Abstract
Interleukin-6 (IL-6) is a cytokine implicated in proinflammatory as well as regenerative processes and acts via receptor complexes consisting of the ubiquitously expressed, signal-transducing receptor gp130 and the IL-6 receptor (IL-6R). The IL-6R is expressed only on hepatocytes and subsets of leukocytes, where it mediates specificity of the receptor complex to IL-6 as the subunit gp130 is shared with all other members of the IL-6 cytokine family such as IL-11 or IL-27. The amount of IL-6R at the cell surface thus determines the responsiveness of the cell to the cytokine and might therefore be decisive in the development of inflammatory disorders. However, how the expression levels of IL-6R and gp130 at the cell surface are controlled is largely unknown. Here, we show that IL-6R and gp130 are constitutively internalized independent of IL-6. This process depends on dynamin and clathrin and is temporally controlled by motifs within the intracellular region of gp130 and IL-6R. IL-6 binding and internalization of the receptors is a prerequisite for activation of the Jak/STAT signaling cascade. Targeting of gp130, but not of the IL-6R, to the lysosome for degradation depends on stimulation with IL-6. Furthermore, we show that after internalization and activation of signaling, both the IL-6R and gp130 are recycled back to the cell surface, a process that is enhanced by IL-6. These data reveal an important function of IL-6 beyond the pure activation of signaling.
Keywords: interleukin-6, interleukin-6 receptor, gp130, recycling, internalization
Abbreviations: ADAM, a disintegrin and metalloprotease; DMEM, Dulbecco's modified Eagle's medium; DMSO, dimethyl sulfoxide; FCS, fetal calf serum; gp130, glycoprotein 130; IL-6R, interleukin 6-receptor; IL, interleukin; Jak, Janus kinase; PBS, phosphate buffered saline; sIL-6R, soluble IL-6 receptor; STAT, signal transducer and activator of transcription
The cytokine interleukin-6 (IL-6) is implicated in mucosal immunity and the hepatic acute-phase response (1). Furthermore, it is involved in the development of sepsis, multiple sclerosis, and rheumatoid arthritis, making IL-6 an attractive therapeutic target (2, 3). The monoclonal antibody tocilizumab, which prevents binding of IL-6 to its IL-6 receptor (IL-6R), has been approved for the treatment of rheumatoid arthritis in more than 160 countries (3, 4).
Binding of IL-6 to the interleukin-6 receptor (IL-6R) and the subsequent recruitment of a gp130 homodimer lead to activation of intracellular signaling cascades, most importantly the janus kinase/signal transducer and activator of transcription (Jak/STAT) pathway (5, 6). Besides negative feedback inhibitors, e.g., SOCS3, internalization of transmembrane receptors is an important regulatory cellular process, which not only regulates the amount of protein on the cell surface, but can also be critical for signal termination. This has been described e.g., for receptor tyrosine kinases such as the endothelial growth factor receptor (7), but is not the case for the IL-6 signaling complex. Internalization routes can be divided into clathrin-dependent and clathrin-independent pathways (8). Internalized receptors are either degraded intracellularly or recycled back to the cell surface.
At present, there are only few data available on the internalization of gp130 and the IL-6R and the intracellular fate of the two receptors (reviewed in (9, 10)). An early study showed rapid clearance of IL-6 and a reduction of binding sites on the cell surface, suggesting ligand-induced internalization that was independent of the intracellular domain (ICD) of the IL-6R, as an ICD-deficient mutant of the IL-6R showed no internalization defect (11). However, the ICD of the IL-6R contains sorting signals that are responsible for transport of the receptor to the basolateral membrane of polarized Madin-Darby canine kidney cells. Mutation of the membrane-proximal tyrosine-based motif (Y408SLG) or the di-leucine-like motif (L427I) disrupts this differential sorting and leads to a localization of the IL-6R mainly at the apical membrane (12). The receptor subunit gp130 has also been shown to be sorted to the basolateral membrane in polarized cells because of a di-leucine motif in its ICD (13).
These results are consistent with the view that IL-6 binding is a prerequisite for endocytosis of its receptors. However, Thiel et al. showed constitutive and ligand-independent internalization of gp130 (14). Whether the IL-6R is also constitutively endocytosed and whether its endocytosis occurs in complex with gp130 are questions that have not been addressed so far.
Furthermore, several findings suggest that internalization is not only a mechanism that terminates IL-6 signaling, but is rather required for gp130 signaling. A mutant variant of this receptor found in inflammatory hepatocellular adenomas is constitutively active and activates STAT3 in the absence of the ligand (15, 16). When HepG2 cells were transfected with this variant and treated with dynasore, an inhibitor of dynamin, phosphorylation of STAT3 was reduced, indicating that the constitutive signal transduction was localized to endosomes (17). In contrast, coexpression of this gp130 variant and dominant-negative dynamin in HEK293 cells did not reduce STAT3 phosphorylation although cell surface levels of gp130 were increased due to impaired clathrin-mediated endocytosis (18).
In the present study, we show that the IL-6R and gp130 are internalized constitutively and independently of IL-6. Both receptors are internalized by clathrin-mediated endocytosis, which is a prerequisite for IL-6-mediated signal transduction. We further show that the internalized receptors are either degraded within the lysosome or recycled back to the cell surface, the latter of which is enhanced in the presence of IL-6.
Results
Regulation of IL-6R levels on the cell surface by limited proteolysis and internalization
IL-6 is a potent proinflammatory cytokine that exerts its actions through a receptor complex containing the α-receptor IL-6R and the signal-transducing receptor gp130. While gp130 is ubiquitously expressed and is also a receptor for all other cytokines of the IL-6 family, the surface expression of the α-receptor renders cells specifically responsive to IL-6. Thus, IL-6R expression has to be tightly regulated to control excessive inflammatory responses that might be harmful to the tissue (19).
We and others have previously shown that the amount of IL-6R on the cell surface is controlled by limited proteolysis by different proteases, among them the metalloprotease ADAM17 (20, 21). Accordingly, activation of ADAM17 by the phorbol ester phorbol 12-myristate 13-acetate (PMA) reduced IL-6R cell-surface levels on the monocytic cell line THP-1 as judged by flow cytometry (Fig. 1A and (22)).
Figure 1.
Cell surface expression of IL-6R over time.A, THP-1 cells were incubated at 37 °C in the presence of 100 nM PMA or DMSO as a control for 2 h. Remaining cell-surface levels of IL-6R were determined via flow cytometry. B and C, Twice washed THP-1 cells were preincubated in serum-free medium for 30 min with or without 10 μM marimastat at 37 °C and subsequently stained for 1 h with the specific antibody against the IL-6R on ice. Cells were washed again twice and incubated for the indicated time periods at 37 °C in serum-free medium containing marimastat or not. Secondary antibody was added for 1 h on ice. Cells were washed and analyzed by flow cytometry. One experiment out of three with similar outcome is shown in panel B, and the quantification of three independent experiments in panel C (mean ± SD, n = 3). D, The sIL-6R in the supernatant of the experiment described in panel (B) was quantified via ELISA. The mean ± SD from three independent experiments is shown. Statistically significant differences were analyzed using one-tailed Welch's t-test. ∗: p < 0.05; ns: not significant. DMSO, dimethyl sulfoxide.
Besides proteolysis, cytokine receptors are often also regulated by internalization. In order to determine a contribution of internalization to the cell-surface amounts of the IL-6R, we stained THP-1 cells with a specific antibody for the IL-6R and incubated the cells for different time periods at 37 °C. The used antibody recognizes an epitope located within the D1 domain of the IL-6R and does not interfere with IL-6 signaling or proteolytic cleavage of the IL-6R. We then visualized the IL-6R at the cell surface with a secondary antibody coupled to a fluorescent dye. As shown in Figure 1B (left panel), IL-6R levels were reduced over time, indicating a constitutive process. Inhibition of proteolysis with the broad-spectrum metalloproteinase inhibitor marimastat (Fig. 1B, right panel) did not significantly prevent IL-6R reduction on the cell surface (Fig. 1C), although sIL-6R in the cell culture supernatant was reduced after 90 min and 120 min of treatment with marimastat (Fig. 1D). We concluded from these experiments that constitutive internalization and proteolysis act simultaneously in THP-1 cells to regulate the amount of IL-6R at the cell surface, but that proteolysis is not the dominant mechanism.
