Abstract
The assessment of tobacco withdrawal is important for both research and clinical purposes. This study describes the psychometric development of a revised version of the 28-item Wisconsin Smoking Withdrawal Scale (WSWS; Welsch et al., 1999). Because the different contexts of use sometimes permit only brief assessment, this revision has produced both a brief and longer form using an updated pool of candidate items. For the revised WSWS2, a candidate pool of 37 items was developed to measure nine putative withdrawal constructs. The stem and wording of items were revised as was the response scale. Data for psychometric analyses were derived from three smoking cessation randomized clinical trials conducted at the University of Wisconsin Center for Tobacco Research and Intervention. Dimensionality, internal consistency, and item characteristic analyses of the candidate items were conducted in a derivation sample to ascertain the factor structure and to identify items that could be used in the WSWS2 scales. Confirmatory factor analyses (CFAs) of reduced item sets and factor structure were conducted in two validation samples along with reliability and validity analyses. Derivation and validation sample analyses yielded a longer version of the WSWS2 (WSWS2-L) with 19 items and six subscales (Craving, Negative Affect, Hunger, Sleep, Restlessness, and Concentration) and a brief 6-item version (WSWS2-B). In validation sample analyses, both the WSWS2-L and the WSWS2-B demonstrated good reliability and validity as well as good fit in CFAs. The WSWS2-L and WSWS2-B possess improved construct coverage, fewer items, and other enhancements relative to the WSWS.
Keywords: nicotine withdrawal, tobacco withdrawal, smoking cessation, tobacco dependence
The accurate assessment of tobacco withdrawal is important for both research and clinical applications. Withdrawal is a core feature of the tobacco dependence construct as it has been formulated over time (Baker, Breslau, et al., 2012; Baker, Piper, et al., 2012; Solomon, 1977; Wikler, 1980) and its assessment can provide vital information about the severity of dependence and its type (Baker, Piper, et al., 2012; Piper et al., 2008). Thus, its accurate assessment should result in more informative tests of motivational models of cigarette dependence and its treatment. For instance, withdrawal symptoms yield important information about the mechanisms of smoking treatment (Bolt et al., 2012; Ferguson et al., 2006; McCarthy et al., 2010; Piper et al., 2008) and can serve as an important phenotype for genetic analyses of tobacco dependence (Baker et al., 2009; Hancock et al., 2018; Hancock et al., 2015; Kubota et al., 2006; Pergadia et al., 2006). The accurate assessment of withdrawal is also clinically important. Not only does it provide a valuable index of abstinence-related distress, but it is one of the most informative predictors of likelihood of smoking cessation success (Cofta-Woerpel et al., 2011; Javitz et al., 2012; Piasecki et al., 2003; Piper et al., 2011; Schnoll et al., 2016) and thus may provide useful information for medication dosing, personalized treatment, and just-in-time and adaptive treatments.
The original Wisconsin Smoking Withdrawal Scale (WSWS; Welsch et al., 1999) was initially designed to measure eight theoretically-based constructs including Anger, Anxiety, Concentration, Craving, Hunger, Sadness, Sleep, and Somatic Symptoms using a set of 37 candidate items derived, in part, from existing withdrawal questionnaires (Hughes & Hatsukami, 1986; Schneider et al., 1984; Shiffman & Jarvik, 1976). Items were rated on a 5-point agreement response scale (0 = strongly disagree to 4 = strongly agree) and included the use of reverse-scored items. Welsh et al. conducted psychometric analyses on data from two independent smoking cessation randomized clinical trials (RCTs) which yielded a 28-item questionnaire with seven subscales (Somatic Symptoms was not retained as a subscale; see Supplemental Table 1). One thing that distinguished the WSWS from some other withdrawal questionnaires is that it comprised multi-item subscales, permitting internal consistency analyses and confirmatory factor analyses (CFAs). All seven WSWS subscales demonstrated good internal consistency ranging from .75 to .93 for postquit ratings and CFAs supported a 7-factor structure (a second-order model also fit the data well). Weekly subscale ratings over the first 4 weeks post-quit showed the expected pattern of an increase in withdrawal ratings in the first week after quitting followed by decreases in weeks 2–4. In addition, post-quit week 1 subscale ratings were predictive of continuous abstinence at week 4, especially the Craving subscale, illustrating the validity of the WSWS.
Subsequent psychometric studies have largely replicated the factorial structure, reliability, and validity of the 28-item WSWS (Castro et al., 2011; Etter & Hughes, 2006; West et al., 2006) and it has been used in numerous experimental studies (e.g., Abdolahi et al., 2015; Gloria et al., 2009; Hendricks et al., 2006; Piper et al., 2008; Robinson et al., 2014).
One limitation of the WSWS is its length. It comprises a relatively large number of items (28), which may limit its use in studies where assessment burden is a concern. This calls for a brief version of the WSWS supported by psychometric analyses. Also, the length of some items (e.g., “I have been bothered by negative moods such as anger, frustration, and irritability”) may not only add to participant burden but also challenge comprehension. Further, the use of a 5-point response scale may be too coarse, thereby reducing sensitivity. Finally, research done since construction of the original scale has suggested additional withdrawal constructs such as restlessness and physical symptoms that might be included in a revision. For example, restlessness is a key DSM-5 (American Psychiatric Association, 2013) diagnostic criterion for Tobacco Withdrawal (ICD-10-CM code F17.203; U.S. National Center for Health Statistics, 2020) and, in addition, DSM-5 notes that tobacco withdrawal may also include associated features such as constipation, coughing, dizziness, dreaming/nightmares, nausea, and sore throat.
Accordingly, a new candidate withdrawal item set was developed in 2014 and administered in three smoking cessation RCTs conducted at the University of Wisconsin Center for Tobacco Research and Intervention (UW-CTRI) that permitted psychometric analyses aimed at developing two revised versions of the WSWS: a longer version comprising multi-item subscales (the WSWS2-L) and a brief version (the WSWS2-B) designed to be appropriate for use where assessment burden or time is an important concern.
Method
The approach to revising the WSWS set several goals related to item selection and construct coverage. These goals included: (1) reliable and valid measurement and representation of key putative withdrawal constructs; (2) retention of a minimum of three items per construct subscale to increase reliability and to avoid under-identification (Kline, 2005; Marsh et al., 1998; Nunnally & Bernstein, 1994); (3) creation of a brief version with one item per subscale to permit use where scale length is a concern (e.g., in studies collecting daily ecological momentary assessment [EMA] data); (4) internal consistency estimates of at least .70 in derivation and validation samples (Nunnally & Bernstein, 1994); (5) identification of factor structure reflecting simple structure (i.e., all items for a factor would have high factor loadings on that factor and low loadings on other factors); (6) acceptable or better goodness of fit indices for the factor structure in validation sample CFAs; (7) good test-retest stability; and (8) evidence of convergent, discriminant, and predictive validity.
