Abstract
Background:
The observed deficit of lung cancer in farmers has been partly attributed to exposure to organic dusts and endotoxins based largely on surrogate metrics. To move beyond these surrogates for etiologic studies, we characterized task-based and time-weighted average (TWA) exposure to inhalable endotoxin, (1→3)-β-D-glucan, and dust in Iowa farmers.
Methods:
We collected 320 personal inhalable dust samples from 32 farmers during 69 sample days in 2015 and 2016. Samples were collected using Button aerosol samplers and analyzed for endotoxin using a kinetic chromogenic amebocyte lysate assay, and for (1→3)-β-D-glucan using a Limulus endpoint assay. We assessed relationships between bioaerosol concentrations and selected tasks and farm characteristics using linear mixed-effects models.
Results:
Bedding work, hog handling, and working in barn/confinement buildings, grain bins, and grain elevators were associated with higher endotoxin exposure. We found a monotonic trend between higher endotoxin concentrations and increasing number of animals. Bedding work, cleaning, and feed/grain storage work were associated with higher (1→3)-β-D-glucan concentrations. The median concentrations by task spanned a 400-fold range for endotoxin, a 600-fold range for (1→3)-β-D-glucan, and an 8-fold range for inhalable dust. Pearson correlations between endotoxin and glucan concentrations were 0.22 for TWA exposure and 0.56 for task samples.
Conclusions:
This characterization of exposure factors that influence bioaerosol concentrations can support the development of refined bioaerosol exposure metrics for future etiologic analyses of cancer and other health outcomes in farmers.
Keywords: agriculture, bioaerosols, endotoxin, glucan, occupational exposure
Introduction
Several studies have shown a deficit of lung cancer in farmers that is not fully attributable to lower rates of smoking compared to the general population (Lenters et al., 2010; Lerro et al., 2019; Schenker et al., 1998). One hypothesis for this inverse association is the higher exposures to bioaerosols, which are airborne particulates originating from microorganisms, fungi, plants, or animals (Douwes et al., 2003; Lindsley et al., 2017) that may contain biologically active components with proinflammatory properties (Basinas et al., 2017). Most studies have focused on endotoxins, which are lipopolysaccharides and lipooligosaccharides found on the cell walls of Gram-negative bacteria (Hoppe Parr et al., 2017). Exposure to endotoxins may modulate circulating levels of inflammatory and immunologic response markers potentially involved in lung carcinogenesis (Lenters et al., 2010; Lundin and Checkoway, 2009). However, bioaerosols may also contain other agents contributing to immune and inflammatory responses, such as (1→3)-β-D-glucans, which are glucose polymers located on the cell walls of fungi and some bacteria (Basinas et al., 2017; Douwes et al., 2003). Exposure to bioaerosols is also associated with a diversity of allergic and non-allergic respiratory diseases (Douwes et al., 2003; Thorne et al., 2015).
The inverse association between bioaerosol exposure and lung cancer risk has been observed in studies of textile workers, with some studies having access to organic dust and/or endotoxin measurements collected in the workplace that allowed for the exploration of exposure-response relationships (Astrakianakis et al., 2007; Xu et al., 2016). In contrast, the associations observed in farmers were mainly inferred from surrogates such as job title, farm type, and size (Lenters et al., 2010). For example, in the Agricultural Health Study (AHS) cohort in the United States, the largest deficits of lung cancer occurred among male farmers raising over 1000 heads of livestock (relative risk (RR)=0.5; 95% confidence interval (CI) 0.3–1.0) or raising poultry (RR=0.63; 95%CI 0.4–0.97) compared to farmers that did not raise animals (Beane Freeman et al., 2012). In the Biomarkers of Exposure and Effect in Agriculture (BEEA) Study, a molecular sub-study within the AHS cohort, hog farmers had altered levels of immune and inflammatory biomarkers potentially associated with lung carcinogenesis, compared to non-hog farmers (Hofmann et al., 2018). However, while livestock farmers have a higher potential for bioaerosol exposure compared to non-livestock farmers (Basinas et al., 2017), the bioaerosol concentrations of AHS participants have not yet been characterized, preventing the investigation of lung cancer risk based on quantitative exposure levels. Additionally, although several studies have assessed exposure to bioaerosols in Europe or in the United States (Basinas et al., 2015; Kullman et al., 1998; Thorne et al., 2009), few studies have documented the exposure levels encountered during crop-related activities on U.S. Midwest farms, which typically have a high prevalence of row crops and large acreage. Moreover, few studies have evaluated factors associated with glucan exposure in agriculture (Cyprowski et al., 2012; Roy and Thorne, 2003; Samadi et al., 2012; Samadi et al., 2009; Singh et al., 2011).
As a first step in the development of estimates for bioaerosol exposure for the AHS and BEEA participants, we undertook a field study to monitor airborne concentration levels of inhalable dust, endotoxin, and (1→3)-β-D-glucans in Iowa farmers. Our primary aim was to characterize task-based factors associated with endotoxin and glucan exposure in both crop and livestock farming activities. We plan to use the results of this exposure characterization to support the future development of task-based models applicable to the BEEA and AHS populations.
Methods
Study population
The AHS (Alavanja et al., 1996) prospective cohort study includes 52,394 licensed private pesticide applicators (mostly farmers) from Iowa and North Carolina enrolled between 1993 and 1997. We conducted bioaerosol exposure characterization on participants within the BEEA sub-study, which investigates the biologic plausibility and potential mechanisms of action underlying the associations between farming exposures and cancer (Hofmann et al., 2015). We recruited 32 farmers to participate in bioaerosol personal monitoring on the day before their interview and biospecimen collection for the BEEA study. All met the eligibility criteria of the BEEA study. That is, they were male, aged 50 years or older, never diagnosed with a primary site of cancer excluding non-melanoma skin cancer, and had completed questionnaires for all phases of the AHS up to 2010. All bioaerosol monitoring participants resided in Iowa and were either actively farming or were expected to be farming during the three months following initial contact.
Sample collection and analysis
Field study design
We intended to visit each participant twice, once in the fall (in 2015 or 2016) and once in the spring (2016 only). Sample days typically ranged from 8:00 to 14:00 (exclusive of sample preparation), although some visits started as early as 6:00 or ended as late as 17:00. Because of the driving distance between the farms and the laboratory and the irregular farming hours, we were not always able to monitor exposure during the entire time spent farming. During each visit, a field researcher recorded farm and task characteristics using two data collection methods. First, we used free-text activity logs of real-time observational data to record information on the tasks performed, their start and end times, the farmer’s location, and use of personal protective equipment. Second, we used standardized modules (n=5; presented in Appendix A) to systematically collect structured information on farming characteristics identified in the literature as associated with endotoxin exposure. For example, the questionnaire for crops included crop type, equipment, location on the farm (e.g., field, grain bin), acreage worked, production phase, and field conditions. Questionnaires for beef cattle, dairy, hog, and poultry included location on the farm (e.g., barn, feedlot), production phase (e.g., farrowing, finishing), feed type, use of antibiotics, manure collection methods, floor type, bedding type, number of animals, and ventilation.
Sampling methods
We collected personal inhalable dust samples using Button aerosol samplers (SKC Inc, No. 225–360, Eighty Four, PA) containing pre-weighed 25 mm binder-free glass fiber filters connected to air sampling pumps (BGI OMNI-400, MesaLabs, Butler, NJ, or SKC model 224-PCXR4, SKC Inc, Eighty Four, PA) operating at a flow rate of 4 L/min. The sampling equipment was carried by the farmers in a backpack or waist pack, depending on the activity performed; for instance, a waist pack was typically more suitable for driving. We calibrated each sampling device before and after the visit using a primary standard (TetraCal, BGI Inc, Waltham, MA). We collected field blanks at a rate of approximately 1 per 10 samples. We also recorded the ambient temperature and relative humidity at sample start time using a digital hygro-thermometer pen (Extech Instruments Corp., no. 445580, Waltham, MA).
We aimed to collect two types of samples during each visit (Figure 1). First, we collected short-term samples representing exposure for a single activity (hereafter referred to as “task samples”). These targeted activities where large contrasts in exposure were expected, such as animal handling, milking, or grain storage, and activities underrepresented in the literature. We also collected samples to characterize daily time-weighted average (TWA) exposure, which had a longer sampling duration and encompassed several activities, including break periods. The task samples were collected on a separate pump generally at the same time as the TWA samples. Occasionally, TWA sampling was paused during very dusty activities while a task sample was collected, in order to prevent filter overloading (Figure 1).
Figure 1.
