Abstract
Duck meat consumption in South Korea has increased in recent years, but no standard about duck farm-specific biosecurity and hygiene guidelines have yet been established. We here investigated Salmonella contamination levels in duck farms to evaluate biosecurity and hygiene practices. We collected 1,116 environmental samples from 31 duck farms in Jeonnam Province, South Korea. The Salmonella-positive farm rate dramatically increased, from 22.6 to 71.0%, on introduction of ducklings. As the ducklings aged 4–6 wk, the positive rate slightly decreased to 64.5%. The Salmonella detection rate on each sampled surface, such as the feed pan (34.4%), wall (33.9%), litter (32.3%), and nipples (24.2%), was highest at 3 wk of age. The most frequently detected Salmonella serovars were Salmonella London (22.2%), Salmonella Albany (21.6%), Salmonella Bareilly (17.0%), and Salmonella Indiana (16.5%). Implementation of cleaning and disinfection procedures, rodent control, and metal house walls significantly lowered the prevalence of Salmonella (P < 0.001, P < 0.01, and P < 0.05, respectively). A high proportion of Salmonella isolates exhibited antimicrobial resistance: 100 and 62.9% exhibited resistance to erythromycin and nalidixic acid, respectively. Furthermore, a majority of S. Albany and all Salmonella Enteritidis isolates were multidrug resistant. These results indicate the level of Salmonella contamination in duck farm environments in Korea is high. Good biosecurity and hygiene practices are the most effective measures for controlling Salmonella contamination.
Key words: prevalence, Salmonella, duck, biosecurity factor, antimicrobial resistance
Introduction
Global duck production has grown steadily from 3 million tons to almost 4.4 million tons between 2000 and 2017 (FAO, 2019). Korea is a country with the seventh highest duck consumption rate in the world. Duck consumption in Korea increased from 46,000 tons in 2004 to 71,000 tons in 2017 (FAO, 2019). Such an increase in duck meat consumption suggests that salmonellosis outbreaks in humans will occur. For example, a survey conducted in the United Kingdom revealed that Salmonella contamination of duck meat (29.0%) was much higher than that of chicken (5.0%) or other poultry meats (8.0%) (Little et al., 2008). The Health Protection Agency in the United Kingdom reported 81 cases of salmonellosis associated with duck. This emphasizes that duck products are becoming more popular among consumers and commonly associated with outbreaks of salmonellosis in human (Noble et al., 2012). As per reports from Korea, Salmonella is frequently isolated from ducks and duck slaughterhouses (Bae et al., 2013; Cha et al., 2013; Lee et al., 2016).
Ducks infected with Salmonella do not show any apparent clinical symptoms but rather exist as asymptomatic carriers (Yu et al., 2008). The only way to prevent outbreaks of salmonellosis is through monitoring and good hygiene practice (Sylejmani et al., 2016). However, Salmonella surveillance in duck farms in Korea is not extensive, and no control guidelines specific to duck farms have been implemented in Korea to date (Kim et al., 2018).
Considering the aforementioned informentioned, in the present study, we investigated the prevalence of Salmonella on duck farms in Korea and attempted to assess the biosecurity factors that could affect Salmonella prevalence. In addition, we evaluated the antimicrobial susceptibility of the isolated Salmonella strains to assess the severity of antibiotic resistances in the duck farm environment.
Materials and methods
Farm Selection
As per the livestock statistics for the last quarter of 2017 (Korea, 2018), there were 416 commercial duck farms in Korea, with an average holding size of 10,000. The Jeonnam Province alone accounts for 54.5% of total duck production in Korea, with 245 duck farms located in close proximity. For the present study, 31 broiler duck farms from the Jeonnam Province were selected as representative of most duck farms in South Korea. The selected farms from Jeonnam Province are located in the cities of Naju (9 farms), Jangheung (8 farms), Yeongam (6 farms), Damyang (4 farms), Suncheon (3 farms), and Hampyeong (1 farm), with a farm holding size of between 8,000 and 30,000.
Sample Collection
For the study, 1,116 samples were collected from 31 duck farms between December 2017 and April 2018, in accordance with the National Poultry Improvement Plan (USDA, 2012), with minor modifications. Briefly, various environmental samples were collected using a sterile surgical gauze moistened with buffered peptone water (Difco) on farms when the ducks were absent (during the prerearing period) and on farms housing 1- to 3-wk-old and 4- to 6-wk-old ducks. Six random sites on the wall and nipple, and nine random sites on the feed pan were swabbed, covering an equivalent surface area of the duck house. Three samples from the same site were pooled into 1 test sample. The litter of 10 g that treated with 1 litter test sample was collected from 15 random sites, and 3 litter samples were collected. Fifteen random site were also swabbed to collect 10 g for 1 dust test sample. Consequently, 3 feed pan, 2 wall, 2 nipple, 1 dust, and 3 litter samples were individually collected for each flock.
