Abstract
Background:
Lenvatinib is the first-line treatment for advanced hepatocellular carcinoma, but prognosis is still unsatisfactory. Recently, hepatic arterial infusion chemotherapy (HAIC), and immune checkpoint inhibitors showed promising results for advanced hepatocellular carcinoma. Considering different anti-malignancy mechanisms, combining these three treatments may improve outcomes. This study aimed to compare the efficacy and safety of lenvatinib, toripalimab, plus HAIC versus lenvatinib for advanced hepatocellular carcinoma.
Methods:
This was a retrospective study including patients treated with lenvatinib [8 mg (⩽60 kg) or 12 mg (>60 kg) once daily] or lenvatinib, toripalimab plus HAIC [LeToHAIC group, lenvatinib 0–1 week prior to initial HAIC, 240 mg toripalimab 0–1 day prior to every HAIC cycle, and HAIC with FOLFOX regimen (oxaliplatin 85 mg/m2, leucovorin 400 mg/m2, 5-fluorouracil bolus 400 mg/m2 on day 1, and 5-fluorouracil infusion 2400 mg/m2 for 46 h, every 3 weeks)]. Progression-free survival, overall survival, objective response rate, and treatment-related adverse events were compared.
Results:
From February 2019 to August 2019, 157 patients were included in this study: 71 in the LeToHAIC group and 86 in the lenvatinib group. The LeToHAIC group showed longer progression-free survival (11.1 versus 5.1 months, p < 0.001), longer overall survival (not reached versus 11 months, p < 0.001), and a higher objective response rate (RECIST: 59.2% versus 9.3%, p < 0.001; modified RECIST: 67.6% versus 16.3%, p < 0.001) than the lenvatinib group. In addition, 14.1% and 21.1% of patients in the LeToHAIC group achieved complete response of all lesions and complete response of the intrahepatic target lesions per modified RECIST criteria, respectively. Grade 3/4 treatment-related adverse events that were more frequent in the LeToHAIC group than in the lenvatinib group included neutropenia (8.5% versus 1.2%), thrombocytopenia (5.6% versus 0), and nausea (5.6% versus 0).
Conclusions:
Lenvatinib, toripalimab, plus HAIC had acceptable toxic effects and might improve survival compared with lenvatinib alone in advanced hepatocellular carcinoma.
Keywords: FOLFOX, hepatic arterial infusion chemotherapy, hepatocellular carcinoma, lenvatinib, toripalimab
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common malignancy and the third leading cause of tumor-related deaths.1 Despite recent improvements in surveillance programs, approximately 50% of patients with HCC are diagnosed at advanced disease stages, with symptoms and/or present vascular invasion or extrahepatic spread.2,3 Lenvatinib or sorafenib, as one of the tyrosine kinase inhibitors (TKIs), is the standard systemic therapy in the treatment of advanced HCC and was demonstrated to be effective and well tolerated in randomized phase III trials.4,5 However, the outcome of these patients remains poor, with a median survival time of 10.7–11.8 months and a response rate of 2–18.8%.4,5
Immune checkpoint inhibitors, for example, programmed cell death protein-1 (PD-1) inhibitors, have yielded a promising clinical efficacy and safety in patients with advanced HCC.6,7 Toripalimab, a PD-1 inhibitor, received conditional approval in China for the treatment of unresectable or metastatic melanoma.8 However, phase III trials of PD-1 inhibitors monotherapy in first and second-line settings for HCC failed to meet the primary endpoint.9,10 In addition, hepatic arterial infusion chemotherapy (HAIC) with oxaliplatin, 5-fluorouracil, and leucovorin (FOLFOX) was reported to improve the survival of patients with advanced HCC,11,13 and HAIC was recommended as a treatment option in patients with advanced HCC in Asia.14,15 Considering the different anti-malignancy mechanisms of TKIs, PD-1 inhibitors, and HAIC, combining these three modalities may show a potential synergic effect and promising preliminary efficacy results in advanced HCC. Recently, the combined therapy of apatinib (a TKI), toripalimab, and HAIC has been reported as an abstract in the American Society of Clinical Oncology meeting, with a response rate of 100%;16 however, only six patients were included in the analysis.
Hence, we conducted this retrospective study to compare the combination of lenvatinib, toripalimab, and HAIC with lenvatinib monotherapy for advanced HCC.
Methods
Patients
This was a retrospective study that was conducted in accordance with the Declaration of Helsinki and performed at three hospitals in China. The study was approved by the Institutional Review Board of Sun Yat-sen University Cancer Center (no. B2019-080-01), First People’s Hospital of Foshan (no. 202011), and Guangzhou No.12 People’s Hospital (no. 2020052). The study protocol is available in Supplement 1. Written informed consent (including, for example, the description of the study, risks and discomforts, benefits, confidentiality) was provided by all patients before conducting the treatment. Once advanced HCC was confirmed, the patient was informed that lenvatinib was the recommended treatment. In addition, HAIC and PD-1 inhibitors were also recommended based on previous studies,6,7,12 and the triple combination therapy of lenvatinib, PD-1 inhibitor, and HAIC, which could achieve promising antitumor activity. The final decision was principally made by the patient. Some patients received the triple combination therapy due to the promising antitumor activity, while others refused the combination therapy due to high cost and regular hospitalization. The abstract (#431) of this study has been accepted for E-Poster presentation (display) at the European Society for Medical Oncology Asia Virtual Congress 2020, and partial results have been shown at this congress.17
Consecutive patients were identified via the electronic medical records based on the following eligibility criteria: 18 years or older with unresectable HCC staged at Barcelona Clinic Liver Cancer (BCLC) C; treated with lenvatinib monotherapy or a combination of lenvatinib, toripalimab, and HAIC with FOLFOX; an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0–1; Child–Pugh class A liver function; at least one measurable intrahepatic lesion according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1,18 and adequate organ function (absolute neutrophil count ⩾1.2 × 109/l, platelet count ⩾60 × 109/l, total bilirubin <30 μmol/l, albumin ⩾30 g/l, aspartate transaminase and alanine transaminase ⩽5 × upper limit of the normal, creatinine clearance rate of ⩽1.5 × upper limit of the normal, and left ventricular ejection ⩾45%). The exclusion criteria included the following: combined with other malignant tumors; incomplete medical information; and loss to follow-up.
