracycline-inhibited transactivator, tetracycline-inhibited transactivator-regulated construct expressing BTX-SUR1, and Kir6.27. The multiplicity of infection for each virus was determined empirically. After 2 h, the infection mixture was replaced with RPMI 1640 supplemented cell culture media (described above) and were incubated at 37 °C. Twenty-four hours postinfection, cells were plated on 15-mm, number 1.5 glass coverslips (Thermo Fisher Scientific) and allowed to adhere overnight. For experiments shown in Figure 3B, cells were preincubated at 37 °C for 30 min in RPMI 1640 without serum and an additional 30 min with 0.01% DMSO or 50 μM st-Ht31 before being treated with 10 nM leptin or vehicle for 30 min. A prior study by our lab determined that leptin stimulation for 30 min is the optimal treatment duration for immunocytochemistry and biochemical experiments (7). Following leptin or vehicle treatment surface BTX-SUR1 was labeled with 1 μg/ml Alexa Fluor 555 α-bungarotoxin (555-BTX, Thermo Fisher Scientific) for 1 h at 4 °C. For experiments shown in Figure 5B, cells were cotransfected with AKAP150 shRNAi and EGFP or pSilencer and EGFP at a 5:1 plasmid DNA ratio respectively using Lipofectamine 2000. The next day cells were infected with BTX-SUR1 and Kir6.2. These cells were treated with vehicle or 10 nM leptin for 30 min and surface BTX-SUR1 was labeled as described for Figure 3B (minus the preincubation with DMSO or st-Ht31). All imaging experiments were performed on a Zeiss LSM780 confocal microscope equipped with a 63×, 1.4 NA, oil immersion, PlanApochromat objective (Carl Zeiss). During imaging for Figure 5B, EGFP expression was used to distinguish which cells had been transfected. Images were processed with Fiji (NIH).
Immunoblotting
INS-1 832/13 cells were lysed in triple lysis buffer (50 mM Tris-HCl, 2 mM EDTA, 2 mM EGTA, 100 mM NaCl, 1% Triton X-100, pH 7.4, with complete EDTA-free protease inhibitor cocktail [Roche]) for 30 min at 4 °C with rotation, and cell lysates were cleared by centrifugation at 21,000g for 10 min at 4 °C. Proteins were separated by SDS-PAGE (8% acrylamide gel) and transferred to nitrocellulose membranes (Millipore). Membranes were incubated overnight at 4 °C with a primary antibody diluted in Tris-buffered saline plus 0.1% Tween 20 (TBST). The antibody for SUR1 (1:500) was generated in rabbit using a C-terminal peptide (KDSVFASFVRADK) of hamster SUR1 as described previously (7). The antibody against AKAP150 (1:1000) was made as described previously (79). The antibody against GFP (1:100) was from Thermo Fisher Scientific. The antibody for tubulin (1:2000) was from Sigma-Aldrich. After three 10 min washes with TBST, blots were incubated for 1 h at room temperature with horseradish-peroxidase-conjugated secondary antibodies in TBST buffer as follows: 1:20,000 donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories) for SUR1, AKAP150, and GFP; 1:20,000 donkey anti-mouse IgG (Jackson ImmunoResearch Laboratories) for AKAP220 and tubulin. After washing three times for 10 min with TBST, blots were developed using Super Signal West Femto (Pierce) and imaged with FluorChemE (ProteinSimple) or Sapphire Biomolecular Imager (Azure Biosystems). The blots were quantified using Fiji software (NIH) and normalized to corresponding controls.