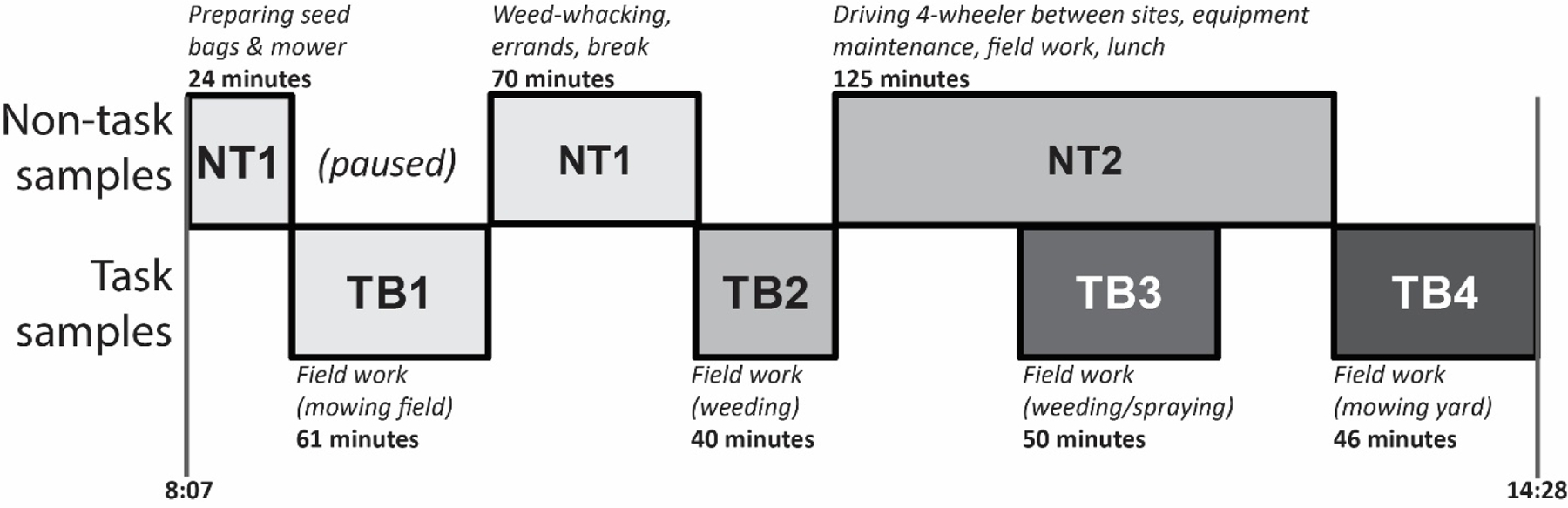
Sampling collection scheme for one of the 69 sample days, comprising four task-based (TB) samples and two non-task (NT) samples.
Analytical methods
We weighed the air sampling filters before and after sample collection in a temperature- and humidity-controlled gravimetrics laboratory (Mettler Toledo XP26 Microbalance, Columbus, OH). We equilibrated all filters and assessed microbalance accuracy prior to weighing using calibration weights (200, 100, and 20 mg). We divided the filters in half, using one half for endotoxin and glucan analysis, and storing the other half for future analyses. The half-filters for endotoxin and glucan analysis were reweighted separately and stored in pyrogen-free 15ml tubes at −20°C until analysis.
We analyzed the half-filters for endotoxin and glucan using a previously described method (Behbod et al., 2013). Briefly, we extracted filters in 3 mL of Limulus Amebocyte Lysate (LAL) reagent water with shaking for 30 minutes (MAXQ 2500, Thermo Scientific), sonicating for 30 minutes at 26°C (FS20D with 40kHz frequency, Fisher Scientific), re-shaking for 10 minutes, and centrifuging for 5 minutes (600 ×g at 4°C;Eppendorf Centrifuge 5810R) to remove debris. We transferred 1.5 mL to pyrogen-free borosilicate tubes for endotoxin analysis and retained the remaining extract and filter for glucan analysis. We evaluated the filter extract for endotoxin at three dilutions using the kinetic chromogenic (LAL) assay as previously described (Thorne, 2000). We measured the rate of 405 nm absorbance increase at every 30 seconds over 90 minutes using a microplate reader (Molecular Devices SpectraMax 384 Plus, Sunnyvale, CA, with Softmax PRO 5.4 analysis software) and evaluated against a 12-point standard curve using E. coli 055:B5 control standard endotoxin.
We measured (1→3)-β-D-glucan using the LAL factor G pathway (Glucatell Reagent Kit, Associates of Cape Cod). Sodium hydroxide was added to the second vial containing extract and filter to yield 0.3N NaOH in pyrogen-free water. The sample was shaken on ice for 25 minutes and then centrifuged (5 minutes, 600 ×g, 4°C). We transferred the extract to a pyrogen-free tube and analyzed for glucan per the manufacturer’s instructions. We adjusted both endotoxin and glucan concentrations for the mean values of the field blanks.
Statistical analyses
Data preparation
We imputed sample values for measurements below the limit of detection (LOD; 0.006 mg/filter for dust, 0.024 EU/ml for endotoxin, and 2.5 pg/ml for (1→3)-β-D-glucans) and above an upper detection limit of 0.8 mg/sample for inhalable dust, using the procedure described by Lubin et al. (2004). We graphically confirmed the lognormality of the sample mass and concentration values before and after imputation. We conducted five iterations of the imputation procedure, resulting in five datasets. For samples with missing ambient temperature or relative humidity values, we assigned the daily average value for Iowa City for the date of the visit reported by the AccuWeather weather forecast service (State College, PA).
Descriptive analyses
We calculated TWA concentrations from the sequential samples for each visit. We then calculated the geometric mean (GM) and geometric standard deviation (GSD) of the TWA and task-based concentrations, overall and by level of selected categorical variables. We also calculated pairwise Pearson correlations between the log-transformed endotoxin, (1→3)-β-D-glucans, and inhalable dust concentrations, separately for task and TWA samples. We also computed the between and within-farmer variance in the log-transformed endotoxin, (1→3)-β-D-glucans, and inhalable dust TWA concentrations using random-effects models. We calculated each of these metrics for each of the five imputed datasets independently and then used the average of the five iterations as the final estimate of that metric.
Statistical modeling of characteristics of task-based exposure
Some tasks and farm characteristics had few measurements and were collinear with other characteristics. As a result, we developed three separate models per analyte. The first model included all task samples and had task as the main variable, using the most frequently sampled task (harvest) as the reference level. The second model included only samples from livestock-related activities and had location on the farm (barn or confinement; feedlot, corrals, or pen) and number of animals in the location as main variables. We categorized the number of animals as <100, 100 to <1000, and ≥1000 animals, as per Beane Freeman et al. (2012). We also conducted tests for trend by treating the three categories of number of animals as a continuous, ordinal variable (0, 1, 2). As a sensitivity analysis, we also converted the number of animals into animal units (AU) standardized by livestock type, with 1 AU equivalent to 1 head of beef cattle, 0.7 dairy cows, 0.4 hogs weighting over 55 lbs, and 0.1 hogs weighting under 55 lbs. We log-transformed AUs prior to its inclusion in the model. The third model included all crop-related samples and had location on the farm (field; grain bin or elevator; other/unknown), production phase (eg., field work, harvest), and crop type as the main variables. In addition, each model included covariates comprising sampling season (fall 2015, spring 2016, fall 2016) as a categorical variable, and relative humidity, temperature, and sampling duration as continuous variables. We centered relative humidity and temperature around their overall mean. We included farmer and sampling day as random effect terms in all models.
We used multimodel averaging (Burnham and Anderson, 2002) to estimate the model coefficients and standard errors. For each model, we first fit sub-models corresponding to all possible combinations of the fixed effects. We then calculated model weights for each sub-model based on the Akaike information criterion corrected for small sample size (Hurvich and Tsai, 1989). The model weights were then used to calculate a weighted average of each coefficient, which takes the value 0 in the sub-models when a variable was absent. In this approach, the multimodel averaged coefficient for a variable is shrunk towards zero when the models that include this variable have small model weights. Shrinkage tends to be minimal for a variable consistently associated with models with high weights. The standard errors of multimodel averaged coefficients account for model selection uncertainty as an additional source of variation. The R package MuMin version 1.42.1 was used to apply the multimodel procedure.
We applied the models to each of the five imputed datasets independently. We then combined the estimates across the five iterations to derive the overall parameter estimates and standard errors, accounting for between- and within-imputation variance (Marshall et al., 2009). We exponentiated the model coefficients to express the estimates as geometric mean ratios (GMR) relative to the reference category. Since there is currently no standard method available to compute multimodel averaged estimates of variance components, we used the variance estimates from the model containing all fixed effects terms. Similarly, we conducted the test for trend for the number of animals using the model with all fixed effects terms.
