Abstract
Background:
Anterior cruciate ligament (ACL) injury has been reported to have a higher incidence in women than in men.
Purpose/Hypothesis:
The purpose was to examine the relationship of anterior knee laxity (AKL), stiffness, and generalized joint laxity (GJL) with respect to the menstrual cycle. It was hypothesized that AKL and GJL would increase during the ovulation phase, when estrogen levels are high.
Study Design:
Descriptive laboratory study.
Methods:
A total of 15 female university students aged >20 years and with normal menstrual cycles were evaluated. AKL was measured as anterior tibial displacement of the femur after application of 44-, 89-, and 133-N loads to the tibia. Stiffness was calculated as Δ force/Δ displacement at loads between 44 and 89 N and between 89 and 133 N. The University of Tokyo joint laxity test was used for evaluation of GJL. The participants’ menstrual cycle was divided into the early follicular, late follicular, ovulation, and luteal phases using the basal body temperature method and an ovulation kit; AKL and GJL were measured once during each phase. Participants were also stratified according to the presence or absence of genu recurvatum (GR).
Results:
There was no significant difference in AKL, stiffness, or GJL among the menstrual phases. In the GR group, AKL values at 89 N and 133 N were significantly higher in the ovulation phase than in the early follicular phase (P = .025 and P =.018, respectively); there were no significant differences in AKL among the phases in the non-GR group. In addition, the GR group in the ovulation phase had significantly higher AKL values at 44 N, 89 N, and 133 N compared with the non-GR group (P = .013, P = .005, and P = .010, respectively). There were no significant differences in GJL among the phases in the GR or non-GR groups.
Conclusion:
Women with GR may have increased AKL in the ovulation phase when compared with the early follicular phase, which may be a risk factor for ACL injury.
Clinical Relevance:
The results of this study suggest that the ovulation phase may be related to the greater incidence of ACL injuries in women.
Keywords: menstrual cycle, anterior knee laxity, generalized joint laxity, genu recurvatum
Anterior cruciate ligament (ACL) injury, which is one of the most common sports injuries, has been reported to have a higher incidence in women than in men.4,7 Sex differences in the incidence of ACL injury include extrinsic factors (physical and visual perturbations, bracing, and shoe-surface interaction) and intrinsic factors (anatomic, neuromuscular, and biomechanical differences between the sexes), which may be multifactorial in nature.9 Among the intrinsic factors, the effects of female hormones are also thought to play a role in sex differences in the incidence of ACL injury.1,3,9,29,30 The menstrual cycle is controlled mainly by cyclic fluctuations of estradiol and progesterone3 and is classified primarily into the follicular, ovulatory, and luteal phases. Several studies investigating the timing of ACL injury in the menstrual cycle have reported that ACL injuries often occur during the follicular1,3 and ovulation phases.29,30 Therefore, understanding the relationship between the menstrual cycle and ACL injury may help prevent the occurrence of ACL injuries in female athletes.
Regarding the relationship between the menstrual cycle and tissue structure of ACL, it has been reported that estrogen receptors are present in the human ACL17 and that female hormones affect the tissue structure of the ACL.31,32 In vitro tissue culture studies with human ACLs have shown that fibroblast proliferation and procollagen type 1 synthesis are increased within 1 to 3 days of increases in estradiol concentration.31,32 In vivo studies have reported that anterior knee laxity (AKL) increases during the ovulation16,26 and luteal phases.25,26 However, other in vivo studies have found no cyclic changes in AKL.2,6,11 Thus, although estrogen affects collagen structure and metabolism, there is no consensus on the effects of female hormones on AKL.
In recent years, generalized joint laxity (GJL) also has been shown to be a risk factor for ACL injury.13,21,28 Stettler et al27 reported higher values for AKL in individuals with higher GJL scores compared with those with normal mobility. In addition, GJL scores are higher in women than in men10; this difference between men and women has been attributed to differences in sex hormone levels. Shultz et al25 examined changes in AKL, genu recurvatum (GR), and GJL during the early follicular phase and early luteal phase of the menstrual cycle, but they did not examine these parameters throughout the menstrual cycle. Furthermore, changes in GJL during the menstrual cycle and the relationship of GJL with AKL remain poorly understood.