Treatments
In the lenvatinib group, patients received oral lenvatinib 12 mg/day (for bodyweight ⩾60 kg) or 8 mg/day (for bodyweight <60 kg).4
In the LeToHAIC (lenvatinib, toripalimab plus HAIC) group, patients received oral lenvatinib as described above 0–1 week prior to the initial HAIC, and lenvatinib was not discontinued before or after each HAIC session. Additionally, patients received 240 mg toripalimab intravenously 0–1 day prior to HAIC. Toripalimab was administered for each HAIC session, and toripalimab was repeated every 3 weeks after HAIC was discontinued. HAIC was performed every 3 weeks as described in our previous study: a catheter/microcatheter was placed in the main feeding hepatic artery, and then the following regimen was administered via the hepatic artery: oxaliplatin 85 mg/m2 from hour 0 to 2 on day 1; leucovorin 400 mg/m2 from hour 2 to 3 on day 1; 5-fluorouracil 400 mg/m2 bolus at hour 3; and 2400 mg/m2 over 46 h on days 1 and 2.12
Dose reduction, interruption, and discontinuation of therapy
A dose reduction of lenvatinib due to lenvatinib-related toxicities (to 8 mg or 4 mg/day or to 4 mg every other day) was permitted.4 The decision to delay lenvatinib and toripalimab follow local standards of care as guided by the locally approved product label.
Dose reductions, treatment interruptions, and discontinuations of HAIC were according to our previous study.12 HAIC was delayed until recovery if neutrophil count less than 1200 cells/μl, platelet count less than 60,000 platelets/μl, a total bilirubin level exceeding 30 μmol/l, an albumin level less than 30 g/l, or serum creatinine up to 1.5 times the institutional upper limit of normal. The 5-fluorouracil dose was decreased to 300 mg/m2 bolus and 1800 mg/m2/cycle continuous infusion in cases of grade 3 or 4 diarrhea or stomatitis, skin toxicity, or other grades 3 major organ drug-related toxicity. The oxaliplatin dose was decreased to 65 mg/m2/cycle in cases of grade 3 or 4 neutropenia or thrombocytopenia, any other grade 3 major organ drug-related toxicity, or paresthesia associated with pain.
In addition, treatments were discontinued due to tumor progression, unacceptable toxicity, the need for an operation and ablation owing to tumor shrinkage, or patient choice. HAIC was also discontinued due to technical difficulties in repeating the HAIC (stenosis or occlusion of tumor-feeding artery or supplied only by extrahepatic collateral arteries), the disappearance of any intratumoral arterial enhancement in all intrahepatic lesions. After HAIC was discontinued alone, patients were allowed to continue lenvatinib or toripalimab in the LeToHAIC group.
Data collection and study objectives
Clinical and radiological data for diagnosis were retrospectively collected from the medical record. The following data were collected and analyzed: sex, age, ECOG PS score, positive or negative of hepatitis B surface antigen, α-fetoprotein (AFP) level, des-γ-carboxy prothrombin, albumin-bilirubin (ALBI) grade, alanine aminotransferase, aspartate aminotransferase, albumin, total bilirubin, tumor size, tumor number, absence or presence of portal vein tumor thrombus (PVTT), absence or presence of hepatic vein tumor thrombus (HVTT), absence or presence of extrahepatic metastasis. All imaging data were independently assessed by two radiologists. If there was a discrepancy between the two radiologists, the final classification was made by another more experienced radiologist.
The primary endpoint was progression-free survival (PFS), defined as the time from the commencement of lenvatinib to progression according to the RECIST criteria or death from any cause, whichever occurred first. The secondary endpoints were overall survival (OS), defined as the time from the commencement of lenvatinib to death from any cause, the objective response rate (ORR), defined as the proportion of patients with complete response or partial response that was maintained for at least 4 weeks from the first radiological confirmation of that rate, and the disease control rate (DCR), defined as the proportion of patients with ORR plus stable disease. The DCR and ORR were evaluated according to RECIST version 1.1 and modified RECIST (mRECIST), respectively.18,19 Adverse events were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03.
Statistics analysis
The results were compared with Chi-squared tests. Survival outcomes were calculated with the Kaplan–Meier method and compared with log-rank tests. Any factors that were statistically significant at p-value <0.10 in the univariate analysis were candidates for entry into a multivariable Cox proportional-hazards model. All p-values were two-sided, with p-values <0.05 considered significant. The statistical package used to perform the analyses was SAS, version 9.0 (SAS Institute).
Results
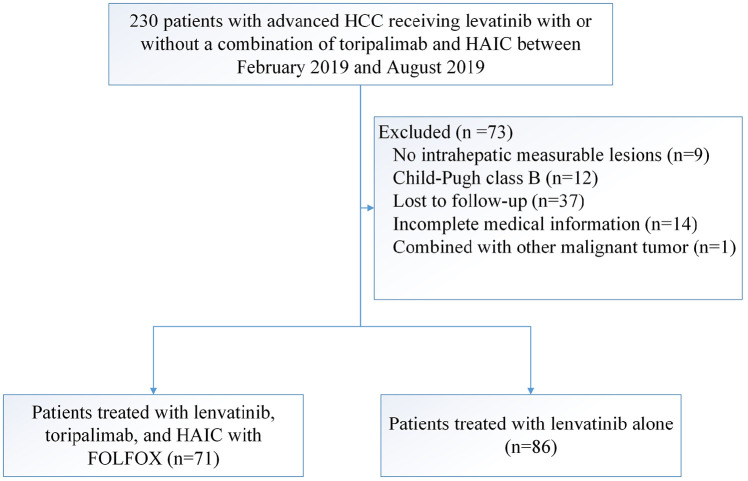
Between 24 February 2019 and 2 August 2019, 157 patients met the criteria for inclusion in this study: 71 patients received triple combination therapy with lenvatinib, toripalimab plus HAIC, and 86 patients received lenvatinib monotherapy (Figure 1). The follow-up was finished on 29 July 2020. The baseline characteristics are summarized in Table 1, and no difference was observed. The study population was predominantly male (86.6%). The main etiology of HCC was hepatitis B virus (89.2%), and these patients all received antiviral therapy, including entecavir and tenofovir, before patients received lenvatinib. All patients had BCLC stage C HCC, 117 (74.5%) patients were diagnosed with PVTT, and 41 (26.1%) patients had extrahepatic spread. The median size of the maximum tumor was 10.9 cm.
Figure 1.
Patient selection flow.
FOLFOX, oxaliplatin, 5-fluorouracil, and leucovorin; HAIC, hepatic arterial infusion chemotherapy; HCC, hepatocellular carcinoma.
Table 1.
