Abstract
Objective:
In 2007, the Ministry of Health (MoH) in Mexico implemented a multidisciplinary health-care model (MHC) for patients with type-2 diabetes (T2D), which has proven more effective in controlling this condition than the conventional health-care model (CHC).
Research design and methods:
We compared the cost-effectiveness of the MHC vs. the CHC for patients with T2D using a quasi-experimental, retrospective design. Epidemiologic and cost data were obtained from a randomly selected sample of health-care units, using medical records as well as patient- and facility-level data. We modelled the cost-effectiveness of the MHC at one, 10 and 20 years using a simulation model.
Results:
The average cumulative costs per patient at 20 years were US$4,225 for the MHC and US$4,399 for the CHC. With a willingness to pay one gross domestic product (GDP) per capita per quality-adjusted life year (QALY) (US$8,910), the incremental net benefits per patient were US$1,450 and US$3,737 at 10 and 20 years, respectively. The MHC was cost-effective from the third year onward; however, increasing coverage to 500 patients per year rendered it cost-effective at year one.
Conclusions:
The MHC is cost-effective at 10 and 20 years. Cost-effectiveness can be achieved in the short term by increasing MHC coverage.
Keywords: Cost-effectiveness, Multidisciplinary care, Diabetes, Low- and middle-income countries
1. Introduction
Type 2 diabetes (T2D) is a global public health challenge that is disproportionately affecting people living in low- and middle-income countries (LMICs) [1]. An estimated 79% of people with diabetes currently live in LMICs, where unmet need is growing rapidly [2]. In Mexico, T2D is growing at an alarming rate, with a current estimated prevalence of 14% [3]. As the second leading cause of mortality, the leading cause of disability-adjusted life years (DALYs), and a major contributor to health system expenditure, T2D poses an unprecedented burden on the Mexican health system [4–6]. As such, developing cost-effective, high-quality models of care delivery for individuals with T2D is one of the most pressing challenges to improve the management of this condition, ameliorate associated complications, and reduce health system costs.
Over the last two decades, there has been an important shift in the care delivery structure for T2D from a traditional physician-led model to that of multidisciplinary health care (MHC) [7,8]. MHC aims to respond to the pluralistic needs of individuals with T2D by engaging the patient and a team of health professionals with complementary skills to work unified towards achieving optimal diabetes management [7]. Since their widespread introduction to clinical practice, MHC teams have been associated with improvement in all-cause mortality, reduction in glycated haemoglobin (HbA1c) [9,10], gains in quality-adjusted life years (QALYs) [11], greater adherence to medical care and proper self-care [5–7]. MHC has also been shown to be cost-effective when compared with traditional care models but only a few studies have been conducted in low resource settings [9].
In response to the growing T2D epidemic in Mexico and to improve the effectiveness of T2D management, in 2007, the Ministry of Health (MoH) implemented a comprehensive model of care for patients with T2D being cared for in the public sector. These multidisciplinary health care units (MHCUs) operate within the MoH, which provides services to the uninsured, usually most socioeconomically vulnerable individuals, which corresponds to about 50% of the Mexican population. MHCU teams are usually comprised of a physician, nurse, psychologist, nutritionist, social worker, and a physical therapist. According to the MoH rules of operation, each team is expected to provide services to 500 patients with T2D per year [12]. Patients are expected to achieve glycemic control within 12 months of establishing care within these units, after which they return to their respective primary-health-care units (PHCUs) [13]. Despite robust evidence on the effectiveness of MHC models of care for patients with T2D in high-income countries [7], no studies to date have evaluated the effectiveness and cost-effectiveness of MHCUs in Mexico. In this study, we: (1) evaluate the effectiveness and cost-effectiveness of MHCUs in Mexico using a quasi-experimental design; and (2) project the cost-effectiveness of MHCUs using a simulation model of diabetes.
2. Methods
We conducted a cost-effectiveness analysis based on a retrospective, quasi-experimental design. Patients with T2D were identified from chart review based on the corresponding T2D diagnostic information. We selected patients who had suboptimal glycemic control and that were followed over a period of 12 months. We estimated the cost-effectiveness of MHC compared to CHC by estimating costs at the public health-care-unit level and using individual-level data extracted from medical records. We gathered epidemiologic and cost data covering the period 2016–2017 from a randomly selected, stratified sample of 40 PHCUs: 20 treatment units (MHCUs that utilized MHC for patients with T2D) and 20 control units (PHCUs utilizing CHC for patients with T2D).