Participants
Derivation Sample
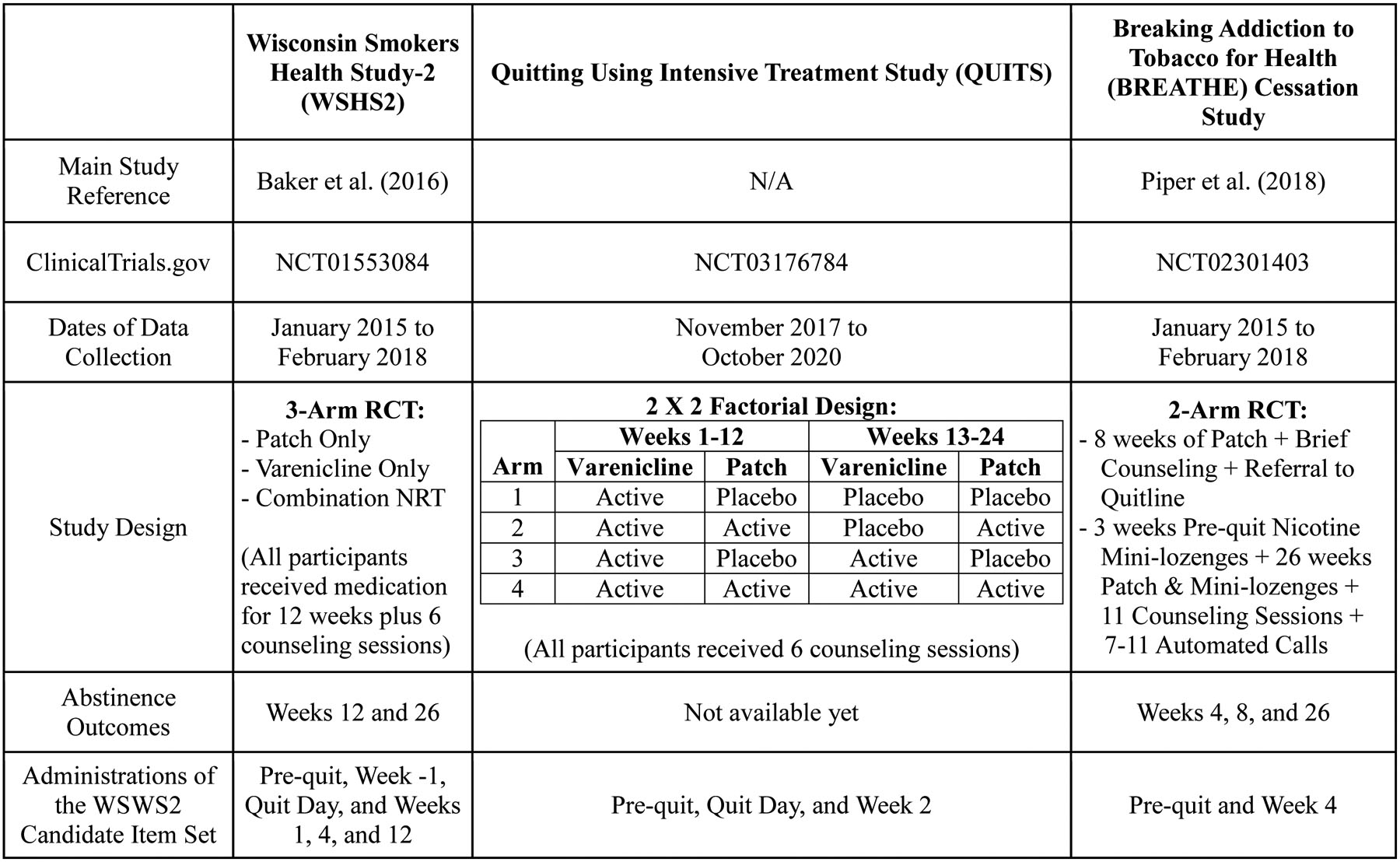
Participants (N=1086) in the Wisconsin Smokers Health Study 2 (WSHS2; ClinicalTrials.gov, NCT01553084), a 3-year longitudinal health and smoking cessation study, constituted the Derivation sample. Participants in this three-group RCT were randomly assigned to receive 12 weeks of open-label varenicline, combination nicotine replacement therapy (CNRT), or the nicotine patch only. The revised set of 37 candidate WSWS items was administered at the WSHS2 pre-quit study initiation visit, the week prior to the target quit day (TQD; pre-quit baseline), on the TQD, and at post-TQD weeks 1, 4, and 12. Biochemically-confirmed 7-day point-prevalence abstinence outcomes, assessed at post-TQD weeks 4 and 26, were used for predictive validity analyses based on baseline to week 1 change in WSWS2 scores. Table 1 provides key details about WSHS2; full study details and results were reported by Baker et al. (2016).
Table 1.
Study Information for the Derivation and Validation Samples
|
Note. WSWS2 = Revised Wisconsin Smoking Withdrawal Scale 2; Patch = Nicotine Patch; Combination NRT = Nicotine Patch + Nicotine Lozenge; RCT=randomized clinical trial.
Validation Samples
In the Quitting Using Intensive Treatment Study (QUITS; ClinicalTrials.gov, NCT03176784), 1251 participants received three months of varenicline and half of these were randomized to one of two levels of two factors: adjuvant nicotine patches (vs. placebo patches) and extended pharmacotherapy duration of 24 weeks (vs. standard duration of 12 weeks), constituting a 2 (Adjuvant) × 2 (Duration) factorial design cessation trial. In QUITS, the set of candidate WSWS items was administered at the pre-quit study visit, on the TQD, and at week 2 post- TQD. Table 1 provides key details about QUITS. As of May, 2020 (when psychometric analyses were completed), the QUITS study was still collecting follow-up data for the primary abstinence outcome; as such, predictive validity analyses are not reported for QUITS.
The Breaking Addiction to Tobacco for Health - Cessation study (BREATHE-C; ClinicalTrials.gov, NCT02301403) was a two-arm RCT that tested an optimized smoking cessation treatment package identified in prior factorial screening experiments (Piper et al., 2016; Schlam et al., 2016) based on the Multiphase Optimization Strategy (MOST; Collins, Dziak, et al., 2014; Collins et al., 2007; Collins, Trail, et al., 2014). As shown in Table 1, 623 participants were randomized either to Recommended Usual Care (R-UC) or Abstinence-Optimized Treatment (A-OT). In the BREATHE-C study, the set of candidate WSWS items was administered at the pre-quit study initiation visit and at week 4 post- TQD. The lack of immediate post-quit (week 1) WSWS2 data precluded predictive validity analyses based on change in WSWS2 scores (e.g., week −1 to week 1). Full study details and primary outcome results were reported by Piper et al. (2018).
Procedures
Participants in all three studies (WSHS2, QUITS, and BREATHE-C) were adult smokers (>4 cigarettes/day) who were motivated to quit smoking and had no contraindications to study pharmacotherapies. All three studies were approved by the University of Wisconsin Institutional Review Board, and all participants provided written informed consent.
Assessments
Development of the Revised WSWS (WSWS2) Candidate Items
One revision to the WSWS involved changing the response scale from a 5-point scale agreement scale (i.e., 0=Strongly disagree to 4=Strongly agree) to a 7-point scale from 1 = Not at all to 7 = Extremely. This change was made to increase the variability and precision of measurement and to avoid response set tendencies that often occur with agreement-type response scales (DeCastellarnau, 2018; Krosnick, 2018; Krosnick & Presser, 2010).
The second revision was to the stem, which was revised to read “In the last 24 hours, how much have you been bothered by… [item]”. This change allowed item wording to be shortened to increase readability and administration efficiency. Finally, we revised the items to fit the new stem and developed new items on substantive bases. Nine a priori withdrawal constructs were identified including seven from the original WSWS (Anger, Anxiety, Concentration, Craving, Hunger, Sadness, and Sleep) and two additional constructs, Restlessness and Physical Symptoms (candidate items and constructs provided in Supplemental Table 2). The final set of 37 candidate items was administered to participants in all three studies at baseline (pre-quit) visits and at additional post-quit visits. Times of post-quit administration of the WSWS2 items differed across studies as shown in Table 1.
Baseline Measures
All participants completed baseline measures including demographic variables (gender, age, and race) and smoking-related assessments including cigarettes per day (CPD), the Fagerstrom Test for Cigarette Dependence (FTCD; Fagerstrom, 2012; Heatherton et al., 1991), and the Brief Wisconsin Inventory of Smoking Dependence Motives (Brief WISDM; Smith et al., 2010), a multifactorial measure of dependence (Piper et al., 2008; Piper et al., 2004). In addition, all participants completed the Positive and Negative Affect Schedule (PANAS), an assessment of positive and negative affect (Watson et al., 1988).
Abstinence Outcomes for Predictive Validity Analyses
The relations of WSWS2 scores with abstinence were determined in the WSHS2 study. Seven-day point-prevalence abstinence was assessed via self-report at post-TQD weeks 4 and 26 with biochemical confirmation of self-reported abstinence determined via exhaled carbon monoxide (CO) tests with values <6 ppm confirming abstinence.
Analytic Plan
The analytic plan included: (1) dimensionality analyses (e.g., parallel analyses; Horn, 1965) and exploratory factor analyses (EFAs) of the candidate items in the Derivation sample to determine the number of factors to retain; (2) examination of factor loadings in EFAs as well as item-to-total correlations and scale reliability in internal consistency analyses (omega; McDonald, 1999) in the Derivation sample to identify the best-performing items to retain for testing in the validation samples; and (3) CFAs, reliability analyses, and validity analyses of WSWS2 scores based on the retained factors and items in the validation samples. With a few exceptions, all participants in the Derivation and Validation samples were used in the analyses regardless of treatment group or abstinence status.
Analyses to Determine Dimensionality (Number of Factors to Retain)
Auerswald and Moshagen (Auerswald & Moshagen, 2019) recommended using multiple approaches to determine dimensionality such as sequential chi-square model tests (SMTs) and parallel analysis (PA; Horn, 1965). In initial analyses, we found that SMTs were overly sensitive (e.g., too many weak factors were identified), thus, we used Empirical Kaiser Criterion (EKC) analyses (Braeken & van Assen, 2017), combined with PA. There are many variants of PA but recent research supports the use of Horn’s (1965) version of PA (Lim & Jahng, 2019). PA was computed via Mplus (Muthén & Muthén, 1998–2017). In EKC, the number of factors to retain is based on the number of sample eigenvalues that are greater than a set of reference eigenvalues of randomly generated correlation matrices under the assumption of independence (i.e., no factor structure). EKC was computed via an online web applet based on Braeken & van Assen (Braeken & van Assen, 2017) and available at https://cemo.shinyapps.io/EKCapp.
