Abstract
Childhood obesity is a major health concern in the United States (US) and those living in rural communities are at higher risk than their urban counterparts. Few prevention trials have engaged whole families of school-age children in community settings, and none to date have promoted family meals, family activity and healthful home environments in rural settings through a rigorous, randomized controlled trial (RCT). The New Ulm at HOME (NU-HOME) study recruited 114 parent/child dyads in a two-arm (intervention versus wait-list control) RCT to test the efficacy of a family meals-focused program aimed to prevent excess weight gain among 7–10 year-old children in rural Minnesota. The NU-HOME program was adapted from a previously tested program for urban families through a unique community collaboration. The program included 7 monthly in-person sessions for all family members. Parents also participated in 4 motivational goal-setting phone calls. The primary outcome measures were age- and sex-adjusted child body mass index (BMI) z-score, percent body fat, and incidence of overweight and obesity post-intervention. Secondary outcomes included quality of food and beverage availability in the home, family meals and snacks; children’s dietary intake quality (e.g., Healthy Eating Index (HEI)-2015, fruits and vegetables, sugar-sweetened beverages, snacks); and children’s screen time and weekly minutes of moderate-to-vigorous physical activity, total physical activity, and sedentary behavior. The NU-HOME RCT was a collaborative effort of academic and health system researchers, interventionists and community leaders that aimed to prevent childhood obesity in rural communities through engagement of the whole family in an interactive intervention.
Keywords: rural, obesity, home, family meals, family, intervention
1. Introduction
Childhood obesity is a major health concern in the United States (US); more than 31% of youth aged 2–19 years have overweight or obesity.1–3 Moreover, obesity tracks from childhood to adulthood4 and is associated with premature morbidity,5 social stigmatization6,7 and increased medical expenditures.8,9 Nationally, compared to urban youth, rural children have about 20–25% higher odds for overweight/obesity10,11 and are thus at greater risk for adult obesity and diabetes.5
In the last decade in the US, few obesity prevention programs for school-age youth have been conducted and evaluated in rural communities. Programs promoting healthy diets and physical activity have been school-based and focused on environmental change to improve the dietary quality of lunches and food service preparation12–15 and/or school-level changes to promote healthful eating and physical activity.14–17 The few studies that did include family involvement was often limited to special events,12 informational materials,14 or limited internet interactions.15
Parents are primary role models for healthful eating and activity and gatekeepers for food and beverage availability and screen time at home.18 Moreover, the home setting is where most children’s calories and energy dense foods are consumed19 and where much screen time occurs.20 Although children in rural communities are at increased risk for obesity and diabetes,21 rural family-focused obesity prevention studies (with weight-related outcomes) outside of school settings are limited, particularly for school-age children. Brown and colleagues conducted an obesity prevention pilot study of 6–9 year-old youth on a rural American Indian reservation and saw promising effects on BMI z-scores, however, the parental component was limited to take-home toolkits (3 per week), 11 weekly text messages, and 3 family dining and activity events.22 Janicke and colleagues conducted the E-FLIP for Kids trial for 8–12 year-old children with overweight or obesity and their parents and saw no significant differences in BMI z-scores between family-based, parent-based and health education groups.23 The iCook 4-H randomized control trial (RCT) of 9–10 year-old youth and their adult food preparers (dyads) that promoted cooking, eating and playing together saw increased BMI z-scores for the intervention group compared to the control group.24 Thus, given limited testing of programs to address rural childhood obesity and lack of family-focused programming, more research is needed to address childhood obesity in high-risk rural environments.
The New Ulm at HOME (NU-HOME) study is innovative as the first RCT to test the effectiveness of a childhood obesity prevention program for school-age children by engaging whole families to promote quality of the home food environment, quality of family meals and snacks, and family physical activity with gold standard dietary and anthropometry assessments. The NU-HOME intervention program was a universal (not just youth with obesity) prevention program intended to support children in healthy weight maintenance, excess weight gain prevention as they grow, and prevention to more severe obesity among those with who already have overweight or obesity. The objective of this report is to describe the NU-HOME study design; recruitment, screening and enrollment of participants; methods and assessments; intervention development and delivery; and sample baseline demographic and weight-related characteristics.
2. Methods
2.1. Study design and overview
The NU-HOME study included two stages: 1) formative work with community partners, and 2) a two-arm RCT (intervention and wait-list control). Community-based participatory research (CBPR) principles25–27 were used with community partners to adapt the existing HOME Plus program that was piloted (HOME pilot: 2006–2008, NIH R21-DK007299728) and tested at full-scale (HOME Plus: 2010–2015, NCT01538615, NIH R01-DK08400029) in urban communities by the Principal Investigator and research teams. The previous HOME Plus study was the first family meals-focused RCT designed to engage whole families to prevent excess weight gain among children,30 with significant effects on body mass index (BMI) z-score prior to pubertal onset.31 The HOME Plus study also demonstrated increased parent self-efficacy for identifying appropriate portion sizes and decreased child intake of sugar-sweetened beverages (SSB).32 The NU-HOME RCT expanded and adapted the urban HOME Plus program to address the needs of a higher-risk rural community.10,11 The NU-HOME trial design mirrored the previous HOME Plus RCT with use of a staggered, two-cohort design and baseline, post-intervention and follow-up assessments. Based on lessons learned from our previous research,31 the NU-HOME intervention program was designed to target slightly younger children (7–10 years of age instead of 8–12 years of age) and delivered during seven months of the elementary school academic year rather than over a 10-month period. In addition, adaptations were made to specifically address barriers unique to a rural environment (detailed below).
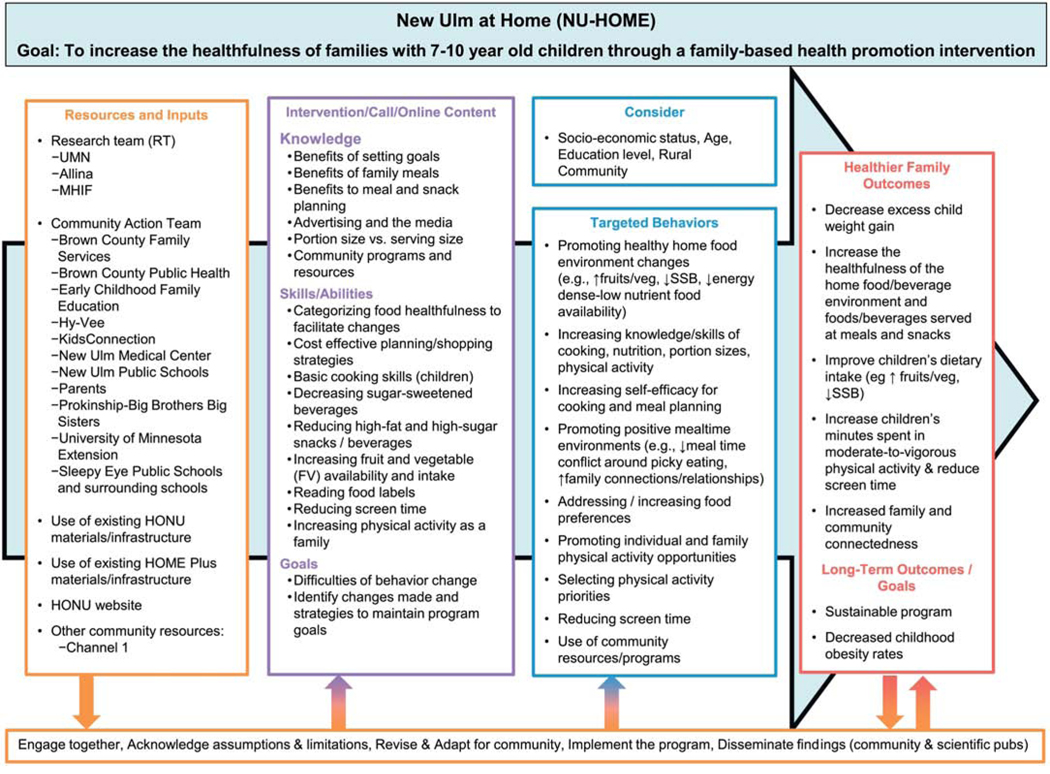
The NU-HOME study was unique in its community partnerships.33 The primary partnership was between the University of Minnesota (UMN) research team and collaborators from the Hearts Beat Back: Heart of New Ulm (HONU) Project. HONU was a 10-year community demonstration project that launched in 2009 and aimed to reduce adult myocardial infarctions and improve modifiable heart disease risk factors in rural New Ulm, Minnesota.34 HONU was a collaborative partnership of the Allina Health System (which operates the New Ulm Medical Center (NUMC)), the Minneapolis Heart Institute Foundation (MHIF), and New Ulm community leaders. HONU interventions were delivered through health care, worksite and community settings and were open to adults living or working in New Ulm. The NU-HOME study research team built on the established HONU partnerships and relationships to promote synergy with existing adult-focused HONU interventions35,36 and family-friendly community resources to increase the likelihood of long-term sustainability.37 The NU-HOME research team worked intensively with a NU-HOME Action Team which was comprised of HONU members from area schools, Allina Health/NUMC physicians and leadership/staff, UMN Extension Service, Brown County Public Health and community organizations. The Action Team provided consultation on the study design, program development and implementation, and research dissemination. With input from the Action Team, the research team created a logic model (see Figure 1) of program resources, activities, outputs and outcomes using a graphical depiction to facilitate common understanding.38
Figure 1.
