Abstract
The autoantibody profile of primary biliary cholangitis (PBC) includes antinuclear antibodies (ANA) which are detectable by indirect immunofluorescence in more than 50% of PBC patients. One of the two immunofluorescence patterns which are historically considered “PBC-specific” is the so-called “multiple nuclear dots” (MND) targeting nuclear body proteins such as Sp100, Sp140, Sp140L proteins, promyelocytic leukemia protein (PML) and small ubiquitin-related modifier proteins (SUMO). It has been hypothesized a role of nuclear body protein alterations in immune disorders such as PBC, thus suggesting novel and more refined therapeutic approaches.
Keywords: Speckled proteins, Multiple nuclear dots, Antinuclear antibodies, Primary biliary cholangitis
To the editor
Patients with primary biliary cholangitis (PBC) produce antinuclear antibodies (ANA) directed against structural components of promyelocytic leukemia protein (PML) and Sp100-containing nuclear bodies (NBs) [1].
Sp100, PML, Sp140, Sp140L, and small ubiquitin-related modifier (SUMO) proteins are PML NB-related proteins that are identified as target antigens in PBC patients [2–6]. These autoantibodies are of clinical relevance in PBC due to their very high disease specificity and as surrogate markers in anti-mitochondrial antibody (AMA) negative PBC cases (Table 1) [1, 2].
Table 1.
Relationship between nuclear pore complex proteins, antinuclear antibodies giving the “multiple nuclear dots” immunofluorescence pattern, and clinical significance in patients with primary biliary cholangitis
| Nuclear structures | Indirect immunofluorescence pattern | Autoantigen targets | Autoantibodies in PBC | ||
|---|---|---|---|---|---|
| Prevalence (%) | Specificity | Worse prognosis | |||
| Nuclear Bodies | MNDa | Sp100 | 17–41 | High | To be confirmed |
| PML | 19 | High | No | ||
| Sp140 | 15 | High | No | ||
| Sp140L | 1.5 | Not assessed | Not assessed | ||
| SUMO-1 | 15 | Not assessed | Not assessed | ||
| SUMO-2 | 42 | Not assessed | Not assessed | ||
Sp100 Sp100 nuclear body component, PML promyelocytic leukemia protein, Sp140 Sp140 nuclear body component, Sp140L Sp140L protein, MND multiple nuclear dots pattern, SUMO small ubiquitin-related modifiers
aMND pattern detected by indirect immunofluorescence using fluorescein-conjugate anti-human total Ig and anti-human IgG subclasses (IgG1, IgG2, IgG3, IgG4) as secondary antibody was found in 16% and 31%, respectively [1, 2]
Within the spectrum of ANA staining patterns by indirect immunofluorescence, the “multiple nuclear dots” (MND) pattern is therefore historically considered as highly specific for PBC [1, 7].
The MND staining pattern is characterized by the presence of 5–20 dots of variable size distributed throughout the cell nucleus but sparing the nucleoli; it is distinguishable from the centromere staining pattern by the presence of fewer nuclear dots per cell, and by the absence of dots in cells that are undergoing mitosis (Fig. 1) [7]. The MND staining pattern is also distinct from the anti-p80 coilin/Cajal body staining pattern, which is characterized by the presence of 2–8 dots per cell nucleus [8].
Fig. 1.