Strategy for the Selection of the Number of Factors and Items to Retain
Following dimensionality analyses, we conducted preliminary internal consistency analyses (alpha coefficient) of item sets based on a priori assignment of specific items to each of the nine withdrawal constructs under consideration. Subscales with alpha coefficient < .50 were eliminated from further testing along with their targeted items. Thus, in the Derivation Sample the Physical Symptoms subscale had an alpha coefficient of .488; the other eight candidate subscales had alpha coefficients > .50. Accordingly, we eliminated the five Physical Symptoms items, leaving 32 candidate items to submit to further evaluation. Welsch (Welsch et al., 1999) also found low alpha coefficients for the set of somatic symptom items and eliminated the subscale and items from further consideration.
We then used Derivation Sample data to conduct PA and EKC analyses of the 32 candidate items to identify the number of factors followed by EFAs to define the factors. Agreement between PA and EKC in terms of number of factors identified was used to target an initial range of number of factors for testing in EFAs. We then conducted EFAs (with oblique factor rotation), varying the number of factors extracted based on the PA and EKC results, to ascertain correspondence with a priori withdrawal constructs. Determination of the optimal factor structure was based on consistency with simple structure. Items were eliminated based on cross-loadings or low factor loadings. Following elimination of poorly performing items for each factor, three or four items were selected based on: (1) high factor loadings; (2) minimizing of semantic overlap; (3) broad substantive coverage of the construct factor; and (4) reading level with emphasis on brevity and clarity. For the brief version of the WSWS2 (the WSWS2-B), one item per factor was selected based on magnitude of CFA factor loadings and apparent factor representativeness of an item.
Exploratory and Confirmatory Factor Analyses
Mplus with the maximum likelihood (ML) estimator was used for all EFAs and CFAs. For EFAs, geomin oblique rotation was specified reflecting the correlated nature of withdrawal constructs. For CFAs, factors were allowed to correlate. The following model goodness of fit statistics were used to evaluate CFA models (Hu & Bentler, 1999): (1) root mean square error of approximation (RMSEA) with values <.06 indicative of good fit; (2) comparative fit index (CFI) with values >.95 indicative of good fit; (3) Tucker-Lewis index (TLI) with values >.95 indicative of good fit; and (4) standardized root mean residual (SRMR) with values <.08 indicative of good fit.
Internal Consistency and Test-Retest Stability
Scale and subscale internal consistency reliability in all three studies was primarily assessed with Omega (McDonald, 1999) given the role of the common factor model in our analyses. Mplus was used to compute Omega along with bootstrap-corrected 95% confidence intervals (CIs). For comparison purposes, SAS/STAT PROC CORR (SAS Institute Inc, Cary, NC; Version 13.2) was used to compute the alpha coefficient (Cronbach, 1951) along with item-to-total correlations. Test-retest stability was computed for the WSWS2-L total scale and subscales as well as the brief version (WSWS2-B) using quit day and week 1 data (to assess stability early in the quit attempt) as well as week 4 and week 12 data (to assess stability later in the quit attempt) in the Derivation Sample.
Convergent, Discriminant, and Predictive Validity Analyses
Validity analyses of Validation Samples’ (BREATHE-C, QUITS) data were computed using SAS/STAT PROC CORR and PROC LOGISTIC (SAS Institute Inc, Cary, NC; Version 13.2). Convergent validity was assessed via correlations between the revised WSWS (WSWS2-L and WSWS2-B) and measures that would be expected to correlate positively with withdrawal. These measures include CPD, CO, FTCD total score, PANAS Negative Affect score, and WISDM scores (total score, primary dependence motives [PDM], secondary dependence motives [SDM], and selected subscales such as the Craving subscale). Discriminant validity was assessed via correlations of the revised WSWS (WSWS2-L and WSWS2-B) with the PANAS Positive Affect score.
Predictive validity was analyzed in the WSHS2 sample via logistic regression with WSWS2 residualized change scores (post-quit week 1 scores residualized on pre-quit week −1 scores) predicting binary abstinence outcomes at weeks 4 and 26. Logistic regression models also included two dummy-coded variables to control for effects of the three active treatment groups (nicotine patch only, varenicline only, and combination NRT) with the nicotine patch condition coded as the reference group.
Results
Table 2 provides participant characteristics for the Derivation Sample (WSHS2) and Validation Samples (QUITS, BREATHE-C). Across the three studies, the samples were similar in terms of percent female, mean age, race, and smoking-related variables (mean CPD and mean FTCD score).
Table 2.
Participant Characteristics in the Derivation and Validation Samples
| Variable | Wisconsin Smokers Health Study-2 (WSHS2) | Quitting Using Intensive Treatment Study (QUITS) | Breaking Addiction to Tobacco for Health Cessation Study (BREATHE-C) |
|---|---|---|---|
| Study N | 1086 | 1251 | 623 |
| Gender (% Female) | 52.0% | 53.9% | 57.3% |
| Age, Mean (SD) | 48.1 (11.6) | 49.2 (12.0) | 49.7 (12.7) |
| % Other | 4.5% | 6.4% | 5.0% |
| CPD, Mean (SD) | 17.0 (8.3) | 16.0 (7.5) | 16.8 (9.4) |
| FTCD Total Score, Mean (SD) | 4.8 (2.1) | 5.0 (2.0) | 4.8 (2.2) |
| Exhaled Carbon Monoxide, ppm Mean (SD) | 15.1 (8.4) | 16.7 (9.5) | 17.9 (11.0) |
| PANAS Positive Affect | 9.8 (3.1) | 9.9 (3.1) | 10.4 (2.8) |
| PANAS Negative Affect | 5.0 (2.6) | 7.3 (3.6) | 7.4 (3.2) |
| Craving Subscale | 4.7 (1.5) | 5.2 (1.4) | 5.2 (1.2) |
Note. SD = standard deviation. PANAS = Positive and Negative Affect Schedule. Brief WISDM = Brief Wisconsin Inventory of Smoking Dependence Motives; PDM = primary dependence motives; SDM = secondary dependence motives.
Derivation Sample Analyses
Dimensionality of the New WSWS Candidate Item Set
As noted above, the WSWS2 candidate item set for analysis in the Derivation Sample consisted of 32 items representing eight constructs (the five Physical Symptoms items were eliminated due to a low alpha coefficient). EKC analysis of the 32 items rated on the quit day in the Derivation Sample yielded a 6-factor structure whereas PA identified a 5-factor structure. As an additional check on dimensionality, we computed an EFA which yielded six eigenvalues greater than one (Guttman, 1954; Kaiser, 1960), supporting a 6-factor structure.
To ascertain the optimal number of factors to retain, factor loadings from EFAs with oblique rotation were examined in separate models with five and six factors extracted. In both the 5- and 6-factor models, three factors (Sleep, Craving, and Hunger) were clearly defined by the expected items. In the 5-factor model, Concentration and Restlessness items loaded on a single factor as did items written for the Sad/Depressed, Anxious/Stressed, and Anger constructs. In the 6-factor model, items for Sad/Depressed, Anxious/Stressed, and Anger loaded on one factor (similar to the 5-factor model) but Concentration and Restlessness items loaded on separate factors. Given the importance of Restlessness as a withdrawal construct that reflects incentive salience, arousal or agitation, smoking motivation, and cessation (Bold et al., 2016; Chandra et al., 2011; Leventhal & Zimmerman, 2010; Wong & Leventhal, 2015), the 6-factor model was preferred. In addition, the factor loadings for the Concentration and Restlessness items were higher in the 6-factor model. Supplemental Table 3 provides factor loadings for the 6-factor model.
Item Selection for the WSWS2
As noted above, a goal for the WSWS2-L was to select a minimum of three items per factor. For two factors, Sleep and Concentration, only three items each were included in the larger set of 32 candidate WSWS items. Thus, the three items for each of these factors were retained. There were four candidate items for the Restlessness factor but one item, “Having trouble calming down,” had a low factor loading (.233) on the Restlessness factor and a high loading on the hybrid Sad/Depressed-Anxious/Stressed-Anger factor. Thus, this item was eliminated from consideration leaving three items for the Restlessness factor. Craving was represented by five items, all of which had factor loadings >.75; the three items with the highest factor loadings were selected for the Craving factor. Hunger was also represented by five items with factor loadings ranging from .604 to .907. Two of the Hunger items with higher factor loadings were selected (“Eating a lot” [.907] and “Thinking about food a lot” [.816]) along with the item “Feeling hungry” that was selected as a better choice than “Overeating” due to less semantic overlap with the “Eating a lot” item thus yielding improved construct coverage.
