Highlights
-
•
Abnormality in blood cholesterol level is significantly correlated with risk of different cancers.
-
•
Majority of tumor tissue from cancer patient exhibits overexpression of LDLR and ACAT for supporting rapid cancer cell proliferation.
-
•
Alteration of the cholesterol metabolism in cancer cells hampers therapeutic response.
-
•
Targeting cholesterol metabolism for treatment of cancer with other conventional chemotherapeutic drugs appears to be beneficial.
Keywords: LDLc, LDLR, Cancer, Cholesterol, Chemoresistance
Abbreviations: LDLc, Low Density Lipoprotein Cholesterol; HDLc, High Density Lipoprotein Cholesterol; VLDL, Very Low Density Lipoprotein; LDLR, Low Density Lipoprotein Receptor; ACAT, Acyl-CoA: cholesterol acyl transferase; HC, Hydroxy Cholesterol; HMG-CoA, 3-hydroxy-3-methylglutaryl-CoA
Abstract
Cholesterol is a fundamental molecule necessary for the maintenance of cell structure and is vital to various normal biological functions. It is a key factor in lifestyle-related diseases including obesity, diabetes, cardiovascular disease, and cancer. Owing to its altered serum chemistry status under pathological states, it is now being investigated to unravel the mechanism by which it triggers various health complications. Numerous clinical studies in cancer patients indicate an alteration in blood cholesterol level (either decreased or increased) in comparison to normal healthy individuals. This article elaborates on our understanding as to how cholesterol is being hijacked in the malignancy for the development, survival, stemness, progression, and metastasis of cancerous cells. Also, it provides a glimpse of how cholesterol derived entities, alters the signaling pathway towards their advantage. Moreover, deregulation of the cholesterol metabolism pathway has been often reported to hamper various treatment strategies in different cancer. In this context, attempts have been made to bring forth its relevance in being targeted, in pre-clinical and clinical studies for various treatment modalities. Thus, understanding the role of cholesterol and deciphering associated molecular mechanisms in cancer progression and therapy are of relevance towards improvement in the management of various cancers.
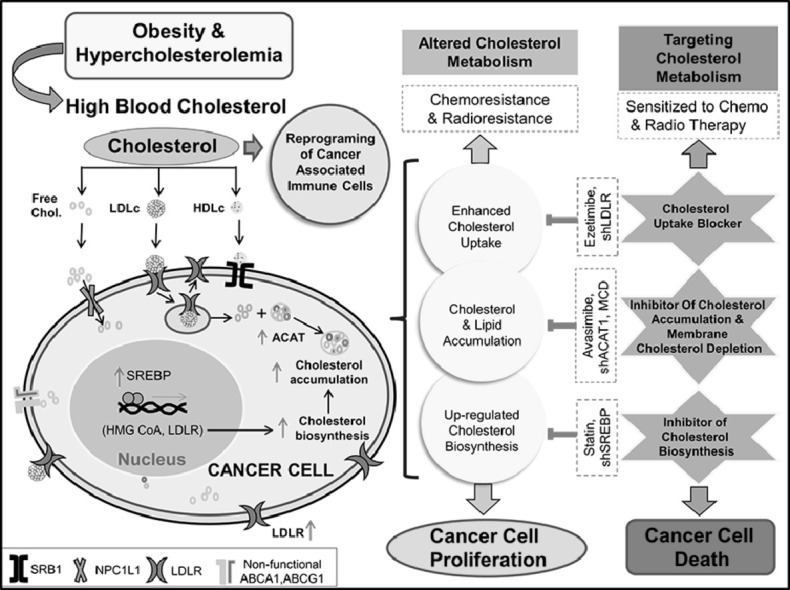
Graphical abstract
Introduction
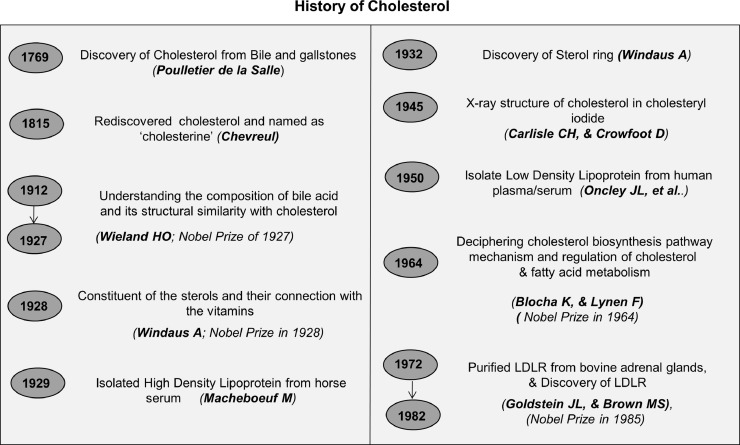
Cholesterol was first discovered by a French Doctor Poulletier de la Salle from bile and gallstones in 1769. It was later on rediscovered by Chevreul in 1815 and termed as ‘cholesterine’ [1]. To date 13 Nobel Prizes have been awarded for studies related to cholesterol [2]. In the early 1900s, Wieland H, & Windaus A, made immense contributions towards delineating the structural and functional role of cholesterol. Notably, Wieland was awarded the Nobel Prize (1927) for his work on deciphering the composition of bile acid and its structural similarity to cholesterol [3]. Windaus A, in 1928 was also awarded the Nobel Prize for his contribution towards understanding “the constitution of the sterols and their connection with the vitamins” [4]. He was the first person who proposed the empirical formula of cholesterol. Later, in 1945 Carlisle CH, and Crowfoot D, deciphered the crystal structure of cholesterol through X-ray analysis in cholesteryl iodide [5,6]. A schematic representation of historical discoveries in cholesterol biology is shown in Fig. 1.
Fig. 1.
Major breakthroughs of cholesterol-related discoveries in chronological order. Landmark discoveries in cholesterol biology [[1], [2], [3], [4], [5], [6], [7], [8], [9],255].
Cholesterol is an organic molecule consisting of steroid rings with a hydroxyl group, two methyl groups, and a hydrogen tail; altogether it is formed by 27 carbon atoms, 45 hydrogen atoms, and 1 hydroxyl group (C27H46O) having a molecular weight of 386.664 g/mol. Cholesterol is present in all animals as well as in plants with slight modification in their molecular structure [7]. Studies were carried out to identify various cholesterol carriers and Macheboeuf M, (1929) first isolated high density lipoprotein (HDL) from horse serum followed by the isolation of low density lipoprotein (LDL) from human plasma/serum by Oncley JL, et al., [8]. The discovery of low density lipoprotein receptor (LDLR) by Goldstein JL, and Brown MS, led to a paradigm shift in the understanding of cholesterol metabolism and they were awarded the Nobel Prize in 1985 for their discovery with regard to “the regulation of cholesterol metabolism” [9]. They demonstrated that LDL is recruited through receptor-mediated uptake followed by endocytosis and recycling of LDLR [10].
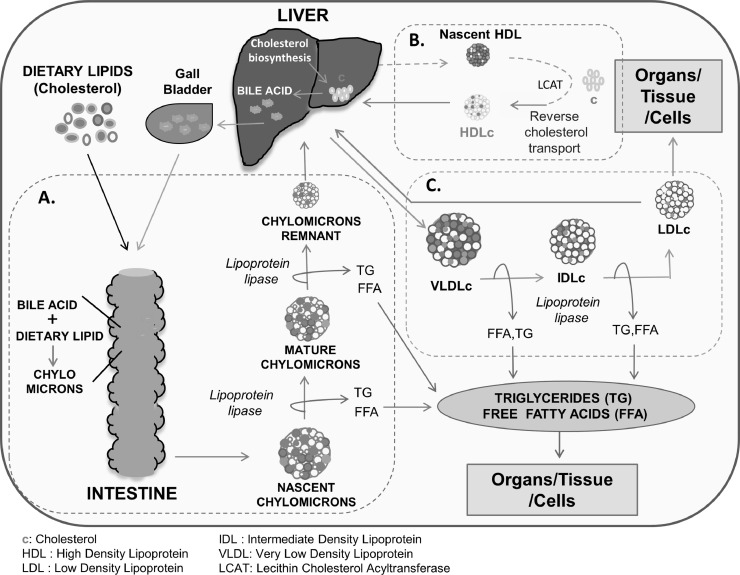
Cholesterol metabolism is tightly regulated in normal cells. Approximately 80% of total cholesterol in humans is normally biosynthesized in the body and 20% is secured from the diet [11]. It is either present in free form or as a cholesterol ester with fatty acids. Most of the dietary cholesterol is in the form of cholesterol ester and its transportation in the body is carried out in bound form with different Apo proteins forming an Apo-lipoprotein complex (cholesterol carrier/transporter) [12]. Depending on the size, density, lipid composition, and Apo protein binding partners, plasma lipoproteins (cholesterol bodies) are classified into various types; i.e., chylomicrons, chylomicron remnants, very low density lipoprotein (VLDL), intermediate low density lipoprotein (ILDL), low density lipoprotein (LDL), lipoprotein A (Lp (a)), high density lipoprotein (HDL) cholesterol. Cholesterol either synthesized or taken up from the diet is released into the circulation as chylomicrons and VLDL from the intestine and liver respectively. For transport of cholesterol to various organs/tissues/cells, chylomicrons and VLDL are further condensed to chylomicron remnant and LDL, which are more enriched with cholesterol. Among all the lipoproteins, LDL is the major cholesterol carrier whereas HDL performs the function of reverse cholesterol transport by delivering cholesterol to the liver from peripheral tissues [12]. A schematic representation of cholesterol transport in our body is given in Fig. 2.
Fig. 2.
Pictorial representation of cholesterol transport in the human body. (A) Transport of dietary lipid in the form of TG, FFA, and cholesterol from the intestine to various organs, tissues, and cells. Dietary lipids or cholesterol are converted into chylomicrons with the help of bile acid. Chylomicrons shed TG & FFA, and the remaining chylomicron remnant containing cholesterol reaches the liver. (B) Reverse cholesterol transport from organs, tissues, or cells and back to the liver. Reverse cholesterol transport is mediated by HDL to remove excess cholesterol from different organs/tissue, small “c” represents cholesterol (C) Transport of TG, FFA, and cholesterol from the liver to other organs, tissues, and cells. The liver releases VLDLc consisting of different lipid components i.e., TG, FFA, and cholesterol. VLDLc shed TG & FFA forming IDLc and LDLc, cholesterol from LDLc is either used up by organs/tissue/cells or being taken up by the liver.
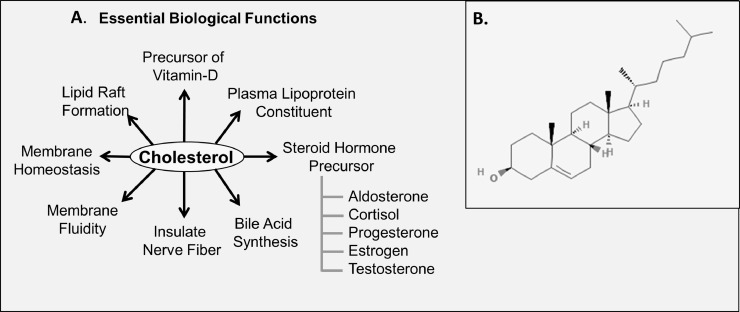
Functionally, the role of cholesterol is indispensable for the growth and development of animal cells. As a component of the mammalian cell membrane, it has an essential role in maintaining the structural integrity of animal cells [13]. It facilitates the stabilization of membrane fluidity and is involved in membrane trafficking processes important for the regulation of numerous trans-membrane signaling pathways [14]. Cholesterol is also involved in bile acid synthesis, serves as a precursor of vitamin D synthesis and steroid hormones (cortisol, cortisone, aldosterone, progesterone, estrogen, testosterone, etc.). Additionally, it is vital for sperm development, immune system defense as well as in the development and functioning of the central nervous system [7,15]. Indispensable biological functions of cholesterol are depicted in Fig. 3.
Fig. 3.
Structure of cholesterol and its indispensable biological functions. (A) Important biological functions of cholesterol. (B) Structure of cholesterol adopted from PubChem. Cholesterol is formed by 27 carbon atoms, 45 hydrogen atoms, and 1 hydroxyl group (C27H46O).
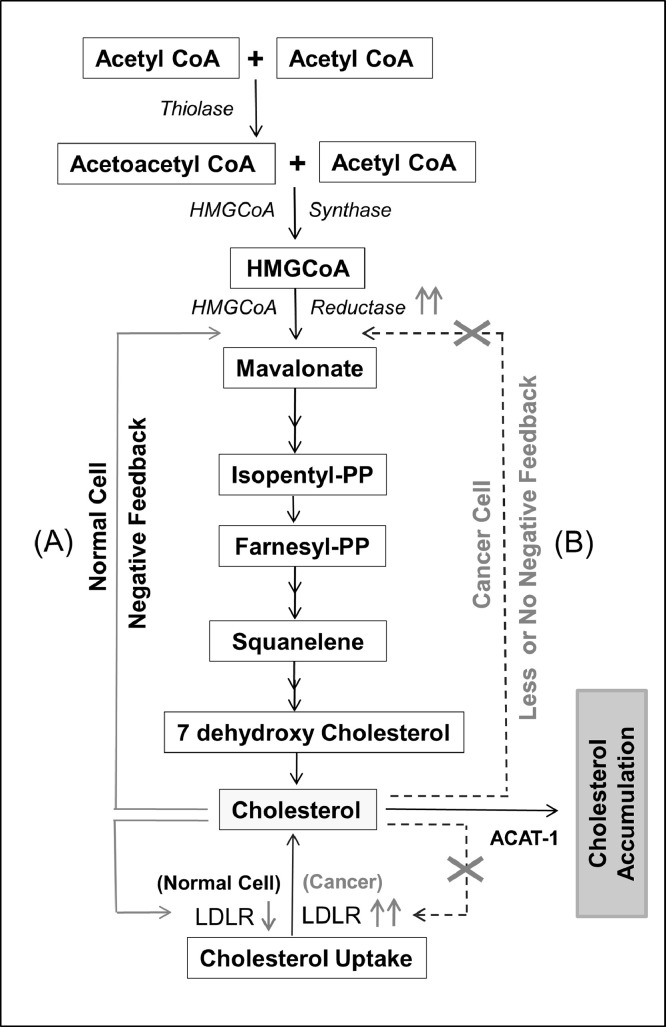
Evidence for the possible role of cholesterol in cancer cell proliferation dates backs to the early 1900s [16]. In 1901, a study by John Holden Webb suggested that the crystallization of cholesterine from living cells causes malignancy [17]. Later on, various pre-clinical and clinical studies report that alteration in blood cholesterol level is a critical phenomenon in many malignancies [18,19]. Recently, cholesterol has been linked to risk factors, occurrences, prognosis and therapeutic outcome of various cancers [20], [21], [22], [23]. Molecular studies reported accumulation of cholesterol or higher cholesterol content in tumor tissues of breast, thyroid, uterine, ovarian, and renal cancers as compared to normal tissues [24,25]. Cancer cells require cholesterol for rapid proliferation and tend to accumulate a high amount of cholesterol for which they either rely on up-regulated cholesterol biosynthesis or by enhancing uptake of cholesterol. Many of the cancer cells lack a feedback mechanism for controlling cholesterol uptake through LDLR expression as compared to normal cells/ tissue (Fig. 4) [26]. Majorities of tumor tissues exhibit a higher expression of LDLR for rapid uptake of cholesterol which is believed to be a more energy-saving process as compared to cholesterol biosynthesis. Overexpression of LDLR facilitates rapid uptake of LDL cholesterol (LDLc) and thus contributes to the accumulation of lipid components including cholesterol, free fatty acids, and triglycerides [26], [27], [28], [29]. Pre-clinical studies highlighted the importance of cholesterol in supporting the growth and proliferation of different cancer types by tuning numerous signaling pathways (Akt, ERK, etc.) [29], [30], [31]. Moreover, recent findings also highlighted the relevance of cholesterol derivatives such as oxysterols which are a byproduct of cholesterol metabolism i.e., the oxidized form of cholesterol. Notable oxysterol includes 25‑hydroxy cholesterol (25-HC), 27‑hydroxy cholesterol (27-HC), 22(R)‑hydroxy cholesterol (22-(R) HC), 6-oxo-cholestan-3b, 5a-diol (OCDO), dendrogenin A (DDA)) and these influence proliferation of cancer cells by altering various cellular functions [32,33].
Fig. 4.
Cholesterol biosynthesis pathway and its feedback mechanism. Cholesterol biosynthesis starts with the condensation of two acetyl CoA molecules into acetoacetyl CoA. (A) Cholesterol biosynthesis in normal cells is regulated through a negative feedback mechanism of cholesterol availability. (B) The cholesterol biosynthesis pathway in cancer cells is not/less regulated by cholesterol availability and stored as cholesterol ester with the help of enzymes like ACAT-1.
The significance of targeting cholesterol metabolic pathways in altering the growth of cancers has been recently emphasized in several studies. It has been demonstrated that targeting cholesterol biosynthesis and cholesterol uptake by a combination of specific drugs sensitized cancer cells to therapy [28,34]. Statin is one such FDA-approved drug that inhibits 3‑hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase which is involved in cholesterol biosynthesis. Other than its cholesterol-lowering effect, it has been reported that statin also has pleiotropic effects on cancer cell proliferation. In vitro and in vivo studies suggest that statin alone or in combination with other drugs inhibits the proliferation and survival of various cancer cells [35,36]. Targeting cholesterol uptake through downregulation/ inhibition of LDLR also increases the efficacy of chemotherapeutic drugs [28].
Cholesterol metabolism in normal and cancer cells
Cholesterol metabolism is a complex process, starting from biosynthesis, efflux, uptake, and, its utilization by the cells. A maximum amount of cholesterol is synthesized in the liver and intestine; however, cancer cells can synthesize cholesterol and also have the ability for rapid uptake of cholesterol to support growth [37]. Before the onset of the cholesterol biosynthesis pathway, citrate is converted to oxaloacetic acid and acetyl-CoA by ATP citrate lyase (ACLY) enzyme, an important step commonly considered as a link between glucose metabolism and lipid biogenesis. Interestingly, majorities of the cancers (glioblastoma, colorectal cancer, breast cancer, non-small cell lung cancer, hepatocellular carcinoma, etc.) exhibit up-regulated ACLY expression thereby supporting cancer cell proliferation [38]. Cholesterol biosynthesis is a multi-step process involving more than 20 enzymes that occurs in the cytosol, mitochondria, endoplasmic reticulum (ER), and peroxisome [39]. The first step of cholesterol biosynthesis is the condensation of two acetyl CoA molecules into acetoacetyl CoA by thiolase enzyme followed by the formation of HMG-CoA which is catalyzed by HMG-CoA synthase (Fig. 4) [39]. HMG-CoA is then converted to mevalonate with the help of HMG-CoA reductase which is the rate-limiting enzyme in the cholesterol biosynthesis pathway, whose regulation is dependent on cholesterol availability. Before the formation of the final product (cholesterol), various intermediate compounds such as isopentenyl pyrophosphate (IPP), farnesyl pyrophosphate (FPP), squalene, 7 dehydroxy cholesterol, etc. are formed [39]. Synthesized cholesterol is secreted to the bloodstream as apolipoproteins complex and/or inserted as part of plasma membrane complex or used for bile acid production in the liver as well as steroids synthesis in adrenal gonads [40].
