Abstract
BACKGROUND
Brain metastases are a common cause of disabling neurologic complications and death in patients with metastatic melanoma. Previous studies of nivolumab combined with ipilimumab in metastatic melanoma have excluded patients with untreated brain metastases. We evaluated the efficacy and safety of nivolumab plus ipilimumab in patients with melanoma who had untreated brain metastases.
METHODS
In this open-label, multicenter, phase 2 study, patients with metastatic melanoma and at least one measurable, nonirradiated brain metastasis (tumor diameter, 0.5 to 3 cm) and no neurologic symptoms received nivolumab (1 mg per kilogram of body weight) plus ipilimumab (3 mg per kilogram) every 3 weeks for up to four doses, followed by nivolumab (3 mg per kilogram) every 2 weeks until progression or unacceptable toxic effects. The primary end point was the rate of intracranial clinical benefit, defined as the percentage of patients who had stable disease for at least 6 months, complete response, or partial response.
RESULTS
Among 94 patients with a median follow-up of 14.0 months, the rate of intracranial clinical benefit was 57% (95% confidence interval [CI], 47 to 68); the rate of complete response was 26%, the rate of partial response was 30%, and the rate of stable disease for at least 6 months was 2%. The rate of extracranial clinical benefit was 56% (95% CI, 46 to 67). Treatment-related grade 3 or 4 adverse events were reported in 55% of patients, including events involving the central nervous system in 7%. One patient died from immune-related myocarditis. The safety profile of the regimen was similar to that reported in patients with melanoma who do not have brain metastases.
CONCLUSIONS
Nivolumab combined with ipilimumab had clinically meaningful intracranial efficacy, concordant with extracranial activity, in patients with melanoma who had untreated brain metastases. (Funded by Bristol-Myers Squibb and the National Cancer Institute; CheckMate 204 ClinicalTrials.gov number, NCT02320058.)
BRAIN METASTASES ARE A COMMON COMplication of solid tumors and remain a major cause of disabling neurologic complications and death in patients with cancer.1 Among primary cancers in adults, melanoma has one of the highest propensities to metastasize to the brain.2 More than one third of patients with advanced melanoma have brain metastases at diagnosis,3 and up to 75% have brain metastases at the time of death.2
Surgical resection and stereotactic radiotherapy are highly effective treatments for local control of brain oligometastases.2,4 Whole-brain radiation therapy is used for multiple brain metastases and leptomeningeal disease, albeit with limited therapeutic efficacy. Systemic chemotherapeutic agents, including those that cross the blood–brain barrier (e.g., temozolomide), have minimal antitumor activity in the brain.5 None of these treatment methods have an effect on the risk of the development of new brain metastases, on the control of extracranial disease, or on overall survival.2,4 Thus, the prognosis of patients with melanoma who have brain metastases has remained poor, with a median overall survival of 4 to 5 months and only 5% surviving in the long term (≥5 years).4
Studies have shown that immune checkpoint inhibitors and targeted therapies (BRAF–MEK inhibitors) have intracranial activity in patients with melanoma who have untreated brain metastases, and both types of therapy may improve survival outcomes in these patients.6,7 Ipilimumab, which blocks cytotoxic T-lymphocyte antigen 4, and pembrolizumab, an anti–programmed death 1 (PD-1) agent, have been shown to have activity against brain metastases from melanoma when each agent is used individually as monotherapy.6,8,9 In phase 2 and phase 3 studies of advanced melanoma, ipilimumab combined with the anti–PD-1 agent nivolumab was shown to have efficacy superior to that of ipilimumab alone.10,11 However, these studies excluded patients with untreated brain metastases. Here, we report the results of CheckMate 204, a phase 2 study to evaluate the efficacy and safety of nivolumab combined with ipilimumab in patients with melanoma who have asymptomatic, untreated brain metastases.
Methods
Patients
Adult patients who had histologically confirmed malignant melanoma with metastases to the brain and an Eastern Cooperative Oncology Group performance status of 0 or 1 (on a 5-point scale, with higher numbers reflecting greater disability) were eligible for this study. All patients had at least one measurable brain metastasis (tumor diameter, 0.5 to 3 cm, as assessed by magnetic resonance imaging [MRI]) that had not been previously irradiated, was not judged to require an immediate local intervention (surgery or radiosurgery), did not result in neurologic signs and symptoms, and had not been treated with systemic glucocorticoid therapy within 10 days before the initiation of study treatment (asymptomatic patients). Previous stereotactic radiosurgery and excision of up to three brain metastases were permitted at least 3 weeks before study treatment, provided that neurologic sequelae had completely resolved and that measurable untreated lesions remained.