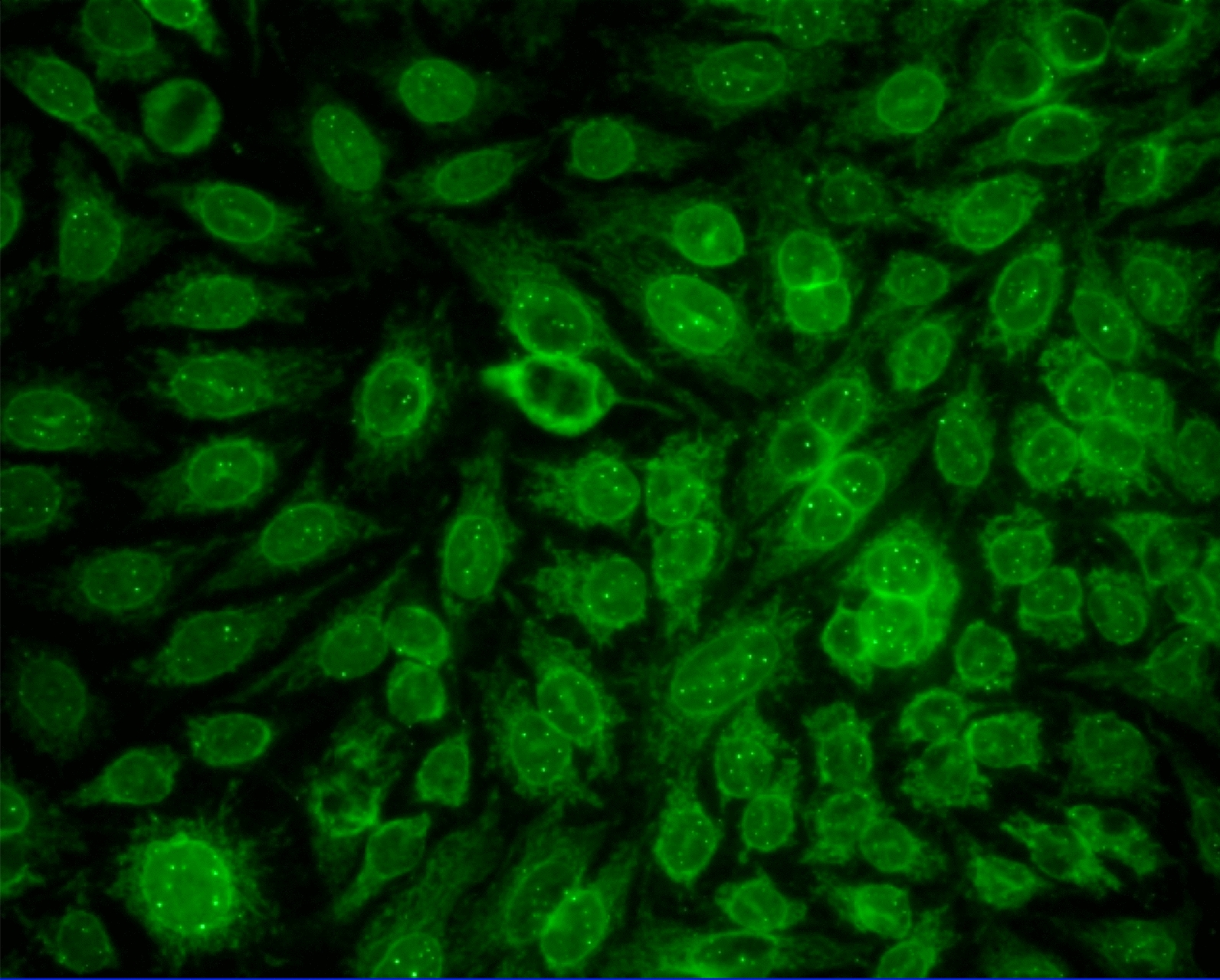
Multiple nuclear dot staining pattern by indirect immunofluorescence on HEp-2 cells (magnification 20). Anti-multiple nuclear dots react with 3–20 nuclear dots distinct from nucleoli and from the anticentromere targets. Punctate staining of chromosomes in mitosis clearly distinguishes anticentromere from anti-multiple nuclear dots
The mechanism leading to ANA production in PBC is still an unsolved question. It has been suggested that xenobiotics and molecular mimicry between microbial agents and self-antigens might be involved in the triggering of disease as well as in the appearance of autoantibodies [9, 10].
Previous data suggest that PML-NBs may have a role in transcriptional events [11]. Moreover, it has been shown that PML, Sp100, and Sp140 are upregulated in response to interferons, a group of proteins with antiviral activities, indicating that PML NBs could also have an important function in antiviral defense [12]. Results from a recent study suggest an implication of Sp140 protein in an innate response to HIV-1 by its interaction with the vif protein encoded by the virus [13].
