Abstract
The aim was to assess the clinical effectiveness of drugs used in hospitalized patients with COVID-19 infection. We conducted a systematic review of randomized clinical trials assessing treatment with remdesivir, chloroquine, hydroxychloroquine, lopinavir, ritonavir, dexamethasone, and convalescent plasma, for hospitalized patients with a diagnosis of SARS-CoV-2 infection. The outcomes were mortality, clinical improvement, duration of ventilation, duration of oxygen support, duration of hospitalization, virological clearance, and severe adverse events. A total of 48 studies were retrieved from the databases. Eleven articles were finally included in the data extraction and qualitative synthesis of results. The meta-analysis suggests a benefit of dexamethasone versus standard care in the reduction of risk of mortality at day 28; and the clinical improvement at days 14 and 28 in patients treated with remdesivir. We can conclude that dexamethasone would have a better result in hospitalized patients, especially in low-resources settings. The analysis of the main treatments proposed for hospitalized patients is of vital importance to reduce mortality in low-income countries, since the COVID-19 pandemic had an economic impact worldwide with the loss of jobs and economic decline in countries with scarce resources.
The reviews of this paper are available via the supplemental material section.
Keywords: antivirals, clinical improvement, COVID-19, drugs, mortality, SARS-CoV-2
Introduction
In December 2019, the first cases of an emerging disease, currently called COVID-19, were presented. The spread of SARS-CoV-2 was declared a pandemic in March 2019, which generated a global health emergency.1 From the first cases, treatments based on drug repositioning were implemented.2
The disease has different degrees of severity, having asymptomatic infected people, people with a mild disease without pneumonia, or mild pneumonia. The severe degree, with dyspnea, bradypnea, hypoxia, pulmonary infiltrates, and the critical clinical condition, with respiratory failure, septic shock, or multi-organ failure, requires optimal treatment and hospital care.3
The fatality rates of this infection vary throughout the world, being higher in Africa, India, the USA, Mexico, and Brazil, where various comorbidities in the population such as hypertension, obesity, and diabetes increase fatality.4,5 Despite implementing recommended control measures in Latin America, the countries have been affected differently, with high fatality rates related to the differences in health services in different countries.6
The use of antivirals or other repositioning drugs is essential for clinical improvement and survival. In the absence of a specific treatment, in vitro and in vivo studies have been proposed to use existing drugs such as tocilizumab (monoclonal antibodies),7 remdesivir (antiviral),8 chloroquine and hydroxychloroquine (antimalarial),9,10 lopinavir and ritonavir (antiretrovirals),11 dexamethasone (glucocorticoid),12 convalescent plasma (neutralizing antibody),13 and traditional medicine.14,15 All of them showed beneficial effects in preclinical studies and some clinical studies; however, evaluating the treatments used in hospitalized patients is required. The aim was to assess the clinical effectiveness of antivirals used in hospitalized patients with COVID-19 infection.
Methods
A systematic review was carried out adhering to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines for conducting systematic reviews.16 The question in this review was:
What is the clinical effectiveness of different drugs employed for COVID-19 treatment in hospitalized patients? To conduct the review, the PICOS structure was followed according to these points:
Patients: adults hospitalized with a diagnosis of SARS-CoV-2 infection;
Intervention: treatment with the following drugs: remdesivir, chloroquine, hydroxychloroquine, lopinavir, ritonavir, dexamethasone, and convalescent plasma;
Comparison: standard care or placebo;
Outcomes: early mortality, late mortality, 28 days mortality, clinical improvement at 7 days, clinical improvement at 14 days, clinical improvement at 28 days, duration of ventilation (days), duration of oxygen support (days), duration of hospitalization (days), virological clearance, and severe adverse events;
Studies (type of): clinical trials published in peer-reviewed journals.
The search was carried out in PubMed, Scopus, and Web of Science databases, between 20 August 2020 and 9 September 2020. The references of the selected articles were also reviewed for an integral reading to include additional studies not indexed in these databases. The clinicaltrials.gov website was also scanned to obtain potential published reports of registered trials. The search strategies included the following keywords: remdesivir, chloroquine, hydroxychloroquine, lopinavir, ritonavir, dexamethasone, convalescent plasma, COVID-19, SARS-CoV2, and hospitalized. See the Supplemental Material file online for more details on the search strategies.
Studies that met the following criteria were included: (I) controlled clinical trials, (II) studies that included hospitalized patients with SARS-CoV-2 infection, (III) published in 2020, (IV) published in English, Chinese, Spanish, or Portuguese. The exclusion criteria were: (I) not being a clinical trial, (II) not treating hospitalized patients.