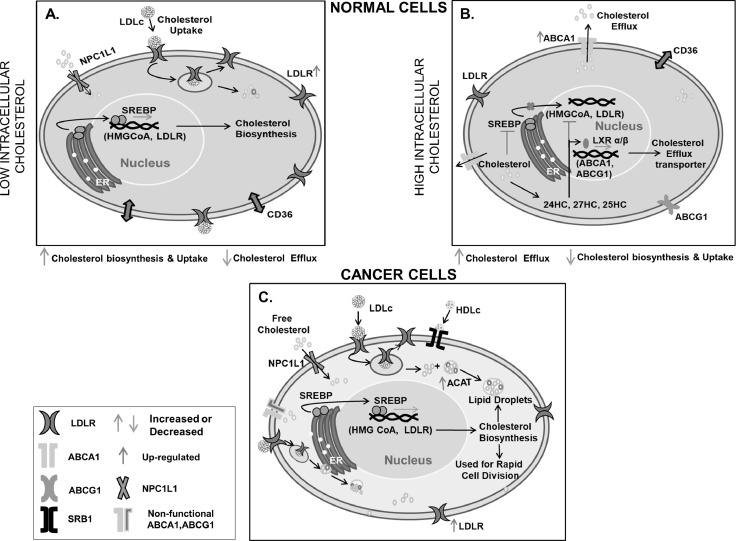
Cholesterol metabolism is regulated in normal cells, for example in the liver cholesterol biosynthesis decreases when blood cholesterol level increases due to a high cholesterol diet and vice versa. When the level of intracellular cholesterol or cholesterol derivative is high; cholesterol biosynthesis is checked through a feedback mechanism by inhibiting HMG-CoA reductase, and downregulation of LDLR through inactivation of Sterol regulatory element-binding protein 2 (SREBP 2) pathway [41]. It activates liver X receptor-α/β (LXRα/β) signaling which in turn helps in the expression of transporters like ATP-binding cassette subfamily A member 1 (ABCA1) for efflux of excess cholesterol [42]. Similarly, when the intracellular cholesterol is low its biosynthesis and uptake are enhanced. However, in cancer cells, this feedback mechanism is deregulated and LDLR, as well as cholesterol biosynthesis, is mostly up-regulated irrespective of cholesterol availability [26,43]. Also, to compensate for enhanced cholesterol uptake or biosynthesis, various cancer cells readjust their cellular mechanism by increasing the expression of acyl-CoA: cholesterol acyltransferase 1 (ACAT1) enzyme. This enzyme catalyzes the conversion of cholesterol to cholesterol ester, which is a more stable form of cholesterol and subsequently stored inside the cancer cell [44]. Stored cholesterol esters may function as a reservoir for rapid cell division. A pictorial representation of the difference in cholesterol metabolism of normal and cancer cells is documented in Fig. 5.
Fig. 5.
Differential response of cancer and normal cells in cholesterol metabolism on cholesterol availability. The presence of cholesterol regulates its metabolism in normal cells but not/less in cancer cells. (A) Low intracellular cholesterol in the normal cell triggers SREBP mediated cholesterol biosynthesis and up-regulates expression of LDLR for cholesterol uptake. (B) High intracellular cholesterol in normal cells blocks SREBP mediated cholesterol biosynthesis and up-regulates LXRα/β mediated cholesterol efflux transporter expression. (C) In cancer cells, the presence of intracellular cholesterol does not affect cholesterol biosynthesis and uptake but increases cholesterol accumulation through overexpression or increased activity of ACAT.
Role of cholesterol in various cancer
As evident from numerous studies including pre-clinical, clinical, meta-analysis, and population-based studies, cholesterol plays a vital role in cancer risk, progression, survival, prognosis, and therapy [[21], [22], [23],37,45]. Alteration in blood cholesterol level is a very common phenomenon in majorities of cancers [22,37,45]; however, it is still not clear for most of the cancers whether decreased blood cholesterol level in the cancer patient is a cause or consequence of the disease.
Deregulated cholesterol metabolism leading to up-regulation of cholesterol uptake, biosynthesis, and accumulation is very prominent to support cancer cell proliferation, invasion, metastasis, and chemotherapeutic drug resistance [22,23,41]. Besides, the role of dietary cholesterol as a risk factor for different cancers, it is also not the same for all. Various studies show an increase in the risk of breast, gastric, ovarian, pancreatic cancer, etc. with increased dietary cholesterol [20]. A brief description of the varied role of cholesterol in different cancer is discussed below.
Colorectal cancer
As reported by the world health organization (WHO) 2018, colorectal cancer is the 2nd deadliest cancer next to lung cancer [46]. Pre-clinical and clinical studies have highlighted the role of cholesterol in colorectal cancer progression [27,29]. Earlier study suggests that, high blood cholesterol level (near to diagnosis of cancer) may reduce the risk of colon cancer independently [47]. However, other groups have reported that increased blood cholesterol level does not have any protective effect on the occurrence and progression of colon cancer instead; it increases the risk of colon cancer [48,49,50]. The decrease in blood cholesterol level in colon cancer patients may be a consequence of increased uptake of blood cholesterol by colon cancer cells and as such it is less likely to be a cause for colon cancer initiation. Various clinical case studies have shown that blood cholesterol level decreases significantly in colorectal cancer patients and exhibits a negative correlation with a higher grade of tumors/polyps when compared to healthy subjects [51,52,53]. Studies also demonstrate that the reduction in serum cholesterol significantly correlates with an increase in the level of cancer-specific antigen, and it is therefore proposed to be a novel biomarker for colon cancer detection [53]. Another clinical study reported that the blood cholesterol level drops even before the onset or diagnosis of adenocarcinoma in the colon [47,48]. Intriguingly colon tumor tissue expresses a higher level of LDLR as compared to normal colon tissue. Increased expression of LDLR might play an important role in enhanced cholesterol uptake from the blood by colon cancer cells to support its rapid division [27,29] which may in part contribute to a decrease in blood cholesterol level in colon cancer patients. Furthermore, it has been reported that in cases followed for 12 months after curative surgery, the blood cholesterol level gradually increases in colon cancer patients [27]. The risk of colon cancer is more with altered blood cholesterol levels, though various studies suggest a difference in the risk of colon cancer in male and female populations [54]. It has been reported that total cholesterol and LDLc is inversely associated with a higher risk of colon cancer mortality in male as compared to female [54]. Another report from Törnberg SA, et al., showed a positive correlation of cholesterol and beta-lipoprotein level with increased risk of rectal and colon cancer in the male population whereas non-significant correlations were detected in the female population [55].
Pre-clinical studies reported that LDLc increases the proliferation of colon cancer cells through the MAPK signaling pathway. It also helps in intestinal inflammation by generating reactive oxygen species (ROS). Furthermore, LDLc supports migration, sphere formation, and stemness of colon cancer cells in vitro conditions [29]. Likewise, Bo W, et al., reported that cholesterol can directly act as a mitogen for intestinal stem cells (ISC). Interestingly, supplementation of cholesterol to mouse intestinal crypt isolate increases cellular cholesterol content thereby supporting crypt organoid proliferation/formation [56,57]. These lines of studies highlight a possible role of cholesterol in the stemness of colon cancer cells thereby supporting cancer progression.
Despite several clinical and epidemiological studies indicating a relationship between cholesterol and colon cancer, a clear understanding of the mechanism or molecular events linking these two entities is still lacking. More insight into the relationship between cholesterol and colon cancer, as well as probing into the molecular events leading to an alteration in growth signaling is likely to contribute towards better management of colon cancer.
Breast cancer
Breast cancer is the most commonly occurring cancer in females. A recent report suggests that the increase in body mass index (BMI) enhances the risk of breast cancer [58]. Since obese people generally have a high level of cholesterol, it is important to investigate the relationship between cholesterol and breast cancer. Several epidemiological studies suggest a significant association of dietary cholesterol intake with increased risk of breast cancer [20,59]. Numerous clinical studies have been carried out for deciphering the correlation between serum cholesterol level and breast cancer; however, the results of these studies are inconsistent. Earlier, it has been reported that blood cholesterol level increases in breast cancer patients irrespective of their BMI when compared to their normal counterparts [60,61]. These suggest that breast cancer per se may be causing an increase in blood cholesterol level. However, another study performed by Sharma V, et al., reported decreased blood cholesterol levels in breast cancer patients (193.07 mg/dl (+/- 36.39)) with respect to healthy controls (216.32 mg/dl (+/- 30.51)) [62].
In a large-scale population study done in Korea, it has been shown that high blood cholesterol (≥ 240 mg/dL) increases the risk of breast cancer (HR, 1.17; 95% CI, 1.03 to 1.33; P trend =0.03) [45]. Also, a positive correlation has been reported between HDLc and increased risk of breast cancer [63,64] along with the increase in percent mammographic density [65]. Clinical study indicates a positive correlation of blood cholesterol level (LDLc) and breast cancer progression i.e., patient with higher blood cholesterol level exhibits greater proliferative capacity with a higher grade of differentiation which is often Her2-neu (ErbB2) positive subtype. Increased blood cholesterol (LDLc) level at the time of diagnosis was associated with poor disease-free survival [66]. In addition, breast cancer patients with increased expression of mevalonate pathway genes in mutant p53 breast tumors were correlated with the worst prognosis and survival [67]. Clinicopathological studies have shown that with the increase in proliferation marker (Ki67), LDLR, scavenger receptor class B member 1 (SCARB1), and ACAT1, accumulation of cholesterol ester increases with the advancement of tumor grade [68].
In postmenopausal women, HDLc exhibits a protective effect and decreases the risk of breast cancer as compared to that in premenopausal women [69]. Each 1-year delay in menopause increases the risk of breast cancer by 3%. [70]. This suggests a probable role of hormonal status linking the functionality of cholesterol in breast cancer. Besides, there are numerous reports which suggest no association [71,72] or negative association of blood cholesterol with breast cancer risk [73].
There are also reports which show the role of mutant p53 in sterol biosynthesis. Mutant p53 derived from the tumor is being recruited at the promoter site of different genes involved in sterol biosynthesis thereby increasing their expression in breast cancer [67]. Additionally, in the xenograft model of breast cancer, diet-induced hypercholesterolemia increases tumor size and angiogenesis [74]. By in vitro experiments, it has been demonstrated that LDLc, VLDLc, or overexpression of LDLR enhances proliferation of breast cancer cells by elevating the expression of pAKT and pERK or through activation of beta-catenin signaling pathway [31,75]. Furthermore, LDLR knockdown in breast cancer cells decreases tumor growth in hypercholesterolemic LDLR-/- and ApoE-/- mice model [76]. Additionally, exogenous lipids promote metastasis, loss of adhesion, and migration of breast cancer cells through Akt-induced EMT and angiogenesis [31,77,78]. It has been previously reported that cholesterol metabolite i.e., 27-HC acts as a selective estrogen receptor modulator (SERM) and helps in breast cancer progression [32]. The enzyme (CYP27A1) responsible for the formation of 27-HC from cholesterol is up-regulated in breast tumor tissue [79]. 27-HC downregulates p53 expression in ER+ breast cancer cells thereby abrogating p53 mediated signaling and contributing to the proliferation of breast cancer cells [32]. Another study also reported that supplementation of 25-HC promotes breast cancer cell proliferation through activation of ERα along with up-regulation of estrogen target genes [80]), On the contrary, Wolfe AR, et al., reported that HDLc decreases mammosphere formation by inhibiting phosphorylation of EGFR, AKT, and FOXO3a signaling molecules [81]. In most of the studies, the role of ACAT, LDLR, and associated signaling intermediates like ERK or AKT has been highlighted, which cannot be overlooked, and the importance of hormonal status in breast cancer and cholesterol interrelationship cannot be neglected.
In summary, the blood cholesterol level and its derivatives seem to have a positive correlation with the risk of breast cancer occurrence, its progression and worsening of patient survival. However, it is not apparent to us as to why there is a differential response in breast cancer risk, proliferation, and progression in various studies with different types of cholesterol /intermediates, and therefore may warrant further exploration.
Prostate cancer
The relation between serum cholesterol and prostate cancer is complex. Few clinical studies indicate a link between high blood cholesterol towards increased risk of high-grade prostate cancer and the status of prostate specific antigen (PSA) [45,82]. The majority of epidemiological studies show weak evidence or no relation between blood cholesterol level and the risk of prostate cancer [83,84,85]. Even though the reports on the correlation of cholesterol with prostate cancer are conflicting, various studies suggest that non-identical races (black and white people) show a differential correlation between serum cholesterol and PSA levels. Increased blood cholesterol is highly correlated with increased PSA levels in the white population [86]. A population-based study from Stopsack KH, et al., demonstrate that lethal prostate cancer relies more on de-novo cholesterol biosynthesis instead of extracellular cholesterol uptake [87], whereas Singh G, et al., have shown up-regulated expression of LDLR in patient's tumor tissue samples. The increase in intracellular cholesterol inside the nucleus along with cyclin E overexpression in prostate tumor tissue indicates a potential role of cholesterol in regulating the cell cycle [88]. Also, metastasis of prostate cancer in bone causes an increase in cholesterol content as compared to the normal bone and these phenomena can be attributed to de novo cholesterol biosynthesis, supported by the influx of cholesterol through LDLR and SRB1 [89].
Studies in the xenograft model indicate the role of cholesterol in promoting tumor growth. Mice fed on a hypercholesterolemic diet with increased blood cholesterol level shows enhanced tumor progression along with induction of angiogenesis [90,91]. Moreover, in vitro conditions, upon treatment of prostate cancer cells with LDLc or cholesterol increased proliferation, migration, and invasion were observed [92,93,94]. Interestingly, prostate cancer cells lack a feedback mechanism for LDLR expression, even in the presence of excess LDLc, suggestive of the involvement of both pathways. In normal prostate cells, the expression of LDLR is tightly regulated and it decreases with an increase in LDLc availability [26]. Increased expression of LDLR is advantageous to prostate cancer cells as it facilitates enhanced uptake of fatty acids and cholesterol, which supports prostaglandin synthesis in cells [26]. Other than LDLR, Scavenger receptor class B type 1 (SRB1, HDLc receptor) was also found to increase in some prostate cancer patient samples [95]. Apart from these, androgen is required for normal epithelial prostate cell proliferation and differentiation. However, in advanced prostate cancer, cells can synthesize androgen by themselves in the presence of cholesterol, thereby making them self-sufficient for rapid cell division, independent of testicular and/or adrenal androgens [91,96].
Above mentioned studies illustrate both positive and negative correlation of cholesterol with prostate cancer. Clinical and preclinical studies highlight the role of cholesterol metabolism and LDLR in enhancing prostate cancer cell proliferation. The differential findings in clinical studies might be contributed in part by variability in the race and hormonal status, which needs further exploration.
Hepatocellular cancer
Hepatocellular carcinoma (HCC) is a malignancy of the liver, which is a primary site of cholesterol biosynthesis and where the majority of lipoproteins are synthesized [97]. A large-scale population-based study in Korea reported that with increase in blood cholesterol level incidence of liver cancer decreases (men: HR, 0.42; 95% CI, 0.38 to 0.45; P trend < 0.001; women: HR, 0.32; 95% CI, 0.27 to 0.39; P trend < 0.001) [45]. Also, numerous studies have reported a positive association of HCC with hypercholesteremia, which is likely to be a consequence of HCC. Alteration in the blood lipid profile of HCC patients can be attributed to an abnormality in lipid metabolism and biosynthesis being influenced by the tumor [98,99,100,101,102]. Elevated total blood cholesterol was also reported in hepatoblastoma of children and those HCC patients with a cirrhotic liver, but not in those patients without HCC [102,103,104]. Increased serum blood cholesterol levels in HCC patients can be attributed to abrogation of cholesterol feedback mechanism or enhanced cholesterol biosynthesis by HCC cells in the liver [98,105]. Even though the majority of the reports show a link between increased blood cholesterol and HCC, interestingly in Hepatitis-B and Hepatitis-C associated HCC, a decrease in serum lipid level including cholesterol, LDLc, HDLc, and triglycerides in comparison to normal counterparts were reported [106,107]. Few reports also suggest a correlation between low LDLc in serum with an elevated risk of liver cancer mortality [108]. Additionally, a decrease in pre-operative or pre-transplant (liver) serum cholesterol level (<100 mg/dl) was associated with decreased HCC reoccurrence free survival in comparison to higher pre-operative serum cholesterol level (>100 mg/dL) [109]. Similarly, another cohort study in Taiwan has reported an association of poorer prognosis of HCC patients with low BMI and low serum cholesterol as compared to high BMI and high serum cholesterol patients after curative surgery [110]. Experimentally, it has been demonstrated that rats bearing hepatoma exhibit alteration in cholesterol feedback mechanism [105]. In high cholesterol diet (HCD) or genetic knockout model (ApoE) of hypercholesterolemia, a decrease in tumor growth or development of fewer tumors in the chemically induced model when compared to their normal counterparts has been reported, which is in support of earlier clinical studies from Kitahara CM, et al., group [111].
Most of the clinical and pre-clinical studies show a strong correlation between alteration of blood cholesterol level and HCC. Though few studies suggest a decrease in HCC incidence and progression with the increase in blood cholesterol, it is unlikely that high blood cholesterol inhibits HCC initiation and progression since the consequence of HCC itself can lead to high blood cholesterol. It will be relevant to study and validate the role of high blood cholesterol level due to other factors including high fat diet or metabolic disorders at the clinical and preclinical level before HCC occurrence.
Pancreatic adenocarcinoma
The five-year survival rate of the pancreatic cancer patient is 8.5% and therefore it is one of the most fatal cancers. Recently, a population-based study reported that a higher dietary intake of cholesterol (100 mg/day) increases the risk of pancreatic cancer by 8% [20,112]. Although in another meta-analysis study it has been reported that a decrease in total serum cholesterol is related to a higher risk of pancreatic cancer occurrence [113]. However, the mechanistic exploration of the relationship between pancreatic cancer and cholesterol is yet to be studied. Increased ACAT1 (level) has also been linked to enhanced cholesterol ester accumulation in the tumor tissue samples and poor survival rate in patients. Interestingly, abrogation of ACAT-activity causes enhanced endoplasmic stress (ER stress), reduced cholesterol esters accumulation and induction of apoptosis, leading to a decrease in tumor progression in mice [114]. Furthermore, a preclinical study in mice bearing spontaneous pancreatic adenocarcinoma (PDAC) (Pdx1-Cre; Ink4a/Arffl/fl; LSL-KrasG12D) demonstrated that cholesterol uptake is increased in the tumor and is associated with increased LDLR expression. Silencing LDLR reduces cholesterol uptake and decreased cell proliferation by abrogating the ERK1/2 pathway [28].
Available literature suggests an important role of ACAT and LDLR in pancreatic cancer progression. Overall, patient sample data and mice model studies indicate a possible link of cholesterol signaling with the progression and prognosis of pancreatic cancer. Though the mechanism is still not clear, it is likely that the bioavailability of cholesterol and its accumulation affects the survival and progression of cancer cells.
Lung cancer
According to the International Agency for Research on Cancer 2018, lung cancer is the most common and one of the deadliest among all cancer types [115]. A recent study from Lyu ZY, et al., group has reported that abnormally higher or lower blood cholesterol level enhances the risk of lung cancer [116]. Though, the decrease in risk of lung cancer has been linked to higher blood cholesterol level whereas lower blood cholesterol level has been associated with increased risk [63,117,118,119,120]. Furthermore, lung cancer patients with lower blood cholesterol levels show worse survival [119,121,122]. Interestingly, compared to normal individuals in lung cancer patients’ lower level of total blood cholesterol, HDLc or LDLc has been reported [123,124,125]. Various reports suggest that dietary cholesterol does not have any considerable role in lung cancer risk [126,127,128,129] though Shekelle RB, et al., have reported that an increase in dietary cholesterol by 500 mg/day enhances the relative risk of lung cancer by 1.9% [130]. In in vitro studies Chen L, et al., has reported an increase in migration and invasion of lung adenocarcinoma cells in the presence of 25-HC [131].
With this available literature, it is difficult to draw a conclusion about the concrete correlation between cholesterol and lung cancer. The contradictory findings from clinical studies and non-linear relationships by in vitro or preclinical studies warrant more planned studies in this area.
