Abstract
Background and aims:
Oxidative stress (OS) induces the production of fibroblast growth factor 21 (FGF21). Previous data have revealed that FGF21 protects cells from OS injury and death, making it a potential therapeutic option for many diseases with increased OS. However, the association of this growth factor with OS markers in humans with chronic kidney disease (CKD) remains unknown. This study aims to evaluate the association of serum FGF21 with serum total antioxidant capacity (TAC) and oxidized low-density lipoproteins (OxLDL) in subjects in different stages of kidney disease.
Methods:
This is a cross-sectional study that included 382 subjects with different stages of CKD, irrespective of type 2 diabetes (T2D) diagnosis. Associations of serum FGF21 with OxLDL, TAC, sex, age, body mass index (BMI), fasting plasma glucose, estimated glomerular filtration rate (eGFR), T2D, and smoking, were evaluated through bivariate and partial correlation analyses. Independent associations of these variables with serum FGF21 were evaluated using multiple linear regression analysis.
Results:
Serum FGF21 was significantly and positively correlated with age (r = 0.236), TAC (lnTAC) (r = 0.217), and negatively correlated with eGFR (r = −0.429) and male sex (r = −0.102). After controlling by age, sex, BMI, T2D, smoking, and eGFR; both TAC and OxLDL were positively correlated with FGF21 (r = 0.117 and 0.158 respectively, p < 0.05). Using multiple linear regression analysis, eGFR, male sex, T2D, OxLDL, and TAC were independently associated with serum FGF21 (STDβ = −0.475, 0.162, −0.153, 0.142 and 0.136 respectively; p < 0.05 for all) adjusted for age, BMI, smoking, and fasting plasma glucose.
Conclusion:
A positive association between serum FGF21 and OS has been found independently of renal function in humans. Results from the present study provide novel information for deeper understanding of the role of FGF21 in OS in humans with CKD and T2D; mechanistic studies to explain the association of serum FGF21 with oxidative stress in CKD are needed.
Keywords: chronic kidney disease, fibroblast growth factor 21, glomerular filtration rate, oxidative stress, serum total antioxidant capacity
Introduction
Fibroblast growth factor 21 (FGF21), is a peptide hormone formed by 209 amino acids in humans 1 mainly expressed by the liver, and white and brown adipose tissues.2,3 In the last few years, the study of this growth factor has been of particular interest since it has been found that its serum concentration is elevated in many metabolic diseases such as the metabolic syndrome, obesity, and non-alcoholic fatty liver disease,4–6 and FGF21 is upregulated in physiological conditions like fasting, 7 protein deprivation, 8 and exercise. 6 In rodent models of diabetes and obesity, administration of FGF21 improved fat metabolism, serum triglycerides, and glycemia, and prevented high-fat-diet-induced obesity.9,10 On the other hand, outcomes of FGF21 administration in humans have been controversial. 11 Likewise, recent data have shown that oxidative stress (OS), a common pathologic mechanism among metabolic and chronic-degenerative diseases, induces FGF21 production through the activating transcription factor 4 (ATF4).8,12 Thus, a direct association between FGF21 and OS has been suggested. 13 In fact, novel data from pre-clinical studies have proved that the administration of FGF21 protects cells of different origins from OS injury.14,15
In chronic kidney disease (CKD), the serum concentration of FGF21 is also elevated16,17 and it gradually increases as estimated glomerular filtration rate (eGFR) declines. 18 Previous data from pharmacokinetics studies in rodents have revealed that nearly 80% of FGF21 is excreted through urine, 12% from feces, and less than 7% from bile. 19 Thus, it is possible that an increase of FGF21 could be directly attributed to the reduction in glomerular filtration in healthy humans, but the pharmacokinetics of endogenous FGF21 in patients with CKD has not been fully elucidated. To date, it is known that OS is involved in the development and progression of kidney disease.20,21 Hence, knowing that FGF21 is produced in response to OS, it is possible that the elevated serum concentration of FGF21 in subjects with CKD could be partially associated to their increased serum oxidative status, independently of its excretion rate. However, to the best of our knowledge, an association between FGF21 and OS in humans with CKD has not been fully yielded.
The prevalence of CKD and its complications are a major burden of disease worldwide. 22 Therapeutic options that effectively prevent its progression, unfortunately, are limited. Thus, prevention of CKD is one of the major health challenges worldwide. 23 Determining whether FGF21 has an independent association with OS markers in CKD could support its therapeutic potential targeting OS for CKD.
OS can be assessed both by the pro-oxidant and the antioxidant perspective, and serum oxidized low-density lipoproteins (OxLDL) are used as a pro-oxidant biomarker of oxidative stress in many metabolic diseases. 24 Likewise, total antioxidant capacity (TAC) is the measure of the overall effect of the antioxidant mechanisms in the oxidative status of serum. 25 Although high antioxidant activity reflects protection or benefit over oxidative stress, it also reflects upregulation of antioxidant mechanisms in response to chronic OS. Thus, TAC assays have been used as an overall indicator of the oxidative status.26–29
Therefore, the objective of this study is to evaluate the association of serum FGF21 with the OS biomarkers serum OxLDL and TAC in subjects with different stages of CKD, with or without type 2 diabetes (T2D), according to the Kidney Disease: Improving Global Outcomes (KDIGO) classification. The hypothesis is that as eGFR deteriorates, there is an increase in OS (demonstrated through the OxLDL and TAC), and that serum concentration of FGF21 is positively associated with these markers, independently of renal function and T2D diagnosis.
Methods
Participants
The subjects studied were randomly selected from the cohorts of three different protocols: The first cohort consisted of patients who participated in the Salt and Mexico (SALMEX) Study 30 (a cross-sectional study aimed to assess the average sodium, potassium, and iodine intake in the Mexican population); from this cohort 112 subjects were selected. The second cohort consisted of subjects diagnosed with T2D who attended the outpatient endocrinology clinic at the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (INCMNSZ), from which 166 subjects were selected. The third cohort consisted of subjects from Municipio de Hidalgo, Michoacan, Mexico [a town with the highest prevalence of CKD of Michoacan, with over 1000 subjects identified (Unpublished data, López-Cervantes M)]; from this cohort 104 subjects were included. Inclusion criteria for all participants were: Adults from 18 to 80 years old, irrespective of sex, T2D diagnosis or stage of renal function. Patients were excluded if they had any hospitalization in the past 3 months and within enrollment of the study, active cancer, febrile illness 6 weeks before enrollment, HIV, Hepatitis B or C infection, pregnancy or lactation, diagnosis of other type of diabetes (such as type 1, latent autoimmune diabetes in the adult or maturity onset diabetes of the young), and diagnosis of any other chronic disease.
This protocol was approved by the Research and Ethics Committee of the INCMNSZ (Protocol reference number 635). The study was conducted according to the Declaration of Helsinki. All participants signed an informed consent before the enrollment in the study.
Clinical evaluation
A clinical history and physical examination were obtained from all participants. Blood pressure was measured after 10 min of sitting rest; weight and height were assessed with an Omron® HBF-514C scale and a wall-mounted height measuring tape A.D.E® 10017 respectively. Body mass index (BMI) was calculated as weight in kg/(height in m) 2 .