Constitutive internalization of the IL-6R and gp130 depends on clathrin and dynamin
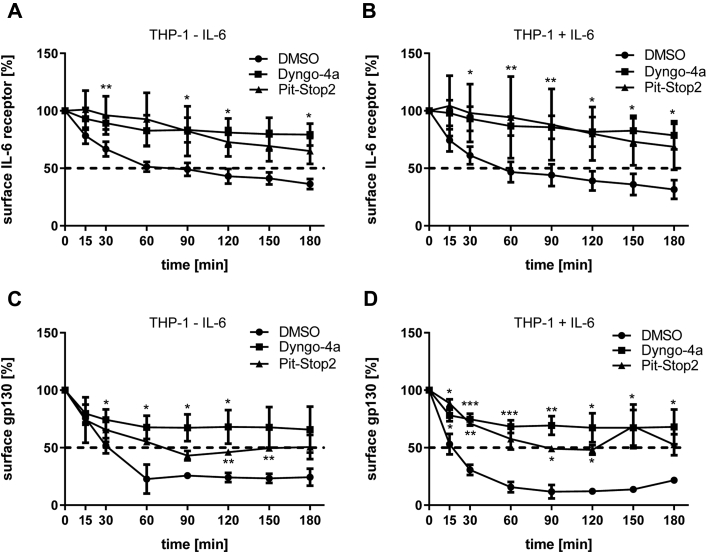
To further analyze the role of internalization and characterize the involved pathways, we incubated THP-1 cells with the inhibitors Dyngo-4a and Pit-Stop2, thereby specifically blocking dynamin and clathrin, respectively, or treated cells with the solvent dimethyl sulfoxide (DMSO) as control. All experiments were done in the presence of marimastat to avoid simultaneous proteolytic cleavage. As before, we observed a constitutive decline of the IL-6R over time, and the cell surface amount was halved after 60 min (Fig. 2A). Blockade of dynamin by Dyngo-4a significantly prevented a decrease of IL-6R on the cell surface (Fig. 2A), and the same was true for the clathrin inhibitor Pit-Stop2 (Fig. 2A). Importantly, the internalization process appeared to be completely independent of the presence or absence of the ligand IL-6 (Fig. 2B and S1, A–C).
Figure 2.
The IL-6R and gp130 are constitutively internalized in a clathrin- and dynamin-dependent manner.A and B, THP-1 cells were washed and afterward preincubated with marimastat in serum-free medium for 30 min at 37 °C. Additionally, dynamin-dependent internalization was inhibited by preincubation with 50 μM Dyngo-4a or clathrin-dependent internalization by adding 25 μM Pit-Stop2. Cells were treated with DMSO as control. In total, 10 ng/ml IL-6 was added where indicated. Subsequently, cells were incubated with the specific antibodies against the IL-6R for 1 h on ice. None-bound antibody was washed away, and internalization was performed at 37 °C for the indicated time periods. Secondary antibody was added for 1 h on ice before cells were washed and analyzed by flow cytometry. C and D, The experiment was performed as described for panels A and B, but gp130 was stained with a specific antibody. Data in all panels were analyzed by two-way ANOVA followed by multiple comparison analysis (both inhibitors were tested against the DMSO control, Dunnett's test). ∗: p < 0.05; ∗∗: p < 0.01; ∗∗∗: p < 0.001. DMSO, dimethyl sulfoxide.
In addition, we furthermore analyzed the internalization of the β-receptor gp130 in the same experimental setup. Like the IL-6R, gp130 was constitutively internalized (Fig. 2C). In agreement with the data obtained for the IL-6R, the addition of both Dyngo-4a and PitStop2 significantly prevented gp130 internalization (Fig. 2C), and no influence of IL-6 on this process was observable (Fig. 2D and S1, D–F). These data show that both the IL-6R and gp130 are constitutively internalized from the plasma membrane in a dynamin- and clathrin-dependent manner, which is in agreement with a recent report (23).
ADAM17-mediated proteolysis of the IL-6R requires clathrin and dynamin
Having shown that the internalization of the IL-6R requires clathrin and dynamin, we sought to determine whether IL-6R proteolysis by ADAM17 is also dependent on these proteins. Activation of ADAM17-mediated proteolysis by the phorbol ester PMA resulted in significantly increased sIL-6R levels compared with the DMSO control (Fig. 3A). Indeed, preincubation of the cells with Pit-Stop2 was able to abolish PMA-induced sIL-6R generation (Fig. 3A). A similar experiment using the dynamin inhibitor Dyngo-4a showed strong and significant reduction of sIL-6R generation compared with PMA treatment alone and no PMA-induced induction when Dyngo-4a is used (Fig. 3B). Furthermore, treatment with Dyngo-4a either alone or in combination with PMA reduced sIL-6R below the unstimulated control. Because this constitutive cleavage of the IL-6R is mediated by ADAM10 (24, 25), these results suggest that inhibition of dynamin also interferes with IL-6R cleavage by ADAM10. In conclusion, our results show that clathrin and dynamin are not only crucial for endocytosis of the IL-6R, but also required for IL-6R proteolysis by ADAM17.
Figure 3.
ADAM17-mediated proteolysis of the IL-6R requires clathrin and dynamin.A and B, 1 x 106 THP-1 were seeded in 1 ml serum-free RPMI in 24-well plates. Subsequently, the cells were incubated with either (A) 25 μM Pit-Stop2 or (B) 50 μM Dyngo-4a or DMSO as control for 30 min. Afterward, the cells were stimulated with 100 nM PMA or DMSO as control for additional 120 min. The amount of sIL-6R in the cell supernatants was quantified via ELISA. Shown is the mean ± SD from three independent experiments. Statistically significant differences were analyzed using one-way ANOVA and Dunnett's multiple comparisons test. ∗: p < 0.05. DMSO, dimethyl sulfoxide.
Gp130 is not essential, but increases IL-6R internalization
The IL-6R itself contains internalization motifs in its intracellular domain that have been shown to be crucial for correct sorting of the receptor (12). Nevertheless, internalization from the plasma membrane of an IL-6R that lacks its ICD occurs in the presence of gp130 (11). This raises the question whether the IL-6R can also be internalized independently of gp130 and if this depends on the presence of its ligand IL-6.
In order to analyze the influence of gp130 on IL-6R internalization, we retrovirally transduced Ba/F3 cells with the IL-6R alone, with the IL-6R in combination with gp130, or with an IL-6R variant whose ICD was replaced with the ICD of gp130 (Fig. 4A). These cells were subjected to an internalization assay in the presence or absence of IL-6. We observed constitutive internalization of the IL-6R when gp130 was coexpressed (Ba/F3-gp130-IL-6R cells), and consistent with our data from THP-1 cells with endogenous IL-6R, IL-6 had no significant influence on IL-6R internalization (Fig. 4B). In cells lacking gp130 (Ba/F3-IL-6R), the IL-6R was also internalized constitutively irrespective of the ligand, but the IL-6R internalization appeared slower compared with the Ba/F3-gp130-IL-6R cells (Fig. 4C). In contrast, the chimeric IL-6R variant (Ba/F3-IL-6 R/gp130-ICD) closely resembled the internalization kinetics of the Ba/F3-gp130-IL-6R, but not of the Ba/F3-IL-6R cells (Fig. 4D). We concluded from these data that the IL-6R can be internalized without the presence of gp130, but that the ICD of gp130 accelerates the turnover of the IL-6R.
Figure 4.
