Abstract
Displaced rib fractures can injure intercostal vessels leading to chest wall hematomas. As the bleeding occurs within the vessel, compression of the vessel wall helps in preventing further bleeding. Therefore, chest wall hematomas rarely result in shock. A thin 78-year-old man transferred to the emergency department with complaints of left dorsal pain due to an injury. He had a history of hypertension and aorta dissection. He arrived at the ED in a state of shock and presented with a large left dorsal wall mass. Subsequent imaging using computed tomography angiography revealed a large hyperdense hematoma at the left dorsal-flank wall along with rib fracture (11th intercostal artery). Moreover, a large fusiform aneurysm was detected from the abdominal aorta to the iliac arteries. Extravasation of the contrast agent was detected at the branch of the 11th intercostal artery, and hence, embolization was performed. The dermis, which comprises collagen and elastin fibers, plays an important role in vessel compression to prevent bleeding. The aortic media also comprises collagen and elastin fibers. Cell turnover, loss of collagen, and excessive elastolysis are associated with the formation of abdominal aortic aneurysms. The systemic degeneration of connecting tissue (collagen and elastin fiber) appears to be progress in patients with an aortic aneurysms and history of aortic dissection compared with other healthy older individuals. Physicians should be cognizant of the potential unexpected large hematoma complications if a risk of systemic connecting tissue degradation exists, as seen in patients with aortic aneurysm or aortic dissection.
Keywords: Chest wall hematoma, Rib fracture, Elderly, Collagen, Elastin fiber, Degeneration
Introduction
Falls are the most common cause of orthopedic injury in patients aged over 65 years. Rib fractures are the most common chest injuries accompanied by blunt chest trauma and are associated with an increased risk for complications and death [1]. The mortality rate increases by approximately 19% for each rib fracture [2], and increased rib fractures lead to higher complications [1]. Hemothorax, pneumothorax, pulmonary contusions and chest wall hematomas are known complications of rib fractures [3]. Hemothorax occurs in the pleural cavity and sometimes induces massive hemorrhage and shock. However, it is rare that chest wall hematomas induce shock. Herein, we report a case of hypovolemic shock induced by a large chest wall hematoma due to an intercostal artery injured by a rib fracture in an elderly patient.
Case Report
This work was conducted in accordance with the Declaration of Helsinki.
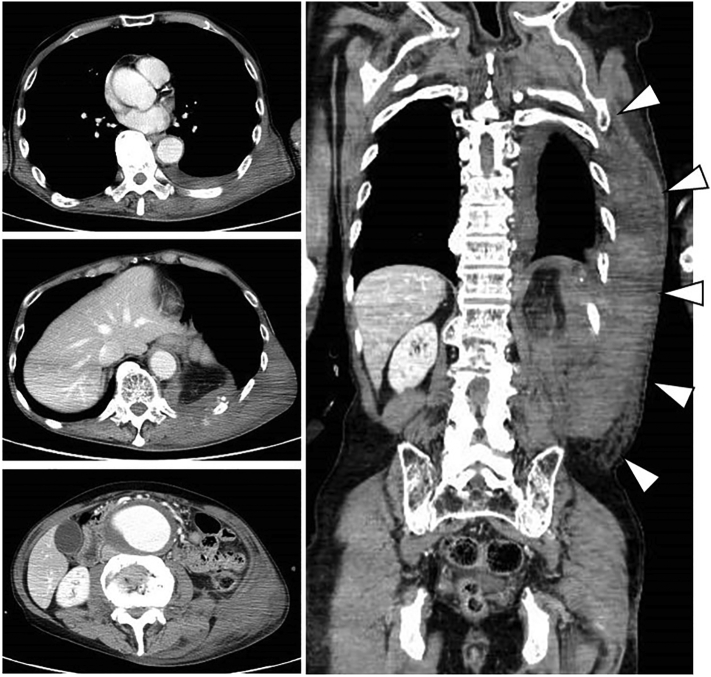
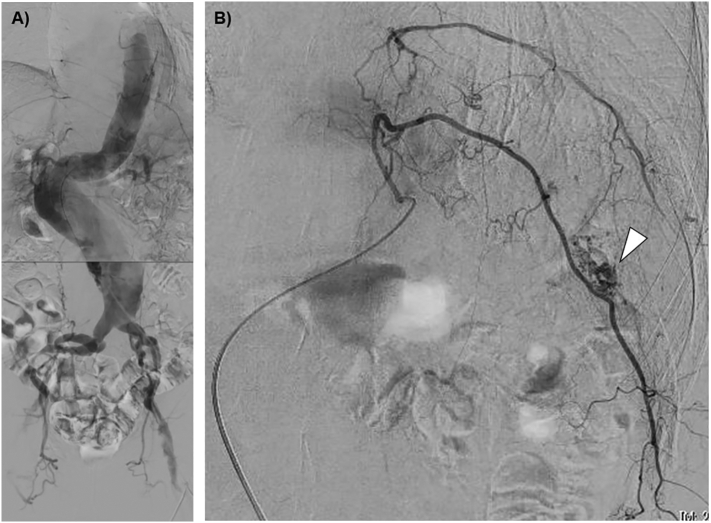
