Abstract
Magnetic resonance neurography allows for the selective visualization of peripheral nerves and is increasingly being investigated. Whereas in the past, the imaging of the extracranial cranial and occipital nerve branches was inadequate, more and more techniques are now available that do allow nerve imaging. This basic review provides an overview of the literature with current state of the art, anatomical landmarks and future perspectives. Furthermore, we illustrate the possibilities of the three-dimensional CRAnial Nerve Imaging (3D CRANI) MR-sequence by means of a few case studies.
Introduction
Magnetic resonance neurography (MRN) refers to dedicated MRI sequences that selectively enhance the visualization of peripheral nerves. Several techniques have been described in the literature including two-dimensional (2D) and three-dimensional (3D) T2 weighted (T2W) fat suppressed and diffusion-weighted imaging.1 The first reports on MRN date from 1992 by Howe and Filler and have much evolved since then.2 At present, MRN is gaining importance due to the introduction of high-field MRI devices and improved imaging techniques.
The skull base course of cranial nerve MRI anatomy has been extensively reviewed.3–6 In this article, we will review the state of the art and relevant MRN anatomy of the extracranial cranial and occipital nerve branches with illustrative pathologic cases. The author’s 3D CRANI MRN sequence will be shared together with its clinical application. This sequence makes useful use of the latest technical developments in MRI research such as compressed sensing and black blood imaging. Assessment methods and benchmark values are cited. Finally, we will discuss some future directions.
State of the art
Although there is well-supported literature on MRN in musculoskeletal imaging, the original research articles are rather limited for the head-neck area. There are several factors why MRN is more difficult to implement in this region. First, the cranial nerves have small calibers and have a complex tortuous course, passing tissues with very different physical properties. The close proximity of fat pads, sinuses and vessels with slow and fast flows require more performant sequences. Ideally, a cranial nerve MRN sequence has a large field of view (FOV) with 3D thin slice thickness, high signal- (SNRs) and contrast-to-noise ratios (CNRs), with uniform fat, venous and arterial suppression and minimal magic angle artifacts. All these requirements should be met within reasonable acquisition times and minimum chance for motion artifacts. Also, when considering nerve-related pathology we can expect surgical and pathology-induced susceptibility artifacts such as edema, increased vascularity and metal particles, which should be accounted for when possible. Previous reports described cranial nerve anatomy using various MRI sequences such as 3D bFFE (3D balanced fast-field echo sequences), T2W TSE (turbo spin echo), STIR (short tau inversion recovery) and CISS (constructive interference in steady state).3–5,7 Although these sequences nicely demonstrate the anatomy, they are not nerve-specific as surrounding structures are not suppressed. In true MRN sequences we try to obtain a heavily T2W image to achieve high soft tissue contrast with homogenous fat, arterial and venous suppression. Several authors published on available techniques for inferior alveolar, lingual, as well as occipital nerve imaging mainly based on 3D PSIF (reversed fast imaging in steady-state free precession).8,9 PSIF combines a steady state with a water excitation pulse and fat suppression, selectively enhancing neural anatomy with excellent vascular suppression. A disadvantage of PSIF is the lower SNR and risk for susceptibility artifacts compared to STIR sequences (Figure 1). A protocol suggested by Chhabra et al9 is further complemented by STIR, CISS, bFFE and DTI (diffusion tensor imaging). By adding multiple sequences, one reduces the risk of non-diagnostic images but loses time and cost-efficiency, which are becoming increasingly important in a health-care environment under financial pressure and with increasing demand for MRI. The authors apply the 3D CRANI (CRAnial Nerve Imaging) sequence which is based on contrast-enhanced black blood 3D STIR TSE preceded by an MSDE (motion-sensitized driven equilibrium) pulse in combination with a pseudo-steady-state sweep and compressed sensing.10,11 Advantages of 3D CRANI are high SNR and CNR and less susceptibility artifacts. By combining 3D PSIF and 3D CRANI, a cranial MRN examination can be performed in a total acquisition time of 12 min. Table 1 describes in detail the author’s MRN protocol including 3D PSIF and 3D CRANI sequences. Routine T1, T2W and 3D FLAIR (fluid attenuated inversion recovery) brain sequences could be added as well to exclude intracranial pathology. DTI is increasingly being used but, for the time being, mostly remains of scientific value.10,12,13 In order to obtain a diagnostic MRN acquisition, adequate patient positioning and coil selection is necessary.6 Thorough patient fixation in mild hyperextension using a 32 channel head coil plays an important role in optimization of the SNR (Figure 2). Others have advocated the use of a 16 channel head neck spine coil.14 Finally, post-processing using maximum intensity projection (MIP) and multiplanar reformatting (MPR) renders the necessary viewing windows to evaluate the attenuation-enhanced cranial nerves along their trajectory or in non-axial planes according to the radiologist’s discretion (Figure 3).15,16 Routine post-processing software packages allow the necessary reformatting to be carried out such as Philips Volume post-processing package (Philips, Best, Netherlands). Freeware software, e.g. Horos (Nimble Co LLC, Annapolis, USA), offers MPR and MIP tools as well.
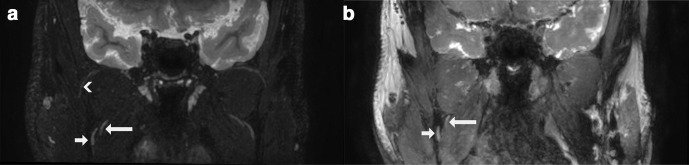
Figure 1.
Coronal thick slab (5 mm) MIP/MPR images in the same subject comparing two magnetic resonance neurography techniques. Short arrow: lingual nerve (V3); long arrow: inferior alveolar nerve (V3); arrowhead: masseteric nerve (V3). (a) 3D CRANI sequence. (b) 3D PSIF sequence. 3D, three-dimensional; CRANI, CRAnial Nerve Imaging; MIP, maximum intensity projection; MPR, multiplanar reformatting; PSIF, reversed fast imaging in steady-state free precession.
Table 1.
