Abstract
Objective:
To study the efficacy and safety of repeat transarterial radioembolization (TARE) to similar hepatic arterial territories.
Methods:
Between 3/2011 and 4/2019, 26 patients (25 males and 1 Female, Mean Age: 65 yo, SD: 11.7 yo, Range: 18–83.0 yo) received TARE with Y90 glass microspheres to treat recurrent or residual primary disease in similar hepatic arterial lobe or segments. Tumor response was evaluated by imaging using the modified-RECIST criteria. Incidence of RILD and adverse events were categorized by a standardized scale using the Common Terminology Criteria for Adverse Events (CTCAE) v.4.0.
Results:
Mean cumulative activity after the first treatment was 2.50 GBq (SD:1.04 GBq, Range:0.61–4.93 GBq) and second treatment was 2.27 GBq (SD:1.01 GBq, Range:0.92–5.46 GBq). Mean interval time between initial and repeat treatments was 9.6 months (Range: 1–53 months). Tumor responses were complete, partial, or progression in 73% (n = 19/26), 23% (n = 6/26), and 4% (n = 1/26) in repeat treatment patients, respectively. The incidence of RILD was 0%. Toxicity after first and second treatment was seen in 19% (n = 5/26) & 23% (n = 6/26) patients, respectively, and were all of CTCAE Grade 2. No significant predictors of treatment toxicity for repeat treatment were identified except increased MELD score (p = 0.04). Kaplan-Meier survival analysis in patients with repeat treatment showed a median survival of 15.0 months (95% CI 8.8–21.1 months) and 19.0 months (95% CI 8.1–29.9 months) in patients who only received one treatment with a p value of 0.485.
Conclusion:
Repeat TARE with glass microspheres was an effective and safe treatment strategy for disease management in patients with residual or recurrent disease to the similar hepatic arterial territories without any major treatment related toxicity.
Advances in knowledge:
Although safety and efficacy of repeat radioembolism has been studied, no study has focused on repeat treatment to similar hepatic arterial territories. The current study shows that repeat treatment to the same hepatic arterial territory is as safe as single treatment to the same territory.
Introduction
Yittrium-90 (Y90) radioembolization has been proven to be an effective and safe treatment option for hepatocellular carcinoma (HCC) by inducing tumor necrosis and prolonging time-to-progression.1,2 While radioembolization is generally safe and well tolerated, there is a potential concern for treatment-induced hepatotoxicity, especially radiation-induced liver disease (RILD).3 While most radiation-induced liver effects are self-limited, a small minority of patients will progress onto RILD, which is characterized by ascites, jaundice, and decreased liver function.4
Repeat radioembolization may be indicated in recurrent patients with HCC but can also carry increased risk for RILD due to increased cumulative radiation exposure.5 Current safety data on repeated use of Y90 treatment for recurrent HCC, especially in the same hepatic region, are conflicted. Some studies have reported no incidence of RILD following repeat treatments,6,7 whereas another study found elevated risk of RILD in repeated Y90 radioembolization.5 Thus, the purpose of this study was to investigate the efficacy and safety of repeated Y90 radioembolization to similar hepatic territories with respect to toxicity and treatment response on imaging.
Methods and materials
Patient selection
Institutional review board approval was attained for this single institution retrospective study. Between March 2011 and April 2019, a total of 26 patients (25 males and one female, mean age 65, SD 11.7, range 18–82) underwent a second radioembolization treatment to the same hepatic arterial territory for progressive and recurrent or residual tumor at an urban tertiary-care academic center. All patients were reviewed at a weekly liver tumor board with participation from hepatology, medical oncology, diagnostic/interventional radiology, and transplant surgery. Patients were scheduled for repeat radioembolization based on multi-disciplinary consensus with a prior objective response and/or lack of suitable alternative treatments. Since 60% of initially treated patients had an objective response (Complete or Partial as described in the Response section), repeat treatment for recurrent disease was deemed appropriate for residual and recurrent disease. For progressive disease, repeat treatment was deemed appropriate with a higher Y90 dosage as there was no suitable alternative form of locoregional treatments or surgical resection. The cohorts’ baseline demographics and characteristics are summarized in Table 1. Patients that received initial whole liver radioembolization, repeat whole liver radioembolization, or repeat treatment to an entirely different arterial territory were excluded from this study. Inclusion and exclusion criteria are visualized in Figure 1. Patient data acquisition was in agreement with the Health Insurance Portability and Accountability Act. Retrospective oncologic, clinical, and radiological data were recorded.
