Abstract
This study was conducted to investigate the beneficial effects of bisdemethoxycurcumin (BDC) on growth performance, glutathione (GSH) redox potential, antioxidant enzyme defense, and gene expression in lipopolysaccharide (LPS)-challenged broilers. A total of 320, male, 1-day-old broilers were randomly assigned to 4 treatment groups including 8 replicates with 10 birds per cage in a 2 × 2 factorial arrangement: BDC supplementation (a basal diet with 0 or 150 mg/kg BDC) and LPS challenge (intraperitoneal injection of 1 mg/kg body weight saline or LPS at 16, 18, and 20 d of age). Results showed that dietary BDC supplementation prevented the LPS-induced decrease in ADG of broilers (P < 0.05). Compared to the saline-challenged group, LPS-challenged broilers showed higher jejunal and ileal malondialdehyde (MDA), protein carbonyl (PC), and 8-hydroxy-2′-deoxyguanosine (8-OHdG) contents (P < 0.05). Dietary BDC supplementation alleviated LPS-induced increases in jejunal 8-OHdG, ileal MDA, and PC contents (P < 0.05). LPS challenge impaired the small intestinal antioxidant system, as evident by the decreases of GSH and total thiol contents, as well as superoxide dismutase (SOD), glutathione peroxidase, glutathione reductase (GR), and glutathione S-transferase (GST) activities. On the other hand, LPS challenge also increased GSH redox potential and oxidized glutathione (GSSG) contents (P < 0.05). Dietary BDC supplementation increased jejunal and ileal GSH contents, SOD activities, jejunal GR activity, and ileal GST activity, while it decreased jejunal and ileal redox potential, and jejunal GSSG contents (P < 0.05). Dietary BDC supplementation significantly alleviated the downregulation of mRNA expression levels of jejunal and ileal copper and zinc superoxide dismutase, catalytic subunit of γ-glutamylcysteine ligase, nuclear factor erythroid-2-related factor 2, heme oxygenase 1, NAD(P)H quinone oxidoreductase 1, and jejunal catalase and GR induced by LPS challenge (P < 0.05). In conclusion, BDC demonstrated favorable protection against LPS-induced small intestinal oxidative damages, as indicated by the improved growth performance, decreased GSH redox potential, enhanced antioxidant enzyme activities, and upregulated antioxidant-related gene expression.
Key words: bisdemethoxycurcumin, intestinal oxidative damage, antioxidant, lipopolysaccharide, broiler
Introduction
In the last few decades, the incidence of oxidative stress has been increasing due to the demand for intensive production and economic interests in poultry. Oxidative stress originates from an imbalance of the excessive free radical production and limited redox buffer capacity, which leads to tissue and cellular damage. Oxidative stress can be induced by many factors, such as high temperature, feed contamination, parasitic infection, and xenobiotic exposure (Lauridsen, 2019). Lipopolysaccharide (LPS) is the outer membrane of most Gram-negative bacteria and used as a well-recognized inducer of oxidative stress in animal models (Hong et al., 2018). Broilers experimentally challenged with LPS displayed obvious symptoms of oxidative damages, such as morphological alternations of organs, reduction of antioxidant enzymatic activities, and dysregulation of intracellular redox status (Zheng et al., 2016; Lv et al., 2020). More importantly, LPS-induced, reactive oxygen species (ROS)-mediated small intestinal damages result in impaired digestion and absorption of nutrients, the loss of antioxidant defense, and the progressive disruption of mucosal redox homeostasis (Garrett et al., 2010; Mishra and Jha, 2019). Dietary antioxidant additives have great application in the poultry industry since improvement of antioxidant defense function is involved in the modulation of growth performance, health care, and stress responses (Kurutas, 2016). However, results from previous studies of synthesized antioxidants are often conflicting and even negative in long-term or high-dose regimes (Kahl and Kappus, 1993; Saito et al., 2003). There is increasing public interest in natural antioxidants that are present in herbs and plants, which might help in preventing oxidative damage without toxic or side effects.
The perennial herb Curcuma longa, commonly known as the golden spice turmeric, has been extensively used as a dietary spice and traditional medicine against a variety of diseases for centuries (Kunnumakkara et al., 2017; Soleimani et al., 2018). Commercial curcumin or curcuminoids often contain 60 to 70% curcumin, 20 to 25% demethoxycurcumin, and 10 to 15% bisdemethoxycurcumin (BDC, Goel et al., 2008). Curcuminoids demonstrate a wide range of biological activities, including antioxidant, anti-inflammatory, and free radical scavenging effects (Teymouri et al., 2018; Li et al., 2019a). Among the 3 curcuminoids, controversy remains regarding their antioxidant efficiencies both in vitro and in vivo (Ahsan et al., 1999; Somparn et al., 2007; Zhang et al., 2010).
Bisdemethoxycurcumin is a demethoxy derivative of curcumin and much more stable than curcumin in physiological media (Luo et al., 2015). BDC can scavenge free radicals and regulate cellular redox balance as a result of its antioxidant property. A study conducted on C57BL/6 mice showed that dietary BDC administration counteracted oxidative stress by increasing superoxide dismutase (SOD) activity and glutathione (GSH) content (Xu et al., 2020). Pei et al. (2016) reported that supplementation with BDC prevented the overproduction of ROS. BDC could upregulate the expression of nuclear factor erythroid-2-related factor 2 (Nrf2) (Jin et al., 2020). Nrf2 responds to oxidative stress and is a master regulator of the transcription of many antioxidant enzymes such as SOD, catalase (CAT), glutathione peroxidase (GSH-Px), γ-glutamylcysteine ligase (γ-GCL), glutathione reductase (GR), and phase II enzymes including heme oxygenase-1 (HO-1), and NAD(P)H quinone oxidoreductase 1 (NQO1) (Klaassen and Reisman, 2010). Furthermore, studies have suggested that BDC can improve mitochondrial function by inhibiting ROS accumulation and the collapse of mitochondrial membrane potential, showing promising potential for the treatment of intestinal oxidative injury under adverse conditions.
Recently, a comparative study from our laboratory found that BDC is more potent than curcumin in alleviating circulating lipid peroxidation and facilitating the antioxidant gene expression of the liver and small intestine in broilers (Zhang et al., 2019). Based on the above results, it is necessary to further investigate the possible antioxidant potential of BDC to alleviate intestinal oxidant injury in broiler chickens. However, little is known about the effects of dietary BDC supplementation on the redox potential and redox status in response to LPS challenge in broilers. Thus, the present study was conducted to determine the possible antioxidant protection of BDC against LPS-induced intestinal oxidative injury in broiler chickens and highlight the application of BDC as a dietary antioxidant intervention in animals.
Materials and methods
Ethics Statement
The experimental protocol in the present study was approved by the Nanjing Agricultural University Institutional Animal Care and Use Committee, China, and conducted in accordance with the Guidelines for Experimental Animals of the Ministry of Science and Technology (Beijing, China).