NU-HOME Logic Model
2.2. NU-HOME study aims
The NU-HOME trial aimed to prevent excess weight gain among 7–10 year-old children living in rural communities by promoting nutritionally-sound and appropriately-portioned snacks and family meals, healthful home food environments, and positive family activities (e.g., participating in family physical activity, reducing screen time, cooking together as a family).
The primary outcome measure was age- and sex-adjusted child body mass index (BMI) z-score. Additional supportive analyses included body fat percentage at post-intervention, and given the higher prevalence of overweight and obesity in rural communities, we evaluated changes in percent over 50th percentile BMI as indicated by the treatment literature for samples with negatively skewed BMI distributions.39–41 We also evaluated incidence (new cases) of overweight and obesity to estimate progression to more severe levels of obesity.42 We hypothesized that relative to children in the wait-list control group, children in the intervention group would have significantly lower post-intervention BMI z-scores, percent body fat and percent over 50th percentile BMI, and lower incidence of overweight or obesity relative to children in the wait-list control group.
Secondary outcomes included quality of food and beverage availability in the home and at family meals and snacks; children’s dietary intake (e.g., Healthy Eating Index (HEI)-2015, fruits and vegetables, sugar-sweetened beverages); children’s weekly screen time; and children’s weekly minutes of moderate-to-vigorous physical activity (MVPA), total physical activity, and sedentary behavior. We hypothesized that children in the intervention group would have significantly higher post-intervention food and beverage quality available in the home and at family meals and snacks, higher HEI scores and fruit and vegetable intake, lower intake of SSB, higher MVPA, and lower screen time relative to children in the wait-list control group.
2.3. Study inclusion and exclusion criteria
All 7–10 year-old children and their primary meal-preparing parent/guardian (hereafter referred to as parents) who lived within a 50-mile radius of the New Ulm or Sleepy Eye communities were eligible for screening. Inclusion criteria included willingness to be randomized to either the intervention or wait-list control group; willingness to attend baseline, post-intervention, and follow-up data collection visits; availability to attend one of four monthly session offerings in each month from October to April; and requirement that the parent live with the child at least half-time and prepare most of the family’s meals. We considered restricting eligibility to overweight/obese children but did not do so based on the universal prevention approach in the HOME Plus study,31 the literature regarding perceptions of exclusion33 and a belief that a universal prevention approach is more sustainable in communities (i.e., it is less feasible for communities to screen for weight status and more acceptable to offer a program for all interested parties). If more than one child in a family met study criteria, a coin was flipped to choose which child would participate in assessments. Exclusion criteria were planning on moving from the area in the next six months and the existence of a medical condition(s) or food allergies contraindicating intervention program participation.
2.4. Recruitment
Together the UMN staff and the Action Team developed comprehensive recruitment strategies. Letters were mailed directly to parents of children in the targeted age range as identified by the Allina Health informatics team using NUMC electronic health record data; the letter was signed by the pediatrician on the Action Team. We also distributed flyers at the NUMC pediatric clinic and throughout the community and sent them home from school with children. We also placed a program description in community education brochures, conducted presentations in the community, published a study informational article in two local newspapers, and promoted the study through other public announcement delivery channels. Interested parents were directed to the Evaluation Director (by phone, email, or in person) for eligibility screening. We recruited two cohorts, one year apart (2017 and 2018).
An institutional agreement was created for human subject research oversight between the Quorum Review Institutional Review Board (IRB), an external IRB contracted to review on behalf of Allina Health, and the Institutional Review Board at the UMN. The signed agreement stated Quorum’s IRB would review and approve all materials and protocols for recruitment and intervention delivery since these activities would be conducted primarily by community partners under Allina staff oversight, and the UMN IRB would review and approve all data collection materials and protocols as these activities were conducted by UMN staff. The approval letters between the two review boards were shared.
2.5. Data collection
Research staff called parents to schedule data collection visits that were held in the local public schools; reminder calls were made one day prior. Data collection visits were held at the school district community building. Prior to data collection visits, parent participants were mailed consent and assent forms to review and also a Home Food Inventory to complete at home the night before their data collection visit. At the baseline data collection visit, study procedures were described, questions answered and parent and child participants provided written consent/assent for participation, respectively. We collected household- and individual-level data from parents and individual-level data from children through electronic surveys; parents completed the surveys on iPads or cell phones and staff assisted children by asking survey questions in an interview style and directly entering the children’s responses on iPads. Trained and certified research staff measured parent and child height, weight and body fat. We used UMN supported Research Electronic Data Capture (REDCap) software (http://www.project-redcap.org/) and data were saved directly to UMN password-protected servers. Certified staff conducted 24-hour dietary recalls of child’s intake by interviewing parents (children were present), and children were outfitted with accelerometers to wear for one week. This in-person data collection visit was followed by one 24-hour dietary recall interview conducted by phone in the next two weeks. Nightly text surveys, created in REDCap software, were also sent to parents’ cell phones via Twilio software, to query about frequency and quality of family meals and snacks in real time (parents were randomly assigned to receive a snack survey on seven nights and meals surveys on the other seven nights over the two-week period). The baseline in-person data collection visit took 1.5–2 hours, with shorter data collection visits at post-intervention and follow-up. Families received a $25 gift card for each in-person data collection visit, a $25 gift card for completing the dietary recall interview by phone and returning the ActiGraph monitor via US mail, and a $25 gift card for completing at least four of seven of each of the meal and snack surveys. Thus, families were eligible to receive a total of $75 in gift cards for each data collection period (baseline, post-intervention and follow-up). All data collection procedures and materials were approved by the UMN IRB.
2.6. Randomization
For each cohort, after baseline data collection, families were randomized to the intervention program or wait-list control group by the study statistician using a computer-generated randomization schedule using Stata version 15 (StataCorp LLC, College Station, TX). Given the nature of the behavioral intervention, the research team was not blinded to assignment. Families randomized to the wait-list control group received an abbreviated program after the final follow-up data collection for their cohort was completed.
3. Assessment and outcome measures
Measures associated with the primary and secondary aims were collected at each of the three data collection visits. Demographic characteristics were collected at baseline.
3.1. Anthropometry and puberty
Trained and certified research staff measured child and parent height and weight and body fat percentage using Tanita scales (TBF400-Total Body Composition Analyzer), a stadiometer and standardized procedures.43 Height and weight were then calculated into BMI [weight (kg)/height (cm)2] as a standard indicator of overweight for school-age children44 and adults. Age- and sex-adjusted BMI (kg/m2) using CDC’s growth charts were calculated to determine child BMI percentiles and standardized z-scores (ANTHRO 1.02 software-CDC). Also, percent over 50th percentile BMI was calculated by finding the percentage of each child’s BMI above the age and sex matched BMI of a child at the 50th percentile, based on the CDC BMI percentiles. Child weight status categories were created based on CDC BMI percentiles for age and sex (Underweight=less than 5th percentile, Normal or Healthy weight=5th percentile to less than 85th percentile, Overweight=85th percentile to less than 95th percentile, Obese=95th percentile or greater). Parent weight status categories were based on BMI (Underweight=BMI less than 18.5, Normal or Healthy weight=BMI 18.5 to less than 25, Overweight=BMI 25 to less than 30; Obese=BMI 30 or more). Parent’s reported on their child’s puberty status by answering the question “Has your child entered puberty?” with response options of Yes, No, and Unsure.
3.2. Home food availability
Home food availability was assessed with the Home Food Inventory (HFI). The inventory includes 13 major food categories (e.g., fruits) and allows for calculation of an obesogenic score that counts the number of foods available that may contribute to obesity and a healthy food score that counts the number of healthy foods available in the home. The instrument has substantial criterion and construct validity.45 Data were double entered in REDCap and verified within the software system.
3.3. Quality of foods served at family meals/snacks
Meal quality was assessed with the Evening Meal Screener (EMS), developed and validated by our team,46 and the Snack Screener, respectively. For the EMS, two scale scores were created: one to assess major food group offerings and another to assess the healthfulness of foods served based on food types, preparation method, and added fats. Our previous research showed high criterion validity and adequate one week test-retest reliability for the EMS.46 Outcomes for the Snack Screener include children’s consumption of fruit/vegetables, high-sugar foods and beverages, and prepared/processed foods as snacks.
3.4. Child dietary intake
Trained staff assessed child dietary intake with 24-hour recalls using the Automated Self-Administered 24-hour (ASA24) Dietary Assessment Tool, version 2016, developed by the National Cancer Institute, Bethesda, MD.47 At each data collection timepoint, we completed two 24-hour recall interviews (one weekday and one weekend day) using the multiple pass method with the parent with assistance from their child given the age of the child participants.48–50 The first recall interview occurred in person at the data collection visit and the second recall interview was completed by phone with the target parent/child dyad. Dietary recall interviews were reviewed according to guidelines for reviewing and cleaning data provided by ASA24 and all known issues were examined and corrected as outlined by ASA24 instructions (https://epi.grants.cancer.gov/asa24/resources/cleaning). At each data collection timepoint, data were averaged across the two days to compile average calories and servings of food groups (fruit and vegetables). In addition, we calculated variables to estimate sugar-sweetened beverage consumption and overall dietary quality (using HEI 2015 criteria).51
3.5. Child physical activity,sedentary behavior and screen time
3.5.1. Objective Child Assessment.
Child physical activity was assessed using objective measures and self-report tools from both children and parents. Children wore ActiGraph wGT3X-BT or GT3XP-BTLE accelerometers (Pensacola, FL), a valid and reliable objective measure of physical activity in children, for 7 days using a standard protocol.52,53 Children wore the monitors on their right hip during most waking hours, except when doing water-related activities (e.g., bathing or swimming). Monitors were initialized prior to data collection and were set to begin collecting data at 6:00am on the day after they were distributed to participants. Data were collected and stored in 10-second epochs. Non-wear time (i.e., any period of 60 or more minutes of consecutive zeros) was recoded to missing. Children with three or more days of at least 480 minutes/day of valid wear time were included in analysis. Physical activity intensity was defined by cut points from Evanson:54 sedentary (<100 counts/minute), light (110–2295 counts/minute), moderate (2296–4011 counts/minute), and vigorous (≥4012 counts/minute). Daily average minutes engaged in MVPA (>2296 counts/minute), total physical activity (>101 counts/minute) and sedentary behavior were the outcomes. To increase compliance in wearing the accelerometer and to provide context to the objective accelerometry data, children also completed a 7-day physical activity log. They recorded their physical activities during expected “free time” (3:30–6pm on weekdays and 7am-11pm on weekends.
