Abstract
Introduction
The objective of this study was to investigate associations between dementia in World Trade Center (WTC) responders and in vivo volumetric measures of hippocampal subfield volumes in WTC responders at midlife.
Methods
A sample of 99 WTC responders was divided into dementia and unimpaired groups. Participants underwent structural T1‐weighted magnetic resonance imaging. Volumetric measures included the overall hippocampus and eight subfields. Regression models examined volumetric measure of interest adjusting for confounders including intracranial volume.
Results
Dementia was associated with smaller hippocampal volume and with reductions across hippocampal subfields. Smaller hippocampal subfield volumes were associated with longer cumulative time worked at the WTC. Domain‐specific cognitive performance was associated with lower volumetric measures across hippocampal subregions.
Conclusions
This is the first study to investigate hippocampal subfield volumes in a sample of WTC responders at midlife. Selective hippocampal subfield volume reductions suggested abnormal cognition that were associated with WTC exposure duration.
Keywords: cognitive impairment, hippocampal subfields, post‐traumatic stress disorder, World Trade Center responder
1. INTRODUCTION
The men and women who responded to the World Trade Center (WTC) attacks on September 11, 2001 (9/11) were exposed to multiple traumatic and physical experiences during rescue and then recovery and clean‐up efforts. 1 , 2 Since 2016, we have reported results of our research revealing that WTC responders, now in their mid‐fifties, are apparently at elevated relative risk for aging‐related cognitive impairment (CI). 3 , 4 Lower cognitive function in WTC responders was associated with higher levels of WTC exposures, WTC‐related post‐traumatic stress disorder (PTSD), and apolipoprotein E ε4 genotype. 3 , 4 , 5 These relationships bear a remarkable similarity to reported characteristics of mild cognitive impairment and dementia. 1 , 6 , 7 , 8 , 9 , 10 Recent studies have further identified differences in plasma‐based biomarkers 11 and reduced cortical thickness in WTC responders with CI. 12
The human hippocampus is an important limbic structure located in the medial temporal lobe that plays a pivotal role in emotional processing and memory formation via its reciprocal connections to cortical and subcortical areas and has shown to be vulnerable to a range of degenerative conditions. 13 , 14 Hippocampal atrophy has long been thought to be an early hallmark characterization of Alzheimer's disease (AD) pathology and has been consistently reported in autopsy and in vivo neuroimaging studies of individuals suffering from mild cognitive impairment (MCI) and dementia. 13 , 15 , 16
The hippocampus is a heterogeneous structure composed of the hippocampal formation, which can be subdivided to three major subfields: the cornu ammonis (CA1‐4), dentate gyrus (DG) and subiculum, and fimbria/fornix. These interconnected subfields have distinctive histological characteristics and are thought to mediate different memory functions. 14 Indeed, functional and structural magnetic resonance imaging (MRI) studies suggested that CA3 and the DG are involved in pattern separation, memory encoding, and retrieval of short‐term memory, while CA1 is involved in consolidation, spatial encoding, as well as retrieval of long‐term, autobiographical, and episodic memory. 17 , 18 , 19 , 20 Studies investigating hippocampal subfields in relation to AD agree that the CA1 and subiculum are often the most affected area. 15 , 16 , 21 , 22 Moreover, studies examining atrophy patterns in hippocampal subfields have shown that subfield volumes (such as CA1 and subregions of the subiculum complex) can better differentiate MCI and AD from normal controls than total hippocampal volume. 15 , 21 , 23
WTC responders provide an excellent opportunity to study the association between CI and neurodegeneration, and the potential overlapping contributions of WTC‐related PTSD and/or WTC exposures because this group experienced the same traumatic event at the same time and have experienced similar co‐exposures to varying levels of neurotoxicants carried by ultrafine WTC airborne particles and intensive psychological trauma. 24 , 25 The main objective of this study was to investigate the possible existence of a relationship among WTC responders linking CI with reductions in the volume of whole hippocampus and specific hippocampal subfields and, if such relationships were identified, to define particular patterns of hippocampal subfield atrophy linked to CI. Secondary analyses examined the role of both WTC‐related PTSD and/or WTC exposure severity to the associations between CI and hippocampal volumetric measures, and to determine levels of association between domain‐specific cognitive dysfunction in WTC responders and hippocampal volumetry.
2. METHODS
2.1. Study design and participants
Participants were recruited from a single clinic‐based monitoring program in the WTC Health Program 2 and participated in an epidemiologic study of cognitive aging involving serial administration of the Montreal Cognitive Assessment (MoCA) in WTC responders. 4 Responders were contacted based on information available from previous research studies and monitoring visits and had previously consented to be contacted regarding participation in research studies.
The study employed a two‐by‐two between‐subjects factorial design including cognitive status (unimpaired/impaired) and PTSD diagnosis (present/absent). Participants met inclusion criteria if they were fluent in English, were between 44 and 65 years of age, and had body mass index (BMI) < 40 at recruitment. Exclusion criteria included: history of diagnosed neurological conditions including diagnosed AD and Parkinson's disease, multiple sclerosis, epilepsy, stroke, or brain cancer; current severe renal or liver disease; uncontrolled diabetes; current use of cognitively active medications; and any conditions that would preclude MRI scans including non‐MR‐safe metal in the body, pacemaker, claustrophobia, pregnancy, and breastfeeding. More information about recruitment efforts can be found in the Appendix. Cognitive status groups were matched by group means for continuous variables and frequencies for categorical variables for main covariates including age at scan (years), sex (male vs. female), race/ethnicity (White, Black, Hispanic, and other), educational attainment at time of scan (high school or less, some college, and university degree), and occupation before 9/11 (police vs. other [e.g., non‐traditional responders including, for example, construction workers and volunteers]).
2.2. Ethics
The Institutional Review Boards at both Stony Brook University and the Icahn School of Medicine at Mount Sinai approved study procedures in accordance with the Declaration of Helsinki. All participants signed informed consents at enrollment after all study procedures were fully explained.
2.3. Data availability
Medical diagnoses are considered protected health information so processed de‐identified data will be made available upon receipt of a reasonable request to the corresponding author; raw image files can be accessed upon completion of a data use agreement.
2.4. Measures
2.4.1. Cognitive status
The diagnosis of CI was provided algorithmically following standard criteria for dementia. 26 Global cognitive status was objectively assessed using the MoCA, an instrument developed to identify age‐related CI. 2 The eligibility criterion used to identify CI was a conservative cutoff score (MoCA ≤20) at a level consistent with possible mild dementia. 12 Eligible responders who had no diagnoses of non‐WTC neurological conditions or medical diagnosis consistent with loss in cognitive performance were included. All imaged individuals fit diagnostic criteria consistent with multidomain cognitive impairment in addition to mild functional limitations consistent with possible dementia. Eligibility criterion for cognitively unimpaired (CU) controls was scoring within the normal range (MoCA ≥26).