Gastric or stomach cancer
Gastric cancer which originates from the inner lining of the stomach is the third deadliest and the fifth most commonly occurring cancer world-wide [46]. Important risk factors include age, gender, ethnicity, bacterial infection (Helicobacter pylori), smoking, alcohol consumption diet, obesity, etc. [132]. Previous studies have shown a positive correlation of dietary cholesterol with the risk of gastric cancer [132,133,134,135,136], whereas report from Lucenteforte E, et al., suggests no correlation [137]. Interestingly, infection of Helicobacter pylori, an auxotrophic bacterium, for cholesterol increases the risk of gastric cancer occurrence. It is reported that Helicobacter pylori obtain cholesterol from the gastric epithelia and convert it to cholesteryl 6′-O-acyl-α-d-glucopyranoside (CAG) for lipid raft clustering in gastric epithelial [138]. Also, the increase in dietary cholesterol consumption has been shown to decrease the success rate of Helicobacter pylori eradication [139], therefore it is likely that dietary cholesterol supports Helicobacter pylori infection and growth. Although several studies indicate a positive association between dietary cholesterol and gastric cancer, the relation between serum cholesterol and the risk of gastric cancer is still unclear. The majority of the clinical studies have reported that the level of blood cholesterol decreases in gastric cancer patients both at the time of diagnosis or at terminal/ advanced stages [140,141,142]. Moreover, the risk of gastric cancer also increases with a decrease in blood cholesterol level showing an inverse association [143,144,145,146]. Shin HJ, et al., reported a link between lower serum cholesterol with a greater incidence of complications (severe complication rate: 15.2% (Low Total Cholesterol) vs. 4.7% (Normal Total Cholesterol) vs 5.5% (High Total Cholesterol); p = 0.004) along with poorer overall survival and relapse-free survival [147]. An increase in the lymphatic and vascular invasion was also reported along with a worse prognosis in the gastric cancer patients with low HDL cholesterol levels as compared to normal HDL cholesterol levels who have undergone gastrectomy [148].
There are also reports which show a positive association of high blood cholesterol with an increased risk of gastric cancer. The risk of gastric dysplasia (pre-cancerous lesion which is often considered as a penultimate stage of gastric cancer) was increased in patients with elevated blood cholesterol levels [149,150]. Earlier studies from Pih GY, et al., group have also reported an increase in the risk of gastric cancer with increase LDL cholesterol and decreased HDL cholesterol [151]. Moreover, a rise in blood cholesterol level also increases the rate of lymph node metastasis in early gastric cancer patients [152].
Alteration in the expression of various genes involved in lipid metabolism is a very common phenomenon in different cancers. Molecular studies in patient tissue samples decipher the mechanism of lipid accumulation including cholesterol in gastric cancer [153]. Genes involved in β-oxidation, lipoprotein excretion, fatty acid transport, and lipid droplets (LD) degradation/lipolysis were significantly down-regulated in neoplasms as compared to normal tissue. Whereas, the genes involved in de novo lipogenesis such as sterol regulatory element-binding protein 1c, acyl-CoA:diacylglycerol acyltransferase 2 were significantly up-regulated in the tumor tissue sample of gastric cancer [153]. Also, HMGCR was up-regulated in gastric cancer tissue as compared to normal tissues [154,155]. Overexpression of HMGCR regulates Hedgehog/Gli1 signaling thereby supporting gastric cancer cell proliferation and migration. Consequently, inhibition of HMGCR by knockdown approach shows a decrease in proliferation and migration of gastric cancer [155]. In addition, a web-based gene survival analysis of low/high-density lipoprotein receptors (LDLR; LRP6; SR-B1), lipoprotein lipase (LPL), and steroidogenic enzymes such as CYP11A1, HSD3B1, CYP17, HSD17B1, CYP19A1, etc. promotes gastric cancer progression. The importance of lipoprotein-mediated cholesterol uptake and steroidogenesis were considered as biosignatures of gastric cancer progression [156].
Numerous clinical studies highlight the relation between serum cholesterol or dietary cholesterol to gastric cancer, however, few studies have been undertaken to untangle the cholesterol-gastric cancer correlation at the preclinical level. In vitro studies in gastric cancer cells shows an increased invasion when supplemented with 25-HC through up-regulation of TLR2/NF-κB-mediated MMP expression without affecting gastric cancer cell proliferation and apoptosis [157]. A study from Lim SC, et al., have also reported that gastric cancer cells exposed to water-soluble cholesterol in vitro conditions undergo autophagy and apoptosis through transient inactivation of ERK1/2 [158], which may likely be due to the inability of gastric cancer cells to utilize excess free cholesterol directly.
The majority of the findings indicate a strong association of low blood cholesterol with gastric cancer. It is still unclear whether the decrease in serum cholesterol level in gastric cancer patients can be a cause or effect of cancer and therefore needs to be investigated. Collectively, it is likely that increased dietary cholesterol may act directly or indirectly by supporting Helicobacter pylori infection in the stomach for gastric cancer initiation or progression, but not necessarily only through increased blood cholesterol levels. However dietary cholesterol factors may be of relevance for those populations or age groups who are vulnerable to gastric cancer.
Ovarian cancer
Ovarian cancer is a common gynecological cancer caused by abnormal growth and division of ovarian cells leading to malignancy. Major risk factors of ovarian cancer include old age, faulty genes inheritance, earlier history of breast cancer, etc. Besides, obesity and dietary factors are also considered as a risk factor for ovarian cancer [159]. Numerous case-control and cohort studies have reported the link between dietary cholesterol with increased risk of ovarian cancer [160,161,162,163,164]. Risch HA, et al., has also suggested that dietary cholesterol may up-regulate the biosynthesis of the circulatory estrogen or progesterone which in turn increases the risk of ovarian cancer [160]. However, a study from Genkinger JM, et al., group indicates no role of dietary cholesterol in the risk of ovarian cancer [165].
A case-control study in Washington County, MD reported that higher blood cholesterol level (greater than 200 mg/dl) increases the risk of ovarian cancer [166]. Besides Delimaris I, et al., also reported that the level of oxLDL was increased in ovarian cancer patients in comparison to the control subjects [167]. However, the majority of the findings including a large-scale meta-analysis showed decreased blood cholesterol level in ovarian cancer patient as compared to normal subjects [168,169,170,171]. Furthermore, an ovarian cancer patient with low blood cholesterol level at the time of diagnosis shows improvement in blood cholesterol level after successful primary surgery and chemotherapy [172].
Several studies concerning serum cholesterol and ovarian cancer survival were also carried out, in which normal LDL cholesterol level shows better survival than those patients having high LDL cholesterol level [173]. However, studies from Zhu F, et al., group reported that elevated preoperative LDL cholesterol improves the 5 years relapse free survival (RFS) of ovarian cancer patients [174].
Additionally, studies in ovarian cancer patients show elevated cholesterol levels in malignant ascites as compared to normal counterparts, which also contributes to poor prognosis [25,175]. Similarly, Grignon DJ, et al., also reported that the cyst fluid from ovarian cancer patients shows a higher concentration of free cholesterol [176]. Increased accumulation of cholesterol ester through an up-regulated expression of ACAT-1 was also reported in ovarian cancer cells (OC-314, SKOV-3, IGROV-1, etc.) as compared to its normal counterpart. Inhibition of ACAT-1 with avasimibe or knockdown of ACAT-1 with shRNA decreases proliferation, invasion, and migration of ovarian cancer cells. Inhibition of ACAT-1 induces apoptosis through up-regulation of reactive oxygen species (ROS) generation along with overexpression of p53 [177]. Besides, studies were also carried out for understanding the role of HMGCR in ovarian cancer. Genetically proxied inhibition of HMGCR (through Single-nucleotide polymorphisms (SNPs) in HMGCR) significantly decreases the odds of epithelial ovarian cancer, suggestive of an important role in ovarian cancer [178].
Despite numerous clinical and case-control studies, the correlation between blood cholesterol and ovarian cancer is still puzzling and needs more in-depth studies. However, the consequences of dietary cholesterol in ovarian cancer cannot be overlooked and need more comprehensive studies. Additionally, the metabolic alteration such as overexpression of ACAT-1 or HMGCR or any other changes involved in lipid or cholesterol metabolism of ovarian cancer needs more insights for a better understanding of the relationship between cholesterol and ovarian cancer.
Blood cancer
Blood cancers are malignancies of hematological origin which mainly include leukemia, lymphoma, and myeloma. Leukemia is the cancer of white blood cells that originate in the bone marrow, lymphoma is the cancer of the lymphatic system and it involves lymphocytes whereas myeloma is the cancer of plasma cells [179]. Owing to the fact that blood cancer cells are directly exposed to blood cholesterol, it is very pertinent to study the direct relationship between the two. Various clinical shreds of evidence indicate that abnormal tumor metabolism can lead to changes in serum cholesterol level. Blood lipid profile analysis of newly diagnosed chronic lymphocytic leukemia (CLL) patients indicates that the levels of total cholesterol, HDL cholesterol, and LDL cholesterol are decreased as compared to the control group [180]. A similar study indicates that total serum cholesterol along with other serum lipoproteins decreases in acute non-lymphocytic leukemia (ANLL) and acute lymphocytic leukemia (ALL) patients. However, total cholesterol levels were found to be increased in the patient groups which respond to the treatment in both the leukemia types [181].
Clinical studies in lymphoma also suggest a similar trend. It has been observed in various subtypes of lymphoma that the total serum cholesterol, HDL cholesterol, and LDL cholesterol levels decrease drastically 5–10 years before the diagnosis of the disease. Interestingly, high HDL cholesterol levels were found to be associated with a decreased risk of non-Hodgkin lymphoma (NHL). However, HDL cholesterol or free cholesterol levels don't influence the prognosis [182,183].
Similarly, in myeloma patients, it has been shown that total cholesterol; HDL cholesterol, and LDL cholesterol levels decrease significantly as compared to healthy subjects. The level of cholesterol further drops with an increase in the tumor stage [184].
On the contrary, increased cholesterol levels were detected in 75% of patients attending a specialized clinic for Chronic Lymphocytic Leukemia (CLL) [185]. It has been reported that hypercholesterolemia precedes the diagnosis of CLL and the use of cholesterol-lowering drugs increases the survival of CLL patients [186].
At the cellular level, many molecules associated with cholesterol metabolism were found to be deregulated in blood cancer cells. Lymphocytes isolated from chronic lymphocytic leukemia (CLL) patients show increased expression of LDLR, SREBP-2, and a nuclear cholesterol channel protein PBR as compared to control. As a result, cholesterol accumulation in the cytoplasm and nucleus was also found to be increased in these cells [187]. Studies in the xenograft model (SCID mice), show an increase in cholesterol accumulation at the leukemia rich sites of the bone marrow, which increases VEGF receptor 1 (FMS like tyrosine kinase-1 or FLT-1) localization in the leukemic cells. Furthermore, their ligand PlGF/VEGF mainly increases FLT-1 accumulation at cholesterol-rich sites. The increased localization of FLT-1 enhances cell migration and viability. Interestingly the removal of membrane cholesterol by methyl-β-cyclodextrin (MCD) also decreased FLT-1 accumulation and VEGF: VEGFR-1 signaling [188].
In Multiple myeloma (MM) exogenous cholesterol may serve as an anti-apoptotic factor and support survival, whereas the absence of lipoproteins in serum induces apoptosis in MM cell lines and primary cultures, which was reversed by exogenous supplementation with LDL cholesterol [189].
The dependence of white blood cells on cholesterol for various functions including lipid raft mediated signaling, membrane dynamics, antibody and cytokine release, etc. indicates the possible role of cholesterol in hematological malignancies. More in-depth analysis of various studies points towards a strong correlation of serum cholesterol with various blood cancer types. Strategies for controlling the accumulation of cholesterol in blood cancer cells need to be explored for better management of this disease. Additionally, various molecules are involved in cholesterol metabolism i.e., LDLc, LDLR, SREBP-2, and PBR can also be targeted along with traditional therapies.
Cholesterol and cancer-associated immune cells functionality
Cholesterol is an important part of the cell membrane and is essential for receptor-mediated signaling and therefore may have implications in the functionality of immune cells. Various groups have stressed the functional importance of cholesterol in the regulation of cancer linked immune cells. Studies from Zhou X, et al., suggested that hypercholesterolemia can drive Th2 response in T cells in the spleen of atherosclerotic Apo E knockout mice [190]. Also, it can induce the differentiation of regulatory T cells in the liver during atherosclerosis development [191]. Therefore, cholesterol may influence tumor growth through the modulation of the immune system. It has been reported that hypercholesterolemia induces epigenetic changes in the hematopoietic stem cells (HSC) which can further interfere with the development of natural killer T cell (NKT) and γδ T-cells. Cholesterol increases miR101c expression which down-regulates Tet1 in HSCs. Downregulation of Tet1 reduces the expression of genes involved in NKT and γδ T-cells differentiation causing a reduction in cancer immunosurveillance which contributes to increased cancer incidence and progression [192]. Moreover, a derivative of cholesterol i.e., 27-HC promotes the metastasis of breast cancer cells by decreasing the number of CD8+ T cells and increasing the number of polymorphonuclear-neutrophils as well as γδ-T cells at distal metastatic sites [193]. In addition, Yang W, et al., observed that inhibition of ACAT1, by avasimibe increases plasma membrane cholesterol availability which enhances T cell receptor clustering and antitumor signaling in CD8+ T cells. Furthermore, the combination of avasimibe and anti-PD1 therapy has a higher efficacy than monotherapy [194]. Cholesterol availability in plasma membrane affects the pro-tumorigenic phenotype of macrophages. It has been reported that ovarian cancer cells promote cholesterol efflux from the plasma membrane which depletes lipid rafts and increases the IL-4 induced pro-tumor phenotype of macrophages. The ablation of specific transporters which helps in cholesterol efflux restores the anti-tumor phenotype of macrophages [195]. In addition to that cholesterol can affect the antigen presentation by dendritic cells (DCs). One of the master regulators of cholesterol metabolism liver X receptor-α (LXR-α) was found to be activated in DCs when exposed to condition media of many cancer cells [41]. This, in turn, decreases the expression of CC chemokine receptor-7 (CCR7) on DCs which hampers its migration from tumor to the draining lymph node and tumor antigen presentation to cytotoxic T cells. Furthermore, the antitumor response was restored in tumor-bearing mice after inhibiting cholesterol synthesis or LXR-α activation. Additionally, tumor-derived factors induce the accumulation of oxidized neutral lipids: triglycerides, cholesterol esters, and fatty acids in DCs which hinders the exogenous antigen presentation by DCs [41]. Recent reports suggest that cholesterol can influence the efficacy of T cell-based immunotherapy for cancer treatment [196]. CD8+ T cells present in the cholesterol-rich tumor microenvironment accumulate cholesterol. This increases the endoplasmic reticulum stress in the T cells leading to up-regulation of immune checkpoint proteins like PD-1, 2B4, TIM-3, LAG-3, etc., and causes exhaustion of T cells. The adoptively transferred CD8+ T cells follow the same fate and hence the efficacy of immunotherapy decreases. Interestingly, the reduction of cholesterol in the T cells restores the antitumor activity of these cells [196].
The effect of cholesterol on the cancer-associated immune system is multidimensional and not restricted to a particular cell type. The available literature suggests that cholesterol metabolism can influence antitumor immunity and affect the immunotherapeutic outcome. However, the differential response of cholesterol in antitumor immunity in various cancers cannot be undermined.
Targeting cholesterol metabolism for the treatment of cancer
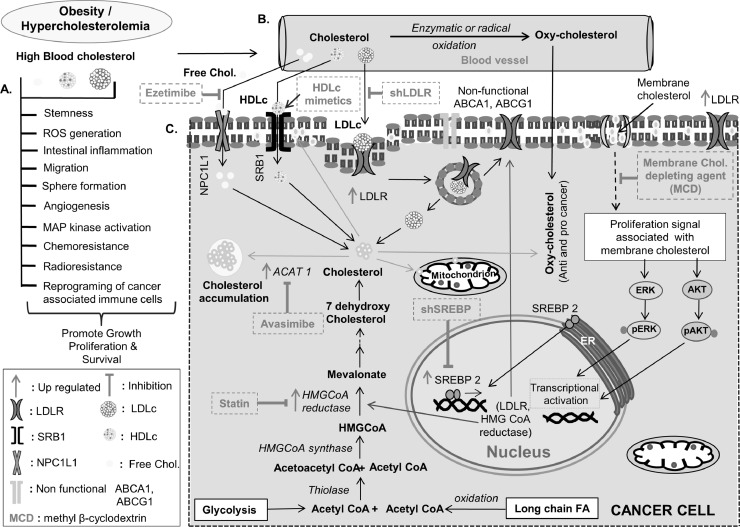
Alteration in cancer cell metabolism is regarded as one of the emerging hallmarks of cancer [197] and one such alteration being a change in lipid metabolism, particularly cholesterol metabolism. Pre-clinical and clinical studies emphasize targeting cholesterol metabolism in combination with approved chemotherapeutic drugs for better management of various cancers (Table 1). A few of the strategies include aiming cholesterol biosynthesis pathway, particularly through HMG-CoA reductase (rate-limiting enzyme) inhibitor i.e., statin or inhibition of cholesterol uptake or targeted delivery of drugs through encapsulation with liposomes or through membrane cholesterol depletion (Fig. 6) [35,36,198,199].
Table 1.
