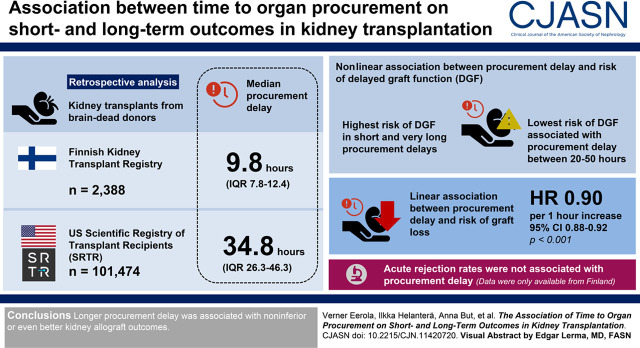
Visual Abstract
Keywords: delayed graft function, chronic allograft failure, acute rejection, kidney transplantation, Tissue and Organ Procurement
Abstract
Background and objectives
Transplant centers in Europe aim to minimize the time from brain death to organ procurement (procurement delay), but evidence to justify this is scarce. In the United States, procurement times are significantly longer. Our objective was to analyze how procurement delay associates with kidney allograft outcomes.
Design, setting, participants, & measurements
Kidney transplantations from brain-dead donors were retrospectively analyzed from the Finnish Kidney Transplant Registry and the Scientific Registry of Transplant Recipients in the United States. Multivariable models were adjusted with donor and recipient characteristics, and the relationship between procurement delay and outcomes was modeled with cubic spline functions.
Results
In total, 2388 and 101,474 kidney transplantations in Finland and the United States were included, respectively. The median procurement delay was 9.8 hours (interquartile range, 7.8–12.4) in Finland and 34.8 hours (interquartile range, 26.3–46.3) in the United States. A nonlinear association was observed between procurement delay and the risk of delayed graft function, with highest risk seen in short and very long procurement delays. In multivariable models, the lowest risk of delayed graft function was associated with procurement delay between 20 and 50 hours. In multivariable models, longer procurement delay was linearly associated with lower risk of graft loss (hazard ratio, 0.90/1 h longer; 95% confidence interval, 0.88 to 0.92; P<0.001). Acute rejection rates, for which data were only available from Finland, were not associated with procurement delay.
Conclusions
Longer procurement delay was associated with noninferior or even better kidney allograft outcomes.
Introduction
The vast majority of organ donations are carried out in brain-dead donors (donation after brain death [DBD]). Brain death causes excretion of cytokines (so-called “cytokine storm”), which leads to initial tachycardia and hypertension followed by a hypotensive phase. Cytokines increase oxygen consumption and inflammatory activation, and they may lead to increase in oxygen free radicals, which may cause cell damage (1–4). Furthermore, hypotensive phase may decrease oxygen supply to already compromised cells. This is further supported by data from animal experiments, in which prolongation of time after brain death has led to increased inflammation, coagulation, and organ dysfunction in kidneys (1,5–7). In addition, organ function is thought to deteriorate and eventually fail if procurement is excessively postponed. Because of these detrimental effects of brain death, it is generally considered that organs should be procured as soon as possible after brain death, and European practices aim to minimize time from brain death to organ cold perfusion (procurement delay). However, usually the cytokine storm settles within hours, and brain-dead organ donors are hemodynamically stable thereafter (8).
Contrary to these beliefs, some retrospective studies have demonstrated an advantageous correlation of longer time before organ retrieval in kidney transplant early function (9,10) and survival (9–11). These studies may have attributed to the increasingly longer procurement times in the United States, although retrospective and with some having small cohort size (9,11) and lack of adjustment for confounders (9). As such, procurement delays vary greatly between countries, and optimal time is currently unknown. Knowing the ideal procurement delay has great implications in transplantation logistics, work shifts, resource allocation, and, ultimately, patient and graft survival.
The aim of this study was to examine the association of procurement delay on kidney allograft early function and survival in two different transplant populations with different median times from brain death to organ procurement (Finland and the United States).
Materials and Methods
Donors and Patients
Consecutive deceased-donor kidney transplantations in Finland from June 2004 to December 2017 were included and followed until death, graft loss, or August 2018. The data were collected from the Finnish Kidney Transplant Registry and from donor medical documents. Only donors in whom the procurement was done within Finland were included, and kidneys that were received from other Scandiatransplant countries were excluded. Similarly, kidneys procured in Finland but sent for transplantation to another country were excluded. All transplantations in Finland are performed in the Helsinki University Hospital, wherefrom a team of transplant surgeons is also responsible for the procurement surgery in the whole country. All donations were from DBD. No donation after cardiac death (DCD) occurred in Finland during the study period, and kidney transplantations from a living donor were not included.
Kidney transplantations recorded in the Scientific Registry of Transplant Recipients (SRTR) database in the United States between January 2008 and August 2018 were included. This study used data from SRTR. The SRTR data system includes data on all donors, waitlisted candidates, and transplant recipients in the United States submitted by the members of the Organ Procurement and Transplantation Network (OPTN). The Health Resources and Services Administration, US Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. Standard Analysis Files (Q3 2018 release) and DEATHS file were used. Data of recipients were linked with donor data using unique donor identification numbers. Only kidneys transplanted from DBD donors were included, and kidneys from DCD or living donors were excluded.
This study was approved by the Institutional Review Board of Helsinki University Hospital (HUS/459/2018) and SRTR. The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the “Declaration of Istanbul on Organ Trafficking and Transplant Tourism.”
The following donor variables were collected for both Finnish and US cohorts: donor sex and age, the time of declaration of brain death, the start time of cold perfusion in organ procurement surgery, cause of death, body mass index, race, use of kidney machine perfusion, resuscitation, laboratory results, donor history of hypertension, diabetes, smoking, alcohol and drug use, hepatitis C status, and number of organs transplanted from the same donor in addition to kidneys. Data on discarded organs were not available for the purpose of this study. Regarding the recipient and transplantation, the following data were collected: recipient sex and age, cause of kidney failure, body mass index, history of hypertension, time in dialysis, maximum panel-reactive antibody status, HLA mismatches, graft cold ischemia time, delayed graft function (DGF), rejection episodes, and graft survival. Kidney Donor Profile Index (KDPI) was also calculated from these variables according to the OPTN/United Network for Organ Sharing mapping table of 2017 (12) for both Finnish and US donors. KDPI is calculated on the basis of donor age, body mass index, hypertension, diabetes, kidney function, cause of death, race, and status of hepatitis C virus. When KDPI was included in the models, the donor factors used to calculate KDPI were left out of the model due to possible multicollinearity. Procurement delay was defined as the time from the declaration of brain death to the start of in situ cold perfusion.