Salmonella Isolation and Serotype Identification
Pre-enrichment broth (Difco) was added to all samples at a 1:10 sample-to-broth ratio, and the mixtures were incubated at 37°C for 22 to 26 h. Then, 0.1 mL of the pre-enriched sample was transferred to 10 mL of Rappaport–Vassiliadis enrichment broth (Difco) and again incubated at 42°C for 24 to 48 h. After incubation, the Rappaport–Vassiliadis enriched samples were streaked onto RAMBACH agar (Difco) and xylose lysine tergitol 4 agar (Difco) by using a 3-mm inoculation loop and incubated at 37°C for 22 to 26 h. Three suspected Salmonella colonies were picked from each plate, and their identities were confirmed by detecting the invA gene by PCR (Rahn et al., 1992). Using the White–Kauffmann–Le Minor scheme (Grimont and Weill, 2007), Salmonella colonies (1–3 per plate) were serotyped by using commercial Salmonella O and H antisera (Difco). If several colonies isolated from the same sample had an identical serotype and antimicrobial susceptibility pattern (see the following), only 1 of the colonies was selected, at random, and included in subsequent analysis.
Antimicrobial Susceptibility Testing
Antimicrobial susceptibility was determined by using the disk diffusion method on Mueller–Hinton agar, as detailed by the Clinical and Laboratory Standards Institute (CLSI, 2017). Eighteen antimicrobial agents were tested, at the following concentrations: ampicillin (10 μg), amoxicillin/clavulanic acid (20/10 μg), amikacin (30 μg), ceftazidime (30 μg), cefotaxime (30 μg), cephalothin (30 μg), ciprofloxacin (5 μg), cefuroxime (30 μg), cefazolin (30 μg), erythromycin (15 μg), cefepime (30 μg), cefoxitin (30 μg), gentamicin (10 μg), kanamycin (30 μg), nalidixic acid (30 μg), streptomycin (10 μg), tetracycline (30 μg), and trimethoprim/sulfamethoxazole (1.25/23.75 μg). The antimicrobial susceptibility test results were interpreted in accordance with CLSI M02 and M07 criteria (CLSI, 2017). If a Salmonella colony was resistant to at least 3 antibiotic classes, it was defined as multidrug resistant.
Biosecurity Factor Analysis
Selected factors pertaining to biosecurity were categorized and evaluated as independent “biosecurity factors.” These factors included geographical location, cleaning and disinfection (C&D), dog presence, flock size, the type of duck house, rodent control, distance to other farms, and history of avian influenza outbreaks (based on personal interviews with the growers). The statistical package SPSS 23 was used for biosecurity factor analysis for each farm. Two-sample t test and ANOVA were used to evaluate the associations between biosecurity factors and Salmonella-positive farms in each production cycle. Differences were considered significant at P < 0.05. If the P-value is less than 0.05, it was determined to be an important biosecurity factor related to the prevalence of Salmonella.
Results
Prevalence of Salmonella on Duck Farms
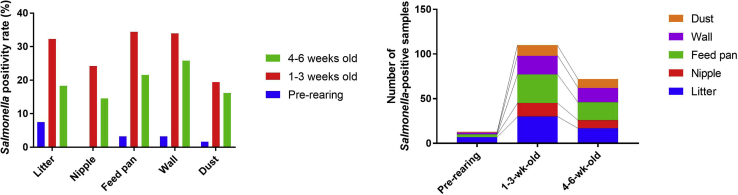
We tested various environmental samples (n = 1,116) collected on duck farms for the presence of Salmonella. Before the introduction of new ducklings, the detection rate of Salmonella was relatively low (7.5, 0.0, 3.2, 3.2, and 1.6% in the litter, nipple, feed pan, wall, and dust samples, respectively) (Figure 1A). After the introduction of ducklings, the Salmonella-positivity rate increased up to 35.9% (Figure 1A). From 4 wk after the introduction of ducklings, the detection rate decreased by approximately 11.4% (4.9–15.0%) (Figure 1A). Similarly, the actual number of Salmonella-positive samples was highest when the ducklings were 1–3 wk of age, followed by when they were 4–6 wk of age.
Figure 1.
Salmonella isolation rate from various sampling sites at 3 time points. The fluctuation in the Salmonella isolation rate at each time point and sampling site is shown as the Salmonella positivity rate (A) and the number of positive samples (B).