All references were managed with Mendeley® software. The selection of the articles began with the removal of duplicate articles and proceeded with the reading of the title and abstract, carried out independently by reviewers 1, 2, and 3. The final decision in cases of disagreement was based on the criteria of a fourth reviewer. In the second phase, the same reviewers read the full text of the studies to define which would be included for the extraction and synthesis of data. The data were stored in Microsoft Office Excel spreadsheets and organized in an instrument constructed by the authors considering: characteristics of the study (author, year, country), sample, study design, and characteristics of the results.
The risk of bias of the studies was evaluated using the ROB2 tool.17 The included studies were independently assessed by reviewers 1 and 2 (see Supplemental file).
The qualitative synthesis was developed following the assessed outcomes: early mortality, late mortality, 28 days mortality, clinical improvement at 7 days, clinical improvement at 14 days, clinical improvement at 28 days, duration of ventilation (days), duration of oxygen support (days), duration of hospitalization (days), virological clearance, and severe adverse events.
Statistical analysis
While use of meta-analyses was precluded for most relationships due to an insufficient number of studies, meta-analyses of inverse variance were conducted for three drugs (remdesivir, dexamethasone, and hydroxychloroquine) and four outcomes (clinical improvement, mortality at day 28, virological clearance, and severe adverse events). Meta-analyses were conducted with Revman v5.3 using pooled fixed effects odds ratios. The significance and the magnitude of heterogeneity across studies were calculated using the Q and I2 statistics. Odds ratios with 95% confidence intervals (CIs) were plotted for the association between drugs, compared with standard care or placebo.
Subgroup analyses were performed to examine differences according to clinical improvement at day 7, 14, or 28 in the treatment with remdesivir.
The review protocol was registered on the PROSPERO platform (CRD42020184436).
Results
Following the described PICOS structure, this systematic review retrieved 48 studies from the databases. After the removal of six duplicates, 42 articles were read in title and abstract. Twenty-seven were eliminated, resulting in 15 articles for full-text reading. Eleven articles were finally included in the data extraction and qualitative synthesis of results (Figure 1).
Figure 1.

Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart of the inclusion process in the systematic review.
The overall risk of bias in the reviewed articles was established at low-risk in two studies.10,18 The remaining eight studies were established at high risk or some concerns. More details can be seen in the Supplemental file.
Two articles reported using lopinavir–ritonavir mixtures, two studies reported remdesivir, three articles reported hydroxychloroquine, one study treated patients with chloroquine, two studies reported dexamethasone, and one study reported convalescent plasma. Patient samples ranged from 30 (the study with the fewest patients) to 6425 (the study with the most patients). The retrieved results were: early mortality (defined as mortality before 12 days), late mortality (defined as mortality after the 12th day), 28 days mortality, clinical improvement at 7, 14, and 28 days (defined by clinical scales), the mean duration of ventilation (in days), the mean duration of oxygen support (in days), the mean duration of hospitalization (in days), virological clearance (by laboratory tests), and severe adverse events (Table 1).
Table 1.
Main characteristics of the included studies.
