Abstract
Background
The preoperative prediction of post hepatectomy liver failure (PHLF) is essential, but there is no gold standard for the prediction at present, and the efficacy of different methods for the prediction has not been compared systematically. In this study, we aimed to compare the efficacy of preoperative two-dimensional shear wave elastography (2D-SWE), indocyanine green (ICG) clearance test and biomarkers for PHLF prediction in patients with hepatocellular carcinoma (HCC).
Methods
We retrospectively studied 215 patients with HCC, who had undergone major liver resection in our hospital. Preoperative data of each patient, including liver stiffness value (LSV) of underlying hepatic parenchyma measured by 2D-SWE, ICG retention rate at 15 min (ICG-R15) measured by ICG clearance test, albumin-bilirubin (ALBI) scores, aspartate aminotransferase–platelet ratio index (APRI), and Fibrosis-4 (FIB-4) were collected for analysis. Post hepatectomy outcomes of study patients were also recorded for assessment of PHLF. The study patients were divided into development cohort (133 patients without PHLF, and 17 patients with PHLF) and validation cohort (59 patients without PHLF, and 6 patients with PHLF) randomly.
Results
In the development cohort, LSV, ICG-R15 and ALBI scores were significantly different between patients with and without PHLF, while no significant difference of APRI and FIB-4 scores was found. LSV had higher AUC (the area under the receiver operating characteristic curve) (AUC = 0.795) for PHLF prediction than ICG-R15 (AUC = 0.619) and ALBI scores (AUC = 0.686) (p < 0.05 for all comparisons). In the validation cohort, the cutoff value of LSV obtained from the development cohort, 10.35 kPa, revealed higher specificity (76.3%) for PHLF prediction than ICG-R15 (specificity: 66.1%) and ALBI scores (specificity: 69.5%) (p < 0.0001).
Conclusions
Compared with ICG-R15, ALBI scores, APRI and FIB-4, LSV measured by 2D-SWE may demonstrate better efficacy for preoperative PHLF prediction in patients with HCC.
Keywords: Ultrasound, Elastography, Hepatectomy, Liver failure, Indocyanine green
Introduction
Liver resection is one of the main treatment options for hepatocellular carcinoma (HCC). However, post hepatectomy liver failure (PHLF) remains the major complication, which leads to the high risk of death [1]. Patients with HCC would develop PHLF more easily due to their liver injury of underlying parenchyma (especially caused by cirrhosis and steatosis) and the large extent of liver resection [2]. Therefore, preoperative prediction of PHLF is important for the strategy of treatment. However, there is no gold standard for the prediction.
Indocyanine green (ICG) clearance test is the most commonly used and is considered relatively reliable for liver function reserve assessment [3]. However, this method is not feasible in patients with an iodine allergy or thyrotoxicosis, because ICG contains iodine. Cases of anaphylactic reaction were reported [4]. And the efficacy of ICG clearance test for PHLF prediction was reported to be dissatisfactory [5, 6].
In recent years, two-dimensional shear wave elastography (2D-SWE) for measurement of liver stiffness value (LSV), has been reported high accuracy and reproducibility for quantitative assessment of liver fibrosis [7, 8], and has been adopted for clinical use [9]. On the other hand, in the same stage of liver fibrosis, the presence of severe liver steatosis seems to reveal higher liver stiffness value [10]. On the basis that both liver fibrosis/cirrhosis and severe liver steatosis have great effects on the liver function [2, 11], N Heucke et al. found that liver stiffness measured by 2D-SWE was strongly correlated with parameters of 13C-methacetin Liver MAximum capacity (LiMAx) test (a liver function test) [12]. In addition, Shen et al. demonstrated that preoperative liver stiffness measured by 2D-SWE could effectively predict PHLF (AUC = 0.715, p < 0.001) [13]. Thus, 2D-SWE may be a promising method for PHLF prediction, with safe, non-invasive, and convenient mode of examination. Another imaging modality for PHLF prediction, Gd-EOB-DTPA-enhanced MRI, demonstrated precise evaluations of regional liver function and estimation of PHLF in smaller study groups [14, 15]. However, this method suffers from limited availability, high cost of time and money.