A thin 78-year-old man was transferred to the emergency department (ED) with complaints of left dorsal pain. He injured the left dorsal region when he bumped it against the table corner the previous day by tripping over a power cord. His medical history revealed hypertension and aorta dissection (Stanford type B), for which he received conservative treatment 6 years ago. He did not take any anticoagulant or antiplatelet medications. The patient arrived at the ED in a state of shock, with an arterial blood pressure of 71/53 mmHg, pulse rate of 98 beats per minute, respiration rate of 32 breaths per minute, and oxygen saturation of 94% under a reservoir mask. He had a large left dorsal-flank wall mass with subcutaneous bleeding. Subsequent imaging with computed tomography angiography (CTA) of the chest-to-pelvis revealed a mild hemothorax and a new large hematoma at the left dorsal-flank wall (Fig. 1) along with a single rib fracture (11th intercostal artery) (Fig. 2). We detected extravasation of the contrast agent from the branch of the intercostal artery. Moreover, a large aneurysm with severe arteriosclerosis demonstrating fusiform expansion was detected from the abdominal aorta (54 mm) to the iliac arteries (36 mm) (Fig. 3A). Initial hospital laboratory evaluation revealed a hemoglobin level of 5.5 g/dL and lactate level of 4.51 mmol/L on presentation. The patient was immediately resuscitated with crystalloid, red blood cells, and fresh frozen plasma. He recovered from shock and received transcatheter arterial embolization. For the extravasation of the contrast agent, embolization was performed using a gelatin sponge (Fig. 3B). He was discharged 13 days after admission without complications.
Fig. 1.
Imaging with CT angiography (CTA) from the chest to the pelvis (cross-section, coronal section). The image shows a mild hemothorax and new large hematoma at the left dorsal-flank wall (Arrowhead).
Fig. 2.
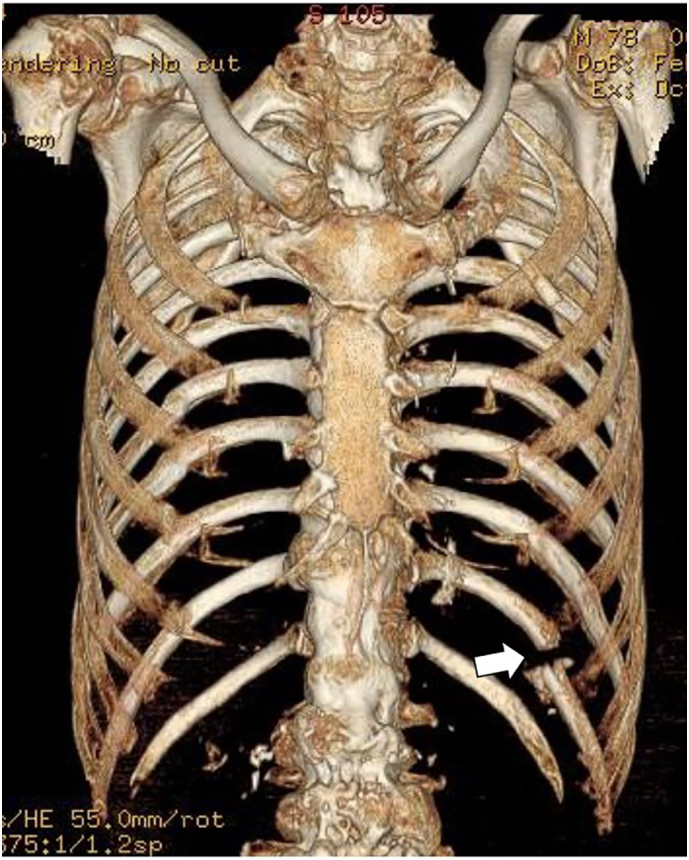
3D images of rib reconstruction. A left rib fracture (11th) (arrow) was detected.
Fig. 3.
Angiography. A) A large aneurysm with severe arteriosclerosis and fusiform expansion was detected from the abdominal aorta to iliac arteries. B) Extravasation of the contrast agent (arrowhead) was detected at the branch of the 11th intercostal artery and embolization was performed using a gelatin sponge.
Discussion
The posterior intercostal arteries supply blood to the intercostal spaces; they exist at the costal groove on the inferior surface of the rib body [3]. Three layers of intercostal muscle, skin and fascial layers that include the parietal pleura surround the arteries. Displaced rib fractures increase the injury risk to the intercostal vessels that can cause a chest wall hematoma. Chest wall hematomas occur in a closed tissue space and compression of the bleeding vessel usually stops further bleeding. Therefore, mortality rates from chest wall hematomas are low [4].
However, in our case, the displaced rib fracture injured proximal intercostal blood vessels and persistent bleeding lead to a large chest wall hematoma and shock. In this case, compression of the surrounding tissue did not stop the bleeding.