Previous adjuvant therapies were permitted (including ipilimumab, with the last dose given >6 months before enrollment); inhibitors of MEK or BRAF were allowed with a 4-week washout period. Patients were excluded from the study if they had known leptomeningeal involvement or autoimmune disease or if they had received systemic treatment with glucocorticoids or other immunosuppressive medication within 14 days before study therapy. A complete list of eligibility criteria is provided in the protocol, available with the full text of this article at NEJM.org.
A later amendment to the study protocol allowed enrollment of 20 symptomatic patients with brain metastases. Patients were enrolled in this cohort at a later time, and most of these patients did not have a minimum follow-up of 6 months at the time of the last data cutoff; therefore, these patients could not be evaluated for the primary end point (defined below). The current report includes only the asymptomatic patients who could be evaluated for the primary end point (94 of 101 treated patients had a minimum follow-up of 6 months). Follow-up of patients with symptomatic brain metastases is ongoing, and results from this cohort are not reported here.
Study Design and Treatment
In this open-label, multicenter, single-group, phase 2 study, patients were treated with nivolumab at a dose of 1 mg per kilogram of body weight every 3 weeks plus ipilimumab at a dose of 3 mg per kilogram intravenously every 3 weeks for four doses (induction phase), followed by nivolumab at a dose of 3 mg per kilogram every 2 weeks (maintenance phase) (Fig. S1 in the Supplementary Appendix, available at NEJM.org). Treatment continued for a maximum of 24 months or until disease progression, unacceptable toxic effects, or withdrawal of consent. Patients who had grade 3 or 4 toxic effects during the induction phase could be retreated during the maintenance phase with nivolumab monotherapy if the toxic effects had resolved and immunosuppressive medications were no longer considered necessary. Guidelines for the discontinuation of treatment and the management of immune-related adverse events are described in the protocol.
End Points
The primary end point was the rate of intracranial clinical benefit, defined as the percentage of patients who had stable disease for at least 6 months after the initiation of treatment, complete response, or partial response. The secondary end points were the rate of extracranial (systemic) and global clinical benefit; intracranial, extracranial (systemic), and global progression-free survival and rate of overall response (defined as the rate of complete or partial response); overall survival; and measures of the central nervous system (CNS)–specific safety of the study treatment.
Assessments
Treatment response was determined in the brain and systemic compartments by serial radiographic assessment every 6 weeks for the first year and every 12 weeks thereafter, for up to 24 months. All brain lesions were assessed with MRI scans, whereas the assessment of extracranial disease was performed by means of computed tomography.11 All assessments of response reported herein were conducted by the study investigators.
Extracranial lesions (a minimum of 10 mm in diameter for measurable nonnodal lesions) were assessed according to Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1. For the assessment of brain lesions, the RECIST criteria were modified to allow up to five intracranial target lesions of 5 to 30 mm in diameter and to include target lesions measuring 5 to 10 mm in their longest diameter, as described previously.7 Intracranial lesions could be measured only by gadolinium-enhanced MRI, with a scan-slice thickness of 1 mm for metastases that had a longest diameter of 5 to 10 mm. Global responses were assessed with the use of a combination of modified RECIST criteria for brain lesions and RECIST criteria for systemic disease and encompassed all index lesions in the brain and systemic compartments. Complete response, partial response, or progression was confirmed by MRI approximately 4 weeks after the initial assessment, and stable disease was evaluated at one assessment at least 6 months after the initiation of treatment. The expression of programmed death ligand 1 (PD-L1) was assessed in extracranial tumor tissue with the use of a validated immunohistochemical assay, as described previously.11
The Common Terminology Criteria for Adverse Events, version 4.0, was used to evaluate safety for all treated patients. Decisions about withholding or discontinuation of treatment were based on investigator judgment in consultation with the study primary investigator and the study medical monitor.
Study Oversight
The study was conducted in accordance with Good Clinical Practice guidelines as defined by the International Conference on Harmonisation and in compliance with the protocol, which was approved by the institutional review board of each study center. The study was originally developed by the Cytokine Working Group and was then expanded under the sponsor, Bristol-Myers Squibb, to include additional participating centers. All the patients provided written informed consent before enrollment.
In lieu of a data and safety monitoring committee, a steering committee was established, consisting of a core group of study investigators who are experts in treating patients with melanoma and brain metastases, along with the sponsor physicians and staff. The study was designed by members of the steering committee (all of whom were academic authors) and the study sponsor. An agreement was made between members of the steering committee and the study sponsor to protect the confidentiality of the data before publication. Data were collected by the sponsor and analyzed in collaboration with the authors. An interim safety analysis was conducted after 20 patients had completed induction treatment or discontinued treatment.
The initial draft of the manuscript was written by the first and last authors, and all the authors contributed to subsequent drafts and provided final approval to submit for publication. Professional medical writing and editorial assistance were provided by StemScientific and funded by the sponsor. The authors vouch for the accuracy and completeness of the data and the analyses, as well as for the adherence of the study to the protocol.
