Abstract
Background:
The intensive study of predictive factors has strongly ameliorated the therapeutic flow-chart of metastatic colorectal cancer (mCRC) by allowing the selection of patients who benefit from specific therapies. For instance, in mRAS (mutated RAS) mCRC patients, anti-EGFR drugs (cetuximab and panitumumab) are not recommended; in this group of patients, the use of anti-angiogenic drugs (bevacizumab and aflibercept) is predominant. However, at progression to standard bevacizumab-based first-line chemotherapy, still to date, there are no studies to guide oncologists in the choice of the best second-line anti-angiogenic drug (bevacizumab beyond progression versus aflibercept).
Methods:
ARBITRATION is a prospective, observational study assessing efficacy differences between second-line fluorouracil/irinotecan (FOLFIRI)/bevacizumab versus FOLFIRI/aflibercept at progression to fluoropyrimidines, oxaliplatin and bevacizumab in mRAS mCRC patients. A test power of 80%, a median survival of 9 months from second-line treatment start and a hazard ratio of 0.67 between the two schedules were the basis for statistical design. The final sample will be 220 patients (110 per treatment). The significance will be verified with a two-tailed log-rank test with an alpha value of the I-type error of 5%. Time-to-outcome will be described by Kaplan–Meier curves and prognostic factors studied through multivariable analyses based on the Cox model. Secondary objectives include safety, responses’ duration and progression-free survival. A translational research will be conducted to measure several angiogenic proteins in patients’ serum before starting the therapy in order to evidence any angiogenic factor patterns related to outcome.
Discussion:
We present a large, prospective, observational study aiming to answer two scientific questions: (1) outcome differences between second-line treatments with FOLFIRI/bevacizumab beyond progression versus FOLFIRI/aflibercept in mRAS mCRC patients, (2) angiogenic factors’ patterns that could associate with efficacy and help oncologists to apply best the therapeutic anti-angiogenic strategies.
Trial registration:
The ARBITRATION trial (version 0.0, 13 April 2020) has been registered into the clinicaltrials.gov registry on 20 May 2020 with identifier NCT04397601.
Keywords: aflibercept, bevacizumab, colorectal cancer, irinotecan, RAS, second-line treatment
Background
Colorectal cancer is the third most frequent neoplasm after prostate and lung in men and breast and lung cancers in women from Western countries.1,2 About 30% of patients presents at diagnosis with advanced and unresectable disease; in these cases, administration of systemic chemotherapies is the mainstay treatment. In recent years, the association with new biologic therapies including anti-angiogenic (bevacizumab and aflibercept), anti-EGFR (panitumumab and cetuximab) and multi-kinase inhibitors (regorafenib) has improved results obtained with chemotherapies (fluorouracil, irinotecan and oxaliplatin).3 However, despite this progress in the cure of metastatic colorectal cancer (mCRC) patients, the prognosis is still dismal, with median survivals that rarely encompass 24–30 months.
The intensive study of predictive factors has strongly ameliorated the therapeutic flow-chart by allowing the selection of patients who benefit from specific therapies. In this context, the assessment of RAS (N- and K-) oncogene mutations is able to predict the response to anti-EGFR agents being mutated RAS (mRAS) mCRC patients resistant to these drugs.4–6 The reason is the constitutive activation of mRAS that does not sense the ligand-receptor disruption induced by anti-EGFR monoclonal antibodies (panitumumab and cetuximab). Thus, both European Society for Medical Oncology and National Comprehensive Cancer Network guidelines do not recommend administration of panitumumab or cetuximab in mRAS mCRC patients.7,8 In this group of patients, the use of anti-angiogenic drugs (bevacizumab and aflibercept) is predominant.
Bevacizumab is indicated in association with chemotherapy in both first- and second-line chemotherapies of mCRC9,10 and it has been demonstrated to be active independently from RAS status.11,12 Two pivotal trials have demonstrated that it can be continued with second-line chemotherapy (bevacizumab beyond progression), being associated with improved survival compared with chemotherapy alone.13,14 Furthermore, aflibercept plus FOLFIRI, another available anti-angiogenic schedule, is able to improve survival after progression to oxaliplatin-based chemotherapy15 with activity independent from the RAS status.16 Thus, FOLFIRI/aflibercept is widely accepted and recommended as a suitable option in second-line treatment of mRAS mCRC patients.
Still to date there are no studies to guide oncologists in the selection of the best anti-angiogenic drug (bevacizumab beyond progression versus aflibercept) after failure of the first-line chemotherapy in mRAS mCRC patients. The present study is the first observational, pragmatic, prospective study aimed to report outcomes of mCRC patients treated with FOLFIRI plus bevacizumab versus FOLFIRI plus aflibercept in second-line treatment of mRAS mCRC.