Thirteen items loaded on the hybrid Sad/Depressed-Anxious/Stressed-Anger factor which was renamed “Negative Affect”. Given the importance of negative affect in models of addiction (Baker et al., 2004; Cambron et al., 2019; Mathew et al., 2017) and the multiple affective states and large number of candidate items, a decision was made to select four items for the Negative Affect factor rather than three. Two items tapping Sad/Depressed (“Feeling unhappy” and “Feeling upset”) were selected along with one Anxious/Stressed item (“Feeling stressed”) and one Anger item (“Feeling angry”). Table 3 provides the set of 19 selected items reflecting the six factors (WSWS2-L) that resulted from the factor and item selection analyses as well as the single item for each subscale selected for the WSWS2-B. In general, the WSWS2-B items were ones with the highest factor loading for a given subscale. The correlation between the WSWS2-L and the WSWS2-B at baseline in the derivation sample was 0.96 (p<.0001); the correlation was similarly high in both validation studies (r = .96, p<.0001, in the BREATHE-C study; r=.97, p<.0001, in the QUITS study).
Table 3.
Set of Six Factors and 19 Items Selected in the Derivation Sample Analyses for the WSWS2-L and WSWS2-B)
| Subscale | Items |
|---|---|
| Negative Affect | Feeling unhappy Feeling upset Feeling stressed Feeling angry |
| Hunger | Eating a lot Feeling hungry Thinking about food a lot |
| Craving | Having urges to smoke Wanting to smoke Thinking about smoking |
| Sleep | Feeling tired Waking frequently during the night Troubled sleep |
| Restlessness |
Feeling restless
Having trouble sitting still Feeling fidgety |
| Concentration | Having trouble paying attention Having trouble thinking clearly Having trouble concentrating |
Note. WSWS2-L = Wisconsin Smoking Withdrawal Scale 2. Underlined items are the items selected for the brief version of the WSWS2 (WSWS2-B).
Validation Sample Analyses
Internal Consistency and Test-Retest Stability
Table 4 provides internal consistency estimates for the WSWS2-L subscale scores and total scores for the Derivation Sample (WSHS2) and the Validation Samples (QUITS, BREATHE-C). Five of six subscales had omega values exceeding .840 in all three samples; the exception was the Sleep subscale with omega values of .773, .790, and .812 in WSHS2, QUITS, and BREATHE-C samples, respectively. The lower omega value for the Sleep subscale is likely attributable to the “Feeling tired” item that had a low factor loading in the Derivation Sample 6-factor EFA. Omegas for the 19-item WSWS2-L exceeded .911 in all three samples whereas omegas for the 6-item WSWS2-B were considerably lower at .738 in WSHS2, .781 in QUITS, and .801 in BREATHE-C.
Table 4.
Internal Consistency Estimates for the WSWS2-L Subscales, Total, and Brief Version (WSWS2-B) for Derivation and Validation Samples
| Subscale | Derivation Sample | Validation Samples | ||||
|---|---|---|---|---|---|---|
| WSHS2 Quit Day (N=941) | QUITS Quit Day (N=1027) | BREATHE-C Week 4 (N=431) | ||||
| Alpha Coefficient | Omega (Bootstrap Corrected; 95% CI) | Alpha Coefficient | Omega (Bootstrap Corrected; 95% CI) | Alpha Coefficient | Omega (Bootstrap Corrected; 95% CI) | |
| Negative Affect | .903 | .905 (.890, .918) | .899 | .903 (.890, .914) | .913 | .913 (.896, .928) |
| Hunger | .851 | .857 (.837, .874) | .869 | .871 (.855, .885) | .876 | .877 (.850, .897) |
| Craving | .955 | .956 (.949, .961) | .950 | .951 (.943, .957) | .934 | .937 (.919, .951) |
| Sleep | .729 | .773 (.747, .796) | .764 | .790 (.770, .810) | .771 | .812 (.783, .838) |
| Restlessness | .870 | .871 (.850, .888) | .852 | .853 (.832, .868) | .847 | .845 (.813, .869) |
| Concentration | .929 | .930 (.916, .941) | .941 | .941 (.930, .949) | .899 | .899 (.869, .920) |
| WSWS2-L Total | .911 | .907 (.897, .914) | .922 | .917 (.908, .924) | .920 | .919 (.906, .929) |
| WSWS2-B | .738 | .744 (.720, .768) | .780 | .781 (.759, .800) | .791 | .801 (.774, .825) |
Note. WSWS2-L = 19-item Wisconsin Smoking Withdrawal Scale 2. WSWS2-RB = 6-item Brief Wisconsin Smoking Withdrawal Scale 2.
Table 5 provides means, SDs, and test-retest stability coefficients (correlations) for WSWS2-L subscales scores, WSWS2-L total scores, and WSWS2-B scores early in the quit attempt (quit day and week 1) and later in the quit attempt (weeks 4 and 12) based on WSHS2 sample data. Only participants in the nicotine patch condition (the least intensive treatment group) were included in the analyses to minimize treatment effects on withdrawal severity. Test-retest stability coefficients for the WSWS2-L total scores exceeded .70 with only a slight decrease for the WSWS2-B. Individual subscale stability coefficients ranged mostly from .60 to .73 with only two values less than .60 (Negative Affect, r[quit day, week 1]=.55; Sleep, r[quit day, week 1]=.53).
Table 5.
Test-Retest Correlations for the WSWS2-L Subscales, Total, and Brief Version (WSWS2-B) for Participants in the WSHS2 Sample Nicotine Patch Only Condition
| Subscale | Means (SD) and Test-Retest Stability | |||||
|---|---|---|---|---|---|---|
| Quit Day and Post-quit Week 1 Correlation (p-value) | Post-quit Weeks 4 and 12 Correlation (p-value) | |||||
| Means (SDs) | Test-Retest Correlation (N=193) | Means (SDs) | Test-Retest Correlation (N=168) | |||
| Quit Day | Post-quit Week 1 | Post-quit Week 4 | Post-quit Week 12 | |||
| Negative Affect | 2.16 (1.53) | 2.29 (1.58) | .55 | 2.21 (1.57) | 2.19 (1.44) | .65 |
| Hunger | 2.45 (1.35) | 2.70 (1.57) | .64 | 2.65 (1.44) | 2.63 (1.37) | .73 |
| Craving | 4.26 (1.76) | 3.94 (1.90) | .61 | 3.60 (1.74) | 3.54 (1.89) | .62 |
| Sleep | 2.44 (1.43) | 2.80 (1.61) | .53 | 2.64 (1.49) | 2.72 (1.43) | .61 |
| Restlessness | 2.30 (1.42) | 2.16 (1.38) | .70 | 2.00 (1.33) | 2.12 (1.38) | .70 |
| Concentration | 2.03 (1.52) | 1.90 (1.35 | .62 | 1.83 (1.20) | 2.03 (1.38) | .60 |
| WSWS2-L Total | 2.59 (1.10) | 2.61 (1.20) | .72 | 2.47 (1.13) | 2.52 (1.16) | .74 |
| WSWS2-B | 2.56 (1.15) | 2.60 (1.29) | .68 | 2.45 (1.17) | 2.49 (1.25) | .73 |
Note. WSWS2-L = 19-item Wisconsin Smoking Withdrawal Scale 2. WSWS2-B = 6-item Brief Wisconsin Smoking Withdrawal Scale 2. All test-retest correlations p<.0001.
Confirmatory Factor Analysis Results
Table 6 provides results for CFAs of the 19-item, six-factor WSWS2-L and the 6-item WSWS2-B in the two Validation Samples. In each sample, an initial model (Model 1) with 19 items loading on six factors yielded good model fit. In both of these CFAs, examination of modification indices (MIs) showed that two items, “Troubled sleep” and “Waking frequently during the night,” had large residual covariances (MI=76.874 in the QUITS CFA; MI=35.861 in the BREATHE-C CFA). A second CFA model (Model 2) was computed that freed the correlated error for these two items and model fit improved. In particular, SRMR improved noticeably in Model 2. Overall, the CFA results confirm good model fit for the 6-factor structure. CFAs for the 6-item WSWS2-B modeled as a single factor also yielded good fit suggesting that the WSWS2-B measures a unidimensional withdrawal construct.
Table 6.
Confirmatory Factor Analyses of the Six-Factor, 19-Item WSWS2-L and the One-Factor, 6-Item WSWS2-B
| QUITS Quit Day Data (N=1027) | BREATHE-C Week 4 Data (N=431) | |||
|---|---|---|---|---|
| Goodness of Fit Index | WSWS-R Model 1: 6 Factors, 19 Items | WSWS-RB: 1 factor, 6 Items | WSWS-R Model 1: 6 Factors, 19 Items | WSWS-RB: 1 factor, 6 Items |
| RMSEA | .057 | .076 | .066 | .079 |
| CFI | .970 | .968 | .957 | .969 |
| TLI | .962 | .946 | .947 | .948 |
| SRMR | .052 | .027 | .053 | .031 |
Note. WSWS2-L = 19-item Wisconsin Smoking Withdrawal Scale 2. WSWS2-B = 6-item Brief Wisconsin Smoking Withdrawal Scale 2. RMSEA=Root Mean Square Error of Approximation, good fit <.06; CFI=Comparative Fit Index (CFI), good fit>.95; TLI=Tucker-Lewis Index, good fit >.95; and SRMR=Standardized Root Mean Residual (SRMR), good fit <.08.
