Abstract
Background
Postoperative atrial fibrillation (PoAF) is a common complication after cardiac surgery. A pre-existing atrial substrate appears to be important in postoperative development of dysrhythmia, but its preoperative estimation is challenging. We tested the hypothesis that combining clinical predictors, noninvasive surrogate markers for atrial fibrosis defining abnormal left atrial (LA) mechanics, and biomarkers of collagen turnover is superior to clinical predictors alone in identifying patients at-risk for PoAF.
Methods
In patients without prior AF undergoing coronary artery bypass grafting, concentrations of biomarkers reflecting collagen synthesis and degradation, extracellular matrix, and regulatory microRNA29s were determined in serum from preoperative blood samples and correlated to atrial fibrosis extent, alteration in atrial deformation properties determined by 3D speckle-tracking echocardiography, and AF development.
Results
Of 90 patients without prior AF, 34 who developed PoAF were older than non-PoAF patients (72.04±10.7 y; P=0.043) with no significant difference in baseline comorbidities, LA size, or ventricular function. Global (P=0.007) and regional longitudinal LA strain and ejection fraction (P=0.01) were reduced in PoAF vs. non-PoAF patients. Preoperative amino-terminal-procollagen-III-peptide (PIIINP) (103.1±39.7 vs. 35.1±19.3; P=0.041) and carboxy-terminal-procollagen-I-peptide levels were elevated in PoAF vs. non-PoAF patients with a reduction in miR-29 levels and correlated with atrial fibrosis extent. Combining age as the only significant clinical predictor with PIIINP and miR-29a provided a model that identified PoAF patients with higher predictive accuracy.
Conclusions
In patients without previous history of AF, using age and biomarkers of collagen synthesis and regulation, a noninvasive tool was developed to identify those at risk for new-onset PoAF.
Keywords: atrial fibrillation, aging, miR-29, fibrosis, biomarkers, speckle-tracking echocardiography
1. Introduction
Postoperative atrial fibrillation (PoAF), an arrhythmic complication after cardiac surgery occurring in up to 16–60% of patients [1], remains a major challenge owing to associated morbidity, increased hospital stay, and health care costs [2–4]. Despite advances in medical management and surgical care, the overall incidence of PoAF has not changed significantly [5,6]. This is partly owing to limited understanding of the underlying mechanisms and, therefore, availability of only partially effective prophylactic therapies [5]. AF after cardiac surgery results from a combination of acute and chronic factors—such as myocardial injury, inflammation, sympathetic activation, and oxidative stress superimposed on an underlying substrate of structural, electrical, and metabolic alterations within the atria—that increase predisposition to AF [5–8]. Recognition of higher-risk individuals and definition of underlying mechanisms may allow development of preventive strategies and therapies that could be individualized for each patient. Clinical predictors currently used to stratify patients at risk for PoAF have only limited sensitivity and specificity and do not fully reflect the pathophysiological substrate responsible for PoAF [5,9]. Therefore, prediction of PoAF development in patients without previous history of AF and heart failure (HF) remains challenging.
Excessive extracellular matrix (ECM) protein synthesis and deposition in the atria promotes interstitial fibrosis, a complex process regulated by various cytokines, growth factors, and regulatory microRNA (miRNA) that modulate activation of cardiac fibroblasts [5]. Biomarkers associated with activation of fibroblasts and collagen turnover, therefore, could be used to identify patients at risk of PoAF [5,7]. Elevated levels of carboxyl-terminal pro-collagen I (PICP), amino-terminal procollagen III (PIIINP), and carboxyl-terminal telopeptide of collagen I (CITP) in plasma have been associated with the increased rate of collagen and ECM turnover during atrial fibrosis [7] and proposed as noninvasive biomarkers to identify patients at risk of PoAF; however, contradictory results reported by different groups (recently reviewed [5]) reduce their utility as sole markers for predicting development of PoAF [5,7,10]. Cardiac fibrosis also is tightly regulated by non-coding miRNA, and recently, various miRNAs have been associated with AF, including the miR-29 family that has an inhibitory effect on fibrosis [5,11–14]. We therefore hypothesized that combining clinical risk factors for AF with pathophysiology-based markers reflecting atrial substrate progression as elevated levels of peptides reflecting collagen-synthesis with regulatory miR-29 could have an incremental value in identifying patients at risk of PoAF who otherwise would be missed on preoperative evaluation.
2. Methods
2.1. Patient recruitment
Patients scheduled for elective coronary artery bypass graft (CABG) surgery at Aurora St. Luke’s Medical Center between April 2013 and November 2014 gave written consent for participation in the study. Inclusion criteria were: 18–90 years of age with no history of HF or AF, preserved left ventricular function, and undergoing CABG only as an elective procedure. Patients with a previous history of AF or HF, those requiring preoperative inotropic support, and those undergoing concomitant valvular, vascular, or emergency bypass surgeries were excluded. Clinical information was obtained from the electronic medical records (Epic; Verona, WI). PoAF, defined as new-onset irregular rhythm without distinct P waves lasting for at least 30 seconds, was detected by continuous telemetry-based electrocardiographic monitoring or 12-lead electrocardiography of patients during the hospital stay. The study complies with the Helsinki protocol, was approved by the Aurora Institutional Review Board, and conforms to the Health Insurance Portability and Accountability Act of 1996.
2.2. Speckle-tracking echocardiography (STE)
A Toshiba Aplio Artida echocardiography system (Tochigi, Japan) was utilized to acquire left atrial (LA) images using a PST-255×2.5 MHz 3D transducer with 3D strain capability. Preoperative LA images were obtained during sinus rhythm (SR) from the apical 4-chamber, 2-chamber, and long-axis views including 1 cardiac cycle. For optimal resolution, ≥20 volumes/second was achieved (see Supplementary Methods).