Distribution of Salmonella Serotypes
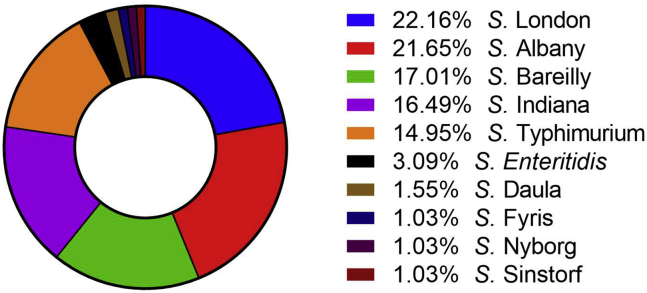
In this study, 194 Salmonella isolates were classified into 10 serovars of Salmonella enterica subspecies. The relative serotype frequency is illustrated as a pie chart in Figure 2. The two most frequently isolated serotypes were Salmonella London (43 isolates; 22.2%) and Salmonella Albany (42 isolates; 21.6%), followed by Salmonella Bareilly (33 isolates; 17.0%), and Salmonella Indiana (32 isolates; 16.5%). The detection rate of Salmonella Typhimurium and Salmonella Enteritidis, the 2 major zoonotic serotypes subject to stringent governmental intervention, was considerably high (14.9 and 3.1%, respectively). Because all Salmonella serotypes cause serious salmonellosis, a poultry farm with any Salmonella contamination could become a serious threat to public health. In Table 1, we summarize the numbers of Salmonella-positive farms identified in the present study, with data presented as per rearing period and Salmonella serotype. Alarmingly, 12.9 and 25.8% of farms rearing 1- to 3-wk-old ducklings were contaminated with S. Enteritidis and S. Typhimurium (Table 1). Minor serotypes, such as Salmonella Daula, Salmonella Fyris, Salmonella Nyborg, and Salmonella Sinstorf, were identified on several farms.
Figure 2.
Salmonella serotype frequency (n = 194). All Salmonella isolates were assigned to 10 serovars. The proportion (%) of each serovar is illustrated as a donut pie chart.
Table 1.
Prevalence of Salmonella serotypes in 31 commercial duck farms.
| Serotype | Prerearing |
1–3 wk of age |
4–6 wk of age |
|---|---|---|---|
| No. of positive farm1 /Total farm (%) |
No. of positive farm1 /Total farm (%) |
No. of positive farm1 /Total farm (%) |
|
| Salmonella London | 0/31 (0.0) | 6/31 (19.4) | 5/31 (16.1) |
| Salmonella Albany | 1/31 (3.2) | 4/31 (12.9) | 5/31 (16.1) |
| Salmonella Bareilly | 1/31 (3.2) | 4/31 (12.9) | 2/31 (6.5) |
| Salmonella Indiana | 1/31 (3.2) | 5/31 (16.1) | 3/31 (9.7) |
| Salmonella Typhimurium | 2/31 (6.5) | 8/31 (25.8) | 9/31 (29.0) |
| Salmonella Enteritidis | 0/31 (0.0) | 4/31 (12.9) | 0/31 (0.0) |
| Salmonella Daula | 2/31 (6.5) | 0/31 (0.0) | 1/31 (3.2) |
| Salmonella Fyris | 0/31 (0.0) | 2/31 (6.5) | 0/31 (0.0) |
| Salmonella Nyborg | 0/31 (0.0) | 0/31 (0.0) | 1/31 (3.2) |
| Salmonella Sinstorf | 0/31 (0.0) | 1/31 (3.2) | 0/31 (0.0) |
A positive farm is a farm from which one or more Salmonella-positive samples were retrieved from the litter, nipple, peed pan, wall, or dust.
Antimicrobial Susceptibility Analysis
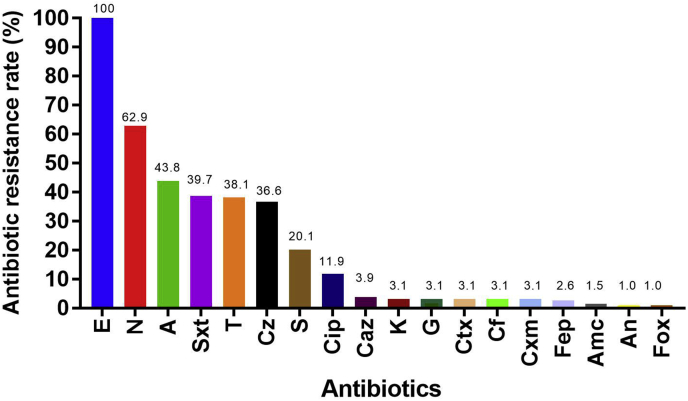
Antimicrobial resistance of the isolates is summarized in Figures 3 and 4. All isolates were resistant to erythromycin (194 isolates; 100%). A large proportion of the Salmonella isolates were resistant to nalidixic acid (122 isolates; 62.9%), followed by ampicillin (85 isolates; 43.8%), trimethoprim/sulfamethoxazole (77 isolates; 39.7%), tetracycline (74 isolates; 38.1%), cefazolin (39 isolates; 36.6%), streptomycin (39 isolates; 20.1%), and ciprofloxacin (23 isolates; 11.9%).
Figure 3.