Experimental Animals and Diets
A total of 320, male, 1-day-old Arbor Acres broilers were randomly assigned to 4 treatment groups containing 8 replicates with 10 birds per replicate. The experiment was designed to have a 2 × 2 factorial arrangement with BDC treatment (supplementation with 0 or 150 mg/kg BDC) and LPS treatment (injection of sterile saline or 1 mg/kg body weight LPS). The supplemental level of 150 mg/kg BDC was determined based on our previous report (Zhang et al., 2019). LPS (serotype O55.B5; Sigma-Aldrich, St. Louis, MO) was dissolved in 0.86% (w/v) sterile saline solution and intraperitoneally injected at a dosage of 1 mg/kg of body weight, according to previous studies (Gonzalez et al., 2011; Chen et al., 2018). At 16, 18, and 20 d of age, broilers were intraperitoneally injected with LPS or an equal amount of 0.86% sterile saline solution. The whole experiment lasted for 20 d. During the whole rearing period, broilers were housed in wire-floored battery cages with a 3-level battery and they had free access to water and mash feed. The ingredient composition and nutrient content of the basal diet were formulated to meet nutrient requirements of broilers (NRC, 1994; Table 1). Broilers were subjected to a 12-hour light–dark cycle and kept in a temperature- and humidity-controlled room (Zhang et al., 2015). The contents of BDC in the diet were determined by an improved HPLC method according to Jayaprakasha et al. (2002). The analyzed value was 0.016% in the diet with BDC treatment alone and 0.015% with BDC and LPS treatments.
Table 1.
Formulation and calculated composition of the basal diet.
| Period | 1–20 d |
|---|---|
| Ingredient (%) | |
| Corn | 57.00 |
| Soybean meal (44.2%, crude protein) | 31.30 |
| Corn gluten meal (60%, crude protein) | 3.90 |
| Soybean oil | 3.10 |
| Dicalcium phosphate | 1.80 |
| Limestone | 1.30 |
| L-Lysine | 0.15 |
| DL-Methionine | 0.15 |
| Premix1 | 1.00 |
| Salt | 0.30 |
| Total | 100.00 |
| Calculation of nutrients | |
| Metabolizable energy, kcal/kg | 3,033 |
| Crude protein, % | 21.52 |
| L-Lysine, % | 1.14 |
| Methionine, % | 0.50 |
| Calcium, % | 1.00 |
| Total phosphorus, % | 0.65 |
| Available phosphorus, % | 0.46 |
| Arginine, % | 1.36 |
| Methionine + cystine, % | 0.85 |
| Analyzed composition | |
| Crude protein, % | 21.38 |
| Calcium, % | 1.06 |
| Total phosphorus, % | 0.68 |
Provided per kg of diet: vitamin A (trans-retinyl acetate), 10,000 IU; vitamin D3 (cholecalciferol), 3,000 IU; vitamin E (all-rac-α-tocopherol acetate), 30 IU; menadione, 1.3 mg; thiamin, 2.2 mg; riboflavin, 8 mg; nicotinamide, 40 mg; choline chloride, 600 mg; calcium pantothenate, 10 mg; pyridoxine·HCl, 4 mg; biotin, 0.04 mg; folic acid, 1 mg; vitamin B12 (cobalamin), 0.013 mg; Fe (from ferrous sulfate), 80 mg; Cu (from copper sulfate), 8 mg; Mn (from manganese sulfate), 110 mg; Zn (from zinc oxide), 65 mg; I (from calcium iodate), 1.1 mg; and Se (from sodium selenite), 0.3 mg.
Sample Collection
At 20 d of age, 1 bird (8 birds per replicate), close to the average body weight, was selected and euthanized by exsanguination within 2 h after LPS or sterile saline injection. The jejunum (from the end of the duodenum to the Meckel's diverticulum) and ileum (from Meckel's diverticulum to 5 cm above the ileo-cecal junction) samples were collected and gently rinsed with 0.86% saline to remove the contents. The mucosa of mid-jejunum and mid-ileum was scraped off by using a glass slide according to the method described by Chen et al. (2018), and stored in liquid nitrogen for further analysis.
Growth Performance
The body weight and feed intake of the birds in each replicate were recorded at 1, 15, and 20 d of age. The ADG, ADFI, and feed conversion rate (FCR) were calculated and adjusted by mortality.
Preparation of Small Intestinal Mucosa Homogenates
The small intestinal mucosa (jejunum and ileum) samples were homogenized with an ice-cold buffer containing 0.86% sodium chloride buffer (w/v 1:9). The supernatant was collected by centrifugation at 3,500 × g for 10 min at 4°C and stored for further analysis. The protein contents of the intestinal homogenates were determined by using a bicinchoninic acid assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Assay of Lipid, Protein, and DNA Oxidation
Lipid peroxidation, protein, and DNA oxidation were expressed as malondialdehyde (MDA), protein carbonyl (PC), and 8-hydroxy-2′-deoxyguanosine (8-OHdG) contents, respectively. The MDA and PC contents were determined using commercial kits purchased from Nanjing Jiancheng Bioengineering Institute. The 8-OHdG contents were measured by ELISA with a commercial kit purchased from Nanjing Jiancheng Bioengineering Institute. The MDA and PC contents were expressed as nanomole per milligram (nmol/mg) protein; the 8-OHdG content was expressed as nanogram per milligram (ng/mg) protein.
Determination of GSH Redox Potential
The reduced GSH and oxidized glutathione (GSSG) contents were spectrophotometrically determined with commercial kits purchased from Nanjing Jiancheng Bioengineering Institute. The GSH redox potential was determined using the method of Nkabyo et al. (2006). Briefly, the GSH redox potential (Eh) values were calculated by using appropriate forms of the Nernst equation (mV) for the respective GSH/GSSG and pools: Eh = −64 + 30 log ([GSSG]/[GSH]2) for pH 7.4.
Determination of Total Thiol (T-SH) Contents and Antioxidant Enzyme Activities
The T-SH contents, and the activities of SOD, GSH-Px, GR, and glutathione S-transferase (GST) were determined spectrophotometrically with commercial kits purchased from Nanjing Jiancheng Bioengineering Institute. The T-SH content was expressed as micromole/milligram (μmol/mg) protein. The results of SOD, GSH-Px, GR, and GST activities were expressed as unit/milligram (U/mg) protein.
Real-Time PCR Analysis
Trizol reagent (Takara, Dalian, China) was administered to extract total RNA from the jejunum and ileum samples. cDNA was acquired by reverse transcription using the PrimeScript RT Reagent kit (Takara). Total cDNA was amplified by quantitative real-time PCR in an ABI 7300 Fast Real-Time PCR detection system (Applied Biosystems, Foster City, CA) and using the SYBR Premix Ex Taq II kit (Takara). The primer sequences for the target and housekeeping genes (CAT, copper and zinc superoxide dismutase [CuZnSOD], catalytic subunit of γ-glutamylcysteine ligase [γ-GCLc], modified subunit of γ-glutamylcysteine ligase [γ-GCLm], GSH-Px, GR, Nrf2, HO-1, NQO1, and β-actin) are provided in Table 2. β-Actin was used as an internal reference.
Table 2.
