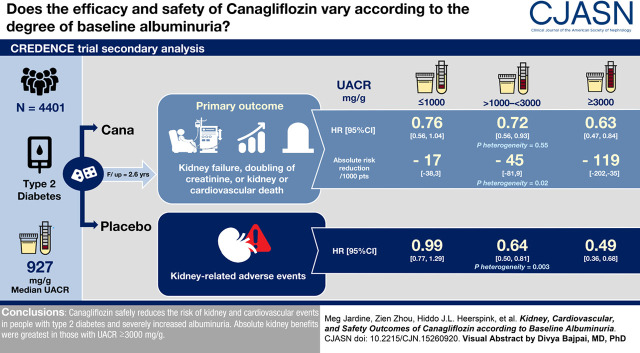
Visual Abstract
Keywords: SGLT2 inhibitors, canagliflozin, chronic kidney disease progression, albuminuria, randomized controlled trials, cardiovascular system, diabetes
Abstract
Background and objectives
The kidney protective effects of renin-angiotensin system inhibitors are greater in people with higher levels of albuminuria at treatment initiation. Whether this applies to sodium-glucose cotransporter 2 (SGLT2) inhibitors is uncertain, particularly in patients with a very high urine albumin-to-creatinine ratio (UACR; ≥3000 mg/g). We examined the association between baseline UACR and the effects of the SGLT2 inhibitor, canagliflozin, on efficacy and safety outcomes in the Canagliflozin and Renal Endpoints in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) randomized controlled trial.
Design, setting, participants, & measurements
The study enrolled 4401 participants with type 2 diabetes, an eGFR of 30 to <90 ml/min per 1.73 m2, and UACR of >300 to 5000 mg/g. Using Cox proportional hazards regression, we examined the relative and absolute effects of canagliflozin on kidney, cardiovascular, and safety outcomes according to a baseline UACR of ≤1000 mg/g (n=2348), >1000 to <3000 mg/g (n=1547), and ≥3000 mg/g (n=506). In addition, we examined the effects of canagliflozin on UACR itself, eGFR slope, and the intermediate outcomes of glycated hemoglobin, body weight, and systolic BP.
Results
Overall, higher UACR was associated with higher rates of kidney and cardiovascular events. Canagliflozin reduced efficacy outcomes for all UACR levels, with no evidence that relative benefits varied between levels. For example, canagliflozin reduced the primary composite outcome by 24% (hazard ratio [HR], 0.76; 95% confidence interval [95% CI], 0.56 to 1.04) in the lowest UACR subgroup, 28% (HR, 0.72; 95% CI, 0.56 to 0.93) in the UACR subgroup >1000 to <3000 mg/g, and 37% (HR, 0.63; 95% CI, 0.47 to 0.84) in the highest subgroup (Pheterogeneity=0.55). Absolute risk reductions for kidney outcomes were greater in participants with higher baseline albuminuria; the number of primary composite events prevented across ascending UACR categories were 17 (95% CI, 3 to 38), 45 (95% CI, 9 to 81), and 119 (95% CI, 35 to 202) per 1000 treated participants over 2.6 years (Pheterogeneity=0.02). Rates of kidney-related adverse events were lower with canagliflozin, with a greater relative reduction in higher UACR categories.
Conclusions
Canagliflozin safely reduces kidney and cardiovascular events in people with type 2 diabetes and severely increased albuminuria. In this population, the relative kidney benefits were consistent over a range of albuminuria levels, with greatest absolute kidney benefit in those with an UACR ≥3000 mg/g.
Clinical Trial registry name and registration number:
ClinicalTrials.gov: CREDENCE, NCT02065791.
Podcast
This article contains a podcast at https://www.asn-online.org/media/podcast/CJASN/2021_02_22_CJN15260920_final.mp3
Introduction
Agents that offer kidney protection often have greater relative benefits in those with higher albuminuria (or proteinuria) at treatment initiation. For example, the protective effect of renin-angiotensin system (RAS) inhibitors on the progression of CKD is modified by baseline proteinuria in people with (1,2) and without (3,4) diabetes. Similarly, the relative benefits of tolvaptan, a vasopressin v2 receptor antagonist, on eGFR decline in people with autosomal-dominant polycystic kidney disease increases with baseline albuminuria (5). The relationship between albuminuria and treatment effects in these studies was demonstrated in populations with normal-to-moderate albuminuria. Whether this holds true at very high levels (including nephrotic-range) and whether albuminuria modifies the effects of sodium-glucose cotransporter 2 (SGLT2) inhibitors are unclear.
Before the demonstration of their benefits for kidney and cardiovascular outcomes (6,7), it was clear that SGLT2 inhibitors reduced albuminuria in patients with type 2 diabetes (8). Albuminuria is a strong predictor of kidney disease progression and cardiovascular disease (9–11) and, together with eGFR, is the foundation for the Kidney Disease Improving Global Outcomes (KDIGO) kidney disease risk classification system (9,12). Consequently, people with higher albuminuria might derive greater absolute benefit from albuminuria-lowering treatments.
SGLT2 inhibitors prevent kidney and cardiovascular events in people with type 2 diabetes (13–16). In the kidney outcome trial, Canagliflozin and Renal Endpoints in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE), canagliflozin reduced the risk of the primary composite outcome of kidney failure, a doubling of serum creatinine, or kidney or cardiovascular death by 30% (hazard ratio [HR], 0.70; 95% confidence interval [95% CI], 0.59 to 0.82). Canagliflozin also reduced the risk of numerous kidney- and cardiovascular-specific outcomes (e.g., kidney failure and the composite outcome of myocardial infarction, stroke, or cardiovascular death).
The CREDENCE trial recruited participants with severely increased albuminuria (urine albumin-to-creatinine ratio [UACR] >300 to 5000 mg/g), including >500 participants with nephrotic-range albuminuria who were already stabilized on RAS blockade. In this population of people at high risk of progressive kidney and cardiovascular disease, we assessed the relative and absolute effects of canagliflozin according to baseline UACR.
Materials and Methods
CREDENCE was an event-driven, double-blind, randomized controlled trial whose design and main results have been previously described (15,17). Ethical approval was obtained at each participating site before commencement of recruitment. The trial was conducted in accordance with the principles of the Declaration of Helsinki.
Participants and Albuminuria Assessment
Trial eligibility criteria were designed to recruit participants at high risk of progression of diabetic kidney disease. Participants were aged ≥30 years, with type 2 diabetes, a glycated hemoglobin (HbA1c) level of 6.5%–12.0%, an eGFR of 30–<90 ml/min per 1.73 m2 (calculated using the CKD Epidemiology Collaboration formula) (18), and a UACR of >300 to 5000 mg/g. Key exclusion criteria included nondiabetic kidney disease, type 1 diabetes, and prior treatment of kidney disease with immunosuppression or KRT. Participants were required to have received treatment with a stable maximum-labeled/tolerated dose of an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker for ≥4 weeks prior to randomization.
In the CREDENCE study, albuminuria was assessed at multiple timepoints. First, to be eligible for screening, participants were required to have a UACR >300 mg/g (>33.9 mg/mmol) or equivalent, confirmed by a local laboratory result within 6 months of screening. At screening, a UACR of >300 to 5000 mg/g (>33.9 to 565.6 mg/mmol) on central laboratory measurement was required. Third, albuminuria was measured at randomization through a central laboratory, but, notably, this was not used to judge eligibility. Thus, participants with a UACR <300 mg/g by randomization could be enrolled.
Participants were randomized in a 1:1 ratio to receive double-blinded oral canagliflozin 100 mg or placebo daily, until initiation of KRT (dialysis or kidney transplantation), occurrence of diabetic ketoacidosis, pregnancy, receipt of disallowed therapy, or study end.
Outcomes
The efficacy outcomes for the current analyses were the same as those reported for the overall trial (15). All efficacy outcomes and selected safety outcomes were independently adjudicated by blinded expert committees.
The primary outcome was the composite of kidney failure (initiation of dialysis for ≥30 days, kidney transplantation, or eGFR<15 ml/min per 1.73 m2 sustained for ≥30 days by central laboratory assessment), a doubling of serum creatinine from baseline (average of randomization and prerandomization value) sustained for ≥30 days by central laboratory assessment, or death due to kidney or cardiovascular disease. Secondary kidney and cardiovascular efficacy outcomes are shown in Table 1.
Table 1.
