Visual Abstract
Keywords: sickle cell trait, sickle cell disease, AKI, Black race, hazard ratio, incidence, eGFR decline
Abstract
Background and objectives
Sickle cell trait and sickle cell disease are associated with faster GFR decline compared with normal hemoglobin phenotypes. We sought to compare the AKI risk in sickle cell trait/disease to normal hemoglobin phenotypes and investigate the association between AKI and GFR decline in sickle cell trait/disease.
Design, setting, participants, & measurements
This multicenter observational study used registry data (January 2005–June 2018) of adult Black patients with sickle cell trait/disease (exposures) and normal hemoglobin phenotype (reference) ascertained by hemoglobin electrophoresis. Outcomes of interest (incident AKI [1.5 times baseline serum creatinine or higher], incident severe AKI [doubling of baseline serum creatinine or higher], and incident sustained AKI [AKI persisting for ≥72 hours]) were adjudicated by chart review and evaluated by Cox regression. The association between AKI and GFR decline (linear mixed models) was also investigated.
Results
We identified 8968 reference patients, 1279 patients with sickle cell trait, and 254 patients with sickle cell disease with a median follow-up of 7.6 years and mean baseline serum creatinine of 0.8 mg/dl. We observed 796 AKI events, 452 sustained AKI events, and 466 severe AKI events. Compared with people with a normal hemoglobin phenotype, sickle cell trait was associated with higher risk for sustained AKI (adjusted hazard ratio, 1.64; 95% confidence interval, 1.27 to 2.11), but not AKI (adjusted hazard ratio, 1.11; 95% confidence interval, 0.91 to 1.36) or severe AKI (adjusted hazard ratio, 1.26; 95% confidence interval, 0.96 to 1.64). Sickle cell disease was associated with AKI (adjusted hazard ratio, 2.85; 95% confidence interval, 2.13 to 3.81), severe AKI (adjusted hazard ratio, 2.38; 95% confidence interval, 1.65 to 3.42), and sustained AKI (adjusted hazard ratio, 2.50; 95% confidence interval, 1.68 to 3.71). Post-AKI GFR decline was significantly faster in sickle cell trait (0.37 ml/min per 1.73 m2 per year faster, P<0.01) and disease (1.69 ml/min per 1.73 m2 per year faster, P<0.01) compared with the reference.
Conclusions
Sickle cell trait and disease are associated with higher risk of AKI, which is associated with accelerated decline in eGFR.
Introduction
Sickle cell trait is a genetic carrier state that has been associated with a higher risk for eGFR decline and incident CKD (1,2). However, mechanisms of eGFR decline in sickle cell trait remain understudied. In particular, data on the risk for AKI in sickle cell trait to date are contradictory (3,4).
In contrast, sickle cell disease is a severe condition characterized by recurrent pain crises and is associated with a markedly higher risk for eGFR decline and incident CKD (1,5–7). CKD is associated with nearly one in five deaths in sickle cell disease (7,8). The relationship between sickle cell disease and AKI and the contribution of AKI to eGFR decline in sickle cell disease is also understudied.
Regardless of severity or duration, AKI has been associated with a higher risk for incident CKD and eGFR decline (9). Therefore, given the higher risk for eGFR decline and incident CKD in sickle cell trait/disease (1,2,7) and the preponderance of evidence for chronic recurrent damage to the kidney medulla in sickle cell trait/disease (10,11), understanding the risks for AKI and the association between AKI and eGFR decline is important in sickle cell trait/disease.
The aim of this study was to compare AKI in sickle cell trait/disease to a reference population and subsequently investigate the association between having at least one AKI event and the rate of eGFR decline over follow-up. Finally, we investigated risk factors for AKI in each study group.
Materials and Methods
Study Population
This study used data from the Research Patient Data Registry, Boston, Massachusetts, collected between January 1, 2005 and June 30, 2018. Research Patient Data Registry is an electronic medical record registry that has been described in detail in previously published studies (1,12,13). The final study cohort used in this manuscript is identical to the study cohort used by the authors in a previous publication (1). However, patients with a baseline eGFR between 15 and 29 ml/min per 1.73 m2 were added to this study.
This study was approved by the Institutional Review Board at Partners Healthcare, Boston, Massachusetts, and the need for informed consent was waived.
Inclusion and Exclusion Criteria
We applied the following inclusion criteria to the entire cohort: age ≥18 years at baseline, at least three serum creatinine values, at least 1 year between first and last serum creatinine dates, self-reported Black race, and a hemoglobin electrophoresis at any time. Patients on dialysis, with a kidney transplant, and/or with an eGFR <15 ml/min per 1.73 m2 at baseline were excluded.
Baseline Determination
The baseline serum creatinine was determined by the average of the first three stable values that were also the lowest serum creatinine values after January 1, 2005.
Exposures
We defined our exposure as patients with sickle cell trait or sickle cell disease confirmed by a hemoglobin electrophoresis test interpreted by a pathologist. The reference group was defined as patients with a normal hemoglobin phenotype confirmed by a hemoglobin electrophoresis test interpreted by a pathologist. These hemoglobin electrophoresis tests were ordered by physicians for clinical indications and not for research.