Results
Descriptive statistics
Study population
We conducted a total of 69 visits, with 23 days in fall 2015 (October-November), 34 days in spring 2016 (April-July), and 12 days in fall 2016 (September-October). Of the 32 farmers visited, twelve farmers were visited twice, with a median interval of 179 days between visits. Eleven farmers were visited three times, and one farmer was visited four times. These additional visits were made to increase the number of samples per task, with a focus on livestock activities. Eight farmers had only one visit, mostly because of study time constraints; only one farmer refused a second visit.
Overall, we collected 320 samples, including 204 task samples (average of 3 per visit). We also collected 36 field blanks. Four of the 69 visits only contained task samples, all of which occurred in November 2015 and were not paired to the BEEA interview. Another visit had no task sample collected because of rain. The overall proportion of samples with a non-detected concentration was below 10% for all agents, while 42 samples (13%) had a dust sample mass above 0.8 mg, our definition of overload. We imputed ambient temperature values for nine samples, and relative humidity for 15 samples, using the daily averages for Iowa City.
Task exposure
The 204 task samples collected included 105 crop-related activities and 50 related to livestock, mostly beef (n=25) and hogs (n=21). The remaining 49 samples involved activities such as driving or maintenance. The average sampling duration was 34 minutes (range 4–105 minutes). Harvesting (n=44) and field work (n=43) were the most-frequently sampled tasks. Harvesting was predominantly conducted with the farmer driving a combine, and the vast majority of samples involved corn (34 samples) and soybeans (6 samples). Field work covered activities such as mowing, weeding, applying fertilizer, and soil cultivation. Figure 2 shows the distribution of endotoxin, (1→3)-β-D-glucans, and inhalable dust concentrations by task using boxplots ordered by decreasing median concentration for endotoxin. Hog handling, bedding work, and cleaning building/equipment had the highest median concentrations for both endotoxin and (1→3)-β-D-glucans, with different rank orders. The ratios between the highest and the lowest concentrations were much smaller for inhalable dust (8-fold difference) than for endotoxin (400-fold) or glucan (600-fold). Table B.1 (Appendix B) presents the number of task samples and the GM and GSD of the endotoxin, (1→3)-β-D-glucans, and dust concentrations by strata of categorical variables. Seven tasks (out of 11) had a GSD greater than 10 for (1→3)-β-D-glucans, compared to two for endotoxin and none for dust.
Figure 2.
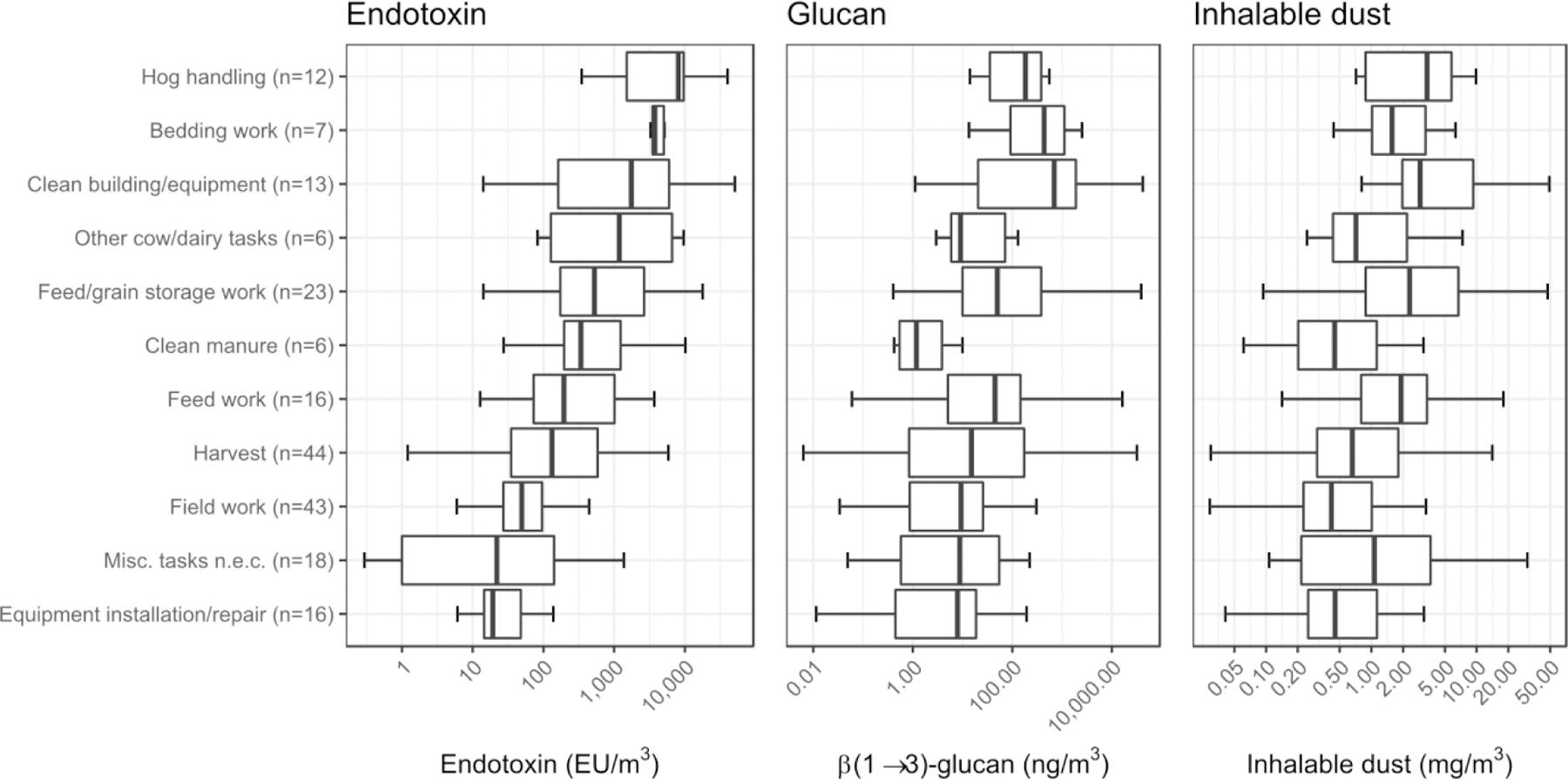
Distribution of endotoxin, glucan, and inhalable dust concentration levels by task. The boxes and whiskers represent the average values of the quartiles across the five imputations. Outliers (ie., values outside the whiskers) are omitted.
Use of dust mask or respirators was recorded for six task samples involving cleaning building/equipment (n=2), feed/grain storage work (n=2), equipment maintenance (n=1) and miscellaneous tasks (cutting concrete, n=1). Compared to the remaining task samples (n=198), these activities were associated with higher concentrations for endotoxin (GM 732 EU/m3 vs. 155 EU/m3), (1→3)-β-D-glucans (GM 225.0 ng/m3 vs. 14.7 ng/m3), and inhalable dust (5.0 mg/m3 vs. 0.85 mg/m3).
TWA exposure
We assessed TWA exposure for 65 visits. Seven visits had a single sample covering the entire sampling period. For the remaining 58 visits, we calculated TWA concentrations from sequential samples (maximum seven samples/visit). The median total sampling duration ranged between 129 and 486 minutes (median 380 minutes). The overall GM TWA concentrations of endotoxin, (1→3)-β-D-glucans, and dust were 167 EU/m3 (GSD 5.2), 19.7 ng/m3 (GSD 8.9), and 0.77 mg/m3 (GSD 2.8), respectively. The GMs and GSDs of TWA concentrations by season and farm type are listed in Appendix B, table B.2. Between-farmer variance explained 28%, <1%, and 3% of the total variance in endotoxin, (1→3)-β-D-glucan, and inhalable dust concentrations, respectively, compared to models that included only random effects.
Correlations between endotoxin, (1→3)-β-D-glucans, and inhalable dust concentrations
The Pearson correlation between the log-transformed endotoxin and (1→3)-β-D-glucans concentrations was 0.56 for task samples and 0.22 for TWAs (Figure 3). The pairwise correlations involving inhalable dust were moderate (r≈0.60), except with (1→3)-β-D-glucans for TWA concentrations (r=0.23).
Figure 3.
