Abstract
Pancreatic ductal adenocarcinoma is the seventh leading cause of cancer death worldwide with an estimated 4329242 deaths occurring in 2018. This estimate, in conjunction with the findings that pancreatic ductal adenocarcinoma incidence is rising and that pancreatic ductal adenocarcinoma has the highest case-fatality rate of any solid tumour, highlights the urgency for designing novel therapeutic strategies to combat this deadly disease. Through the efforts of the global research community, our knowledge of the factors that lead to the development of pancreatic ductal adenocarcinoma, its progression, and the interplay between tumour cells and their surrounding microenvironment have improved substantially. Although these scientific advances have not yet translated into targeted or immunotherapy strategies that are effective for most patients with pancreatic ductal adenocarcinoma, important incremental progress has been made particularly for the treatment of specific molecular subgroups of tumours. Although PD-1 inhibitors for mismatch-repair-deficient tumours and NTRK inhibitors for tumours containing NTRK gene fusions are the most recent targeted agents approved by the US Food and Drug Administration, olaparib for germline BRCA-mutated pancreatic ductal adenocarcinoma is expected to be approved soon in the maintenance setting. These recent advances show the accelerated pace at which pancreatic ductal adenocarcinoma drugs are achieving successful clinical outcomes. Here we review the current understanding of the pathophysiology of pancreatic ductal adenocarcinoma, recent advances in the understanding of the stromal microenvironment, current standard-of-care treatment, and novel therapeutic targets and strategies that hold promise for improving patient outcomes. We predict that there will be major breakthroughs in the treatment of pancreatic ductal adenocarcinoma in the next 5–10 years. These breakthroughs will result from the increased understanding of the treatment barriers imposed by the tumour-associated stroma, and from the development of novel approaches to re-engineer the tumour microenvironment in favour of effective anticancer responses.
Introduction
Pancreatic ductal adenocarcinoma is the seventh leading cause of cancer death worldwide with an estimated 4329242 deaths occurring in 2018. With a 5-year survival rate of only 9% and the highest incidence-to-mortality ratio of any solid tumour, there is a tremendous need for the development of novel therapeutic strategies for this deadly disease.1 In this Review, we will discuss progress in understanding the biology of pancreatic ductal adenocarcinoma, current treatment frameworks, and emerging therapeutic options for advanced or unresectable pancreatic ductal adenocarcinoma, which constitute more than 80% of pancreatic ductal adenocarcinoma at diagnosis.
Improving the current standard of care
The mainstay of current treatment for metastatic pancreatic ductal adenocarcinoma is combination cytotoxic chemotherapy (figure 1). There are two multidrug regimens that are first-line treatment options for patients with metastases who have a good performance status: FOLFIRINOX (fluorouracil, leucovorin, irinotecan, and oxaliplatin) and gemcitabine combined with nanoparticle albumin-bound paclitaxel (nab-paclitaxel).2,3
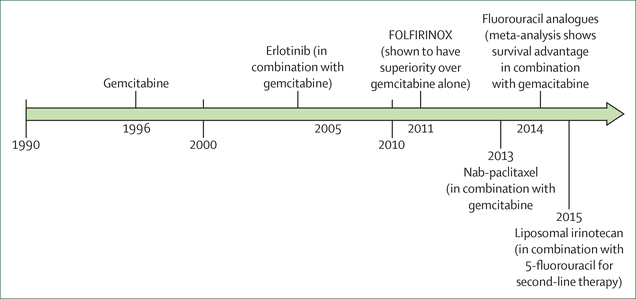
Figure 1: Timeline of US Food and Drug Administration approvals for the treatment of metastatic pancreatic cancer.
Treatment options for metastatic pancreatic cancer placed on a timeline on the basis of their year of US Food and Drug Administration (FDA) approval. FOLFIRINOX (leucovorin, fluorouracil, irinotecan, and oxaliplatin) and fluorouracil analogues in combination with gemcitabine are also included given their use in clinical practice, although no specific FDA approval has been obtained. Nab=nanoparticle albumin-bound.
There are no published, large, prospective, head-to-head trials directly comparing FOLFIRINOX with gemcitabine–nab-paclitaxel, and either regimen is a reasonable upfront option for patients with metastatic pancreatic ductal adenocarcinoma with good performance. GATA6 expression represents a predictive marker that might help to decide between these options by differentiating between classical and basal-like pancreatic ductal adenocarcinoma subtypes.4 As basal-like pancreatic ductal adenocarcinoma did not respond as well as classical subtypes to fluorouracil-based therapy in the ESPAC-3 and COMPASS trials, absence of GATA6 expression might assist selection between these two first-line options.5 A new prospective study, the PASS study, is being planned to address this question. It will be done in the USA and Canada and funded by the charity organisations Stand Up 2 Cancer and the Lustgarten Foundation.
Attempts have been made to improve these regimens by modifying the sequence of approved combination regimens or adding other cytotoxic agents. A quadruplet regimen involving the addition of cisplatin and capecitabine to gemcitabine and nab-paclitaxel was tested in 24 patients; 16 patients achieved partial responses and eight patients had stable disease.6 An ongoing follow-up study is testing all five active cytotoxic agent classes at lower doses (gemcitabine, nab-paclitaxel, cisplatin, irinotecan, and capecitabine) with results expected next year (NCT03535727).
In the second-line setting, patients with good performance status are often, in practice, given the alternative first-line regimen that they did not receive initially. The only approved second-line therapy is a combination of fluorouracil and protein-encapsulated irinotecan for patients who received a gemcitabine-based therapy in the first line. To the best of our knowledge, no study has shown an improvement in outcomes for any drug regimen compared with gemcitabine alone among patients with an Eastern Cooperative Oncology Group performance status greater than 1. This is a key point, as many patients with pancreatic ductal adenocarcinoma present with poor (≥2) performance status.
It is important to mention that studies done in Asia have shown good efficacy using S-1 either as a monotherapy or together with other chemotherapy agents.7 However, these results were not replicated in European and North American cohorts, suggesting population-specific differences.
As with other cancer types, cytotoxic therapy has been tested in combination with immune checkpoint blockade. Gemcitabine and nab-paclitaxel were evaluated in combination with pembrolizumab in 17 patients, three of whom had a partial response.8 Looking specifically at the 11 patients treated in the first-line setting evaluable for response, all achieved stable disease or a partial response with an overall survival of 15 months, creating enthusiasm for further evaluation of this regimen.8
Biological pathways driving pancreatic ductal adenocarcinoma development and progression and new opportunities for therapeutic interventions
Molecularly targeted therapy
Targeting KRAS
Pancreatic ductal adenocarcinoma typically arises in the background of a precancerous lesion, pancreatic intraepithelial neoplasia (PanIN), which becomes more dysplastic with successive accumulation of genetic aberrations (table).9 Although pancreatic ductal adenocarcinoma represents a highly heterogeneous disease, there are recurrent alterations commonly seen within four key oncogene or tumour suppressor genes (KRAS, TP53, SMAD4, and CDKN2A).9
Table:
Genetic mutations present in pancreatic cancer
| Frequency (%)* | Role in tumorigenesis | Potential targeted approaches | Level of clinical evidence | |
|---|---|---|---|---|
| KRAS | 91% | Constitutive activation of RAS–MAPK pathway promoting proliferation | KRAS inhibitors, vaccines, bispecific antibodies, MEK inhibitors | Ongoing trials, encouraging early phase data in other cancer types |
| TP53 | 70% | Impaired recognition of DNA damage and cell cycle arrest | None currently available | NA |
| CDKN2A | 46% | Loss of regulation of the CDK4 and CDK6 growth checkpoint | CDK4 and CDK6 inhibitors | Ongoing trials |
| SMAD | 38% | Alteration in TGFβ signalling permitting increased proliferation | TGFβ inhibitors | Encouraging phase 2 data |
| ATM | 3% | Dysfunctional homologous recombination repair | ATR inhibitors, platin chemotherapy, PARP inhibitors† | Ongoing trials |
| BRAF | 2·2% | Constitutive activation of RAS–MAPK pathway promoting proliferation | BRAF inhibitors with or without MEK inhibitors | Case reports and series suggest efficacy |
| BRCA1 | 1·3% | Dysfunctional homologous recombination repair | Platin chemotherapy, PARP inhibitors† | Positive phase 3 data for PARP inhibitors in germline mutations |
| BRCA2 | 1·3% | Dysfunctional homologous recombination repair | Platin chemotherapy, PARP inhibitors† | Positive phase 3 data for PARP inhibitors in germline mutations |
| MSI family | 0·8% | Impaired mismatch repair | PD-1 blockade | FDA-approved |
| PALB2 | 0·7% | Dysfunctional homologous recombination repair | Platin chemotherapy, PARP inhibitors† | Ongoing trials |
| NRG1 fusion | 0·5% | Increased HER3 dimerisation with HER2 causing RAS–MAPK activation | EGFR inhibitors: afatinib or pertuzumab | Case reports and series suggest efficacy |
| NTRK fusion | 0·3% | Upregulation of TRK activity | TRK inhibitors: larotrectinib | FDA-approved |
| ALK amplification | 0·16% | Constitutive activation of RAS–MAPK pathway promoting proliferation | ALK inhibitors: crizotinib | Case reports and series suggest efficacy |
FDA=US Food and Drug Administration. PARP=poly (ADP-ribose) polymerase.