End Points
Long-term dependent outcome measure was graft survival, in which graft failure was defined as the need of retransplant, return to dialysis, or recipient death. DGF was defined as the need for dialysis during the first week after transplantation. Data regarding acute rejection (AR), available only for the Finnish cohort, were defined as the need for rejection treatment in a biopsy-proven borderline or acute cellular or antibody-mediated rejection.
Statistical Analyses
For presentation of the data, transplantations were divided into quartiles on the basis of the length of procurement delay. We report frequencies and percentages for categorical data and medians and interquartile ranges (IQRs) for continuous data. Numbers of patients with missing values are stated in Table 1.
Table 1.
Characteristics of donors and kidney transplantations in Finland from June 2004 to December 2017 and the United States from January 2008 to August 2018
| Baseline Characteristics | Finland: 2388 Kidney Transplantations; 1311 Donors | United States: 101,474 Kidney Transplantations; 58,792 Donors | Missing Finland, n (%) | Missing United States, n (%) |
|---|---|---|---|---|
| Donor time from brain death to organ perfusion, median (IQR), h | 9.8 (7.8–12.4) | 34.8 (26.3–46.3) | 0 | 0 |
| Donor age, median (IQR), yr | 55 (45–63) | 37 (23–50) | 0 | 0 |
| Donor BMI, median (IQR), kg/m2 | 24.8 (23.1–27.7) | 26.1 (22.6–30.5) | 2 (0.2) | 0 |
| Donor sex, men | 674 (56%) | 35,396 (60%) | 0 | 0 |
| Donor cardiac arrest prior to brain death | 229 (18%) | 4427 (8%) | 0 | 9 (0.0) |
| Donor medical conditions | ||||
| Hypertension | 377 (29%) | 15,481 (26%) | 0 | 375 (0.6) |
| Diabetes | 57 (4%) | 4031 (7%) | 0 | 0 |
| Donor cause of death | 0 | 0 | ||
| Cerebrovascular accident | 896 (68%) | 17,822 (30%) | ||
| Trauma | 340 (26%) | 22,009 (37%) | ||
| Anoxia | 37 (3%) | 17,447 (30%) | ||
| Other | 75 (6%) | 1514 (3%) | ||
| Donor need of inotropic medication | 1210 (92%) | 30,355 (52%) | 0 | 123 (0.2) |
| Donor creatinine, median (IQR), mg/dl | 0.7 (0.5–0.8) | 1.0 (0.7–1.3) | 1 (0.1) | 0 |
| KDPI,a median (IQR) | 60 (36–80) | 44 (20–69) | 4 (0.3) | 436 (0.7) |
| Donor organ yield,b median (IQR) | 3 (2–3) | 4 (3–4) | 0 | 0 |
| Cold ischemia time, median (IQR), h | 20.3 (17.2–23.4) | 15.5 (10.4–21.9) | 7 (0.3) | 1985 (2) |
| Recipient age, median (IQR), yr | 53 (42–62) | 54 (42–63) | 0 | 0 |
| Recipient BMI, median (IQR), kg/m2 | 25.0 (22.1–28.1) | 27.4 (23.6–31.6) | 527 (22) | 2524 (3) |
| Recipient sex, men | 1566 (66%) | 60,867 (60%) | 0 | 0 |
| Retransplantation | 249 (10%) | 13,308 (13%) | 0 | 0 |
| Cause of kidney failure | 0 | 0 | ||
| Diabetic kidney disease | 659 (28%) | 25,722 (25%) | ||
| GN | 623 (26%) | 20,021 (20%) | ||
| Polycystic kidney disease | 427 (18%) | 7570 (8%) | ||
| Other | 679 (28%) | 47,702 (47%) | ||
| Unknown | 0 (0.0%) | 459 (0.5%) | ||
| Recipient time in dialysis before transplantation, median (IQR), mo | 22 (13–38) | 46 (23–73) | 8 (0.3) | 10,962 (11) |
| Preemptive transplantations | 0 (0.0%) | 12,343 (12%) | 0 | 958 (1) |
| No. of HLA A and B mismatches, median (IQR) | 2 (1–2) | 3 (2–4) | 0 | 738 (0.7) |
| No. of HLA DR mismatches, median (IQR) | 1 (0–1) | 1 (1–2) | 0 | 739 (0.7) |
| No. of HLA mismatches, median (IQR) | 3 (2–3) | 4 (3–5) | 0 | 739 (0.7) |
| Delayed graft function | 849 (36%) | 23,007 (23%) | 0 | 596 (0.6) |
| Acute rejection | 430 (18%) | NA | 0 | NA |
| Graft survival at 1 yr | 95% | 93% | 1 (0.0) | 0 |
| Graft survival at 3 yr | 90% | 85% | 1 (0.0) | 0 |
| Graft survival at 5 yr | 82% | 76% | 1 (0.0) | 0 |
| Graft survival at 10 yr | 61% | 50% | 1 (0.0) | 0 |
Median (interquartile range [IQR]) and n (%) are shown unless otherwise indicated. All variables were significantly different between the cohorts (P<0.001 for every variable; recipient age P=0.003). In graft survival, outcome is defined as death, retransplantation, or return to dialysis. BMI, body mass index; KDPI, Kidney Donor Profile Index; NA, not available.
Percentiles 0–100 indicating higher risk of graft failure relative to other kidneys with increasing percentage.
Organ yield from multiorgan donor: maximum of seven (heart, lungs, liver, pancreas, intestine, and two kidneys).
In the main analysis, we assessed the association between procurement delay (hours) and DGF, as well as procurement delay and kidney graft survival by fitting unadjusted and multivariable logistic regression models and Cox proportional hazards models, respectively. In multivariable analysis, we controlled for potential confounders, which we identified on the basis of directed acyclic graphs (13). For both DGF and graft survival, we considered KDPI, recipient age (years), diabetes, and dialysis vintage (months) as confounders (Supplemental Figure 1). Of these, all except diabetes were used in analysis as continuous variables. All models were fitted on the complete cases data formed by excluding observations with missing data on response variables and/or covariates. To account for the clustering nature of the data due to the relationship between kidneys from the same donor, we calculated cluster-robust SEMs of the estimates by using the Huber–White method (14).
