Abstract
Objectives:
Defining the posterior extent of breast cancer prior to surgery has clinical implications. However, there are limited data available to guide the interpretation of breast cancers seen on MRI that abut the pectoralis muscle but lack associated muscle enhancement.
Methods:
In this retrospective study of breast MRIs performed between May 2008 and July 2019, 43 female patients demonstrated breast cancers abutting the pectoralis muscle without enhancement of the muscle itself. Imaging features of the cancers as well as pathologic and clinical outcomes were recorded. Statistical analyses of associations between imaging findings and clinical outcomes were performed using Fisher’s exact test, logistic regression, a Mann–Whitney U test and/or Student’s t-test.
Results:
The pectoralis major muscle was pathologically invaded by carcinoma in 4/43 (9.3%). There was no significant association between pectoralis muscle invasion and any MR imaging feature of the breast cancer. Tumors causing deformation of the muscle contour by MRI, tumors larger in size, tumors with a larger extent abutting the muscle and tumors in which the imaging feature abutting the muscle was a mass or non-mass enhancement (rather than a spicule) were more commonly seen in patients with muscle invasion, although these did not reach statistical significance (p > 0.05).
Conclusion:
In this study, a lack of pectoralis muscle enhancement by MRI did not exclude pathologic muscle invasion by breast cancers abutting the muscle.
Advances in knowledge:
Knowledge of the likelihood of pectoralis muscle involvement for breast cancers abutting the pectoralis muscle on MRI may guide accurate interpretation and definition of the posterior extent of disease.
Introduction
Defining the posterior extent of breast cancer prior to surgery has clinical implications, as it may affect surgical and oncologic treatment planning. Specifically, according to the American Joint Committee on Cancer staging guidelines for breast cancer, direct tumor extension to the chest wall is classified as T4 disease regardless of the tumor size.1 In contrast, tumor extension into the pectoralis major or minor muscles without involvement of the underlying chest wall does not directly change the TNM staging.1 Additionally, knowledge of pectoralis muscle tumor involvement prior to surgery may alter surgical management in efforts to obtain clear histologic margins, which has important prognostic implications.2–7
Despite the clinical implications of detecting breast cancer invasion of the pectoralis muscles pre-operatively, there are limited data in the current literature to guide the interpretation of a very posterior breast tumor seen on breast MRI. In 2000, Morris et al8 described a series of 19 posterior breast cancers seen on MRI. Of the five patients with pectoralis muscle enhancement, all five had muscle invasion at surgical pathology, compared to none of the six patients with a tumor abutting the pectoralis muscle without enhancement of the muscle and none of the eight patients with a preserved posterior fat plane. In 2005, another study reported 33 breast MRI exams (some of which were performed after neoadjuvant chemotherapy) on which there was obliteration of the fat plane between the breast cancer and the pectoralis muscle by MRI.9 In this study, 7 of 33 cases showed muscle enhancement on MRI (5/7 showed muscle invasion at surgery) and 26/33 showed no muscle enhancement (0/26 had muscle invasion at pathology).
A related study by Samreen et al10 reviewed breast MRI exams on which the associated report described chest wall invasion in 23 patients. While both contrast enhancement and restricted diffusion of the pectoralis major muscle and/or chest wall were found to be helpful to diagnose invasion of those structures in that study, only four patients in this series did not show enhancement of the pectoralis muscle itself and specific pathology for these cases was not reported. Additional studies have reported subgroup data regarding breast cancers abutting the pectoralis muscle, with most reports describing an association between muscle enhancement on MRI and muscle invasion at surgery, and a lack of muscle enhancement with a lack of muscle invasion; however, these reports include only three or fewer patients.11–14
Accurate interpretation of posterior breast cancer extent on MRI, including pectoralis muscle involvement, may impact surgical and oncologic treatment. However, there are limited data to guide interpretation for exams where the tumor abuts the pectoralis muscle on MRI without enhancement of the muscle itself. In fact, the combined sample size from published studies designed to evaluate the implications of this MRI finding consist of only 32 patients in entirety.8,9 The aim of this study was to report clinical and pathology outcomes for patients with breast cancers that abut the pectoralis major muscle on MRI without enhancement of the muscle itself.