Furthermore, some serum biomarker panels of diffuse liver diseases and liver function, were also reported for non-invasive and convenient prediction of PHLF. Zou et al. identified albumin-bilirubin (ALBI) scores as an independent predictor of PHLF [16]. In the study of Zhang et al., AUCs of aspartate aminotransferase–platelet ratio index (APRI) and Fibrosis-4 (FIB-4) for preoperative PHLF prediction were 0.697 and 0.696, respectively (p < 0.001 for both) [17].
However, the efficacy of different methods for preoperative PHLF prediction has not been compared systematically.
In this study, we aimed to compare the efficacy of preoperative 2D-SWE, ICG clearance test and biomarkers for PHLF prediction in patients with HCC.
Materials and methods
Patients
Between February 2019 and December 2019, 334 patients with HCC were retrospectively and consecutively recruited from the general surgery department of our hospital (the diagnosis of HCC was pathologically confirmed after liver resection). All of these patients had adequate data of preoperative assessment (including 2D-SWE examination, ICG clearance test and laboratory testing), operative factors (including hilar occlusion, operative time and blood loss) and post hepatectomy outcomes (including histological inflammation grade and fibrosis stage of underlying hepatic parenchyma, tumor size, parameters of laboratory testing).
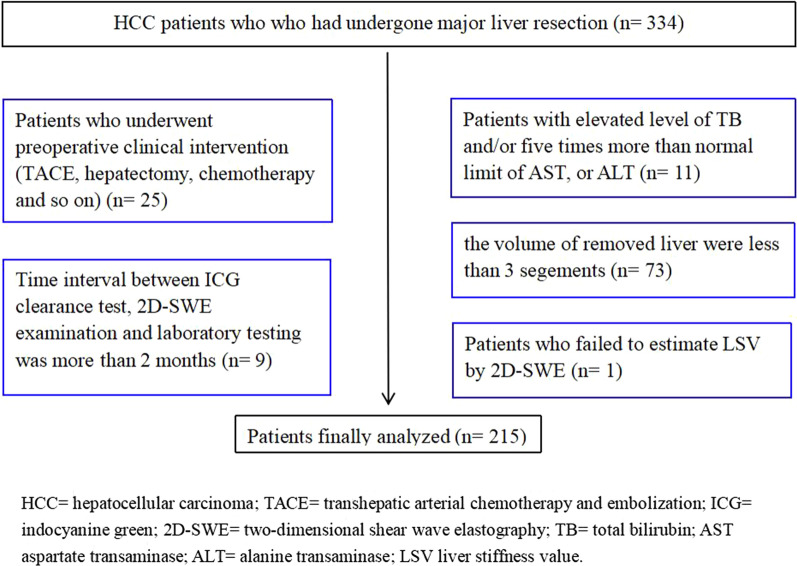
The exclusion criteria were: (a) patients who underwent preoperative clinical intervention (for example, transhepatic arterial chemotherapy and embolization/TACE, hepatectomy and chemotherapy) (n = 25), (b) patients with elevated level of total bilirubin (TB, normal range: 5.0–28.0 μmol/L) and/or evident elevated level (five times more than normal limit) of aspartate transaminase (AST, normal range: < 35 IU/L), or alanine transaminase (ALT, normal range: < 40 IU/L) (n = 11), (c) the volume of removed liver were less than 3 segements (n = 73), (d) patients who failed to estimate LSV by 2D-SWE (n = 1), (e) time interval between ICG clearance test, 2D-SWE examination and laboratory testing was more than 2 months (n = 9).
Thus, 215 patients were finally analyzed in our study (Fig. 1) (mean age: 54 years, range: 23–78 years). Study patients were divided into two cohorts randomly. In the development cohort (n = 150), the cutoff values of different methods for predicting PHLF were calculated. In the validation cohort (n = 65), validation of the developed cutoff values was performed.
Fig. 1.
Flow chart of patient selection
LSV measurement by 2D-SWE examination
Aixplorer US imaging system (Supersonic Imagine, Axi-en-Provence, France) equipped with a 1-6 MHz transducer (SC6-1), was employed in our study.
LSV measurement was conducted after B-mode and Doppler-mode US examination of the whole liver.