As the logistic regression and Cox models involve the assumption of linearity for the continuous data, we used restricted cubic spline function to account for potentially nonlinear association between the outcome of interest and procurement delay, KDPI, recipient age, and dialysis vintage. We tested for nonlinearity and modeled the associations as either linear or nonlinear. Linear associations between procurement delay and the outcome of interest were reported using the odds ratio (OR) or hazard ratio (HR) with 95% confidence interval (95% CI), as appropriate. The associations assessed with spline function were reported by plotting the predicted probability of DGF or the predicted relative hazard of graft survival as a function of procurement delay. As the Cox regression model is on the basis of the assumption of proportional hazards, we tested for the potential nonproportionality. We accounted for the nonproportionality by splitting the follow-up time into smaller intervals and by assessing time-varying coefficients and/or including interactions with follow-up time.
In addition, we performed several sensitivity analyses using the US data, which included enough observations for stratified analysis. We checked for consistency of the results with respect to implementation of kidney allocation system (KAS) in December 2014, number of organs transplanted from the same donor (organ yield), and cold ischemia time by dividing observations into strata and repeating the main analysis within each stratum. The number of strata varied between two for KAS (before and after KAS) to four for organ yield (only kidney[s]; kidney[s]; or one, two, or more than two other organs) and cold ischemia time (<12, 12–18, 18–24, or ≥24 hours).
We set the significance level at 5%. All analyses were performed using either IBM SPSS version 25 for Windows (Armonk, NY), or R software, including survival and rms packages (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patients
Between June 1, 2004 and December 31, 2017, 2660 kidneys were procured in Finland, from which 74 were discarded and 198 were sent abroad, leaving 2388 kidney transplantations from 1356 donors in the final analyses.
Between January 1, 2008 and August 31, 2018, 125,595 kidney transplantations from 72,290 deceased donors were recorded in the SRTR database. Altogether, 20,874 kidney transplantations, which were from DCD donors, were excluded. Furthermore, procurement delay could not be determined in 2889 kidney transplant recipients because of missing date of brain death, and thus, these were excluded from the analysis. In addition, we excluded 349 transplantations with extreme (>120-hour) procurement delay values. The final cohort from the US SRTR database included 101,474 kidney transplantations.
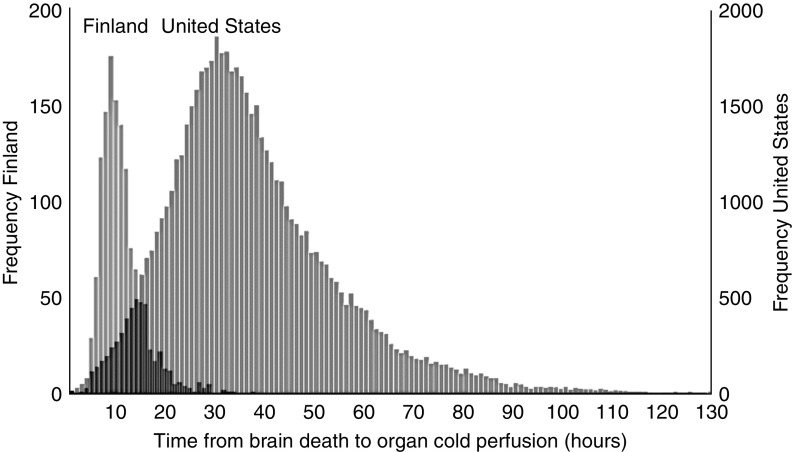
Basic donor and recipient characteristics among the cohorts from Finland and the United States are depicted in Table 1. The median procurement delay was 9.8 hours (IQR, 7.8–12.4) in Finland and 34.9 hours (IQR, 26.3–46.5) in the United States (Figure 1).
Figure 1.
Time from declaration of brain death to cold perfusion.
Patients were divided into quartiles on the basis of procurement delay. There were significant differences in basic donor and recipient characteristics between the delay quartiles (Table 2). Outcomes of patients, divided into quartiles, are presented in Table 3.
Table 2.
Differences in characteristics of quartiles of kidney transplantations in Finland from June 2004 to December 2017 and the United States from January 2008 to August 2008 by quartiles of time from brain death to organ perfusion (procurement delay)
| Variable | Finland: Quartiles of Time from Brain Death to Organ Perfusion in Organ Donors | United States: Quartiles of Time from Brain Death to Organ Perfusion in Organ Donors | ||||||
|---|---|---|---|---|---|---|---|---|
| Quartile, n of donors, n of recipients | First, n: 321, n: 596 | Second, n: 326, n: 598 | Third, n: 330, n: 597 | Fourth, n: 334, n: 597 | First, n: 14,689, n: 25,366 | Second, n: 14,686, n: 25,371 | Third, n: 14,711, n: 25,369 | Fourth, n: 14,706, n: 25,368 |
| Time from brain death to organ perfusion, median (IQR), h | 6.5 (5.8–7.2) | 8.7 (8.2–9.1) | 10.8 (10.2–11.5) | 15.1 (13.5–18.0) | 20.5 (16.1–23.8) | 30.6 (28.5–32.7) | 39.6 (37.1–42.6) | 57.1 (50.9–67.1) |
| KDPI, median (IQR), % | 76 (55–89) | 63 (43–81) | 57 (34–79) | 43 (23–62) | 53 (25–77) | 45 (20–69) | 41 (19–66) | 37 (17–62) |
| Donor age, median (IQR), yr | 61 (54–67) | 56 (47–63) | 53 (40–61) | 48 (37–56) | 43 (26–55) | 37 (23–50) | 35 (22–48) | 34 (22–47) |
| Donor BMI, median, kg/m2 | 25.7 (23.9–27.8) | 25.0 (23.3–27.6) | 24.7 (22.9–27.7) | 24.5 (22.9–27.5) | 26.4 (22.8–30.9) | 26.0 (22.4–30.6) | 26.0 (22.5–30.4) | 25.9 (22.5–30.2) |
| Donor creatinine, median, mg/dl | 0.68 (0.55–0.81) | 0.67 (0.53–0.80) | 0.63 (0.50–0.80) | 0.64 (0.53–0.80) | 1.00 (0.71–1.36) | 1.00 (0.70–1.30) | 1.00 (0.70–1.33) | 0.99 (0.70–1.40) |