Antibiotic resistance rate of Salmonella isolates. The antibiotic resistance rate (%) of Salmonella isolates is presented as a bar graph. Abbreviations: A, ampicillin; Amc, amoxicillin/clavulanic acid; An, amikacin; Caz, ceftazidime; Cf, cephalothin; Cip, ciprofloxacin; Ctx, cefotaxime; Cxm, cefuroxime; Cz, cefazolin; E, erythromycin; Fep, cefepime; Fox, cefoxitin; G, gentamicin; K, kanamycin; N, nalidixic acid; S, streptomycin; Sxt, trimethoprim/sulfamethoxazole; T, tetracycline.
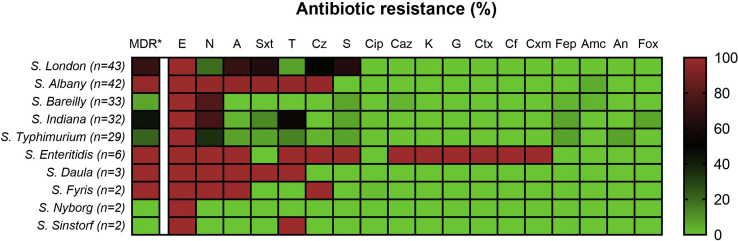
Figure 4.
Antibiotic resistance profiles of Salmonella isolates. The antibiotic resistance profiles of Salmonella isolates are illustrated as a heat-map. The color of each cell represents the % of isolates resistant to the indicated antibiotic, as shown in the color legend on the right. Multidrug resistance (MDR∗) was defined as resistance to at least 3 antibiotic classes. Abbreviations: A, ampicillin; Amc, amoxicillin/clavulanic acid; An, amikacin; Caz, ceftazidime; Cf, cephalothin; Cip, ciprofloxacin; Ctx, cefotaxime; Cxm, cefuroxime; Cz, cefazolin; E, erythromycin; Fep, cefepime; Fox, cefoxitin; G, gentamicin; K, kanamycin; N, nalidixic acid; S, streptomycin; Sxt, trimethoprim/sulfamethoxazole; T, tetracycline.
The majority of S. Bareilly (24 isolates; 72.0%) and S. Indiana (39.0%; 13 isolates) isolates were only resistant to nalidixic acid and erythromycin. However, S. Albany (42 isolates; 100.0%) isolates were resistant to at least 6 antimicrobials. S. Enteritidis (6 isolates; 100.0%) isolates were resistant to at least 12 antibiotics, including gentamicin and third-generation cephalosporins such as ceftazidime and cefotaxime.
Analysis of Biosecurity Factors
We next analyzed the correlation between the biosecurity factors and the numbers of Salmonella-positive farms in each production cycle. The serotypes and the origin of samples were not included for the analysis.
Salmonella prevalence was significantly higher when C&D were not conducted (P < 0.001) and when nonmetal duck houses were used (P < 0.001), regardless of the production cycle. Similarly, rodent control had a significant impact on reducing Salmonella prevalence (P < 0.001) in farms housing 1- to 3-wk-old and 4- to 6-wk-old ducks. On the other hand, the geographical region, flock size, dog presence, avian influenza history, and distance to the nearest poultry farm did not significantly affect Salmonella prevalence.
Discussion
In this study, we evaluated the Salmonella contamination status of the duck farm environment and assessed the effect of biosecurity factors on bacterial contamination, to explore critical impact points for the managing contamination load.
Interestingly, Salmonella positivity was significantly higher in the farm environment samples collected 1–3 wk after introducting ducklings than those collected at 3–6 wk in all sample types (Figure 1). This finding is consistent with that of previous studies (Tsai and Hsiang, 2005; Flament et al., 2012; Cha et al., 2013), which demonstrated that the frequency of Salmonella isolation from a cloacal swab or liver culture is highest in 1- to 3-wk-old ducklings. The observed peak of Salmonella positivity at 1–3 wk after duckling introduction is likely from a high amount of Salmonella in excrement by the ducklings because the ducklings' adaptive immune system is not yet fully mature. The lower level of Salmonella positivity on farms housing 4- to 6-wk-old ducks is likely because the immunologically mature ducks excrete much less Salmonella than ducklings (Tsai and Hsiang, 2005; Yu et al., 2008). Alternatively, a routine change in husbandry procedures between the rearing periods could artificially lower the Salmonella contamination load. Special litter treatments, primarily with 3-wk-old ducks, is a typical duck farming practice on South Korean duck farms. Special litter treatment, such as mechanical replacement of caked litter, for example, using a tractor rotavator, and top-dressing of the old litter with a light layer of new litter, is reported to occur more frequently as ducklings grow (Ritz et al., 2005).