Outcomes
Our primary outcomes were (1) incident AKI, defined as an increase in the baseline serum creatinine of ≥1.5 times (14), (2) incident severe AKI, defined as doubling of baseline serum creatinine or higher (14), and (3) incident sustained AKI, defined as a serum creatinine sustained at ≥1.5 times the baseline serum creatinine for at least 72 hours (15). In patients with few creatinine values and a rise in serum creatinine over time that did not occur within a discernible time frame for AKI, nonacute eGFR decline or CKD progression was presumed to have occurred. Only first AKI events in the outpatient and inpatient settings were utilized. AKI events were adjudicated by a nephrologist (K.O.O.) by direct examination of dates and trends of serum creatinine values for each patient over follow-up.
Among patients with sickle cell trait and sickle cell disease, we reported causes of AKI and classified them as (1) hemodynamic (emesis, diarrhea, poor oral intake, hyperglycemia, overdiuresis, cardiorenal, postop, hypertensive urgency/emergency, hypotension, or sepsis), (2) nephrotoxic (contrast, medications, rhabdomyolysis, autoimmune, or idiopathic glomerulopathies), (3) obstructive, (4) related to pain crisis/acute chest syndrome, or (5) undetermined (if workup/documentation was not performed).
We also investigated the difference between reference and sickle cell trait/disease in the mean annual change in eGFR (1) pre-AKI (including patients who never had AKI) and (2) post-AKI. Subsequently, we compared the difference in the mean annual change in eGFR pre-AKI (including patients who never had AKI) to the mean annual change in eGFR post-AKI among normal hemoglobin phenotype, sickle cell trait, and sickle cell disease groups separately. GFR was estimated using the CKD Epidemiology Collaboration creatinine equation (16,17).
Covariates
Baseline characteristics were determined at the time of baseline serum creatinine determination. Demographics were obtained by chart review. Comorbidities (hypertension, diabetes mellitus, and cardiovascular disease [stroke or coronary artery disease]) were obtained using algorithms on the basis of International Classification of Disease, 10th edition (ICD-10) diagnosis codes and their ICD-9 equivalents (Supplemental Appendix) (18). Laboratory values and prevalent medication use were obtained by chart review.
Statistical Analyses
All analyses were conducted using STATA 14.2 (StataCorp, College Station, TX). Incidence rates per 1000 person-years for each AKI outcome in each study group were calculated using the number of outcome events and total person-time. The association between sickle cell trait/disease and time to first AKI event (any, severe, and sustained) was then evaluated using Cox regression models, with results summarized using hazard ratios (HR) and Wald asymptotic 95% confidence intervals (95% CI). Start times were at the establishment of the baseline serum creatinine. End of follow-up occurred at time of first AKI event, last available serum creatinine, June 30, 2018, maintenance dialysis initiation, or death. Incident AKI events were first identified and then subsequently categorized as “severe AKI” and/or “sustained AKI.” If a first AKI event was neither severe nor sustained, the subsequent first severe and/or first sustained AKI event was captured by chart review as well. Censoring occurred at the event of interest (any, severe, or sustained AKI), last available serum creatinine, June 30, 2018, maintenance dialysis initiation, or death.
Multivariable models were adjusted for baseline age, sex, hypertension, diabetes mellitus, cardiovascular disease, baseline eGFR <60 ml/min per 1.73 m2, and renin-angiotensin-aldosterone system inhibitors. Hemoglobin electrophoresis indication was included in the sickle cell trait models. The covariates used for model adjustment were selected on the basis of prior literature and clinical relevance to the analyses (9,19,20). Schoenfeld residuals were used to test proportional hazard assumptions. Subgroup analysis by baseline eGFR <60 ml/min per 1.73 m2 was also performed.
The mean annual change in eGFR was evaluated using linear mixed models, with random intercepts and slopes to estimate the linear trend in eGFR over time in the exposure groups (sickle cell trait, sickle cell disease), and compared to that of the reference group (normal hemoglobin phenotype), while accounting for correlations of observations within each patient (21). The following analyses were performed: (1) the difference in the mean annual change in eGFR pre-AKI (including patients who never had AKI) between sickle cell trait and normal hemoglobin phenotype and between sickle cell disease and normal hemoglobin phenotype; (2) the difference in the mean annual change in eGFR post-AKI between sickle cell trait and normal hemoglobin phenotype and between sickle cell disease and normal hemoglobin phenotype; and (3) the difference in the mean annual change in eGFR pre-AKI compared with post-AKI within normal hemoglobin phenotypes, sickle cell trait, and sickle cell disease groups separately. All of the above analyses excluded AKI eGFR values. Interaction terms between time and the exposures of interest (sickle cell trait or sickle cell disease or pre- versus post-AKI as appropriate) were included to determine the difference in eGFR slopes (i.e., normal hemoglobin phenotype slope versus sickle cell trait/disease slope, and pre-AKI eGFR slope versus post-AKI eGFR slope) over time. End of follow-up was last available serum creatinine, June 30, 2018, maintenance dialysis initiation, or death. The estimated difference in eGFR slopes and their 95% CIs were reported.