Percentage of pancreatic cancers with genomic aberrations.
PARP inhibitors include olaparib or veliparib.
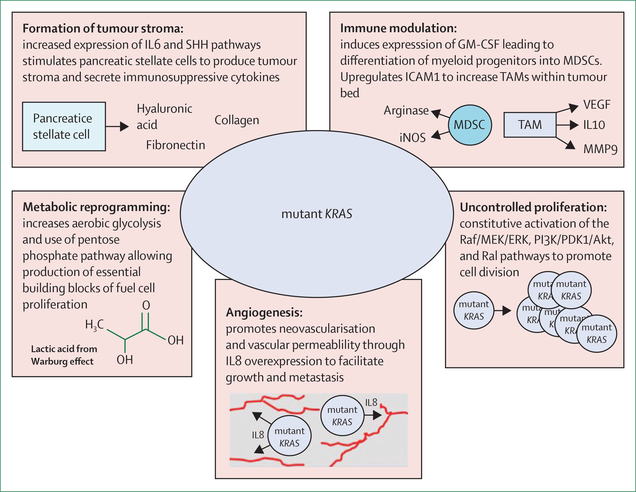
One of the earliest alterations found in PanIN lesions is activation of the RAS–MAPK pathway, typically through acquisition of a KRAS codon 12, 13, or 61 mutation, leading to multiple cancer-promoting effects (figure 2).10 Despite the attractiveness of this target and substantial efforts, it has been challenging to develop clinically effective direct inhibitors of KRAS.11,12 Murine models suggest that KRAS inhibition (or direct blockade of downstream MEK) and resultant MAPK repression results in the activation of AKT, EGFR, HER2, and PDGFRα, and AXL, potentially explaining the inefficacy of these agents.13 Despite these setbacks, continued efforts are being made to develop novel means of inhibiting KRAS or its downstream effectors.
Figure 2: The consequences of mutated KRAS.
Outline of the multiple pathways through which mutant KRAS facilitates pancreatic cancer formation and growth, and prevents an effective antitumour immune response. GM-CSF=granulocyte-macrophage colony-stimulating factor. IL=interleukin. iNOS=inducible nitric oxide synthase. MDSC=myeloid-derived suppressor cell. MMP9=matrix metallopeptidase 9. SHH=sonic hedgehog. TAM=tumour-associated macrophage.
In the case of KRAS Gly12Cys mutations, there are early indications that inhibitors might be effective in patients with non-small-cell lung cancer.14 Seven of 13 patients with KRAS Gly12Cys mutations who received AMG510, a KRAS Gly12Cys inhibitor, acheived a partial response, whereas the other six patients had stable disease.14 Gly12Cys is an uncommon mutational change found in only 2% of pancreatic tumours, and activity of these agents in pancreatic ductal adenocarcinoma is presently unknown. However, if effective, this approach might inform efforts to inhibit more common mutations (ie, KRAS Gly12Asp, Gly12Val, or Gly12Arg).
Alternatively, the ubiquitous nature of KRAS mutations and their specificity to tumour cells make KRAS a good candidate for immune targeting. Historically, KRAS mutations were not considered immunogenic, with minimal KRAS-mutation-specific T-cell responses detectable in patients with pancreatic ductal adenocarcinoma. However, Tran and colleagues15 reported on a patient with colon cancer who was treated successfully with administration of ex-vivo expanded tumour infiltrating lymphocytes targeting KRAS Gly12Asp, suggesting that further investigation of KRAS as an antigen is warranted. Our group is starting a clinical trial (NCT04117087) using a peptide vaccination strategy with immune checkpoint inhibition against the six most common KRAS mutations in patients with pancreatic ductal adenocarcinoma in the adjuvant setting.
KRAS wild-type tumours
Although few pancreatic ductal adenocarcinomas are KRAS wild-type, these tumours deserve special attention given their enrichment for currently targetable genetic alterations. Erlotinib was tested in combination with gemcitabine for the first-line treatment of unselected patients with metastatic pancreatic ductal adenocarcinoma and showed a statistically but not clinically significant increased overall survival (6.2 months with erlotinib plus gemcitabine vs 5·9 months with gemcitabine alone), leading to its US Food and Drug administration (FDA) approval.16 However, as other combination treatments were shown to have more promising results than erlotinib plus gemcitabine, this combination is rarely clinically utilised. One exception is in KRAS wild-type disease, where it was observed in a phase 2 trial evaluating nimotuzumab, an EGFR inhibitor, in combination with gemcitabine, that EGFR inhibition might provide increased benefit. Subgroup analyses that specifically looked at patients with KRAS wild-type tumours, including 13 patients in the nimotuzumab plus gemcitabine group and 20 patients in the group receiving gemcitabine only, showed an improvement in overall survival (11.6 months in the nimotuzumab plus gemcitabine vs 5.6 months in the gemcitabine group).17 These findings prompted a phase 3 trial in China for patients with KRAS wild-type tumours. This trial finished enrolment in March, 2018, and results are anticipated imminently.
Other actionable mutations found to be enriched in the small subset of patients with KRAS wild-type pancreatic ductal adenocarcinoma include ALK amplifications (0·16%), BRAF mutations (2·2%), NRG1 fusions (0·5%), and NTRK gene fusions (0·3%).18–22 Case reports and series suggest that these tumours might respond to targeted inhibition of these altered pathways.18,19 These rare but targetable molecular abnormalities form the basis for the recommendation that all patients with pancreatic ductal adenocarcinoma undergo genetic profiling of their tumours, so that appropriate treatment options can be explored.
DNA damage repair modulators
Pancreatic ductal adenocarcinoma is associated with germline and somatic mutations within the homologous recombination repair pathway (BRCA2, ATM, BRCA1, or PALB2).23 Recognition of these mutations is clinically valuable as homologous recombination deficiency might predict increased sensitivity to platinum chemotherapy and could be therapeutically targetable.
For example, the subset of homologous recombination deficient tumours with BRCA1/2 mutations display increased sensitivity to poly (ADP-ribose) polymerase (PARP) inhibitors. A pivotal phase 3 randomised trial showed that PARP inhibitors prolonged progression-free survival as maintenance therapy in patients with germline BRCA1/2 mutations who had not progressed on first-line platinum-based chemotherapy.24 We think that this trial provides the most exciting clinical data to date for a mutation-driven subset of pancreatic ductal adenocarcinoma, and FDA approval is expected imminently.24 It is, however, important to note that the comparator group was placebo, and that overall survival data are not yet mature enough to fully understand the true benefit. Still, these data further emphasise the need for patients with metastatic pancreatic ductal adenocarcinoma to get molecular testing at diagnosis.