Methods and materials
Subjects
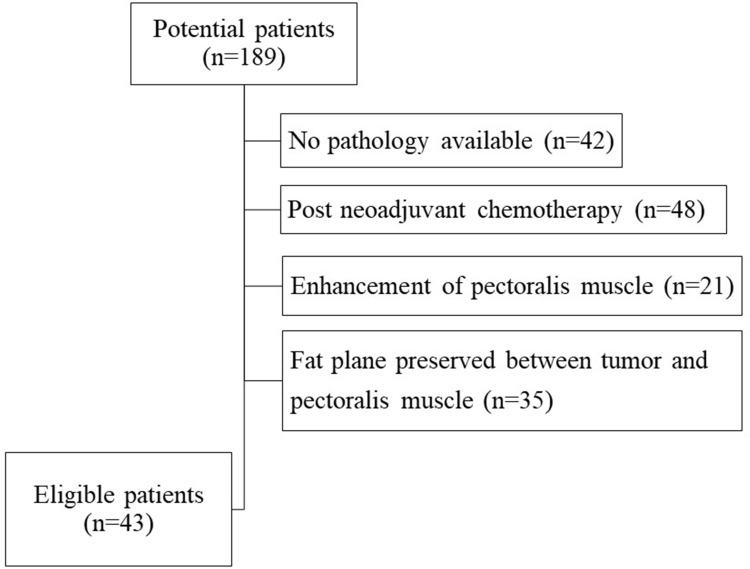
This HIPAA-compliant study was approved by the Johns Hopkins School of Medicine Institutional Review Board, which waived the need for informed consent. A retrospective review of medical records from May 2008 to July 2019 identified 189 breast MRI exams in which the final report described enhancement associated with a biopsy-proven breast cancer abutting or extending to the pectoralis major muscle. A fellowship trained dedicated breast imaging radiologist with 6 years of experience reviewed the images for each case blinded to the pathology and clinical outcomes. Exams with obliteration of the fat plane between the breast cancer and the pectoralis major muscle were included, and exams with a preserved fat plane or with enhancement of the pectoralis muscle itself were excluded. MRI exams performed after neoadjuvant chemotherapy were also excluded. 43 breast cancer patients with MRI findings abutting the pectoralis major muscle, without enhancement of the muscle, were included (Figure 1).
Figure 1.
Flowchart shows the number of patients excluded from the study population for each exclusion criterion. A total of 43 eligible patients were included in the study.
Image acquisition
The breast MRI technique for the 43 exams was variable, as the population included patients referred to our institution (Johns Hopkins School of Medicine) after the MRI was obtained at an outside institution and MRIs performed within our institution may have been performed at any of our multiple locations where there is variation in scanner types. Furthermore, our institutional imaging protocols have evolved over the 11 year study period. All included studies, however, involved at least three post-contrast series and the first post-contrast series was able to be viewed in both the axial and sagittal projections.
Data collection and image interpretation
Imaging features for these 43 eligible patients were recorded according to the Breast Imaging Reporting and Data System (BI-RADS) lexicon by the single reader blinded to clinical and pathology outcomes.15 This included the specific MRI finding type abutting the pectoralis muscle: a mass, a spicule of a mass (defined as an extension from the margin of a spiculated mass), or non-mass enhancement. The presence of associated mass effect from the tumor on the pectoralis muscle (tenting and/or mass effect) was also assessed. For each eligible patient, clinical history and surgical pathology details were also recorded, including whether the posterior margins were clear, close (within 1 mm) or positive for tumor involvement, as well as whether tumor invasion of the pectoralis muscle was seen on surgical pathology. If posterior margins were clear (>1 mm) and no skeletal muscle was present on surgical pathology, the case was recorded as having no muscle invasion. The number of months of clinical follow-up for each patient and the clinical status of the patient at follow-up, including whether recurrence or the development of metastasis occurred during follow-up, was also recorded.