Risk factors for incident AKI evaluated by Cox regression were age, sex, hypertension, diabetes mellitus, cardiovascular disease, and baseline hemoglobin. We also investigated hemoglobin S, F, and A2 levels among patients with sickle cell trait. Among patients with sickle cell disease, we investigated prevalent hydroxyurea use. Each risk factor was adjusted (except where the covariate was the risk factor of interest) for age, sex, hypertension, diabetes mellitus, cardiovascular disease, renin-angiotensin-aldosterone system inhibitors, and, in the reference and sickle cell trait groups, hemoglobin electrophoresis indication. This risk factor analysis was performed using separate models by sickle cell trait/disease status.
Cox regression analysis of AKI, severe AKI, and sustained AKI was repeated in the baseline eGFR ≥60 and eGFR <60 ml/min per 1.73 m2 subgroups.
Results
Baseline Characteristics
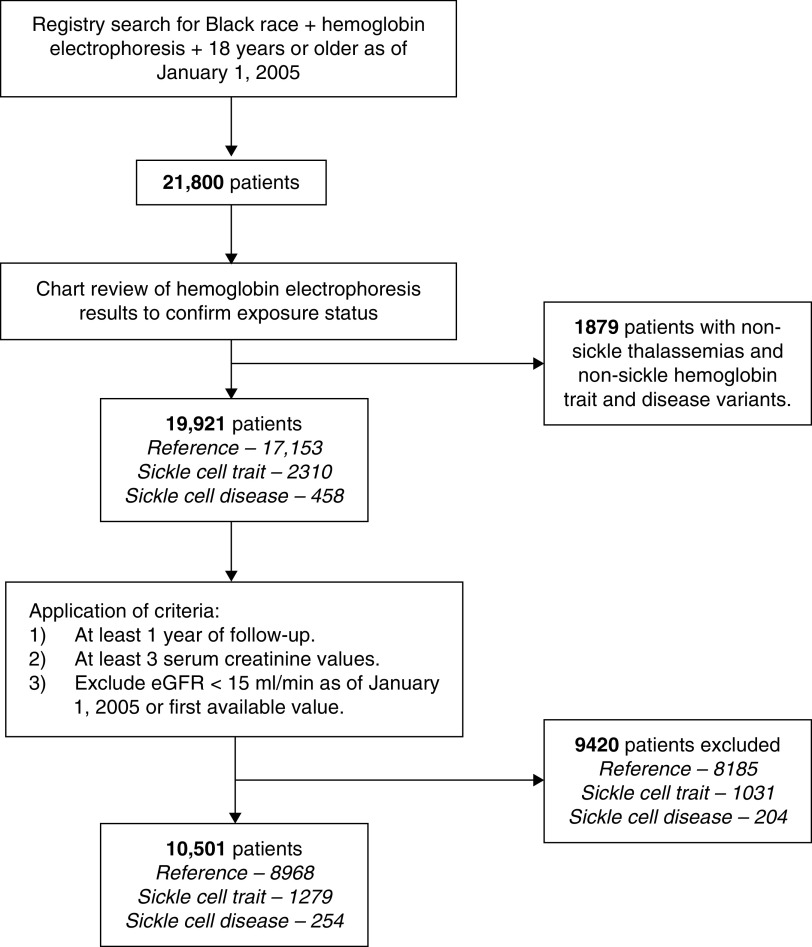
We identified 10,501 patients (8968 reference, 1279 sickle cell trait, and 254 sickle cell disease) (Figure 1). Baseline characteristics are displayed in Table 1. The mean age of all patients was 36±13 years. Median follow-up for the entire cohort was 7.6 (interquartile range 4.1–10.9) years. The mean baseline serum creatinine for all patients was 0.8±0.3 mg/dl. Hemoglobin electrophoresis indications were predominantly routine perinatal testing (49%) and anemia workup (37%).
Figure 1.
Flow chart of patient inclusion.
Table 1.