Patient baseline demographic and clinical characteristics.
| LeToHAIC group (n = 71) | Lenvatinib group (n = 86) | p-value | |
|---|---|---|---|
| Sex | 0.24 | ||
| Male | 59 | 77 | |
| Female | 12 | 9 | |
| Age, years | 0.35 | ||
| ⩽50 | 40 | 42 | |
| >50 | 31 | 44 | |
| ECOG | 0.38 | ||
| 0 | 14 | 22 | |
| 1 | 57 | 64 | |
| HBsAg | 0.5 | ||
| Positive | 62 | 78 | |
| Negative | 9 | 8 | |
| AFP, ng/ml | 0.98 | ||
| ⩽400 | 26 | 31 | |
| >400 | 45 | 55 | |
| DCP, mAU/ml | 0.91 | ||
| ⩽10,000 | 32 | 38 | |
| >10,000 | 39 | 48 | |
| ALBI grade | 0.43 | ||
| 1 | 31 | 43 | |
| 2 | 40 | 43 | |
| Albumin, g/l | 0.45 | ||
| <40 | 37 | 50 | |
| ⩾40 | 34 | 36 | |
| Total bilirubin, μmol/l | 0.35 | ||
| ⩽20 | 55 | 61 | |
| >20 | 16 | 25 | |
| ALT, U/l | 0.47 | ||
| ⩽50 | 33 | 35 | |
| >50 | 38 | 51 | |
| AST, U/l | 0.35 | ||
| ⩽60 | 23 | 34 | |
| >60 | 48 | 52 | |
| Tumor size, cm | 0.21 | ||
| ⩽10 | 26 | 40 | |
| >10 | 45 | 46 | |
| Tumor number | 0.14 | ||
| ⩽3 | 3 | 9 | |
| >3 | 68 | 77 | |
| PVTT | 0.44 | ||
| Absent | 16 | 24 | |
| Present | 55 | 62 | |
| HVTT | 0.82 | ||
| Absent | 45 | 53 | |
| Present | 26 | 33 | |
| Metastasis | 0.35 | ||
| Absent | 55 | 61 | |
| Present | 16 | 25 |
Calculated using the following equation: linear predictor = (log10 bilirubin μmol/l × 0.66) + (albumin g/L × −0.085). The continuous linear predictor was further categorized into three different grades for prognostic stratification purposes: grade 1 (less than −2.60), grade 2 (between −2.60 and −1.39) and grade 3 (above −1.39).
Statistical significance was assessed with the Chi-square test.
AFP, α-fetoprotein; ALBI, albumin-bilirubin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; DCP, des-γ-carboxy prothrombin; ECOG, Eastern Cooperative Oncology Group; HBsAg, hepatitis B surface antigen; HVTT, hepatic vein tumor thrombus; PVTT, portal vein tumor thrombus.
Treatment administration is listed in Table 2. In the LeToHAIC group, 71 patients were treated with a total of 269 cycles of HAIC (median 4) and 583 cycles of toripalimab (median 8). The median duration of lenvatinib was 9.1 months. In the lenvatinib group, the median duration of lenvatinib was 4.7 months. In the LeToHAIC group, five patients continued lenvatinib or lenvatinib plus toripalimab on the cut-off date. After the termination of the study treatment, 3 patients refused second-line-therapy, and 63 patients received second-line-therapy, including curative surgical resection (9), ablation (2), sorafenib (8), regorafenib (20), other PD-1 antibody (10), transarterial chemoembolization (TACE) (10), and radiotherapy (4). In the lenvatinib group, one patient continued lenvatinib on the cut-off date. After the termination of the study treatment, 2 patients refused second-line-therapy, and 83 patients received second-line-therapy, including sorafenib plus HAIC (10), HAIC monotherapy (2), sorafenib (6), regorafenib (31), PD-1 antibody (27), and TACE (7). After patients discontinued the second-line-therapy, patients also received other subsequent treatments. The detailed subsequent treatments are shown in Table 2. More patients in the LeToHAIC group than those in the lenvatinib group received curative surgical resection owing to tumor shrinkage (9 versus 0, p = 0.001). Instead, more patients in the lenvatinib group received subsequent PD-1 antibody, HAIC, and regorafenib (p < 0.05) than those in the LeToHAIC group.
Table 2.
Treatment administration.
| LeToHAIC group (n = 71) | Lenvatinib group (n = 86) | p-value | |
|---|---|---|---|
| Study treatment, median (range) | |||
| HAIC cycle | 4 (1–6) | – | |
| Toripalimab cycle | 8 (1–17) | – | |
| Duration of lenvatinib, months | 9.1 (1.4–16.6) | 4.7 (0.9–16.4) | |
| Number of patients who received subsequent treatments | |||
| HAIC | 0 | 12 | 0.001 |
| Resection | 9 | 0 | 0.001 |
| Ablation | 3 | 0 | 0.18 |
| Sorafenib | 9 | 17 | 0.23 |
| PD-1 antibody | 15* | 40 | 0.001 |
| Regorafenib | 20 | 39 | 0.03 |
| Transarterial chemoembolization | 13 | 16 | 0.96 |
| Radiotherapy | 8 | 5 | 0.22 |
Patient in the LeToHAIC group receive other PD-1 antibodies, such as nivolumab, pembrolizumab, sintilimab, camrelizumab.
HAIC, hepatic arterial infusion chemotherapy; LeToHAIC, lenvatinib, toripalimab plus HAIC; PD-1, programmed cell death protein-1.
Efficacy
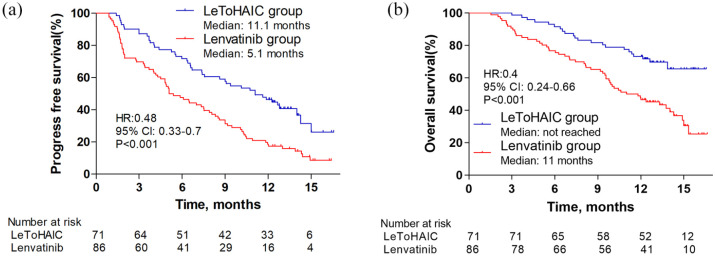
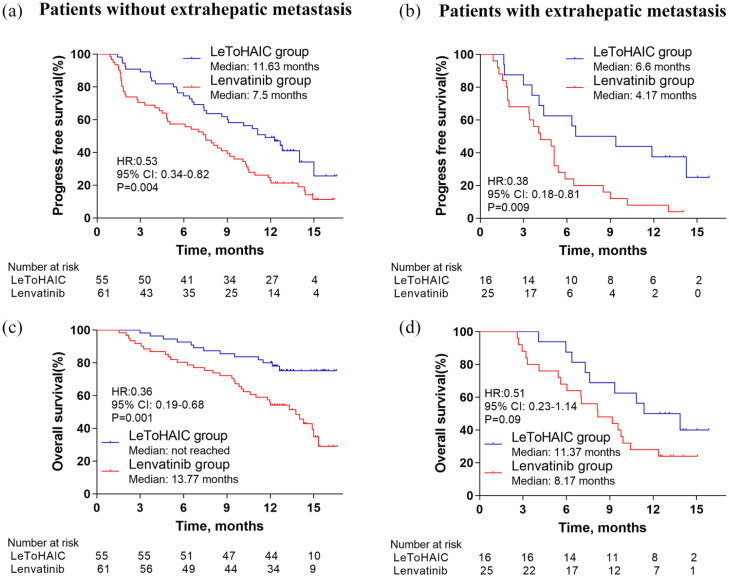
At the time of analysis, 44 patients had disease progression and 22 patients had died in the LeToHAIC group, while 76 patients had disease progression and 54 patients had died in the lenvatinib group. The median PFS in the LeToHAIC group was 11.1 months [95% confidence interval (CI), 7.85–14.35] compared with 5.1 months (95% CI, 3.62–6.58) in the lenvatinib group [hazard ratio (HR) = 0.48; 95% CI, 0.33−0.7; p < 0.001; Figure 2(a)]. The median OS was not reached in the LeToHAIC group, while the median OS was 11 months (95% CI, 8.14–13.86) in the lenvatinib group [HR = 0.4; 95% CI, 0.24−0.66; p < 0.001; Figure 2(b)]. After stratification by absence or presence of extrahepatic metastasis, the median PFS in the LeToHAIC group was significantly longer than that in the lenvatinib group [absence of extrahepatic metastasis: 11.63 (95% CI, 8.88–14.38) versus 7.5 (95% CI, 5.35–9.65) months, p = 0.004, Figure 3(a); presence of extrahepatic metastasis: 6.6 (95% CI, 0.66–12.55) versus 4.17 (95% CI, 2.21–6.13) months, p = 0.009, Figure 3(b)], and the median OS in the LeToHAIC group was longer than that in the lenvatinib group [absence of extrahepatic metastasis: not reached versus 13.77 (95% CI, 11.35–16.19) months, p = 0.001, Figure 3(c); presence of extrahepatic metastasis: 11.37 (95% CI, 6.79–15.94) versus 8.17 (95% CI, 4.69–11.65) months, p = 0.09, Figure 3(d)].