2.4.2. Cognitive functioning
Domains of cognitive functioning for a variety of domains were collected using a validated computer‐based battery, 28 which consists of repeated game‐like tasks administered in a laptop‐based format. 29 , 30 , 31 A total of five tasks were used to measure cognitive dysfunction across domains of throughput (One‐Card Learning accuracy compared to testing speed), visual memory (One‐Card Learning), episodic memory (Continuous Paired Associate Learning), visuospatial learning (Groton Maze learning test), visuospatial recall (Groton Maze learning test delayed recall), intra‐individual item‐response variability (Detection response variability), reaction speed (Detection responses per second), and processing speed (Identification responses per second). Tasks included 28 to 140 independent trials with overall scores averaged across valid trials.
RESEARCH IN CONTEXT
Systematic Review: The authors reviewed the literature using PubMed and Google Schoolar, as well as meeting abstracts and presentations. Evidence suggests that World Trade Center (WTC) responders are experiencing high levels of cognitive impairment and decline that is associated with both lengthier exposures to the WTC and the presence of post‐traumatic stress disorder, and that cortical atrophy is present in WTC responders with dementia. To date, nothing is known about the extent to which hippocampal atrophy is associated with dementia at midlife in WTC responders.
Interpretation: In this study of 99 WTC responders at midlife, hippocampal volume across six subfields including the presubiculum were reduced in WTC responders with dementia. Long‐term exposure to the WTC sites factors was associated with atrophy in the molecular layer, presubiculum, and subiculum.
Future Directions: Results suggest that hippocampal atrophy may be a good indicator of risk of early onset dementia in WTC responders. Follow‐up is necessary with positron emission tomography to determine whether responders have biomarkers consistent with Alzheimer's disease or another related dementia.
2.5. Clinical assessments
PTSD was assessed using the Structured Clinical Interview for the DSM‐IV (SCID‐IV), 32 a semi‐structured interview schedule administered by trained clinical interviewers. The PTSD module used WTC exposures as the index trauma. 4 Eligibility criterion for PTSD status was the presence/absence of current PTSD diagnosis.
Major depression disorder was assessed using the SCID‐IV 32 and the presence or absence of current (i.e., active in the past month) major depressive disorder (MDD) diagnosis was determined. MDD was not an exclusion criterion and was measured as a possible covariate.
2.5.1. WTC exposure duration
We have previously reported that CI, longitudinal analyses of MCI incidence, 3 and cognitive dysfunction across domains of memory and throughput 5 are associated with longer duration of physical presence at the WTC sites. In this study, we followed prior efforts by examining cumulative time working on the WTC site (expressed in months and collected at enrollment in the parent study) to indicate WTC exposures. This exposure variable was not available for 10 participants, therefore analyses including this variable was done using a sample of 89 WTC responders. There is no significant difference between the participants removed (n = 10) and the participants included in the analysis (n = 89) in any of the demographic characteristics. There was also no group difference in cognitive status and PTSD status.
Other measures of interest included alcohol use examined using the Alcohol Use Disorders Identification Test (AUDIT) 10‐item questionnaire. 33 Alcohol use disorder was not an exclusion criterion but was measured as a possible covariate. BMI (kg/m2) was calculated in a standard way. Demographic information included age in years, sex, race/ethnicity, occupation before 9/11, and educational attainment.
2.6. Image acquisition and processing
Study subjects underwent one MRI (high‐resolution 3‐Tesla SIEMENS mMR Biograph scanner) using 20 channel head and neck coil and a three‐dimensional T1‐weighted magnetization‐prepared rapid gradient echo (MPRAGE) sequence with the following parameters: repetition time (TR) = 1900 s, echo time (TE) = 2.49 ms, inversion time (TI) = 900 ms, flip angle = 9°, acquisition matrix = 256 × 256 and 224 slices with final voxel size = 0.89 × 0.89 × 0.89 mm to detect microstructural reorganization of hippocampal subfields.
MPRAGE images were visually inspected to rule out gross motion artifacts and low gray–white matter contrast, which can undermine segmentation accuracy. No scans were excluded as a result. The standard cross‐sectional pipeline in FreeSurfer V.6.0 was used to perform subcortical reconstruction and segmentation. 34 , 35 , 36 , 37 Briefly, processing includes intensity normalization, automated topology corrections, and automatic segmentation of cortical and subcortical regions. 36 Total intracranial volumes (TIV) were also extracted to correct for head size variability. 38 , 39
2.7. Hippocampal subfield volumes
A cross‐sectional pipeline for automated hippocampal subfield segmentation of T1‐weighted MRI, released by FreeSurfer V.6.0, was applied to compute volumetric estimations of the hippocampal subregion as previously demonstrated. 40 In this study, we included the following eight hippocampal subfields of interest: cornus ammonis (CA)1, CA2 + CA3 (noted as CA2/3), CA4, granule cell layer of dentate gyrus (noted as CG‐DG), molecular layer (noted as ML), subiculum, presubiculum, and fimbria/fornix (noted as fimbria). These subregions are commonly reported to undergo volume reduction in neurodegenerative conditions such as CI and dementia across numerous studies with a range of image acquisition and segmentation protocols. 41 As we did not have a priori hypotheses regarding laterality in subfield volumes, left and right subfield volumes were averaged and examined bilaterally. Left and right whole hippocampal volumes were extracted using the same pipeline and analyzed separately in the same manner.
Quality control was completed in two steps: segmented images were overlaid on corresponding structural images and checked visually by research staff who were blinded to the subject's status to ensure good co‐registration and correct assignment of subfield labels; hippocampal subfield volumes that were above the 75th or below the 25th percentile by a factor of 1.5 times (i.e., 1.5 x interquartile range [IQR]) were identified as possible outliers. Subjects that had one or more of the hippocampal subfields fail both steps of the quality‐control process could be removed from the analysis, though we identified no exclusion candidates.
2.8. Statistical analyses
Descriptive sample statistics are provided using mean and standard deviations, or frequencies and percentages where noted. Sample characteristics are reported for the overall sample and separately for CU and CI subsamples. Results for continuous variables are reported throughout the text as mean and standard deviation (mean ± SD) unless mentioned otherwise. Bivariate analyses for clinical and demographic variables relied on two‐tailed independent‐samples t‐tests and nonparametric tests to calculate P‐values (P). The threshold for significance was set at P < 0.05. Because main covariates were included in matching efforts, main multivariable‐adjusted analyses did not include additional adjustments for matched variables (i.e., age at scan, sex, race/ethnicity, educational attainment, and occupation before 9/11). Normality was confirmed using the adjusted Jarque–Bera test. Ordinary least square regression was fit to volumetric measures of interest. Because hippocampal volume differs by head size, TIV was included as covariate in all regression analyses. Cognitive status (CU vs. CI), throughput scores, PTSD status (absent vs. present PTSD diagnosis), and cumulative time worked on WTC site (months) were used as predictors. Sensitivity analyses used regression models to examine the independent contribution of cognitive status or throughput scores as predictors adjusting for matched variables and TIV. Exploratory analyses examined the relationships between domains of cognitive dysfunction and the volume of hippocampal subfields of interest. Standardized beta coefficient (β) and standard error for the standardized coefficient (SE) were reported. We accounted for multiple testing bias using the false rate discovery (FDR) 42 for hippocampal subfield volumes analyses. Whole volume analyses were done separately therefore not corrected for FDR. Statistical analyses were performed using R programming language V 3.5.2.