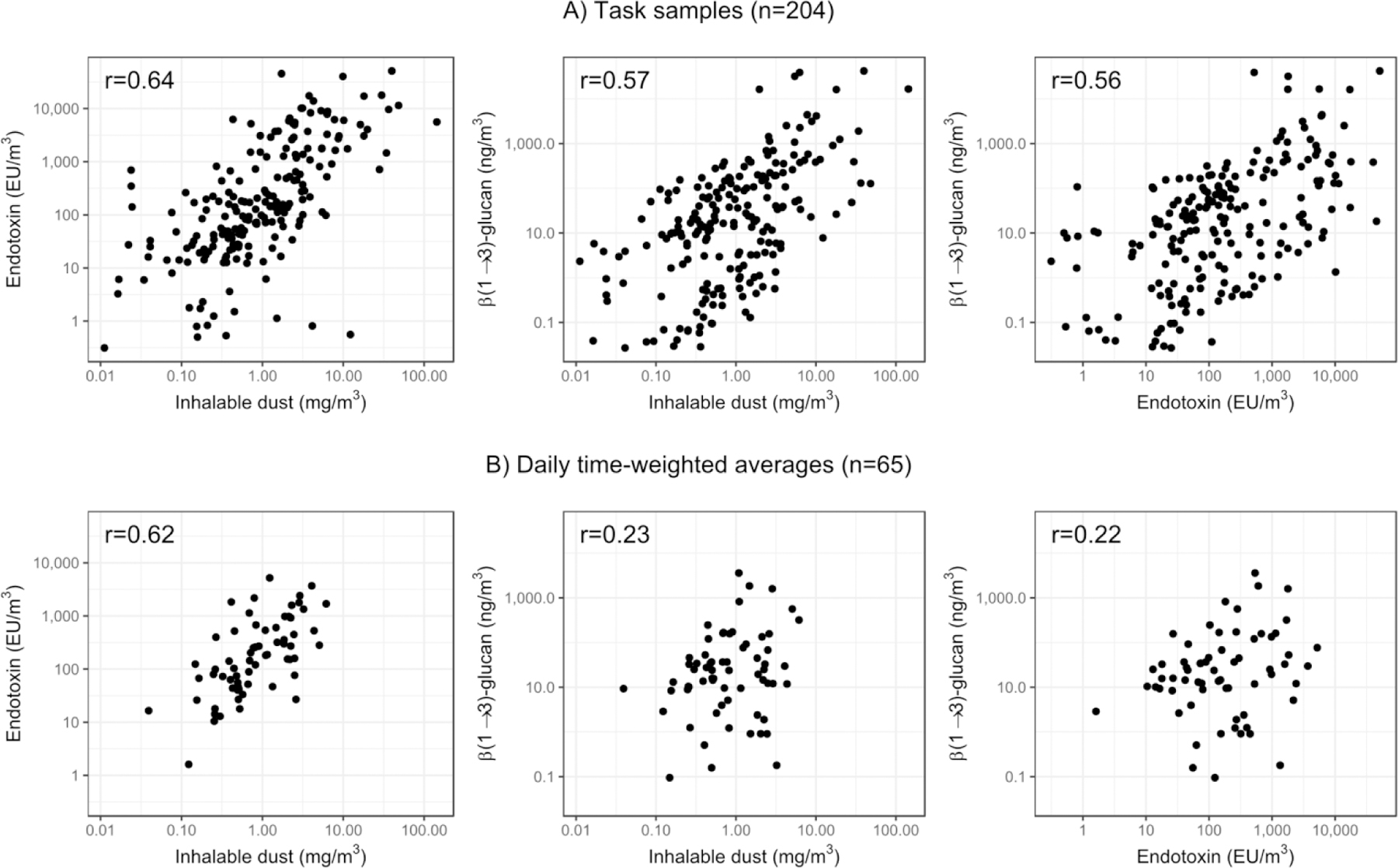
Bivariate distributions of log-transformed endotoxin, glucan, and inhalable dust concentrations and pairwise Pearson’s correlations (r), for task samples and daily TWAs. The correlations and data points reported are the average values of the five imputations
Determinants of task-based exposure
The multimodel averaged coefficients of the overall model for tasks, expressed as ratios of increase or decrease in GM exposure relative to a reference category, are shown in Table 1. Bedding work had the highest exposure to endotoxin, with GMs approximately 25 times higher than the reference of harvest, followed by hog handling (17-fold increase) and other cow/dairy tasks (5-fold increase). Increased concentrations were also found for cleaning building/equipment, feed/grain storage work, and cleaning manure. The tasks with the highest average (1→3)-β-D-glucans exposure were bedding work and cleaning building or equipment with a 26-fold and a 3.5-fold increase relative to harvest, respectively. Feed/grain storage was the only task with a statistically significant increase in exposure to inhalable dust relative to harvesting (2.4-fold increase). A 1-minute increase in sampling duration was associated with a 3% decrease in GM dust concentrations (95% confidence interval (CI) 2–4%). Fixed effects explained 40%, 15%, and 24% of the total variance in endotoxin, glucan, and dust concentrations.
Table 1.
All task samples (n=204): geometric mean ratios and 95% confidence intervals of model parameters
| Endotoxin | Glucan | Inhalable dust | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fixed effects | N | EXP(β) | 95%CI | EXP(β) | 95%CI | EXP(β) | 95%CI | |||
| Intercept | 202 | (71.8–568) | 20.4 | (5.8–71.7) | 1.92 | (0.99–3.75) | ||||
| Task (reference: Harvest) | 44 | |||||||||
| Bedding work | 7 | 24.8 | (4.55–135) | 26.1 | (2.13–320) | 2.22 | (0.64–7.73) | |||
| Clean building/equipment | 13 | 3.94 | (0.91–17.0) | 3.46 | (0.39–30.7) | 1.99 | (0.67–5.93) | |||
| Clean manure | 6 | 2.34 | (0.43–12.7) | 0.11 | (0.01–1.14) | 0.51 | (0.15–1.78) | |||
| Equipment installation, maintenance and repair | 16 | 0.18 | (0.05–0.59) | 0.18 | (0.03–1.02) | 0.59 | (0.25–1.38) | |||
| Feed work | 16 | 1.32 | (0.38–4.59) | 1.37 | (0.23–8.34) | 1.33 | (0.55–3.21) | |||
| Feed/grain storage work | 23 | 3.75 | (1.25–11.3) | 2.90 | (0.58–14.5) | 2.41 | (1.06–5.47) | |||
| Field work | 43 | 0.41 | (0.15–1.14) | 0.41 | (0.10–1.63) | 0.90 | (0.44–1.84) | |||
| Hog handling | 12 | 17.28 | (4.29–69.6) | 2.83 | (0.40–20.3) | 1.61 | (0.59–4.38) | |||
| Miscellaneous tasks n.e.c.a | 18 | 0.14 | (0.04–0.46) | 0.41 | (0.07–2.28) | 1.50 | (0.64–3.51) | |||
| Other cow/dairy tasks | 6 | 5.15 | (0.90–29.3) | 0.60 | (0.05–7.34) | 1.36 | (0.38–4.88) | |||
| Sampling duration (minutes) | 0.99 | (0.97–1.01) | 0.99 | (0.97–1.01) | 0.97 | (0.96–0.98) | ||||
| Temperature (°C)b | 0.99 | (0.94–1.03) | 0.95 | (0.88–1.04) | 0.98 | (0.94–1.02) | ||||
| Relative humidity (%)c | 1.00 | (0.99–1.01) | 1.01 | (0.98–1.03) | 1.00 | (0.99–1.01) | ||||
| Season (reference: Fall 2015) | 64 | |||||||||
| Fall 2016 | 104 | 0.73 | (0.27–1.96) | 0.98 | (0.60–1.61) | 0.82 | (0.43–1.57) | |||
| Spring 2016 | 36 | 1.06 | (0.63–1.78) | 0.97 | (0.64–1.47) | 1.07 | (0.72–1.58) | |||
| Nulld | Fulle | Null | Full | Null | Full | |||||
| Variance components | VC | VC | %f | VC | VC | % | VC | VC | % | |
| Between farmer | 1.95 | 0.30 | 85 | 0.33 | 0.02 | 95 | 0.40 | 0.04 | 89 | |
| Between day | 0.37 | 0.51 | −36 | 2.62 | 2.09 | 20 | 0.18 | 0.28 | −61 | |
| Residual | 4.16 | 3.11 | 25 | 6.74 | 6.12 | 9 | 2.12 | 1.73 | 19 | |
| Total | 6.49 | 3.91 | 40 | 9.69 | 8.22 | 15 | 2.71 | 2.06 | 24 | |
N: Number of samples, β: Point estimate; CI: Confidence interval, n.e.c.: Not elsewhere classified; VC: Variance component
Includes tasks such as concrete cutting, office work, and woodworking
Variable centered by subtracting the average temperature value of samples (19.7°C)
Variable centered by subtracting the average relative humidity value of samples (53.0%)
Variance components of the model containing only random-effects
Variance components of the model containing all fixed-effect variables
Proportion of variance explained by the full model relative to a null model
In the model for livestock-related samples (Table 2), we found a monotonic positive trend between endotoxin exposure and the number of animals: compared to a building or feedlot with fewer than 100 animals, the GM endotoxin concentration was 2.3 times higher for 100 to 999 animals (95%CI 0.53–9.97), and 4 times higher for 1000 animals or more (95%CI 0.38–42.4), with a statistically significant trend (p-trend=0.02). Using a model containing animal units instead of the number of animals, endotoxin concentrations were 1.5 times higher for each doubling of the number of animal units (Appendix B, table B.3). Barns or confinement buildings were associated with a 2.9-fold increase in endotoxin exposure relative to feedlot, corral or pen. A 5.8-fold increase in (1→3)-β-D-glucans exposure was found for barn and confinement units (95%CI 1.08–31.02). For inhalable dust, barn/confinement units also had elevated exposure (GMR 2.72, 95%CI 1.26–5.89). The between-farmer variance estimated for the full model was very close to zero for all three analytes, as well as for the null model with (1→3)-β-D-glucans. The proportion of total variance explained by the fixed effects ranged from 22% for (1→3)-β-D-glucans to 41% for endotoxin.