Internalization of IL-6R depends on gp130-ICD.A, schematic depiction of Ba/F3 cells, which express endogenously neither IL-6R nor gp130, which were stably transduced with the IL-6R and gp130, the IL-6R alone, or a genetic fusion construct consisting of the extracellular and transmembrane domain (ECD/TM) of the IL-6R and the intracellular domain (ICD) of gp130. B–D, An internalization assay was performed as described in the legend to Figure 2. Briefly, the individual cell lines were cultured in the presence or absence of IL-6, and IL-6R was labeled with a specific antibody. Internalization was performed at 37 °C for the indicated time points, and a secondary antibody was used for staining of remaining surface receptor before cells were analyzed by flow cytometry. E, schematic depiction of Ba/F3-gp130 cells, which were stably transduced with IL-6RΔICD, IL-6R-Y/A, or IL-6R-LI/AA. F–H, Internalization assays of the three Ba/F3-gp130 cell lines were performed as described for panel (B). Data shown are the mean ± SD from three independent experiments.
To further decipher the role of IL-6R inherent internalization motifs, we either removed the ICD of the IL-6R (termed IL-6RΔICD) or mutated the tyrosine residue at position 408 (termed IL-6R-Y/A) or the leucine/isoleucine residues at positions 427/428 (termed IL-6R-LI/AA) and generated stably transduced Ba/F3-gp130 cell lines (Fig. 4E). Afterwards, we performed a similar internalization assay with these three cell lines. As shown in Figure 4, F–H, addition of IL-6 had no influence on IL-6R internalization in all three cell lines. Interestingly, however, IL-6R internalization was decreased in all cell lines, suggesting a regulatory role for the two motifs and the intracellular region as a whole (Fig. 4, F–H). Because all generated cell lines are derived from individual clones, a more detailed comparison of the cell lines is not permissible.
Collectively, the data shown argue against ligand-induced internalization of IL-6 R/gp130 and favor constitutive internalization of both receptors. Furthermore, the IL-6R can be internalized in the absence of gp130, although gp130 and especially its ICD enhance IL-6R internalization.
Internalization of the IL-6R is a prerequisite for STAT3 activation
As we could show that the inhibition of clathrin and dynamin prevented IL-6R internalization, we next sought to analyze the consequences on IL-6 signal transduction. Therefore, we analyzed STAT3 phosphorylation by Western blot in THP-1 cells that were stimulated for different time periods with IL-6 and pretreated with Dyngo-4a (blocking dynamin), Pit-Stop2 (blocking clathrin), or DMSO as a control (Fig. 5, A and B). In cells pretreated with DMSO only, IL-6 stimulation induced a strong phosphorylation of STAT3 after 15 min that was almost abrogated after 60 min. In contrast, when cells were pretreated with Dyngo-4a, STAT3 phosphorylation after IL-6 stimulation was detectable, but strongly reduced compared with the control (Fig. 5A). The treatment with Pit-Stop2 before IL-6 stimulation prevented any subsequent phosphorylation of STAT3 within the time period of 120 min (Fig. 5B). However, both inhibitors slightly reduced total STAT3 levels, which might contribute to the reduction in STAT3 phosphorylation. In a further approach we transfected HeLa cells with the C-terminal part of AP180, which is a dominant-negative form of this clathrin-binding protein (DN-AP180). This variant still binds to clathrin but leads to its mislocalization and thereby impedes the formation of clathrin-coated pits (26). In line with our previous results, overexpression of DN-AP180 led to a strongly reduced STAT3 activation upon IL-6 stimulation in comparison with GFP-transfected cells (Fig. 5C).
Figure 5.
STAT3 activation depends on internalization of IL-6R and gp130.A and B, THP-1 cells were starved in serum-free medium for 4 h. 30 min before stimulation cells were pretreated with 50 μM Dyngo-4a or 25 μM Pit-Stop2 or DMSO as a control. In total, 10 ng/ml IL-6 was added to the culture medium for the indicated times and cells were subsequently lysed in lysis buffer. Phosphorylated STAT3, total STAT3, and GAPDH were analyzed by western blot. C, HeLa cells were transiently transfected with expression plasmids encoding either DN-AP180_BFP2 or GFP, and the experiment was performed as described in (A). D–F, the HeLa cells were transiently transfected with expression plasmids expressing GFP or Endo-A1, Endo-A2, or Endo-A3. The experiment was performed as described in (A). One experiment out of three with similar outcome is shown. DMSO, dimethyl sulfoxide.
In addition to clathrin-mediated endocytosis, endophilin-mediated endocytosis, which is completely independent of clathrin, has recently been described (27, 28). Hence, we investigated whether this pathway had any influence on IL-6-mediated signaling. Therefore, we overexpressed the BAR domains of endophilin A1, A2, and A3 lacking their N-terminal amphipathic helix, which proved to be efficient inhibitors for the endophilin-dependent internalization (27). We expressed these constructs transiently in HeLa cells, stimulated them with IL-6, and analyzed STAT3 phosphorylation via Western blot (Fig. 5, D–F). We could not detect a major difference in STAT3 activation in these cells compared with GFP-expressing cells, although STAT3 phosphorylation appeared slightly enhanced when one of the endophilin constructs was overexpressed. However, our results exclude a major role for endophilin-mediated endocytosis in IL-6 signaling. Thus, we concluded that internalization of the IL-6 R/gp130 complex is necessary for STAT3 activation, but that this is not dependent on endophilin, but on clathrin- and dynamin-dependent mechanisms.
The IL-6R is constitutively targeted to the lysosome following internalization
Following internalization, receptors are directed from the early endosomes to lysosomes for degradation or to recycling endosomes to be redirected to the plasma membrane. In order to analyze the intracellular fate of the IL-6R and gp130, we did a pulse-chase experiment to analyze a possible targeting of both receptors to lysosomes following internalization. For this purpose, we labeled surface IL-6R on overexpressing HeLa cells with an antibody at 4 °C and shifted the cells afterward to 37 °C in the presence or absence of IL-6 for different periods of time. After fixation and permeabilization, localization of the receptor to lysosomes was detected by adding a fluorescently labeled secondary antibody and analyzing colocalization with the lysosome-associated marker protein 2 (LAMP-2). We could clearly detect colocalization of the internalized IL-6R with LAMP-2, indicating targeting of the receptor to lysosomes after endocytosis (Fig. 6A). Interestingly, the amount of lysosomal IL-6R increased over time, but was not affected by the presence of IL-6 (Fig. 6B). Thus, ligand binding did not induce lysosomal degradation, indicating that the IL-6R was constitutively directed to lysosomes.
Figure 6.
Internalized IL-6R and gp130 are targeted to the lysosome independent of IL-6.A, localization of the IL-6R to the lysosome was analyzed in IL-6R-transfected HeLa cells, which were seeded onto coverslips and labeled with a primary IL-6R-specific antibody for 1 h at 4 °C. Following a wash step, the cells were stained with a secondary AlexaFlour488-labeled anti-goat antibody for 1 h at 4 °C. Cells were washed and incubated for 15, 30, or 60 min at 37 °C in the presence or absence of 10 ng/ml IL-6. Subsequently, cells were fixed with 4% PFA, permeabilized with 0.2% saponin, and finally stained with a primary LAMP-2-specific antibody and a secondary AlexaFlour594 anti-mouse antibody. At last, cells were mounted onto microscopy slides and analyzed using the Olympus FV1000 confocal laser scanning microscope. Scale bar: 50 μm. B, colocalization of the IL-6R with LAMP-2 was quantified using ImageJ and the JACoP plugin calculating Mander’s coefficient. C and D, Colocalization of gp130 with the lysosomal marker LAMP-2 at different time points was analyzed as described for the IL-6R before. Mander’s coefficient was calculated from 20 cells out of three independent experiments, and statistical significant differences were determined using unpaired t-tests and the two-stage linear step-up procedure of Benjamini, Krieger, and Yekutieli. ns: not significant; ∗: p < 0.05.