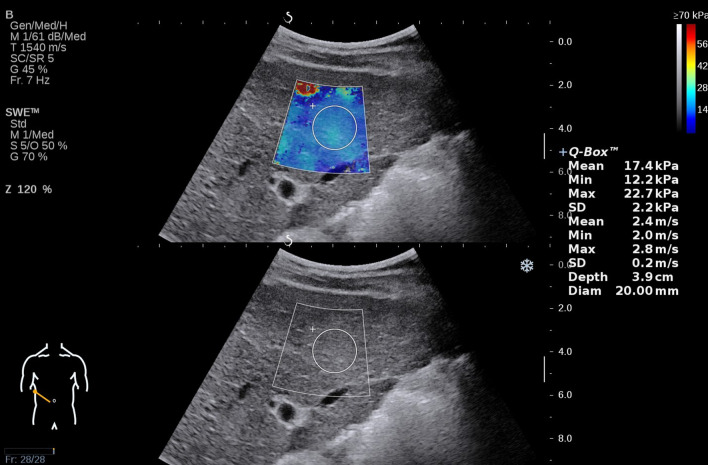
Performance of 2D-SWE examination was on the basis of “European Federation for Ultrasound in Medicine and Biology (EFSUMB) Guidelines and Recommendations on the Clinical Use of Ultrasound Elastography” [9]. Patients fasted for more than 6 h before the measurement. They were in supine position, with right arm above the head. The transducer was aligned along a right intercostal space, to illustrate the right lobe of liver in gray scale imaging. An optimal cross-sectional image was considered when no large vessels and no liver masses were observed. Then the patient was asked to hold breath for 3–5 s in natural breathing cycle, and SWE mode was switched on subsequently. A sector region of elasticity imaging (approximately 4 × 3 cm) was located 1–2 cm below the liver capsule. When elasticity signal was stabilized and filled more than 90% of the sector region, a circular region of interest (ROI) with a diameter of 2 cm was placed in the position of homogeneous elasticity signal, and the mean elasticity value (kPa) of ROI was calculated as a result of the measurement (Fig. 2). Failure of measurement was considered if elasticity signal was filled less than 50% of the elasticity imaging region, or minimum value of elasticity in ROI was no more than 1.0 kPa. Five valid results for each patient were acquired, and the median elasticity value of these results was recorded as the data of our study. Patients who were not able to hold their breath for measurement were excluded in our study.
Fig. 2.
Liver stiffness value (LSV) measured by two-dimensional shear wave elastography (2D-SWE)
ICG clearance test
Patients were measured in supine position, with a probe fixed to the nose. A standard dose of ICG (0.5 mg/kg) was prepared for ICG solution of 5 mg/ml (sterile water/ ICG = 5 mL:25 mg). The solution was injected rapidly via median cubital vein, and then ICG-R15 was recorded by DDG-3300 K system (pulse-dye densitometry).
Serum biomarker panels
Serum biomarkers including AST (IU/L), ALT (IU/L), platelet count (109 /L), age (years), TB (μ mol/L) and albumin (g/L) were used for panels calculation.
Diagnostic criteria
According to the International Study Group of Liver Surgery (ISGLS) criteria, patients with elevated serum values of international normalized ratio (INR) and TB on or after postoperative day 5 are considered a diagnosis of PHLF. Grade A PHLF is diagnosed based on deterioration from the preoperative baseline, which does not require a change in the clinical management. Grade B PHLF results in a deviation from the regular clinical pathway but manageable with noninvasive treatment. Patients who develop PHLF requiring an invasive procedure are classified as having grade C PHLF [18]. It should be specially explained that patients with grade B and C PHLF were divided into “patients with PHLF group” in our study.
Histological inflammation and fibrosis of liver parenchyma were evaluated based on the Scheuer scoring system (published in 1994) [19].
Statistical analysis
Statistical analysis was performed by SPSS 24.0 and medcalc. Mann–Whitney U test and chi-square test were used to evaluate the significant difference between categories. The area under the receiver operating characteristic (ROC) curve (AUC) was performed for analyzing diagnostic performance, with a calculation of optimal cut-off values (the point that maximized the sum of sensitivity and specificity). Delong test was conducted for comparison between AUCs. Accuracy, sensitivity, specificity were calculated in the validation cohort. Cochran’s Q test was used to compare sensitivity and specificity among groups. Significant difference was considered if p < 0.05.