Targeting lipid or cholesterol metabolism for cancer treatment.
| S. No | Cancer types | Drug /Inhibitors Under Investigation | in vitro/ in vivo/Clinical Studies | Cell line/ Animal model/Patient Information | Outcome of Treatment/ Effect / Treatment modalities | Ref. |
|---|---|---|---|---|---|---|
| 1 | Colon | Lovastatin | in vitro & in vivo | SW480, SW620 cellsBalb/c mice | Decreases MCA-26 tumor colonies in the liver of Balb/c mice compared to untreated mice | [35] |
| 2 | Colon Lung Breast | Liposomal Doxorubicin and Simvastatin | in vitro | HT29-dx (drug-resistant), HT29, MCF-7, A549 cells | Combination treatment shows better anticancer efficacy in multidrug-resistant tumor cells, without cardiomyocytes toxicity | [36] |
| 3 | Colon | Atorvastatin & Phloretin | in vitro | SW620 and HCT116 cells | The synergistic effect resulted in apoptosis and cell cycle arrest at the G2/M checkpoint. Combined treatment enhanced the anti-cancer activity of Atorvastatin at a relatively low dosage | [203] |
| 4 | Colon | Long-circulating Liposomal Simvastatin (LCL-SIM) | in vitro & in vivo | C26 cell and Balb/c mice | Anti-angiogenic and anti-inflammatory Antitumor efficacy | [204] |
| 5 | Colon | Nanoliposomal anti PCSK9 | in vivo | CT26 cells and Balb/c mice | Inhibits tumor growth and increases survival | [217] |
| 6 | Colon | SREBP 1 & SREBP 2Knockdown | in vitro & in vivo | DLD1 and HCT116 cellsNOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ mice;Pt130 cells | Knockdown decreased fatty acids level. Silencing of SREBP1 or SREBP2 expression also decreases mitochondrial respiration, glycolysis, as well as fatty acid oxidation. Inhibit xenograft colon tumor growth in vivo | [218] |
| 7 | Colon | Pioglitazone (PPAR gamma) agonist | in vivo | C57BL/6-ApcMin /+mice | A decrease in intestinal polyps was observed in Min mice, 63–9% lesser than the control value. | [221] |
| 8 | Colon | HDL mimetics: L-4F (an apolipoproteinA-I mimetic peptide) & G*(an apolipoprotein J mimetic peptide) | in vivo | CT26 cellBALB/c mice | HDL mimetics decrease lysophosphatidic acid (LPA), a serum biomarker of colon cancer in mice.L-4F reduced size and number of polyps in APC (min/+) mice | [222] |
| 9 | Prostate | Ezetimibe | in vivo | LNCaP cellsSCID mice | Inhibit tumor angiogenesis and decrease tumor growth | [90] |
| 10 | Prostate | Statin | Clinical | 6655 participants (men) | Statin decreases chronic inflammation and lowers the risk of advanced prostate cancer | [199] |
| 11 | Prostate | Avasimibe, Sandoz 58–035 &shRNA (ACAT-1) | in vitro | PC-3 and LNCaP-HP cells.Athymic nude mice | Targeting cholesterol esterification pathway inhibits proliferation of prostate cancer cells both in vitro and in vivo | [225] |
| 12 | Hepato-cellular carcinoma | Eicosapentaenoic acid (EPA) & Lovastatin | in vitro | HepG2 cells | Combination treatment regulates HMGCoA reductase and LDL receptor gene expression in the HepG2 cell line. Synergistically inhibits cancer cell proliferation | [201] |
| 13 | Hepato-cellular carcinoma | Naringenin (NGEN)(High Cholesterol Diet) | in vivo | Rat | NGEN decreased plasma fatty acid composition, hepatic pro-inflammatory mediators, and the expression of tumor necrosis factor-α, interleukin-6, interleukin-1β, inducible nitric oxide synthase, and matrix metalloproteinases (MMP-2, 9) | [220] |
| 14 | Hepato cellular carcinoma, Breast | Methyl-β-cyclodextrin (MCD), Doxorubicin | in vitro & in vivo | MCF-7, Hepa1–6C57Bl/6 | MCD sensitize MCF-7 and Hepa1–6cells to Doxorubicin | [224] |
| 15 | Pancreatic ductal adeno-carcinoma (PDAC) | LDLR shRNA | in vitro & in vivo | Pdx1-Cre, Ink4a/ Arffl/fl; LSL-KrasG12DPK4A cell lines | Decreases cholesterol uptake sensitized PDAC to gemcitabine | [28] |
| 16 | Pancreatic ductal adeno-carcinoma (PDAC) | Simvastatin, Lovastatin, Atorvastatin, Pravastatin, Rosuvastatin | Clinical | 2142 patients | Statin lowers the risk of mortality in pancreatic cancer patients independent of cholesterol level | [211] |
| 17 | Pancreatic | Simvastatin, Atorvastatin, Rosuvastatin, Pravastatin, Fluvastatin | Clinical | 1761 patients with pancreatic adeno-carcinoma | The use of simvastatin and atorvastatin increases the survival of non-metastatic pancreatic cancer patients | [212] |
| 18 | Pancreatic | Simvastatin | in vivo | Mice model -LsL-Kras (G12D); Pdx1-Cre and LsL-Kras(G12D); LsL-Trp53(R172H); Pdx1-Cre | Simvastatin acts as a chemopreventive agent as well as inhibits pancreatic cancer formation in mice | [213] |
| 19 | Pancreatic | Zoledronic acid (inhibitor of farnesyl diphosphate synthase) | in vitro & in vivo | Panc-1, BxPC3, L3.6pl, UN-KPC-961 cell lines. LSL-KrasG12D/+, LSL-Trp53R172H/+, Pdx-1-Cre (KPC) mouse model | Inhibition of farnesyl diphosphate synthase involved in cholesterol biosynthesis radio sensitized pancreatic cancer cell lines. | [228] |
| 20 | Pancreatic ductal adenocarcinoma (PDA) | JQ1 (BET inhibitor), Atorvastatin | in vitro | PANC-1, AsPC-1, MIA PAC-2 cells | Combination treatment of JQ1 and atorvastatin inhibits PDA cell proliferation in vitro conditions. | [248] |
| 21 | Melanoma | Methyl-β-cyclodextrin (MCD), Tamoxifen | in vitro & in vivo | A375, B16F10 and B16F1 cell lines. | MCD sensitizes melanoma cells to tamoxifen | [198] |
| 22 | Epithelial Ovarian cancer (EOC) | Statin | Clinical | 2040 EOC cases2100 cases without disease | 32% decrease in the risk of ovarian cancer in statin users in comparison to non-statin user females | [205] |
| 23 | Ovarian | Simvastatin | in vitro, in vivo & Clinical | Hey and SKOV3 cells;K18-gT121+/- p53fl/fl Brca1fl/fl (KpB) mouse model | Simvastatin decreases ovarian cancer cell proliferation and tumor growth. Induced G1 cell cycle arrest and apoptosis | [249] |
| 24 | Ovarian | Simvastatin, Lovastatin, Fluvastatin, 25-hydroxycholesterol, 22(S)- hydroxycholesterol, 22(R)-hydroxycholesterol | in vitro | SKOV-3 and ES-2, OVCAR-8 | Oxysterol potentiate statin treatment by inhibiting SREBP-2 | [250] |
| 25 | Ovarian | Methyl-β-cyclodextrin (MCD) | in vitro & Clinical | PA-1, OVCAR-3, and SKOV-3 cells | MCD sensitizes cisplatin-resistant ovarian cancer cells to cisplatin. | [25] |
| 26 | Ovarian | Avasimibe, Cisplatin | in vitro | H-6036, OC-314, and SKOV-3 cells | Inhibition of ACAT-1 enhances the chemosensitivity of cisplatin to ovarian cancer cells | [177] |
| 27 | Ovarian | HSP27 and HER2 inhibitor encapsulation into LDL | in vitro | SKOV3 | Treatment of LDL encapsulated HSP27 and HER2 inhibitor inhibits SKOV3 proliferation | [251] |
| 28 | Breast | Methyl-β-cyclodextrin (MCD),Carboplatin, 5-flurouracil | in vitro & in vivo | MCF-7, MDA-MD-231 | MCD sensitize breast cancer cells to Carboplatin and 5-flurouracil | [223] |
| 29 | Breast | Paclitaxel-cholesterol complex (PTX-CH Emul) | in vitro & in vivo | MCF7, MDA-MB-231 cells | PTX-CH Emul shows more antineoplastic effect on TNBC cells (MDA-MB-231) as compared to non-TNBC (MCF7). | [234] |
| 30 | Breast | Atorvastatin | Clinical (rando-mise) | 63 Women, (Age 35–50)16 (25%) Women withdrew. | Significant increase in serum IGF-1 in the statin group but no effect of atorvastatin on Mammographic Density (MD) | [235] |
| 31 | Breast | Simvastatin & MBCD (cholesterol depleting drug) | in vitro | RAW264.7 and MCF-7 cells | Combination treatment prevents breast cancer-induced osteoclast activity. | [236] |
| 32 | Breast | Cholesterol lowering medication, Tamoxifen & Letrozole | Clinical | 8010 (Postmenopausal women) | Cholesterol lowering medication during adjuvant endocrine therapy may prevent the recurrence of hormone receptor-positive breast cancer. | [237] |
| 33 | Lung and Breast | Cepharanthine, Cisplatin | in vitro & in vivo | Human umbilical Vein endothelial cells (HUVEC) A549, MDA-MB-231 & HEK293T cells.NOD/SCID mice, Transgenic zebrafish line Tg (fli1a:EGFP)y1 | Blocking cholesterol trafficking with cepharanthine inhibits angiogenesis and sensitizes breast and lung cancer cells to chemotherapy. | [238] |
| 34 | Lung | Cholesterol Cisplatin, Oxaliplatin, Carboplatin Pravastatin and Nicardipine | in vitro & Clinical | A549 cell64 patients | Patients showing quick chemoresistance have elevated serum cholesterol levels and found to have upregulated ABCG expression in their tumors. The use of ABCG blocker (nicardipine) increases the efficacy of platinum base drugs in vitro conditions. | [229] |
| 35 | Lung | All-trans retinoic acid (ATRA), DOTAP/cholesterolliposomes & DSPC/cholesterol liposomes | in vitro | A549 cells | Cationic liposome (DOTAP/cholesterol) incorporation with ATRA increases apoptotic cell death of A549. | [239] |
| 36 | Lung | Statin | Clinical | 483,733 patients | The use of statin reduces lung cancer risk by 55% irrespective of race, age group, diabetic, alcoholic, or even smoking. | [240] |
| 37 | Lung | Cholesterol oxidaseBordetella species cholesterol oxidase (COD-B) | in vitro & in vivo | A549 and SPC-A-1 cellsBALB/c nude mice | COD-B oxidizes membrane cholesterol thereby decreasing cholesterol content and increases ROS. COD-B causes apoptosis of lung cancer cells by interfering with AKT and ERK pathway. | [241] |
| 38 | Lung | Betulin, Fatostatin, 25-HC & Gefitinib | in vitro & in vivo | A549 and PC9 cellsBALB/c SCID mice | Combination treatment of SREBP inhibitor with EGFR inhibitor (Gefitinib) increases the non-small cell lung cancer death in comparison to alone treatment. | [242] |
| 39 | Lung | Pirarubicin, Ellipticine & MCD | in vitro | A549 and CHO-K1 cells | Membrane cholesterol depletion with MCD enhances drug uptake of pirarubicin but not Ellipticine in A549 and CHO-K1 cells. | [243] |
| 40 | Lung | Zaragozic acids | in vitro & in vivo | C57Bl/6 CD45.1 or CD45.2 and NOD–SCID mice RMA and LLC cells | Zaragozic acids, an inhibitor of the downstream mevalonate pathway enhances the antitumor effects of active and adoptive immunotherapy. Increases overall survival of tumor-bearing mice on treatment with zaragozic acids and TAA-loaded DCs. | [244] |
| 41 | Lung | Gemcitabine-cholesterol (Gem-Chol) liposome | in vitro & In vivo | H22 and S180 tumor xenograftWistar male rats | Gem-Chol conjugate enhances the efficacy of Gemcitabine. | [245] |
| 42 | Lung | siRNA/curcumin loaded, Cholesterol conjugated chitosan | in vitro | A549 cell | Cholesterol conjugated chitosan can be used for hydrophobic drug delivery. Cellular uptake of drug is more efficient in Cholesterol conjugated chitosan. | [246] |
| 43 | Lung | LXR agonistT0901317,Gefitinib | in vivo & in vitro | A549 cellHCC827–8–1 (gefitinib-sensitive) cells | Combination treatment of T0901317 & gefitinib inhibits migration and invasion of lung cancer. | [247] |
| 44 | Gastric | Simvastatin | in vitro | MKN45 and MGC803 cells | Inhibits migration, invasion, proliferation, and induced apoptosis in gastric cancer cells by interfering with YAP and β-catenin activity. | [206] |
| 45 | Gastric | Simvastatin | in vitro & Clinical | AGS, ATCC CRL 1739 cells | Statin reduces the incidence of gastric cancer by attenuating Helicobacter pylori CagA translocation | [202] |
| 46 | Stomach | Statin | Clinical | 17,737 statin users and 13,412 statin non-user | The use of statin decreases the incidence of stomach cancer on hypercholesterolemic individuals. | [252] |
| 47 | Blood (chronic myelogenous leukemia) | Avasimibe, Imatinib | in vitro & in vivo | K562R (imatinib-resistant) | Avasimibe sensitized K562R to imatinib | [226] |
| 48 | Blood (lymphoblasts and myeloma cells) | Lovastatin Simvastatin Cerivastatin Leverkusen, Atorvastatin | in vitro | Jurkat, CEM, IM9, U266 cell MCC-2 | Statin induces mitochondrial apoptosis pathway in human T, B, and myeloma tumor cells causing cell death | [200] |
| 49 | Blood (leukemia and lymphoma) | Simvastatin, Atorvastatin, Rosuvastatin, Fluvastatin,Venetoclax | in vitro, in vivo, & Clinical | AML cells (OCI-AML2, OCI-AML3, MOLM13), DLBCL cells (OCI-LY8, SU-DHL4).C57BL/6 N mice | Statin increases the pro-apoptotic activity of Venetoclax (B cell lymphoma-2 inhibitor) in primary leukemia and lymphoma cells | [253] |
Fig. 6.
Schematic representation of cholesterol associated changes in cancer cells and available therapeutic targets. (A) Role of cholesterol in stemness, ROS generation, intestinal inflammation, migration, sphere formation, activation of different signaling molecules, increased angiogenesis, imparting chemo and radioresistance as well as reprogramming of cancer-associated immune cells. (B) Conversion of blood cholesterol to oxycholesterol, through enzymatic or radical oxidation. (C) Different mode of cholesterol uptake; pathway associated with altered cholesterol biosynthesis and storage of cholesterol along with different agents targeting cholesterol metabolism for treatment.
Collectively, various studies reveal a positive effect of statin use in colon, breast, lung, liver, prostate, ovarian, gastric, blood, and pancreatic cancers [35,36,199,200,201,202,203,204,205,206]. However, few reports suggest cancer-promoting or chemoresistant effect on statin treatment [207,208], (209). Low dose treatment of statin promotes its aggressiveness in prostate cancer [208], likewise treatment of lovastatin in ovarian cancer decreases chemosensitivity towards platinum drugs [209]. Interestingly, in human subjects’ use of statin particularly simvastatin and atorvastatin not only reduce the risk of mortality but also increases survival among pancreatic cancer patients [210], [211], [212]. Likewise, in in vivo studies, it has been reported that simvastatin administration in LsL-Kras (G12D); Pdx1-Cre, and LsL-Kras (G12D); LsL-Trp53 (R172H) mice model delayed the spontaneous formation of pancreatic cancer lesions [213]. Similar findings were reported in colon cancer, in which the use of lovastatin decreased the metastatic potential of colon cancer cells [35]. In another study with colon cancer cells, atorvastatin in combination with phloretin exhibited a synergistic effect by arresting the cell cycle at the G2/M checkpoint and enhancing apoptotic cell death [203]. Furthermore, in lung cancer, atorvastatin also sensitizes gefitinib resistant KRAS mutant human non-small cell lung carcinoma cells by interrupting Kras/PI3K and Kras/Raf complexes causing AKT and ERK activity suppression [214]. Meta-analysis conducted by different groups shows a significant decreased in the risk of gastric cancer ranging from 27 to 32% in statin users [215,216]. Similarly, a 32% decreased in ovarian cancer risk was also reported in statin users as compared to non-users [205]. Also, in hepatocellular carcinoma, lovastatin in combination with eicosapentaenoic acid (EPA) regulate HMG-CoA and LDLR expression thereby inhibiting the proliferation of HepG2 cells [202]. Moreover, administration of anti-proprotein convertase subtilisin/kexin 9 (PCSK9) antibody nanoliposomes in mice helps in decreasing colorectal cancer progression. Targeting PCSK9 is among the recent steerages used for reducing blood cholesterol levels [217].
Other than statin various blockers/inhibitor/knockdown approaches were explored for controlling tumor progression. Use of FDA approved cholesterol uptake blocker drug ezetimibe retards in vivo prostate cancer progression by inhibiting angiogenesis. Ezetimibe specifically blocks NPC1L1 which plays an important role in dietary cholesterol uptake; inhibition of NPC1L1 decreases the level of blood cholesterol [90]. Knockdown of SREBP1 and SREBP2 causes a decreased in, fatty acid synthesis, mitochondrial respiration, fatty acid oxidation as well as glycolysis, which reduces colon cancer xenograft tumor formation [218]. In breast cancer cells (ER+) cholesterol biosynthesis genes were up-regulated and targeting these genes through siRNA decreased proliferation [219]. Other compounds like naringenin (NGEN) were reported to inhibit hepatocellular carcinoma cell proliferation by decreasing plasma fatty acid composition [220]. Use of a peroxisome proliferator-activated receptor gamma (PPAR gamma) agonist (pioglitazone) or HDL mimetics l-4F (an apolipoprotein A-I mimetic peptide) were also shown to have an inhibitory effect on colon cancer progression. Both pioglitazone and l-4F decreases the incidence of intestine polyp's occurrence. Moreover, treatment with HDL mimetics L-4F also decreases serum biomarker (lysophosphatidic acid (LPA)) of colon cancer in mice [221,222]. Likewise targeting cholesterol associated metabolic pathway together with chemotherapeutic drugs increases the cytotoxic potential of these drugs. Earlier studies from our laboratory have reported that membrane cholesterol depletion through MCD enhances the cytotoxic potential of tamoxifen, doxorubicin, carboplatin, and 5-fluorouracil in melanoma, breast, and hepatocellular carcinoma cells [198,223,224]. Also, targeting cholesterol uptake by using shRNA for LDLR increases the efficacy of gemcitabine in pancreatic cancer [28]. Another aspect of targeting cholesterol metabolism is through the inhibition of the cholesterol esterification process. Inhibition of cholesterol esterification enzyme ACAT1 through avasimibe or by using ACAT1 shRNA inhibits proliferation of prostate cancer and myelogenous leukemia (CML) cells [225]. Moreover, in the xenograft model of CML (K562R cell, resistant to imatinib) the combination treatment of avasimibe and imatinib shows a synergistic effect in reducing the tumor burden [226].
The role of lipids/cholesterol in chemotherapeutic response warrants more in-depth studies. HMG-CoA reductase inhibitors (i.e., statin) are commonly used for the treatment of cardiovascular diseases, however, its role as an anti-cancer agent is still debatable. For proper management of various cancers, it would be interesting to see how targeting altered lipid/cholesterol metabolism with specific inhibitors either alone or in combination with chemotherapeutic drugs could influence tumor progression. A schematic representation of different approaches for targeting cholesterol metabolism of cancer cells, along with different functions of cholesterol in helping cancer progression is depicted in Fig. 6.
Cholesterol and resistance to cancer therapy
Targeting cholesterol/lipid metabolism has a profound effect on cancer cell proliferation and progression. One of the major hurdles faced by cancer patients in conventional therapy is resistance to chemotherapy or radiotherapy. Numerous reports suggest that alteration in lipid/cholesterol metabolism contributes to the ineffectiveness of various anticancer drugs and radiation therapy (Table 2) [25,227,228,229]. Montero J, et al., group, reported that the increase in mitochondrial cholesterol level causes chemoresistance in hepatocellular carcinoma [227]. In hepatocellular carcinoma cells, it has been shown that 7-Ketocholesterol (7-KC) up-regulates the expression of P-glycoprotein (P-gp) through PI3K/mTOR signaling thereby diminishing the efficacy of doxorubicin [230]. Studies in chemoresistant ovarian cancer cells show more cholesterol uptake via up-regulation of LDLR and a decrease in endogenous cholesterol biosynthesis through downregulation of enzymes involve in cholesterol biosynthesis i.e., Farnesyl Diphosphate Synthase (FDPS) and oxidosqualene cyclase (OSC) [209].
Table 2.