Efficacy and safety end points of the CREDENCE study that are included in this study
| Primary end point | Composite of kidney failure, a doubling of serum creatinine from baseline, or death due to kidney or cardiovascular disease |
| Secondary kidney end points | Composite of kidney failure, a doubling of serum creatinine, or kidney death |
| Composite of kidney failure or kidney death | |
| Composite of KRT initiation (dialysis for ≥30 days or kidney transplantation) or kidney death | |
| Kidney failure | |
| Doubling of serum creatinine | |
| Composite of kidney failure, or kidney or cardiovascular death | |
| Secondary cardiovascular end points | Composite of cardiovascular death or hospitalization for heart failure |
| Major adverse cardiovascular events composite of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke | |
| Hospitalization for heart failure | |
| Cardiovascular death | |
| Death from any cause | |
| Composite of cardiovascular death, myocardial infarction, stroke, or hospitalization for heart failure or unstable angina | |
| Intermediate outcomes | Change in urine albumin-to-creatinine ratio |
| Acute, chronic, and total eGFR slope | |
| Change in glycated hemoglobin | |
| Change in body weight | |
| Change in systolic BP | |
| Safety outcomes | |
| Kidney-related safety outcomes | Composite of AKI, anuria, azotemia, blood creatinine increased, blood urea increased, eGFR decreased, nephropathy toxic, renal failure, and renal impairment |
| AKI | |
| Other safety outcomes | Volume depletion |
| Hyperkalemia | |
| Urinary tract infections | |
| Hypoglycemia | |
Table adapted from efficacy and end points of Canagliflozin and Renal Endpoints in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE).
Safety outcomes with ten or more events in each albuminuria subgroup were examined, and included all kidney-related adverse events combined, AKI, volume depletion, hyperkalemia, urinary tract infections (UTIs), and hypoglycemia (Table 1). Similar to other CREDENCE secondary analyses, kidney-related adverse events were defined as those that were coded as primarily involving the kidney according to Medical Dictionary for Regulatory Activities terminology, and which were investigator-reported (Table 1).
Percentage and absolute change in albuminuria was calculated as the difference between baseline UACR and the average of all UACR measurements to week 182. eGFR slope was assessed as the acute change in eGFR from baseline to week 3 (acute slope), the annualized change in eGFR from week 3 until treatment end (chronic slope), and the annualized change in eGFR from baseline to week 130 (total slope). Finally, we assessed the intermediate outcomes of HbA1c, body weight, and systolic BP.
Statistical Analyses
The effects of canagliflozin were analyzed according to the baseline UACR categories ≤1000, >1000 to <3000, and ≥3000 mg/g. These broadly equate to a urine protein-to-creatinine ratio of ≤1920 mg/g, >1920 to <5000 mg/g, and ≥5000 mg/g, albeit with some uncertainty around these values (http://ckdpcrisk.org/pcr2acr/; accessed on 24 July 2020) (19). Baseline UACR was used in this analysis as it represents the pretreatment measurement at which all participants had been treated with a stable dose of maximally tolerated angiotensin-converting enzyme inhibitor/angiotensin receptor blocker.
For all event-based outcomes, an intention-to-treat approach was used. Annualized incidence rates were calculated per 1000 patient-years of follow-up. HRs and 95% CIs were estimated using a Cox proportional hazards regression model, stratified by screening eGFR (30 to <45, 45 to <60, and 60 to <90 ml/min per 1.73 m2). The heterogeneity of relative effects across UACR subgroups was assessed by including UACR group as a model covariate, together with an interaction term for treatment and baseline UACR. To calculate absolute risk differences, the number of participants with an outcome (per 1000 patients over median follow-up) in those assigned to canagliflozin was subtracted from the corresponding number in those assigned to placebo. The heterogeneity in absolute risk reduction was estimated using a fixed-effect meta-analysis, with a chi-squared test.
To assess the relative effects of canagliflozin on albuminuria, HbA1c, body weight, and systolic BP, linear mixed-effects models for repeated measures were used to analyze the percentage change in the outcome (log-transformed for UACR) over time. Models were adjusted for baseline value and trial visit. Time was included as a categorical factor such that the geometric means were modeled for each visit separately. The residuals from the mean model were assumed to have an unstructured covariance matrix.
eGFR slope analyses were conducted using on-treatment eGFR measurements only. This was to avoid the expected distortions from modifications of the hemodynamic effect after cessation of study drug. On-treatment eGFR measurements comprised all measurements available between day 1 and the last dose of study medication (+2 days), from a central laboratory. To estimate the effects of canagliflozin on the mean eGFR slope, a two-slope, mixed-effects, linear spline model was fitted to eGFR measurements (with a knot at week 3, the first postrandomization eGFR measure), with a random intercept and random slopes for treatment. Similar to previous CREDENCE subgroup analyses (20), the mean total slope was computed as a weighted combination of the acute and chronic slopes. Heterogeneity in the effect of canagliflozin on acute, chronic, and total eGFR slope between UACR subgroups was estimated by comparing the subgroup-level effects, using a chi-squared test with two degrees of freedom, accounting for the standard error of the mean (SEM) in each subgroup. Change in mean eGFR according to treatment and baseline UACR is graphically presented using a restricted maximum likelihood, repeated measures approach.
No adjustment for multiplicity of testing was made. Importantly, given the post hoc nature of these analyses, the presented P values should be interpreted with caution and have been presented for descriptive rather than inferential purposes. Analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
The 4401 participants of the CREDENCE trial were followed for a median of 2.6 years (range, 0.0–4.5) (15). Overall, median baseline UACR was 927 mg/g (105 mg/mmol). Around half (53%) had a baseline UACR ≤1000 mg/g, 35% had a UACR between >1000 and <3000 mg/g, and 12% had a UACR ≥3000 mg/g (Table 2). Mean age was lower in patients with a higher baseline UACR compared with those with a lower UACR. The proportion of women and patients of Asian ethnicity were higher among participants with a higher UACR compared with those with a lower UACR, whereas the opposite was true for the proportion of White patients. Higher UACR subgroups were also more likely to be using glucose-lowering regimens reflecting higher diabetes severity (i.e., more receiving insulin and fewer receiving a sulphonylurea or biguanide), and more likely to have microvascular disease, higher BP, and lower eGFR, compared with lower UACR subgroups (Table 2).
Table 2.
Baseline characteristics of participants in the CREDENCE trial, according to baseline UACR
| Characteristic | Baseline UACR, mg/g | ||
|---|---|---|---|
| ≤1000 | >1000 to <3000 | ≥3000 | |
| n=2348 (53%) | n=1547 (35%) | n=506 (12%) | |
| Age, yr, mean (SD) | 64 (9) | 63 (9) | 60 (9) |
| Women, n (%) | 756 (32%) | 534 (35%) | 204 (40%) |
| Race, n (%) | |||
| Asian | 422 (18%) | 331 (21%) | 124 (25%) |
| Black | 138 (6.0%) | 65 (4%) | 21 (4%) |
| White | 1596 (68%) | 1018 (66%) | 317 (63%) |
| Othera | 192 (8%) | 133 (9%) | 44 (9%) |
| Region, n (%) | |||
| North America | 666 (28%) | 381 (25%) | 135 (27%) |
| Central/South America | 523 (22%) | 314 (20%) | 104 (21%) |
| Europe | 457 (20%) | 328 (21%) | 79 (16%) |
| Rest of the world | 702 (30%) | 524 (34%) | 188 (37%) |
| Current smoker, n (%) | 325 (14%) | 242 (16%) | 72 (14%) |
| History of hypertension, n (%) | 2273 (97%) | 1501 (97%) | 486 (96%) |
| History of heart failure, n (%) | 319 (14%) | 247 (16%) | 86 (17%) |
| Duration of diabetes, yr, mean (SD) | 16 (9) | 16 (9) | 15 (8) |
| Drug therapy, n (%) | |||
| Insulin | 1463 (62%) | 1057 (68%) | 364 (72%) |
| Sulfonylurea | 727 (31%) | 427 (28%) | 114 (23%) |
| Biguanide | 1433 (61%) | 865 (56%) | 247 (49%) |
| GLP-1 receptor agonist | 108 (5%) | 56 (5%) | 19 (4%) |
| DPP-4 inhibitor | 419 (18%) | 267 (17%) | 65 (13%) |
| Statin | 1628 (69%) | 1077 (70%) | 331 (65%) |
| Antithrombotic agentb | 1448 (62%) | 915 (59%) | 261 (52%) |
| RAS inhibitor | 2345 (100%) | 1545 (100%) | 505 (100%) |
| β-blocker | 938 (340%) | 631 (41%) | 201 (40%) |
| Diuretic | 913 (39%) | 708 (46%) | 261 (52%) |
| Microvascular disease history, n (%) | |||
| Neuropathy | 1106 (47%) | 765 (50%) | 276 (55%) |
| Retinopathy | 913 (39%) | 708 (46%) | 261 (52%) |
| History of cardiovascular disease, n (%) | 1198 (51%) | 758 (49%) | 264 (52%) |
| Body mass index, kg/m2, mean (SD)c | 31.4 (6.1) | 31.3 (6.1) | 31.2 (6.6) |
| Systolic BP, mm Hg, mean (SD) | 138 (15) | 142 (16) | 143 (16) |
| Diastolic BP, mm Hg, mean (SD) | 77 (9) | 79 (9) | 80 (9) |
| Glycated hemoglobin, %, mean (SD) | 8.3 (1.3) | 8.2 (1.3) | 8.4 (1.5) |
| Triglycerides, mg/dl, mean (SD)c | 186 (133) | 204 (159) | 221 (142) |
| Cholesterol, mg/dl, mean (SD)c | 174 (46) | 182 (50.3) | 201 (58) |
| HDL cholesterol, mg/dl, mean (SD)c | 43 (12) | 46 (12) | 46 (16) |
| LDL cholesterol, mg/dl, mean (SD)c | 93 (39) | 97 (39) | 112 (50) |
| Ratio of LDL to HDL, mean (SD)c | 2.2 (1.0) | 2.3 (1.1) | 2.6 (1.3) |
| eGFR, ml/min per 1.73 m2, mean (SD) | 58 (18) | 55 (18) | 53 (18) |
| UACR, mg/g, median (IQR)d | 489 (321–693) | 1630 (1254–2167) | 3893 (3408–4765) |
| UACR, mg/mmol, median (IQR)d | 55 (36–78) | 184 (142–245) | 440 (385–539) |
CREDENCE, Canagliflozin and Renal Endpoints in Diabetes with Established Nephropathy Clinical Evaluation; UACR, urine albumin-to-creatinine ratio; GLP-1, glucagon-like peptide-1; DPP-4, dipeptidyl peptidase-4; RAS, renin-angiotensin system; IQR, interquartile range.