Statistical Analysis
Statistical analyses of the primary end point, the rate of intracranial clinical benefit, were based on an originally planned sample size of 110 patients for the entire study population. Rates of clinical benefit were calculated to yield clinically meaningful results with respect to the lower bounds of the Clopper–Pearson exact two-sided 90% confidence interval and 95% confidence interval. The planned sample size ensured that the maximum width of the exact 90% confidence interval for any given estimate of the rate of clinical benefit did not exceed 18% and that the maximum width of the exact 95% confidence interval for any given estimate of the rate of clinical benefit did not exceed 20%.
Time-to-event analyses were conducted with the use of the Kaplan–Meier method, with medians presented along with 95% confidence intervals based on the Brookmeyer and Crowley method. Analyses were performed using SAS software, version 9.2 (SAS Institute).
Results
Patients and Treatment
This trial was conducted at 28 sites in the United States, with enrollment from February 2015 through June 2017 (for the cohort with asymptomatic brain metastases). A total of 101 patients were treated in the asymptomatic cohort. As of November 15, 2017, of the 101 patients who had been enrolled, 94 had a minimum follow-up of 6 months (median follow-up, 14.0 months) and could be evaluated for the primary end point (Fig. S2 in the Supplementary Appendix).
The median number of doses received during the induction phase was 3 (range, 1 to 4); 33 of 94 patients (35%) received all 4 scheduled doses of both nivolumab and ipilimumab. A total of 55 patients (59%) received nivolumab as maintenance therapy (median number of doses, 15; range, 1 to 48). The overall median duration of therapy was 3.4 months. At the cutoff date for the current analysis, 70 patients (74%) were no longer receiving treatment and 24 (26%) were still receiving the study treatment; 21 (22%) had died. Radiographic progression was documented in 33 patients (35%): 17 (18%) had intracranial progression only, 4 (4%) had extracranial progression only, and 12 (13%) had both intracranial and extracranial progression. A total of 11 (12%) of the 33 patients had progression with new lesions in the brain. After discontinuation of study treatment, 25 patients (27%) received subsequent anticancer therapy (Table S1 in the Supplementary Appendix).
Of the 94 patients who could be evaluated for the primary end point, 16 (17%) had received previous systemic anticancer therapy, with the most common being a BRAF inhibitor (11%), a MEK inhibitor (9%), or both (Table S2 in the Supplementary Appendix). Eight patients (9%) had received stereotactic radiotherapy before study entry, and 2 patients (2%) received stereotactic radiotherapy during the study. Of the 94 patients, 72 (77%) had one or two intracranial target lesions and 21 (22%) had three or more intracranial target lesions (Table 1); 36 patients (38%) had one or two extracranial target lesions, and 43 (46%) had three or more extracranial target lesions (Table S3 in the Supplementary Appendix).
Table 1.
Demographic and Clinical Characteristics of the Patients at Baseline.*
| Characteristic | Nivolumab plus Ipilimumab (N = 94) |
|---|---|
| Median age (range) — yr | 59 (22–81) |
| Sex — no. (%) | |
| Male | 65 (69) |
| Female | 29 (31) |
| Lactate dehydrogenase — no. (%) | |
| At or below the upper limit of the normal range | 55 (59) |
| Above the upper limit of the normal range | 39 (41) |
| PD-L1 expression — no. (%) | |
| ≥1% | 41 (44) |
| <1% | 34 (36) |
| Could not be evaluated | 19 (20) |
| Stereotactic radiotherapy before study entry — no. (%) | |
| Yes | 8 (9) |
| No | 86 (91) |
| No. of target lesions at pretreatment tumor assessment — no. of patients (%) | |
| No lesions | 1 (1)† |
| 1 Lesion | 49 (52) |
| 2 Lesions | 23 (24) |
| ≥3 Lesions | 21 (22) |
Percentages may not total 100 because of rounding. PD-L1 denotes programmed death ligand 1.
The inclusion of this patient was a protocol deviation.
Efficacy
According to modified RECIST, version 1.1, criteria, 24 patients (26%) had a complete response and 28 (30%) a partial response in the brain, yielding a rate of intracranial objective response of 55% (95% confidence interval [CI], 45 to 66) (Table 2).
Table 2.