Biochemical evaluation
Blood samples were obtained after 8–12 h of fasting. Total blood was drained into a BD® Vacutainer SST tube, then was centrifuged and the serum was frozen at −80°C until analysis. Serum creatinine and fasting plasma glucose were analyzed using the automated Beckman Coulter SYNCHRON® system. The eGFR was calculated using the Chronic Kidney Disease Epidemiology (CKD-EPI) formula. 31 Hemoglobin A1c (HbA1c) was analyzed only in subjects with diabetes (due to budget and practicality of the test) through high-performance liquid chromatography using a Bio-Rad® Variant II Turbo Hemoglobin testing system.
OS was assessed both by the pro-oxidant and the antioxidant perspective; OxLDL was used as the pro-oxidant OS biomarker and TAC as the antioxidant OS biomarker. OxLDL was assessed using a solid-phase two-site enzyme immunoassay (Mercodia® Oxidized LDL ELISA) following the use directions specified by the developer and read spectrophotometrically. TAC was measured using a QuantiChrom® kit (DTAC-100, BioAssay Systems) in which Cu2+ (Copper) is reduced by antioxidants to Cu+; the resulting Cu+ specifically forms a colored complex with a dye reagent, and the color intensity at 570 nm is proportional to the serum TAC of the sample. 32 Smoking status was defined as current if the subject self-reported that have smoked any tobacco in the previous 12 months and included those who had quit a year earlier; non-smokers were defined as those who have more than 1 year without smoking or who have never smoked. 33 None of the participants were taking any antioxidant or vitamin supplement.
Finally, Human FGF21 in serum was quantified with an FGF21 Sandwich ELISA kit (Millipore® ELISA kit) following the assay procedure recommended by the developer.
Statistical analysis
Subjects were categorized according to the KDIGO classification into six stages (G1, G2, G3a, G3b, G4, and G5). 33 Descriptive statistics were used to estimate means and standard deviation for normally distributed variables, and median and interquartile range for variables with skewed distribution. Frequencies were expressed in percentages. Differences in frequencies from categorical variables were assessed using the Chi 2 test. Means and medians were compared using ANOVA or Kruskal–Wallis tests. To evaluate the correlation between serum FGF21 and age, sex, BMI, T2D diagnosis, smoking status, TAC, OxLDL, and eGFR, Pearson’s correlation coefficients were performed. Finally, a multiple linear regression analysis was made to evaluate which variables were independently associated with the increase of serum FGF21. Serum FGF21, OxLDL, and TAC were log-transformed to improve discrimination, calibration, and to minimize influence of extreme observations in correlation and multilinear regression analyzes. Statistical significance was considered with a p < 0.05. SPSS v. 24.0 statistical package (SPSS Chicago, IL, US) was used for all analyses.
Results
Demographic, clinical, and biochemical characteristics of the subjects classified by stages of kidney disease (G1 to G5) are summarized in Table 1. Mean age for all subjects was 55.0 ± 9.7 years, and those in stage G1 were significantly younger than the rest of patients. Overall, 49% of all subjects were males, and the proportion was similar among the six groups (p = 0.147). T2D was present in 43% of the total population; ~96% of patients with eGFR lower than 45 ml/min/1.73 m2 had T2D, and was higher compared with the rest of patients (p < 0.05). Duration of T2D was also higher in subjects from the final stages of kidney disease (G3b, G4, and G5), but the difference was only significant when compared with subjects from stage G1 (p < 0.05 versus G3b and G4, p < 0.001 versus G5). Regarding smoking status, 22% of all participants were current smokers, and among all groups, the proportion of smokers was higher in the G5 stage group (47.6%), and the lowest proportion was on the G4 stage group (4.2%). In addition, BMI, systolic, and diastolic blood pressure were similar among all groups. Hemoglobin concentration was significantly lower in subjects from G3B, G4, and G5 stages when compared with those from G1, G2, and G3a (p < 0.05 for all). As commented above, HbA1c was only measured in subjects with T2D diagnosis, and it was significantly lower in subjects from G5 stage when compared with the other five groups, possibly due to decreased hemoglobin concentration in these patients. Median FGF21 serum concentration for all subjects was of 406 (234–683) pg/ml, and it tended to increase as renal function declined, except in G4 stage, where it presented a non-significant decrease when compared with G3b [746 (512–1343) versus 584 (352–887) pg/ml; p < 0.05], but it increased again in G5 stage [1313 (627–1735) pg/ml] (p < 0.05). Regarding the oxidative marker TAC, a visible increasing trend was observed throughout kidney disease stages, and there was only a significant difference in its median serum concentration between G1 [421 (350–549) µM/Trolox Eq.] and G4 stages [593 (452–690) µM/Trolox Eq.] (p < 0.05). Mean serum concentration of OxLDL for all subjects was 53.2 ± 18.8 IU/L; among groups, OxLDL serum concentration was similar (p = 0.148).
Table 1.
Demographic, clinical, and biochemical characteristics of participants categorized according to KDIGO stages of chronic kidney disease.
| Variable | G1 (n = 185) | G2 (n = 96) | G3a (n = 30) | G3b (n = 23) | G4 (n = 24) | G5 (n = 24) | p |
|---|---|---|---|---|---|---|---|
| Age (years) | 52 ± 9 | 57 ± 10 a | 59 ± 11 a | 60 ± 12 a | 59 ± 10 a | 58 ± 7 | <0.001 |
| Male sex (%) | 53.0 | 39.6 a | 36.7 | 52.2 | 58.3 | 58.3 | 0.147 |
| Type 2 diabetes (%) | 31.9 | 19.8 a | 66.7a,b | 95.7a,b,c | 95.8a,b,c | 95.8a,b,c | <0.001 |
| Diabetes duration (y) | 10 (5–16) | 18 (7–21) | 20 (12–24) a | 24 (15–31)a,b | 20 (10–32) a | 27 (16–35)a,b | <0.001 |
| Current smokers (%) | 24.3 | 26.1 | 7.7 | 9.1 | 4.2a,b | 47.6a,c,d,e | 0.002 |
| BMI (kg/m2) | 28.5 ± 5.4 | 27.7 ± 4.4 | 29.2 ± 5.6 | 26.1 ± 3.2 | 28.8 ± 5.2 | 28.5 ± 4.2 | 0.184 |
| SBP (mm Hg) | 125 ± 18 | 131 ± 21 | 127 ± 25 | 136 ± 22 | 134 ± 26 | 134 ± 28 | 0.011 |
| DBP (mm Hg) | 75 ± 11 | 78 ± 11 | 77 ± 13 | 77 ± 13 | 81 ± 13 | 77 ± 11 | 0.216 |
| Hemoglobin (g/dl) | 14.3 ± 2.9 | 14.8 ± 2.6 | 14.2 ± 1.8 | 12.9 ± 1.7 | 12.1 ± 2.0a,b | 11.5 ± 2.4a,b,c | <0.001 |
| Hemoglobin A1c (%) | 8.7 ± 2.0 | 8.5 ± 2.1 | 9.0 ± 2.3 | 8.1 ± 2.1 | 7.7 ± 2.4 | 6.7 ± 1.0a,c | 0.001 |
| Fasting glucose (mg/dl) | 97 (85–150) | 91 (83–106) a | 105 (84–143) b | 124 (94–159) b | 98 (81–29) | 109 (86–154) b | 0.170 |
| Serum creatinine (mg/dl) | 0.7 (0.6–0.8) | 0.9 (0.8–1.0) a | 1.3 (1.1–1.4)a,b | 1.7 (1.5–1.8)a,b,c | 2.9 (2.7–3.7)a,b,c,d | 6.1 (5.1–11.4)a,b,c,d,e | <0.001 |
| eGFR (ml/min/1.73 m2) | 104 (99–111) | 78 (70–83) a | 51 (49–55)a,b | 38 (32–41)a,b,c | 19 (16–22)a,b,c,d | 7 (4–11)a,b,c,d,e | <0.001 |
| Serum FGF21 (pg/ml) | 336 (204–541) | 370 (249–578) | 452 (227–810) | 746 (512–1343)a,b,c | 584 (352–887)a,b | 1313 (627–1735)a,b,c,e | <0.001 |
| Serum total antioxidant capacity (µM/Trolox Eq.) | 421 (350–549) | 462 (369–623) a | 481 (425–650) a | 506 (407–485) | 593 (452–690) a | 521 (368–644) | 0.003 |
| OxLDL (IU/L) | 55.4 ± 18.3 | 53.5 ± 18.9 | 49.6 ± 17.1 | 50 ± 20.4 | 47.9 ± 17.1 | 47.8 ± 19.5 | 0.148 |
Variables are shown as mean ± SD or median (interquartile range) or percentages. p value: ANOVA or Kruskal–Wallis, or chi2.ap < 0.05 versus G1.