| Author | Study site | Design | Sample | Intervention | Control | Outcomes reported |
|---|---|---|---|---|---|---|
| Cao19 | Hubei | Randomized, open-label, clinical trial | 99 intervention, 100 control | Lopinavir–ritonavir | Standard care | Mortality at day 28, early mortality, late mortality, clinical improvement at days 7, 14, and 28, duration of ventilation, duration of oxygen support, duration of hospitalization, virological clearance, and adverse events |
| Hung20 | Hong Kong | Randomized, open-label, clinical trial | 86 intervention, 41 control | Lopinavir–ritonavir–ribavirin–interferon Beta-1b | Lopinavir–ritonavir | Mortality at day 28, clinical improvement at day 7, duration of hospitalization, virological clearance, and adverse events |
| Wang18 | Hubei | Randomized, double-blinded, clinical trial | 158 intervention, 79 control | Remdesivir | Placebo | Mortality at day 28, early mortality, late mortality, clinical improvement at days 7, 14, and 28, duration of ventilation, duration of oxygen support, duration of hospitalization, virological clearance, and adverse events |
| Chen9 | Shanghai | Randomized, open-label, clinical trial | 15 intervention, 15 control | Hydroxychloroquine | Standard care | Virological clearance and adverse events |
| Gautret21 | France | Non-randomized, open-label, clinical trial | 20 intervention, 16 control | Hydroxychloroquine | Standard care | Virological clearance |
| Tang22 | China | Randomized, open-label, clinical trial | 75 intervention, 75 control | Hydroxychloroquine | Standard care | Virological clearance and adverse events |
| Borba10 | Brazil | Randomized, double-blinded, clinical trial | 41 intervention, 40 control | Chloroquine high dosage | Chloroquine low dosage | Mortality at day 28, early mortality, and adverse events |
| RECOVERY23 | United Kingdom | Randomized, open-label, clinical trial | 2104 intervention, 4321 control | Dexamethasone | Standard care | Mortality at day 28 |
| Li24 | Hubei | Randomized, open-label, clinical trial | 52 intervention, 50 control | Convalescent plasma | Standard care | Mortality at day 28, clinical improvement at days 7, 14, and 28, virological clearance, and adverse events |
| Spinner8 | USA | Randomized, open-label, clinical trial | 197 intervention A, 199 intervention B, 200 control | Remdesivir | Standard care | Clinical improvement at days 7, 14, and 28, and adverse events |
| Tomazini25 | Brazil | Randomized, open-label, clinical trial | 151 intervention, 148 control | Dexamethasone | Standard care | Mortality at day 28, and adverse events |
28 days mortality
Six clinical trials assessed the mortality of hospitalized patients at day 28,10,18,19,23–25 and one study reported mortality at day 30.20 The drugs applied as an intention of treatment reporting mortality were: lopinavir–ritonavir,19 lopinavir–ritonavir–ribavirin–interferon Beta-1b,20 remdesivir,18 chloroquine at high doses (600 mg),10 dexamethasone,23,25 and convalescent plasma24 (Table 2).
Table 2.
Reported outcomes in the included studies.
| Author | Intervention | Mortality at day 28 %(I)–%(C) | Early mortality %(I)–%(C) | Late mortality %(I)–%(C) | Clinical improvement at day 7 %(I)–%(C) | Clinical improvement at day 14 %(I)–%(C) | Clinical improvement at day 28 %(I)–%(C) | Duration of ventilation Median (I)–(C) |
Duration of oxygen support Median (I)–(C) |
Duration of hospital stay, days Median (I)–(C) |
Virological clearance %(I)–%(C) | Adverse events %(I)–%(C) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cao19 | Lopinavir–ritonavir | 19.2–25 | 19–27.1 | 19.3–23.1 | 6.1–2 | 45.5–30 | 78.8–70 | 4–5 | 12–13 | 14–16 | 60.3–58.6 (day 28) | 20–32.3 |
| Hung20 | Lopinavir–ritonavir–ribavirin–interferon Beta-1b | 0–0 | – | – | 4–8 (days to NEWS2 = 0) | – | – | – | – | 9–14.5 | 8–13 (median days) | 0–2 |
| Wang18 | Remdesivir | 14–13 | 11–15 | 14–10 | 3–2 | 27–23 | 65–58 | 7–15.5 | 19–21 | 25–24 | 75.6–83.1 (day 28) | 18–26 |
| Chen9 | Hydroxychloroquine | – | – | – | – | – | – | – | – | – | 86.7–93.3 (day 7) | 26.7–20 |
| Gautret21 | Hydroxychloroquine | – | – | – | – | – | – | – | – | – | 70–12.5 (day 6) | – |
| Tang22 | Hydroxychloroquine | – | – | – | – | – | – | – | – | – | 70.7–74.7 (day 28) | 3–0 |
| Borba10 | Chloroquine high dosage | – | 39–15 | – | – | – | – | – | – | – | – | 18.9–11.1 |
| RECOVERY23 | Dexamethasone | 22.9–25.7 | – | – | – | – | – | – | – | – | – | – |
| Li24 | Convalescent plasma | 15.7–24 | – | – | 9.6–9.8 | 32.7–17.6 | 51.9–43.1 | – | – | – | 87.2–37.5 (day 3) | 3.8–0 |
| Spinner8 | Remdesivir | – | – | – | 48*
56** 47*** |
77*
76** 68*** |
90*
90** 83*** |
– | – | – | – | 5*
5** 9*** |
| Tomazini25 | Dexamethasone | 56.3–61.5 | – | – | – | – | – | – | – | – | – | 3.3–6.1 |
Intervention A (10 days remdesivir).
Intervention B (5 days remdesivir).
Control.