Figure 2.
Kaplan–Meier curves for progression-free survival (a) and overall survival (b).
CI, confidence interval; HR, hazard ratio; LeToHAIC, lenvatinib, toripalimab plus hepatic arterial infusion chemotherapy.
Figure 3.
Kaplan–Meier curves for progression-free survival (a, b) and overall survival (c, d) after stratification by the absence or presence of extrahepatic metastasis.
CI, confidence interval; HR, hazard ratio; LeToHAIC, lenvatinib, toripalimab plus hepatic arterial infusion chemotherapy.
The results of univariate and multivariate analyses of PFS and OS are listed in Table 3. Multivariate analysis showed that independent risk factors for PFS were type of treatment (lenvatinib, toripalimab plus HAIC versus lenvatinib, HR = 0.47; 95% CI, 0.32–0.68; p < 0.001), AFP level (⩽400 versus >400 ng/ml, HR = 0.67; 95% CI, 0.45–0.99; p = 0.04), ALBI grade (1 versus 2, HR = 0.69; 95% CI, 0.48–0.99; p = 0.047), and extrahepatic metastasis (absence versus presence, HR = 0.63; 95% CI, 0.42–0.94; p = 0.02). Furthermore, the independent risk factors for OS were type of treatment (lenvatinib, toripalimab plus HAIC versus lenvatinib, HR = 0.39; 95% CI, 0.24–0.64; p < 0.001), PVTT (absence versus presence, HR = 0.49; 95% CI, 0.27–0.87; p = 0.01), and extrahepatic metastasis (absence versus presence, HR = 0.44; 95% CI, 0.27–0.72; p = 0.001).
Table 3.
Univariate and multivariate analysis of progression-free survival and overall survival.
| Progression-free survival |
Overall survival |
|||||
|---|---|---|---|---|---|---|
| Univariate analysis |
Multivariate analysis |
Univariate analysis |
Multivariate analysis |
|||
| P1 | HR (95% CI) | P2 | P1 | HR | P2 | |
| Group (lenvatinib, toripalimab plus HAIC versus lenvatinib) | <0.001 | 0.47 (0.32–0.68) | <0.001 | <0.001 | 0.39 (0.24–0.64) | <0.001 |
| Sex (male/female) | 0.54 | 0.36 | ||||
| Age, year (⩽50 versus >50) | 0.19 | 0.33 | ||||
| ECOG (0 versus 1) | 0.93 | 0.35 | ||||
| HBsAg (positive versus negative) | 0.7 | 0.11 | ||||
| AFP, ng/ml (⩽400 versus >400) | 0.02 | 0.67 (0.45–0.99) | 0.04 | 0.03 | 0.71 (0.43–1.19) | 0.19 |
| DCP, mAU/ml (⩽10,000 versus >10,000) | 0.2 | 0.03 | 0.66 (0.41–1.06) | 0.08 | ||
| ALBI grade (1 versus 2) | 0.07 | 0.69 (0.48–0.99) | 0.047 | 0.28 | ||
| Tumor size, cm (⩽10 versus >10) | 0.72 | 0.38 | ||||
| Tumor number (⩽3 versus >3) | 0.68 | 0.81 | ||||
| PVTT (absent versus present) | 0.23 | 0.098 | 0.49 (0.27–0.87) | 0.01 | ||
| HVTT (absent versus present) | 0.22 | 0.52 | ||||
| Metastasis (absent versus present) | 0.02 | 0.63 (0.42–0.94) | 0.02 | 0.001 | 0.44 (0.27–0.72) | 0.001 |
P1 value was calculated with two-sided log-rank test. Any factors that were statistically significant at P <10% in the univariate analysis were candidates for entry into a multivariable Cox analysis.
P2 value was calculated by multivariable Cox proportional-hazards analysis.
AFP, α-fetoprotein; ALBI, albumin-bilirubin; CI, confidence interval; DCP, des-γ-carboxy prothrombin; ECOG, Eastern Cooperative Oncology Group; HAIC, hepatic arterial infusion chemotherapy; HBsAg, hepatitis B surface antigen; HR, hazard ratio; HVTT, hepatic vein tumor thrombus; PVTT, portal vein tumor thrombus.
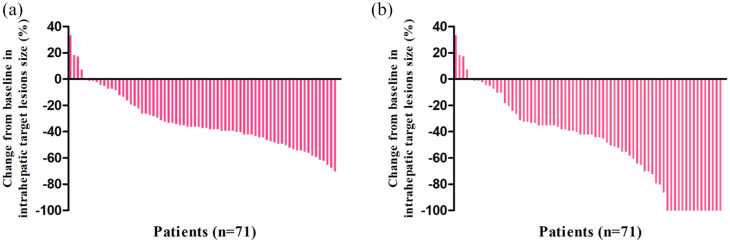
The tumor responses are listed in Table 4. Based on the RECIST criteria, the DCR and ORR were significantly higher in the LeToHAIC group than those in the lenvatinib group (90.1% versus 72.1%, p = 0.005; 59.2% versus 9.3%, p < 0.001, respectively). Based on the mRECIST criteria, the DCR and ORR were also significantly higher in the LeToHAIC group than those in the lenvatinib group (90.1% versus 72.1%, p = 0.005; 67.6% versus 16.3%, p < 0.001). In addition, 10 (14.1%) patients in the LeToHAIC group achieved complete response according to the mRECIST criteria. A waterfall plot was constructed to show the change in the intrahepatic target lesion size of patients in the LeToHAIC group (Figure 4). Complete response of the intrahepatic target lesions according to the mRECIST criteria was noted in 15 (21.1%) patients. Computed tomography or magnetic resonance imaging scans of five representative patients who received lenvatinib, toripalimab, plus HAIC are shown in supplemental figures.