Convergent and Discriminant Validity Results
In the BREATHE-C sample, the baseline WSWS2-L total score was significantly correlated with the FTCD score (r=.18, p<.001), WISDM total score (r=.460, p<.0001), WISDM PDM (r=.375, p<.0001), WISDM SDM (r=.442, p<.0001), and the Negative PANAS score (r=.685, p<.0001). The WSWS2-L total score was not significantly correlated with CPD (r=.047, p=.2452), CO (r=.041, p=.3057), or the Positive PANAS score (r= −.033, p=.4111). The WSWS2-B showed an identical pattern of significant and non-significant correlations but the magnitude of the correlations was somewhat smaller. The WSWS2-L Craving subscale was significantly correlated with the WISDM Craving subscale, r=.650, p<.0001.
Predictive Validity Results
Table 7 provides results of logistic regression analyses predicting abstinence outcomes (week 4 and week 26 separately) via residualized WSWS2-L scores (total, brief, and subscales in separate analyses) in the WSHS-2 study. The residualized change scores were computed as week 1 scores residualized on week −1 (pre-quit) scores. Dummy-coded treatment variables were included in all models. The residualized change scores for both the WSWS2-L total score and the WSWS2-B significantly predicted abstinence at week 4 but not at week 26. For analyses of WSWS2-L subscale scores, two sets of models were computed: (1) univariable models that tested single subscales (along with treatment) in separate models; and (2) multivariable models that tested all six subscales (along with treatment) in a single model. The multivariable model allowed determination of significant subscale predictors of abstinence controlling for the other subscales. Univariable analyses showed that the Negative Affect and Craving subscale scores significantly predicted week 4 abstinence status but only Craving predicted week 26 abstinence status. In the multivariable analyses, the Craving subscale was the only statistically significant predictor of week 4 and week 26 abstinence although the Negative Affect subscale approached significance for week 4 abstinence (p=.0554).
Table 7.
Predictive Validity of Residualized WSWS2 Change Scores: Logistic Regressions of Week 4 and Week 26 Abstinence in the WSHS2 Study
| Residualized Composite Scores | Week 4 Self-Reported Abstinence | Week 26 CO-Confirmed Abstinence | ||
|---|---|---|---|---|
| Wald (p-value) | Wald (p-value) | |||
| WSWS2-L Total Score (19 Items) | 9.66 (p=.0019) | 1.11 (p=.2918) | ||
| WSWS2-B (6 Items) | 7.40 (p=.0065) | 1.07 (p=.3000) | ||
| Week 4 Self-Reported Abstinence | Week 26 CO-Confirmed Abstinence | |||
| Residualized WSWS2-L Subscales | Univariate Model | Multivariate Model | Univariate Model | Multivariate Model |
| Wald (p-value) | Wald (p-value) | Wald (p-value) | Wald (p-value) | |
| Negative Affect | 11.97 (p=.0005) | 3.67 (p=.0544) | 0.90 (p=.3416) | 0.04 (p=.8367) |
| Hunger | 0.62 (p=.4305) | 0.21 (p=.6444) | 0.23 (p=.6322) | 0.01 (p=.9316) |
| Craving | 31.98 (p<.0001) | 24.65 (p<.0001) | 9.83 (p=.0017) | 10.53 (p=.0012) |
| Sleep | 1.03 (p=.3097) | 0.14 (p=.7092) | 0.47 (p=.4940) | 0.12 (p=.7247) |
| Restlessness | 0.61 (p=.4362) | 2.05 (p=.1514) | 0.50 (p=.4792) | 3.25 (p=.0715) |
| Concentration | 1.32 (p=.2502) | 0.58 (p=.4477) | 0.07 (p=.7897) | 0.02 (p=.8953) |
Note. WSWS2-L = 19-item Wisconsin Smoking Withdrawal Scale 2. WSWS2-B = 6-item Brief Wisconsin Smoking Withdrawal Scale 2. All models include treatment as a predictor. The residualized change scores were computed as post-quit week 1 scores residualized on pre-quit week −1 scores. The Univariate Model includes only the single subscale and treatment as predictors. The Multivariate Model includes all six subscale and dummy-coded treatment as predictors.
Discussion
This research used rigorous scale development methodology to produce two smoking withdrawal assessments. One, the WSWS2-L (Appendix 1), is a 19-item multi-dimensional withdrawal assessment that includes six subscales, each comprising multiple items. The second, WSWS2-B (Appendix 2), is a brief assessment comprising 6 items that are designed to measure the same six withdrawal related constructs assessed in the WSWS2-L: i.e., craving, negative affect, sleep, restlessness, concentration, and hunger. The two scales were developed using psychometric analyses that: 1) identified the optimal number of factors to include in the assessments; 2) used both substantive and analytic methods to identify best-performing or appropriate items; and 3) assessed scores from both scales and the individual subscales for internal consistency and test-retest stability. These psychometric analyses illustrate that the WSWS2-L and WSWS2-B scales as well as the six WSWS2-L subscales have acceptable internal consistency and good test-retest stability across early and late post-TQD time points.
In validity analyses, the WSWS2-L and WSWS2-B were found to be positively related to measures of cigarette dependence (albeit not measures of smoking heaviness i.e., CPD, CO), negative affect, and short-term abstinence (e.g., 4 weeks post-TQD), demonstrating convergent and predictive validity. Conversely, the WSWS2-L and WSWS2-B were unrelated to positive affect, demonstrating divergent validity. These findings suggest that both measures are indeed tapping the critical construct of tobacco withdrawal that is positively related to tobacco dependence and inversely related to ability to quit smoking. Finally, CFAs revealed robust goodness of fit across multiple indices of fit.
While both questionnaires are fairly brief and should produce little assessment burden, there may be circumstances where using the briefer WSWS2-B would be preferable; e.g., when assessments occur over multiple occasions, when responding to numerous items would be difficult (e.g., in studies collecting EMA data), or when it is a component of a large assessment battery. However, there may be potential costs to using a brief assessment that comprises only single items to measure each of six withdrawal constructs. The WSWS2-L in general had somewhat stronger associations with abstinence outcomes than did the WSWS2-B. Moreover, when individual types of withdrawal symptoms are of interest, only the WSWS2-L permits assessment of internal consistency at any given point of usage. Moreover, the use of multi-item subscales will probably show stronger relations with important validity criteria than will single items. However, it is important to bear in mind that the WSWS2-L and the WSWS2-B total scores are highly correlated, r >.95, in all three studies.
The multi-dimensional nature of the WSWS2-L allows for meaningful tests of associations with individual withdrawal dimensions. For instance, the predictive validity analyses illustrated that negative affect and craving were the withdrawal constructs that were most strongly related to 4-week abstinence. Further, only the Craving subscale was predictive of 26-week abstinence. Having reliable subscales should facilitate other analyses that clarify how different withdrawal facets are associated with theoretically and clinically meaningful criteria: types of dependence, candidate genes, treatment responsivity, and physiologic responses (e.g., imaging). Thus, reliable assessments of withdrawal facets should aid bootstrapping addiction theory and understanding treatment effects.
This research did not directly compare the WSWS2 scales with alternative withdrawal assessments. However, the WSWS2 scales can be compared in terms of coverage of the withdrawal constructs. For example, the Minnesota Tobacco Withdrawal Scale-Revised (MTWS-R; Hughes, 2020) comprises eight well-validated, primary withdrawal symptom descriptors (single items) based on DSM-5 (“Angry, irritable, frustrated;” “Anxious, nervous;” “Depressed mood, sad;” “Difficulty concentrating;” “Increased appetite, hungry, weight gain;” “Insomnia, sleep problems, awakening at night;” “Restless;” and “Impatient”) as well as nine additional symptom candidates (e.g., “Craving to smoke;” “Decreased pleasure from events;” and “Dizziness”). MTWS-R items are rated as 0 = none, 1 = slight, 2 = mild, 3 = moderate, 4 = severe. Compared to the WSWS2-L which has multi-item subscale scores in addition to a total score and a brief version score, the MTWS-R yields only a total score as the sum of the 17 items. Future research should compare the revised versions of these two different withdrawal instruments in terms of their construct validity.