| Donor hypertension | 125 (39%) | 98 (30%) | 82 (25%) | 72 (22%) | 4741 (32%) | 3948 (27%) | 3589 (25%) | 3203 (22%) |
| Donor cause of death: Cerebrovascular accident | 236 (74%) | 227 (70%) | 221 (67%) | 211 (63%) | 5367 (37%) | 4675 (32%) | 4189 (29%) | 3909 (27%) |
| Donor resuscitated | 53 (17%) | 60 (18%) | 56 (17%) | 60 (18%) | 1149 (8%) | 1142 (8%) | 1122 (8%) | 1014 (7%) |
| Donor diabetes | 23 (7%) | 15 (5%) | 6 (2%) | 13 (4%) | 1292 (9%) | 1028 (7%) | 903 (6%) | 808 (6%) |
| Donor organ yield,a median | 2 (2–2) | 3 (2–3) | 3 (2–3) | 3 (2–4) | 3 (2–4) | 3 (3–4) | 4 (3–5) | 4 (3–5) |
| Cold ischemia time, median, h | 20.9 (18.1–23.6) | 20.6 (18.0–23.8) | 20.3 (16.9–23.3) | 19.4 (14.5–22.9) | 16.3 (10.8–23.0) | 16.0 (11.1–21.1) | 14.1 (9.7–21.2) | 15.5 (10.2–21.5) |
| Recipient age, median, yr | 57 (48–65) | 54 (42–62) | 51 (40–60) | 48 (37–58) | 56 (45–64) | 54 (42–63) | 53 (41–62) | 52 (40–62) |
| Recipient diabetes | 147 (25%) | 165 (28%) | 162 (27%) | 185 (31%) | 9232 (36%) | 8594 (34%) | 8315 (33%) | 8167 (32%) |
| HLA MM total, median | 3 (2–3) | 3 (2–3) | 3 (2–3) | 3 (2–3) | 4 (3–5) | 4 (3–5) | 4 (3–5) | 4 (3–5) |
| Previous kidney transplant | 65 (11%) | 58 (10%) | 64 (11%) | 62 (10%) | 2960 (12%) | 3204 (13%) | 3481 (14%) | 3663 (14%) |
| Donor Black | NA | NA | NA | NA | 1987 (14%) | 2405 (16%) | 2634 (18%) | 2655 (18%) |
| Recipient Black | NA | NA | NA | NA | 8737 (34%) | 8499 (34%) | 8421 (33%) | 7545 (30%) |
| Recipient BMI, median, kg/m2 | 25.5 (22.7–28.5) | 25.2 (22.1–28.1) | 24.8 (22.3–28.0) | 24.3 (21.6–28.1) | 27.6 (23.9–31.7) | 27.5 (23.6–31.7) | 27.3 (23.5–31.6) | 27.1 (23.3–31.3) |
| Recipient time in dialysis, mo, median (IQR) | 23 (15–38) | 23 (14–38) | 22 (12–38) | 19 (12–36) | 43 (23–67) | 43 (23–69) | 46 (23–74) | 51 (25–83) |
Time from brain death to organ perfusion: time from declaration of brain death to in situ organ cold perfusion. Kidney Donor Profile Index (KDPI) is calculated from donor age, height, weight, history of diabetes and hypertension, cause of death, creatinine, and race. IQR, interquartile range; BMI, body mass index; MM, mismatch; NA, not available.
Organ yield from multiorgan donor: maximum of seven (heart, lungs, liver, pancreas, intestine, and two kidneys).
Table 3.
Outcomes of kidney transplantations in Finland from June 2004 to December 2017 and the United States from January 2008 to August 2008 by quartiles of time from brain death to organ perfusion (procurement delay)
| Variable | Finland: Quartiles of Time from Brain Death to Organ Perfusion in Organ Donors | United States: Quartiles of Time from Brain Death to Organ Perfusion in Organ Donors | ||||||
|---|---|---|---|---|---|---|---|---|
| Quartile, n of transplantations | First, n: 596 | Second, n: 598 | Third, n: 597 | Fourth, n: 597 | First, n: 14,635 | Second, n: 14,828 | Third, n: 14,838 | Fourth, n: 14,641 |
| Delayed graft function | 233 (39%) | 222 (37%) | 204 (34%) | 190 (32%) | 6059 (24%) | 5614 (22%) | 5458 (22%) | 5944 (24%) |
| Acute rejection | 115 (19%) | 107 (18%) | 106 (18%) | 102 (17%) | NA | NA | NA | NA |
| 1-yr graft survival | 93% | 96% | 94% | 95% | 92% | 93% | 94% | 94% |
| 5-yr graft survival | 75% | 85% | 84% | 84% | 74% | 76% | 77% | 78% |
| 10-yr graft survival | 54% | 63% | 62% | 69% | 47% | 50% | 53% | 51% |
| Follow-up, median (IQR), yr | 4.1 (2.0–8.0) | 5.2 (2.7–9.0) | 5.3 (2.3–8.8) | 4.1 (1.7–7.0) | 4.2 (2.0–6.9) | 3.8 (1.7–6.0) | 2.9 (1.0–5.0) | 1.9 (0.7–3.8) |
| Graft survival, median, yr | 10.9 | 13.0 | >13.5a | 13.5 | 9.7 | 10.0 | 10.3 | 10.1 |
Graft survival is defined as alive with a functioning graft. NA, not available for the US cohort; IQR, interquartile range.
Over 50% of grafts were still functioning at the end of follow-up. Median (>13, 5 years) was thus not reached in follow-up. In graft survival, outcome is defined as death, retransplantation, or return to dialysis.
Short-Term Outcomes
Complete DGF response and confounder data were available on 2371 (99% of the initial cohort) and 89,337 (88%) transplantations in the Finnish and the US cohorts, respectively. In the Finnish cohort, unadjusted analysis on the association between procurement delay and the probability of DGF demonstrated a linear relationship (crude OR, 0.85; 95% CI, 0.77 to 0.95; P=0.003; P=0.67 for nonlinearity) (Figure 2A), which was, however, attenuated after adjustment (adjusted OR, 0.99; 95% CI, 0.89 to 1.11; P=0.88). In contrast, a strongly nonlinear relationship was observed between procurement delay and the probability of DGF in the US cohort in both unadjusted (Figure 2B) (P<0.001 for nonlinear relationship) and multivariable analyses (Figure 2C) (P<0.001). The lowest probability of DGF was associated with procurement delay between 20 and 50 hours, with higher probability in shorter or very long procurement delays.
Figure 2.