Table 4.
Summary of best response.
| RECIST |
mRECIST |
|||||
|---|---|---|---|---|---|---|
| LeToHAIC group (%) | Lenvatinib group (%) | p-valuea | LeToHAIC group (%) | Lenvatinib group (%) | p-valuea | |
| CR | 0 | 0 (0) | – | 10 (14.1) | 0 (0) | <0.001 |
| PR | 42 (59.2) | 8 (9.3) | <0.001 | 38 (53.5) | 14 (16.3) | <0.001 |
| SD | 22 (31) | 54 (62.8) | <0.001 | 16 (22.5) | 48 (55.8) | <0.001 |
| PD | 7 (9.9) | 24 (27.9) | 0.005 | 7 (9.9) | 24 (27.9) | 0.002 |
| DCR | 64 (90.1) | 62 (72.1) | 0.005 | 64 (90.1) | 62 (72.1) | 0.005 |
| ORR | 42 (59.2) | 8 (9.3) | <0.001 | 48 (67.6) | 14 (16.3) | <0.001 |
Statistical significance was assessed with the Chi-square test.
CR, complete response; DCR, disease control rate; HAIC, hepatic arterial infusion chemotherapy; LeToHAIC, lenvatinib, toripalimab plus HAIC; mRECIST, modified Response Evaluation Criteria in Solid Tumors; NA, not assessable; ORR, objective response rate; PD, progressive disease; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumors; SD, stable disease.
Figure 4.
Best percentage changes from baseline in size of the intrahepatic target lesions of patients receiving lenvatinib, toripalimab plus hepatic arterial infusion chemotherapy. (a) Assessed with RECIST in patients with image measurements before and after treatment; (b) Assessed with mRECIST in patients with image measurements before and after treatment.
mRECIST, modified Response Evaluation Criteria in Solid Tumors; RECIST, Response Evaluation Criteria in Solid Tumors.
Safety
Treatment-related deaths did not occur in this study, and treatment-related adverse events (TRAEs), which occurred in ⩾10% of patients, are shown in Table 5. The following grade 3–4 adverse events were more frequent in the LeToHAIC group than in the lenvatinib group: neutropenia [6 (8.5%) versus 1 (1.2%), p = 0.03], thrombocytopenia [4 (5.6%) versus 0, p = 0.04], and nausea [4 (5.6%) versus 0, p = 0.04]. Any-grade liver dysfunction, including elevated alanine aminotransferase, elevated aspartate aminotransferase, hyperbilirubinemia, hypoalbuminemia was more frequent in the LeToHAIC group than in the lenvatinib group. These liver dysfunctions were mainly mild to moderate and returned to normal within 1 week in most patients. No patient had liver failure. The most common potentially immune-related TRAE was grade 1–2 hypothyroidism (19.7%). Moreover, one patient developed a grade 3 immune-related rash, and one patient developed a grade 3 immune-related hepatitis. After treatment with corticosteroids and suspending toripalimab, the patient with immune-related dermatitis returned to normal immediately, and the patient with immune-related hepatitis recovered after 1 month. Moreover, specific abdominal pain associated with oxaliplatin infusion occurred in 18 (25.4%) patients in the LeToHAIC group. This pain could be acute and severe but was quickly relieved by slowing or stopping the infusion of oxaliplatin. In addition, no treatment-related deaths were observed in the two groups. Serious adverse events occurred in 11 (15.5%) of 71 patients in the LeToHAIC group (6 gastrointestinal bleeding, 1 thrombocytopenia, 1 renal failure, and 3 ascites), and 9 (10.5%) of 86 patients in the lenvatinib group (3 gastrointestinal bleeding, 2 hypertensions, and 4 ascites) (p = 0.35).
Table 5.
Treatment-related adverse eventsa.
| Adverse event | LeToHAIC group (n = 71) |
Lenvatinib group (n = 86) |
p-value |
|||
|---|---|---|---|---|---|---|
| Any grade (%) | Grade 3–4 (%) | Any grade (%) | Grade 3–4 (%) | Any grade | Grade 3–4 | |
| Neutropenia | 33 (46.5) | 6 (8.5) | 15 (17.4) | 1 (1.2) | <0.001 | 0.03 |
| Thrombocytopenia | 36 (50.7) | 4 (5.6) | 14 (16.3) | 0 | <0.001 | 0.04 |
| Fatigue | 44 (62) | 3 (4.2) | 31 (36) | 2 (2.3) | 0.001 | 0.66 |
| Hypertension | 28 (39.4) | 8 (11.3) | 31 (36) | 8 (9.3) | 0.66 | 0.69 |
| Weight loss | 29 (40.8) | 2 (2.8) | 28 (32.6) | 1 (1.2) | 0.28 | 0.59 |
| Hypothyroidism | 14 (19.7) | 0 | 13 (15.1) | 0 | 0.45 | – |
| Hand–foot skin reaction | 22 (31) | 3 (4.2) | 21 (24.4) | 2 (2.3) | 0.36 | 0.66 |
| Rash | 10 (14.1) | 0 | 11 (12.8) | 0 | 0.81 | – |
| Nausea | 30 (42.3) | 4 (5.6) | 19 (22) | 0 | 0.007 | 0.04 |
| Vomiting | 24 (33.8) | 3 (4.2) | 16 (18.6) | 1 (1.2) | 0.03 | 0.33 |
| Diarrhea | 24 (33.8) | 4 (5.6) | 28 (32.6) | 3 (3.4) | 0.87 | 0.7 |
| Abdominal pain | 21 (29.6) | 2 (2.8) | 13 (15.1) | 1 (1.2) | 0.03 | 0.59 |
| Sensory neuropathy | 20 (28.2) | 0 | 0 | 0 | <0.001 | – |
| Proteinuria | 23 (32.4) | 4 (5.6) | 20 (23.3) | 3 (3.4) | 0.2 | 0.7 |
| Elevated ALT | 46 (64.8) | 6 (8.5) | 19 (22.1) | 2 (2.3) | <0.001 | 0.14 |
| Elevated AST | 49 (69) | 8 (11.2) | 21 (24.4) | 3 (3.5) | <0.001 | 0.11 |
| Hyperbilirubinemia | 34 (47.9) | 2 (2.8) | 20 (23.3) | 1 (1.2) | 0.001 | 0.59 |
| Hypoalbuminemia | 47 (66.2) | 2 (2.8) | 3 (3.4) | 0 | <0.001 | 0.2 |
p-value was calculated by a two-sided Chi-square test.