Sequences for real-time PCR primers.1
| Gene | GeneBank ID | Primer sequence (5′→3′) | Product size (bp) |
|---|---|---|---|
| β-Actin | NM_205518.1 | TGCTGTGTTCCCATCTATCG | 150 |
| TTGGTGACAATACCGTGTTCA | |||
| CAT | NM_001031215.1 | GGTTCGGTGGGGTTGTCTTT | 211 |
| CACCAGTGGTCAAGGCATCT | |||
| CuZnSOD | NM_205064.1 | CCGGCTTGTCTGATGGAGAT | 124 |
| TGCATCTTTTGGTCCACCGT | |||
| γ-GCLc | XM_419910.3 | TGCGGTTCTGCACAAAATGG | 272 |
| TGCTGTGCGATGAATTCCCT | |||
| γ-GCLm | NM_001007953.1 | CCAGAACGTCAAAGCACACG | 187 |
| TCCTCCCATCCCCCAGAAAT | |||
| GSH-Px | NM_001277853.1 | GACCAACCCGCAGTACATCA | 205 |
| GAGGTGCGGGCTTTCCTTTA | |||
| GR | XM 004943130.1 | ACGGCTCCTCACATCCTCATT | 109 |
| CCAGGTCGAAGAACCCATCAC | |||
| Nrf2 | NM_205117.1 | GATGTCACCCTGCCCTTAG | 215 |
| CTGCCACCATGTTATTCC | |||
| HO-1 | HM237181.1 | GGTCCCGAATGAATGCCCTTG | 138 |
| ACCGTTCTCCTGGCTCTTGG | |||
| NQO1 | NM_001277621.1 | CCTCTACGCCATAGGGTTCA | 165 |
| TCAGCCGCTTCAATCTTCTT |
Catalase (CAT), copper and zinc superoxide dismutase (CuZnSOD), catalytic subunit of γ-glutamylcysteine ligase (γ-GCLc), modified subunit of γ-glutamylcysteine ligase (γ-GCLm), glutathione peroxidase (GSH-Px), glutathione reductase (GR), nuclear factor erythroid-2-related factor 2 (Nrf2), heme oxygenase 1 (HO-1), and NAD(P)H quinone oxidoreductase 1 (NQO1).
Statistical Analysis
Data were analyzed by two-way ANOVA using the general linear model procedure of SPSS 17.0 (SPSS Inc., Chicago, IL) with dietary BDC and LPS challenge as the main effects. The significant differences among groups were determined using Tukey's multiple-range tests. A P-value of less than 0.05 was considered statistically significant. Results are presented as means with their pooled standard errors.
Results
Growth Performance
In the present study, mortality was about 1.03% and not related to treatments (data not shown). The results of the growth performance are shown in Table 3. Before LPS challenge, dietary BDC supplementation had no effect on the growth performance of broilers from 1 to 15 d of age (P > 0.05). From 16 to 20 d of age, LPS challenge significantly decreased the ADG compared with the saline-challenged group (P < 0.05). Dietary BDC supplementation significantly increased ADG compared with the unsupplemented group (P < 0.05). There was a significant BDC–LPS interaction on ADG (P < 0.05), but no significant interaction was observed for ADFI and FCR (P > 0.05). When broilers were challenged with LPS, dietary BDC supplementation significantly increased the ADG by 14.95% compared to those in the unsupplemented group (P < 0.05).
Table 3.
Effects of bisdemethoxycurcumin on growth performance in LPS-challenged broilers.1
| Items | Basal diet |
BDC diet |
SEM |
P-value |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Saline | LPS | Saline | LPS | BDC | LPS | Interaction | |||
| 1–15 d | ADG (g/bird/day) | 24.83 | 26.39 | 25.40 | 25.84 | 0.456 | 0.992 | 0.284 | 0.545 |
| ADFI (g/bird/day) | 33.73 | 36.13 | 34.62 | 35.80 | 0.684 | 0.843 | 0.200 | 0.658 | |
| FCR | 1.36 | 1.37 | 1.36 | 1.39 | 0.008 | 0.483 | 0.238 | 0.661 | |
| 16–20 d | ADG (g/bird/day) | 55.16a | 44.41c | 57.99a | 51.05b | 0.409 | <0.001 | <0.001 | 0.027 |
| ADFI (g/bird/day) | 78.83 | 70.24 | 82.08 | 75.63 | 2.098 | 0.312 | 0.084 | 0.799 | |
| FCR | 1.43 | 1.58 | 1.42 | 1.48 | 0.038 | 0.457 | 0.182 | 0.568 | |
a–cMeans with no common superscript within each row are significantly (P < 0.05) different.
Abbreviation: FCR, feed conversion rate.
BDC, basal diet fed with 150 mg/kg bisdemethoxycurcumin; LPS, lipopolysaccharide with a dose of 1 mg/kg of body weight.
Lipid, Protein, and DNA Oxidation
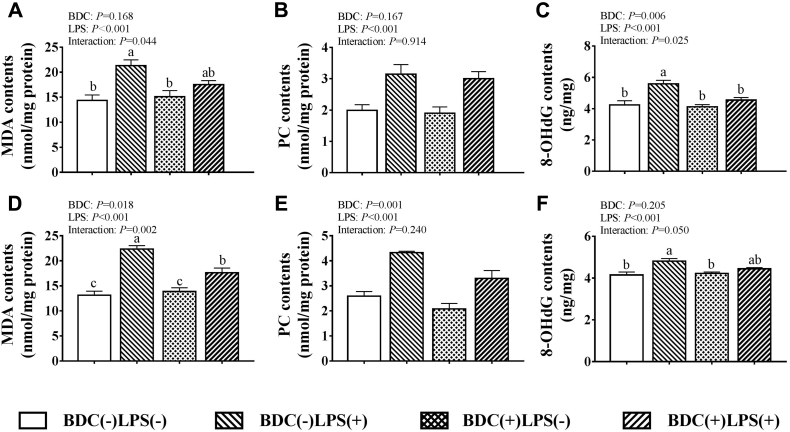
On day 20, the LPS-challenged groups had higher (P < 0.05) MDA, PC, and 8-OHdG contents in the jejunum and ileum than the saline-challenged groups (Figure 1). Dietary BDC supplementation significantly decreased jejunal 8-OHdG, ileal MDA, and PC contents as compared to those fed a basal diet (P < 0.05). The interaction between LPS challenge and dietary BDC supplementation significantly influenced the 8-OHdG contents in jejunum, and MDA contents in jejunum and ileum (P < 0.05). When broilers were challenged with LPS, dietary BDC supplementation significantly decreased the jejunal 8-OHdG contents by 18.60% compared with those in the unsupplemented group (P < 0.05). The ileal MDA content was 21.08% lower in broilers that received both BDC supplementation and LPS challenge than those that received the LPS challenge alone (P < 0.05).
Figure 1.
Effects of bisdemethoxycurcumin on the MDA, PC, and 8-OHdG contents of jejunum (A, B, C) and ileum (D, E, F) in LPS-challenged broilers. Values are means (n = 8), with their standard errors represented by vertical bars. Bars with unlike letters (a–c) are significantly different according to Tukey's multiple comparison test at P < 0.05. BDC, basal diet fed with 150 mg/kg bisdemethoxycurcumin; LPS, lipopolysaccharide with a dose of 1 mg/kg of body weight. Abbreviations: 8-OHdG, 8-hydroxy-2′-deoxyguanosine; MDA, malondialdehyde; PC, protein carbonyl.