Rationale for evaluating angiogenic factors
Neoangiogenesis has a crucial role in tumour progression. Among many soluble factors involved in this process [including vascular endothelial growth factors (VEGFs), transforming growth factor-β and angiopoietin-1 and -2, fibroblast growth factors (FGFs), placental growth factor (PlGF)], VEGF-A and its receptors have a pivotal role and are understood best.17,18
Blockage of VEGF-A is responsible for the anti-tumor effects of bevacizumab, which is a humanized IgG1 monoclonal antibody specifically sequestering the VEGF-A and, in turn, inhibiting its angiogenic effects into tumors.9,10 However, aflibercept, a recombinant fusion protein between the Fc portion of IgG1 and binding portions of VEGFR 1 and 2 (VEGF Receptors 1 and 2), is an anti-angiogenic agent targeting both VEGF-A and PlGF;19 the last has been implicated in promoting angiogenesis in later phases of tumor progression.18,20 Additionally, PlGF is one of the pivotal angiogenic factors implicated in resistance to bevacizumab.21–24
Thus, the aim of the ARBITRATION study will be to evaluate VEGF-A and PlGF levels in patients’ serum before starting second-line chemotherapy with bevacizumab or aflibercept in order to evidence any pattern related to response and/or prognosis. The hypothesis is that knowledge of relative levels of VEGF and PIGF could direct the choice for the best anti-angiogenic drug in second-line treatment of mRAS mCRC patients.
Methods and design
ARBITRATION is an observational, prospective, no-profit, two-arm, open-label study, conducted under real-word conditions and designed to describe any risk of death differences among FOLFIRI/bevacizumab and FOLFIRI/aflibercept, two suitable options in second-line treatment of mRAS mCRC patients progressing at first-line chemotherapy based on fluoropyrimidines, oxaliplatin and bevacizumab. It will be conducted in an academic center (Istituto Nazionale Tumori di Napoli, IRCCS “G. Pascale” in Naples, Italy). The study includes a biomarker analysis (characterization of angiogenic factors before second-line treatment start).
Objectives
The primary objective is patients’ survival (overall survival; OS) according to the different schedules. Activity will be evaluated according to Response Evaluation Criteria In Solid Tumors (RECIST) criteria, version 1.1. Secondary objectives include: safety, responses’ duration, and progression-free survival (PFS). Toxicity will be graded according to the Common Terminology Criteria for Adverse Events the National Cancer Institute, version 4.0, 14 June 2010. Response duration is defined as the time elapsing from documentation of objective response (Complete Response [CR] or Partial Response [PR]) to tumor progression. PFS will be calculated from the treatment start until progression (according to RECIST), OS until death from any cause. Tertiary objective is the study of angiogenic biological markers (VEGF-A and PlGF) as predictors of outcome (see the “Translational research” section below). Intent-to-treat population and per-protocol population will be investigated simultaneously.
Ethical considerations
The protocol has been designed and developed according to the principles of the Good Clinical Practice guidelines of the International Conference on Harmonization and of the Declaration of Helsinki. The study has been approved by the Ethical Committee of the National Cancer Institute of Naples, Italy (No. 04/20). All patients will provide a written informed consent before starting treatment and blood samples collection. The privacy of patients included in the ARBITRATION study will be carefully protected by the Structure that has the responsibility for registration, collection and management of personal data. Patient names will be not provided to persons not involved in the study, with the exception of the Ministry of Health or Ethics Committees (as required by the current legislation only for inspection and control purposes). After registration in the study, a progressive numerical code will be assigned to the patients and will be used to identify them in all documents, electronic data systems or communications. A list to translate numerical codes in patients’ names will exist exclusively at the Secretariat of the ARBITRATION study.
Study design and statistical considerations
Assumptions for sample size calculation were derived from the literature and internal dataset reviews in plenary consensus discussion. A test power of 80% and a median survival of 9 months in second-line treatment of mCRC patients were chosen for statistical design. The final sample will be 220 patients (110 per arm) considering a hazard ratio of 0.67 between the two schedules as indicated by our bio-statisticians. The significance will be verified with a two-tailed log-rank test with an alpha value of the I-type error of 5%. Time-to-outcome will be described by Kaplan–Meier curves. Prognostic factors will be studied through multivariable analyses based on the Cox model. Descriptive statistics and associations between variables (including angiogenic biomarkers’ levels) will be conducted with χ2, Mann–Whitney U or analysis of variance tests, where appropriate. Confidence intervals will be calculated at 95%.
The basic characteristics of patients will be described for the categorical variables as total number and percentage and for the continuous variables as mean and standard deviation or median and interquartile range. Summary statistics on dose changes, interruptions, non-compliance, and treatment duration will be done.
