Abstract
The composition of dynamic covalent imine libraries (DCL) adapts to the presence of the hexameric resorcinarene capsule. In the presence of the self-assembled capsule, a kinetic and thermodynamic modulation of the imine constituents of the DCLs was observed, which was induced by an unusual predatory action of the capsule on specific imine constituents. More complex 2 × 2 DCLs also adapt to the presence of the hexameric capsule, showing a thermodynamic and kinetic modulation of the constituents induced by the predatory action of the capsule. By cross-referencing experimental data, a good selectivity (up to 66%) for one constituent can be induced in a 2 × 2 DCL.
Introduction
Nature is a continual source of inspiration for those scientists interested in mimicking the strict level of selectivity and efficiency that are the basis of living systems.1 Biomimicry2,3 starts from the inspiration of natural processes which include the modus operandi of natural enzymes, one of the most amazing phenomena in biological chemistry.3 Enzymes are able to work selectively in the presence of complex mixture of substrates, leading to the selective formation of specific products at once. On this basis, one of the aims of enzyme mimicry3 is the synthesis of artificial systems able to work selectively in the presence of a complex mixture of reagents.
In the past decades, dynamic covalent chemistry (DCC)4−6 has aroused a particular interest. In DCC, simple building blocks are held together by reversible covalent bonds to form a library of products that, under thermodynamic equilibrium conditions, are continuously interconverting.6 Under these conditions, the library is usually able to respond to an external stimulus (Figure 1) by changing its equilibrium composition according to Le Châtelier’s principle. Examples were reported in the literature in which dynamic covalent libraries (DCL) undergo reorganization as a response to a physical stimulus such as temperature,6a crystallization,6b,6c distillation,7 or phase separation.8 Interesting examples of supramolecular modulation of DCLs have been also reported in the literature.9a Sanders and Pantoş pointed out that a dynamic library of naphthalenediimide-based macrocycles responded to the presence of different complementary naphthalene guests.9b Another example of supramolecular modulation of a DCL was reported by Sanders and co-workers in which a dynamic library of hydrazone-based pseudopeptides changed in the product distribution after addition of acetylcholine.9c In the presence of the ammonium guest, the equilibrium shifted toward the cyclic trimer, which was able to selectively bind acetylcholine.9c Analogously, a dynamic library of cyclic pseudopeptide receptors changed its equilibrium distribution in the presence of a Li+ guest, which was able to convert a complex mixture of about 10 macrocycles into one that contains 98% of the Li+ receptor.10
Figure 1.
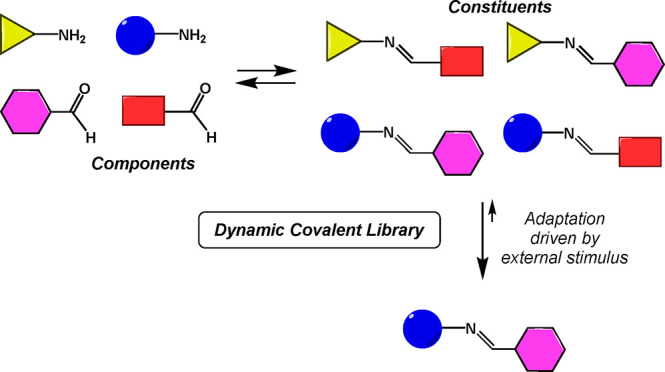
Adaptation of a dynamic covalent library of imines to an external stimulus.
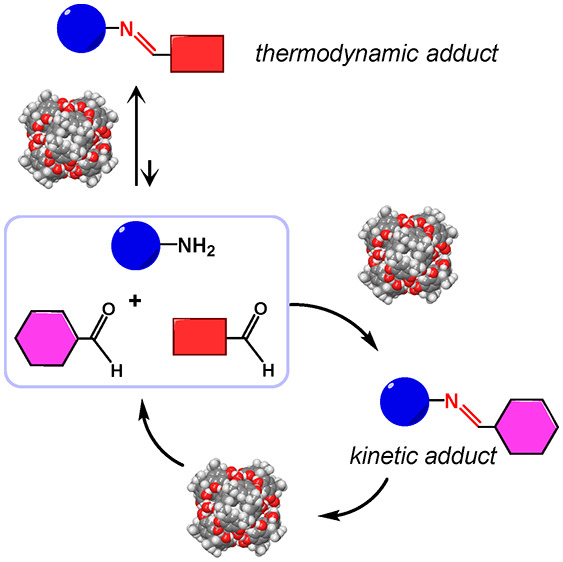
Among the major subjects explored in DCC, surely the imines have attracted particular attention.11 Formation of an imine bond is a dynamic process where a carbonyl compound reacts in a reversible manner with an amino group with the loss of water (Figure 1). Usually, upon response to an external stimulus, an imine-based dynamic library reorganizes the composition of its constituents and drives it toward the preferential formation of only selected members (Figure 1).
In particular, the reversible formation of imine bonds is affected by external factors such as temperature, pH, and concentration but also by internal factors such as steric and electronic features of the substrates. Dynamic imines have been used in different applications, including the synthesis of complex molecular architectures, such as cages,12 and in self-sorting systems.13
Recently, in biomimicry, much attention has been devoted to catalytic processes in a nanoconfined space using self-assembled capsules.14 The confined space inside the self-assembled containers looks like an enzyme pocket and provides interesting catalytic features. Among the self-assembled architectures, the hexameric resorcinarene capsule CR6 (Figure 2) has become increasingly important in catalysis.15 The formation of a hexameric resorcin[4]arene capsule CR6 (Figure 2) in the solid state was originally reported by Atwood,16 whereas evidence for its formation in water-saturated chloroform or wet benzene solution was provided by Cohen17 and co-workers by diffusion NMR experiments. The capsule is obtained by self-assembly of six resorcinarene 1 and eight water molecules, sealed by 60 H-bonding interactions. The container CR6 shows some features that make it a useful tool in biomimetic catalysis:3 (a) the internal π···electron-rich cavity of 1375 Å3 is able to recognize neutral and cationic species and to stabilize transition states, due to secondary interactions; (b) the capsule CR6 behaves as a mild Brønsted acid with a pKa value of about 5.5–6.0;18a (c) four bridging water molecules show a H-bond-donating free valence, which is catalytically relevant.18b,19 In addition, previously reported data20 show that the CR6 capsule is able to exert a substrate selectivity, whereas stereo- and regioselectivity toward the products are also generally observed.
Figure 2.
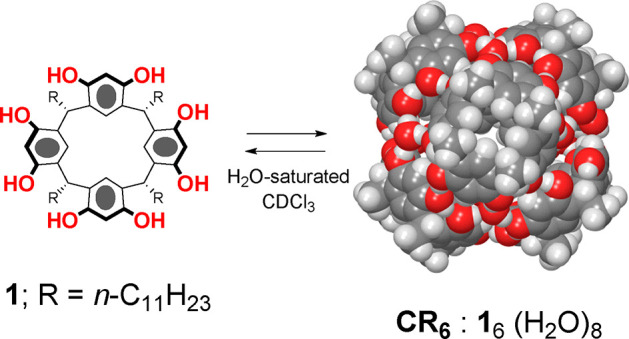
Self-assembly of resorcinarene 1 in water-saturated CDCl3 forming the hexameric resorcinarene capsule CR6.
Tiefenbacher and co-workers reported the first example of catalysis of formation of an iminium group inside CR6,21 exploiting its mild acidity and its ability to stabilize cationic intermediates and transition states. Successively, our group reported experimental and computational evidence of the formation of an iminium specie inside CR6.22
These considerations prompted us to investigate the behavior of dynamic imine libraries in the presence of CR6. As stated by Lehn,7a “changes in expression of the different constituents as a factor of external parameters represent an adaptation of the system to environmental conditions”. On this basis, we wonder if the composition of dynamic imine libraries adapts to the presence of CR6, which, in addition to catalytic abilities, usually also shows a substrate and product selectivity.
Results and Discussion
Adaptation of the DCL of Imines A2a and A2b to the Presence of the Hexameric Capsule
We start this study by investigating the formation of imines A2a and A2b in single experiments (Scheme 1) in the presence or in the absence of the hexameric capsule CR6.
Scheme 1. Synthesis of Imines A2a and A2b in the Presence of Capsule CR6.
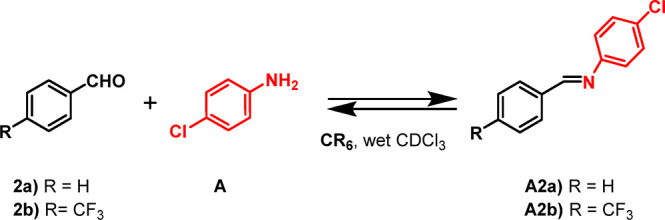
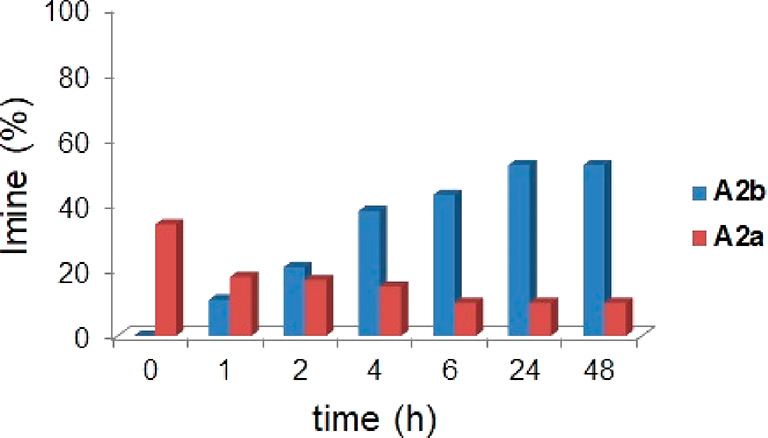
When benzaldehyde 2a and p-chloroaniline A were mixed in an equimolar ratio (42.3 mM) in water-saturated CDCl3 at 30 °C in the presence of capsule CR6 (1 equiv), the formation of imine A2a was detected in the reaction mixture after 30 min (Figure 3). The equilibrium was reached after 2 h, leading to 34% of A2a (Figure 3).