p < 0.05 versus G2.
p < 0.05 versus G3a.
p < 0.05 versus G3b.
p < 0.05 versus G4.
BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; FGF21, fibroblast growth factor 21; TAC, total antioxidant capacity; OxLDL, oxidized low-density lipoproteins; SBP, systolic blood pressure.
Pharmacological treatment of participants categorized according to KDIGO stages is presented in Table 2. There was a significant difference in subjects treated with metformin and insulin among groups, metformin being extensively used by patients in G1, G2, and G3 stages and poorly used in the final stages (p < 0.001). On the other hand, as the eGFR decreases, insulin treatment gradually increased (p < 0.001). Treatment with statins was present in 43.3% of the total population, and it tended to increase its use among eGFR lower than 90 ml/min/1.73 m2, but it was not significant (0.105). Angiotensin receptor blockers were significantly lower in stage G1 compared with G3b, G4, and G5.
Table 2.
Pharmacological treatment of the participants categorized according to KDIGO stages of chronic kidney disease.
| Variable | G1 (n = 185) | G2 (n = 96) | G3a (n = 30) | G3b (n = 23) | G4 (n = 24) | G5 (n = 24) | p |
|---|---|---|---|---|---|---|---|
| Metformin (%) | 94.9 | 94.4 | 70.0 a | 31.8a,b,c | 17.4a,b,c | 4.5a,b,c,d | <0.001 |
| Insulin treatment (%) | 22.8 | 20.3 | 44.4a,b | 77.3 | 69.6a,b | 81.8a,b,c | <0.001 |
| Sulfonylureas (%) | 25.4 | 16.7 | 25.0 | 9.1 | 13.0 | 9.1 | 0.352 |
| DPP4 inhibitors (%) | 3.4 | 0.0 | 5.0 | 13.6 | 13.0 | 4.5 | 0.289 |
| Statins (%) | 28.8 | 61.1 a | 50.0 | 54.5 a | 47.8 | 45.5 | 0.105 |
| Fibrates (%) | 15.3 | 33.3 | 25.0 | 31.8 | 39.1 a | 4.5b,d,e | 0.037 |
| ACEI (%) | 18.6 | 33.3 | 35.0 | 36.4 | 26.1 | 18.2 | 0.420 |
| ARB (%) | 8.5 | 22.2 | 10.0 | 40.9 a | 30.4 a | 45.5a,c | 0.001 |
Variables are shown as percentages. p value: chi2.
p < 0.05 versus G1.
p < 0.05 versus G2.
p < 0.05 versus G3a.
p < 0.05 versus G3b.
p < 0.05 versus G4.
ACEI, angiotensin converting enzyme inhibitors; ARB, angiotensin receptor blockers; DPP4, dipeptidyl peptidase-4 inhibitors.
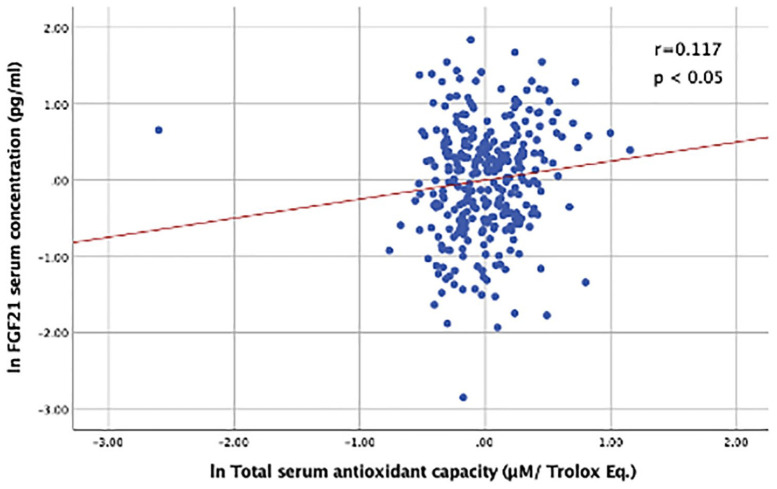
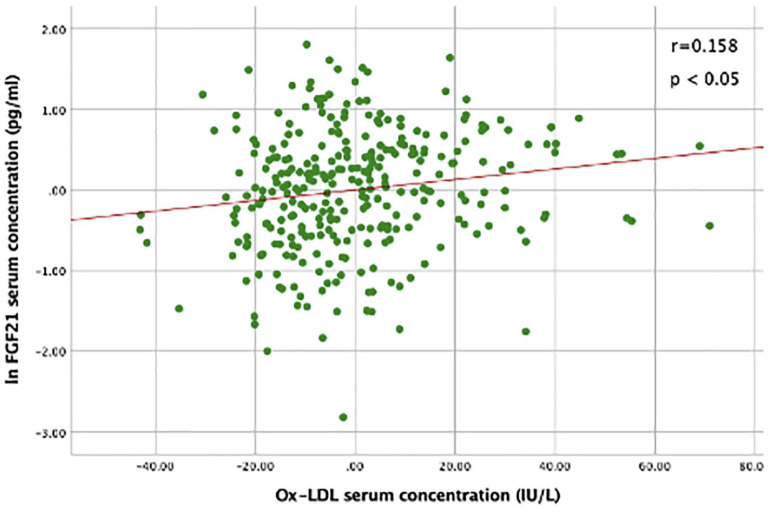
Bivariate correlation analyses are shown in Table 3. Overall, FGF21 serum concentration had a significant positive correlation with age (r = 0.236), and lnTAC (r = 0.217), and was negatively correlated with the eGFR (r = −0.429) and male sex (r = −0.102); (p < 0.05 for all). There was no significant correlation of serum FGF21 with OxLDL. The positive association of FGF21 with lnTAC remained significant (r = 0.117, p < 0.05) after controlling by age, sex, BMI, eGFR, smoking status, and T2D diagnosis (Figure 1). Additionally, after controlling by age, sex, BMI, T2D, smoking status and eGFR, a significant positive association between FGF21 and OxLDL was found (Figure 2) (r = 0.158, p < 0.05).