In addition, we also analyzed lysosomal targeting of gp130 following stimulation with IL-6 (Fig. 6C). As for the IL-6R, colocalization of gp130 with LAMP-2 increased over time (Fig. 6D). However, the process was accelerated when the cells were stimulated with IL-6, and significantly more gp130 colocalized with LAMP-2. There was an increase of gp130/LAMP-2 colocalization already 15 min after addition of cytokine. These results suggest that the IL-6/IL-6 R/gp130 complex is disassembled following endocytosis, and the two receptor subunits are differentially targeted to lysosomal degradation.
The IL-6R is recycled back to the cell surface in an IL-6-dependent manner
Having analyzed the trafficking of the IL-6 signaling complex to lysosomes, we next wanted to examine whether IL-6R and gp130 were recycled back to the cell surface. For this purpose, we adapted a procedure from the Kveiborg lab (29) and stained IL-6R-transfected HeLa cells with a specific antibody against the IL-6R at 37 °C for 1 h to allow internalization of the IL-6R–antibody complexes. The remaining antibody bound to the cell surface was then removed by stripping with an acidic buffer before AlexaFlour488-conjugated secondary antibody was applied for 1 h at 37 °C to stain recycled receptor molecules that reappeared on the surface of the cells. As negative control, only secondary but not primary antibody was applied. To confirm the performance of the stripping buffer (strip control), cells were stained with secondary antibody after stripping at 4 °C to impede recycling. When cells were not stimulated with IL-6, only few cells showed fluorescent staining at the cell-surface (Fig. 7B, upper panels). In contrast, stimulation of the cells with IL-6 increased IL-6R recycling as indicated by an increase of AlexaFlour488-positive cells compared with unstimulated cells (Fig. 7A, lower panels), and quantification revealed a statistically significant increase in IL-6R recycling when cells were stimulated with IL-6 (Fig. 7B). Importantly, due to the absence of signal at the cell surface in the strip control cells, we ensured that IL-6R detected at the cell surface indeed underwent internalization and recycling.
Figure 7.
The IL-6R is recycled to the cell surface in an IL-6-dependent manner.A, HeLa cells were transfected with pcDNA3.1-IL-6R and seeded onto coverslips. Surface IL-6R was stained with a specific antibody for 1 h at 37 °C in the presence (lower panels) or absence (upper panel) of 10 ng/ml IL-6. Controls were stained at 4 °C except negative control, where no primary antibody was added. After washing, noninternalized antibody was stripped with an acidic buffer (except surface staining control) and cells were washed again. For recycling, cells were incubated again 1 h at 37 °C adding AlexaFlour488-conjugated anti-mouse IgG antibody. Finally, cells were washed, fixed in 4% PFA for 10 min at room temperature, washed again, and mounted onto microscopy slides. Analysis was performed using the Olympus FV1000 confocal laser scanning microscope. Scale bar: 50 μm. B, percentage of fluorescently labeled cells shown in (B) was quantified with ImageJ and 11 pictures taken from three individual experiments were analyzed. Data were analyzed by Student’s t-test. C, localization of the IL-6R to recycling endosomes was analyzed in IL-6R-transfected HeLa cells, which were seeded onto coverslips and labeled with a primary IL-6R-specific antibody for 1 h at 4 °C. Following a wash step, cells were stained with a secondary AlexaFlour488-labeled anti-goat antibody for 1 h at 4 °C. Cells were washed and incubated for 15, 30, or 60 min at 37 °C in the presence or absence of 10 ng/ml IL-6. Subsequently, cells were fixed with 4% PFA, permeabilized with 0.2% saponin, and finally stained with a primary Rab11-specific antibody and a secondary AlexaFlour594 anti-mouse antibody (Rab11). Lastly, cells were mounted onto microscopy slides and analyzed using the Olympus FV1000 confocal laser scanning microscope. Scale bar: 50 μm. D, colocalization of the IL-6R with Rab11 at different time points was quantified using ImageJ and the JACoP plugin. Mander's coefficient of 20 cells was calculated, and statistical significant differences were determined using unpaired t-tests and the two-stage linear step-up procedure of Benjamini, Krieger, and Yekutieli. ns: not significant; ∗: p < 0.05.
These results suggest that recycling of the IL-6R, in contrast to internalization, was increased in the presence of the ligand. In order to further verify this observation, we did immunofluorescence stainings in HeLa cells of the IL-6R and Rab11, a marker for recycling endosomes (30). In accordance with our previous results, stimulation of the cells with IL-6 led to a statistically significant increase of colocalization of both proteins at 15, 30, and 60 min (Fig. 7, C and D).
IL-6 increases gp130 recycling to the cell surface
In analogy to the IL-6R, gp130 has previously been shown to be ligand-independently and constitutively internalized (14). Therefore, we tried to answer the question whether gp130 also succumbs to ligand-induced recycling and performed a recycling assay similar to the one described above with HeLa cells overexpressing gp130 (Fig. 8A). As for the IL-6R, IL-6 treatment increased the number of fluorescently labeled cells, indicating ligand-induced recycling of gp130 (Fig. 8A, lower panels). As seen for the IL-6R, the percentage of cells with recycled receptor was about twice as high in the presence of IL-6 compared with unstimulated cells (Fig. 8B). This was further corroborated by an increase in colocalization with Rab11 when cells were stimulated with IL-6, which was statistically significant at all time points analyzed (Fig. 8, C and D). Thus, like the IL-6R, gp130 can also be recycled back to the cell surface, and this process is also increased in the presence of IL-6.
Figure 8.
gp130 is recycled to the cell surface in an IL-6-dependent manner.A, p409-gp130-transfected HeLa cells were seeded onto cover slips. A gp130-specific antibody was added to the culture for 1 h at 37 °C in the presence (lower panels) or absence (upper panels) of 10 ng/ml IL-6 to enable internalization of the receptor and bound antibody. Cells were washed, and noninternalized antibody was stripped off applying an acidic buffer. Afterward, cells were incubated for 1 h at 37 °C with an AlexaFour488-coupled secondary antibody staining receptor molecules that were recycled back to the cell surface. Negative control was performed without primary antibody, for surface staining, the antibodies were applied at 4 °C, and for strip control secondary antibody was applied at 4 °C. Scale bar: 50 μm. B, Percentage of fluorescently labeled cells shown in (A) was analyzed with ImageJ. Eleven pictures taken from three individual experiments were analyzed. Data were analyzed by Student’s t-test. C, HeLa cells were transfected with p409-gp130 and seeded onto cover slips. A gp130-specific primary antibody was used to stain receptor molecules on the surface, which were allowed to internalize for 15, 30, or 60 min at 37 °C in the presence or absence of IL-6. Afterward, cells were fixed with 4% PFA, permeabilized with 0.2% saponin, and stained with a specific Rab11 antibody and secondary antibodies that were AlexaFlour488- (gp130) or AlexaFlour594-coupled (Rab11). Finally, cells were mounted onto microscopy slides and analyzed using the Olympus FV1000 confocal laser scanning microscope. Scale bar: 50 μm. D, Colocalization of gp130 and Rab11 was quantified using ImageJ and the JACoP plugin. Mander's coefficient of 20 cells from three individual experiments was calculated, and statistical significant differences were determined using unpaired t-tests and the two-stage linear step-up procedure of Benjamini, Krieger, and Yekutieli. ns: not significant; ∗: p < 0.05.
Discussion
IL-6 is a pleiotropic cytokine involved in regenerative and pathophysiological processes. Its signal transduction occurs via gp130 and the IL-6R in its membrane-bound or soluble form. The regulation of cell surface levels and release of the soluble form of the IL-6R have to be tightly controlled, which is executed by internalization and proteolytic ectodomain release (31).
We could verify that proteolysis is a strong regulator of IL-6R surface levels. Shedding and subsequent ectodomain release could be induced by PMA as expected, confirming our own and other previous results (20, 21, 32). Still, when shedding was blocked by the broad-spectrum metalloproteinase inhibitor marimastat, labeled IL-6R disappeared from the cell surface over time, and this could be blocked by small chemical clathrin and dynamin inhibitors. This pointed to clathrin-mediated endocytosis as the main mechanism of endocytosis of the IL-6R and is in line with previous data (14, 33). There might also be a vast interplay between both surface-level regulating processes.