Figure 3.
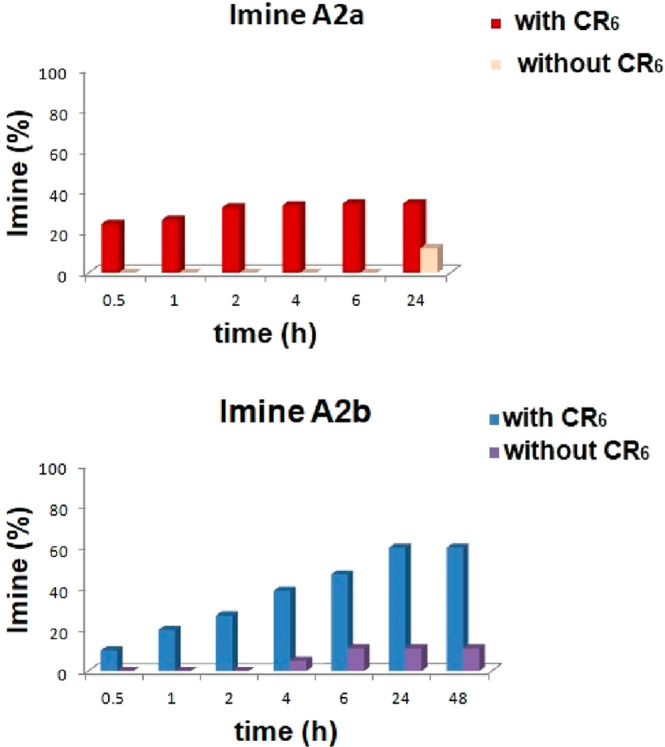
Formation of imines A2a (top) and A2b (bottom) during the single experiments in the presence or in the absence of capsule CR6 (Scheme 1).
When the reaction in Scheme 1 was performed under the same conditions but in the absence of capsule CR6, the formation of A2a was slowed and only 12% of it was detected in the reaction mixture after 24 h.
When p-trifluoromethylbenzaldehyde 2b was used with p-chloroaniline A in the presence of capsule CR6, imine A2b reached an equilibrium value of 60% after 24 h (Figure 3). Also, in this case, the formation of A2b was slowed in the absence of CR6 (Figure 3).
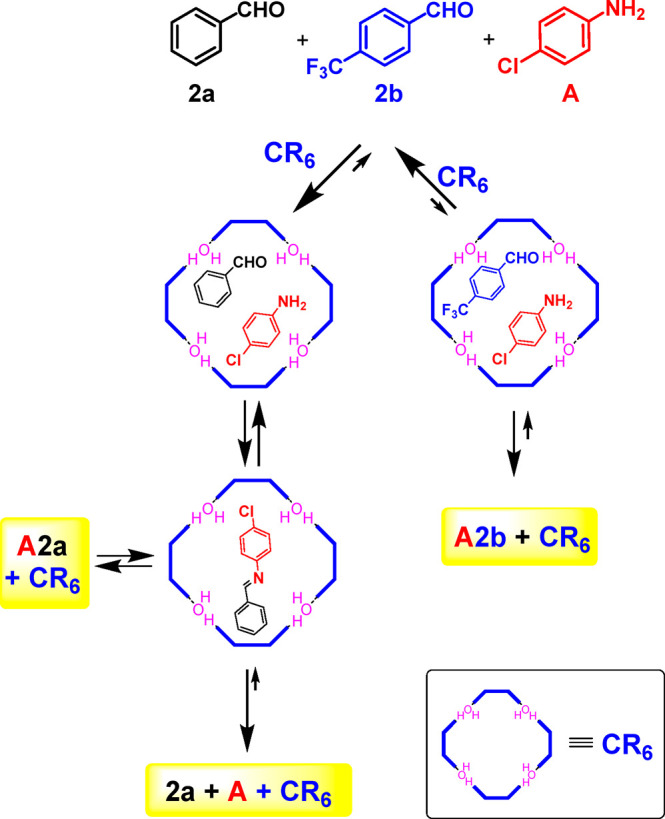
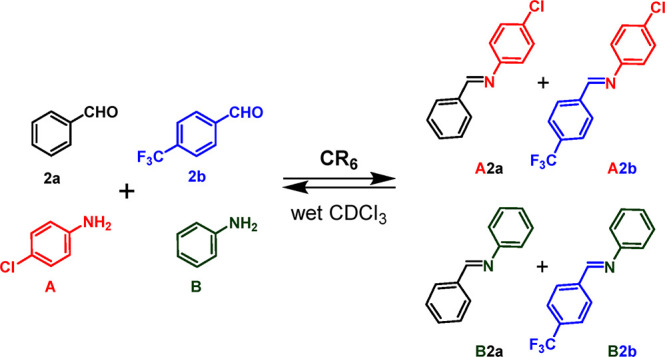
With these results in hand, we then investigated an imine-based DCL of two imine constituents, A2a and A2b (Scheme 2), formed by the three components of benzaldehyde 2a, p-trifluoromethylbenzaldehyde 2b, and p-chloroaniline A (in an equimolar ratio, Scheme 2). Experiments were performed either in the presence or in the absence of capsule CR6 in water-saturated CDCl3 using a concentration of 42.3 mM each of 2a/2b/A/CR6. The reactions were conducted at 30 °C. The formation of imine products was monitored as a function of time by quantitative 1H NMR (qNMR) spectroscopy using 1,1,2,2-tetracloroethane (TCE) as an internal standard. Aliquots of the reaction mixtures were added to DMSO (Supporting Information) in order to disaggregate CR6, and the imine signals were integrated with respect to the signal of TCE. In the absence of the CR6 capsule, two imine constituents, A2a and A2b, were formed in equal quantities up to 48 h (Figure 4a) when the conversion was about 20% for each.
Scheme 2. Dynamic Library Formed by the Three Components 2a, 2b, and A and by the Two Constituents A2a and A2b in the Presence of CR6.
Figure 4.
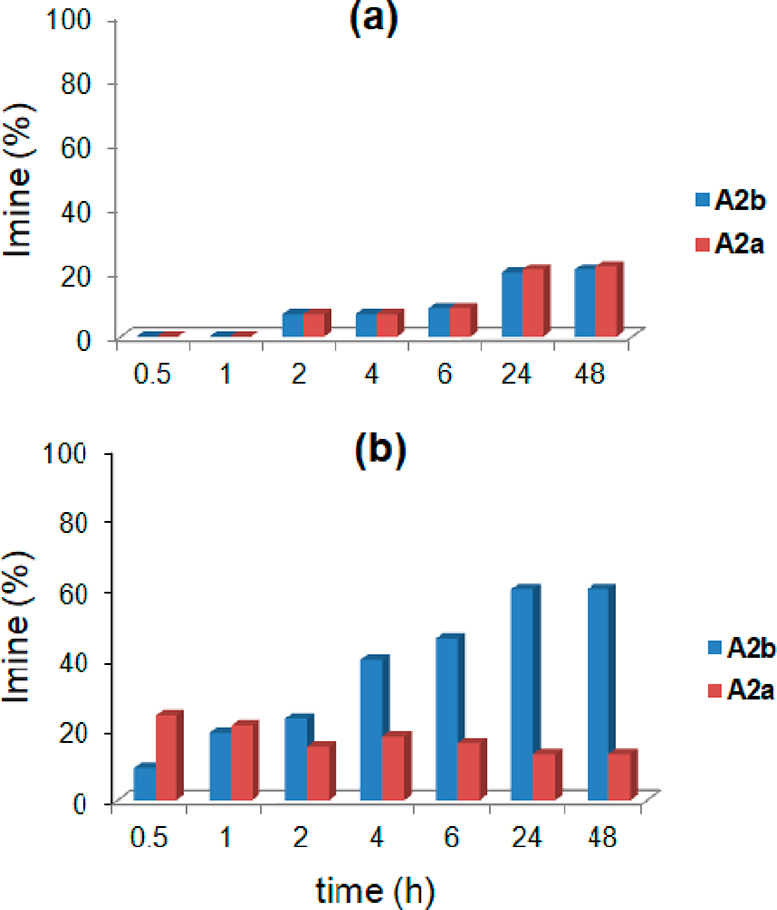
Distribution of imine constituents A2a and A2b in the DCL in Scheme 2, without (a) and with (b) capsule CR6.
Interestingly, the DCL in Scheme 2 adapts to the presence of the capsule CR6 (Figure 4b). In fact, imines A2a and A2b were formed immediately after being mixed with a conversion of 30 and 10%, respectively.