Includes American Indian or Alaska Native, Native Hawaiian or other Pacific Islander, multiple, other, unknown, and not reported.
Includes anticoagulation and antiplatelet agents, including aspirin.
≤1% missing data.
Eligibility was on the basis of a screening UACR of >300 to 5000 mg/g (33.9–565.6 mg/mmol). By baseline, 527 participants had a UACR <300 mg/g, including 31 with normoalbuminuria (UACR<30 mg/g, or <3 mg/mmol) and 496 with microalbuminuria (UACR 30–300 mg/g, or 3–30 mg/mmol) (15).
Higher baseline UACR was consistently associated with a higher rate of kidney and cardiovascular events in both the placebo and canagliflozin groups (Figures 1 and 2, Supplemental Table 1). The rates at which participants with baseline UACR ≥3000 mg/g randomized to placebo experienced at least one event was 201.5 events per 1000 patient-years for the primary outcome, and 126.9 events per 1000 patient-years for the composite of kidney failure or kidney death. The rates at which this same population experienced at least one cardiovascular or fatal event was 87.2 events per 1000 patient-years for the composite of cardiovascular death or hospitalization for heart failure; 77.0 for the composite of cardiovascular death, myocardial infarction, or stroke; and 71.4 for all-cause mortality.
Figure 1.
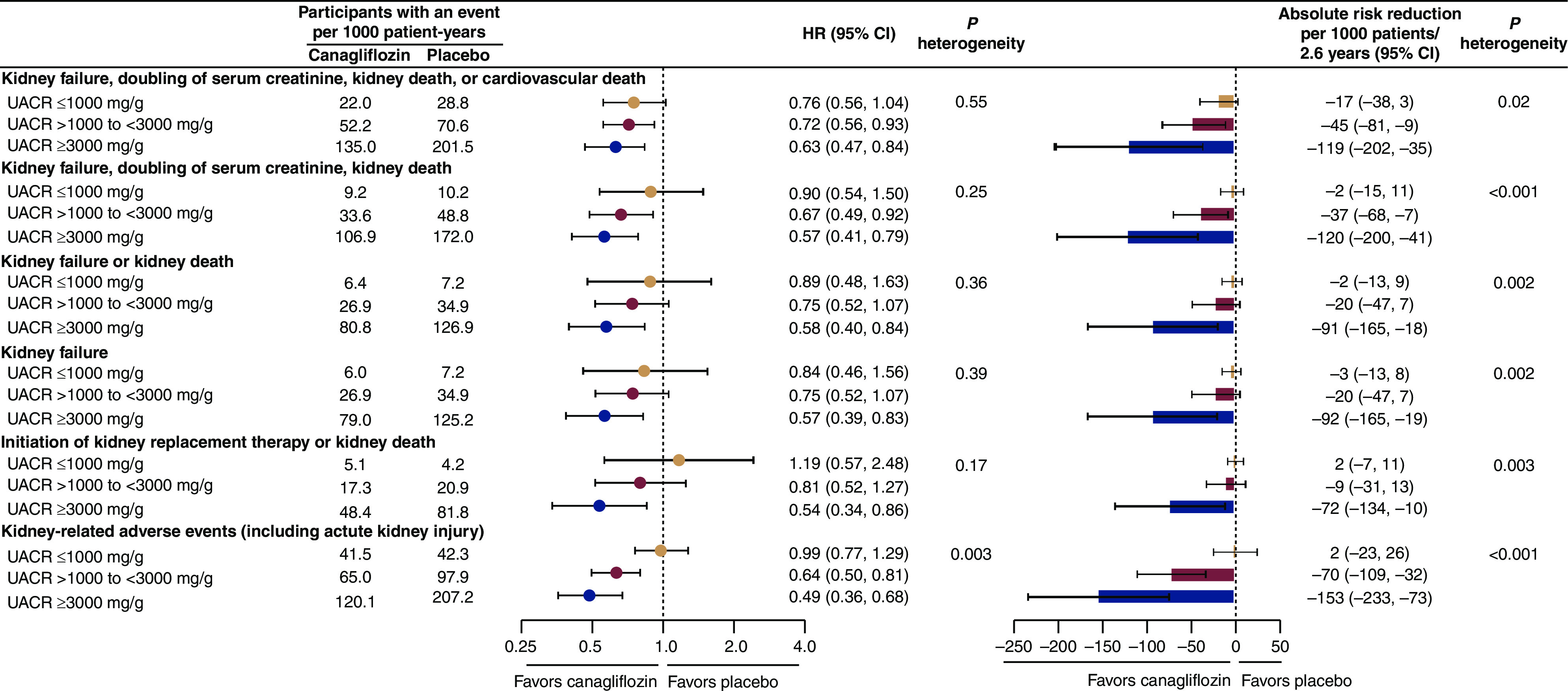
For all kidney-related efficacy outcomes, the relative benefits of canagliflozin were consistent across all urine albumin-to-creatinine ratio (UACR) categories. For kidney-related adverse events, a greater relative risk reduction was observed in higher UACR categories. For all kidney-related outcomes, absolute benefits were greatest in individuals with higher levels of UACR. 95% CI, 95% confidence interval; HR, hazard ratio.
Figure 2.
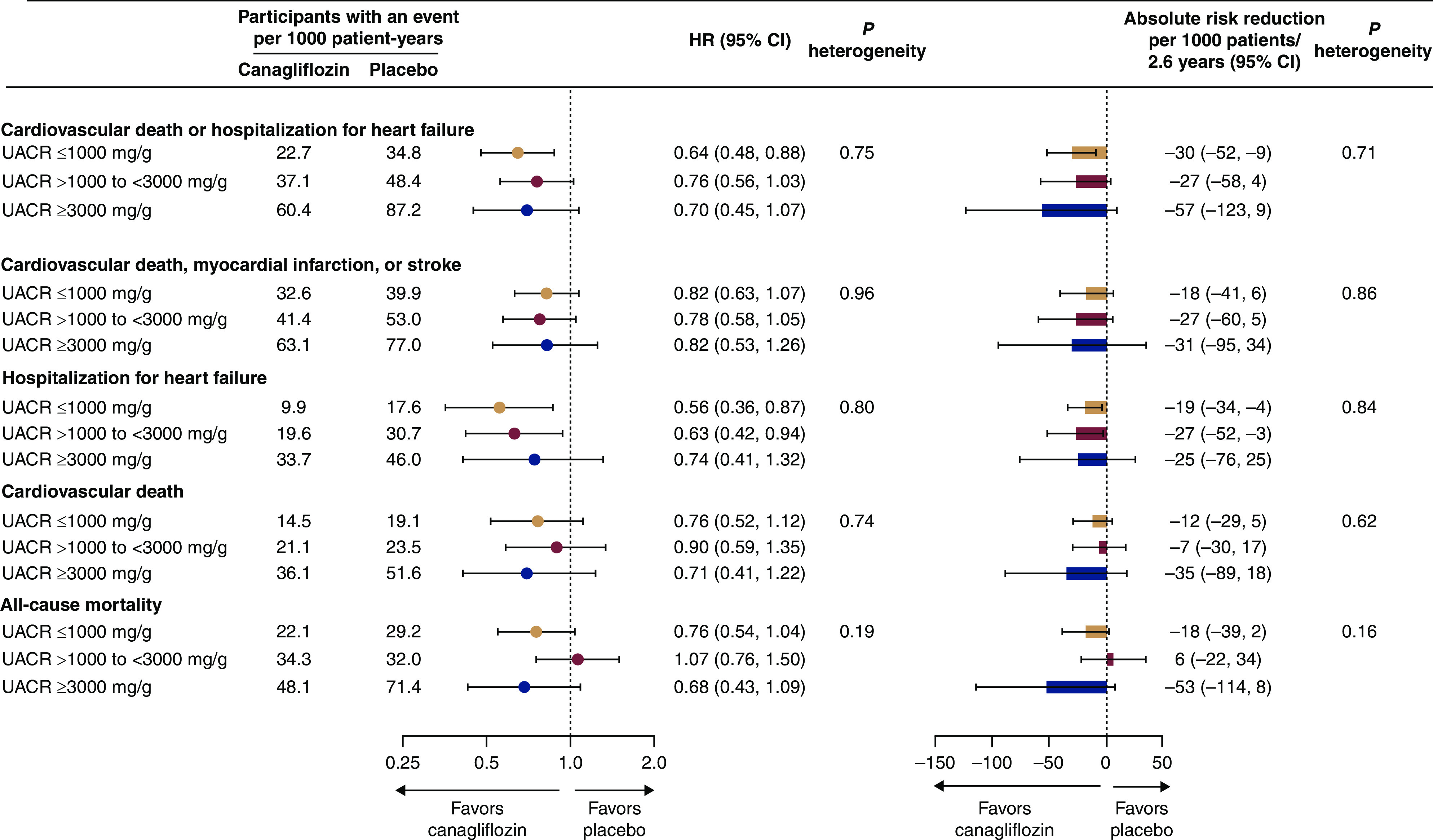
For all cardiovascular-related efficacy outcomes and all-cause mortality, the relative and absolute benefits of canagliflozin were consistent across UACR categories.