3. RESULTS
3.1. Demographic and clinical characteristics
WTC responders who completed T1‐weighted imaging in this study were in their mid‐fifties at the time of the imaging (56.37 ± 5.19), and most were male (78.8%). Table 1 summarizes the clinical and demographic characteristics for the overall sample and for responders with and without cognitive impairment. By design, cognitive status groups were matched in terms of age at scan, sex, race/ethnicity, educational attainment, and occupation before 9/11. BMI, major depression diagnosis, WTC exposure, and TIV did not differ significantly between the cognitive status groups.
TABLE 1.
Demographic and clinical characteristics of N = 99 participants in the WTC neurocognitive imaging study
| Overall | CU | CI | ||
|---|---|---|---|---|
| Characteristic | (N = 99) | (N = 51) | (N = 48) | P |
| Age, y | 56.37 ± 5.19 | 56.37 ± 4.59 | 56.36 ± 5.81 | 0.993 |
| Sex | 0.876 | |||
| Male | 78 (78.8%) | 41 (80%) | 37 (77%) | |
| Female | 22 (22.2%) | 10 (20%) | 11 (23%) | |
| Race/ethnicity | 0.096 | |||
| White | 73 (73.7%) | 43 (84.3%) | 30 (62.5%) | |
| Black | 10 (10.1%) | 3 (5.9%) | 7 (14.6%) | |
| Hispanic | 12 (12.1%) | 4 (7.8%) | 8 (16.7%) | |
| Other | 4 (4.0%) | 1 (2.0%) | 3 (6.2%) | |
| Occupation before 9/11 | 0.186 | |||
| Police | 73 (73.7%) | 41 (80.4%) | 32 (66.7%) | |
| Other | 26 (26.3%) | 10 (19.6%) | 16 (33.3%) | |
| Educational attainment | 0.359 | |||
| High school or less | 23(23.2%) | 9 (17.6%) | 14 (29.2%) | |
| Some college | 47 (47.5%) | 25 (49.0%) | 22 (45.8%) | |
| University degree | 29 (29.3%) | 17 (33.4%) | 12 (25.0%) | |
| BMI, kg/m2 | 29.22 ± 4.03 | 28.69 ± 4.00 | 29.79 ± 4.04 | 0.175 |
| PTSD Dx a | 1.000 | |||
| No | 52 (52.5%) | 27 (52.9%) | 25 (52.1%) | |
| Yes | 47 (47.5%) | 24 (47.1%) | 23 (47.9%) | |
| MDD Dx a | 0.906 | |||
| No | 81 (81.8%) | 41 (80.4%) | 40 (83.3%) | |
| Yes | 18 (18.2%) | 10 (19.6%) | 8 (16.7%) | |
| AUDIT | 3.04 ± 3.77 | 3.20 ± 3.79 | 2.87 ± 3.78 | 0.671 |
| Total months on site | 4.05 ± 3.31 | 3.99 ± 3.38 | 4.10 ± 3.27 | 0.882 |
| TIV, cm3 | 1578.5 ± 147 | 1583.26 ± 128.25 | 1573.44 ± 165.86 | 0.742 |
Notes: Means (± standard deviations) or percentages (%) reported. P‐values examine the extent to which noted characteristics differ across cognitive status groups and were derived using Student's t‐tests for continuous variables and χ2 or Fisher's exact tests for categorical variables.
The rates reflect the 2 × 2 design of the study.
Abbreviations: AUDIT, Alcohol Use Disorders Identification Test.; BMI, body mass index, CI, WTC responders with cognitive impairment; CU, WTC responders with unimpaired cognition; MDD Dx, lifetime major depression disorder diagnosis; PTSD Dx, WTC‐related posttraumatic stress disorder diagnosis; TIV, total intracranial volume; WTC, World Trade Center.
3.2. Between group differences of hippocampal subfield volumes
Whole hippocampal volume was reduced in WTC responders with CI compared to responders with unimpaired cognition (CU: 3648.62 ± 301.1 mm3 vs. CI: 3516.4 ± 377.93 mm3, β = –0.17, SE = 0.08, P = 0.031). Table 2, Panel A, includes a summary of raw hippocampal subfield volumes. Volumetric differences between the CI and CU, adjusted for TIV (Figure 1 and Appendix Table A1, Panel A), suggested that CI was significantly associated with lower volume in CA4 (β = –0.18, SE = 0.08, P = 0.047), CG‐DG (β = –0.19, SE = 0.08, P = 0.047), presubiculum (β = –0.25, SE = 0.09, P = 0.042), and fimbria (β = –0.23, SE = 0.09, P = 0.047). Sensitivity analysis results from a series of regression models additionally adjusting for matched variables showed similar patterns of associations (Table 2, Panel B [2.B]). Moreover, CI was also significantly associated with lower volume in ML (β = –0.20, SE = 0.08, P = 0.018) and subiculum (β = –0.19, SE = 0.08, P = 0.031) when adjusting for matched variables.
TABLE 2.
Association between cognitive status and hippocampal subfield volumes
| Panel A: Mean Raw Volumes (mm3) by cognitive impairment status | Panel B: Standardized regression coefficients indicating size of cognitive impairment association | ||||
|---|---|---|---|---|---|
| Subregion name | CU | CI | β | SE | P |
| CA1 | 678.17 ± 65.19 | 660.83 ± 80.21 | –0.12 | 0.080 | 0.144 |
| CA2/3 | 226.91 ± 28.90 | 221.03 ± 26.56 | –0.11 | 0.089 | 0.207 |
| CA4 | 271.58 ± 26.06 | 260.46 ± 27.72 | –0.21 | 0.082 | 0.018* |
| CG‐DG | 318.66 ± 29.55 | 305.42 ± 33.20 | –0.21 | 0.078 | 0.018* |
| ML | 603.94 ± 54.86 | 580.50 ± 64.66 | –0.20 | 0.078 | 0.018* |
| Subiculum | 455.01 ± 47.77 | 435.31 ± 54.96 | –0.19 | 0.084 | 0.031* |
| Presubiculum | 319.36 ± 32.95 | 299.57 ± 40.33 | –0.23 | 0.088 | 0.018* |
| Fimbria | 97.93 ± 12.48 | 90.67 ± 16.91 | –0.24 | 0.089 | 0.018* |
Raw volumes of hippocampal subfields of interest in WTC responders with CU and WTC responders with CI.
Standardized beta coefficients (β) represent the change in standard deviation units of the subregion volume that is associated with CI after adjusting for with CI after adjusting for matched variables (i.e., age at scan, sex, race/ethnicity, educational attainment, and occupation before 9/11) and TIV. P‐values (P) were corrected for false discovery rate. *P < 0.05.