The purpose of the present study was to examine the relationship of AKL, stiffness, and GJL with respect to the menstrual cycle. For the purpose of this study, the menstrual cycle was divided into 4 phases: early follicular, late follicular, ovulation, and luteal phases. We also examined the presence or absence of GR with respect to menstrual phase. Our hypothesis was that AKL and GJL would increase during the ovulation phase, when estrogen levels are high.
Methods
Participants
This study was approved by a university ethics review committee. In addition, this study complied with the Declaration of Helsinki and was conducted only after written informed consent was obtained from the study participants, who had been fully informed (in both oral and written form) of the nature of the experiment.
Between February and April 2019, we recruited female students from a single university for this study. The inclusion criteria for these students were as follows: (1) no history of injury involving the osteochondral surface, ligament, tendon, capsule, or menisci; (2) no use of oral contraceptives or other hormone-stimulating medications in the preceding 6 months25; and (3) physically active less than 3 times per week. A total of 49 students were initially recruited and surveyed using a questionnaire and interview.
Menstrual Cycle Recording
Of the initial 49 participants, 26 who had regular menstrual cycles were asked to measure and record their basal body temperature (BBT) every morning for 1 to 2 months before the start of the experiment. The participants were provided with basal thermometers (Citizen Electronic Thermometer CTEB503L; Citizen Systems Co. Ltd) for this purpose. To estimate the ovulation date, the participants were provided with ovulation kits (Doctor’s Choice One Step Ovulation Test Clear; Beauty and Health Research, Inc) that were used from the day after the end of menstruation. As luteinizing hormone (LH) levels in urine and serum have been shown to be correlated,12 the ovulation date was estimated using the ovulation kit results as a substitute for blood sampling.
A BBT table was prepared as a recording sheet, and daily BBT, menstrual period, and ovulation kit results were recorded. Based on these data, the first day of menstruation was assigned as the nominal day 1, and the mean BBT up to day 6 was calculated. When the BBT for 3 consecutive days after ovulation (as determined via the ovulation kit) was at least 0.2°C greater than this mean value, it was judged that the participant showed a biphasic cycle of low and high temperatures.18
Participants with biphasic cycles were classified as having a normal ovulatory pattern, whereas those with monophasic cycles were considered to have an anovulatory pattern.8,18 Of the 26 participants whose menstrual cycles were monitored, 2 were excluded because their BBT was monophasic; AKL and GJL were measured in the remaining 24 participants. In addition, 9 other participants were excluded for the reasons indicated in Figure 1.
Figure 1.
Participant selection flowchart. AKL, anterior knee laxity; GJL, generalized joint laxity.
The final enrolled study population consisted of 15 participants. The participants had a menstrual cycle length of 25 to 38 days20 (mean ± SD, 30.3 ± 2.4 days) and biphasic BBTs during the cycle in which AKL and GJL measurements were performed (Figure 1).
Timing of Measurements
Measurements were taken once in each of the 4 phases of the menstrual cycle (early follicular, late follicular, ovulation, and luteal phases). AKL and GJL were measured in the early follicular phase, from 3 to 4 days after the start of menstruation; in the late follicular phase, from 3 to 4 days after the end of menstruation; in the ovulation phase, from 2 to 4 days after the day when the ovulation kit gave a positive result; and in the luteal phase, from 5 to 10 days after the start of the high temperature phase. Taking into account possible diurnal variations, all measurements in all participants were performed between 8 AM and noon.24
Measurement Methods
AKL was measured as the anterior displacement (in millimeters) of the tibia relative to the femur when loads of 44 N, 89 N, and 133 N were applied to the tibia26; displacement was measured using the KS Measure (cruciate ligament function tester, KS Measure KSM-100; Japan Sigmax Co, Ltd). The measurement was performed on the pivot (nondominant) foot. Participants were placed in the supine position per the manufacturer’s guidelines, and knee joint flexion was set to about 30° using a goniometer (Nishikawashinwa). A knee support was placed on the posterior part of the distal thigh, and a foot support was placed under the foot. The position of the KS Measure was adjusted so that the patella-contacting part was at the knee center and the ankle-fixing part was at the ankle center; the ankle was fixed using a lower limb–fixing belt, and the lower leg was fixed using a traction belt. After instructing the participant to relax the leg muscles, the load handle was operated to perform the measurement (Figure 2). Measurement was performed 5 times, the high and low values were excluded, and the average of the 3 remaining values was used. Knee joint angle measurement and load handle operation were performed by a single researcher (S.M.), and the KS Measure panel was performed by another researcher (T.Y.). Stiffness was recorded as Δ force/Δ displacement at loads between 44 N and 89 N and between 89 N and 133 N.