Early phase trials have also shown that PARP inhibitors are efficacious in later lines of treatment for homologous recombination deficient pancreatic ductal adenocarcinoma or in combination with platinum agents in the first-line setting.25 These results are in the process of being tested in later phase clinical trials. This approach is one of the most promising areas of research in pancreatic ductal adenocarcinoma therapy, albeit for a small subset of patients, with the hope that novel and combination approaches will show efficacy in the first-line setting.
In addition, ATM is a key component of the homologous recombination pathway, although there are conflicting reports on whether tumours with ATM mutations respond to treatment strategies targeting homologous recombination deficient tumours.23 Cells can compensate for loss of ATM through upregulation of ATR, suggesting that inhibitors of ATR might show efficacy in ATM-deficient tumours.23 Tissue agnostic early studies of ATR inhibitors in ATM-deficient tumours are ongoing.
Cell cycle inhibitors
CDKN2A acts as a checkpoint to cell cycle progression through its inhibition of the cyclin-dependent kinases CDK4 and CDK6, providing a rationale for targeting CDKN2A-mutated tumours with CDK4 and CDK6 inhibitors.26 Dual inhibition of CDK4 and CDK6 with palbociclib and of MEK (which is downstream of KRAS) with trametinib, has shown efficacy in xenograft models.27 An early phase study of palbociclib with a PI3K/mTOR inhibitor is being done in multiple cancers, including pancreatic ductal adenocarcinoma (NCT03065062). Still, with the feedback loops inherent in CDK and PI3K–AKT signalling, these trials have a considerable biological hurdle to overcome, likely accounting for the absence of positive results for the combination targeted therapy approaches.
Pancreatic tumour stroma
Pancreatic ductal adenocarcinoma is characterised by the presence of a dense fibrous stroma that represents up to 90% of the tumour volume. This pancreatic extracellular matrix, produced by cancer-associated fibroblasts, is predominantly made of collagen, hyaluronic acid, and fibronectin. The full implications of this extracellular matrix and associated cells are still under investigation, but this dense stroma has been shown to limit efficacy of standard cytotoxic, immune, and targeted agents.28
Increased hyaluronic acid is associated with decreased survival in patients with pancreatic ductal adenocarcinoma, probably through increased interstitial pressure that impedes diffusion of therapeutic agents and nutrients into the tumour microenvironment.29 Hyaluronidase was investigated to break down hyaluronic acid. A phase 1b study of PEGylated hyaluronidase (PEGPH20) in combination with gemcitabine showed an overall survival of 6·6 months in all-comers, but an overall survival of 13 months in the six patients with elevated hyaluronic acid concentrations.30 This study was followed by a randomised phase 2 trial of gemcitabine and nab-paclitaxel with or without PEGPH20. The PEGPH20 group of this trial had an improvement in progression-free survival (6·0 months vs 5·3 months in patients who did not receive PEGPH20), which was amplified in patients with tumours with high hyaluronic acid expression (9·2 months for patients receiving PEGPH20 vs 5·2 months for those that did not).31 However, enthusiasm lessened after a randomised phase 2 trial, which used FOLFIRINOX with or without PEGPH20, showed reduced survival for patients receiving PEGPH20 and FOLFIRINOX (overall survival 7·7 months) compared with patients solely receiving FOLFIRINOX (overall survival 14·4 months).32 A phase 3 trial evaluating PEGPH20 in combination with gemcitabine and nab-paclitaxel in patients who have tumours with high hyaluronic acid concentrations reported no improvement in overall survival compared with gemcitabine alone, leading to a suspension of further exploration of PEGPH20.
SHH signalling facilitates pancreatic stellate cell transdifferentiation to myofibroblasts, and matrix metallopeptidase and nerve growth factor secretion.33 Experimental models inhibiting the SHH pathway were promising, with a reduction of tumour stroma and improved chemotherapy delivery. A single-arm phase 2 trial of gemcitabine, nab-paclitaxel, and vismodegib showed a 43% response in 49 patients.34 However, interest for this approach has lessened after a clinical trial using an SHH inhibitor in combination with gemcitabine was stopped early because of worsened outcomes. This result was later explained by animal modelling, which revealed that although SHH inhibitors reduced stroma formation, tumours became more aggressive and poorly differentiated. Further testing of this strategy is not presently being explored.
The concept that microvascular invasion and neovasculature are needed for pancreatic ductal adenocarcinoma growth and spread resulted in many trials testing antiangiogenic agents in combination with chemotherapy, including multiple negative phase 3 trials. A systematic review has underscored these findings and showed no benefit across antiangiogenic agents tested in combination with gemcitabine-based therapy.35
Tumour-associated macrophages
Tumour-associated macrophages are abundant in the pancreatic ductal adenocarcinoma stroma where they promote tumour formation, treatment resistance, and suppression of antitumour immune responses.36 Strategies have been developed to target tumour-associated macrophages and convert them into tumouricidal macrophages that promote rather than suppress antitumour immunity (figure 3).
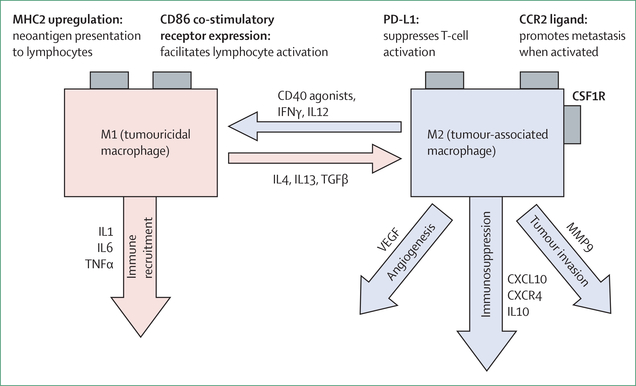
Figure 3: Plasticity of macrophages.
Illustration of the factors that convert a macrophage between a tumouricidal (M1) and immunosuppressive (M2) state, and the consequences of these changes. MHC=major histocompatibility complex. IL=interleukin.
CD40 agonist therapy has been proposed as a strategy to facilitate reprogramming of tumour stroma to improve penetration by chemotherapy and promote antitumour immune responses.36 This target was identified on the basis of the normal interaction of CD40 with CD40L on CD4 T cells, which activates macrophages to promote antigen presentation and effective priming of cytotoxic T cells.37 CD40 agonist therapy was tested in combination with gemcitabine in a cohort of 21 patients with pancreatic ductal adenocarcinoma and had a 19% response, with 52% of patients achieving stable disease at their 2-month evaluation.37 A follow-up study testing the combination of chemotherapy, a CD40 agonist, and anti-PD-1 blockade is ongoing; early results suggest a significant increase in early response compared with chemotherapy plus CD40 agonist therapy alone.38 A follow-up randomised controlled study is being planned and should begin enrolling in 2020.
CCR2 inhibitors are also being developed to prevent accumulation of tumour-associated macrophages. CCL2 binds to CCR2 on inflammatory monocytes to attract them to the tumour stroma where they differentiate into immunosuppressive tumour-associated macrophages.39 A phase 1b trial showed that 16 of 33 patients with borderline resectable pancreatic ductal adenocarcinoma receiving a CCL2–CCR2 inhibitor (PF-04136309) in combination with FOLFIRINOX achieved a partial response.39 Post-treatment biopsies revealed a significant reduction in tumour-associated macrophages and T regulatory cells (Tregs), and an increase in both CD4 and CD8 T cells.