Table 3.
Correlation between FGF21, age, sex, BMI, T2D diagnosis, smoking status, TAC, OxLDL, and eGFR.
| Age (years) | Sex (%) | BMI (kg/m2) | T2D (%) | Smoking (%) | TAC (µM/Trolox Eq.) | OxLDL (IU/L) | eGFR (ml/min) | |
|---|---|---|---|---|---|---|---|---|
| FGF21 † | 0.236 ** | −0.102 * | 0.056 | 0.044 | 0.045 | 0.217 ** | 0.084 | −0.429 ** |
Pearson’s correlation coefficient. n = 382.
p < 0.05.
p < 0.001.
log-transformed variable.
BMI, body mass index; eGFR, estimated glomerular filtration rate; FGF21, fibroblast growth factor 21; OxLDL, oxidized low-density lipoproteins; T2D, type 2 diabetes; TAC, serum total antioxidant capacity.
Figure 1.
Correlation between serum FGF21 and TAC, controlled by age, sex, BMI, T2D diagnosis, smoking status, and eGFR.
Serum FGF21 and TAC were log-transformed.
Pearson’s Partial Correlation showed a positive correlation between FGF21 and serum total antioxidant capacity. n = 382; r = 0.117; p < 0.05.
Figure 2.
Correlation between serum FGF21 and OxLDL, controlled by age, sex, BMI, T2D diagnosis, smoking status, and eGFR.
Serum FGF21 was log-transformed.
Pearson’s Partial Correlation showed a positive correlation between FGF21 and OxLDL, n = 382; r = 0.158; p < 0.05.
Finally, a multiple linear regression was calculated to predict FGF21 serum concentration based on age, sex, BMI, T2D diagnosis, smoking status, fasting plasma glucose, TAC, OxLDL, and eGFR (Table 4). A significant regression equation was found (F = 13.154, p < 0.001, constant 6.0), with an R2 of 0.288. The eGFR, male sex, T2D diagnosis, OxLDL, and TAC were the independent variables that significantly influenced the serum concentration of FGF21 (STDβ = −0.475, 0.162, −0.153, 0.142, and 0.136 for eGFR, male sex, T2D diagnosis, OxLDL, and TAC respectively; p < 0.05 for all).
Table 4.
Multiple linear regression analysis using log-transformed serum FGF21 as dependent variable.
| Independent variables | Standardized β | t | p |
|---|---|---|---|
| eGFR (ml/min/1.73 m2 ) | −0.475 | −7.488 | <0.001 |
| Sex (male) | 0.162 | −3.060 | 0.002 |
| T2D diagnosis (diagnosis) | −0.153 | −2.415 | 0.016 |
| OxLDL (IU/L) | 0.142 | 2.776 | 0.006 |
| Serum total antioxidant capacity (µM/Trolox Eq.) | 0.136 | 2.605 | 0.010 |
| Age (years) | 0.077 | 1.424 | 0.156 |
| BMI (kg/m2) | 0.017 | 0.321 | 0.748 |
| Smoking | 0.089 | 1.699 | 0.090 |
| Fasting plasma glucose (mg/dL) | 0.002 | 0.037 | 0.970 |
n = 382.
Constant = 6.008; F = 13.154; R2 = 0.288; p < 0.001.
Variables in bold had statistical significance in the regression model.
BMI, body mass index; FGF21, fibroblast growth factor 21; eGFR, estimated glomerular filtration rate; OxLDL, oxidized low-density lipoproteins; T2D, type 2 diabetes.
Discussion
In this cross-sectional study, a positive significant association between serum FGF21 and OS independently of renal function in humans was found. It is well known that excessive generation of reactive oxygen and nitrogen species (ROS and RNS) are contributors involved in the initiation and progression of CKD, 34 and that oxidized lipoproteins and TAC are markers of OS that are elevated in CKD and accumulate as kidney function deteriorates.27,35 Nevertheless, in this study, serum concentrations of OxLDL and TAC did not show significant increases as stages of kidney disease progressed. In an additional regression performed (Supplementary Table 1), smoking was positively associated with the serum concentration of OxLDL (STDβ = 0.144, p = 0.012). Hence, smoking status could influence in not having the expected difference of OxLDL between stages of kidney disease, since in G1 and G2 stages there were more active smokers than in the G3a, G3b, and G4 stages. Additionally, it has been reported that, in subjects with end-stage renal disease, type and form of substitutive renal therapy influences the serum oxidative status;36,37 unfortunately, given that the data of participants in this study were extracted from other previous protocols, no information was available about substitutive renal therapy of participants in the final stages of kidney disease. Therefore, it is not known whether this factor could intervene in the non-observed difference in OxLDL and TAC between CKD stages.
Although no significant increases in OxLDL and TAC were observed as eGFR decreased, serum FGF21 did have a notable tendency to increase throughout kidney disease stages. This finding, along with similar results reported previously,18,38,39 and as it has also been described that serum FGF21 is associated with an increased risk of developing nephropathy, 40 suggests that in CKD, FGF21 elevates as a compensating protection mechanism. 41
In both partial correlation and multiple linear regression analyses, serum FGF21 was positively associated with TAC and OxLDL. Actually, in the regression analysis it was found that the increase in one unit (µM/Trolox Eq.) of TAC was associated with the increase of 0.136 standardized units (pg/ml) of FGF21, and that the increase in one unit (IU/L) of OxLDL was associated with the increase of 0.142 standardized units (pg/ml) of FGF21. These associations were independent of age, sex, BMI, T2D diagnosis, smoking, fasting plasma glucose, and the eGFR. Taken together, these findings suggest that the elevated serum concentration of FGF21 in CKD is at least partially influenced by the serum oxidative status.
To date, evidence suggests that OS stimulates FGF21 production,8,12,13 and other studies have demonstrated that FGF21 protects cells from OS injury and death in cardiac cells exposed to hydrogen peroxide; 42 neuroblastoma cells (SH-SY5Y) exposed to amyloid-beta1-42; 43 mesenchymal stem cells exposed to hydrogen peroxide and tumor necrosis factor alpha (TNFa); 44 and in vivo in a rodent model of sciatic crush injury, 45 FGF21 has shown an antioxidant and protective effect.