Table 1.
Summary of baseline characteristics of patients receiving repeat radioembolization.
| Characteristic | Repeat TreatmentNN = 26 | Single treatment onlyNN = 158 |
|---|---|---|
| Sex, M/F | 25 (96%)/1 (4%) | 142 (90%)/ 16 (10%) |
| Age | 65 yo (18–83.0) | 65 yo (14–88.0) |
| BCLC | ||
| A | 59.3% (n = 15/26) | 68.3% (n = 108/158) |
| B | 25.9% (n = 7/26) | 26.1% (n = 41/158) |
| C | 14.8% (n = 4/26) | 5.5% (n = 9/158) |
| CPS | ||
| A | 85.2% (n = 22/26) | 75.5% (n = 119/158) |
| B | 14.8% (4/26) | 24.5% (39/158) |
| MELD | 8.5 (SD: 2.36, Range: 6 to 14) | 9.8 (SD: 3.77, Range: 6 to 26) |
| Primary tumor Hepatocellular carcinoma Cholangiocarcinoma | 96.2% (n = 25/26) 3.8% (n = 1/26) | 94.3% (n = 149/158) 5.7% (n = 8/158) |
| Extra Hepatic Disease | 19.2% (n = 5/26) | 13.9% (n = 22/158) |
| Splenic Vein Thrombosis | ||
| Average time interval between first and second radioembolization | 9.6 months (1.0–52.0) | NA |
Figure 1.
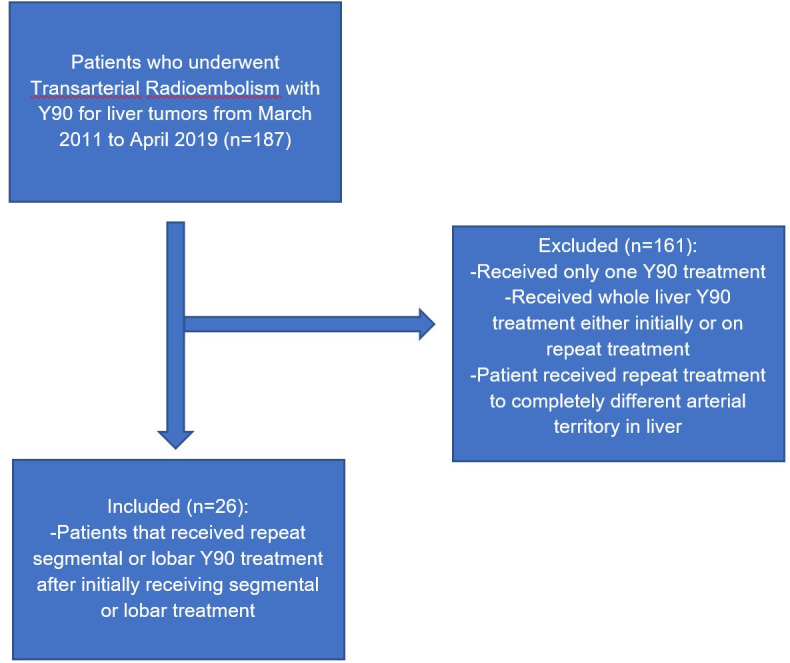
Inclusion and exclusion criteria for study. Patients were excluded if they initially received whole liver treatment or if they received whole liver treatment during subsequent treatments.