GSH Redox Potential, GSH, and GSSG Contents
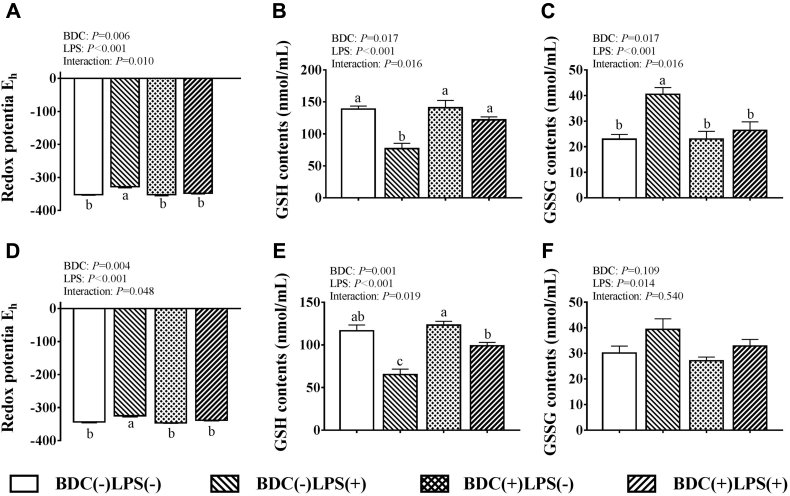
As shown in Figure 2, LPS challenge significantly decreased the GSH contents but increased the Eh and GSSG contents in jejunum and ileum compared with the saline-challenged groups (P < 0.05). Dietary BDC supplementation significantly decreased jejunal and ileal Eh, and jejunal GSSG contents while it increased jejunal and ileal GSH contents compared with the unsupplemented group (P < 0.05). There was a significant interaction between dietary BDC supplementation and LPS challenge on the Eh and GSH contents in jejunum and ileum, and GSSG contents in jejunum (P < 0.05). When broilers were challenged with LPS, dietary BDC supplementation significantly decreased jejunal and ileal Eh by 5.90 and 4.22% compared with those in the unsupplemented group (P < 0.05), respectively. The jejunal and ileal GSH contents were 58.89 and 51.89% higher in broilers that received both BDC supplementation and LPS challenge than those that received LPS challenge alone (P < 0.05), respectively. When broilers were challenged with LPS, dietary BDC supplementation significantly decreased jejunal GSSG content by 53.28% compared with those in the unsupplemented group (P < 0.05).
Figure 2.
Effects of bisdemethoxycurcumin on the glutathione redox potential, GSH, and GSSG contents of jejunum (A, B, C) and ileum (D, E, F) in LPS-challenged broilers. Values are means (n = 8), with their standard errors represented by vertical bars. Bars with unlike letters (a, b) are significantly different according to Tukey's multiple comparison test at P < 0.05. BDC, basal diet fed with 150 mg/kg bisdemethoxycurcumin; LPS, lipopolysaccharide with a dose of 1 mg/kg of body weight. Abbreviations: GSH, reduced glutathione; GSSG, oxidized glutathione.
T-SH Contents and Antioxidant Enzyme Activities
The small intestinal T-SH contents and antioxidant enzyme activities of broilers are shown in Table 4. Compared with the saline-challenged broilers, the LPS-challenged groups showed significantly decreased T-SH contents and GSH-Px, GR, and GST activities in both the jejunum and ileum, and SOD activities in the ileum (P < 0.05). Dietary BDC supplementation significantly increased jejunal and ileal SOD activities, jejunal GR activities, and ileal GST activities (P < 0.05) as compared to those fed the basal diet. Significant interaction between dietary BDC supplementation and LPS challenge was observed for jejunal and ileal GSH-Px activities, jejunal GST activities, and ileal SOD activities (P < 0.05). When broilers were challenged with LPS, dietary BDC supplementation significantly increased jejunal GST activity by 30.86% and ileal GSH-Px activity by 34.21% compared with those in the unsupplemented group (P < 0.05).
Table 4.
Effects of bisdemethoxycurcumin on the T-SH contents and antioxidant enzymes activities of jejunum and ileum in LPS-challenged broilers.1
| Item | Basal diet |
BDC diet |
SEM |
P-value |
||||
|---|---|---|---|---|---|---|---|---|
| Saline | LPS | Saline | LPS | BDC | LPS | Interaction | ||
| Jejunum | ||||||||
| T-SH (μmol/mg protein) | 9.85 | 6.64 | 9.51 | 8.61 | 0.483 | 0.404 | 0.042 | 0.242 |
| SOD (U/mg protein) | 162.37 | 100.62 | 177.67 | 173.75 | 8.891 | 0.019 | 0.075 | 0.115 |
| GSH-Px (U/mg protein) | 56.55a | 22.30b | 52.56a | 39.13a,b | 2.447 | 0.200 | <0.001 | 0.042 |
| GR (U/mg protein) | 17.65 | 14.34 | 18.05 | 17.06 | 0.310 | 0.018 | 0.002 | 0.071 |
| GST (U/mg protein) | 7.61a | 5.25b | 7.07a | 6.87a | 0.203 | 0.192 | 0.004 | 0.013 |
| Ileum | ||||||||
| T-SH (μmol/mgprot) | 12.42 | 9.77 | 12.26 | 10.05 | 0.399 | 0.936 | 0.005 | 0.780 |
| SOD (U/mg protein) | 178.64a | 156.61b | 179.86a | 167.71b | 1.184 | 0.015 | <0.001 | 0.046 |
| GSH-Px (U/mg protein) | 45.70a | 26.95c | 44.11a,b | 36.17b | 1.060 | 0.083 | <0.001 | 0.017 |
| GR (U/mg protein) | 15.81 | 13.59 | 16.36 | 13.61 | 0.562 | 0.802 | 0.036 | 0.815 |
| GST (U/mg protein) | 8.85 | 5.35 | 12.90 | 8.97 | 0.488 | 0.001 | 0.001 | 0.827 |
a–cMeans with no common superscript within each row are significantly (P < 0.05) different.
Abbreviations: GR, glutathione reductase; GSH-Px, glutathione peroxidase; GST, glutathione S-transferase; SOD, superoxide dismutase; T-SH, total thiol.
BDC, basal diet fed with 150 mg/kg bisdemethoxycurcumin; LPS, lipopolysaccharide with a dose of 1 mg/kg of body weight.
Expression of Antioxidant-Related Genes
As shown in Tables 5 and 6, the LPS challenge significantly downregulated the mRNA expression levels of CAT, CuZnSOD, γ-GCLc, γ-GCLm, GSH-Px, GR, Nrf2, HO-1, and NQO1 in jejunum and ileum (P < 0.05). Dietary BDC supplementation significantly upregulated the mRNA expression levels of jejunal and ileal CuZnSOD, γ-GCLc, Nrf2, HO-1, NQO1, and jejunal CAT and GR as compared to the unsupplemented groups (P < 0.05). Significant interactions between dietary BDC supplementation and LPS challenge were observed for the mRNA expression levels of jejunal and ileal γ-GCLc, Nrf2, HO-1, and NQO1, jejunal CAT, and ileal CuZnSOD (P < 0.05). The mRNA expression levels of jejunal CAT, γ-GCLc, Nrf2, HO-1, and NQO1 were 21.26, 35.26, 16.30, 28.07, and 31.84% higher in broilers that received both BDC supplementation and LPS challenge than those that received the LPS challenge alone (P < 0.05), respectively. When broilers were challenged with LPS, dietary BDC supplementation significantly increased the mRNA expression levels of ileal γ-GCLc, Nrf2, and CuZnSOD by 20.76, 13.49, and 16.71% compared with those in the unsupplemented group (P < 0.05), respectively.
Table 5.