2.3. Histology of right atrial appendage (RAA) tissue
Sections (5.0 μm) of RAA tissues removed from patients undergoing elective coronary artery bypass grafting (CABG) were used for Masson’s trichrome staining, and the ratio of collagen to myocardial tissue or percentage fibrosis were calculated as described earlier [15].
2.4. Collection of preoperative blood
Preoperative venous blood samples were collected in the fasting state on the morning of the surgeries. The blood sample was kept in ethylenediaminetetraacetic acid (EDTA)-coated venous blood collection tubes (Becton and Dickinson) for evaluation of biomarkers. Plasma was extracted within 1 hour of the peripheral blood withdrawal and centrifuged for 1,500 rpm/15 minutes (4°C); aliquots were stored in nuclease/proteinase-free tubes at −80°C until their use.
2.5. Statistical analysis
An initial step in the statistical analysis entailed review of all variables for each patient to assess if there were any inconsistencies such as extreme values for both continuous and categorical variables. Exploratory data analysis was performed to provide insight into the patterns and trends in the data. Patients’ characteristics are presented as proportions for categorical variables and mean ± standard deviation (SD) and/or median [(lower quartile (Q1) - upper quartile (Q3)] for continuous variables. Comparison of categorical variables was performed using the chi square and Fisher’s exact tests, and the two-sample t-test and Wilcoxon rank-sum test were utilized to assess differences in continuous patient characteristics. The relationship between the variables also was evaluated, using a linear model with goodness of fit and F-test. The odds ratios and associated 95% confidence limit (CL) were estimated to quantify the magnitude and direction of the risk of developing PoAF. Model discrimination was assessed using the receiver-operating characteristic (ROC) area under the curve (AUC) with the associated 95% CL by fitting logistic regression models with the outcome as PoAF. In addition, model fit was assessed using the likelihood ratio test. Odds ratios were estimated to quantify the magnitude and direction of the independent predictors of PoAF. All statistical analyses were performed using SAS Version 9.4 (Cary, NC), or GraphPad Prism version 8.0.0 (San Diego, CA). All tests were done at a 5% level of significance.
See Supplementary Methods for details.
3. Results
3.1. Baseline characteristics of the studied population
The baseline characteristics of 90 patients who met inclusion and exclusion criteria are summarized in Table 1.
TABLE 1.
Baseline characteristics of patients who developed or remained free of PoAF after CABG
| Characteristics | Overall % (n=90) | No PoAF (n=56) | PoAF (n=34) | P value |
|---|---|---|---|---|
| Demographics | ||||
| Age | 69.1±11.0 (90) | 67.3±10.7 | 72.4±10.8 | 0.043 |
| ≤59 years | 20.0% (18) | 53.7± 4.4 | 53.1±3.7 | 0.7139 |
| 60–69 years | 32.2% (29) | 64.2±3.6 | 65.1±2.5 | 0.5204 |
| 70–79 years | 25.6% (23) | 74.9±3.1 | 74.4±3.2 | 0.7364 |
| 80–90 years | 22.2% (20) | 83.8±2.6 | 83.3±1.4 | 0.6074 |
| Body mass index | 0.4042 | |||
| Normal (18–25 kg/m2) | 22.2% (20) | 12 (21.4%) | 8 (23.5%) | 0.5459 |
| Overweight (25–30 kg/m2) | 30.0% (27) | 17 (30.3%) | 10 (29.4%) | 0.1623 |
| Obese (≥31 kg/m2) | 47.8% (43) | 27 (48.3%) | 16 (47.1 %) | 0.6070 |
| Sex | ||||
| Male | 72.7% (40) | 16 (66.7%) | 24 (77.4%) | 0.3525 |
| History | ||||
| Hypertension | 78.9% (71) | 43 (76.7%) | 28 (82.3%) | 0.5304 |
| Diabetes mellitus | 45.6% (41) | 26 (46.4%) | 15 (44.1%) | 0.831 |
| AV regurgitation/AV insufficiency | 13.3% (12) | 7 (12.5%) | 5 (14.7%) | 0.7653 |
| Chronic kidney disease | 23.3% (21) | 13 (23.2%) | 8 (23.5%) | 0.9727 |
| COPD | 17.7% (16) | 8 (14.2%) | 8 (23.5%) | 0.2661 |
| Stroke | 12.7% (7) | 6 (10.7%) | 4 (2.9%) | 0.8778 |
| Sleep apnea | 11.1% (10) | 5 (8.9%) | 5 (14.7%) | 0.3978 |
| Myocardial infarction | 12.2% (11) | 6 (10.7%) | 5 (14.7%) | 0.5751 |
| Left ventricular ejection fraction | 55.6 ± 11.2 | 55.4±11.5 | 58.3±9.4) | 0.737 |
| ≥ 50% | 73.3% (66) | 40 (71.4%) | 26 (76.4%) | 0.491 |
| 40–49% | 26.7% (24) | 16 (28.6 %) | 8 (23.5%) | 0.713 |
| Medications | ||||
| Aspirin | 100.0% (90) | 55 (98.2%) | 34 (100%) | 0.4333 |
| Beta blocker | 100.0% (90) | 56 (100.0%) | 34 (100%) | >9.9999 |
| Calcium channel blocker | 0.0% (0) | 0 (0.0%) | 0 (0.0%) | n.a. |
AV, aortic valve; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; PoAF, postoperative atrial fibrillation.