Procedural technique
Therasphere® (Boston Scientific; Marlborough, MA) glass microspheres synthesized with radioactive Y-90 particles was the embolic agent of choice for all initial and repeat radioembolization procedures. Dose calculations for both treatments were made with the help of Technetium-99 macroaggregated albumin (MAA) mapping procedures to define tumor vascular distribution, isolate and embolize potential non-target enterohepatic vessels, and compute hepatopulmonary shunt fractions. All of the treatments were either lobar (n = 14/26) or segmental (n = 12/26) and no whole liver treatment was performed. Y90 administration was performed by four interventional radiologists with five or more years of experience with transarterial radioembolization (TARE). Dosimetry using the medical internal radiation dose (MIRD) model for lobar treatments was calculated at 120 Gy and 120 Gy for segmental treatments. All doses were delivered the subsequent Tuesday through Friday from the date of calibration. As per protocol, all treatments were calculated to deliver less than 30 Gy to the lungs per single treatment and less than 50 Gy cumulatively. Treatment volumes were calculated using prior cross-sectional imaging or cone beam CT obtained at mapping using proprietary software Visage 7.1 (Pro Medicus Limited; Richmond, Australia).
Repeat radioembolization was classified as Y-90 treatment to the same target territory as identified on imaging and point of radioembolic administration on angiography. The majority of patients were treated for hepatocellular carcinoma (96%, n = 25/26) while the remaining (4%, n = 1/26) were treated for cholangiocarcinoma. HCC was diagnosed following the American College of Radiology guidelines using the Liver Imaging Reporting and Data System (LI-RADS) with multiphase CT and MRI imaging evaluation. Lesions that were not diagnosed as HCC were usually investigated further with biopsy. The average time between treatments was 9.6 months (range 1–52 months).
Response
Each patient had outpatient follow-up appointments for bloodwork and clinical evaluation at 1, 4, and 7 months. In addition, imaging was obtained on each patient at 3 months and 6 months post-treatment. Tumor response was rated as either progression, partial, or complete based on contrast enhancement in follow-up imaging based on the American Association for the Study of Liver Diseases and Journal of the National Cancer Institute (AASLD-JNCI) guidelines for grading liver tumor response using the Modified Response Evaluation Criteria in Solid Tumors (mRECIST) evaluation tool.8 Both MRI and CT modalities were used for post-treatment response evaluation. Prior to,2 at this tertiary center, post-treatment imaging studies were performed at 1 month post-treatment but this was discontinued at the discretion of abdominal imagers since post-treatment changes prohibited declaration of true response.9
Toxicity analysis
Post-treatment toxicity was graded by the Common Terminology Criteria for Adverse Events (CTCAE) v.4.03 for 6 months after each treatment.10 The incidence of RILD was evaluated within 6 months of treatment. RILD was diagnosed in patients by evaluating clinical documentation for the following adverse outcomes as described by Lawerence et al11: anicteric ascites and increasing alkaline phosphatase out of proportion to other liver enzymes.
Statistical analysis
The purpose of statistical analysis in this study was to analyze the efficacy and safety of patients receiving repeat treatment to similar hepatic arterial territories. Safety was evaluated by comparing the incidence of specific toxicities such as abdominal pain after each procedure. A student t-test was used to determine if there was significantly more toxicity associated with repeat treatments compared to initial. The proportion of treated liver to total liver volume was also calculated to quantify relatively how much liver was treated with each therapy. Efficacy was reported by evaluating therapy response on imaging. The proportion of complete, partial, and tumor progression was reported for each treatment. Kaplan-Meier survival analysis was performed to measure evaluate and visualize the mean survival time. Patients who received only one radioembolization treatment were analyzed only for cumulative survival.
Statistical analysis was also performed to investigate potential prognostic factors of toxicity in patients receiving repeat radioembolization treatment. Multiple factors were considered including age, gender, tumor distribution, lung shunt fraction, tumor volume, MELD score, and albumin-bilirubin grade (ALBI). ALBI is a well-established prognostic factor for survival in patients receiving radioembolization. ALBI grade is computed using the following formula: ALBI score = (log10 bilirubin [µmol/L] × 0.66) + (albumin [g/L] × −0.0852).12 Fisher exact test was used for nominal characteristics such as gender and tumor distribution and Kruskal-Wallis test was used for the remaining continuous characteristics. RStudio v.3.6.1 (RStudio Inc., Vienna, Austria) was used for univariate statistical analysis.