Limitations and Future Directions
Limitations of this research include a lack of comparison withdrawal measures (e.g., MTWS-R) to permit examination of relative validities. This is a goal for our future research. Another limitation is that all of the participants in this research received smoking cessation medication over the course of the quit attempt. This may have reduced withdrawal severity; thus, reported symptoms are likely somewhat weaker than ones that would have been reported by participants receiving no medication; this certainly may have affected the relations of the withdrawal measures with criterion measures. Likewise, these analyses collapsed across smokers who were and were not abstinent post-TQD (except for test-retest stability analyses) to maximize the sample size for psychometric analyses. Future research should explore WSWS2 psychometric properties in smokers who are completely abstinent. Additionally, future studies should examine measurement invariance across a wide variety of important participant characteristics (e.g., sex, race) and treatment-relevant states (e.g., abstinent vs smoking; medicated vs. unmedicated withdrawal) (Hussey & Hussey, 2020).
Another limitation is that the WSWS2-B was not evaluated through administration as a separate standalone scale. Additional research is needed to verify that the six WSWS2-B items will function similarly to the 19-item WSWS2-L when administered by themselves. Further, this study did not examine WSWS2 performance over enough post-quit time points to permit the analysis of withdrawal waveforms. Thus, this study cannot determine the temporal patterning of withdrawal symptoms. Finally, the WSWS2 does not assess physical symptoms. Such symptoms may be important manifestations of withdrawal severity and dependence but may not be optimally measured via self-report scales.
Given the importance of craving in assessing risk for relapse, future research should examine predictive and convergent validity of the WSWS2-L craving subscale in comparison with established measures of nicotine craving such as the Questionnaire of Smoking Urges-Brief version (Cox, Tiffany, & Christen, 2001) and the Tobacco Craving Questionnaire-Short Form (Heishman, Singleton, & Pickworth, 2008). Also, this study focused on assessing tobacco withdrawal among cigarette smokers but may have important implications for assessment of withdrawal from other forms of nicotine and tobacco. For instance, future research may examine the reliability and validity of the WSWS2 as a tool to assess withdrawal from other forms of smoked tobacco (e.g., cigars, hookah) and smokeless tobacco products (e.g., chew, snus). Furthermore, as e-cigarettes, vaping, and emerging nicotine delivery systems continue to change the landscape of nicotine use it will be important to evaluate whether current measures such as WSWS2 provide a valid and reliable measure of nicotine withdrawal from these products or if new assessment tools are needed.
In summary, relative to the original WSWS scale, the WSWS2-L and WSWS2-B offer greater construct coverage, fewer items, and other enhancements. These versions should prove useful in tobacco cessation and other smoking-related research.
Supplementary Material
Figure 1.
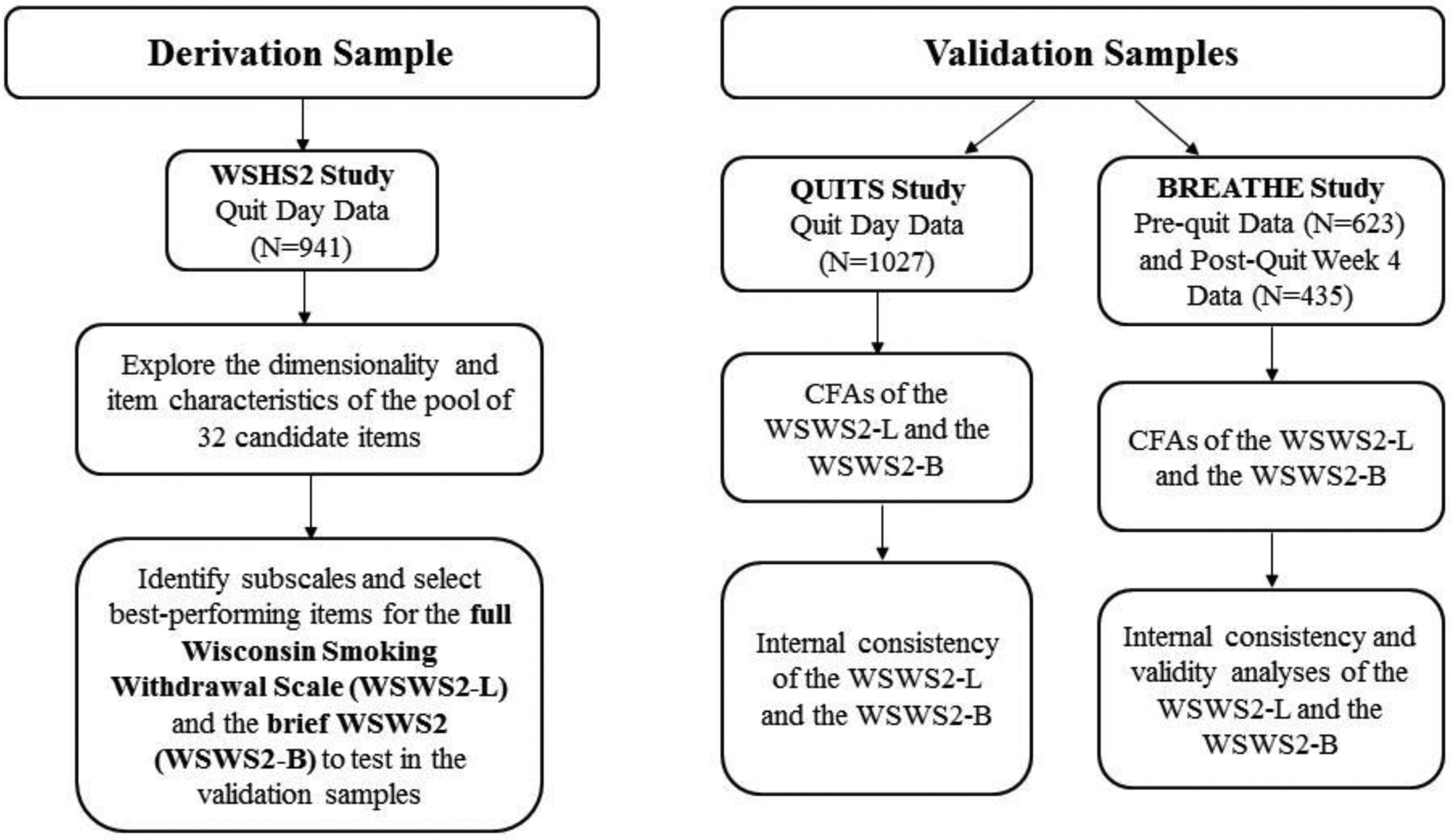
Analyses Conducted on the Derivation and Validation Samples
Public Significance Statement:
This research yielded revised long and brief versions of the Wisconsin Smoking Withdrawal Scale (WSWS). These revised versions (19-item standard version, WSWS2-L; 6-item brief version, WSWS2-B) should prove useful in tobacco cessation and other smoking-related research.
Acknowledgments
This research was supported by grants R01HL109031 and R01HL109031 from the National Heart, Lung, and Blood Institute, and P50CA143188, P01CA180945, and K05CA139871 from the National Cancer Institute to the University of Wisconsin Center for Tobacco Research and Intervention, and by the Wisconsin Partnership Program. The views expressed in this article are those of the authors and do not necessarily represent the views of the National Institutes of Health or the Department of Veterans Affairs.
We would like to acknowledge the staff at Aurora Health Care, Dean Medical Group, and Epic Systems Corporation for their collaboration in this research. We are very grateful to the staff and students at the Center for Tobacco Research and Intervention in the University of Wisconsin School of Medicine and Public Health for their help with this research.
Appendix 1. Wisconsin Smoking Withdrawal Questionnaire 2 (WSWS2-L)
| In the last 24 hours, using a scale from 1=not at all to 7=extremely, how much have you been bothered by… | ||||||||
|---|---|---|---|---|---|---|---|---|
| Not at all 1 | 2 | 3 | 4 | 5 | 6 | Extremely 7 | Refuse to Answer | |
| Feeling upset | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ |
| Thinking about food a lot | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ |
| Wanting to smoke | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ |
| Troubled sleep | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ |
| Feeling restless | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ |
| Having trouble concentrating | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ |
| Feeling unhappy | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ |
| Eating a lot | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ |
| Having urges to smoke | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ |
| Feeling tired | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ |
| Having trouble sitting still | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ |
| Having trouble paying attention | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ |
| Feeling stressed | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ |
| Feeling hungry | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ |
| Thinking about smoking | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ |
| Waking frequently during the night | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ |
| Feeling fidgety | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ |
| Having trouble thinking clearly | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ |
| Feeling angry | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ |
Appendix 2. Brief Wisconsin Smoking Withdrawal Questionnaire 2 (WSWS2-B)
| In the last 24 hours, using a scale from 1=not at all to 7=extremely, how much have you been bothered by… | ||||||||
|---|---|---|---|---|---|---|---|---|
| Not at all 1 | 2 | 3 | 4 | 5 | 6 | Extremely 7 | Refuse to Answer | |
| Feeling upset | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ |
| Thinking about food a lot | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ |
| Wanting to smoke | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ |
| Troubled sleep | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ |
| Feeling restless | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ |
| Having trouble concentrating | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ | ❍ |
Footnotes
The authors declare that they have no conflicts of interest. Studies providing data for the analyses in this article were approved by the University of Wisconsin Health Sciences Institutional Review Board, and all participants gave written informed consent.