Imine A2a, obtained by benzaldehyde 2a and p-chloroaniline A, was formed faster than A2b, but after 1 h, A2a started to decrease as A2b increased. This trend continued up to 24 h, when the A2a/A2b ratio reached a value of 15/60 and remained constant (48 h). The results in Figure 4 showed that A2a was kinetically favored, whereas A2b was the thermodynamic product under these conditions.
As known, in an imine-based DCL, the imine constituents exchange their components between them by reversible formation of chemical bonds. Usually, these processes are under thermodynamic control, and in this way, the most stable constituent prevails. Thus, we have envisioned a new experiment, reported in Scheme 3, in which benzaldehyde 2a and p-chloroaniline A were reacted in the presence of CR6 in order to form only imine A2a. After 24 h, A2a was formed in 34% yield (Scheme 3). At this point, the mixture was added to 1 equiv of aldehyde 2b, and 1 h later, A2b started to increase as A2a decreased (Scheme 3 and Figure 5). The equilibrium was reached 24 h later (Figure 5), showing a distribution pattern close to that obtained in the experiment in Figure 4b.
Scheme 3.
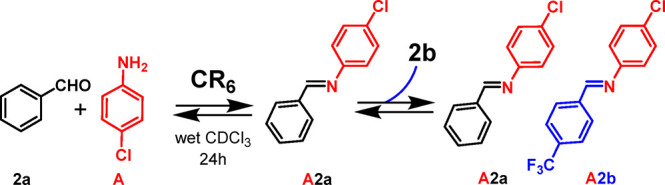
Figure 5.
The evolution of the imine composition in Figure 4 clearly indicates that the capsule CR6 shows two effects:
-
(a)
CR6 acts as a catalyst by accelerating the formation of imine constituents A2a and A2b, due to its mild acidity and capability to stabilize cationic intermediates.
-
(b)
CR6 acts as an external stimulus because the DCL composition of A2a and A2b adapts to its presence. The formation of imine A2a is initially favored, whereas A2b prevails at longer time. Thus, under these conditions, A2a and A2b represent the kinetic and the thermodynamic adducts, respectively.
In order to get more insights on the mechanism of this kinetic and thermodynamic modulation of the DCL in Scheme 2, we performed uptake experiments.23 In detail, a competition experiment was carried out in which benzaldehyde 2a and p-trifluoromethylbenzaldehyde 2b were in competition to occupy the inner cavity of CR6. The uptake of 2a/2b inside CR6 was measured by quantitative 1H NMR experiments, in which the aldehydes 2a and 2b (42.3 mM each one) were mixed in the presence of 1 equiv of CR6 in water-saturated CDCl3. The quantity of encapsulated aldehyde was obtained by determining the difference between its initial concentration and the concentration of the free aldehyde in solution. The 1H NMR signal of the free aldehyde was integrated with respect to the signal of the internal standard (TCE). After equilibration, a 52% uptake of benzaldehyde 2a inside CR6 was measured, a value significantly higher than that obtained for the aldehyde 2b (5%). Thus the hexameric capsule CR6 shows a higher affinity for benzaldehyde 2a with respect to p-CF3-benzaldehyde 2b. Clearly, this result is in accord with the finding that A2a is preferentially formed in the early stage of the reaction, where the capsule is filled to a greater extent with benzaldehyde 2a.
Proof of the encapsulation of benzaldehyde 2a inside CR6 was obtained by 1D and 2D NMR studies and, in particular, by HSQC experiments (Supporting Information, Figures S48–S54). From these studies, it emerges that the benzaldehyde is encapsulated inside CR6 with slow kinetics with respect to the NMR time scale (600 MHz). Analogously, the encapsulation of the aldehyde 2b inside CR6 was studied by 1D and 2D NMR experiments (Supporting Information, Figures S55–S59). Analogous studies were reported in the Supporting Information in order to show the encapsulation of p-chloroaniline A inside CR6 (Supporting Information, Figures S60–S63).
The fate of the two imines A2a and A2b remains to be understood. In detail, we wonder why A2b prevails for a long time whereas A2a decreases with respect to its initial percentage.
When A2a was dissolved in water-saturated CDCl3 solution in the presence of CR6 (1 equiv), after 30 min, 62% of A2a was hydrolyzed to 2a and A (Figure 6). After 4 h, the hydrolysis of A2a was close to the equilibrium (Figure 6), with a 65% conversion of A2a to constituents 2a and A. Interestingly, with respect to the total quantity of benzaldehyde 2a obtained by hydrolysis of A2a after 4 h, a 29% uptake of 2a inside CR6 was measured. The uptake of 2a inside CR6 suggests that probably the benzaldehyde 2a behaves like a reversible inhibitor for the capsule CR6, slowing down its catalytic activity. Under the same conditions but in the absence of CR6, imine A2a was stable over time and no hydrolysis products were detected (Supporting Information). These data strongly indicate that the hydrolysis of A2a occurs inside CR6 due to its catalytic activity. This was confirmed by the finding that, in the presence of DMSO, a solvent able to break down the capsule,15 no conversion of A2a into 2a and A was observed. In contrast, the hydrolysis of A2b to 2b and A in the presence of CR6 was slower (Figure 6); in fact, the equilibrium was reached after 72 h with a 40% of conversion of A2b to 2b and A.
Figure 6.
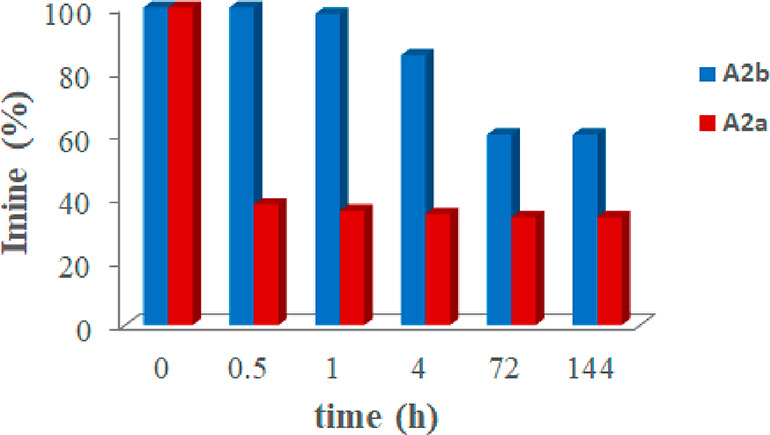
Hydrolysis of imines A2a and A2b in the presence of capsule CR6.
On this basis, we can explain the origin of the kinetic and thermodynamic modulation of the DCL in Scheme 2 (Figure 4b). Imine A2a is accumulated preferentially during the early stage of the reaction in Scheme 2 (Figures 7 and 8), due to the preferential encapsulation of 2a inside the cavity of CR6 (Figures 5–8), which catalyzes the formation of A2a. In the presence of a significant quantity of A2a, its hydrolysis starts quickly inside the CR6 capsule (Figure 8), catalyzed by the inherent Brønsted acidity of the capsule and its ability to stabilize cationic intermediates and transition states.24 On the other hand, the hydrolysis of imine A2b inside CR6 is less favored, probably because of the lower affinity of the capsule for the imine A2b. In fact, qNMR experiments revealed a very low level of uptake of A2b inside CR6 of 5%, immediately after mixing of A2b and CR6, whereas imine A2a is encapsulated to a greater extent (45%). In silico calculations were in accord with these results (Figure 7 and Supporting Information). Quantum-mechanical calculations (Supporting Information) indicate an enthalpic stabilization of −22.14 kcal/mol and a Gibbs free energy stabilization of −8.08 kcal/mol for the formation of the A2a⊂CR625 complex. However, the formation of the complex A2b⊂CR6 is unfavored in enthaplic as well as Gibbs free energy terms.25
Figure 7.
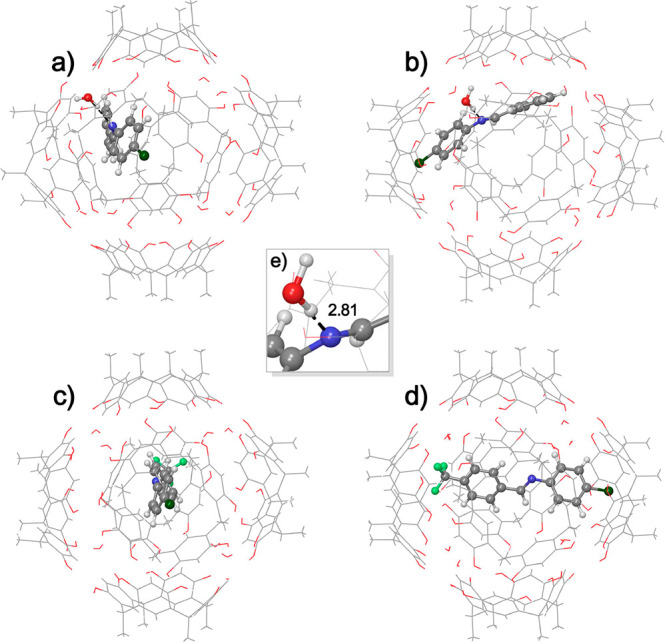
Different views of the optimized geometries of complexes (a,b) A2a⊂CR6 and (c,d) A2a⊂CR6. (e) Particular H-bonding interaction of A2a with the bridged water molecule of CR6.
Figure 8.
“Predatory” mechanism proposed for the adaptation of imine constituents in the DCL in Scheme 1 and Figure 3b.