Results
Mean cumulative activity after the first treatment were 2.50 GBq (SD: 1.02 GBq, Range: 0.61–4.93 GBq) and second treatment were 2.27 GBq (SD: 1.02 GBq, Range: 0.92–5.46 GBq). The mean hepatopulmonary shunt fraction after initial treatment was 3.56% (SD: 2.70%, Range: 0.90 to 13.20%) and after repeat treatment was 6.22% (SD: 6.40%, Range: 0.00 to 27.10%). The average diameter of largest lesion was 5.64 cm (SD: 2.99 cm, Range: 1.3 to 12.5 cm) and average tumor treatment volume was 1029.0 cm3 (SD: 452.0 cm3, Range: 563 to 2158 cm3) for initial treatment and average diameter of the largest lesion was 5.24 cm (SD: 3.15 cm, Range: 1.6 to 14.3 cm) and average tumor treatment volume was 849.60 cm3 (SD: 408.60 cm3, Range: 295 to 1948 cm3) for repeat treatment. The mean proportion of treated liver to non-treated liver was 0.65 (SD: 0.17, Range: 0.33 to 0.89) for the initial treatment and 0.58 (SD: 0.22, Range: 0.21 to 0.88) for repeat treatment. These findings are summarized in Table 2.
Table 2.
Treatment characteristics for initial and repeat treatments
| Y90 Treatment Characteristics | |
|---|---|
| Mean Treatment Volume for first Treatment | 1029.0 cm3 |
| Proportion of Treated to total liver | 0.65 |
| Mean Treatment Volume for second Treatment | 849.6 cm3 |
| Proportion of Treated to total liver | 0.58 |
| Calculated Activity at first Treatment | 2.50 GBq |
| Calculated Activity at second Treatment | 2.27 GBq |
| Mean Lung Shunt Fraction after first Treatment | 3.56% |
| Mean Lung Shunt Fraction after second Treatment | 6.22% |
After initial treatment, 40.0% of patients had complete response, 20.0% had partial response, and 20.0% had tumor progression. Treatment administration was segmental in 46% (n = 12/26) of patients and in this group, 66% (n = 8/12) had complete response, 25% (n = 3/12) had partial, and 8% (n = 1/12) had tumor progression. The other 54% (n = 14/26) of patients received lobar treatment with 79% (n = 11/14) exhibiting complete response and 21% (n = 3/14) exhibiting partial response. The occurrence of RILD was 0% (n = 0/26). Toxicity after first and second treatment was seen in 19% (5/26) & 23% (n = 6/26) patients, respectively, and were all of CTCAE Grade 2. Toxicities included nausea, pain, and fever. There was no statistically significant difference between the initial and repeat treatment toxicities. These findings along are summarized in Table 3. Kaplan-Meier survival analysis in initial treatment patients demonstrated a median survival of 19.0 months [95% CI (8.1–29.9)] and 15.0 months [95% CI (8.8–21.1)] in patients who only received repeat treatment with a p value of 0.485. The Kaplan-Meier survival plot is seen in Figure 2.
Table 3.
Each toxicity reported for initial and repeat treatments. T-test used to evaluate for statistical significance.
| CTCAE | RE treatment # | All grades (n) | Grade 3 (n) | P value (for all grades) |
|---|---|---|---|---|
| Fever | First treatment Second treatment | 2 1 | 0 0 | p >> 0.05 |
| Fatigue | First treatment Second treatment | 0 1 | 0 0 | p >> 0.05 |
| Nausea and/or vomiting | First treatment Second treatment | 0 3 | 0 0 | p >> 0.05 |
| Abdominal pain | First treatment Second treatment | 3 4 | 0 0 | p >> 0.05 |
Figure 2.