Human skin is organized into 3 layers: the epidermis, dermis and hypodermis. Shear forces and breaking strength of the dermis are 27 MPa . By contrast, force resistance in the hypodermis is only 1–5 MPa [5]. The dermis plays an important role in compression of bleeding. They consists of elastin fibers and interstitial collagen (mainly types I and III) which are synthesized by fibroblasts. With aging, the number of fibroblasts reduces, and the diameter of collagen fiber bundles decrease causing dermic atrophy and weakness [6]. Dermis degeneration may have been associated with the development of a large hematoma in our patient.
Aortic integrity and stability are also determined by medial elastin fibers and interstitial collagens (types I and III) in the media [7]. The etiology of abdominal aortic aneurism can be genetic; for example elastin and collagen degradation often correlate with genetically unstable proteins or increased protease production [8]. Satta et al. [9] reported that increased turnover and loss of types I and III fibrillar collagens is evident in patients with abdominal aortic aneurysms. In addition, Thompson [10] et al. reported that excessive elastolysis caused by increased collagenase, elastase, and especially MMP expression are associated with aortic dilation. Exposure of elastase to the aortic wall culminates in aortic aneurysm formation by producing MCP-1 and RANTES in animals.
In our case, the patient had a large aneurysm extending from the abdominal aorta to iliac arteries and a past medical history of aortic dissection. Both the dermis and aortic media are composed with collagen (types I and III) and elastin fibers. The systemic degeneration of connecting tissue (collagen and elastin fiber) progressed not only in the abdominal artery but in other connective tissue such as the skin as well.
Conclusion
Elderly adult patients with a large aortic aneurysm or aortic dissection may experience complications due to systemic degradation of connecting tissue (collagen and elastin fibers) compared to that observed with other healthy elderly patients. Physicians should be aware of unexpected large hematoma complications in such patients.
Sources of support
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors have no conflicts of interest to declare.
Footnotes
This case has not presented at any meeting.
References
- 1.Battle C.E., Hutchings H., Evans P.A. Risk factors that predict mortality in patients with blunt chest wall trauma: a systematic review and meta-analysis. Injury. 2012;43:8–17. doi: 10.1016/j.injury.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Bulger E.M., Arneson M.A., Mock C.N., Jurkovich G.J. Rib fractures in the elderly. J. Trauma. 2000;48:1040–1046. doi: 10.1097/00005373-200006000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Talbot B.S., Gange C.P., Jr., Chaturvedi A., Klionsky N., Hobbs S.K., Chaturvedi A. Traumatic rib injury: patterns, imaging pitfalls, complications, and treatment. Radiographics. 2017;37:628–651. doi: 10.1148/rg.2017174003. [DOI] [PubMed] [Google Scholar]
- 4.Rashid M.A., Wikström T., Ortenwall P. Nomenclature, classification, and significance of traumatic extrapleural hematoma. J. Trauma. 2000;49:286–290. doi: 10.1097/00005373-200008000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Saulis A.S., Lautenschlager E.P., Mustoe T.A. Biomechanical and viscoelastic properties of skin, SMAS, and composite flaps as they pertain to rhytidectomy. Plast. Reconstr. Surg. 2002;110:590–598. doi: 10.1097/00006534-200208000-00035. [DOI] [PubMed] [Google Scholar]
- 6.Lovell C.R., Smolenski K.A., Duance V.C., Light N.D., Young S., Dyson M. Type I and III collagen content and fibre distribution in normal human skin during ageing. Br. J. Dermatol. 1987;117:419–428. doi: 10.1111/j.1365-2133.1987.tb04921.x. [DOI] [PubMed] [Google Scholar]
- 7.O’Brien J.P. A concept of diffuse actinic arteritis. The role of actinic damage to elastin in ‘age change’ and arteritis of the temporal artery and in polymyalgia rheumatica. Br. J. Dermatol. 1978;98:1–13. doi: 10.1111/j.1365-2133.1978.tb07327.x. [DOI] [PubMed] [Google Scholar]
- 8.Shimizu K., Mitchell R.N., Libby P. Inflammation and cellular immune responses in abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 2006;26:987–994. doi: 10.1161/01.ATV.0000214999.12921.4f. [DOI] [PubMed] [Google Scholar]
- 9.Satta J., Haukipuro K., Kairaluoma M.I., Juvonen T. Aminoterminal propeptide of type III procollagen in the follow-up of patients with abdominal aortic aneurysms. J. Vasc. Surg. 1997;25:909–915. doi: 10.1016/s0741-5214(97)70222-2. [DOI] [PubMed] [Google Scholar]
- 10.Thompson R.W., Parks W.C. Role of matrix metalloproteinases in abdominal aortic aneurysms. Ann. N. Y. Acad. Sci. 1996;800:157–174. doi: 10.1111/j.1749-6632.1996.tb33307.x. [DOI] [PubMed] [Google Scholar]