%(C), % in the control group; %(I), % in the intervention group; C, control; I, intervention; NEWS, National Early Warning Score.
Early mortality
The early mortality, measured as the death produced before 12 days from patients allocation, was reported by a study using lopinavir–ritonavir,19 one trial using remdesivir,18 and one trial using chloroquine at high doses (600 mg)10 (Table 2).
Late mortality
The late mortality, measured as the death produced after 12 days from patients allocation, was only reported by two studies, one of them using lopinavir–ritonavir,19 and the other one using remdesivir18 (Table 2).
Clinical improvement
The clinical improvement was measured using the National Early Warning Score (NEWS) 2.26 It is an aggregate scoring system including six physiological parameters: respiration rate, oxygen saturation, systolic blood pressure, pulse rate, level of consciousness, and temperature. Clinical improvement at day 7 was reported by three studies,18,19,24 while three studies reported clinical improvement at days 14 and 28.18,19,24 One study reported the median time (in days) to reach a NEWS2 score of zero20 (Table 2). The study published by Spinner8 also reported clinical improvement at days 7, 14, and 28, but it is not declared which scale was used to assess the clinical improvement.
Duration of ventilation
This outcome was measured as the median number of days of duration of mechanical ventilation. It was reported by two studies using lopinavir–ritonavir19 and remdesivir18 (Table 2).
Duration of oxygen support
Two studies measured this outcome as the need for oxygen support through the nasal duct or mask, high-flow oxygen, or non-invasive ventilation.18,19 The duration of oxygen support was reported in median days (Table 2).
Duration of hospital stay
This outcome was reported in median days by three studies using lopinavir–ritonavir,19 lopinavir–ritonavir–ribavirin–interferon Beta-1b,20 and remdesivir18 (Table 2).
Virological clearance
This outcome was measured as the respiratory tract sample that was positive on Real Time-Polimerase Chain Reaction, and it was reported as the virus clearance in respiratory samples in days after the allocation. One study reported this outcome at day 3,24 one study at day 6,21 two studies at day 7,9,22 two at day 28,18,19 and one study reported as the median days to reach a zero viral load20 (Table 2).
Adverse events
In this review, the data were extracted from nine studies reporting any severe adverse events8–10,18,19,20,24,25,22 (it must be noted that a patient can develop one or more than one adverse event). Severe (or serious) adverse events were extracted as dichotomous data (Table 2). Of the nine studies that reported adverse events, only one has recorded no adverse events in any patient undergoing the intervention with lopinavir–ritonavir–ribavirin–interferon Beta-1b.20 It is necessary to highlight the incidence of adverse events in studies with lopinavir–ritonavir,19 hydroxychloroquine,9 remdesivir,18 and chloroquine.10
The conclusions reported by seven studies suggest that there is no benefit with the use of lopinavir–ritonavir,19 remdesivir,8,18 hydroxychloroquine,9,22 and chloroquine at high dosages.10 However, two studies reported that dexamethasone resulted in lower mortality at day 28 among patients with severe clinical conditions23 and a higher mean number of days alive and free from mechanical ventilation;25 both studies together make up a total sample of 6724 patients. Another trial suggests that triple viral treatment (lopinavir–ritonavir–ribavirin–interferon Beta-1b) was superior to lopinavir–ritonavir alone in a sample of 127 patients.20 Finally, one study suggests that hydroxychloroquine is significantly associated with viral load reduction in a sample of 36 patients21 (Table 3).
Table 3.
Primary outcomes and main conclusions of the included studies.