Baseline characteristics of Black patients with a hemoglobin electrophoresis seen between 2005 and 2018 with at least 1 yr of follow-up and three serum creatinine measurements
| Covariate | Normal Hemoglobin Phenotype (N=8968) | Sickle Cell Trait (N=1279) | Sickle Cell Disease (N=254) |
|---|---|---|---|
| Demographics | |||
| Mean age (SD), yr | 36 (±13) | 40 (±15) | 32 (±12) |
| Age ≥65 yr, N (%) | 310 (3) | 94 (7) | 2 (1) |
| Female, N (%) | 7876 (88) | 999 (78) | 133 (52) |
| Median follow-up (IQR), yr | 7.6 (4.1–10.9) | 7.8 (4.0–10.9) | 6.0 (3.0–9.8) |
| Comorbidities, N (%) | |||
| Hypertension | 1814 (20) | 385 (30) | 30 (12) |
| Diabetes mellitus | 1394 (16) | 320 (25) | 24 (9) |
| Cardiovascular disease | 882 (10) | 187 (15) | 71 (28) |
| Hemoglobin electrophoresis indicationsa, N (%) | |||
| Anemia | 3495 (39) | 315 (25) | — |
| Perinatal testing | 4629 (52) | 482 (37) | — |
| Other | 329 (3) | 215 (17) | — |
| Unknown | 515 (6) | 267 (21) | — |
| Medications, N (%) | |||
| Renin-angiotensin-aldosterone inhibitors | 1790 (20) | 368 (29) | 52 (20) |
| Hydroxyurea | 14 (0.2) | 4 (0.3) | 125 (49) |
| Laboratory values | |||
| Mean serum creatinine (SD), mg/dl | 0.8 (±0.3) | 0.9 (±0.3) | 0.8 (±0.5) |
| Mean eGFR (SD), ml/min | 114 (±27) | 104 (±28) | 128 (±36) |
| eGFR categories, N (%) | |||
| ≥60 ml/min | 8681 (97) | 1202 (94) | 240 (94) |
| 30–59 ml/min | 246 (2) | 70 (5) | 9 (4) |
| 15–29 ml/min | 41 (1) | 7 (1) | 5 (2) |
| Mean hemoglobin (SD), g/dl | 12.3 (1.6) | 12.6 (1.7) | 9.9 (2.2) |
| Mean hemoglobin A2 percentage levels (SD) | — | 3.2 (±0.7) | — |
| Hemoglobin F >0.4%, N (%) | — | 299 (25) | — |
| Mean hemoglobin S percentage (SD) | — | 37 (±4.3) | — |
IQR, interquartile range.
Indications for hemoglobin electrophoresis in patients with sickle cell disease were all to assess sickle cell disease–modifying treatment response (e.g., follow-up after exchange transfusion to assess sickle hemoglobin levels or to assess fetal hemoglobin levels on hydroxyurea).
Compared with the reference, patients with sickle cell trait were older (40±15 versus 36±13 years), with fewer women (78% versus 88%), and a higher prevalence of comorbidities.
Patients with sickle cell disease were younger than the reference (32±12 versus 36±13 years), with fewer females (52% versus 88%), and a lower prevalence of comorbidities, except for cardiovascular disease, where the prevalence was higher in sickle cell disease (28% versus 10%).
Time to Incident AKI in Sickle Cell Trait and Sickle Cell Disease
Results are summarized in Table 2. Patients with sickle cell trait experienced 133 incident AKI events, 65% of which were categorized as hemodynamic (Supplemental Table 1). No events were pregnancy related. The incidence rate of AKI events in sickle cell trait was 13.85 out of 1000 person-years. Sickle cell trait was associated with a significantly higher risk for AKI and severe AKI compared with the reference in unadjusted analysis; however, these were both attenuated in adjusted analysis. Sickle cell trait was associated with a higher risk for incident sustained AKI compared with the reference before and after adjustment (adjusted HR 1.64; 95% CI, 1.27 to 2.11).
Table 2.
Risks for incident AKI by hemoglobin phenotype
| Outcomes | AKI | Severe AKI | Sustained AKI |
|---|---|---|---|
| Number of events | |||
| Normal hemoglobin phenotype | 611 | 354 | 333 |
| Sickle cell trait | 133 | 78 | 91 |
| Sickle cell disease | 52 | 34 | 28 |
| Incidence rate (per 1000 person-yr) | |||
| Normal hemoglobin phenotype | 9.1 | 5.3 | 5.0 |
| Sickle cell trait | 13.9 | 8.1 | 9.4 |
| Sickle cell disease | 31.8 | 20.6 | 17.0 |
| Time to first event (unadjusted HR, 95% CI) | |||
| Normal hemoglobin phenotype | 1 (reference) | 1 (reference) | 1 (reference) |
| Sickle cell trait | 1.50 (1.24 to 1.81) | 1.51 (1.18 to 1.93) | 1.87 (1.48 to 2.36) |
| Sickle cell disease | 3.48 (2.63 to 4.63) | 3.89 (2.74 to 5.54) | 3.39 (2.30 to 4.99) |
| Time to first event (adjusteda HR, 95% CI) | |||
| Normal hemoglobin phenotype | 1 (reference) | 1 (reference) | 1 (reference) |
| Sickle cell trait | 1.11 (0.91 to 1.36) | 1.26 (0.96 to 1.64) | 1.64 (1.27 to 2.11) |
| Sickle cell disease | 2.85 (2.13 to 3.81) | 2.38 (1.65 to 3.42) | 2.50 (1.68 to 3.71) |
HR, hazard ratio; 95% CI, 95% confidence interval.
Adjusted for age, sex, hypertension, diabetes mellitus, cardiovascular disease, baseline eGFR <60 ml/min per 1.73 m2, and prevalent use of renin-angiotensin-aldosterone system inhibitors. For violations of the proportional hazard assumption, specific covariates were fitted with time interactions (see Supplemental Appendix). Hemoglobin electrophoresis indication was included in the sickle cell trait model.