Kidney Outcomes
The relative risk reduction for the primary composite outcome of kidney failure, doubling of serum creatinine, or kidney or cardiovascular death (HR, 0.70; 95% CI, 0.59 to 0.82 in the primary analysis previously published; see Supplemental Table 2) was consistent across the baseline albuminuria subgroups (Pheterogeneity=0.55). Similarly consistent effects were observed for all other kidney outcomes (all Pheterogeneity>0.17), including the secondary kidney composite outcome of kidney failure, doubling of serum creatinine, or kidney death (HR, 0.66; 95% CI, 0.53 to 0.81 in the primary analysis); the secondary kidney composite outcome of kidney failure or kidney death (HR, 0.69; 95% CI, 0.54 to 0.87 in the primary analysis); the exploratory composite outcome of KRT initiation or kidney death (HR, 0.72; 95% CI, 0.54 to 0.97 in the primary analysis) (Figure 1); the individually assessed outcomes of kidney failure and doubling of serum creatinine; and the composite outcome of kidney failure or kidney or cardiovascular death (Supplemental Table 1).
For almost all kidney outcomes, the absolute benefit was greatest in the highest UACR category (≥3000 mg/g) (all Pheterogeneity<0.05, except for the composite of kidney failure or kidney or cardiovascular death, for which Pheterogeneity=0.11) (Figure 1, Supplemental Table 1). For every 1000 patients with the highest level of baseline albuminuria treated over a median of 2.6 years, canagliflozin would be expected to prevent 119 participants experiencing the primary composite outcome (number needed to treat [NNT], 9; 95% CI, 5 to 29); 120 experiencing the composite of kidney failure, doubling of serum creatinine, or kidney death (NNT, 9; 95% CI, 5 to 25); 91 experiencing kidney failure or kidney death (NNT, 11; 95% CI, 7 to 56); and 72 experiencing KRT initiation or kidney death (NNT, 11; 95% CI, 9 to 100) (Figure 1).
Cardiovascular Outcomes and All-Cause Death
For all cardiovascular outcomes where canagliflozin had an effect in the primary analyses (Supplemental Tables 1 and 2), the relative benefit was consistent across UACR subgroups (Figure 2, Supplemental Table 1; all Pheterogeneity>0.75). This included the composite of cardiovascular death or hospitalization for heart failure (HR, 0.69; 95% CI, 0.57 to 0.83 in the primary analysis); major adverse cardiovascular event; and the extended cardiovascular composite of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for heart failure or unstable angina (Figure 2, Supplemental Table 1). Canagliflozin did not reduce cardiovascular death or all-cause mortality in any of the UACR subgroups (Figure 2).
Although the rates of cardiovascular events were higher in those groups with higher UACR at baseline, there were no clear differences in the absolute benefits of canagliflozin on cardiovascular outcomes across albuminuria categories (all Pheterogeneity>0.16) (Figure 2, Supplemental Table 1).
Effects on UACR
Overall, canagliflozin reduced UACR by 31% (95% CI, 26% to 35%), with an absolute reduction of 239.5 mg/g (95% CI, 207.0 to 270.2 mg/g) (Figure 3). The relative reduction was higher in individuals with a lower baseline UACR (Pheterogeneity=0.03; Figure 3). However, the opposite was true for absolute albuminuria reduction, which was 162.9 mg/g (137.9–186.0 mg/g), 355.2 mg/g (263.3–438.5 mg/g), and 340.9 mg/g (−51.2 to 669.0 mg/g) in those with baseline UACR ≤1000 mg/g, >1000 to <3000 mg/g, and ≥3000 mg/g, respectively.
Figure 3.
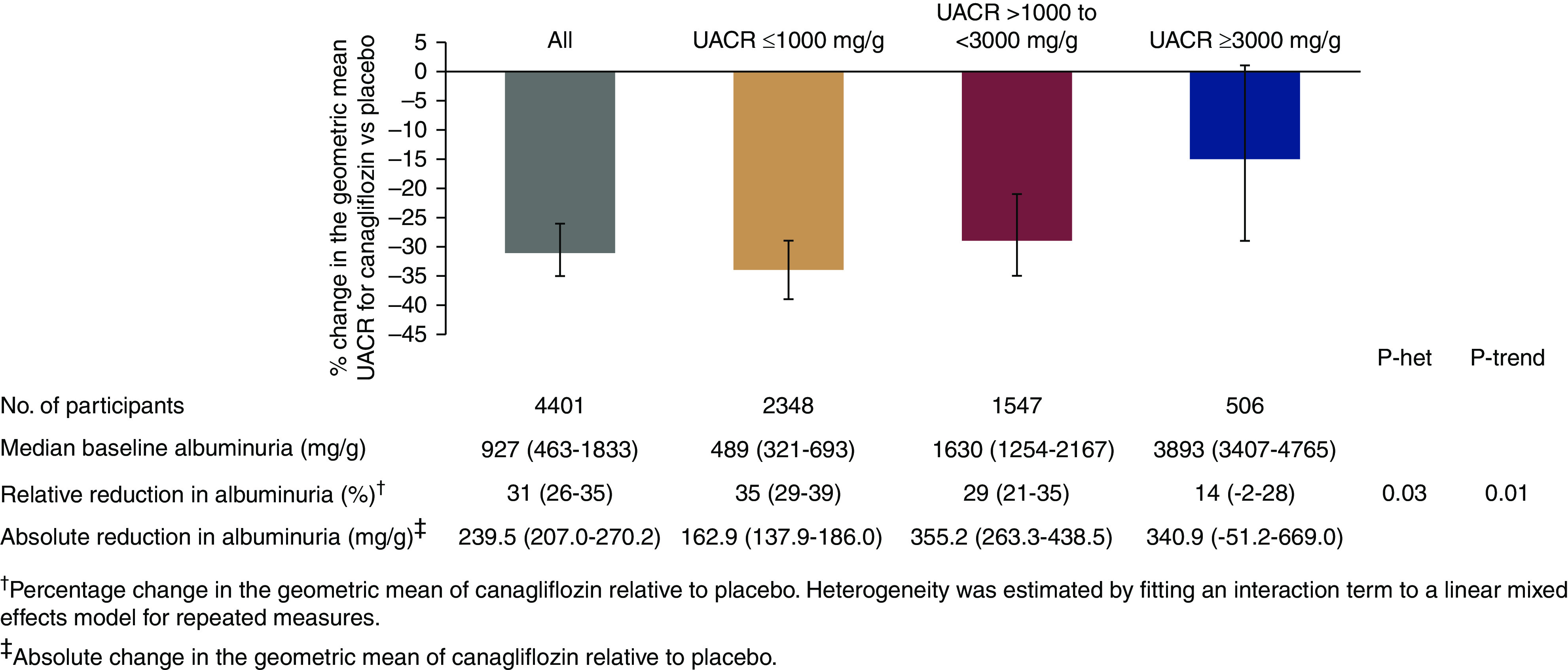
The relative reduction in albuminuria was greater in individuals with a lower baseline UACR, while the absolute reduction was greater in individuals with a higher UACR. het, heterogeneity.