Listed are adverse events, as defined by the National Cancer Institute Common Terminology Criteria (version 4.03), that occurred in at least 10% of patients in either study group.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; HAIC, hepatic arterial infusion chemotherapy; LeToHAIC, lenvatinib, toripalimab plus HAIC.
Discussion
In this study, patients who received lenvatinib, toripalimab plus HAIC achieved significantly better PFS (11.1 versus 5.1 months), OS (not reached versus 11 months), and ORR (59.2% versus 9.3% according to the RECIST criteria; 67.6% versus 16.3% according to the mRECIST criteria) than patients who received lenvatinib alone. Notably, 14.1% complete response of all lesions and 21.1% complete response of intrahepatic target lesions according to the mRECIST criteria were observed in patients receiving lenvatinib, toripalimab plus HAIC. In addition, both groups were found to have a manageable toxicity profile. In the multivariate analysis, the type of treatment, AFP level, ALBI grade, and extrahepatic metastasis were the independent factors for PFS. Moreover, the type of treatment, PVTT, and extrahepatic metastasis were the independent factors for OS. A previous study concerning sorafenib in combination with HAIC reported that AFP level, PVTT, and extrahepatic metastasis were the independent factors.12 The ALBI grade, based solely on albumin and bilirubin levels, is a described index of liver dysfunction and related to survival in HCC.20 After stratification by absence or presence of extrahepatic metastasis, the median PFS and OS in the LeToHAIC group was significantly longer than that in the lenvatinib group except for the OS in the patients with the presence of extrahepatic metastasis (p = 0.09). However, one should note that there were wide 95% CI ranges for these patients, which might be attributed to a low number of patients [n = 41 (26.1%)]. It seemed that additional treatment of HAIC might be also effective even though the patients have extrahepatic metastasis.
In the LeToHAIC group, patients achieved an approximately 60% ORR according to the RECIST criteria, which seemed higher than previous studies investigating other treatments for advanced HCC. 4,5,9,12,13,21 The ORR according to the RECIST criteria in patients with advanced HCC treated with lenvatinib, PD-1 inhibitor, HAIC, and sorafenib plus HAIC ranged from 18.3% to 40.8%.4,9,12,13 In addition, the PFS (11.1 months) observed in this study seemed better than that observed in previous studies about lenvatinib, PD-1 inhibitor, HAIC, and sorafenib plus HAIC (range from 3 to 7.3 months).4,9,12,13 Furthermore, the PFS and ORR (11.1 months and 59.2%, respectively) of patients receiving lenvatinib, toripalimab plus HAIC in this study might be better than those of patients receiving first-line systemic treatment (sorafenib, lenvatinib, and atezolizumab plus bevacizumab) for advanced HCC, with a PFS of 3.7–7.3 months and an ORR of 2% to 27.3%.4,5,21 Notably, our study population may be considered to have a poor prognosis because the median size of the maximum tumor was 10.9 cm, and 74.5% of patients had PVTT.
Recently, a conference abstract showed that six patients receiving HAIC, apatinib (one of TKIs), and toripalimab achieved promising clinical benefit and safety, with a response rate of 100%.16 However, the sample size was not large enough, and our study included more detailed data and more patients in the evaluation of efficacy and safety. In addition, apatinib selectively inhibits vascular endothelial growth factor (VEGF)2,22 and lenvatinib, which were approved by the US Food and Drug Administration (FDA) for the treatment of advanced HCC, acts as a multiple kinase inhibitor against VEGF receptor (VEGFR)1, VEGFR2, and VEGFR3.23 HCC is a complex disease with multiple signaling pathways involved in its carcinogenesis and varying according to HCC etiology.24 Thus, lenvatinib may be a more appropriate TKI that was chosen in the triple combination therapy than apatinib for HCC. Moreover, toripalimab was chosen in the triple combination therapy, whereas nivolumab and pembrolizumab, which have been approved by the US FDA for the treatment of advanced HCC, were not selected. The reason was that patients with HCC in China were relatively poor, and toripalimab is about four times cheaper than nivolumab or pembrolizumab. Thus, patients preferred to choose toripalimab. On the other hand, HAIC rather than TACE was chosen due to the following reasons: (1) TACE may be an alternative treatment for advanced HCC, but a randomized phase III study showed that the addition of TACE to sorafenib did not improve OS compared with sorafenib alone;25 (2) our previous studies have certified HAIC improved survival of patients with advanced HCC;12,13 (3) our previous studies showed that HAIC was superior to TACE in OS, PFS, and tumor response in intermediate stage HCC.26,27
The high ORR and PFS that were observed in the patients receiving lenvatinib, toripalimab plus HAIC may be due to the synergistic antitumor effect of lenvatinib, toripalimab, and HAIC. First, chemotherapy may activate the adaptive immune system by increasing human leucocyte antigen expression and augmenting T-cell stimulation,28 and help recover immunosurveillance by disrupting signal transducer and activator of transcription 6-mediated immunosuppression.29 In addition, chemotherapy can increase antigenicity via immunogenic cell death of tumor cells as well as reduction of ‘off-target’ immunosuppression in the tumor microenvironment.30 Second, lenvatinib has more potent inhibitory activities against VEGFRs and fibroblast growth factor receptors than sorafenib,23 and inhibition of these pathways can enhance the efficacy of PD-1 inhibitors via mitigating immunosuppression within the tumor and its microenvironment.31,33 Moreover, in HCC, miR-29b directly suppresses matrix metalloproteinase-2 expression and, in turn, impairs VEGFR2 signaling in endothelial cells. Its overexpression inhibits angiogenesis and tumorigenesis in vivo and represses the ability of HCC cells to promote capillary tube formation of endothelial cells.34 Anti-VEGF drugs can decrease CD4+ regulatory T-lymphocytes and myeloid-derived suppressor cells as well as the activation and differentiation of dendritic cells, and combination of PD-1 inhibitor and anti-VEGF drug may change a cold tumor into a hot one.35 Recently, a prospective trial has shown that lenvatinib plus PD-1 inhibitor has promising antitumor activity in HCC, with a response rate of 36%.36 Finally, lenvatinib and PD-1 inhibitor can increase chemotherapeutic drug delivery via promoting vascular normalization.23,37 A conference abstract showed that lenvatinib plus HAIC of modified FOLFOX regime had shown promising ORR (58.3%) and PFS (8.1 months).38
On the other hand, the adverse events in the lenvatinib group were consistent with those observed in the REFLECT trial.4 Although patients treated with lenvatinib, toripalimab plus HAIC had significantly elevated frequencies of grade 3–4 neutropenia, thrombocytopenia, and nausea, these TRAEs were not unexpected and were manageable by treatment interruption or dose modification. In addition, treatment-related deaths did not occur. In the LeToHAIC group, the lenvatinib-related TRAEs that occurred in our study, such as hypertension and hand–foot skin reactions, were consistent with those that occurred in a previous study,4 and HAIC-related TRAEs, such as gastrointestinal toxicity, myelosuppression, and liver dysfunction were also consistent with those that occurred in the previous studies.13,26 Hypothyroidism, the most common immune-related adverse event, occurred in 19.7% of the patients, which was in line with that reported for toripalimab.39,40 No potentially synergistic toxicity was observed, and the combination of these three treatments was clinically feasible and safe.