Statistical analysis
Using SPSS v. 25 (IBM, Armonk, NY), the association between pectoralis muscle invasion on pathology and the imaging features of the cancer by MRI was assessed using Fisher’s Exact test with a p-value of less than 0.05 declared as significant. The association between pectoralis muscle invasion and patient age was assessed using Student’s t-test (SPSS v. 25). The association between pectoralis muscle invasion and both the size of MRI enhancement and the extent of tumor abutting the muscle were assessed with a Mann–Whitney U test using mean ranks (SPSS v. 25). The odds ratio of developing cancer recurrence or metastasis during the follow-up period as a function of posterior margin status was evaluated using binary logistic regression (using R 2017, R Foundation for Statistical Computing, Vienna, Austria).
Results
Clinical and imaging characteristics
43 female breast cancer patients with MRI enhancement abutting the pectoralis major muscle, without enhancement of the muscle itself, were included in this study. The mean patient age was 57 years (standard deviation 11.2 years). Invasive ductal carcinoma (IDC) was the most common tumor type (30/43, 69.8%), with most cancers representing a primary tumor (38/43, 88.4%) rather than recurrent disease (Table 1).
Table 1.
Frequency of clinical and imaging features within the study population
| Characteristic | Frequency |
|---|---|
| Primary or recurrent tumor | |
| Primary | 38/43 (88.4%) |
| Recurrence | 5/43 (11.6%) |
| Tumor type | |
| IDC | 30/43 (69.8%) |
| ILC | 3/43 (7%) |
| IMC | 6/43 (14%) |
| Micro-invasive IDC | 2/43 (4.7%) |
| DCIS | 2/43 (4.7%) |
| Clinical status at follow-up | |
| No evidence of disease | 36/43 (83.7%) |
| Alive with disease | 3/43 (7%) |
| Deceased | 3/43 (7%) |
| No follow-up | 1/43 (2.3%) |
| MRI finding type that abuts the muscle | |
| Non-mass enhancement | 13/43 (30.2%) |
| Mass | 21/43 (48.8%) |
| Spicule of a mass | 9/43 (20.9%) |
| Deformed contour of pectoralis muscle | |
| Tenting of the muscle | 10/43 (23.3%) |
| Mass effect on the muscle | 2/43 (4.7%) |
| Both tenting and mass effect | 4/43 (9.3%) |
| No deformation of the muscle contour | 27/43 (62.8%) |
| Margin status on surgical pathology | |
| Clear margins > 1 mm | 26/43 (60.5%) |
| Close margins within 1 mm | 9/43 (20.9%) |
| Involved posterior margins | 8/43 (18.6%) |
| Involvement of pectoralis major muscle on surgical pathology | |
| No involvement of muscle | 34/43 (79.1%) |
| Muscle involvement | 4/43 (9.3%) |
| Unknown status of muscle involvement | 5/43 (11.6%) |
DCIS, Ductal carcinoma in situ; IDC, Invasive ductal carcinoma; ILC, Invasive lobular carcinoma; IMC, Invasive mammary carcinoma with ductal and lobular features.
Five patients had a personal history of ipsilateral breast cancer 3–15 years prior to the recurrence that now abuts the pectoralis muscle. The previous cancer was treated with lumpectomy and radiation therapy in four patients and mastectomy in one patient. The recurrence was at the lumpectomy site in two patients, in the same quadrant but not within the lumpectomy bed in two patients, and in a different quadrant from the original cancer in one patient. At surgery for the current cancer diagnosis which abuts the pectoralis muscle on breast MRI, one patient had clear margins, two patients had close (<1 mm) posterior margins, one patient had positive posterior margins and one patient had pectoralis muscle invasion.
The most common type of MRI enhancement abutting the pectoralis muscle was a mass (21/43, 48.8%), with non-mass enhancement or a spicule of a mass less common. The total extent of abnormal enhancement varied widely and was not associated with pathologic muscle invasion, with a median size of 41 mm (interquartile range 19–77 mm) for tumors with pathologic muscle invasion compared to a median size of 24 mm (interquartile range 4–130 mm) for tumors without pathologic muscle invasion (U = 51, p = 0.447). There was a trend for tumors with a larger portion of tumor abutting the pectoralis muscle to be associated with muscle invasion. Specifically, median length of abutment for tumors with pathologic invasion was 22.0 mm (interquartile range 13.5–35.0 mm, range 12.0–38.0 mm) compared to a median of 10.5 mm (interquartile range 6.0–17.25 mm, range 2.0–36 mm) for tumors without pathologic muscle invasion, although this did not reach statistical significance (U = 27, p = 0.051).