Sample size was calculated in order to detect change in HbA1c of up to two percentage points [14]. Given that CHCUs could refer patients with T2D and suboptimal glycemic control to MHCUs and since the MHC model had not been implemented at the national level before 2016–2017, we used MHCUs that were located far enough (at least a 2-hour commute from CHCUs) to avoid contamination. Given that the model of care covers only 8% of the total patient population with T2D who are followed in public health MoH facilities, we were able to identify PHCs at which patients were not exposed to multidisciplinary care. Hence, we utilize these health units as the counterfactual arm. The units were also selected based on the number of registered patients with T2D in order to standardize the size of the health units in the analysis. At each health-care unit, we selected the medical records of patients with T2D and suboptimal glycemic control (defined as equal or greater than a HbA1c of 7.0% following the national diabetes guidelines [15]) who had at least 12 months of follow-up. We extracted data on gender, age, HbA1c, year of diabetes diagnosis, years with diabetes, body mass index, blood pressure and serum lipid levels. We included only patients with T2D who had suboptimal glycemic control for two main reasons. First, MHC units only see patients with T2D with suboptimal glycemic control. These patients are followed for 12 months, after which those who have achieved glycemic control are referred back to their PHCUs. Secondly, patients with T2D and suboptimal glycemic control are at the highest risk to develop micro- and macrovascular complications, which are associated with greater health system costs.
2.1. Costs
Short-term costs.
We used a micro-costing technique to estimate the cost per patient year of individuals with poorly controlled T2D. Estimates were developed at the health-unit level in MHCUs and PHCUs. We collected data on prices and utilization of health resources at the health-unit level to estimate the costs of medications, staff, equipment, general services, and training.
Medium- and long-term costs.
For the 10- and 20-year horizons, we included the cost of monitoring patients with T2D and suboptimal glycemic control for both MHCUs and PHCUs, given that patients from MHCUs must be counter-referred to the PHCUs after one year linked to a MHC. We included the treatment costs of diabetes-related complications (ischemic heart disease, myocardial infarction, heart failure, stroke, amputation, blindness and renal failure) based on the cost of care for complications within the public health sector in Mexico, as reported in the literature (see Table S1, Supplementary Material) [16–23].
2.2. Effectiveness
Short-term outcome.
We defined “effectiveness in the short term” as any improvement in HbA1c after 12 months of exposure to multidisciplinary care, since the MHC standards of operation establish that patients should achieve glycemic control within one year [24]. We used linear, fixed effects regression and latent class models to estimate the causal effect of MHC on glycemic control. However, using observational data to estimate the effectiveness parameter raised two concerns. First, the non-random assignment to MHC at the patient level could create confounding bias, so we used inverse probability of treatment weighting (IPTW) assuming selection on observables, which was implemented as a reweighting step in the outcome regression. Second, selection bias could occur as a result of loss to follow-up, because we estimated effectiveness from a subset of patients with records of their HbA1c levels after one year. We therefore implemented the Heckman correction for this source of endogeneity between exposure to MHC and changes in HbA1c [25].
We analysed the effect of one year of exposure to the MCH model on the change in HbA1c, assuming that these patients could receive MHC for only one year according to the rules of operation of MHCUs. We assumed selection on observables to estimate the average treatment effect on the treated (ATET).
Medium- and long-term outcomes.
Considering that sub optimally controlled diabetes can markedly affect the quality of life of individuals with T2D, we estimated QALY expectancy to evaluate effectiveness in the medium and long terms. QALYs were estimated from utility decrements obtained from a dataset of 1,093 Mexican patients with T2D with and without complications [26]. Data were collected as part of this study using the EQ-5D-5L instrument (see Table S1, Supplementary Material) [27].
2.3. Cost-effectiveness analysis
We utilized the United Kingdom Prospective Diabetes Study (UKPDS) model [28], a microsimulation model of diabetes progression, to calculate the cost-effectiveness of the treatment of patients in the intervention (n = 455) and control (n = 201) arms over 1, 10 and 20 years, using an annual discount rate of 5%. The model was based on a system of parametric equations that predicted the annual absolute risk of presenting seven health complications associated with T2D (myocardial infarction, heart failure, ischemic heart disease, stroke, blindness, amputation and renal failure), as well as death. These equations were estimated through Weibull, logistic and Gompertz regressions. We adjusted the short- and long-term analyses for age, gender, age at T2D diagnosis and biomarkers at the end of the first year. We used final post-intervention biomarkers and costs of care from the short-term analysis as baseline indicators to model the long-term costs and effectiveness (see Table S2, Supplementary Material). The costs of each complication were obtained from the literature (see Table S1, Supplementary Material), while the QALYs were drawn from a survey of Mexican patients with T2D (see Table S1, Supplementary Material) [26]. The results of the analyses are presented as the incremental cost-effectiveness ratio (ICER) between the two arms of the study. The ICER represents the difference in cost per QALY improvement per patient between the intervention (MHC model) and control (CHC model) arms. We also show results concerning the net monetary benefit (NMB), which indicates the value of each intervention (MHC and CHC) in monetary terms, using a cost-effectiveness threshold of US$8,910 per QALY gain, representing one GDP per capita in Mexico [29]. Finally, we present the incremental net benefit per patient (INBP), which is the difference between the NMB per patient in the MHC model and the NMB in the CHC model.