Effects of bisdemethoxycurcumin on the expression of antioxidant genes of jejunum in LPS-challenged broilers.1
| Items2 | Basal diet |
BDC diet |
SEM |
P-value |
||||
|---|---|---|---|---|---|---|---|---|
| Saline | LPS | Saline | LPS | BDC | LPS | Interaction | ||
| CAT | 100.00b | 67.67d | 108.44a | 82.06c | 0.662 | <0.001 | <0.001 | 0.033 |
| CuZnSOD | 100.00 | 66.66 | 105.07 | 77.86 | 1.801 | 0.032 | <0.001 | 0.402 |
| γ-GCLc | 100.00a | 65.48c | 100.74a | 80.10b | 1.703 | 0.032 | <0.001 | 0.051 |
| γ-GCLm | 100.00 | 68.93 | 99.15 | 71.46 | 0.995 | 0.677 | <0.001 | 0.402 |
| GSH-Px | 100.00 | 66.42 | 98.16 | 70.02 | 1.304 | 0.738 | <0.001 | 0.306 |
| GR | 100.00 | 61.92 | 100.66 | 68.30 | 0.799 | 0.036 | <0.001 | 0.084 |
| Nrf2 | 100.00a | 65.076c | 101.83a | 75.69b | 0.841 | 0.001 | <0.001 | 0.014 |
| HO-1 | 100.00b | 66.36d | 110.05a | 84.99c | 1.044 | <0.001 | <0.001 | 0.049 |
| NQO1 | 100.00a | 58.32c | 101.67a | 76.89b | 1.041 | <0.001 | <0.001 | 0.000 |
a–dMeans with no common superscript within each row are significantly (P < 0.05) different.
BDC, basal diet fed with 150 mg/kg bisdemethoxycurcumin; LPS, lipopolysaccharide with a dose of 1 mg/kg of body weight.
Catalase (CAT), copper and zinc superoxide dismutase (CuZnSOD), catalytic subunit of γ-glutamylcysteine ligase (γ-GCLc), modified subunit of γ-glutamylcysteine ligase (γ-GCLm), glutathione peroxidase (GSH-Px), glutathione reductase (GR), nuclear factor erythroid-2-related factor 2 (Nrf2), heme oxygenase 1 (HO-1), and NAD(P)H quinone oxidoreductase 1 (NQO1).
Table 6.
Effects of bisdemethoxycurcumin on the expression of antioxidant genes of ileum in LPS-challenged broilers.1
| Items2 | Basal diet |
BDC diet |
SEM |
P-value |
||||
|---|---|---|---|---|---|---|---|---|
| Saline | LPS | Saline | LPS | BDC | LPS | Interaction | ||
| CAT | 100.00 | 73.27 | 110.11 | 93.63 | 6.082 | 0.221 | 0.087 | 0.677 |
| CuZnSOD | 100.00a | 73.01c | 104.05a | 85.30b | 0.937 | <0.001 | <0.001 | 0.036 |
| γ-GCLc | 100.00b | 74.94d | 109.30a | 90.50c | 0.660 | <0.001 | <0.001 | 0.025 |
| γ-GCLm | 100.00 | 77.05 | 98.63 | 80.36 | 0.715 | 0.502 | <0.001 | 0.112 |
| GSH-Px | 100.00a | 70.62b | 97.35a | 76.85b | 1.015 | 0.386 | <0.001 | 0.037 |
| GR | 100.00 | 70.16 | 99.76 | 69.04 | 0.758 | 0.659 | <0.001 | 0.775 |
| Nrf2 | 100.00b | 74.08d | 117.40a | 84.07c | 0.905 | <0.001 | <0.001 | 0.050 |
| HO-1 | 100.00 | 76.11 | 111.66 | 84.13 | 0.697 | <0.001 | <0.001 | 0.201 |
| NQO1 | 100.00 | 74.86 | 110.17 | 87.81 | 0.850 | <0.001 | <0.001 | 0.420 |
a–dMeans with no common superscript within each row are significantly (P < 0.05) different.
BDC, basal diet fed with 150 mg/kg bisdemethoxycurcumin; LPS, lipopolysaccharide with a dose of 1 mg/kg of body weight.
Catalase (CAT), copper and zinc superoxide dismutase (CuZnSOD), catalytic subunit of γ-glutamylcysteine ligase (γ-GCLc), modified subunit of γ-glutamylcysteine ligase (γ-GCLm), glutathione peroxidase (GSH-Px), glutathione reductase (GR), nuclear factor erythroid-2-related factor 2 (Nrf2), heme oxygenase 1 (HO-1), and NAD(P)H quinone oxidoreductase 1 (NQO1).
Discussion
LPS is the major cell wall component derived from Gram-negative bacteria, and routinely used to induce immune and oxidative stress models in rodents and animals (Hong et al., 2018). In the present study, LPS caused severe oxidative injury in the small intestine and decreased growth performance in broilers. Also, other beneficial effects were observed in the modulation of the redox potential, redox status, and antioxidant defense in the jejunum and ileum in LPS-challenged broilers, which were probably attributed to the known antioxidant property of BDC and supported its protective role for antioxidant defense activation against stress-induced oxidative damage.
After LPS challenge, dietary BDC supplementation significantly increased the ADG in broilers. To the best of our knowledge, little information is available about the effect of BDC on growth performance in LPS-challenged broilers. It has been reported that dietary curcumin supplementation (500 mg/kg) elicits no effect in broilers under normal healthy circumstances (Xie et al., 2019). Our previous study has reported that dietary BDC administration induces a numerical increase in the ADG of broilers during 1 to 21 d of age, without reaching a statistically significant level (Zhang et al., 2019). Since no LPS challenge was included in these studies, the potential benefits of BDC in stressed broilers cannot be ruled out. In the present study, we observed an increased ADG and unchanged ADFI and FCR following BDC treatment, which indicated a possible beneficial effect of BDC on growth performance in broilers.
Most studies of antioxidant effects have focused on determining the severity of lipid peroxidation, but there is strong evidence that 8-OHdG offers a specific biomarker of the oxidative injury in humans and animals (Patel et al., 2007). DNA bases are prone to be oxidatively modified, and 8-OHdG is one of the common products by ROS attack (Kasai, 1997). 8-OHdG accumulation has been linked to many cellular metabolic disorders including small intestinal injury, and therefore the maintenance of DNA integrity following oxidative damage is vital. In the present study, the 8-OHdG contents of jejunum were significantly decreased by dietary BDC supplementation in LPS-challenged broilers. Obaidi et al. (2018) investigated the potential of curcumin as an alternative chemopreventive agent to prevent oxidant stress and discovered that curcumin treatment reduced the 8-OHdG levels. Kim et al. (2019) reported that coadministration of curcumin (200 mg/kg) with benzo[a]pyrene significantly reduced the formation of 8-OHdG adducts in liver, kidney, and stomach tissues. Biswas et al. (2019) found that diabetic mice supplemented with curcumin and curcumin analogs exhibited decreased 8-OHdG levels. It was believed that the antioxidant property of curcumin against oxidant-induced DNA damage was attributed to its structure, in which the β-diketone and phenolic hydroxyl groups possessed radical trapping activities (Masuda et al., 2001). Thus, we speculated that BDC, which shares the same chemical structure of β-diketone and phenolic hydroxyl groups of curcumin, exerts antioxidant effects by modulating the biomacromolecule DNA implicated in the oxidation process.