| Author | Sample | Intervention | Control | Primary outcomes | Conclusions |
|---|---|---|---|---|---|
| Cao19 | 99 intervention, 100 control | Lopinavir–ritonavir | Standard care | Time to clinical improvement | No benefit was observed with lopinavir–ritonavir |
| Hung20 | 86 intervention, 41 control | Lopinavir–ritonavir–ribavirin–interferon Beta-1b | Lopinavir–ritonavir | Time to virological clearance | Triple viral treatment was superior to lopinavir–ritonavir alone |
| Wang18 | 158 intervention, 79 control | Remdesivir | Placebo | Time to clinical improvement | No benefit was observed with remdesivir |
| Chen9 | 15 intervention, 15 control | Hydroxychloroquine | Standard care | Virological clearance | No benefit was observed with hydroxychloroquine |
| Gautret21 | 20 intervention, 16 control | Hydroxychloroquine | Standard care | Virological clearance | Hydroxychloroquine is significantly associated with viral load reduction |
| Tang22 | 75 intervention, 75 control | Hydroxychloroquine | Standard care | Virological clearance | No benefit was observed with hydroxychloroquine |
| Borba10 | 41 intervention, 40 control | Chloroquine high dosage | Chloroquine low dosage | Reduction in lethality | Higher doses of chloroquine should not be administered |
| RECOVERY23 | 2104 intervention, 4321 control | Dexamethasone | Standard care | Mortality at day 28 | Dexamethasone resulted in lower mortality |
| Li24 | 52 intervention, 50 control | Convalescent plasma | Standard care | Time to clinical improvement | No benefit was observed with convalescent plasma |
| Spinner8 | 197 intervention A, 199 intervention B, 200 control |
A: remdesivir 10 days B: remdesivir 5 days |
Standard care | Time to clinical improvement | No difference was observed with the remdesivir 10-days group |
| A difference was observed in the remdesivir 5-days group, with uncertain clinical importance | |||||
| Tomazini25 | 151 intervention 148 control |
Dexamethasone | Standard care | The mean number of days alive and free from mechanical ventilation during the first 28 days | The mean number of days alive and free from mechanical ventilation during the first 28 days was higher in the intervention group |
Meta-analysis
After discarding the individual articles that did not show conclusions in favor of the drugs used, five articles were included in the quantitative synthesis.
The result of two studies was integrated into the fixed-effects meta-analysis for comparing dexamethasone versus standard care in the reduction of mortality at day 28.23,25 This drug shows a low benefit for patients in severe clinical conditions [odds ratio (OR): 0.86; CI: 0.76–0.96] (Figure 2).
Figure 2.
Forest plot of drugs employed in hospitalized patients with SARS-CoV-2 infection. Comparison: dexamethasone versus standard care. Outcome: mortality at day 28.
CI, confidence interval; IV, inverse variance.
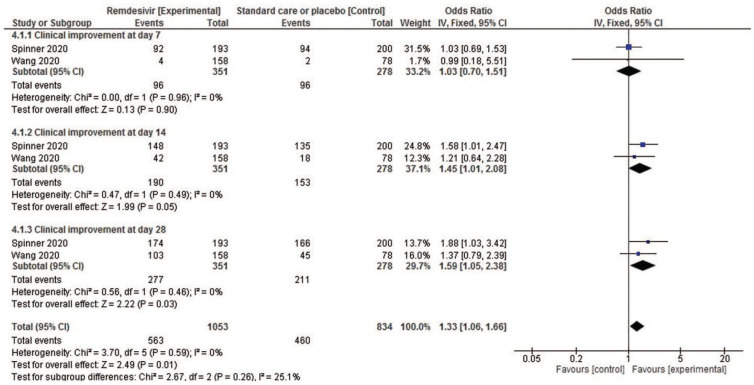
Two studies reporting remdesivir outcomes were compared to test the overall effect of this antiviral on clinical improvement on days 7, 14, and 28. The results of the fixed-effects meta-analysis show no association with clinical improvement at day 7 (OR: 1.03; CI: 0.70–1.51), but a very slight association with clinical improvement at day 14 (OR: 1.45; CI: 1.01–2.08) and at day 28 (OR: 1.59; CI: 1.05–2.38) (Figure 3). The drug was not associated with the presence of severe adverse events in the 10-days treatment group (OR: 0.57; CI: 0.36–0.92) (Figure S.1 in Supplemental file).
Figure 3.
Forest plot of drugs employed in hospitalized patients with SARS-CoV-2 infection. Comparison: remdesivir versus standard care or placebo. Outcome: clinical improvement.
CI, confidence interval; IV, inverse variance.
The results of three studies9,21,22 were meta-analyzed to establish comparisons between the use of hydroxychloroquine and standard care, using the outcome “virological clearance at day 7”. High heterogeneity was observed in the studies, so the meta-analysis of random effects suggests no benefits using this drug (OR: 1.64; CI: 0.17–15.67) (Figure S.2 in Supplemental file). Also, the results of two trials9,22 were meta-analyzed for the outcome of “severe adverse events” of hydroxychloroquine. No heterogeneity was observed; therefore, a fixed-effects meta-analysis was run. The results show no differences in the risk of using the drug or the standard care (OR: 1.96; CI: 0.44–8.71) (Figure S.3 in Supplemental file).
Discussion
With the focus on adult hospitalized patients, following the PICOS strategy, this systematic review was able to identify nine clinical trials that were very heterogeneous among themselves, due to experimentation with different drugs and different administration regimens. In total, 8282 patients were included in hospitals in China, France, Brazil, the United Kingdom, and the United States.