There were several limitations in this study. First, its retrospective design and nonrandomized nature made it vulnerable to a variety of potential biases even though there was no difference in the baseline characteristics. The findings in this study needed prospective randomized controlled trials to verify. Second, subsequent treatments may be a confounding factor. The number of patients in the LeToHAIC group who underwent subsequent hepatic resection was greater than that in the lenvatinib group (12.7% versus 0%). However, this can be explained by the better treatment response in the LeToHAIC group, resulting in more translation to resectable HCC. Third, the follow-up time was relatively short for OS because an insufficient number of OS events was observed. A total of 49 (69%) patients in the LeToHAIC group were still alive at the last data cut-off, and long-term survival data are still lacking. However, the follow-up time was sufficient for short-term efficacy (PFS and tumor response), and these indicators are not affected by subsequent treatment and can more accurately reflect the efficacy compared with OS. Finally, this study was performed only in China. The main etiology of HCC in this area was the hepatitis B virus. Whether HAIC is suitable for patients with hepatitis C virus needs further study.
In summary, our study indicated that, compared with lenvatinib alone, triple combination therapy with lenvatinib, toripalimab, and HAIC might be associated with acceptable toxicities and better survival benefits in patients with advanced HCC. A prospective trial is ongoing to evaluate triple combination therapy (ClinicalTrials.gov identifier: NCT04044313).
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359211002720 for Lenvatinib, toripalimab, plus hepatic arterial infusion chemotherapy versus lenvatinib alone for advanced hepatocellular carcinoma by Min-Ke He, Run-Bin Liang, Yang Zhao, Yu-Jie Xu, Huan-Wei Chen, Yuan-Min Zhou, Zhi-Cheng Lai, Li Xu, Wei Wei, Yao-Jun Zhang, Min-Shan Chen, Rong-Ping Guo, Qi-Jiong Li and Ming Shi in Therapeutic Advances in Medical Oncology
Supplemental material, sj-pdf-2-tam-10.1177_17588359211002720 for Lenvatinib, toripalimab, plus hepatic arterial infusion chemotherapy versus lenvatinib alone for advanced hepatocellular carcinoma by Min-Ke He, Run-Bin Liang, Yang Zhao, Yu-Jie Xu, Huan-Wei Chen, Yuan-Min Zhou, Zhi-Cheng Lai, Li Xu, Wei Wei, Yao-Jun Zhang, Min-Shan Chen, Rong-Ping Guo, Qi-Jiong Li and Ming Shi in Therapeutic Advances in Medical Oncology
Footnotes
Authors contributions: Ming Shi, Qi-Jiong Li, and Min-Ke He conceived and designed the study. Min-Ke He, Yu-Jie Xu, Run-Bin Liang, and Yang Zhao participated in the acquisition of the data. Min-Ke He, Yu-Jie Xu, Run-Bin Liang, Qi-Jiong Li participated in the analysis, or interpretation of the data. Min-Ke He and Ming Shi participated in the drafting of the article or critical revision for important intellectual content. All authors were involved in the approval of the version to be published and agreement to be accountable for all aspects of the work.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Key R&D Program of China (2017YFA0505803), National Natural Science Foundation of China (No. 81625017, 82072610), National Science and Technology Major Project of China (2018ZX10302205).
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Min-Ke He, Department of Hepatobiliary Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, China.
Run-Bin Liang, Department of Hepatobiliary Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, China.
Yang Zhao, Department of Hepatobiliary Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, China.
Yu-Jie Xu, Department of Hepatobiliary Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, China.
Huan-Wei Chen, First People’s Hospital of Foshan, Foshan, Guangdong, China.
Yuan-Min Zhou, Guangzhou No.12 People’s Hospital, Guangzhou, China.
Zhi-Cheng Lai, Department of Hepatobiliary Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, China.
Li Xu, Department of Hepatobiliary Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, China.
Wei Wei, Department of Hepatobiliary Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, China.
Yao-Jun Zhang, Department of Hepatobiliary Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, China.
Min-Shan Chen, Department of Hepatobiliary Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, China.
Rong-Ping Guo, Department of Hepatobiliary Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, China.
Qi-Jiong Li, Department of Hepatobiliary Oncology, Cancer Center, Sun Yat-sen University, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, 510060, P.R. China.
Ming Shi, Department of Hepatobiliary Oncology, Cancer Center, Sun Yat-sen University, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, 510060, P.R. China.