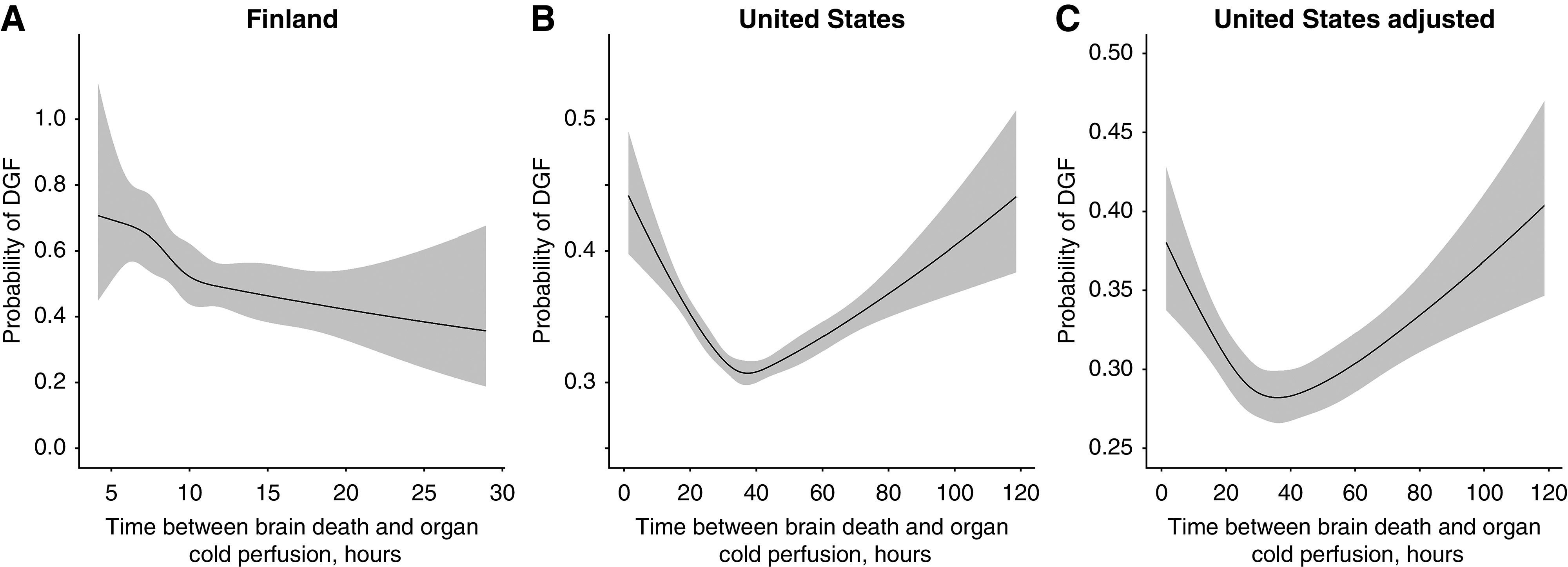
Association of procurement delay with delayed graft function. Gray areas correspond to 95% confidence bands. (A) Finnish cohort: P=0.67 for nonlinear association. (B) US cohort: P<0.001 for nonlinear association. (C) US cohort: P<0.001 for nonlinear association. Predicted values were calculated by setting confounder values to their median (Kidney Donor Profile Index =45, recipient age =53 years, dialysis vintage =45.57 months) or the most frequent category (no diabetes). DGF, delayed graft function.
Acute Rejection
AR rates were not affected by procurement delay in the Finnish cohort (Table 3) (OR, 0.92; 95% CI, 0.81 to 1.05; P=0.22 in unadjusted logistic regression). Data concerning ARs were not available for the US cohort.
Graft Survival
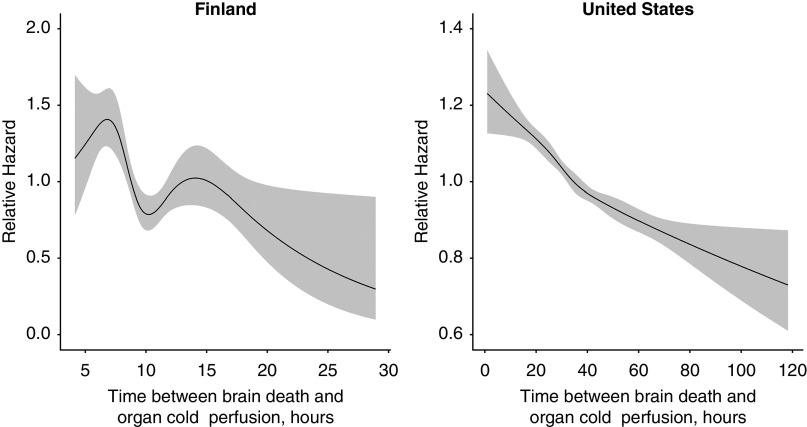
Complete response and confounder data for graft survival analyses were available on 2371 (99% of the initial cohort) and 89,814 (89%) transplantations in the Finnish and the US cohorts, respectively (Figure 3). Unadjusted graft survival rates in different quartiles of procurement delay are presented in Figure 3 and Table 3. In unadjusted analyses, we found a nonlinear association between procurement delay and kidney graft survival in the Finnish cohort (Figure 4) (P=0.005 for nonlinearity) but a linear association in the US cohort (Figure 4) (P=0.24 for nonlinearity), both showing lower hazard for graft loss associated with longer procurement delay. After adjustment for confounders, nonlinearity persisted in the Finnish cohort (P=0.03) but was not present in the US cohort (P=0.67 for nonlinearity). In the US cohort, modeling a linear association yielded the crude and adjusted HRs of 0.90 (95% CI, 0.88 to 0.92; P<0.001) and 0.93 (95% CI, 0.92 to 0.95; P<0.001) per 1-hour longer procurement delay, respectively. No significant interactions were found between procurement delay and recipient characteristics (P=0.05–0.73 and P=0.41–0.98), KDPI (P=0.96 and P=0.50), or cold ischemia time (P=0.14 and P=0.41) for the Finnish and the US cohorts, respectively.
Figure 3.