Response to Treatment.
| Variable | Intracranial (N = 94) | Extracranial (N = 94) | Global (N = 94) |
|---|---|---|---|
| Best overall response — no. (%)* | |||
| Complete response | 24 (26) | 7 (7) | 8 (9) |
| Partial response | 28 (30) | 40 (43) | 40 (43) |
| Stable disease for ≥6 mo | 2 (2) | 6 (6) | 5 (5) |
| Progressive disease | 31 (33) | 28 (30) | 33 (35) |
| Could not be evaluated† | 9 (10) | 13 (14) | 8 (9) |
| Objective response‡ | |||
| No. of patients | 52 | 47 | 48 |
| Percent of patients (95% CI) | 55 (45–66) | 50 (40–60) | 51 (40–62) |
| Clinical benefit§ | |||
| No. of patients | 54 | 53 | 53 |
| Percent of patients (95% CI) | 57 (47–68) | 56 (46–67) | 56 (46–67) |
R esponse was assessed by the investigators in accordance with the Response Evaluation Criteria in Solid Tumors, version 1.1 (modified criteria were used for intracranial response). Confirmed responses are reported. Some patients may have had global responses that were greater than responses in intracranial or extracranial lesions alone. In these patients, the calculated tumor burden showed a greater decrease when the intracranial and extracranial responses were added together, whereas decreases in intracranial or extracranial lesions alone may not have been sufficient to be considered a response. Percentages may not total 100 because of rounding.
This category included patients who withdrew consent or did not have a tumor assessment during the study. Seven patients did not have extracranial lesions and thus were categorized as not able to be evaluated for the extracranial assessments. The percentage of patients who could not be evaluated for response was consistent with previous studies of nivolumab plus ipilimumab in advanced melanoma.11,12
This category included patients with a complete response and those with a partial response. The calculation of 95% confidence interval was based on the Clopper–Pearson method.
This category included patients with a complete response, those with a partial response, and those with stable disease for 6 months or longer. The 95% confidence interval was based on the Clopper–Pearson method.
An additional 2 patients (2%) had stable disease lasting 6 months or longer, which resulted in a rate of intracranial clinical benefit of 57% (95% CI, 47 to 68) (Table 2). Similar rates of objective response (50%) and clinical benefit (56%) were observed for extracranial lesions, although a lower proportion of patients (7%) had a complete extracranial response. Intracranial responses were observed across the range of target lesions, including in patients with three or more intracranial target lesions (Table S4 in the Supplementary Appendix).
Subgroup analyses of the rate of intracranial clinical benefit showed results consistent with those of the primary analysis (Table S5 in the Supplementary Appendix). The rate of clinical benefit was higher among patients with lactate dehydrogenase levels above the upper limit of the normal range than in patients with levels at or below the upper limit of the normal range (67% vs. 51%). Nivolumab plus ipilimumab was associated with a higher rate of clinical benefit among patients with tumor PD-L1 expression that was at least 5% than among patients with tumor PD-L1 expression that was below 5% (76% vs. 48%), a finding consistent with the results of previous studies in extracranial disease.11 The rate of clinical benefit was similar across target-lesion sizes.
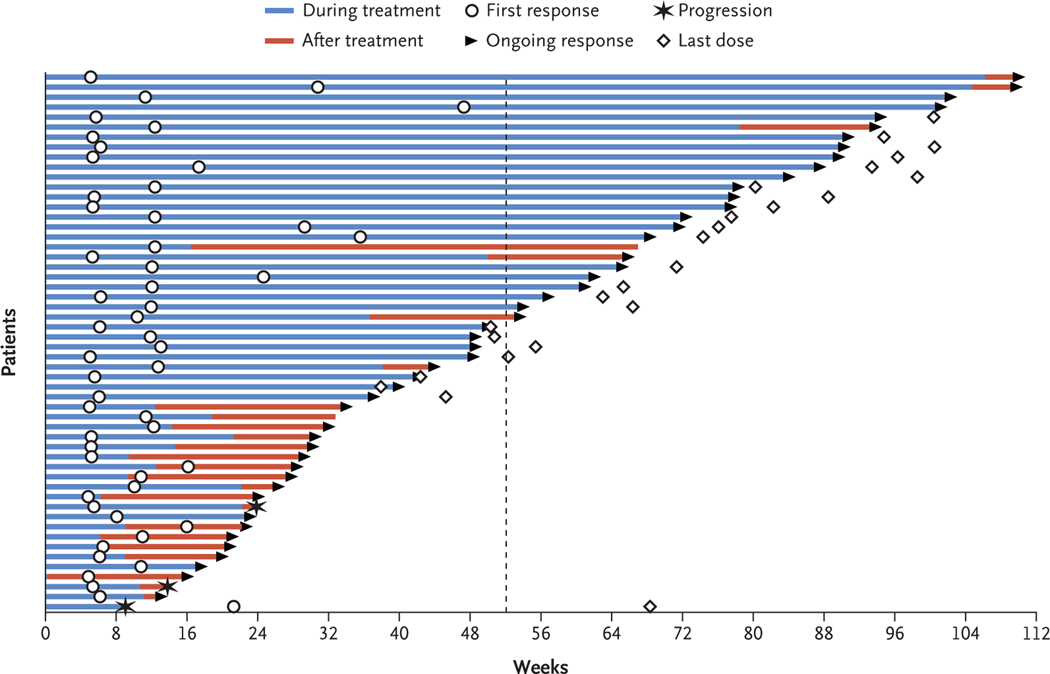
Among patients who had an objective response in the brain, 47 of 52 responses (90%) were ongoing at the time of the current analysis (Fig. 1). The median time to response was 2.3 months (range, 1.1 to 10.8). For extracranial responses, 43 of 47 (91%) were ongoing at the time of the current analysis (Fig. S3 in the Supplementary Appendix). The median time to response was 2.1 months (range, 1.1 to 15.0).
