Abstract
Background:
Significant achievements in the treatment of chronic thromboembolic pulmonary hypertension (CTEPH) have provided effective therapeutic options for most patients. However, the true impact of the changed landscape of CTEPH therapies on patients’ management and outcomes is poorly known. We aimed to characterize the incidence, clinical characteristics, and outcomes of CTEPH patients in the modern era of CTEPH therapies.
Methods:
We analyzed the data of CTEPH adults enrolled in the prospective multicenter registry.
Results:
We enrolled 516 patients aged 63.8 ± 15.4 years. The incidence rate of CTEPH was 3.96 per million adults per year. The group was burdened with several comorbidities. New oral anticoagulants (n = 301; 58.3%) were preferred over vitamin K antagonists (n = 159; 30.8%). Pulmonary endarterectomy (PEA) was performed in 120 (23.3%) patients and balloon pulmonary angioplasty (BPA) in 258 (50%) patients. PEA was pretreated with targeted pharmacotherapy in 19 (15.8%) patients, and BPA in 124 (48.1%) patients. Persistent CTEPH was present in 46% of PEA patients and in 65% of patients after completion of BPA. Persistent CTEPH after PEA was treated with targeted pharmacotherapy in 72% and with BPA in 27.7% of patients. At a mean time period of 14.3 ± 5.8 months, 26 patients had died. The use of PEA or BPA was associated with better survival than the use of solely medical treatment.
Conclusions:
The modern population of CTEPH patients comprises mostly elderly people significantly burdened with comorbid conditions. This calls for treatment decisions that are tailored individually for every patient. The combination of two or three methods is currently a frequent approach in the treatment of CTEPH.
Clinical Trial Registration:
clinicaltrials.gov/ct2/show/NCT03959748
Keywords: chronic thromboembolic pulmonary hypertension, epidemiology, registry
Introduction
Chronic thromboembolic pulmonary hypertension (CTEPH) is a rare and progressive disease caused by obstruction of the pulmonary arteries (PAs) by organized thrombi with accompanying precapillary arteriopathy.1–4 Prognosis in this disease has significantly improved5 with the advent of pulmonary endarterectomy (PEA), which has provided hemodynamic normalization and improved clinical outcomes,2,6 especially in patients with central localization of pulmonary obstruction.
The group of CTEPH patients was well characterized in a large prospective international registry which enrolled patients between February 2007 and January 2009.7 As the phenotype of CTEPH patients may be population dependent,8 several national reports as summarized in Table 17,9–29 were of added value. Most of them, however, recruited patients before the marketing authorization of riociguat by the European Medicines Agency through the European Union (27/03/2014), and before the first experience with balloon pulmonary angioplasty (BPA) outside of Japan.30 Significant achievements in the treatment of CTEPH in the last decade have provided effective therapeutic options for most CTEPH patients, even those with distal localization of the thrombi. This was mainly due to refinement of the technique of BPA by Japanese physicians.31,32 However, the true impact of the changed landscape of CTEPH therapies on patients’ management and outcomes is poorly known and thus far the results of only one prospective CTEPH registry enrolling patients in the BPA era have been published.25 Therefore, in the present study based on the multicenter national registry (Polish Registry of Pulmonary Hypertension; BNP-PL) we assessed the prevalence, incidence, clinical characteristics and outcomes of patients with CTEPH recruited in the modern era of CTEPH therapies.
Table 1.
Summary of national and international CTEPH registries.
| Country (registry name) | Author | Time period | Prospective/ retrospective | International | Centers (n) | Patients (n) | Age (years) | Women (n, %) | Operable disease (n, %) | Treatment (n, %) | Prevalence (1/1000,000) | Incidence (1/1000,000/year) | Survival (year, %) | Follow-up (years) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PEA | BPA | PH-specific drugs | ||||||||||||||
| Switzerland | Fischler et al.9 | January 1999–December 2004 | P | No | 9 | 63 | 63 ± 13 | 37 (59) | – | 7 (11) | 0 | 46 (73) | 7 | – | 1: 90 3: 78 5: 62 |
2 ± 1.4a |
| UK | Condliffe et al.10,11 | January 2001–June 2006 | P | No | 5 | 469 | 58 ± 15 | 234 (50) | 321 (68) | 236 (50) | 0 | 228 (49) | – | 1.1–1.75b | 1: 88 versus 82c
3: 76 versus 70c |
Till Jan 2007 |
| Austriag | Bonderman et al.12 | 1996–2007 | R | Yes | 4 | 433 | 58 (46–67) | 227 (52) | 282 (65) | 248 (57) | 0 | – | – | – | – | – |
| International Registry | Pepke-Zaba et al.7, Delcroix et al.13 | February 2007–January 2009 | P | Yes | 27 | 679 | 63 (51–72) | 339 (50) | 442 (65) | 404 (59) | 0 | 313 (46) | – | – | 1: 93 (90–95) versus 88 (83–91)c
2: 91 (87–93) versus 79 (74–83)c 3: 89 (86–92) versus 70 (64–76)c |
3.5 |
| Spain (REHAP) | Escribano-Subias et al.14 | January 1998–June 2008 | R | No | 31 | 162 | 61 ± 15 | 97 (60) | – | 49 (30) | 0 | – | 3.2 | 0.9 | 1: 93 3: 75 5: 65 |
3.7 ± 4.2a |
| UK (ASPIRE) | Hurdman et al.15 | February 2001–February 2010 | P | No | 1 | 242 | 61 ± 15 | 131 (54) | 180 (74) | 128 (53) | 0 | 208 (86) | – | 0.3–3.7b | 1: 89 3: 71 |
2.9 ± 2.1a |
| Portugal | Baptista et al.16 | 2008–2010 | P | No | 5 | 33 | 60 ± 12.5 | 23 (70) | – | 5 (15) | 0 | 22 (67) | – | 1.1 | 1: 93.9 | – |
| Switzerland | Mueller-Mottet et al.17 | 1998–2012 | P | No | 13 | 249 | 63 ± 14 | 129 (52) | – | 35 (14) | 0 | 217 (87) | – | – | 1: 91 2: 84 3: 77 4: 73 |
3.6a |
| Spain (REHAP) | Escribano-Subias et al.18 | January 2006–December 2013 | R + P | No | 31 | 391 | 63.7 (48.0–73.3) | 227 (58) | – | 122 (31) | 0 | 274 (70) | – | – | 1: 97 (90–95) versus 93 (83–91)c
2: 91 (87–93) versus 81 (74–83)c 3: 86 (86–92) versus 65 (64–76)c |
– |
| Korea | Park et al.19 | September 2008–October 2011 | R | No | 5 | 134 | 58.3 ± 15.9 | 76 (57) | 105 (78) | 28 (21) | 0 | 99 (74) | – | – | 96 versus 82.4c | Till Dec 2011 |
| Sweden (SPAHR) | Radegran et al.20 | 2008–2014 | P | No | 7 | 183 | 70 ± 14 | 92 (50) | – | 60 (33) | 0 | – | 19 | 2 | 1: 96 versus 91c
3: 89 versus 75c 5: 86 versus 69c |
Till Dec 2014 |
| Germany (Giessen PH) | Gall et al.21 | March 1993–October 2011 | R + P | No | 1 | 459 | 62 ± 13 | 258 (56) | – | 91 (20) | 0 | 256 (83)d | – | – | 1: 96.1 versus 84.5c
3: 87.1 versus 72.5c 5: 76.7 versus 61.8c |
3.1e |
| Portugal | Santos et al.22 | January 2005–December 2016 | R | No | 1 | 47 | 64 ± 14 | 35 (74) | 37 (79) | 14 (30) | 3 (6) | 32 (68) | – | – | 1: 95 (83–99) 3: 81 (63–91) 5: 81 (63–91) |
4.4 ± 3.3a |
| UK | Martinez et al.23 | January 2001–March 2012 | R | No | – | 283 | 68.2f | 153 (54) | – | – | – | – | – | – | 2: 73.2 | – |
| Latvia | Skride et al.24,28,29 | September 2007–December 2016 | P | No | 1 | 44 | 67 (47–73) | 27 (61) | – | 7 (16) | 0 | 44 (100) | 15.7 | 5.1 | 1: 83.8 3: 59.0 5: 44.2 |
1.5 (0.6–2.8)e |
| Germany | Kramm et al.25 | January 2016–December 2016 | P | No | 3 | 392 | 63.5 ± 15 | 196 (50) | – | 197 (50) | 49 (13) | 220 (56) | – | 5.7 | – | – |
| Greece | Bazmpani et al.26 | September 2009–October 2016 | R | No | 2 | 27 | 59.3 ± 15.1 | 16 (59) | 18 (67) | 10 (37) | 2 (7) | 20 (74) | – | 1.9e | ||
| Ukraine | Radchenko et al.27 | June 2014–July 2018 | P | No | 1 | 52 | – | – | – | 6 (12) | 0 | 50 (96) | – | – | 1: 88 3: 83 5: 83 |
4.3e |
| Poland | Kopeć et al. (current paper) | March 2018–August 2019 | R + P | No | 16 | 516 | 63.8 ± 15.4 | 260 (50) | 154 (29.8) | 120 (23.3) | 258 (50.0) | 378 (74) | 16.4 | 3.96 | 1: 98 versus 92c | 1.2 ± 0.5a |
Mean ± standard deviation.