In their review article, Fraschilla and Jeffrey posit that the speckled protein (SP) family are central chromatin regulators of gene silencing that establish immune cell identity and function [14]. They correctly point out that: (1) mutations in human SP140 associate with three immunological diseases: Crohn’s disease, chronic lymphocytic leukemia, and multiple sclerosis; (2) mutations in human SP110 associate with veno-occlusive disease with immunodeficiency; (3) finally, many viruses have evolved mechanisms to inhibit SP function in PML-NBs, organelles associated with viral gene repression, suggesting that SPs mediate protective viral defense mechanisms [15].
They conclude that all SPs are associated with autoimmune, inflammatory, or infectious diseases, underscoring their role in maintaining immune homeostasis and proper functional response to pathogens.
Regarding their role in PBC, it has been widely established the high diagnostic value of autoantibodies direct against SPs, especially in patients lacking antimitochondrial antibodies. Moreover, a prognostic role for MND/anti-Sp100 antibodies has also been suggested: Zuchner et al. described a faster disease progression among anti-Sp100-positive patients with PBC [16]. Rigopoulou et al. reported that anti-MND-positive patients had significantly more severe liver disease than those that were anti-MND negative, as shown by the higher frequency of cirrhosis and worse outcome [17]. However, these observations still need to be confirmed in larger series of patients, possibly recruited from different centers and with different ethnic and genetic backgrounds.
We would like also to add further relevant and unmentioned evidence that supports a potential role of infections as a potential trigger of PBC and anti-SPs autoantibodies development in genetically predisposed individuals. Specifically, it has been demonstrated a possible role of microorganisms that are responsible for recurrent urinary tract infections, as triggers of PBC and ANAs generation, has long been suggested [16–19]. Moreover, a possible molecular mimicry between the epitopic regions of Escherichia coli and Sp100 has been hypothesized on the basis of a strong correlation between the presence of anti-Sp100 antibodies and AMA positivity in women with recurrent urinary tract infections, with or without evidence of PBC [20].
A different mimicry mechanism has been proposed by Shinoda et al. who have found that peptides from gp210 and Sp100 proteins are recognized by T-cell clones responsive to the major autoepitope of E2 subunit of the pyruvate dehydrogenase complex. The investigators thus hypothesized that the PBC-specific antinuclear reactivities could be the result of intermolecular spreading involving mitochondrial antigens and mimicry sequences of nuclear proteins [21].
It is noteworthy that more than 80% of anti-NB positive patients has two or three simultaneous anti-NB reactivities, suggesting a “clustering” of autoantigens, an observation supporting the hypothesis that intermolecular epitope-spreading mechanisms might be operative in the propagation of several reactivities in the same patient [4, 22–25].
Given the observation that anti-Sp140, as well as anti-PML antibodies, almost exclusively occur in anti-Sp100-positive patients, but not vice versa, it could be hypothesized that the NB is a multiantigenic complex in which the immune response might involve first Sp100, and only later spread to Sp140 and PML that share the same subnuclear localization.
We agree with Fraschilla and Jeffrey that further studies elucidating mechanisms of SPs alteration, contributing to immune disorders, will aid in the design of new therapies associated with SPs role and function.
Furthermore, immune-expressed SPs may offer novel and more refined therapeutic avenues for taming hyperactive autoimmune responses.
Acknowledgements
None.
Abbreviations
- PBC
Primary biliary cholangitis
- MND
Multiple nuclear dots
- NBs
Nuclear bodies
- PML
Promyelocytic leukemia protein
- SP
Speckled protein
- SUMO
Small ubiquitin-related modifier proteins
Authors’ contributions
AG and PM reviewed the literature and drafted the manuscript; AG, PM, LM and FT contributed to manuscript drafting; all authors were involved in acquisition of data. All authors read and approved the final manuscript.
Funding
The authors received no financial support to produce this manuscript.