Table 2.
Livestock-related task samples (n=50): geometric mean ratios and 95% confidence intervals of model parameters
| Endotoxin | Glucan | Inhalable dust | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | N | EXP(β) | 95%CI | EXP(β) | 95%CI | EXP(β) | 95%CI | |||
| Intercept | 1370 | (203–9290) | 47.5 | (2.75–820) | 3.60 | (1.16–11.2) | ||||
| Location on the farm (reference: Feedlot, corral, pen) | 23 | |||||||||
| Barn, confinement | 27 | 2.94 | (0.73–12.0) | 5.79 | (1.08–31.0) | 2.72 | (1.26–5.89) | |||
| Number of animals (reference: <100) | 28 | |||||||||
| 100–999 | 15 | 2.30 | (0.53–9.97) | 1.06 | (0.56–2.01) | 1.00 | (0.83–1.21) | |||
| ≥1000 | 7 | 4.01 | (0.38–42.4) | 1.11 | (0.42–2.94) | 0.99 | (0.74–1.33) | |||
| Sampling duration (minutes) | 0.96 | (0.92–1.00) | 0.98 | (0.93–1.03) | 0.96 | (0.94–0.98) | ||||
| Temperature (°C)a | 0.99 | (0.95–1.04) | 0.98 | (0.91–1.06) | 1.00 | (0.98–1.02) | ||||
| Relative humidity (%)b | 1.00 | (0.98–1.01) | 1.00 | (0.98–1.03) | 1.00 | (0.99–1.01) | ||||
| Season (reference: Fall 2015) | 13 | |||||||||
| Fall 2016 | 29 | 0.43 | (0.06–3.37) | 0.26 | (0.02–3.03) | 0.31 | (0.07–1.44) | |||
| Spring 2016 | 8 | 0.57 | (0.14–2.33) | 0.20 | (0.02–2.14) | 0.62 | (0.27–1.43) | |||
| Nullc | Fulld | Null | Full | Null | Full | |||||
| Variance components | VC | VC | %e | VC | VC | % | VC | VC | % | |
| Between farmer | 0.32 | <0.01 | 100 | <0.01 | <0.01 | — | 0.20 | <0.01 | 100 | |
| Between day | 1.26 | 0.69 | 46 | 0.71 | 0.70 | 2 | 0.28 | 0.16 | 44 | |
| Residual | 2.55 | 1.73 | 32 | 6.28 | 4.76 | 24 | 1.65 | 1.24 | 25 | |
| Total | 4.13 | 2.42 | 41 | 7.00 | 5.45 | 22 | 2.13 | 1.39 | 35 | |
N: Number of samples, β: Point estimate; CI: Confidence interval; VC: Variance component
Variable centered by subtracting the average temperature value of samples (19.7°C)
Variable centered by subtracting the average relative humidity value of samples (53.0%)
Variance components of the model containing only random-effects
Variance components of the model containing all fixed-effect variables
Proportion of variance explained by the full model relative to a null model
In the models for crop-related samples (Table 3), activities conducted in or near grain bins or grain elevators were associated with a 5-fold increase in endotoxin exposure relative to working in a field (95% CI 1.33–21.2). For (1→3)-β-D-glucans, a 1°C increase in ambient temperature was associated with a 11% reduction in average concentration levels (95%CI 1–20%). We found no evidence of a relationship between production phase or crop type and exposure to endotoxin, (1→3)-β-D-glucans, or inhalable dust. Between-farmer variance in endotoxin and (1→3)-β-D-glucans concentrations was close to zero. Fixed effects explained 19%, 24%, and 34% of the total variance in the endotoxin, (1→3)-β-D-glucans, and inhalable dust concentrations, respectively.
Table 3.
Crop-related samples (n=105): geometric mean ratio and 95% confidence intervals of model parameters
| Endotoxin | Glucan | Inhalable dust | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | N | EXP(β) | 95%CI | EXP(β) | 95%CI | EXP(β) | 95%CI | |||
| Intercept | 89.8 | (27.7–291) | 17.6 | (2.56–122) | 1.71 | (0.53–5.57) | ||||
| Location on the farm (Reference: Field) | 57 | |||||||||
| Grain bin/elevator | 27 | 5.31 | (1.33–21.2) | 1.30 | (0.34–4.93) | 1.37 | (0.50–3.73) | |||
| Other | 21 | 1.72 | (0.59–5.02) | 1.11 | (0.43–2.87) | 1.10 | (0.60–2.01) | |||
| Production phase (reference: Harvest, mechanical) | 21 | |||||||||
| Field work (excluding harvest) | 20 | 0.97 | (0.62–1.51) | 0.45 | (0.04–5.44) | 1.78 | (0.33–9.52) | |||
| Other/missing | 30 | 1.01 | (0.67–1.54) | 2.43 | (0.34–17.7) | 1.52 | (0.45–5.11) | |||
| Post-harvest | 34 | 1.00 | (0.67–1.49) | 2.17 | (0.18–26.6) | 1.52 | (0.37–6.35) | |||
| Crop type (reference: Corn) | 43 | |||||||||
| Grain | 7 | 0.97 | (0.56–1.67) | 0.84 | (0.16–4.47) | 0.63 | (0.14–2.79) | |||
| Hay | 4 | 1.16 | (0.40–3.33) | 0.41 | (0.02–7.14) | 0.71 | (0.20–2.57) | |||
| None | 9 | 0.93 | (0.50–1.73) | 0.42 | (0.04–4.33) | 0.72 | (0.23–2.19) | |||
| Soy | 16 | 0.99 | (0.68–1.46) | 1.88 | (0.32–10.9) | 0.98 | (0.59–1.62) | |||
| Straw/stalk | 10 | 0.90 | (0.40–1.99) | 0.48 | (0.06–4.05) | 0.71 | (0.22–2.32) | |||
| Other or mixed crops | 16 | 0.87 | (0.36–2.13) | 0.40 | (0.04–4.18) | 0.61 | (0.13–2.75) | |||
| Sampling duration (minutes) | 1.00 | (0.98–1.01) | 0.99 | (0.96–1.02) | 0.97 | (0.96–0.99) | ||||
| Temperature (°C)a | 0.97 | (0.89–1.04) | 0.89 | (0.80–0.99) | 0.95 | (0.90–1.00) | ||||
| Relative humidity (%)b | 1.01 | (0.98–1.03) | 1.01 | (0.97–1.04) | 1.00 | (0.99–1.01) | ||||
| Season (reference: Fall 2015) | 33 | |||||||||
| Fall 2016 | 55 | 0.97 | (0.61–1.55) | 1.05 | (0.46–2.39) | 0.71 | (0.29–1.77) | |||
| Spring 2016 | 17 | 0.99 | (0.71–1.38) | 1.27 | (0.35–4.70) | 1.16 | (0.64–2.12) | |||
| Nullc | Fulld | Null | Full | Null | Full | |||||
| Variance components | VC | VC | %e | VC | VC | % | VC | VC | % | |
| Between farmer | <0.01 | <0.01 | — | <0.01 | <0.01 | — | 0.24 | 0.04 | 85 | |
| Between day | 1.94 | 1.10 | 43 | 2.56 | 0.19 | 93 | 0.35 | <0.01 | 100 | |
| Residual | 2.71 | 2.68 | 1 | 7.62 | 7.59 | 0 | 2.13 | 1.75 | 18 | |
| Total | 4.66 | 3.79 | 19 | 10.19 | 7.78 | 24 | 2.72 | 1.79 | 34 | |
N: Number of samples, β: Point estimate; CI: Confidence interval; VC: Variance component
Variable centered by subtracting the average temperature value of samples (19.7°C)
Variable centered by subtracting the average relative humidity value of samples (53.0%)
Variance components of the model containing only random-effects
Variance components of the model containing all fixed-effect variables
Proportion of variance explained by the full model relative to a null model
Discussion
Our study adds to the limited information available on factors associated with exposure to endotoxin and (1→3)-β-D-glucans in farming activities prevalent in the U.S. Midwest. Overall, the exposures measured were highly variable and spanned several orders of magnitude, in agreement with the literature on bioaerosol exposure in agriculture (Basinas et al., 2015; Kullman et al., 1998; Spaan et al., 2006). Livestock-related activities generally had higher exposures to endotoxin and (1→3)-β-D-glucans, although most of these tasks had significant exposure variability. We observed contrasts in exposure between tasks and other farming characteristics; however, the confidence intervals were often wide because of small sample sizes and large exposure variability. The use of respiratory protection was low as previously observed by studies in the United States (Carpenter et al., 2002; Kearney et al., 2016) and Europe (Basinas et al., 2015). Lastly, our study represents one of the few characterizations of (1→3)-β-D-glucans exposure in agricultural activities.