3.2. PoAF incidence and clinical characteristics
Out of 90 patients with no prior history of HF or AF undergoing CABG, 34 patients (37.7%) developed PoAF before discharge from the hospital after surgery. Patients with PoAF were older than those who remained free of PoAF (72.04 ± 10.7 years vs. 67.43 ± 9.9; p < 0.05). Otherwise, there were no significant differences in preoperative comorbidities, risk factors for AF, use of medications, underlying atrial or ventricular dimensions, or other echocardiographic parameters as summarized in Tables 1 and Supplementary Table 1.
3.3. Reduced global longitudinal and regional strain in patients at risk of PoAF
Differences in LA global and regional longitudinal strain on preoperative imaging were assessed using STE and compared between patients who subsequently developed PoAF and those who remained in SR during hospitalization. Despite the absence of any significant differences in atrial or ventricular dimension or function between the 2 groups on routine echocardiography (Supplementary Table 1), patients who developed PoAF had greater reduction in global longitudinal strain compared with non-PoAF patients (6.9 ± 0.69 vs. 10.9 ± 0.93, p = 0.008; Fig. 1A). There was a significant reduction in regional LA longitudinal strain in the anteroseptal (p = 0.035), anterior (p = 0.006), and lateral segments (p = 0.023) as well as in the lateral (p = 0.041) and posterior roof segments (p = 0.007) in patients who subsequently developed PoAF when compared with those who did not (Fig. 1B–1D). Patients who developed PoAF also had significantly reduced atrial ejection fraction on preoperative echocardiogram compared with those who remained free of PoAF (32 ± 2% vs. 42 ± 2%, respectively; p = 0.010, Fig. 1E), an increase in end-systolic volume (60 ± 6 mL vs. 42 ± 4 mL, respectively, p = 0.04), and no significant difference in end-diastolic volume (88 ± 8 mL vs. 72 ± 6 mL, respectively, p = 0.16). Overall, these findings indicate the presence of an underlying atrial substrate abnormality manifesting as reduced global and regional myocardial strain with depressed LA function in patients who developed PoAF that otherwise was not revealed on routine echocardiogram.
FIGURE 1.
Abnormal preoperative global and regional left atrial strain in postoperative atrial fibrillation (PoAF) patients determined by 3D speckle-tracking echocardiography (STE). (A) Representative figure of preoperative 3D STE during sinus rhythm showing reduced global diastolic peak positive (+), signifying reservoir and conduit function and unchanged atrial contractile function [global systole peak negative (−)] with 16-segmental atrial strain traces represented as a bull’s-eye plot (B) of affected segments demonstrating reduced regional strain values of coronary artery bypass graft patient. (C) Bar graphs representing (mean±SD) regional left atrial strains corresponding to 16-segmented tracings showed significant reduction (*P<0.05; ϯP<0.01) in anteroseptal, anterior, lateral, lateral roof, and posterior roof of left atrial region a-f (see inset) of patients who developed PoAF. (D) Bar graph representing (mean±SD) reduced global longitudinal strain in patients who developed PoAF. (E) Data (mean±SD) representing the relationship between preoperative recordings of end-diastolic volume, significant reduction in end-systolic volume, and left atrial ejection fraction and development of PoAF. EDV, end-diastolic volume; EF, ejection fraction; ESV, end-systolic volume.
3.4. Increased right atrial appendage (RAA) collagen burden is associated with PoAF
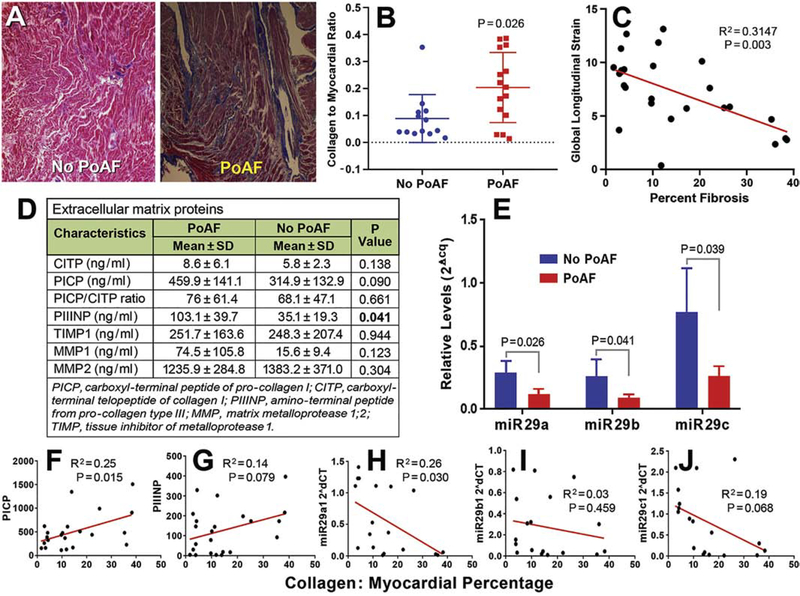
Of the total 90 study participants who underwent surgery, atrial appendages of 28 patients could be used for histological assessment of atrial fibrosis. Masson’s trichrome staining revealed increased collagen deposition in those who developed PoAF (n = 15), with a significant increase in blue collagen-stained area compared with those who remained in SR (collagen/myocardial ratio 0.20 ± 0.09 vs. 0.09 ± 0.01, p = 0.026; Fig. 2A, 2B). This increased collagen deposition observed in PoAF patients was associated with impaired LA global longitudinal strain (GLS) with an AUC of 78.3% (C statistics = 0.783; 95% CI, 0.659–0.909) (Fig. 2C).
FIGURE 2.