Eligibility criteria
The inclusion criteria for enrollment in the ARBITRATION study overlap with the institutional clinical practice and are:
Cytological or histological diagnosis of mRAS colorectal adenocarcinoma;
Progression at first-line chemotherapy with fluoropyrimidines, oxaliplatin and bevacizumab;
Stage IV;
Age <75 years;
ECOG Performance Status 0 or 1;
Adequate organ system functions defined as follows: absolute neutrophil count ⩾1.5 × 109/L; hemoglobin ⩾9.0 g/dL; platelets ⩾90 × 109/L; total bilirubin ⩽1.5 × upper limit of normal (ULN); alanine aminotransferase ⩽2.5 × ULN; estimated glomerular filtration rate ⩾30 mL/min per 1.73 m2; spot urine [albumin/creatinine ratios (spot urine)] <500 mg/g (56 mg per/mmol); left ventricular ejection fraction ⩾45%;
Life expectancy >3 months;
Negative pregnancy test for all potentially childbearing women.
The main exclusion criteria are as follows:
Presence of primary non-treated stenosing colorectal neoplasm;
Active or uncontrolled infections;
Recent or active bleedings;
Coagulopathy of any cause;
Cardiovascular diseases including inadequately controlled hypertension, coronary artery disease, ischemic or hemorrhagic stroke, moderate/severe arrhythmias, aortic aneurysm requiring surgical repair, moderate/sever valvular heart diseases, recent deep vein thrombosis with or without pulmonary embolism, recent arterial thrombosis;
Other concomitant uncontrolled or uncompensated diseases (diabetes, chronic obstructive pulmonary disease, asthma, etc.);
Blood laboratory values contraindicating the study drugs at clinician evaluation;
Presence of brain metastases;
Refusal or inability to provide informed consent;
Impossibility to guarantee follow-up.
Therapeutic schedules
ARBITRATION is a prospective, observational, non-randomized study. The therapeutic schedules will be administered at oncologist discretion following the best clinical practice and are represented by FOLFIRI/bevacizumab and FOLFIRI/aflibercept. These schedules will consist of irinotecan (180 mg/m2), leucovorin (400 mg/m2), fluorouracil as an intravenous bolus of 400 mg/m2, and fluorouracil as a continuous 46-h infusion of 2400 mg/m2 in combination with either bevacizumab (5 mg/kg) (FOLFIRI/bevacizumab) or aflibercept (4 mg/kg) (FOLFIRI/aflibercept) on day 1 of each 14-day cycle. Atropine 0.25 mg sub cutis will be administered for the prevention of acute cholinergic events before irinotecan infusion. Premedication with antihistamines at standard doses and dexamethasone 4 mg will be applied before bevacizumab and aflibercept infusions. Administration of chemotherapy will be allowed until progression or unacceptable toxicity. In the case of unacceptable toxicity related to irinotecan the patients will continue bevacizumab or aflibercept and 5-FU until progression. In the case of unacceptable toxicity related to bevacizumab or aflibercept the patient will continue FOLFIRI until progression. In the case of unacceptable toxicity related to 5-FU the patients will continue with bevacizumab or aflibercept and irinotecan until progression.
Drugs’ dose modifications
Dose modifications will be applied in relation to occurrence of toxicities and, once modified, the dose will be the same in all subsequent cycles. Toxicity recovery (grade ⩽1) will be mandatory for re-challenge of therapy. A 20% reduction of chemotherapy will be allowed in the case of grade 2 hematologic or non-hematologic toxicities (except for alopecia). A dose reduction of 50% will be applied at the second relapse of grade 2 or at first grade 3 toxicities; however, after a third grade 2 or a second grade 3 toxicity or after the first appearance of grade ⩾2 cardiovascular chemotherapy will be permanently interrupted. Chemotherapy will be also stopped in the case of first occurrence of grade 4 haematologic or non-haematologic toxicity. Bevacizumab or aflibercept will be permanently stopped in the case of one of the following side effects: gastrointestinal perforation, grade ⩾3 thromboembolism, grade 4 hemorrhage, grade ⩾3 hypertension or proteinuria, congestive heart failure. Anti-hypertensive medications (angiotensin-converting enzyme inhibitors and/or calcium antagonists at standard doses) will be applied in the case of grade ⩾2 hypertension, or in the case of any symptomatic blood pressure increase of both diastolic and systolic values as compared with baseline. Chemotherapy will be discontinued in the case of administration delay for more than 4 weeks; in this case, the exclusive administration of anti-angiogenic drugs will be permitted. No prophylactic use of G-CSF or erythropoetin is planned.
Timing of exams and procedures
Screening phase
The screening phase of the ARBITRATION study will begin after signing of the informed consent and it will consist in the evaluation of the inclusion and exclusion criteria and in the collection of a venous blood sample (10 mL) for the determination of angiogenic biomarkers. According to our clinical practice, baseline visit and exams will be performed within 28 days before therapy start (clinical history, clinical examination, ECOG Performance Status, vital signs). Blood count, clinical biochemistry, CarcinoEmbryonic Antigen (CEA), Carcinoma Antigen 19.9 (CA19.9), electrocardiogram (ECG) and cardiac ultrasonography with evaluation of the ventricular ejection fraction will be performed in our local facilities. Total-body computed tomography (CT) with intravenous (i.v.) contrast or, if contraindicated, abdomen magnetic resonance imaging (MRI) and chest CT without i.v. contrast will be obtained. Pregnancy test will be done for all potentially childbearing women and must be negative. The test can also be repeated during second-line treatment if requested by the local Ethics Committee. However, all patients will be invited to use barrier methods for anti-conception.