Figure 2.
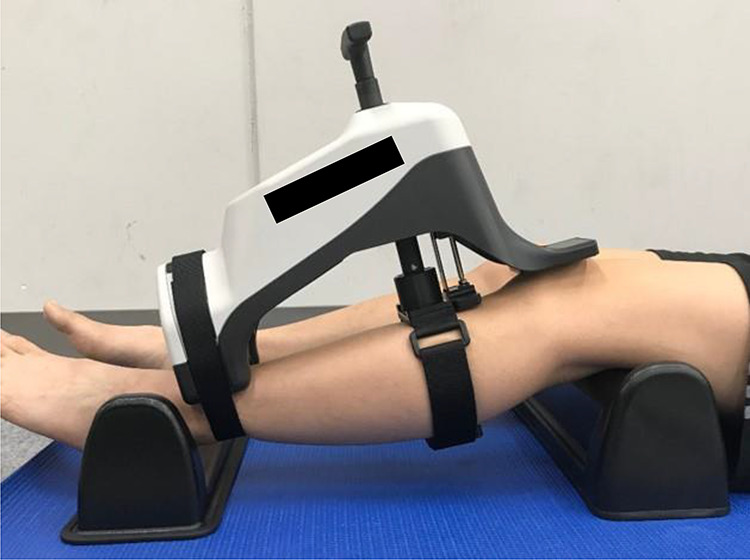
Joint angle measurement position. Supports were placed under the thigh and foot to position the knee at a flexion angle of approximately 30°. The KS Measure (cruciate ligament function tester, KS Measure KSM-100; Japan Sigmax Co, Ltd) was positioned so that the patellar contact area was in the center of the knee and the ankle fixation area was in the center of the ankle. After instructing the participant to relax, the load handle was operated, and measurements were recorded.
GJL was measured using the University of Tokyo joint laxity test19 (Figure 3). Mobility was measured at the spine and bilaterally at the hip, knee, ankle, shoulder, elbow, and wrist, for a total of 7 positions. The assessment criteria were (1) thumb touches the forearm, (2) hyperextension of the elbows ≥15°, (3) gripping fingers behind the back, (4) hyperextension of the knee ≥10°, (5) passive dorsiflexion of the ankle ≥ 45°, (6) forward flexion of the trunk with the knees straight in the standing position and the palms of the hands resting on the floor, and (7) external rotation of the hip in the standing position and the toes open ≥180°. Each item was assigned a value of 1 point; for bilateral positions, the left and right sides were assigned a value of 0.5 point each. For items with joint angle as the criterion, the joint angle was measured using a goniometer. Joint angle measurements were performed by 1 operator (T.Y.) and recorded by another operator (S.M.).
Figure 3.
The University of Tokyo joint laxity test.19 The laxity at 6 major joints of the body (bilaterally at the hip, knee, leg, shoulder, elbow, wrist) and of the spine was examined. The assessment criteria were (1) thumb touches the forearm, (2) hyperextension of the elbows ≥15°, (3) gripping fingers behind the back, (4) hyperextension of the knee ≥10° (genu recurvatum), (5) passive dorsiflexion of the ankle ≥45°, (6) forward flexion of the trunk with the knees straight in the standing position and the palms of the hands resting on the floor, and (7) external rotation of the hip in the standing position and the toes open ≥180°. Each item was assigned a value of 1 point (0.5 point each for the left and right sides for bilateral joints), for a total of 7 points.
GR was defined in this study as hyperextension of the knee ≥10° (item 4 in the GJL test). Depending on the presence or absence of GR, participants were classified into the GR group or non-GR group. Participants with GR in the measured limb (nondominant foot) with AKL were included in the GR group.