In CKD, the approach of ameliorating OS in order to prevent or limit the progression of kidney deterioration has been explored for several years.34,46,47 Administration of some antioxidants has been associated with a significant reduction of the progression to end-stage renal disease and the improvement of the glomerular filtration rate; 48 the use of the nuclear factor erythroid 2-related factor 2 (Nrf2) activator, bardoxolone methyl, was shown to improve eGFR in patients with diabetic nephropathy;49,50 however, it increased albuminuria and blood pressure in patients with stage 4 CKD in the BEACON trial, and mortality was higher in the group receiving bardoxolone methyl,51,52 thus further study of this agent was suspended. It is discussed whether bardoxolone methyl could be more effective in subjects with earlier stages of CKD (G3) and if safety concerns might be associated with drug interactions. 53 On the other hand, in some antihypertensive and anti-hyperglycemic medications it has been shown that among the mechanisms that confer them their renal protective effects is the reduction of OS.54,55
Particularly, the use of FGF21 as a treatment for OS in CKD has been scarcely studied but it has had favorable results. For example, in rodent models of nephropathy associated to lipotoxicity and type 1 diabetes, this growth factor prevented kidney damage by suppressing 3-nitrotyrosine and 4-hydroxynonenal production in addition to reducing the inflammatory process and fibrosis; 56 indirectly, induction of the production of FGF21 through fenofibrate, and the consequent activation of the Akt-Nrf2 antioxidant pathway, prevented the death of tubular human cells exposed to high glucose concentrations and reduced OS markers. 57 Unfortunately, the therapeutic potential of FGF21 for OS in CKD in humans has not yet been studied, possibly due to its instability and short half-life. 58 Furthermore, to be able to consider FGF21 as a treatment in CKD, it is necessary to better understand the role of this growth factor in the mechanisms of the disease. The results of this study suggest that serum FGF21 is associated to OS markers in CKD; however, longitudinal studies that allow evaluation of causal associations would bring valuable information in this topic.
Although diagnosis of T2D was not correlated to FGF21 serum concentration in Spearman’s test, it was associated with increased serum concentration of FGF21 in the multiple regression analysis. Previous studies suggested that FGF21 serum concentration is elevated in subjects with T2D possibly due to increased visceral fat resistance to this growth factor 59 or the influence of BMI, total cholesterol, or triglyceride levels; 60 in our analysis, the association of FGF21 with T2D diagnosis was independent of BMI, but the lipid profile was not evaluated. Fasting plasma glucose did not show a significant association with FGF21 either in correlation or in regression analysis, possibly because there was no significant variance of glucose between groups.
In the correlation analysis, age was positively correlated with FGF21. Higher serum concentration of FGF21 in the elderly has been reported before; 61 furthermore, in healthy subjects, FGF21 has been found to increase with advancing age 62 and it has been suggested that it can improve lifespan. 63 However, in this study, the association of age with serum FGF21 was lost in the multiple linear regression, possibly due to the influence of the other independent variables studied. Sex did not show a significant correlation with FGF21, but in the multiple linear regression male sex was associated with an increase of 0.162 standardized units (pg/ml) of serum FGF21. Previous evidence had found that the concentration of FGF21 was higher in female subjects and it was associated with a higher risk of developing end-stage renal disease in women but not in men; 64 also in other study, serum concentration of FGF21 in women with T2D was associated with an increased risk of lower extremity atherosclerotic disease, but not in men. 65 It has been shown that estrogen influences liver production of FGF21; 66 however, the exact mechanisms of sex dimorphism in FGF21 regulation have not yet been fully elucidated.
The authors acknowledge the following limitations. First, the authors acknowledge that besides T2D, data regarding the etiology of CKD were not recorded. Second, it is known that TAC quantification is influenced by many biological compounds with chain-breaking antioxidant activity like urate, ascorbate, bilirubin, alfa tocopherol, carotenoids, and flavonoids; therefore, to make a more accurate interpretation of its values, the measurement of these compounds is recommended. 67 However, this assessment was not possible for us and we could only report that participants were not taking any vitamin or antioxidant supplements. Third, in some of the patients, total cholesterol, LDL-C, and HDL-C levels were not recorded, thus these variables were not included in the regression models, although lipid profile could provide more interesting information about the associations seen. Finally, due to budget limitations, TAC and OxLDL were the only biomarkers available to assess the association of OS and FGF21 serum concentration. Using other well-known biomarkers of OS in humans, such as serum lipid hydroperoxides, plasma malondialdehyde, or F2-isoprostanes, 68 to assess its association with serum FGF21 could provide valuable information regarding this research topic in future studies.
Conclusion
A positive association between FGF21 serum concentration and OS markers has been found independently of renal function in humans. Nevertheless, the present study cannot conclude that FGF21 serum values could make significant changes in OS. To the best of our knowledge, this study is the first to report this association. Since there is a great interest in the potential therapeutic use of FGF21 in relieving OS, the present study provides valuable information for the deeper understanding of the role of FGF21 in OS in humans with CKD with or without diabetes. Further mechanistic studies to explain the association of serum FGF21 with oxidative stress in CKD are needed.
Supplemental Material
Supplemental material, sj-pdf-1-tae-10.1177_20420188211001160 for Fibroblast growth factor 21 is associated with increased serum total antioxidant capacity and oxidized lipoproteins in humans with different stages of chronic kidney disease by Miguel Ángel Gómez-Sámano, Valerie Paola Vargas-Abonce, Froylan David Martínez-Sánchez, Lucía Palacios-Báez, Juan Mauricio Vera-Zertuche, María Fernanda Navarro-Flores, Mariana Guadalupe Morales-García, Jorge Ignacio Fonseca-Correa, Julia María Zuarth-Vázquez, Olynka Vega-Vega, Ricardo Correa-Rotter, Rodolfo Rincón-Pedrero, Luis E. Morales-Buenrostro, Josefina Alberú-Gómez, Julia Berenice Ramírez-González, Reyna Lizette Pacheco-Domínguez, Malaquías López-Cervantes, María de los Ángeles Mendoza-de-la-Garza, Yolanda Victoria Baeza-Arias, Ángeles Espinosa-Cuevas, Guadalupe López-Carrasco, Angelina López-Estrada, Luz Elizabeth Guillén-Pineda, Francisco Javier Gómez-Pérez and Daniel Cuevas-Ramos in Therapeutic Advances in Endocrinology and Metabolism
Footnotes
Author contribution(s): Miguel Ángel Gómez-Sámano: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writingoriginal draft; Writing-review & editing.
Valerie Paola Vargas-Abonce: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Supervision; Validation; Visualization; Writing-original draft; Writing-review & editing.
Froylan David Martínez-Sánchez: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Supervision; Writing-original draft; Writing-review & editing.
Lucía Palacios-Báez: Funding acquisition; Methodology; Project administration; Resources; Supervision; Writing-review & editing.
Juan Mauricio Vera-Zertuche: Investigation; Methodology; Project administration; Resources; Validation; Writing-review & editing.
María Fernanda Navarro-Flores: Data curation; Investigation; Methodology; Project administration; Resources; Software; Writing-review & editing.
Mariana Guadalupe Morales-García: Investigation; Methodology; Resources; Supervision; Writing-review & editing.
Jorge Ignacio Fonseca-Correa: Investigation; Methodology; Resources; Writing-review & editing.
Julia María Zuarth-Vázquez: Investigation; Methodology; Project administration; Validation; Writing-review & editing.
Olynka Vega-Vega: Investigation; Methodology; Visualization; Writing-review & editing.
Ricardo Correa-Rotter: Investigation; Methodology; Writing-review & editing.
Rodolfo Rincón-Pedrero: Investigation; Methodology; Writing-review & editing.
Luis E. Morales-Buenrostro: Data curation; Formal analysis; Methodology; Writing-original draft.
Josefina Alberú-Gómez: Investigation; Methodology; Writing-review & editing.
Julia Berenice Ramírez-González: Investigation; Methodology; Writing-review & editing.