2.4. Scenario and sensitivity analyses
In the base case, we found considerable heterogeneity in the scale of service utilization across health-care units in both models of care. The scale of provision of health services directly affects the unitary costs of operation; thus, in addition to the base case, we modelled two additional scenarios of cost-effectiveness that took into account the current annual coverage of patients with T2D in the health-care units (approximately 455 patients): (1) a normative-capacity scenario, which referred to the minimum number of patients with T2D required to be cared for annually in the MHCUs to meet the goals established by the MoH: approximately 500; and (2) a maximum-capacity scenario, which referred to the largest number of patients with T2D that MHC teams could serve based on the best-performing MCHU in our dataset: approximately 800 [26].
We performed a deterministic sensitivity analysis to explore results pertaining to cost-effectiveness over plausible parameter ranges for the MCH model, such as the cost of medicines, the cost of staff and treatment effect.
3. Results
3.1. Characteristics of the analytical sample
Baseline characteristics of the individuals in the study sample are summarized in Table 1. The final study sample included 656 individuals across 40 health units. When compared with CHC, MHC had a lower percentage of male patients. Participants in MHCs were farther out of a T2D diagnosis and had a higher number of years with a T2D diagnosis than participants in CHC units. There were no differences in HbA1c between the MHC and CHC groups. On average, results at the end of 1-year of exposition showed that systolic and diastolic blood pressure measurements as well as triglycerides were higher in the CHC group when compared with the MHC group. Comparison between final levels of these biomarkers of CHC and MHC groups are included in the supplementary material (Table S6).
Table 1 –
Baseline characteristics of the analytical sample.
| General (n = 656) (A) |
CHC (n = 201) (B) |
MHC (n = 455) (C) |
Difference (C - B) |
|
|---|---|---|---|---|
| Mean (SD) | ||||
| Individual characteristics | ||||
| Sex (% of male) | 0.29 (0.45) | 0.36 (0.48) | 0.26 (0.44) | −0.10* |
| Age (years) | 55.73 (11.23) | 56.98 (12.26) | 55.17 (10.72) | −1.73* |
| Year of Dx (years) | 2007 (7.04) | 2008 (7.21) | 2006 (6.93) | −2.00* |
| Years with the disease (years) | 7.80 (7.01) | 6.80 (7.21) | 8.24 (6.89) | 1.40* |
| Biomarkers | ||||
| HbA1c (%) | 8.20 (2.20) | 8.40 (2.24) | 8.11 (2.17) | −0.29 |
| Weight (kg) | 73.36 (16.06) | 73.95 (13.91) | 73.10 (16.94) | −0.85 |
| Height (cm) | 156.36 (8.48) | 157.49 (8.36) | 155.85 (8.50) | −1.60* |
| BMI (kg/m2) | 29.96 (5.88) | 29.85 (5.37) | 30.01 (6.09) | 0.16 |
| Systolic pressure (mmHg) | 120.17 (17.42) | 122.36 (15.97) | 119.20 (17.96) | −3.10* |
| Diastolic pressure (mmHg) | 75.39 (10.36) | 77.77 (10.86) | 74.34 (9.96) | −3.43* |
| Cholesterol (mg/dL) | 180.83 (42.34) | 185.01 (40.06) | 178.99 (43.22) | −6.01* |
| Triglycerides (mg/dL) | 175.73 (105.83) | 192.61 (98.88) | 168.27 (108.03) | −24.4* |
Note: Statistical significance level < 0.05.
Chi-Squared and t-student tests for categorical and continuous variables respectively.
3.2. Average MHC and CHC costs per patient
Table 2 displays the short-, medium- and long-term costs for both MHC and CHC models. Broken down by short-term costs per patient, health staff costs represented the highest share of the total cost per patient (72.8%), followed by equipment, medicines and general services. The costs of MHC per patient were more than three times higher than the CHC costs over the short term. In contrast, the medium- and long-term costs per patient were higher for CHC. When compared with MHC, the cost per patient under CHC was 5.2% higher after 10 years and 4.1% higher after 20 years.
Table 2 –
Disaggregation of total annual average costs per patient (US$, 2017).
| Short-term | CHC (n = 20) (A) |
MHC (n = 20) (B) |
|||||
|---|---|---|---|---|---|---|---|
| Media | Median | SD | Mean | Median | SD | Difference(B – A) | |
| Staff | $52.10 | $34.40 | $60.70 | $152.30 | $122.80 | $130.10 | $100.20 |
| Equipment | $5.30 | $0.70 | $17.00 | $46.70 | $2.70 | $92.10 | $41.40 |
| Drugs | $1.20 | $0.30 | $2.10 | $12.40 | $9.40 | $11.70 | $11.20 |
| Services | $4.80 | $3.30 | $5.20 | $6.20 | $4.70 | $4.60 | $1.40 |
| Training | $0.20 | $ - | $0.30 | $0.30 | $ - | $0.90 | $0.10 |
| Total mean annual cost per patient | $63.60 | $217.88 | $154.28 | ||||
| Medium and Long-term | |||||||
| Average cumulative cost per patient (10 y) | $2,302.51 | $2,188.97 | −$113.54 | ||||
| Average cumulative cost per patient (20 y) | $4,398.71 | $4,225.37 | −$173.34 | ||||
Note: Note: Statistical significance level < 0.05.