Abbreviations: CA, Cornu Ammonis; CG‐DG, granule cell layer of dentate gyrus; CI, cognitive impairment; CU, unimpaired cognition; ML, molecular layer; se, standard error for the standardized coefficient; TIV, total intracranial volume; WTC, World Trade Center.
FIGURE 1.
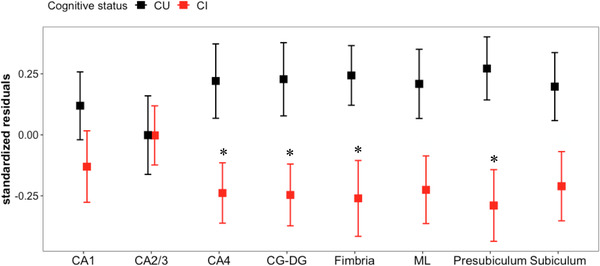
Effects of cognitive status on hippocampal subfield volumes. Difference between cognitively unimpaired (CU, black) and cognitively impaired (CI, red) WTC responders was determined using linear regression models for each hippocampal subfield of interest. Y‐axis shows standardized residuals of hippocampal subfield volumes after controlling total intracranial volume (TIV). Error bars represent ± standard error of the mean. All significant effects passed the false discovery rate. *P < 0.05. Abbreviations: CA, cornu ammonis; CG‐DG, granule cell layer of dentate gyrus; ML, molecular layer, P = P‐value; WTC, World Trade Center
Examining WTC exposures, regression models using cognitive status and cumulative WTC exposure duration simultaneously as predictors revealed that whole hippocampal volume was reduced in responders with longer exposure duration (β = –0.20, SE = 0.08, P = 0.020). Analyses additionally identified significant associations between WTC exposure duration and smaller ML, subiculum, and presubiculum (Table 3.B). However, similar models using PTSD status as predictor of interest revealed no associations with subregions (Appendix Table A1.B).
TABLE 3.
Association between both cognitive status and length of time worked on the WTC site and hippocampal subfield volumes
| Panel A: Standardized regression coefficients showing size of cognitive impairment association | Panel B: Standardized regression coefficients showing size of association with total months on site | |||||
|---|---|---|---|---|---|---|
| Subregion name | β | SE | P | β | SE | P |
| CA1 | –0.10 | 0.086 | 0.245 | –0.16 | 0.087 | 0.148 |
| CA2/3 | –0.11 | 0.087 | 0.218 | 0.09 | 0.088 | 0.333 |
| CA4 | –0.22 | 0.084 | 0.031* | –0.07 | 0.085 | 0.394 |
| CG‐DG | –0.21 | 0.083 | 0.031* | –0.1 | 0.084 | 0.333 |
| ML | –0.18 | 0.083 | 0.056 | –0.2 | 0.084 | 0.045* |
| Subiculum | –0.18 | 0.086 | 0.056 | –0.24 | 0.087 | 0.031* |
| Presubiculum | –0.28 | 0.085 | 0.013* | –0.29 | 0.086 | 0.0095** |
| Fimbria | –0.19 | 0.097 | 0.074 | –0.11 | 0.098 | 0.333 |
Notes: Regression models included both cognitive impairment and total time worked at the WTC site (months) simultaneously as predictors were used to test associations with hippocampal subfield volumes adjusted for TIV. Resulted standardized beta coefficients (β) represent the change in standard deviation units of subregion volume associated with CI (Panel A) and length of time worked on the WTC site (Panel B). P‐values (P) were corrected for false discovery rate. *P < 0.05 and **P < 0.01.
Abbreviations: β, standardized beta coefficient; CA, cornu ammonis; CG‐DG, granule cell layer of dentate gyrus; CI, WTC responders with cognitive impairment; CU, WTC responders with unimpaired cognition; ML, molecular layer; se, standard error for the standardized coefficient; TIV, total intracranial volume; WTC, World Trade Center.
3.3. Cognitive functioning and hippocampal subfield volumes
Next, we evaluated relationships between cognitive functioning and whole hippocampal volume and hippocampal subfield volumes in this WTC responder population. Lower cognitive throughput performance was associated with smaller whole hippocampal volume after adjusting for TIV (β = 0.26, SE = 0.08, P = 0.001). Results from regression models also showed that poorer throughput scores were significantly associated with lower volumetric measures in most hippocampal subregions studied including CA1, CA4, GC‐DG, ML, subiculum, and presubiculum (Figure 2, Table 4.A). Sensitivity analyses revealed that associations remained significant after adjusting for matching information (Table 4.B).
FIGURE 2.
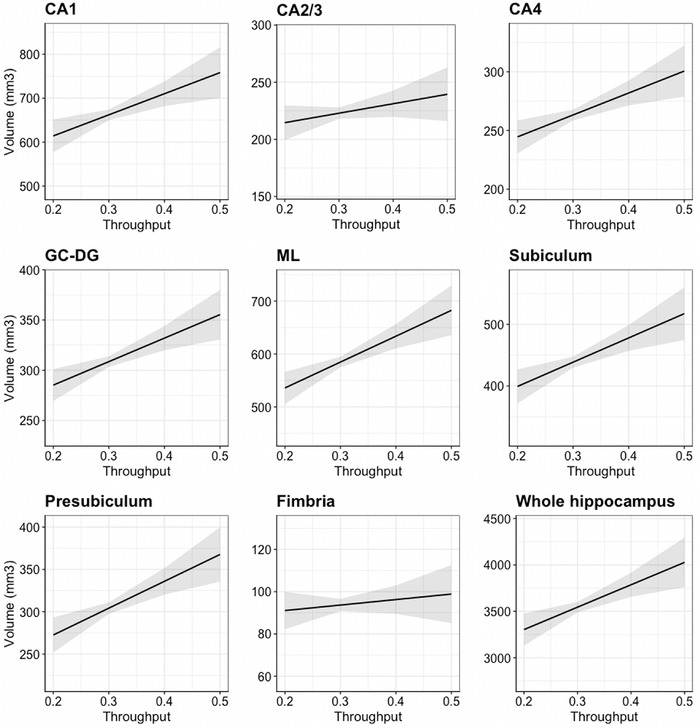
Association between cognitive throughput performances of hippocampal subfield volumes and whole hippocampal volume adjusted for total intracranial volume (TIV). Regression models show the associations between throughput scores (higher score = better outcome) and hippocampal subfields or whole hippocampal volume adjusted for TIV. Y‐axis is scaled to mean ± 2.5 standard deviations of each subregion absolute volume. Sample size used in this analysis was 98 participants because one participant failed to complete the One Card Learning test that was used to calculate the throughput score and therefore was not included in this analysis. Abbreviations: CA, cornu ammonis; CG‐DG, granule cell layer of dentate gyrus; ML, molecular layer
TABLE 4.