Increased during observation period.
Operated (PEA and/or BPA) versus non-operated.
Data available for only 310 patients.
Median and interquartile range.
Excluded patients ⩾85 years.
Also centers from Germany, Czech Republic and Slovakia.
BPA, balloon pulmonary angioplasty; CTEPH, chronic thromboembolic pulmonary hypertension; P, prospective analysis; PEA, pulmonary endarterectomy; PH, pulmonary hypertension; R, retrospective analysis.
Methods
Registry design and CTEPH diagnosis
The design of the BNP-PL registry (https://clinicaltrials.gov/ct2/show/NCT03959748), enrollment criteria, and data collection were recently described in detail.33–35 The CTEPH adult arm of the registry enrolls adults with CTEPH from all 16 CTEPH reference centers in Poland accredited by the National Health Fund (NHF) for medical treatment of CTEPH. This ensures that all diagnosed patients in Poland are included in the registry. Among the enrolled centers, eight are actively involved in the BPA programme. Patients qualifying for PEA are referred to one of two cardiac surgery centers experienced in PEA. The protocol of the study was reviewed and accepted by the Bioethical Committee of Physicians and Dentists Chamber in Krakow (L.dz.OIL/KBL/27/2018). All patients signed an informed consent before enrollment in the study.
For the purposes of the present study, we analyzed the data of newly and previously diagnosed CTEPH adult (>18 years old) patients who were managed in the participating centers between 1 March 2018 and 31 August 2019. The diagnostic algorithm of CTEPH was based on current European Society of Cardiology (ESC) and European Respiratory Society (ERS) recommendations.2 Newly diagnosed patients were those whose diagnoses had been established since 1 March 2018 (termed ‘incident cases’). At the time of enrollment they had not been treated for CTEPH. Patients who were diagnosed earlier have been classified as ‘prevalent cases’. All patients were categorized as operable or non-operable by one of two CTEPH teams.36 The diagnosis of CTEPH was at the discretion of co-investigators who managed the enrolled patients in their centers. However, the diagnosis had to fulfill the criteria recommended by the ESC and ERS. The validity and credibility of the diagnostic algorithms were ensured by the experience of the investigators, CTEPH team evaluation, and an audit of the NHF programme.
Baseline and follow-up assessment
We analyzed patients’ characteristics at three timepoints: the time of diagnosis, enrollment, and follow-up. In incident cases the time of diagnosis was the same as the time of enrollment. The minimum set of data at diagnosis included: World Health Organization functional class (WHO-FC), CTEPH team opinion (operable versus non-operable), results of the imaging tests to diagnose CTEPH and data obtained at right heart catheterization such as mean pulmonary artery pressure (mPAP), right atrial pressure, cardiac index, pulmonary vascular resistance, PA wedge pressure, and PA oxygen saturation.37 At enrollment we gathered the following data: WHO-FC, 6-minute walk distance (6MWD), N-terminal pro-brain natriuretic peptide (NT-proBNP) or brain natriuretic peptide (BNP), and right atrial area (RAA) as assessed by echocardiography, medical, surgical, or interventional treatments, and comorbidities. All follow-up visits were scheduled according to the NHF programme criteria, which require the assessment of treatment efficacy at 3 to 6-month intervals. Patients were followed from the time of enrollment until 31 August 2019.
Statistical analysis
The period prevalence of CTEPH was calculated as the number of patients diagnosed with CTEPH in every center who were alive on 1 March 2018, and all new patients diagnosed between 1 March 2018 and 31 August 2019 (numerator) per the total number of Polish adults (denominator, n = 31,512,906; 16,471,228 adult women and 15,041,678 adult men) based on the most recent data (31 December 2017) obtained from Statistics Poland (Central Statistical Office, https://stat.gov.pl/en). The incidence was calculated as the number of new CTEPH cases per year (numerator) per the number of Polish adults (denominator). For the comparison of continuous variables between the two groups, we used the Student’s t test, and for categorical variables the chi2 test with Yates’s correction as needed. A Kaplan–Meier survival curve was used to delineate patient survival starting at the time of enrollment. The significance level was set at an alpha level of 0.05. Statistical analysis was performed with the use of Dell Inc. (2016), Dell Statistica (data analysis software system), (version 13; Dell, Texas, USA) software.dell.com.
Results
Study group and diagnostics
We enrolled in the present study 516 patients with CTEPH aged 63.8 ± 15.4 years, including 329 (63.8%) prevalent and 187 (36.2%) incident cases. At enrollment a significant number of patients (n = 286; 55.4%) were ⩾65 years old. Most patients were at WHO-FC II and III (n = 235; 45.5% and n = 225; 43.6%, respectively) followed by patients at WHO-FC I and IV (n = 39; 7.6% and n = 17; 3.3%, respectively). The mean 6MWD was 334 ± 166.4 m, the mean NT-proBNP level was 1482 ± 3130 pg/ml, and the mean RAA was 28.1 ± 17 cm2. At enrollment the study patients were treated with riociguat (n = 247; 47.9%), sildenafil (n = 93; 18%), treprostinil (n = 12; 2.3%), macitentan (n = 3; 0.6%) and bosentan (n = 1; 0.2%).
At diagnosis (Table 2) most patients were at WHO-FC III; however, incident cases were less functionally impaired than prevalent cases. The period prevalence of CTEPH was 16.4 per million adults, including 15.5 per million women and 17.3 per million men. The incidence rate was estimated at 3.96 new CTEPH patients per million per year.
Table 2.
Characterization of the study group at the time of diagnosis of chronic thromboembolic pulmonary hypertension.
| Variable | All n = 516 | Prevalent cases n = 329 | Incident cases n = 187 | p-Value | |
|---|---|---|---|---|---|
| Age (years) | 61.7 ± 15.6 | 61.2 ± 15.9 | 63.2 ± 14.8 | 0.27 | |
| WHO-FC | I (n, %) | 4 (0.8%) | 4 (1.2%) | 0 | 0.0006 |
| II (n, %) | 133 (25.8%) | 66 (20.1%) | 67 (35.8%) | ||
| III (n, %) | 348 (67.4%) | 237 (72.0%) | 111 (59.4%) | ||
| IV (n, %) | 31 (6%) | 22 (6.7%) | 9 (4.8%) | ||
| RHC | mPAP (mmHg) | 45.5 ± 11.8 | 45.9 ± 11.7 | 44.1 ± 12.2 | 0.2 |
| PAWP (mmHg) | 9.3 ± 3.5 | 9.4 ± 3.6 | 8.7 ± 3.3 | 0.08 | |
| RAP (mmHg) | 7.7 ± 4.6 | 7.8 ± 4.6 | 7.2 ± 4.7 | 0.27 | |
| Pa-SpO2 (%) | 63.8 ± 8.7 | 63.6 ± 8.6 | 64.4 ± 8.8 | 0.47 | |
| Ao-SpO2 (%) | 93 ± 4.3 | 92.3 ± 4.4 | 93.2 ± 4.2 | 0.1 | |
| CI (l/min/m2) | 2.2 ± 0.7 | 2.2 ± 0.7 | 2.2 ± 0.7 | 0.55 | |
| PVR (WU) | 8.8 ± 4.6 | 8.7 ± 4.4 | 8.9 ± .4 | 0.8 | |
Ao-SpO2, blood oxygen saturation in aorta; CI, cardiac index; mPAP, mean pulmonary artery pressure; NT-proBNP, N terminal pro b-type natriuretic peptide; Pa-SpO2, blood oxygen saturation in pulmonary artery; PAWP, pulmonary artery wedge pressure; PVR, pulmonary vascular resistance; RAA, right atrial area; RAP, right atrial pressure; RHC, right heart catheterization; WHO-FC, World Health Organization functional class.
CTEPH was confirmed by classic PA arteriography in most patients (n = 496; 96.1%). In the other 20 (3.9%) patients the diagnosis was based on computed tomography pulmonary artery angiography (CTPA).