Chi-Squared and t-student tests for categorical and continuous variables respectively.
Exchange rate: 1 US$ =18.89 MXN (Bank of Mexico, 2019). Staff: health-care employees who had contact with T2D patients, namely general practitioners, medical specialists, nurses, nurse specialists, administrative employees, psychologists, nutritionists and physical therapists. Services: water, gas, electricity and telephone. Drugs: Metformin, Fast-acting and Intermediate Insulin, Lispro, Glargine, Acarbose and Linagliptin. Equipment: Cobas equipment, impedance cardiogram equipment, microalbumin equipment, centrifuge, weighing machine, examination tables, electrocardiogram, glucometer and clinical devices. Training: workshops, courses and other activities related to the care of patients with T2D. Note that medium- and long-term costs included the cost of medical following-up of patients with diabetes and the costs of complications at 10 and 20 years as regards the probability of each individual developing a complication according to their final HbA1c levels after one year of exposure to either of the care models analysed.
3.3. Cost-effectiveness results
Table 3 shows the cost-effectiveness results at 1, 10, and 20 years. Results at one year showed that the MHC model was not cost-effective with an ICER greater than the willingness to pay (WTP): US$12,166.2 > US$8,910 per QALY. The MHC model became cost-effective from the third year of operation onward with an ICER of US$1,423.2 (see Table S3). Over the medium and long term, MHC led to 0.15 and 0.40 additional QALYs than CHC at 10 and 20 years, respectively. The average cumulative costs per patient at 10 years were US$2,189 for MHC and US$2,302 for CHC. The average cumulative costs of MHC versus CHC per patient at 20 years were US$4,225 and US$4,399, respectively. Given a cost-effectiveness threshold of one GDP per capita (US$8,910) per QALY, the INBP of MHC compared to CHC were US$1,450 and US$ 3,737 at 10 and 20 years, respectively. Based on negative incremental costs combined with positive incremental QALYs, this indicated that MHC generated cost savings during these periods.
Table 3 –
Cost-effectiveness analysis over the short, medium, and long terms (US$, 2017).
| Average cumulative cost per patient (US$) |
Average cumulative effectiveness per patient |
Increment cost (US$) |
Incremental effectiveness |
ICER (US$) | Net monetary benefit (US$) |
Incremental net benefit per patient (US$) |
|
|---|---|---|---|---|---|---|---|
| At one year | |||||||
| CHC | 364.24 | 1.74 | 97.33 | 0.01 | 12,166.25 | 15,156.98 | |
| MHC | 461.57 | 1.75 | 15,130.93 | −26.05 | |||
| At 10 years | |||||||
| CHC | 2,302.51 | 6.63 | −113.54 | 0.15 | Cost Saving | 56,770.79 | |
| MHC | 2,188.97 | 6.78 | 58,220.83 | 1,450.04 | |||
| At 20 years | |||||||
| CHC | 4,398.71 | 9.35 | −173.34 | 0.40 | Cost Saving | 78,909.79 | |
| MHC | 4,225.37 | 9.75 | 82,647.13 | 3,737.34 |
Note: MHC refers to Multidisciplinary health care and CHC to Conventional health care. Exchange rate: 1 US$ = 18.89 MXN (Bank of Mexico, 2019). Data retrieved from the UKPDS model v 1.3. Effectiveness units: quality-adjusted life years (QALYs). Willingness to pay (WTP): 1 GDP = US $8,910 per capita per QALY (Bank of Mexico, 2019). Discount rate: 5% for cost and effectiveness. ICER: incremental cost / incremental effectiveness ratio. Net monetary benefit (NMB): WTP* QALYs - costs. Incremental net benefit per patient (INBP): net monetary benefit (NMB) of MHC - NMB of CHC.
3.4. Sensitivity analysis
Fig. 1 shows the results of the one-way sensitivity analysis, including variations in costs (staff and drugs), effectiveness (HbA1c levels) and discount rates. We divided the analysis into maximum (dark colors) and minimum values (light colors). The minimum costs were US$153 for MHC and US$34 for CHC; the minimum changes in Hb1Ac levels were <1 percentage point and the minimum discount rate was 3%. The maximum costs were US$281 for MHC and US$91 for CHC; the maximum changes in Hb1Ac levels were 1–2 percentage points and the maximum discount rate was 7%. Panels A and B show the results at 10 years, and panels C and D at 20 years. All panels show that the discount rate had the greatest impact on the NMB, whereas the impact of variation in effectiveness and care costs per patient on the NMB was minimal.