Reyna Lizette Pacheco-Domínguez: Investigation; Methodology; Writing-review & editing.
Malaquías López-Cervantes: Investigation; Methodology; Writing-review & editing.
María de los Ángeles Mendoza-de-la-Garza: Investigation; Methodology; Writing-review & editing.
Yolanda Victoria Baeza-Arias: Investigation; Methodology; Writing-review & editing.
Ángeles Espinosa-Cuevas: Conceptualization; Investigation; Methodology; Resources; Supervision; Validation; Writing-original draft.
Guadalupe López-Carrasco: Investigation; Methodology; Writing-review & editing.
Angelina López-Estrada: Investigation; Methodology; Writing-review & editing.
Luz Elizabeth Guillén-Pineda: Investigation; Methodology; Validation; Writing-review & editing.
Francisco Javier Gómez-Pérez: Funding acquisition; Investigation; Methodology; Project administration; Writing-original draft; Writing-review & editing.
Daniel Cuevas-Ramos: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Writing-review & editin
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received funding from the department of Endocrinology and Metabolism of the Instituto Nacional de Ciencias Medicas y Nutricion Salvador Zubiran
ORCID iDs: Miguel Ángel Gómez-Sámano  https://orcid.org/0000-0002-8596-6669
https://orcid.org/0000-0002-8596-6669
Froylan David Martínez-Sánchez  https://orcid.org/0000-0002-2719-1105
https://orcid.org/0000-0002-2719-1105
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Miguel Ángel Gómez-Sámano, Instituto Nacional de Ciencias Medicas y Nutricion Salvador Zubiran, Mexico City, Mexico.
Valerie Paola Vargas-Abonce, Instituto Nacional de Ciencias Medicas y Nutricion Salvador Zubiran, Mexico City, Mexico.
Froylan David Martínez-Sánchez, Instituto Nacional de Ciencias Medicas y Nutricion Salvador Zubiran, Mexico City, Mexico.
Lucía Palacios-Báez, Instituto Nacional de Ciencias Medicas y Nutricion Salvador Zubiran, Mexico City, Mexico.
Juan Mauricio Vera-Zertuche, Instituto Nacional de Ciencias Medicas y Nutricion Salvador Zubiran, Mexico City, Mexico.
María Fernanda Navarro-Flores, Instituto Nacional de Ciencias Medicas y Nutricion Salvador Zubiran, Mexico City, Mexico.
Mariana Guadalupe Morales-García, Instituto Nacional de Ciencias Medicas y Nutricion Salvador Zubiran, Mexico City, Mexico.
Jorge Ignacio Fonseca-Correa, Instituto Nacional de Ciencias Medicas y Nutricion Salvador Zubiran, Mexico City, Mexico.
Julia María Zuarth-Vázquez, Instituto Nacional de Ciencias Medicas y Nutricion Salvador Zubiran, Mexico City, Mexico.
Olynka Vega-Vega, Instituto Nacional de Ciencias Medicas y Nutricion Salvador Zubiran, Mexico City, Mexico.
Ricardo Correa-Rotter, Instituto Nacional de Ciencias Medicas y Nutricion Salvador Zubiran, Mexico City, Mexico.
Rodolfo Rincón-Pedrero, Instituto Nacional de Ciencias Medicas y Nutricion Salvador Zubiran, Mexico City, Mexico.
Luis E. Morales-Buenrostro, Instituto Nacional de Ciencias Medicas y Nutricion Salvador Zubiran, Mexico City, Mexico
Josefina Alberú-Gómez, Instituto Nacional de Ciencias Medicas y Nutricion Salvador Zubiran, Mexico City, Mexico.
Julia Berenice Ramírez-González, Department of Preventive Medicine and Public Health, Universidad Nacional Autónoma de México, Mexico City, Mexico.
Reyna Lizette Pacheco-Domínguez, Department of Preventive Medicine and Public Health, Universidad Nacional Autónoma de México, Mexico City, Mexico.
Malaquías López-Cervantes, Department of Preventive Medicine and Public Health, Universidad Nacional Autónoma de México, Mexico City, Mexico.
María de los Ángeles Mendoza-de-la-Garza, Instituto Nacional de Ciencias Medicas y Nutricion Salvador Zubiran, Mexico City, Mexico.
Yolanda Victoria Baeza-Arias, Instituto Nacional de Ciencias Medicas y Nutricion Salvador Zubiran, Mexico City, Mexico.
Ángeles Espinosa-Cuevas, Instituto Nacional de Ciencias Medicas y Nutricion Salvador Zubiran, Mexico City, Mexico.
Guadalupe López-Carrasco, Instituto Nacional de Ciencias Medicas y Nutricion Salvador Zubiran, Mexico City, Mexico.
Angelina López-Estrada, Instituto Nacional de Ciencias Medicas y Nutricion Salvador Zubiran, Mexico City, Mexico.
Luz Elizabeth Guillén-Pineda, Instituto Nacional de Ciencias Medicas y Nutricion Salvador Zubiran, Mexico City, Mexico.
Francisco Javier Gómez-Pérez, Instituto Nacional de Ciencias Medicas y Nutricion Salvador Zubiran, Mexico City, Mexico.
Daniel Cuevas-Ramos, Department of Endocrinology and Metabolism, Instituto Nacional de Ciencias Medicas y Nutricion Salvador Zubiran, Vasco de Quiroga # 15, Sección XVI Tlalpan 14000, Mexico City, Mexico.