Altered cholesterol metabolism and drug resistance.
| S. No | Cancer Types | Resistant Drugs /Inhibitors | in vitro/in vivo/ Clinical Studies | Cell line/ Animal model/ Patient Information | Outcome of Treatment/ Effect /Treatment modalities | Ref. |
|---|---|---|---|---|---|---|
| 1 | Prostate | Simvastatin | in vitro & in vivo | PC3, LNCaP & 22RV1 cells,Hsd: Athymic Nude-Foxn1 nu/nu mice. | Low dose treatment of simvastatin increases the aggressiveness of prostate cancer in mice models. | [208] |
| 2 | Ovarian | Cisplatin, Paclitaxel | in vitro & Clinical | PA-1, OVCAR-3, SKOV-3 cells | Cholesterol increases expression of MDR1 through LXRɑ/β activation and imparts chemoresistance of cisplatin and paclitaxel | [25] |
| 3 | Ovarian | Cisplatin | in vitro | A2780 cell | SREBP2 imparts cisplatin resistance via upregulation of LDLR, FDFT1, and HMGCR in A2780 cell | [254] |
| 4 | Ovarian | Cisplatin, Lovastatin | in vitro | PEA1, PEA2, PEO14 cells | Inhibition of cholesterol biosynthesis with statin increases cisplatin chemoresistance in ovarian cancer | [209] |
| 5 | Chronic myelogenous leukemia | Imatinib | in vitro | K562R (imatinib resistant) cell | A high level of cholesterol ester accumulation is associated with resistance to imatinib in chronic myelogenous leukemia cells | [226] |
| 6 | Lung | Cisplatin, Carboplatin,Oxaliplatin,Cholesterol | in vitro & Clinical | A549 cell,Patient sample | Pretreatment of cholesterol increases the expression of ABCG2 which imparts chemoresistance to platinum-based drugs. | [229] |
| 7 | Hepato-cellular Carcinoma | Doxorubicin, | in vitro | Huh-7, HepG2 cells | 7-Ketocholesterol regulate P-gp through PI3K/mTOR signaling and decreases the efficacy of Doxorubicin | [230] |
| 8 | Hepato-cellular carcinoma | Sorafenib | in vitro | HepG2, HUH7 cells | Pre-treatment of LDLc decreases HCC cell death from sorafenib. | [232] |
Clinicopathological studies in ovarian cancer have shown a significant correlation of high ascites cholesterol content with poor prognosis and chemoresistance. High cholesterol content hampers the sensitivity of paclitaxel and cisplatin through up-regulation of ABCG2 and MDR1 (drug efflux pump) along with an increase in cholesterol receptor (LXRɑ/β) expression [25]. Moreover, the increase in cholesterol biosynthesis genes imparts resistance to estrogen deprivation in ER+ breast cancer cells [219]. It also contributes to statin resistance along with a decrease in the survival of breast cancer patients [231]. Furthermore, the accumulation of cholesterol esters due to ACAT-1 enzyme was correlated with reduced efficacy of imatinib in CLM cells (226). Earlier, our group has reported by in vitro studies that pretreatment of hepatocellular carcinoma cells with LDLc decreases sorafenib induced cell death [232]. A similar observation was reported in lung adenocarcinoma where pretreatment or co-treatment of cholesterol with oxaliplatin or carboplatin decreased the cytotoxic potential of these drugs in A549 cells [229]. In gastric cancer supplementation of 25-HC in both in vitro and in vivo models decreases the sensitivity of 5-FU mediated cytotoxicity (157). Additionally, studies from Souchek JJ (2014) group had shown that genes involved in radioresistance (FDPS, ACAT2, AG2, CLDN7, DHCR7, ELFN2, FASN, SC4MOL, SIX6, SLC12A2, and SQLE) are mostly associated with the cholesterol biosynthesis pathway [228]. Therefore, it is evident from the available literature that cholesterol has an important role in imparting resistance to various therapeutic interventions. However, it is needful to focus more on clinical studies for understanding the relevance of high blood cholesterol towards the therapeutic response of various cancers. Moreover, the underlying molecular mechanism needs further investigation to understand the mode of action through which cholesterol or its metabolism directly or indirectly interferes with the efficacy of various drugs.
Conclusion
Global Health Observatory (GHO) data indicates that there has been a substantial increase in the percentage of the adult population with elevated total blood cholesterol (≥ 5.0 mmol/l) level worldwide which is up to 39% of the adult population [233]. Increased blood cholesterol is very common in obesity and familial hypercholesterolemia patients. Thus, with the increasing population of hypercholesterolemic people, elevated blood cholesterol levels may have a profound effect on various cancers. Enormous work has been done towards understanding the structural and functional aspects of cholesterol in human physiology as well as its role in various diseases. Previous studies have already hinted at the importance of cholesterol in cancer however the correlation between these two is not fully elucidated to date. In-spite of several intriguing studies there are still many unsolved questions that need to be answered. The ability of cancer cells in reprogramming cholesterol metabolism through intrinsic cues and the impact of extracellular cholesterol in providing extrinsic signals to the cancer cells needs to be investigated at the molecular level. It will be quite fascinating to understand the interrelationship between the availability of cholesterol or its intermediate, with cholesterol metabolism of cancer cells and how they regulate each other. It is evident from earlier findings that cholesterol may directly or indirectly help in the progression of various cancers. Additionally, in preclinical and clinical studies the expression of LDLR was up-regulated in most of the cancers which could be an important target for further studies. However, the role of cholesterol in cancer initiation is still elusive and needs further investigation. Recent studies emphasize targeting cholesterol metabolism for the treatment of various cancers. Therefore, targeting cholesterol metabolism in hypercholesteremia and obesity might open yet unexplored avenues for the management of various cancers.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgments
Acknowledgments
The authors thank National Center for Cell Science, Pune, India; University Grants Commission (UGC), New Delhi, India; Department of Biotechnology (DBT), New Delhi, India, and Savitribai Phule Pune University, Pune, India for their support.
Credit author statement
Shyamananda Singh Mayengbam.: Conceptualization, data researched & literature survey, writing - original draft preparation, discussion of the content, writing - reviewing & editing, generation of figures & table Abhijeet Singh.: Data researched, literature survey, writing- reviewing, editing & making table Ajay D Pillai.: Discussion, writing - reviewing and editing Manoj Kumar Bhat.: Supervision, conceptualization, discussion of the content, writing - reviewing and editing. All authors read and approved the manuscript before submission.
Funding
This work was supported by an Intramural grant from National Center for Cell Science (NCCS), an autonomous institution funded by the Department of Biotechnology (DBT), Government of India. The funding agencies had no involvement in data collection, interpretation, and analysis, the decision to publish, or writing of the manuscript.
References
- 1.Olson R.E. Discovery of the lipoproteins, their role in fat transport and their significance as risk factors. J. Nutr. 1998;128:439S–443S. doi: 10.1093/jn/128.2.439S. [DOI] [PubMed] [Google Scholar]
- 2.Brown M.S., Goldstein J.L. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232(4746):34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- 3.Heinrich Wieland – Biographical. NobelPrize.org. Nobel Media AB. https://www.nobelprize.org/prizes/chemistry/1927/wieland/biographical/; [accessed 17 September 2020].
- 4.Adolf Windaus – Facts. NobelPrize.org. Nobel Media AB. https://www.nobelprize.org/prizes/chemistry/1928/windaus/facts/; [accessed 17 September 2020].
- 5.Wolf G. The discovery of vitamin D: the contribution of Adolf Windaus. J. Nutr. 2004;134(6):1299–1302. doi: 10.1093/jn/134.6.1299. [DOI] [PubMed] [Google Scholar]
- 6.Carlisle C.H., Crowfoot D. The crystal structure of cholesteryl iodide. Proc. R. Soc. Ser. A. 1945;184:64–82. doi: 10.1098/rspa.1943.0040. [DOI] [Google Scholar]
- 7.National Center for Biotechnology Information. PubChem Database. Cholesterol, CID=5997, https://pubchem.ncbi.nlm.nih.gov/compound/Cholesterol; [accessed 17 September 2020].
- 8.Oncley J.L., Gurd F.R.N., Melin M. Preparation and properties of serum and plasma proteins. XXV. Composition and properties of human serum β-lipoprotein1, 2, 3. Am. Chem. Soc. 1950;72(1):458–464. doi: 10.1021/ja01157a121. [DOI] [Google Scholar]
- 9.The Nobel Prize in physiology or medicine 1985. NobelPrize.org. Nobel Media AB. https://www.nobelprize.org/prizes/medicine/1985/summary; [accessed 17 September 2020].
- 10.Goldstein J.L., Brown M.S. The LDL receptor. Arterioscler. Thromb. Vasc. Biol. 2009;29(4):431–438. doi: 10.1161/ATVBAHA.108.179564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harvard health publishing, Harvard medical school. How it's made: cholesterol production in your body. https://www.health.harvard.edu/heart-health/how-its-made-cholesterol-production-in-your-body. 2019 [accessed 18 September 2020].
- 12.Feingold K.R., Grunfeld C. MDText.com, Inc.; South Dartmouth (MA): 2000. Introduction to Lipids and Lipoproteins. Endotext [Internet]http://www.ncbi.nlm.nih.gov/books/NBK305896 [accessed 17 September 2020] [Google Scholar]
- 13.Cooper G.M. 2nd Ed. Sinauer Associates; Sunderland (MA): 2000. The Cell: A Molecular Approach. [Google Scholar]
- 14.Ikonen E. Cellular cholesterol trafficking and compartmentalization. Nat. Rev. Mol. Cell Biol. 2008;9(2):125–138. doi: 10.1038/nrm2336. [DOI] [PubMed] [Google Scholar]
- 15.Javitt N.B. Bile acid synthesis from cholesterol: regulatory and auxiliary pathways. FASEB J. 1994;8(15):1308–1311. doi: 10.1096/fasebj.8.15.8001744. [DOI] [PubMed] [Google Scholar]
- 16.Luden G. The blood cholesterol-its importance, and the value of its determination in cancer research. Can. Med. Assoc. J. 1922;12(3):147–152. [PMC free article] [PubMed] [Google Scholar]
- 17.Webb H.J. Cancer, its nature and its treatment. Lancet. 1901;158:976–978. doi: 10.1016/S0140-6736(01)73258-8. [DOI] [Google Scholar]
- 18.Currie A.N. The cholesterol of blood in malignant disease. Br. J. Exp. Pathol. 1924;5(5):293–299. [Google Scholar]
- 19.Mattick W.L., Buchwald K. Blood cholesterol studies in cancer, II. With investigations as to possible diagnostic relations. Cancer Res. 1928;12(3):236–245. doi: 10.1158/jcr.1928.236. [DOI] [Google Scholar]
- 20.Hu J., La Vecchia C., de Groh M., Negri E., Morrison H., Mery L. Dietary cholesterol intake and cancer. Ann. Oncol. 2012;23(2):491–500. doi: 10.1093/annonc/mdr155. [DOI] [PubMed] [Google Scholar]
- 21.Zhou P., Li B., Liu B., Chen T., Xiao J. Prognostic role of serum total cholesterol and high-density lipoprotein cholesterol in cancer survivors: a systematic review and meta-analysis. Clin. Chim. Acta. 2018;477:94–104. doi: 10.1016/j.cca.2017.11.039. [DOI] [PubMed] [Google Scholar]
- 22.Ding X., Zhang W., Li S., Yang H. The role of cholesterol metabolism in cancer. Am. J. Cancer Res. 2019;9(2):219–227. [PMC free article] [PubMed] [Google Scholar]
- 23.Yan A., Jia Z., Qiao C., Wang M., Ding X. Cholesterol metabolism in drug resistant cancer (Review) Int. J. Oncol. 2020;57:1103–1115. doi: 10.3892/ijo.2020.5124. [DOI] [PubMed] [Google Scholar]
- 24.Edwin A.D., Gray T.M., Myrton F.B. Tumor sterols. Metabolism. 1969;18(8):646–651. doi: 10.1016/0026-0495(69)90077-8. [DOI] [PubMed] [Google Scholar]
- 25.Kim S., Lee M., Dhanasekaran D.N., Song Y.S. Activation of LXRɑ/β by cholesterol in malignant ascites promotes chemoresistance in ovarian cancer. BMC Cancer. 2018;18(1):1232. doi: 10.1186/s12885-018-5152-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y., Hughes-Fulford M. Human prostate cancer cells lack feedback regulation of low-density lipoprotein receptor and its regulator, SREBP2. Int. J. Cancer. 2001;91(1):41–45. doi: 10.1002/1097-0215(20010101)91. https://doi.org/1<41::aid-ijc1009>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 27.Niendorf A., Nägele H., Gerding D., Meyer-Pannwitt U., Gebhardt A. Increased LDL receptor mRNA expression in colon cancer is correlated with a rise in plasma cholesterol levels after curative surgery. Int. J. Cancer. 1995;61(4):461–464. doi: 10.1002/ijc.2910610405. [DOI] [PubMed] [Google Scholar]
- 28.Guillaumond F., Bidaut G., Ouaissi M., Servais S., Gouirand V., Olivares O. Cholesterol uptake disruption, in association with chemotherapy, is a promising combined metabolic therapy for pancreatic adenocarcinoma. Proc. Natl. Acad. Sci. 2015;112(8):2473–2478. doi: 10.1073/pnas.1421601112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang C., Li P., Xuan J., Zhu C., Liu J., Shan L. Cholesterol Enhances Colorectal Cancer Progression via ROS Elevation and MAPK Signaling Pathway Activation. Cell. Physiol. Biochem. 2017;42(2):729–742. doi: 10.1159/000477890. [DOI] [PubMed] [Google Scholar]
- 30.Gorin A., Gabitova L., Astsaturov I. Regulation of cholesterol biosynthesis and cancer signaling. Curr. Opin. Pharmacol. 2012;12(6):710–716. doi: 10.1016/j.coph.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.dos Santos C.R., Domingues G., Matias I., Matos J., Fonseca I., de Almeida J.M. LDL-cholesterol signaling induces breast cancer proliferation and invasion. Lipids Health Dis. 2014;13:16. doi: 10.1186/1476-511X-13-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raza S., Ohm J.E., Dhasarathy A., Schommer J., Roche C., Hammer K.D. The cholesterol metabolite 27-hydroxycholesterol regulates p53 activity and increases cell proliferation via MDM2 in breast cancer cells. Mol. Cell. Biochem. 2015;410(1–2):187–195. doi: 10.1007/s11010-015-2551-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silvente-Poirot S., Dalenc F., Poirot M. The effects of cholesterol-derived oncometabolites on nuclear receptor function in cancer. Cancer Res. 2018;78(17):4803–4808. doi: 10.1158/0008-5472.CAN-18-1487. [DOI] [PubMed] [Google Scholar]
- 34.Gu L., Saha S.T., Thomas J., Kaur M. Targeting cellular cholesterol for anticancer therapy. FEBS J. 2019;286(21):192–4208. doi: 10.1111/febs.15018. [DOI] [PubMed] [Google Scholar]
- 35.Mehta N., Hordines J., Sykes D., Doerr R.J., Cohen S.A. Low density lipoproteins and Lovastatin modulate the organ-specific transendothelial migration of primary and metastatic human colon adenocarcinoma cell lines in vitro. Clin. Exp. Metastasis. 1998;16(7):587–594. doi: 10.1023/A:1006548902592. [DOI] [PubMed] [Google Scholar]
- 36.Kopecka J., Campia I., Olivero P., Pescarmona G., Ghigo D., Bosia A. A LDL-masked liposomal-doxorubicin reverses drug resistance in human cancer cells. J. Control. Release. 2011;149(2):196–205. doi: 10.1016/j.jconrel.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Kuzu O.F., Noory M.A., Robertson G.P. The role of cholesterol in cancer. Cancer Res. 2016;76(8):2063–2070. doi: 10.1158/0008-5472.CAN-15-2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khwairakpam A.D., Shyamananda M.S., Sailo B.L., Rathnakaram S.R., Padmavathi G., Kotoky J. ATP citrate lyase (ACLY): a promising target for cancer prevention and treatment. Curr. Drug Targets. 2015;16(2):156–163. doi: 10.2174/1389450115666141224125117. [DOI] [PubMed] [Google Scholar]
- 39.Cerqueira N.M., Oliveira E.F., Gesto D.S., Santos-Martins D., Moreira C., Moorthy H.N. Cholesterol Biosynthesis: a Mechanistic Overview. Biochemistry. 2016;55(39):5483–5506. doi: 10.1021/acs.biochem.6b00342. (2016) [DOI] [PubMed] [Google Scholar]
- 40.Hu J., Zhang Z., Shen W., Azhar S. Cellular cholesterol delivery, intracellular processing and utilization for biosynthesis of steroid hormones. Nutr. Metab. (Lond) 2010;7:47. doi: 10.1186/1743-7075-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang B., Song B., Xu C. Cholesterol metabolism in cancer: mechanisms and therapeutic opportunities. Nat. Metab. 2020;2:132–141. doi: 10.1038/s42255-020-0174-0. [DOI] [PubMed] [Google Scholar]
- 42.Wang B., Tontonoz P. Liver X receptors in lipid signalling and membrane homeostasis. Nat. Rev. Endocrinol. 2018;14(8):452–463. doi: 10.1038/s41574-018-0037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siperstein M.D., Fagan V.M. Deletion of the cholesterol-negative feedback system in liver tumors. Cancer Res. 1964;24(7):1108–1115. [PubMed] [Google Scholar]
- 44.Antalis C.J., Arnold T., Rasool T., Lee B., Buhman K.K., Siddiqui R.A. High ACAT1 expression in estrogen receptor negative basal-like breast cancer cells is associated with LDL-induced proliferation. Breast Cancer Res. Treat. 2010;122(3):661–670. doi: 10.1007/s10549-009-0594-8. [DOI] [PubMed] [Google Scholar]
- 45.Kitahara C.M., Berrington de González A., Freedman N.D., Huxley R., Mok Y., Jee S.H. Total cholesterol and cancer risk in a large prospective study in Korea. J. Clin. Oncol. 2011;29(12):1592–1598. doi: 10.1200/JCO.2010.31.5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 47.Mamtani R., Lewis J.D., Scott F.I., Ahmad T., Goldberg D.S., Datta J., Yang Y.X., Boursi B. Disentangling the association between statins, cholesterol, and colorectal cancer: a Nested Case-Control Study. PLoS Med. 2016;13(4) doi: 10.1371/journal.pmed.1002007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mannes G.A., Maier A., Thieme C., Wiebecke B., Paumgartner G. Relation between the frequency of colorectal adenoma and the serum cholesterol level. N. Engl. J. Med. 1986;315(26):1634–1638. doi: 10.1056/NEJM198612253152602. [DOI] [PubMed] [Google Scholar]