Significant fibrosis in the right atrial appendage of coronary artery bypass graft patients with reduced circulating miR29s and increased procollagen I and III peptide levels in those who developed postoperative atrial fibrillation (PoAF). (A) Representative photographs of Masson’s trichrome staining of right atrial appendage (RAA) histology sections display severe fibrosis (blue) in a PoAF patient (right) compared with a No PoAF patient (left). The individual data points-column scatter graph (B) displays Masson’s trichrome stained collagen (blue) to myocardium (red, total area) ratio, depicting a significantly higher fibrosis (mean±SD and each sample value) in coronary artery bypass graft (CABG) patients who developed PoAF as quantified using ImageJ macro. P ≤ 0.05 was considered significant, n=13 (No PoAF), 15 (PoAF). Linear regression between global longitudinal strain and fibrosis in CABG patients’ RAA histology sections determined by Masson’s trichrome staining and quantified using ImageJ macro to obtain collagen to myocardium ratio (C). Preoperative levels (mean±SD) of markers of collagen and extracellular matrix in patients who developed PoAF versus no-PoAF development after CABG (D). Relative preoperative levels of circulating miR-29a, -b, and -c determined by quantitative polymerase chain reaction were significantly reduced in patients who developed PoAF versus those who remained free of PoAF after CABG during their hospital stay (E). Linear regression between the percentage of right atrial appendage tissue fibrosis and plasma levels of PICP (F), PIIINP (G), and circulating preoperative miR-29a (H), miR-29b (I), and miR-29c (J) levels in coronary artery bypass graft patients.
3.5. Circulating levels of markers of collagen synthesis and degradation and PoAF
The level of protein biomarkers indicative of collagen turnover was assessed in the preoperative blood samples of patients who subsequently developed PoAF or remained in SR. The level of PIIINP, a marker of collagen III synthesis, was significantly increased in patients who developed PoAF versus those who remained free of PoAF (103.1 ± 39.7 vs. 35.1 ± 19.3, p = 0.041; Fig. 2D). Collagen I synthesis biomarker PICP showed a trend toward higher levels without reaching statistical significance, whereas collagen I degradation marker CITP was not significantly different between the groups. The overall ratio of PICP: CITP, indicative of greater collagen synthesis, was elevated in those who developed PoAF but was not statistically significant (76 ± 61.4 vs 68.1 ± 47.1; p = 0.661; Fig. 2D). No significant differences were observed in the circulating levels of MMP1, MMP2, or TIMP1 between the two groups (Fig. 2D).
3.6. MicroRNA-29 involved in regulating fibrosis is attenuated in patients who developed PoAF
Preoperative circulating levels of miR-29s (miR-29a, −29b, −29c), known to modulate fibrosis [11,12,14], were compared between the two groups. MiR 29a, -b, and -c were significantly reduced in patients who subsequently developed PoAF compared with those who remained in SR (Fig. 2E).
3.7. Circulating collagen propeptides, miR-29s and extent of fibrosis in RAA tissue
There was a correlation between increasing levels of circulating PICP and extent of atrial appendage fibrosis that was statistically significant (p = 0.015, Fig. 2F) and a weaker correlation between PIIINP levels and RAA tissue fibrosis (p = 0.08, Fig. 2G). Circulating miR-29a (R = −0.26, p = 0.03) and miR-29c (R = −0.19, p = 0.07) levels showed a weak inverse correlation with RAA fibrosis, whereas miR-29b showed no correlation (R = −0.03, p = 0.46) (Fig. 2H–J).
3.8. Risk prediction for PoAF based on clinical risk factors and incremental value of circulatory and imaging biomarkers
In this population at low risk of PoAF, age was the only independent risk factor associated with PoAF in a multivariate analysis that included age, body mass index, chronic obstructive pulmonary disease, hypertension, and sleep apnea. Considering age as a continuous variable, a 10-year increase in age was associated with an overall 2-fold risk of developing PoAF (OR=1.83, 95% CL 1.12–2.58, p = 0.02). Other known risk factors for AF—including history of hypertension, obstructive sleep apnea, body mass index, diabetes mellitus, and myocardial infarction—were not predictive of PoAF in these patients, as summarized in Supplementary Table 2. On preoperative cardiac imaging, none of the standard echocardiographic features were predictive of PoAF except for the left atrial ejection fraction (LAEF), which was reduced in patients who subsequently developed PoAF and was a strong independent predictor of PoAF development (C statistics = 0.837; 95% CI, 0.648–0.980; likelihood ratio test, p = 0.0237; Supplementary Figure 1A). A cutoff LAEF of 42% had 85% sensitivity and 50% specificity.
Among circulating biomarkers, the predictive value of PICP for the whole group (C statistics = 0.615; 95% CI, 0.425–0.805, p = 0.0994) and that of PIIINP (C statistics = 0.640; 95% CI, 0.458–0.821; p = 0.0724) alone in identifying patients who developed PoAF was low (Fig. 3A). Similarly, miR-29s showed modest associations with the development of PoAF, with an AUC of 61.6% for miR-29a (C statistics = 0.616; 95% CI, 0.466–0.765; p = 0.0957; Supplementary Figure 1B), 59.6% for miR-29b (C statistics = 0.596; 95% CI, 0.442–0.750; p = 0.1226; Supplementary Figure 1C), and 56.3% for miR-29c (C statistics = 0.563; 95% CI, 0.404–0.721, p = 0.2889; Supplementary Figure 1D). Thus, individually the circulating biomarkers had modest predictive power to identify individuals at risk for PoAF. However, when individual biomarkers PIIINP and PICP were added to the clinical predictor of age the predictability of the model increased with C statistic = 0.703 (95% CI, 0.522–0.884; p = 0.064) in a continuous model (Fig. 3A). The greatest C statistic was observed when age, PIIINP, and miR-29a were combined; that further improved the model predictability with C statistics = 0.799 (95% CI, 0.617–0.980; p = 0.024) (Fig. 3B) with 91% specificity and 69% sensitivity and Youden Y = 0.603. The cutoff values with best sensitivity and specificity for identifying those who developed PoAF were age = 69 years, PIIINP = 92 ng/ml, PICP = 176 ng/ml, and miR29a 2ΔCq = 0.205 (Fig 3C). The elevated levels of PIIINP and PICP and reduced miR-29a identified all PoAF patients who were missed by LAEF criteria (Supplementary Table 3).