During the diagnostic work-up, ventilation–perfusion lung scintigraphy (V/Q scan) was performed in 155 (30%) patients and perfusion scintigraphy in 57 (11%) patients. In most patients (n = 133; 25.8%) the single photon edition computed tomography (SPECT-CT) technique was used. In the remaining 22 (4.3%) patients, planar lung scintigraphy was applied. V/Q scan showed at least one unmatched segmental perfusion defect in 118 (76.1%) patients and at least one unmatched subsegmental perfusion defect in an additional 37 (23.9%) out of the 155 patients who had been tested with this technique.
Using PA arteriography as the reference method to detect CTEPH, we found that the criterion of one or more mismatched segmental perfusion defect in V/Q scan had a sensitivity to diagnose CTEPH of 81.1% (95% confidence interval (CI): 72.5–87.9%), the criterion of any mismatched perfusion defects had a sensitivity of 100% (95% CI: 96.7–100%), and CTPA had a sensitivity of 76.6% (95% CI: 67.6−84.1%). Central lesions were present in 157 (30.4%) patients, peripheral lesions were present in 206 (39.9%) patients, and mixed (central and peripheral) lesions were present in 144 (27.9%) patients.
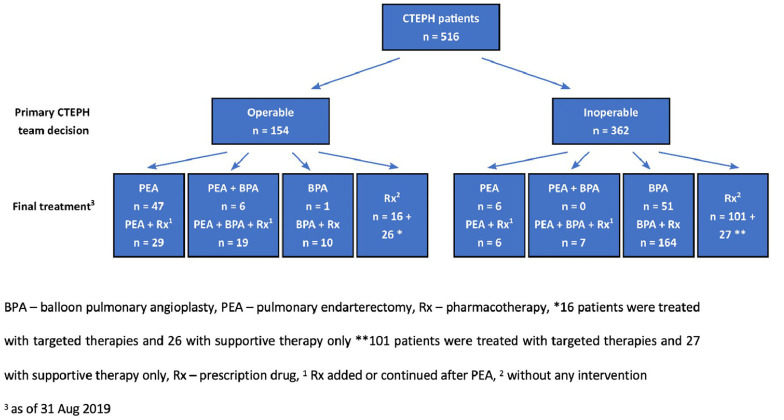
At the time of diagnosis 362 (70.2%) patients were classified as inoperable and 154 (29.8%) were considered operable. Inoperability resulted from distal localization of the thromboembolic lesions (n = 225; 62.2%), poor clinical status, low benefit-to-risk ratio or comorbidities (n = 88; 24.3%), or a lack of consent (n = 49; 13.5%).
Therapy
At enrollment to the study PEA or BPA were completed in 216 patients. In 100 patients BPA treatment was ongoing, and the remaining 200 patients had had no previous intervention. At follow-up PEA was performed in an additional 13 patients and BPA in an additional 19 patients. Overall, PEA was performed in 120 (23.3%) patients and BPA in 258 (50%) patients (Figure 1). Analysis of the treatment status at study enrollment showed that the near normalization of mPAP (<25 mmHg) was present in 44.3% of patients after PEA and 35.3% of patients after BPA (Table 3). Additional BPA after PEA resulted in hemodynamic near-normalization in 35% of patients (Table 3).
Figure 1.
Assessment of operability by the chronic thromboembolic pulmonary hypertension (CTEPH) teams and final treatment of the study group.
Table 3.
Treatment status of study patients at enrollment.
| Category | No. of patients | No. of patients using targeted pharmacotherapy after completion of treatment with PEA or BPA (%) | |
|---|---|---|---|
| PEA/BPA completed | |||
| Hemodynamic near-normalization (mPAP <25 mmHg) | After PEA | 43 | 7 (16.3) |
| After BPA | 42 | 19 (45.2) | |
| After PEA + BPA | 7 | 3 (42.9) | |
| Persistent CTEPH (mPAP ⩾25 mmHg) | After PEA | 34 | 22 (64.7) |
| After BPA | 77 | 64 (83.1) | |
| After PEA and BPA | 13 | 12 (92.3) | |
| Ongoing interventional treatment | |||
| BPA treatment | After PEA (persistent CTEPH) | 10 | – |
| As first choice intervention | 90 | – | |
| No interventional treatment at enrollment | |||
| CTEPH without PEA or BPA at enrollment to the study | 200 | – | |
BPA, balloon pulmonary angioplasty; CTEPH, chronic thromboembolic pulmonary hypertension; mPAP, mean pulmonary artery pressure; PEA, pulmonary endarterectomy.
Before PEA 19 (15.8%) patients were pretreated with targeted pharmacotherapy, while BPA was pretreated in 124 (48.1%) patients. Table 3 shows that almost half of the patients treated with BPA still continued targeted pharmacotherapy despite mPAP near-normalization, which is in contrast to the group of patients after PEA. Targeted pharmacotherapy included monotherapy in most patients (n = 343; 97.4%), and a combination therapy in a small group of nine (2.6%) patients who participated in the open-label phases of clinical trials. The therapies included: riociguat (n = 253; 71.9%), sildenafil (n = 83; 23.6%), subcutaneous treprostinil (n = 5; 1.4%), macitentan (n = 2; 0.6%) and combinations of sildenafil and treprostinil (n = 1; 0.3%), sildenafil and macitentan (n = 1; 0.3%), sildenafil and bosentan (n = 1; 0.3%), and riociguat and treprostinil (n = 6; 1.7%).
Associated medical conditions and additional therapies
Most patients had a history of acute pulmonary embolism (APE) and were of a blood group other than O (Table 4). They also frequently presented with conditions characteristic of left heart disease. The operable patients, as compared to the non-operable patients, were younger, more frequently of a blood group other than O and had a history of deep vein thrombosis and APE. In turn, non-operable patients more frequently had thyroid disorders including hypothyroidism and hyperthyroidism. Most patients were using anticoagulation at enrollment, usually in the form of non-vitamin K antagonist oral anticoagulants (NOACs) (Table 5). The other frequently used therapies reflected the burden of comorbidities.
Table 4.
Associated medical conditions and additional therapies in patients with chronic thromboembolic pulmonary hypertension at enrollment to the study.