- 49.Park S.K., Joo J.S., Kim D.H., Kim Y.E., Kang D., Yoo K.Y. Association of serum lipids and glucose with the risk of colorectal adenomatous polyp in men: a case-control study in Korea. J. Korean Med. Sci. 2000;15(6):690–695. doi: 10.3346/jkms.2000.15.6.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodriguez-Broadbent H., Law P.J., Sud A., Palin K., Tuupanen S., Gylfe A. Mendelian randomisation implicates hyperlipidaemiaasa risk factor for colorectal cancer. Int. J. Cancer. 2017;140(12):2701–2708. doi: 10.1002/ijc.30709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neugut A.I., Johnsen C.M., Fink D.J. Serum cholesterol levels in adenomatous polyps and cancer of the colon. A case-control study. JAMA. 1986;255(3):365–367. [PubMed] [Google Scholar]
- 52.Zhang X., Zhao X.W., Liu D.B., Han C.Z., Du L.L., Jing J.X. Lipid levels in serum and cancerous tissues of colorectal cancer patients. World J. Gastroenterol. 2014;20(26):8646–8652. doi: 10.3748/wjg.v20.i26.8646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li T., Qian Y., Li H., Deng J. Combination of serum lipids and cancer antigens as a novel marker for colon cancer diagnosis. Lipids Health Dis. 2018;17(1):261. doi: 10.1186/s12944-018-0911-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cowan L.D., O'Connell D.L., Criqui M.H., Barrett-Connor E., Bush T.L., Wallace R.B. Cancer mortality and lipid and lipoprotein levels. Lipid Research Clinics Program Mortality Follow-up Study. Am. J. Epidemiol. 1990;131(3):468–482. doi: 10.1093/oxfordjournals.aje.a115521. [DOI] [PubMed] [Google Scholar]
- 55.Törnberg S.A., Holm L.E., Carstensen J.M., Eklund G.A. Risks of cancer of the colon and rectum in relation to serum cholesterol and beta-lipoprotein. N. Engl. J. Med. 1986;315(26):1629–1633. doi: 10.1056/NEJM198612253152601. [DOI] [PubMed] [Google Scholar]
- 56.Wang B., Rong X., Palladino E.N.D., Wang J., Fogelman A.M., Martín M.G. Phospholipid remodeling and cholesterol availability regulate intestinal stemness and tumorigenesis. Cell Stem Cell. 2018;22(2):206–220. doi: 10.1016/j.stem.2017.12.017. e4http://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beverly A.P. Cholesterol and stem cell proliferation. Science. 2018;359(6378):881–882. doi: 10.1126/science.359.6378.881-b. [DOI] [Google Scholar]
- 58.Liu K., Zhang W., Dai Z., Wang M., Tian T., Liu X. Association between body mass index and breast cancer risk: evidence based on a dose-response meta-analysis. Cancer Manag. Res. 2018;10:143–151. doi: 10.2147/CMAR.S144619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li C., Yang L., Zhang D., Jiang W. Systematic review and meta-analysis suggest that dietary cholesterol intake increases risk of breast cancer. Nutr. Res. 2016;36(7):627–635. doi: 10.1016/j.nutres.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 60.Ostroumova M.N., Kovalenko I.G., Bershteĭn L.M., Tsyrlina E.V. Dil'man VM. Osobennosti dislipidemii u onkologicheskikh bol'nykh [Characteristics of dyslipidemia in cancer patients] Vopr. Onkol. 1986;32(1):34–43. [PubMed] [Google Scholar]
- 61.Wei L.J., Zhang C., Zhang H., Wei X., Li S.X., Liu J.T. A case-control study on the association between serum lipid level and the risk of breast cancer. Zhonghua Yu Fang Yi Xue Za Zhi. 2016;50(12):1091–1095. doi: 10.3760/cma.j.issn.0253-9624.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 62.Sharma V., Sharma A. Serum cholesterol levels in carcinoma breast. Indian J. Med. Res. 1991;94:193–196. [PubMed] [Google Scholar]
- 63.Boyd N.F., McGuire V. Evidence of association between plasma high-density lipoprotein cholesterol and risk factors for breast cancer. J. Natl. Cancer Inst. 1990;82(6):460–468. doi: 10.1093/jnci/82.6.460. [DOI] [PubMed] [Google Scholar]
- 64.Martin L.J., Melnichouk O., Huszti E., Connelly P.W., Greenberg C.V. Serum lipids, lipoproteins, and risk of breast cancer: a nested case-control study using multiple time points. J. Natl. Cancer Inst. 2015;107(5) doi: 10.1093/jnci/djv032. djv032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Flote V.G., Frydenberg H., Ursin G., Iversen A., Fagerland M.W., Ellison P.T. High-density lipoprotein-cholesterol, daily estradiol and progesterone, and mammographic density phenotypes in premenopausal women. Cancer Prev. Res. (Phila.) 2015;8(6):535–544. doi: 10.1158/1940-6207.CAPR-14-0267. [DOI] [PubMed] [Google Scholar]
- 66.Rodrigues Dos Santos C., Fonseca I., Dias S., Mendes de Almeida J.C. Plasma level of LDL-cholesterol at diagnosis is a predictor factor of breast tumor progression. BMC Cancer. 2014;14:132. doi: 10.1186/1471-2407-14-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Freed-Pastor W.A., Mizuno H., Zhao X., Langerød A., Moon S.H., Rodriguez-Barrueco R. Mutant p53 disrupts mammary tissue architecture via the mevalonate pathway. Cell. 2012;148(1–2):244–258. doi: 10.1016/j.cell.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de Gonzalo-Calvo D., López-Vilaró L., Nasarre L., Perez-Olabarria M., Vázquez T. Intratumor cholesteryl ester accumulation is associated with human breast cancer proliferation and aggressive potential: a molecular and clinicopathological study. BMC Cancer. 2015;15:460. doi: 10.1186/s12885-015-1469-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ni H., Liu H., Gao R. Serum lipids and breast cancer risk: a meta-analysis of prospective cohort studies. PLoS ONE. 2015;10(11) doi: 10.1371/journal.pone.0142669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun Y.S., Zhao Z., Yang Z.N., Xu F., Lu H.J., Zhu Z.Y. Risk factors and preventions of breast cancer. Int. J. Biol. Sci. 2017;13(11):1387–1397. doi: 10.7150/ijbs.21635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Elkhadrawy T.M., Ahsan H., Neugut A.I. Serum cholesterol and the risk of ductal carcinoma in situ: a case-control study. Eur. J. Cancer Prev. 1998;7(5):393–396. doi: 10.1097/00008469-199810000-00004. [DOI] [PubMed] [Google Scholar]
- 72.Ha M., Sung J., Song Y.M. Serum total cholesterol and the risk of breast cancer in postmenopausal Korean women. Cancer Causes Control. 2009;20(7):1055–1060. doi: 10.1007/s10552-009-9301-7. [DOI] [PubMed] [Google Scholar]
- 73.Törnberg S.A., Holm L.E., Carstensen J.M. Breast cancer risk in relation to serum cholesterol, serum beta-lipoprotein, height, weight, and blood pressure. Acta Oncol. 1988;27(1):31–37. doi: 10.3109/02841868809090315. [DOI] [PubMed] [Google Scholar]
- 74.Pelton K., Coticchia C.M., Curatolo A.S., Schaffner C.P., Zurakowski D., Solomon K.R. Hypercholesterolemia induces angiogenesis and accelerates growth of breast tumors in vivo. Am. J. Pathol. 2014;184(7):2099–2110. doi: 10.1016/j.ajpath.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu J., Xu A., Lam K.S., Wong N.S., Chen J., Shepherd P.R. Cholesterol-induced mammary tumorigenesis is enhanced by adiponectin deficiency: role of LDL receptor upregulation. Oncotarget. 2013;4(10):1804–1818. doi: 10.18632/oncotarget.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gallagher E.J., Zelenko Z., Neel B.A., Antoniou I.M., Rajan L., Kase N. Elevated tumor LDLR expression accelerates LDL cholesterol-mediated breast cancer growth in mouse models of hyperlipidemia. Oncogene. 2017;36(46):6462–6471. doi: 10.1038/onc.2017.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Antalis C.J., Uchida A., Buhman K.K., Siddiqui R.A. Migration of MDA-MB-231 breast cancer cells depends on the availability of exogenous lipids and cholesterol esterification. Clin. Exp. Metastasis. 2011;28(8):733–741. doi: 10.1007/s10585-011-9405-9. [DOI] [PubMed] [Google Scholar]
- 78.Lu C.W., Lo Y.H., Chen C.H., Lin C.Y., Tsai C.H., Chen P.J. VLDL and LDL, but not HDL, promote breast cancer cell proliferation, metastasis and angiogenesis. Cancer Lett. 2017;388:130–138. doi: 10.1016/j.canlet.2016.11.033. [DOI] [PubMed] [Google Scholar]
- 79.Nelson E.R., Wardell S.E., Jasper J.S., Park S., Suchindran S., Howe M.K. 27-Hydroxycholesterol links hypercholesterolemia and breast cancer pathophysiology. Science. 2013;342(6162):1094–1098. doi: 10.1126/science.1241908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lappano R., Recchia A.G., De Francesco E.M., Angelone T., Cerra M.C., Picard D. The cholesterol metabolite 25-hydroxycholesterol activates estrogen receptor α-mediated signaling in cancer cells and in cardiomyocytes. PLoS ONE. 2011;6(1):e16631. doi: 10.1371/journal.pone.0016631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wolfe A.R., Atkinson R.L., Reddy J.P., Debeb B.G., Larson R., Li L. High-density and very-low-density lipoprotein have opposing roles in regulating tumor-initiating cells and sensitivity to radiation in inflammatory breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 2015;91(5):1072–1080. doi: 10.1016/j.ijrobp.2014.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jamnagerwalla J., Howard L.E., Allott E.H., Vidal A.C., Moreira D.M., Castro-Santamaria R. Serum cholesterol and risk of high-grade prostate cancer: results from the REDUCE study. Prostate Cancer Prostatic Dis. 2018;21(2):252–259. doi: 10.1038/s41391-017-0030-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jacobs E.J., Stevens V.L., Newton C.C., Gapstur S.M. Plasma total, LDL, and HDL cholesterol and risk of aggressive prostate cancer in the cancer prevention study II nutrition cohort. Cancer Causes Control. 2012;23(8):1289–1296. doi: 10.1007/s10552-012-0006-y. [DOI] [PubMed] [Google Scholar]
- 84.YuPeng L., YuXue Z., PengFei L., Cheng C., YaShuang Z., DaPeng L. Cholesterol levels in blood and the risk of prostate cancer: a meta-analysis of 14 prospective studies. Cancer Epidemiol. Biomarkers Prev. 2015;24(7):1086–1093. doi: 10.1158/1055-9965.EPI-14-1329. [DOI] [PubMed] [Google Scholar]
- 85.Bull C.J., Bonilla C., Holly J.M., Perks C.M., Davies N., Haycock P. Blood lipids and prostate cancer: a Mendelian randomization analysis. Cancer Med. 2016;5(6):1125–1136. doi: 10.1002/cam4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zapata D., Howard L.E., Allott E.H., Hamilton R.J., Goldberg K., Freedland S.J. Is PSA related to serum cholesterol and does the relationship differ between black and white men? Prostate. 2015;75(16):1877–1885. doi: 10.1002/pros.23069. [DOI] [PubMed] [Google Scholar]
- 87.Stopsack K.H., Gerke T.A., Andrén O., Andersson S.O., Giovannucci E.L., Mucci L.A. Cholesterol uptake and regulation in high-grade and lethal prostate cancers. Carcinogenesis. 2017;38(8):806–811. doi: 10.1093/carcin/bgx058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Singh G., Sankanagoudar S., Dogra P., Chandra N.C. Interlink between cholesterol & cell cycle in prostate carcinoma. Indian J. Med. Res. 2017;146(Suppl):S38–S44. doi: 10.4103/ijmr.IJMR_1639_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thysell E., Surowiec I., Hörnberg E., Crnalic S., Widmark A., Johansson A.I. Metabolomic characterization of human prostate cancer bone metastases reveals increased levels of cholesterol. PLoS ONE. 2010;5(12):e14175. doi: 10.1371/journal.pone.0014175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Solomon K.R., Pelton K., Boucher K., Joo J., Tully C., Zurakowski D. Ezetimibe is an inhibitor of tumor angiogenesis. Am. J. Pathol. 2009;174(3):1017–1026. doi: 10.2353/ajpath.2009.080551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mostaghel E.A., Solomon K.R., Pelton K., Freeman M.R., Montgomery R.B. Impact of circulating cholesterol levels on growth and intratumoral androgen concentration of prostate tumors. PLoS ONE. 2012;7(1):e30062. doi: 10.1371/journal.pone.0030062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Awad A.B., Fink C.S., Williams H., Kim U. In vitro and in vivo (SCID mice) effects of phytosterols on the growth and dissemination of human prostate cancer PC-3 cells. Eur. J. Cancer Prev. 2001;10(6):507–513. doi: 10.1097/00008469-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 93.Murtola T.J., Syvälä H., Pennanen P., Bläuer M., Solakivi T., Ylikomi T. The importance of LDL and cholesterol metabolism for prostate epithelial cell growth. PLoS ONE. 2012;7(6):e39445. doi: 10.1371/journal.pone.0039445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Alfaqih M.A., Nelson E.R., Liu W., Safi R., Jasper J.S., Macias E. CYP27A1 loss dysregulates cholesterol homeostasis in prostate cancer. Cancer Res. 2017;77(7):1662–1673. doi: 10.1158/0008-5472.CAN-16-2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schörghofer D., Kinslechner K., Preitschopf A., Schütz B., Röhrl C., Hengstschläger M. The HDL receptor SR-BI is associated with human prostate cancer progression and plays a possible role in establishing androgen independence. Reprod. Biol. Endocrinol. 2015;13:88. doi: 10.1186/s12958-015-0087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dillard P.R., Lin M.F., Khan S.A. Androgen-independent prostate cancer cells acquire the complete steroidogenic potential of synthesizing testosterone from cholesterol. Mol. Cell. Endocrinol. 2008;295(1–2):115–120. doi: 10.1016/j.mce.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bell A.W. Lipid metabolism in liver and selected tissues and in the whole body of ruminant animals. Prog. Lipid Res. 1979;18(3):117–164. doi: 10.1016/0163-7827(79)90013-4. [DOI] [PubMed] [Google Scholar]
- 98.Venturini I., Amedei R., Modonesi G., Cosenza R., Miglioli L., Cioni G. May plasma cholesterol level be considered a neoplastic marker in liver disease from cirrhosis to hepatocellular carcinoma? Ital. J. Gastroenterol. Hepatol. 1999;31(1):61–65. [PubMed] [Google Scholar]
- 99.Antoniello S., Auletta M., Cigolari S., Vecchione R., Cacciatore L. Epatocarcinoma associato ad ipoglicemia ed ipercolesterolemia LDL [Hepatocarcinoma associated with hypoglycemia and LDL hypercholesterolemia] Minerva Dietol. Gastroenterol. 1986;32(1):79–85. [PubMed] [Google Scholar]
- 100.Hirayama C., Yamanishi Y., Irisa T. Serum cholesterol and squalene in hepatocellular carcinoma. Clin. Chim. Acta. 1979;91(1):53–57. doi: 10.1016/0009-8981(79)90470-4. [DOI] [PubMed] [Google Scholar]
- 101.Rubiés-Prat J., Masana L., Masdeu S., Nubiola A.R., Chacón P. Hipercolesterolemia asociada a hepatocarcinoma [hypercholesterolemia associated with hepatocarcinoma] Med. Clin. (Barc) 1983;80(4):175–176. [PubMed] [Google Scholar]
- 102.Venturini I., Zeneroli M.L., Corsi L., Baraldi C., Ferrarese C., Pecora N. Diazepam binding inhibitor and total cholesterol plasma levels in cirrhosis and hepatocellular carcinoma. Regul. Pept. 1998;74(1):31–34. doi: 10.1016/s0167-0115(98)000135. [DOI] [PubMed] [Google Scholar]
- 103.Ahaneku J.E., Taylor G.O., Olubuyide I.O., Agbedana E.O. Abnormal lipid and lipoprotein patterns in liver cirrhosis with and without hepatocellular carcinoma. J. Pak. Med. Assoc. 1992;42(11):260–263. [PubMed] [Google Scholar]
- 104.Muraji T., Woolley M.M., Sinatra F., Siegel S.M., Isaacs H. The prognostic implication of hypercholesterolemia in infants and children with hepatoblastoma. J. Pediatr. Surg. 1985;20(3):228–230. doi: 10.1016/s0022-3468(85)80108-1. [DOI] [PubMed] [Google Scholar]
- 105.Bricker L.A., Morris H.P., Siperstein M.D. Loss of the cholesterol feedback system in the intact hepatoma-bearing rat. J. Clin. Invest. 1972;51(2):206–215. doi: 10.1172/JCI106805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhao J., Zhao Y., Wang H., Gu X., Ji J., Gao C. Association between metabolic abnormalities and HBV related hepatocelluar carcinoma in Chinese: a cross-sectional study. Nutr. J. 2011;10:49. doi: 10.1186/1475-2891-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Khattab M.A., Eslam M., Mousa Y.I., Ela-adawy N., Fathy S., Shatat M. Association between metabolic abnormalities and hepatitis C-related hepatocellular carcinoma. Ann. Hepatol. 2012;11(4):487–494. [PubMed] [Google Scholar]
- 108.Saito N., Sairenchi T., Irie F., Iso H., Iimura K., Watanabe H. Low serum LDL cholesterol levels are associated with elevated mortality from liver cancer in Japan: the Ibaraki Prefectural health study. Tohoku J. Exp. Med. 2013;229(3):203–211. doi: 10.1620/tjem.229.203. [DOI] [PubMed] [Google Scholar]
- 109.Colhoun E.D., 5th, Forsberg C.G., Chavin K.D., Baliga P.K., Taber D.J. Incidence and risk factors of hepatocellular carcinoma after orthotopic liver transplantation. Surgery. 2017;161(3):830–836. doi: 10.1016/j.surg.2016.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee Y.L., Li W.C., Tsai T.H., Chiang H.Y., Ting C.T. Body mass index and cholesterol level predict surgical outcome in patients with hepatocellular carcinoma in Taiwan - a cohort study. Oncotarget. 2016;7(16):22948–22959. doi: 10.18632/oncotarget.8312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Qin W.H., Yang Z.S., Li M., Chen Y., Zhao X.F., Qin Y.Y. High serum levels of cholesterol increase antitumor functions of nature killer cells and reduce growth of liver tumors in mice. Gastroenterology. 2020;158(6):1713–1727. doi: 10.1053/j.gastro.2020.01.028. [DOI] [PubMed] [Google Scholar]
- 112.Chen H., Qin S., Wang M., Zhang T., Zhang S. Association between cholesterol intake and pancreatic cancer risk: evidence from a meta-analysis. Sci. Rep. 2015;5:8243. doi: 10.1038/srep08243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen W.C., Boursi B., Mamtani R., Yang Y.X. Total serum cholesterol and pancreatic cancer: a nested case-control study. Cancer Epidemiol. Biomarkers Prev. 2019;28(2):363–369. doi: 10.1158/1055-9965.EPI-18-0421. [DOI] [PubMed] [Google Scholar]
- 114.Li J., Gu D., Lee S.S., Song B., Bandyopadhyay S., Chen S. Abrogating cholesterol esterification suppresses growth and metastasis of pancreatic cancer. Oncogene. 2016;35(50):6378–6388. doi: 10.1038/onc.2016.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.International agency for research on cancer, WHO. https://gco.iarc.fr/today/data/factsheets/cancers/15-Lung-fact-sheet.pdf; 2018 [accessed 19 September 2020].