Figure 1. Time to and Duration of Intracranial Response.
The plot shows the onset and durability of intracranial objective responses to the combination of nivolumab and ipilimumab, according to modified Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1, criteria. Open circles indicate the first evidence of objective response (complete or partial response), and arrows indicate an ongoing response; 47 of 52 responses (90%) were ongoing at the time of the analysis. The dashed line indicates 1 year after treatment initiation. The median time to response was 2.3 months (range, 1.1 to 10.8). The median duration of intracranial response has not been reached.
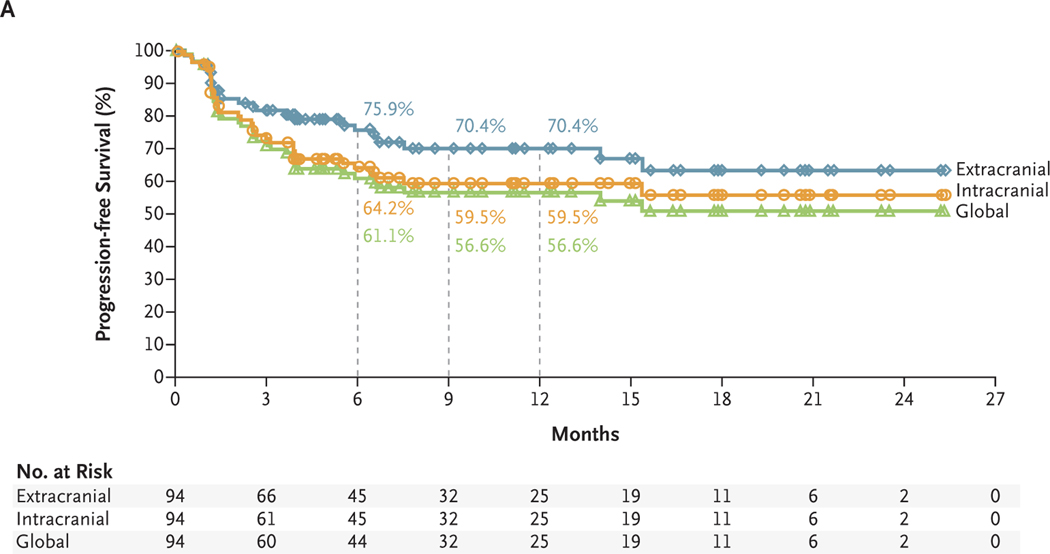
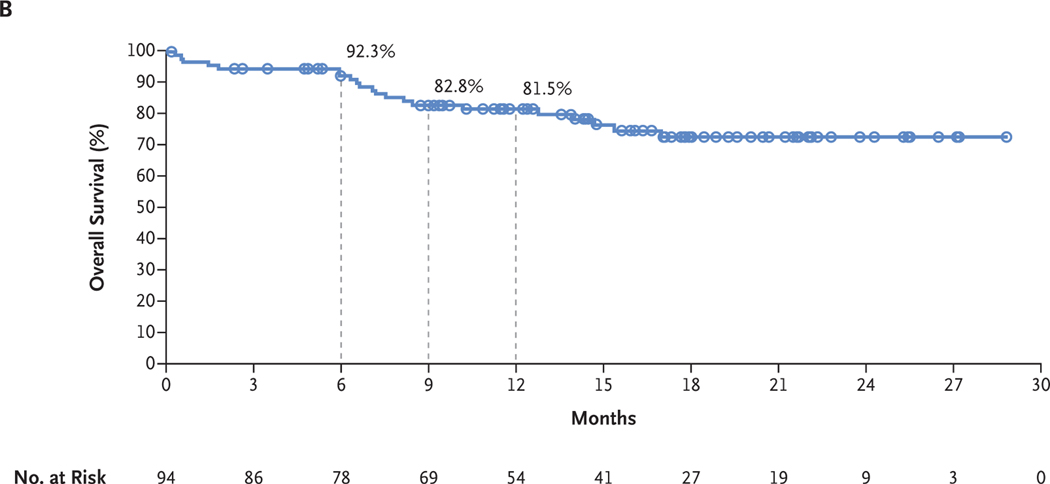
With a minimum follow-up of 6 months and median follow-up of 14.0 months, the 6-month and 9-month rates of progression-free survival were 64.2% and 59.5%, respectively, for intracranial assessments, 75.9% and 70.4% for extracranial assessments, and 61.1% and 56.6% for global assessments (Fig. 2A). In an initial assessment of overall survival, the 6-month and 9-month survival rates were 92.3% and 82.8%, respectively, and the estimated 12-month survival rate was 81.5% (Fig. 2B).