CSF1R is another immune signalling pathway protein expressed by tumour-associated macrophages. Inhibitors of CSF1R have shown success in producing tumour regression and improved survival in mouse models through reprogramming of tumour-associated macrophages to enhance antigen presentation and promote an antitumour T-cell response.36 A preliminary study reported durable responses in five of 31 heavily pretreated patients with pancreatic ductal adenocarcinoma who received a CSF1R inhibitor in combination with nivolumab.40 These early findings have led to multiple studies testing CSF1R inhibitors as single agents and in combination with other immune checkpoint inhibitors or chemotherapy.
Myeloid-derived suppressor cells
Pancreatic tumour cells secrete granulocyte-macrophage colony-stimulating factor (GM-CSF), resulting in differentiation and migration of myeloid-derived suppressor cells (MDSCs) to tumour stroma where they act to attenuate cytotoxic T lymphocyte infiltration and upregulate PD-L1 expression on tumour cells (figure 4).39,41,42 Murine studies suggested that ibrutinib, a BTK inhibitor, can reduce the number and immunosuppressive function of MDSCs present in the tumour microenvironment.42 However, despite this strong preclinical rationale, a phase 3 trial combining ibrutinib with gemcitabine–nab-paclitaxel did not yield a survival advantage.
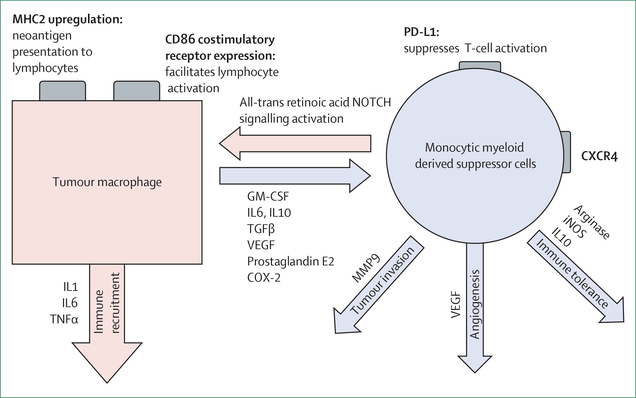
Figure 4: Plasticity of myeloid-derived suppressor cells.
Monocytic myeloid-derived suppressor cells are able to convert to tumouricidal macrophages that facilitate tumour clearance. Mechanisms to catalyse this transformation remain an active and important area of investigation. MHC=major histocompatibility complex. GM-CSF=granulocyte-macrophage colony-stimulating factor. IL=interleukin. iNOS=inducible nitric oxide synthase.
An alternative approach targets the interaction between CXCL12, a chemokine protein produced by cancer-associated fibroblasts, and the chemokine receptor CXCR4, found on T cells, leading to recruitment of MDSCs into the tumour microenvironment.43,44 Combining inhibition of CXCR4 and PD-1 in mice showed infiltration of activated T cells; and, in cultured tumour biopsies, this approach showed increased tracking of T lymphocytes to tumour cells and induction of apoptosis.43 Alternatively, the use of PEGPH20 in combination with a GM-CSF-conjugated whole-cell pancreatic ductal adenocarcinoma vaccine (GVAX) has been shown to reduce both CXCR4 and PD-L1 expression in animal models.44 In both instances, the reduction in CXCR4 was associated with enhanced CD8 T-lymphocyte infiltration into tumours. Studies of these agents in combination with anti-PD-1 therapy are enrolling presently, and their utility in pancreatic ductal adenocarcinoma remains a particularly exciting area of exploration.
Regulatory T cells
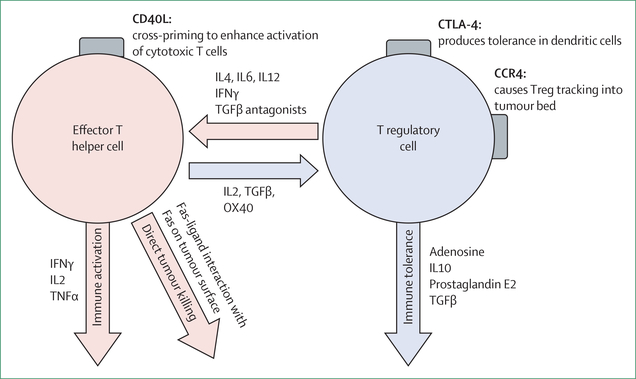
Tregs suppress inflammation and dampen antitumour responses through secretion of the cytokines interleukin-10 and TGFβ, and by CTLA-4 signalling. However, subsets of Tregs have great plasticity and can convert to effector CD4 T cells under appropriate conditions (figure 5). Multiple approaches are being tested to target Tregs through altering both membrane signalling and chemokine–cytokine signalling within the tumour microenvironment.
Figure 5: Plasticity of T lymphocytes.
Schematic of transition that can occur between effector T helper cells and T regulatory cells, the factors that influence these transitions, and the effects that result. IL=interleukin. Treg=T regulatory cell.
TGFβ has a pleotropic set of actions. Whereas some actions are antitumour, others facilitate immune evasion, epithelial-mesenchymal transition, and differentiation of T lymphocytes to Tregs.45 TGFβ antagonism is being combined with traditional chemotherapy to test whether the stromal-reducing properties of TGFβ inhibition would facilitate delivery of chemotherapy. A phase 1b clinical trial, which combined galunisertib (a TGFβ inhibitor) with gemcitabine, had a 42·9% response.46 This trial was followed by a randomised phase 2 cohort in which patients received gemcitabine with or without galunisertib. The trial showed an overall survival of 8·9 months in patients receiving the combination versus 7·1 months in patients receiving gemcitabine alone, revealing a significant but modest improvement.46 The increased haematological toxicity seen among patients in the galunisertib group (grade 3–4 neutropenia in 29% of the patients and grade 3–4 thrombocytopenia in 21% of patients) might limit its use as an adjunct to currently used first-line combinations.
An alternative method is the use of a bispecific antibody, M7824, targeting TGFβ and PD-L1. This antibody inhibits two complementary immunosuppressive pathways simultaneously via two distinct functional domains by binding PD-L1 expressed on the cell surface and acting like a trap for soluble TGFβ. This approach showed activity in a phase 1 study of multiple tumour types, with two of five patients with pancreatic ductal adenocarcinoma having durable benefit.47 This agent was also tested in 30 patients with biliary tract cancers, of whom 27% had durable responses, although 33% had grade 3 or higher toxicities.48
FAK is another protein in the microenvironment that is being targeted by inhibitory agents. Tumour cells overexpress FAK, which leads to Treg and MDSC recruitment, macrophage conversion to tumour-associated macrophages, and fibroblast activation and proliferation.49 A FAK inhibitor (VS-4718) given in a mouse model increased survival through tumour stasis, decreased fibrosis, and reduced tumour-associated macrophage, MDSC, and Treg infiltration.49 Unfortunately, the positive effects of monotherapy were transient, with resistance developing via upregulation of the STAT3 pathway.50 These data show how loss of one pathway can be compensated by another, and that FAK inhibition in combination with STAT3 pathway inhibition might be more successful in extending antitumour responses.50
Finally, tumour-infiltrating Tregs highly express CCR4 on their surface. Mogamulizumab, an anti-CCR4 antibody, is under investigation in hopes that the removal of Tregs from the tumour microenvironment will produce a more robust antitumour immune response.51
Epigenetics, tumour plasticity, metabolism, and cancer stem cells
Epigenetic therapy
The transcriptional profile of human cells is governed in part by the open nature of the DNA chromatin: DNA methylation leads to tightening of these complementary strands whereas acetylation weakens DNA interstrand binding, opening up the DNA for transcription.
Epigenetic therapy aims to exogenously influence this process to promote responses to immunotherapy in solid tumours via uncovering of neoantigens and reprogramming of the tumour microenvironment.52 Entinostat, a histone deacetylase inhibitor, in combination with checkpoint blockade, facilitated responses in murine pancreatic ductal adenocarcinoma models through suppression of MDSC activity, leading to improved responses to immune checkpoint inhibitor therapy.52 This strategy is undergoing evaluation in clinical trials (NCT03250273).