References
- Abdolahi A, Williams GC, Benesch CG, Wang HZ, Spitzer EM, Scott BE, … van Wijngaarden E (2015). Damage to the insula leads to decreased nicotine withdrawal during abstinence. Addiction, 110(12), 1994–2003. 10.1111/add.13061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders, 5th ed. Washington, D.C.: American Psychiatric Association. [Google Scholar]
- Auerswald M, & Moshagen M (2019). How to determine the number of factors to retain in exploratory factor analysis: A comparison of extraction methods under realistic conditions. Psychological Methods, 24(4), 468–491. 10.1037/met0000200 [DOI] [PubMed] [Google Scholar]
- Baker TB, Breslau N, Covey L, & Shiffman S (2012). DSM criteria for tobacco use disorder and tobacco withdrawal: a critique and proposed revisions for DSM-5. Addiction, 107(2), 263–275. 10.1111/j.1360-0443.2011.03657.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, & Fiore MC (2004). Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychological Review, 111(1), 33–51. 10.1037/0033-295X.111.1.33 [DOI] [PubMed] [Google Scholar]
- Baker TB, Piper ME, Schlam TR, Cook JW, Smith SS, Loh WY, & Bolt D (2012). Are tobacco dependence and withdrawal related amongst heavy smokers? Relevance to conceptualizations of dependence. Journal of Abnormal Psychology, 121(4), 909–921. 10.1037/a0027889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Weiss RB, Bolt D, von Niederhausern A, Fiore MC, Dunn DM, … Cannon DS (2009). Human neuronal acetylcholine receptor A5-A3-B4 haplotypes are associated with multiple nicotine dependence phenotypes. Nicotine & Tobacco Research, 11(7), 785–796. 10.1093/ntr/ntp064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bold KW, McCarthy DE, Minami H, Yeh VM, Chapman GB, & Waters AJ (2016). Independent and interactive effects of real-time risk factors on later temptations and lapses among smokers trying to quit. Drug and Alcohol Dependence, 158, 30–37. 10.1016/j.drugalcdep.2015.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolt DM, Piper ME, Theobald WE, & Baker TB (2012). Why two smoking cessation agents work better than one: role of craving suppression. Journal of Consulting and Clinical Psychology, 80(1), 54–65. 10.1037/a0026366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braeken J, & van Assen M (2017). An empirical Kaiser criterion. Psychological Methods, 22(3), 450–466. 10.1037/met0000074 [DOI] [PubMed] [Google Scholar]
- Cambron C, Haslam AK, Baucom BRW, Lam C, Vinci C, Cinciripini P, … Wetter DW (2019). Momentary precipitants connecting stress and smoking lapse during a quit attempt. Health Psychology, 38(12), 1049–1058. 10.1037/hea0000797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro Y, Kendzor DE, Businelle MS, Mazas CA, Cofta-Woerpel L, Cinciripini PM, & Wetter DW (2011). Structural and predictive equivalency of the Wisconsin Smoking Withdrawal Scale across three racial/ethnic groups. Nicotine & Tobacco Research, 13(7), 548–555. 10.1093/ntr/ntr039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, Scharf D, & Shiffman S (2011). Within-day temporal patterns of smoking, withdrawal symptoms, and craving. Drug and Alcohol Dependence, 117(2–3), 118–125. 10.1016/j.drugalcdep.2010.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cofta-Woerpel L, McClure JB, Li Y, Urbauer D, Cinciripini PM, & Wetter DW (2011). Early cessation success or failure among women attempting to quit smoking: trajectories and volatility of urge and negative mood during the first postcessation week. Journal of Abnormal Psychology, 120(3), 596–606. 10.1037/a0023755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins LM, Dziak JJ, Kugler KC, & Trail JB (2014). Factorial experiments: efficient tools for evaluation of intervention components. American Journal of Preventive Medicine, 47(4), 498–504. 10.1016/j.amepre.2014.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins LM, Murphy SA, & Strecher V (2007). The Multiphase Optimization Strategy (MOST) and the Sequential Multiple Assignment Randomized Trial (SMART): new methods for more potent eHealth interventions. American Journal of Preventive Medicine, 32(5 Suppl), S112–118. 10.1016/j.amepre.2007.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins LM, Trail JB, Kugler KC, Baker TB, Piper ME, & Mermelstein RJ (2014). Evaluating individual intervention components: making decisions based on the results of a factorial screening experiment. Translational Behavioral Medicine, 4(3), 238–251. 10.1007/s13142-013-0239-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, & Christen AG (2001). Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & Tobacco Research, 3(1), 7–16. 10.1080/14622200020032051 [DOI] [PubMed] [Google Scholar]
- Cronbach LJ (1951). Coefficient alpha and the internal structure of tests. Psychometrika, 16, 297–334. https://link.springer.com/article/10.1007/BF02310555 [Google Scholar]
- DeCastellarnau A (2018). A classification of response scale characteristics that affect data quality: a literature review. Quality & Quantity, 52(4), 1523–1559. 10.1007/s11135-017-0533-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etter JF, & Hughes JR (2006). A comparison of the psychometric properties of three cigarette withdrawal scales. Addiction, 101(3), 362–372. 10.1111/j.1360-0443.2005.01289.x [DOI] [PubMed] [Google Scholar]
- Fagerstrom K (2012). Determinants of tobacco use and renaming the FTND to the Fagerstrom Test for Cigarette Dependence. Nicotine & Tobacco Research, 14(1), 75–78. 10.1093/ntr/ntr137 [DOI] [PubMed] [Google Scholar]
- Ferguson SG, Shiffman S, & Gwaltney CJ (2006). Does reducing withdrawal severity mediate nicotine patch efficacy? A randomized clinical trial. Journal of Consulting and Clinical Psychology, 74(6), 1153–1161. 10.1037/0022-006X.74.6.1153 [DOI] [PubMed] [Google Scholar]
- Gloria R, Angelos L, Schaefer HS, Davis JM, Majeskie M, Richmond BS, … Baker TB (2009). An fMRI investigation of the impact of withdrawal on regional brain activity during nicotine anticipation. Psychophysiology, 46, 681–693. 10.1111/j.1469-8986.2009.00823.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman L (1954). Some necessary conditions for common-factor analysis. Psychometrika, 19, 149–161. [Google Scholar]
- Hancock DB, Guo Y, Reginsson GW, Gaddis NC, Lutz SM, Sherva R, … Johnson EO (2018). Genome-wide association study across European and African American ancestries identifies a SNP in DNMT3B contributing to nicotine dependence. Molecular Psychiatry, 23(9), 1911–1919. 10.1038/mp.2017.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock DB, Reginsson GW, Gaddis NC, Chen X, Saccone NL, Lutz SM, … Stefansson K (2015). Genome-wide meta-analysis reveals common splice site acceptor variant in CHRNA4 associated with nicotine dependence. Translational Psychiatry, 5, e651. 10.1038/tp.2015.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, & Fagerstrom KO (1991). The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction, 86(9), 1119–1127. 10.1111/j.1360-0443.1991.tb01879.x [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Singleton EG, & Pickworth WB (2008). Reliability and validity of a Short Form of the Tobacco Craving Questionnaire. Nicotine Tob Res, 10(4), 643–651. 10.1080/14622200801908174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks PS, Ditre JW, Drobes DJ, & Brandon TH (2006). The early time course of smoking withdrawal effects. Psychopharmacology (Berl), 187(3), 385–396. 10.1007/s00213-006-0429-9 [DOI] [PubMed] [Google Scholar]
- Horn JL (1965). A rationale and test for the number of factors in factor analysis. Psychometrika, 30, 179–185. 10.1007/BF02289447 [DOI] [PubMed] [Google Scholar]
- Hu L. t., & Bentler PM (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling, 6(1), 1–55. 10.1080/10705519909540118 [DOI] [Google Scholar]
- Hughes JR (2020). Minnesota Tobacco Withdrawal Scale - Revised (MTWS-R. Retrieved 20 July 2020 from: http://www.med.uvm.edu/docs/behavior_rating_scale_self/behavior-and-health-documents/behavior_rating_scale_self.pdf?sfvrsn=372e8344_2
- Hughes JR, & Hatsukami D (1986). Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry, 43(3), 289–294. 10.1001/archpsyc.1986.01800030107013 [DOI] [PubMed] [Google Scholar]