Results
Characteristics of study patients
In our study, 215 patients with HCC were finally enrolled. The mean age of study patients was 54 years (range, 23–78 years), and 179 (83.2%) of them were male.
17 patients in the development cohort and 6 patients in the validation cohort developed PHLF.
Basic characteristics of study patients were described including age, gender, body mass index (BMI), platelet count, TB, ALT, AST, albumin, and international normalized ratio of prothrombin time (INR) (Table 1).
Table 1.
Characteristics of study patients
| Variable | All patients (n = 215) | Development cohort | Validation cohort | P value* | ||||
|---|---|---|---|---|---|---|---|---|
| Patients without PHLF (n = 133) | Patients with PHLF (n = 17) | P value | Patients without PHLF (n = 59) | Patients with PHLF (n = 6) | P value | |||
| Age (years) | 54 ± 11 | 55 ± 10 | 52 ± 11 | 0.528 | 52 ± 9 | 54 ± 11 | 0.366 | 0.556 |
| Numbers of male patients (%) | 179 (83.2) | 119 (89.5) | 15 (88.2) | 0.876 | 51 (86.4) | 5 (83.3) | 0.834 | 0.504 |
| Body mass index (kg/m2) | 23.0 ± 3.2 | 23.2 ± 3.3 | 21.5 ± 2.3 | 0.060 | 22.7 ± 3.0 | 22.3 ± 2.7 | 0.320 | 0.679 |
| Serum biomarker levels | ||||||||
| Platelet count (109/L) | 137 | 143 | 147 | 0.921 | 138 | 141 | 0.098 | 0.053 |
| TB (μ mol/L) | 14.3 | 14.3 | 15.1 | 0.785 | 14.2 | 14.7 | 0.112 | 0.685 |
| AST (U/L) | 34 | 34 | 41 | 0.232 | 33 | 40 | 0.304 | 0.656 |
| ALT (U/L) | 33 | 33 | 34 | 0.787 | 32 | 45 | 0.145 | 0.966 |
| Albumin (g/L) | 42.2 | 42.2 | 38.5 | 0.170 | 42.7 | 39.2 | 0.169 | 0.521 |
| INR | 1.03 | 1.03 | 1.05 | 0.171 | 1.06 | 1.04 | 0.765 | 0.072 |
| Histological inflammation grade | ||||||||
| G0 | 20 | 14 | 3 | 0.547 | 3 | 0 | 0.910 | 0.415 |
| G1 | 55 | 35 | 2 | 16 | 2 | |||
| G2 | 87 | 51 | 7 | 26 | 3 | |||
| G3 | 53 | 33 | 5 | 14 | 1 | |||
| Fibrosis stage | ||||||||
| S0 | 21 | 13 | 1 | 0.567 | 7 | 0 | 0.670 | 0.757 |
| S1 | 10 | 6 | 0 | 3 | 1 | |||
| S2 | 32 | 21 | 1 | 9 | 1 | |||
| S3 | 52 | 30 | 4 | 17 | 1 | |||
| S4 | 100 | 63 | 11 | 23 | 3 | |||
| Tumor size > 5 cm (%) | 91 (42.3) | 47 (35.3) | 9 (52.9) | 0.158 | 31 (52.5) | 4 (66.7) | 0.508 | 0.024 |
| Operative factors | ||||||||
| Hilar occlusion (min) | 14 | 14 | 15 | 0.378 | 13 | 14 | 0.102 | 0.067 |
| Operative time (min) | 256 | 256 | 254 | 0.138 | 254 | 251 | 0.645 | 0.122 |
| Blood loss (mL) | 300 | 300 | 400 | 0.059 | 200 | 300 | 0.496 | 0.061 |
Data were described as means ± standard deviations or medians as appropriate. Comparison between patients with and without post hepatectomy liver failure (PHLF) groups was conducted respectively in the development cohort and validation cohort. Asterisk (*) revealed comparison between development cohort and validation cohort
TB = total bilirubin; AST = aspartate transaminase; ALT = alanine aminotransferase; INR = international normalized ratio of prothrombin time
No significant difference of operative factors (hilar occlusion, operative time and blood loss), histological inflammation grade and fibrosis stage of underlying hepatic parenchyma, tumor size was found between patients with and without PHLF, neither in the development cohort nor in the validation cohort (Table 1).