Mechanisms that regulate shedding by ADAM proteases are still under investigation, but a strong induction of ectodomain release is able to drastically reduce the availability of the receptor on the surface. It is plausible to assume that both mechanisms, proteolysis and internalization, act in concert to regulate the amount of transmembrane proteins on the cell surface. Indeed, this has been described for the vascular endothelial growth factor receptor 2, which also undergoes constitutive endocytosis. Endocytosis was proposed in this context to act as a protective mechanism against shedding (34). Consequently, mechanisms that reduce internalization would also interfere with shedding and subsequently could elevate serum levels of sIL-6R. However, such mechanism will certainly also affect the activity of membrane-bound proteases and not only influence their substrates.
Opposing results have been published on whether the internalization of the IL-6R was rather ligand-induced or constitutive. Zohlnhöfer et al. and Dittrich et al. reported that binding sites for IL-6 on the cell surface were reduced after application of the cytokine (35, 36). In contrast, our results point to a constitutive ligand-independent internalization of the receptor. This has also been proposed by Gerhartz et al., who analyzed the half-life of the IL-6R in cycloheximide-treated Madin-Darby canine kidney and HepG2 cells and found it to be unaffected by the presence of IL-6, and by Fujimoto et al., who obtained the same result for GFP-tagged IL-6R in HeLa cells (33, 37). In the latter study, analysis of the intracellular fate of tocilizumab (TCZ), a therapeutic monoclonal antibody against the IL-6R, failed to prove any significant direction of the IL-6R to recycling endosomes under TCZ treatment. As TCZ blocks IL-6 binding to the IL-6R, this is consistent with our results that recycling is induced by IL-6 and occurs only to a small extent in the absence of the cytokine. Our finding that IL-6 induces recycling of the IL-6R may also explain why IL-6 has not previously been demonstrated to induce increased degradation of the IL-6R although the receptor is internalized (36). However, the molecular mechanism of how IL-6 induces IL-6R recycling in detail warrants further investigation.
Further controversy exists on whether internalization motifs of the IL-6R are responsible and sufficient for IL-6R internalization or if rather the ICD of gp130 is necessary for the internalization of the IL-6R. Cells expressing a truncated version of the IL-6R that consists only of the extracellular and transmembrane domain are able to internalize IL-6R, whereas mutation of gp130 internalization motifs reduced IL-6R endocytosis (36, 38). Our data point toward a constitutive internalization that requires neither IL-6 nor gp130. Consequently, the internalization of the IL-6R has to be mediated by its inherent internalization motifs when no gp130 is present. Although this situation might seem artificial, granulocytes have recently been identified as the first cell type that expresses IL-6R but no gp130 (39). The different kinetics of the colocalization with Rab11 of the IL-6R and gp130 under IL-6 stimulation in the present study might also be a hint that these receptor subunits, after signal transduction from early endosomes, do not undergo trafficking in complex but rather independently.
Experimental procedures
Cells and reagents
HEK293 cells, THP-1 cells, Phoenix cells, and HeLa cells were cultured in Dulbecco's modified Eagle's medium (DMEM) high-glucose culture medium (Gibco/Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal calf serum (FCS), penicillin (60 mg/l), and streptomycin (100 mg/l). Ba/F3-derived cell lines were also cultured in DMEM high-glucose culture medium (Gibco/Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% FCS, penicillin (60 mg/l), and streptomycin (100 mg/l) and additional 10 ng/ml Interleukin-6 (IL-6), 10 ng/ml Hyper-IL-6 or 10 ng/ml Interleukin-3, depending on the receptor composition of the individual cell line. All cells were kept at 37˚C and 5% CO2 in a standard incubator with a water-saturated atmosphere.
The broad-spectrum matrix-metalloprotease inhibitor marimastat was purchased from Sigma-Aldrich, St Louis, MO, USA, as well as the protein kinase C activator PMA. The inhibitors Pit-Stop2 (clathrin inhibitor) and Dyngo-4a (dynamin inhibitor) were purchased from abcam (Cambridge, United Kingdom).
The following antibodies were used: 4–11 (IL-6R-specific antibody) was expressed and purified in-house. pSTAT3, STAT3, and GAPDH-specific antibodies were purchased from Cell Signaling Technology (Frankfurt/M., Germany), B-P4 (gp130-specific antibody) and the Rab11-specific antibody were obtained from abcam (Cambridge, United Kingdom), H4B4 (LAMP-2-specific antibody from mouse) was purchased from Developmental Studies Hybridoma Bank (Iowa City, IA, USA), the LAMP-2-specific antibody derived from rabbit was obtained from Thermo Scientific (Waltham, MA, USA), and the IL-6R-specific antibody BAF227 was purchased from R&D systems (Minneapolis, MN, USA). The secondary antibodies anti-mouse-IgG-APC, anti-mouse-IgG-HRP, and anti-rabbit-IgG-HRP were obtained from Dianova (Hamburg, Germany). The fluorescently labeled antibodies anti-mouse-IgG-AlexaFlour488, anti-goat-IgG-AlexaFlour488, anti-mouse-IgG-AlexaFlour594, and anti-rabbit-IgG-AlexaFlour594 were obtained from life technologies at Thermo Scientific (Waltham, MA, USA).
IL-6R and gp130 internalization assay
The internalization assay using different Ba/F3 cell lines was performed as previously described (20). For the analysis of IL-6R internalization, the cells were stained with primary antibody against IL-6R (4, 5, 6, 7, 8, 9, 10, 11) and APC-conjugated anti-mouse-IgG antibody. For gp130 internalization, the cells were stained with primary antibody B-P4 (abcam, Cambridge, United Kingdom) against gp130 and APC-conjugated anti-mouse-IgG antibody. The staining on THP-1 cells was performed in the same way, but these cells were not starved before staining. Where indicated, marimastat was added to prevent proteolytic cleavage of the IL-6R. One milliliter of the cell suspension was transferred into 1.5 ml tubes, centrifuged at 1.200 g for 10 min at 4 °C, and the supernatants were transferred into a new tube for clearance via centrifugation at 18.000 g for 20 min at 4 °C. Following, the supernatants were stored at –20 °C until further analysis.
For the analysis of dynamin or clathrin involvement in internalization, the cells were incubated with the inhibitors Pit-Stop2 (clathrin inhibitor) or Dyngo-4a (dynamin inhibitor) for 40 min at 37 °C before staining with primary antibody. The inhibitors were also added during the time course of internalization.
IL-6R proteolysis
THP-1 cells were washed with phosphate buffered saline (PBS) twice and 1 x 106 cells per inhibitor, and controls were seeded in 1 ml serum-free RPMI in 24-well plates. Subsequently, the cells were incubated with either 25 μM Pit-Stop2 (abcam, Cambridge, UK) or 50 μM Dyngo-4a (abcam, Cambridge, UK) or DMSO as control for 30 min at 37 °C and 5% CO2. Afterward, the cells were stimulated with 100 nM PMA (Sigma-Aldrich, St Louis, USA) or DMSO as control and incubated at 37 °C and 5% CO2 for additional 120 min. The cells were then transferred into 1.5 ml tubes and centrifuged at 1.200 g for 10 min at 4 °C. The supernatants were transferred into a new tube, cleared via centrifugation at 18.000 g for 20 min at 4 °C, and stored at –20 °C until further analysis.
ELISA
The amount of sIL-6R in the supernatant was detected using the DuoSet Human IL-6Rα ELISA kit (R&D systems, Minneapolis, USA). The ELISA was performed according to the supplier’s instructions, except for the adaption of the used volumes. Fifty microliters of capture antibody, detection antibody, and undiluted samples were used as well as 60 μl of substrate solution and stop solution. Streptavidin–horseradish peroxidase (R&D Systems, Minneapolis, USA) and peroxidase substrate BM blue POD (Roche, Basel, Switzerland) were used for the enzymatic reaction, which was stopped by addition of 2 N H2SO4. The absorbance was measured at 450 nm on FLUOstar Omega (BMG labtech, Offenburg, Germany).