Association between cognitive throughput performances and hippocampal subfield volumes
| Panel A: Standardized regression coefficients showing size of association with throughputa | Panel B: Standardized regression coefficients indicating size of association with throughputb | |||||
|---|---|---|---|---|---|---|
| Subregion name | β | SE | P | β | SE | P |
| CA1 | 0.24 | 0.078 | 0.004** | 0.22 | 0.077 | 0.007** |
| CA2/3 | 0.11 | 0.084 | 0.226 | 0.13 | 0.089 | 0.171 |
| CA4 | 0.25 | 0.079 | 0.003** | 0.25 | 0.080 | 0.004** |
| CG‐DG | 0.27 | 0.077 | 0.002** | 0.26 | 0.077 | 0.004** |
| ML | 0.29 | 0.077 | 0.002** | 0.28 | 0.076 | 0.003** |
| Subiculum | 0.28 | 0.082 | 0.002** | 0.27 | 0.082 | 0.004** |
| Presubiculum | 0.31 | 0.085 | 0.002** | 0.28 | 0.086 | 0.004** |
| Fimbria | 0.06 | 0.092 | 0.489 | 0.03 | 0.093 | 0.783 |
Notes: Regression models show the association between throughput scores (n = 98; higher score = better outcome) and hippocampal subfields aadjusted for TIV or badjusted for matching variables (i.e., age at scan, sex, race/ethnicity, educational attainment, and occupation before 9/11) and TIV. Standardized beta coefficients (β) represent the change in standard deviation units of the subregion volume that is associated with 1 standard deviation increase throughput score. P‐values (P) were corrected for false discovery rate. *P < 0.05 and **P < 0.01.
Abbreviations: CA, cornu ammonis; CG‐DG, granule cell layer of dentate gyrus; ML, molecular layer; se, standard error for the standardized coefficient; TIV, total intracranial volume; WTC, World Trade Center.
3.4. Exploratory analyses for additional cognitive functions
Exploratory analyses were performed to assess the potential presence of relationships linking various domains of cognitive dysfunction and the volume of hippocampal subfields of interest. Responders with CI had significantly poorer performance in all cognitive domains tested compared to unimpaired responders (Appendix Table A2). However, only a number of cognitive domains were associated with changes in hippocampal subfield volumes (Figure 3). For example, delayed recall performance on visuospatial task was found to be associated with volume in selective hippocampal subfields including the CA1 (β = –0.20, SE = 0.08, P = 0.040), ML (β = –0.21, SE = 0.08, P = 0.040), presubiculum (β = –0.20, SE = 0.09, P = 0.049), and subiculum (β = –0.21, SE = 0.08, P = 0.040).
FIGURE 3.
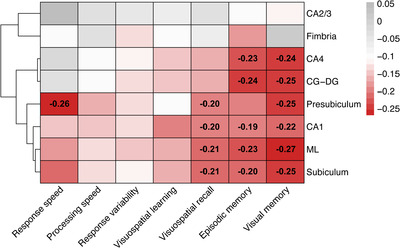
Heat map showing levels of association among various cognitive domains and volumetric measures of hippocampal subfields. Standardized beta coefficient (β) from linear regression modeling adjusting for total intracranial volume (TIV). Coefficients were transformed so that increases in cognitive domains scores are consistent with worse outcomes. All coefficients deemed statistically significant upon adjusting for the false discovery rate (FDR = 0.05) were reported (black font, P < 0.05). Red filling indicates reduced volume was associated with poorer performances; gray filling indicates increased volume was associated with poorer performances. Abbreviations: CA, cornu ammonis; CG‐DG, granule cell layer of dentate gyrus; ML, molecular layer, P = P‐value
4. DISCUSSION
This study examined whether CI in WTC responders was characterized by hippocampal volume loss consistent with cognitively impairing neurodegenerative diseases, with secondary goals including examining exposure‐related correlates of hippocampal atrophy and of neurocognitive dysfunction evident in this population. This is the first study to investigate whether CI was associated with volumetric differences in the whole hippocampus and/or specific hippocampal subfields. In this study, we observed associations linking reduced hippocampal volumetric measures and CI. Moreover, lower cognitive throughput and a number of memory functions were associated with lower volumes in most hippocampal subregions studied, while no significant associations were observed for attention, executive function, or intra‐individual response variability.
The regional pattern of CI‐related hippocampal volume reductions in WTC responders revealed that reductions were most prominent in the presubiculum, but these changes were also observed in the CA4, CG‐DG and fimbria. Volume reductions were also statistically significant in the subiculum and the ML after additionally adjusting to matched variable. No diagnosis‐related differences were observed in either CA1 or CA2/3 subfields. Our findings are consistent with those of Carlesimo et al., 21 who reported smaller hippocampal subfield volumes in those with amnestic MCI and dementia compared to healthy controls, with the largest volume reductions located in the presubiculum. Interestingly, that study also reported volume reductions in dementia as a result of AD compared to MCI in the presubiculum and subiculum suggestive of progressive focal atrophy of the hippocampus in AD. 21 Our study identified CI‐related volume reductions in hippocampal subregions commonly reported in AD‐related dementia and MCI such as the presubiculum–subiculum complex. 15 , 16 , 21 , 23
We did not identify differences in the CA1 subfield across cognitive status groups, an observation that we found surprising because several volumetric studies in dementia revealed early and consistent CA1 volume reduction. 15 , 16 , 23 , 41 One explanation for this unusual null finding may be that CA1 volume is across the WTC responder population. If so, these results may suggest that the screening tool used to assign participants to cognitive status groups (CU vs. CI) was not sensitive to early cognitive decline resulting from CA1 volume loss. This explanation is supported by identifying associations between reduced CA1 volume and poorer throughput and memory.
We observed smaller overall hippocampal volume, and specific hippocampal subfield volumes, among responders with CI. This finding is consistent with studies identifying reduced cortical thickness in WTC responders. 12 Similarly to the cortical thinning finding, the pattern of hippocampal subfields volume reductions only partially overlapped with patterns of subregions commonly known to be affected in late‐onset AD and related dementias. 41 Future studies using molecular imaging investigating the association between amyloid beta (Aβ) deposits and/or neuronal neurofibrillary tangles containing hyperphosphorylated tau protein and the volumetric changes in the brain (and specifically in the hippocampus and hippocampal subfields) should enable a better understanding of the etiology of the CI observed in WTC responders. 43
Long‐term WTC exposure was independently associated with a reduced whole hippocampal volume. This finding is consistent with results of previous studies that found association between increased exposure to fine particulate matter and smaller left hippocampal volume, 44 though studies are far from consistent on this association. 45 , 46 , 47 As noted in a recent literature review, 48 in addition to converging evidence suggesting strong associations between cognition and exposure to particulate matter < 2.5 μm in size, 49 WTC responders were also exposed to a number of pollutants including lead, polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), and dioxins 50 that may also play important roles in the brain structure. 51 To the best of our knowledge, this is the first study to examine possible associations between measures of WTC exposure duration and hippocampal subfield volumes. Our findings of significant association between long‐term WTC exposure and volumetric reductions in specific hippocampal subregions including ML, subiculum, and presubiculum suggest a long‐term and selective hippocampal vulnerability to environmental insults experienced in the WTC site.