References
- 1. Kharitonenkov A, Shiyanova TL, Koester A, et al. FGF-21 as a novel metabolic regulator. J Clin Invest 2005; 115: 1627–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nishimura T, Nakatake Y, Konishi M, et al. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim Biophys Acta 2000; 1492: 203–206. [DOI] [PubMed] [Google Scholar]
- 3. Hondares E, Iglesias R, Giralt A, et al. Thermogenic activation induces FGF21 expression and release in brown adipose tissue. J Biol Chem 2011; 286: 12983–12990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang X, Yeung DCY, Karpisek M, et al. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes 2008; 57: 1246–1253. [DOI] [PubMed] [Google Scholar]
- 5. Dushay J, Chui PC, Gopalakrishnan GS, et al. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology 2010; 139: 456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cuevas-Ramos D, Almeda-Valdes P, Gómez-Pérez FJ, et al. Daily physical activity, fasting glucose, uric acid, and body mass index are independent factors associated with serum fibroblast growth factor 21 levels. Eur J Endocrinol 2010; 163: 469–477. [DOI] [PubMed] [Google Scholar]
- 7. Gälman C, Lundåsen T, Kharitonenkov A, et al. The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPAR alpha; activation in man. Cell Metab 2008; 8: 169–174. [DOI] [PubMed] [Google Scholar]
- 8. De Sousa-Coelho AL, Marrero PF, Haro D. Activating transcription factor 4-dependent induction of FGF21 during amino acid deprivation. Biochem J 2012; 443: 165–171. [DOI] [PubMed] [Google Scholar]
- 9. Coskun T, Bina HA, Schneider MA, et al. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 2008; 149: 6018–6027. [DOI] [PubMed] [Google Scholar]
- 10. Berglund ED, Li CY, Bina HA, et al. Fibroblast growth factor 21 controls glycemia via regulation of hepatic glucose flux and insulin sensitivity. Endocrinology 2009; 150: 4084–4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Staiger H, Keuper M, Berti L, et al. Fibroblast growth factor 21—metabolic role in mice and men. Endocr Rev 2017; 38: 468–488. [DOI] [PubMed] [Google Scholar]
- 12. Kim KH, Jeong YT, Oh H, et al. Autophagy deficiency leads to protection from obesity and insulin resistance by inducing FGF21 as a mitokine. Nat Med 2013; 19: 83–92. [DOI] [PubMed] [Google Scholar]
- 13. Gómez-Sámano MÁ, Grajales-Gómez M, Zuarth-Vázquez JM, et al. Fibroblast growth factor 21 and its novel association with oxidative stress. Redox Biol 2017; 11: 335–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wente W, Efanov AM, Brenner M, et al. Fibroblast growth factor-21 improves pancreatic β-cell function and survival by activation of extracellular signal–regulated kinase 1/2 and akt signaling pathways. Diabetes 2006; 55: 2470–2478. [DOI] [PubMed] [Google Scholar]
- 15. Planavila A, Redondo-Angulo I, Ribas F, et al. Fibroblast growth factor 21 protects the heart from oxidative stress. Cardiovasc Res 2015; 106: 19–31. [DOI] [PubMed] [Google Scholar]
- 16. Stein S, Bachmann A, Lössner U, et al. Serum levels of the adipokine FGF21 depend on renal function. Diabetes Care 2009; 32: 126–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Han SH, Choi SH, Cho BJ, et al. Serum fibroblast growth factor 21 concentration is associated with residual renal function and insulin resistance in end-stage renal disease patients receiving long-term peritoneal dialysis. Metabolism 2010; 59: 1656–1662. [DOI] [PubMed] [Google Scholar]
- 18. Lin Z, Zhou Z, Liu Y, et al. Circulating FGF21 levels are progressively increased from the early to end stages of chronic kidney diseases and are associated with renal function in Chinese. PLoS One 2011; 6: e18398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. He Y, Li Y, Wei Z, et al. Pharmacokinetics, tissue distribution, and excretion of FGF-21 following subcutaneous administration in rats. Drug Test Anal 2018; 10: 1061–1069. [DOI] [PubMed] [Google Scholar]
- 20. Daenen K, Andries A, Mekahli D, et al. Oxidative stress in chronic kidney disease. Pediatr Nephrol 2019; 34: 975–991. [DOI] [PubMed] [Google Scholar]
- 21. Himmelfarb J. Relevance of oxidative pathways in the pathophysiology of chronic kidney disease. Cardiol Clin 2005; 23: 319–330. [DOI] [PubMed] [Google Scholar]
- 22. Bikbov B, Purcell CA, Levey AS, et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet 2020; 395: 709–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Luyckx VA, Cherney DZI, Bello AK. Preventing CKD in developed countries. Kidney Int Rep 2020; 5: 263–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Trpkovic A, Resanovic I, Stanimirovic J, et al. Oxidized low-density lipoprotein as a biomarker of cardiovascular diseases. Crit Rev Clin Lab Sci 2015; 52: 70–85. [DOI] [PubMed] [Google Scholar]
- 25. Ghiselli A, Serafini M, Natella F, et al. Total antioxidant capacity as a tool to assess redox status: critical view and experimental data. Free Radic Biol Med 2000; 29: 1106–1114. [DOI] [PubMed] [Google Scholar]
- 26. Lalongo C. Preanalytic of total antioxidant capacity assays performed in serum, plasma, urine and saliva. Clin Biochem 2017; 50: 356–363. [DOI] [PubMed] [Google Scholar]
- 27. Dounousi E, Papavasiliou E, Makedou A, et al. Oxidative stress is progressively enhanced with advancing stages of CKD. Am J Kidney Dis 2006; 48: 752–760. [DOI] [PubMed] [Google Scholar]
- 28. Chuang C-C, Shiesh S-C, Chi C-H, et al. Serum total antioxidant capacity reflects severity of illness in patients with severe sepsis. Crit Care 2006; 10: R36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Petelin A, Tedeschi P, Maietti A, et al. Total serum antioxidant capacity in healthy normal weight and asymptomatic overweight adults. Exp Clin Endocrinol Diabetes 2017; 125: 470–477. [DOI] [PubMed] [Google Scholar]
- 30. Vega-Vega O, Fonseca-Correa JI, Mendoza-De la, Garza A, et al. Contemporary dietary intake: too much sodium, not enough potassium, yet sufficient iodine: the SALMEX cohort results. Nutrients 2018; 10: 816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huang D, Ou B, Prior RL. The chemistry behind antioxidant capacity assays. J Agric Food Chem 2005; 53: 1841–1856. [DOI] [PubMed] [Google Scholar]
- 33. Medina-Urrutia AX, Martínez-Sánchez FD, Posadas-Romero C, et al. Metabolic control achievement in a population with premature coronary artery disease: results of the genetics of atherosclerotic disease study. Ther Adv Endocrinol Metab 2020; 11: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stevens PE, Levin A. Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 2013; 158: 825–830. [DOI] [PubMed] [Google Scholar]
- 35. Duni A, Liakopoulos V, Roumeliotis S, et al. Oxidative stress in the pathogenesis and evolution of chronic kidney disease: untangling Ariadne’s thread. Int J Mol Sci 2019; 20: 3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tucker PS, Dalbo VJ, Han T, et al. Clinical and research markers of oxidative stress in chronic kidney disease. Biomarkers 2013; 18: 103–115. [DOI] [PubMed] [Google Scholar]
- 37. Ogunleye A, Akinbodewa AA, Adejumo OA, et al. Changes in antioxidant status associated with haemodialysis in chronic kidney disease. Ghana Med J 2018; 52: 29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Roumeliotis S, Eleftheriadis T, Liakopoulos V. Is oxidative stress an issue in peritoneal dialysis? Semin Dial 2019; 32: 463–466. [DOI] [PubMed] [Google Scholar]
- 39. Crasto C, Semba RD, Sun K, et al. Serum fibroblast growth factor 21 is associated with renal function and chronic kidney disease in community-dwelling adults. J Am Geriatr Soc 2012; 60: 792–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hindricks J, Ebert T, Bachmann A, et al. Serum levels of fibroblast growth factor-21 are increased in chronic and acute renal dysfunction. Clin Endocrinol (Oxf) 2014; 80: 918–924. [DOI] [PubMed] [Google Scholar]