Patients with sickle cell disease experienced 52 incident AKI events, 38% of which were hemodynamic and 37% occurred in the setting of pain crisis/acute chest syndrome (Supplemental Table 1). No events were pregnancy related. The incident rate for AKI events in sickle cell disease was 31.81 out of 1000 person-years. Sickle cell disease was only associated with AKI, severe AKI, and sustained AKI before and after adjusted analysis (adjusted AKI HR, 2.85; 95% CI, 2.13 to 3.81; adjusted severe AKI HR, 2.38; 95% CI, 1.65 to 3.42); adjusted sustained AKI HR, 2.50; 95% CI, 1.68 to 3.71).
Temporal Association between Mean Annual Change in Estimated Glomerular Filtration Rate and AKI
The difference in the pre-AKI mean annual change in eGFR (including patients who never had AKI) between sickle cell trait and the reference and sickle cell disease and the reference is summarized in Table 3. The pre-AKI mean annual change in eGFR was negative (i.e., eGFR declined) in all three study groups, and this mean annual eGFR decline was significantly faster in sickle cell trait (0.28 ml/min per 1.73 m2 per year faster, P<0.01) and sickle cell disease (0.86 ml/min per 1.73 m2 per year faster, P<0.01) compared with normal hemoglobin phenotypes.
Table 3.
Temporal associations between AKI and the mean annual change in eGFR by hemoglobin phenotype
| Outcomes | Normal Hemoglobin Phenotype | Sickle Cell Trait | Sickle Cell Disease |
|---|---|---|---|
| Median (IQR) number of eGFR values over follow-up | 44 (22–120) | 65 (25–157) | 237 (113–563) |
| Mean (SD) eGFR at baseline | 114.4 (27.4) | 103.7 (27.7) | 127.9 (35.7) |
| Adjusted mean annual change in eGFR pre-AKI (including patients who never had AKI) within each group (95% CI)a | −0.82 (−0.80 to −0.84) | −1.09 (−1.05 to −1.14) | −1.68 (−1.60 to −1.76) |
| Adjusted difference in mean annual change in eGFR pre-AKI (including patients who never had AKI) between groups (95% CI)a | 0 (reference) | −0.28 (−0.22 to −0.33) | −0.86 (−0.79 to −0.94) |
| Adjusted mean annual change in eGFR post-AKI within each group (95% CI)a | −1.63 (−1.56 to −1.69) | −2.00 (−1.85 to −2.14) | −3.31 (−3.10 to −3.53) |
| Adjusted difference in mean annual change in eGFR post-AKI between groups (95% CI)a | 0 (reference) | −0.37 (−0.21 to −0.54) | −1.69 (−1.51 to −1.88) |
| Adjusted difference between mean annual change in eGFR pre-AKI (including patients who never had AKI) and mean annual change in eGFR post-AKI within each group (95% CI)a | −0.81 (−0.76 to −0.87) | −0.90 (−0.78 to −1.03) | −1.63 (−1.44 to −1.82) |
IQR, interquartile range; 95% CI, 95% confidence interval.
All models were adjusted for baseline age, sex, hypertension, diabetes mellitus, cardiovascular disease, and renin-angiotensin-aldosterone system inhibitors. Hemoglobin electrophoresis indication was included in the sickle cell trait and normal hemoglobin phenotype (reference) models.
The post-AKI mean annual change in eGFR was also negative (i.e., eGFR declined) in all three study groups (Table 3). The post-AKI mean annual eGFR decline was significantly faster in sickle cell trait (0.37 ml/min per 1.73 m2 per year faster, P<0.01) and sickle cell disease (1.69 ml/min per 1.73 m2 per year faster, P<0.01) compared with normal hemoglobin phenotypes (Table 3).
In all three groups, the post-AKI mean annual change in eGFR declined significantly faster than the pre-AKI mean annual change in eGFR (Table 3).
Risk Factors for Incident AKI in Sickle Cell Trait/Disease
Among patients with sickle cell trait, diabetes mellitus, cardiovascular disease, baseline eGFR <60 ml/min per 1.73 m2, and lower hemoglobin were significantly associated with AKI (Supplemental Table 2). Among patients with sickle cell disease, cardiovascular disease, baseline eGFR <60 ml/min per 1.73 m2, hydroxyurea, and lower hemoglobin were significantly associated with a higher risk for AKI.
Discussion
In this longitudinal study of Black patients in a multicenter health care system in the United States, we found that sickle cell trait was associated with a higher risk for sustained AKI compared with reference patients, and sickle cell disease was associated with a higher risk for AKI, severe AKI, and sustained AKI compared with reference patients. Furthermore, sickle cell trait and sickle cell disease were associated with faster post-AKI eGFR decline compared with the reference. To our knowledge, this granular analysis of AKI in patients with sickle cell trait/disease is the first to utilize nondiagnosis code criteria to determine AKI outcomes, and the first to examine the association between AKI and the mean annual change in eGFR in sickle cell trait/disease.