- 116.Lyu Z.Y., Li N., Wang G., Su K., Li F., Guo L.W. Association between total cholesterol and risk of lung cancer incidence in men: a prospective cohort study. Zhonghua Liu Xing Bing Xue Za Zhi. 2018;39(5):604–608. doi: 10.3760/cma.j.issn.0254-6450.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 117.Ahn J., Lim U., Weinstein S.J., Schatzkin A., Hayes R.B., Virtamo J. Prediagnostic total and high-density lipoprotein cholesterol and risk of cancer. Cancer Epidemiol. Biomarkers Prev. 2009;18(11):2814–2821. doi: 10.1158/1055-9965.EPI-08-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang Y., Xu J., Lou Y., Hu S., Yu K., Li R. Pretreatment direct bilirubin and total cholesterol are significant predictors of overall survival in advanced non-small-cell lung cancer patients with EGFR mutations. Int. J. Cancer. 2017;140(7):1645–1652. doi: 10.1002/ijc.30581. [DOI] [PubMed] [Google Scholar]
- 119.Sok M., Ravnik J., Ravnik M. Preoperative total serum cholesterol as a prognostic factor for survival in patients with resectable non-small-cell lung cancer. Wien. Klin. Wochenschr. 2009;121(9–10):314–317. doi: 10.1007/s00508-009-1169-8. [DOI] [PubMed] [Google Scholar]
- 120.Lin X., Lu L., Liu L., Wei S., He Y., Chang J. Blood lipids profile and lung cancer risk in a meta-analysis of prospective cohort studies. J. Clin. Lipidol. 2017;11(4):1073–1081. doi: 10.1016/j.jacl.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 121.Chang A.K., Barrett-Connor E., Edelstein S. Low plasma cholesterol predicts an increased risk of lung cancer in elderly women. Prev. Med. 1995;24(6):557–562. doi: 10.1006/pmed.1995.1089. [DOI] [PubMed] [Google Scholar]
- 122.Li J.R., Zhang Y., Zheng J.L. Decreased pretreatment serum cholesterol level is related with poor prognosis in resectable non-small cell lung cancer. Int. J. Clin. Exp. Pathol. 2015;8(9):11877–11883. [PMC free article] [PubMed] [Google Scholar]
- 123.Umeki S. Decreases in serum cholesterol levels in advanced lung cancer. Respiration. 1993;60(3):178–181. doi: 10.1159/000196195. [DOI] [PubMed] [Google Scholar]
- 124.Siemianowicz K., Gminski J., Stajszczyk M., Wojakowski W., Goss M., Machalski M. Serum HDL cholesterol concentration in patients with squamous cell and small cell lung cancer. Int. J. Mol. Med. 2000;6(3):307–311. doi: 10.3892/ijmm.6.3.307. [DOI] [PubMed] [Google Scholar]
- 125.Chi P.D., Liu W., Chen H., Zhang J.P., Lin Y., Zheng X. High-density lipoprotein cholesterol is a favorable prognostic factor and negatively correlated with C-reactive protein level in non-small cell lung carcinoma. PLoS ONE. 2014;9(3):e91080. doi: 10.1371/journal.pone.0091080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Knekt P., Seppänen R., Järvinen R., Virtamo J., Hyvönen L., Pukkala E. Dietary cholesterol, fatty acids, and the risk of lung cancer among men. Nutr. Cancer. 1991;16(3–4):267–275. doi: 10.1080/01635589109514165. [DOI] [PubMed] [Google Scholar]
- 127.Wu Y., Zheng W., Sellers T.A., Kushi L.H., Bostick R.M., Potter J.D. Dietary cholesterol, fat, and lung cancer incidence among older women: the Iowa Women's Health Study (United States) Cancer Causes Control. 1994;5(5):395–400. doi: 10.1007/BF01694752. [DOI] [PubMed] [Google Scholar]
- 128.Smith-Warner S.A., Ritz J., Hunter D.J., Albanes D., Beeson W.L., van den Brandt P.A. Dietary fat and risk of lung cancer in a pooled analysis of prospective studies. Cancer Epidemiol. Biomarkers Prev. 2002;11(10Pt 1):987–992. [PubMed] [Google Scholar]
- 129.Lin X., Liu L., Fu Y., Gao J., He Y., Wu Y. Dietary cholesterol intake and risk of lung cancer: a meta-analysis. Nutrients. 2018;10(2):185. doi: 10.3390/nu10020185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shekelle R.B., Rossof A.H., Stamler J. Dietary cholesterol and incidence of lung cancer: the Western Electric Study. Am. J. Epidemiol. 1991;134(5):480–484. doi: 10.1093/oxfordjournals.aje.a116119. discussion 543-4. [DOI] [PubMed] [Google Scholar]
- 131.Chen L., Zhang L., Xian G., Lv Y., Lin Y., Wang Y. 25-Hydroxycholesterol promotes migration and invasion of lung adenocarcinoma cells. Biochem. Biophys. Res. Commun. 2017;484(4):857–863. doi: 10.1016/j.bbrc.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 132.Zhu Y.H., Jeong S., Wu M., Jin Z.Y., Zhou J.Y., Han R.Q. Dietary intake of fatty acids, total cholesterol, and stomach cancer in a chinese population. Nutrients. 2019;11(8):1730. doi: 10.3390/nu11081730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.González C.A., Riboli E., Badosa J., Batiste E., Cardona T., Pita S. Nutritional factors and gastric cancer in Spain. Am. J. Epidemiol. 1994;139(5):466–473. doi: 10.1093/oxfordjournals.aje.a117029. [DOI] [PubMed] [Google Scholar]
- 134.López-Carrillo L., López-Cervantes M., Ward M.H., Bravo-Alvarado J., Ramírez-Espitia A. Nutrient intake and gastric cancer in Mexico. Int. J. Cancer. 1999;83(5):601–605. doi: 10.1002/(sici)1097-0215(19991126)83. https://doi.org/5<601::aid-ijc5>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 135.Mayne S.T., Risch H.A., Dubrow R., Chow W.H., Gammon M.D., Vaughan T.L. Nutrient intake and risk of subtypes of esophageal and gastric cancer. Cancer Epidemiol. Biomarkers Prev. 2001;10(10):1055–1062. [PubMed] [Google Scholar]
- 136.Hu J., La Vecchia C., Negri E., de Groh M., Morrison H., Mery L. Canadian cancer registries epidemiology research group. Macronutrient intake and stomach cancer. Cancer Causes Control. 2015;26(6):839–847. doi: 10.1007/s10552-015-0557-9. [DOI] [PubMed] [Google Scholar]
- 137.Lucenteforte E., Bosetti C., Gallus S., Bertuccio P., Pelucchi C., Tavani A. Macronutrients, fatty acids and cholesterol intake and stomach cancer risk. Ann. Oncol. 2009;20(8):1434–1438. doi: 10.1093/annonc/mdp009. [DOI] [PubMed] [Google Scholar]
- 138.Jan H.M., Chen Y.C., Yang T.C., Ong L.L., Chang C.C., Muthusamy S. Cholesteryl α-D-glucoside 6-acyltransferase enhances the adhesion of Helicobacter pylori to gastric epithelium. Commun. Biol. 2020;3(1):120. doi: 10.1038/s42003-020-0855-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ikezaki H., Furusyo N., Jacques P.F., Shimizu M., Murata M., Schaefer E.J. Higher dietary cholesterol and ω-3 fatty acid intakes are associated with a lower success rate of Helicobacter pylori eradication therapy in Japan. Am. J. Clin. Nutr. 2017;(2):581–588. doi: 10.3945/ajcn.116.144873. [DOI] [PubMed] [Google Scholar]
- 140.Chao F.C., Efron B., Wolf P. The possible prognostic usefulness of assessing serum proteins and cholesterol in malignancy. Cancer. 1975;35(4):1223–1229. doi: 10.1002/10970142(197504)35. https://doi.org/4<1223::aid−cncr2820350429>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 141.Kim T.H., Ahn S.J., Jung W.T., Lee O.J., Ha W.S., Jang J.S. Clinical Significance of the Levels of Serum Cholesterol in Patients with Gastric Cancer. Cancer Res. Treat. 2003;35(4):335–340. doi: 10.4143/crt.2003.35.4.335. [DOI] [PubMed] [Google Scholar]
- 142.Tomiki Y., Suda S., Tanaka M., Okuzawa A., Matsuda M., Ishibiki Y. Reduced low-density-lipoprotein cholesterol causing low serum cholesterol levels in gastrointestinal cancer: a case control study. J. Exp. Clin. Cancer Res. 2004;23(2):233–240. [PubMed] [Google Scholar]
- 143.Törnberg S.A., Carstensen J.M., Holm L.E. Risk of stomach cancer in association with serum cholesterol and beta-lipoprotein. Acta Oncol. 1988;27(1):39–42. doi: 10.3109/02841868809090316. PMID:3365353. [DOI] [PubMed] [Google Scholar]
- 144.Kang R., Li P., Wang T., Li X., Wei Z., Zhang Z. Apolipoprotein E epsilon 2 allele and low serum cholesterol as risk factors for gastric cancer in a Chinese Han population. Sci. Rep. 2016;6:19930. doi: 10.1038/srep19930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Asano K., Kubo M., Yonemoto K., Doi Y., Ninomiya T., Tanizaki Y. Impact of serum total cholesterol on the incidence of gastric cancer in a population-based prospective study: the Hisayama study. Int. J. Cancer. 2008;122(4):909–914. doi: 10.1002/ijc.23191. [DOI] [PubMed] [Google Scholar]
- 146.Iso H., Ikeda A., Inoue M., Sato S., Tsugane S.JPHC Study Group Serum cholesterol levels in relation to the incidence of cancer: the JPHC study cohorts. Int. J. Cancer. 2009;125(11):2679–2686. doi: 10.1002/ijc.24668. [DOI] [PubMed] [Google Scholar]
- 147.Shin H.J., Roh C.K., Son S.Y., Hoon H., Han S.U. Prognostic value of hypocholesterolemia in patients with gastric cancer. Asian J. Surg. 2020 doi: 10.1016/j.asjsur.2020.08.014. S1015-9584(20)30261-X. [DOI] [PubMed] [Google Scholar]
- 148.Tamura T., Inagawa S., Hisakura K., Enomoto T., Ohkohchi N. Evaluation of serum high-density lipoprotein cholesterol levels as a prognostic factor in gastric cancer patients. J. Gastroenterol. Hepatol. 2012;27(10):1635–1640. doi: 10.1111/j.1440-1746.2012.07189.x. [DOI] [PubMed] [Google Scholar]
- 149.Jung M.K., Jeon S.W., Cho C.M., Tak W.Y., Kweon Y.O., Kim S.K., et al. Hyperglycaemia, hypercholesterolaemia and the risk for developing gastric dysplasia. Dig Liver Dis. 2008;40(5):361–5. https://doi.org/ 10.1016/j.dld.2007.12.002. [DOI] [PubMed]
- 150.Huang Y.K., Kang W.M., Ma Z.Q., Liu Y.Q., Zhou L., Yu J.C. Body mass index, serum total cholesterol, and risk of gastric high-grade dysplasia: a case-control study among Chinese adults. Medicine (Baltimore). 2016;95(35):e4730. doi: 10.1097/MD.0000000000004730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pih G.Y., Gong E.J., Choi J.Y., Kim M.J., Ahn J.Y., Choe J. Associations of serum lipid level with gastric cancer risk, pathology, and prognosis. Cancer Res. Treat. 2020 doi: 10.4143/crt.2020.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kitayama J., Hatano K., Kaisaki S., Suzuki H., Fujii S., Nagawa H. Hyperlipidaemia is positively correlated with lymph node metastasis in men with early gastric cancer. Br. J. Surg. 2004;91(2):191–198. doi: 10.1002/bjs.4391. [DOI] [PubMed] [Google Scholar]
- 153.Enjoji M., Kohjima M., Ohtsu K., Matsunaga K., Murata Y., Nakamuta M. Intracellular mechanisms underlying lipid accumulation (white opaque substance) in gastric epithelial neoplasms: a pilot study of expression profiles of lipid-metabolism-associated genes. J. Gastroenterol. Hepatol. 2016;31(4):776–781. doi: 10.1111/jgh.13216. [DOI] [PubMed] [Google Scholar]
- 154.Caruso M.G., Notarnicola M., Cavallini A., Di Leo A. 3-Hydroxy-3-methylglutaryl coenzyme A reductase activity and low-density lipoprotein receptor expression in diffuse-type and intestinal-type human gastric cancer. J. Gastroenterol. 2002;37(7):504–508. doi: 10.1007/s005350200078. PMID: 12162407. [DOI] [PubMed] [Google Scholar]
- 155.Chushi L., Wei W., Kangkang X., Yongzeng F., Ning X., Xiaolei C. HMGCR is up-regulated in gastric cancer and promotes the growth and migration of the cancer cells. Gene. 2016;587(1):42–47. doi: 10.1016/j.gene.2016.04.029. [DOI] [PubMed] [Google Scholar]
- 156.Chang W.C., Huang S.F., Lee Y.M., Lai H.C., Cheng B.H., Cheng W.C. Cholesterol import and steroidogenesis are biosignatures for gastric cancer patient survival. Oncotarget. 2017;8(1):692–704. doi: 10.18632/oncotarget.13524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang S., Yao Y., Rao C., Zheng G., Chen W. 25-HC decreases the sensitivity of human gastric cancer cells to 5-fluorouracil and promotes cells invasion via the TLR2/NF-κB signaling pathway. Int. J. Oncol. 2019;54(3):966–980. doi: 10.3892/ijo.2019.4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lim S.C., Parajuli K.R., Duong H.Q., Choi J.E., Han S.I. Cholesterol induces autophagic and apoptotic death in gastric carcinoma cells. Int. J. Oncol. 2014;44(3):805–811. doi: 10.3892/ijo.2014.2246. [DOI] [PubMed] [Google Scholar]
- 159.Cancer Research UK. https://www.cancerresearchuk.org/about-cancer/ovarian-cancer/what-is-ovarian-cancer. [Accessed 25th December 2020].
- 160.Risch H.A., Jain M., Marrett L.D., Howe G.R. Dietary fat intake and risk of epithelial ovarian cancer. J. Natl. Cancer Inst. 1994;86(18):1409–1415. doi: 10.1093/jnci/86.18.1409. [DOI] [PubMed] [Google Scholar]
- 161.Kushi L.H., Mink P.J., Folsom A.R., Anderson K.E., Zheng W., Lazovich D. Prospective study of diet and ovarian cancer. Am. J. Epidemiol. 1999;149(1):21–31. doi: 10.1093/oxfordjournals.aje.a009723. PMID:9883790. [DOI] [PubMed] [Google Scholar]
- 162.Pan S.Y., Ugnat A.M., Mao Y., Wen S.W., Johnson K.C. Canadian cancer registries epidemiology research group. A case-control study of diet and the risk of ovarian cancer. Cancer Epidemiol. Biomarkers Prev. 2004;13(9):1521–1527. [PubMed] [Google Scholar]
- 163.Khodavandi A., Alizadeh F., Razis A.F.A. Association between dietary intake and risk of ovarian cancer: a systematic review and meta-analysis. Eur. J. Nutr. 2020 doi: 10.1007/s00394-020-02332-y. [DOI] [PubMed] [Google Scholar]
- 164.Zhang D., Xi Y., Feng Y. Ovarian cancer risk in relation to blood lipid levels and hyperlipidemia: a systematic review and meta-analysis of observational epidemiologic studies. Eur. J. Cancer Prev. 2020 doi: 10.1097/CEJ.0000000000000597. [DOI] [PubMed] [Google Scholar]
- 165.Genkinger J.M., Hunter D.J., Spiegelman D., Anderson K.E., Beeson W.L., Buring J.E. A pooled analysis of 12 cohort studies of dietary fat, cholesterol and egg intake and ovarian cancer. Cancer Causes Control. 2006;17(3):273–285. doi: 10.1007/s10552-005-0455-7. [DOI] [PubMed] [Google Scholar]
- 166.Helzlsouer K.J., Alberg A.J., Norkus E.P., Morris J.S., Hoffman S.C., Comstock G.W. Prospective study of serum micronutrients and ovarian cancer. J. Natl. Cancer Inst. 1996;88(1):32–37. doi: 10.1093/jnci/88.1.32. [DOI] [PubMed] [Google Scholar]
- 167.Delimaris I., Faviou E., Antonakos G., Stathopoulou E., Zachari A., Dionyssiou-Asteriou A. Oxidized LDL, serum oxidizability and serum lipid levels in patients with breast or ovarian cancer. Clin. Biochem. 2007;40(15):1129–1134. doi: 10.1016/j.clinbiochem.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 168.Novikov A.M., Merabishvili N.V., Bassalyk L.S. Lipidy syvorotki krovi u bol'nykh épitelial'nymi opukholiami iaichnikov [Serum lipids in epithelial ovarian tumor patients] Vopr. Onkol. 1982;28(3):67–73. [PubMed] [Google Scholar]
- 169.Gadomska H., Janecki J., Marianowski L., Nowicka G. Lipids in serum of patients with malignant ovarian neoplasms. Int. J. Gynaecol. Obstet. 1997;57(3):287–293. doi: 10.1016/s0020-7292(97)00071-4. [DOI] [PubMed] [Google Scholar]
- 170.Qadir M.I., Malik S.A. Plasma lipid profile in gynecologic cancers. Eur. J. Gynaecol. Oncol. 2008;29(2):158–161. [PubMed] [Google Scholar]
- 171.Onwuka J.U., Okekunle A.P., Olutola O.M., Akpa O.M., Feng R. Lipid profile and risk of ovarian tumours: a meta-analysis. BMC Cancer. 2020;20(1):200. doi: 10.1186/s12885-020-6679-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Avall-Lundqvist E.H., CO Peterson. Serum cholesterol and apolipoprotein B levels may reflect disease activity in ovarian cancer patients. Acta Oncol. 1996;35(8):1007–1010. doi: 10.3109/02841869609100719. [DOI] [PubMed] [Google Scholar]
- 173.Li A.J., Elmore R.G., Chen I.Y., Karlan B.Y. Serum low-density lipoprotein levels correlate with survival in advanced stage epithelial ovarian cancers. Gynecol. Oncol. 2010;116(1):78–81. doi: 10.1016/j.ygyno.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhu F., Xu X., Shi B., Zeng L., Wang L., Wu X. The positive predictive value of low-density lipoprotein for recurrence-free survival in ovarian cancer. Int. J. Gynaecol. Obstet. 2018;143(2):232–238. doi: 10.1002/ijgo.12645. [DOI] [PubMed] [Google Scholar]
- 175.Rana S.V., Babu S.G., Kocchar R. Usefulness of ascitic fluid cholesterol as a marker for malignant ascites. Med. Sci. Monit. 2005;11(3):CR136–CR142. [PubMed] [Google Scholar]
- 176.Grignon D.J., Kirk M.E., Haines D.S. Cholesterol granulomas in lymph nodes draining a benign ovarian neoplasm. Arch. Pathol. Lab. Med. 1985;109(12):1124–1126. [PubMed] [Google Scholar]
- 177.Ayyagari V.N., Wang X., Diaz-Sylvester P.L., Groesch K., Brard L. Assessment of acyl-CoA cholesterol acyltransferase (ACAT-1) role in ovarian cancer progression-An in vitro study. PLoS ONE. 2020;15(1) doi: 10.1371/journal.pone.0228024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yarmolinsky J., Bull C.J., Vincent E.E., Robinson J., Walther A., Smith G.D. Association between genetically proxied inhibition of HMG-CoA reductase and epithelial ovarian cancer. JAMA. 2020;323(7):646–655. doi: 10.1001/jama.2020.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yale medicine. https://www.yalemedicine.org/conditions/blood-cancers [accessed 10 January 2021].