Regarding the risk of bias of the included studies, it is essential to note that there were included eight with a high risk of bias or some concerns. The lack of blinding affected the risk of bias, mainly in studies launched under emergency conditions due to the international health crisis.
This study differs from another recent systematic review that evaluated antiviral drugs in patients with suspected, probable, or confirmed diagnosis of SARS-CoV-2 infection.27 Our study focuses only on hospitalized patients since, in some low-income Latin American countries, the epidemic has not yet reached its peak, and hospitals are experiencing saturation in their facilities.28–30
The only drugs reported by more than one article published in peer-reviewed journals were hydroxychloroquine, remdesivir, and dexamethasone.
Hydroxychloroquine did not show benefits in virological clearance in our meta-analysis. Also, serious adverse events reported in another systematic review27 have led to the conclusion that the use of hydroxychloroquine is not recommended.
Regarding remdesivir, our meta-analysis has shown some association with clinical improvement on days 14 and 28. Furthermore, we observed that there were no association of this drug with adverse events.
Concerning the use of dexamethasone, it has shown low benefits in our meta-analysis in patients with severe clinical conditions for mortality at day 28.
In general, individual studies have concluded that no benefit was observed with lopinavir–ritonavir,19 chloroquine,10 or convalescent plasma.24
Although the meta-analyzed results of remdesivir may seem encouraging, its use in low-resource countries is determined by the cost of this drug. So, it can be assumed that up to now, the only drug with a large sample and demonstrated effectiveness has been dexamethasone, based on clinical trials conducted by the RECOVERY Collaborative Group and Tomazini.23,25 Although only one of these two studies has reported adverse events, their conclusions are encouraging, mainly due to its low cost and easy accessibility in low-resource settings, as in Latin American countries. This result is similar to that reported by Siemieniuk in a review published a few months ago;27 this would indicate that studies may continue to produce relevant results for low-resource countries until a vaccine is available.
Among the limitations of this study, the rapid generation of new knowledge in times of the pandemic can potentially affect the timeliness of this review in a short time. Another limitation is the heterogeneity and high risk of bias in the studies. In this review, we chose not to issue recommendations with the GRADE methodology, due to heterogeneity and high risk of bias. Another limitation is that not all studies assessed mortality outcomes in the same way. Some evaluated only early mortality, others evaluated late mortality, and others, mortality at day 28; this is another crucial point on heterogeneity.
Among the strengths of this study, focusing solely on inpatient studies allowed us to review a larger volume of outcomes in these studies. The analysis of the main treatments proposed for hospitalized patients is of vital importance to reduce mortality in low-income countries, since the COVID-19 pandemic had an economic impact worldwide with the loss of jobs and economic decline31 in countries with scarce resources. In these settings, the use of dexamethasone may be an affordable option. While there is no vaccine available, in the meantime other studies are still being developed all over the world from different therapeutic focus, as part of a joint effort by all academics, clinicians, and scientists around the world.32–41 As of today, social distancing is so far the most crucial measure in controlling the spread of the disease.5
Conclusion
Dexamethasone would have a better result in hospitalized patients, although a detailed report of its adverse events is necessary. In Latin American countries, it is necessary to wait for the conclusion of some studies in the recruitment phase in Argentina and Mexico.
Supplemental Material
Supplemental material, sj-pdf-1-tar-10.1177_17534666211007214 for Clinical effectiveness of drugs in hospitalized patients with COVID-19: a systematic review and meta-analysis by Roberto Ariel Abeldaño Zuñiga, Silvia Mercedes Coca, Giuliana Florencia Abeldaño and Ruth Ana María González-Villoria in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-2-tar-10.1177_17534666211007214 for Clinical effectiveness of drugs in hospitalized patients with COVID-19: a systematic review and meta-analysis by Roberto Ariel Abeldaño Zuñiga, Silvia Mercedes Coca, Giuliana Florencia Abeldaño and Ruth Ana María González-Villoria in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-3-tar-10.1177_17534666211007214 for Clinical effectiveness of drugs in hospitalized patients with COVID-19: a systematic review and meta-analysis by Roberto Ariel Abeldaño Zuñiga, Silvia Mercedes Coca, Giuliana Florencia Abeldaño and Ruth Ana María González-Villoria in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-4-tar-10.1177_17534666211007214 for Clinical effectiveness of drugs in hospitalized patients with COVID-19: a systematic review and meta-analysis by Roberto Ariel Abeldaño Zuñiga, Silvia Mercedes Coca, Giuliana Florencia Abeldaño and Ruth Ana María González-Villoria in Therapeutic Advances in Respiratory Disease
Footnotes
Author contributions: RAAZ contributed to the development of the research project.