Figure 2. Kaplan–Meier Estimates of Survival.
Panel A shows the Kaplan–Meier estimates of progression-free survival as assessed by the investigators. Patients were followed for a minimum of 6 months. The median progression-free survival has not been reached for intracranial, extracranial, or global disease. For intracranial, extracranial, and global disease, respectively, the rates of progression-free survival were 64.2% (95% CI, 53.0 to 73.4), 75.9% (95% CI, 65.0 to 83.9), and 61.1% (95% CI, 50.0 to 70.5) at 6 months and 59.5% (95% CI, 47.9 to 69.3), 70.4% (95% CI, 58.4 to 79.6), and 56.6% (95% CI, 45.2 to 66.5) at 9 months. The corresponding estimated rates of progression-free survival at 12 months were 59.5% (95% CI, 47.9 to 69.3), 70.4% (95% CI, 58.4 to 79.6), and 56.6% (95% CI, 45.2 to 66.5). Panel B shows the Kaplan–Meier estimates of overall survival. The median overall survival has not been reached. The overall survival rates were 92.3% (95% CI, 84.5 to 96.3) at 6 months and 82.8% (95% CI, 73.1 to 89.3) at 9 months. The estimated rate of overall survival at 12 months was 81.5% (95% CI, 71.5 to 88.2). Symbols indicate censored data.
Safety
Adverse events of grade 3 or 4 that were evaluated by the investigator to be related to the study treatment were reported in 55% of patients; the most common of these events were an increase in levels of alanine aminotransferase or aspartate aminotransferase (Table 3). During the trial, 19 patients (20%) discontinued treatment because of an adverse event of grade 3 or 4. Treatment-related adverse events of any grade that affected the CNS occurred in 34 patients (36%), with grade 3 or 4 events occurring in 7 patients (7%) (Table S6 in the Supplementary Appendix). The most common treatment-related adverse event of any grade in the nervous system was headache (21 patients [22%]), with 3 patients (3%) having headache of grade 3 or 4. Other treatment-related neurologic adverse events of grade 3 or 4 were brain edema (2 patients [2%]), intracranial hemorrhage (1 patient [1%]), peripheral motor neuropathy (1 [1%]), and syncope (1 [1%]). Each of these adverse events led to treatment discontinuation, and the one reported case of peripheral motor neuropathy was irreversible. One death was evaluated by the investigator to be related to the study treatment (grade 5 immune-related myocarditis).13
Table 3.
Adverse Events.*
| Event | Any Grade (N = 94) | Grade 3 or 4 (N = 94) |
|---|---|---|
| no. of patients (%) | ||
| Any adverse event | 91 (97) | 56 (60) |
| Treatment-related adverse event | 90 (96) | 52 (55) |
| Fatigue | 45 (48) | 4 (4) |
| Increased ALT level | 35 (37) | 15 (16) |
| Maculopapular rash | 34 (36) | 7 (7) |
| Diarrhea | 33 (35) | 6 (6) |
| Increased AST level | 32 (34) | 14 (15) |
| Nausea | 26 (28) | 2 (2) |
| Headache | 21 (22) | 3 (3) |
| Hypothyroidism | 20 (21) | 1 (1) |
| Decreased appetite | 16 (17) | 1 (1) |
| Increased lipase level | 14 (15) | 8 (9) |
| Hyperthyroidism | 12 (13) | 3 (3) |
| Vomiting | 12 (13) | 2 (2) |
| Increased amylase level | 11 (12) | 6 (6) |
| Hypophysitis | 11 (12) | 5 (5) |
| Pneumonitis | 8 (9) | 2 (2) |
| Rash | 8 (9) | 2 (2) |
| Anemia | 8 (9) | 1 (1) |
| Colitis | 7 (7) | 7 (7) |
| Abdominal pain | 7 (7) | 1 (1) |
| Adrenal insufficiency | 6 (6) | 1 (1) |
| Increased blood bilirubin level | 6 (6) | 1 (1) |
| Hyponatremia | 5 (5) | 1 (1) |
| Treatment-related adverse event leading to discontinuation of treatment | 25 (27) | 19 (20) |
Shown are treatment-related adverse events of any grade that occurred in at least 5% of the patients who had one or more treatment-related adverse events of grade 3 or 4. One patient died from grade 5 myocarditis. The severity of adverse events was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. ALT denotes alanine aminotransferase, and AST aspartate aminotransferase.