- Hussey I, & Hussey S (2020). Hidden invalidity among 15 commonly used measures in social and personality psychology. Advances in Methods and Practices in Psychological Science, advance online publication. Retrieved 20 July 2020 from: 10.1177/2515245919882903 [DOI] [Google Scholar]
- Javitz HS, Lerman C, & Swan GE (2012). Comparative dynamics of four smoking withdrawal symptom scales. Addiction, 107(8), 1501–1511. 10.1111/j.1360-0443.2012.03838.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser HF (1960). The application of electronic computers to factor analysis. Educational and Psychological Measurement, 20, 141–151. 10.1177/001316446002000116 [DOI] [Google Scholar]
- Kline RB (2005). Methodology in the social sciences. Principles and practice of structural equation modeling (2nd ed.). New York, N.Y.: Guilford Press. [Google Scholar]
- Krosnick JA (2018). Questionnaire design. In Vannette DL & Krosnick JA (Eds.), The Palgrave handbook of survey research (pp. 439–455). Cham, Switzerland: Palgrave Macmillan. [Google Scholar]
- Krosnick JA, Presser S (2010). Question and questionnaire design. In Marsden PV & Wright JD (Eds.), Handbook of survey research (2nd ed., pp. 263–313). Bingley, UK: Emerald. [Google Scholar]
- Kubota T, Nakajima-Taniguchi C, Fukuda T, Funamoto M, Maeda M, Tange E, … Azuma J (2006). CYP2A6 polymorphisms are associated with nicotine dependence and influence withdrawal symptoms in smoking cessation. Pharmacogenomics J, 6(2), 115–119. 10.1038/sj.tpj.6500348 [DOI] [PubMed] [Google Scholar]
- Leventhal AM, & Zimmerman M (2010). The relative roles of bipolar disorder and psychomotor agitation in substance dependence. Psychology of Addictive Behaviors, 24(2), 360–365. 10.1037/a0019217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S, & Jahng S (2019). Determining the number of factors using parallel analysis and its recent variants. Psychological Methods, 24(4), 452–467. 10.1037/met0000230 [DOI] [PubMed] [Google Scholar]
- Marsh HW, Hau KT, Balla JR, & Grayson D (1998). Is more ever too much? The number of indicators per factor in confirmatory factor analysis. Multivariate Behavioral Research, 33(2), 181–220. 10.1207/s15327906mbr3302_1 [DOI] [PubMed] [Google Scholar]
- Mathew AR, Hogarth L, Leventhal AM, Cook JW, & Hitsman B (2017). Cigarette smoking and depression comorbidity: systematic review and proposed theoretical model. Addiction, 112(3), 401–412. 10.1111/add.13604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy DE, Piasecki TM, Jorenby DE, Lawrence DL, Shiffman S, & Baker TB (2010). A multi-level analysis of non-significant counseling effects in a randomized smoking cessation trial. Addiction, 105(12), 2195–2208. 10.1111/j.1360-0443.2010.03089.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald RP (1999). Test theory: A unified treatment. Mahwah, NJ: Lawrence Erlbaum. [Google Scholar]
- Muthén LK, & Muthén BO (1998–2017). Mplus user’s guide. Eigth eEdition (Vol. Muthén & Muthén.): Los Angeles, CA. [Google Scholar]
- Nunnally JC, & Bernstein IH (1994). Psychometric theory, 3rd edition. New York: McGraw Hill. [Google Scholar]
- Pergadia ML, Heath AC, Martin NG, & Madden PA (2006). Genetic analyses of DSM-IV nicotine withdrawal in adult twins. Psychological Medicine, 36(7), 963–972. 10.1017/S0033291706007495 [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Jorenby DE, Smith SS, Fiore MC, & Baker TB (2003). Smoking withdrawal dynamics: II. Improved tests of withdrawal-relapse relations. Journal of Abnormal Psychology, 112(1), 14–27. 10.1037/0021-843X.112.1.14 [DOI] [PubMed] [Google Scholar]
- Piper ME, Bolt DM, Kim SY, Japuntich SJ, Smith SS, Niederdeppe J, Cannon DS, Baker TB (2008). Refining the tobacco dependence phenotype using the Wisconsin Inventory of Smoking Dependence Motives. Journal of Abnormal Psychology, 117(4), 747–761. 10.1037/a0013298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Federman EB, McCarthy DE, Bolt DM, Smith SS, Fiore MC, & Baker TB (2008). Using mediational models to explore the nature of tobacco motivation and tobacco treatment effects. Journal of Abnormal Psychology, 117(1), 94–105. 10.1037/0021-843X.117.1.94 [DOI] [PubMed] [Google Scholar]
- Piper ME, Fiore MC, Smith SS, Fraser D, Bolt DM, Collins LM, … Baker TB (2016). Identifying effective intervention components for smoking cessation: a factorial screening experiment. Addiction, 111(1), 129–141. 10.1111/add.13162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Piasecki TM, Federman EB, Bolt DM, Smith SS, Fiore MC, & Baker TB (2004). A multiple motives approach to tobacco dependence: the Wisconsin Inventory of Smoking Dependence Motives (WISDM-68). Journal of Consulting and Clinical Psychology, 72(2), 139–154. 10.1037/0022-006X.72.2.139 [DOI] [PubMed] [Google Scholar]
- Piper ME, Schlam TR, Cook JW, Sheffer MA, Smith SS, Loh WY, Bolt DM, Kim SY, Kaye JT, Hefner KR, Baker TB (2011). Tobacco withdrawal components and their relations with cessation success. Psychopharmacology (Berl), 216(4), 569–578. 10.1007/s00213-011-2250-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson CD, Pickworth WB, Heishman SJ, & Waters AJ (2014). The acute tobacco withdrawal syndrome among black smokers. Psychology of Addictive Behaviors, 28(1), 173–181. 10.1037/a0031950 [DOI] [PubMed] [Google Scholar]
- Schlam TR, Fiore MC, Smith SS, Fraser D, Bolt DM, Collins LM, … Baker TB (2016). Comparative effectiveness of intervention components for producing long-term abstinence from smoking: a factorial screening experiment. Addiction, 111(1), 142–155. 10.1111/add.13153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider NG, Jarvik ME, & Forsythe AB (1984). Nicotine vs. placebo gum in the alleviation of withdrawal during smoking cessation. Addictive Behaviors, 9(2), 149–156. 10.1016/0306-4603(84)90052-2 [DOI] [PubMed] [Google Scholar]
- Schnoll RA, Hitsman B, Blazekovic S, Veluz-Wilkins A, Wileyto EP, Leone FT, & Audrain-McGovern JE (2016). Longitudinal changes in smoking abstinence symptoms and alternative reinforcers predict long-term smoking cessation outcomes. Drug and Alcohol Dependence, 165, 245–252. 10.1016/j.drugalcdep.2016.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman SM, & Jarvik ME (1976). Smoking withdrawal symptoms in two weeks of abstinence. Psychopharmacology (Berl), 50(1), 35–39. 10.1007/BF00634151 [DOI] [PubMed] [Google Scholar]
- Smith SS, Piper ME, Bolt DM, Fiore MC, Wetter DW, Cinciripini PM, & Baker TB (2010). Development of the Brief Wisconsin Inventory of Smoking Dependence Motives. Nicotine & Tobacco Research, 12(5), 489–499. 10.1093/ntr/ntq032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon RL (1977). An opponent-process theory of acquired motivation: The affective dynamics of addiction. In Maser JD & Seligman MEP (Eds.), Psychopathology: Experimental models (pp. 66–103). San Francisco: Witt Freeman. [Google Scholar]
- U.S. National Center for Health Statistics. (2020). International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM). https://www.cdc.gov/nchs/icd/icd10cm.htm [PubMed]
- Watson D, Clark LA, & Tellegen A (1988). Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology, 54(6), 1063–1070. 10.1037/0022-3514.54.6.1063 [DOI] [PubMed] [Google Scholar]
- Welsch SK, Smith SS, Wetter DW, Jorenby DE, Fiore MC, & Baker TB (1999). Development and validation of the Wisconsin Smoking Withdrawal Scale. Experimental and Clinical Psychopharmacology, 7(4), 354–361. 10.1037/1064-1297.7.4.354 [DOI] [PubMed] [Google Scholar]
- West R, Ussher M, Evans M, & Rashid M (2006). Assessing DSM-IV nicotine withdrawal symptoms: a comparison and evaluation of five different scales. Psychopharmacology (Berl), 184(3–4), 619–627. 10.1007/s00213-005-0216-z [DOI] [PubMed] [Google Scholar]
- Wikler A (1980). Opioid dependence: Mechanisms and treatment. New York: Plenum. [Google Scholar]
- Wong JA, & Leventhal AM (2015). Smoking-related correlates of psychomotor restlessness and agitation in a community sample of daily cigarette smokers. American Journal on Addictions, 24(2), 166–172. 10.1111/ajad.12158 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