In addition to these more established epigenetic targets, coactivators of BET, a bromodomain protein, have gained prominence because of their importance to MYC transcription and activity, which is of particular interest given that MYC signalling is consistently upregulated in pancreatic ductal adenocarcinoma.53 Mazur and colleagues reported the synergistic effects of BET and histone deacetylase inhibition in pancreatic ductal adenocarcinoma mouse models through decreasing MYC activity and inflammation.53 BET inhibitors are currently undergoing therapeutic evaluation in patients with pancreatic ductal adenocarcinoma (NCT03925428).
Tumour metabolism
Pancreatic ductal adenocarcinomas have markedly altered cellular metabolism typified by increased utilisation of aerobic glycolysis and upregulation of the pentose phosphate pathway. Although previous attempts to exploit this process with metformin proved unsuccessful, newer more targeted approaches have shown early signs of favourable outcomes.54 One notable example is the use of CPI-613, an inhibitor of the pyruvate dehydrogenase and α-ketoglutarate dehydrogenase complexes, which—in combination with FOLFIRINOX—had a 61% response, including 17% complete response, in a small phase 1 trial.55 This combination is currently undergoing evaluation in a phase 3 trial (NCT03504423).
Cancer stem cells
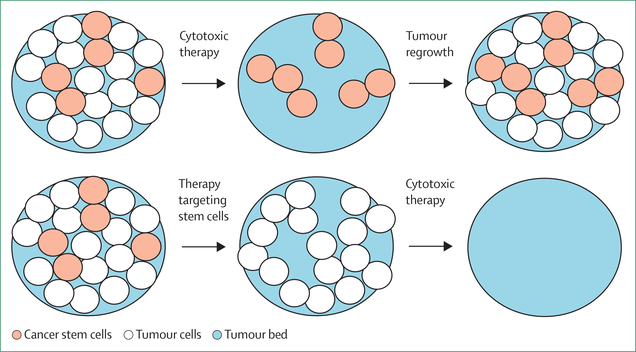
Pancreatic cancer stem cells represent a small minority of tumour cells and are characterised by self-renewal, the ability to differentiate, and chemotherapy resistance (figure 6).56 Pancreatic cancer stem cells are noted to express CD44, CD24, and CD326 on their cell surface, and these molecules interact with the tumour stroma to support their self-renewal and treatment resistance properties. Many strategies have attempted to target this treatment-resistant population without substantial success. One notable example tested napabucasin, a STAT3 inhibitor, and showed 78% disease control in a phase 1b/2 trial of patients with metastatic pancreatic ductal adenocarcinoma in combination with gemci-tabine–abraxane.57 This prompted a phase 3 trial, which unfortunately did not replicate the earlier favourable outcomes.
Figure 6: Targeting cancer stem cells.
Given their chemotherapy-resistant properties, cancer stem cells often survive traditional chemotherapy to repopulate the tumour bed. Effective targeting of cancer stem cells with current and future cytotoxic therapies would therefore lead to better clearance of tumours.
These disappointing results might relate to the heterogeneous nature of these stem cells, their plasticity between stem cell states, and the reversion of mature pancreatic ductal adenocarcinoma cells. Despite the setbacks, cancer stem cells are an important and active area of investigation with regards to treatment resistance and disease aggressiveness. Durable responses will probably require the targeting of multiple stem cell pathways or their use in combination with agents targeting the tumour microenvironment components that nurture cancer cell stemness. The use of single cell sequencing is facilitating our understanding of the factors that influence tumour cell heterogeneity.58 These efforts and the valuable information they yield should help to inform both traditional and stem cell targeting therapies moving forward.58
Immunotherapy
We and others have been developing immunotherapy approaches to activate the immune system against pancreatic ductal adenocarcinomas. Before the development of checkpoint inhibitors, immunotherapy relied on vaccines to induce a T-cell response against shared antigens (including mutant KRAS and mesothelin), which are expressed by the majority of pancreatic ductal adenocarcinomas. Several vaccine approaches (including peptide vaccines, dendritic cell vaccines, and whole tumour cell vaccines) have been explored in small studies, yielding mixed results.
Some of these studies did report the induction of both CD4 and CD8 T cells against the target antigens; however, these changes usually did not translate into high rates of clinical responses.59
GVAX has been the most well studied of these vaccine approaches in patients with pancreatic ductal adenocarcinoma. GVAX was produced by stably transfecting two cancer cell lines with GM-CSF expression constructs to activate T cells against a wide variety of potential tumour-specific antigens.60 Initial clinical investigations with GVAX revealed an expansion of mesothelin-specific CD8 T cells.60 This led to the development of a mesothelin-expressing Listeria monocytogenes (LM-mesothelin) vaccine for use in a prime–boost approach with GVAX. A randomised phase 1 trial, which compared the combination of GVAX as prime and LM-mesothelin as boost with GVAX alone, revealed that the combination is efficacious as a second-line and third-line therapy. Specifically, this study showed vaccine-induced immune responses that were associated with a survival benefit in patients receiving the combination; additionally, survival was higher in either group among individuals who received at least three doses (rather than two or fewer doses) of the vaccine (overall survival 9·7 months vs 4·6 months).61 Unfortunately, a randomised phase 2b study evaluating this combination in the second-line and third-line metastatic setting versus chemotherapy did not show an improvement in overall survival.62 With recognition of immune checkpoints, it became clear that even though these vaccines were able to augment T-cell immune responses, these T cells would then be turned off by one or more checkpoint signals on the tumour, stromal cells, or immune cells. This hypothesis was evaluated in a small study (of 25 patients) in which patients were randomly assigned to receive the GVAX vaccine plus ipilimumab, or ipilimumab alone. Although it was a small study, prolonged survival was observed in 27% of patients who received the combination. Furthermore, long-term survivors (>6 months) were noted to have greater T-cell diversification before therapy and were more likely to have an expansion of more than 100 diverse T-cell clones with therapy, as measured by T-cell receptor sequencing. These data provide the first evidence that vaccine induction of T cells is a necessary first step for checkpoint inhibitors to have activity in patients with pancreatic ductal adenocarcinoma, which suggests that these approaches might be complementary. Additional studies testing GVAX and the combination of GVAX and Listeria vaccination with anti-PD-1 and other combinations of checkpoint inhibitors are currently ongoing or under development.59,63
Peptide vaccine strategies are also undergoing reevaluation in this new era of checkpoint inhibitor therapy. A randomised phase 2 study of survivin 2B peptide vaccination with interferon β (including 83 patients in total) showed a significant immunological response, with subgroup analysis suggesting a potential survival benefit.64 Zaidi and colleagues took a different approach and evaluated a neoantigen vaccine in a mouse model of pancreatic ductal adenocarcinoma. This study showed that a vaccine targeting just 12 expressed neoantigens can cure tumour-bearing mice when the vaccine is given in combination with checkpoint inhibitors.65 On the basis of these data, a clinical trial is in development that will administer personalised neoantigen peptide vaccines with immune checkpoint inhibition to metastatic patients with pancreatic ductal adenocarcinoma in the maintenance setting.
As discussed above, vaccines have shown promise when given with checkpoint inhibitors. These investigations are necessary because pancreatic ductal adenocarcinoma has not been shown to be responsive to either single agent or combination checkpoint approaches. The immunosuppressive tumour microenvironment and low tumour mutation burden probably contribute to this unresponsiveness, culminating in a shortage of good quality T cells available for activation by these checkpoint inhibitors. A notable exception to this rule are tumours with mismatch repair deficiency, which results in substantial tumour mutation burden and production of novel antigens.66 Mismatch repair deficiency is uncommon in pancreatic ductal adenocarcinoma (0·8%), but confers sensitivity to immune checkpoint blockade with pembrolizumab, which represent a highly effective FDA-approved therapy with a response of 30–50%, including durable complete responses.66 All patients with pancreatic ductal adenocarcinoma should be tested for mismatch repair deficiency as standard of care. The favourable outcome seen in mismatch repair deficiency pancreatic ductal adenocarcinoma and other tumour types treated with pembrolizumab have made immune checkpoint blockade one of the largest areas of therapeutic exploration in pancreatic ductal adenocarcinoma. In addition to vaccines, agents targeting the immunosuppressiv tumour microenvironment, including CD40 agonists, FAK inhibitors, and CSF1R inhibitors represent potential partners to checkpoint therapy in an effort to activate an antitumour immune response against pancreatic ductal adenocarcinoma.