We identified 10 different Salmonella serotypes in the present study. The most predominant serovar was S. London (43 isolates; 22.2%). This finding is consistent with that of a previous report that S. London is one of the most prevalent serotypes in Korean duck slaughterhouses (Bae et al., 2013). Other common isolates in this study were S. Albany (42 isolates; 21.6%) and S. Bareilly (33 isolates; 17.0%) (Figure 2). Although S. Albany and S. Bareilly are rarely isolated on duck farms in other countries, they are frequently observed in the chicken industry (Zaidi et al., 2006; Cleary et al., 2010; Im et al., 2015). S. Indiana (32 isolates; 16.5%), S. Typhimurium (29 isolates; 14.9%), and S. Enteritidis (6 isolates; 3.1%) were also isolated and are regularly reported (Tsai and Hsiang, 2005; Pan et al., 2010; Flament et al., 2012; Cha et al., 2013). Each of these serotypes causes food poisoning, and therefore, contamination of the duck environment could pose a high potential risk for human food safety (Flament et al., 2012). Notably, we observed a significant level of contamination with S. Typhimurium and S. Enteritidis 1–3 wk after the introduction of ducklings (Table 1), which is significantly higher than the levels observed at duck farms in developed countries, such as Belgium (1.1%), Estonia (2.3%), and Norway (0.0%) (Flament et al., 2012; ESFA and ECDC, 2017). The reason for the substantially lower contamination in European countries could be because duck farms in the European Union have been under systemic surveillance, via the Salmonella monitoring system, by the European Food Safety Authority (ESFA and ECDC, 2017). Consequently, to reduce the risk of salmonellosis outbreaks, the Korean government should develop advanced biosecurity (Martelli et al., 2017) and hygiene measures (Sylejmani et al., 2016) and a surveillance system for Salmonella on duck farms.
Identifying the primary source of Salmonella contamination on farms is critical for designing an effective biosecurity plan. In this study, we analyzed the antimicrobial resistance patterns and the point at which most farms became Salmonella-positive to determine the primary contamination source. The antimicrobial resistance patterns on the surveyed farms were similar, though each farm used a different antibiotic practice. This result suggests that Salmonella likely originated from a common source. Salmonella incidence of each serotype on most farms was negative or very low (0–6.5%) until ducklings were introduced (Table 1), Therefore, a likely source is the introduction of ducklings and is probably associated with the breeding farm or hatchery. Breeding farms and hatcheries have been reported to be sources of the spread of Salmonella to multiple farms (Flament et al., 2012; Choi et al., 2014). However, the present study was primarily aimed at screening for the prevalence of Salmonella on Korean duck farms, it does not provide direct evidence to support this notion. The Salmonella contamination status of the duck breeder hatchery should be investigated in future studies to determine whether they are contamination sources.
Erythromycin is a commonly used antibiotic that has been widely used for the treatment of Riemerella anatipestifer infections in the duck industry. Notably, all Salmonella serotypes isolated in the present study were resistant to erythromycin, probably because of its frequent usage on duck farms (Figure 3). The next highest resistance was to nalidixic acid (Figure 3), as reported previously (Lee et al., 2016). Almost all S. Albany isolates were multidrug resistant, that is, resistant to at least 6 antibiotics, including trimethoprim/sulfamethoxazole and tetracycline (Figure 4). S. Enteritidis isolates were resistant to at least 12 antibiotics (Figure 4), including third-generation cephalosporins and gentamicin. This is a serious concern because third-generation cephalosporins are critical antibiotics for the treatment of salmonellosis. Multidrug resistant S. Enteritidis isolates are reported infrequently (Flament et al., 2012; Cha et al., 2013). Instead, Korean duck slaughter houses are reported to have Salmonella spp. that produce extended-spectrum β-lactamases (Lee et al., 2016). These findings suggest that future outbreaks of multidrug resistant and extended-spectrum β-lactamase–producing Salmonella will likely occur in South Korea.
We also investigated several biosecurity factors related to the prevalence of Salmonella in the duck farm environment. Compared with chickens, ducks moisten the surrounding environment as part of preening behavior, and this damp environment favors the proliferation of Salmonella (Murray, 1991). Therefore, C&D is crucial for preventing the recirculation of Salmonella on a duck farm during different production cycles (Shang et al., 2018). Our results confirmed that C&D was the primary biosecurity factor affecting the prevalence of Salmonella on duck farms regardless of the production cycle (Table 2). In this context, using metal-type housing, which is easily cleaned and disinfected, also contributed to lowering the Salmonella positivity of farms (P < 0.001) (Table 2). In the case of metal-type duck houses, cleaning with high-pressure water to remove dirt and grime is easy, and the metal facilitates drying, which prevents dilution of the disinfectant. In addition, metal-type housing is similar to closed ventilation housing on broiler farms. Prevalence of Salmonella in closed-house broiler farms is significantly lower than that in open-house broiler farms (Soliman et al., 2020). Therefore, the prevalence of Salmonella is correlated with housing type. Another important biosecurity factor that affected Salmonella prevalence was rodent control (Table 2). Rodents play an important role in transmitting Salmonella within duck farms and between farms because they are potent vectors of Salmonella (Hulaj et al., 2016). Therefore, appropriate biosecurity measures, such as C&D and rodent control, should be considered to minimize the spread of Salmonella within the duck industry.