Determinants of task exposure
We observed the highest average exposure to endotoxin and (1→3)-β-D-glucans during hog handling and bedding work. Hog handling included moving or loading/unloading animals, which have previously been associated with very high exposures (GM=12,150 EU/m3) in a study conducted in Iowa (O’Shaughnessy et al., 2012). Two European studies also found increased endotoxin concentrations for controlling or moving hogs (Basinas et al., 2013; Preller et al., 1995). Our findings of higher exposure during bedding work mostly concerned beef cattle farming, but studies conducted on dairy farms also found elevated endotoxin concentrations for this task (Basinas et al., 2014; Garcia et al., 2013; Pfister et al., 2018). We were unable to evaluate the influence of the type of bedding material on bioaerosol concentrations because only straw or corn stalks were used in the farms visited.
Our study confirmed the potential for high exposure to bioaerosols during cleaning activities, as previously reported in the literature (Basinas et al., 2013; Madsen and Matthiesen, 2013; Preller et al., 1995; Straumfors et al., 2015). Contributing factors to the high concentrations measured include cleaning in small spaces (e.g., inside combines), and use of compressed air or high-pressure washers that increased the likelihood of airborne concentrations. For the latter, we observed endotoxin concentrations of up to 45,000 EU/m3 for power washing a pig nursery, comparable to the GM of 40,000 EU/m3 observed by O’Shaughnessy et al. (2012) in Iowa.
Among the livestock-related factors, endotoxin concentrations were higher in barn and confinement buildings than in exterior environments. This is consistent with an earlier survey of two swine confinement units in Iowa (O’Shaughnessy et al., 2010) that found a median work shift concentration of approximately 1000 EU/m3. However, we were unable to assess contrasts in exposure for barn and confinement buildings separately because of small sample sizes and because animal location was correlated with the number of animals. We also observed a monotonic trend between increasing endotoxin concentrations and increasing number of animals, which provides support for the use of herd size as a surrogate measure for endotoxin exposure in epidemiological studies (Beane Freeman et al., 2012; Hofmann et al., 2018). In contrast, we did not observe an association with the number of animals (or animal units) for (1→3)-β-D-glucans. Patterns were similar using animal units (Appendix B, table B.3), suggesting that the trends observed using the number of animals were not necessarily due to differences in livestock type between the categories.
The increase in endotoxin exposure we observed in relation to working in or near grain bins or grain elevators has also been noted in a study from Colorado that reported total dust endotoxin concentrations above 50,000 EU/m3 in corn bins in farms and in grain elevators (Buchan et al., 2002). This pattern was not observed here for (1→3)-β-D-glucan which could be due to the relatively low concentrations (GM=54 ng/m3). In contrast, a Norwegian study found an inhalable glucan GM of 1500 ng/m3 on commercial grain elevator workers (Halstensen et al., 2013), whereas our study was mostly conducted on farms.
The influence of temperature and relative humidity on bioaerosol concentrations was generally negligible. One exception was a trend of lower bioaerosol concentrations, especially for (1→3)-β-D-glucans, that was associated with increasing temperature observed in crop-related samples, which could be due to the type of task performed. For example, harvesting and field work tended to be conducted during warmer temperatures compared to equipment cleaning and maintenance. Sampling duration was only negatively associated with inhalable dust concentrations, which could reflect the use of shorter sampling duration in situations of high exposures to avoid overloading the filter.
TWA exposure
Overall, the large GSDs indicate significant between-day variability in exposure, especially for endotoxin and (1→3)-β-D-glucans. This observation of high exposure variability is in line with a literature review on exposure in livestock farms (Basinas et al., 2015) and a large survey conducted in the Netherlands (Spaan et al., 2006). This represents a challenge for assessing exposures and supports the use of a task-based approach. Unfortunately, we were not always able to sample farmers for the entire work day because of long working hours and the logistics of collecting samples in remote areas. Thus, these TWAs may underestimate the farmers’ true daily exposure if he performed high exposure tasks before or after sample collection, or overestimate it if the unmeasured periods did not entail exposure. In addition, between-farmer factors only represented a small proportion of the overall variability in TWA concentrations. This suggests that collecting information on task and task frequency is important to characterizing the variability of bioaerosol exposure in farmers, similar to assessing occupations with large exposure variability such as construction workers and hospital cleaners (Sauvé and Friesen, 2019), as well as pesticide exposure in farmers (Fenske, 2005; Thomas et al., 2010).
There are currently no regulatory occupational exposure limits (OELs) in the U.S. or elsewhere for either endotoxin or (1→3)-β-D-glucans; however, a health-based OEL for endotoxin of 90 EU/m3 has been proposed to protect against acute respiratory effects (Health Council of the Netherlands and Dutch Expert Committee on Occupational Safety, 2010). Overall, 40 visits (62% of total) had endotoxin TWA concentrations above this OEL. This proportion was higher for farms with livestock (66%) than for crop-only farms (47%). Our findings are consistent with a recent U.S. study in dairy farms that found that 89% of workers had work-shift exposures exceeding this OEL (Davidson et al., 2018).
Correlations between analytes
The relatively low correlations between endotoxin and (1→3)-β-D-glucans concentrations, especially for TWA samples, and the differences observed in the effects of exposure determinants support the development of separate exposure estimates for the two components for our population of farmers. Compared to the correlations observed in our study (r=0.56 for task samples and r=0.22 for TWA samples), studies that reported correlations in the agricultural sector varied from a Pearson correlation of r=0.32 in vegetable and flower greenhouses (Madsen et al., 2016), r=0.86 in horse stables (Samadi et al., 2009), and r=0.93 in grain elevators and feed mills (Halstensen et al., 2013). The correlations of approximately 0.6 observed here between inhalable dust and endotoxin were similar to the Spearman correlation of 0.69 found by Spaan et al. (2006) in a large survey in the agricultural industry. The moderate correlations suggest that inhalable dust is not a reliable surrogate of inhalable endotoxin exposure in the farms studied.
Strengths and limitations
Strengths of this study include a sampling strategy representative of usual exposure conditions, because we did not select sample days or farmers based on expectations of high exposure. For example, our study included rainy days where the farmers spent most of the time inside doing maintenance or office related activities. Our study also covered exposures during activities involving production types less represented in the literature, such as row crops and beef cattle farming. In addition, we incorporated independent observations of the farmers’ activities and the real time recording of tasks and ancillary farm characteristics.
Our study also has some limitations. First, nearly half of all visits had at least one sample with dust overloading, most of which were non-task samples with a longer sampling duration. We chose to retain these overloaded samples in the analysis using an unbiased multiple imputation approach. We believe that the additional variability from the imputation procedure is small relative to the overall environmental variation in exposure levels. Second, some farm characteristics recorded in our study, such as bedding type, ventilation, or feeding method, had few samples. We also did not measure additional meteorological parameters such as wind speed, which has been shown to influence endotoxin concentrations in another study (Dungan et al., 2011) Thus, we were unable to include these exposure variables in our models. Third, large hog, poultry, and dairy farms were underrepresented, thereby limiting the generalization of our findings for these farm types. However, several studies have documented exposures in these farm types (eg., Basinas et al., 2015; Douglas et al., 2018; O’Shaughnessy et al., 2010), which could serve to fill in data gaps for future exposure assessment efforts. Fourth, we had several instances of between-farmer variance close to zero in the models for endotoxin and glucan. This situation has been observed in studies of occupational exposures (Rappaport et al., 1995) and, in our case, is likely due to a combination of very large GSDs and a relatively small number of samples per farmer, especially in the analyses restricted to crop or livestock samples. This may have impacted our ability to estimate the proportion of between-farmer variance explained by the fixed effects in the models. Fifth, our study was conducted in a single geographic area, which could limit the generalizability of our findings on factors affecting exposure levels for the same tasks conducted in a different area. We also did not collect samples during the winter season, which has been associated with higher bioaerosols exposures during livestock activities in other studies (Basinas et al., 2015). Lastly, we limited our characterization of bioaerosols to endotoxin and to (1→3)-β-D-glucans. However, there is increasing attention towards the role on the modulation of immune and inflammatory responses played by specific bacterial or fungal species and by the extent of microbial diversity (Heederik and von Mutius, 2012; Poole and Romberger, 2012). For this reason, we used one half of each filter to quantify the endotoxin and (1→3)-β-D-glucans concentrations in dusts, and froze the other half for future analyses.