Surface biotinylation
INS-1 832/13 cells cultured in 10 cm dishes at 50–60% confluency were transfected with 20 μg of pSilencer or AKAP150 shRNAi plasmid DNA and 40 μl of lipofectamine 2000. Forty-eight hours following transfection, the cells were replated in new 10 cm dishes. Seventy-two hours posttransfection, cells at 70–80% confluency were incubated in RPMI 1640 without serum for 1 h at 37 °C prior to a 30 min treatment with vehicle or 10 nM leptin. Cells were then washed four times with cold PBS containing 9 mM CaCl2 and 5.9 mM MgCl2 (DPBS) and incubated with 1 mg/ml EZ-Link Sulfo-NHS-SS-Biotin (Pierce) in DPBS for 30 min at 4 °C. The reaction was terminated by incubating cells twice with DPBS containing 50 mM glycine for 5 min at 4 °C, followed by two washes with cold DPBS. Cells were then lysed with 300 μl triple lysis buffer as described above, and 500 μg of total lysate was incubated with 50 μl of 50% slurry Neutravidin-agarose beads (Pierce) overnight at 4 °C. Biotinylated proteins were eluted with 2× protein loading buffer for 10 min at 37 °C. Both eluent and input samples (50 μg total cell lysate) were analyzed by immunoblotting using anti-SUR1. These experiments were repeated with slight variations in the transfection protocol. For AKAP220 KD experiments 30 nM of AKAP220 or scramble siRNA was used for transfection. The transfections for AKAP150 KD rescue experiments with WT AKAP79-GFP, AKAP79ΔPKA, or AKAP79ΔPIX were performed using 20 μg of AKAP150 shRNAi plasmid DNA and 5 μg of WT AKAP79-GFP, AKAP79ΔPKA, or AKAP79ΔPIX plasmid DNA. In addition to immunoblotting for anti-SUR1, input samples were analyzed using anti-AKAP150, anti-AKAP220, and anti-GFP to confirm transfections were successful. Surface SUR1 bands were normalized to the upper band of total SUR1, as the upper band represents the mature population of SUR1 incorporated into KATP channels that may traffic to the cell membrane. AKAP150 and AKAP220 bands were normalized to the tubulin loading control.
Statistical analysis
All data were analyzed with the program GraphPad Prism. Results were expressed as mean ± standard error of the mean (SEM). Two-way analysis of variance (ANOVA) followed by the post hoc Bonferroni's test or one-way ANOVA followed by the post hoc Dunnet's test or Tukey's test were used for multiple comparisons as detailed in figure legends. When only two groups were compared, unpaired Student's t-tests were used. The level of statistical significance was set at p < 0.05.
Data availability
All data are contained in the article.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
We thank Dr Christopher Newgard for the rat insulinoma INS-1 clone 832/13 cells. We also thank Dr Manuela Zaccolo for the cAMP CUTie sensor plasmids and Dr John Scott for the AKAP150 shRNAi plasmids. Finally, we thank the Advanced Light Microscopy Core at Jungers (OHSU, Portland, OR) staff for their expertise in image acquisition and analysis
Author contributions
V. A. C. conceived the project, designed and performed the experiments, analyzed the data, and wrote the article; Z. Y. performed the experiments; M. L. D. provided the reagents and edited the article; S.-L. S. conceived the project, analyzed the data, and wrote the article. All the authors have full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All the authors reviewed the results and approved the final version of the article.
Funding and additional information
This work was supported by National Institutes of Health grant R01DK057699 and 3R01DK057699-14S1 (to S.-L. S.). Dr M. L. D. acknowledges the support of NIH grant NS040701. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Edited by Roger Colbran
Contributor Information
Veronica A. Cochrane, Email: cochranv@ohsu.edu.
Show-Ling Shyng, Email: shyngs@ohsu.edu.