Construction of expression plasmids
For the generation of the IL-6 R/gp130-ICD chimera and for the insertion of point mutations into the IL-6R internalization motifs, splicing by overlapping extension (SOE)-PCR was performed using the expression plasmids pcDNA3.1-IL-6R and p409-gp130 as templates. The IL-6R constructs were subcloned into the pMOWS-puro vector to allow retroviral transduction of Ba/F3 cells.
Retroviral transduction of Ba/F3 and Ba/F3-gp130 cells
Retroviral transduction of Ba/F3 and Ba/F3-gp130 cells via Phoenix-Eco cell supernatant was performed as described previously (40, 41, 42). Cells stably expressing different IL-6R constructs were selected with puromycin (1.5 μg/ml) and cultivated with 10 ng/ml recombinant IL-6 or IL-3.
Activation of signaling pathways and analysis via western blot
In order to analyze the effect of inhibition of internalization pathways on STAT3 signaling, 2 x 106 THP-1 cells per time point were starved in 6-well plates in 2 ml serum-free DMEM for 4 h at 37 °C before they were incubated with the internalization inhibitors Pit-Stop2 or Dyngo-4a and as a control DMSO for 40 min at 37 °C. After incubation, 10 ng/ml IL-6 was added, and the cells were harvested at different time points. In a second approach, HeLa cells were transfected with 5 μg plasmid of pN1-eGFP or DN-AP180_BFP2 or one of the endophilin constructs (Δhelix0-BAR-A1, -A2, or -A3 in pEGFP) (27). The next day, the cells were distributed on six wells of a 6-well plate. Two days after transfection, the cells were starved for 3 h at 37 °C in 2 ml serum-free DMEM before 10 ng/ml of IL-6 was added, and the cells were harvested at different time points.
The cells were washed in PBS and finally lysed in 80 μl lysis buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 2 mM EDTA, 1 mM NaF, 1 mM Na3VO4, 1% IGEPAL (NP-40), 1% Triton-X-100, complete protease inhibitor cocktail (Roche Applied Science, Mannheim, Germany)).
For analysis via western blot, 30 μg total protein of each sample was loaded onto a 10% SDS gel, run for 2 h at 110 V, and transferred onto a PVDF membrane (Merck Millipore, Darmstadt, Germany) via semidry blot using the Trans-Blot-Turbo (Bio-Rad, Hercules, CA, USA) at 1 A and constant 25 V for 40 min. The membrane was blocked in 5% milk powder in TBS-T for 1 h at room temperature, washed in TBS-T, and subsequently incubated with antibody against phosphorylated STAT3 or GAPDH as loading control over night at 4 °C. The following day, the membrane was washed in TBS-T and incubated with HRP-conjugated secondary antibody for 1 h at room temperature. The membrane was washed again in TBS-T, and proteins were detected with the ECL Chemocam Imager (Intas Science Imaging, Göttingen, Germany) using the EMD Millipore Immobilon Western Chemiluminescent HRP Substrate (Merck Millipore, Darmstadt, Germany). For the detection of total STAT3 levels, the membrane was stripped for 40 min at room temperature using the Restore Western Blot Stripping Buffer (Thermo Fisher Scientific, Waltham, MA, USA). The membrane was washed with TBS-T and incubated in 5% milk powder in TBS-T for 1 h at room temperature. The membrane was then washed again in TBS-T and incubated with primary antibody against total STAT3 over night at 4 °C. Incubation and detection were performed as described above.
IL-6R and gp130 recycling assay using immunofluorescence staining
This experiment was conducted in a manner similar to that described by Stautz et al. (29). HeLa cells were transfected either with pcDNA3.1-IL-6R or p409-gp130 plasmid before being seeded onto coverslips. The cells were incubated with 0.12% (w/v) glycine in 1xPBS for 10 min, washed in PBS, and blocked in 10% FCS in PBS for 1 h at room temperature before staining with 4–11 antibody (anti-IL-6R) or B-P4 (anti-gp130) for 1 h at 37 °C in the presence or absence of 10 ng/ml IL-6. After washing the cells with PBS, noninternalized primary antibody was stripped from the cell surface by incubation in serum-free DMEM pH 2.0 for 30 min at 4 °C. Subsequently, the cells were washed again in PBS and were incubated with AlexaFlour488-conjugated anti-mouse secondary antibody for 1 h at 37 °C. Finally, the cells were washed, fixed in 4% PFA in PBS for 10 min at room temperature, washed again in PBS and additionally once in ddH2O, and mounted onto microscopy slides using ProLong Gold Antifade Mountant with DAPI (Thermo Fisher Scientific, Waltham, MA, USA). As controls surface staining and a strip control were additionally analyzed. For these, the cells were incubated with primary antibody for 1h at 4 °C in the presence or absence of 10 ng/ml IL-6. The strip control was then incubated in serum-free DMEM pH 2.0 for 30 min at 4 °C, whereas the surface staining control was incubated in serum-free DMEM pH 7.4 for 30 min at 4 °C. Staining with AlexaFlour488-conjugated anti-mouse secondary antibody was performed for 1 h at 4 °C. The negative control was treated as the sample staining but was excluded from staining with primary antibody. Recycling was analyzed using the Olympus FV1000 confocal laser scanning microscope and ImageJ (NIH, Bethesda, MD, USA).
Colocalization of IL-6R or gp130 with lysosome (LAMP-2) and recycling endosome (Rab11)
For the colocalization experiment of IL-6R or gp130, HeLa cells were transfected with either pcDNA3.1-IL-6R or p409-gp130wt and seeded onto coverslips the next day. Two days after transfection cells were washed in PBS, incubated for 10 min at room temperature in 0.12 % (w/v) glycine in 1xPBS, washed again, and blocked in 10% FCS in PBS for 30 min at room temperature. Surface IL-6R or gp130 was stained with BAF227 or B-P4 antibody for 1 h at 4 °C, respectively. To remove unbound antibody, the cells were washed three times in PBS before the cells were incubated with fluorescently labeled anti-goat-IgG-AlexaFlour488 (IL-6R) or anti-mouse-IgG-AlexaFlour488 (gp130) antibody for 1 h at 4 °C. To allow internalization of the receptors, the cells were incubated in serum-free DMEM for 15, 30, or 60 min at 37 °C in the presence or absence of 10 ng/ml IL-6. The cells were washed in PBS, fixed in 4% PFA/PBS for 10 min at room temperature, washed again, incubated in 0.12 % (w/v) glycine in 1x PBS for 10 min at room temperature, and blocked in 10% FCS and 0.2% saponin in PBS for 30 min at room temperature. Subsequently, the cells were incubated with antibody directed against the lysosomal marker LAMP-2 or against the recycling endosome marker Rab11 for 1 h at room temperature. After three washing steps in 10% FCS, 0.2% Saponin in PBS, the cells were incubated with fluorescently labeled antibodies anti-mouse-IgG-AlexaFlour594 or anti-rabbit-IgG-AlexaFlour594 for 1 h at room temperature. Finally, the cells were washed in 10% FCS, 0.2% saponin in PBS, and once in ddH2O and mounted onto microscopy slides using ProLong Gold Antifade Mountant with DAPI (Thermo Fisher Scientific, Waltham, MA, USA). Images were taken using the Olympus FV1000 confocal laser scanning microscope, and colocalization was analyzed using ImageJ (NIH, Bethesda, MD, USA).
Statistical analyses
Statistical analyses were carried out with GraphPad Prism 8 (GraphPad Software, San Diego, CA, USA). The used methods are described in the legends of the respective figures.
Data availability
All data are contained within the article.