Natural bond orbital (NBO)26 and noncovalent interaction (NCI)27 (see Supporting Information) analyses were performed on complexes A2a⊂CR6 and A2b⊂CR6 to identify the second-order interactions between the capsule and the imine. Second-order perturbation theory (SOPT) analysis of the FOCK matrix in NBO basis clarified that the better binding affinity of A2a is principally due to a strong hydrogen bonding interaction (Figure 7e) between the nitrogen atom of the imine moiety of A2a and a bridged water molecule of CR6. This strong28 H-bonding interaction shows a N···OH2 distance of 2.81 Å (Figure 7e) and a N···H–OH angle of 167° and accounts for 49% of the total stabilization energy of the A2a⊂CR6 complex (51% represents the van der Waals interactions). Regarding the A2b⊂CR6 complex, because of the steric demand imposed by the trifluoromethyl group, A2b is forced to stay on the axis that joins two vertexes of the capsule (Figure 7c,d), with the −CF3 group pointing inside the cavity of a resorcinarene macrocycle (Figure 7c,d). In this position, the imine moiety of A2b is too far from the bridged water molecules of CR6 and cannot establish any H-bonding interactions.
In summary, these results show that this is a rare example of kinetic and thermodynamic adaptation of a DCL, in which the intraspecific “predatory” effect29 of the catalyst (CR6) on one of the constituents (A2a) plays a crucial role.
Now, in order to corroborate this assumption, we studied the distribution of the constituents A2a and A2b in the presence of lower quantities of “predator” CR6 (Figure 9). When the reaction in Scheme 2 was performed in the presence of a lower quantity of CR6 (0.5 equiv), the kinetically favored imine A2a was prevalent up to 2 h (Figure 9), a time significantly longer than that observed in the presence of 1 equiv of CR6 (0.5 h). Under these conditions, the thermodynamic imine A2b began to prevail at 4 h, and finally, the quantity of A2a after 48 h was slightly higher than that obtained in the presence of 1 equiv of the capsule (see Figure 9). Decreasing the quantity of capsule CR6 to 0.1 equiv, the imine A2a prevailed for up to about 20 h, with a yield of about 40%, higher than that observed in the presence of 0.5 (23%) and 1.0 equiv (15%) of CR6. This result clearly indicates that the stability of the imine A2a in the DCL increases by decreasing the quantity of capsule CR6, showing in this way its predatory effect on A2a.
Figure 9.
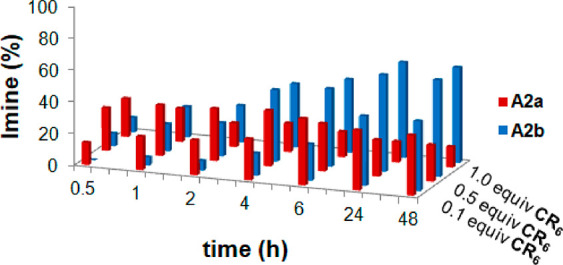
Evolution of the distribution of A2a and A2b with different amounts of capsule CR6.
Interestingly, when the p-nitrobenzaldehyde 2c was used instead of 2b, the DCL of the components A/2a/2c showed an analogous behavior (see Supporting Information). In detail, in the presence of CR6, an adaptation of constituents was thermodynamically driven by the hexameric capsule toward the imine A2c derived by aldehyde bearing an electron-withdrawing group on the phenyl ring, whereas the constituent A2a remained the kinetically favored one (Figures S20–S23).
In contrast, when the p-OMe-benzaldehyde 2d was used together with 2a and A as a component of the DCL, the formation of imines A2a and A2d was observed in very low yields in the presence of CR6.
Interestingly, in this case, imine A2a was favored over time (Figures S24–S27).
Adaptation of the 2 × 1 DCL of Imines B2a and B2b to the Presence of the Hexameric Capsule
Next, we investigated a DCL starting with aniline B and aldehydes 2a and 2b (R = CF3) (Scheme 4) as components. Imine constituents B2a and B2b were formed immediately after mixing (Figure 10b), whereas in the absence of a capsule, the reaction proceeded more slowly (Figure 10a). With regard to the imine distribution, B2a was kinetically favored, reaching 20% conversion after 0.5 h (Figure 10b). After 30 min, B2a started to decrease as B2b increased, whereas the equilibrium was reached after 6 h with a B2b/B2a ratio of 52/10. In summary, even when the aniline components are changed, the benzaldehyde-derived imine B2a results in the kinetically favored product and the imine obtained by 2b results in the thermodynamic one.
Scheme 4. Dynamic Library of Three Components 2a, 2b, and A–C and Two Constituents in the Presence of CR6.
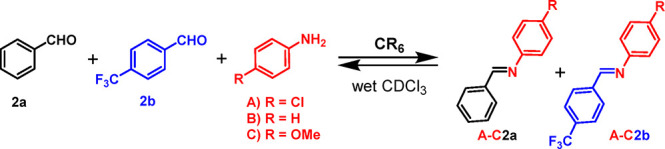
Figure 10.
Distribution of imine constituents B2a and B2b in the DCL in Scheme 4, without (a) and with (b) capsule CR6.
The decrease of the quantity of imine B2a in the experiment in Figure 10b suggests a possible predatory action of the capsule CR6 on the imine B2a. Encouraged by this hypothesis, we evaluated the stability of B2a in water-saturated CDCl3 in the presence or in the absence of capsule CR6 (Figure 11). Imine B2a was hydrolyzed in the presence of capsule CR6, and the reaction reached equilibrium with a 73% conversion of B2a after 1 h (Figure 11), whereas in the absence of capsule CR6, imine B2a was stable in water-saturated CDCl3 at room temperature.
Figure 11.
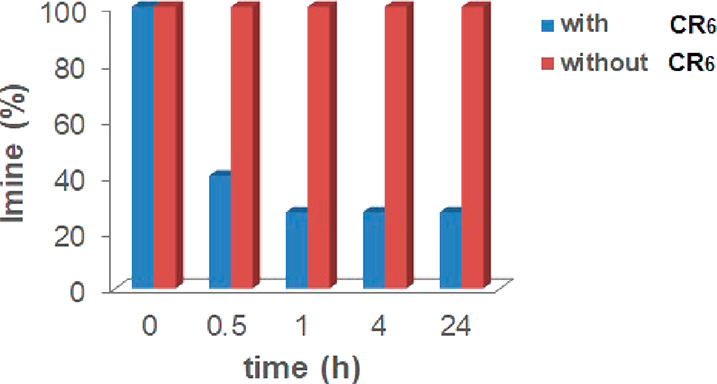
Hydrolysis of imine B2a in the presence and in the absence of capsule CR6.
Adaptation of the 2 × 1 DCL of Imines C2a and C2b to the Presence of the Hexameric Capsule
Starting from p-methoxyaniline C, which shows a basicity (pKa = 5.36) higher than that of A and B, and aldehydes 2a/2b in Scheme 4, the DCL adapts in the presence of CR6 (Figure 12), but smaller kinetic effects were observed.
Figure 12.
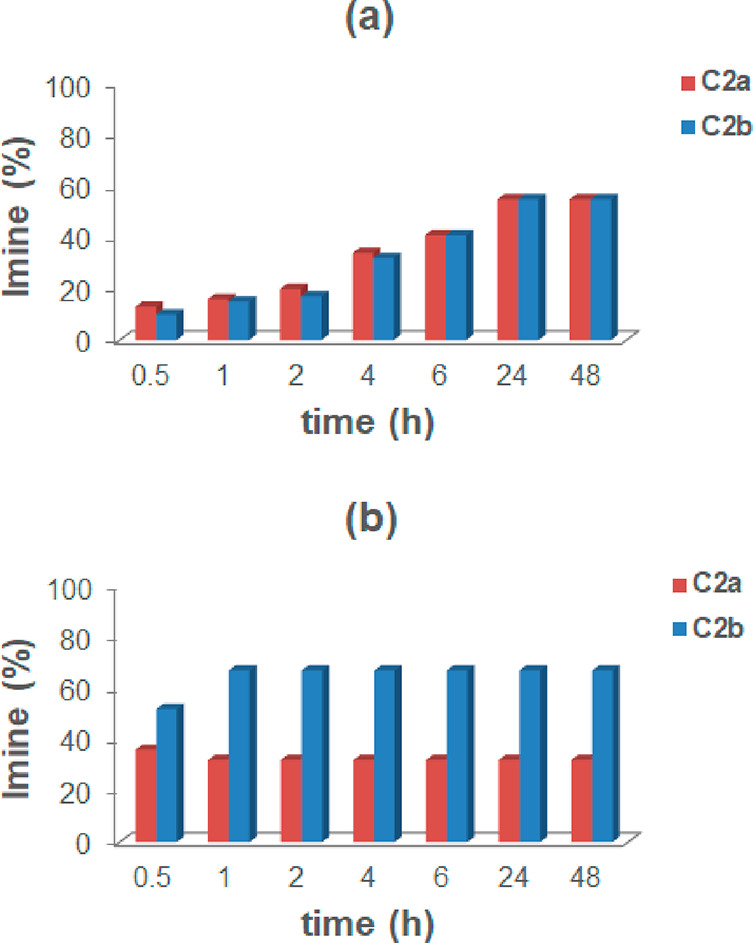
Distribution of imines C2a and C2b in the dynamic system generated by aldehydes 2a and 2b and aniline C, without (a) and with (b) capsule CR6.
A comparison of the distribution over the time of the constituents C2a and C2b (Figure 12), with and without capsule, clearly shows that, in the presence of CR6 (Figure 12b), the equilibrium was reached after 1 h with a C2b/C2a ratio of 67/32.