References
- 1.Seufert J., Kieffer T.J., Leech C.A., Holz G.G., Moritz W., Ricordi C., Habener J.F. Leptin suppression of insulin secretion and gene expression in human pancreatic islets: Implications for the development of adipogenic diabetes mellitus. J. Clin. Endocrinol. Metab. 1999;84:670–676. doi: 10.1210/jcem.84.2.5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kieffer T.J., Heller R.S., Leech C.A., Holz G.G., Habener J.F. Leptin suppression of insulin secretion by the activation of ATP-sensitive K + channels in pancreatic p-cells. Diabetes. 1997;46:1087–1093. doi: 10.2337/diab.46.6.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Emilsson V., Liu Y.L., Cawthorne M. a, Morton N.M., Davenport M. Expression of the functional leptin receptor mRNA in pancreatic islets and direct inhibitory action of leptin on insulin secretion. Diabetes. 1997;46:313–316. doi: 10.2337/diab.46.2.313. [DOI] [PubMed] [Google Scholar]
- 4.Kulkarni R.N., Wang Z.L., Wang R.M., Hurley J.D., Smith D.M., Ghatei M.A., Withers D.J., Gardiner J.V., Bailey C.J., Bloom S.R. Leptin rapidly suppresses insulin release from insulinoma cells, rat and human islets and, in vivo, in mice. J. Clin. Invest. 1997;100:2729–2736. doi: 10.1172/JCI119818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ookuma K., Ookuma M., York D.A. Effects of leptin on insulin secretion from isolated rat pancreatic islets. Diabetes. 1998;47:219–223. doi: 10.2337/diab.47.2.219. [DOI] [PubMed] [Google Scholar]
- 6.Park S.-H., Ryu S.Y., Yu W.J., Han Y.E., Ji Y.S., Oh K., Sohn J.W., Lim A., Jeon J.P., Lee H., Lee K.H., Lee S.H., Berggren P.O., Jeon J.H., Ho W.K. Leptin promotes KATP channel trafficking by AMPK signaling in pancreatic -cells. Proc. Natl. Acad. Sci. U. S. A. 2013;110:12673–12678. doi: 10.1073/pnas.1216351110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen P.-C., Kryukova Y.N., Shyng S.-L. Leptin regulates K ATP channel trafficking in pancreatic β-cells by a signaling mechanism involving AMP-activated protein kinase (AMPK) and cAMP-dependent protein kinase (PKA) J. Biol. Chem. 2013;288:34098–34109. doi: 10.1074/jbc.M113.516880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Y., Shyng S.L., Chen P.C. Concerted trafficking regulation of Kv2.1 and KATP channels by leptin in pancreatic beta-cells. J. Biol. Chem. 2015;290:29676–29690. doi: 10.1074/jbc.M115.670877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Y., Fortin D.A., Cochrane V.A., Chen P.-C., Shyng S.-L. NMDA receptors mediate leptin signaling and regulate potassium channel trafficking in pancreatic β-cells. J. Biol. Chem. 2017;292:15512–15524. doi: 10.1074/jbc.M117.802249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cochrane V.A., Wu Y., Yang Z., ElSheikh A., Dunford J., Kievit P., Fortin D.A., Shyng S.L. Leptin modulates pancreatic β-cell membrane potential through Src kinase-mediated phosphorylation of NMDA receptors. J. Biol. Chem. 2020;295:17281–17297. doi: 10.1074/jbc.RA120.015489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor S.S., Zhang P., Steichen J.M., Keshwani M.M., Kornev A.P. PKA: Lessons learned after twenty years. Biochim. Biophys. Acta. 2013;1834:1271–1278. doi: 10.1016/j.bbapap.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim C., Cheng C.Y., Saldanha S.A., Taylor S.S. PKA-I holoenzyme structure reveals a mechanism for cAMP-dependent activation. Cell. 2007;130:1032–1043. doi: 10.1016/j.cell.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 13.Seino S., Shibasaki T. PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis. Physiol. Rev. 2005;85:1303–1342. doi: 10.1152/physrev.00001.2005. [DOI] [PubMed] [Google Scholar]