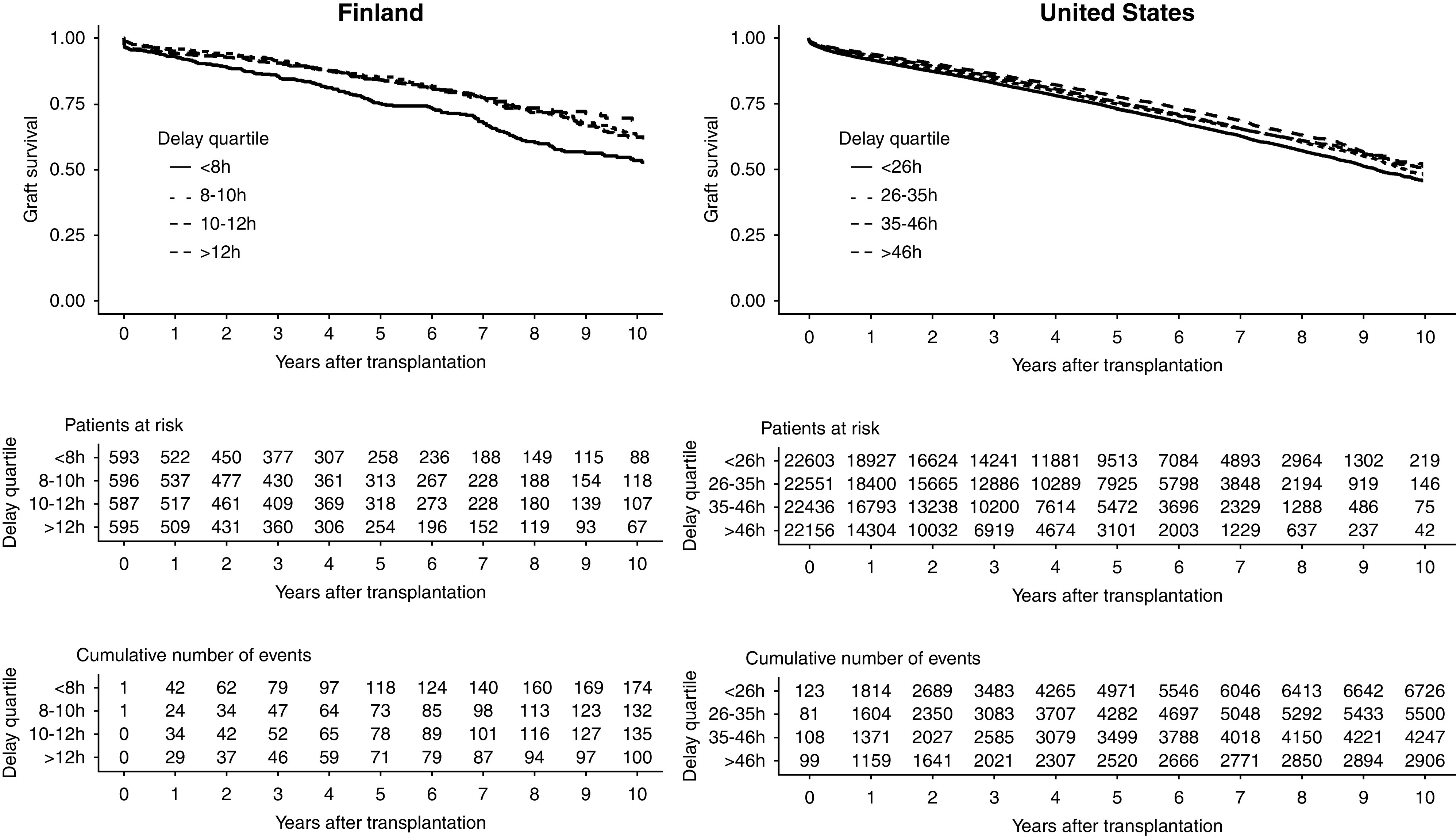
Longer procurement delay is associated with better graft survival. Outcome is defined as death, return to dialysis, or retransplantation.
Figure 4.
Relative hazard of graft failure or death associated with procurement delay. Gray areas correspond to 95% confidence bands: P=0.005 for nonlinear association in the Finnish cohort and P=0.11 for nonlinear association in the US cohort.
In the unadjusted and multivariable Cox models fitted to the Finnish cohort, we found the assumption of proportional hazards to hold for all variables except dialysis vintage. In the unadjusted and multivariable Cox models fitted to the US cohort, we found nonproportionality for all variables including procurement delay. After accounting for nonproportionality by splitting the follow-up time and including interaction between dialysis vintage and follow-up time, a nonlinear association between procurement delay and graft survival persisted (P=0.03 for nonlinearity) in the Finnish cohort. In the US cohort, the association between procurement delay and hazard attenuated over time (Supplemental Table 1). After restricting the follow-up to 1.5 years, we found the assumption of proportional hazards to hold and the association to be nonlinear in the unadjusted analysis (P=0.04) but linear in the multivariable analysis (HR, 0.90; 95% CI, 0.87 to 0.93).
In the US cohort, we performed sensitivity analysis in ten strata and found the results to be similar to that of the main analysis in all except two strata (Supplemental Figures 2–12). Before the implementation of the KAS in 2014, the median procurement delay was 31 hours (IQR, 24–40), compared with 42 hours (IQR, 32–55) after the implementation of KAS. However, in sensitivity analyses, the association between procurement delay and graft outcomes remained similar in both groups (Supplemental Figures 2 and 3). When the number of organs transplanted from the same donor was taken into account, no interaction was recorded between the organ yield and procurement delay (P=0.37–0.94) in either of the cohorts. In the sensitivity analysis within each stratum, the findings remained similar if maximum two other organs were transplanted in addition to kidneys from the same donor (Supplemental Figures 4–7). In the stratum including kidney transplants procured with more than two other transplanted organs, we found no statistically significant association between procurement delay and hazard (HR, 0.96; 95% CI, 0.91 to 1.00; P=0.08). When restricting cold ischemia time to 18–24 hours, we observed a nonlinear relationship (P=0.04) between procurement delay and hazard in multivariable analysis (Supplemental Figure 11).
Discussion
In this study of two countries, longer procurement delay was not associated with lower long-term kidney allograft survival. On the contrary, very short delay (<8 hours) was associated with worse graft survival, whereas increasing delay was associated with improved graft survival to a smaller extent but without apparent upper limit. Of note, the median procurement delay in the longest delay quartile in Finland (15 hours) was shorter than the median delay in the shortest quartile in the United States (21 hours). The probability of DGF was lowest between 20 and 50 hours of procurement delay, whereas procurement delay was not associated with AR rates in Finnish transplants. These results together imply a sweet spot of approximately 24–48 hours after brain death for organ procurement.
In concordance with our results, three earlier studies reported association of increasing procurement delay with improved kidney graft survival (9–11), while one smaller study did not find an association (15). Two of these studies found no association between procurement delay and the risk of DGF in multivariable models (10,11), whereas in our study, the risk of DGF was higher with very long procurement delays. AR was not affected by procurement delay (10,11), and none of the studies reported any benefits of shorter delay (9–11,15). All studies conducted have been retrospective and, therefore, possibly limited by the same confounding factors. Although the findings in this study are not novel, our study confirmed the findings of the previous studies in the largest cohort to date across two continents, with statistical methods taking account of the nonlinear association between procurement delay and graft outcomes. We also demonstrate that the association between slightly better outcomes and longer procurement delay remains also in the current era, where times from brain death to organ procurement in the United States are longer than in the earlier studies.