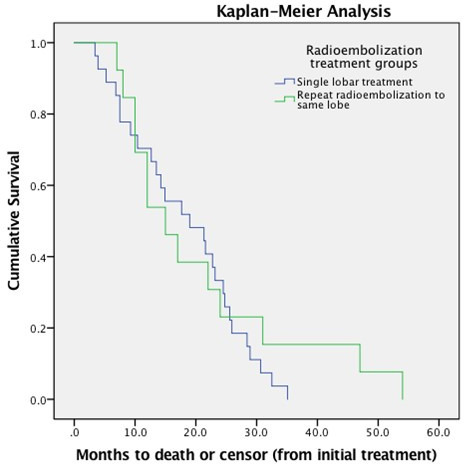
Kaplan-Meier survival plot for single and repeat radioembolization treatment. Patients who received only one radioembolization treatment were analyzed only for cumulative survival.
Bivariate analysis was performed to determine the association of prognostic factors with toxicity. Of the factors tested, only MELD score analysis yielded a statistically significant p value of 0.040. Total activity administered (p = 0.70) and treatment volume (p = 0.64) did not show any statistically significant association. All of the factors tested are listed in Table 4.
Table 4.
Prognostic factors evaluated to determine and association with increased toxicity. Fisher exact test was used for nominal characteristics such as gender and tumor distribution and Kruskal Wallis test was used for the remaining continuous characteristics.
| P-Values for Prognostic Factors | |
|---|---|
| Variable | P-Value |
| Gender | 0.95 |
| Age | 0.24 |
| MELD | 0.04 |
| Total Dose | 0.7 |
| Shunt | 0.82 |
| Treatment Distribution | 0.67 |
| Treatment Volume | 0.64 |
| ALBI | 0.31 |
| Total Liver Volume | 0.57 |
Discussion
Current dosing for radioembolization uses the information from the Tc-99 MAA mapping to predict the distribution of Y90. However, Tc-99 MAA and Y90 embolic particles have been reported to differ in distribution properties by over 10%.13,14 Microspheres in general have a heterogeneous distribution pattern, which leads to uncertainty behind dosage calculations and can potentially lead to larger amounts of radiation reaching non-tumorous tissue. RILD is a well-known complication, albeit rare with a 4% incidence, and increases with age, poor liver baseline function, and the volume of liver treated. Other serious complications include radiation pneumonitis for patients that have significant hepatopulmonary shunts and GI ulceration secondary to hepaticoenteric arterial communications.15 As such, with uncertainty regarding target and non-target radioactivity, these toxicities are a deterrent for repeat radioembolization treatments.
Lam et al evaluated the safety of repeated Y90 treatment with resin spheres and found that there was an increased incidence of RILD for the repeat group. The authors advised caution with repeat treatment due to increased incidence of RILD but also from the uncertainty of dosing.5 It is worthwhile to note however that whole liver treatment was performed in five out of the eight repeat treatments.5 In addition, Currie et al looked at long-term safety in patients receiving radioembolization treatments. They found that there was an increased incidence (13%) of radiation-induced chronic hepatotoxicity 6 or more months after treatment. There were a total of 69 toxicity events in the 13/98 patients that developed radiation induced chronic hepatotoxicity. They reported that tumor involvement of more than 50% of liver and previous cirrhosis were predisposing factors to developing radiation-induced chronic hepatotoxicity.16 While this study investigated long-term outcomes of single radioembolization, it did not quantify the amount of liver treated as well as assess the safety of re-treatment.