| Variables | All N = 516 | Operable N = 154 | Non-operable N = 362 | p-Value | |
|---|---|---|---|---|---|
| Male sex (n, %) | 256 (49.6) | 92 (59.7) | 164 (45.3) | 0.003 | |
| Age (mean ± SD) | 63.8 ± 15.4 | 58.7 ± 14.1 | 65.9 ± 15.5 | <0.0001 | |
| Comorbidities | |||||
| Diabetes | 88 (17%) | 28 (18.2%) | 60 (16.6%) | 0.67 | |
| Smoking | Present | 24 (4.7%) | 17 (11%) | 7 (1.9%) | <0.0001 |
| Past | 143 (27.7%) | 43 (27.9%) | 100 (27.6%) | 0.96 | |
| Obesity (n, %) | 163 (31.6) | 57 (37) | 106 (29.3) | 0.08 | |
| Hypertension (n, %) | 303 (58.7) | 88 (57.1) | 215 (59.4) | 0.59 | |
| Coronary artery disease (n, %) | 96 (18.6) | 28 (18.2) | 68 (18.8) | 0.86 | |
| Myocardial infarction history (n, %) | 38 (7.4) | 14 (9.1) | 24 (6.6) | 0.33 | |
| Atrial fibrillation (any) (n, %) | 76 (14.7) | 20 (13) | 56 (15.5) | 0.46 | |
| Atrial flutter (any) (n, %) | 23 (4.5) | 8 (5.2) | 15 (4.1) | 0.6 | |
| Depression (n, %) | 27 (5.2) | 7 (4.6) | 20 (5.5) | 0.64 | |
| COPD (n, %) | 49 (9.5) | 16 (10.4) | 33 (9.1) | 0.66 | |
| Asthma (n, %) | 30 (5.8) | 10 (6.5) | 20 (5.5) | 0.67 | |
| Interstitial lung disease (n, %) | 8 (1.6) | 0 | 8 (2.2) | 0.06 | |
| Sleep apnea (n, %) | 7 (1.4) | 2 (1.3) | 5 (1.4) | 0.94 | |
| Hypothyroidism (n, %) | 62 (12) | 12 (7.8) | 50 (13.8) | 0.05 | |
| Hyperthyroidism (n, %) | 29 (5.6) | 3 (2) | 26 (7.2) | 0.02 | |
| Liver cirrhosis (n, %) | 5 (1) | 3 (2) | 2 (0.6) | 0.14 | |
| Chronic kidney disease (n, %) | 127 (24.6) | 24 (15.6) | 103 (28.5) | 0.002 | |
| Acute PE history and PE risk factors | |||||
| Acute pulmonary embolism history (n, %) | ⩾1 | 427 (83) | 137 (89) | 290 (80.1) | 0.02 |
| 1 | 361 (70) | 105 (76.6) | 256 (70.7) | 0.57 | |
| >1 | 66 (12.8) | 32 (20.8) | 34 (9.4) | 0.0004 | |
| Deep vein thrombosis history (n, %) | 239 (46.3) | 85 (55.2) | 154 (42.5) | 0.009 | |
| Thrombolytic treatment for acute thromboembolic disease (n, %) | 14 (2.7) | 6 (3.9) | 8 (2.2) | 0.28 | |
| Implantation of inferior vena cava filter in history (n, %) | 44 (8.5) | 19 (12.3) | 25 (6.9) | 0.04 | |
| Varicose veins in the lower extremities (n, %) | 178 (34.5) | 51 (33.1) | 127 (35.1) | 0.65 | |
| Major surgery in history (n, %) | 90 (17.4) | 27 (17.5) | 63 (17.4) | 0.98 | |
| Splenectomy (n, %) | 18 (3.5) | 4 (2.6) | 14 (3.9) | 0.47 | |
| Cancer (n, %) | Present | 10 (1.9) | 3 (2) | 7 (1.9) | 0.99 |
| Past | 39 (7.6) | 7 (4.6) | 32 (8.8) | 0.09 | |
| Fractures requiring immobilization (n, %) | 15 (2.9) | 7 (4.6) | 8 (2.2) | 0.15 | |
| Prolonged hospitalization in history (n, %) | 48 (9.3) | 12 (7.8) | 36 (9.9) | 0.44 | |
| Ventriculoatrial shunt (n, %) | 8 (1.6) | 1 (0.7) | 7 (1.9) | 0.28 | |
| Inflammatory bowel disease (n, %) | 7 (1.4) | 2 (1.3) | 5 (1.4) | 0.94 | |
| Permanent cardiac pacing (n, %) | 22 (4.3) | 5 (3.3) | 17 (4.7) | 0.45 | |
| Infective endocarditis history (n, %) | 4 (0.8) | 0 | 4 (1.1) | 0.19 | |
| Blood group other than 0 (n, %) | 445 (86.2) | 141 (91.2) | 304 (84) | 0.02 | |
COPD, chronic obstructive pulmonary disease; PE, pulmonary embolism.
Table 5.
Pharmacotherapy in the study patients at enrollment to the registry.
| Treatments | No. (%) |
|---|---|
| Anticoagulation (n, %) | 508 (98.4) |
| NOACs (non-vitamin K antagonist oral anticoagulants) (n, %) | 301 (58.3) |
| Vitamin K antagonists (n, %) | 159 (30.8) |
| Low molecular weight heparin (n, %) | 48 (9.3) |
| Beta-blockers (n, %) | 270 (52.3) |
| Angiotensin-converting enzyme inhibitors (n, %) | 119 (23.1) |
| Angiotensin receptor blockers (n, %) | 43 (8.3) |
| Ivabradine (n, %) | 8 (1.6) |
| Amiodaron (n, %) | 15 (2.9) |
| Furosemide (n, %) | 201 (39) |
| Torasemide (n, %) | 186 (36) |
| Thiazide diuretics (n, %) | 42 (8.1) |
| Aldosterone antagonists (n, %) | 153 (29.7) |
| Selective serotonin reuptake inhibitors (n, %) | 15 (2.9) |
| Other antidepressants (n, %) | 14 (2.7) |
| Acetylsalicylic acid | 20 (3.9) |
| Clopidogrel (n, %) | 12 (2.3) |
| Proton-pump inhibitors (n, %) | 275 (53.3) |
| Statins (n, %) | 222 (43) |
| Systemic corticosteroids (n, %) | 17 (3.3) |
| Immunosuppressive drugs (n, %) | 10 (1.9) |
| Antiarrhythmics other than beta-blockers (n, %) | 8 (1.6) |
Follow-up
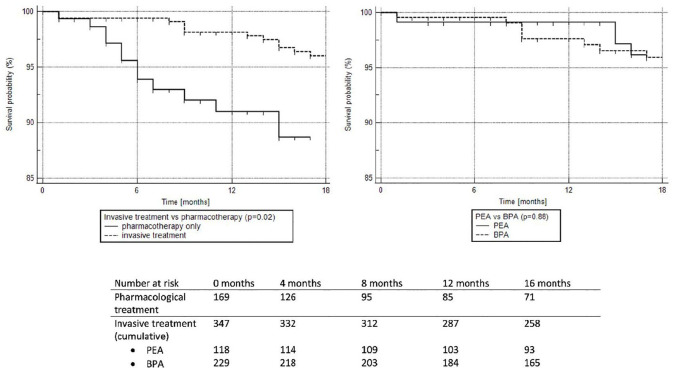
Patients were followed for a mean of 14.3 ± 5.8 months. During that time 26 (5%) patients died due to progression of right heart failure (10 cases; 38.5%), cancer (three cases; 11.5%), infections (three cases; 11.5%), sudden cardiac death (two cases; 7.7%), respiratory failure (two cases; 7.7%), bleeding (one case; 3.8%), renal failure (one case; 3.8%) and unknown causes (four cases; 15.4%). Patients treated only with pharmacotherapy had more severe disease at enrollment and worse survival than patients treated with PEA or BPA (Table 6 and Figure 2). We did not find any difference in survival (p = 0.88) between patients treated with PEA and patients treated with BPA. Table 6 presents characteristics of patients in relation to the mode of treatment. As compared to invasive methods (PEA or BPA), medical treatment was chosen more frequently in more compromised patients defined by higher WHO-FC and NT-proBNP levels and shorter 6MWD, while BPA was preferred over PEA in older patients and in those with less centrally located lesions. Table 7 shows that patients who died at follow-up had higher NT-proBNP blood levels, greater RAA, and shorter 6MWD at enrollment than those who survived.
Table 6.
Comparison of study patients in relation to treatment modalities.
| Variables | Medical treatment* (n = 170) | PEA** (n = 118) | BPA*** (n = 228) | p-Value | |
|---|---|---|---|---|---|
| Men (n, %) | 81 (47.9) | 70 (59.3) | 105 (45.9) | 0.05 | |
| Age at study enrollment (years) | 67.3 ± 15.6 | 54.5 ± 14.4 | 63.6 ± 15.2 | 0.0003a | |
| Localizations of thrombotic lesions | Central (%) | 46 (27.2) | 73 (61.9) | 38 (16.6) | <0.0001b |
| Central or mixed (%) | 88 (52.1) | 100 (84.7) | 113 (49.3) | <0.0001c | |
| Data obtained at enrollment | |||||
| WHO-FC | I (%) | 4 (2.4) | 15 (12.7) | 20 (8.7) | 0.0003d |
| II (%) | 65 (38.1) | 51 (43.2) | 119 (52.4) | ||
| III (%) | 96 (56.5) | 48 (40.7) | 81 (34.4) | ||
| IV (%) | 5 (3) | 3 (2.5) | 8 (3.5) | ||
| 6MWD (m) | 294.1 ± 154.2 | 360 ± 180 | 351.6 ± 162 | <0.0001e | |
| RAA (cm2) | 29 ± 15.5 | 31 ± 22.8 | 26.5 ± 15.1 | 0.03f | |
| NT-proBNP (pg/mL) | 2067.4 ± 3508.3 | 947 ± 1404 | 1321 ± 3397 | 0.002g | |
Patients in whom only pharmacological therapy was used.
Patients in whom PEA was used as a first-line invasive treatment.
Patients in whom BPA was used as a first-line invasive treatment.
In post hoc analysis significant differences were found between: (a) PEA group and medical treatment group (p < 0.0001) and between PEA group and BPA group (p < 0.0001); (b) all groups (p < 0.0001); (c) PEA group and medical treatment group (p < 0.0001) and between PEA group and BPA group (p < 0.0001); (d) medical treatment group and PEA group (p = 0.002) and medical treatment group and BPA (p = 0.0001) group; (e) medical treatment group and PEA group (p = 0.001) and medical treatment group and BPA (p = 0.0005) group; (f) medical treatment group and PEA group (p = 0.048); (g) medical treatment and PEA group (p = 0.001), and medical treatment and BPA (p = 0.03) group.
6MWD, 6-minute walk distance; BPA, balloon pulmonary angioplasty; NT-proBNP, N terminal pro B-type natriuretic peptide; PEA, pulmonary endarterectomy; RAA, right atrial area; WHO-FC, World Health Organization functional class.