- 14.Renström E., Eliasson L., Rorsman P. Protein kinase A-dependent and -independent stimulation of exocytosis by cAMP in mouse pancreatic B-cells. J. Physiol. 1997;502:105–118. doi: 10.1111/j.1469-7793.1997.105bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gromada J., Bokvist K., Ding W.G., Holst J.J., Nielsen J.H., Rorsman P. Glucagon-like peptide 1 (7-36) amide stimulates exocytosis in human pancreatic beta-cells by both proximal and distal regulatory steps in stimulus-secretion coupling. Diabetes. 1998;47:57–65. doi: 10.2337/diab.47.1.57. [DOI] [PubMed] [Google Scholar]
- 16.Suga S., Kanno T., Nakano K., Takeo T., Dobashi Y., Wakui M. GLP-I(7-36) amide augments Ba2+ current through L-type Ca2+ channel of rat pancreatic beta-cell in a cAMP-dependent manner. Diabetes. 1997;46:1755–1760. doi: 10.2337/diab.46.11.1755. [DOI] [PubMed] [Google Scholar]
- 17.Kaihara K.A., Dickson L.M., Jacobson D.A., Tamarina N., Roe M.W., Philipson L.H., Wicksteed B. β-Cell-specific protein kinase A activation enhances the efficiency of glucose control by increasing acute-phase insulin secretion. Diabetes. 2013;62:1527–1536. doi: 10.2337/db12-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang S.N., Wenna N.D., Yu J., Yang G., Qiu H., Yu L., Juntti-Berggren L., Köhler M., Berggren P.O. Glucose recruits KATP channels via non-insulin-containing dense-core granules. Cell Metab. 2007;6:217–228. doi: 10.1016/j.cmet.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Wong W., Scott J.D. AKAP signalling complexes: Focal points in space and time. Nat. Rev. Mol. Cell Biol. 2004;5:959–970. doi: 10.1038/nrm1527. [DOI] [PubMed] [Google Scholar]
- 20.Hinke S.A., Navedo M.F., Ulman A., Whiting J.L., Nygren P.J., Tian G., Jimenez-Caliani A.J., Langeberg L.K., Cirulli V., Tengholm A., Dell'Acqua M.L., Santana L.F., Scott J.D. Anchored phosphatases modulate glucose homeostasis. EMBO J. 2012;31:3991–4004. doi: 10.1038/emboj.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Willoughby D., Masada N., Wachten S., Pagano M., Halls M.L., Everett K.L., Ciruela A., Cooper D.M. AKAP79/150 interacts with AC8 and regulates Ca 2+ -dependent cAMP synthesis in pancreatic and neuronal systems. J. Biol. Chem. 2010;285:20328–20342. doi: 10.1074/jbc.M110.120725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Josefsen K., Lee Y.C., Thams P., Efendic S., Nielsen J.H. AKAP 18 alpha and gamma have opposing effects on insulin release in INS-1E cells. FEBS Lett. 2010;584:81–85. doi: 10.1016/j.febslet.2009.10.086. [DOI] [PubMed] [Google Scholar]
- 23.Lester L.B., Langeberg L.K., Scott J.D. Anchoring of protein kinase A facilitates hormone-mediated insulin secretion. Proc. Natl. Acad. Sci. U. S. A. 1997;94:14942–14947. doi: 10.1073/pnas.94.26.14942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lester L.B., Faux M.C., Nauert J.B., Scott J.D. Targeted protein kinase A and PP-2B regulate insulin secretion through reversible phosphorylation. Endocrinology. 2001;142:1218–1227. doi: 10.1210/endo.142.3.8023. [DOI] [PubMed] [Google Scholar]
- 25.Fraser I.D.C. A novel lipid-anchored A-kinase Anchoring Protein facilitates cAMP-responsive membrane events. EMBO J. 1998;17:2261–2272. doi: 10.1093/emboj/17.8.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aunis D., Bader M.F. The cytoskeleton as a barrier to exocytosis in secretory cells. J. Exp. Biol. 1988;139:253–266. doi: 10.1242/jeb.139.1.253. [DOI] [PubMed] [Google Scholar]