Our findings add significantly to the sparse literature on AKI in sickle cell trait. Only two prior studies we are aware of have examined AKI in sickle cell trait and produced contradictory results (3,4). Both studies were limited by the use of diagnosis codes to determine AKI outcomes, which is associated with low sensitivity and a tendency to select for more severe AKI episodes (22). Our findings that sickle cell trait is associated with sustained AKI may suggest that certain types of AKI events in sickle cell trait could adversely affect an already compromised kidney microvasculature (23–25), thus delaying recovery. In our sickle cell trait cohort, 65% of AKI events were classified as hemodynamic, and this may have influenced our findings. Furthermore, our subgroup analysis (Supplemental Tables 3 and 4) suggests the observed association of higher risk for AKI in sickle cell trait is primarily driven by patients with an eGFR >60 ml/min per 1.73 m2. Given that sustained AKI has been associated with poorer outcomes in other disease states (26,27), further study is required in sickle cell trait.
Notably, post-AKI eGFR decline in sickle cell trait was faster than post-AKI eGFR decline in the reference group. It is unclear whether kidney abnormalities in sickle cell trait (11,28–30) may have additive negative effects to AKI. This novel observation warrants further study.
We examined several risk factors for AKI in sickle cell trait. Lower hemoglobin was associated with AKI risk; however, higher levels of hemoglobin A2 and F, which inhibit red blood cell sickling and have been linked with reduced eGFR decline in sickle cell trait (1,31,32), were not associated with a lower risk for AKI in sickle cell trait. Higher hemoglobin S levels were not associated with a higher risk for AKI in this cohort. Therefore, the role of hemoglobin subtypes in sickle cell trait kidney injury requires further study.
In contrast to sickle cell trait, our sickle cell disease cohort demonstrated markedly higher risk for all AKI outcomes examined. Two prior studies have described a higher risk for AKI in sickle cell disease, although both studies focused on prevalent AKI and used ICD diagnosis codes to determine AKI outcomes (4,33). Our data provide a granular examination of incident AKI defined by Kidney Disease Improving Global Outcomes (KDIGO) creatinine criteria in sickle cell disease (14). Nearly half of all sickle cell disease AKI events in our cohort occurred during pain crises or acute chest syndrome. Exploratory subanalysis by sickle cell disease phenotype severity (Supplemental Table 5) showed that the severe phenotypes (predominantly hemoglobin SS disease) drove the associations seen in this study (Supplemental Table 6). The association between hydroxyurea and AKI risk in sickle cell disease must be interpreted with caution. Hydroxyurea was previously prescribed only in sickle cell disease patients with more severe clinical presentations (34), and therefore, confounding by indication may be a factor.
This study significantly contributes to the limited literature on AKI in sickle cell trait/disease, but its limitations should be acknowledged. Some of the main strengths of this study are the granular longitudinal data, use of KDIGO creatinine criteria to adjudicate AKI outcomes, and the large sample size. However, the presence of ascertainment bias created by a tendency for patients who are sicker to have more laboratory values means these results should be interpreted with caution (35). The significant difference in baseline serum creatinine noted in Table 3 may infer residual confounding due to significant differences in susceptibility for AKI. Also, this was a retrospective study dependent on hemoglobin electrophoresis testing to identify patients, which limited generalizability and led to a preponderance of females. We attempted to address this by adding hemoglobin electrophoresis indications to the appropriate multivariable models. Other limitations in this study include the use of only the first AKI event without accounting for repeat events, the assumption of linear eGFR decline, the use of ICD codes to verify comorbidities, and absence of data on albuminuria, nonsteroidal anti-inflammatory drug use, blood transfusions, APOL1 genotype, hemoglobin at AKI event, and socioeconomic status. Although there are limitations to creatinine-based GFR estimations in sickle cell disease due to hyperfiltration (36–38), the CKD Epidemiology Collaboration creatinine estimation is the most reliable creatinine-based GFR estimation in sickle cell disease and was used in this study (36,38).
In conclusion, our study observed a significantly higher risk for incident-sustained AKI among Black patients with sickle cell trait compared with Black patients with a normal hemoglobin phenotype. We also observed a significantly higher risk for all examined AKI outcomes among Black patients with sickle cell disease compared with Black patients with a normal hemoglobin phenotype. Post-AKI eGFR decline was significantly faster in both sickle cell trait and disease patients compared with patients with a normal hemoglobin phenotype. These findings should be investigated in prospective studies that focus on potential mechanisms and the natural history of AKI in sickle cell trait/disease. Such studies would better inform preventive measures and interventions to attenuate AKI risk in Black patients with sickle cell trait/disease.
Disclosures
A. Allegretti reports consultancy agreements with Mallinckrodt Pharmaceuticals. S. Kalim reports being supported by National Institutes of Health award K23 DK 106479. S. Nigwekar reports having consultancy agreements with Allena Pharma, Becker Professional Education, Epizon Pharma, and Laboratoris Sanifit; reports receiving research funding from Allena Pharma and Hope Pharma; reports being supported by the American Heart Association Career Development Award 18CDA34110131; reports receiving honoraria from Guidpoint and Sanofi-Aventis; reports serving as a scientific advisor or member of Vifor Pharma; and reports being supported by the National Center for Research Program Winter 2015 Fellow-to-Faculty Transition Award 15FTF25980003 from the American Heart Association and by the KL2/Catalyst Medical Research Investigator Training award TR001100 (an appointed KL2 award) from Harvard Catalyst, The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health). All remaining authors have nothing to disclose.