PTSD status was not observed to independently contribute to changes in whole hippocampal and/or subfield volumes. These findings may seem inconsistent with the conclusion of several studies and meta‐analyses in patients suffering from PTSD that generally reported significant atrophy of whole hippocampus 52 , 53 , 54 or hippocampal subfield, 55 , 56 , 57 but consistent with studies reporting no difference in hippocampal volumetric measures in PTSD. 58 , 59 , 60 A key difference between this study and prior studies was the reliance on individuals who varied not only in PTSD status but also in CI. This selection procedure allowed us to focus our investigation on whether PTSD was associated with hippocampal volumetric measures independently from cognitive status. Our results suggest that associations with PTSD on hippocampal atrophy are not independent of cognitive status.
Finally, the current study showed that poorer performance in throughput, working memory, visuospatial delayed recall, and delayed visual memory were moderately but significantly associated with hippocampal subfield volumes, while processing speed, response speed, visuospatial learning, and response variability were not. Lower performance in memory‐related function was apparently associated with reduced volume in subregions such as CA1, CA4, CG‐DG, ML, subiculum, and presubiculum whereas no such associations were observed to involve the CA2/3, and fimbria. Findings support the hypothesis that cellular and molecular mechanisms associated with memory impairment may be localized to specific hippocampal subfields. 14
4.1. Limitations
In matching the population's structure, this study includes mostly non‐Hispanic Whites and relatively few females. Additionally, though relying on well‐validated indicators and studying information that is crucial to WTC responders, this study lacks a non‐exposed comparison group collected using the same protocol. Generalizability is diminished because this population, being composed of WTC responders, is relatively homogeneous in terms of occupational and educational structure. Yet, this also represents a key strength because many studies of PTSD rely on individuals recruited from disadvantaged populations. The cross‐sectional design of the present study could not demonstrate whether a smaller hippocampal volume is a pre‐existing factor that puts individuals at risk for early development of CI or is a consequence of neuropathological processes leading to cognitive dysfunction. The targeted nature of this study meant that while we could ensure a larger proportion of responders who completed imaging had CI, it also meant that the study excluded many responders who did not meet exacting eligibility criteria resulting in potential bias. Further research is needed with a larger group of responders from many backgrounds to determine the extent of hippocampal atrophy attributable to WTC exposures. In focusing on CI and PTSD, this study did not identify individuals who were most severely exposed. Future research should seek to conduct prospective studies to examine imaging findings in relation to specific types of exposures and to examine changes in cognition over time among those with exposure‐related hippocampal atrophy. Such studies could help determine whether volumetric reductions that were detectable at baseline reflect early or premorbid and potentially predisposing factors. Such temporal sequencing will enable us to move closer toward any inferences of exposure risks and of causality. Last, future studies including larger sample sizes are needed to confirm these results.
5. CONCLUSIONS
In this study, we provide novel evidence that CI is independently associated with both whole hippocampus and selective hippocampal subfield volumes in a sample of WTC responders. Selective hippocampal subfield volume reductions may contribute to the development of WTC‐related CI. Furthermore, these findings are in line with our previous reports that one or more CI‐related neurodegenerative processes in WTC responders may be, at least in part, consistent with the amyloidosis and/or tauopathy associated with AD. 11 , 12 Future studies using multimodal imaging approaches that are not limited to structural imaging may provide supplementary information, and, when taken together, this series of investigations should facilitate elucidation of factors contributing to the etiology of the CI observed in WTC responders.
CONFLICTS OF INTEREST
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
ACKNOWLEDGMENTS
The authors would like to acknowledge support from the Centers for Disease Control and Prevention for supporting the neuroimaging study (CDC/NIOSH U01 OH011314), the National Institutes for Health (NIH/NIA P50 AG005138, NIH/NIA R01 AG049953), and for the clinical monitoring program for World Trade Center responders (CDC 200‐2011‐39361). The authors would like to thank all the participants. We also thank Dr. Monika A. Waszczuk for her assistance in formulating the statistical analysis plan for this manuscript.
1.
As previously described, 12 a total of 598 responders were contacted as they fit the preliminary inclusion criteria based on available information. After initial screening, 176 responders were interested in participation and were invited to an in‐person screening visit. Of those who were screened, 88.6% (n = 156) completed the screening visit, of whom 27.6% (n = 43) were deemed ineligible after exclusion criteria were applied, and 7.7% declined further participation. The most frequent reason for screened participants being deemed ineligible was failure to meet the inclusion criteria for both CI and PTSD statuses. For example, more than one‐third (34.9%; n = 15) of responders who had previously been diagnosed with PTSD failed to endorse PTSD diagnosis at screening interviews. Of those who were eligible, 100 WTC responders underwent a neuroimaging scan. One participant exited the scanner before completing the T1‐weighted sequence, leaving a final analytic sample of 99 responders. The average time between screening and scanning was 26.2 (SD = 17.2) days (median = 22 days, IQR] = 14–35 days).
TABLE A1.
Cognitive status regressed on hippocampal subfield volumes
| Panel A: Standardized regression coefficients showing size of association with cognitive impairment statua | Panel B: Standardized regression coefficients showing size of association with cognitive impairment statusb | |||||
|---|---|---|---|---|---|---|
| Subregion name | β | SE | P | β | SE | P |
| CA1 | –0.10 | 0.081 | 0.256 | –0.10 | 0.082 | 0.260 |
| CA2/3 | –0.09 | 0.084 | 0.301 | –0.09 | 0.084 | 0.305 |
| CA4 | –0.18 | 0.080 | 0.047* | –0.18 | 0.081 | 0.049* |
| CG‐DG | –0.19 | 0.079 | 0.047* | –0.19 | 0.079 | 0.049* |
| ML | –0.17 | 0.080 | 0.052 | 0.17 | 0.081 | 0.052# |
| Subiculum | –0.17 | 0.084 | 0.058 | –0.17 | 0.084 | 0.055# |
| Presubiculum | –0.25 | 0.086 | 0.042* | –0.25 | 0.086 | 0.037* |
| Fimbria | –0.23 | 0.089 | 0.047* | –0.23 | 0.089 | 0.049* |
Notes: Regression models show the size of the association between cognitive impairment on hippocampal subfields adjusted for TIV (Panel A) or adjusted for PTSD status and TIV (Panel B). Standardized beta coefficients (β) represent the change in standard deviation units of subregion volume associated with CI. P‐values (P) were corrected for false discovery rate.*P < 0.05.
Abbreviations: β, standardized beta coefficient; CA, cornu ammonis; CG‐DG, granule cell layer of dentate gyrus; CI, WTC responders with cognitive impairment; CU, WTC responders with unimpaired cognition; ML, molecular layer; se, standard error for the standardized coefficient; WTC, World Trade Center.
TABLE A2.