FIGURE 3.
Receiver operating characteristic (ROC) curves for logistic regression model improves the prediction accuracy of preoperative plasma levels of PIIINP and miR-29a with age for the development of PoAF. Predictive accuracy of PICP and PIINP with age (A), with age, PIIINP, and miR-29a in ROC model (B), and sensitivity, specificity, and cutoff value for age, LAEF, circulating PIIINP, PICP, and miR-29a levels at 95% CI to identify patients at risk of PoAF development (C).
4. Discussion
The main finding of our study in patients undergoing CABG with no previous history of AF is that, despite similar comorbidities and standard echocardiographic features, patients who developed PoAF had an underlying abnormal atrial substrate that could be identified with more sensitive imaging modalities such as STE in combination with biochemical and molecular biomarkers noninvasively. Reduced preoperative myocardial longitudinal LA strain assessed by STE, a surrogate for atrial fibrosis [16]; elevated circulating levels of PIIINP, a marker of collagen synthesis [5]; and reduced miR-29a, a regulatory microRNA modulating fibrosis [17] showed association with PoAF. Individual clinical factors or biomarkers had modest predictability toward PoAF; however, when clinical factors (age >65 y) were combined with PIIINP and miR-29a, a stronger predictive tool was developed that identified patients who subsequently developed PoAF with higher predictive accuracy than clinical factors or biomarkers in isolation. Reduced LAEF also was a strong predictor of PoAF, and in patients in whom LAEF ≥ 42%, circulating biomarkers were useful in identifying patients who developed PoAF.
Although natriuretic peptides, cardiac troponin, and various other biomarkers have been used to identify patients at risk of PoAF, these markers in patients without a history of AF, HF, or significant ventricular dysfunction are often not sensitive and lack predictive accuracy owing to variability in the underlying pathophysiology that increases the risk for developing PoAF [5,18]. Postoperative elevated NT-pro BNP has been suggested to be a predictor of PoAF compared to BNP, but association with preoperative NT-pro BNP levels and PoAF has not been consistently demonstrated [19]. Similarly, levels of preoperative high-sensitive cardiac troponin T, a marker of myocardial injury, have not been shown to predict PoAF, and postoperative levels are not useful for preoperative screening of patients [20–22]. Therefore, presurgical identification of patients without a prior history of AF who are likely to develop PoAF after cardiac surgery continues to be a challenge. PoAF after cardiac surgery results from complex interactions of underlying substrate, presence of comorbidities, surgery-related factors, and postoperative care, and contributes to morbidity and increased hospital stay, expense, and mortality [5,8,23,24]. Recent studies indicate advanced age and aging-associated atrial fibrosis independently contributing to recurrence of AF after cardiac interventions [18,25]. This is in agreement with our findings, in which age was the only clinical factor that independently predicted risk of incident PoAF, with a 2-fold increase in the odds of developing PoAF for every 10-year increment in age [18]. The observation that patients who developed PoAF also had increased interstitial fibrosis suggests that underlying atrial fibrosis plays an important role in the development of AF following cardiac surgery even in this group of patients who are without a prior history of AF, HF, or significant left ventricular dysfunction or atrial enlargement [7,9,26,27]. One of the important findings of the study suggestive of impaired atrial mechanical function, that reduced LAEF was a strong predictor of PoAF, suggests that this should be determined in every patient undergoing cardiac surgery to identify those who otherwise would be considered at low risk of PoAF. In addition, on STE, reduced global and regional LA strain indicative of impaired LA reservoir function, which is influenced by atrial relaxation, chamber stiffness, and reduced contractility [28], was predictive of PoAF. The regional differences in LA strain suggest the presence of patchy fibrosis that can promote heterogeneity in electrical conduction leading to reentrant arrhythmias manifesting as PoAF [29,30]. In these patients, PoAF appears to be a manifestation of a concealed atrial abnormality not detected by routine clinical exam but unmasked by acute surgical stress triggering electrophysiological instability of an otherwise stable substrate. This abnormal substrate, however, can be identified by more sensitive imaging modalities such as STE or with circulating biomarkers reflective of fibrosis and collagen synthesis and its regulation. This also is suggested by Masson’s trichrome staining of atrial tissue and with elevated levels of markers of collagen synthesis. These results provide reasonable correlation of reduced LA GLS with histological assessment of myocardial collagen deposition in atrial appendage tissue. These results are consistent with previous studies using STE, cardiac magnetic resonance imaging, or intra-cardiac voltage mapping that correlated fibrosis with recurrence of AF after ablation [28–34].