Effects on eGFR Slope
An acute drop in eGFR with treatment commencement was apparent and similar at week 3 in every baseline albuminuria category (Pheterogeneity=0.44) (Figure 4, Supplemental Table 3). Thereafter, canagliflozin attenuated annual eGFR decline in every albuminuria category, with some evidence that this protective effect varied by baseline UACR (Pheterogeneity=0.04) in a nonlinear way (Supplemental Table 3). The absolute reduction in chronic eGFR slope was 2.31 (95% CI, 1.88 to 2.73), 3.29 (95% CI, 2.67 to 3.91), and 2.49 (95% CI, 1.00 to 3.99) ml/min per 1.73 m2 per year in the UACR subgroups ≤1000, >1000 to <3000, and ≥3000 mg/g, respectively. Those with baseline UACR ≥3000 mg/g assigned to placebo had the largest chronic eGFR slope, with a loss of 8.92 (SEM 0.53) ml/min per 1.73 m2 per year, which canagliflozin reduced by 28% to a loss of 6.43 (SEM 0.55) ml/min per 1.73 m2 per year. Results were similar for total eGFR slope (to week 130) (Supplemental Table 3).
Figure 4.
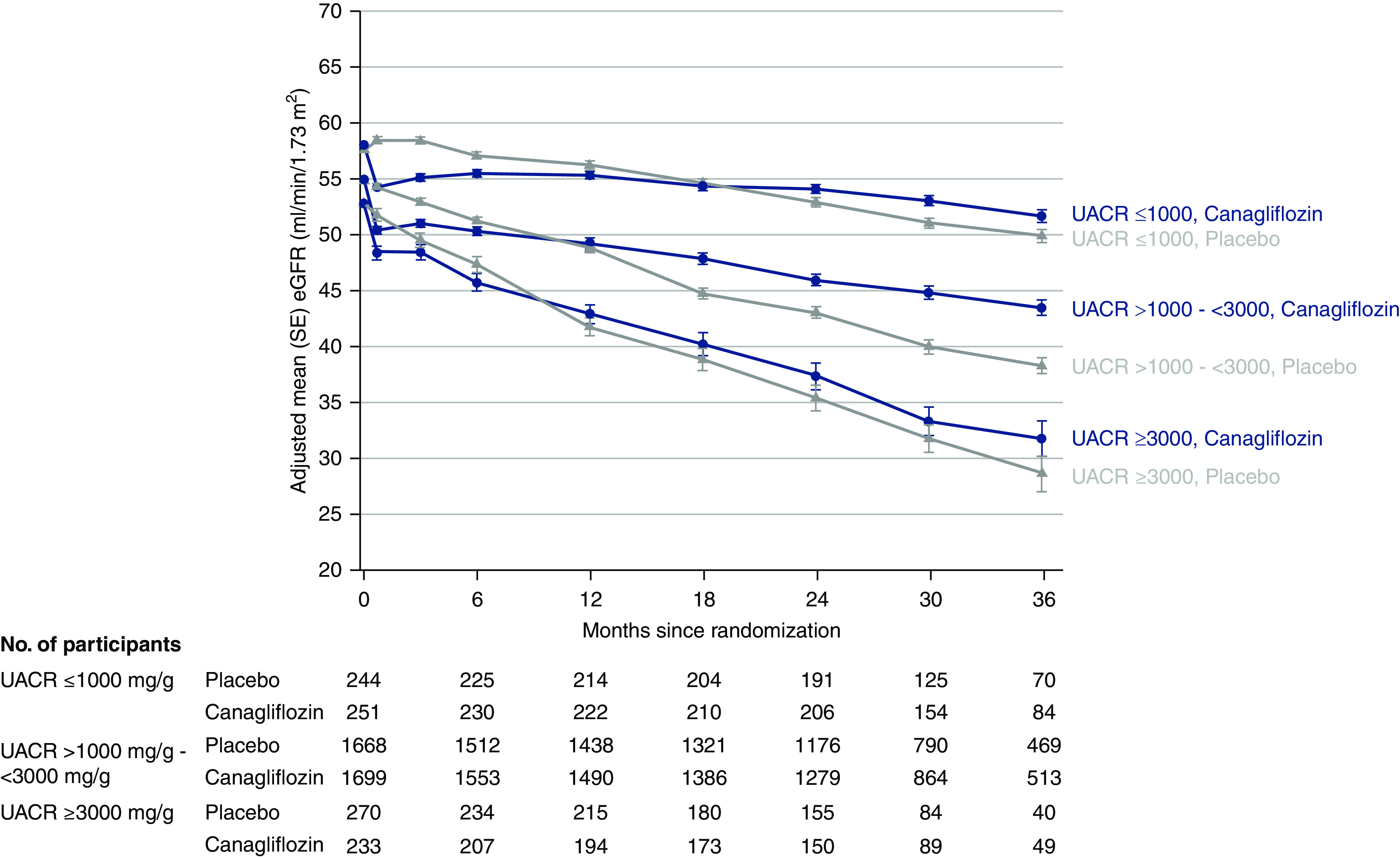
After an acute drop in eGFR between treatment commencement and week 3 in all baseline categories of albuminuria, canagliflozin attenuated annual eGFR decline from week 3 to last available measurement in every albuminuria subgroup. The absolute reduction in chronic eGFR slope was greatest in individuals with a UACR of >1000 to <3000 mg/g. SE, standard error.
Kidney Safety Outcomes
In the primary CREDENCE study paper, canagliflozin reduced the risk of reported kidney-related adverse events overall (HR, 0.71; 95% CI, 0.61 to 0.82). Stratified by baseline UACR, the relative and absolute protective effects were greater in people with higher baseline albuminuria (Pheterogeneity =0.003 and <0.001 for relative and absolute effects, respectively) (Supplemental Table 4).
There was no statistical evidence that the relative or absolute effect of canagliflozin, or lack thereof, on other safety outcomes varied according to baseline albuminuria (Supplemental Table 4).
Effects on Intermediate Outcomes
In the two lower UACR categories, reductions in mean HbA1c, mean body weight, and mean systolic BP were greater in the group treated with canagliflozin compared with the placebo group (Supplemental Table 5). For the higher UACR category, only body weight was reduced by treatment with canagliflozin. Across all intermediate outcomes, the largest reductions were observed in those with a baseline UACR ≤1000 mg/g.
Discussion
In the CREDENCE trial, canagliflozin produced better kidney outcomes in adults with type 2 diabetes at high risk of kidney disease progression (15). In our study, the relative benefit was consistent across all baseline albuminuria levels, including those in the nephrotic range. However, individuals with UACR ≥3000 mg/g, and therefore at greatest risk of progression of kidney disease, derived greater absolute benefit for kidney outcomes from canagliflozin during the median follow-up of 2.6 years. Canagliflozin also reduced a range of cardiovascular events, including hospitalization for heart failure and major adverse cardiovascular event. For cardiovascular outcomes, both relative and absolute treatment effects were consistent across the UACR categories. Canagliflozin appears safe for a range of albuminuria levels and, indeed, provided greater relative and absolute protection against kidney-related adverse events in those with a baseline UACR ≥3000 mg/g. These findings support the value of canagliflozin treatment for kidney and cardiovascular protection in people with diabetes and severely increased albuminuria (>300 mg/g).
The well-established association between albuminuria and the risk of CKD progression, kidney failure, and AKI (9–11,21,22) is on the basis of pooled analyses involving more than 1 million people, including >100,000 with diabetes (23,24). These analyses form the rationale for classifications of the KDIGO CKD risk classification system, which grades albuminuria in categories of A1 (0–29 mg/g), A2 (30–299 mg/g), and A3 (≥300 mg/g) (9,23). Within these combined cohorts, mean albuminuria in those with available quantitative albuminuria measurements was around 17 mg/g (1.9 mg/mmol), with only 2% of participants having a UACR ≥300 mg/g (or 2+ on dipstick) (23), which is more moderate than the albuminuria levels seen in the CREDENCE study. The CREDENCE cohort extends these analyses, providing capacity to examine kidney and cardiovascular risk in individuals with nephrotic-range albuminuria (≥3000 mg/g). Risk continues to increase well beyond UACR levels of 300 mg/g, most notably for kidney end points. Among placebo-treated participants, the rate of the composite outcome of kidney failure, a doubling of serum creatinine, or kidney death increased from 10.2 events per 1000 patient-years in those with baseline UACR ≤1000 mg/g, to 172 events per 1000 patient-years in those with UACR ≥3000 mg/g. Cardiovascular risk also increased, although not as steeply, with, for example, rates of cardiovascular death rising from 19.1 deaths per 1000 patient-years in placebo-treated participants with UACR ≤1000 mg/g, to 51.6 deaths per 1000 patient-years in those with UACR ≥3000 mg/g. The relative clinical kidney and cardiovascular benefit was as strong, and the absolute kidney benefit greater, in those with nephrotic-range albuminuria, making this population a priority group for treatment.
We also examined the effect of canagliflozin on albuminuria. The relative reduction in albuminuria appeared less marked in those with a baseline value ≥3000 mg/g than in those with lower UACR. Not surprisingly, absolute reductions were lower in those with UACR ≤1000 mg/g than in those with higher levels. It is possible that there are multiple causes of albuminuria in those with nephrotic-range albuminuria, not all of which may be amenable to the effects of SGLT2 inhibitors. These causes potentially include hemodynamic mechanisms, alternations in albuminuria handling, fixed structural injury, and others. These findings should be regarded as speculative, given the relatively small number of participants with nephrotic-range albuminuria recruited, and require confirmation in other trials enrolling high-risk patients. Nevertheless, they raise the possibility that SGTL2 inhibitors confer kidney protection in patients with diabetes through mechanisms independent of albuminuria reduction.