3.5.2. Child Self-Report.
Children completed a self-report physical activity checklist (PAQ-C) to assess their unstructured and structured play during the school year.55 Focusing on the past 7 days, children reported on frequency (none, 1–2, 304, 5–6 or 7+ times) of 22 different activities; activity during physical education class, recess, lunch, after school, evenings, and weekend; and whether anything prevented them from doing their normal physical activities. This checklist has good internal consistency reliability for both girls and boys (Cronbach’s alpha=0.83 and 0.80, respectively).
3.5.3. Parent Report.
Via survey, parents estimated the amount of time children spent, in minutes and hours, exposed to media during free time in the previous week (e.g., texting, TV, movies/DVDs, gaming devices using a validated instrument.20
3.6. Parent and child psychosocial surveys
Parents reported on the following content as secondary outcomes for the study: family evening meal frequency, meal planning self-efficacy, self-efficacy to prepare a healthy meal, confidence in understanding portion sizes, family meal routines, and parental perception of child cooking skill/frequency. These scales have been used in our previous research with acceptable internal consistency reliability (see statistics for each scale below). Children completed questions regarding their own cooking skills.
3.6.1. Family Evening Meal frequency.
Family dinner frequency items have been used extensively in our previous research and refer to the meal at the end of the day which is the family meal we target for increase in our programming.31,32,56 We modified this item by replacing “dinner” with “evening meal” as our Action Team indicated many people in the rural community refer to the midday meal as “dinner”. Therefore, parents were asked “During the past 7 days, how many times were most members of your family sitting and eating the evening meal together?” Response options ranged from 0 (none) to 7 (7 days per week), with M=5.3, SD=1.95. Cronbach alpha in current sample=0.92.
3.6.2. Family Meal Planning Self-Efficacy.
Meal planning self-efficacy was assessed with a 3-item scale by Storfer-Isser57 and measured fatigue and time pressure as barriers to planning meals. Response options ranged from 0 (never) to 4 (always) with one negative item reverse coded. Sample items include “I plan meals for my child at least one day in advance.” and “I go with the flow and do not plan meals for my child or my family,” and “I plan meals for my child ahead of time when I know I am going to be busy.” Possible/observed range (0–12); M=6.9, SD=2,7; Cronbach alpha in current sample=0.87.
3.6.3. Self-Efficacy to Prepare a Healthy Meal.
A 4-item scale was adapted from Beshara et al.58 and Nothwehr59 by making minor wording changes. Response options range from 1 (Not at all likely) to 4 (Very likely). Sample items included “How likely are you to prepare a healthy meal after a tiring day?” and “How likely are you to prepare a healthy meal when you have not been to the store lately?” Possible/observed range (4–20); M=11.5, SD=3.6; Cronbach alpha in current sample=0.83.
3.6.4. Confidence in Understanding Portion Sizes.
Parent report of their confidence in knowing recommended portion and serving sizes of food was assessed with a 4-item scale created as part of our previous research.32 Response options ranged from 1 (Strongly disagree) to 4 (Strongly agree). Sample items included “I feel confident I know appropriate portion sizes for my child’s meals.” and “I feel confident that I know what a serving size is compared to a portion size.” Possible/observed range (4–16); M=10.8, SD=2.5; Cronbach alpha in current sample=0.89.
3.6.5. Family Meal Routines.
We adapted a 6-item scale by Fiese and Kline60 (Cronbach alpha=0.64; test-retest reliability r=0.69). The scale was adapted by changing the original two sentence format to only one sentence and changing from two response options to three. Response options ranged from 1 (Not true) to 3 (True) with three negative items reverse coded. Sample items include “In my family, everyone is expected to be home for the evening meal.” and “In my family, people feel strongly about eating the evening meal together.” Possible range (6–18); observed range (7–18), M=13.9, SD=2.7; Cronbach alpha in current sample=0.76.
3.6.6. Child Self-Report of Cooking Skill Use/Frequency.
Children’s report of their own use and frequency of cooking skills was assessed with adaptations of scale by Lohse (Cronbach alpha=0.75; test-retest reliability r=0.80).61 We adapted the scale by adding two items (peeling fruits and vegetables and mixing ingredients) and deleting one item (with help, I can use a recipe). Response options ranged from 1 (NO!) to 5 (YES!). Thus, our adapted 9-item scale included the following items: “Can you….” 1) get fruit for a snack for him/herself, 2) get vegetables as a snack for him/herself, 3) help the family make a meal, 4) make a leafy-green salad, 5) cut up food, 6) measure ingredients, 7) follow recipe directions, 8) peel fruit/vegetables, 9) mix ingredients together. Possible range (9–45); observed range (18–45); M=34.3, SD=6.3; Cronbach alpha in current sample = 0.68.
3.6.7. Parent Report of Child Cooking Skill Use/Frequency.
Parent perception of their child’s use of cooking skills was assessed by adding the question stem “How often does your child….” to the items described above for children’s self-report. Response items ranged from 0 (never) to 3 (often). Possible range (0–27); observed range (3–23); M=13.1, SD=4.1; Cronbach alpha in current sample =0.77.
3.7. Demographic characteristics
Rural-Urban Commuting Area (RUCA) Codes were designated for each household based on census tract and zip codes to describe the rural communities as isolated small rural, small rural and large rural and metropolitan areas.62,63 All other demographic data were reported by parents via survey. Age was calculated from child or parent date of birth and date of data collection. Parents reported on their own and their child’s sex and ethnicity (Hispanic or Latino or not Hispanic or Latino). Parents could choose more than one race category (Asian, African American, Native American, Pacific Islander, white) and categories were collapsed into white or not white/multiracial for reporting purposes. Parent marital status was collected with the question “What is your marital status?” with response options of Married; Not married, Living with significant other; Separated; Divorced; Widowed; Single, Never married. Parent education was assessed with the question “What is the highest level of education you have completed?” with the response options of Attends or has attended high school; Completed high school or GED; Attends or has attended college or technical school; Completed an associate’s school in college or completed technical school; Completed a bachelor’s degree in college; Attends or has attended graduate school; Completed graduate school. Response options were collapsed into less than or equal to high school; some college; Associate’s degree; Bachelor’s degree or higher for reporting purposes. Food Insecurity was measured with the 6-item Short form of the Household Food Security Scale.64 A family was considered to receive economic assistance if they answered YES to one of the following two questions “Does your child receive free or reduced priced school lunches at school?” or “Does your household receive public assistance?”
4. NU-HOME Intervention Program Description
4.1. Overview
Although the NU-HOME program was adapted from our previously evaluated curriculum30 specifically for a rural community, the NU-HOME program goals remained similar because of their consistency with effective obesity intervention components as described in the literature48,65,66 and from the advice of our Action Team who considered fruit and vegetable intake, reductions of SSBs and portion control to be key areas needed in family-focused intervention in their communities. Family physical activity was also added to the NU-HOME intervention program. Like our previous research, the NU-HOME program, goals, and behavioral messages were guided by Social Cognitive Theory (SCT)67 and a socio-ecological framework.68,69 SCT is useful in understanding and explaining health behavior changes and external influences on behaviors.70,71 Ecological models emphasize the importance of multiple levels of environmental influences on health behavior.68 We proposed that home environmental and family behavioral factors would impact children’s eating and physical activity behaviors that then would contribute to excess weight gain. Efforts to change modifiable behaviors under parents’ control such as types of foods served and accessible at home, meal preparation, and children’s physical activity were logical targets for obesity prevention.44,72 In particular, intervention session activities were designed to enhance child and parent skills, self-efficacy, and outcome expectations related to trying new vegetables, cooking healthful meals, sharing mealtimes, and participating in physical activities together.
4.2. Program staff
A Registered Dietitian and a health educator from MHIF were the lead NU-HOME interventionists and were trained in intervention delivery using a scripted curriculum, goal-setting phone call methods, food safety, and first aid in accordance with the study protocol. During sessions, the lead interventionists were assisted by undergraduate nursing and elementary education students who completed training in human subjects research (i.e., CITI), food safety and intervention delivery. Students assisted with the study as part of a service-learning component to an existing course they were taking at their institution which was located close to the rural community where the research took place.73 Students assisted with set-up, cooking station assistance, family meal preparation, parent and child group activities, and clean-up.