Median procurement delays vary markedly in the reported series. Shortest procurement delays were reported in Germany (median 8 hours [9]) and longest in the United States (median 24 hours [10]). Interestingly, Nijboer et al. (10) analyzed US data from 1994 to 2007, whereas our data included years 2008–2018, showing that the median delay increased from 24 to 35 hours between these two eras in the United States. The reasons behind the different delays in different countries and the increase in median delay in the United States over time seem logistics driven, but no clear additional harm was observed from prolonging procurement over the years.
Some of the concerns of longer procurement delay are the potential deterioration of the donor and the potential loss of viable organs. However, evidence to justify these concerns is scarce and possibly derives from hemodynamically unstable donors with insufficient donor management protocols in the past. A study from Brazil found over 30-hour procurement time to be a possible risk factor for losing a donor (16), whereas studies from the United States found no difference in organ procurement rates up to over 60 hours after brain death (8,17).
In heart transplantation, prolonged donor management time, but not time after brain death, has been associated with poorer outcome (18). However, newer studies found no significant survival difference in hearts (19) and a positive association of longer delay with better lung AR– and bronchiolitis obliterans–free survival (20). Interestingly, no animal studies favoring longer procurement were found.
A limitation of this study is that causality cannot be established from this observational registry analysis. Also, because of the retrospective and nonrandomized nature of the study, it is susceptible to residual confounding and distortion of the association due to nonrandom allocation. The latter cannot be controlled using standard statistical methods, such as model adjustment (21). Moreover, the studied associations seem to be more complex than in the simplified directed acyclic graph that was used to identify confounders. In fact, some covariates are likely to play the role of both confounder and mediator of the effect of interest. Only kidney transplantations were analyzed in this study, and further studies are needed to assess correlation of procurement delay on other organs. There are also several strengths. We tried to limit the bias by conducting multivariable analyses using multiple confounding variables, which should account for better-quality organs distributing unevenly between procurement times. Another strength is the sample size, which is five times larger than in the biggest earlier report (10). Analysis of two different cohorts from two continents with different organ procurement practices and large differences in times from brain death to organ procurement gives a broader perspective to this effect, although the relatively small sample size of the Finnish cohort limits our possibilities to adjust for confounding factors in this cohort in all analyses. The optimal delay seems to be over 8 hours, but procurement delays up to 50 hours do not seem harmful for kidney transplants. When considering the optimal timing of the organ procurement, multiple factors have to be taken into account. In these data, longer procurement delays were associated with increasing number of organs transplanted from the same donor, which is indeed expected as the logistics of both the organ procurement and allocation take more time. In addition to medical factors related to the outcome of the grafts, although beyond the scope of this study, economical costs of prolonging the procurement operation have to be considered.
The mechanisms by which longer procurement delay is associated with better graft survival can only be speculated. Brain death (and its associated cytokine storm) and ischemia may be considered as “hits” that affect kidney allografts negatively. In this two-hit theory, it could be beneficial for the kidney to recover from the first hit (brain death) before it is exposed to the second hit (ischemia). Also, protective mechanisms, such as heat-shock proteins and systemic mediators upregulated by ischemia, could play a role in allograft preservation (22–24). However, no serial data about the trend in urine output or kidney function in the donors were available, limiting our possibilities to further explore this hypothesis.
Disclosures
A. But reports employment with the University of Helsinki. V. Eerola reports employment with Helsinki University and Helsinki University Hospital. I. Helanterä reports employment with Helsinki University Central Hospital; consultancy agreements with Aplagon, Astellas, Hansa Biopharma, and Novartis; receiving grants from Finska Läkaresällskapet and Helsinki University Hospital; and serving as an editorial fellow for American Journal of Transplantation. H. Isoniemi, M. Lempinen, H. Mäkisalo, and A. Nordin report employment with Helsinki University Hospital. V. Sallinen reports employment with Helsinki University Hospital; receiving grants from the Academy of Finland, the Finnish Cancer Foundation (Syöpäsäätiö), the Finnish Gastroenterological Society, the Finnish Surgical Society, and Finska Läkaresällskapet; and receiving lecture fees from the City of Vantaa, the Finnish Gastroenterological Society, and the University of Helsinki, outside the submitted work. V. Sallinen reports serving as an editor of BJS Open, an editor of Scandinavian Journal of Surgery, and an editor of Duodecim.
Funding
This study was funded by grants from Helsinki University Hospital (Valtion Tutkimusrahoitus; to I. Helanterä, H. Isoniemi, and V. Sallinen) and Finska Läkaresällskapet (to I. Helanterä and V. Sallinen).
Supplementary Material
Acknowledgments
We thank the transplantation coordinators Ms. Siv Ansa, Mr. Eero Hartikka, Mr. Heikki Norio, Ms. Carola Schaumann, and Ms. Leena Toivonen from Helsinki University Hospital and Ms. Nina Ask for their help with the transplantation registry of Finland.
The data reported here have been supplied by the Hennepin Healthcare Research Institute as the contractor for SRTR. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by SRTR or the US Government.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Time to Procurement and Post-Kidney Transplant Outcomes: How Do We Provide a Personalized Medicine Approach to Optimizing Organ Donation?” on pages 340–342.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.11420720/-/DCSupplemental.
Supplemental Table 1. US cohort: time-dependent hazard ratio.
Supplemental Figure 1. Graphical presentation of confounding and mediators in our study.
Supplemental Figure 2. The relative hazard predicted from the univariate model with a restricted cubic spline. Sensitivity analysis before KAS, US cohort.
Supplemental Figure 3. The relative hazard predicted from the univariate model with a restricted cubic spline. Sensitivity analysis after KAS, US cohort.
Supplemental Figure 4. The relative hazard predicted from the univariate model with a restricted cubic spline. Sensitivity analysis of organ yield, kidneys only, US cohort.
Supplemental Figure 5. The relative hazard predicted from the univariate model with a restricted cubic spline. Sensitivity analysis of organ yield, kidneys and one other organ, US cohort.
Supplemental Figure 6. The relative hazard predicted from the univariate model with a restricted cubic spline. Sensitivity analysis of organ yield, kidneys and two other organs, US cohort.
Supplemental Figure 7. The relative hazard predicted from the univariate model with a restricted cubic spline. Sensitivity analysis of organ yield, kidneys and more than two other organs, US cohort.
Supplemental Figure 8. The relative hazard predicted from the univariate model with a restricted cubic spline. Sensitivity analysis of cold ischemia time <12h, US cohort.
Supplemental Figure 9. The relative hazard predicted from the multivariable model with a restricted cubic spline. Sensitivity analysis of cold ischemia time <12h, US cohort.