Magnetic resonance neurography sequences for a 3T Philips system (Philips, Best, Netherlands). 3D CRANI and 3D PSIF (reversed fast imaging in steady-state free precession) sequences
| 3D CRANI | 3D PSIF | |
|---|---|---|
| Basic MRI technique | 3D STIR (TSE) | 3D FFE (GE) |
| TR/TE (ms) | 2300/188 | 12/2.5 |
| FOV (AP/RL/FH mm) | 200/200/100 | 200/164/200 |
| Acquired voxel size (AP/RL/FH mm) | 0.9/0.9/0.9 (isotropic) | 0.9/0.9/0.9 (isotropic) |
| Reconstructed voxel size (AP/RL/FH mm) | 0.5/0.5/0.45 | 0.45/0.4/0.4 |
| Slice thickness (mm) | 0.5 | 0.45 |
| Slice oversampling | 1.5 | 1.4 |
| Acquisition time (min:sec) | 5:17 | 6:45 |
| Compressed sensing (acceleration rate) | Yes (3) | No |
| Flip angle | N/A | 35° |
| Fat suppression technique | STIR | Proset |
| TSE factor | 43 (Startup echoes: 2, linear in Y direction) | N/A |
| Additional techniques |
|
|
| Post-processing | MIP/MPR | |
| Multiplanar reformatting | Orthogonal plane: 5 mm slab thickness with 4.5 mm overlap Curved/oblique planes: 9 mm slap thickness with 8.5 mm overlap |
|
CRANI, CRAnial Nerve Imaging; FFE, Fast field echo; FOV, Field-of-view; GE, Gradient echo; MIP, Maximum-intensity-projection; MPR, Multiplanar reformatting; MSDE, Motion-sensitized driven equilibrium; N/A, Not applicable; PSIF, reversed fast imaging in steady-state free precession; STIR, Short tau inversion recovery; TE, Echo time; TR, Repetition time; TSE, Turbospin echo.
These can be further supplemented with routine brain T1W, T2W, CISS and FLAIR images.
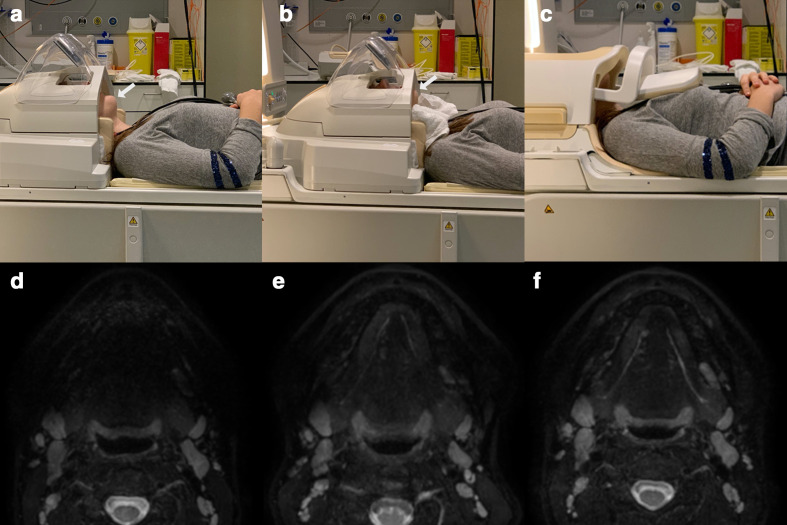
Figure 2.
(a) Patient positioning in a standard 32 channel head coil without additional measures. Note the anterior mandible is located outside the coil. (b) Patient positioning after fixation by means of an inflatable pillow with the head in slight hyperextension using a towel roll. The mandible is now well positioned within the coil. (c) Alternative coil, being a 16 channel neck coil. (d) Imaging output after patient positioning as in example (a) Signal loss is seen at the anterior segment. (e) Imaging output after slight hyperextension and thorough fixation as in example (b). (f) Imaging output using a neck coil after patient positioning as in example (c).
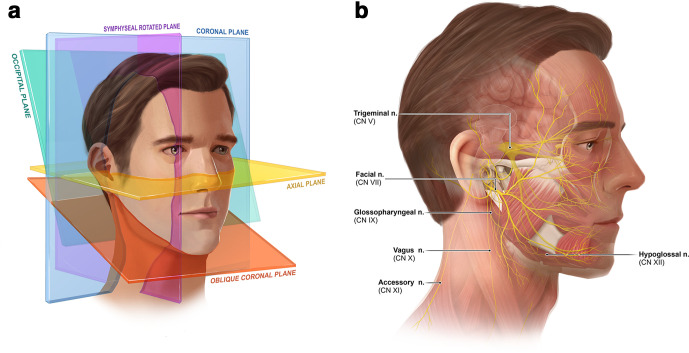
Figure 3.
(a) Orthogonal and additional planes constructed in evaluating the cranial and occipital peripheral nerves. Multiplanar reformatting and maximum intensity projection post-processing is applied to visualize the tortuous nerves in the necessary viewing planes. (b) Overall nerve anatomy discussed in this review.
In the next paragraphs, the cranial and occipital nerve imaging anatomy is described and further illustrated by a Supplementary Video 1.
Trigeminal nerve
Anatomy
The trigeminal nerve has extensive sensory, motor and (para-)sympathetic functions in the orofacial area. The nerve splits into three main divisions before it leaves the skull: ophthalmic nerve (V1), maxillary nerve (V2) and mandibular nerve (V3). The ophthalmic division (V1) splits into three branches (lacrimal, frontal and nasociliary nerve) which enter the orbit via the superior orbital fissure. The maxillary division (V2) leaves the cranial cavity via the foramen rotundum and reaches the pterygopalatine fossa. It innervates the teeth of the upper jaw and part of the nasal mucosa. Its dermal branches, the zygomatic nerves and infraorbital nerve, enter via the inferior orbital fissure. The infraorbital nerve runs over the floor of the orbit, it passes through the infraorbital foramen to the skin of the lower eyelid, the side of the nose and part of the upper lip (Figure 4).
Figure 4.
(a) Ophthalmic division of the trigeminal nerve (V1) using the 3D CRANI sequence. (b) 3D CRANI sequence. Increased caliber of the right infra orbital nerve (V2) in a patient with Short lasting Unilateral Neuralgiform headache attacks with Conjunctival injection and Tearing (SUNCT) rhinorrhea and forehead sweating. (c) 3D CRANI sequence. Increased signal intensity is noted of the Vidian nerve (V2) in the same patient as seen in (b). (d) 47-year-old male diagnosed with empty nose syndrome after repeated sinonasal procedures. Marked increase of caliber and signal intensity of both maxillary and infraorbital nerves (arrows) is seen. CRANI, CRAnial Nerve Imaging; SUNCT, short lasting Unilateral Neuralgiform headache attacks with Conjunctival injection and Tearing rhinorrhea and forehead sweating
The mandibular nerve (V3) runs through the foramen ovale from the middle cranial fossa to the infratemporal fossa. Three of its four large branches (buccal nerve, inferior alveolar nerve and auriculotemporal nerve) reach the lower skin of the face and are responsible for the cutaneous innervation of the face but it also carries the smaller motor part (radix motoria) which supplies the muscles of mastication. Approximately, 1 cm below the foramen ovale, the trunk of the mandibular nerve splits into an anterior and a posterior division. Its branches are described in three groups (trunk, anterior and posterior division). Branches of the trunk of the mandibular nerve include: the medial pterygoid nerve for the chewing muscle of the same name and meningeal rami.