Recently, the GSH redox potential has begun gaining increasing importance and has been characterized in monitoring the abnormal fluctuation as a redox-sensitive indicator (Wu et al., 2004). In animal cells, redox potential is typically more negative than the surrounding environment, which is essential for the maintenance of tissue redox homeostasis, metabolic functions, and cellular integrity (Dooley et al., 2004; Mallikarjun et al., 2012). Intracellular redox potential is highly regulated by several redox couples including GSH/GSSG, cysteine/cystine (Cys/CySS), and thioredoxin/thioredoxin disulfide (Trx/TrxSS) (Circu and Aw, 2011). Among these, GSH/GSSG is easily detectable and flexible in response to electrophilic substances, oxidative stress, and various pathological conditions. GSH is the most abundant low molecular weight thiol, and readily oxidized to GSSG (Mirzahosseini and Noszál, 2016). Therefore, we determined the GSH redox potential of jejunum and ileum through the GSH/GSSG redox couple in the present study. Our results suggested that broilers fed a BDC-supplemented diet had higher GSH contents in the jejunum and ileum than those fed a basal diet after LPS challenge. Xu et al. (2020) showed that BDC intervention (5 μg/kg/day) reduces oxidative stress in APP/PS1 mice by increasing GSH contents. The GSSG contents of jejunum were decreased in BDC-supplemented and LPS-challenged broilers, which was in agreement with the results of Zhang et al. (2019). Of note, there was a parallel drop of GSH redox potential with the changed GSH and GSSG contents in our study, suggesting a shift of redox balance toward the reduction of cellular microenvironment after LPS challenge. Bray (2000) and Salami et al. (2015) revealed that there is a significant correlation between dietary antioxidant administration and the improvement of GSH redox potential. Thus, dietary BDC treatment may be directly responsible for the improved redox status including the decreased GSH redox potential, GSSG contents, and the increased GSH contents. These overall further verified the antioxidant protection of BDC against LPS-induced oxidant injury and supported the role of BDC as a promising dietary antioxidant.
Inefficient removal of intracellular ROS is a debilitating feature of the antioxidant system in the small intestine. Pei et al. (2016) have found that BDC reduces cellular superoxide in a dose-dependent manner, confirming an obvious antioxidant property of BDC. Although BDC is proven to be a strong free radical quencher in vitro, it is not clear whether the decreased ROS levels after BDC treatment were attributed to the direct scavenging ability of ROS in vivo (Jayaprakasha et al., 2006). The antioxidant defense system that consists of enzymatic and nonenzymatic antioxidants plays a key role in the elimination of the extra ROS. Numerous studies have identified the inhibitory effect of curcumin on ROS levels by enhancing enzymatic antioxidant enzymes, such as SOD, GSH-Px, GST, and GR (Molina-Jijón et al., 2011; Sahin et al., 2012). However, there is little evidence in the literature for the potential effects of BDC on the enzymatic and nonenzymatic antioxidants. Our results showed that BDC significantly increased the SOD activities of jejunum and ileum, GR activities of jejunum, and GST activities of ileum, regardless of the LPS challenge. Consistent with our findings, Xu et al. (2020) demonstrated a similar increase of SOD activity after BDC treatment in mice. In the present study, BDC supplementation significantly increased the GST activities of jejunum and GSH-Px activities of ileum in LPS-challenged broilers. The induction of GST and GSH-Px activities by BDC was similar to the findings of the curcumin research, which demonstrated that increased GST and GSH-Px activities were crucial in cell antioxidant protection following dietary curcumin administration (Zhang et al., 2018; Uzunhisarcikli and Aslanturk, 2019). GST and GSH-Px belong to members of the GSH-related antioxidant enzymes and are involved in the detoxification of xenobiotics and peroxides by reducing GSH as substrate (Lapenna et al., 2018). The increased GSH-Px and GST activities following BDC treatment suggested that there was an intensive interaction between GSH and the electrophilic substance with the increasing depletion of intracellular GSH, leading to the alleviated oxidant damage. Meanwhile, significant elevation in jejunal and ileal GSH contents was observed by BDC administration in our study. Based on these results, we speculated that the increased GSH content induced by BDC was partly derived from the increased GSH-related metabolic enzyme activities. This positive feedback loop involved in the induction of GSH-related antioxidant enzymes was a possible candidate for the antioxidant mechanism of BDC in broilers.
Antioxidant protection of BDC is also manifested by the higher expression of antioxidant-related genes following LPS challenge. Nrf2 is a key nuclear transcription factor and serves as a master regulator of phase II detoxification and antioxidant genes (Tonelli et al., 2018). BDC, like curcumin, is a novel Nrf2 activator with a cytoprotective potential for oxidative damages in animals (Kim et al., 2010). In the present study, we identified the induction of BDC on these antioxidant genes of the small intestine combined with LPS challenge. Li et al. (2019b) reported that BDC attenuated the decline of nuclear Nrf2 level and HO-1 expression in the cardiomyocyte injury model. A possible mechanism may be medicated through the induction of Nrf2 and its downtown genes by BDC to suppress oxidative stress, just like curcumin. γ-GCL is a key rate-limiting enzyme in de novo synthesis cascade of GSH (Ferguson and Bridge, 2016). γ-GCL consists of a catalytic subunit γ-GCLc, and a modulatory subunit γ-GCLm. Our results showed that BDC significantly increased the mRNA expression levels of its catalytic subunit γ-GCLc in both the jejunum and ileum in LPS-challenged broilers. A study reported that the γ-GCLc subunit played a more important role in the regulation of γ-GCL activity since the γ-GCLc subunit still showed catalytic activity in the absence of γ-GCLm. Moreover, the activity of the γ-GCLc subunit was found to increase substantially by covalent interactions with γ-GCLm (Orr et al., 2005). Thus, BDC, acting as a potential regulator of GSH, not only increased the intracellular GSH contents but also improved the de novo synthesis of GSH. It was suggested that induction of BDC on GSH-related gene expression contributed to the antioxidant effect of BDC to some extent.
Conclusion
Our results demonstrate that BDC exerts antioxidant protection on LPS-induced small intestine injury and its potential mechanism may be associated with the reduced GSH redox potential, enhanced antioxidant system, and upregulated mRNA expression levels of Nrf2 and antioxidant-related genes. Moreover, the increase of growth performance might be attributed to the improved redox homeostasis by dietary BDC supplementation following LPS challenge. BDC can be a promising candidate for dietary antioxidant intervention in the poultry industry.
Acknowledgments
This research was financially supported by The National Experimental Teaching Demonstration Center of Animal Science.
Disclosures
The authors declare no competing financial interest.