Pathology outcomes
Surgical pathology demonstrated the posterior margins to be involved by carcinoma in 8/43 (18.6%) patients, close (i.e. within 1 mm of carcinoma) in 9/43 (20.9%) and clear in 26/43 (60.5%) patients. The pectoralis major muscle was invaded by carcinoma in 4/43 (9.3%) patients (Figures 2 and 3) and uninvolved in 34/43 (79.1%) patients. In 5/43 (11.6%) patients, surgical pathology reported close posterior margins and did not specify whether any skeletal muscle was included in the specimen, and therefore these cases were excluded from subsequent analysis regarding pectoralis muscle invasion outcomes (Table 1).
Figure 2.
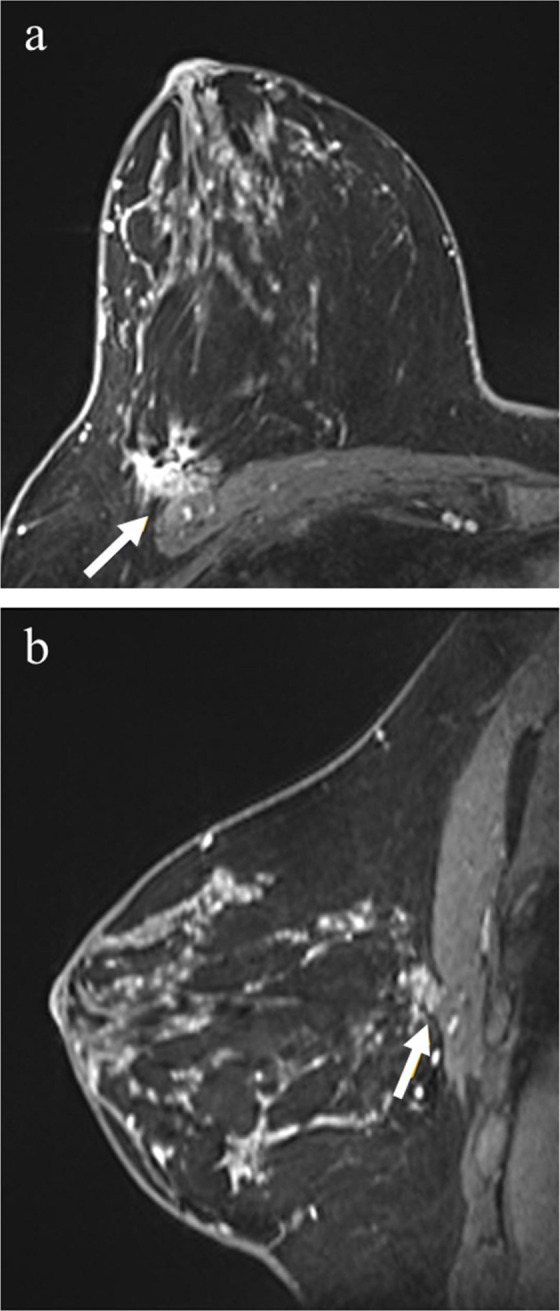
40-year-old female with right breast cancer. Post-contrast fat-saturated axial images (a) and sagittal images (b) demonstrate a spiculated mass in the outer right breast, abutting the pectoralis major muscle (arrows) but with no enhancement of the muscle itself. Surgical pathology yielded tumor invasion of the pectoralis major muscle. During 29 months of clinical follow-up, the patient remained cancer free.
Figure 3.