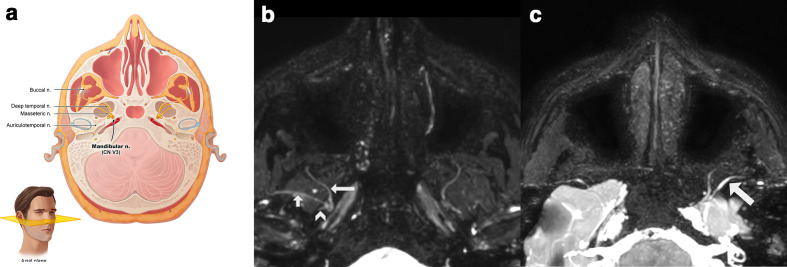
Branches of the anterior division are all motoric except the buccal nerve which appears between the two heads of lateral pterygoid muscle (Figure 5). The masseteric nerve runs superior from the lateral pterygoid muscle. It passes between the tendon of temporal muscle and the temporomandibular joint (TMJ) and reaches the masseter muscle via the mandibular incisura. On the way, it supplies small branches towards the TMJ. The deep temporal branches, two or three in number, run over the lateral pterygoid muscle to innervate the temporal muscle. The lateral pterygoid nerve innervates the muscle of the same name.
Figure 5.
(a) Anatomic relation of the anterior division of the mandibular nerve (V3) best seen in the axial plane. (b) 3D CRANI sequence illustrating the buccal nerve (long arrow), the masseteric nerve (short arrow) and the stem of the auriculotemporal nerve (arrowhead). (c) 3D CRANI sequence in a patient with post-traumatic trigeminal neuropathy of the mandibular division after placement of a titanium temporomandibular joint prosthesis. The left masseteric nerve is thickened and shows an increased signal intensity. CRANI, CRAnial Nerve Imaging
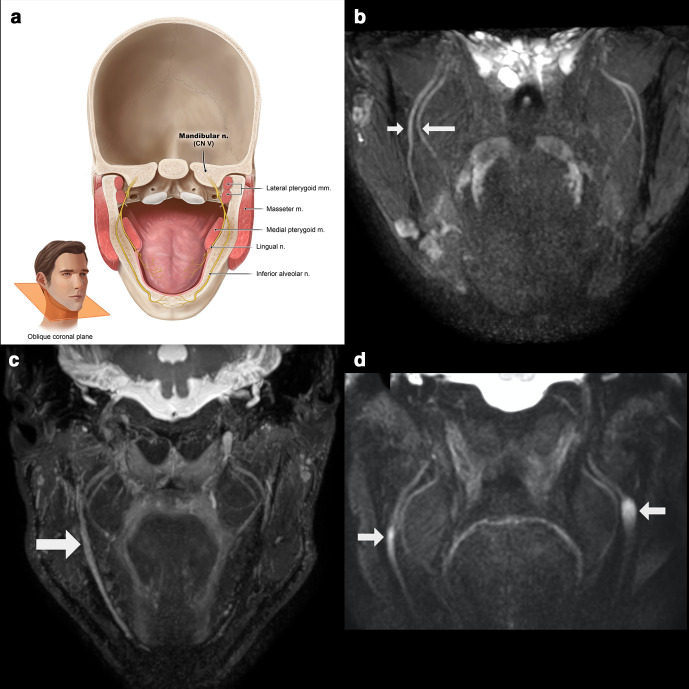
The posterior division of the mandibular nerve constitutes the auriculotemporal, lingual, inferior alveolar and mylohyoid nerve (Figures 5 and 6). The auriculotemporal nerve innervates most of the temporal region and a small part of the auricle (leading edge) and the outer ear canal. The lingual nerve, providing sensory and gustatory innervation to the tongue, appears in the infratemporal fossa between both pterygoid muscles and runs anteroinferiorly over the lateral side of medial pterygoid muscle. The inferior alveolar nerve also ends up between the two pterygoid muscles in the infratemporal fossa. There, it lies behind the lingual nerve. Together with the artery of the same name, it runs between the sphenomandibular ligament towards the inferior alveolar canal or mandibular canal. Just before it enters the mandibular foramen, it releases the mylohyoid nerve that innervates the mylohyoid muscle and anterior belly of the digastric muscle. The inferior alveolar nerve innervates all the teeth of the lower jaw, the adjacent gums and, via its end branch (mental nerve), the skin of the chin and the skin and mucosa of the lower lip.17–19
Figure 6.
(a) Anatomic overview of the posterior division of the mandibular nerve (V3) on a coronal oblique plane. (b) Normal appreciation of the lingual (long arrow) and inferior alveolar (short arrow) nerve running between the pterygoid muscles. (c) Right sided post-traumatic trigeminal neuropathy of the inferior alveolar nerve after ramus bone grafting. (d) Patient with neurofibromatosis Type 1, showing bilateral neurofibromas of the inferior alveolar nerve at the level of the mandibular foramen.
Imaging
The brainstem, cisternal and cavernous trigeminal segments can be imaged using conventional brain sequences including CISS and balanced FFE sequences and have been extensively reviewed in the past.6,19 The peripheral trigeminal nerve branches are best viewed in multiple planes after thick-slab MIP. The first (V1), second (V2) and anterior division of the third division (V3) can be well depicted on axial views whereas the lingual and inferior alveolar nerve are best seen in a coronal oblique direction (Figures 4–6).