Figure 2.
Kaplan–Meier survival curves for patients with chronic thromboembolic pulmonary hypertension treated with pulmonary endarterectomy (PEA) or balloon pulmonary angioplasty (BPA) (invasive treatment) and with only pharmacotherapy including targeted treatment.
Table 7.
Comparison of patients with chronic thromboembolic pulmonary hypertension with respect to survival at the end of follow-up.
| Variables | Survivors (n = 490) | Non-survivors (n = 26) | p-Value | |
|---|---|---|---|---|
| Men (n, %) | 245 (50) | 11 (42.3) | 0.45 | |
| Age at diagnosis (years) | 63.7 ± 15.4 | 65.9 ± 15.7 | 0.48 | |
| Age at study enrollment (years) | 64.5 ± 16.4 | 66.8 ± 15.7 | 0.48 | |
| Incident cases (n, %) | 176 (35.9) | 11 (42.3) | 0.51 | |
| Final method of treatment | PEA (%) | 114 (23.3) | 4 (15.4) | 0.38 |
| PEA or BPA (%) | 334 (68.2) | 13 (50) | 0.05 | |
| Localizations of thrombotic lesions | Central versus mixed or peripheral (%) | 151 (30.8) | 6 (23.1) | 0.4 |
| Central or mixed versus peripheral (%) | 285 (58.2) | 16 (61.5) | 0.73 | |
| Data obtained at diangosis | ||||
| WHO-FC | I (%) | 4 (1) | 0 | 0.82 |
| II (%) | 128 (26.1) | 5 (19.2) | ||
| III (%) | 329 (67.1) | 19 (73.1) | ||
| IV (%) | 29 (5.9) | 2 (7.7) | ||
| 6MWD (m) | 306.4 ± 150.8 | 258.6 ± 117.2 | 0.14 | |
| RHC | mPAP (mmHg) | 45.4 ± 11.9 | 46.6 ± 10.8 | 0.63 |
| PAWP (mmHg) | 9.3 ± 3.5 | 9 ± 3.1 | 0.65 | |
| RAP (mmHg) | 7.7 ± 4.7 | 7.2 ± 3.5 | 0.58 | |
| Pa-SpO2 (%) | 63.8 ± 8.6 | 62.5 ± 9.1 | 0.5 | |
| Ao-SpO2 (%) | 93.1 ± 4.3 | 92.2 ± 3.4 | 0.42 | |
| CI (l/min/m2) | 2.2 ± 0.7 | 2 ± 0.8 | 0.3 | |
| PVR (WU) | 8.7 ± 4.7 | 9.4 ± 3.9 | 0.46 | |
| Data obtained at enrollment | ||||
| WHO-FC | I (%) | 39 (8) | 0 | 0.03 |
| II (%) | 228 (42.4) | 7 (26.9) | ||
| III (%) | 208 (42.4) | 17 (65.4) | ||
| IV (%) | 15 (3.1) | 2 (7.7) | ||
| 6MWD (m) | 342 ± 164 | 192 ± 142 | <0.0001 | |
| RAA (cm2) | 27.5 ± 16.2 | 38.9 ± 25.9 | 0.002 | |
| NT-proBNP (pg/mL) | 1411 ± 3111 | 2806 ± 3271 | 0.03 | |
6MWD, 6-minute walk distance; Ao-SpO2, blood oxygen saturation in aorta; CI, cardiac index; mPAP, mean pulmonary artery pressure; NT-proBNP, N terminal pro B-type natriuretic peptide; Pa-SpO2, blood oxygen saturation in pulmonary artery; PAWP, pulmonary artery wedge pressure; PVR, pulmonary vascular resistance; RAA, right atrial area; RAP, right atrial pressure; RHC, right heart catheterization; WHO-FC, World Health Organization functional class.
Discussion
In the present study we have shown the most recent data on characteristics, diagnostic work-up, therapeutic decisions and outcomes of CTEPH patients in the era of advanced surgical, interventional, and pharmacological therapies. This population at its mean age over 60 years was diagnosed as being in poor functional status and burdened with several comorbidities. Most patients were classified as inoperable based on the CTEPH team decisions. Despite the fact that PEA remains a treatment of choice in CTEPH targeted pharmacotherapies and BPA have been used more frequently than PEA. Importantly, a hybrid use of two or three of the available treatment methods is typical, and pharmacotherapy has frequently been retained even after successful interventional treatment. Unexpectedly, we noted a preference for NOACs over vitamin K antagonists in this group.
CTEPH is considered a rare disease, but its prevalence and incidence are poorly known. Most data come from follow-up studies in patients presenting with APE.2 However, a significant number of patients with CTEPH do not have a history of APE, which suggests that the true incidence of CTEPH is likely to be underestimated.7 More robust data may come from observational studies that include all patients with a CTEPH diagnosis made in a general population of a given territory. In this way,10 involving all pulmonary hypertension centers in the UK the incidence of CTEPH was estimated at 1.75 cases per million per year in 2006. Reports from other countries show similar (Spain 0.9 cases per million per year, Portugal 1.1 cases per million per year, Sweden two cases per million per year) or higher (Latvia 5.1 cases per million per year, Germany 5.7 cases per million per year) incidence of CTEPH, which locates our results in the middle. In a recent study using data from epidemiologic studies, registries and national databases, it has been estimated that diagnosed CTEPH patients account for only 7–29% of all CTEPH patients, which means that most CTEPH patients are still undiagnosed in our country and others.38
In our study most patients had their final diagnosis of CTEPH made based on classic pulmonary angiography, which is a diagnostic gold standard, and rarely were they based on CTPA and lung V/Q scan only. The use of V/Q scan was relatively low, which results from the low availability of this diagnostic tool in Poland. However, the use of PA angiography was higher than in some previous reports7 and was dictated by preferences of CTEPH teams, especially PEA and BPA specialists. In a subgroup of patients we could have found that the criterion of any perfusion lung defect in V/Q scan identified all patients who had CTEPH, but neither a lack of any segmental perfusion defect in V/Q scan nor a lack of angio-computed tomography signs of CTEPH were able to exclude the disease reliably. This observation is in line with previous reports39,40 and supports the recommendations of the nuclear medicine societies and reports of the Sixth World Symposium on Pulmonary Hypertension2 to use binary interpretation of the lung V/Q scan.
In our study most patients were determined as inoperable due to distal localization of the thromboembolic lesions, poor clinical status, comorbidities, or lack of consent. This was in contrast to other national and international CTEPH registries in which operable patients accounted for 60% to 70% of the assessed groups (Table 1). Those studies, however, recruited patients before the wide availability of approved medical therapies and BPA, which may have influenced the decisions of the CTEPH teams. This was recently illustrated by a group at the French National Reference Center,41 where the proportion of patients with CTEPH who had been operated on decreased after the advent of BPA (in 2014), from 82% in 2012–2013 to 51% in 2015–2016. Importantly, the total number of surgically treated patients with CTEPH remained unchanged because referrals increased during this time. Another analysis of the UK CTEPH center shows that the number of patients determined primarily as technically operable is significantly higher than the number of patients who are operated on (60% of patients qualifying for PEA).42 In a recent German registry of CTEPH which enrolled newly diagnosed patients in 2016, only 50% were treated with PEA.25 The Spanish Registry of Pulmonary Arterial Hypertension (REHAP), which started in 2007, shows that the chance of being operated on for CTEPH depended on the expertise of the qualifying center.18 In designated CTEPH expert centers 47.9% of patients were operated on, while in other centers it was only 4.6% of patients. In Poland, where determination of CTEPH patients is centralized (conducted by two CTEPH teams) and regulated by a reimbursement policy of the NHF, 70% of patients were determined as inoperable. An important proportion of the patients in this group had poor clinical status and comorbidities which in the opinion of the CTEPH teams excluded them from operation. In fact our CTEPH patients had more comorbidities than those reported in the international registry7 (e.g. obesity 31.6% versus 17.6%; coronary artery disease 18.6% versus 11.8%; diabetes 17% versus 5.2%) despite being of a similar age at study enrollment. When differences in the proportions of operable and inoperable patients in different studies are analyzed, it should be noted that there is no current method for standardizing operability or surgical risk for CTEPH, which to a large extent is a subjective decision of surgeons based on their experience.