Discussion
The results of our study show that systemic therapy with combined nivolumab and ipilimumab has clinically meaningful efficacy in patients with asymptomatic, untreated melanoma metastases to the brain. Intracranial responses were observed in more than half the patients treated, were detected at the first disease assessment (which is indicative of a rapid response), and were durable. Intracranial activity was largely concordant with extracranial activity, as was previously shown with ipilimumab alone.6 Most importantly, therapy with nivolumab plus ipilimumab prevented intracranial progression for more than 6 months in 64% of patients. These results are relevant in a population in whom progression can quickly result in substantial neurologic symptoms, functional impairment, and the need for glucocorticoid therapy. Although current practice is to start with surgery, stereotactic radiotherapy, or both followed by immunotherapy or targeted agents, our results support the initiation of immunotherapy to achieve prompt control of both extracranial and brain metastases. This approach could also lead to a decrease in or avoidance of complications of whole-brain radiation therapy and stereotactic radiotherapy (e.g., cognitive decline and radiation necrosis, respectively).
We found a rate of intracranial clinical benefit of 57% in association with combined nivolumab and ipilimumab, which was higher than previously reported with ipilimumab alone (12 of 51 patients [24%])6 or pembrolizumab alone (4 of 18 patients [22%]),9 and similar to that reported with ipilimumab plus fotemustine (10 of 20 patients [50%]),14 in similar patient populations. However, we cannot account for potential differences among these studies that are inherent to singlegroup phase 2 studies, such as selection bias and differences in the number and sizes of intracranial target lesions, that may have affected the results. In a population similar to that in our study, the results of a contemporaneous randomized, phase 2 study of nivolumab alone or nivolumab combined with ipilimumab in patients with melanoma who had brain metastases (led by the Anti-PD1 Brain Collaboration) were consistent with the current findings and showed a higher rate of intracranial response with the combination than with nivolumab alone (46% vs. 20%).15 In that study as well as our own, one limitation of the analyses is the lack of central independent review of the data.
Data from studies that directly compare nivolumab plus ipilimumab with combinations of BRAF and MEK inhibitors in patients with BRAF-mutant melanoma and brain metastases are lacking. Among patients who have melanoma with the BRAF V600 mutation and asymptomatic brain metastases, a phase 2 study of dabrafenib plus trametinib (COMBI-MB) showed a rate of intracranial response of 58%.7 Within the limitations of cross-trial comparisons, including different eligibility criteria and whether the use of glucocorticoids is allowed, the intracranial response rate with nivolumab plus ipilimumab in our study was similar to that of dabrafenib plus trametinib in the COMBI-MB study. Combined therapy with BRAF and MEK inhibitors resulted in a duration of intracranial response of 6.5 months, which was shorter than that observed for extracranial disease, in which the duration of response was 10.2 months. Moreover, the intracranial progression-free survival of 5.6 months in the COMBI-MB study was substantially shorter than the extracranial progression-free survival of 10.1 months reported in a phase 3 study of dabrafenib plus trametinib.16 In our study, the use of immunotherapy seemed capable of inducing intracranial responses that were very similar to extracranial responses in character, depth, and duration.
The safety profile that was observed in our study was consistent with those in earlier studies of nivolumab and ipilimumab involving patients with melanoma who did not have brain metastases.10–12,17 Moreover, the incidence of Treatment-related grade 3 or 4 adverse events in our study was similar to that reported in the phase 2 COMBI-MB study (56%).7 Among our concerns in designing this study was the possibility that the inflammatory events accompanying an effective T-cell response could result in peritumoral intracranial edema, but in this population of patients with asymptomatic brain metastases who were not receiving glucocorticoids, the incidence of brain edema during therapy was only 2%. The majority of adverse events of grade 3 or 4 resolved when established safety guidelines were followed, and no patient died from Treatment-related neurotoxic effects. Although an ongoing phase 3b–4 study (CheckMate 511; ClinicalTrials.gov number, NCT02714218) is evaluating the safety profile of nivolumab at a dose of 3 mg per kilogram plus ipilimumab at a dose of 1 mg per kilogram as compared with nivolumab at 1 mg per kilogram plus ipilimumab at 3 mg per kilogram, the currently available evidence does not suggest that lower doses of ipilimumab are superior to the doses used in our study. The dosing regimen we used is the current recognized standard and Food and Drug Administration–approved first-line regimen for the treatment of advanced melanoma.
In conclusion, our results show that nivolumab combined with ipilimumab was an effective treatment for patients with asymptomatic, untreated brain metastases from melanoma. In our study, the regimen had a spectrum of toxic effects similar to that associated with these agents in patients who do not have brain metastases.
Supplementary Material
Acknowledgments
Supported by Bristol-Myers Squibb and a grant from the National Cancer Institute (Cancer Center Support Grant P30 CA008748, to Dr. Postow).