In addition to the neurovascular conflict seen in trigeminal neuralgia cases, more and more neurological abnormalities are becoming detectable. The branches that are mostly involved in pathological conditions are the lingual and inferior alveolar nerve. Their course makes these nerves vulnerable to numerous dental and oromaxillofacial procedures. MRN techniques can aid in grading and clinical decision-making if trauma has occurred.20 Interested readers are referred to a recent systematic review summarizing the available knowledge base on MRN in post-traumatic trigeminal neuropathies.21 There is also increasing interest for the use of MRN in orofacial pain patients and more specifically in migraine and trigeminal autonomic cephalalgia (Figure 4).22
Facial nerve
The facial nerve consists out of motor, sensory and parasympathetic fibers. The sensory fibers innervate a part of the inner ear and the special sensory fibers transport the taste stimuli from the anterior two-thirds of the tongue via the chorda tympani. The parasympathetic fibers innervate the submandibular, sublingual and minor salivary glands, as well as the lacrimal glands. The motor fibers innervate the muscles responsible for the facial expression.
The primary or cisternal segment of the facial nerve leaves the brainstem close to the dorsal pons, transverses the cerebellopontine angle and enters the temporal bone by the porus acousticus in proximity to the vestibulocochlear nerve branches: superior to the cochlear nerve and anterior to the superior and inferior vestibular nerves. The trajectory through the temporal bone is subdivided in a meatal, labyrinthine, tympanic and mastoid segment. The sensory fibers, coming from the intermediate nerve, give on the one hand sensibility to the posterior concha and external auditory canal, on the other hand, the special sensory fibers will form the chorda tympani.
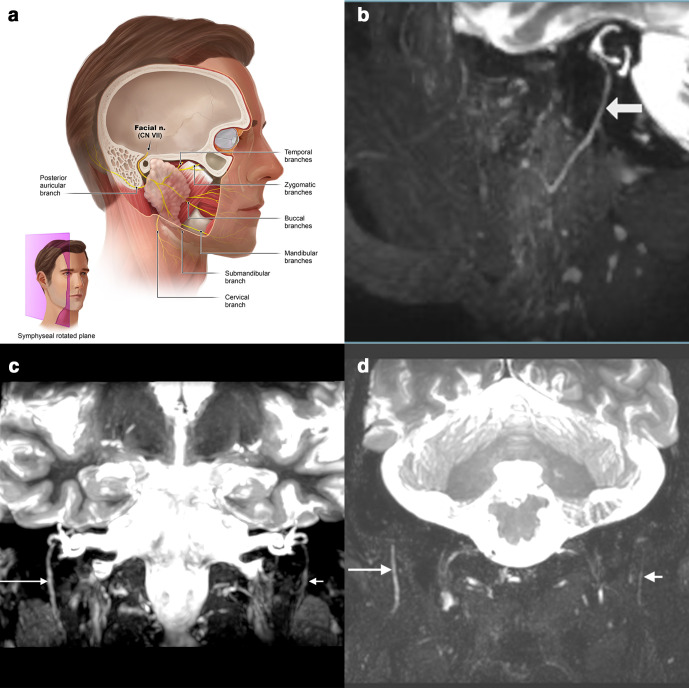
The main trunk of the facial nerve leaves skull base via the stylomastoid foramen, it immediately releases the smaller posterior auricular r. auricularis. Subsequently, the nerve enters the craniomedial part of the parotid gland. Over the intraglandular course the nerve subdivides into five branches, which appear separately at the upper, front and lower edges of this gland. These end branches spread from here to the facial mimic muscles like the spread fingers of a hand resting on the parotid area (temporal, zygomatic, buccal, marginal mandibular and cervical, Figure 6).23,24
Imaging
When considering MRI-imaging of the facial nerve, two segments need to be distinguished from each other: the skull base and the extracranial nerve segments. The intracranial and the temporal facial nerve segment is best visualized using 3D CISS, axial T1W and fat-suppressed T2W images.25 Visualization of the facial nerve within the stylomastoid canal, the extracranial and intraparotid part of the VII cranial nerve can be made using thick-slab MIP/MPR reconstructions of 3D PSIF, and in case of extensive artifacts a black blood 3D-STIR such as 3D CRANI.9 In case of nerve pathology, an increase of signal intensity and nerve caliber changes can be identified (Figure 7). The use of a 3D PSIF sequence in combination with microsurface coils resulted in superior visualization of the peripheral facial nerve branches, in comparison to a standard head and neck coil, as was reported by Meng et al26. The 3D-DESS-WE (double-echo steady state with water excitation) sequence is another established option to be considered for peripheral facial nerve neurography.27–29
Figure 7.
(a) Anatomic overview of the facial nerve and its branches which are best seen on a sagittal slightly rotated or coronal plane. (b) Sagittal view of a normal extracranial facial nerve entering the parotid gland on a 3D CRANI thick-slab MIP/MPR-image. (c) Coronal image with bilateral visualization of the intratemporal and extraforaminal facial nerve after iatrogenic damage on the right side. A slowly recuperative facial nerve paresis occurred after an infiltration with local anesthesia. (d) 60-year-old male with a right sided Bell’s palsy. The right extracranial facial (long arrow) nerve shows increased caliber and signal intensity compared to the contralateral facial nerve (short arrow). Discrepancies are noted all the way to the intraparotid course. 3D, three-dimensional; CRANI,CRAnial Nerve Imaging; MIP, maximum intensity projection; MPR, multiplanarreformatting.
Cranial nerves IX to XII
The trajectory of the IX, X and XII cranial nerves is anatomically closely intercalated, moreover, a lot of imaging characteristics are similar and therefore they are discussed together (Figure 8).
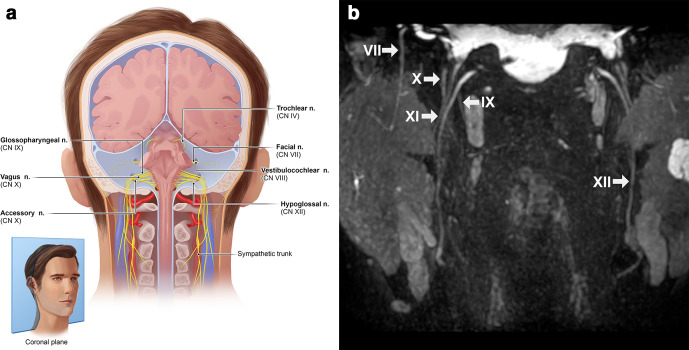
Figure 8.
(a) Anatomic overview of the facial (VII), hypoglossal (XII), glossopharyngeal (IX), vagus (X) and accessory (XI) nerves which can be seen in close relation to each other on a coronal plane. (b) Coronal view after a 3D CRANI sequence indicating the aforementioned peripheral nerves without pathological characteristics. 3D, three-dimensional; CRANI,CRAnial Nerve Imaging.