On the other hand, the reaction without a capsule progressed more slowly and gave an equimolar mixture of imines C2a and C2b along the reaction time (Figure 12a).
A close inspection of the kinetics in Figure 12a,b indicates that, in the absence of CR6, imine C2a increases over the time until it reaches an equilibrium value of 55% after 24 h. In contrast, when capsule CR6 was present, a conversion of 36% of C2a was obtained after 30 min, but after 1 h, C2a started to decrease until it reached an equilibrium value of 32% after 6 h. Again, this behavior suggests a predatory action of the capsule CR6 on C2a. Thus, in order to confirm this assumption, we evaluated the stability of C2a in water-saturated CDCl3 in the presence or in the absence of capsule CR6 (Figure 13).
Figure 13.
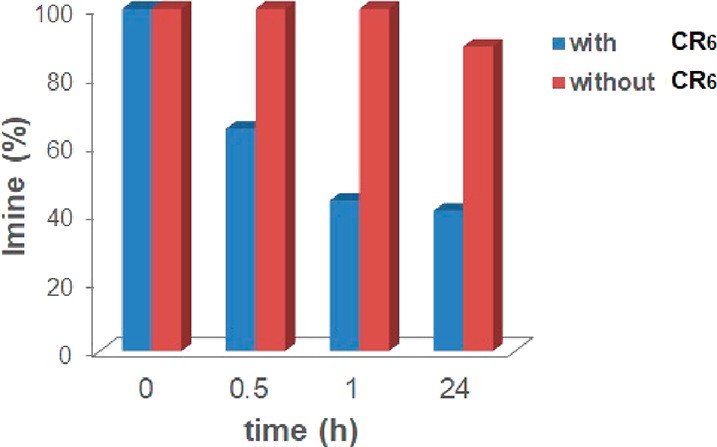
Hydrolysis of imine C2a in the presence and in the absence of capsule CR6.
Imine C2a was rapidly hydrolyzed in the presence of capsule CR6, and the reaction reached the equilibrium with a 59% conversion of C2a after 1 h, whereas in the absence of capsule CR6, imine C2a was stable in water-saturated CDCl3 at room temperature.
In summary, these results (Figures 12 and 13) indicate that the DCL of imines C2a and C2b also adapts in the presence of capsule CR6 by a predatory effect of the capsule on one of the imine constituent.
Adaptation of 2 × 1 DCLs of Imines E2a/E2b and A2a/E2a to the Presence of the Hexameric Capsule. Substrate Selectivity: Aromatic versus Aliphatic Amine
One of the aims of the enzyme mimicry is to achieve the substrate selectivity typical of natural systems. Thus, we envisioned to study the substrate selectivity of the hexameric capsule in the presence of a mixture constituted by aromatic and aliphatic amines. First, we analyzed the modulation of the DCL in Scheme 5 starting with benzaldehyde 2a, p-trifluoromethylbenzaldehyde 2b, and n-butylamine E in the presence or in the absence of a capsule in water-saturated CDCl3 using a concentration of 42.3 mM each of 2a/2b/E/CR6 at 30 °C.
Scheme 5. Dynamic Library of Three Components 2a, 2b, and E and Two Constituents in the Presence or in the Absence of CR6.
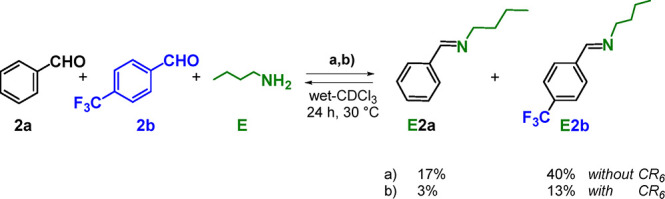
The results in Table S9 and Scheme 5 clearly show that the formation of imines E2a and E2b is favored in the absence of CR6, with 17 and 40% yield, respectively, after 24 h. However, in the presence of CR6, E2a and E2b were obtained in 3 and 13% yield, respectively. This is in contrast to the results reported in Figure 4, in which the formation of imines A2a and A2b starting by an aromatic amine such as p-chloroaniline A and aldehydes 2a and 2b is favored in the presence of CR6.
Thus, these results indicate that the capsule suppresses the reactivity of an aliphatic amine such as the n-butylamine E toward the aldehydes 2a and 2b. This conclusion can be explained on the basis of the data previously reported by Tiefenbacher.18a In fact, n-butylamine E is protonated by the capsule to an extent of 80%, and the resulting n-butylammonium cation is stabilized inside the capsule by cation···π interactions.18a,20a Consequently, the percentage of free neutral n-butylamine is low, and the imine formation (Scheme 5) is suppressed. In contrast, p-chloroaniline A, which shows a lower basicity (pKa = 3.8), is not protonated by CR6(18a) and consequently shows a remarkable reactivity when co-confined with aldehydes.
With these results in hand, we performed a competition experiment (Scheme 6) in which p-chloroaniline A and n-butylamine E compete for benzaldehyde 2a. In detail, A, E, and 2a were mixed in 1/1/1 ratio (42.3 mM) in wet CDCl3 in the presence or in the absence of CR6. As reported in Supporting Information (Table S10 and Figures S38–S40) and Scheme 6, in the absence of capsule CR6, imines E2a and A2a were formed in 50 and 25% yield, respectively (Figure 14). However, in the presence of CR6, the selectivity order was reversed to 15/25 in favor of A2a (Figure 14).
Scheme 6. Dynamic Library of Three Components 2a, A, and E in the Presence of CR6: p-Chloroaniline versus n-Butylamine Substrate Selectivity.
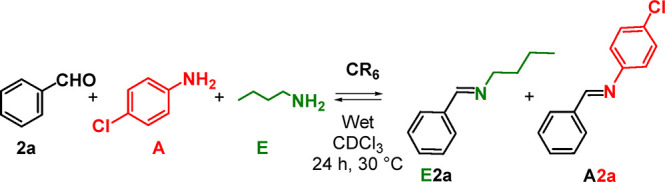
Figure 14.
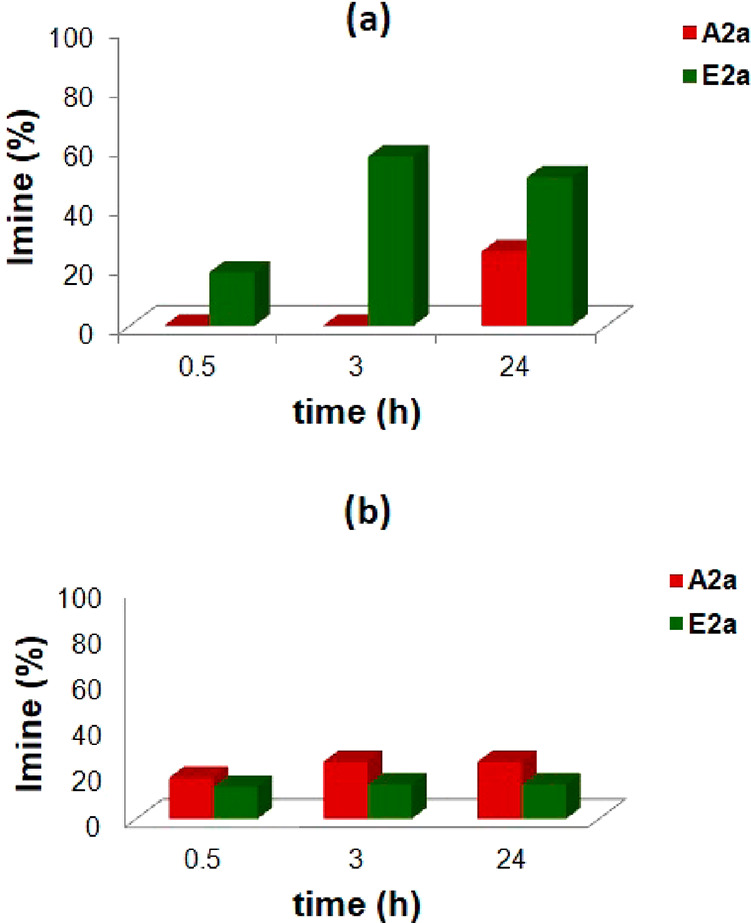
Distribution of imine constituents A2a and E2a in the DCL, without (a) and with (b) capsule CR6.
In summary, is clear that the CR6 capsule is able to host the scarcely basic p-chloroaniline A in its neutral form, thus promoting the formation of the corresponding imine in the presence of an aldehyde. When the more basic n-butylamine is used, the corresponding ammonium form is obtained after protonation inside the capsule stabilized by cation···π interactions. In this way, the formation of imine is suppressed. This is an intriguing example of substrate selectivity that the CR6 capsule exerts toward aliphatic versus aromatic amines, by decreasing the reactivity of the former toward the formation of imines.
Adaptation of the 2 × 2 DCL of Imines A2a, A2b, B2a, and B2b to the Presence of the Hexameric Capsule
At this point, our attention was focused on a more complex DCL formed by four constituents derived by four components (Scheme 7). We mixed equimolar amounts of aldehydes 2a and 2b with anilines A (p-Cl) and B (p-H) (Scheme 7), and we monitored the adaptation of the DCL of the four imine constituents in the presence of CR6.
Scheme 7. Dynamic Library of Four Components 2a, 2b, A, and B and Four Constituents in the Presence of CR6.