Most patients enrolled in the registry were treated with one targeted drug, usually riociguat, which was the only treatment accepted for reimbursement by the NHF and which was supported by data from clinical trials.43,44 An important and new finding of our study is that targeted pharmacotherapies in some patients were used despite a good result of BPA. This might have resulted from the fact that BPA has usually been supported by pharmacotherapy and that the clinical significance of its withdrawal or maintenance has not been documented. Almost all CTEPH patients were anticoagulated at enrollment, usually with NOACs. Although the safety and efficacy of NOACs in CTEPH patients is unknown and their use has not been supported by pulmonary hypertension guidelines, the number of patients using NOACs presenting to CTEPH centers is rising,45,46 which results from the potential for a lower side-effect profile, and the greater convenience of dosing of NOACs over vitamin K antagonists. A multicenter retrospective analysis of patients with CTEPH after PEA showed that post-PEA functional and hemodynamic outcomes and bleeding risk were unaffected by the type of anticoagulation, but recurrent venous thromboembolism rates were significantly higher in those receiving NOACs.45 Still, prospective studies are needed to understand the clinical usefulness of NOACs in CTEPH.
The most frequent comorbidities of CTEPH patients were hypertension, coronary artery disease (CAD), diabetes, thyroid disorders, and atrial fibrillation. The prevalence of cardiovascular disease (CVD) risk factors and CAD is high in our group, exceeding that reported in the international CTEPH registry, which had recruited patients 10 years earlier. This may result from the fact that Polish adults are generally burdened with a higher incidence of CVD risk factors and a higher risk of CVD mortality than their counterparts in western European countries.47,48 Thyroid disorders, especially hypothyroidism and treatment with levothyroxine, have previously been linked to CTEPH,49,50 and our results complement the previous findings. Our new observation is that hyperthyroidism was over two times more prevalent in non-operable patients as compared to operable patients. This association has never been analyzed and requires further research, but data from preclinical studies link elevated T3 to vascular remodeling, which plays an important role in patients with distal disease.51 Most of our CTEPH patients had at least one APE in their history, but fewer than half had experienced deep vein thrombosis, which is in line with previous European reports.7 Similarly to these reports, a history of deep vein thrombosis and APE, especially recurrent pulmonary embolism, were associated with the operable phenotype of CTEPH. Implantation of a vena cava filter was generally low. Its insertion was more commonly applied in operable patients, as it was previously a routine perioperative procedure in some centers.
We found that 64.7% of patients after completed BPA treatment and 55.7% of patients after PEA still had CTEPH (persistent or recurrent). These PEA results were similar in a recent study of the Papworth Hospital where 51% of patients still had mPAP ⩾25 mmHg at the time of follow-up right heart catheterization 3–6 months after PEA.52 Based on our results, we do not conclude about the superiority of BPA or PEA, as in our study these two groups were not adjusted for such comparisons, but we underscore the need for careful observation of CTEPH patients after BPA and PEA. In addition, the relatively high rates of persistent CTEPH may support the role of adjunctive medical therapy in patients treated with PEA or BPA. However, PEA or BPA are associated with better survival than pharmacotherapy alone.
At diagnosis, the prevalent cases had more severe disease than incident cases. This could suggest a trend towards earlier diagnosis of CTEPH in recent years.
Strengths and limitations
Our study has several strengths. First of all, we present the results of the largest multicenter CTEPH registry recruiting patients in the modern era of CTEPH treatment, including pharmacological, surgical, and percutaneous methods. Second, by including all centers which diagnose and treat CTEPH patients we were able to estimate the incidence and prevalence of this disease. Third, we assessed the diagnostic value of lung V/Q scan and CTPA in real-world clinical practice.
The main limitation of our study is the risk of selection bias when we present the outcome results, as prevalent patients who died before 1 March 2018 could not be enrolled into our study. To reduce this risk we started the observation at enrollment to the study instead of at CTEPH diagnosis. The methodological limitations inherent to registry-based studies have recently been acknowledged.53 We also acknowledge that due to a low number of end points, our survival analysis was not adjusted for confounding factors; for example, age, severity of CTEPH, and comorbid conditions which besides the treatment method could have impacted the status of the patients at follow-up.
Conclusions
The modern population of CTEPH patients comprises mostly elderly people significantly burdened with comorbid conditions. The combination of two or three therapeutic methods is currently a typical approach in the management of CTEPH. This calls for treatment decisions that are individually tailored to every patient.
Footnotes
Author contributions: GK is the guarantor of the paper and takes responsibility for the integrity of the work as a whole, from inception to publication. Conception and design: GK. Acquisition of data: all authors. Analysis and interpretation of data: GK, MW, WM, MK, PPr, WJ, EL. Drafting the manuscript: GK, WM, MW. Critical revision for intellectual content and final approval of the version to be published: all authors.
Conflict of interest statement: Grzegorz Kopeć reports grants and personal fees from Actelion Pharma, personal fees from Janssen-Cilag Polska, personal fees from AOP Orphan, personal fees from MSD. Olga Dzikowska-Diduch has nothing to declare. Ewa Mroczek has nothing to declare. Tatiana Mularek-Kubzdela reports fees for lectures from MSD, Bayer, Jansen, AOP Orphan, Beringher, Pfizer, grants from MSD, Bayer, Jansen, AOP Orphan, Beringher. Łukasz Chrzanowski reports fees for lectures from MSD, non-financial support from MSD. Ilona Skoczylas has nothing to declare. Michał Tomaszewski has nothing to declare. Małgorzata Peregud-Pogorzelska has nothing to declare. Danuta Karasek has nothing to declare. Ewa Lewicka reports fees for lectures from Bayer, MSD, AOP Orphan, Janssen-Cilag, Pfizer and Boehringer-Ingelheim. Wojciech Jacheć has nothing to declare. Zbigniew Gąsior has nothing to declare. Piotr Błaszczak has nothing to declare. Katarzyna Ptaszyńska-Kopczyńska has nothing to declare. Katarzyna Mizia-Stec has nothing to declare. Andrzej Biederman has nothing to declare. Dariusz Zieliński has nothing to declare. Roman Przybylski has nothing to declare. Piotr Kędzierski has nothing to declare. Marcin Waligóra has nothing to declare. Marek Roik has nothing to declare. Marek Grabka has nothing to declare. Joanna Orłowska has nothing to declare. Aleksander Araszkiewicz has nothing to declare. Marta Banaszkiewicz has nothing to declare. Sylwia Sławek-Szmyt has nothing to declare. Szymon Darocha reports grants and personal fees from Janssen-Cilag, MSD, Bayer, and AOP Orphan. Wojciech Magoń has nothing to declare. Alicja Dąbrowska-Kugacka reports fees for lectures from MSD, Actelion, AOP Orphan and Bayer. Jakub Stępniewski has nothing to declare. Kamil Jonas has nothing to declare. Karol Kamiński has nothing to declare. Jarosław D. Kasprzak reports fees for lectures from Janssen/Actelion, AOP Orphan. Piotr Podolec reports personal fees from Actelion, personal fees from Jansen, personal fees from AOP ORPHAN, personal fees from Bayer. Piotr Pruszczyk reports fees for lectures from Merck. Adam Torbicki reports grants and personal fees from Actelion Pharma, personal fees from Janssen-Cilag Polska, personal fees from AOP Orphan, personal fees from MSD, grants and personal fees from Bayer Healthcare and the Chairperson of the Foundation on Pulmonary Hypertension. The foundation receives donations including those from industry. The chairmanship is an honorary function and is unrelated to financial or other benefits. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results. Marcin Kurzyna reports fees for lectures from MSD, AOP Orphan and Janssen-Cilag.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported by the Polish Cardiac Society.
ORCID iD: Grzegorz Kopeć  https://orcid.org/0000-0001-9921-2801
https://orcid.org/0000-0001-9921-2801
Contributor Information
Grzegorz Kopeć, Pulmonary Circulation Centre, Department of Cardiac and Vascular Diseases, Institute of Cardiology, Jagiellonian University Medical College, John Paul II Hospital, ul. Pradnicka 80, Krakow 31-202, Poland.
Olga Dzikowska-Diduch, Department of Internal Medicine and Cardiology, Medical University of Warsaw, Warszawa, Poland.
Ewa Mroczek, Department of Cardiology, Provincial Specialist Hospital Research and Development Center, Wrocław, Poland.
Tatiana Mularek-Kubzdela, Department of Cardiology, Poznan University of Medical Sciences, Poznań, Poland.
Łukasz Chrzanowski, I Department and Chair of Cardiology, Medical University of Lodz, Łódź, Poland.
Ilona Skoczylas, 3rd Department of Cardiology, Faculty of Medical Sciences in Zabrze, Medical University of Silesia, Katowice, Poland.
Michał Tomaszewski, Department of Cardiology, Medical University of Lublin, Lublin, Poland.