Funding
K. Olaniran was supported by the American Society of Nephrology Ben J. Lipps Research Fellowship Award.
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.06960520/-/DCSupplemental.
Supplemental Table 1. Distribution of causes of AKI among patients with sickle cell trait and sickle cell disease.
Supplemental Table 2. Risk factors for incident AKI among patients with sickle cell trait and sickle cell disease.
Supplemental Table 3. Risks for incident AKI by hemoglobin phenotype among patients with a baseline eGFR ≥60 ml/min per 1.73 m2.
Supplemental Table 4. Risks for incident AKI by hemoglobin phenotype among patients with a baseline eGFR <60 ml/min per 1.73 m2.
Supplemental Table 5. Sickle cell disease genotypes.
Supplemental Table 6. Risks for incident AKI by severity of sickle cell disease phenotype.
References
- 1.Olaniran KO, Allegretti AS, Zhao SH, Achebe MM, Eneanya ND, Thadhani RI, Nigwekar SU, Kalim S: Kidney function decline among Black patients with sickle cell trait and sickle cell disease: An observational cohort study. J Am Soc Nephrol 31: 393–404, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naik RP, Derebail VK, Grams ME, Franceschini N, Auer PL, Peloso GM, Young BA, Lettre G, Peralta CA, Katz R, Hyacinth HI, Quarells RC, Grove ML, Bick AG, Fontanillas P, Rich SS, Smith JD, Boerwinkle E, Rosamond WD, Ito K, Lanzkron S, Coresh J, Correa A, Sarto GE, Key NS, Jacobs DR, Kathiresan S, Bibbins-Domingo K, Kshirsagar AV, Wilson JG, Reiner AP: Association of sickle cell trait with chronic kidney disease and albuminuria in African Americans. JAMA 312: 2115–2125, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu J, Nelson DA, Deuster PA, Marks ES, O’Connor FG, Kurina LM: Sickle cell trait and renal disease among African American U.S. Army soldiers. Br J Haematol 185: 532–540, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bucknor MD, Goo JS, Coppolino ML: The risk of potential thromboembolic, renal and cardiac complications of sickle cell trait. Hemoglobin 38: 28–32, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Rees DC, Williams TN, Gladwin MT: Sickle-cell disease. Lancet 376: 2018–2031, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Piel FB, Steinberg MH, Rees DC: Sickle cell disease. N Engl J Med 376: 1561–1573, 2017 [DOI] [PubMed] [Google Scholar]
- 7.Powars DR, Elliott-Mills DD, Chan L, Niland J, Hiti AL, Opas LM, Johnson C: Chronic renal failure in sickle cell disease: Risk factors, clinical course, and mortality. Ann Intern Med 115: 614–620, 1991 [DOI] [PubMed] [Google Scholar]
- 8.Powars DR, Chan LS, Hiti A, Ramicone E, Johnson C: Outcome of sickle cell anemia: A 4-decade observational study of 1056 patients. Medicine (Baltimore) 84: 363–376, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Chawla LS, Kimmel PL: Acute kidney injury and chronic kidney disease: An integrated clinical syndrome. Kidney Int 82: 516–524, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Statius van Eps LW, Pinedo-Veels C, de Vries GH, de Koning J: Nature of concentrating defect in sickle-cell nephropathy. Microradioangiographic studies. Lancet 1: 450–452, 1970 [DOI] [PubMed] [Google Scholar]
- 11.Nath KA, Katusic ZS: Vasculature and kidney complications in sickle cell disease. J Am Soc Nephrol 23: 781–784, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nalichowski R, Keogh D, Chueh HC, Murphy SN: Calculating the benefits of a research patient data repository. AMIA Annu Symp Proc 2006: 1044, 2006 [PMC free article] [PubMed] [Google Scholar]
- 13.Nigwekar SU, Solid CA, Ankers E, Malhotra R, Eggert W, Turchin A, Thadhani RI, Herzog CA: Quantifying a rare disease in administrative data: The example of calciphylaxis. J Gen Intern Med 29[Suppl 3]: S724–S731, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kellum JA, Lameire N; KDIGO AKI Guideline Work Group: Diagnosis, evaluation, and management of acute kidney injury: A KDIGO summary (Part 1). Crit Care 17: 204, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freda BJ, Knee AB, Braden GL, Visintainer PF, Thakar CV: Effect of transient and sustained acute kidney injury on readmissions in acute decompensated heart failure. Am J Cardiol 119: 1809–1814, 2017 [DOI] [PubMed] [Google Scholar]
- 16.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS; CKD-EPI Investigators: Estimating glomerular filtration rate from serum creatinine and cystatin C [published correction appears in N Engl J Med 367: 681, 2012]. N Engl J Med 367: 20–29, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levey AS, Stevens LA, Schmid CH, Zhang Y, Castro AF, Feldman HI, Kusek JW, Eggers P, Lente FV, Greene T, Coresh J: A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olaniran KO, Eneanya ND, Allegretti AS, Zhao SH, Achebe MM, Thadhani RI: Cardiovascular outcomes in African Americans with sickle cell trait and chronic kidney disease. Am J Nephrol 49: 93–102, 2019 [DOI] [PubMed] [Google Scholar]