In the presence of a capsule (Figure 15b), a mixture of all four imines was formed immediately after mixing. Imine constituents A2a and B2b were the main components, followed by B2a and A2b in a distribution of 25, 20, 17, and 11%, respectively (Figure 15b). Two hours later, imines A2b and B2b started to increase as A2a and B2a decreased. Thus, imines A2a and B2a resulted in the kinetic products, whereas products A2b and B2b emerged under thermodynamic conditions.
Figure 15.
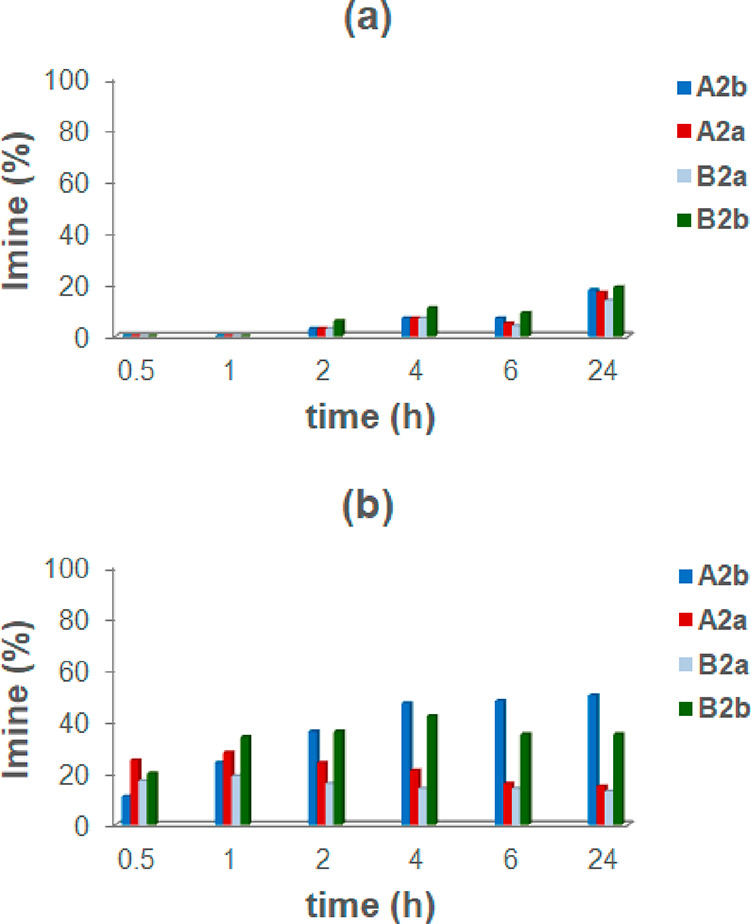
Distribution of imines in the dynamic systems from 2a, 2b, A, and B, without (a) and with (b) capsule CR6.
When the reaction was performed without the capsule, the composition of the library showed no substantial preference for the distribution of the components (Figure 15a). When the reaction in Scheme 7 was performed in the presence of a lower quantity of CR6 (0.5 equiv, Figure S42), the kinetically favored imine A2a survived longer, thus also in this case, the predatory effect of the capsule toward A2a was suppressed (Figure S42).
In accordance with one of the aims of enzyme mimicry,3 to work selectively in the presence of a complex mixture of reagents, the results reported in Figure 15 clearly show that the hexameric capsule CR6 is able to work selectively in the presence of complex mixtures of substrates, due to a fine control of the encapsulated species, leading to the selective formation of specific imines. In order to further corroborate this result, we performed a new 2 × 2 experiment, changing the amine and aldehyde components.
Adaptation of the 2 × 2 DCL of Imines C2b, C2d, D2b, and D2d to the Presence of the Hexameric Capsule
Finally, we focused our attention toward a 2 × 2 DCL starting with components bearing an electron-donating OMe group (2d/C) and an electron-withdrawing trifluoromethyl group (2b/D) (Scheme 8). As in all of the above cases, the formation of imines was more efficient in the presence of the CR6 capsule (Figure 16).
Scheme 8. Dynamic Library of Four Components 2d, 2b, C, and D and Four Constituents in the Presence of CR6.
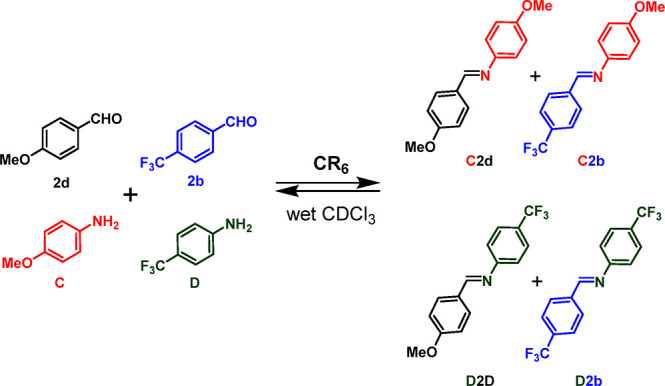
Figure 16.
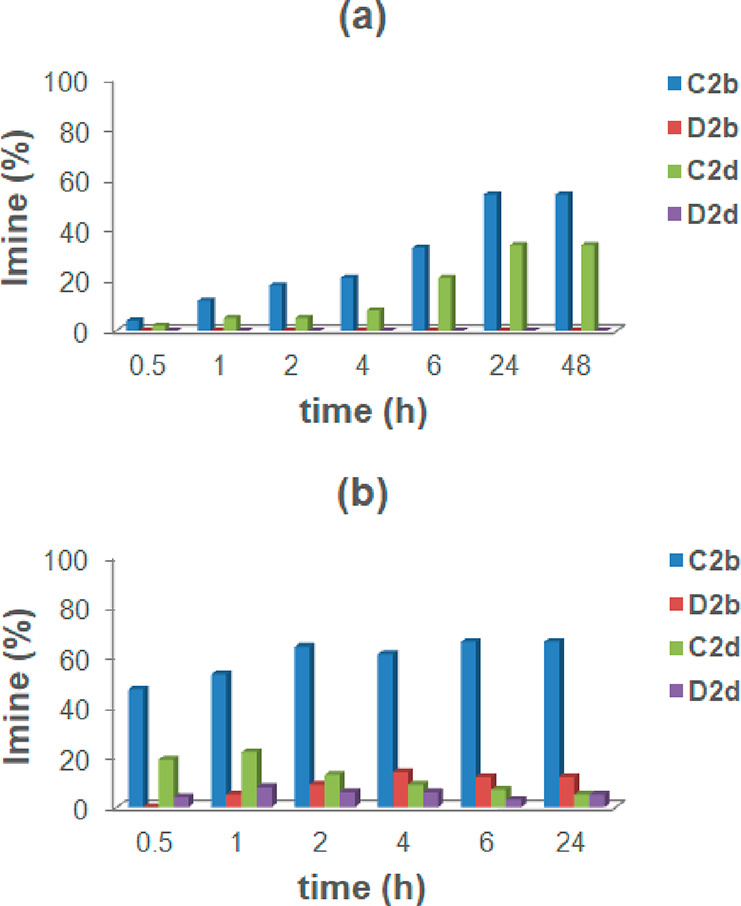
Distribution of imines in the dynamic systems from 2b, 2d, C, and D, without (a) and with (b) capsule CR6.
After 0.5 h, imine constituents C2b and C2d from p-methoxyaniline C were detected as the main components of the mixture in a C2b/C2d ratio of 47/19, whereas imines D2b and D2d, obtained from the less reactive p-trifluoromethylaniline D, were present in almost negligible quantities. Over time, an increase in the quantity of C2b was observed, which after 24 h was the most abundant constituent of the mixture, with a composition of C2b, D2b, C2d, and D2d of 66, 12, 5, and 5%, respectively. In summary, by cross-referencing the data in Figures 12 and 15, it becomes clear that no kinetic preference was observed in DCL systems in which the p-OMe-benzaldehyde 2d or p-OMe-aniline C are present as components.
Conclusions
In conclusion, we have demonstrated that dynamic covalent libraries of imine constituents are able to adapt their composition in response to the presence of the hexameric resorcinarene capsule CR6. The DCL of imines A2a and A2b formed by benzaldehyde 2a, p-CF3-benzaldehyde 2b, and p-chloroaniline A adapts its composition in the presence of CR6, showing a kinetic and thermodynamic preference of the constituents. In particular, the kinetically favored constituent A2a, obtained from benzaldehyde 2a, is preferentially formed immediately after mixing, due to the preferred inclusion of 2a inside CR6. Surprisingly, the capsule shows a predatory behavior toward imine A2a, which is quickly hydrolyzed to components A and 2a inside the capsule. On the other hand, imine constituent A2b, obtained from p-CF3-benzaldehyde 2b, is hydrolyzed slower than A2a. Uptake studies show that, after the hydrolysis of imine A2a, the benzaldehyde component 2a remains included in the capsule CR6. Interestingly, the hexameric capsule CR6 shows an analogous predatory action on other benzaldehyde-based imines such as B2a and C2a derived from aniline B and p-OMe-aniline C, respectively. Finally, more complexes of 2 × 2 DCL systems adapt to the presence of the hexameric capsule, showing a thermodynamic and kinetic modulation of the constituents and leading to a good selectivity (up to 66%) for one of them.
Acknowledgments
This work was supported by the University of Salerno (FARB 2018 and PhD funding).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.0c04705.