Fig. 1 –
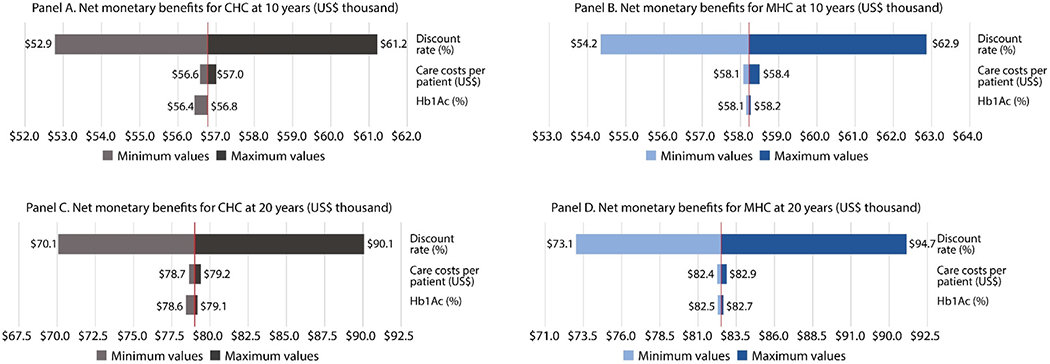
Results of the sensitivity analysis (US$, 2017). Note. Minimum costs: US$153 for MHC and US$34 for CHC; minimum Hb1Ac levels: 7.91% for MHC and 8.09% for CHC; minimum discount rate: 3%; maximum costs: US$ 281 for MHC and US$91 for CHC; maximum Hb1Ac levels: 8.31% for MHC and 8.71% for CHC; and maximum discount rate: 7%.
3.5. Scenario analysis
We estimated different levels of cost-effectiveness for three scenarios (see Figure S4, Supplementary Material): the base case represents the current annual coverage for the MHC model per team that treated an average of 455 patients and yielded a cost per patient of approximately US$217.8; the institutional-target coverage (500 patients per year); and the maximum-capacity coverage (800 patients per year). Using 10 and 20 years as time horizons, we found that raising the scale of production to the institutional target (500 patients per year) reduced the cost per patient to US$165.6. Compared to the base case, this coverage scenario raised the INBP by 3.93% at 10 years and by 1.53% at 20 years. In addition, where the scale of production rose to the maximum annual capacity per year per team, the cost per patient dropped to US$103.5 and the INBP increased by 8.12% and 3.15% at 10 and 20 years, respectively, with respect to the base case (see more details in Tables S3 and S4, Supplementary Material).
4. Discussion
Our study demonstrated that the implementation of the MHC model for patients with T2D and suboptimal glycemic control over the course of 12 months is a high-value strategy that is cost saving in the medium and long term at current levels of service utilization: approximately 455 patients per year. These results are attributable to averting the health complications associated with sub optimally controlled T2D. This study represents the first evaluation of effectiveness and cost-effectiveness of MHC models for T2D management in Mexico. Our findings have important policy implications by highlighting the cost-effectiveness of MHC, which can guide restructuring of primary care delivery throughout Mexico. The positive health outcomes and quality of life gains observed for patients with T2D coupled with substantial potential cost savings for the Mexican health system can guide the implementation of effective and cost-efficient models of care delivery for patients with T2D.
Although the MHC intervention was not cost-effective in the short-term period, MHC was shown to be cost-effective from the third year onward, at which point the incremental cost-effectiveness ratio (ICER) per QALY (US$1,423.21 in our study) becomes lower than the cost-effectiveness threshold willingness to pay (WTP) per QALY (US$8,910 in our study). This is attributed to the cost per patient in the short term (one year of MHC exposure), which is approximately three times greater for the MHC when compared to the CHC model. The long-term cost saving observed with the MHC model is explained by the economic benefits of preventing costly diabetes complications, which develop over the course of years. This finding represents an example of cost-saving associated with preventive interventions, which require greater investment at the outset but then yield benefits in the medium and long term. Therefore, in the context of preventing diabetes-related complications, health and cost benefits are usually seen over the long term [11]. Importantly, while there are other metabolic risk factors to consider more broadly as they relate to cardiovascular disease, including hypertension and hyperlipidaemia, baseline levels of these indicators in our study were on average in the normal range, with minor changes throughout the study period. As such, while it is important to consider these risk factors in the cost-effectiveness of MHC, the primary endpoint in this study was HbA1c, both due to the well-documented success of multidisciplinary care models in T2D management and the lack of evidence of their cost-effectiveness in Mexico.
In the sensitivity analysis, we found that discount-rate variations have the greatest impact on the net monetary benefit (NMB). This can be explained by the fact that the largest benefits occur in the future and, depending on how much we discount in the future, present gains vary considerably.
According to the results of the different MHC-coverage scenarios, the performance of the MHC model can be improved in the short term. In a scenario featuring annual increases in levels of coverage for MHCUs, we observed a considerable reduction in the cost per patient for the MHC model in the short term, rendering the model more cost-effective in the short, medium and long terms.