Table 2.
Biosecurity factor analysis for Salmonella on duck farms.
| Biosecurity factors | No. of farms | Prerearing |
1–3 wk of age |
4–6 wk of age |
|---|---|---|---|---|
| No. Salmonella-positive farms (%) | ||||
| Overall | 31 | 7 (22.6) | 22 (71.0) | 20 (64.5) |
| Geographic region | ||||
| Damyang | 4 | 1 (25.0) | 4 (100) | 4 (100.0) |
| Suncheon | 3 | 1 (33.3) | 1 (33.3) | 1 (33.3) |
| Yeongam | 6 | 0 (0.0) | 4 (66.6) | 3 (50.0) |
| Jangheung | 8 | 1 (12.5) | 5 (62.5) | 6 (75.0) |
| Naju | 9 | 4 (44.4) | 7 (77.8) | 5 (55.5) |
| Hampyeong | 1 | 0 (0.0) | 1 (100.0) | 1 (100.0) |
| P-value1 | 0.664 | 0.088 | 0.081 | |
| Flock size | ||||
| Small (<15,000) | 11 | 3 (27.3) | 8 (72.7) | 7 (63.6) |
| Medium (15,000–20,000) | 9 | 1 (11.1) | 4 (44.4) | 4 (44.4) |
| Large (>20,000) | 11 | 3 (27.3) | 10 (90.9) | 9 (81.8) |
| P-value1 | 0.645 | 0.076 | 0.237 | |
| Type of house | ||||
| Iron | 8 | 0 (0.0) | 3 (37.5) | 3 (37.5) |
| Noniron (fabric mesh) | 23 | 7 (30.4) | 19 (82.6) | 17 (73.9) |
| P-value2 | 0.0113 | 0.0143 | 0.00053 | |
| Cleaning and disinfection | ||||
| Not practiced | 8 | 5 (62.5) | 8 (100.0) | 8 (100.0) |
| Either cleaning or disinfection | 11 | 2 (18.2) | 9 (81.8) | 9 (81.8) |
| Both cleaning or disinfection | 12 | 0 (0.0) | 5 (41.7) | 3 (25.0) |
| P-value1 | 0.00023 | 0.0093 | <0.00013 | |
| Dog presence | ||||
| Yes | 14 | 2 (14.3) | 9 (64.3) | 8 (57.1) |
| No | 17 | 5 (29.4) | 13 (76.5) | 12 (70.6) |
| P-value2 | 0.321 | 0.474 | 0.453 | |
| Distance to the nearest poultry farm | ||||
| <50 m | 11 | 4 (36.4) | 9 (81.8) | 7 (63.6) |
| 50–500 m | 15 | 2 (13.3) | 9 (60.0) | 9 (60.0) |
| 500 m– 3 km | 5 | 1 (20.0) | 4 (80.0) | 4 (80.0) |
| P-value1 | 0.403 | 0.454 | 0.74 | |
| History of past avian influenza outbreak | ||||
| Yes | 7 | 2 (28.6) | 4 (57.1) | 5 (71.4) |
| No | 24 | 5 (20.8) | 18 (75.0) | 15 (62.5) |
| P-value2 | 0.679 | 0.377 | 0.677 | |
| Rodent control | ||||
| Yes | 18 | 2 (11.1) | 9 (50.5) | 7 (38.9) |
| No | 13 | 5 (38.5) | 13 (100.0) | 13 (100.0) |
| P-value2 | 0.076 | 0.0013 | <0.00013 | |
P-value based on two-sample t test.
P-value based on ANOVA.
Differences were considered significant at P < 0.05.
One limitation of this study was that there was about 2–3 yr lag from sample collection (2017–2018) to report. Despite the lag in data release, our report provides the most updated information on Salmonella prevalence of Korean duck farm, which has not actively reported since the last report in 2014 (Kim et al., 2016). Such delay in data publication is often observed in duck farm prevalence studies, as the characteristics of Salmonella isolated from duck farms between 2016 and 2017 were published in 2019 and 2020 (Yang et al., 2019; Mridha et al., 2020). The inherent technical issues to investigate duck farms, such as reluctance to release sensitive information and scarcity of baseline information, appear to hamper the update process. Our study could be a baseline setting for future duck farm prevalence studies to shorten the gap between sampling and data publication.
In conclusion, we reported a high level of Salmonella contamination on duck farms, highlighting the need to establish an effective surveillance system of Salmonella in the duck industry. The findings could help to develop critical control points in biosecurity plans specific to duck farms.
Acknowledgments
This work was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, and Forestry (IPET) through Animal Disease Management Technology Development Program funded by the Ministry for Agriculture, Food, and Rural Affairs (MAFRA) [grant number 316047-03]. The sponsors did not play any role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Disclosures
The authors declare no conflicts of interest.