In other tumour types, monoclonal antibodies have been shown to be efficient in binding tumour cells, eliciting complement activation, evoking antibody-dependent cellular cytotoxicity, or delivering a conjugated chemo-radiotherapy payload. Thus, there is interest in applying monoclonal antibodies for the treatment of pancreatic ductal adenocarcinoma.67 The aforementioned evidence of immune activation against mesothelin in pancreatic ductal adenocarcinoma made mesolthelin a top candidate for this approach.67 Amatuximab, a chimeric antibody against mesothelin, showed evidence of responses in patients with mesothelioma and preclinical pancreatic ductal adenocarcinoma models.68 However, enthusiasm was dampened when a randomised phase 2 trial in patients with pancreatic ductal adenocarcinoma did not show an improvement in overall survival with the addition of amatuximab to gemcitabine versus gemcitabine alone.69 Use of radiolabelled amatuximab showed superior tumour penetration in patients with ovarian cancer compared with patients with pancreatic ductal adenocarcinoma, which suggests that the unique pancreatic ductal adenocarcinoma stroma might be an obstacle to this approach.69 Ongoing trials of drug-conjugated mesothelin antibodies and antibodies against cancer stem cells remain active and we await these results. Successful use of monoclonal antibodies will probably require concomitant use of stromal-modifying agents to improve penetration of these monoclonal antibodies into the pancreatic ductal adenocarcinoma tumour bed.
The use of oncolytic viruses provides an alternate method of immune activation within pancreatic ductal adenocarcinoma tumours since these viruses can infect tumour cells, induce innate immune responses, and directly kill infected cancer cells.70 Several different constructs have been used for this approach, including human herpes virus 1, reoviruses, and adenoviruses.70 Reovirus-based trials have shown antitumour activity in early phase pancreatic ductal adenocarcinoma trials, including a 34 patient phase 2 trial that showed a clinical benefit rate (stable disease or partial response at 12 weeks) of 58%.71 Genetic engineering has played a prominent role in improving the safety profile of these agents; modifications were developed that only allow replication in some cell-based contexts, such as absence of functional p53 or presence of inflammation via the COX-2 pathway.72 It is likely that these oncolytic viruses have both direct effects on the tumours and indirect effects on the tumour microenvironment, creating the potential for combining these viruses with other tumour microenvironment modulators.
The advent of genetically engineering cells has also facilitated the development of chimeric antigen receptor (CAR) T-cell therapy. CAR T cells targeting several candidate antigens have been studied preclinically, including CAR T cells expressing mesothelin, MUC1, carcinoembryonic antigen, prostate stem cell antigen, CD24, and HER2.73,74 Given CD24’s purported role asa cancer stem cell marker, it is noteworthy that targeting of CD24, which is present on only a small subpopulation of pancreatic ductal adenocarcinoma cells, slowed tumour growth and prolonged survival in mice models.74 Promising preclinical findings have created enthusiasm for pushing these treatments forward, and these strategies are currently being assessed in clinical trials with some early evidence of efficacy with mesothelin-targeted lymphocytes.75 However, there is still much to be learned about this approach in solid tumours. It is likely that the stromal barriers and immune checkpoint pathways present in pancreatic ductal adenocarcinoma will require reprogramming in combination with CAR T-cell therapy to achieve beneficial outcomes.
Conclusion
Pancreatic ductal adenocarcinoma is an important and growing global health problem that mandates the highest priority for improving efficacy and the dismal 5-year survival offered by currently available treatment options. Despite grim statistics, substantial progress has been made in better understanding the unique features of pancreatic ductal adenocarcinoma and its surrounding microenvironment that inhibit effective penetration and tumour killing by chemotherapeutic and immunotherapy agents. There is more optimism now than ever before that advances will be made by combining chemotherapy more effectively with agents that target the unique features of pancreatic ductal adenocarcinoma tumours. The next 5–10 years should deliver major improvements in outcomes through the use of novel agents that specifically target pathological signalling pathways and genetic alterations unique to individuals with pancreatic ductal adenocarcinomas. These novel agents will probably need to be used as part of combination approaches (ie, with other novel agents, traditional chemotherapy, or immunotherapy) to prevent early resistance mechanisms from emerging. Importantly, targeted agents are being developed with reduced toxicities. These new advances should achieve considerable survival benefits with reduced side-effects, thereby converting pancreatic ductal adenocarcinoma from a death sentence into a manageable chronic disease.
Search strategy and selection criteria.
To review the current state of treatment development in pancreatic cancer, we searched PubMed using the search terms “pancreatic cancer and immunotherapy”, “pancreatic cancer and genetics”, “pancreatic cancer and stroma”, “pancreatic cancer and cytotoxics”, “pancreatic cancer and chemotherapy”, and “pancreatic cancer and targeted” for articles published between Sept 1, 2014, and Aug 31, 2019. We reviewed the abstracts of articles that were written in English and seemed relevant based on the title. Articles that were deemed appropriate on the basis of this evaluation were read and included as pertinent to the aims of this manuscript. Additional resources were uncovered via citations within these articles and targeted searches on specific emerging treatment strategies. Abstracts from the 2018–19 meeting of the European Society for Medical Oncology, the 2019 Gastrointestinal Cancer American Society of Clinical Oncology meeting, the 2019 American Society of Clinical Oncology meeting, and the 2019 American Association of Cancer Research meeting were also reviewed to incorporate recent advances in this dynamic field.
Acknowledgments
EJ receives US National Institutes of Health (NIH) funding (grant number P50-CA062924, P50-CA062924, R01-CA197296). NSA receives NIH funding (grant number R01-CA228414).