The present study demonstrated acceptable efficacy and safety for repeat radioembolization treatment to the similar hepatic arterial territories. Both repeat lobar as well as segmental treatments were evaluated for post procedure toxicity as well as therapeutic response. The study did not identify any major toxicity from repeat treatments as all toxicities were graded two or less. Additionally, no instances of RILD were observed, likely reflecting the safety of repeat treatments when less than whole liver treatment is pursued. This is further explained by looking at the proportion of treated liver to whole liver. The study found that after repeat treatment the proportion of treated to whole liver was 0.58 and after initial treatment was 0.65, likely indicative of contra lateral hypertrophy of the untreated liver following initial radioembolization. By treating a smaller proportion of liver, there was likely a larger reserve volume that could have compensated for liver function. The study also investigated for potential prognostic factors and found only an increased MELD score as a significant risk factor for post-treatment toxicity. This finding is likely explained by the fact that MELD score is a predictor of liver disease severity and therefore those with higher MELD scores are susceptible to adverse outcomes from limited hepatic reserve. Of note, total activity and treatment volume were not statistically significant predictors of adverse events.
In addition to toxicity, the efficacy of repeat treatment was also evaluated. In the total repeat treatment cohort (segmental and lobar), 73% (n = 19/26) had a complete response on imaging while 23% (n = 6/26) had partial response and 4% (n = 1/26) had tumor progression. This favorable response profile was observed without a significant change in toxicity in repeat treatment in repeat lobar and segmental treatment.
This study is limited by many factors, including those inherent to a study with a retrospective design and limited sample size. A larger sample size would be paramount in developing a stronger statistical analysis regarding prognostic factors. Furthermore, the chronic long-term effects of repeat treatment were not investigated (6 months past treatment). Patient data past 6 months was not reported given lack of ideal follow-up to evaluate long-term toxicity profile. Additionally, this study is limited to radioembolization using glass microspheres. These findings may not be applicable to those using resin microspheres with respect toward repeat safety and toxicity.
In conclusion, repeat radioemoblization using glass microspheres to the same hepatic arterial territory can be performed with an acceptable safety profile and objective tumor response in patients receiving either repeat lobar or segmental treatment as long as whole liver treatment is avoided. There are predictable prognostic factors such as an elevated MELD score that may caution physicians when deciding to administer repeat treatment. Variation in MELD score which measures the change in MELD score pre- and post-procedure can also be considered in future studies to evaluate deterioration of liver function with TARE.17 Larger samples are needed to better elucidate other prognostic factors that can be used to predict repeat treatment toxicity.
Footnotes
Conflict of interest: OA reports consulting role with Cook Medical®, Argon Medical®, Cardiva Medical®.
Contributor Information
Wali Badar, Email: wali.badar@my.rfums.org.
Thuong Van Ha, Email: tvh@uchicago.med.edu.
Steven Zangan, Email: szangan@uchicago.med.edu.
Rakesh Navuluri, Email: rnavuluri@uchicago.med.edu.
Anjana Pillai, Email: apillai@uchicago.med.edu.
Talia Baker, Email: tbaker@uchicago.med.edu.
Leonard Dalag, Email: ldalag@uchicago.med.edu.
Ross Han, Email: rhan@uchicago.med.edu.
Osman Ahmed, Email: oahmed@uchicago.med.edu.