Development cohort
LSV, ICG-R15, ALBI scores, APRI, and FIB-4 of patients
The median LSV of patients with PHLF was 13.0 kPa, which was significantly higher than patients without PHLF (8.5 kPa) (p < 0.0001). The median ICG-R15 values of patients with PHLF (6.4%) was significantly higher than patients without PHLF (5.2%) (p = 0.009). The median ALBI scores of patients with PHLF was -2.5, which was significantly higher than patients without PHLF (-2.8) (p = 0.012). And no significant difference of APRI and FIB-4 scores was shown between patients with and without PHLF (Table 2).
Table 2.
Parameters of development cohort
| Variable | All patients (n = 150) | Patients without PHLF (n = 133) | Patients with PHLF (n = 17) | P value |
|---|---|---|---|---|
| LSV (kPa) | 9.0 (6.9, 11.9) | 8.5 (6.8, 11.0) | 13.0 (10.5, 16.0) | < 0.0001 |
| ICG-R15 (%) | 5.4 (3.4, 7.2) | 5.2 (3.4, 7.1) | 6.4 (4.0, 8.8) | 0.009 |
| ALBI scores | − 2.8 (− 3.0, − 2.5) | − 2.8 (− 3.0, − 2.6) | − 2.5 (− 2.8, − 2.2) | 0.012 |
| APRI | 0.63 (0.41, 1.16) | 0.62 (0.38, 1.14) | 0.62 (0.50, 1.29) | 0.488 |
| FIB-4 | 9.17 (6.81, 11.55) | 9.17 (6.79, 11.60) | 8.88 (6.76, 11.30) | 0.704 |
Data were described as medians (interquartile range). Statistical analysis was conducted for comparison between patients with and without post hepatectomy liver failure (PHLF) groups. LSV = liver stiffness value; ICG-R15 = indocyanine green retention rate at 15 min; ALBI = albumin-bilirubin; APRI = aspartate aminotransferase–platelet ratio index; FIB-4 = Fibrosis-4
Performance of preoperative LSV, ICG-R15, ALBI scores for prediction of PHLF
The AUC of LSV for predicting PHLF was 0.795, which was significantly higher (p < 0.0001) than AUCs of ICG-R15 (AUC = 0.619) and ALBI scores (AUC = 0.686) (Table 3, and Fig. 3).
Table 3.
Liver stiffness, ICG-R15 and ALBI scores in predicting PHLF
| Variable | Development cohort | Validation cohort | |||||||
|---|---|---|---|---|---|---|---|---|---|
| AUC (95% CI) | P value | Criterion | Accuracy (%) | Sensitivity (%) | P value | Specificity (%) | P value | ||
| LSV | 0.795 (0.714, 0.877) | < 0.0001 | > 10.35 kPa | 76.9 | 83.3 | 0.223 | 76.3 | < 0.0001 | |
| ICG-R15 | 0.619 (0.487, 0.752) | 0.109 | > 6.4% | 66.1 | 66.7 | 66.1 | |||
| ALBI scores | 0.686 (0.541, 0.832) | 0.012 | > − 2.547 | 70.8 | 83.3 | 69.5 | |||
In the development cohort, AUCs were compared with AUC = 0.5, in the validation cohort, comparison was conducted between LSV, ICG-R15 and ALBI scores. LSV = liver stiffness value; PHLF = post hepatectomy liver failure; ICG-R15 = indocyanine green retention rate at 15 min; ALBI = albumin-bilirubin; NPV = negative predictive value; PPV = positive predictive value
Fig. 3.