Intrarater Reliability
To assess the interrater reliability of AKL measurements, we recruited 10 adult male participants (mean age, 21.0 years ± 0.0; mean ± SD height, 176 ± 6.5 cm; mean ± SD weight, 68.9 ± 6.3 kg) without orthopaedic diseases or pain in the lower limbs. AKL measurements were performed using the above-described method; again, the measurement was performed 5 times, the high and low values were excluded, and the average (mean) of the remaining 3 measurements was used. The measurement was repeated on 2 or more separate days within 1 week, and the intraclass correlation coefficient (ICC) (1,3) was calculated.
Statistical Analysis
One-way repeated-measures analysis of variance was used to compare AKL, stiffness, and GJL for each menstrual phase as well as to compare AKL and GJL of each phase between the GL and non-GL groups. The Bonferroni method was used as a post hoc test. Comparisons of AKL in the ovulation phase between the GR and non-GR groups were performed using the Student t test. The level of significance was 5%. Statistical analyses were performed using SPSS (Version 24.0; SPSS Japan Inc).
Results
The mean ± SD age of the 15 study participants was 21.0 ± 0.2 years, their mean ± SD height was 159.0 ± 4.4 cm, and their mean ± SD weight was 51.9 ± 5.9 kg. AKL and stiffness did not vary significantly among the phases (Table 1). In GJL, no significant differences were found among the menstrual phases, and there were no differences in the rate of the GR items in each phase (Table 2).
Table 1.
Changes in AKL and Stiffness During the Menstrual Cycle of Participants (N = 15)a
| Early Follicular Phase | Late Follicular Phase | Ovulation Phase | Luteal Phase | |
|---|---|---|---|---|
| AKL, mm | ||||
| 44 N | 2.1 ± 0.9 | 2.6 ± 1.1 | 2.8 ± 1.3 | 2.6 ± 1.2 |
| 89 N | 4.2 ± 1.6 | 4.6 ± 1.9 | 5.0 ± 1.8 | 5.0 ± 1.9 |
| 133 N | 6.2 ± 2.1 | 6.5 ± 2.6 | 7.0 ± 2.2 | 7.0 ± 2.5 |
| Stiffness, N/mm | ||||
| 44-89 N | 29.4 ± 26.6 | 34.7 ± 35.7 | 25.7 ± 15.6 | 22.9 ± 11.1 |
| 89-133 N | 28.3 ± 18.0 | 33.6 ± 30.6 | 27.8 ± 21.9 | 26.7 ± 14.9 |
aData are presented as mean ± SD. There were no significant differences in results among the phases. AKL, anterior knee laxity.
Table 2.
Changes in GJL During the Menstrual Cycle of Participants (N = 15)a
| Early Follicular Phase | Late Follicular Phase | Ovulation Phase | Luteal Phase | |
|---|---|---|---|---|
| GJL score, mean ± SD | 2.2 ± 1.0 | 2.6 ± 1.0 | 2.8 ± 1.2 | 2.6 ± 1.1 |
| Participants with GR, n | 6 | 4 | 5 | 4 |
| GR-positive rate, % | 40.0 | 26.7 | 33.3 | 26.7 |
aThere were no significant differences in results among the phases. GJL, generalized joint laxity; GR, genu recurvatum.
Seven participants were classified into the GR group, and 8 were classified into the non-GR group. However, the phase in which each participant in the GR group showed GR varied (Table 3). In the GR group, there were no significant differences in AKL at 44 N, but AKL values at 89 N and 133 N were significantly higher in the ovulation phase than in the early follicular phase (P = .025 and .018, respectively) (Table 4). In contrast, there were no significant differences in AKL among the phases in the non-GR group. In addition, the GR group had significantly higher AKL values at 44 N, 89 N, and 133 N than did the non-GR group (P = .013, P = .005, P = .010, respectively) only in the ovulation phase. There were no significant differences in GJL among the phases in the GR and non-GR groups.
Table 3.
Presence of GR During the Menstrual Cycle for Participants in the GR Group (n = 7)a
| Participant | Early Follicular Phase | Late Follicular Phase | Ovulation Phase | Luteal Phase |
|---|---|---|---|---|
| 1 | – | + | – | – |
| 13 | + | – | + | + |
| 17 | + | – | + | – |
| 18 | + | + | + | + |
| 19 | + | – | – | – |
| 21 | + | + | + | + |
| 24 | + | + | + | + |
aGR, genu recurvatum; +, presence of GR; –, absence of GR.