- 27.Muallem S., Kwiatkowska K., Xu X., Yin H.L. Actin filament disassembly is a sufficient final trigger for exocytosis in nonexcitable cells. J. Cell Biol. 1995;128:589–598. doi: 10.1083/jcb.128.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson J.L., Monfregola J., Napolitano G., Kiosses W.B., Catz S.D. Vesicular trafficking through cortical actin during exocytosis is regulated by the Rab27a effector JFC1/Slp1 and the RhoA-GTPase–activating protein Gem-interacting protein. Mol. Biol. Cell. 2012;23:1902–1916. doi: 10.1091/mbc.E11-12-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomas A., Yermen B., Min L., Pessin J.E., Halban P.A. Regulation of pancreatic beta-cell insulin secretion by actin cytoskeleton remodelling: Role of gelsolin and cooperation with the MAPK signalling pathway. J. Cell Sci. 2006;119:2156–2167. doi: 10.1242/jcs.02942. [DOI] [PubMed] [Google Scholar]
- 30.Depry C., Allen M.D., Zhang J. Visualization of PKA activity in plasma membrane microdomains. Mol. Biosyst. 2011;7:52–58. doi: 10.1039/c0mb00079e. [DOI] [PubMed] [Google Scholar]
- 31.Pawson T., Scott J.D. Signaling through scaffold, anchoring, and adaptor proteins. Science. 1997;278:2075–2080. doi: 10.1126/science.278.5346.2075. [DOI] [PubMed] [Google Scholar]
- 32.Smith F.D., Langeberg L.K., Scott J.D. The where's and when's of kinase anchoring. Trends Biochem. Sci. 2006;31:316–323. doi: 10.1016/j.tibs.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 33.Carr D.W., Hausken Z.E., Fraser I.D., Stofko-Hahn R.E., Scott J.D. Association of the type II cAMP-dependent protein kinase with a human thyroid RII-anchoring protein. Cloning and characterization of the RII-binding domain. J. Biol. Chem. 1992;267:13376–13382. [PubMed] [Google Scholar]
- 34.Mason M.J., Simpson A.K., Mahaut-Smith M.P., Robinson H.P.C. The interpretation of current-clamp recordings in the cell-attached patch-clamp configuration. Biophys. J. 2005;88:739–750. doi: 10.1529/biophysj.104.049866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perkins K.L. Cell-attached voltage-clamp and current-clamp recording and stimulation techniques in brain slices. J. Neurosci. Methods. 2006;154:1–18. doi: 10.1016/j.jneumeth.2006.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dell'Acqua M.L., Faux M.C., Thorburn J., Thorburn A., Scott J.D. Membrane-targeting sequences on AKAP79 bind phosphatidylinositol-4, 5-bisphosphate. EMBO J. 1998;17:2246–2260. doi: 10.1093/emboj/17.8.2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nauert J.B., Rigas J.D., Lester L.B. Identification of an IQGAP1/AKAP79 complex in beta-cells. J. Cell. Biochem. 2003;90:97–108. doi: 10.1002/jcb.10604. [DOI] [PubMed] [Google Scholar]
- 38.Purkey A.M., Woolfrey K.M., Crosby K.C., Stich D.G., Chick W.S., Aoto J., Dell'Acqua M.L. AKAP150 palmitoylation regulates synaptic incorporation of Ca2+-permeable AMPA receptors to control LTP. Cell Rep. 2018;25:974–987.e4. doi: 10.1016/j.celrep.2018.09.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Colledge M., Dean R.A., Scott G.K., Langeberg L.K., Huganir R.L., Scott J.D. Targeting of PKA to glutamate receptors through a MAGUK-AKAP complex. Neuron. 2000;27:107–119. doi: 10.1016/s0896-6273(00)00013-1. [DOI] [PubMed] [Google Scholar]
- 40.Lee J., Chung M.-K., Ro J.Y. Activation of NMDA receptors leads to phosphorylation of TRPV1 S800 by protein kinase C and A-Kinase anchoring protein 150 in rat trigeminal ganglia. Biochem. Biophys. Res. Commun. 2012;424:358–363. doi: 10.1016/j.bbrc.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoshi N., Langeberg L.K., Scott J.D. Distinct enzyme combinations in AKAP signalling complexes permit functional diversity. Nat. Cell Biol. 2005;7:1066–1073. doi: 10.1038/ncb1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oliveria S.F., Dell'Acqua M.L., Sather W.A. AKAP79/150 anchoring of calcineurin controls neuronal L-type Ca2+ channel activity and nuclear signaling. Neuron. 2007;55:261–275. doi: 10.1016/j.neuron.