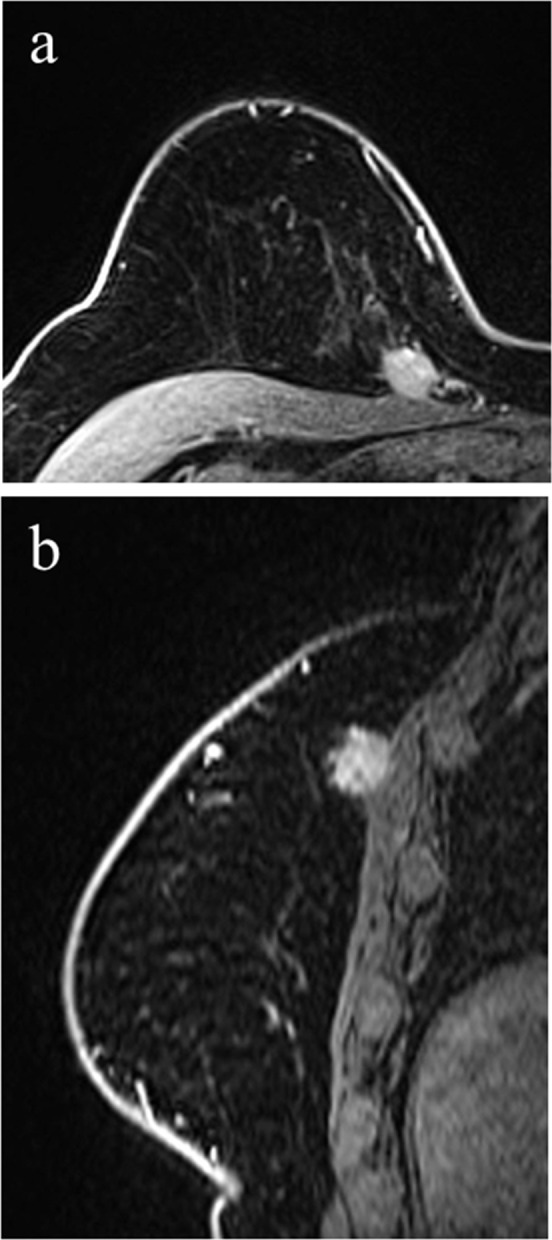
75-year-female with right breast cancer. Post-contrast fat-saturated axial images (a) and sagittal images (b) demonstrate, in the upper inner quadrant of the right breast, a mass with irregular margins abutting the pectoralis major muscle. There is mild mass effect and tenting of the muscle but without enhancement of the muscle itself. Surgical pathology demonstrated tumor invasion of the pectoralis major muscle. During 12 months of clinical follow-up, the patient remained cancer free.
There was no association with MRI finding type and the presence of pectoralis muscle invasion at pathology, as 1/11 (9.2%) of nonmass enhancement abutting the muscle proved to have invasion at pathology compared to 3/20 (15.0%) of masses (p = 0.802, Table 2). None of the nine patients with a spicule abutting with the pectoralis muscle by MRI demonstrated muscle invasion at surgical pathology.
Table 2.
Association of tumor features and tumor invasion of pectoralis major muscle at surgical pathology
| Tumor feature | n (%) with invasion of the pectoralis major muscle at surgical pathology | p-value |
|---|---|---|
| MRI finding type abutting the muscle | ||
| Non-mass enhancement | 1/11 (9.1%) | p = 0.802 |
| Mass | 3/20 (15.0%) | |
| Spicule of a mass | 0/7 (0%) | |
| Deformed contour of pectoralis muscle | ||
| Tenting of the muscle | 1/8 (12.5%) | p = 0.099 |
| Mass effect on the muscle | 1/2 (50.0%) | |
| Both tenting and mass effect | 1/4 (25%) | |
| No deformation of the muscle contour | 1/24 (4.2%) | |
| Tumor type | ||
| IDC | 4/38 | n/a |
| ILC | 0/38 (0%) | |
| IMC | [td] | |
| Microinvasive IDC | 0/38 (0%) | |
| DCIS | 0/38 (0%) | |
DCIS, Ductalcarcinoma in situ; IDC, Invasive ductal carcinoma; ILC, invasive lobular carcinoma; IMC, Invasive mammary carcinoma with ductal and lobular features.
There was a trend for tumors with a deformed pectoralis muscle contour to be associated with muscle invasion but this did not reach statistical significance. Three of the 14 tumors (21.4%) with pectoralis muscle tenting, mass effect, or both demonstrated muscle invasion at pathology, compared to 1/24 (4.2%) of tumors without deformation of the muscle contour (p = 0.099, Table 2). The majority of tumors with pectoralis muscle tenting, mass effect or both demonstrated no muscle invasion at surgery (11/14, 78.6%). There was no significant association between a deformed pectoralis muscle contour and involvement of the posterior margin at surgical pathology (odds ratio 0.36, 95% confidence interval 0.09–1.43).