Table 4.
Changes in AKL and GJL During the Menstrual Cycle in the GR and Non-GR Groupsa
| Early Follicular Phase | Late Follicular Phase | Ovulation Phase | Luteal Phase | |
|---|---|---|---|---|
| GR group (n = 7) | ||||
| AKL, mm | ||||
| 44 N | 2.2 ± 1.0 | 2.7 ± 1.1 | 3.6 ± 1.4b | 2.6 ± 1.4 |
| 89 N | 4.2 ± 1.7 | 4.8 ± 2.1 | 6.3 ± 1.6b,c | 5.0 ± 2.1 |
| 133 N | 6.1 ± 2.1 | 7.0 ± 2.8 | 8.5 ± 1.8b,c | 7.0 ± 2.5 |
| GJL score | 2.3 ± 1.1 | 2.8 ± 1.0 | 3.0 ± 1.0 | 2.6 ± 1.3 |
| Non-GR group (n = 8) | ||||
| AKL, mm | ||||
| 44 N | 2.0 ± 1.0 | 2.4 ± 1.2 | 2.1 ± 0.6 | 2.6 ± 1.1 |
| 89 N | 4.2 ± 1.7 | 4.4 ± 1.9 | 3.9 ± 1.1 | 5.0 ± 2.0 |
| 133 N | 6.2 ± 2.2 | 6.1 ± 2.6 | 5.7 ± 1.7 | 7.1 ± 2.6 |
| GJL score | 2.1 ± 1.0 | 2.4 ± 1.1 | 2.7 ± 1.4 | 2.6 ± 1.1 |
aData are presented as mean ± SD. AKL, anterior knee laxity; GJL, generalized joint laxity; GR, genu recurvatum.
bStatistically significant difference compared with the non-GR group (P < .05).
cStatistically significant difference compared with early follicular phase (P < .05).
The resulting ICC (1,3) for AKL measurements was 0.866 to 0.956. According to the criteria of Landis and Koch,14 reproducibility is considered to be almost perfect when the ICC is 0.81 or more. Therefore, the reproducibility of AKL measurement in this study was considered to be high.
Discussion
This study investigated the relationship between AKL and GJL during the menstrual cycle in female university students who had a regular menstrual cycle and a biphasic BBT. Several previous studies have examined the relationship between ACL injury and laxity of the knee joint,9,13,28 but the relationship between the menstrual cycle and GJL and the relationship between AKL and GJL have not been sufficiently investigated. The main findings of this study were that AKL, stiffness, and GJL did not change during the menstrual cycle. However, when the participants were classified into a GR group and a non-GR group, AKL of the GR group was significantly higher in the ovulation phase than in the early follicular phase.
The results of this study showed that, compared with the respective values, AKL and stiffness did not change significantly among the phases of the menstrual cycle. Regarding AKL, the results were consistent with those of previous studies2,6,11 indicating that AKL does not change during the menstrual cycle. It has been reported that ACL injuries often occur during the follicular phase1,3 and ovulation phase,29,30 but such injuries do not always coincide with the time of peak AKL. Therefore, the cyclic fluctuation of risk factors other than AKL may be involved in the occurrence of ACL injury during the menstrual cycle. Regarding stiffness, the present results were consistent with those of Shultz et al26 who indicated that stiffness also does not change during the menstrual cycle. Therefore, the menstrual cycle may not affect the changes in the mechanical properties of ligament structure.
In GJL, no significant differences were found among the phases of the menstrual cycle, and there were no differences in the presence of GR in each phase. This result was different from that of Shultz et al,25 who reported that the largest mean difference in GJL occurred between day 1 of menses and day 5 of the early luteal phase.