Synthesis and characterization of imines, experimental procedures for the synthesis of dynamic imine libraries, kinetic experiments on DCLs, 1H NMR spectra of DCLs of imines as a function of time, proof of encapsulation of aldehydes by 1D and 2D experiments, 1H NMR experiments as proof of the predatory effect of the capsule (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Berg J. M.; Stryer L.; Tymoczko J.; Gatto G.. Biochemistry, 9th ed.; W.H. Freeman & Company: New York, 2019. [Google Scholar]
- a Benyus J. M.Biomimicry: Innovation Inspired by Nature; Harper Perennial, 2002. [Google Scholar]; b Swiegers G. F.Bioinspiration and Biomimicry in Chemistry: Reverse-Engineering Nature; John Wiley & Sons, Inc.: Hoboken, NJ, 2012. [Google Scholar]
- a Breslow R. Biomimetic Chemistry and Artificial Enzymes: Catalysis by Design. Acc. Chem. Res. 1995, 28, 146–153. 10.1021/ar00051a008. [DOI] [Google Scholar]; b Marchetti L.; Levine M. Biomimetic Catalysis. ACS Catal. 2011, 1, 1090–1118. 10.1021/cs200171u. [DOI] [Google Scholar]; c Raynal M.; Ballester P.; Vidal-Ferran A.; van Leeuwen P. W. N. M. Supramolecular catalysis. Part 2: artificial enzyme mimics. Chem. Soc. Rev. 2014, 43, 1734–1787. 10.1039/C3CS60037H. [DOI] [PubMed] [Google Scholar]
- a Lehn J.-M. Dynamic Combinatorial Chemistry and Virtual Combinatorial Libraries. Chem. - Eur. J. 1999, 5, 2455–2463. . [DOI] [Google Scholar]; b Jin Y.; Yu C.; Denman R. J.; Zhang W. Recent advances in dynamic covalent chemistry. Chem. Soc. Rev. 2013, 42, 6634–6654. 10.1039/c3cs60044k. [DOI] [PubMed] [Google Scholar]; c Zhang W.; Jin Y.. Dynamic Covalent Chemistry: Principles, Reactions, and Applications, 1st ed.; John Wiley & Sons Ltd.: Chichester, UK, 2018. [Google Scholar]; d Frei P.; Hevey R.; Ernst B. Dynamic Combinatorial Chemistry: A New Methodology Comes of Age. Chem. - Eur. J. 2019, 25, 60–73. 10.1002/chem.201803365. [DOI] [PubMed] [Google Scholar]; e Ji Q.; Lirag R. C.; Miljanić O. Š. Kinetically controlled phenomena in dynamic combinatorial libraries. Chem. Soc. Rev. 2014, 43, 1873–1884. 10.1039/C3CS60356C. [DOI] [PubMed] [Google Scholar]; f Hsu C.-W.; Miljanić O. Š.. Self-Sorting through Dynamic Covalent Chemistry. In Dynamic Covalent Chemistry: Principles, Reactions, and Applications, 1st ed.; Zhang W., Jin Y., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2018. [Google Scholar]
- a Giuseppone N.; Lehn J.-M. Protonic and Temperature Modulation of Constituent Expression by Component Selection in a Dynamic Combinatorial Library of Imines. Chem. - Eur. J. 2006, 12, 1715–1722. 10.1002/chem.200501038. [DOI] [PubMed] [Google Scholar]; b Folmer-Andersen J. F.; Lehn J.-M. Thermoresponsive Dynamers: Thermally Induced, Reversible Chain Elongation of Amphiphilic Poly(acylhydrazones). J. Am. Chem. Soc. 2011, 133, 10966–10973. 10.1021/ja2035909. [DOI] [PubMed] [Google Scholar]
- a Baxter P. N. W.; Lehn J.-M.; Rissanen K. Generation of an equilibrating collection of circular inorganic copper (I) architectures and solid-state stabilization of the dicopper helicate component. Chem. Commun. 1997, 1323–1324. 10.1039/a703083e. [DOI] [Google Scholar]; b Chow C.; Fujii S.; Lehn J.-M. Cristallization-driven constitutional changes of dynamic polymers in response to neat/solution conditions. Chem. Commun. 2007, 4363–4365. 10.1039/b713413d. [DOI] [PubMed] [Google Scholar]; c Barboiu M.; Dumitru F.; Legrand Y.-M.; Petit E.; van der Lee A. Self-sorting of equilibrating metallosupramolecular DCLs via constitutional crystallization. Chem. Commun. 2009, 2192–2194. 10.1039/b900155g. [DOI] [PubMed] [Google Scholar]
- a Osowska K.; Miljanić O. Š. Self-Sorting of Dynamic Imine Library during Distillation. Angew. Chem., Int. Ed. 2011, 50, 8345–8349. 10.1002/anie.201102813. [DOI] [PubMed] [Google Scholar]; b Ji Q.; Miljanić O. Š. Distillative Self-Sorting of Dynamic Ester Libraries. J. Org. Chem. 2013, 78, 12710–12716. 10.1021/jo402305p. [DOI] [PubMed] [Google Scholar]; c Buchs néè Levrand B.; Godin G.; Trachsel A.; de Saint Laumer J.-Y.; Lehn J.-M.; Herrmann A. Reversible Aminal Formation: Controlling the Evaporation of Bioactive Volatiles by Dynamic Combinatorial/Covalent Chemistry. Eur. J. Org. Chem. 2011, 2011, 681–695. 10.1002/ejoc.201001433. [DOI] [Google Scholar]
- a Pérez-Fernández R.; Pittelkow M.; Belenguer A. M.; Sanders J. K. M. Phase-transfer dynamic combinatorial chemistry. Chem. Commun. 2008, 1738–1740. 10.1039/b718075f. [DOI] [PubMed] [Google Scholar]; b Hafezi N.; Lehn J.-M. Adaptation of Dynamic Covalent Systems of Imine Constituents to Medium Change by Component Redistribution under Reversible Phase Separation. J. Am. Chem. Soc. 2012, 134, 12861–12868. 10.1021/ja305379c. [DOI] [PubMed] [Google Scholar]; c Osypenko A.; Dhers S.; Lehn J.-M. Pattern Generation and Information Transfer through a Liquid /Liquid Interface in 3D Constitutional Dynamic Networks of Imine Ligands in Response to Metal Cation Effectors. J. Am. Chem. Soc. 2019, 141, 12724–12737. 10.1021/jacs.9b05438. [DOI] [PubMed] [Google Scholar]
- a Rowan S. J.; Cantrill S. J.; Cousins G. R. L.; Sanders J. K. M.; Stoddart J. F. Dynamic Covalent Chemistry. Angew. Chem., Int. Ed. 2002, 41, 898–952. . [DOI] [PubMed] [Google Scholar]; b Au-Yeung H. Y.; Cougnon F. B.L.; Otto S.; Pantoş G. D.; Sanders J. K.M. Exploiting donor-acceptor interactions in aqueous dynamic combinatorial libraries: exploratory studies of simple systems. Chem. Sci. 2010, 1, 567–574. 10.1039/c0sc00307g. [DOI] [Google Scholar]; c Cousins G. R. L.; Furlan R. L. E.; Ng Y.-F.; Redman J. E.; Sanders J. K. M. Identification and Isolation of a Receptor for N-Methyl Alkylammonium Salts: Molecular Amplification in a Pseudo-Peptide Dynamic Combinatorial Library. Angew. Chem., Int. Ed. 2001, 40, 423–428. . [DOI] [PubMed] [Google Scholar]
- Furlan R. L. E.; Ng Y.-F.; Otto S.; Sanders J. K.M. A new Cyclic Pseudopeptide Receptor for Li+ from a Dynamic Combinatorial Library. J. Am. Chem. Soc. 2001, 123, 8876–8877. 10.1021/ja0160703. [DOI] [PubMed] [Google Scholar]
- Belowich M. E.; Stoddart J. F. Dynamic imine chemistry. Chem. Soc. Rev. 2012, 41, 2003–2024. 10.1039/c2cs15305j. [DOI] [PubMed] [Google Scholar]
- a Ono K.; Iwasawa N. Dynamic Behavior of Covalent Organic Cages. Chem. - Eur. J. 2018, 24, 17856–17868. 10.1002/chem.201802253. [DOI] [PubMed] [Google Scholar]; b Ding H.; Chen R.; Wang C.. Organic Cages through Dynamic Covalent Reactions. In Dynamic Covalent Chemistry: Principles, Reactions and Applications; Zhang W., Jin Y., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2018; pp 165–205. [Google Scholar]; c Acharyya K.; Mukherjee P. S. Organic Imine Cages: Molecular Marriage and Applications. Angew. Chem., Int. Ed. 2019, 58, 8640–8653. 10.1002/anie.201900163. [DOI] [PubMed] [Google Scholar]; d Greenaway R. L.; Santolini V.; Pulido A.; Little M. A.; Alston B. M.; Briggs M. E.; Day G. M.; Cooper A. I.; Jelfs K. E. From Concept to Crystals via prediction: Multi-Component Organic Cage Pots by Social Self-Sorting. Angew. Chem., Int. Ed. 2019, 58, 16275–16281. 10.1002/anie.201909237. [DOI] [PubMed] [Google Scholar]