Supporting information
This article contains supporting information.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
The authors thank Alyn Gerneth and Christian Bretscher for excellent technical assistance. This work was funded by grants from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—Projektnummer 125440785—SFB 877 (project A14) and by the Bundesministerium für Bildung und Forschung (BMBF grant “InTraSig”, project B). The expression plasmid encoding DN-AP180_BFP2 was kindly provided by Harvey McMahoon (MRC Laboratory of Molecular Biology, Cambridge), and the expression plasmids encoding the different endophilin constructs were kindly provided by Emmanuel Boucrot (MRC Laboratory of Molecular Biology, Cambridge).
Author contributions
C. M. F., B. K., and T. D. performed the experiments and analyzed the results. Y. G. helped with and advised the statistical analyses. S. D. and S. R.-J. provided critical reagents and analyzed the results. J. H. and J. L. analyzed the results. C. G. and S. A. S. jointly conceived and coordinated the study and wrote the paper. All the authors approved the final version of the article.
Edited by Peter Cresswell
Supporting information
References
- 1.Hunter C.A., Jones S.A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 2015;16:448–457. doi: 10.1038/ni.3153. [DOI] [PubMed] [Google Scholar]
- 2.Jones S.A., Scheller J., Rose-John S. Therapeutic strategies for the clinical blockade of IL-6/gp130 signaling. J. Clin. Invest. 2011;121:3375–3383. doi: 10.1172/JCI57158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garbers C., Heink S., Korn T., Rose-John S. Interleukin-6: Designing specific therapeutics for a complex cytokine. Nat. Rev. Drug Discov. 2018;17:395–412. doi: 10.1038/nrd.2018.45. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka T., Narazaki M., Kishimoto T. Therapeutic targeting of the interleukin-6 receptor. Annu. Rev. Pharmacol. Toxicol. 2012;52:199–219. doi: 10.1146/annurev-pharmtox-010611-134715. [DOI] [PubMed] [Google Scholar]
- 5.Lütticken C., Wegenka U.M., Yuan J., Buschmann J., Schindler C., Ziemiecki A., Harpur A.G., Wilks A.F., Yasukawa K., Taga T., Kishimoto T., Barbieri G., Pellegrini S., Sendtner M., Heinrich P.C. Association of transcription factor APRF and protein kinase Jak1 with the interleukin-6 signal transducer gp130. Science (New York, NY) 1994;263:89–92. doi: 10.1126/science.8272872. [DOI] [PubMed] [Google Scholar]
- 6.Stahl N., Boulton T.G., Farruggella T., Ip N.Y., Davis S., Witthuhn B.A., Quelle F.W., Silvennoinen O., Barbieri G., Pellegrini S., Ihle J.N., Yancopoulos G.D. Association and activation of Jak-Tyk kinases by CNTF-LIF-OSM-IL-6 beta receptor components. Science (New York, NY) 1994;263:92–95. doi: 10.1126/science.8272873. [DOI] [PubMed] [Google Scholar]
- 7.Thiel S., Sommer U., Kortylewski M., Haan C., Behrmann I., Heinrich P.C., Graeve L. Termination of IL-6-induced STAT activation is independent of receptor internalization but requires de novo protein synthesis. FEBS Lett. 2000;470:15–19. doi: 10.1016/s0014-5793(00)01276-x. [DOI] [PubMed] [Google Scholar]
- 8.Le Roy C., Wrana J.L. Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nat. Rev. Mol. Cell Biol. 2005;6:112–126. doi: 10.1038/nrm1571. [DOI] [PubMed] [Google Scholar]
- 9.Cendrowski J., Maminska A., Miaczynska M. Endocytic regulation of cytokine receptor signaling. Cytokine Growth Factor Rev. 2016;32:63–73. doi: 10.1016/j.cytogfr.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Hermanns H.M., Wohlfahrt J., Mais C., Hergovits S., Jahn D., Geier A. Endocytosis of pro-inflammatory cytokine receptors and its relevance for signal transduction. Biol. Chem. 2016;397:695–708. doi: 10.1515/hsz-2015-0277. [DOI] [PubMed] [Google Scholar]
- 11.Dittrich E., Rose-John S., Gerhartz C., Mullberg J., Stoyan T., Yasukawa K., Heinrich P.C., Graeve L. Identification of a region within the cytoplasmic domain of the interleukin-6 (IL-6) signal transducer gp130 important for ligand-induced endocytosis of the IL-6 receptor. J. Biol. Chem. 1994;269:19014–19020. [PubMed] [Google Scholar]
- 12.Martens A.S., Bode J.G., Heinrich P.C., Graeve L. The cytoplasmic domain of the interleukin-6 receptor gp80 mediates its basolateral sorting in polarized madin-darby canine kidney cells. J. Cell Sci. 2000;113(Pt 20):3593–3602. doi: 10.1242/jcs.113.20.3593. [DOI] [PubMed] [Google Scholar]
- 13.Doumanov J.A., Daubrawa M., Unden H., Graeve L. Identification of a basolateral sorting signal within the cytoplasmic domain of the interleukin-6 signal transducer gp130. Cell Signal. 2006;18:1140–1146. doi: 10.1016/j.cellsig.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 14.Thiel S., Dahmen H., Martens A., Müller-Newen G. Constitutive internalization and association with adaptor protein-2 of the interleukin-6 signal transducer gp130. FEBS Lett. 1998;441:231–234. doi: 10.1016/s0014-5793(98)01559-2. [DOI] [PubMed] [Google Scholar]
- 15.Rebouissou S., Amessou M., Couchy G., Poussin K., Imbeaud S., Pilati C., Izard T., Balabaud C., Bioulac-Sage P., Zucman-Rossi J. Frequent in-frame somatic deletions activate gp130 in inflammatory hepatocellular tumours. Nature. 2009;457:200–204. doi: 10.1038/nature07475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sommer J., Effenberger T., Volpi E., Waetzig G., Bernhardt M., Suthaus J., Garbers C., Rose-John S., Floss D., Scheller J. Constitutively active mutant gp130 receptor protein from inflammatory hepatocellular adenoma is inhibited by an anti-gp130 antibody that specifically neutralizes interleukin 11 signaling. J. Biol. Chem. 2012;287:13743–13751. doi: 10.1074/jbc.M111.349167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt-Arras D., Müller M., Stevanovic M., Horn S., Schütt A., Bergmann J., Wilkens R., Lickert A., Rose-John S. Oncogenic deletion mutants of gp130 signal from intracellular compartments. J. Cell Sci. 2013;127:341–353. doi: 10.1242/jcs.130294. [DOI] [PubMed] [Google Scholar]
- 18.Rinis N., Kuster A., Schmitz-Van de Leur H., Mohr A., Muller-Newen G. Intracellular signaling prevents effective blockade of oncogenic gp130 mutants by neutralizing antibodies. Cell Commun. Signal. 2014;12:14. doi: 10.1186/1478-811X-12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolf J., Rose-John S., Garbers C. Interleukin-6 and its receptors: A highly regulated and dynamic system. Cytokine. 2014;70:11–20. doi: 10.1016/j.cyto.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 20.Riethmueller S., Somasundaram P., Ehlers J.C., Hung C.-W.W., Flynn C.M., Lokau J., Agthe M., Düsterhöft S., Zhu Y., Grötzinger J., Lorenzen I., Koudelka T., Yamamoto K., Pickhinke U., Wichert R. Proteolytic Origin of the soluble human IL-6R in Vivo and a decisive role of N-Glycosylation. Plos Biol. 2017;15:e2000080. doi: 10.1371/journal.pbio.2000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Müllberg J., Schooltink H., Stoyan T., Günther M., Graeve L., Buse G., Mackiewicz A., Heinrich P., Rose-John S. The soluble interleukin-6 receptor is generated by shedding. Eur. J. Immunol. 1993;23:473–480. doi: 10.1002/eji.1830230226. [DOI] [PubMed] [Google Scholar]