- 19.Ronco C, Bellomo R, Kellum JA: Acute kidney injury. Lancet 394: 1949–1964, 2019 [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, James MT: Acute kidney injury [published correction appears in Ann Intern Med 168: 84, 2018 10.7326/L17-0682]. Ann Intern Med 167: ITC66–ITC80, 2017 [DOI] [PubMed] [Google Scholar]
- 21.Laird NM, Ware JH: Random-effects models for longitudinal data. Biometrics 38: 963–974, 1982 [PubMed] [Google Scholar]
- 22.Grams ME, Waikar SS, MacMahon B, Whelton S, Ballew SH, Coresh J: Performance and limitations of administrative data in the identification of AKI. Clin J Am Soc Nephrol 9: 682–689, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nath KA, Vercellotti GM: Renal functional decline in sickle cell disease and trait. J Am Soc Nephrol 31: 236–238, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nath KA, Hebbel RP: Sickle cell disease: Renal manifestations and mechanisms. Nat Rev Nephrol 11: 161–171, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nath KA, Katusic ZS, Gladwin MT: The perfusion paradox and vascular instability in sickle cell disease. Microcirculation 11: 179–193, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Choi JS, Kim YA, Kim MJ, Kang YU, Kim CS, Bae EH, Ma SK, Ahn YK, Jeong MH, Kim SW: Relation between transient or persistent acute kidney injury and long-term mortality in patients with myocardial infarction. Am J Cardiol 112: 41–45, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Hoste EAJ, Kellum JA: AKI severity class doesn’t tell all: The case for transient AKI. Nephrol Dial Transplant 25: 1738–1739, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Diaw M, Pialoux V, Martin C, Samb A, Diop S, Faes C, Mury P, Sall Diop N, Diop SN, Ranque B, Mbaye MN, Key NS, Connes P: Sickle cell trait worsens oxidative stress, abnormal blood rheology, and vascular dysfunction in type 2 diabetes. Diabetes Care 38: 2120–2127, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tripette J, Connes P, Hedreville M, Etienne-Julan M, Marlin L, Hue O, Hardy-Dessources MD: Patterns of exercise-related inflammatory response in sickle cell trait carriers. Br J Sports Med 44: 232–237, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Monchanin G, Serpero LD, Connes P, Tripette J, Wouassi D, Francina A, Massarelli R, Gozal D, Thiriet P, Martin C: Plasma levels of adhesion molecules ICAM-1 and VCAM-1 in athletes with sickle cell trait with or without alpha-thalassemia during endurance exercise and recovery. Clin Hemorheol Microcirc 40: 89–97, 2008 [PubMed] [Google Scholar]
- 31.Griffin PJ, Sebastiani P, Edward H, Baldwin CT, Gladwin MT, Gordeuk VR, Chui DHK, Steinberg MH: The genetics of hemoglobin A2 regulation in sickle cell anemia. Am J Hematol 89: 1019–1023, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akinsheye I, Alsultan A, Solovieff N, Ngo D, Baldwin CT, Sebastiani P, Chui DHK, Steinberg MH: Fetal hemoglobin in sickle cell anemia. Blood 118: 19–27, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yeruva SLH, Paul Y, Oneal P, Nouraie M: Renal failure in sickle cell disease: Prevalence, predictors of disease, mortality and effect on length of hospital stay. Hemoglobin 40: 295–299, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shah N, Bhor M, Xie L, Halloway R, Arcona S, Paulose J, Yuce H: Treatment patterns and economic burden of sickle-cell disease patients prescribed hydroxyurea: A retrospective claims-based study. Health Qual Life Outcomes 17: 155, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rifkin DE, Coca SG, Kalantar-Zadeh K: Does AKI truly lead to CKD? J Am Soc Nephrol 23:979–984, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arlet J-B, Ribeil J-A, Chatellier G, Eladari D, De Seigneux S, Souberbielle J-C, Friedlander G, de Montalembert M, Pouchot J, Prié D, Courbebaisse M: Determination of the best method to estimate glomerular filtration rate from serum creatinine in adult patients with sickle cell disease: A prospective observational cohort study. BMC Nephrol 13: 83, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ware RE, Rees RC, Sarnaik SA, Iyer RV, Alvarez OA, Casella JF, Shulkin BL, Shalaby-Rana E, Strife CF, Miller JH, Lane PA, Wang WC, Miller ST; BABY HUG Investigators: Renal function in infants with sickle cell anemia: Baseline data from the BABY HUG trial. J Pediatr 156: 66–70.e1, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yee MEM, Lane PA, Archer DR, Joiner CH, Eckman JR, Guasch A: Estimation of glomerular filtration rate using serum cystatin C and creatinine in adults with sickle cell anemia. Am J Hematol 92: E598–E599, 2017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.