Glossopharyngeal nerve anatomy
The glossopharyngeal nerve or IX cranial nerve is composed of a combination of motor, sensory and parasympathetic fibers. Firstly, the sensory, gustatory and visceral stimuli are transported via afferent fibers from the retroauricular region, the posterior third of the tongue, the pharynx wall and the tonsils, the soft palate and the eardrum. Secondly, the motor efferents innervate the stylopharyngeus muscle. Thirdly, the parasympathetic fibers stimulate the production of saliva within the parotid gland.30
The origin of the IX cranial nerve is strongly associated with the vagus nerve, sharing three functional nuclei in the upper medulla oblongata. The nerve branches from the medulla oblongata within the cerebellomedullary cistern, slightly superior to the vagus nerve. The IX, X and XI cranial nerves course in an anterolateral direction through the cistern to the jugular foramen, exiting the foramen anteriorly from the internal jugular vein. Within the foramen, the glossopharyngeal nerve is known for two focal expansions: a superior node handling general sensible information and a lower node handling visceral sensory, taste and carotid innervations. The extratemporal course of the IX cranial nerve continues in a caudal direction within the carotid space and disperses in five major branches. Firstly, the tympanic nerve branches from the inferior node, carrying sensory information from the external and middle ear and parasympathetic stimuli to the parotid gland via the lesser petrosal nerve. Secondly, the stylopharyngeus branch gives motor input to the stylopharyngeus muscle. Thirdly, the pharyngeal branches associate with branches from the vagus nerve, forming the pharyngeal plexus. Fourthly, the carotid sinus branch mediates parasympathetic information to the carotid body. Finally, the lingual branch conveys general and gustatory sensory input from the posterior third of the tongue.30,31
Vagus nerve anatomy
The n. X forms the pharyngeal plexus and mediates the motor function of the soft palate. The parasympathetic fibers of the dorsal motor core of the n. X innervate pharynx, esophagus, trachea, bronchi, lungs, heart, intestines, liver and pancreas.
Multiple rootlets exiting the ventrolateral sulcus, formed by the olive and interior cerebellar peduncle, fuse together in the vagus nerve. The vagus and glossopharyngeal nerve progress closely intercalated through the cerebellopontine angle. Noteworthy, is the small meningeal branch coming from the vagus nerve, innervating the dura within the posterior cranial fossa. Subsequently, the vagus nerve travels through the center of the jugular foramen: superficial to the internal jugular vein and caudal to the glossopharyngeal nerve. Caudally progressing within the carotid space between, however, slightly posterior to the internal carotid artery and internal jugular vein. The internal jugular vein remains lateral and superficial to the vagus nerve; the common carotid artery travels medial and slightly anterior to the nerve.
There are four major extracranial branches leaving the vagus nerve in the head and neck area. Firstly, the auricular branch or Arnold nerve, exiting from the main nerve when passing through the jugular foramen, this nerve receives sensory input coming from the external auditory canal and tympanic membrane. Secondly, the pharyngeal branches leave the vagus nerve below the skull base and form, together with the IX cranial nerve, the pharyngeal plexus innervating the muscles of the soft palate and pharynx. Besides the motor function, the plexus conveys sensory stimuli coming from the epiglottis, trachea and esophagus. Thirdly, the superior laryngeal nerve has a sensory, as well as a motor component. The internal sensory branch conducts sensory input from the hypopharynx, larynx and vocal cords, the external motor branch innervates the cricothyroid and inferior pharyngeal constrictor muscles. And finally, the recurrent laryngeal nerve (RLN) is identified with its renowned asymmetrical anatomical morphology. Bilaterally, the RLN branches from the vagus nerve, it loops around the subclavian artery on the right side and on the left side around the aortic arch. The RLN mediates somatic and visceral sensory input coming from below the vocal cords, moreover, conveying motor output to all laryngeal musculature with exception of the cricothyroid muscle. Hereafter, the vagus nerve continues the trajectory into the thorax.6,32,33
Accessory nerve anatomy
The accessory nerve solely contains motor fibers, innervating the sternocleidomastoid as well as the trapezius muscle. The accessory nerve is composed from of both cranial and spinal (C1-5) rootlets. The main trunk of the accessory nerve subsequently travels in a lateral direction, before the nerve leaves the skull via the jugular foramen wherein connections with the vagus nerve can be found. The extracranial accessory nerve runs through the center of the carotid space between the medial internal carotid artery and laterally positioned internal jugular vein (IJV). Subsequently, the nerve divides again in the cranial and spinal roots. The cranial rootlets or internal branches fuse together with the vagus nerve and the spinal rootlets or external branches laterally cross the IJV, passing the transverse process of atlas mostly anteriorly and advancing medially from the styloid process and digastric and stylohyoid muscles. Further progression of the nerve follows an anterolateral direction before reaching the sternocleidomastoid muscle and subsequent formation of a nerve plexus with the ventral rami of C2 to C4, mediating the innervation of the trapezius muscle.34–36
Hypoglossal nerve anatomy
The hypoglossal nerve is a purely motor nerve, innervating the extrinsic and intrinsic musculature of the tongue, with exception of the palatoglossus muscle. The XII cranial nerve is formed by two bundles of 10–15 rootlets coming from the ventrolateral sulcus at the medulla oblongata. The bundles pierce through the dura mater separately and fuse together after passing through the hypoglossal canal. The extracranial hypoglossal nerve is joined by efferent C1 motor fibers and progresses laterally and inferiorly to the vagus nerve and internal carotid artery, initially closely associated with the carotid space. Subsequently, after passing the occipital artery, the hypoglossal nerve will turn and mostly pass through the space between carotid arteries and internal jugular vein. After progressing medially to the hyoid tendon of the digastric muscle, the nerve will enter the submandibular space medial to the submandibular gland and hyoglossus muscle (Figure 9). Some of the C1-fibers branch off more cranially and innervate the superior root of the ansa cervicalis, however, other C1 nerve fibers conveying motor input for the geniohyoid and thyrohyoid muscles will remain associated with the XII cranial nerve.31,37,38
Figure 9.