Treatment phase
Clinical examination, evaluation of vital parameters, blood count and clinical biochemistry will be performed at day 1 of each cycle before administration of both chemotherapy schedules (every 2 weeks). Cardiological evaluation, CEA, CA19.9 and assessment of response to treatment through total-body CT with i.v. contrast (if contraindicated: abdomen MRI and chest CT without i.v. contrast) will be performed every 3 months.
End-of-treatment phase
The end-of-study visit should be done within 30 days of the last occurrence of administration of therapy for progression, toxicity and/or upon patient request. Additional therapies or follow-up procedures will be permitted at the discretion of the oncologist responsible for the medical treatment. The following data will be collected at the final visit: ECOG Performance Status, clinical examination, tumor status, vital signs, blood count and clinical biochemistry. The “patient death form” must be completed when the patient dies.
Follow-up
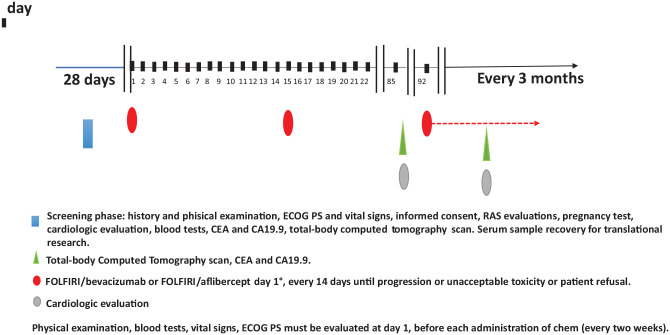
All patients included in the ARBITRATION study will be followed up through monitoring visits planned at our center every 3 months. At each visit, considering the high-risk of progression, all patients will perform clinical examination, blood count and clinical biochemistry, cardiological evaluation (ECG and cardiac ultrasonography), CEA, CA19.9 and total-body CT with i.v. contrast (if contraindicated: abdomen MRI and chest CT without i.v. contrast). Timing of exams and procedures is summarized in Figure 1 and Table 1.
Figure 1.
Ideal timeline of ARBITRATION study.
CA19.9, Carcinoma Antigen; CEA, CarcinoEmbryonic Antigen; ECOG PS, ECOG Performance Status; FOLFIRI, Fluorouracil, folinic acid and irinotecan; RAS, RAt Sarcoma.
Table 1.
Schedule of assessments for ARBITRATION study.
| Study assessments | Screening phase | Within 28 days | Second-line start | Every 2 weeks | Every 3 months | End-of-treatment | Follow-up |
|---|---|---|---|---|---|---|---|
| Informed consent | X | ||||||
| Eligibility criteria | X | ||||||
| Pregnancy test | X | X | |||||
| Concurrent medications | X | X | X | X | |||
| Cardiologic evaluation | X | X | X | ||||
| Anamnesis | X | X | X | X | |||
| Height | X | ||||||
| Weight | X | X | X | X | X | ||
| Clinical examination, ECOG PS, vital signs | X | X | X | X | X | ||
| Blood count and clinical biochemistry, CEA and CA19.9 | X | X | X | X | |||
| Urinalysis | X | X | X | X | |||
| Creatinine clearance | X | X | X | ||||
| Assessment of angiogenic factors | X | ||||||
| Total-body computed tomography with i.v. contrast (if contraindicated: abdomen MRI and chest computed tomography without i.v. contrast) | X | X | X | ||||
| Adverse events evaluation | X | X | X | X | X | ||
| Survival | X | X |
CA19.9, Carcinoma Antigen; CEA, CarcinoEmbryonic Antigen; i.v., intravenous; MRI, magnetic resonance imaging; ECOG PS, ECOG Performance Status.
Data management
All information related to the protocol will be registered in electronic Case Report Forms (eCRFs). Institutional Standard Operating Procedures (SOPs) have been applied for writing and presenting this protocol to the Ethical Committee and will ensure patient safety and the integrity of the clinical data (integral SOPs can be requested at monitoraggioscc@istitutotumori.na.it). Audits will be planned by the Director of Scientific Monitoring and Research Quality Assurance (Gianfranco De Feo). The Principal Investigator will be responsible for clarifying or responding to any eventual queries. At the end of enrollment, after resolution of all queries the database will be locked and analysed.