Footnotes
Declaration of interests
EJ reports a licensing agreement with Aduro Biotech, and Johns Hopkins University has the potential to receive royalties in the future. EJ receives grant funding from Bristol-Myers Squibb, Amgen, and Aduro Biotech, is a consultant for DragonFly, CSTONE, and Geneocea, and is the medical adviser for Lustgarten Foundation for pancreatic cancer research and receives grant funding from Lustgarten Foundation. ESC and NSA report no competing interests.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 2.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011; 364: 1817–25. [DOI] [PubMed] [Google Scholar]
- 3.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013; 369: 1691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinelli P, Carrillo-de Santa Pau E, Cox T, et al. GATA6 regulates EMT and tumour dissemination, and is a marker of response to adjuvant chemotherapy in pancreatic cancer. Gut 2017; 66: 1665–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Kane GM, Fischer S, Denroche R, et al. Integrative molecular profiling and response to chemotherapy on the COMPASS trial. Proc Am Soc Clin Oncol 2019; 37: 188 (asbtr). [Google Scholar]
- 6.Reni M, Balzano G, Zanon S, et al. Phase 1B trial of nab-paclitaxel plus gemcitabine, capecitabine, and cisplatin (PAXG regimen) in patients with unresectable or borderline resectable pancreatic adenocarcinoma. Br J Cancer 2016; 115: 290–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamada C, Okusaka T, Ikari T, et al. Efficacy and safety of gemcitabine plus S-1 in pancreatic cancer: a pooled analysis of individual patient data. Br J Cancer 2017; 116: 1544–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiss GJ, Blaydorn L, Beck J, et al. Phase Ib/II study of gemcitabine, nab-paclitaxel, and pembrolizumab in metastatic pancreatic adenocarcinoma. Invest New Drugs 2018; 36: 96–102. [DOI] [PubMed] [Google Scholar]
- 9.Hidalgo M. Pancreatic cancer. N Engl J Med 2010; 362: 1605–17. [DOI] [PubMed] [Google Scholar]
- 10.Sparmann A, Bar-Sagi D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell 2004; 6: 447–58. [DOI] [PubMed] [Google Scholar]
- 11.Athuluri-Divakar SK, Vasquez-Del Carpio R, Dutta K, et al. A small molecule ras-mimetic disrupts ras association with effector proteins to block signaling. Cell 2016; 165: 643–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Neil BH, Scott AJ, Ma WW, et al. A phase II/III randomized study to compare the efficacy and safety of rigosertib plus gemcitabine versus gemcitabine alone in patients with previously untreated metastatic pancreatic cancer. Ann Oncol 2016; 27: 1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kessler D, Gmachl M, Mantoulidis A, et al. Drugging an undruggable pocket on KRAS. Proc Natl Acad Sci USA 2019; 116: 15823–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin C, Bunn B. Phase 1 study shows novel KRAS inhibitor well tolerated by patients with adenocarcinoma and nonsmall cell lung cancer. September 8, 2019. https://www.iaslc.org/About-IASLC/News-Detail/phase-1-study-shows-novel-kras-inhibitor-well-tolerated-by-patients-with-adenocarcinoma-and-nonsmall-cell-lung-cancer (accessed Sept 25, 2019). [Google Scholar]
- 15.Tran E, Robbins PF, Lu Y-C, et al. T-cell transfer therapy targeting mutant KRAS in cancer. N Engl J Med 2016; 375: 2255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007; 25: 1960–66. [DOI] [PubMed] [Google Scholar]
- 17.Schultheis B, Reuter D, Ebert MP, et al. Gemcitabine combined with the monoclonal antibody nimotuzumab is an active first-line regimen in KRAS wildtype patients with locally advanced or metastatic pancreatic cancer: a multicenter, randomized phase IIb study. Ann Oncol 2017; 28: 2429–35. [DOI] [PubMed] [Google Scholar]
- 18.Singhi AD, Ali SM, Lacy J, et al. Identification of targetable ALK rearrangements in pancreatic ductal adenocarcinoma. J Natl Compr Canc Netw 2017; 15: 555–62. [DOI] [PubMed] [Google Scholar]
- 19.Pishvaian MJ, Diego Rolfo C, Liu SV, Multani PS, Maneval EC, Garrido-Laguna I. Clinical benefit of entrectinib for patients with metastatic pancreatic cancer who harbor NTRK and ROS1 fusions. Proc Am Soc Clin Oncol 2018; 36: 521 (abstr). [Google Scholar]
- 20.Jonna S, Feldman RA, Swensen J, et al. Detection of NRG1 gene fusions in solid tumors. Clin Cancer Res 2019; 25: 4966–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heining C, Horak P, Uhrig S, et al. NRG1 fusions in KRAS wild-type pancreatic cancer. Cancer Discov 2018; 8: 1087–95. [DOI] [PubMed] [Google Scholar]
- 22.Jones MR, Williamson LM, Topham JT, et al. NRG1 gene fusions are recurrent, clinically actionable gene rearrangements in KRAS wild-type pancreatic ductal adenocarcinoma. Clin Cancer Res 2019; 25: 4674–81. [DOI] [PubMed] [Google Scholar]
- 23.Shindo K, Yu J, Suenaga M, et al. Deleterious germline mutations in patients with apparently sporadic pancreatic adenocarcinoma. J Clin Oncol 2017; 35: 3382–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Golan T, Hammel P, Reni M, et al. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N Engl J Med 2019; 381: 317–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Reilly EM, Lee JW, Lowery MA, et al. Phase 1 trial evaluating cisplatin, gemcitabine, and veliparib in 2 patient cohorts: germline BRCA mutation carriers and wild-type BRCA pancreatic ductal adenocarcinoma. Cancer 2018; 124: 1374–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pihlak R, Weaver JMJ, Valle JW, McNamara MG. Advances in molecular profiling and categorisation of pancreatic adenocarcinoma and the implications for therapy. Cancers (Basel) 2018; 10: E17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ziemke EK, Dosch JS, Maust JD, et al. Sensitivity of KRAS-mutant colorectal cancers to combination therapy that cotargets MEK and CDK4/6. Clin Cancer Res 2016; 22: 405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DuFort CC, DelGiorno KE, Hingorani SR. Mounting pressure in the microenvironment: fluids, solids, and cells in pancreatic ductal adenocarcinoma. Gastroenterology 2016; 150: 1545–57.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blair AB, Kim VM, Muth ST, et al. Dissecting the stromal signalling and regulation of myeloid cells and memory effector T cells in pancreatic cancer. Clin Cancer Res 2019; 25: 5351–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hingorani SR, Harris WP, Beck JT, et al. Phase Ib study of PEGylated recombinant human hyaluronidase and gemcitabine in patients with advanced pancreatic cancer. Clin Cancer Res 2016; 22: 2848–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hingorani SR, Zheng L, Bullock AJ, et al. HALO 202: randomized phase II study of PEGPH20 plus nab-paclitaxel/gemcitabine versus nab-paclitaxel/gemcitabine in patients with untreated, metastatic pancreatic ductal adenocarcinoma. J Clin Oncol 2018; 36: 359–66. [DOI] [PubMed] [Google Scholar]
- 32.Ramanathan RK, McDonough SL, Philip PA, et al. Phase Ib/II randomized study of FOLFIRINOX plus pegylated recombinant human hyaluronidase versus folfirinox alone in patients with metastatic pancreatic adenocarcinoma: SWOG S1313. J Clin Oncol 2019; 37: 1062–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katagiri T, Kobayashi M, Yoshimura M, et al. HIF-1 maintains a functional relationship between pancreatic cancer cells and stromal fibroblasts by upregulating expression and secretion of sonic hedgehog. Oncotarget 2018; 9: 10525–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Jesus-Acosta A, O’Dwyer PJ, Ramanathan RK, et al. A phase II study of vismodegib, a hedgehog (Hh) pathway inhibitor, combined with gemcitabine and nab-paclitaxel (nab-P) in patients (pts) with untreated metastatic pancreatic ductal adenocarcinoma (PDA). Proc Am Soc Clin Oncol 2014; 32: 257 (abstr). [Google Scholar]
- 35.Tong M, Wang J, Zhang H, et al. Efficacy and safety of gemcitabine plus anti-angiogenesis therapy for advanced pancreatic cancer: a systematic review and meta-analysis of clinical randomized phase III trials. J Cancer 2019; 10: 968–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Candido JB, Morton JP, Bailey P, et al. CSF1R+ macrophages sustain pancreatic tumor growth through T cell suppression and maintenance of key gene programs that define the squamous subtype. Cell Rep 2018; 23: 1448–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beatty GL, Chiorean EG, Fishman MP, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 2011; 331: 1612–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Hara M, O’Reilly E, Rosemarie M, et al. A phase Ib study of CD40 agonistic monoclonal antibody APX005M together with gemcitabine (Gem) and nab-paclitaxel (NP) with or without nivolumab (Nivo) in untreated metastatic ductal pancreatic adenocarcinoma (PDAC) patients. Cancer Res 2019; 79 (suppl 13): CT004 (abstr). [Google Scholar]