- 22.Schumacher N., Meyer D., Mauermann A., von der Heyde J., Wolf J., Schwarz J., Knittler K., Murphy G., Michalek M., Garbers C., Bartsch J.W.W., Guo S., Schacher B., Eickholz P., Chalaris A. Shedding of endogenous interleukin-6 receptor (IL-6R) is Governed by A Disintegrin and metalloproteinase (ADAM) proteases while a Full-length IL-6R Isoform Localizes to Circulating Microvesicles. J. Biol. Chem. 2015;290:26059–26071. doi: 10.1074/jbc.M115.649509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monhasery N., Moll J., Cuman C., Franke M., Lamertz L., Nitz R., Gorg B., Häussinger D., Lokau J., Floss D.M., Piekorz R., Dimitriadis E., Garbers C., Scheller J. Transcytosis of IL-11 and apical redirection of gp130 is mediated by IL-11alpha receptor. Cell Rep. 2016;16:1067–1081. doi: 10.1016/j.celrep.2016.06.062. [DOI] [PubMed] [Google Scholar]
- 24.Garbers C., Jänner N., Chalaris A., Moss M.L., Floss D.M., Meyer D., Koch-Nolte F., Rose-John S., Scheller J. Species specificity of ADAM10 and ADAM17 proteins in interleukin-6 (IL-6) trans-signaling and novel role of ADAM10 in inducible IL-6 receptor shedding. J. Biol. Chem. 2011;286:14804–14811. doi: 10.1074/jbc.M111.229393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matthews V., Schuster B., Schutze S., Bussmeyer I., Ludwig A., Hundhausen C., Sadowski T., Saftig P., Hartmann D., Kallen K.J., Rose-John S. Cellular cholesterol depletion triggers shedding of the human interleukin-6 receptor by ADAM10 and ADAM17 (TACE) J. Biol. Chem. 2003;278:38829–38839. doi: 10.1074/jbc.M210584200. [DOI] [PubMed] [Google Scholar]
- 26.Zhao X., Greener T., Al-Hasani H., Cushman S.W., Eisenberg E., Greene L.E. Expression of auxilin or AP180 inhibits endocytosis by mislocalizing clathrin: Evidence for formation of nascent pits containing AP1 or AP2 but not clathrin. J. Cell Sci. 2001;114:353–365. doi: 10.1242/jcs.114.2.353. [DOI] [PubMed] [Google Scholar]
- 27.Boucrot E., Ferreira A.P.A., Almeida-Souza L., Debard S., Vallis Y., Howard G., Bertot L., Sauvonnet N., McMahon H.T. Endophilin marks and controls a clathrin-independent endocytic pathway. Nature. 2015;517:460–465. doi: 10.1038/nature14067. [DOI] [PubMed] [Google Scholar]
- 28.Renard H.-F., Simunovic M., Lemière J., Boucrot E., Garcia-Castillo M., Arumugam S., Chambon V., Lamaze C., Wunder C., Kenworthy A.K., Schmidt A.A., McMahon H.T., Sykes C., Bassereau P., Johannes L. Endophilin-A2 functions in membrane scission in clathrin-independent endocytosis. Nature. 2015;517:493–496. doi: 10.1038/nature14064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stautz D., Leyme A., Grandal M.V., Albrechtsen R., van Deurs B., Wewer U., Kveiborg M. Cell-surface metalloprotease ADAM12 is internalized by a clathrin- and Grb2-dependent mechanism. Traffic. 2012;13:1532–1546. doi: 10.1111/j.1600-0854.2012.01405.x. [DOI] [PubMed] [Google Scholar]
- 30.Ullrich O., Reinsch S., Urbe S., Zerial M., Parton R.G. Rab11 regulates recycling through the pericentriolar recycling endosome. J. Cell Biol. 1996;135:913–924. doi: 10.1083/jcb.135.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chalaris A., Garbers C., Rabe B., Rose-John S., Scheller J. The soluble interleukin 6 receptor: Generation and role in inflammation and cancer. Eur. J. Cell Biol. 2011;90:484–494. doi: 10.1016/j.ejcb.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Riethmueller S., Ehlers J.C., Lokau J., Düsterhöft S., Knittler K., Dombrowsky G., Grötzinger J., Rabe B., Rose-John S., Garbers C. Cleavage site localization differentially controls interleukin-6 receptor proteolysis by ADAM10 and ADAM17. Sci. Rep. 2016;6:25550. doi: 10.1038/srep25550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujimoto K., Ida H., Hirota Y., Ishigai M., Amano J. Intracellular Dynamics and fate of a Humanized anti–interleukin-6 receptor monoclonal antibody, tocilizumab. Mol. Pharmacol. 2015;88:660–675. doi: 10.1124/mol.115.099184. [DOI] [PubMed] [Google Scholar]
- 34.Basagiannis D., Christoforidis S. Constitutive endocytosis of VEGFR2 Protects the receptor against shedding. J. Biol. Chem. 2016;291:16892–16903. doi: 10.1074/jbc.M116.730309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zohlnhöfer D., Graeve L., Rose-John S., Schooltink H., Dittrich E., Heinrich P.C. The hepatic interleukin-6 receptor. Down-regulation of the interleukin-6 binding subunit (gp80) by its ligand. FEBS Lett. 1992;306:219–222. doi: 10.1016/0014-5793(92)81004-6. [DOI] [PubMed] [Google Scholar]
- 36.Dittrich E., Gerhartz C., Rose-John S., Müllberg J., Stoyan T., Heinrich P.C., Graeve L. A region within the cytoplasmic domain of the interleukin-6 signal transducer gp130 important for ligand-induced endocytosis of the IL-6 receptor. Ann. N. Y Acad. Sci. 1995;762:410–412. doi: 10.1111/j.1749-6632.1995.tb32350.x. [DOI] [PubMed] [Google Scholar]
- 37.Gerhartz C., Dittrich E., Stoyan T. Biosynthesis and half-life of the interleukin-6 receptor and its signal transducer gp130. Eur. J. Biochem. 1994;223:265–274. doi: 10.1111/j.1432-1033.1994.tb18991.x. [DOI] [PubMed] [Google Scholar]
- 38.Dittrich E., Haft C.R., Muys L., Heinrich P.C., Graeve L. A di-leucine motif and an upstream serine in the interleukin-6 (IL-6) signal transducer gp130 mediate ligand-induced endocytosis and down-regulation of the IL-6 receptor. J. Biol. Chem. 1996;271:5487–5494. doi: 10.1074/jbc.271.10.5487. [DOI] [PubMed] [Google Scholar]
- 39.Wilkinson A.N., Gartlan K.H., Kelly G., Samson L.D., Olver S.D., Avery J., Zomerdijk N., Tey S.K., Lee J.S., Vuckovic S., Hill G.R. Granulocytes are Unresponsive to IL-6 due to an absence of gp130. J. Immunol. 2018;200:3547–3555. doi: 10.4049/jimmunol.1701191. [DOI] [PubMed] [Google Scholar]
- 40.Ketteler R., Glaser S., Sandra O., Martens U.M., Klingmüller U. Enhanced transgene expression in primitive hematopoietic progenitor cells and embryonic stem cells efficiently transduced by optimized retroviral hybrid vectors. Gene Ther. 2002;9:477–487. doi: 10.1038/sj.gt.3301653. [DOI] [PubMed] [Google Scholar]
- 41.Garbers C., Thaiss W., Jones G.W., Waetzig G.H., Lorenzen I., Guilhot F., Lissilaa R., Ferlin W.G., Grötzinger J., Jones S.A., Rose-John S., Scheller J. Inhibition of classic signaling is a novel function of soluble glycoprotein 130 (sgp130), which is controlled by the ratio of interleukin 6 and soluble interleukin 6 receptor. J. Biol. Chem. 2011;286:42959–42970. doi: 10.1074/jbc.M111.295758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suthaus J., Tillmann A., Lorenzen I., Bulanova E., Rose-John S., Scheller J. Forced homo- and heterodimerization of all gp130-type receptor complexes leads to constitutive ligand-independent signaling and cytokine-independent growth. Mol. Biol. Cell. 2010;21:2797–2807. doi: 10.1091/mbc.E10-03-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are contained within the article.