In a scenario in which MHCUs use teams comprised of a doctor, a nurse, a dietician, a psychologist, and a physical therapist, a total of 500 patients can be covered annually (as required by MoH operating rules [26]). According to our findings, the MHC intervention is cost-effective from the first year onward with an ICER of US$5,037.5 per QALY (WTP: US$8,910 per QALY) and cost saving from the third year onward. Moreover, in a scenario of maximum capacity that is providing coverage to 800 patients per unit per year, the MHC model is cost saving from the first year onward (see Table S2, Supplementary Material).
4.1. Strengths and limitations
Our study has several strengths. For the cost-effectiveness analysis reported in this paper, we used costs and health output data of patients with diabetes collected retrospectively. We used a robust quasi-experimental design taking advantage of the fact that the multidisciplinary care introduced by the MoH in 2011 had not been widely implemented in the country. In 2012, public-sector coverage for this model represented only 6%1 of patients with diabetes covered by the MoH [30]. We randomly selected health units that covered patients with diabetes under two different models of care: MHC and CHC, and compared the effectiveness of each model after one year of exposure.
Although we found the MHC model to be effective in the short term (one year) and cost-effective in the medium and long terms, an improvement in MHC coverage should be considered in order to reduce the costs of this care model in the short term and render it cost-effective from the first year on. Specific strategies to increase the coverage of the model should be designed in order to achieve more rapid short-term cost-effectiveness. One option is to integrate the MHC model within the primary health units so as to enhance the adequate reference to MHC and to facilitate the access to this type of model of care to patients with T2D with suboptimal glycemic control.
This study has several limitations. First, one major assumption underlying the medium- and long-term effects of the MHC model was that once patients are exposed to the MHC model and have achieved glucose control, they maintain these levels of glucose control in the future. Although the literature has not yet supported the sustainability of the short-term results of the MHC model, preliminary results of patients exposed to this model in Mexico show that patients who achieve control after 12 months of exposure can maintain this level of control at 31 months after completing the intervention. Second, the UKPDS model is based on the life expectancy of individuals from the UK (80 years) and other multi-ethnic groups from Asia, Europe, and some regions from North America, whereas the life expectancy of the Mexican population is 77 years. Although we introduced biomarkers into the UKPDS model at baseline and follow-up, QALYs from Mexican patients with T2D, and costs from the specific context of the Mexican public-health-care system, the differences in life expectancy could have resulted in an overestimation of the effects of the MHC model, particularly with respect to other causes of death. Third, given that we included only patients with T2D who also had suboptimal glycemic control based on HbA1c data, we applied inverse probability of treatment weighting (IPTW) to adjust for differences among patients with T2D attending PHCUs and those attending MHCUs, to avoid potential selection bias attributable to the specific profile of the patients attending MHCUs. We also controlled for the inverse Mills-ratio2 to adjust for potential selection bias attributable to differences among health-care units and their capacity to retain patients.
Finally, in order to identify the effect of the MHC model, we defined our analytical sample applying a match technique using patients’ baseline characteristics. However, in order to identify variations of the effect of MHC model between the analytical sample exposed to multidisciplinary model (467 T2D patients) and the overall T2D patients (7,598 T2D patients) exposed also to the same model of care from the 20 sampled MHCUs of this study, we compared the difference of the effect of MHC in the two samples. We found that although the overall sample started with patients with worse levels of HbA1c (mean baseline: 8.9%) compared to the T2D patients from the analytical sample (mean baseline: 8.1%), the effect of the multidisciplinary model of care was a reduction of around 1 percentage points in the HbA1c in both samples after one to 18 months of exposure to the MHC model of care. This shows that although the analytical sample excluded patients with worse levels of HbA1c, the potential effect of the MHC model could be the same (see Table S5, supplementary material).
In light of the alarming rise in T2D prevalence in Mexico and other LMICs, there is an urgent need to develop and implement cost-effective models of care for patients with T2D. In Latin American countries, evidence on effectiveness and cost-effectiveness of multidisciplinary health care models for patients with T2D is lacking. In this study we demonstrate the cost-effectiveness of multidisciplinary-team-based care in Mexico after three years of MHC implementation and show that with expansion of this model of care, cost-effectiveness can be achieved at the outset of MHC implementation. These findings have important implications for improving care delivery for T2D in Mexico and potentially other LMICs and may inform policies to improve outcomes for patients with T2D, reduce the risk of complications and decrease health system costs.
Supplementary Material
Acknowledgements
We gratefully acknowledge the contributions of all participants including those who collected information for the study. We especially thank Roxana Rodríguez-Franco for her valuable support in the literature review, as well as Dr. Martín Romero for his advice and estimation of sample size. We also wish to thank the CENAPRECE-Ministry of Health team for their assistance in the collection of information and for their comments regarding service operation. Special appreciation is expressed to Dr. Arantxa Colchero for her advice on the development of several of our collection instruments. We acknowledge to Consejo Nacional de Ciencia y Tecnología (CONACYT) to fund the research project number 000000000273258- ANALISIS DE COSTO-EFECTIVIDAD.