Among the biomarkers of collagen synthesis studied, PIIINP was significantly elevated and PICP showed a trend toward elevation in the preoperative blood samples of those who developed PoAF. In patients who had preserved LAEF, elevated PICP and PIIINP levels and reduced miR29a were able to identify all patients who developed PoAF and who were otherwise missed by imaging criteria, thus supporting the hypothesis that combining biomarkers with different sensitivity (PIIINP 93%) and sensitivity (PICP 95%) helps to identify patients at risk of PoAF and that even when subclinical fibrosis is not detected by routine echocardiography, PoAF can still be recognized by markers of collagen turnover. In our study, other markers of ECM turnover, including MMP1, MMP2, and TIMP1, showed no significant differences between the groups that did or did not develop PoAF, which agrees with the previous report [35]. Previous studies reported that an increase in the levels of PICP and PIIINP correlate with persistent AF [36], PoAF [7], and ventricular fibrosis [37]. But, this was not universally observed by all [10,38]; this likely may result from the heterogeneity of the populations, with a spectrum of LA substrate abnormalities ranging from those with active collagen production in an early stage to a burned out state without ongoing collagen synthesis.
The family of miR-29, composed of three members: miR-29a, miR-29b, and miR-29c [17], is known for regulating several genes modulating different biological processes and diseases, including ECM turnover and fibrosis in cardiac tissue [11,17,39] in association with hypertrophic cardiomyopathy and AF [11–14]. Their role as a circulating biomarker useful in identifying patients at risk of PoAF, however, had not been explored previously. The data in the present study revealed the decrease in the preoperative levels of miR-29s in patients who developed PoAF; therefore, this is the first report demonstrating miR-29s and their association with PoAF. This agrees with previous studies in which reduced plasma miR-29a and miR-29b levels were associated with fibrosis-linked AF, HF, and hypertrophic cardiomyopathy [11,12,14].
MiR-29 has been shown to negatively regulate the expression of collagens, as previously reported, and the potential benefit of elevating miR-29 levels has been demonstrated in fibrosis disease models in various organs, including the kidneys, lungs, and liver [17,39]. However, this is not universal and depends on the model and species studied, as in the pressure overload murine model, anti-miR-29 therapy or miR-29 deletion was shown to prevent ventricular hypertrophy and fibrosis [39]. This discrepancy could be the result of differences in species, organs, heart chamber, targeted cell type (cardiomyocytes vs. fibroblasts), or stimuli initiating fibrosis studied, with differences in fibroblast responsiveness to condition previously recognized [40]. In our study, reduced miR-29 levels correlated with atrial appendage fibrosis mainly in patients who developed PoAF in line with a previous report of decreased miR-29 in AF patients [11].
Among the circulating biomarkers tested, propeptide biomarker of collagen synthesis PIIINP and the regulatory miR-29a showed a statistically significant association with the development of PoAF. However, individually they had only a modest predictive power to identify patients who developed PoAF. When the individual peptide biomarkers PIIINP and PICP were added to the clinical predictor of age, the predictability of the model increased, with the greatest C statistic achieved when age, PIIINP, and miR-29a were combined. The elevated collagen propeptides and reduced miR29a were also useful in identifying those at risk with preserved LAEF without overt mechanical dysfunction, suggesting that these biomarkers could identify early pathophysiological changes before overt atrial contractility is affected.
4.1. Study limitations
We confined our study to only CABG surgery patients with no history of AF. Limitations to our study include a relatively small sample size of patients undergoing CABG, all with underlying coronary artery disease; however, the number of patients reported is comparable or higher than previous reports [7]. We limited the study to patients who were at low risk for PoAF and free of AF, HF, and left ventricular dysfunction or severe valve diseases. These are known predictors of PoAF that also contribute to cardiac fibrosis and could have confounded the result. Circulating biomarkers were correlated with histological fibrosis in the RAA in a limited number of patients to prove the correlations of these circulating biomarkers with fibrosis in the RAA and with STE myocardial deformation abnormalities in the LA. Clinical predictors of AF were included in the univariate and multivariate analysis, and age, which came out as an independent predictor for this study population, was included in the final model along with miR-29a, PICP, and PIIINP. The strength of our study includes the correlation of markers of collagen synthesis (PICP, PIIINP levels) with histological evidence of fibrosis, miR-29a levels, and deformation properties to detect subtle atrial substrate abnormalities in those who subsequently developed PoAF. The cutoff values of these biomarkers determined in this study need to be validated in other cohorts at intermediate and higher risk of PoAF, but the utility is highest in a population that could not be identified by standard clinical practice.
5. Conclusion
In summary, we report a novel predictive tool incorporating age, LA strain, LA ejection fraction on LA imaging, and biomarkers of collagen synthesis and regulation that increased our ability to identify patients with concealed AF substrate that was unmasked by the stress of cardiac surgery, resulting in the development of PoAF. Patients with preserved global and regional LA strain, lower PIIINP levels, and higher miR-29a apparently have not reached a critical substrate threshold to develop PoAF. Further studies are warranted to validate the utility of this novel noninvasive approach toward identifying patients at risk for the development of PoAF. This is important because nearly 7 million cardiac and vascular surgeries are performed annually in the US, with the majority in the growing elderly population that is at risk for PoAF [4]. The ability to identify patients at high risk for this complication could be useful to selectively deploy prophylactic intervention or to reduce hospital stays, cost, comorbidities, and mortality associated with PoAF.
Supplementary Material
Highlights.
Postoperative AF is a common problem in patients post cardiac surgery
Clinical predictors used to distinguish patients at risk of PoAF have limitations
Available biomarkers do not fully reflect the substrate responsible for PoAF
We developed a model better able to find CABG patients at risk of PoAF
Further studies are warranted to validate the utility of this noninvasive approach
Acknowledgement
The authors are grateful to Jennifer Pfaff, Susan Nord, Brian Miller and Brian Schurrer for editorial and graphic assistance.