Performance across various cognitive domains by cognitive impairment status
| Cognitive domains | CU | CI | Cohen's D | P |
|---|---|---|---|---|
| Throughput a | 0.33 ± 0.04 | 0.30 ± 0.03 | 0.87 | <0.0001** |
| Processing speed b | 2.54 ± 0.09 | 2.64 ± 0.13 | 0.81 | 0.0002** |
| Response speed b | 2.73 ± 0.07 | 2.80 ± 0.1 | 0.76 | 0.0003** |
| Intra‐individual response variability b | 0.09 ± 0.03 | 0.12 ± 0.04 | 0.89 | <0.0001** |
| Episodic memory b | 94.52 ± 59.04 | 157.67 ± 62.64 | 1.04 | <0.0001** |
| Visuospatial learning b | 57.08 ± 15.42 | 95.04 ± 61.21 | 0.87 | <0.0001** |
| Visuospatial recall b | 9.47 ± 4.33 | 17.5 ± 10.99 | 0.98 | <0.0001** |
| Visual memory a | 0.99 ± 0.12 | 0.91 ± 0.10 | 0.75 | 0.0003** |
Notes: Means and standard deviations of cognitive domains outcome measures for WTC responders with and without cognitive impairment are reported. P‐values (P) examine the extent to which cognitive performances differ across cognitive status using Student's t tests or Mann Whitney test as appropriate and effect size (Cohen's D) are also reported. P presented here are corrected for the false discovery rate. *P < 0.05 and **P < 0.01j
aHigher score = better performance.
bLower score = better performance.
Abbreviations: CI, WTC responders with cognitive impairment; CU, WTC responders with unimpaired cognition; WTC, World Trade Center.
Deri Y, Clouston SAP, DeLorenzo C, et al. Selective hippocampal subfield volume reductions in World Trade Center responders with cognitive impairment. Alzheimer's Dement. 2021;13:ed212165. 10.1002/dad2.12165
REFERENCES
- 1. Greenberg MS, Tanev K, Marin M‐F, Pitman RK. Stress, PTSD, and dementia. Alzheimer's Dement. 2014;10:S155‐S165. [DOI] [PubMed] [Google Scholar]
- 2. Dasaro CR, Holden WL, Berman KD, et al. Cohort profile: world trade center health program general responder cohort. Int J Epidemiol. 2017;46:e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Clouston SAP, Diminich ED, Kotov R, et al. Incidence of mild cognitive impairment in World Trade center responders: long‐term consequences of re‐experiencing the events on 9/11/2001. Alzheimers Dement (Amst). 2019;11:628‐636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Clouston SA, Kotov R, Pietrzak RH, et al. Cognitive impairment among World Trade Center responders: long‐term implications of re‐experiencing the 9/11 terrorist attacks. Alzheimers Dement (Amst). 2016;4:67‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clouston S, Pietrzak RH, Kotov R, et al. Traumatic exposures, posttraumatic stress disorder, and cognitive functioning in World Trade Center responders. Alzheimers Dement (N Y). 2017;3:593‐602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yaffe K, Vittinghoff E, Lindquist K, et al. Posttraumatic stress disorder and risk of dementia among us veterans. Arch General Psychiatry. 2010;67:608‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Saunders AM, Strittmatter WJ, Schmeche D, et al. Association of apolipoprotein E allele epsilon 4 with late‐onset familial and sporadic Alzheimer's disease. Neurology. 1993;43:1467‐1472. [DOI] [PubMed] [Google Scholar]
- 8. Boyle PA, Buchman AS, Wilson RS, Kelly JF, Bennett DA. The APOE epsilon4 allele is associated with incident mild cognitive impairment among community‐dwelling older persons. Neuroepidemiology. 2010;34:43‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gatto NM, Henderson VW, Hodis HN, et al. Components of air pollution and cognitive function in middle‐aged and older adults in Los Angeles. Neurotoxicology. 2014;40:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Calderon‐Garciduenas L, Reed W, Maronpot RR, et al. Brain inflammation and Alzheimer's‐like pathology in individuals exposed to severe air pollution. Toxicol Pathol. 2004;32:650‐658. [DOI] [PubMed] [Google Scholar]
- 11. Kritikos M, Clouston SAP, Diminich ED, et al. Pathway analysis for plasma β‐amyloid, tau and neurofilament light (ATN) in World trade center responders at midlife. Neurol Ther. 2020;9:159‐171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clouston SAP, Deri Y, Horton M, et al. Reduced cortical thickness in World trade center responders with cognitive impairment. Alzheimers Dement (Amst). 2020;12:e12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jack CR, Dickson DW, Parisi JE, et al. Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology. 2002;58:750‐757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bartsch T, Wulff P. The hippocampus in aging and disease: from plasticity to vulnerability. Neuroscience. 2015;309:1‐16. [DOI] [PubMed] [Google Scholar]
- 15. Joie LR, Perrotin A, Sayette VDL, et al. Hippocampal subfield volumetry in mild cognitive impairment, Alzheimer's disease and semantic dementia. Neuroimage Clin. 2013;3:155‐162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mueller SG, Schuff N, Yaffe K, et al. Hippocampal atrophy patterns in mild cognitive impairment and Alzheimer's disease. Hum Brain Mapp. 2010;31:1339‐1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mueller SG, Chao LL, Berman B, Weiner MW. Evidence for functional specialization of hippocampal subfields detected by MR subfield volumetry on high resolution images at 4 T. Neuroimage. 2011;56:851‐857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bartsch T, Döhring J, Rohr A, Jansen O, Deuschl G. CA1 neurons in the human hippocampus are critical for autobiographical memory, mental time travel, and autonoetic consciousness. Proc Natl Acad Sci U S A. 2011;108:17562‐17567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bakker A, Kirwan CB, Miller M, Stark CE. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319:1640‐1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zammit AR, Ezzati A, Zimmerman ME, et al. Roles of hippocampal subfields in verbal and visual episodic memory. Behav Brain Res. 2017;317:157‐162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carlesimo GA, Piras F, Orfei MD, et al. Atrophy of presubiculum and subiculum is the earliest hippocampal anatomical marker of Alzheimer's disease. Alzheimers Dement (Amst). 2015;1:24‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mueller SG, Weiner MW. Selective effect of age, Apo e4, and Alzheimer's disease on hippocampal subfields. Hippocampus. 2009;19:558‐564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li YD, Dong HB, Xie GM, Zhang LJ. Discriminative analysis of mild Alzheimer's disease and normal aging using volume of hippocampal subfields and hippocampal mean diffusivity: an in vivo magnetic resonance imaging study. Am J Alzheimers Dis Other Demen. 2013;28:627‐633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lippmann M, Cohen MD, Chen LC. Health effects of World Trade Center (WTC) dust: an unprecedented disaster's inadequate risk management. Crit Rev Toxicol. 2015;45:492‐530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cohen MD, Chen L, Lippmann M. In: Lippmann M & G D L, eds. Environmental Toxicants: Human Exposures and Their Health Effects. John Wiley and Son; 2019. [Google Scholar]
- 26. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's Dement. 2011;7:263‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nasreddine ZS, Phillips NA, Bãdirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695‐699. [DOI] [PubMed] [Google Scholar]