- 41. Ong K-L, Januszewski AS, O’Connell R, et al. The relationship of fibroblast growth factor 21 with cardiovascular outcome events in the fenofibrate intervention and event lowering in diabetes study. Diabetologia 2015; 58: 464–473. [DOI] [PubMed] [Google Scholar]
- 42. Anuwatmatee S, Tang S, Wu BJ, et al. Fibroblast growth factor 21 in chronic kidney disease. Clin Chim Acta 2019; 489:196–202. [DOI] [PubMed] [Google Scholar]
- 43. Yan J, Wang J, Huang H, et al. Fibroblast growth factor 21 delayed endothelial replicative senescence and protected cells from H2O2-induced premature senescence through SIRT1. Am J Transl Res 2017; 9: 4492–4501. [PMC free article] [PubMed] [Google Scholar]
- 44. Amiri M, Braidy N, Aminzadeh M. Protective effects of fibroblast growth factor 21 against amyloid-beta1–42-induced toxicity in SH-SY5Y cells. Neurotox Res 2018; 34: 574–583. [DOI] [PubMed] [Google Scholar]
- 45. Linares GR, Leng Y, Maric D, et al. Overexpression of fibroblast growth factor-21 (FGF-21) protects mesenchymal stem cells against caspase-dependent apoptosis induced by oxidative stress and inflammation. Cell Biol Int 2020; 44: 2163–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lu Y, Li R, Zhu J, et al. Fibroblast growth factor 21 facilitates peripheral nerve regeneration through suppressing oxidative damage and autophagic cell death. J Cell Mol Med 2019; 23: 497–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Small DM, Coombes JS, Bennett N, et al. Oxidative stress, anti-oxidant therapies and chronic kidney disease. Nephrology 2012; 17: 311–321. [DOI] [PubMed] [Google Scholar]
- 48. Zhang R, Saredy J, Shao Y, et al. End-stage renal disease is different from chronic kidney disease in upregulating ROS-modulated proinflammatory secretome in PBMCs - a novel multiple-hit model for disease progression. Redox Biol 2020; 34: 101460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jun M, Venkataraman V, Razavian M, et al. Antioxidants for chronic kidney disease. Cochrane Database Syst Rev 2012; 10: CD008176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pergola PE, Krauth M, Huff JW, et al. Effect of bardoxolone methyl on kidney function in patients with T2D and stage 3b–4 CKD. Am J Nephrol 2011; 33: 469–476. [DOI] [PubMed] [Google Scholar]
- 51. Pergola PE, Raskin P, Toto RD, et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med 2011; 365: 327–336. [DOI] [PubMed] [Google Scholar]
- 52. Rossing P. Could problems with bardoxolone methyl have been predicted? Nat Rev Nephrol 2013; 9: 128–130. [DOI] [PubMed] [Google Scholar]
- 53. De Zeeuw D, Akizawa T, Audhya P, et al. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med 2013; 369: 2492–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. de Zeeuw D, Akizawa T, Audhya P, et al.; BEACON Trial Investigators. Bardoxolone methyl in type 2 diabetes and advanced chronic kidney disease. N Engl J Med 2014; 370: 1767–1769. [DOI] [PubMed] [Google Scholar]
- 55. Jing W, Vaziri ND, Nunes A, et al. LCZ696 (sacubitril/valsartan) ameliorates oxidative stress, inflammation, fibrosis and improves renal function beyond angiotensin receptor blockade in CKD. Am J Transl Res 2017; 9: 5473–5484. [PMC free article] [PubMed] [Google Scholar]
- 56. Kimura Y, Kuno A, Tanno M, et al. Canagliflozin, a sodium–glucose cotransporter 2 inhibitor, normalizes renal susceptibility to type 1 cardiorenal syndrome through reduction of renal oxidative stress in diabetic rats. J Diabetes Investig 2019; 10: 933–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhang C, Shao M, Yang H, et al. Attenuation of hyperlipidemia- and diabetes-induced early-stage apoptosis and late-stage renal dysfunction via administration of fibroblast growth factor-21 is associated with suppression of renal inflammation. PLoS One 2013; 8: e82275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cheng Y, Zhang X, Ma F, et al. The role of Akt2 in the protective effect of fenofibrate against diabetic nephropathy. Int J Biol Sci 2020; 16: 553–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhen EY, Jin Z, Ackermann BL, et al. Circulating FGF21 proteolytic processing mediated by fibroblast activation protein. Biochem J 2016; 473: 605–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hong ES, Lim C, Choi HY, et al. Plasma fibroblast growth factor 21 levels increase with ectopic fat accumulation and its receptor levels are decreased in the visceral fat of patients with type 2 diabetes. BMJ Open Diabetes Res Care 2017; 7: e000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wang Y-S, Ye J, Cao Y-H, et al. Increased serum/plasma fibroblast growth factor 21 in type 2 diabetes mellitus: a systematic review and meta-analysis. Postgrad Med J 2019; 95: 134–139. [DOI] [PubMed] [Google Scholar]
- 62. Conte M, Ostan R, Fabbri C, et al. Human aging and longevity are characterized by high levels of mitokines. J Gerontol A Biol Sci Med Sci 2019; 74: 600–607. [DOI] [PubMed] [Google Scholar]
- 63. Hanks LJ, Gutiérrez OM, Bamman MM, et al. Circulating levels of fibroblast growth factor-21 increase with age independently of body composition indices among healthy individuals. J Clin Transl Endocrinol 2015; 2: 77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Salminen A, Kaarniranta K, Kauppinen A. Regulation of longevity by FGF21: interaction between energy metabolism and stress responses. Ageing Res Rev 2017; 37: 79–93. [DOI] [PubMed] [Google Scholar]
- 65. Liu J-J, Liu S, Choo RWM, et al. Sex modulates the association of fibroblast growth factor 21 with end-stage renal disease in Asian people with type 2 diabetes: a 6.3-year prospective cohort study. Diabet Med 2018; 35: 880–886. [DOI] [PubMed] [Google Scholar]
- 66. Zhang X, Hu Y, Zeng H, et al. Serum fibroblast growth factor 21 levels is associated with lower extremity atherosclerotic disease in Chinese female diabetic patients. Cardiovasc Diabetol 2015; 14: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chukijrungroat N, Khamphaya T, Weerachayaphorn J, et al. Hepatic FGF21 mediates sex differences in high-fat high-fructose diet-induced fatty liver. Am J Physiol Metab 2017; 313: E203–E212. [DOI] [PubMed] [Google Scholar]
- 68. Young IS. Measurement of total antioxidant capacity. J Clin Pathol 2001; 54: 339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lee R, Margaritis M, Channon KM, et al. Evaluating oxidative stress in human cardiovascular disease: methodological aspects and considerations. Curr Med Chem 2012; 19: 2504–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tae-10.1177_20420188211001160 for Fibroblast growth factor 21 is associated with increased serum total antioxidant capacity and oxidized lipoproteins in humans with different stages of chronic kidney disease by Miguel Ángel Gómez-Sámano, Valerie Paola Vargas-Abonce, Froylan David Martínez-Sánchez, Lucía Palacios-Báez, Juan Mauricio Vera-Zertuche, María Fernanda Navarro-Flores, Mariana Guadalupe Morales-García, Jorge Ignacio Fonseca-Correa, Julia María Zuarth-Vázquez, Olynka Vega-Vega, Ricardo Correa-Rotter, Rodolfo Rincón-Pedrero, Luis E. Morales-Buenrostro, Josefina Alberú-Gómez, Julia Berenice Ramírez-González, Reyna Lizette Pacheco-Domínguez, Malaquías López-Cervantes, María de los Ángeles Mendoza-de-la-Garza, Yolanda Victoria Baeza-Arias, Ángeles Espinosa-Cuevas, Guadalupe López-Carrasco, Angelina López-Estrada, Luz Elizabeth Guillén-Pineda, Francisco Javier Gómez-Pérez and Daniel Cuevas-Ramos in Therapeutic Advances in Endocrinology and Metabolism