Patients’ study withdrawals
Patients’ withdrawals from study therapy will be registered in eCRFs. The information will include date and reason for cessation. Patients’ off study therapy will be followed up until death. Point censoring will be applied at a reasonably very low rate if the patient is lost to follow-up or the event does not occur within the study duration. Patients receiving an “incorrect” treatment or stopping the treatment early for any reason will be not excluded from the time-to-outcome analysis; all patients receiving at least one dose of treatment will be included in the descriptive statistics as well as in safety analysis.
Translational research
Biomarkers levels (VEGF-A and PlGF) will be evaluated in patients’ serum before starting the second-line chemotherapy and correlated with outcomes and therapy effectiveness. If available, they will be also assessed in Formalin-Fixed Paraffin Embedded (FFPE) specimens from primary and metastatic tumor tissues. These investigations will be conducted in the laboratories of the Bersagli Molecolari del Microambiente of the National Cancer Institute of Naples, IRCCS “G. Pascale”. Serum will be separated from blood and stored at −80°C. The levels of the VEGF-A and PlGF will be measured using a Quantikine ELISA kit (R&D Systems) according to the manufacturer’s instructions. In brief, 100 μL of samples will be added to 96-well plates coated with antibody to VEGF-A or PlGF and incubated for 2 h. After washing with wash buffer, wells will be filled with 200 μL of horseradish peroxidase-conjugated antibody for 1 h, then washed, and finally treated with substrate solution. Absorbance of the samples will be detected at 450 nm as well as 570 nm using a microplate spectrophotometer (BioRAD). The samples’ concentrations expressed in pg/mL will be assessed in triplicate and the average values used for statistical analyses. Comparisons between VEGF-A and PlGF levels will be evaluated by using the two-sided non-parametric Wilcoxon test and Cox proportional hazard model will be adopted to estimate their impact on OS (see also “Study design and statistical considerations” section above). Results will be also compared with referral values of VEGF-A and PlGF as derived from previous published studies. The serum levels of other cytokines [i.e. angiopoietin-1, angiopoietin-2, VEGF-C, stromal cell-derived factor-1, platelet-derived growth factor beta, basic fibroblast growth factor, interleukin-8, chemokine (C-C motif) ligand 2 and chemokine (C-C motif) ligand 5] will be subsequently evaluated in an independent ancillary study beyond the scope of the present one.
Data dissemination
Final results of the study will be presented at national and international meetings. The findings of the ARBITRATION study will be submitted for publication to peer-reviewed open access medical/scientific journals. If appropriate, upon Scientific Director authorization, the results of the study will be disseminated by press releases by the Istituto Nazionale Tumori di Napoli, IRCCS “G. Pascale”.
Discussion
The identification of predictive and prognostic biomarkers in mCRC patients is under intensive investigation. To date, RAS oncogene status (mutated versus wild-type) represents the only validated biological factor driving the therapeutic choices in mCRC. The large part of mRAS patients do not respond to anti-EGFR drugs (panitumumab and cetuximab) so that in their therapeutic flow-chart the use of anti-angiogenesis drugs is prevalent. Unfortunately, there are no biomarkers to guide the choice of anti-angiogenesis drugs in second-line treatment of mCRC after failure of standard folfox/bevacizumab chemotherapy. Furthermore, there are no prospective studies in the literature aiming to address this issue.
We have recently reported a real practice experience,25 which is on the basis of the ARBITRATION study design. We found that the two schedules (FOLFIRI/bevacizumab versus FOLFIRI/aflibercept) were well tolerated with median survivals of 8.9 and 12.1 months, respectively. Interestingly, although the administration of aflibercept was not allowed as maintenance therapy after a 6-month induction phase, FOLFIRI/aflibercept was associated with a lower risk of death. We hypothesized, at least theoretically, that the higher inhibition of other pro-angiogenic factors than bevacizumab could have accounted for better second-line results. In fact, aflibercept binds to VEGF-A with higher affinity than bevacizumab, and also it binds to PlGF. This additional target may be important in promoting tumor progression in bevacizumab-pretreated mCRC.21–24 Furthermore, the evidence that doubling bevacizumab concentration in second-line treatment does not overcome resistance to bevacizumab26 could indicate that other pro-angiogenic factors are involved in later phases of mCRC. Unfortunately, there are no clinical studies prospectively assessing VEGFs and PlGF and their relationships during treatment with sequential anti-angiogenic therapies; this is a great limit of the scientific literature about mCRC treatment. Furthermore, after the ARBITRATION results, we will take advantage of a large, independent and more complex analysis of many other angiogenic soluble factors from serum samples prospectively collected and stored in our institute.