4.3. Intervention materials
The intervention materials that emphasized the key goals, content, and behavioral messages for families included a leader’s curriculum, participant guidebook, and participant website. The leader’s NU-HOME intervention curriculum included a list of needed supplies and hand-outs, intervention script with timings, points for discussion, and activities. The participant guidebook, Promoting Healthy Behaviors in the Home! –Your Family’s Guide to NU-HOME was given to families at the first session of the intervention. Families referred to the guidebook throughout the program as it included session topics, goal-setting activities related to the behavioral messages, session recipes and additional resources to supplement the topics discussed at sessions. Program messages were also reinforced on a secure, password-protected website created for NU-HOME participants. Session materials, additional recipes and physical activity videos were made available on the website after each monthly session as well as recipes to highlight the featured vegetable of the month. Links to websites and materials for download that supported and reinforced the nutrition and physical activity message of each session, including community resources, were also featured on the website. Finally, a physical activity and a nutrition challenge were featured each month for the family to complete and record on the website blog.
4.4. Intervention delivery
In-person family intervention sessions were held monthly for seven months (October-April). Sessions were held in the Family and Consumer Science classrooms at the local public schools to accommodate cooking space for multiple families, and other adjacent rooms were used for physical activity breaks, parent and child groups and childcare. Every session was offered four times a month in the early evening to allow for scheduling flexibility and ensure the families fit in the kitchen space. All household members of parents and children enrolled in the study were encouraged to attend and participate. Childcare was available for children less than 6 years old, and families received a $10 gas card for each session to help with transportation cost owing to the travel necessary in these rural communities and to enhance retention and adherence. To encourage session attendance, each time families came to a session or completed a website challenge, they received entries for a drawing for a personal home visit by a local chef. Families unable to attend a session received a telephone call from their interventionist who recapped the session and were able to download all session materials from the website.
4.5. NU-HOME program intervention components
4.5.1. Multiple family group sessions.
The monthly, two-hour in-person NU-HOME sessions included multiple family groups and consisted of interactive educational and hands-on experiential learning activities about key nutrition, cooking and physical activity topics. Topic content was designed to facilitate family achievement of intervention goals and session objectives as shown in Table 1.
Table 1.
NU-HOME study program intervention session components
| Month | Session Type | Topics Covered | Parent Group (40 minutes) | Child Group (40 minutes) |
|---|---|---|---|---|
| 1: Let’s get Started | In-person Group | •Program goals and goal setting •Benefits and strategies for family meals •How to make positive family meals •Cooking skills •Family physical activity – line dancing •Highlight a community resource |
•Benefits to family meals discussion •Barriers and strategies for family meals •Goal setting discussion |
•Knife skills demonstration and practice •Making a salad activity •Brainstorming ideas for family meal discussion topics |
| Online augmentation session | ||||
| Telephone call | •Family and individual goals | |||
| 2: Think Outside the Box | In-person Group | •Components of a healthful meal (MyPlate) •Meal planning strategies •Shopping strategies to save money and promote meal planning •Cooking skills •Family physical activity –wheel of warm-up •Highlight a community resource |
•Meal planning •Shopping strategies for buying healthful foods •Planning in advance for left-overs |
•Peeling demonstration and practice •MyPlate discussion and meal planning activity •Kids role in shopping |
| Online augmentation session | ||||
| 3: Balance, Balance, Keep the Balance | In-person Group | •Food labels and meal planning •Portion size versus serving size •Eating healthfully away from home •Basic cooking skills •Family physical activity – team hustle •Highlight a community resource |
•Label reading •Using nutrition information for eating out •Portion sizes versus serving sizes •Strategies to decrease portion sizes |
•Portion sizes vs serving size •Portion size and common object matching •Food labels •Prepare a fruit parfait |
| Online augmentation session | ||||
| Telephone call | •Family and individual goals | |||
| 4: Power Down to Power up | In-person Group | •Reducing screen time and increasing positive conversations at meals •Increasing physical activity as a family •Healthful snacking •Family physical activity – garbage ball game •Highlight a community resource |
•Strategies to decrease screen time and increase family physical activity •Fat and sugar in common snacks •Strategies to limit unhealthy snacks and ideas for healthful snack foods |
•Strategies to decrease screen time and increase family physical activity •Measuring fat and sugar in common snacks •Strategies to limit unhealthy snacks and ideas for healthful snack foods |
| Online augmentation session | ||||
| 5: Less Sugar – A sweet deal | In-person Group | •Increase physical activity as a family •Decrease sugar-sweetened beverages (SSB) •Family physical activity – yoga •Highlight a community resource |
•Goals for family physical activity •Sugar in beverages matching game •Strategies to reduce SSB intake and rehydrate |
•Family physical activity •Sugar in beverages matching game •Alternatives to SSs and taste testing |
| Online augmentation session | ||||
| Telephone call | •Family and individual goals | |||
| 6: “Agreenable” meals and snacks | In-person Group | •Increasing FV availability at home •Eat more FV each day •Family physical activity – 2 × 2 fitness •Highlight a community resource |
•Increasing home FV availability •Increasing FV served at meals and snacks |
•Identify FV from every color of the rainbow •Taste test FV •Make a fruit kabob |
| Online augmentation session | ||||
| 7: The future is bright | In-person | •Reflect on program goals •Identify changes made and maintenance •Family physical activity – charades •Highlight a community resource |
•Reflect on changes made and maintenance •Brainstorm strategies to improve the healthfulness of “go-to” meals •Create family “recipe” to describe how each family was successful in making healthful changes and plans to sustain those changes |
•Review of behavioral messages and cooking skills learned •Use stickers to plan a healthful meal using MyPlate “race” •Make a smoothie |
| Telephone call | •Follow-up on goals/maintenance | |||
4.5.2. Introduction and Taste-Testing.
Each session began with an introduction to the session topic, and for sessions 2–7, the introduction also included a review of the past month’s topic, family goal setting and progress, and time for participant questions. Next, to promote vegetable exposure, participants were offered tastings of a seasonal vegetable and were asked to try it and rate it on a written form in their binders. Participants received the vegetable of the month to take home to encourage repeated exposures before the next session and to work towards the program goals of increasing availability of fruits/vegetables at home and serving them at family meals and snacks.
4.5.3. Meal Preparation.
Following taste-testing, participants prepared a meal together. This meal preparation activity allowed parents and children to build confidence in cooking skills and practice skills together (e.g., meal planning, reading recipes, measuring ingredients, knife skills). The recipes families prepared were selected a priori by the research team to feature vegetables and fruits, be family- and budget-friendly, tasty, and simple to make with a limited number of readily available ingredients. Recipes at each session included a meat entrée, a vegetarian entrée, a salad, and a side dish of fruit or vegetables.
4.5.4. Physical Activity Breaks.
The physical activity break allowed parents and children to move their bodies together after meal preparation (but before eating). These breaks lasted between 5 and 10 minutes, often included music, and were designed as activities they could do inside their homes year-round with no additional equipment or cost (i.e., yoga, line dancing, and games).
4.5.5. Small Groups.
To accommodate different adult and child learning styles, parents and children were separated for some activities (see Table 1). Parent group activities focused on family behavior change related to program goals and session objectives, which often included discussions, strategizing around barriers/obstacles for change, learning new information, and practicing skills (e.g., meal planning). Children’s small group activities focused on similar content but delivery was more developmentally-appropriate through experiential activities (e.g., game format). In addition, children were given time to continue practicing kitchen skills like using a knife and measuring ingredients with staff supervision.
4.5.6. Family Meal and Conclusion.
At the end of the small group component, parents and children came together to eat the family meal made by all participating families. Staff served family members and encouraged families to try all foods as they went through a buffet line. A sample plate was available for viewing to demonstrate serving sizes and to reinforce healthy portion sizes. After the meal, a physical activity or nutrition resource in the community was highlighted (i.e., programs at the nearby state park, grocery store tours designed to help families shop smarter for healthful foods), and families completed session evaluations regarding session content, food and activities, and selected family-level goals for the next month.
4.5.7. Parent Goal-Setting Telephone Calls.
Within a couple of weeks of each session 1, 3, 5 and 7 (see Table 1), lead interventionists conducted four brief (~20 minute) telephone calls with parents. The individual calls were tailored to give parents the opportunity to focus on a key NU-HOME goal that was specific to their family that they may have wanted to address in more detail. Parents could choose a new goal at any point or continue to work on one goal throughout the program. The interventionists followed established protocols using motivational interviewing principles74–76 and used open-ended questions and reflections to elicit participant’s motivation and desire for change.77
4.5.8. Data safety and monitoring plan.
Because of the low risk status of this study, the Data Safety Monitoring Plan (DSMP) for this trial focused on close monitoring by the Principal Investigator (PI) in conjunction with a safety officer. The plan includes prompt reporting of any serious adverse events to the NIH and the University of Minnesota IRB. An injury log and a checklist for the Safety Officer were developed to meet the goals of this plan. Data regarding treatment duration for intervention subjects (number of sessions attended), adverse events, serious adverse events, deaths, and other situations that might be of safety concern were continually collected and documented. The Evaluation Director, in conjunction with the MHIF intervention staff, were responsible for assembling the data, producing these reports, and providing them to the PI as well as assuring that all parties obtain copies of these reports.