Conclusion
On average, we found higher endotoxin and (1→3)-β-D-glucans exposure levels for tasks associated with livestock, especially for bedding work and hog handling, and observed a positive trend between endotoxin concentrations and increasing number of livestock. The substantial variability in bioaerosol exposure levels observed in our study is also consistent with other exposure studies in agriculture and highlights the importance of collecting information on task and task frequency in characterizing exposures. The relatively weak correlations found between endotoxin and (1→3)-β-D-glucan concentrations provide support for the development of separate exposure estimates for these two bioaerosol constituents for the BEEA and AHS study participants.
Supplementary Material
Acknowledgments
Amy Miller, Kate Torres, Emily Tristani, Linda Gowen, Himanshi Singh, Marsha Dunn, and other staff at Westat, Inc. (Rockville, MD) contributed to the study coordination and data management. We would also like to thank the field research team in Iowa, including Charles Lynch, Debra Lande, Debra Podaril, and Jennifer Hamilton. Finally, we gratefully acknowledge the participation of the Biomarkers of Exposure and Effect in Agriculture Study participants that made this work possible.
Funding
Funding for this work was provided by the Intramural Research Program of the National Institutes of Health, with support from National Cancer Institute Director’s Intramural Innovation Award Program, and by the National Institute of Environmental Health Sciences [grant number NIH P30 ES005605].
Abbreviations:
- AHS
Agricultural Health Study
- BEEA
Biomarkers of Exposure and Effect in Agriculture
- CI
Confidence interval
- GM
Geometric mean
- GMR
Geometric mean ratio
- GSD
Geometric standard deviation
- OEL
Occupational exposure limit
- RR
Relative risk
- TWA
Time-weighted average
Footnotes
Declaration of interest: none
Institution and Ethics approval and informed consent
Recruitment and study protocols were approved by the Institutional Review Boards of the National Cancer Institute, the University of Iowa, and Westat (Rockville, MD). All participating farmers provided written consent.
References
- Alavanja MC, Sandler DP, McMaster SB, Zahm SH, McDonnell CJ, Lynch CF, Pennybacker M, Rothman N, Dosemeci M, Bond AE, Blair A, 1996. The Agricultural Health Study. Environ. Health Perspect 104, 362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrakianakis G, Seixas NS, Ray R, Camp JE, Gao DL, Feng Z, Li W, Wernli KJ, Fitzgibbons ED, Thomas DB, Checkoway H, 2007. Lung Cancer Risk Among Female Textile Workers Exposed to Endotoxin. J. Natl. Cancer Inst 99, 357–364. [DOI] [PubMed] [Google Scholar]
- Basinas I, Elholm G, Wouters IM, 2017. Endotoxins, glucans and other microbial cell wall agents, in: Viegas C, Viegas S, Gomes A, Täubel M, Sabino R (Eds.), Exposure to Microbiological Agents in Indoor and Occupational Environments Springer Berlin Heidelberg, New York, NY, pp. 159–190. [Google Scholar]
- Basinas I, Schlunssen V, Takai H, Heederik D, Omland O, Wouters IM, Sigsgaard T, Kromhout H, 2013. Exposure to inhalable dust and endotoxin among Danish pig farmers affected by work tasks and stable characteristics. Ann. Occup. Hyg 57, 1005–1019. [DOI] [PubMed] [Google Scholar]
- Basinas I, Sigsgaard T, Erlandsen M, Andersen NT, Takai H, Heederik D, Omland O, Kromhout H, Schlunssen V, 2014. Exposure-affecting factors of dairy farmers’ exposure to inhalable dust and endotoxin. Ann. Occup. Hyg 58, 707–723. [DOI] [PubMed] [Google Scholar]
- Basinas I, Sigsgaard T, Kromhout H, Heederik D, Wouters IM, Schlunssen V, 2015. A comprehensive review of levels and determinants of personal exposure to dust and endotoxin in livestock farming. J. Expo. Sci. Environ. Epidemiol 25, 123–137. [DOI] [PubMed] [Google Scholar]
- Beane Freeman LE, DeRoos AJ, Koutros S, Blair A, Ward MH, Alavanja M, Hoppin JA, 2012. Poultry and livestock exposure and cancer risk among farmers in the agricultural health study. Cancer Causes Control 23, 663–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behbod B, Urch B, Speck M, Scott JA, Liu L, Poon R, Coull B, Schwartz J, Koutrakis P, Silverman F, Gold DR, 2013. Endotoxin in concentrated coarse and fine ambient particles induces acute systemic inflammation in controlled human exposures. Occup. Environ. Med 70, 761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchan RM, Rijal P, Sandfort D, Keefe T, 2002. Evaluation of airborne dust and endotoxin in corn storage and processing facilities in Colorado. Int. J. Occup. Med. Environ. Health 15, 57–64. [PubMed] [Google Scholar]
- Burnham KP, Anderson DR, 2002. Model selection and multimodel inference : a practical information-theoretic approach, 2nd ed. Springer, New York. [Google Scholar]
- Carpenter WS, Lee BC, Gunderson PD, Stueland DT, 2002. Assessment of personal protective equipment use among Midwestern farmers. Am. J. Ind. Med 42, 236–247. [DOI] [PubMed] [Google Scholar]
- Cyprowski M, Buczyńska A, Kozajda A, Sowiak M, Bródka K, Szadkowska-Stańczyk I, 2012. Exposure to (1 → 3)-β-D-glucans in swine farms. Aerobiologia 28, 161–168. [Google Scholar]
- Davidson ME, Schaeffer J, Clark ML, Magzamen S, Brooks EJ, Keefe TJ, Bradford M, Roman-Muniz N, Mehaffy J, Dooley G, 2018. Personal exposure of dairy workers to dust, endotoxin, muramic acid, ergosterol and ammonia on large-scale dairies in the high plains western United States. J. Occup. Environ. Hyg 15, 182–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas P, Robertson S, Gay R, Hansell AL, Gant TW, 2018. A systematic review of the public health risks of bioaerosols from intensive farming. Int. J. Hyg. Environ. Health 221, 134–173. [DOI] [PubMed] [Google Scholar]
- Douwes J, Thorne P, Pearce N, Heederik D, 2003. Bioaerosol health effects and exposure assessment: progress and prospects. Ann. Occup. Hyg 47, 187–200. [DOI] [PubMed] [Google Scholar]
- Dungan RS, Leytem AB, Bjorneberg DL, 2011. Concentrations of airborne endotoxin and microorganisms at a 10,000-cow open-freestall dairy. J. Anim. Sci 89, 3300–3309. [DOI] [PubMed] [Google Scholar]
- Fenske RA, 2005. State-of-the-art measurement of agricultural pesticide exposures. Scand. J. Work. Environ. Health 31 Suppl 1, 67–73; discussion 63–65. [PubMed] [Google Scholar]
- Garcia J, Bennett DH, Tancredi D, Schenker MB, Mitchell D, Reynolds SJ, Mitloehner FM, 2013. Occupational exposure to particulate matter and endotoxin for California dairy workers. Int. J. Hyg. Environ. Health 216, 56–62. [DOI] [PubMed] [Google Scholar]
- Halstensen AS, Heldal KK, Wouters IM, Skogstad M, Ellingsen DG, Eduard W, 2013. Exposure to grain dust and microbial components in the Norwegian grain and compound feed industry. Ann. Occup. Hyg 57, 1105–1114. [DOI] [PubMed] [Google Scholar]
- Health Council of the Netherlands, Dutch Expert Committee on Occupational Safety, 2010. Endotoxins: health-based recommended occupational exposure limit Health Council of the Netherlands, The Hague. [Google Scholar]
- Heederik D, von Mutius E, 2012. Does diversity of environmental microbial exposure matter for the occurrence of allergy and asthma? J. Allergy Clin. Immunol 130, 44–50. [DOI] [PubMed] [Google Scholar]