Małgorzata Peregud-Pogorzelska, Department of Cardiology, Pomeranian Medical University, Szczecin, Poland.
Danuta Karasek, 2nd Department of Cardiology, Faculty of Health Sciences, Collegium Medicum, Nicolaus Copernicus University, Bydgoszcz, Poland.
Ewa Lewicka, Department of Cardiology and Electrotherapy, Medical University of Gdańsk, Gdańsk, Poland.
Wojciech Jacheć, 2nd Department of Cardiology, School of Medicine with Dentistry Division in Zabrze, Medical University of Silesia, Zabrze, Poland.
Zbigniew Gąsior, Department of Cardiology, School of Health Sciences, Medical University of Silesia, Katowice, Poland.
Piotr Błaszczak, Department of Cardiology, Cardinal Wyszynski Hospital, Lublin, Poland.
Katarzyna Ptaszyńska-Kopczyńska, Department of Cardiology, Medical University of Białystok, Białystok, Poland.
Katarzyna Mizia-Stec, 1st Department of Cardiology, School of Medicine in Katowice, Medical University of Silesia, Katowice, Poland.
Andrzej Biederman, Department of Cardiac Surgery, Medicover Hospital, Warszawa, Poland.
Dariusz Zieliński, Department of Cardiac Surgery, Medicover Hospital, Warszawa, Poland.
Roman Przybylski, Department of Heart Diseases, Wroclaw Medical University, Clinic of Cardiac Transplantation and Mechanical Circulatory Support, Wrocław, Poland.
Piotr Kędzierski, Department of Pulmonary Circulation, Thromboembolic Diseases and Cardiology, Centre of Postgraduate Medical Education, European Health Centre, Otwock, Poland.
Marcin Waligóra, Pulmonary Circulation Centre Department of Cardiac and Vascular Diseases, Institute of Cardiology, Jagiellonian University Medical College, John Paul II Hospital, Kraków, Poland, Department of Medical Education, Center for Innovative Medical Education, Jagiellonian University Medical College, Krakow, Poland.
Marek Roik, Department of Internal Medicine and Cardiology, Medical University of Warsaw, Warszawa, Poland.
Marek Grabka, 1st Department of Cardiology, School of Medicine in Katowice, Medical University of Silesia, Katowice, Poland.
Joanna Orłowska, Department of Cardiology, Provincial Specialist Hospital Research and Development Center, Wrocław, Poland.
Aleksander Araszkiewicz, Department of Cardiology, Poznan University of Medical Sciences, Poznań, Poland.
Marta Banaszkiewicz, Department of Pulmonary Circulation, Thromboembolic Diseases and Cardiology, Centre of Postgraduate Medical Education, European Health Centre, Otwock, Poland.
Sylwia Sławek-Szmyt, Department of Cardiology, Poznan University of Medical Sciences, Poznań, Poland.
Szymon Darocha, Department of Pulmonary Circulation, Thromboembolic Diseases and Cardiology, Centre of Postgraduate Medical Education, European Health Centre, Otwock, Poland.
Wojciech Magoń, Pulmonary Circulation Centre, Department of Cardiac and Vascular Diseases, Institute of Cardiology, Jagiellonian University Medical College, John Paul II Hospital, Krakow, Poland.
Alicja Dąbrowska-Kugacka, Department of Cardiology and Electrotherapy, Medical University of Gdańsk, Gdańsk, Poland.
Jakub Stępniewski, Pulmonary Circulation Centre Department of Cardiac and Vascular Diseases, Institute of Cardiology, Jagiellonian University Medical College, John Paul II Hospital, Kraków, Poland, Department of Medical Education, Center for Innovative Medical Education, Jagiellonian University Medical College, Krakow, Poland.
Kamil Jonas, Pulmonary Circulation Centre Department of Cardiac and Vascular Diseases, Institute of Cardiology, Jagiellonian University Medical College, John Paul II Hospital, Kraków, Poland, Department of Medical Education, Center for Innovative Medical Education, Jagiellonian University Medical College, Krakow, Poland.
Karol Kamiński, Department of Population Medicine and Lifestyle Diseases Prevention, Medical University of Białystok, Białystok, Poland.
Jarosław D. Kasprzak, I Department and Chair of Cardiology, Medical University of Lodz, Łódź, Poland
Piotr Podolec, Department of Cardiac and Vascular Diseases, Institute of Cardiology, Jagiellonian University Medical College, John Paul II Hospital, Kraków, Poland.
Piotr Pruszczyk, Department of Internal Medicine and Cardiology, Medical University of Warsaw, Warszawa, Poland.
Adam Torbicki, Department of Pulmonary Circulation, Thromboembolic Diseases and Cardiology, Centre of Postgraduate Medical Education, European Health Centre, Otwock, Poland.
Marcin Kurzyna, Department of Pulmonary Circulation, Thromboembolic Diseases and Cardiology, Centre of Postgraduate Medical Education, European Health Centre, Otwock, Poland.
References
- 1. Galiè N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 2. Kim NH, Delcroix M, Jais X, et al. Chronic thromboembolic pulmonary hypertension. Eur Respir J 2019; 53: 1801915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Magoń W, Stępniewski J, Waligóra M, et al. Virtual histology to evaluate mechanisms of pulmonary artery lumen enlargement in response to balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension. J Clin Med 2020; 9: 1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Magoń W, Stępniewski J, Waligóra M, et al. Pulmonary artery elastic properties after balloon pulmonary angioplasty in patients with inoperable chronic thromboembolic pulmonary hypertension. Can J Cardiol 2019; 35: 422–429. [DOI] [PubMed] [Google Scholar]
- 5. Riedel M, Stanek V, Widimsky J, et al. Longterm follow-up of patients with pulmonary thromboembolism. Late prognosis and evolution of hemodynamic and respiratory data. Chest 1982; 81: 151–158. [DOI] [PubMed] [Google Scholar]
- 6. Mayer E, Jenkins D, Lindner J, et al. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. J Thorac Cardiovasc Surg 2011; 141: 702–710. [DOI] [PubMed] [Google Scholar]
- 7. Pepke-Zaba J, Delcroix M, Lang I, et al. Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation 2011; 124: 1973–1981. [DOI] [PubMed] [Google Scholar]
- 8. Chausheva S, Naito A, Ogawa A, et al. Chronic thromboembolic pulmonary hypertension in Austria and Japan. J Thorac Cardiovasc Surg 2019; 158: 604–614. [DOI] [PubMed] [Google Scholar]
- 9. Fischler M, Speich R, Dorschner L, et al. Pulmonary hypertension in Switzerland: treatment and clinical course. Swiss Med Wkly 2008; 138: 371–378. [DOI] [PubMed] [Google Scholar]
- 10. Condliffe R, Kiely DG, Gibbs JSR, et al. Improved outcomes in medically and surgically treated chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med 2008; 177: 1122–1127. [DOI] [PubMed] [Google Scholar]
- 11. Condliffe R, Kiely DG, Gibbs JSR, et al. Prognostic and aetiological factors in chronic thromboembolic pulmonary hypertension. Eur Respir J 2009; 33: 332–337. [DOI] [PubMed] [Google Scholar]
- 12. Bonderman D, Wilkens H, Wakounig S, et al. Risk factors for chronic thromboembolic pulmonary hypertension. Eur Respir J 2009; 33: 325–331. [DOI] [PubMed] [Google Scholar]
- 13. Delcroix M, Lang I, Pepke-Zaba J, et al. Long-term outcome of patients with chronic thromboembolic pulmonary hypertension : results from an international prospective registry. Circulation 2016; 133: 859–871. [DOI] [PubMed] [Google Scholar]
- 14. Escribano-Subias P, Blanco I, López-Meseguer M, et al. Survival in pulmonary hypertension in Spain: insights from the Spanish registry. Eur Respir J 2012; 40: 596–603. [DOI] [PubMed] [Google Scholar]
- 15. Hurdman J, Condliffe R, Elliot CA, et al. ASPIRE registry: assessing the spectrum of pulmonary hypertension identified at a REferral centre. Eur Respir J 2012; 39: 945–955. [DOI] [PubMed] [Google Scholar]
- 16. Baptista R, Meireles J, Agapito A, et al. Pulmonary hypertension in Portugal: first data from a nationwide registry. Biomed Res Int 2013; 2013: 489574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mueller-Mottet S, Stricker H, Domeninghetti G, et al. Long-term data from the Swiss pulmonary hypertension registry. Respiration 2015; 89: 127–140. [DOI] [PubMed] [Google Scholar]
- 18. Escribano-Subías P, Del Pozo R, Román-Broto A, et al. Management and outcomes in chronic thromboembolic pulmonary hypertension: from expert centers to a nationwide perspective. Int J Cardiol 2016; 203: 938–944. [DOI] [PubMed] [Google Scholar]