4.5.9. Wait-List Control Delayed Intervention.
The wait-list control group families received an abbreviated NU-HOME program in a three-session format that was delayed until after follow-up data collection. Our program design purposefully included a “train the trainers” model to increase good will, stakeholder satisfaction, build capacity and facilitate sustainability. Thus, our collaborators from the University of Minnesota Extension’s Food, Health and Nutrition and 4-H units co-facilitated delayed intervention delivery with MHIF staff. The same school facilities were used as intervention sites and the abbreviated program included the primary topics of the benefits of family meals, healthful meal planning and shopping, child food preparation skills, portion sizes, and healthful food and beverage choices as these topics were determined by the research team to be the most enjoyed by participants in the main trial and those likely most influential for weight-related behavior change. Recipes used for the wait-list control delayed program were either the most highly rated recipes by the first cohort in the primary intervention delivery or they were aligned with food orders for the primary intervention when delivery overlapped between the cohort 1 wait-list control program and NU-HOME primary intervention for cohort 2.
5. Process measures
Process variables related to recruitment, intervention program fidelity, validity and dose are important to assess in health behavior change programs.78,79 We assessed these variables regularly and they are shown in Table 2. A recruitment log assessed external validity. Intervention program fidelity occurred through several activities: 1) intervention staff completed checklists of activities after each session to ensure all activities were completed, 2) noninterventional research staff and students were trained to observe and record intervention session fidelity at sessions 2, 4, and 6. Fidelity checklists were reviewed by the PI and findings were discussed with the interventionists to reinforce study goals and provide course correction if needed. Participant attendance (number of adults, number of children and number at childcare for each family) at each session was maintained throughout the program to facilitate calculations of intervention dose. Motivational/goal-setting call completion and topics were also documented in REDCap software notes.
Table 2.
NU-HOME study process evaluation measures
| Intervention component | Data collected | Method | Process evaluation conceptual category |
|---|---|---|---|
| Recruitment | •Referral methods, participation rate by site | •Recruitment log | •External validity (recruitment bias) |
| Treatment delivery | •Delivery of individual components •Direct observation (3 sessions) |
•Intervention staff checklist •Evaluation staff observation checklist |
•Fidelity •Fidelity •Fidelity |
| Receipt of treatment | •Family- and individual level attendance •Encouragement/goal setting call completion •Sustained use of program materials and activities |
•Attendance log •Phone call log and goal information •Parent surveys |
•Dose •Dose and fidelity •Dose and fidelity |
| Session satisfaction and acceptance | •Participant satisfaction and acceptance | •Parent and child session evaluations | •Fidelity |
| Contamination | •Competing/complementary nutrition and activity programs | •Parent survey •Cross-check with HONU program lists |
•Internal validity •Internal validity •Internal validity |
| Self-assessed change (intervention group only) | •Reported behavior changes behavior | •Parent surveys | •Internal validity |
At the post-intervention measurement, parents in the intervention group completed a survey to measure process outcomes of the following: 1) program satisfaction, 2) use of the Family Guidebook outside of sessions, 3) if/which session recipes were used at home, and 4) behavioral changes they attributed to NU-HOME. Children also completed a short process survey on which they also reported on program satisfaction and behavioral changes from the NU-HOME program. All parents in both the intervention and wait-list control groups completed surveys to assess exposure to other nutrition and physical activity programming to evaluate possible program contamination.
According to CBPR principles, early stage work should include an ongoing evaluation of the extent to which CBPR principles are followed.25 Thus, we developed guidelines to describe the academic/community partnership and how decisions were made. Action Team members periodically complete a CBPR instrument that assessed decision-making processes for: 1) participant involvement, 2) shaping the purpose/scope of research, 3) research implementation and context, and 4) nature of research outcomes.
6. Sample size and power considerations
Power calculations for the study outcome of age and sex-adjusted child BMI z-score were conducted to estimate sample size. Power calculations were based on two assessment time points, correlation over time, and variability of age- and sex-adjusted BMI z-scores. Using child (<11 years old) data from the HOME Plus trial, we estimated a within-child correlation (ρ) between the primary outcome measurements over time of 0.9. Utilizing a baseline-adjusted analysis approach, with a sample size of 96 (48 per group), we will be able to detect an effect size (ES) of 0.25 for age- and sex-adjusted BMI z-scores at 80% power (type 1 error rate is 0.05). This ES corresponds to approximately 1.4 kg decrease in average weight gain between intervention and control groups (similar to our significant post-hoc findings with prepubescent children in the HOME Plus study).31 This corresponding decrease was estimated using BMIi=M(1+LSzi)1/L formula with age-and sex-specific L,M,S parameters (L=power in the Box-Cox transformation, M=median, S=generalized coefficient of variation) and average weight and height values for 8–10 year-old boys and girls [at 50th or higher percentile for weight].80 With an estimated attrition rate of 15%, our enrollment goal was 120 families (240 parent/child dyads) to allow for a final effective sample of about 100 families at final follow-up.
7. Statistical analysis
Multiple linear regression models will be constructed for the child outcomes of post intervention BMI-z score, percent over 50th percentile BMI, and percent body fat, with the predictor of interest being the treatment group. All models will be adjusted for the outcome measure at baseline, child sex, baseline child age, and a baseline indicator of family economic assistance. Incidence of overweight or obesity will be evaluated by defining a binary variable indicating whether or not a child changed weight categories from baseline to post intervention into either the overweight or obese weight status categories. Logistic regression will be used with this indicator as the outcome, adjusting for an indicator of baseline overweight or obese weight status, treatment group, child sex, baseline child age, and baseline family economic assistance status. For secondary outcomes, multiple linear regression models will be constructed for the following post-intervention outcomes: quality of food and beverage availability in the home and at family meals and snacks; children’s dietary intake; children’s weekly minutes of MVPA, total physical activity and sedentary behavior; and children’s weekly screen time, with the predictor of interest being the treatment group. All models will be adjusted for the outcome measure at baseline, child sex, baseline child age, and baseline indicator of family economic assistance.
All comparisons will be performed under the intent-to-treat principle (i.e., all randomized participants will be included and analyzed according to group assignment, regardless of compliance). All p-values will be two-sided and considered at the 0.05 level for statistical significance. Analyses will be carried out in R, version 3.6.1. (R Core Team (2019).81
8. Results
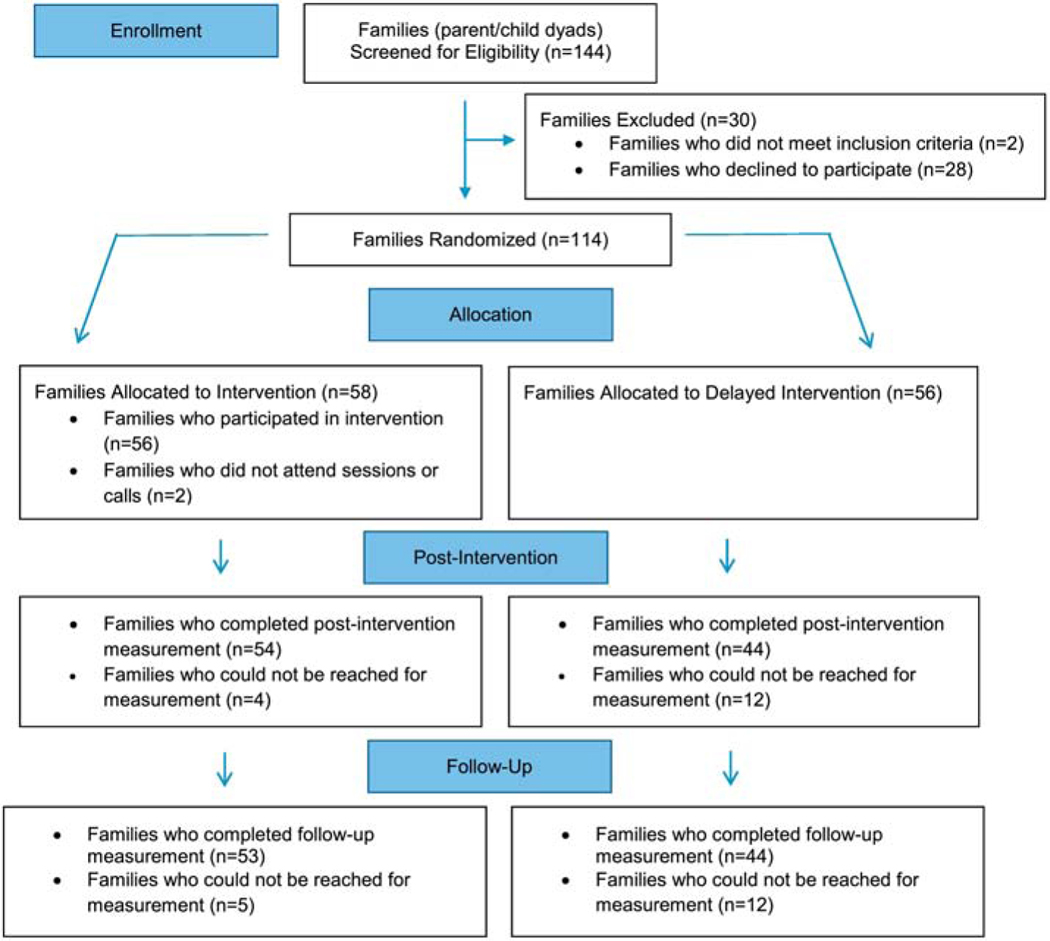
Figure 2 shows the results of the NU-HOME recruitment, eligibility status, and enrollment for 2016 (cohort 1) and 2017 (cohort 2) combined. A total of 144 parent/child dyads completed screening and 30 did not meet eligibility criteria or were not interested in study participation after screening. One hundred-fourteen parent/child dyads agreed to participate in the study, provided written consent/assent, completed baseline measurement and were randomized to the NU-HOME intervention group (n=58) or the wait-list control group (n=56). Of the 114 enrolled parent/child dyads, 53% were recruited from the letter sent from or information provided by the NUMC, 19% were recruited from flyers sent home by the schools, 12% found out about the study from a friend of family member, and the remaining 16% were recruited at community events, the newspaper articles or newsletters, social media or their employer.