REFERENCES
- 1.Saini A, Wallace A, Alzubaidi S, Knuttinen MG, Naidu S, Sheth R, et al. History and evolution of yttrium-90 radioembolization for hepatocellular carcinoma. J Clin Med 2019; 8: 55: E5507 01 2019. doi: 10.3390/jcm8010055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salem R, Gordon AC, Mouli S, Hickey R, Kallini J, Gabr A, et al. Y90 radioembolization significantly prolongs time to progression compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology 2016; 151: 1155–63. doi: 10.1053/j.gastro.2016.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riaz A, Lewandowski RJ, Kulik LM, Mulcahy MF, Sato KT, Ryu RK, et al. Complications following radioembolization with yttrium-90 microspheres: a comprehensive literature review. J Vasc Interv Radiol 2009; 20: 1121–30. doi: 10.1016/j.jvir.2009.05.030 [DOI] [PubMed] [Google Scholar]
- 4.Braat MNGJA, van Erpecum KJ, Zonnenberg BA, van den Bosch MAJ, Lam MGEH, Braat M, Lam M. Radioembolization-induced liver disease: a systematic review. Eur J Gastroenterol Hepatol 2017; 29: 144. doi: 10.1097/MEG.0000000000000772 [DOI] [PubMed] [Google Scholar]
- 5.Lam MGEH, Louie JD, Iagaru AH, Goris ML, Sze DY. Safety of repeated yttrium-90 radioembolization. Cardiovasc Intervent Radiol 2013; 36: 1320–8. doi: 10.1007/s00270-013-0547-9 [DOI] [PubMed] [Google Scholar]
- 6.Filippi L, Di Costanzo GG, Tortora R, Pelle G, Cianni R, Schillaci O, et al. Repeated treatment with 90Y-microspheres in intrahepatic cholangiocarcinoma relapsed after the first radioembolization. Cancer Biother Radiopharm 2019; 34: 231–7. doi: 10.1089/cbr.2018.2718 [DOI] [PubMed] [Google Scholar]
- 7.Zarva A, Mohnike K, Damm R, Ruf J, Seidensticker R, Ulrich G, et al. Safety of repeated radioembolizations in patients with advanced primary and secondary liver tumors and progressive disease after first selective internal radiotherapy. J Nucl Med 2014; 55: 360–6. doi: 10.2967/jnumed.113.127662 [DOI] [PubMed] [Google Scholar]
- 8.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010; 30: 052–60. doi: 10.1055/s-0030-1247132 [DOI] [PubMed] [Google Scholar]
- 9.Young S, Taylor A, Golzarian J, Flanagan S, D'Souza D, Sanghvi T. Clinical utility of one month imaging following selective internal radiation therapy. Diagn Interv Imaging 2019; 100: 39–46. doi: 10.1016/j.diii.2018.07.005 [DOI] [PubMed] [Google Scholar]
- 10.NIH . Common terminology criteria for adverse events (CTCAE) | Protocol development | CTEP. 2019. Available from: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm.
- 11.Lawrence TS, Robertson JM, Anscher MS, Jirtle RL, Ensminger WD, Fajardo LF. Hepatic toxicity resulting from cancer treatment. Int J Radiat Oncol Biol Phys 1995; 31: 1237–48. doi: 10.1016/0360-3016(94)00418-K [DOI] [PubMed] [Google Scholar]
- 12.Gui B, Weiner AA, Nosher J, Lu S-E, Foltz GM, Hasan O, et al. Assessment of the albumin-bilirubin (ALBI) grade as a prognostic indicator for hepatocellular carcinoma patients treated with radioembolization. Am J Clin Oncol 2018; 41: 861–6. doi: 10.1097/COC.0000000000000384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tong AKT, Kao YH, Too CW, Chin KFW, Ng DCE, Chow PKH. Yttrium-90 hepatic radioembolization: clinical review and current techniques in interventional radiology and personalized dosimetry. Br J Radiol 2016; 89: 20150943. doi: 10.1259/bjr.20150943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kao YH. Results confounded by a disregard for basic dose-response radiobiology. J Nucl Med 2013; 54: 1682–3. doi: 10.2967/jnumed.113.122846 [DOI] [PubMed] [Google Scholar]
- 15.Riaz A, Awais R, Salem R. Side effects of yttrium-90 radioembolization. Front Oncol 2014; 4: 198. doi: 10.3389/fonc.2014.00198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Currie BM, Hoteit MA, Ben-Josef E, Nadolski GJ, Soulen MC. Radioembolization-Induced chronic hepatotoxicity: a single-center cohort analysis. J Vasc Interv Radiol 2019; 30: 1915–23. doi: 10.1016/j.jvir.2019.06.003 [DOI] [PubMed] [Google Scholar]
- 17.Delicque J, Hermida M, Piron L, Allimant C, Belgour A, Pageaux G-P, et al. Intra arterial treatment of hepatocellular carcinoma: comparison of MELD score variations between radio-embolization and chemo-embolization. Diagn Interv Imaging 2019; 100: 689–97. doi: 10.1016/j.diii.2019.05.006 [DOI] [PubMed] [Google Scholar]