On the other hand, in the GR group, AKL at 89 N and 133 N was significantly higher in the ovulation phase than in the early follicular phase. In addition, the GR group had significantly higher AKL than did the non-GR group only in the ovulation phase. Furthermore, there were no significant differences in GJL between each phase in the GR and non-GR groups. Therefore, women with GR who show cyclic changes in AKL may be more sensitive to the effects of estrogen. Shultz et al25 reported that the average daily pattern of change in AKL, GR, and GJL was quite similar among variables but the magnitude and pattern of cyclic changes varied considerably among women. Shultz et al25 also observed that the correlation coefficients for the relationships between laxity variables at the individual level were –0.07 for AKL and GJL and 0.16 for GR and GJL but were 0.43 for AKL and GR. Therefore, it will be necessary to further investigate the relationship between AKL and GR as a parameter of local laxity around the knee joint.
Regarding the method of measuring GR, the present study evaluated the GR of the GJL test while the participants were in a standing position; in contrast, Shultz et al25 conducted measurements while the participants were in a supine position contracting their quadriceps and maximally extending their knees. However, given that quadriceps muscle strength is high during the ovulation phase,22 it is possible that quadriceps muscle strength affects the GR value. Therefore, we should measure GR using a method that excludes the contraction of the quadriceps, permitting examination of the relationship between AKL and GR.
Uhorchak et al28 found that relative risk ratios associated with having 1 or more risk factors ranged from approximately 1.0 to 37.7, indicating that some combinations of factors led to a risk of noncontact ACL injury that was 40 times higher than was observed when those combinations of factors were not present. Thus, the present results suggest that changes in AKL during the menstrual cycle may be associated with the presence or absence of GR; this observation indicates that these parameters may represent risk factors for ACL injury.
Several limitations must be considered in this study. First, the menstrual cycle was not classified by measurements of hormone concentrations. Instead of measuring hormone concentrations by blood sampling, the present study performed cycle classification by the combination of an ovulation kit, which is an inexpensive and noninvasive method, and the BBT method. As a correlation has been shown between urinary LH and serum LH levels, it was inferred that the ovulation phase could be defined clearly by using the ovulation kit in this study. In addition, the use of the BBT procedure enables the identification of ovulatory and anovulatory cycles.8,18 Thus, by including participants whose BBT was biphasic, it was expected that those with normal ovulatory cycles, whose menstrual cycle could be classified into 4 phases, would be selected. However, the timing and phases of estrogen and progesterone concentration changes vary considerably across the menstrual cycle.23,24,26 For future studies, it may be necessary to classify the menstrual cycle according to hormone concentrations in serum, urine, or saliva.
The second limitation is the selection of participants who are not at risk of ACL injury. To observe the simple effects of the menstrual cycle, female students with a normal menstrual cycle, no use of oral contraceptives, and low levels of physical activity were selected for this study. Lee et al15 reported that women who used oral contraceptives had significantly lower AKL values than those who did not use oral contraceptives. De Crée et al5 reported lower plasma estradiol levels after strenuous exercise in untrained female participants. The results of the present study may be applicable to a nonactive cohort of individuals, but they do not provide any insight into those at risk for ACL injury. In addition, AKL increased during the ovulation phase in women with GR, but it is unclear whether the incidence of ACL injury also increased during the same period. Prospective observation of women with risk factors for ACL injury is necessary to determine the relationship between changes in women’s body structure during the menstrual cycle and the incidence or timing of ACL injury.
The third limitation is the sample size. The total number of participants in this study was 15. The GR group was also small, with only 7 participants. This may limit the interpretation and robustness of the results of this study; therefore, a larger sample size is needed.
Conclusion
In this study, the relationships of AKL, stiffness, and GJL in menstrual cycle were investigated; the cycle itself was divided into 4 phases based on a combination of the BBT method and the results of an ovulation kit. AKL, stiffness, and GJL did not vary significantly among the phases of the menstrual cycle. However, in the GR group, AKL values at 89 N and 133 N were significantly higher in the ovulation phase than in the early follicular phase. In addition, the GR group had significantly higher AKL values than did the non-GR group only in the ovulation phase. These results suggested that changes in AKL during the menstrual cycle in women with GR may be a risk factor for ACL injury. In the future, it will be necessary to classify the menstrual cycle by measuring hormone concentrations and to include participants at risk of ACL injury in order to fully examine the effects of the menstrual cycle on other risk factors for ACL injury.
Acknowledgment
The authors thank those anonymous individuals who generously donated their bodies and so enabled this study to be performed.
Footnotes
Final revision submitted September 18, 2020; accepted November 13, 2020.