Figure 2.
NU-HOME Consort Flow Diagram for Randomized Clinical Trial
Child and primary parent/household demographic characteristics at baseline are summarized as mean (SD) for continuous and count (%) for categorical variables (Table 3). Child participants were about 9 years old on average. More than half the child sample were girls and slightly less than half the sample were overweight or obese. Participating parents were almost exclusively female, most were married, and were on average in their late 30s. About half of parents were college educated. Racial/ethnic diversity of both the child and parent samples were limited. More than three-quarters of the parents were overweight or obese. Slightly less than one-third of households received economic assistance but about one in five were food insecure. Household RUCA codes identified the following areas for our sample: large rural (code 4 or 5; 73%), small rural town (code 7; 16%), isolated small rural (code 10; 9%) and metropolitan (code 2; 2%).
Table 3.
NU-HOME study baseline child, parent and household characteristics (n=114 parent/child dyads)
| CHILD Characteristics | |
|---|---|
| Age (years) | |
| Mean (SD) | 8.9 (1.1) |
| Median (range) | 8.8 (6.9, 11.0) |
| Gender, N (%) | |
| Female | 67 (58.8) |
| Male | 47 (41.2) |
| Ethnicity, N (%) | |
| Not Hispanic/Latino | 106 (93.0) |
| Hispanic/Latino | 8 (7.0) |
| Race, N (%) | |
| Not white or multiracial | 8 (7.0) |
| White | 106 (93.0) |
| BMI Category, N (%) | |
| Healthy weight | 62 (54.4) |
| Overweight | 24 (21.1) |
| Obese | 28 (24.6) |
| BMI z-score | |
| Mean (SD) | 0.9 (1.0) |
| Median (Range) | 0.9 (−1.4–3.0) |
| % Over 50th percentile BMI | |
| Mean (SD) | 19.6 (24.3) |
| Median (Range) | 12.5 (−14.9, 107.8) |
| % Body Fat | |
| Mean (SD) | 22.9 (7.4) |
| Median (Range) | 20.9 (13.5, 46.6) |
| Puberty, N (%) | |
| Not started puberty | 100 (87.7) |
| Started puberty | 3 (2.6) |
| Unsure | 11 (9.6) |
| PARENT/HOUSEHOLD Characteristics | |
| Age (years) | |
| Mean (SD) | 37.8 (5.3) |
| Median (Range) | 38.1 (27.3–55.0) |
| Gender, N (%) | |
| Female | 111 (97.4) |
| Male | 3 (2.6) |
| Ethnicity, N (%) | |
| Not Hispanic/Latino | 109 (95.6) |
| Hispanic/Latino | 5 (4.4) |
| Race, N (%) | |
| Not white or multiracial | 4 (3.5) |
| White | 110 (96.5) |
| Marital Status, N (%) | |
| Married or not married living with significant other | 97 (85.0) |
| Separated, divorced, widowed, never married | 17 (15.0) |
| Education, N (%) | |
| Less than or equal to High School | 13 (11.5) |
| Some college | 25 (22.1) |
| Associated degree | 16 (14.2) |
| Bachelor’s degree or higher | 59 (52.2) |
| Missing | 1 |
| BMI Category, N (%) | |
| Healthy weight | 27 (24.1) |
| Overweight | 39 (34.8) |
| Obese | 46 (41.1) |
| Missing | 2 |
| BMI | |
| Mean (SD) | 29.9 (6.75) |
| Median (Range) | 28.7 (19.0, 51.6) |
| Missing (pregnant) | 2 |
| % Body Fat | |
| Mean (SD) | 34.7 (8.6) |
| Median (Range) | 33.7 (19.8–53.2) |
| Missing (pregnant or had pacemaker) | 3 |
| Food Insecurity, N (%) | |
| Insecure | 21 (18.4) |
| Secure | 93 (81.6) |
| Economic Assistance, N (%) | |
| Receives assistance | 32 (28.1) |
| Does not receive assistance | 82 (71.9) |
9. Discussion
The NU-HOME study used a randomized controlled trial (RCT) design to test the effectiveness of a childhood obesity prevention program for school-age children and their families living in rural communities. The study is unique in its focus on the quality of the home food environment, the frequency and quality of family meals and snacks, an emphasis on family physical activity, and program delivery in community settings. Furthermore, measurement was conducted with gold standard procedures and assessment measures of dietary quality, physical activity and weight-related outcomes. The NU-HOME intervention program is a universal prevention program intended to impact health by supporting children in the maintenance of a healthy weight trajectory and prevention of excess weight gain as they grow, develop and mature while also preventing progression to more severe obesity among children already experiencing overweight or obesity. Our attention to high-risk rural children attempts to address the continued health disparities of rural communities, and our universal approach increases reach of programmatic content beyond just those children identified as being at higher risk for excess weight gain.
If the NU-HOME program is effective in preventing excess weight gain or impacting secondary outcomes related to dietary intake and/or physical activity among the rural study participants, there is great potential for translating the intervention to be broadly implemented throughout rural communities nationally to increase knowledge and build skills that could lead to behavior change and eventual health equity among rural youth. The NU-HOME program has high translation potential through our collaboration with engaged and knowledgeable community partners and our implementation in a real-world setting. As part of our academic-community partnership we purposefully used a “train the trainers” model for intervention delivery to facilitate sustainability. Our plans for engagement with UMN Extension Service partners in program development and delayed programming for control participants further facilitates program sustainability through potential delivery of a program for rural youth throughout the state(s) by Extension Service faculty and staff. Although Extension staff routinely deliver meal preparation and cooking demonstrations, additional tangible and financial resources for staffing, equipment (stoves/ovens, refrigeration, cooking utensils), food costs, kitchen and childcare space would be needed to implement the hands-on skill-based learning provided in the NU-HOME program.
In addition, synergy between the NU-HOME program and HONU programs will provide other rural communities with examples of effective, community-wide health initiatives for children and adults. With our use of Social Cognitive Theory and a social ecological framework, we combined individual-level behavior change from the NU-HOME program with policy, systems and environmental changes occurring in the broader community as part of the HONU program to promote health. This approach reaches people where they are at within their environmental context and may have the potential to have a broader impact on the community at large.
An important component of the NU-HOME trial is the dissemination phase. We will begin this phase by conducting meetings with the Action Team and Extension colleagues to decide on timing of and venues for dissemination. We expect venues will include traditional academic journals, research conferences, technical and lay reports (to parents, health care professionals, stakeholders), and local non-research conferences at schools, community events, and media.82 It is likely all academic and community partners will collaboratively author all dissemination products.
The NU-HOME RCT was a unique collaborative effort of academic and health system researchers, interventionists and community leaders that aimed to prevent childhood obesity in rural communities through engagement of the whole family in an interactive intervention. The NU-HOME trial design, high-quality measurement, and focus on promoting nutritionally-sound and appropriately-portioned snacks and family meals, healthful home food environments, and positive family activities all provide ways to extend the limited existing family-focused programming in rural communities.
Acknowledgements
The following members of our Action Team deserve special thanks for their contributions to the design, development, adaptation and implementation of the NU-HOME study: Antonio Alba Meraz, Kari Beran, Jeff Bertrang, Mikayla Bruggeman, Carisa Buegler, Daryn Collins, Lisa Compton, Nadia Crooker, Stef Dietz, Denise Kamm, Kelly Kunkel, Jennifer Mauer, Jasmine Moreno, Laura Schmidt, Betty Uehling, and Cindy Winters. We thank the following research team members and student volunteers who contributed to the large and multiple data collection efforts several hours away from the University of Minnesota: Lori Rathburn, Yazmin Cespedes, Jessica Ramos, Christie Martin, Eydie Kramer, Sam Sommerness, Brooke Wagner, Stephanie Grace, Amanda Folk, Amanda Schmid, Sean Vercellone, Claudia Murray, Chloe Perrizo, Kelly Kruse, Helayna Sjoberg, Natalia Cismesia, Rachel Groklags, Carly Molenaar, Krista Gapp, Katherine Bastain, Michael Urvig, Hannah Coleman, and Zac Stepanski. We thank the following interventionists and support staff for their expertise and professionalism in intervention implementation: Jennifer Beaudette, Kate Callahan Schmitz, Shawn Hildebrandt, Hannah Hunziker, Rachel Jones, Kendra Newman, Abigail Schwab, Mary Story, Haley Braun, and Emily Ray. This project would have been much more difficult to complete without the generosity and flexibility of School Superintendents Jeff Bertrang and John Cselovszki for their support of the study and use of their school facilities. Our collaboration with faculty instructors Laurel Ostrow and Pat Beierwaltes from the Minnesota State University at Mankato and their students contributed significantly to the success of the study. We also thank Jackie Boucher for her contributions to the development of the NU-HOME study and Justin Clark for his assistance with baseline descriptive analyses for this manuscript.