Funding: This work was supported in part by NIH R01 HL101240 (to A.J.); intramural grants from Aurora Foundation, Aurora Research Institute (grant no. 505-3975 to F.R. and M.M., grant no. 505-3976 to F.R.); David V Uihlein Foundation (grant no. 570-5025 to F.R.).
ABBREVIATIONS
- AF
atrial fibrillation
- AUC
area under the curve
- BMI
body mass index
- CABG
coronary artery bypass graft
- CITP
carboxyl-terminal telopeptide of collagen I
- CL
confidence limit
- ECM
extracellular matrix
- GLS
global longitudinal strain
- HF
heart failure
- LA
left atrium/left atrial
- miRNA
microRNA
- PICP
carboxyl-terminal pro-collagen I
- PIIINP
amino-terminal procollagen III
- PoAF
postoperative atrial fibrillation
- RAA
right atrial appendage
- ROC
receiver operating characteristic
- SR
sinus rhythm
- STE
speckle-tracking echocardiography
Footnotes
Declarations of interest: none
All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Lowres N, Mulcahy G, Jin K, Gallagher R, Neubeck L, Freedman B, Incidence of postoperative atrial fibrillation recurrence in patients discharged in sinus rhythm after cardiac surgery: a systematic review and meta-analysis, Interact. Cardiovasc. Thorac. Surg 26 (2018) 504–511. [DOI] [PubMed] [Google Scholar]
- [2].Mathew JP, Fontes ML, Tudor IC, et al. , A multicenter risk index for atrial fibrillation after cardiac surgery, JAMA. 291 (2004) 1720–1729. [DOI] [PubMed] [Google Scholar]
- [3].Echahidi N, Pibarot P, O’Hara G, Mathieu P, Mechanisms, prevention, and treatment of atrial fibrillation after cardiac surgery, J. Am. Coll. Cardiol 51 (2008) 793–801. [DOI] [PubMed] [Google Scholar]
- [4].Benjamin EJ, Virani SS, Callaway CW, et al. , Heart disease and stroke statistics-2018 update: A report from the American Heart Association, Circulation 137 (2018) e67–e492. [DOI] [PubMed] [Google Scholar]
- [5].Turagam MK, Mirza M, Werner PH, et al. , Circulating biomarkers predictive of postoperative atrial fibrillation, Cardiol. Rev. 24 (2016) 76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Shen J, Lall S, Zheng V, Buckley P, Damiano RJ Jr., Schuessler RB, The persistent problem of new-onset postoperative atrial fibrillation: a single-institution experience over two decades, J. Thorac. Cardiovasc. Surg. 141 (2011) 559–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Swartz MF, Fink GW, Sarwar MF, et al. , Elevated pre-operative serum peptides for collagen I and III synthesis result in post-surgical atrial fibrillation, J. Am. Coll. Cardiol 60 (2012) 1799–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ramlawi B, Otu H, Mieno S, et al. , Oxidative stress and atrial fibrillation after cardiac surgery: a case-control study, Ann. Thorac. Surg 84 (2007) 1166–1172; discussion 1172–1163. [DOI] [PubMed] [Google Scholar]
- [9].Estes NA 3rd, Sacco RL, Al-Khatib SM, et al. , American Heart Association atrial fibrillation research summit: a conference report from the American Heart Association, Circulation 124 (2011) 363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Neuberger HR, Cacciatore A, Reil JC, et al. , Procollagen propeptides: serum markers for atrial fibrosis?, Clin. Res. Cardiol. 101 (2012) 655–661. [DOI] [PubMed] [Google Scholar]
- [11].Dawson K, Wakili R, Ordog B, et al. , MicroRNA29: a mechanistic contributor and potential biomarker in atrial fibrillation, Circulation 127 (2013) 1466–1475, 1475e1461–1428. [DOI] [PubMed] [Google Scholar]
- [12].Luo X, Yang B, Nattel S, MicroRNAs and atrial fibrillation: mechanisms and translational potential, Nat. Rev. Cardiol. 12 (2015) 80–90. [DOI] [PubMed] [Google Scholar]
- [13].Condorelli G, Latronico MV, Cavarretta E, microRNAs in cardiovascular diseases: current knowledge and the road ahead, J. Am. Coll. Cardiol. 63 (2014) 2177–2187. [DOI] [PubMed] [Google Scholar]
- [14].Roncarati R, Viviani Anselmi C, Losi MA, et al. , Circulating miR-29a, among other upregulated microRNAs, is the only biomarker for both hypertrophy and fibrosis in patients with hypertrophic cardiomyopathy, J. Am. Coll. Cardiol. 63 (2014) 920–927. [DOI] [PubMed] [Google Scholar]
- [15].Ross GR, Bajwa T Jr., Edwards S, et al. , Enhanced store-operated Ca(2+) influx and ORAI1 expression in ventricular fibroblasts from human failing heart, Biol. Open 6 (2017) 326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Pernigo M, Benfari G, Geremia G, et al. , Atrial function as an independent predictor of postoperative atrial fibrillation in patients undergoing aortic valve surgery for severe aortic stenosis, J. Am. Soc. Echocardiogr 30 (2017) 956–965.e951. [DOI] [PubMed] [Google Scholar]
- [17].van Rooij E, Sutherland LB, Thatcher JE, et al. , Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis, Proc. Natl. Acad. Sci. U. S. A. 105 (2008) 13027–13032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kirchhof P, Benussi S, Kotecha D, et al. , 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS, Eur. Heart J. 37 (2016) 2893–2962. [DOI] [PubMed] [Google Scholar]
- [19].Cai GL, Chen J, Hu CB, Yan ML, Xu QH, Yan J, Value of plasma brain natriuretic peptide levels for predicting postoperative atrial fibrillation: a systemic review and meta-analysis, World J. Surg 38 (2014) 51–59. [DOI] [PubMed] [Google Scholar]