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Logue J.S., Whiting J.L., Tunquist B., Sacks D.B., Langeberg L.K., Wordeman L., Scott J.D. AKAP220 protein organizes signaling elements that impact cell migration. J. Biol. Chem. 2011;286:39269–39281. doi: 10.1074/jbc.M111.277756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Radeva M.Y., Kugelmann D., Spindler V., Waschke J. PKA compartmentalization via AKAP220 and AKAP12 contributes to endothelial barrier regulation. PLoS One. 2014;9 doi: 10.1371/journal.pone.0106733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dell'Acqua M.L., Dodge K.L., Tavalin S.J., Scott J.D. Mapping the protein phosphatase-2B anchoring site on AKAP79. Binding and inhibition of phosphatase activity are mediated by residues 315-360. J. Biol. Chem. 2002;277:48796–48802. doi: 10.1074/jbc.M207833200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oliveria S.F., Gomez L.L., Dell'Acqua M.L. Imaging kinase--AKAP79--phosphatase scaffold complexes at the plasma membrane in living cells using FRET microscopy. J. Cell Biol. 2003;160:101–112. doi: 10.1083/jcb.200209127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murphy J.G., Sanderson J.L., Gorski J.A., Scott J.D., Catterall W.A., Sather W.A., Dell'Acqua M.L. AKAP-anchored PKA maintains neuronal L-type calcium channel activity and NFAT transcriptional signaling. Cell Rep. 2014;7:1577–1588. doi: 10.1016/j.celrep.2014.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dittmer P.J., Dell'Acqua M.L., Sather W.A. Ca2+/calcineurin-dependent inactivation of neuronal L-type Ca2+ channels requires priming by AKAP-anchored protein kinase A. Cell Rep. 2014;7:1410–1416. doi: 10.1016/j.celrep.2014.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taylor S.S., Buechler J.A., Yonemoto W. cAMP-dependent protein kinase: framework for a diverse family of regulatory enzymes. Annu. Rev. Biochem. 1990;59:971–1005. doi: 10.1146/annurev.bi.59.070190.004543. [DOI] [PubMed] [Google Scholar]
- 50.Surdo N.C., Berrera M., Koschinski A., Brescia M., Machado M.R., Carr C., Wright P., Gorelik J., Morotti S., Grandi E., Bers D.M., Pantano S., Zaccolo M. FRET biosensor uncovers cAMP nano-domains at b-adrenergic targets that dictate precise tuning of cardiac contractility. Nat. Commun. 2017;8:1–14. doi: 10.1038/ncomms15031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mehebik N., Jaubert A.-M., Sabourault D., Giudicelli Y., Ribière C. Leptin-induced nitric oxide production in white adipocytes is mediated through PKA and MAP kinase activation. Am. J. Physiol. Physiol. 2005;289:C379–C387. doi: 10.1152/ajpcell.00320.2004. [DOI] [PubMed] [Google Scholar]
- 52.Stone J.D., Narine A., Tulis D.A. Inhibition of vascular smooth muscle growth via signaling crosstalk between AMP-activated protein kinase and cAMP-dependent protein kinase. Front. Physiol. 2012;3 doi: 10.3389/fphys.2012.00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kobashigawa L.C., Xu Y.C., Padbury J.F., Tseng Y.-T., Yano N. Metformin protects cardiomyocyte from doxorubicin induced cytotoxicity through an AMP-activated protein kinase dependent signaling pathway: An in vitro study. PLoS One. 2014;9 doi: 10.1371/journal.pone.0104888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zheng S., Qu Z., Zanetti M., Lam B., Chin-Sang I. C. elegans PTEN and AMPK block neuroblast divisions by inhibiting a BMP-insulin-PP2A-MAPK pathway. Development. 2018;145:dev166876. doi: 10.1242/dev.166876. [DOI] [PubMed] [Google Scholar]
- 55.Kudo N., Gillespie J.G., Kung L., Witters L.A., Schulz R., Clanachan A.S., Lopaschuk G.D. Characterization of 5′AMP-activated protein kinase activity in the heart and its role in inhibiting acetyl-CoA carboxylase during reperfusion following ischemia. Biochim. Biophys. Acta. 1996;1301:67–75. doi: 10.1016/0005-2760(96)00013-6. [DOI] [PubMed] [Google Scholar]