Funding
This study was supported by National Institutes of Health (NIH) awards 1R01HL123699 (National Heart, Lung, and Blood Institute; NHLBI) and UL1TR002494 (National Center for Advancing Translational Sciences; NCATS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NHLBI, the NCATS or the NIH. This study is registered with NIH ClinicalTrials.gov: NCT02973815.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Flegal KM, Ogden CL, Yanovski JA, et al. High adiposity and high body mass index-for-age in US children and adolescents overall and by race-ethnic group. Am J Clin Nutr. 2010;91(4):1020–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity Among Adults and Youth: United States, 2015–2016. NCHS Data Brief. 2017(288):1–8. [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll MD, Fakhouri TH, et al. Prevalence of Obesity Among Youths by Household Income and Education Level of Head of Household - United States 2011–2014. MMWR Morbidity and Mortality Weekly Report. 2018;67(6):186–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh AS, Mulder C, Twisk JW, van Mechelen W, Chinapaw MJ. Tracking of childhood overweight into adulthood: a systematic review of the literature. Obes Rev: an official journal of the International Association for the Study of Obesity. 2008;9(5):474–488. [DOI] [PubMed] [Google Scholar]
- 5.Bjorge T, Engeland A, Tverdal A, Smith GD. Body mass index in adolescence in relation to cause-specific mortality: a follow-up of 230,000 Norwegian adolescents. Am J Epidemiol. 2008;168(1):30–37. [DOI] [PubMed] [Google Scholar]
- 6.Neumark-Sztainer D, Story M, Faibisch L. Perceived stigmatization among overweight African-American and Caucasian adolescent girls. J Adoles Health : official publication of the Society for Adolescent Medicine. 1998;23(5):264–270. [DOI] [PubMed] [Google Scholar]
- 7.Must A, Strauss RS. Risks and consequences of childhood and adolescent obesity. Int J Obes Related Met Dis: journal of the International Association for the Study of Obesity. 1999;23 Suppl 2:S2–11. [DOI] [PubMed] [Google Scholar]
- 8.Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff (Millwood). 2009;28(5):w822–831. [DOI] [PubMed] [Google Scholar]
- 9.Biener A, Cawley J, Meyerhoefer C. The Impact of Obesity on Medical Care Costs and Labor Market Outcomes in the US. Clin Chem. 2018;64(1):108–117. [DOI] [PubMed] [Google Scholar]
- 10.Johnson JA 3rd, Johnson AM Urban-rural differences in childhood and adolescent obesity in the United States: a systematic review and meta-analysis. Child Obes (Print). 2015;11(3):233–241. [DOI] [PubMed] [Google Scholar]
- 11.Davis AM, Bennett KJ, Befort C, Nollen N. Obesity and related health behaviors among urban and rural children in the United States: data from the National Health And Nutrition Examination Survey 2003–2004 and 2005–2006. J Ped Psychol. 2011;36(6):669–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greening L, Harrell KT, Low AK, Fielder CE. Efficacy of a school-based childhood obesity intervention program in a rural southern community: TEAM Mississippi Project . Obesity (Silver Spring). 2011;19(6):1213–1219. [DOI] [PubMed] [Google Scholar]
- 13.Schetzina KE, Dalton WT 3rd, Lowe EF, et al. A coordinated school health approach to obesity prevention among Appalachian youth: the Winning with Wellness Pilot Project. Fam Community Health. 2009;32(3):271–285. [DOI] [PubMed] [Google Scholar]
- 14.Schetzina KE, Dalton WT 3rd, Pfortmiller DT, Robinson HF, Lowe EF, Stern HP. The Winning with Wellness pilot project: rural Appalachian elementary student physical activity and eating behaviors and program implementation 4 years later. Fam Community Health. 2011;34(2):154–162. [DOI] [PubMed] [Google Scholar]
- 15.Williamson DA, Champagne CM, Harsha DW, et al. Effect of an environmental school-based obesity prevention program on changes in body fat and body weight: a randomized trial. Obesity (Silver Spring). 2012;20(8):1653–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ling J, King KM, Speck BJ, Kim S, Wu D. Preliminary assessment of a school-based healthy lifestyle intervention among rural elementary school children. J Sch Health. 2014;84(4):247–255. [DOI] [PubMed] [Google Scholar]
- 17.Cohen JF, Kraak VI, Choumenkovitch SF, Hyatt RR, Economos CD. The CHANGE study: a healthy-lifestyles intervention to improve rural children’s diet quality. J Acad Nutr Diet. 2014;114(1):48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Birch LL, Davison KK. Family environmental factors influencing the developing behavioral controls of food intake and childhood overweight. Pediatr Clinics North Am. 2001;48(4):893–907. [DOI] [PubMed] [Google Scholar]
- 19.Briefel RR, Wilson A, Gleason PM. Consumption of low-nutrient, energy-dense foods and beverages at school, home, and other locations among school lunch participants and nonparticipants. J Am Diet Assoc. 2009;109(2 Suppl):S79–90. [DOI] [PubMed] [Google Scholar]
- 20.Rideout W, Roberts DF, Foehr UG. Generation M: Media in the lives of 8–18 year olds. A Kaiser Family Foundation Study. 2005. [Google Scholar]
- 21.The National Advisory Committee on Rural H, Human S. The 2011 Report to the Secretary: Rural Health and Human Services Issues. NACRHHS;2011. [Google Scholar]
- 22.Brown B, Harris KJ, Heil D, et al. Feasibility and outcomes of an out-of-school and home-based obesity prevention pilot study for rural children on an American Indian reservation. Pilot Feasibility Studies. 2018;4:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janicke DM, Lim CS, Perri MG, et al. Featured Article: Behavior Interventions Addressing Obesity in Rural Settings: The E-FLIP for Kids Trial. J Pediatr Psychol. 2019;44(8):889–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White AA, Colby SE, Franzen-Castle L, et al. The iCook 4-H Study: An Intervention and Dissemination Test of a Youth/Adult Out-of-School Program. J Nutr Educ Behav. 2019;51(3s):S2–s20. [DOI] [PubMed] [Google Scholar]
- 25.Israel BA, Coombe CM, Cheezum RR, et al. Community-based participatory research: a capacity-building approach for policy advocacy aimed at eliminating health disparities. Am J Public Health. 2010;100(11):2094–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coombe CM, Schulz AJ, Guluma L, et al. Enhancing Capacity of Community-Academic Partnerships to Achieve Health Equity: Results From the CBPR Partnership Academy. Health Promot Pract. 2018:1524839918818830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ward M, Schulz AJ, Israel BA, Rice K, Martenies SE, Markarian E. A conceptual framework for evaluating health equity promotion within community-based participatory research partnerships. Eval Program Plann. 2018;70:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fulkerson JA, Rydell S, Kubik MY, et al. Healthy Home Offerings via the Mealtime Environment (HOME): feasibility, acceptability, and outcomes of a pilot study. Obesity (Silver Spring). 2010;18 Suppl 1:S69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fulkerson JA, Neumark-Sztainer D, Story M, et al. The Healthy Home Offerings via the Mealtime Environment (HOME) Plus study: design and methods. Contemp Clin Trials. 2014;38(1):59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flattum C, Draxten M, Horning M, et al. HOME Plus: Program design and implementation of a family-focused, community-based intervention to promote the frequency and healthfulness of family meals, reduce children’s sedentary behavior, and prevent obesity. Int J Behav Nutr Phys Act. 2015;12:53–015-0211–0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fulkerson JA, Friend S, Flattum C, et al. Promoting healthful family meals to prevent obesity: HOME Plus, a randomized controlled trial. Int J Behav Nutr Phys Act. 2015;12:154–015-0320–0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fulkerson JA, Friend S, Horning M, et al. Family Home Food Environment and Nutrition-Related Parent and Child Personal and Behavioral Outcomes of the Healthy Home Offerings via the Mealtime Environment (HOME) Plus Program: A Randomized Controlled Trial. J Acad Nutr Diet. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lim CS, Follansbee-Junger KW, Crawford MS, Janicke DM. Treatment outcome research in rural pediatric populations: The challenge of recruitment. J Pediatr Psychol. 2011;36(6):696–707. [DOI] [PubMed] [Google Scholar]
- 34.Sidebottom AC, Sillah A, Miedema MD, et al. Changes in cardiovascular risk factors after 5 years of implementation of a population-based program to reduce cardiovascular disease: The Heart of New Ulm Project. Am Heart J. 2016;175:66–76. [DOI] [PubMed] [Google Scholar]
- 35.VanWormer JJ, Johnson PJ, Pereira RF, et al. The Heart of New Ulm Project: using community-based cardiometabolic risk factor screenings in a rural population health improvement initiative. Popul Health Manag. 2012;15(3):135–143. [DOI] [PubMed] [Google Scholar]
- 36.Boucher JLP RF, Graham KJ;Pettingill RR;Toscano JV;Henry TD The heart of New Ulm: A vision for the future. J Cardio Trans Res. 2008;1(4):310–316. [DOI] [PubMed] [Google Scholar]
- 37.Bauer UE, Briss PA, Goodman RA, Bowman BA. Prevention of chronic disease in the 21st century: elimination of the leading preventable causes of premature death and disability in the USA. Lancet. 2014;384(9937):45–52. [DOI] [PubMed] [Google Scholar]
- 38.Rhodes SD, Tanner AE, Mann-Jackson L, et al. Promoting Community and Population Health in Public Health and Medicine: A Stepwise Guide to Initiating and Conducting Community-engaged Research. J Health Dispar Res Pract. 2018;11(3):16–31. [PMC free article] [PubMed] [Google Scholar]
- 39.Wilfley DE, Saelens BE, Stein RI, et al. Dose, Content, and Mediators of Family-Based Treatment for Childhood Obesity: A Multisite Randomized Clinical Trial. JAMA Pediatr. 2017; 171(12): 1151–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ho M, Garnett SP, Baur L, Burrows T, et al. Effectiveness of lifestyle interventions in child obesity: Systematic review with meta-analysis. Pediatr. 2012; 130(6): e1647–71. [DOI] [PubMed] [Google Scholar]
- 41.Epstein LH, Paluch Ra, Roemmich JN, Beecher MD Family-based obesity treatment, then and now: Twenty-five years of pediatric obesity treatment. Health Psychol. 2007; 26(4): 381–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Daniels SR, Kelly AS. Pediatric severe obesity: Time to establish serious treatments for a serious disease. Child Obes. 2014;10(4):283–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lohman T, Roche A, Martorell R. Anthropometric Standardization Reference Manual. Champaign, IL: Human Kinetics Books; 1988. [Google Scholar]
- 44.Dietz WH, Gortmaker SL. Preventing obesity in children and adolescents. Annual Review of Public Health. 2001;22:337–353. [DOI] [PubMed] [Google Scholar]
- 45.Fulkerson JA, Nelson MC, Lytle L, Moe S, Heitzler C, Pasch KE. The validation of a home food inventory. Int J Behav Nutr Phys Act. 2008;5:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fulkerson JA, Lytle L, Story M, Moe S, Samuelson A, Weymiller A. Development and validation of a screening instrument to assess the types and quality of foods served at home meals. Int J Behav Nutr Phys Act. 2012;9:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park Y, Dodd KW, Kipnis V, et al. Comparison of self-reported dietary intakes from the Automated Self-Administered 24-h recall, 4-d food records, and food-frequency questionnaires against recovery biomarkers. Am J Clin Nutr. 2018;107(1):80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Story M, Sherwood NE, Himes JH, et al. An after-school obesity prevention program for African-American girls: the Minnesota GEMS pilot study. Ethnic Dis. 2003;13(1 Suppl 1):S54–64. [PubMed] [Google Scholar]
- 49.Johnson RK, Driscoll P, Goran MI. Comparison of multiple-pass 24-hour recall estimates of energy intake with total energy expenditure determined by the doubly labeled water method in young children. J Am Diet Assoc. 1996;96(11):1140–1144. [DOI] [PubMed] [Google Scholar]
- 50.Lytle LA, Nichaman MZ, Obarzanek E, et al. Validation of 24-hour recalls assisted by food records in third-grade children. The CATCH Collaborative Group. J Am Diet Assoc 1993;93(12):1431–1436. [DOI] [PubMed] [Google Scholar]
- 51.USDA. Healthy Eating Index (HEI). U.S. Department of Agriculture. fns.usda.gov/resource/healthy-eating-index-hei. Published 2019. Accessed February, 2019.