One or more of the authors has declared the following potential conflict of interest or source of funding: This study was supported by a Grant-in-Aid for Scientific Research (19K11358) from the Japan Society for the Promotion of Science (JSPS) and commissioned by the Japan Sports Agency (Female Athletes Development and Support Projects 2020). AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from Niigata University of Health and Welfare (study ID: 17946).
References
- 1. Arendt EA, Bershadsky B, Agel J. Periodicity of noncontact anterior cruciate ligament injuries during the menstrual cycle. J Gend Specif Med. 2002;5(2):19–26. [PubMed] [Google Scholar]
- 2. Beynnon BD, Bernstein IM, Belisle A, et al. The effect of estradiol and progesterone on knee and ankle joint laxity. Am J Sports Med. 2005;33(9):1298–1304. [DOI] [PubMed] [Google Scholar]
- 3. Beynnon BD, Johnson RJ, Braun S, et al. The relationship between menstrual cycle phase and anterior cruciate ligament injury: a case-control study of recreational alpine skiers. Am J Sports Med. 2006;34(5):757–764. [DOI] [PubMed] [Google Scholar]
- 4. Beynnon BD, Vacek PM, Newell MK, et al. The effects of level of competition, sport, and sex on the incidence of first-time noncontact anterior cruciate ligament injury. Am J Sports Med. 2014;42(8):1806–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. De Crée C, Ball P, Seidlitz B, Van Kranenburg G, Geurten P, Keizer HA. Responses of catecholestrogen metabolism to acute graded exercise in normal menstruating women before and after training. J Clin Endocrinol Metab. 1997;82(10):3342–3348. [DOI] [PubMed] [Google Scholar]
- 6. Eiling E, Bryant AL, Petersen W, Murphy A, Hohmann E. Effects of menstrual-cycle hormone fluctuations on musculotendinous stiffness and knee joint laxity. Knee Surg Sports Traumatol Arthrosc. 2007;15(2):126–132. [DOI] [PubMed] [Google Scholar]
- 7. Fernandez-Jaen T, Lopez-Alcorocho JM, Rodriguez-Inigo E, Castellan F, Hernandez JC, Guillen-Garcia P. The importance of the intercondylar notch in anterior cruciate ligament tears. Orthop J Sports Med. 2015;3(8):2325967115597882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hatch MC, Figa-Talamanca I, Salerno S. Work stress and menstrual patterns among American and Italian nurses. Scand Work Environ Health. 1999;25(2):144–150. [DOI] [PubMed] [Google Scholar]
- 9. Hewett TE, Myer GD, Ford KR. Anterior cruciate ligament injuries in female athletes, part 1: mechanisms and risk factors. Am J Sports Med. 2006;34(2):299–311. [DOI] [PubMed] [Google Scholar]
- 10. Jansson A, Saartok T, Werner S, Renstrom P. General joint laxity in 1845 Swedish school children of different ages: age- and gender-specific distributions. Acta Paediatr. 2004;93(9):1202–1206. [DOI] [PubMed] [Google Scholar]
- 11. Karageanes SJ, Blackburn K. The association of the menstrual cycle with the laxity of the anterior cruciate ligament in adolescent female athletes. Clin Sports Med. 2000;10:162–168. [DOI] [PubMed] [Google Scholar]
- 12. Kerin JF, Warnes GM, Crocker J, et al. 3-hour urinary radioimmunoassay for luteinising hormone to detect onset of preovulatory LH surge. Lancet. 1980;2(8191):430–431. [PubMed] [Google Scholar]
- 13. Kramer LC, Denegar CR, Buckley WE, Hertel J. Factors associated with anterior cruciate ligament injury: history in female athletes. J Sports Med Phys Fitness. 2007;47(4):446–454. [PubMed] [Google Scholar]
- 14. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- 15. Lee H, Petrofsky JS, Daher N, Berk L, Laymon M. Differences in anterior cruciate ligament elasticity and force for knee flexion in women: oral contraceptive users versus non-oral contraceptive users. Eur J Appl Physiol. 2014;114(2):285–294. [DOI] [PubMed] [Google Scholar]