References
- Ahsan H., Parveen N., Khan N.U., Hadi S.M. Pro-oxidant, anti-oxidant and cleavage activities on DNA of curcumin and its derivatives demethoxycurcumin and bisdemethoxycurcumin. Chem. Biol. Interact. 1999;121:161–175. doi: 10.1016/s0009-2797(99)00096-4. [DOI] [PubMed] [Google Scholar]
- Biswas S., Chen S., Liang G., Feng B., Cai L., Khan Z.A., Chakrabarti S. Curcumin analogs reduce stress and inflammation indices in experimental models of diabetes. Front. Endocrinol. (Lausanne) 2019;10:887. doi: 10.3389/fendo.2019.00887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray T.M. Dietary antioxidants and assessment of oxidative stress. Nutrition. 2000;16:578–581. doi: 10.1016/s0899-9007(00)00365-8. [DOI] [PubMed] [Google Scholar]
- Chen Y., Zhang H., Cheng Y., Li Y., Wen C., Zhou Y. Dietary l-threonine supplementation attenuates lipopolysaccharide-induced inflammatory responses and intestinal barrier damage of broiler chickens at an early age. Br. J. Nutr. 2018;119:1254–1262. doi: 10.1017/S0007114518000740. [DOI] [PubMed] [Google Scholar]
- Circu M.L., Aw T.Y. Redox biology of the intestine. Free Radic. Res. 2011;45:1245–1266. doi: 10.3109/10715762.2011.611509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley C.T., Dore T.M., Hanson G.T., Jackson W.C., Remington S.J., Tsien R.Y. Imaging dynamic redox changes in mammalian cells with green fluorescent protein indicators. J. Biol. Chem. 2004;279:22284–22293. doi: 10.1074/jbc.M312847200. [DOI] [PubMed] [Google Scholar]
- Ferguson G., Bridge W. Glutamate cysteine ligase and the age-related decline in cellular glutathione: the therapeutic potential of γ-glutamylcysteine. Arch. Biochem. Biophys. 2016;593:12–23. doi: 10.1016/j.abb.2016.01.017. [DOI] [PubMed] [Google Scholar]
- Garrett W.S., Gordon J.I., Glimcher L.H. Homeostasis and inflammation in the intestine. Cell. 2010;140:859–870. doi: 10.1016/j.cell.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel A., Kunnumakkara A.B., Aggarwal B.B. Curcumin as "curecumin": from kitchen to clinic. Biochem. Pharmacol. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Gonzalez D., Mustacich D.J., Traber M.G., Cherian G. Early feeding and dietary lipids affect broiler tissue fatty acids, vitamin E status, and cyclooxygenase-2 protein expression upon lipopolysaccharide challenge. Poult. Sci. 2011;90:2790–2800. doi: 10.3382/ps.2011-01452. [DOI] [PubMed] [Google Scholar]
- Hong G.L., Zheng D., Zhang L.L., Ni R., Wang G., Fan G.C., Lu Z.Q., Peng T.Q. Administration of nicotinamide riboside prevents oxidative stress and organ injury in sepsis. Free Radic. Biol. Med. 2018;123:125–137. doi: 10.1016/j.freeradbiomed.2018.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin F., Chen X., Yan H., Xu Z., Yang B., Luo P., He Q. Bisdemethoxycurcumin attenuates cisplatin-induced renal injury through anti-apoptosis, anti-oxidant and anti-inflammatory. Eur. J. Pharmacol. 2020;874:173026. doi: 10.1016/j.ejphar.2020.173026. [DOI] [PubMed] [Google Scholar]
- Jayaprakasha G.K., Lingamullu J.M.R., Sakariah K.K. Improved HPLC method for the determination of curcumin, demethoxycurcumin, and bisdemethoxycurcumin. J. Agric. Food Chem. 2002;50:3668–3672. doi: 10.1021/jf025506a. [DOI] [PubMed] [Google Scholar]
- Jayaprakasha G.K., Rao L.J., Sakariah K.K. Antioxidant activities of curcumin, demethoxycurcumin and bisdemethoxycurcumin. Food Chem. 2006;98:720–724. doi: 10.1021/jf025506a. [DOI] [PubMed] [Google Scholar]
- Kahl R., Kappus H. Toxicology of the synthetic antioxidants BHA and BHT in comparison with the natural antioxidant vitamin E. Z. Lebensm Unters Forsch. 1993;196:329–338. doi: 10.1007/BF01197931. [DOI] [PubMed] [Google Scholar]
- Kasai H. Analysis of a form of oxidative DNA damage, 8-hydroxy-2'-deoxyguanosine, as a marker of cellular oxidative stress during carcinogenesis. Mutat. Res. 1997;387:147–163. doi: 10.1016/s1383-5742(97)00035-5. [DOI] [PubMed] [Google Scholar]
- Kim A. Na, Jeon W.K., Lee J.J., Kim B.C. Up-regulation of heme oxygenase-1 expression through CaMKII-ERK1/2-Nrf2 signaling mediates the anti-inflammatory effect of bisdemethoxycurcumin in LPS-stimulated macrophages. Free Radic. Biol. Med. 2010;49:323–331. doi: 10.1016/j.freeradbiomed.2010.04.015. [DOI] [PubMed] [Google Scholar]
- Kim K.S., Kim N.Y., Son J.Y., Park J.H., Lee S.H., Kim H.R., Kim B., Kim Y.G., Jeong H.G., Lee B.M., Kim H.S. Curcumin ameliorates benzo[a]pyrene-induced DNA damages in stomach tissues of sprague-dawley rats. Int. J. Mol. Sci. 2019;20:5533–5546. doi: 10.3390/ijms20225533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen C.D., Reisman S.A. Nrf2 the rescue: effects of the antioxidative/electrophilic response on the liver. Toxicol. Appl. Pharm. 2010;244:57–65. doi: 10.1016/j.taap.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunnumakkara A.B., Bordoloi D., Padmavathi G., Monisha J., Roy N.K., Prasad S., Aggarwal B.B. Curcumin, the golden nutraceutical: multitargeting for multiple chronic diseases. Br. J. Pharmacol. 2017;174:1325–1348. doi: 10.1111/bph.13621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurutas E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr. J. 2016;15:71–93. doi: 10.1186/s12937-016-0186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapenna D., Ciofani G., Calafiore A.M., Cipollone F., Porreca E. Impaired glutathione-related antioxidant defenses in the arterial tissue of diabetic patients. Free Radic. Biol. Med. 2018;124:525–531. doi: 10.1016/j.freeradbiomed.2018.06.033. [DOI] [PubMed] [Google Scholar]
- Lauridsen C. From oxidative stress to inflammation: redox balance and immune system. Poult. Sci. 2019;98:4240–4246. doi: 10.3382/ps/pey407. [DOI] [PubMed] [Google Scholar]
- Li X., Huo C., Xiao Y., Xu R., Liu Y., Jia X., Wang X. Bisdemethoxycurcumin Protection of cardiomyocyte mainly depends on Nrf2/HO-1 activation mediated by the PI3K/AKT pathway. Chem. Res. Toxicol. 2019;32:1871–1879. doi: 10.1021/acs.chemrestox.9b00222. [DOI] [PubMed] [Google Scholar]
- Li C., Miao X., Li F., Adhikari B.K., Liu Y., Sun J., Zhang R., Cai L., Liu Q., Wang Y. Curcuminoids: implication for inflammation and oxidative stress in cardiovascular diseases. Phytother. Res. 2019;33:1302–1317. doi: 10.1002/ptr.6324. [DOI] [PubMed] [Google Scholar]
- Luo C., Du Z., Wei X., Chen G., Fu Z. Bisdemethoxycurcumin attenuates gastric adenocarcinoma growth by inducing mitochondrial dysfunction. Oncol. Lett. 2015;9:270–274. doi: 10.3892/ol.2014.2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Z.P., Dai H.J., Wei Q.W., Jin S., Wang J., Wei X.H., Yuan Y.W., Yu D.B., Shi F.X. Dietary genistein supplementation protects against lipopolysaccharide-induced intestinal injury through altering transcriptomic profile. Poult. Sci. 2020;99:3411–3427. doi: 10.1016/j.psj.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallikarjun V., Clarke D.J., Campbell C.J. Cellular redox potential and the biomolecular electrochemical series: a systems hypothesis. Free Radic. Biol. Med. 2012;53:280–288. doi: 10.1016/j.freeradbiomed.2012.04.034. [DOI] [PubMed] [Google Scholar]