Availability of data and materials
Data included in this manuscript are available in the following published manuscripts:
1. Muratori P et al. Autoimmunity. 2009;42:224–7.
2. Granito A et al. Am J Gastroenterol 2010;105:125–31.
3. Granito A et al. Aliment Pharmacol Ther. 2006;24:1575–83.
Declarations
Ethics approval and consent to participate
The patients referred to in the manuscript signed a written informed consent form for the purpose of publication of the results and the study was approved by local ethical committee.
Competing interests
The authors declare that they have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Granito A, Muratori P, Quarneti C, Pappas G, Cicola R, Muratori L. Antinuclear antibodies as ancillary markers in primary biliary cirrhosis. Expert Rev Mol Diagn. 2012;12(1):65–74. doi: 10.1586/erm.11.82. [DOI] [PubMed] [Google Scholar]
- 2.Muratori P, Granito A, Ferri S, Pappas G, Volta U, Menichella R, Bianchi FB, Lenzi M, Muratori L. Multiple nuclear dots and rim-like/membranous IgG isotypes in primary biliary cirrhosis. Autoimmunity. 2009;42:224–7. doi: 10.1080/08916930802709133. [DOI] [PubMed] [Google Scholar]
- 3.Bloch DB, Chiche JD, Orth D, de la Monte SM, Rosenzweig A, Bloch KD. Structural and functional heterogeneity of nuclear bodies. Mol Cell Biol. 1999;19(6):4423–30. doi: 10.1128/MCB.19.6.4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Granito A, Yang WH, Muratori L, Lim MJ, Nakajima A, Ferri S, Pappas G, Quarneti C, Bianchi FB, Bloch DB, Muratori P. PML nuclear body component Sp140 is a novel autoantigen in primary biliary cirrhosis. Am J Gastroenterol. 2010;105:125–31. doi: 10.1038/ajg.2009.596. [DOI] [PubMed] [Google Scholar]
- 5.Saare M, Hämarik U, Venta R, Panarina M, Zucchelli C, Pihlap M, Remm A, Kisand K, Toots U, Möll K, Salupere R, Musco G, Uibo R, Peterson P. SP140L, an evolutionarily recent member of the SP100 family, is an autoantigen in primary biliary cirrhosis. J Immunol Res. 2015;2015:526518. doi: 10.1155/2015/526518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janka C, Selmi C, Gershwin ME, Will H, Sternsdorf T. Small ubiquitin-related modifiers: a novel and independent class of autoantigens in primary biliary cirrhosis. Hepatology. 2005;41:609–16. doi: 10.1002/hep.20619. [DOI] [PubMed] [Google Scholar]
- 7.Granito A, Muratori P, Muratori L, Pappas G, Cassani F, Worthington J, Guidi M, Ferri S, Molo DE, C, Lenzi M, Chapman RW, Bianchi FB. Antinuclear antibodies giving the ‘multiple nuclear dots’ or the ‘rim-like/membranous’ patterns: diagnostic accuracy for primary biliary cirrhosis. Aliment Pharmacol Ther. 2006;24:1575–83. doi: 10.1111/j.1365-2036.2006.03172.x. [DOI] [PubMed] [Google Scholar]
- 8.Yang WH, Yu JH, Nakajima A, et al. Do antinuclear antibodies in primary biliary cirrhosis patients identify increased risk for liver failure? Clin Gastroenterol Hepatol. 2004;2:1116–22. doi: 10.1016/S1542-3565(04)00465-3. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan MM. Novosphingobium aromaticivorans: a potential initiator of primary biliary cirrhosis. Am J Gastroenterol. 2004;99:2147–9. doi: 10.1111/j.1572-0241.2004.41121.x. [DOI] [PubMed] [Google Scholar]
- 10.Rieger R, Leung PS, Jeddeloh MR, et al. Identification of 2-nonynoic acid, a cosmetic component, as a potential trigger of primary biliary cirrhosis. J Autoimmun. 2006;27:7–16. doi: 10.1016/j.jaut.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 11.LaMorte VJ, Dyck JA, Ochs RL, Evans RM. Localization of nascent RNA and CREB binding protein with the PML-containing nuclear body. Proc Natl Acad Sci USA. 1998;95:4991–6. doi: 10.1073/pnas.95.9.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Regad T, Chelbi-Alix MK. Role and fate of PML nuclear bodies in response to interferon and viral infections. Oncogene. 2001;20:7274–86. doi: 10.1038/sj.onc.1204854. [DOI] [PubMed] [Google Scholar]