- a Osowska K.; Miljanić O. Š. Oxidative Kinetic Self-Sorting of a Dynamic Imine Library. J. Am. Chem. Soc. 2011, 133, 724–727. 10.1021/ja109754t. [DOI] [PubMed] [Google Scholar]; b Kulchat S.; Chaur M. N.; Lehn J.-M. Kinetic selectivity and Thermodynamic Features of Competitive Imine Formation in Dynamic Covalent Chemistry. Chem. - Eur. J. 2017, 23, 11108–11118. 10.1002/chem.201702088. [DOI] [PubMed] [Google Scholar]; c He M.; Lehn J.-M. Time-Dependent Switching of Constitutional Dynamic Libraries and Networks from Kinetic to Thermodynamic Distributions. J. Am. Chem. Soc. 2019, 141, 18560–18569. 10.1021/jacs.9b09395. [DOI] [PubMed] [Google Scholar]; d Zhou Y.; Li L.; Ye H.; Zhang L.; You I. Quantitative Reactivity Scales for Dynamic Covalent and Systems Chemistry. J. Am. Chem. Soc. 2016, 138, 381–389. 10.1021/jacs.5b11361. [DOI] [PubMed] [Google Scholar]
- a Hof F.; Craig S. L.; Nuckolls C.; Rebek J. Jr. Molecular Encapsulation. Angew. Chem., Int. Ed. 2002, 41, 1488–1508. . [DOI] [PubMed] [Google Scholar]; b Rebek J., Jr.Hydrogen- Bonded Capsules: Molecular Behaviour in Small Spaces; World Scientific: Singapore, 2015. [Google Scholar]
- a Catti L.; Zhang Q.; Tiefenbacher K. Advantages of Catalysis in Self-Assembled Molecular Capsules. Chem. - Eur. J. 2016, 22, 9060–9066. 10.1002/chem.201600726. [DOI] [PubMed] [Google Scholar]; b Borsato G.; Scarso A.. Catalysis within the Self-Assembled Resorcin[4]arene Hexamer. In Organic Nanoreactors; Sadjadi S., Ed.; Academic Press: London, 2016; pp 203–234. [Google Scholar]; c Zhang Q.; Catti L.; Tiefenbacher K. Catalysis inside the Hexameric Resorcinarene Capsule. Acc. Chem. Res. 2018, 51, 2107–2114. 10.1021/acs.accounts.8b00320. [DOI] [PubMed] [Google Scholar]; d Gaeta C.; Talotta C.; De Rosa M.; La Manna P.; Soriente A.; Neri P. The Hexameric Resorcinarene Capsule at Work: Supramolecular Catalysis in Confined Spaces. Chem. - Eur. J. 2019, 25, 4899–4913. 10.1002/chem.201805206. [DOI] [PubMed] [Google Scholar]
- MacGillivray L. R.; Atwood J. L. A chiral spherical molecular assembly held together by 60 hydrogen bonds. Nature 1997, 389, 469–472. 10.1038/38985. [DOI] [Google Scholar]
- Avram L.; Cohen Y. Spontaneous Formation of hexameric Resorcinarene Capsule in Chloroform Solution as Detected by Diffusion NMR. J. Am. Chem. Soc. 2002, 124, 15148–15149. 10.1021/ja0272686. [DOI] [PubMed] [Google Scholar]
- a Zhang Q.; Tiefenbacher K. Hexameric Resorcinarene Capsule is a Brønsted Acid: Investigation and Application to Synthesis and Catalysis. J. Am. Chem. Soc. 2013, 135, 16213–16219. 10.1021/ja4080375. [DOI] [PubMed] [Google Scholar]; b La Manna P.; Talotta C.; Floresta G.; De Rosa M.; Soriente A.; Rescifina A.; Gaeta C.; Neri P. Mild Friedel-Crafts Reactions inside a Hexameric Resorcinarene Capsule: C-Cl Bond Activation through Hydrogen Bonding to Bridging Water Molecules. Angew. Chem., Int. Ed. 2018, 57, 5423–5428. 10.1002/anie.201801642. [DOI] [PubMed] [Google Scholar]; c La Manna P.; De Rosa M.; Talotta C.; Rescifina A.; Floresta G.; Soriente A.; Gaeta C.; Neri P. Synergic Interplay Between Halogen Bonding and Hydrogen Bonding in the Activation of a Neutral Substrate in a Nanoconfined Space. Angew. Chem., Int. Ed. 2020, 59, 811–818. 10.1002/anie.201909865. [DOI] [PubMed] [Google Scholar]
- a Gambaro S.; De Rosa M.; Soriente A.; Talotta C.; Floresta G.; Rescifina A.; Gaeta C.; Neri P. A hexameric resorcinarene capsule as a hydrogen bonding catalyst in the conjugate addition of pyrroles and indoles to nitroalkenes. Org. Chem. Front. 2019, 6, 2339–2347. 10.1039/C9QO00224C. [DOI] [Google Scholar]; b Köster J. M.; Häussinger D.; Tiefenbacher K. Activation of primary and secondary benzylic and tertiary alkyl(sp3)C-F bonds inside a self-assembled molecular container. Front. Chem. 2019, 6, 639. 10.3389/fchem.2018.00639. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Gambaro S.; La Manna P.; De Rosa M.; Soriente A.; Talotta C.; Gaeta C.; Neri P. The Hexameric Resorcinarene Capsule as a Brønsted Acid Catalyst for the Synthesis of Bis(heteroaryl)methanes in a Nanoconfined Space. Front. Chem. 2019, 7, 687. 10.3389/fchem.2019.00687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Giust S.; La Sorella G.; Sperni L.; Strukul G.; Scarso A. Substrate Selective Amide Coupling Driven by Encapsulation of a Coupling Agent Within a Self-Assembled Hexameric Capsule. Chem. Commun. 2015, 51, 1658–1661. 10.1039/C4CC08833F. [DOI] [PubMed] [Google Scholar]; b Cavarzan A.; Reek J. N. H.; Trentin F.; Scarso A.; Strukul G. Substrate Selectivity in the Alkyne Hydration Mediated by NHC–Au(I) Controlled by Encapsulation of the Catalyst Within a Hydrogen Bonded Hexameric Host. Catal. Sci. Technol. 2013, 3, 2898–2901. 10.1039/c3cy00300k. [DOI] [Google Scholar]
- Bräuer T. M.; Zhang Q.; Tiefenbacher K. Iminium Catalysis inside a Self-Assembled Supramolecular Capsule: Modulation of Enantiomeric Excess. Angew. Chem., Int. Ed. 2016, 55, 7698–7701. 10.1002/anie.201602382. [DOI] [PubMed] [Google Scholar]
- La Manna P.; De Rosa M.; Talotta C.; Gaeta C.; Soriente A.; Floresta G.; Rescifina A.; Neri P. The hexameric resorcinarene capsule as an artificial enzyme: ruling the regio and stereochemistry of a 1,3-dipolar cycloaddition between nitrones and unsaturated aldehydes. Org. Chem. Front. 2018, 5, 827–837. 10.1039/C7QO00942A. [DOI] [Google Scholar]
- Köster J. M.; Tiefenbacher K. Elucidating the Importance of Hydrochloric Acid as a Cocatalyst for Resorcinarene-Capsule-Catalyzed Reactions. ChemCatChem 2018, 10, 2941–2944. 10.1002/cctc.201800326. [DOI] [Google Scholar]
- a Zhang Q.; Tiefenbacher K. Terpene cyclization catalysed inside a self-assembled cavity. Nat. Chem. 2015, 7, 197–202. 10.1038/nchem.2181. [DOI] [PubMed] [Google Scholar]; b Zhang Q.; Catti L.; Pleiss J.; Tiefenbacher K. Terpene Cyclizations inside a Supramolecular Catalyst: Leaving-Group-Controlled Product Selectivity and Mechanistic Studies. J. Am. Chem. Soc. 2017, 139, 11482–11492. 10.1021/jacs.7b04480. [DOI] [PubMed] [Google Scholar]; c Zhang Q.; Rinkel J.; Goldfuss B.; Dickschat J. S.; Tiefenbacher K. Sesquiterpene Cyclisations Catalysed inside the Resorcinarene Capsule and Application in the Short Synthesis of Isolongifolene and Isolongifolenone. Nat. Catal. 2018, 1, 609–615. 10.1038/s41929-018-0115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Due to the high computational cost derived from the large number of atoms involved, we choose to conduct an in silico investigation using the ONIOM method on a reduced model of CR6, substituting the undecylic residues (the so-called “feet”) present in the hexameric capsule with the methyl ones. This approach was previously used by us (refs (18b), (18c), (19), and (22)).
- Weinhold F.; Landis C. R.. Valency and Bonding: A Natural Bond Orbital Donor–Acceptor Perspective; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Johnson E. R.; Keinan S.; Mori-Sánchez P.; Contreras-García J.; Cohen A. J.; Yang W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. 10.1021/ja100936w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The H-bond interaction can be classified as strong, medium, and weak as a function of the geometrical parameters such as D–H···A angle and D···A distance. See:Steiner T. The Hydrogen Bond in the Solid State. Angew. Chem., Int. Ed. 2002, 41, 48–76. . [DOI] [PubMed] [Google Scholar]
- a Fujii T.; Rondelez Y. Predator-Prey Molecular Ecosystems. ACS Nano 2013, 7, 27–34. 10.1021/nn3043572. [DOI] [PubMed] [Google Scholar]; b Dhers S.; Holub J.; Lehn J.-M. Coevolution and ratiometric behaviour in metal cation-driven dynamic covalent systems. Chem. Sci. 2017, 8, 2125–2130. 10.1039/C6SC04662B. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