- 16. Lee H, Petrofsky JS, Daher N, Berk L, Laymon M, Khowailed IA. Anterior cruciate ligament elasticity and force for flexion during the menstrual cycle. Med Sci Monit. 2013;29:1080–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu SH, Al-Shaikh R, Panossian V, et al. Primary immunolocalization of estrogen and progesterone target cells in the human anterior cruciate ligament. J Orthop Res. 1996;14(4):526–533. [DOI] [PubMed] [Google Scholar]
- 18. Moghissi KS. Prediction and detection of ovulation. Fertil Steril. 1980;34(2):89–98. [DOI] [PubMed] [Google Scholar]
- 19. Motohashi M. Profile of bilateral anterior cruciate ligament injuries: a retrospective follow-up study. J Orthop Surg (Hong Kong). 2004;12(2):210–215. [DOI] [PubMed] [Google Scholar]
- 20. Nose-Ogura S, Yoshino O, Dohi M, et al. Risk factors of stress fractures due to the female athlete triad: differences in teens and twenties. Scand J Med Sci Sports. 2019;29(10):1501–1510. [DOI] [PubMed] [Google Scholar]
- 21. Ramesh R, Von Arx O, Azzopardi T, Schranz PJ. The risk of anterior cruciate ligament rupture with generalized joint laxity. J Bone Joint Surg Am. 2005;87:800–803. [DOI] [PubMed] [Google Scholar]
- 22. Sarwar R, Niclos BB, Rutherford OM. Changes in muscle strength, relaxation rate and fatiguability during the human menstrual cycle. J Physiol. 1996;493(pt 1):267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shultz SJ, Gansneder BM, Sander TC, Kirk SE, Perrin DH. Absolute serum hormone levels predict the magnitude of change in anterior knee laxity across the menstrual cycle. J Orthop Res. 2006;24(2):124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shultz SJ, Kirk SE, Johnson ML, Sander TC, Perrin DH. Relationship between sex hormones and anterior knee laxity across the menstrual cycle. Med Sci Sports Exerc. 2004;36(7):1165–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shultz SJ, Levine BJ, Nguyen AD, Kim H, Montgomery MM, Perrin DH. A comparison of cyclic variations in anterior knee laxity, genu recurvatum, and general joint laxity across the menstrual cycle. J Orthop Res. 2010;28(11):1411–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shultz SJ, Sander TC, Kirk SE, Perrin DH. Sex differences in knee joint laxity change across the female menstrual cycle. J Sports Med Phys Fitness. 2005;45(4):594–603. [PMC free article] [PubMed] [Google Scholar]
- 27. Stettler M, Luder G, Schmid S, et al. Passive anterior tibial translation in women with and without joint hypermobility: an exploratory study. Int J Rheum Dis. 2018;21(10):1756–1762. [DOI] [PubMed] [Google Scholar]
- 28. Uhorchak JM, Scoville CR, Williams GN, Arcerio RA, St Pierre P, Taylor DC. Risk factors associated with noncontact injury of the anterior cruciate ligament: a prospective four-year evaluation of 859 West Point cadets. Am J Sports Med. 2003;31:831–842. [DOI] [PubMed] [Google Scholar]
- 29. Wojtys EM, Huston LJ, Boynton MD, Spindler KP, Lindenfeld TN. The effect of the menstrual cycle on anterior cruciate ligament injuries in women as determined by hormone levels. Am J Sports Med. 2002;30(2):182–188. [DOI] [PubMed] [Google Scholar]
- 30. Wojtys EM, Huston LJ, Lindenfeld TN, Hewett TE, Greenfield ML. Association between the menstrual cycle and anterior cruciate ligament injuries in female athletes. Am J Sports Med. 1998;26(5):614–619. [DOI] [PubMed] [Google Scholar]
- 31. Yu WD, Liu SH, Hatch JD, Panossian V, Finerman GA. Effect of estrogen on cellular metabolism of the human anterior cruciate ligament. Clin Orthop Relat Res. 1999;366:229–238. [DOI] [PubMed] [Google Scholar]
- 32. Yu WD, Panossian V, Hatch JD, Liu SH, Finerman GA. Combined effects of estrogen and progesterone on the anterior cruciate ligament. Clin Orthop Relat Res. 2001;383:268–281. [DOI] [PubMed] [Google Scholar]