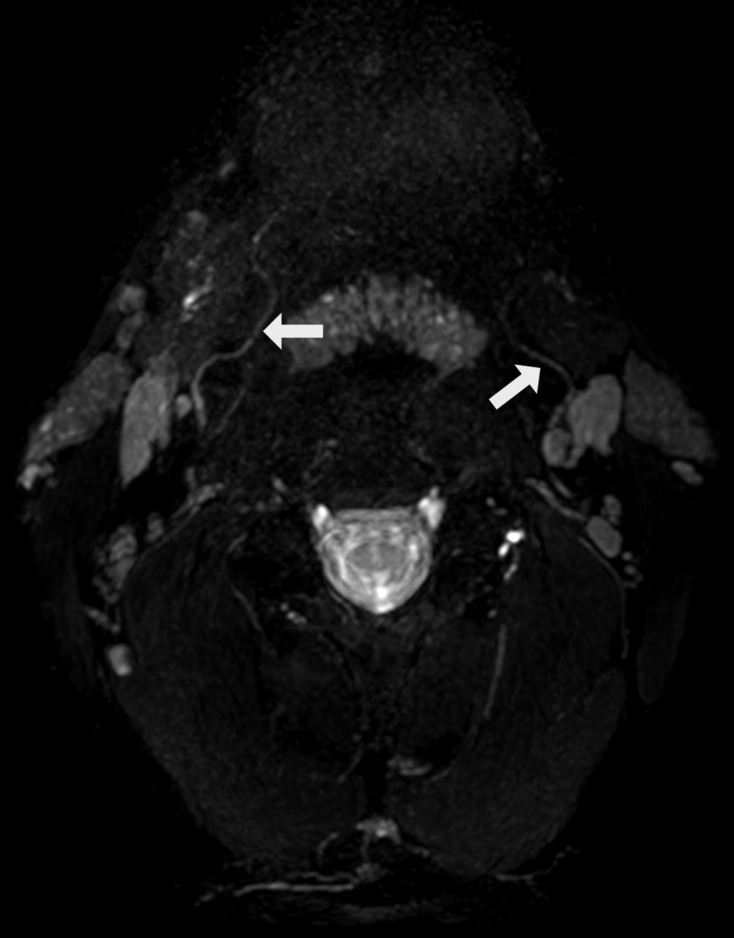
Bilateral normal appreciation of the peripheral hypoglossal nerve (white arrows) on an axial 3D CRANI image after MIP/MPR showing its course around the great vessels before innervating the tongue. 3D, three-dimensional; CRANI, CRAnial Nerve Imaging
Glossopharyngeal, vagus, accessory and hypoglossal nerve imaging
Evaluation of the cisternal IX, X and XI cranial nerve segments is preferably performed using heavily T2W steady-state free precession imaging sequences.25 However, these 2D sequences have mainly been replaced by 3D CISS and 3D FIESTA.3 The distinct foraminal nerve segments are identified using conventional 3D FIESTA, or CE-MRA (contrast-enhanced magnetic resonance angiograph) as described by Linn et al39.5 The below-skull-base-related nerve segments can be nicely differentiated on the MIP/MPR-reformatted 3D CRANI images (Figures 8 and 9). They are conveniently identified on coronal and axial planes. As discussed by Chhabra and colleagues, 3D PSIF is also a valuable technique for neurography of these closely intercalated cranial nerves.20 The typical nerve trajectories can be distinguished as follows, the extracranial vagus nerve is positioned between the medial IX and lateral XII nerve and has the largest diameter.
Occipital nerves (C2)
Anatomy
The greater occipital nerve (GON) ensues from the fusion of nerve fibers coming from the medial branch of the dorsal ramus of the second and, to a lesser degree, the third spinal nerve. At the level of the C1-C2 vertebrae, the nerve travels in the occipital direction between the medial inferior capitis oblique and lateral semispinal muscles. Important anatomical variation is described concerning the penetration of the trapezius, semispinalis capitis and inferior capitis oblique muscles which are pierced by GON in respectively, 45, 7,5 and 90% of cases.40 Next, the nerve loops upwards, joins the occipital artery and subdivides in a medial and lateral branch before terminal branches ensue.40–43
The lesser occipital nerve (LON) typically originates from the ventral rami of spinal nerves C2 and C3. The nerve loops around the sternocleidomastoid muscle (SCM). Its trajectory is parallel to the posterior border of the SCM, piercing the superficial lamina of the cervical fascia, in direction of the occipital area. Finally, the LON divides into medial and lateral branches in the middle between the intermastoid line and inion. Interconnections or overlap of GON and LON branches are frequently present.42–44
The third occipital nerve (TON) derives from the superficial medial branch of the dorsal ramus of the third spinal nerve. The nerve courses on top of the dorsolateral surface of the C2-C3 facet joint. The TON travels deeply to the semispinalis capitis muscle in a posterior direction when a communicating branch to GON exits. The overlying musculature will be pierced by TON before progressing subcutaneously.33,40,42,43
Functionally, GON, LON and TON receive somatic sensory input from the occipital region. The semispinalis muscle will receive motor output via GON, and to a lesser degree from TON (Figure 10).33,42,43
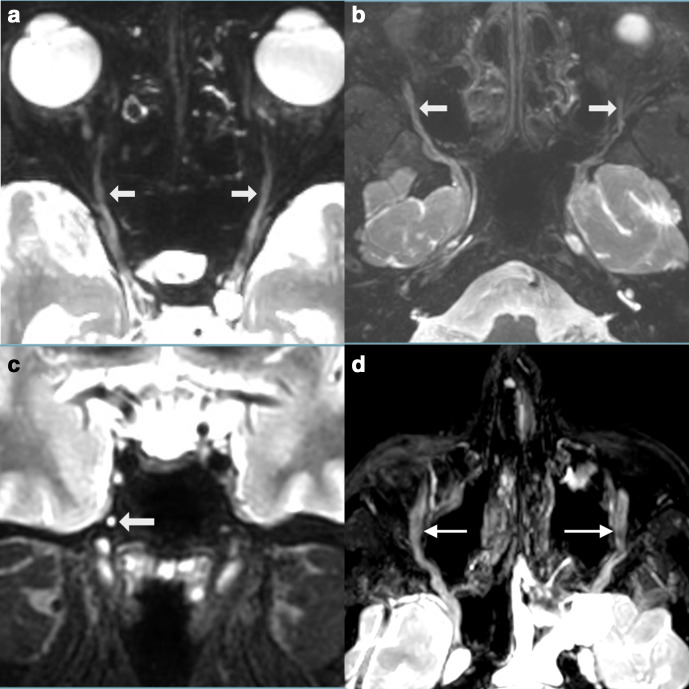
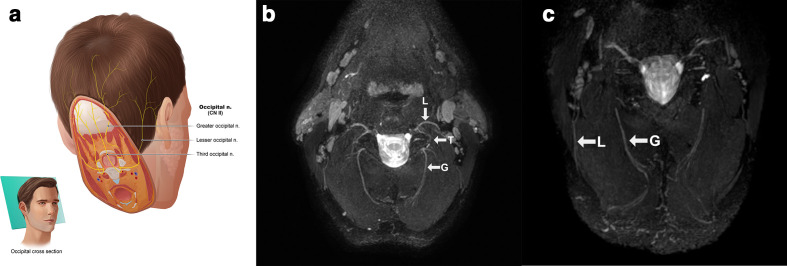
Figure 10.