References
- Bae D.H., Dessie H.K., Baek H.J., Kim S.G., Lee H.S., Lee Y.J. Prevalence and characteristics of Salmonella spp. isolated from poultry slaughterhouses in Korea. J. Vet. Med. Sci. 2013;75:1193–1200. doi: 10.1292/jvms.13-0093. [DOI] [PubMed] [Google Scholar]
- Cha S.Y., Kang M., Yoon R.H., Park C.K., Moon O.K., Jang H.K. Prevalence and antimicrobial susceptibility of Salmonella isolates in Pekin ducks from South Korea. Comp. Immunol. Microbiol. Infect. Dis. 2013;36:473–479. doi: 10.1016/j.cimid.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Choi S.W., Ha J.S., Kim B.Y., Lee D.H., Park J.K., Youn H.N., Hong Y.H., Lee S.B., Lee J.B., Park S.Y. Prevalence and characterization of Salmonella species in entire steps of a single integrated broiler supply chain in Korea. Poult. Sci. 2014;93:1251–1257. doi: 10.3382/ps.2013-03558. [DOI] [PubMed] [Google Scholar]
- Cleary P., Browning L., Coia J., Cowden J., Fox A., Kearney J., Lane C., Mather H., Quigley C., Syed Q. A foodborne outbreak of Salmonella Bareilly in the United Kingdom. Euro Surveill. 2010;15 doi: 10.2807/ese.15.48.19732-en. doi: 10.2807/ese.15.48.19732-en. PMID: 21144449. [DOI] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI) Clinical and Laboratory Standards Institute; Wayne, PA: 2017. Performance Standards for Antimicrobial Susceptibilit Y Testing. M100-S22 Ed. [Google Scholar]
- European Food Safety Authority (ESFA), European Center for Disease Prevention and Control (ECDC) The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2016. EFSA. J. 2017;15:e05077. doi: 10.2903/j.efsa.2017.5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAOSTAT Production of Livestock primary (duck, meat) 2019. http://www.fao.org/faostat/en/#data/QL
- Flament A., Soubbotina A., Mainil J., Marlier D. Prevalence of Salmonella serotypes in male mule ducks in Belgium. Vet. Rec. 2012;170:311. doi: 10.1136/vr.100156. [DOI] [PubMed] [Google Scholar]
- Grimont P.A., Weill F.X. Institut Pasteur; Paris, France: 2007. Antigenic Formulae of the Salmonella Serovars. WHO Collaborating Centre for Reference and Research on Salmonella. [Google Scholar]
- Hulaj B., Cabeli P., Goga I., Taylor N., Hess C., Hess M.l. Survey of the prevalence of Salmonella species on laying hen farms in Kosovo. Poult. Sci. 2016;95:2030–2037. doi: 10.3382/ps/pew149. [DOI] [PubMed] [Google Scholar]
- Im M.C., Jeong S.J., Kwon Y.K., Jeong O.M., Kang M.S., Lee Y.J. Prevalence and characteristics of Salmonella spp. isolated from commercial layer farms in Korea. Poult. Sci. 2015;94:1691–1698. doi: 10.3382/ps/pev137. [DOI] [PubMed] [Google Scholar]
- Kim H.B., Jang J.Y., Chang B.J., Kim A.R., Choe B.H. Prevalence and antimicrobial resistance of Salmonella spp. and Escherichia coli isolated from ducks in Korea. Korean J. Vet. Res. 2016;56:91–95. [Google Scholar]
- Kim W.H., An J.U., Kim J., Moon O.K., Bae S.H., Bender J.B., Cho S. Risk factors associated with highly pathogenic avian influenza subtype H5N8 outbreaks on broiler duck farms in South Korea. Transbound Emerg. Dis. 2018;65:1329–1338. doi: 10.1111/tbed.12882. [DOI] [PubMed] [Google Scholar]
- Lee S.K., Choi D., Chon J.W., Seo K.H. Resistance of strains producing extended-spectrum β-lactamases among Salmonella from duck Carcasses at slaughterhouses in three major Provinces of South Korea. Foodborne Pathog. Dis. 2016;13:135–141. doi: 10.1089/fpd.2015.2042. [DOI] [PubMed] [Google Scholar]
- Little C.L., Richardson J.F., Owen R.J., de Pinna E., Threlfall E.J. Prevalence, characterisation and antimicrobial resistance of Campylobacter and Salmonella in raw poultrymeat in the UK, 2003–2005. Int. J. Environ. Health Res. 2008;18:403–414. doi: 10.1080/09603120802100220. [DOI] [PubMed] [Google Scholar]
- Martelli F., Gosling R.J., Callaby R., Davies R. Observations on Salmonella contamination of commercial duck farms before and after cleaning and disinfection. Avian Pathol. 2017;46:131–137. doi: 10.1080/03079457.2016.1223835. [DOI] [PubMed] [Google Scholar]