The choice to design the ARBITRATION protocol as an observational non-randomized study was mainly due to the fact that both FOLFIRI/aflibercept and FOLFIRI/bevacizumab are largely suitable and approved therapeutic options as second-line treatment in mRAS mCRC. Thus, a “control” group could not be identified and randomization with more stringent eligibility criteria would have limited the patients’ enrollment rate. In this context, observational studies do not require randomization and are an acceptable tool to observe outcomes differences. Although randomized studies (randomized controlled trials; RCTs) are considered more reliable because randomization reduces errors and/or biases, very rarely are well-designed, prospective, observational studies (with a clear and a priori study design, accurate eligibility criteria, large sample size) discordant with RCTs.27,28 However, in order to limit the detrimental effects of lack of randomization, any confounding factors, in particular initial tumor burden, patients’ age, sex and Performance Status (PS), CEA and Lactate DeHydrogenase (LDH) levels, will be carefully described, analyzed (risk adjustment factors through Cox model multivariate analysis) and discussed a posteriori, in order to avoid any interference with clinicians’ choices and patients’ recruitment. This attitude of the ARBITRATION study, along with its mono-institutional nature, will contribute to giving more accurate outcome estimates and to controlling “real practice” confounding factors.
In conclusion, we present a large, prospective, observational protocol aiming to describe: (1) survival differences between second-line treatments with FOLFIRI/bevacizumab beyond progression versus FOLFIRI/aflibercept in mRAS mCRC patients, (2) angiogenic factors patterns that could associate with outcomes and help oncologists to apply best the therapeutic anti-angiogenic strategies.
Acknowledgments
We acknowledge the LILT (Lega Italiana per la Lotta contro i Tumori – sezione di Napoli) for precious collaboration and we are grateful to Dr. Alessandra Trocino, Librarian at IRCCS “G. Pascale” of Naples, Italy, for bibliographic assistance.
Footnotes
Author contributions: All authors have read and approved the manuscript before submission.
AO, SS, GN contributed to study protocol conception and writing.
MN, AT, CDA, LP contributed to planning the translational research.
OC, ADM, FT, CP, MC, AP, VA, FI, AAm, UP, MDM, PD contributed to planning the clinical aspects of the protocol including evaluations of primary and secondary objectives.
MT, PC, GB, GDF contributed to the statistical design and procedural aspects of the protocol.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Ethics approval and consent to participate: The “Comitato Etico IRCCS Pascale” (President Prof. Francesco Paolo Casavola, email: comitatoetico@istitutotumori.na.it, phone +39 081 5903397) approved the ARBITRATION study and its “consent to participate” form on 26 February 2020 (document no. 04/20). Written informed consent will be obtained from all participants to the ARBITRATION study.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The “Istituto Nazionale Tumori di Napoli – IRCCS G. Pascale”, via M. Semmola, 80131, Naples, Italy, will provide funds for translational research and publication costs related to the ARBITRATION study. This funding body had no role in the design of ARBITRATION study and will not have any in collection, execution, analyses or interpretation of data. Furthermore, the funding source will not have any role in the decision to submit results of the study for publication. There is no external or government funding.
ORCID iD: Michele Caraglia  https://orcid.org/0000-0003-2408-6091
https://orcid.org/0000-0003-2408-6091
Contributor Information
Alessandro Ottaiano, Innovative Therapies for Abdominal Metastases Unit, Istituto Nazionale Tumori di Napoli, IRCCS “G. Pascale”, via M. Semmola, Naples, Campania 80131, Italy.
Stefania Scala, Functional Genomics, Istituto Nazionale Tumori di Napoli, IRCCS “G. Pascale”, Naples, Italy.
Mariachiara Santorsola, Innovative Therapies for Abdominal Metastases Unit, Istituto Nazionale Tumori di Napoli, IRCCS “G. Pascale”, Naples, Italy.
Anna Maria Trotta, Functional Genomics, Istituto Nazionale Tumori di Napoli, IRCCS “G. Pascale”, Naples, Italy.
Crescenzo D’Alterio, Functional Genomics, Istituto Nazionale Tumori di Napoli, IRCCS “G. Pascale”, Naples, Italy.
Luigi Portella, Functional Genomics, Istituto Nazionale Tumori di Napoli, IRCCS “G. Pascale”, Naples, Italy.
Ottavia Clemente, Department of Abdominal Oncology, Istituto Nazionale Tumori di Napoli, IRCCS “G. Pascale”, Naples, Italy.
Anna Nappi, Department of Abdominal Oncology, Istituto Nazionale Tumori di Napoli, IRCCS “G. Pascale”, Naples, Italy.
Nicoletta Zanaletti, Department of Abdominal Oncology, Istituto Nazionale Tumori di Napoli, IRCCS “G. Pascale”, Naples, Italy.
Alfonso De Stefano, Department of Abdominal Oncology, Istituto Nazionale Tumori di Napoli, IRCCS “G. Pascale”, Naples, Italy.
Antonio Avallone, Department of Abdominal Oncology, Istituto Nazionale Tumori di Napoli, IRCCS “G. Pascale”, Naples, Italy.
Vincenza Granata, Department of Radiology, Istituto Nazionale Tumori di Napoli, IRCCS “G. Pascale”, Naples, Italy.