(a) Anatomic overview of the occipital nerves which are seen together on an oblique axial plane. (b) Lesser (L), third (T) and greater (G) occipital nerves after MIP/MPR on a 3D CRANI sequence in a healthy subject. (c) More distal course of the lesser (L) and greater (G) occipital nerve in a healthy subject. 3D, three-dimensional; CRANI, CRAnial Nerve Imaging; MIP, maximum intensity projection; MPR, multiplanarreformatting.
Imaging
The occipital nerves are easily visualized on a slightly oblique axial plane using 3D PSIF or 3D CRANI sequences and are of increasing interest to neurologists and pain specialists. Pathological thickening and signal alterations can be noted in cases of occipital neuralgia also referred to as occipital migraine.22 Occasionally, one can also detect pathological changes after trauma or surgery in this region (Figure 10).
MRN assessment
The evaluation of the extraforaminal cranial nerves and possible abnormalities is best performed systematically. A proposal is presented in Table 2. At present, large series with benchmark values for each nerve segment are lacking in order to distinguish pathology from normal. Studies are underway to report these normal values.60–62 However, internal validation is always possible with the contralateral side. When bilateral abnormalities occur, a possible problem arises, but in combination with the clinical picture and focal changes, there is usually no diagnostic problem. Table 2 includes anatomical benchmark values for the discussed cranial and occipital nerves. We should remain cautious with external validation of nerve thickness and signal intensity as MR sequences are not actual anatomical representations but representations of signal intensity. Therefore, caution should be exercised when comparing MR parameters with published reference values. For the time being, internal comparison with the non-pathological side is the most reliable method. Interested readers are referred to the excellent review papers by Chhabra et al5,9,63.
Table 2.
Assessment of normal MRN findings and anatomical benchmark nerve diameters (Attention: this does not necessarily correspond to MRN nerve diameters which reflect signal intensities)
| Assessment of normal MRN findings | |||
|---|---|---|---|
| |||
| Anatomical benchmark nerve diameters | |||
| Nerve | Site of measurement | Mean diameter (mm) ±standard deviation | Reference |
| Trigeminal nerve | |||
| V1: Ophthalmic division | Middle cranial fossa | 1.7 ± 0.1 | Pennisiet al45 |
| V2: Maxillary division | Middle cranial fossa | 4.01 ± 0.52 | Zhang et al46 |
| V2: infraorbital nerve | Infraorbital foramen | 3.30 ± 0.52 | Zhang et al46 |
| V3: Mandibular division | Middle cranial fossa | 7.41 ± 1.41 | Soeira et al47 |
| V3: Inferior alveolar nerve | Mandibular foramen | 2.2 ± 0.4 | Ikeda et al48 |
| V3: Mental nerve | Mental foramen | 1.68–2.37 | Gershenson et al49 |
| V3: Lingual nerve | Third molar region | 3.0 ± 0.5 | VonArx et al50 |
| V3: Auriculotemporal | After fusion of rootlets | 3.18 ± 0.84 | Komarnitki et al51 |
| Facial nerve | |||
| Segment | Labyrinth | 1.13 ± 0.39 | Vianna et al52 |
| Tympanic | 1.09 ± 0.57 | Vianna et al52 | |
| Mastoid | 1.33 ± 0.65 | Vianna et al52 | |
| Average between stylomastoid foramen and intraparotid branching | 1.4 ± 0.2 | Lo et al53 | |
| Peripheral branch | Intraparotid | 0.5 ± 0.1 | Tawfik et al54 |
| Temporal branch | 0.94 ± 0.33 | Martínez Pascual et al55 | |
| Zygomatic | 1.00 ± 0.46 | Martínez Pascual et al55 | |
| Buccal | 0.99 ± 0.40 | Martínez Pascual et al55 | |
| Mandibular | 0.80 ± 0.34 | Martínez Pascual et al55 | |
| Cervical | 0.83 ± 0.15 | Martínez Pascual et al55 | |
| IX-X-XI nerves | |||
| Glossopharyngeal nerve | Midcervical | Not available | |
| Vagus nerve | Midcervical | 5.1 ± 1.5 | Hammer et al56 |
| Accessory nerve | Midcervical | Not available | |
| Hypoglossal nerve | |||
| Segment | Proximal | 1.41 ± 1.01 | Vacher et al57 |
| Above greater horn of hyoid | 1.23 ± 0.77 | Vacher et al57 | |
| Cervical loop | 0.48 ± 0.26 | Vacher et al57 | |
| Occipital nerves | |||
| Greater occipital nerve | After exiting the semispinalis capitis muscle | 3.8 ± 1.6 | Ducic et al58 |
| Lesser occipital nerve | Posterior border of sternocleidomastoid msucle | 1.2 ± 1.6 | Ducic et al58 |
| Third occipital nerve | After exiting the trapezius muscle | 1.5 ± 0.3 | Tubbs et al59 |
MRN, magnetic resonance neurography.