- 13.Madani N, Millette R, Platt EJ, et al. Implication of the lymphocyte-specific nuclear body protein Sp140 in an innate response to human immunodeficiency virus type 1. J Virol. 2002;76:11133–8. doi: 10.1128/JVI.76.21.11133-11138.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fraschilla I, Jeffrey KL. The speckled protein (SP) family: immunity’s chromatin readers. Trends Immunol. 2020;41:572–85. doi: 10.1016/j.it.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scherer M, Stamminger T. Emerging role of PML nuclear bodies in innate immune signaling. J Virol. 2016;90:5850–4. doi: 10.1128/JVI.01979-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Züchner D, Sternsdorf T, Szostecki C, et al. Prevalence, kinetics, and therapeutic modulation of autoantibodies against Sp100 and promyelocytic leukemia protein in a large cohort of patients with primary biliary cirrhosis. Hepatology. 1997;26:1123–30. doi: 10.1002/hep.510260506. [DOI] [PubMed] [Google Scholar]
- 17.Rigopoulou EI, Davies ET, Pares A, et al. Prevalence and clinical significance of isotype specific antinuclear antibodies in primary biliary cirrhosis. Gut. 2005;54:528–32. doi: 10.1136/gut.2003.036558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bogdanos DP, Baum H, Sharma UC, et al. Antibodies against homologous microbial caseinolytic proteases P characterise primary biliary cirrhosis. J Hepatol. 2002;36:14–21. doi: 10.1016/S0168-8278(01)00252-5. [DOI] [PubMed] [Google Scholar]
- 19.Selmi C, Gershwin ME. The role of environmental factors in primary biliary cirrhosis. Trends Immunol. 2009;30:415–20. doi: 10.1016/j.it.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Berg Tanaka A, Leung PSC, Gershwin ME. Pathogen infections and primary biliary cholangitis. Clin Exp Immunol. 2019;195:25–34. doi: 10.1111/cei.13198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kivity S, Agmon-Levin N, Blank M, Shoenfeld Y. Infections and autoimmunity—friends or foes? Trends Immunol. 2009;30:409–14. doi: 10.1016/j.it.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Bogdanos DP, Baum H, Butler P, et al. Association between the primary biliary cirrhosis specific anti-sp100 antibodies and recurrent urinary tract infection. Dig Liver Dis. 2003;35:801–5. doi: 10.1016/S1590-8658(03)00466-3. [DOI] [PubMed] [Google Scholar]
- 23.Shimoda S, Nakamura M, Ishibashi H, et al. Molecular mimicry of mitochondrial and nuclear autoantigens in primary biliary cirrhosis. Gastroenterology. 2003;124:1915–25. doi: 10.1016/S0016-5085(03)00387-1. [DOI] [PubMed] [Google Scholar]
- 24.James JA, Harley JB. B-cell epitope spreading in autoimmunity. Immunol Rev. 1998;164:185–200. doi: 10.1111/j.1600-065X.1998.tb01220.x. [DOI] [PubMed] [Google Scholar]
- 25.Vanderlugt CL, Miller SD. Epitope spreading in immune-mediated diseases: implications for immunotherapy. Nat Rev Immunol. 2002;2:85–95. doi: 10.1038/nri724. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in this manuscript are available in the following published manuscripts:
1. Muratori P et al. Autoimmunity. 2009;42:224–7.
2. Granito A et al. Am J Gastroenterol 2010;105:125–31.
3. Granito A et al. Aliment Pharmacol Ther. 2006;24:1575–83.