Many strengths of this study relate to the design of the original trial. The CREDENCE study recruited people with severely increased albuminuria despite a maximum-tolerated RAS blockade dose, providing the ability to test the effect of canagliflozin in people at very high kidney risk. In addition, kidney outcomes were independently adjudicated and eGFR and UACR assessed centrally. However, the trial did not include patients with screening albuminuria equivalent to the KDIGO stages A1 and A2. Moreover, our findings are limited to people with diabetes and high kidney risk, with the extent of any generalizability to nondiabetic kidney disease still unknown. Future trials are awaited (25,26). The CREDENCE study was stopped early on grounds of clear efficacy for the primary end point. This may limit the power to assess the effect of canagliflozin on secondary and safety outcomes.
Previous SGLT2 inhibitor trials have shown consistent effects on kidney and cardiovascular outcomes across different levels of albuminuria (27–29). However, these trials included few participants with severely increased albuminuria. We extend this observation to individuals with nephrotic-range albuminuria who experienced similar relative, and greater absolute, kidney benefits from canagliflozin. The consistent relative benefit seen across all levels of baseline albuminuria in the CREDENCE (15) and Canagliflozin Cardiovascular Assessment Study (CANVAS) Program trials (7,16) makes it reasonable to assume that absolute benefits would accrue in those at lower risk if followed for a longer time horizon, as would happen in clinical practice. Taken together, these findings provide treatment options for those with diabetes and nephrotic-range albuminuria. Ongoing SGLT2 inhibitor trials will provide complementary evidence for the effects of SGLT2 inhibitors on kidney and cardiovascular outcomes in those with nondiabetic kidney disease (26,30,31).
Disclosures
R. Agarwal reports employment with Indiana University, IU Heath Physicians, and VA Medical Center; research funding from GlaxoSmithKline; consultancy agreements with Abbvie, Akebia, Amgen, AstraZeneca, Bayer, Birdrock Bio, Boehringer Ingelheim, Celgene, Daiichi Sankyo, Eli Lilly, GlaxoSmithKline, Ironwood Pharmaceuticals, Johnson and Johnson, Merck, Novartis, Opko, Otsuka, Reata, Relypsa, Sandoz, Sanofi, Takeda, and ZS Pharma; honoraria from Abbvie, Akebia, Amgen, AstraZeneca, Bayer, Birdrock Bio, Boehringer Ingelheim, Celgene, Daiichi Sankyo, Eli Lilly, GlaxoSmithKline, Ironwood Pharmaceuticals, Johnson and Johnson, Merck, Novartis, Opko, Otsuka, Reata, Relypsa, Sandoz, Sanofi, Takeda, and ZS Pharma; serving as a scientific advisor or member of Akebia, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Hypertension, Ironwood Pharmaceuticals, Johnson and Johnson, Journal of the American Society of Hypertension, Kidney Disease: Improving Global Outcomes, Reata, Relypsa, Sanofi, and Seminars in Dialysis; and has served as Associate Editor of the American Journal of Nephrology and Nephrology, Dialysis, and Transplantation, and as an author on UpToDate. G.L. Bakris reports research funding paid to the University of Chicago for serving as principal investigator on national clinical trials for AbbVie, Bayer, CVRX, Janssen, Novo Nordisk, and Takeda; consultancy for AbbVie, AstraZeneca, Alynalam, AstraZeneca, Bayer, Boehringer Ingelheim, Cyclerion Therapeutics, Daiichi Sankyo, Eli Lilly, Ionis, Janssen, KBP Biosciences, Merck, Novo Nordisk, Nxstage Medical, Pfizer, Relypsa, Sanofi, Takeda, and Vascular Dynamics; honoraria from Alynalam, AstraZeneca, Ionis, Merck, Novo Nordisk, and Relypsa; serving on a steering committee for Vascular Dynamics; and serving as Editor of the American Journal of Nephrology and Nephrology, Editor-in-Chief of UpToDate, and Nephrology and Hypertension Section Editor of UpToDate, and Associate Editor of Diabetes Care, Hypertension Research, and Nephrology, Dialysis, and Transplantation. C.P. Cannon reports research grants from Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Janssen, Merck, Pfizer, and Takeda; and consulting fees from Aegerion, Alnylam, Amarin, Amgen, Applied Therapeutics, AstraZeneca, Ascendia, Boehringer Ingelheim, Bristol-Myers Squibb, Corvidia, Eisai, Eli Lilly, GlaxoSmithKline, HLS Therapeutics, Innovent, Janssen, Kowa, Merck, Pfizer, Regeneron, and Sanofi. D.M. Charytan reports fees paid by Janssen Pharmaceuticals to the Baim Institute for work on the CREDENCE trial Steering Committee and as Scientific Lead; salary support from the Baim Institute for this work through October 2018, after which time, he received consulting fees from the Baim Institute; consulting for Amgen, Daiichi Sankyo, Douglas and London, Eli Lilly/Boehringer Ingelheim, Fresenius, Gilead, GlaxoSmithKline, Janssen (steering committee), Medtronic/Covidien, Merck, Novo Nordisk, and Zoll; serving on Data Safety and Monitoring boards for AstraZeneca and Allena Pharmaceuticals; serving on a Clinical Endpoint Committee (CEC) for Merck and PLC Medical; research funding from Amge, Bioporto (clinical trial support), Gilead, Medtronic (clinical trial support), and NovoNordisk; serving as an Associate Editor of CJASN; and expert witness fees related to proton pump inhibitors. D. de Zeeuw reports serving on advisory boards and/or as speaker for Bayer, Boehringer Ingelheim, Fresenius, Mitsubishi-Tanabe, Mundipharma, and Retrophin; serving on steering committees and/or as a speaker for AbbVie and Janssen; and serving on Data Safety and Monitoring Committees for Bayer. T. Greene reports consulting fees from AstraZeneca, Durect, Invokana, Janssen, Novartis, and Pfizer; and research funding from AstraZeneca, Boehringer Ingelheim, CSL Behring, and Vertex. M. Jardine is supported by a cofunded National Health and Medical Research Council Career Development Fellowship and National Heart Foundation Future Leader Fellowship; is responsible for research projects that have received unrestricted funding from Amgen, Baxter, Eli Lilly, and Merck Sharpe Dohme (MSD); reports receiving research funding from Amgen, Baxter, CSL, Eli Lilly, Dimerix, Gambro, and MSD; reports receiving honoraria from Amgen, AstraZeneca, Janssen, Roche, and Vifor; reports serving as a scientific advisor or member of Akebia, AstraZeneca, Baxter, Bayer, Boehringer Ingelheim, CSL Behring, Janssen, and Vifor; serves on a steering committee sponsored by CSL; has served on advisory boards sponsored by Akebia, Baxter, Boehringer Ingelheim, and Vifor; and has spoken at scientific meetings sponsored by Janssen; with any consultancy, honoraria, or travel support paid to her institution. H.J. Lambers Heerspink reports employment with University Medical Center Groningen; has served as a consultant for Abbvie, Astellas, AstraZeneca, Boehringer Ingelheim, Fresenius, Gilead, Janssen, Merck, and Mitsubishi-Tanabe; and reports grant support from Abbvie, AstraZeneca, Boehringer Ingelheim, and Janssen. C. Hockham and Q. Li report employment with The George Institute for Global Health. A. Levin reports employment with University of British Columbia and British Columbia Provincial Renal Agency; serves as a scientific advisor to AstraZeneca, Boehringer Ingelheim, and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK); is on the Data Safety and Monitoring Board for NIDDK, Kidney Precision Medicine, University of Washington Kidney Research Institute Scientific Advisory Committee; and is funded by Canadian Institute of Health Research and Kidney Foundation of Canada. She has received fees for time as CREDENCE National Coordinator from Janssen, directed to her academic team. She reports consultancy agreements with Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Johnson and Johnson/Jansen, Reata, and Retrophin; receiving research funding from AstraZeneca, Boehringer Ingelheim, Janssen, Johnson and Johnson, Merck, National Institutes of Health (NIH), NIDDK, Ortho Biotech, Otsuka, and Oxford Clinical Trials; and serving as a scientific advisor or member of Bayer, Canadian Journal of Kidney Health and Disease, Certa, Chinook Therapeutics, Canadian Institutes of Health Research, The George Institute, Johnson and Johnson, Kidney Foundation of Canada, NIH NIDDK, Otsuka, Reata, and Retrophin. J.