Clinical outcomes
The median clinical follow-up time for the study population was 29.0 months (interquartile range 12–65 months, range 1–125 months). Most patients (36/43, 83.7%) had no evidence of disease at the most recent follow-up, with 3/43 (7.0%) of patients alive with disease and 3/43 (7.0%) of patients deceased due to metastatic breast cancer. Of the eight patients with positive posterior margins, one patient was found to have metastasis immediately after surgery and two patients developed recurrence or metastasis during follow-up. In comparison, excluding one patient who had no follow-up after surgery, 0/9 of patients with close margins and 3/25 (12%) of patients with clear margins developed recurrence or metastasis during the follow-up period. Although there was a trend for patients with positive posterior margins to have recurrence or develop metastasis during follow-up, this did not reach statistical significance (odds ratio for patients with positive margins compared to those with close or clear margins was 4.13, confidence interval 0.55–31.26, p = 0.17).
All four patients with muscle invasion at pathology had a tumor type of Grade 3 invasive ductal carcinoma. Of the four patients with pectoralis muscle invasion, none developed recurrence or metastasis during follow-up (median follow-up time for these four patients was 18.5 months, interquartile range 13.5–26.5, range 12–29 months). Among the eight patients with positive posterior margins, five patients had invasive ductal carcinoma (IDC), one patient had 1 mm of micro-invasive ductal carcinoma within 2.0 cm of ductal carcinoma in situ (DCIS), one patient had invasive lobular carcinoma (ILC) and one patient had invasive mammary carcinoma with both ductal and lobular features.
Among the four patients with pectoralis invasion, radiation therapy was not performed in two patients in whom final surgical margins were clear and was recommended for the remaining two patients. Among patients who had positive margins and underwent mastectomy, two patients received post-mastectomy radiation therapy and two did not (including one patient who was found to have metastatic disease soon after surgery).
Discussion
This study aimed to describe outcomes for 43 breast cancer patients with tumors abutting the pectoralis muscle on breast MRI without enhancement of the muscle itself. Despite a lack of enhancement of the pectoralis muscle on breast MRI, surgical pathology demonstrated muscle invasion in 4/43 (9.3%) patients in this study. This is in contrast to prior reports of outcomes for this imaging finding, with 0/5 patients demonstrating muscle invasion in one study and 0/26 patients in another.8,9 While this difference could be related to smaller sample sizes in the prior studies, there are additional differences as well. The study by Morris et al8 described early experience for posterior breast cancers on MRI, published in 2000, and there are expected differences in standard breast MRI scanning protocol over the last 20 years. The more recent study by Kazama et al included an undisclosed number of breast MRIs that were performed after neoadjuvant chemotherapy.9 It has been established that the accuracy of breast MRI exams performed after neoadjuvant chemotherapy may be impacted by the specific chemotherapy agent used as well as the breast cancer subtype.14,16,17 This study therefore excluded breast MRIs exams performed after neoadjuvant chemotherapy, and this difference in patient population could contribute to the difference in results. While the limited data available to date suggest that obliteration of the fat plane between a breast cancer and the pectoralis muscle is only indicative of pectoralis muscle invasion if muscle enhancement is present, this study suggests that muscle invasion is still a possibility, as it was present in 9.3% of patients in this study.