SCM, GFA, and RAMGV performed data collection, analyzed, and interpreted the results.
RAAZ, SCM, and RAMGV wrote the article. All authors reviewed and approved the final version.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Supplemental material: The reviews of this paper are available via the supplemental material section.
Contributor Information
Roberto Ariel Abeldaño Zuñiga, Guillermo Rojas Mijangos SN, Ciudad Universitaria, Miahuatlan de Porfirio Diaz, Oaxaca, 70800, Mexico; Postgraduate Department, University of Sierra Sur, Miahuatlan de Porfirio Diaz, Oaxaca, 70800, Mexico.
Silvia Mercedes Coca, Public Health Research Institute, University of Sierra Sur, Miahuatlan de Porfirio Diaz, Oaxaca, Mexico.
Giuliana Florencia Abeldaño, School of Medicine, University of Sierra Sur, Miahuatlan de Porfirio Diaz, Oaxaca, Mexico.
Ruth Ana María González-Villoria, Postgraduate Department, University of Sierra Sur, Miahuatlan de Porfirio Diaz, Oaxaca, Mexico.
References
- 1. Sohrabi C, Alsafi Z, O’Neill N, et al. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19). Int J Surg 2020; 76: 71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sanders JM, Monogue ML, Jodlowski TZ, et al. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA 2020; 323: 1824–1836. [DOI] [PubMed] [Google Scholar]
- 3. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med 2020; 8: e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grech V. Unknown unknowns – COVID-19 and potential global mortality. Early Hum Dev 2020; 144: 105026. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6. Simbana-Rivera K, Gomez-Barreno L, Guerrero J, et al. Interim analysis of pandemic Coronavirus disease 2019 (COVID-19) and the SARS-CoV-2 virus in Latin America and the Caribbean: morbidity, mortality and molecular testing trends in the region. medRxiv 2020. DOI: 10.1101/2020.04.25.20079863. [DOI] [Google Scholar]
- 7. Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci 2020; 117: 10970–10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Spinner CD, Gottlieb RL, Criner GJ, et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA 2020; 324: 1048–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen J, Liu D, Liu L, et al. A pilot study of hydroxychloroquine in treatment of patients with moderate COVID-19. J Zhejiang Univ Med Sci 2020; 49: 215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Borba MGS, Val FFA, Sampaio VS, et al. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. JAMA Netw Open 2020; 3: e208857. [DOI] [PubMed] [Google Scholar]
- 11. Osborne V, Davies M, Lane S, et al. Lopinavir-ritonavir in the treatment of COVID-19: a dynamic systematic benefit-risk assessment. Drug Saf 2020; 43: 809–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Johnson RM, Vinetz JM. Dexamethasone in the management of COVID-19. BMJ 2020; m2648. [DOI] [PubMed] [Google Scholar]
- 13. Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA 2020; 323: 1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ren J, Zhang A-H, Wang X-J. Traditional Chinese medicine for COVID-19 treatment. Pharmacol Res 2020; 155: 104743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xu J, Zhang Y. Traditional Chinese medicine treatment of COVID-19. Complement Ther Clin Pract 2020; 39: 101165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019; l4898. [DOI] [PubMed] [Google Scholar]
- 18. Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020; 395: 1569–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe COVID-19. N Engl J Med 2020; 382: 1787–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hung IF-N, Lung K-C, Tso EY-K, et al. Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet 2020; 395: 1695–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gautret P, Lagier J, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents 2020; 56: 105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tang W, Cao Z, Han M, et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ 2020; 369: m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. The RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with COVID-19 — preliminary report. N Engl J Med 2021; 384: 693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li L, Zhang W, Hu Y, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19. JAMA 2020; 324: 460–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tomazini BM, Maia IS, Cavalcanti AB, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA 2020; 324: 1307–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Royal College of Physicians. National Early Warning Score (NEWS) 2. London: RCP, https://www.rcplondon.ac.uk/projects/outputs/national-early-warning-score-news-2 (2017, accessed 8 September 2020). [Google Scholar]