- 39.Nywening TM, Wang-Gillam A, Sanford DE, et al. Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: a single-centre, open-label, dose-finding, non-randomised, phase 1b trial. Lancet Oncol 2016; 17: 651–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Squibb Bristol-Myers. Bristol-Myers Squibb and Five Prime present phase 1a/1b data evaluating cabiralizumab (anti-CSF-1 receptor antibody) with opdivo (nivolumab) in patients with advanced solid tumors. November 9, 2017. https://news.bms.com/press-release/bristolmyers/bristol-myers-squibb-and-five-prime-present-phase-1a1b-data-evaluating-ca (accessed Sept 14, 2019). [Google Scholar]
- 41.Ma HS, Poudel B, Torres ER, et al. A CD40 agonist and PD-1 antagonist antibody reprogram the microenvironment of nonimmunogenic tumors to allow T-cell-mediated anticancer activity. Cancer Immunol Res 2019; 7: 428–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stiff A, Trikha P, Wesolowski R, et al. Myeloid-derived suppressor cells express Bruton’s tyrosine kinase and can be depleted in tumor bearing hosts by ibrutinib treatment. Cancer Res 2016; 76: 2125–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feig C, Jones JO, Kraman M, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci USA 2013; 110: 20212–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kultti A, Zhao C, Singha NC, et al. Accumulation of extracellular hyaluronan by hyaluronan synthase 3 promotes tumor growth and modulates the pancreatic cancer microenvironment. BioMed Res Int 2014; 2014: 817613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seo YD, Jiang X, Sullivan KM, et al. Mobilization of CD8+ T cells via CXCR4 blockade facilitates PD-1 checkpoint therapy in human pancreatic cancer. Clin Cancer Res 2019; 25: 3934–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Melisi D, Garcia-Carbonero R, Macarulla T, et al. Galunisertib plus gemcitabine vs. gemcitabine for first-line treatment of patients with unresectable pancreatic cancer. Br J Cancer 2018; 119: 1208–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strauss J, Heery CR, Schlom J, et al. Phase I trial of M7824 (MSB0011359C), a bifunctional fusion protein targeting PD-L1 and TGFβ, in advanced solid tumors. Clin Cancer Res 2018; 24: 1287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoo C, Oh D, Choi H, et al. AB053. P-21. M7824 (MSB0011359C), a bifunctional fusion protein targeting transforming growth factor β (TGF-β) and PD-L1, in Asian patients with pretreated biliary tract cancer (BTC): efficacy by BTC subtype. Hepatobiliary Surg Nutr 2019; 8 (suppl 1): AB053. [Google Scholar]
- 49.Jiang H, Hegde S, Knolhoff BL, et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat Med 2016; 22: 851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang H, Liu X, Knolhoff BL, et al. Development of resistance to FAK inhibition in pancreatic cancer is linked to stromal depletion. Gut 2019; published online May 10. DOI: 10.1136/gutjnl-2018-317424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murakami T, Hiroshima Y, Matsuyama R, Homma Y, Hoffman RM, Endo I. Role of the tumor microenvironment in pancreatic cancer. Ann Gastroenterol Surg 2019; 3: 130–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Christmas BJ, Rafie CI, Hopkins AC, et al. Entinostat converts immune-resistant breast and pancreatic cancers into checkpoint-responsive tumors by reprogramming tumor-infiltrating MDSCs. Cancer Immunol Res 2018; 6: 1561–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mazur PK, Herner A, Mello SS, et al. Combined inhibition of BET family proteins and histone deacetylases as a potential epigenetics-based therapy for pancreatic ductal adenocarcinoma. Nat Med 2015; 21: 1163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Noel MS, Wang-Gillam A, Ocean AJ, Chawla SP, Del Priore G, Picozzi VJ. Phase II trial of SM-88 in patients with metastatic pancreatic cancer: preliminary results of the first stage. Proc Am Soc Clin Oncol 2019; 37: 200 (abstr). [Google Scholar]
- 55.Alistar A, Morris BB, Desnoyer R, et al. Safety and tolerability of the first-in-class agent CPI-613 in combination with modified FOLFIRINOX in patients with metastatic pancreatic cancer: a single-centre, open-label, dose-escalation, phase 1 trial. Lancet Oncol 2017; 18: 770–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Evan GI, Hah N, Littlewood TD, et al. Re-engineering the pancreas tumor microenvironment: a “regenerative program” hacked. Clin Cancer Res 2017; 23: 1647–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barati Bagherabad M, Afzaljavan F, ShahidSales S, Hassanian SM, Avan A. Targeted therapies in pancreatic cancer: promises and failures. J Cell Biochem 2019; 120: 2726–41. [DOI] [PubMed] [Google Scholar]
- 58.Ligorio M, Sil S, Malagon-Lopez J, et al. Stromal microenvironment shapes the intratumoral architecture of pancreatic cancer. Cell 2019; 178: 160–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Popovic A, Jaffee EM, Zaidi N. Emerging strategies for combination checkpoint modulators in cancer immunotherapy. J Clin Invest 2018; 128: 3209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jaffee EM, Hruban RH, Biedrzycki B, et al. Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J Clin Oncol 2001; 19: 145–56. [DOI] [PubMed] [Google Scholar]
- 61.Le DT, Wang-Gillam A, Picozzi V, et al. Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J Clin Oncol 2015; 33: 1325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Le DT, Picozzi VJ, Ko AH, et al. Results from a phase IIb, randomized, multicenter study of GVAX pancreas and CRS-207 compared with chemotherapy in adults with previously treated metastatic pancreatic adenocarcinoma (ECLIPSE Study). Clin Cancer Res 2019; 25: 5493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morrison AH, Byrne KT, Vonderheide RH. Immunotherapy and prevention of pancreatic cancer. Trends Cancer 2018; 4: 418–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shima H, Tsurita G, Wada S, et al. Randomized phase II trial of survivin 2B peptide vaccination for patients with HLA-A24-positive pancreatic adenocarcinoma. Cancer Sci 2019; 110: 2378–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zaidi N, Quezada SA, Kuroiwa JMY, et al. Anti-CTLA-4 synergizes with dendritic cell-targeted vaccine to promote IL-3-dependent CD4+ effector T cell infiltration into murine pancreatic tumors. Ann N Y Acad Sci 2019; 1445: 62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017; 357: 409–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nichetti F, Marra A, Corti F, et al. The role of mesothelin as a diagnostic and therapeutic target in pancreatic ductal adenocarcinoma: a comprehensive review. Target Oncol 2018; 13: 333–51. [DOI] [PubMed] [Google Scholar]
- 68.Hassan R, Kindler HL, Jahan T, et al. Phase II clinical trial of amatuximab, a chimeric antimesothelin antibody with pemetrexed and cisplatin in advanced unresectable pleural mesothelioma. Clin Cancer Res 2014; 20: 5927–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mizukami T, Kamachi H, Fujii Y, et al. The anti-mesothelin monoclonal antibody amatuximab enhances the anti-tumor effect of gemcitabine against mesothelin-high expressing pancreatic cancer cells in a peritoneal metastasis mouse model. Oncotarget 2018; 9: 33844–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thomas AM, Santarsiero LM, Lutz ER, et al. Mesothelin-specific CD8(+) T cell responses provide evidence of in vivo cross-priming by antigen-presenting cells in vaccinated pancreatic cancer patients. J Exp Med 2004; 200: 297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Man YKS, Davies JA, Coughlan L, et al. The novel oncolytic adenoviral mutant Ad5–3Δ-A20T retargeted to αvβ6 integrins efficiently eliminates pancreatic cancer cells. Mol Cancer Ther 2018; 17: 575–87. [DOI] [PubMed] [Google Scholar]
- 72.Chesney J, Puzanov I, Collichio F, et al. Randomized, open-label phase II study evaluating the efficacy and safety of talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone in patients with advanced, unresectable melanoma. J Clin Oncol 2018; 36: 1658–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Adusumilli PS, Cherkassky L, Villena-Vargas J, et al. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Sci Transl Med 2014; 6: 261ra151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maliar A, Servais C, Waks T, et al. Redirected T cells that target pancreatic adenocarcinoma antigens eliminate tumors and metastases in mice. Gastroenterology 2012; 143: 1375–84. [DOI] [PubMed] [Google Scholar]
- 75.Beatty GL, O’Hara MH, Lacey SF, et al. Activity of mesothelin-specific chimeric antigen receptor T cells against pancreatic carcinoma metastases in a phase 1 trial. Gastroenterology 2018; 155: 29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]