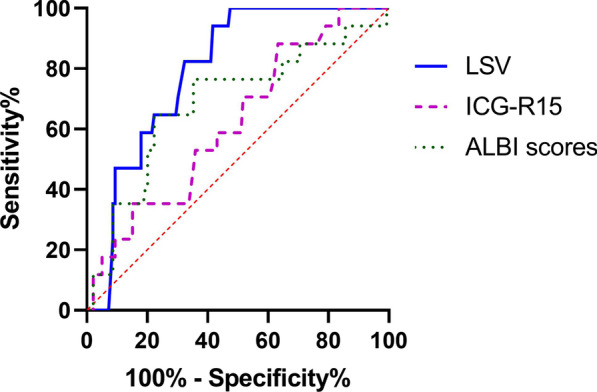
The receiver operating characteristic curves (ROC) of liver stiffness value (LSV), indocyanine green (ICG) retention rate at 15 min (ICG-R15) and albumin-bilirubin (ALBI) scores for predicting post hepatectomy liver failure (PHLF)
Validation cohort
Performances of preoperative LSV, ICG-R15, ALBI scores for PHLF prediction
According to analysis of the development cohort, cutoff values of LSV, ICG-R15 and ALBI scores for predicting PHLF were 10.35 kPa, 6.4%, and − 2.547, respectively. Based on the cutoff values of them, LSV revealed higher specificity (76.3%), than that of ICG-R15 (specificity, 66.1%) and ALBI scores (specificity, 69.5%) in the validation cohort (p < 0.0001), while showed no significant difference of sensitivity among them (Table 3).
Discussion
The preoperative prediction of PHLF is essential for treatment and prognosis. However, the efficacy of various preoperative methods for PHLF prediction including ICG clearance test, biomarkers, imaging techniques has not been compared systematically through literature review. This study compared and validated the performance of LSV, ICG-R15, ALBI scores, APRI and FIB-4 for PHLF prediction. In our study, Child-Turcotte-Pugh (CTP) score, a widely used tool for liver function assessment, was not employed. Because CTP score is a classification system, and two of its criteria (ascites and hepatic encephalopathy) are subjective. Previous studies demonstrated the variability and uncertainty of post hepatectomy outcomes among patients with CTP grade A [20]. And noticeably, the patients of our study were all classified as CTP grade A.
In our development cohort, LSV, ICGR15, and ALBI scores revealed significant difference between patients with/without PHLF, whereas no significant difference was found in APRI and FIB-4 scores. Zhang et al. showed that APRI and FIB-4 scores were weak risk factors for PHLF prediction, which was consistent with our results [17]. Therefore, APRI and FIB-4 scores were not included in our further analysis. On the other hand, LSV (AUC = 0.795, p < 0.0001) showed higher efficacy for predicting PHLF than ICG-R15 (AUC = 0.619, p = 0.109) and ALBI scores (AUC = 0.686, p = 0.012). A possible reason for the result is that LSV is an intrinsic property of liver. ICG clearance test is influenced substantially by the hepatic blood flow. Local (including portal hypertension or arterial thrombosis) and general factors (such as circadian variations or low cardiac output) could affect hepatic blood flow and thus ICG clearance [21]. Chong et al. reported higher efficacy of preoperative LSV (AUC = 0.647) for predicting high-grade PHLF than ICG-R15 (AUC = 0.552), which is in keeping with our study [22]. Therefore, LSV could be an alternative method for preoperative prediction of PHLF.
In our validation cohort, the cutoff value of LSV (10.35 kPa) obtained from the development cohort demonstrated significant higher specificity for predicting PHLF than that of ICG-R15 and ALBI scores. Lei et al. indicated that LSV revealed good efficacy for predicting PHLF (AUC = 0.860, p < 0.001) and its cutoff value (14.0 kPa) had high sensitivity (94.6%) [23]. They presented a higher cutoff value than our study mainly due to the different diagnostic criteria of PHLF and different measurement equipment of LSV.
There were some limitations in our study. It was a retrospective single center analysis. And the patient numbers of PHLF groups were relatively small due to the low morbidity, which results in the noneffective comparison of sensitivity between LSV, ICG-R15 and ALBI scores and the weaker power of the study. There is a call for a long-term, multicenter and large sample size study to draw further conclusions.
In conclusion, compared with ICG-R15, ALBI scores, APRI and FIB-4, LSV measured by 2D-SWE may demonstrate better capability for predicting PHLF before major liver resection in patients with HCC. Although higher LSV may not have an influence on the operation strategy based on the results of present study, it would be a sign of additional attention and management during perioperative period.
Acknowledgements
There is nothing to declare.