- 56.Davies S.P., Helps N.R., Cohen P.T., Hardie D.G. 5′-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase. Studies using bacterially expressed human protein phosphatase-2C alpha and native bovine protein phosphatase-2AC. FEBS Lett. 1995;377:421–425. doi: 10.1016/0014-5793(95)01368-7. [DOI] [PubMed] [Google Scholar]
- 57.Kim K., Baek A., Hwang J.E., Choi Y.A., Jeong J., Lee M.S., Cho D.H., Lim J.S., Kim K.I., Yang Y. Adiponectin-activated AMPK stimulates dephosphorylation of AKT through protein phosphatase 2A activation. Cancer Res. 2009;69:4018–4026. doi: 10.1158/0008-5472.CAN-08-2641. [DOI] [PubMed] [Google Scholar]
- 58.Yuan D., Zhou S., Liu S., Li K., Zhao H., Long S., Liu H., Xie Y., Su Y., Yu F., Li S. The AMPK-PP2A axis in insect fat body is activated by 20-hydroxyecdysone to antagonize insulin/IGF signaling and restrict growth rate. Proc. Natl. Acad. Sci. U. S. A. 2020;117:9292–9301. doi: 10.1073/pnas.2000963117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Johanns M., Lai Y.C., Hsu M.F., Jacobs R., Vertommen D., Van Sande J., Dumont J.E., Woods A., Carling D., Hue L., Viollet B., Foretz M., Rider M.H. AMPK antagonizes hepatic glucagon-stimulated cyclic AMP signalling via phosphorylation-induced activation of cyclic nucleotide phosphodiesterase 4B. Nat. Commun. 2016;7:10856. doi: 10.1038/ncomms10856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Johnstone T.B., Agarwal S.R., Harvey R.D., Ostrom R.S. cAMP signaling compartmentation: Adenylyl cyclases as anchors of dynamic signaling complexes. Mol. Pharmacol. 2018;93:270–276. doi: 10.1124/mol.117.110825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Efendiev R., Samelson B.K., Nguyen B.T., Phatarpekar P.V., Baameur F., Scott J.D., Dessauer C.W. AKAP79 interacts with multiple adenylyl cyclase (AC) isoforms and scaffolds AC5 and -6 to α-Amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) receptors. J. Biol. Chem. 2010;285:14450–14458. doi: 10.1074/jbc.M110.109769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bauman A.L., Soughayer J., Nguyen B.T., Willoughby D., Carnegie G.K., Wong W., Hoshi N., Langeberg L.K., Cooper D.M., Dessauer C.W., Scott J.D. Dynamic regulation of cAMP synthesis through anchored PKA-adenylyl cyclase V/VI complexes. Mol. Cell. 2006;23:925–931. doi: 10.1016/j.molcel.2006.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tenner B., Getz M., Ross B., Ohadi D., Bohrer C.H., Greenwald E., Mehta S., Xiao J., Rangamani P., Zhang J. Spatially compartmentalized phase regulation of a Ca2+-cAMP-PKA oscillatory circuit. Elife. 2020;9 doi: 10.7554/eLife.55013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang M., Patriarchi T., Stein I.S., Qian H., Matt L., Nguyen M., Xiang Y.K., Hell J.W. Adenylyl cyclase anchoring by a kinase anchor protein AKAP5 (AKAP79/150) is important for postsynaptic β-adrenergic signaling. J. Biol. Chem. 2013;288:17918–17931. doi: 10.1074/jbc.M112.449462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Haushalter K.J., Casteel D.E., Raffeiner A., Stefan E., Patel H.H., Taylor S.S. Phosphorylation of protein kinase A (PKA) regulatory subunit RIα by protein kinase G (PKG) primes PKA for catalytic activity in cells. J. Biol. Chem. 2018;293:4411–4421. doi: 10.1074/jbc.M117.809988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Prada M.P., Syed A.U., Reddy G.R., Martín-Aragón Baudel M., Flores-Tamez V.A., Sasse K.C., Ward S.M., Sirish P., Chiamvimonvat N., Bartels P., Dickson E.J., Hell J.W., Scott J.D., Santana L.F., Xiang Y.K. AKAP5 complex facilitates purinergic modulation of vascular L-type Ca2+ channel CaV1.2. Nat. Commun. 2020;11:5303. doi: 10.1038/s41467-020-18947-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wilson A.J., Jabr R.I., Clapp L.H. Calcium modulation of vascular smooth muscle ATP-sensitive K(+) channels: Role of protein phosphatase-2B. Circ. Res. 2000;87:1019–1025. doi: 10.1161/01.res.87.11.1019. [DOI] [PubMed] [Google Scholar]