Supplemental Figure 10. The relative hazard predicted from the univariate model with a restricted cubic spline. Sensitivity analysis of cold ischemia time 12 to 18 hours, US cohort.
Supplemental Figure 11. The relative hazard predicted from the univariate model with a restricted cubic spline. Sensitivity analysis of cold ischemia time 18 to 24 hours, US cohort.
Supplemental Figure 12. The relative hazard predicted from the univariate model with a restricted cubic spline. Sensitivity analysis of cold ischemia time ≥24 hours, US cohort.
References
- 1.van Der Hoeven JAB, Ter Horst GJ, Molema G, de Vos P, Girbes ARJ, Postema F, Freund RL, Wiersema J, van Schilfgaarde R, Ploeg RJ: Effects of brain death and hemodynamic status on function and immunologic activation of the potential donor liver in the rat. Ann Surg 232: 804–813, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Hoeven JAB, Molema G, Ter Horst GJ, Freund RL, Wiersema J, van Schilfgaarde R, Leuvenink HGD, Ploeg RJ: Relationship between duration of brain death and hemodynamic (in)stability on progressive dysfunction and increased immunologic activation of donor kidneys. Kidney Int 64: 1874–1882, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Bos EM, Leuvenink HGD, van Goor H, Ploeg RJ: Kidney grafts from brain dead donors: Inferior quality or opportunity for improvement? Kidney Int 72: 797–805, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Pratschke J, Weiss S, Neuhaus P, Pascher A: Review of nonimmunological causes for deteriorated graft function and graft loss after transplantation. Transpl Int 21: 512–522, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Pratschke J, Wilhelm MJ, Laskowski I, Kusaka M, Beato F, Tullius SG, Neuhaus P, Hancock WW, Tilney NL: Influence of donor brain death on chronic rejection of renal transplants in rats. J Am Soc Nephrol 12: 2474–2481, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Schuurs TA, Morariu AM, Ottens PJ, ’t Hart NA, Popma SH, Leuvenink HGD, Ploeg RJ: Time-dependent changes in donor brain death related processes. Am J Transplant 6: 2903–2911, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Morariu AM, Schuurs TA, Leuvenink HGD, van Oeveren W, Rakhorst G, Ploeg RJ: Early events in kidney donation: Progression of endothelial activation, oxidative stress and tubular injury after brain death. Am J Transplant 8: 933–941, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Lytle FT, Afessa B, Keegan MT: Progression of organ failure in patients approaching brain stem death. Am J Transplant 9: 1446–1450, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Kunzendorf U, Hohenstein B, Oberbarnscheid M, Muller E, Renders L, Schott GE, Offermann G: Duration of donor brain death and its influence on kidney graft function. Am J Transplant 2: 292–294, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Nijboer WN, Moers C, Leuvenink HGD, Ploeg RJ: How important is the duration of the brain death period for the outcome in kidney transplantation? Transpl Int 24: 14–20, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Ergün M, Özdemir-van Brunschot DMD, Donders RART, Hilbrands LB, Hoitsma AJ, Warlé MC: Prolonged duration of brain death was associated with better kidney allograft function and survival: A prospective cohort analysis. Ann Transplant 24: 147–154, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Organ Procurement and Transplantation Network: KDRI to KDPI mapping table of 2017, 2018. Available at: https://optn.transplant.hrsa.gov/media/2150/kdpi_mapping_table.pdf. Accessed March 8, 2020
- 13.Suttorp MM, Siegerink B, Jager KJ, Zoccali C, Dekker FW: Graphical presentation of confounding in directed acyclic graphs. Nephrol Dial Transplant 30: 1418–1423, 2015 [DOI] [PubMed] [Google Scholar]
- 14.White H: Maximum likelihood estimation of misspecified models. Econometrica 50: 1–25, 1982 [Google Scholar]
- 15.Muruve NA, Helling TS, Luger AM, Martinez J, Nelson PW, Pierce GE, Ross G, Shield CF III, Warady BA, Aeder MI, Bryan CF: Effect of donor brain-death duration on graft outcome. Transplant Proc 33: 2980–2981, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Westphal GA, Slaviero TA, Montemezzo A, Lingiardi GT, de Souza FCC, Carnin TC, Soares DR, Hachiya AH, Ferraz LL, de Andrade J: The effect of brain death protocol duration on potential donor losses due to cardiac arrest. Clin Transplant 30: 1411–1416, 2016 [DOI] [PubMed] [Google Scholar]
- 17.Inaba K, Branco BC, Lam L, Salim A, Talving P, Plurad D, Green DJ, Demetriades D: Organ donation and time to procurement: Late is not too late. J Trauma 68: 1362–1366, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Cantin B, Kwok BWK, Chan MCY, Valantine HA, Oyer PE, Robbins RC, Hunt SA: The impact of brain death on survival after heart transplantation: Time is of the essence. Transplantation 76: 1275–1279, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Jawitz OK, Raman V, Barac YD, Anand J, Patel CB, Mentz RJ, DeVore AD, Milano C: Influence of donor brain death duration on outcomes following heart transplantation: A United Network for Organ Sharing Registry analysis. J Thorac Cardiovasc Surg 159: 1345–1353.e2, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jawitz OK, Raman V, Barac Y, Mulvihill MS, Moore C, Choi AY, Hartwig M, Klapper J: Impact of donor brain death duration on outcomes after lung transplantation. Ann Thorac Surg 108: 1519–1526, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Austin PC, Stuart EA: The performance of inverse probability of treatment weighting and full matching on the propensity score in the presence of model misspecification when estimating the effect of treatment on survival outcomes. Stat Methods Med Res 26: 1654–1670, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nijboer WN, Schuurs TA, van der Hoeven JAB, Fekken S, Wiersema-Buist J, Leuvenink HGD, Hofker S, Homan van der Heide JJ, van Son WJ, Ploeg RJ: Effect of brain death on gene expression and tissue activation in human donor kidneys. Transplantation 78: 978–986, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Schuurs TA, Gerbens F, van der Hoeven JAB, Ottens PJ, Kooi KA, Leuvenink HGD, Hofstra RMW, Ploeg RJ: Distinct transcriptional changes in donor kidneys upon brain death induction in rats: Insights in the processes of brain death. Am J Transplant 4: 1972–1981, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Saat TC, Susa D, Roest HP, Kok NFM, van den Engel S, Ijzermans JNM, de Bruin RWF: A comparison of inflammatory, cytoprotective and injury gene expression profiles in kidneys from brain death and cardiac death donors. Transplantation 98: 15–21, 2014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.