- Masuda T., Maekawa T., Hidaka K., Bando H., Takeda Y., Yamaguchi H. Chemical studies on antioxidant mechanism of curcumin: analysis of oxidative coupling products from curcumin and linoleate. J. Agric. Food Chem. 2001;49:2539–2547. doi: 10.1021/jf001442x. [DOI] [PubMed] [Google Scholar]
- Mirzahosseini A., Noszál B. Species-specific standard redox potential of thiol-disulfide systems: a key parameter to develop agents against oxidative stress. Sci. Rep. 2016;6:37596–37606. doi: 10.1038/srep37596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra B., Jha R. Oxidative stress in the poultry gut: potential challenges and interventions. Front. Vet. Sci. 2019;6:60–64. doi: 10.3389/fvets.2019.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Jijón E., Tapia E., Zazueta C., Hafidi M.E., Zatarain-Barrón Z.L., Hernández-Pando R., Medina-Campos O.N., Zarco-Márquez G., Torres I., Pedraza-Chaverri J. Curcumin prevents Cr(VI)-induced renal oxidant damage by a mitochondrial pathway. Free Radic. Biol. Med. 2011;51:1543–1557. doi: 10.1016/j.freeradbiomed.2011.07.018. [DOI] [PubMed] [Google Scholar]
- Nkabyo Y.S., Gu L.H., Jones D.P., Ziegler T.R. Thiol/disulfide redox status is oxidized in plasma and small intestinal and colonic mucosa of rats with inadequate sulfur amino acid intake. J. Nutr. 2006;136:1242–1248. doi: 10.1093/jn/136.5.1242. [DOI] [PubMed] [Google Scholar]
- NRC . 9th ed. Natl. Acad. Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Obaidi I., Higgins M., Bahar B., Davis J.L., McMorrow T. Identification of the multifaceted chemopreventive activity of curcumin against the carcinogenic potential of the food additive, KBrO3. Curr. Pharm. Des. 2018;24:595–614. doi: 10.2174/1381612824666171226143201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr W.C., Radyuk S.N., Prabhudesai L., Toroser D., Benes J.J., Luchak J.M., Mockett R.J., Rebrin I., Hubbard J.G., Sohal R.S. Overexpression of glutamate-cysteine ligase extends life span in Drosophila melanogaster. J. Biol. Chem. 2005;280:37331–37338. doi: 10.1074/jbc.M508272200. [DOI] [PubMed] [Google Scholar]
- Patel P.R., Bevan R.J., Mistry N., Lunec J. Evidence of oligonucleotides containing 8-hydroxy-2'-deoxyguanosine in human urine. Free Radic. Biol. Med. 2007;42:552–558. doi: 10.1016/j.freeradbiomed.2006.11.025. [DOI] [PubMed] [Google Scholar]
- Pei H., Yang Y., Cui L., Yang J., Li X., Yang Y., Duan H. Bisdemethoxycurcumin inhibits ovarian cancer via reducing oxidative stress mediated MMPs expressions. Sci. Rep. 2016;6:28773–28780. doi: 10.1038/srep28773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin K., Orhan C., Tuzcu Z., Tuzcu M., Sahin N. Curcumin ameloriates heat stress via inhibition of oxidative stress and modulation of Nrf2/HO-1 pathway in quail. Food Chem. Toxicol. 2012;50:4035–4041. doi: 10.1016/j.fct.2012.08.029. [DOI] [PubMed] [Google Scholar]
- Saito M., Sakagami H., Fujisawa S. Cytotoxicity and apoptosis induction by butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT) Anticancer Res. 2003;23:4693–4701. [PubMed] [Google Scholar]
- Salami S.A., Majoka M.A., Saha S., Garber A., Gabarrou J.F. Efficacy of dietary antioxidants on broiler oxidative stress, performance and meat quality: science and market. Avian Biol. Res. 2015;8:65–78. [Google Scholar]
- Soleimani V., Sahebkar A., Hosseinzadeh H. Turmeric (Curcuma longa) and its major constituent (curcumin) as nontoxic and safe substances: Review. Phytother. Res. 2018;32:985–995. doi: 10.1002/ptr.6054. [DOI] [PubMed] [Google Scholar]
- Somparn P., Phisalaphong C., Nakornchai S., Unchern S., Morales N.P. Comparative antioxidant activities of curcumin and its demethoxy and hydrogenated derivatives. Biol. Pharm. Bull. 2007;30:74–78. doi: 10.1248/bpb.30.74. [DOI] [PubMed] [Google Scholar]
- Teymouri M., Barati N., Pirro M., Sahebkar A. Biological and pharmacological evaluation of dimethoxycurcumin: a metabolically stable curcumin analogue with a promising therapeutic potential. J. Cell Physiol. 2018;233:124–140. doi: 10.1002/jcp.25749. [DOI] [PubMed] [Google Scholar]
- Tonelli C., Chio I.I.C., Tuveson D.A. Transcriptional regulation by Nrf2. Antioxid. Redox Signal. 2018;29:1727–1745. doi: 10.1089/ars.2017.7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzunhisarcikli M., Aslanturk A. Hepatoprotective effects of curcumin and taurine against bisphenol A-induced liver injury in rats. Environ. Sci. Pollut. Res. Int. 2019;26:37242–37253. doi: 10.1007/s11356-019-06615-8. [DOI] [PubMed] [Google Scholar]
- Wu G., Fang Y.Z., Yang S., Lupton J.R., Turner N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004;134:489–492. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- Xie Z., Shen G., Wang Y., Wu C. Curcumin supplementation regulates lipid metabolism in broiler chickens. Poult. Sci. 2019;98:422–429. doi: 10.3382/ps/pey315. [DOI] [PubMed] [Google Scholar]
- Xu Y., Hu R., He D., Zhou G., Wu H., Xu C., He B., Wu L., Wang Y., Chang Y., Ma R., Xie M., Xiao Z. Bisdemethoxycurcumin inhibits oxidative stress and antagonizes alzheimer's disease by up-regulating SIRT1. Brain Behav. 2020;22:e01655. doi: 10.1002/brb3.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.F., Bai K.W., Su W.P., Wang A.A., Zhang L.L., Huang K.H., Wang T. Curcumin attenuates heat-stress-induced oxidant damage by simultaneous activation of GSH-related antioxidant enzymes and Nrf2-mediated phase II detoxifying enzyme systems in broiler chickens. Poult. Sci. 2018;97:1209–1219. doi: 10.3382/ps/pex408. [DOI] [PubMed] [Google Scholar]
- Zhang J.F., Han H.H., Shen M.M., Zhang L.L., Wang T. Comparative studies on the antioxidant profiles of curcumin and bisdemethoxycurcumin in erythrocytes and broiler chickens. Animals (Basel) 2019;9:953–966. doi: 10.3390/ani9110953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.F., Hu Z.P., Lu C.H., Yang M.X., Zhang L.L., Wang T. Dietary curcumin supplementation protects against heat-stress-impaired growth performance of broilers possibly through a mitochondrial pathway. J. Anim. Sci. 2015;93:1656–1665. doi: 10.2527/jas.2014-8244. [DOI] [PubMed] [Google Scholar]
- Zhang L., Wu C., Zhao S., Yuan D., Lian G., Wang X., Wang L., Yang J. Demethoxycurcumin, a natural derivative of curcumin attenuates LPS-induced pro-inflammatory responses through down-regulation of intracellular ROS-related MAPK/NF-kappaB signaling pathways in N9 microglia induced by lipopolysaccharide. Int. Immunopharmacol. 2010;10:331–338. doi: 10.1016/j.intimp.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Zheng X.C., Wu Q.J., Song Z.H., Zhang H., Zhang J.F., Zhang L.L., Zhang T.Y., Wang C., Wang T. Effects of oridonin on growth performance and oxidative stress in broilers challenged with lipopolysaccharide. Poult. Sci. 2016;95:2281–2289. doi: 10.3382/ps/pew161. [DOI] [PubMed] [Google Scholar]