- 27. Siemieniuk RA, Bartoszko JJ, Ge L, et al. Drug treatments for COVID-19: living systematic review and network meta-analysis. BMJ 2020; 370: m2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Friedman J, Calderón-Villarreal A, Bojorquez I, et al. Excess out-of-hospital mortality and declining oxygen saturation: the sentinel role of EMS data in the COVID-19 crisis in Tijuana, Mexico. Ann Emerg Med. 2020; 76: 413–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ahumada H, Espina S, Navajas F. COVID-19 with uncertain phases: estimation issues with an illustration for Argentina. SSRN Electron J. Epub ahead of print 2020. DOI: 10.2139/ssrn.3633500. [DOI] [Google Scholar]
- 30. Díaz-Guio DA, Villamil-Gómez WE, Dajud L, et al. Will the Colombian intensive care units collapse due to the COVID-19 pandemic? Travel Med Infect Dis 2020; 101746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ayittey FK, Ayittey MK, Chiwero NB, et al. Economic impacts of Wuhan 2019-nCoV on China and the world. J Med Virol 2020; 92: 473–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Saha RP, Sharma AR, Singh MK, et al. Repurposing drugs, ongoing vaccine, and new therapeutic development initiatives against COVID-19. Front Pharmacol. Epub ahead of print 19 August 2020. DOI: 10.3389/fphar.2020.01258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bhattacharya M, Sharma AR, Patra P, et al. A SARS-CoV-2 vaccine candidate: In-silico cloning and validation. Informatics Med Unlocked 2020; 20: 100394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chakraborty C, Sharma AR, Sharma G, et al. Extensive partnership, collaboration, and teamwork is required to stop the COVID-19 outbreak. Arch Med Res. Epub ahead of print May 2020. DOI: 10.1016/j.arcmed.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bhattacharya M, Sharma AR, Patra P, et al. Development of epitope-based peptide vaccine against novel coronavirus 2019 (SARS-COV-2): immunoinformatics approach. J Med Virol 2020; 92: 618–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Saha A, Sharma AR, Bhattacharya M, et al. Tocilizumab: a therapeutic option for the treatment of cytokine storm syndrome in COVID-19. Arch Med Res 2020; 51: 595–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chakraborty C, Sharma AR, Bhattacharya M, et al. COVID-19: consider IL-6 receptor antagonist for the therapy of cytokine storm syndrome in SARS-CoV-2 infected patients. J Med Virol 2020; 92: 2260–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chakraborty C, Sharma AR, Bhattacharya M, et al. Consider TLR5 for new therapeutic development against COVID-19. J Med Virol 2020; 92: 2314–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Saha A, Sharma AR, Bhattacharya M, et al. Probable molecular mechanism of remdesivir for the treatment of COVID-19: need to know more. Arch Med Res 2020; 51: 585–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chakraborty C, Sharma AR, Sharma G, et al. SARS-CoV-2 causing pneumonia-associated respiratory disorder (COVID-19): diagnostic and proposed therapeutic options. Eur Rev Med Pharmacol Sci 2020; 24: 4016–4026. [DOI] [PubMed] [Google Scholar]
- 41. Chakraborty C, Sharma A, Bhattacharya M, et al. The 2019 novel coronavirus disease (COVID-19) pandemic: a zoonotic prospective. Asian Pac J Trop Med 2020; 13: 242–246. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tar-10.1177_17534666211007214 for Clinical effectiveness of drugs in hospitalized patients with COVID-19: a systematic review and meta-analysis by Roberto Ariel Abeldaño Zuñiga, Silvia Mercedes Coca, Giuliana Florencia Abeldaño and Ruth Ana María González-Villoria in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-2-tar-10.1177_17534666211007214 for Clinical effectiveness of drugs in hospitalized patients with COVID-19: a systematic review and meta-analysis by Roberto Ariel Abeldaño Zuñiga, Silvia Mercedes Coca, Giuliana Florencia Abeldaño and Ruth Ana María González-Villoria in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-3-tar-10.1177_17534666211007214 for Clinical effectiveness of drugs in hospitalized patients with COVID-19: a systematic review and meta-analysis by Roberto Ariel Abeldaño Zuñiga, Silvia Mercedes Coca, Giuliana Florencia Abeldaño and Ruth Ana María González-Villoria in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-4-tar-10.1177_17534666211007214 for Clinical effectiveness of drugs in hospitalized patients with COVID-19: a systematic review and meta-analysis by Roberto Ariel Abeldaño Zuñiga, Silvia Mercedes Coca, Giuliana Florencia Abeldaño and Ruth Ana María González-Villoria in Therapeutic Advances in Respiratory Disease