None of the recorded MRI imaging features of the breast cancer tumor itself were associated with pectoralis muscle invasion. Tumors associated with muscle invasion were more commonly masses on MRI rather than nonmass enhancement, however, masses were also the most common imaging appearance for breast cancers in this study and larger studies would be needed to further investigate a possible association. It is notable, however, that none of the cases in which the aspect of the cancer abutting the pectoralis muscle was a spicule of a mass were associated with muscle invasion, suggesting that this imaging finding could be assessed a lower degree of suspicion for invasion compared to a mass or non-mass enhancement abutting the muscle. Additionally, while patients with pectoralis muscle invasion had a larger median size of abnormal MRI enhancement compared to those without muscle invasion (4.1 vs 2.4 cm), this did not reach statistical significance. Similarly, there was a trend for tumors with a larger extent abutting the pectoralis to be associated with pathologic invasion, although this also did not reach statistical significance (p = 0.051). This could be due to a true lack of association, although these results suggest that attention to overall tumor size as well as the extent of tumor abutting the pectoralis muscle will also be of interest in future larger studies. While there was a trend for tumors with associated pectoralis muscle deformation to be associated with muscle invasion, this did not reach statistical significance (p = 0.099). Of the 24 tumors with no deformation of the pectoralis muscle, only 1 (4.2%) showed muscle invasion at surgery, compared to 3/14 (21.4%) tumors with deformation of the pectoralis muscle, suggesting the possibility that a lack of muscle deformation could decrease suspicion for muscle invasion. To our knowledge, the investigation of this possible association has not been reported to date, but may be of interest in future studies.
It is well established that breast cancer involvement of the surgical margins has prognostic implications.2–7 In this study, although there was a trend for patients with positive posterior margins to have recurrence or develop metastasis during follow-up, this did not reach statistical significance, which may be due to the small sample size and a limited mean follow-up time of 37.9 months. Although none of the four patients with muscle invasion developed recurrence or metastasis during follow-up, larger studies with increased follow-up time would be needed to evaluate this outcome.
This study has several limitations. Although this is the largest study to date of posterior breast cancers abutting the pectoralis muscle on breast MRI, the sample size of 43 patients is still relatively small. Additionally, variation in MRI scanning technique and protocols over the 11 year study period limits the consistency of image acquisition.
Conclusions
Accurately defining the posterior extent of breast cancers pre-operatively has important clinical implications. While there is evidence to support that pectoralis muscle enhancement on MRI is associated with muscle invasion, there are extremely limited data regarding breast cancers that abut the pectoralis major muscle on MRI without enhancement of the muscle itself. In this study of 43 females with breast cancers abutting the pectoralis muscle, 4/43 (9.3%) showed pectoralis muscle invasion at surgical pathology, demonstrating that a lack of enhancement of the pectoralis muscle does not exclude the possibility of muscle invasion. While no specific clinical or imaging feature was associated with muscle invasion in this study, our data suggest that tumors causing a deformation of the pectoralis muscle, overall tumor size, extent of the tumor abutting the pectoralis muscle and the specific imaging feature abutting the muscle on MRI may warrant further investigation in future studies. While this study of 43 patients is the largest study to date evaluating outcomes for breast tumors abutting the pectoralis muscle without muscle enhancement on breast MRI, additional larger studies are needed to further define the clinical implications for this MRI finding in order to optimize surgical and oncologic patient care.
Contributor Information
Kelly S Myers, Email: kmyers25@jhmi.edu.
Erica Stern, Email: estern14@jhmi.edu.
Emily B Ambinder, Email: emcinto8@jhmi.edu.
Eniola T Oluyemi, Email: eobadin1@jhmi.edu.