References
- 1. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. [DOI] [PubMed] [Google Scholar]
- 2. Cabibbo G, Enea M, Attanasio M, et al. A meta-analysis of survival rates of untreated patients in randomized clinical trials of hepatocellular carcinoma. Hepatology 2010; 51: 1274–1283. [DOI] [PubMed] [Google Scholar]
- 3. Yoon SM, Ryoo BY, Lee SJ, et al. Efficacy and safety of transarterial chemoembolization plus external beam radiotherapy vs sorafenib in hepatocellular carcinoma with macroscopic vascular invasion: a randomized clinical trial. JAMA Oncol 2018; 4: 661–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018; 391: 1163–1173. [DOI] [PubMed] [Google Scholar]
- 5. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359: 378–390. [DOI] [PubMed] [Google Scholar]
- 6. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017; 389: 2492–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol 2018; 19: 940–952. [DOI] [PubMed] [Google Scholar]
- 8. Keam SJ. Toripalimab: first global approval. Drugs 2019; 79: 573–578. [DOI] [PubMed] [Google Scholar]
- 9. Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol 2020; 38: 193–202. [DOI] [PubMed] [Google Scholar]
- 10. Yau T, Park JW, Finn RS, et al. CheckMate 459: a randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann Oncol 2019; 30: v874–v875. [Google Scholar]
- 11. He MK, Zou RH, Li QJ, et al. Phase II study of sorafenib combined with concurrent hepatic arterial infusion of oxaliplatin, 5-fluorouracil and leucovorin for unresectable hepatocellular carcinoma with major portal vein thrombosis. Cardiovasc Intervent Radiol 2018; 41: 734–743. [DOI] [PubMed] [Google Scholar]
- 12. He M, Li Q, Zou R, et al. Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for hepatocellular carcinoma with portal vein invasion: a randomized clinical trial. JAMA Oncol 2019; 5: 953–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lyu N, Kong Y, Mu L, et al. Hepatic arterial infusion of oxaliplatin plus fluorouracil/leucovorin vs. sorafenib for advanced hepatocellular carcinoma. J Hepatol 2018; 69: 60–69. [DOI] [PubMed] [Google Scholar]
- 14. Kudo M, Matsui O, Izumi N, et al. JSH consensus-based clinical practice guidelines for the management of hepatocellular carcinoma: 2014 update by the Liver Cancer Study Group of Japan. Liver Cancer 2014; 3: 458–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen L, Martinelli E, Cheng A, et al. Pan-Asian adapted ESMO clinical practice guidelines for the management of patients with intermediate and advanced/relapsed hepatocellular carcinoma: a TOS-ESMO initiative endorsed by CSCO, ISMPO, JSMO, KSMO, MOS and SSO. Ann Oncol 2020; 31: 334–351. [DOI] [PubMed] [Google Scholar]
- 16. Gu Y-K, Zhang T-Q, Huang Z-L, et al. Hepatic artery infusion chemotherapy combined with apatinib and toripalimab in advanced hepatocellular carcinoma: real-world data from a single center. J Clin Oncol 2020; 38(Suppl. 15): e16602. [Google Scholar]
- 17. Lai ZC, He MK, Shi M, et al. 172P triple combination therapy of lenvatinib, toripalimab, and hepatic arterial infusion chemotherapy versus lenvatinib for advanced hepatocellular carcinoma. Ann Oncol 2020; 31: S1306. [Google Scholar]
- 18. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 19. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010; 30: 52–60. [DOI] [PubMed] [Google Scholar]
- 20. Pinato DJ, Sharma R, Allara E, et al. The ALBI grade provides objective hepatic reserve estimation across each BCLC stage of hepatocellular carcinoma. J Hepatol 2017; 66: 338–346. [DOI] [PubMed] [Google Scholar]
- 21. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 2020; 382: 1894–1905. [DOI] [PubMed] [Google Scholar]
- 22. Tian S, Quan H, Xie C, et al. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci 2011; 102: 1374–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tohyama O, Matsui J, Kodama K, et al. Antitumor activity of lenvatinib (e7080): an angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J Thyroid Res 2014; 2014: 638747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schulze K, Imbeaud S, Letouze E, et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet 2015; 47: 505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Park JW, Kim YJ, Kim DY, et al. Sorafenib with or without concurrent transarterial chemoembolization in patients with advanced hepatocellular carcinoma: the phase III STAH trial. J Hepatol 2019; 70: 684–691. [DOI] [PubMed] [Google Scholar]
- 26. He MK, Le Y, Li QJ, et al. Hepatic artery infusion chemotherapy using mFOLFOX versus transarterial chemoembolization for massive unresectable hepatocellular carcinoma: a prospective non-randomized study. Chin J Cancer 2017; 36: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shi M, Li Q, He M, et al. 981O Hepatic Arterial Infusion Chemotherapy (HAIC) with oxaliplatin, fluorouracil, and leucovorin (FOLFOX) versus Transarterial Chemoembolization (TACE) for unresectable Hepatocellular Carcinoma (HCC): a randomised phase III trial. Ann Oncol 2020; 31: S688. [Google Scholar]
- 28. Liu WM, Fowler DW, Smith P, et al. Pre-treatment with chemotherapy can enhance the antigenicity and immunogenicity of tumours by promoting adaptive immune responses. Br J Cancer 2010; 102: 115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lesterhuis WJ, Punt CJ, Hato SV, et al. Platinum-based drugs disrupt STAT6-mediated suppression of immune responses against cancer in humans and mice. J Clin Invest 2011; 121: 3100–3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mathew M, Enzler T, Shu CA, et al. Combining chemotherapy with PD-1 blockade in NSCLC. Pharmacol Ther 2018; 186: 130–137. [DOI] [PubMed] [Google Scholar]
- 31. Voron T, Colussi O, Marcheteau E, et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med 2015; 212: 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Courau T, Nehar-Belaid D, Florez L, et al. TGF-beta and VEGF cooperatively control the immunotolerant tumor environment and the efficacy of cancer immunotherapies. JCI Insight 2016; 1: e85974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Welte T, Kim IS, Tian L, et al. Oncogenic mTOR signalling recruits myeloid-derived suppressor cells to promote tumour initiation. Nat Cell Biol 2016; 18: 632–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Leone P, Buonavoglia A, Fasano R, et al. Insights into the regulation of tumor angiogenesis by Micro-RNAs. J Clin Med 2019; 8: 2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Longo V, Brunetti O, Gnoni A, et al. Emerging role of immune checkpoint inhibitors in hepatocellular carcinoma. Medicina (Kaunas) 2019; 55: 698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Finn R, Ikeda M, Zhu A, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol 2020; 38: 2960–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shigeta K, Datta M, Hato T, et al. Dual programmed death receptor-1 and vascular endothelial growth factor receptor-2 blockade promotes vascular normalization and enhances antitumor immune responses in hepatocellular carcinoma. Hepatology (Baltimore, Md) 2020; 71: 1247–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mai Q, Mo Z, Shi F, et al. Lenvatinib plus hepatic arterial infusion of modified FOLFOX regime in patients with advanced hepatocellular carcinoma. J Clin Oncol 2020; 38(Suppl. 15): e16603. [Google Scholar]
- 39. Wang Z, Ying J, Xu J, et al. Safety, antitumor activity, and pharmacokinetics of toripalimab, a programmed cell death 1 inhibitor, in patients with advanced non-small cell lung cancer: a phase 1 trial. JAMA Netw Open 2020; 3: e2013770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tang B, Chi Z, Chen Y, et al. Safety, efficacy, and biomarker analysis of toripalimab in previously treated advanced melanoma: results of the POLARIS-01 multicenter phase II trial. Clin Cancer Res 2020; 26: 4250–4259. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359211002720 for Lenvatinib, toripalimab, plus hepatic arterial infusion chemotherapy versus lenvatinib alone for advanced hepatocellular carcinoma by Min-Ke He, Run-Bin Liang, Yang Zhao, Yu-Jie Xu, Huan-Wei Chen, Yuan-Min Zhou, Zhi-Cheng Lai, Li Xu, Wei Wei, Yao-Jun Zhang, Min-Shan Chen, Rong-Ping Guo, Qi-Jiong Li and Ming Shi in Therapeutic Advances in Medical Oncology
Supplemental material, sj-pdf-2-tam-10.1177_17588359211002720 for Lenvatinib, toripalimab, plus hepatic arterial infusion chemotherapy versus lenvatinib alone for advanced hepatocellular carcinoma by Min-Ke He, Run-Bin Liang, Yang Zhao, Yu-Jie Xu, Huan-Wei Chen, Yuan-Min Zhou, Zhi-Cheng Lai, Li Xu, Wei Wei, Yao-Jun Zhang, Min-Shan Chen, Rong-Ping Guo, Qi-Jiong Li and Ming Shi in Therapeutic Advances in Medical Oncology