Carmen Notariello, Department of Abdominal Oncology, Istituto Nazionale Tumori di Napoli, IRCCS “G. Pascale”, Naples, Italy.
Amalia Luce, Department of Precision Medicine, University of Campania “L. Vanvitelli”, Naples, Italy Biogem Scarl, Institute of Genetic Research, Laboratory of Precision and Molecular Oncology, Ariano Irpino, Italy.
Angela Lombardi, Department of Precision Medicine, University of Campania “L. Vanvitelli”, Naples, Italy Biogem Scarl, Institute of Genetic Research, Laboratory of Precision and Molecular Oncology, Ariano Irpino, Italy.
Carmine Picone, Department of Radiology, Istituto Nazionale Tumori di Napoli, IRCCS “G. Pascale”, Naples, Italy.
Antonella Petrillo, Department of Radiology, Istituto Nazionale Tumori di Napoli, IRCCS “G. Pascale”, Naples, Italy.
Francesco Perri, Head and Neck Cancer Unit, Istituto Nazionale Tumori di Napoli, IRCCS “G. Pascale”, Naples, Italy.
Fabiana Tatangelo, Pathology Unit, Istituto Nazionale Tumori di Napoli, IRCCS “G. Pascale”, Naples, Italy.
Annabella Di Mauro, Pathology Unit, Istituto Nazionale Tumori di Napoli, IRCCS “G. Pascale”, Naples, Italy.
Vittorio Albino, Hepatobiliary Surgical Oncology Unit, Istituto Nazionale Tumori di Napoli, IRCCS “G. Pascale”, Naples, Italy.
Francesco Izzo, Hepatobiliary Surgical Oncology Unit, Istituto Nazionale Tumori di Napoli, IRCCS “G. Pascale”, Naples, Italy.
Daniela Rega, Colorectal Cancer Surgery Unit, Istituto Nazionale Tumori di Napoli, IRCCS “G. Pascale”, Naples, Italy.
Ugo Pace, Colorectal Cancer Surgery Unit, Istituto Nazionale Tumori di Napoli, IRCCS “G. Pascale”, Naples, Italy.
Massimiliano Di Marzo, Colorectal Cancer Surgery Unit, Istituto Nazionale Tumori di Napoli, IRCCS “G. Pascale”, Naples, Italy.
Paolo Chiodini, Medical Statistics Unit, University of Campania, Luigi Vanvitelli, Naples, Italy.
Gianfranco De Feo, Scientific Directorate, Istituto Nazionale Tumori di Napoli, IRCCS “G. Pascale”, Naples, Italy.
Paola Del Prete, Scientific Directorate, Istituto Nazionale Tumori di Napoli, IRCCS “G. Pascale”, Naples, Italy.
Gerardo Botti, Scientific Directorate, Istituto Nazionale Tumori di Napoli, IRCCS “G. Pascale”, Naples, Italy.
Paolo Delrio, Colorectal Cancer Surgery Unit, Istituto Nazionale Tumori di Napoli, IRCCS “G. Pascale”, Naples, Italy.
Michele Caraglia, Department of Precision Medicine, University of Campania “L. Vanvitelli”, Naples, Italy Biogem Scarl, Institute of Genetic Research, Laboratory of Precision and Molecular Oncology, Ariano Irpino, Italy.
Guglielmo Nasti, Innovative Therapies for Abdominal Metastases Unit, Istituto Nazionale Tumori di Napoli, IRCCS “G. Pascale”, Naples, Italy.