- 180.Yavasoglu I., Sargin G., Yilmaz F., Altındag S., Akgun G., Tombak A. Cholesterol levels in patients with chronic lymphocytic leukemia. J. Natl. Med. Assoc. 2017;109(1):23–27. doi: 10.1016/j.jnma.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 181.Baroni S., Scribano D., Zuppi C., Pagano L., Leone G., Giardina B. Prognostic relevance of lipoprotein cholesterol levels in acute lymphocytic and nonlymphocytic leukemia. Acta Haematol. 1996;96(1):24–28. doi: 10.1159/000203710. [DOI] [PubMed] [Google Scholar]
- 182.Lim U., Gayles T., Katki H.A., Stolzenberg-Solomon R., Weinstein S.J., Pietinen P. Serum high-density lipoprotein cholesterol and risk of non-hodgkin lymphoma. Cancer Res. 2007;67(11):5569–5574. doi: 10.1158/0008-5472.CAN-07-0212. [DOI] [PubMed] [Google Scholar]
- 183.Alford S.H., Divine G., Chao C., Habel L.A., Janakiraman N., Wang Y. Serum cholesterol trajectories in the 10 years prior to lymphoma diagnosis. Cancer Causes Control. 2018;29(1):143–156. doi: 10.1007/s10552-017-0987-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yavasoglu I., Tombuloglu M., Kadikoylu G., Donmez A., Cagirgan S., Bolaman Z. Cholesterol levels in patients with multiple myeloma. Ann. Hematol. 2008;87(3):2238. doi: 10.1007/s00277-007-0375-6. [DOI] [PubMed] [Google Scholar]
- 185.Chow S., Buckstein R., Spaner D.E. A link between hypercholesterolemia and chronic lymphocytic leukemia. Leuk. Lymphoma. 2016;57(4):797–802. doi: 10.3109/10428194.2015.1088651. [DOI] [PubMed] [Google Scholar]
- 186.Mozessohn L., Earle C., Spaner D., Cheng S.Y., Kumar M., Buckstein R. The association of dyslipidemia with chronic lymphocytic leukemia: a population-based study. J. Natl. Cancer Inst. 2016;109(3) doi: 10.1093/jnci/djw226. [DOI] [PubMed] [Google Scholar]
- 187.Sh Sankanagoudar, Singh G., Mahapatra M., Kumar L., NCh Chandra. Cholesterol homeostasis in isolated lymphocytes: a differential correlation between male control and chronic lymphocytic leukemia subjects. Asian Pac. J. Cancer Prev. 2017;18(1):23–30. doi: 10.22034/APJCP.2017.18.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Casalou C., Costa A., Carvalho T., Gomes A.L., Zhu Z., Wu Y., Dias S. Cholesterol regulates VEGFR-1 (FLT-1) expression and signaling in acute leukemia cells. Mol. Cancer Res. 2011;9(2):215–224. doi: 10.1158/1541-7786.MCR-10-0155. [DOI] [PubMed] [Google Scholar]
- 189.Tirado-Vélez J.M., Benítez-Rondán A., Cózar-Castellano I., Medina F., Perdomo G. Low-density lipoprotein cholesterol suppresses apoptosis in human multiple myeloma cells. Ann. Hematol. 2012;91(1):83–88. doi: 10.1007/s00277-011-1246-8. [DOI] [PubMed] [Google Scholar]
- 190.Zhou X., Paulsson G., Stemme S., Hansson G.K. Hypercholesterolemia is associated with a T helper (Th) 1/Th2 switch of the autoimmune response in atherosclerotic apo E-knockout mice. J. Clin. Invest. 1998;101(8):1717–1725. doi: 10.1172/JCI1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Mailer R.K.W., Gisterå A., Polyzos K.A., Ketelhuth D.F.J., Hansson G.K. Hypercholesterolemia induces differentiation of regulatory T cells in the liver. Circ. Res. 2017;120(11):1740–1753. doi: 10.1161/CIRCRESAHA.116.310054. [DOI] [PubMed] [Google Scholar]
- 192.Tie G., Yan J., Khair L., Messina J.A., Deng A., Kang J. Hypercholesterolemia increases colorectal cancer incidence by reducing production of NKT and γδ T Cells from hematopoietic stem cells. Cancer Res. 2017;77(9):2351–2362. doi: 10.1158/0008-5472.CAN-16-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Baek A.E., Yu Y.A., He S., Wardell S.E., Chang C.Y., Kwon S. The cholesterol metabolite 27 hydroxycholesterol facilitates breast cancer metastasis through its actions on immune cells. Nat. Commun. 2017;8(1):864. doi: 10.1038/s41467-017-00910-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yang W., Bai Y., Xiong Y., Zhang J., Chen S., Zheng X. Potentiating the antitumour response of CD8(+) T cells by modulating cholesterol metabolism. Nature. 2016;531(7596):651–655. doi: 10.1038/nature17412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Goossens P., Rodriguez-Vita J., Etzerodt A., Masse M., Rastoin O., Gouirand V. Membrane cholesterol efflux drives tumor-associated macrophage reprogramming and tumor progression. Cell Metab. 2019;29(6):1376–1389. doi: 10.1016/j.cmet.2019.02.016. e4. [DOI] [PubMed] [Google Scholar]
- 196.Ma X., Bi E., Lu Y., Su P., Huang C., Liu L. Cholesterol induces CD8+ T cell exhaustion in the tumor microenvironment. Cell Metab. 2019;30(1):143–156. doi: 10.1016/j.cmet.2019.04.002. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Pavlova N.N., Thompson C.B. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23(1):27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Mohammad N., Malvi P., Meena A.S., Singh S.V., Chaube B., Vannuruswamy G. Cholesterol depletion by methyl-β-cyclodextrin augments tamoxifen induced cell death by enhancing its uptake in melanoma. Mol. Cancer. 2014;13:204. doi: 10.1186/1476-4598-13-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Allott E.H., Howard L.E., Vidal A.C., Moreira D.M., Castro-Santamaria R., Andriole G.L. Statin use, serum lipids, and prostate inflammation in men with a negative prostate biopsy: results from the REDUCE Trial. Cancer Prev. Res. (Phila.) 2017;10(6):319–326. doi: 10.1158/1940-6207.CAPR-17-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Cafforio P., Dammacco F., Gernone A., Silvestris F. Statins activate the mitochondrial pathway of apoptosis in human lymphoblasts and myeloma cells. Carcinogenesis. 2005;26(5):883–891. doi: 10.1093/carcin/bgi036. [DOI] [PubMed] [Google Scholar]
- 201.Notarnicola M., Messa C., Refolo M.G., Tutino V., Miccolis A., Caruso M.G. Synergic effect of eicosapentaenoic acid and lovastatin on gene expression of HMGCoA reductase and LDL receptor in cultured HepG2 cells. Lipids Health Dis. 2010;9:135. doi: 10.1186/1476-511X-9-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lin C.J., Liao W.C., Lin H.J., Hsu Y.M., Lin C.L., Chen Y.A. Statins attenuate helicobacter pylori CagA translocation and reduce incidence of gastric cancer: in vitro and population-based case-control studies. PLoS ONE. 2016;11(1) doi: 10.1371/journal.pone.0146432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zhou M., Zheng J., Bi J., Wu X., Lyu J., Gao K. Synergistic inhibition of colon cancer cell growth by a combination of atorvastatin and phloretin. Oncol. Lett. 2018;15(2):1985–1992. doi: 10.3892/ol.2017.7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Luput L., Licarete E., Drotar D.M., Nagy A.L., Sesarman A., Patras L. In Vivo double targeting of C26 colon carcinoma cells and microenvironmental protumor processes using liposomal simvastatin. J. Cancer. 2018;9(2):440–449. doi: 10.7150/jca.21560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Akinwunmi B., Vitonis A.F., Titus L., Terry K.L., Cramer D.W. Statin therapy and association with ovarian cancer risk in the New England case control (NEC) study. Int. J. Cancer. 2019;144(5):991–1000. doi: 10.1002/ijc.31758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Liu Q., Xia H., Zhou S., Tang Q., Zhou J., Ren M. Simvastatin inhibits the malignant behaviors of gastric cancer cells by simultaneously suppressing YAP and β-catenin signaling. Onco Targets Ther. 2020;13:2057–2066. doi: 10.2147/OTT.S237693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Goldstein M.R., Mascitelli L., Pezzetta F. Do statins prevent or promote cancer? Curr. Oncol. 2008;15(2):76–77. doi: 10.3747/co.v15i2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Caro-Maldonado A., Camacho L., Zabala-Letona A., Torrano V., Fernández-Ruiz S., Zamacola-Bascaran K. Low-dose statin treatment increases prostate cancer aggressiveness. Oncotarget. 2017;9(2):1494–1504. doi: 10.18632/oncotarget.22217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Criscuolo D., Avolio R., Calice G., Laezza C., Paladino S., Navarra G. Cholesterol homeostasis modulates platinum sensitivity in human ovarian cancer. Cells. 2020;9(4):828. doi: 10.3390/cells9040828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Walker E.J., Ko A.H., Holly E.A., Bracci P.M. Statin use and risk of pancreatic cancer: results from a large, clinic-based case-control study. Cancer. 2015;121(8):1287–1294. doi: 10.1002/cncr.29256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Huang B.Z., Chang J.I., Li E., Xiang A.H., Wu B.U. Influence of statins and cholesterol on mortality among patients with pancreatic cancer. J. Natl. Cancer Inst. 2016;109(5) doi: 10.1093/jnci/djw275. [DOI] [PubMed] [Google Scholar]
- 212.Lee H.S., Lee S.H., Lee H.J., Chung M.J., Park J.Y., Park S.W. Statin use and its impact on survival in pancreatic cancer patients. Medicine (Baltimore). 2016;95(19):e3607. doi: 10.1097/MD.0000000000003607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Fendrich V., Sparn M., Lauth M., Knoop R., Plassmeier L., Bartsch D.K. Simvastatin delay progression of pancreatic intraepithelial neoplasia and cancer formation in a genetically engineered mouse model of pancreatic cancer. Pancreatology. 2013;13(5):502–507. doi: 10.1016/j.pan.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 214.Chen J., Bi H., Hou J., Zhang X., Zhang C., Yue L. Atorvastatin overcomes gefitinib resistance in KRAS mutant human non-small cell lung carcinoma cells. Cell Death. Dis. 2013;4(9):e814. doi: 10.1038/cddis.2013.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wu X.D., Zeng K., Xue F.Q., Chen J.H., Chen Y.Q. Statins are associated with reduced risk of gastric cancer: a meta-analysis. Eur. J. Clin. Pharmacol. 2013;69(10):1855–1860. doi: 10.1007/s00228-013-1547-z. [DOI] [PubMed] [Google Scholar]
- 216.Singh P.P., Singh S. Statins are associated with reduced risk of gastric cancer: a systematic review and meta-analysis. Ann. Oncol. 2013;24(7):1721–1730. doi: 10.1093/annonc/mdt150. [DOI] [PubMed] [Google Scholar]
- 217.Momtazi-Borojeni A.A., Nik M.E., Jaafari M.R., Banach M., Sahebkar A. Potential anti-tumor effect of a nanoliposomal antiPCSK9 vaccine in mice bearing colorectal cancer. Arch. Med. Sci. 2019;15(3):559–569. doi: 10.5114/aoms.2019.84732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Wen Y.A., Xiong X., Zaytseva Y.Y., Napier D.L., Vallee E., Li A.T. Downregulation of SREBP inhibits tumor growth and initiation by altering cellular metabolism in colon cancer. Cell Death. Dis. 2018;9(3):265. doi: 10.1038/s41419-018-0330-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Simigdala N., Gao Q., Pancholi S., Roberg-Larsen H., Zvelebil M., Ribas R. Cholesterol biosynthesis pathway as a novel mechanism of resistance to estrogen deprivation in estrogen receptor-positive breast cancer. Breast Cancer Res. 2016;18(1):58. doi: 10.1186/s13058-016-0713-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Chtourou Y., Fetoui H., Jemai R., Ben Slima A., Makni M., Gdoura R. Naringenin reduces cholesterol-induced hepatic inflammation in rats by modulating matrix metalloproteinases-2, 9 via inhibition of nuclear factor κB pathway. Eur. J. Pharmacol. 2015;746:96–105. doi: 10.1016/j.ejphar.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 221.Niho N., Takahashi M., Shoji Y., Takeuchi Y., Matsubara S., Sugimura T. Dose-dependent suppression of hyperlipidemia and intestinal polyp formation in Min mice by pioglitazone, a PPAR gamma ligand. Cancer Sci. 2003;94(11):960–964. doi: 10.1111/j.1349-7006.2003.tb01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Su F., Grijalva V., Navab K., Ganapathy E., Meriwether D., Imaizumi S. HDL mimetics inhibit tumor development in both induced and spontaneous mouse models of colon cancer. Mol. Cancer Ther. 2012;11(6):1311–1319. doi: 10.1158/1535-7163.MCT-11-0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Upadhyay A.K., Singh S., Chhipa R.R., Vijayakumar M.V., Ajay A.K., Bhat M.K. Methyl-beta-cyclodextrin enhances the susceptibility of human breast cancer cells to carboplatin and 5-fluorouracil: involvement of Akt, NF-kappaB and Bcl-2. Toxicol. Appl. Pharmacol. 2006;216(2):177–185. doi: 10.1016/j.taap.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 224.Mohammad N., Singh S.V., Malvi P., Chaube B., Athavale D., Vanuopadath M., Nair S.S., Nair B., Bhat M.K. Strategy to enhance efficacy of doxorubicin in solid tumor cells by methyl-β-cyclodextrin: involvement of p53 and Fas receptor ligand complex. Sci. Rep. 2015;5:11853. doi: 10.1038/srep11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Yue S., Li J., Lee S.Y., Lee H.J., Shao T., Song B. Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT activation underlies human prostate cancer aggressiveness. Cell Metab. 2014;19(3):393–406. doi: 10.1016/j.cmet.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Bandyopadhyay S., Li J., Traer E., Tyner J.W., Zhou A., Oh S.T. Cholesterol esterification inhibition and imatinib treatment synergistically inhibit growth of BCR-ABL mutation-independent resistant chronic myelogenous leukemia. PLoS ONE. 2017;12(7) doi: 10.1371/journal.pone.0179558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Montero J., Morales A., Llacuna L., Lluis J.M., Terrones O., Basañez G. Mitochondrial cholesterol contributes to chemotherapy resistance in hepatocellular carcinoma. Cancer Res. 2008;68(13):5246–5256. doi: 10.1158/0008-5472.CAN-07-6161. [DOI] [PubMed] [Google Scholar]
- 228.Souchek J.J., Baine M.J., Lin C., Rachagani S., Gupta S., Kaur S. Unbiased analysis of pancreatic cancer radiation resistance reveals cholesterol biosynthesis as a novel target for radiosensitisation. Br. J. Cancer. 2014;111(6):1139–1149. doi: 10.1038/bjc.2014.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Wu Y., Si R., Tang H., He Z., Zhu H., Wang L. Cholesterol reduces the sensitivity to platinum-based chemotherapy via upregulating ABCG2 in lung adenocarcinoma. Biochem. Biophys. Res. Commun. 2015;457(4):614–620. doi: 10.1016/j.bbrc.2015.01.035. [DOI] [PubMed] [Google Scholar]
- 230.Wang S.F., Chou Y.C., Mazumder N., Kao F.J., Nagy L.D., Guengerich F.P. 7-Ketocholesterol induces P-glycoprotein through PI3K/mTOR signaling in hepatoma cells. Biochem. Pharmacol. 2013;86(4):548–560. doi: 10.1016/j.bcp.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Kimbung S., Lettiero B., Feldt M., Bosch A., Borgquist S. High expression of cholesterol biosynthesis genes is associated with resistance to statin treatment and inferior survival in breast cancer. Oncotarget. 2016;7(37):59640–59651. doi: 10.18632/oncotarget.10746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Athavale D., Chouhan S., Pandey V., Mayengbam S.S., Singh S., Bhat M.K. Hepatocellular carcinoma-associated hypercholesterolemia: involvement of proprotein-convertase-subtilisin-kexin type-9 (PCSK9) Cancer Metab. 2018;6:16. doi: 10.1186/s40170-018-0187-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.World Health Organisation. Global health observatory (GHO) data https://www.who.int/gho/ncd/risk_factors/cholesterol_text/en/; [accessed 19 September 2020].
- 234.Ye J., Xia X., Dong W., Hao H., Meng L., Yang Y. Cellular uptake mechanism and comparative evaluation of antineoplastic effects of paclitaxel-cholesterol lipid emulsion on triple-negative and non-triple-negative breast cancer cell lines. Int. J. Nanomed. 2016;11:4125–4140. doi: 10.2147/IJN.S113638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Ji Y., Rounds T., Crocker A., Sussman B., Hovey R.C., Kingsley F. The effect of atorvastatin on breast cancer biomarkers in high-risk women. Cancer Prev. Res. (Phila.) 2016;9(5):379–384. doi: 10.1158/1940-6207.CAPR-15-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Chowdhury K., Sharma A., Sharma T., Kumar S., Mandal C.C. Simvastatin and MBCD inhibit breast cancer-induced osteoclast activity by targeting osteoclastogenic factors. Cancer Invest. 2017;35(6):403–413. doi: 10.1080/07357907.2017.1309548. [DOI] [PubMed] [Google Scholar]
- 237.Borgquist S., Giobbie-Hurder A., Ahern T.P., Garber J.E., Colleoni M., Láng I. Cholesterol, cholesterol-lowering medication use, and breast cancer outcome in the BIG 1-98 study. J. Clin. Oncol. 2017;35(11):1179–1188. doi: 10.1200/JCO.2016.70.3116. [DOI] [PubMed] [Google Scholar]
- 238.Lyu J., Yang E.J., Head S.A., Ai N., Zhang B., Wu C. Pharmacological blockade of cholesterol trafficking by cepharanthine in endothelial cells suppresses angiogenesis and tumor growth. Cancer Lett. 2017;409:91–103. doi: 10.1016/j.canlet.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Kawakami S., Suzuki S., Yamashita F., Hashida M. Induction of apoptosis in A549 human lung cancer cells by all-trans retinoic acid incorporated in DOTAP/cholesterol liposomes. J. Control. Release. 2006;110(3):514–521. doi: 10.1016/j.jconrel.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 240.Khurana V., Bejjanki H.R., Caldito G., Owens M.W. Statins reduce the risk of lung cancer in humans: a large case-control study of US veterans. Chest. 2007;131(5):1282–1288. doi: 10.1378/chest.06-0931. [DOI] [PubMed] [Google Scholar]
- 241.Liu J., Xian G., Li M., Zhang Y., Yang M., Yu Y. Cholesterol oxidase from Bordetella species promotes irreversible cell apoptosis in lung adenocarcinoma by cholesterol oxidation. Cell Death. Dis. 2014;5(8):e1372. doi: 10.1038/cddis.2014.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Li J., Yan H., Zhao L., Jia W., Yang H., Liu L. Inhibition of SREBP increases gefitinib sensitivity in non-small cell lung cancer cells. Oncotarget. 2016;7(32):52392–52403. doi: 10.18632/oncotarget.10721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Zhang L., Bennett W.F., Zheng T., Ouyang P.K., Ouyang X., Qiu X. Effect of cholesterol on cellular uptake of cancer drugs pirarubicin and ellipticine. J. Phys. Chem. B. 2016;120(12):3148–3156. doi: 10.1021/acs.jpcb.5b12337. [DOI] [PubMed] [Google Scholar]
- 244.Lanterna C., Musumeci A., Raccosta L., Corna G., Moresco M., Maggioni D. The administration of drugs inhibiting cholesterol/oxysterol synthesis is safe and increases the efficacy of immunotherapeutic regimens in tumor-bearing mice. Cancer Immunol. Immunother. 2016;65(11):1303–1315. doi: 10.1007/s00262-016-1884-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Li T., Chen L., Deng Y., Liu X., Zhao X., Cui Y. Cholesterol derivative-based liposomes for gemcitabine delivery: preparation, in vitro, and in vivo characterization. Drug Dev. Ind. Pharm. 2017;43(12):2016–2025. doi: 10.1080/03639045.2017.1361965. [DOI] [PubMed] [Google Scholar]
- 246.Muddineti O.S., Shah A., Rompicharla S.V.K., Ghosh B., Biswas S. Cholesterol-grafted chitosan micelles as a nanocarrier system for drug-siRNA co-delivery to the lung cancer cells. Int. J. Biol. Macromol. 2018;118(PtA):857–863. doi: 10.1016/j.ijbiomac.2018.06.114. [DOI] [PubMed] [Google Scholar]
- 247.Lou R., Cao H., Dong S., Shi C., Xu X., Ma R. Liver X receptor agonist T0901317 inhibits the migration and invasion of non-small-cell lung cancer cells in vivo and in vitro. Anticancer Drugs. 2019;30(5):495–500. doi: 10.1097/CAD.0000000000000758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Carrer A., Trefely S., Zhao S., Campbell S.L., Norgard R.J., Schultz K.C. Acetyl-CoA metabolism supports multistep pancreatic tumorigenesis. Cancer Discov. 2019;9(3):416–435. doi: 10.1158/2159-8290.CD-18-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Stine J.E., Guo H., Sheng X., Han X., Schointuch M.N., Gilliam T.P. The HMG-CoA reductase inhibitor, simvastatin, exhibits anti-metastatic and anti-tumorigenic effects in ovarian cancer. Oncotarget. 2016;7(1):946–960. doi: 10.18632/oncotarget.5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Casella C., Miller D.H., Lynch K., Brodsky A.S. Oxysterols synergize with statins by inhibiting SREBP-2 in ovarian cancer cells. Gynecol. Oncol. 2014;135(2):333–341. doi: 10.1016/j.ygyno.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Alhadad L.J., Harisa G.I., Alanazi F.K. Design and encapsulation of anticancer dual HSP27 and HER2 inhibitor into low density lipoprotein to target ovarian cancer cells. Saudi Pharm. J. 2020;28(4):387–396. doi: 10.1016/j.jsps.2020.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.You H.S., You N., Lee J.W., Lim H.J., Kim J., Kang H.T. Inverse association between statin use and stomach cancer incidence in individuals with hypercholesterolemia, from the 2002-2015 NHIS-HEALS data. Int. J. Environ. Res. Public Health. 2020;17(3):1054. doi: 10.3390/ijerph17031054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Lee J.S., Roberts A., Juarez D., Vo T.T., Bhatt S., Herzog L.O. Statins enhance efficacy of venetoclax in blood cancers. Sci. Transl. Med. 2018;10(445):eaaq1240. doi: 10.1126/scitranslmed.aaq1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Zheng L., Li L., Lu Y., Jiang F., Yang X.A. SREBP2 contributes to cisplatin resistance in ovarian cancer cells. Exp. Biol. Med. (Maywood) 2018;243(7):655–662. doi: 10.1177/1535370218760283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.The nobel prize in physiology or medicine 1964. NobelPrize.org. Nobel Media AB https://www.nobelprize.org/prizes/medicine/1964/summary/ [accessed 22 January 2021]