- 28. Maruff P, Thomas E, Cysique L, et al. Validity of the CogState brief battery: relationship to standardized tests and sensitivity to cognitive impairment in mild traumatic brain injury, schizophrenia, and AIDS dementia complex. Arch Clin Neuropsychol. 2009;24:165‐178. [DOI] [PubMed] [Google Scholar]
- 29. Lim YY, Pietrzak RH, Bourgeat P, et al. Relationships between performance on the Cogstate Brief Battery, neurodegeneration, and Aβ accumulation in cognitively normal older adults and adults with MCI. Arch Clin Neuropsychol. 2015;30:49‐58. [DOI] [PubMed] [Google Scholar]
- 30. Hammers D, Spurgeon E, Ryan K, et al. Reliability of repeated cognitive assessment of dementia using a brief computerized battery. Am J Alzheimer's Dis Other Dement. 2011;26:326‐333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Racine AM, Clark LR, Berman SE, et al. Associations between performance on an abbreviated cogstate battery, other measures of cognitive function, and biomarkers in people at risk for Alzheimer's disease. J Alzheimers Dis. 2016;54:1395‐1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. First MB. Structured clinical interview for the DSM (SCID). Encyclopedia Clin Psychol. 2014:1‐6. [Google Scholar]
- 33. Conigrave KM, Hall WD, Saunders JB. The AUDIT questionnaire: choosing a cut‐off score. Alcohol use disorder identification test. Addiction. 1995;90:1349‐1356. [DOI] [PubMed] [Google Scholar]
- 34. Dale AM, Fischl B, Sereno MI. Cortical surface‐based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179‐194. [DOI] [PubMed] [Google Scholar]
- 35. Fischl B, Sereno MI, Dale AM. Cortical surface‐based analysis. II: inflation, flattening, and a surface‐based coordinate system. Neuroimage. 1999;9:195‐207. [DOI] [PubMed] [Google Scholar]
- 36. Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341‐355. [DOI] [PubMed] [Google Scholar]
- 37. Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050‐11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. O'Brien LM, Ziegler DA, Deutsch CK, et al. Adjustment for whole brain and cranial size in volumetric brain studies: a review of common adjustment factors and statistical methods. Harv Rev Psychiatry. 2006;14:141‐151. [DOI] [PubMed] [Google Scholar]
- 39. Buckner RL, Head D, Parker J, et al. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas‐based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724‐738. [DOI] [PubMed] [Google Scholar]
- 40. Iglesias JE, Augustinack JC, Nguyen K, et al. A computational atlas of the hippocampal formation using ex vivo, ultra‐high resolution MRI: application to adaptive segmentation of in vivo MRI. Neuroimage. 2015;115:117‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. de Flores R, La Joie R, Chételat G. Structural imaging of hippocampal subfields in healthy aging and Alzheimer's disease. Neuroscience. 2015;309:29‐50. [DOI] [PubMed] [Google Scholar]
- 42. Benjamini Y, Hochberg Y. Controlling the false discovery rate ‐ a practical and powerful approach to multiple testing. J Roy Stat Soc B Met. 1995;57:289‐300. [Google Scholar]
- 43. Jack CR, Bennett DA, Blennow K, et al. NIA‐AA research framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14:535‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hedges DW, Erickson LD, Kunzelman J, Brown BL, Gale SD. Association between exposure to air pollution and hippocampal volume in adults in the UK Biobank. Neurotoxicology. 2019;74:108‐120. [DOI] [PubMed] [Google Scholar]
- 45. Power MC, Lamichhane AP, Liao D, et al. The association of long‐term exposure to particulate matter air pollution with brain MRI findings: the ARIC study. Environ Health Perspect. 2018;126:027009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chen JC, Wang X, Wellenius GA, et al. Ambient air pollution and neurotoxicity on brain structure: evidence from women's health initiative memory study. Ann Neurol. 2015;78:466‐476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wilker EH, Preis SR, Beiser AS, et al. Long‐term exposure to fine particulate matter, residential proximity to major roads and measures of brain structure. Stroke. 2015;46:1161‐1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kritikos M, Gandy S, Meliker JR, Luft BJ, Clouston SA. Acute versus chronic exposures to inhaled particulate matter and neurocognitive dysfunction: pathways to Alzheimer's disease or a related dementia. J Alzheimer's Dis. 2020:1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gavett SH. World Trade Center fine particulate matter–chemistry and toxic respiratory effects: an overview. Environ Health Perspect. 2003;111:971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Landrigan PJ, Lioy PJ, Thurston G, et al. Health and environmental consequences of the world trade center disaster. Environ Health Perspect. 2004;112:731‐739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cho J, Sohn J, Noh J, et al. Association between exposure to polycyclic aromatic hydrocarbons and brain cortical thinning: the Environmental Pollution‐Induced Neurological EFfects (EPINEF) study. Sci Total Environ. 2020;737:140097. [DOI] [PubMed] [Google Scholar]
- 52. O'Doherty DC, Chitty KM, Saddiqui S, Bennett MR, Lagopoulos J. A systematic review and meta‐analysis of magnetic resonance imaging measurement of structural volumes in posttraumatic stress disorder. Psychiatry Res. 2015;232:1‐33. [DOI] [PubMed] [Google Scholar]
- 53. Karl A, Schaefer M, Malta LS, et al. A meta‐analysis of structural brain abnormalities in PTSD. Neurosci Biobehav Rev. 2006;30:1004‐1031. [DOI] [PubMed] [Google Scholar]
- 54. Felmingham K, Williams LM, Whitford TJ, et al. Duration of posttraumatic stress disorder predicts hippocampal grey matter loss. Neuroreport. 2009;20:1402‐1406. [DOI] [PubMed] [Google Scholar]
- 55. Chen LW, Sun D, Davis SL, et al. Smaller hippocampal CA1 subfield volume in posttraumatic stress disorder. Depress Anxiety. 2018;35:1018‐1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang Z, Neylan TC, Mueller SG, et al. Magnetic resonance imaging of hippocampal subfields in posttraumatic stress disorder. Arch Gen Psychiatry. 2010;67:296‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hayes JP, Hayes S, Miller DR, et al. Automated measurement of hippocampal subfields in PTSD: evidence for smaller dentate gyrus volume. J Psychiatr Res. 2017;95:247‐252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Golier JA, Yehuda R, Santi SD, et al. Absence of hippocampal volume differences in survivors of the Nazi Holocaust with and without posttraumatic stress disorder. Psychiatry Res. 2005;139:53‐64. [DOI] [PubMed] [Google Scholar]
- 59. Yehuda R, Golier JA, Tischler L, et al. Hippocampal volume in aging combat veterans with and without post‐traumatic stress disorder: relation to risk and resilience factors. J Psychiatr Res. 2007;41:435‐445. [DOI] [PubMed] [Google Scholar]
- 60. Mueller SG, Ng P, Neylan T, et al. Evidence for disrupted gray matter structural connectivity in posttraumatic stress disorder. Psychiatry Res. 2015;234:194‐201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Medical diagnoses are considered protected health information so processed de‐identified data will be made available upon receipt of a reasonable request to the corresponding author; raw image files can be accessed upon completion of a data use agreement.