Funding sources
This study was conducted with funding from the National Council for Science and Technology (CONACYT) in Mexico. The funder played no role in the design of the study nor in the collection, analysis and interpretation of data and in the writing of the manuscript.
Footnotes
Own estimations according to data on active patients in 2018 obtained from the Information System for Chronic Diseases (SIC) and the report of the National Center for Disease Prevention and Control Programs (CENAPRECE) in Mexico.
To correct for selection bias, James Heckman proposed a two-stage estimation procedure using the inverse Mills ratio. First, a probit regression is modeled to observe the positive outcome of the dependent variable, in our case, the probability of belonging to the analytical sample. The inverse Mills ratio must be generated by means of a probit model. Second, the estimated parameters are used to calculate the inverse Mills ratio, which is then included as an additional explanatory variable in the ordinary least squares (OLS) estimation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A.: Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.diabres.2020.108336.
REFERENCES
- [1].IDF Diabetes Atlas 9th edition 2019. [Internet]. [cited 2020 Jan 16]. Available from: https://www.diabetesatlas.org/en/.
- [2].Manne-Goehler J, Geldsetzer P, Agoudavi K, Andall-Brereton G, Aryal KK, Bicaba BW, et al. Health system performance for people withdiabetes in 28 low-and middle-incomecountries: A cross-sectional study of nationallyrepresentative surveys. PLoS Med 2019;16(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Villalpando S, la Cruz V de, Rojas R. Prevalence and distribution of type 2 diabetes mellitus in Mexican adult population. A probabilistic survey [Prevalencia y distribución de la diabetes mellitus tipo 2 en población adulta mexicana. Una encuesta probabilística.]. Salud Pública México; Vol 52 Supl 1 Results Mex Natl Heal Nutr Surv 2006 non-transmissible chronic Dis [Internet]. 2010. February 3; Available from: http://saludpublica.mx/index.php/spm/article/view/4950/9697. [DOI] [PubMed] [Google Scholar]
- [4].Escobedo J, Rodríguez-Abrego G, Aranda J, Zurita B, Ramirez T, Herrera J. Disability-adjusted life-years (DALYs) for diabetes in Mexico in 2005: a cross-sectional burden of disease analysis. Lancet 2013;381:S46. [Google Scholar]
- [5].Barraza-Lloréns M, Guajardo-Barrón V, Picó J, García R, Hernández C, Mora F, Athié J, Crable EUA. Carga económica de la diabetes mellitus en México, 2013. [Internet]. México. D. F,; 201Available from: http://funsalud.org.mx/portal/wp-content/uploads/2015/08/Carga-Economica-Diabetes-en-Mexico-2013.pdf [Google Scholar]
- [6].Jesus Alegre-Díaz, William Herrington, Malaquías López-Cervantes, Louisa Gnatiuc, Raul Ramirez, Michael Hill, et al. Diabetes and cause-specific mortality in Mexico City. N Engl J Med 2016;375(20):1961–71. 10.1056/NEJMoa1605368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].McGill M, Blonde L, Chan JCN, Khunti K, Lavalle FJ, Bailey CJ. The interdisciplinary team in type 2 diabetes management: Challenges and best practice solutions from real-world scenarios. J Clin Transl Endocrinol 2017;7:21–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Margaret McGill, Anne-Marie Felton. New global recommendations: A multidisciplinary approach to improving outcomes in diabetes. Primary Care Diabetes 2007;1(1):49–55. 10.1016/j.pcd.2006.07.004. [DOI] [PubMed] [Google Scholar]
- [9].Siaw MYL, Malone DC, Ko Y, Lee JYC. Cost-effectiveness of multidisciplinary collaborative care versus usual care in the management of high-risk patients with diabetes in Singapore: Short-term results from a randomized controlled trial. J Clin Pharm Ther 2018;43(6):775–83. [DOI] [PubMed] [Google Scholar]
- [10].Tratamiento de la Diabetes Mellitus tipo 2 en el primer nivel de Atención. [Internet]. México; Available from: http://www.cenetec.salud.gob.mx/interior/catalogoMaestroGPC.html%0D [Google Scholar]