- 19. Park SY, Lee SM, Shin JW, et al. Epidemiology of chronic thromboembolic pulmonary hypertension in Korea: results from the Korean registry. Korean J Intern Med 2016; 31: 305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rådegran G, Kjellström B, Ekmehag B, et al. Characteristics and survival of adult Swedish PAH and CTEPH patients 2000–2014. Scand Cardiovasc J 2016; 50: 243–250. [DOI] [PubMed] [Google Scholar]
- 21. Gall H, Felix JF, Schneck FK, et al. The Giessen pulmonary hypertension registry: survival in pulmonary hypertension subgroups. J Heart Lung Transplant 2017; 36: 957–967. [DOI] [PubMed] [Google Scholar]
- 22. Santos M, Gomes A, Cruz C, et al. Long-term survival in pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: insights from a referral center in Portugal. Rev Port Cardiol 2018; 37: 749–757. [DOI] [PubMed] [Google Scholar]
- 23. Martinez C, Wallenhorst C, Teal S, et al. Incidence and risk factors of chronic thromboembolic pulmonary hypertension following venous thromboembolism, a population-based cohort study in England. Pulm Circ 2018; 8: 2045894018791358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Skride A, Sablinskis K, Lejnieks A, et al. Characteristics and survival data from Latvian pulmonary hypertension registry: comparison of prospective pulmonary hypertension registries in Europe. Pulm Circ 2018; 8: 2045894018780521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kramm T, Wilkens H, Fuge J, et al. Incidence and characteristics of chronic thromboembolic pulmonary hypertension in Germany. Clin Res Cardiol 2018; 107: 548–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bazmpani MA, Arvanitaki A, Toumpourleka M, et al. Epidemiology and management of chronic thromboembolic pulmonary hypertension: experience from two expert centers. Hell J Cardiol 2018; 59: 16–23. [DOI] [PubMed] [Google Scholar]
- 27. Radchenko GD, Zhyvylo IO, Sirenko YM. Analysis of pulmonary hypertension patient survival after treatment in referral center (data of first Ukrainian register). Pulm Circ 2019; 9: 2045894019845604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sablinskis K, Sablinskis M, Lejnieks A, et al. Growing number of incident pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension patients in Latvia: a shifting epidemiological landscape? Data from a national pulmonary hypertension registry. Eur J Intern Med 2019; 59: e16–e17. [DOI] [PubMed] [Google Scholar]
- 29. Kigitovica D, Sablinskis M, Sablinskis K, et al. Pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension incidence in Latvia in 2018. Eur J Intern Med 2019; 65: e9–e10. [DOI] [PubMed] [Google Scholar]
- 30. Andreassen AK, Ragnarsson A, Gude E, et al. Balloon pulmonary angioplasty in patients with inoperable chronic thromboembolic pulmonary hypertension. Heart 2013; 99: 1415–1420. [DOI] [PubMed] [Google Scholar]
- 31. Ogawa A, Satoh T, Fukuda T, et al. Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Qual Outcomes 2017; 10: e004029. [DOI] [PubMed] [Google Scholar]
- 32. Yanaka K, Nakayama K, Shinke T, et al. Sequential hybrid therapy with pulmonary endarterectomy and additional balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. J Am Heart Assoc. Epub ahead of print 7 July 2018. DOI: 10.1161/JAHA.118.008838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kopeć G, Kurzyna M, Mroczek E, et al. Characterization of patients with pulmonary arterial hypertension: data from the Polish Registry of Pulmonary Hypertension (BNP-PL). J Clin Med 2020; 9: 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kwiatkowska J, Zuk M, Migdal A, et al. Children and adolescents with pulmonary arterial hypertension: baseline and follow-up data from the Polish Registry of Pulmonary Hypertension (BNP-PL). J Clin Med 2020; 9: 1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kopeć G, Kurzyna M, Mroczek E, et al. Database of pulmonary hypertension in the Polish population (BNP-PL): design of the registry. Kardiol Pol 2019; 77: 972–974. [DOI] [PubMed] [Google Scholar]
- 36. Siennicka A, Darocha S, Banaszkiewicz M, et al. Treatment of chronic thromboembolic pulmonary hypertension in a multidisciplinary team. Ther Adv Respir Dis 2019; 13: 1753466619891529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kurzyna M, Araszkiewicz A, Błaszczak P, et al. Summary of recommendations for the haemodynamic and angiographic assessment of the pulmonary circulation. Joint statement of the Polish Cardiac Society’s working group on pulmonary circulation and Association of Cardiovascular Interventions. Kardiol Pol 2015; 73: 63–68. [DOI] [PubMed] [Google Scholar]
- 38. Gall H, Hoeper MM, Richter MJ, et al. An epidemiological analysis of the burden of chronic thromboembolic pulmonary hypertension in the USA, Europe and Japan. Eur Respir Rev 2017; 26: 160121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tunariu N, Gibbs SJR, Win Z, et al. Ventilation-perfusion scintigraphy is more sensitive than multidetector CTPA in detecting chronic thromboembolic pulmonary disease as a treatable cause of pulmonary hypertension. J Nucl Med 2007; 48: 680–684. [DOI] [PubMed] [Google Scholar]
- 40. He J, Fang W, Lv B, et al. Diagnosis of chronic thromboembolic pulmonary hypertension: comparison of ventilation/perfusion scanning and multidetector computed tomography pulmonary angiography with pulmonary angiography. Nucl Med Commun 2012; 33: 459–463. [DOI] [PubMed] [Google Scholar]
- 41. Amsallem M, Guihaire J, Arthur Ataam J, et al. Impact of the initiation of balloon pulmonary angioplasty program on referral of patients with chronic thromboembolic pulmonary hypertension to surgery. J Heart Lung Transplant 2018; 37: 1102–1110. [DOI] [PubMed] [Google Scholar]
- 42. Quadery SR, Swift AJ, Billings CG, et al. The impact of patient choice on survival in chronic thromboembolic pulmonary hypertension. Eur Respir J 2018; 52: 1800589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Darocha S, Banaszkiewicz M, Pietrasik A, et al. Sequential treatment with sildenafil and riociguat in patients with persistent or inoperable chronic thromboembolic pulmonary hypertension improves functional class and pulmonary hemodynamics. Int J Cardiol 2018; 269: 283–288. [DOI] [PubMed] [Google Scholar]
- 44. Ghofrani HA, D’Armini AM, Grimminger F, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med 2013; 369: 319–329. [DOI] [PubMed] [Google Scholar]
- 45. Bunclark K, Newnham M, Chiu YD, et al. A multicenter study of anticoagulation in operable chronic thromboembolic pulmonary hypertension. J Thromb Haemost 2020; 18: 114–122. [DOI] [PubMed] [Google Scholar]
- 46. Wilkens H, Konstantinides S, Lang IM, et al. Chronic thromboembolic pulmonary hypertension (CTEPH): updated recommendations from the Cologne Consensus Conference 2018. Int J Cardiol 2018; 272S: 69–78. [DOI] [PubMed] [Google Scholar]
- 47. Zdrojewski T, Jankowski P, Bandosz P, et al. A new version of cardiovascular risk assessment system and risk charts calibrated for Polish population. Kardiol Pol 2015; 73: 958–961. [DOI] [PubMed] [Google Scholar]
- 48. Bandosz P, O’Flaherty M, Drygas W, et al. Decline in mortality from coronary heart disease in Poland after socioeconomic transformation: modelling study. BMJ 2012; 344: d8136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Krieg VJ, Hobohm L, Liebetrau C, et al. Risk factors for chronic thromboembolic pulmonary hypertension – importance of thyroid disease and function. Thromb Res 2020; 185: 20–26. [DOI] [PubMed] [Google Scholar]
- 50. Kim NH, Lang IM. Risk factors for chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2012; 21: 27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Richter MJ, Sommer N, Schermuly R, et al. The prognostic impact of thyroid function in pulmonary hypertension. J Heart Lung Transplant 2016; 35: 1427–1434. [DOI] [PubMed] [Google Scholar]
- 52. Cannon JE, Su L, Kiely DG, et al. Dynamic risk stratification of patient long-term outcome after pulmonary endarterectomy: results from the United Kingdom national cohort. Circulation 2016; 133: 1761–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Weatherald J, Reis A, Sitbon O, et al. Pulmonary arterial hypertension registries: past, present and into the future. Eur Respir Rev 2019; 28: 190128. [DOI] [PMC free article] [PubMed] [Google Scholar]