- [20].Masson S, Wu JH, Simon C, et al. , Circulating cardiac biomarkers and postoperative atrial fibrillation in the OPERA trial, Eur. J. Clin. Invest. 45 (2015) 170–178. [DOI] [PubMed] [Google Scholar]
- [21].Hernandez-Romero D, Vilchez JA, Lahoz A, et al. , High-sensitivity troponin T as a biomarker for the development of atrial fibrillation after cardiac surgery, Eur. J. Cardiothorac. Surg 45 (2014) 733–738. [DOI] [PubMed] [Google Scholar]
- [22].Knayzer B, Abramov D, Natalia B, Tovbin D, Ganiel A, Katz A, Atrial fibrillation and plasma troponin I elevation after cardiac surgery: relation to inflammation-associated parameters, J. Card. Surg 22 (2007) 117–123. [DOI] [PubMed] [Google Scholar]
- [23].Pimor A, Galli E, Vitel E, et al. , Predictors of post-operative cardiovascular events, focused on atrial fibrillation, after valve surgery for primary mitral regurgitation, Eur. Heart J. Cardiovasc. Imaging 20 (2019) 177–184. [DOI] [PubMed] [Google Scholar]
- [24].Maesen B, Nijs J, Maessen J, Allessie M, Schotten U, Post-operative atrial fibrillation: a maze of mechanisms, Europace 14 (2012) 159–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hernandez-Romero D, Vilchez JA, Lahoz A, et al. , Galectin-3 as a marker of interstitial atrial remodelling involved in atrial fibrillation, Sci. Rep 7 (2017) 40378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Magnani JW, Rienstra M, Lin H, et al. , Atrial fibrillation: current knowledge and future directions in epidemiology and genomics, Circulation 124 (2011) 1982–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Schotten U, Verheule S, Kirchhof P, Goette A, Pathophysiological mechanisms of atrial fibrillation: a translational appraisal, Physiol. Rev. 91 (2011) 265–325. [DOI] [PubMed] [Google Scholar]
- [28].Hoit BD, Assessment of left atrial function by echocardiography: Novel insights, Curr. Cardiol. Rep 20 (2018) 96. [DOI] [PubMed] [Google Scholar]
- [29].Kuppahally SS, Akoum N, Burgon NS, et al. , Left atrial strain and strain rate in patients with paroxysmal and persistent atrial fibrillation: relationship to left atrial structural remodeling detected by delayed-enhancement MRI, Circ. Cardiovasc. Imaging 3 (2010) 231–239. [DOI] [PubMed] [Google Scholar]
- [30].Leung M, Abou R, van Rosendael PJ, et al. , Relation of echocardiographic markers of left atrial fibrosis to atrial fibrillation burden, Am. J. Cardiol. 122 (2018) 584–591. [DOI] [PubMed] [Google Scholar]
- [31].Ozben B, Akaslan D, Sunbul M, et al. , Postoperative atrial fibrillation after coronary artery bypass grafting surgery: A two-dimensional speckle tracking echocardiography study, Heart Lung Circ. 25 (2016) 993–999. [DOI] [PubMed] [Google Scholar]
- [32].Mirza M, Caracciolo G, Khan U, et al. , Left atrial reservoir function predicts atrial fibrillation recurrence after catheter ablation: a two-dimensional speckle strain study, J. Interv. Card. Electrophysiol 31 (2011) 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kistler PM, Sanders P, Fynn SP, et al. , Electrophysiologic and electroanatomic changes in the human atrium associated with age, J. Am. Coll. Cardiol 44 (2004) 109–116. [DOI] [PubMed] [Google Scholar]
- [34].Olsen FJ, Mogelvang R, Jensen GB, Jensen JS, Biering-Sorensen T, Relationship between left atrial functional measures and incident atrial fibrillation in the general population: The Copenhagen City Heart Study, JACC Cardiovasc. Imaging 12 (2019) 981–989. [DOI] [PubMed] [Google Scholar]
- [35].Huxley RR, Lopez FL, MacLehose RF, et al. , Novel association between plasma matrix metalloproteinase-9 and risk of incident atrial fibrillation in a case-cohort study: the Atherosclerosis Risk in Communities study, PLoS One 8 (2013) e59052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kallergis EM, Manios EG, Kanoupakis EM, et al. , Extracellular matrix alterations in patients with paroxysmal and persistent atrial fibrillation: biochemical assessment of collagen type-I turnover, J. Am. Coll. Cardiol 52 (2008) 211–215. [DOI] [PubMed] [Google Scholar]
- [37].Querejeta R, Varo N, Lopez B, et al. , Serum carboxy-terminal propeptide of procollagen type I is a marker of myocardial fibrosis in hypertensive heart disease, Circulation 101 (2000) 1729–1735. [DOI] [PubMed] [Google Scholar]
- [38].Tziakas DN, Chalikias GK, Papanas N, Stakos DA, Chatzikyriakou SV, Maltezos E, Circulating levels of collagen type I degradation marker depend on the type of atrial fibrillation, Europace 9 (2007) 589–596. [DOI] [PubMed] [Google Scholar]
- [39].Sassi Y, Avramopoulos P, Ramanujam D, et al. , Cardiac myocyte miR-29 promotes pathological remodeling of the heart by activating Wnt signaling, Nat. Commun 8 (2017) 1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Rizvi F, DeFranco A, Siddiqui R, et al. , Chamber-specific differences in human cardiac fibroblast proliferation and responsiveness toward simvastatin, Am. J. Physiol. Cell. Physiol 311 (2016) C330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.