- [11].Tao L, Wilson ECF, Wareham NJ, Sandæk A, Rutten GEHM, Lauritzen T, et al. Cost-effectiveness of intensive multifactorial treatment compared with routine care for individuals with screendetected Type 2 diabetes: Analysis of the ADDITION-UK cluster-randomized controlled trial. Vol. 32, Diabetic Medicine. 2015. p. 907–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Manual de organización para la atención de pacientes en UNEME Enfermedades Crónicas: Sobrepeso, Riesgo Cardiovascular y Diabetes. [Internet]. 2da. Edición; México, D. F.; CENAPRECE: Secretaría de Salud.; Available from: http://www.cenaprece.salud.gob.mx/programas/interior/adulto/descargas/pdf/ManualProcedimientos_atencion_pacientes_UNEME_ECsobrepeso_RCV_DM.pdf [Google Scholar]
- [13].Instituto Mexicano del Seguro Social. Tratamiento de la Diabetes Mellitus tipo 2 en el primer nivel de Atención. México: Instituto Mexicano del Seguro Social; 2014. [Google Scholar]
- [14].Sosa-Rubí SG, Contreras-Loya D, López-Ridaura R, Colchero-Aragonés A, Alarid-Escudero F, Romero-Martinez M. Protocolo de investigación Propuesta CONACYT: Análisis de costo-efectividad del modelo UNEMES-EC para la atención multidisciplinaria de pacientes con diabetes mellitus tipo 2 (DM2). Instituto Nacional de Salud Pública; 2017. [Google Scholar]
- [15].Glycemic targets: standards of medical care in Diabetes 2018. Diabetes Care. 2018;41:S55–64. [DOI] [PubMed] [Google Scholar]
- [16].Centro Nacional de Excelencia Tecnológica en Salud (CENETEC). Diagnóstico, estratificación y tratamiento hospitalario inicial de pacientes con síndrome coronario agudo sin elevación ST. México: Secretaría de Salud; 2010. [Google Scholar]
- [17].Diario Oficial de la Federación (DOF). Aprobacicón de los Costos Unitarios por Nivel de Atención Médica actualizados al año 2018. México: Diario Oficial Federal (DOF); 2010. [Google Scholar]
- [18].Centro Nacional de Excelencia Tecnológica en Salud (CENETEC). Guía de Práctica Clínica de Diagnóstico y Tratamiento de Infarto Agudo de Miocardio con Elevación del Segmento ST en Mayores de 65 Años. México: Secretaría de Salud; 2013. [Google Scholar]
- [19].Meza R, Barrientos-gutierrez T, Rojas-martinez R, Reynosonoverón N, So L, Lazcano-ponce E, et al. Burden of type 2 diabetes in Mexico : past, current and future prevalence and incidence rates. 2015;81:445–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pichon-Riviere A, Reynales-Shigematsu LM, Bardach A, Caporale J, Augustovski F, Alcaraz A, et al. Gallegos-Rivero V H-SRE. Carga de Enfermedad Atribuible al Tabaquismo en México. Documento Ténico IECS N° 10. Buenos Aires, Argentina: 2013. [Google Scholar]
- [21].Catálogo Universal de Servicios de Salud (CAUSES). México: Secretaría de Salud; 2018. [Google Scholar]
- [22].Centro Nacional de Excelencia Tecnológica en Salud (CENETEC). Rehabilitación del Paciente Adulto Amputado de Extremidad inferior por Diabetes Mellitus, en el segundo y tercer nivel de atención. México: Secretaría de Salud; 2019. [Google Scholar]
- [23].Centro Nacional de Excelencia Tecnológica en Salud (CENETEC). Prevención secundaria, diagnóstico, tratamiento y vigilancia de la enfermedad vascular cerebral isquémica. México: Secretaría de Salud. [Google Scholar]
- [24].Secretaría de Salud. Manual de procedimientos para la atención de pacientes en UNEME Enfermedades Crónicas: Sobrepeso, Riesgo Cardiovascular y Diabetes; 2015. [Google Scholar]
- [25].Gerhard T Bias: Considerations for research practice. Am J Heal Pharm 2008;65(22):2159–68. [DOI] [PubMed] [Google Scholar]
- [26].Sosa-Rubí SG, Contreras-Loya D, Gomez-Dantés O. Análisis de costo-efectividad del modelo UNEMES-EC para la atención multidisciplinaria de pacientes con diabetes mellitus tipo 2 (DM2). Unpublished results; 2019. [Google Scholar]
- [27].EuroQol. EQ-5D Instruments [Internet]. [cited 2019 Aug 26]. Available from: https://euroqol.org/eq-5d-instruments/ [Google Scholar]
- [28].Clarke PM, Gray AM, Briggs A, Farmer AJ, Fenn P, Stevens RJ, et al. A model to estimate the lifetime health outcomes of patients with Type 2 diabetes: The United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model (UKPDS no. 68). Diabetologia. 2004;47(10):1747–59. [DOI] [PubMed] [Google Scholar]
- [29].Consejo General de Salubridad. Guía de Evaluación de Insumos para la salud. 2017. [Google Scholar]
- [30].Secretaria de Salud. Programa Sectorial de Salud: Programa de Acción Específico para la “Prevención y Control de la Diabetes Mellitus 2013-2018”. [Internet]. Programa Sectorial de Salud. 2013. p. 78. Available from: http://www.cenaprece.salud.gob.mx/descargas/pdf/PAE_PrevencionControlDiabetesMellitus2013_2018.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