References
- 1. Herszényi L, Tulassay Z. Epidemiology of gastrointestinal and liver tumors. Eur Rev Med Pharmacol Sci 2010; 14: 249–258. [PubMed] [Google Scholar]
- 2. Altekruse SF, Kosary CL, Krapcho M, et al. SEER cancer statistics review, 1975–2007. Bethesda, MD: National Cancer Institute, http://seer.cancer.gov (2009, accessed 20 December 2018). [Google Scholar]
- 3. Lucas AS, O’Neil BH, Goldberg RM. A decade of advances in cytotoxic chemotherapy for metastatic colorectal cancer. Clin Colorectal Cancer 2011; 10: 238–244. [DOI] [PubMed] [Google Scholar]
- 4. Normanno N, Tejpar S, Morgillo F, et al. Implications for KRAS status and EGFR-targeted therapies in metastatic CRC. Nat Rev Clin Oncol 2009; 6: 519–527. [DOI] [PubMed] [Google Scholar]
- 5. Waring P, Tie J, Maru D, et al. RAS mutations as predictive biomarkers in clinical management of metastatic colorectal cancer. Clin Colorectal Cancer 2016; 15: 95–103. [DOI] [PubMed] [Google Scholar]
- 6. Jinesh GG, Sambandam V, Vijayaraghavan S, et al. Molecular genetics and cellular events of K-Ras-driven tumorigenesis. Oncogene 2018; 37: 839–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 2016; 27: 1386–1422. [DOI] [PubMed] [Google Scholar]
- 8. Messersmith WA. NCCN guidelines updates: management of metastatic colorectal cancer. J Natl Compr Canc Netw 2019; 17: 599–601. [DOI] [PubMed] [Google Scholar]
- 9. Venook AP, Niedzwiecki D, Lenz HJ, et al. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: a randomized clinical trial. JAMA 2017; 317: 2392–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Giampieri R, Salvatore L, Del Prete M, et al. Angiogenesis genotyping and clinical outcome during regorafenib treatment in metastatic colorectal cancer patients. Sci Rep 2016; 6: 25195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kubicka S, Greil R, André T, et al. Bevacizumab plus chemotherapy continued beyond first progression in patients with metastatic colorectal cancer previously treated with bevacizumab plus chemotherapy: ML18147 study KRAS subgroup findings. Ann Oncol 2013; 24: 2342–2349. [DOI] [PubMed] [Google Scholar]
- 12. Price TJ, Hardingham JE, Lee CK, et al. Impact of KRAS and BRAF gene mutation status on outcomes from the phase III AGITG MAX trial of capecitabine alone or in combination with bevacizumab and mitomycin in advanced colorectal cancer. J Clin Oncol 2011; 29: 2675–2682. [DOI] [PubMed] [Google Scholar]
- 13. Masi G, Salvatore L, Boni L, et al. Continuation or reintroduction of bevacizumab beyond progression to first-line therapy in metastatic colorectal cancer: final results of the randomized BEBYP trial. Ann Oncol 2015; 26: 724–730. [DOI] [PubMed] [Google Scholar]
- 14. Bennouna J, Sastre J, Arnold D, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol 2013; 14: 29–37. [DOI] [PubMed] [Google Scholar]
- 15. Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol 2012; 30: 3499–3506. [DOI] [PubMed] [Google Scholar]
- 16. Wirapati P, Pomella V, Vandenbosch B, et al. Velour trial biomarkers update: impact of RAS, BRAF, and sidedness on aflibercept activity. J Clin Oncol 2017; 35(Suppl. 15): 3538.28862883 [Google Scholar]
- 17. Angelucci A, Delle Monache S, Cortellini A, et al. “Vessels in the storm”: searching for prognostic and predictive angiogenic factors in colorectal cancer. Int J Mol Sci 2018; 19: pii: E299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Khan K, Cunningham D, Chau I. Targeting angiogenic pathways in colorectal cancer: complexities, challenges and future directions. Curr Drug Targets 2017; 18: 56–71. [DOI] [PubMed] [Google Scholar]
- 19. Tampellini M, Sonetto C, Scagliotti GV. Novel anti-angiogenic therapeutic strategies in colorectal cancer. Expert Opin Investig Drugs 2016; 25: 507–520. [DOI] [PubMed] [Google Scholar]
- 20. Van de Veire S, Stalmans I, Heindryckx F, et al. Further pharmacological and genetic evidence for the efficacy of PlGF inhibition in cancer and eye disease. Cell 2010; 141: 178–190. [DOI] [PubMed] [Google Scholar]
- 21. Fan F, Samuel S, Gaur P, et al. Chronic exposure of colorectal cancer cells to bevacizumab promotes compensatory pathways that mediate tumour cell migration. Br J Cancer 2011; 104: 1270–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kopetz S, Hoff PM, Morris JS, et al. Phase II trial of infusional fluorouracil, irinotecan, and bevacizumab for metastatic colorectal cancer: efficacy and circulating angiogenic biomarkers associated with therapeutic resistance. J Clin Oncol 2010; 28: 453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lieu CH, Tran H, Jiang ZQ, et al. The association of alternate VEGF ligands with resistance to anti-VEGF therapy in metastatic colorectal cancer. PLoS One 2013; 8: e77117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Van Cutsem E, Dochy E, Paccard C, et al. Impact of prior bevacizumab treatment on VEGFA and PlGF levels and patient outcomes: a retrospective analysis of baseline plasma samples from the VELOUR trial. Ann Oncol 2017; 28(Suppl. 3): mdx262.011. [Google Scholar]
- 25. Ottaiano A, Capozzi M, Tafuto S, et al. Folfiri-aflibercept vs. folfiri-bevacizumab as second line treatment of MRAS metastatic colorectal cancer in real practice. Front Oncol 2019; 9: 766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Iwamoto S, Takahashi T, Tamagawa H, et al. FOLFIRI plus bevacizumab as second-line therapy in patients with metastatic colorectal cancer after first-line bevacizumab plus oxaliplatin-based therapy: the randomized phase III EAGLE study. Ann Oncol 2015; 26: 1427–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med 2000; 342: 1887–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Silverman SL. From randomized controlled trials to observational studies. Am J Med 2009; 122: 114–120. [DOI] [PubMed] [Google Scholar]