- 68.Orie N.N., Thomas A.M., Perrino B.A., Tinker A., Clapp L.H. Ca2+/calcineurin regulation of cloned vascular K ATP channels: Crosstalk with the protein kinase A pathway. Br. J. Pharmacol. 2009;157:554–564. doi: 10.1111/j.1476-5381.2009.00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Beguin P., Nagashima K., Nishimura M., Gonoi T., Seino S. PKA-mediated phosphorylation of the human KATP channel: Separate roles of Kir6.2 and SUR1 subunit phosphorylation. EMBO J. 1999;18:4722–4732. doi: 10.1093/emboj/18.17.4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin Y.-F., Jan Y.N., Jan L.Y. Regulation of ATP-sensitive potassium channel function by protein kinase A-mediated phosphorylation in transfected HEK293 cells. EMBO J. 2000;19:942–955. doi: 10.1093/emboj/19.5.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Light P.E., Manning Fox J.E., Riedel M.J., Wheeler M.B. Glucagon-like peptide-1 inhibits pancreatic ATP-sensitive potassium channels via a protein kinase A- and ADP-dependent mechanism. Mol. Endocrinol. 2002;16:2135–2144. doi: 10.1210/me.2002-0084. [DOI] [PubMed] [Google Scholar]
- 72.Zhang J., Bal M., Bierbower S., Zaika O., Shapiro M.S. AKAP79/150 signal complexes in G-protein modulation of neuronal ion channels. J. Neurosci. 2011;31:7199–7211. doi: 10.1523/JNEUROSCI.4446-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nystoriak M.A., Nieves-Cintrón M., Nygren P.J., Hinke S.A., Nichols C.B., Chen C.Y., Puglisi J.L., Izu L.T., Bers D.M., Dell'acqua M.L., Scott J.D., Santana L.F., Navedo M.F. AKAP150 contributes to enhanced vascular tone by facilitating large-conductance Ca 2+ -activated K + channel remodeling in hyperglycemia and diabetes mellitus. Circ. Res. 2014;114:607–615. doi: 10.1161/CIRCRESAHA.114.302168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lim A., Park S.H., Sohn J.W., Jeon J.H., Park J.H., Song D.K., Lee S.H., Ho W.K. Glucose deprivation regulates K ATP channel trafficking via AMP-activated protein kinase in pancreatic β-cells. Diabetes. 2009;58:2813–2819. doi: 10.2337/db09-0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smelt M.J., Faas M.M., de Haan B.J., de Vos P. Pancreatic beta-cell purification by altering FAD and NAD(P)H metabolism. Exp. Diabetes Res. 2008;2008 doi: 10.1155/2008/165360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Van De Winkel M., Pipeleers D. Autofluorescence-activated cell sorting of pancreatic islet cells: Purification of insulin-containing B-cells according to glucose-induced changes in cellular redox state. Biochem. Biophys. Res. Commun. 1983;114:835–842. doi: 10.1016/0006-291x(83)90857-4. [DOI] [PubMed] [Google Scholar]
- 77.Hansen W.A., Christie M.R., Kahn R., Norgaard A., Abel I., Petersen A.M., Jorgensen D.W., Baekkeskov S., Nielsen J.H., Lernmark A. Supravital dithizone staining in the isolation of human and rat pancreatic islets. Diabetes Res. 1989;10:53–57. [PubMed] [Google Scholar]
- 78.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., Tinevez J.Y., White D.J., Hartenstein V., Eliceiri K., Tomancak P. Fiji: An open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brandao K.E., Dell’Acqua M.L., Levinson S.R. A-kinase anchoring protein 150 expression in a specific subset of TRPV1- and CaV 1.2-positive nociceptive rat dorsal root ganglion neurons. J. Comp. Neurol. 2012;520:81–99. doi: 10.1002/cne.22692. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are contained in the article.