-W. Li has nothing to disclose. K. W. Mahaffey has received research support from Afferent, American Heart Association, Amgen, Apple, AstraZeneca, Bayer, Cardiva Medical, Daiichi, Eidos, Ferring, Google (Verily), Johnson and Johnson, Luitpold, Medtronic, Merck, NIH, Novartis, Sanifit, Sanofi, St. Jude, and Tenax; and has served as a consultant (speaker fees for continuing medical education events only) for Abbott, Ablynx, Amgen, Anthos, AstraZeneca, Baim Institute, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, CSL Behring, Elsevier, GlaxoSmithKline, Inova, Intermountain Health, Johnson and Johnson, MedErgy, Medscape, Mitsubishi, Mount Sinai, Mundi Pharma, Myokardia, NIH, Novartis, Novo Nordisk, Otsuka, Portola, Radiometer, Regeneron, Sanofi, SmartMedics, Springer Publishing, Theravance, and University of California, San Fancisco. B. Neal reports employment with The George Institute for Global Health and University of New South Wales Sydney; is supported by an Australian National Health and Medical Research Council Principal Research Fellowship; has consultancy agreements with Janssen Research and Development; holds a research grant for this study from Janssen Research and Development; has received research funding from Bupa Australia; has held research grants for other large-scale cardiovascular outcome trials from Merck Schering Plough, Roche, and Servier; has received honoraria (all paid to institution) from AstraZeneca, Janssen Research and Development, Johnson and Johnson, Merck Sharpe Dohme, Mitsubishi Tanabe Pharma Corporation, Mundipharma International, Novartis, Pfizer, Roche, and Servier; and his institution has received consultancy, honoraria, or travel support for contributions he has made to advisory boards and/or the continuing medical education programs of Abbott, Janssen, Novartis, Pfizer, Roche, and Servier. B.L. Neuen reports employment with The George Institute for Global Health and New South Wales Health; has received travel support from Janssen and consultancy fees from Bayer, with all honoraria paid to his institution; and serves as a Senior Editorial Fellow of JASN. R. Oh is a full-time employee of Janssen Research and Development and has ownership interest in Johnson and Johnson. M. Oshima is supported by the Japan Society for the Promotion of Science Program for Fostering Globally Talented Researchers. V. Perkovic reports employment with University of New South Wales Sydney, The Royal North Shore Hospital; consultancy agreements with AbbVie, Astellas, AstraZeneca, Baxter, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Chinook, Durect, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Merck, Mitsubishi Tanabe, Mundipharma, Novartis, Novo Nordisk, Pfizer, Pharmalink, Relypsa, Retrophin, Roche, Sanofi, Servier, and Vitae; receiving research funding from Pfizer (supplied drug and seed funding for the Therapeutic Evaluation of Steroids in IgA Nephropathy Global Study) and GlaxoSmithKline; receiving honoraria from AbbVie, Astellas, AstraZeneca, Bayer, Baxter, Boehringer Ingelheim, Bristol-Myers Squibb, Chinook, Durect, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Merck, Mitsubishi Tanabe, Mundipharma, Novartis, Novo Nordisk, Pfizer, Pharmalink, Relypsa, Retrophin, Roche, Sanofi, Servier, and Vitae; serving/served on steering committees for trials funded by AbbVie, AstraZeneca, Bayer, Boehringer Ingelheim, Chinook, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Novartis, Novo Nordisk, and Retrophin; and serving as Board Director of George Clinical, George Institute, Garvan Institute, Mindgardens Network, Children’s Cancer Institute, and Victor Chang Cardiac Research Institute. C. Pollock reports honoraria for serving on advisory boards and as a speaker for AstraZeneca, Boehringer Ingelheim/Eli Lilly, and Merck Sharpe Dohme; honoraria from Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lily, MSD, Sanofi, Novartis, Otsuka, and Vifor; copyright as book editor from Elsevier; serving as a scientific advisor or member of AstraZeneca, Boehringer Ingelheim, Eli Lily, Janssen Cilag, Merck Sharp Dohme, Novartis, Otsuka, Pharmaxis, Vifor; speakers bureau for AstraZeneca, Janssen Cilag, Otsuka, and Vifor; and serving as Chair of Kidney Health Australia, Chair of the New South Wales Bureau of Health Information, Deputy Chair Australian Organ Tissue and Transplant Authority, Chair of the New South Wales Cardiovascular Research Network, and Director of Certa Therapeutics. D. C. Wheeler reports consultancy agreements with Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, GlaxoSmithKline, Gilead, Janssen, Mitsubishi, Mundipharma, Napp, Ono Pharma, Tricida, and Vifor Fresenius; receiving honoraria from Amgen, Astellas, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Janssen, Mundipharma, Merck Sharpe Dohme, Napp, Ono Pharma, Reata, and Vifor Fresenius; speakers bureau for Amgen, Astellas, Janssen, Mundipharma, Napp, Merk Sharpe Dohme, and Vifor Fresenius; honorary professorial fellow of George Institute for Global Health; and other interests/relationships with Kidney Health Initiative. H. Zhang reports consultancy agreements with Janssen and Novartis; and serving as Board Committee Member of Chinese Society of Nephrology, Board Committee Member of Nephrology in Chinese Medical Doctor Association, Vice Director of the Nephrology Committee of Beijing Society of Medicine, member of International Society of Nephrology Advancing Clinical Trials Committee, and member of International Society of Nephrology Global Outreach Sister Renal Centre Committee. Z. Zhou reports employment with The George Institute for Global Health, University of New South Wales, Sydney, Australia, and Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China; and receiving a Scientia PhD Scholarship from the University of New South Wales, Sydney. B. Zinman has served as a consultant and received honoraria from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, MSD, Novo Nordisk, and Sanofi; and has received grant support from Boehringer Ingelheim, Novo Nordisk, and AstraZeneca.
Funding
The CREDENCE study was sponsored by Janssen Research and Development, LLC.
Data Sharing Statement
Deidentified individual-level data from this study, together with data dictionaries, will be made available in the public domain via the Yale University Open Data Access Project (YODA; http://yoda.yale.edu/) once the product and relevant indication studied have been approved by regulators in the United States and European Union and the study has been completed for 18 months, with no defined end date. The study protocol and statistical analysis plan are already in the public domain. All requests for data access will need to be made via the YODA Project. Data can be requested by any external researcher who submits a legitimate scientific proposal that promotes research that may advance science or lead to improvements in individual and public health and health care delivery. All proposals will be reviewed by the YODA Project. Once approved, data will be shared via a secure Safe Harbor platform. Requestors must sign a data use agreement before receiving the data.
Supplementary Material
Acknowledgments
We thank all participants, investigators, and trial teams for their participation in the trial. The CREDENCE study was sponsored by Janssen Research & Development, LLC, and was conducted collaboratively by the sponsor, an academic-led steering committee, and an academic research organization, George Clinical. Analyses were performed by George Clinical and independently confirmed by the sponsor. Medical writing support was provided by Dr. Elizabeth Meucci, of MedErgy, and was funded by Janssen Global Services, LLC. Canagliflozin has been developed by Janssen Research & Development, LLC, in collaboration with Mitsubishi Tanabe Pharma Corporation. All authors provided input through the development of this manuscript and approved the final version for submission. Because Dr. David M. Charytan is an Associate Editor of CJASN, he was not involved in the peer review process for this manuscript. Another editor oversaw the peer review and decision-making process for this manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.15260920/-/DCSupplemental.
Supplemental Table 1. Relative and absolute effects of canagliflozin on additional kidney, cardiovascular, and mortality outcomes by baseline UACR.
Supplemental Table 2. Relative and absolute effects of canagliflozin on additional kidney, cardiovascular, and mortality outcomes in the overall CREDENCE cohort. These results have been previously published (1).
Supplemental Table 3. Effects of canagliflozin on eGFR slope (total, acute, and chronic) by baseline UACR. The acute, chronic, and total mean change in eGFR and SEM in each treatment group (canagliflozin or placebo), according to UACR category, are presented.
Supplemental Table 4. Relative and absolute effects of canagliflozin on kidney safety outcomes by baseline UACR.
Supplemental Table 5. Effects of canagliflozin on the intermediate outcomes of HbA1c, body weight, and systolic BP by baseline UACR.