- Mridha D., Uddin M.N., Alam B., Akhter A.H.M.T., Islam S.S., Islam M.S., Khan M.S.R., Lutful Kabir S.M. Identification and characterization of Salmonella spp. from samples of broiler farms in selected districts of Bangladesh. Vet. World. 2020;13:275–283. doi: 10.14202/vetworld.2020.275-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray C.J. Salmonellae in the environment. Rev. Sci. Tech. 1991;10:765–785. [PubMed] [Google Scholar]
- Noble D., Lane C., Little C., Davies R., De Pinna E., Larkin L., Morgan D. Revival of an old problem: an increase in Salmonella enterica serovar Typhimurium definitive phage type 8 infections in 2010 in England and Northern Ireland linked to duck eggs. Epidemiol. Infect. 2012;140:146–149. doi: 10.1017/S0950268811000586. [DOI] [PubMed] [Google Scholar]
- Pan Z., Geng S., Zhou Y., Liu Z., Fang Q., Liu B., Jiao X. Prevalence and antimicrobial resistance of Salmonella spp. isolated from domestic animals in Eastern China. J. Anim. Vet. Adv. 2010;9:2290–2294. [Google Scholar]
- Rahn K., De Grandis S.A., Clarke R.C., McEwen S.A., Galán J.E., Ginocchio C., Curtiss R., Gyles C.L. Amplification of an invA gene sequence of Salmonella typhimurium by polymerase chain reaction as a specific method of detection of Salmonella. Mol. Cell Probes. 1992;6:271–279. doi: 10.1016/0890-8508(92)90002-f. [DOI] [PubMed] [Google Scholar]
- Ritz C., Fairchild B., Lacy M. Bulletin; 2005. Litter quality and broiler performance. Cooperative extension Service/the University of Georgia College of Agricultural and environmental Sciences. United State Department of Agriculture, Washington, DC, 1267:2005. [Google Scholar]
- Shang K., Wei B., Kang M. Distribution and dissemination of antimicrobial-resistant Salmonella in broiler farms with or without enrofloxacin use. BMC Vet. Res. 2018;14:257. doi: 10.1186/s12917-018-1590-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman E.S., Abdallah M.S. Assessment of biosecurity measures in broiler's farms in the Suez Canal area–Egypt using a seasonal prevalence of Salmonellosis. Vet. World. 2020;13:622–632. doi: 10.14202/vetworld.2020.622-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statistics Korea Livestock statistics in the last quarter of 2017. 2018. http://kosis.kr/statHtml/statHtml.do?orgId=101&tblId=DT_1EO112&vw_cd=MT_ZTITLE&list_id=F1A_020&seqNo=&lang_mode=ko&language=kor&obj_var_id=&itm_id=&conn_path=MT_ZTITLE
- Sylejmani D., Musliu A., Ramadani N., Sparagano O., Hamidi A. Associations between the level of biosecurity and occurrence of Dermanyssus gallinae and Salmonella spp. in layer farms. Avian Dis. 2016;60:454–459. doi: 10.1637/11327-111415-Reg. [DOI] [PubMed] [Google Scholar]
- Tsai H.J., Hsiang P.H. The prevalence and antimicrobial susceptibilities of Salmonella and Campylobacter in ducks in Taiwan. J. Vet. Med. Sci. 2005;67:7–12. doi: 10.1292/jvms.67.7. [DOI] [PubMed] [Google Scholar]
- United State Department of Agriculture . 2012. National Poultry Improvement plan and Auxiliary Provisions. USDA-APHIS publication, Washington, DC, 91-55-1066. [Google Scholar]
- Yang J., Ju Z., Yang Y., Zhao X., Jiang Z., Sun S. Antimicrobial susceptibility and genotype profiles of Salmonella isolated from duck farms and a slaughterhouse in Shandong province, China. BMC Microbiol. 2019;19:202. doi: 10.1186/s12866-019-1570-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C.Y., Chu C., Chou S.J., Chao M.R., Yeh C.M., Lo D.Y., Su Y.C., Horng Y.M., Weng B.C., Tsay J.G. Comparison of the association of age with the infection of Salmonella and Salmonella enterica serovar Typhimurium in Pekin ducks and Roman geese. Poul. Sci. 2008;87:1544–1549. doi: 10.3382/ps.2008-00018. [DOI] [PubMed] [Google Scholar]
- Zaidi M.B., McDermott P.F., Fedorka-Cray P., Leon V., Canche C., Hubert S.K., Abbott J., León M., Zhao S., Headrick M. Nontyphoidal Salmonella from human clinical cases, asymptomatic children, and raw retail meats in Yucatan, Mexico. Clin. Infect. Dis. 2006;42:21–28. doi: 10.1086/498508. [DOI] [PubMed] [Google Scholar]