- Hofmann JN, Beane Freeman LE, Lynch CF, Andreotti G, Thomas KW, Sandler DP, Savage SA, Alavanja MC, 2015. The Biomarkers of Exposure and Effect in Agriculture (BEEA) Study: Rationale, Design, Methods, and Participant Characteristics. J. Toxicol. Environ. Health A 78, 1338–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann JN, Shiels MS, Friesen MC, Kemp TJ, Chaturvedi AK, Lynch CF, Parks CG, Pinto LA, Hildesheim A, Alavanja MCR, Beane Freeman LE, 2018. Industrial hog farming is associated with altered circulating immunological markers. Occup. Environ. Med 75, 212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe Parr KA, Hađina S, Kilburg-Basnyat B, Wang Y, Chavez D, Thorne PS, Weiss JP, 2017. Modification of sample processing for the Limulus amebocyte lysate assay enhances detection of inflammogenic endotoxin in intact bacteria and organic dust. Innate Immun 23, 307–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurvich CM, Tsai C-L, 1989. Regression and time series model selection in small samples. Biometrika 76, 297–307. [Google Scholar]
- Kearney GD, Gallagher B, Shaw R, 2016. Respiratory protection behavior and respiratory indices among poultry house workers on small, family-owned farms in North Carolina: A pilot project. J Agromedicine 21, 136–143. [DOI] [PubMed] [Google Scholar]
- Kullman GJ, Thorne PS, Waldron PF, Marx JJ, Ault B, Lewis DM, Siegel PD, Olenchock SA, Merchant JA, 1998. Organic dust exposures from work in dairy barns. Am. Ind. Hyg. Assoc. J 59, 403–413. [DOI] [PubMed] [Google Scholar]
- Lenters V, Basinas I, Beane-Freeman L, Boffetta P, Checkoway H, Coggon D, Portengen L, Sim M, Wouters IM, Heederik D, Vermeulen R, 2010. Endotoxin exposure and lung cancer risk: a systematic review and meta-analysis of the published literature on agriculture and cotton textile workers. Cancer Causes Control 21, 523–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerro CC, Koutros S, Andreotti G, Sandler DP, Lynch CF, Louis LM, Blair A, Parks CG, Shrestha S, Lubin JH, Albert PS, Hofmann JN, Beane Freeman LE, 2019. Cancer incidence in the Agricultural Health Study after 20 years of follow-up. Cancer Causes Control 30, 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley WG, Green BJ, Blachere FM, Martin SB, Law BF, Jensen PA, Schafer MP, 2017. Sampling and characterization of bioaerosols, in: Ashley K, O’Connor PF (Eds.), NIOSH Manual of Analytical Methods (NMAM), 5th Edition. National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention, Department of Health and Human Services, Cincinnati, OH. [Google Scholar]
- Lubin JH, Colt JS, Camann D, Davis S, Cerhan JR, Severson RK, Bernstein L, Hartge P, 2004. Epidemiologic evaluation of measurement data in the presence of detection limits. Environ. Health Perspect 112, 1691–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundin JI, Checkoway H, 2009. Endotoxin and cancer. Environ. Health Perspect 117, 1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen AM, Matthiesen CB, 2013. Exposure to aerosols during high-pressure cleaning and relationship with health effects. Ann. Agric. Environ. Med 20, 420–425. [PubMed] [Google Scholar]
- Madsen AM, Thilsing T, Baelum J, Garde AH, Vogel U, 2016. Occupational exposure levels of bioaerosol components are associated with serum levels of the acute phase protein Serum Amyloid A in greenhouse workers. Env Health 15, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall A, Altman DG, Holder RL, Royston P, 2009. Combining estimates of interest in prognostic modelling studies after multiple imputation: current practice and guidelines. BMC Med. Res. Methodol 9, 57–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shaughnessy P, Peters T, Donham K, Taylor C, Altmaier R, Kelly K, 2012. Assessment of swine worker exposures to dust and endotoxin during hog load-out and power washing. Ann. Occup. Hyg 56, 843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shaughnessy PT, Donham KJ, Peters TM, Taylor C, Altmaier R, Kelly KM, 2010. A task-specific assessment of Swine worker exposure to airborne dust. J. Occup. Environ. Hyg 7, 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister H, Madec L, Cann PL, Costet N, Chouvet M, Jouneau S, Vernhet L, 2018. Factors determining the exposure of dairy farmers to thoracic organic dust. Environ. Res 165, 286–293. [DOI] [PubMed] [Google Scholar]
- Poole JA, Romberger DJ, 2012. Immunological and inflammatory responses to organic dust in agriculture. Curr. Opin. Allergy Clin. Immunol 12, 126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preller L, Heederik D, Kromhout H, Boleij JS, Tielen MJ, 1995. Determinants of dust and endotoxin exposure of pig farmers: development of a control strategy using empirical modelling. Ann. Occup. Hyg 39, 545–557. [PubMed] [Google Scholar]
- Rappaport SM, Lyles RH, Kupper LL, 1995. An Exposure-Assessment Strategy Accounting for Within- and Between-Worker Sources of Variability. Ann. Occup. Hyg 39, 469–495. [DOI] [PubMed] [Google Scholar]
- Roy CJ, Thorne PS, 2003. Exposure to particulates, microorganisms, β(1–3)-glucans, and endotoxins during soybean harvesting. AIHA Journal 64, 487–495. [DOI] [PubMed] [Google Scholar]
- Samadi S, van Eerdenburg FJ, Jamshidifard AR, Otten GP, Droppert M, Heederik DJ, Wouters IM, 2012. The influence of bedding materials on bio-aerosol exposure in dairy barns. J. Expo. Sci. Environ. Epidemiol 22, 361–368. [DOI] [PubMed] [Google Scholar]
- Samadi S, Wouters IM, Houben R, Jamshidifard AR, Van Eerdenburg F, Heederik DJ, 2009. Exposure to inhalable dust, endotoxins, beta(1−>3)-glucans, and airborne microorganisms in horse stables. Ann. Occup. Hyg 53, 595–603. [DOI] [PubMed] [Google Scholar]
- Sauvé J-F, Friesen MC, 2019. Using decision rules to assess occupational exposure in population-based studies. Current Environmental Health Reports 6, 148–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenker MB, Christiani D, Cormier Y, Dimich-Ward H, Doekes G, Dosman J, Douwes J, Dowling K, Enarson D, Green F, 1998. Respiratory health hazards in agriculture. Am. J. Respir. Crit. Care Med 158. [DOI] [PubMed] [Google Scholar]
- Singh U, Reponen T, Cho KJ, Grinshpun SA, Adhikari A, Levin L, Indugula R, Green BJ, 2011. Airborne endotoxin and beta-D-glucan in PM1 in agricultural and home environments. Aerosol Air Qual Res 11, 376–386. [Google Scholar]
- Spaan S, Wouters IM, Oosting I, Doekes G, Heederik D, 2006. Exposure to inhalable dust and endotoxins in agricultural industries. J. Environ. Monit 8, 63–72. [DOI] [PubMed] [Google Scholar]
- Straumfors A, Heldal KK, Wouters IM, Eduard W, 2015. Work tasks as determinants of grain dust and microbial exposure in the norwegian grain and compound feed industry. Ann. Occup. Hyg 59, 724–736. [DOI] [PubMed] [Google Scholar]
- Thomas KW, Dosemeci M, Coble JB, Hoppin JA, Sheldon LS, Chapa G, Croghan CW, Jones PA, Knott CE, Lynch CF, Sandler DP, Blair AE, Alavanja MC, 2010. Assessment of a pesticide exposure intensity algorithm in the Agricultural Health Study. J. Expo. Sci. Environ. Epidemiol 20, 559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne PS, 2000. Inhalation toxicology models of endotoxin- and bioaerosol-induced inflammation. Toxicology 152, 13–23. [DOI] [PubMed] [Google Scholar]
- Thorne PS, Ansley AC, Perry SS, 2009. Concentrations of Bioaerosols, Odors, and Hydrogen Sulfide Inside and Downwind from Two Types of Swine Livestock Operations. J. Occup. Environ. Hyg 6, 211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne PS, Mendy A, Metwali N, Salo P, Co C, Jaramillo R, Rose KM, Zeldin DC, 2015. Endotoxin Exposure: Predictors and Prevalence of Associated Asthma Outcomes in the United States. Am. J. Respir. Crit. Care Med 192, 1287–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L-Y, Wang K, Li W-J, Guo Y-L, Kong J-L, 2016. Effect of endotoxin exposure on lung cancer risk in cotton textile mills and agriculture: a meta-analysis. Translational Cancer Research 5, 250–264. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