References
- 1.Lv J, Perkovic V, Foote CV, Craig ME, Craig JC, Strippoli GF: Antihypertensive agents for preventing diabetic kidney disease. Cochrane Database Syst Rev 12: CD004136, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maione A, Navaneethan SD, Graziano G, Mitchell R, Johnson D, Mann JF, Gao P, Craig JC, Tognoni G, Perkovic V, Nicolucci A, De Cosmo S, Sasso A, Lamacchia O, Cignarelli M, Manfreda VM, Gentile G, Strippoli GF: Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers and combined therapy in patients with micro- and macroalbuminuria and other cardiovascular risk factors: A systematic review of randomized controlled trials. Nephrol Dial Transplant 26: 2827–2847, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Jafar TH, Stark PC, Schmid CH, Landa M, Maschio G, Marcantoni C, de Jong PE, de Zeeuw D, Shahinfar S, Ruggenenti P, Remuzzi G, Levey AS; AIPRD Study Group : Proteinuria as a modifiable risk factor for the progression of non-diabetic renal disease. Kidney Int 60: 1131–1140, 2001. [DOI] [PubMed] [Google Scholar]
- 4.The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia): Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. Lancet 349: 1857–1863, 1997 [PubMed] [Google Scholar]
- 5.Gansevoort RT, Meijer E, Chapman AB, Czerwiec FS, Devuyst O, Grantham JJ, Higashihara E, Krasa HB, Ouyang J, Perrone RD, Torres VE; TEMPO 3:4 Investigators: Albuminuria and tolvaptan in autosomal-dominant polycystic kidney disease: Results of the TEMPO 3:4 trial. Nephrol Dial Transplant 31: 1887–1894, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnott C, Li Q, Kang A, Neuen BL, Bompoint S, Lam CSP, Rodgers A, Mahaffey KW, Cannon CP, Perkovic V, Jardine MJ, Neal B: Sodium-glucose cotransporter 2 inhibition for the prevention of cardiovascular events in patients with type 2 diabetes mellitus: A systematic review and meta-analysis. J Am Heart Assoc 9: e014908, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neuen BL, Young T, Heerspink HJL, Neal B, Perkovic V, Billot L, Mahaffey KW, Charytan DM, Wheeler DC, Arnott C, Bompoint S, Levin A, Jardine MJ: SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: A systematic review and meta-analysis. Lancet Diabetes Endocrinol 7: 845–854, 2019 [DOI] [PubMed] [Google Scholar]
- 8.Heerspink HJ, Desai M, Jardine M, Balis D, Meininger G, Perkovic V: Canagliflozin slows progression of renal function decline independently of glycemic effects. J Am Soc Nephrol 28: 368–375, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU: The definition, classification, and prognosis of chronic kidney disease: A KDIGO controversies conference report [published correction appears in Kidney Int 80: 1000, 2011 10.1038/ki.2011.310]. Kidney Int 80: 17–28, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Woodward M, Hirakawa Y, Kengne AP, Matthews DR, Zoungas S, Patel A, Poulter N, Grobbee R, Cooper M, Jardine M, Chalmers J; ADVANCE Collaborative Group: Prediction of 10-year vascular risk in patients with diabetes: The AD-ON risk score. Diabetes Obes Metab 18: 289–294, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Ninomiya T, Perkovic V, de Galan BE, Zoungas S, Pillai A, Jardine M, Patel A, Cass A, Neal B, Poulter N, Mogensen CE, Cooper M, Marre M, Williams B, Hamet P, Mancia G, Woodward M, Macmahon S, Chalmers J; ADVANCE Collaborative Group: Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol 20: 1813–1821, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, De Zeeuw D, Hostetter TH, Lameire N, Eknoyan G: Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 67: 2089–2100, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME Investigators: Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 373: 2117–2128, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS; DECLARE–TIMI 58 Investigators: Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 380: 347–357, 2019 [DOI] [PubMed] [Google Scholar]
- 15.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW; CREDENCE Trial Investigators: Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 380: 2295–2306, 2019 [DOI] [PubMed] [Google Scholar]
- 16.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR; CANVAS Program Collaborative Group: Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 377: 644–657, 2017 [DOI] [PubMed] [Google Scholar]
- 17.Jardine MJ, Mahaffey KW, Neal B, Agarwal R, Bakris GL, Brenner BM, Bull S, Cannon CP, Charytan DM, de Zeeuw D, Edwards R, Greene T, Heerspink HJL, Levin A, Pollock C, Wheeler DC, Xie J, Zhang H, Zinman B, Desai M, Perkovic V; CREDENCE Study Investigators: The Canagliflozin and Renal Endpoints in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) study rationale, design, and baseline characteristics. Am J Nephrol 46: 462–472, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration): A new equation to estimate glomerular filtration rate [published correction appears in Ann Intern Med 155: 408, 2011]. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sumida K, Nadkarni GN, Grams ME, Sang Y, Ballew SH, Coresh J, Matsushita K, Surapaneni A, Brunskill N, Chadban SJ, Chang AR, Cirillo M, Daratha KB, Gansevoort RT, Garg AX, Iacoviello L, Kayama T, Konta T, Kovesdy CP, Lash J, Lee BJ, Major RW, Metzger M, Miura K, Naimark DMJ, Nelson RG, Sawhney S, Stempniewicz N, Tang M, Townsend RR, Traynor JP, Valdivielso JM, Wetzels J, Polkinghorne KR, Heerspink HJL; Chronic Kidney Disease Prognosis Consortium: Conversion of urine protein-creatinine ratio or urine dipstick protein to urine albumin-creatinine ratio for use in chronic kidney disease screening and prognosis: An individual participant-based meta-analysis. Ann Intern Med 173: 426–435, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jardine MJ, Zhou Z, Mahaffey KW, Oshima M, Agarwal R, Bakris G, Bajaj HS, Bull S, Cannon CP, Charytan DM, de Zeeuw D, Di Tanna GL, Greene T, Heerspink HJL, Levin A, Neal B, Pollock C, Qiu R, Sun T, Wheeler DC, Zhang H, Zinman B, Rosenthal N, Perkovic V; CREDENCE Study Investigators: Renal, cardiovascular, and safety outcomes of canagliflozin by baseline kidney function: A secondary analysis of the CREDENCE randomized trial. J Am Soc Nephrol 31: 1128–1139, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jardine MJ, Hata J, Woodward M, Perkovic V, Ninomiya T, Arima H, Zoungas S, Cass A, Patel A, Marre M, Mancia G, Mogensen CE, Poulter N, Chalmers J; ADVANCE Collaborative Group: Prediction of kidney-related outcomes in patients with type 2 diabetes. Am J Kidney Dis 60: 770–778, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Gansevoort RT, Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J; Chronic Kidney Disease Prognosis Consortium: Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int 80: 93–104, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey A, de Jong P, Gansevoort RT, van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey AS, de Jong PE, Gansevoort RT, Levey A, El-Nahas M, Eckardt KU, Kasiske BL, Ninomiya T, Chalmers J, Macmahon S, Tonelli M, Hemmelgarn B, Sacks F, Curhan G, Collins AJ, Li S, Chen SC, Hawaii Cohort KP, Lee BJ, Ishani A, Neaton J, Svendsen K, Mann JF, Yusuf S, Teo KK, Gao P, Nelson RG, Knowler WC, Bilo HJ, Joosten H, Kleefstra N, Groenier KH, Auguste P, Veldhuis K, Wang Y, Camarata L, Thomas B, Manley T; Chronic Kidney Disease Prognosis Consortium: Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int 79: 1341–1352, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Heerspink HJL, Stefansson BV, Chertow GM, Correa-Rotter R, Greene T, Hou FF, Lindberg M, McMurray J, Rossing P, Toto R, Langkilde AM, Wheeler DC; DAPA-CKD Investigators: Rationale and protocol of the dapagliflozin and prevention of adverse outcomes in chronic kidney disease (DAPA-CKD) randomized controlled trial. Nephrol Dial Transplant 35: 274–282, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herrington WG, Preiss D, Haynes R, von Eynatten M, Staplin N, Hauske SJ, George JT, Green JB, Landray MJ, Baigent C, Wanner C: The potential for improving cardio-renal outcomes by sodium-glucose co-transporter-2 inhibition in people with chronic kidney disease: A rationale for the EMPA-KIDNEY study. Clin Kidney J 11: 749–761, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mosenzon O, Wiviott SD, Cahn A, Rozenberg A, Yanuv I, Goodrich EL, Murphy SA, Heerspink HJL, Zelniker TA, Dwyer JP, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Kato ET, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS, Raz I: Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: An analysis from the DECLARE-TIMI 58 randomised trial. Lancet Diabetes Endocrinol 7: 606–617, 2019 [DOI] [PubMed] [Google Scholar]
- 28.Neuen BL, Ohkuma T, Neal B, Matthews DR, de Zeeuw D, Mahaffey KW, Fulcher G, Li Q, Jardine M, Oh R, Heerspink HL, Perkovic V: Effect of canagliflozin on renal and cardiovascular outcomes across different levels of albuminuria: Data from the CANVAS program. J Am Soc Nephrol 30: 2229–2242, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wanner C, Lachin JM, Inzucchi SE, Fitchett D, Mattheus M, George J, Woerle HJ, Broedl UC, von Eynatten M, Zinman B; EMPA-REG OUTCOME Investigators: Empagliflozin and clinical outcomes in patients with type 2 diabetes mellitus, established cardiovascular disease, and chronic kidney disease. Circulation 137: 119–129, 2018 [DOI] [PubMed] [Google Scholar]
- 30. ClinicalTrials.gov: Effects of dapagliflozin in non-diabetic patients with proteinuria (DIAMOND), 2017. Available at: https://clinicaltrials.gov/ct2/show/NCT03190694. Accessed May 11, 2019.
- 31. ClinicalTrials.gov: EMPA-KIDNEY (the study of heart and kidney protection with empagliflozin), 2018. Available at: https://clinicaltrials.gov/ct2/show/NCT03594110. Accessed February 12, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.