REFERENCES
- 1.American Joint Committee on Cancer . Breast. : Badve S. S, Beitsch P. D, Bose S, AJCC cancer staging manual. 8th ed. Chicago, IL: Springer; 2017. . 589–636. [Google Scholar]
- 2.Lagios MD. Pathologic features related to local recurrence following lumpectomy and irradiation. Semin Surg Oncol 1992; 8: 122–8. [PubMed] [Google Scholar]
- 3.Harris JR, Lippman ME, Veronesi U, Willett W. Breast cancer. N Engl J Med 1992; 327: 390–8. doi: 10.1056/NEJM199208063270606 [DOI] [PubMed] [Google Scholar]
- 4.Lee SC, Jain PA, Jethwa SC, Tripathy D, Yamashita MW. Radiologist's role in breast cancer staging: providing key information for clinicians. Radiographics 2014; 34: 330–42. doi: 10.1148/rg.342135071 [DOI] [PubMed] [Google Scholar]
- 5.Park CC, Mitsumori M, Nixon A, Recht A, Connolly J, Gelman R, et al. Outcome at 8 years after breast-conserving surgery and radiation therapy for invasive breast cancer: influence of margin status and systemic therapy on local recurrence. J Clin Oncol 2000; 18: 1668–75. doi: 10.1200/JCO.2000.18.8.1668 [DOI] [PubMed] [Google Scholar]
- 6.Obedian E, Haffty BG. Negative margin status improves local control in conservatively managed breast cancer patients. Cancer J Sci Am 2000; 6: 28–33. [PubMed] [Google Scholar]
- 7.DiBiase SJ, Komarnicky LT, Schwartz GF, Xie Y, Mansfield CM. The number of positive margins influences the outcome of women treated with breast preservation for early stage breast carcinoma. Cancer 1998; 82: 2212–20. doi: [DOI] [PubMed] [Google Scholar]
- 8.Morris EA, Schwartz LH, Drotman MB, Kim SJ, Tan LK, Liberman L, et al. Evaluation of pectoralis major muscle in patients with posterior breast tumors on breast Mr images: early experience. Radiology 2000; 214: 67–72. doi: 10.1148/radiology.214.1.r00ja1667 [DOI] [PubMed] [Google Scholar]
- 9.Kazama T, Nakamura S, Doi O, Hirose M, Suzuki K, Ito H, Masanori H, Hisao I. Prospective evaluation of pectoralis muscle invasion of breast cancer by MR imaging. Breast Cancer 2005; 12: 312–6. doi: 10.2325/jbcs.12.312 [DOI] [PubMed] [Google Scholar]
- 10.Samreen N, Lee C, Bhatt A, Carter J, Hieken T, Adler K, et al. A clinical approach to diffusion-weighted magnetic resonance imaging in evaluating chest wall invasion of breast tumors. J Clin Imaging Sci 2019; 9: 11–17. doi: 10.25259/JCIS_97_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mango VL, Kaplan J, Sung JS, Moskowitz CS, Dershaw DD, Morris EA. Breast carcinoma in augmented breasts: MRI findings. American Journal of Roentgenology 2015; 204: W599–604. doi: 10.2214/AJR.14.13221 [DOI] [PubMed] [Google Scholar]
- 12.Orel SG, Schnall MD, LiVolsi VA, Troupin RH. Suspicious breast lesions: MR imaging with radiologic-pathologic correlation. Radiology 1994; 190: 485–93. doi: 10.1148/radiology.190.2.8284404 [DOI] [PubMed] [Google Scholar]
- 13.Kerslake RW, Carleton PJ, Fox JN, Imrie MJ, Cook AM, Read JR, et al. Dynamic gradient-echo and fat-suppressed spin-echo contrast-enhanced MRI of the breast. Clin Radiol 1995; 50: 440–54. doi: 10.1016/S0009-9260(05)83159-9 [DOI] [PubMed] [Google Scholar]
- 14.Pasquero G, Surace A, Ponti A, BORTOLINI M, TOTA D, MANO MP, et al. Role of magnetic resonance imaging in the evaluation of breast cancer response to neoadjuvant chemotherapy. In Vivo 2020; 34: 909–15. doi: 10.21873/invivo.11857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris EA, Comstock CE, Lee CH. ACR BI-RADS magnetic resonance imaging. In: ACR BI-RADS atlas, breast imaging reporting and data system. Reston, VA: American College of Radiology; 2013. [Google Scholar]
- 16.Lobbes MBI, Prevos R, Smidt M, Tjan-Heijnen VCG, van Goethem M, Schipper R, et al. The role of magnetic resonance imaging in assessing residual disease and pathologic complete response in breast cancer patients receiving neoadjuvant chemotherapy: a systematic review. Insights Imaging 2013; 4: 163–75. doi: 10.1007/s13244-013-0219-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bouzón A, Acea B, Soler R, Iglesias Ángela, Santiago P, Mosquera J, et al. Diagnostic accuracy of MRI to evaluate tumour response and residual tumour size after neoadjuvant chemotherapy in breast cancer patients. Radiol Oncol 2016; 50: 73–9. doi: 10.1515/raon-2016-0007 [DOI] [PMC free article] [PubMed] [Google Scholar]