Abbreviations
- PHLF
Post hepatectomy liver failure
- 2D-SWE
Two-dimensional shear wave elastography
- ROI
Region of interest
- LSV
Liver stiffness value
- ICG
Indocyanine green
- ICG-R15
Indocyanine green retention rate at 15 min
- HCC
Hepatocellular carcinoma
- ALBI
Albumin-bilirubin
- APRI
Aspartate aminotransferase–platelet ratio index
- FIB-4
Fibrosis-4
- AUC
The area under the receiver operating characteristic curve
- PPV
Positive predictive value
- NPV
Negative predictive value
- TB
Total bilirubin
- AST
Aspartate transaminase
- ALT
Alanine transaminase
- INR
International normalized ratio
Authors' contributions
R.F. and T.Q. wrote the main manuscript text. R.F. prepared Figs. 1 and 2. W.L., Q.L. and Y.L. reviewed the manuscript. All authors read and approved the final manuscript.
Funding
There is nothing to declare.
Availability of data and materials
The datasets analysed during the current study are not publicly available due to conduction of further study but are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This retrospective study was approved by the ethics committee of West China Hospital, Sichuan University and in accordance with the Declaration of Helsinki (approval no. B2015-063). Written informed consents were obtained from all patients.
Consent for publication
Not applicable.
Competing interests
There is nothing to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
First author: Rong Fu
Contributor Information
Rong Fu, Email: 448939373@qq.com.
Yan Luo, Email: luoyanddoc@163.com.
References
- 1.Liu JY, Ellis RJ, Hu QL, Cohen ME, Hoyt DB, FACS et al. PostHepatectomy liver failure risk calculator for preoperativeand early postoperative period following major hepatectomy. Ann Surg Oncol. 2020 doi: 10.1245/s10434-020-08239-6. [DOI] [PubMed] [Google Scholar]
- 2.Van den Broek MAJ, Olde Damink SWM, Dejong CHC, Lang H, Malago M, Jalan R, et al. Liver failure after partial hepatic resection: definition, pathophysiology, risk factors and treatment. Liver Int. 2008 doi: 10.1111/j.1478-3231.2008.01777.x. [DOI] [PubMed] [Google Scholar]
- 3.Wang YY, Zhao XH, Ma L, Ye JZ, Wu FX, Tang J, et al. Comparison of the ability of Child-Pugh score, MELD score, and ICG-R15 to assess preoperative hepatic functional reserve in patients with hepatocellular carcinoma. J Surg Oncol. 2018 doi: 10.1002/jso.25184. [DOI] [PubMed] [Google Scholar]
- 4.Eric L, Eleonore M, Daniela D, Lim C, Dhonneur G, Azoulay D. Current use and perspective of indocyanine green clearance in liver diseases. Anaesth Crit Care Pain Med. 2016 doi: 10.1016/j.accpm.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Tomimaru Y, Eguchi H, Gotoh K, Kawamoto K, Wada H, Asaoka T, et al. Platelet count is more useful for predicting posthepatectomy liver failure atsurgery for hepatocellular carcinoma than indocyanine green clearance test. J Surg Oncol. 2016 doi: 10.1002/jso.24166. [DOI] [PubMed] [Google Scholar]
- 6.Ibis C, Albayrak D, Sahiner T, Soytas Y, Gurtekin B, Sivrikoz N. Value of preoperative indocyanine green clearance test for predicting post-hepatectomy liver failure in noncirrhotic patients. Med Sci Monit. 2017 doi: 10.12659/msm.907306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhuang Y, Ding H, Zhang Y, Sun H, Xu C, Wang W. Two-dimensional shear-wave elastography performance in the noninvasive evaluation of liver fibrosis in patients with chronic hepatitis B: comparison with serum fibrosis indexes. Radiology. 2017 doi: 10.1148/radiol.2016160131. [DOI] [PubMed] [Google Scholar]
- 8.Ferraioli G, Tinelli C, Dal Bello B, Zicchetti M, Filice G, Filice C, et al. Accuracy of real-time shear wave elastography for assessing liver fibrosis in chronic hepatitis C: a pilot study. Hepatology. 2012 doi: 10.1002/hep.25936. [DOI] [PubMed] [Google Scholar]