We thank the patients and investigators who participated in the CheckMate 204 study; Caroline Chung, M.D., University of Texas M.D. Anderson Cancer Center, for contributions to the study; Robin Edwards, M.D., Bristol-Myers Squibb, for study protocol development; Jasmine Rizzo, M.D., M.P.H., Bristol-Myers Squibb, for data analyses and data validation; and Ward A. Pedersen, Ph.D., and Cara Hunsberger, StemScientific, for professional medical writing and editorial assistance (funded by BristolMyers Squibb).
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
Contributor Information
Hussein A. Tawbi, University of Texas M.D. Anderson Cancer Center, Houston California
Peter A. Forsyth, Moffitt Cancer Center and Research Institute, Tampa, FL California
Alain Algazi, University of California–San Francisco, San Francisco California
Omid Hamid, Angeles Clinic and Research Institute, Los Angeles California
F. Stephen Hodi, Dana–Farber Cancer Institute, Boston New York
Stergios J. Moschos, University of North Carolina Lineberger Comprehensive Cancer Center, Chapel Hill New York
Nikhil I. Khushalani, Moffitt Cancer Center and Research Institute, Tampa, FL California New York
Karl Lewis, University of Colorado Comprehensive Cancer Center, Aurora New York
Christopher D. Lao, University of Michigan, Ann Arbor New York
Michael A. Postow, Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College, New York
Michael B. Atkins, Georgetown–Lombardi Comprehensive Cancer Center, Washington DC
Marc S. Ernstoff, Roswell Park Cancer Institute, Buffalo New York
David A. Reardon, Dana–Farber Cancer Institute, Boston New York
Igor Puzanov, Roswell Park Cancer Institute, Buffalo New York
Ragini R. Kudchadkar, Winship Cancer Institute of Emory University, Atlanta
Reena P. Thomas, Stanford University Hospital, Palo Alto California
Ahmad Tarhini, University of Pittsburgh Medical Center, Pittsburgh Cleveland Clinic–Taussig Cancer Institute, Cleveland.
Anna C. Pavlick, New York University, Lake Success New York
Joel Jiang, Bristol-Myers Squibb, Princeton NJ
Alexandre Avila, Bristol-Myers Squibb, Princeton, NJ
Sheena Demelo, Bristol-Myers Squibb, Princeton, NJ
Kim Margolin, Department of Medical Oncology, City of Hope, Duarte. California
References
- 1.Specht HM, Combs SE. Stereotactic radiosurgery of brain metastases. J Neurosurg Sci 2016; 60:3 57–66. [PubMed] [Google Scholar]
- 2.Sloan AE, Nock CJ, Einstein DB. Diagnosis and treatment of melanoma brain metastasis: a literature review. Cancer Control 2009; 16:2 48–55. [DOI] [PubMed] [Google Scholar]
- 3.Cagney DN, Martin AM, Catalano PJ, et al. Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: a population-based study. Neuro Oncol 2017; 19: 1511–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies MA, Liu P, McIntyre S, et al. Prognostic factors for survival in melanoma patients with brain metastases. Cancer 2011; 117: 1687–96. [DOI] [PubMed] [Google Scholar]
- 5.Agarwala SS, Kirkwood JM, Gore M, et al. Temozolomide for the treatment of brain metastases associated with metastatic melanoma: a phase II study. J Clin Oncol 2004; 22: 2101–7. [DOI] [PubMed] [Google Scholar]
- 6.Margolin K, Ernstoff MS, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol 2012; 13: 45965. [DOI] [PubMed] [Google Scholar]
- 7.Davies MA, Saiag P, Robert C, et al. Dabrafenib plus trametinib in patients with BRAFV600-mutant melanoma brain metastases (COMBI-MB): a multicentre, multicohort, open-label, phase 2 trial. Lancet Oncol 2017; 18:8 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363: 711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldberg SB, Gettinger SN, Mahajan A, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol 2016; 17:9 76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015; 372: 2006–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015; 373: 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolchok JD, Chiarion-Sileni V, Gon-zalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2017; 377:1 345–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson DB, Balko JM, Compton ML, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 2016; 375: 1749–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Giacomo AM, Ascierto PA, Pilla L, et al. Ipilimumab and fotemustine in patients with advanced melanoma (NIBIT-M1): an open-label, single-arm phase 2 trial. Lancet Oncol 2012; 13:8 79–86. [DOI] [PubMed] [Google Scholar]
- 15.Long GV, Atkinson V, Lo S, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol 2018;1 9: 672–81. [DOI] [PubMed] [Google Scholar]
- 16.Long GV, Flaherty KT, Stroyakovskiy D, et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a phase 3 study. Ann Oncol 2017; 28: 1631–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hodi FS, Chesney J, Pavlick AC, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol 2016;1 7: 1558–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.