- 52.Trost SG, Pate RR, Freedson PS, Sallis JF, Taylor WC. Using objective physical activity measures with youth: how many days of monitoring are needed? Med Sci Sport Exerc. 2000;32(2):426–431. [DOI] [PubMed] [Google Scholar]
- 53.Cain KL, Sallis JF, Conway TL, Van Dyck D, Calhoon L. Using accelerometers in youth physical activity studies: a review of methods. J Phys Act Health. 2013;10(3):437–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Evenson KR, Catellier DJ, Gill K, Ondrak KS, McMurray RG. Calibration of two objective measures of physical activity for children. J Sports Sci. 2008;26(14):1557–1565. [DOI] [PubMed] [Google Scholar]
- 55.Crocker PR, Bailey DA, Faulkner RA, Kowalski KC, McGrath R. Measuring general levels of physical activity: preliminary evidence for the Physical Activity Questionnaire for Older Children. Med Sci Sport Exerc. 1997;29(10):1344–1349. [DOI] [PubMed] [Google Scholar]
- 56.Horning ML, Fulkerson JA, Friend SE, Neumark-Sztainer D. Associations among Nine Family Dinner Frequency Measures and Child Weight, Dietary, and Psychosocial Outcomes. J Acad Nutr Diet. 2016;116(6):991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Storfer-Isser A, Musher-Eizenman D. Measuring parent time scarcity and fatigue as barriers to meal planning and preparation: quantitative scale development. J Nutr Educ Behav. 2013;45(2):176–182. [DOI] [PubMed] [Google Scholar]
- 58.Beshara M, Hutchinson A, Wilson C. Preparing meals under time stress. The experience of working mothers. Appetite. 2010;55(3):695–700. [DOI] [PubMed] [Google Scholar]
- 59.Self-efficacy Nothwehr F. and its association with use of diet-related behavioral strategies and reported dietary intake. Health Educ Behav. 2008;35(5):698–706. [DOI] [PubMed] [Google Scholar]
- 60.Fiese BHK CA Development of the Family Ritual Questionnaire: Initial reliability and validation studies. J Fam Psychol. 1993;6(3):290–299. [Google Scholar]
- 61.Lohse B, Cunningham-Sabo L, Walters LM, Stacey JE. Valid and reliable measures of cognitive behaviors toward fruits and vegetables for children aged 9 to 11 years. J Nutr Educ Behav. 2011;43(1):42–49. [DOI] [PubMed] [Google Scholar]
- 62.RUCA data. Rural Health Research Center. depts.washington.edu/uwruca/ruca-codes.php. Published 2019. Accessed February, 2019.
- 63.ERS. Rural-urban community area codes. USDA. ers.usda.gov/data-products/rural-urban-commuting-area-codes/. Published 2019. Accessed February, 2019. [Google Scholar]
- 64.Blumberg SJ, Bialostosky K, Hamilton WL, Briefel RR. The effectiveness of a short form of the Household Food Security Scale. Am J Public Health. 1999;89(8):1231–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Story M, Kaphingst KM, Robinson-O’Brien R, Glanz K. Creating healthy food and eating environments: policy and environmental approaches. Ann Rev Pub Health. 2008;29:253–272. [DOI] [PubMed] [Google Scholar]
- 66.Story M, Hannan PJ, Fulkerson JA, et al. Bright Start: Description and main outcomes from a group-randomized obesity prevention trial in American Indian children. Obesity (Silver Spring, Md). 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bandura A Social foundations of thought and action: A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice-Hall; 1986. [Google Scholar]
- 68.Elder JP, Lytle L, Sallis JF, et al. A description of the social-ecological framework used in the trial of activity for adolescent girls (TAAG). Health Educ Res. 2007;22:155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Klein EG, Lytle LA, Chen V. Social ecological predictors of the transition to overweight in youth: results from the Teens Eating for Energy and Nutrition at Schools (TEENS) study. J Am Diet Assoc. 2008;108(7):1163–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Glanz KR,B. Theory at a Glance: A Guide for Health Promotion and Practice. In: National Cancer I, ed. Rockville, MD: US Department of Health and Human Services; 1995. [Google Scholar]
- 71.Story M, Neumark-Sztainer D, French S. Individual and environmental influences on adolescent eating behaviors. J Am Diet Assoc. 2002;102(3 Suppl):S40–51. [DOI] [PubMed] [Google Scholar]
- 72.Maniccia DM, Davison KK, Marshall SJ, Manganello JA, Dennison BA. A meta-analysis of interventions that target children’s screen time for reduction. Pediatrics. 2011;128(1):e193–210. [DOI] [PubMed] [Google Scholar]
- 73.Horning ML, Ostrow L., Beierwaltes P, Beaudette J, Schmitz K, Fulkerson JA Service learning within community-engaged research: Facilitating nursing student learning outcomes. J Prof Nurs. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Resnicow K, DiIorio C, Soet JE, Ernst D, Borrelli B, Hecht J. Motivational interviewing in health promotion: it sounds like something is changing. Health Psychol: Official journal of the Division of Health Psychology, American Psychological Association. 2002;21(5):444–451. [PubMed] [Google Scholar]
- 75.Resnicow K, Jackson A, Wang T, et al. A motivational interviewing intervention to increase fruit and vegetable intake through Black churches: results of the Eat for Life trial. American J Pub Health. 2001;91(10):1686–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Resnicow K, Davis R, Rollnick S. Motivational interviewing for pediatric obesity: Conceptual issues and evidence review. J Am Diet Assoc. 2006;106(12):2024–2033. [DOI] [PubMed] [Google Scholar]
- 77.Greaney ML, Sprunck-Harrild K, Bennett GG, et al. Use of email and telephone prompts to increase self-monitoring in a Web-based intervention: randomized controlled trial. J Med Inter Res. 2012;14(4):e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Durlak JA, DuPre EP. Implementation matters: a review of research on the influence of implementation on program outcomes and the factors affecting implementation. Am J Comm Psychol. 2008;41(3–4):327–350. [DOI] [PubMed] [Google Scholar]
- 79.Bellg AJ, Borrelli B, Resnick B, et al. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol. 2004;23(5):443–451. [DOI] [PubMed] [Google Scholar]
- 80.CDC. Center for Disease Control and Prevention. Overweight and obesity. cdc.gov/obesity. Published 2019. Accessed 06/01/2019.
- 81.R: A language and environment for statistical computing. R Foundation for Statistical Computing. https://www.R-project.org/. Published 2019. Accessed February, 2019.
- 82.Minkler M, Baden AC. Impacts of CBPR on academic researchers, research quality and methodology, and power relations. In: MMW N, ed. Community-Based Participatory Research for Health: From Process to Outcomes. San Francisco, CA: Jossey-Bass; 2008:243–262. [Google Scholar]