- 9.Ferraioli G, Filice C, Castera L, Choi BI, Sporea I, Wilson SR, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 3: liver. Ultrasound Med Biol. 2015 doi: 10.1016/j.ultrasmedbio.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Petta S, Maida M, Macaluso FS, Di Marco V, Cammà C, Cabibi D, et al. The severity of steatosis influences liver stiffness measurement in patients with nonalcoholic fatty liver disease. Hepatology. 2015 doi: 10.1002/hep.27844. [DOI] [PubMed] [Google Scholar]
- 11.Jawahar A, Gonzalez B, Balasubramanian N, Adams W, Goldberg A. Comparison of computed tomography hepatic steatosis criteria for identification of abnormal liver function and clinical risk factors, in incidentally noted fatty liver. Eur J Gastroenterol Hepatol. 2020 doi: 10.1097/MEG.0000000000001502. [DOI] [PubMed] [Google Scholar]
- 12.Heucke N, Wuensch T, Mohr J, Kaffarnik M, Arsenic R, Sinn B, et al. Non-invasive structure-function assessment of the liver by 2D time-harmonic elastography and the dynamic Liver MAximum capacity (LiMAx) test. J Gastroenterol Hepatol. 2019 doi: 10.1111/jgh.14629. [DOI] [PubMed] [Google Scholar]
- 13.Shen Y, Zhou C, Zhu G, Shi G, Zhu X, Huang C, et al. Liver stiffness assessed by shear wave elastography predicts postoperative liver failure in patients with hepatocellular carcinoma. J Gastrointest Surg. 2017 doi: 10.1007/s11605-017-3443-9. [DOI] [PubMed] [Google Scholar]
- 14.Donadon M, Lanza E, Branciforte B, Muglia R, Lisi C, Pedicini V, et al. Hepatic uptake index in the hepatobiliary phase of gadolinium ethoxybenzyl diethylenetriamine penta acetic acideenhanced magnetic resonance imaging estimates functional liver reserve and predicts post-hepatectomy liver failure. Surgery. 2020 doi: 10.1016/j.surg.2020.04.041. [DOI] [PubMed] [Google Scholar]
- 15.Tsujita Y, Sofue K, Komatsu S, Yamaguchi T, Ueshima E, Ueno Y, et al. Prediction of post-hepatectomy liver failure using gadoxetic acid-enhanced magnetic resonance imaging for hepatocellular carcinoma with portal vein invasion. Eur J Radiol. 2020 doi: 10.1016/j.ejrad.2020.109189. [DOI] [PubMed] [Google Scholar]
- 16.Zou H, Yang X, Li QL, Zhou QX, Xiong L, Wen Y. A comparative study of albumin-bilirubin score with Child-Pugh Score, Model for End-Stage Liver Disease Score and indocyanine green R15 in predicting posthepatectomy liver failure for hepatocellular carcinoma patients. Dig Dis. 2018 doi: 10.1159/000486590. [DOI] [PubMed] [Google Scholar]
- 17.Zhang ZQ, Yang B, Zou H, Xiong L, Miao XY, Wen Y, et al. ALBI/ST ratio versus FIB-4 and APRI as a predictor of posthepatectomy liver failure in hepatocellular carcinoma patients. Medicine (Baltimore) 2019 doi: 10.1097/MD.0000000000015168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS) Surgery. 2011 doi: 10.1016/j.surg.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994 doi: 10.1002/hep.1840190629. [DOI] [PubMed] [Google Scholar]
- 20.Philip DS. Preoperative assessment of liver function. Surg Clin N Am. 2004 doi: 10.1016/S0039-6109(03)00224-X. [DOI] [PubMed] [Google Scholar]
- 21.Levesque E, Martin E, Dudau D, Lim C, Dhonneur G, Azoulay D. Current use and perspective of indocyanine green clearance in liver diseases. Anaesth Crit Care Pain Med. 2016 doi: 10.1016/j.accpm.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Chong CC, Wong GL, Chan AW, Wong VW, Fong AK, Cheung YS, et al. Liver stiffness measurement predicts high-grade post-hepatectomy liver failure: aprospective cohort study. J Gastroenterol Hepatol. 2017 doi: 10.1111/jgh.13503. [DOI] [PubMed] [Google Scholar]
- 23.Lei JW, Ji XY, Hong JF, Li WB, Chen Y, Pan Y, et al. Prediction of posthepatectomy liver failure using transient elastography in patients with hepatitis B related hepatocellular carcinoma. BMC Gastroenterol. 2017 doi: 10.1186/s12876-017-0732-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analysed during the current study are not publicly available due to conduction of further study but are available from the corresponding author on reasonable request.