Future perspectives
MRN performance and applications are evolving rapidly. Where 0.5 T systems were available 20 years ago, we are now seeing the arrival of clinical 7 T and higher. The introduction of these high-field MRI devices can further improve spatial resolution and soft tissue contrast. However, there is also a risk of increasing susceptibility artifacts as they increase with increasing field strength and thus, they should not be considered the holy grail in MRN imaging. Rather a combination of high-field systems, specialized coils, improved post-processing and contrast agents will likely evolve this field in the next phase.26,64,65 First, there is a need to further define anatomical benchmarks for the cranial nerves. Several authors have described reference values for the trigeminal nerve or provided classifications to define degree of nerve injury.14,62 Next, there is a whole field of research left to obtain functional information by means of DTI and diffusion tensor tractography (DTT). These techniques are based on differences in diffusion of protons along nerve tracts and allow quantification of diffusion restriction by means of the apparent diffusion coefficient (ADC) and fractional anisotropy (FA) values. In combination with morphological changes, a more detailed description of neural dysfunction and neuroregeneration becomes reality.66 Additionally, they allow for a multiparametric and standardized approach towards nerve injuries and pathology, which is currently lacking. Several studies described the successful application of DTI and DTT in extraforaminal cranial nerve imaging.12,67–70 But, most reports only describe DTT of the proximal nerve branches with varying reference values.68,71 DTT of the small distal cranial nerve branches remains challenging and, for the time being, is mostly of scientific value.13,72 In addition to strong diagnostic value, MRN also offers applications for planning of surgical procedures. Current indications are summarized in Table 3. The surgeon could check in advance where the nerve is located in relation to the neoplasia or when the anatomy deviates from the normal.68 Panoramic reconstructions can aid in dental surgery planning (Figure 11).75 Fusion with CT images could help with the placement of a TMJ prosthesis near these peripheral nerve branches (Figure 12) and it is only a matter of time before artificial intelligence aids find their way to the clinic.76,77 The current paper is based on only a handful studies published by a limited number of centers. Large studies on MRN applications in the head and neck area are lacking. Most studies are still in a feasibility phase. There is a need for more high-quality research to further validate these techniques for the various applications in the coming years. In conclusion, the field of MRN is still in its infancy but a wide range of applications are already under development. It is therefore important that radiologists and anyone involved in cranial nerve pathology become familiar with these techniques and their possibilities.
Table 3.
Possible MRN indications in the head and neck area
| Indication | Reference |
|---|---|
| Chronic demyelinating neuropathies | Own experience, unpublished |
| Cranial neuralgias | Hwang et al22 |
| Follow-up of regeneration (e.g. after nerve surgery) | Not available |
Traumatic neuropathies
|
Burian et al73, Dessouky et al20, Zuniga et al62 |
| Nerve sheath tumors | Chhabra et al74 |
| Surgical planning involving cranial nerves | Dessouky et al20 |
| Viral, bacterial neuritis (e.g. Bell’s palsy) | Own experience, unpublished |
MRN, magnetic resonance neurography.
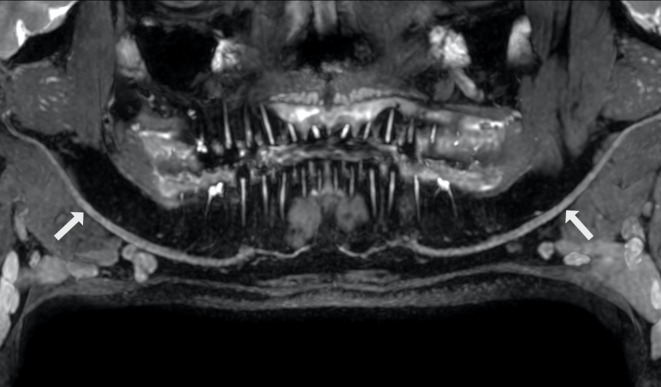
Figure 11.
Panoramic curved reconstruction and MIP of the inferior alveolar nerve using a 3D CRANI sequence allowing a full evaluation at a glance. 3D, three-dimensional; CRANI,CRAnial Nerve Imaging; MIP, maximum intensity projection.
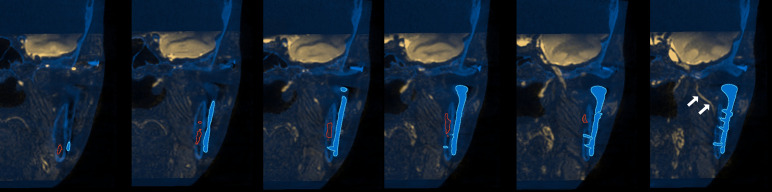
Figure 12.
3D fusion of CT and MRN images which can be valuable in planning the placement of a custom made temporomandibular joint prosthesis (blue outline). The inferior alveolar nerve is segmented (red outline) and indicated (white arrows) before entering the mandibular canal. 3D, three-dimensional; MRN, magnetic resonance neurography.
Contributor Information
Fréderic Van der Cruyssen, Email: frederic.vandercruyssen@uzleuven.be.
Tomas-Marijn Croonenborghs, Email: tomasmarijn@gmail.com.
Tara Renton, Email: tara.renton@kcl.ac.uk.
Robert Hermans, Email: robert.hermans@uzleuven.be.
Constantinus Politis, Email: stan@politis.be.
Reinhilde Jacobs, Email: reinhilde.jacobs@uzleuven.be.
Jan Casselman, Email: jan.casselman@azsintjan.be.
REFERENCES
- 1.Chhabra A, Andreisek G, Soldatos T, Wang KC, Flammang AJ, Belzberg AJ, et al. Mr neurography: past, present, and future. AJR Am J Roentgenol 2011; 197: 583–91. doi: 10.2214/AJR.10.6012 [DOI] [PubMed] [Google Scholar]
- 2.Howe FA, Filler AG, Bell BA, Griffiths JR. Magnetic resonance neurography. Magn Reson Med 1992; 28: 328–38. doi: 10.1002/mrm.1910280215 [DOI] [PubMed] [Google Scholar]
- 3.Blitz AM, Macedo LL, Chonka ZD, Ilica AT, Choudhri AF, Gallia GL, et al. High-Resolution CISS MR imaging with and without contrast for evaluation of the upper cranial nerves: segmental anatomy and selected pathologic conditions of the cisternal through extraforaminal segments. Neuroimaging Clin N Am 2014; 24: 17–34. doi: 10.1016/j.nic.2013.03.021 [DOI] [PubMed] [Google Scholar]
- 4.Hiwatashi A, Yoshiura T, Yamashita K, Kamano H, Honda H. High-resolution stir for 3-T MRI of the posterior fossa: visualization of the lower cranial nerves and arteriovenous structures related to neurovascular compression. AJR Am J Roentgenol 2012; 199: 644–8. doi: 10.2214/AJR.11.6566 [DOI] [PubMed] [Google Scholar]
- 5.Blitz AM, Choudhri AF, Chonka ZD, Ilica AT, Macedo LL, Chhabra A, et al. Anatomic considerations, nomenclature, and advanced cross-sectional imaging techniques for visualization of the cranial nerve segments by MR imaging. Neuroimaging Clin N Am 2014; 24: 1–15. doi: 10.1016/j.nic.2013.03.020 [DOI] [PubMed] [Google Scholar]
- 6.Casselman J, Mermuys K, Delanote J, Ghekiere J, Coenegrachts K. MRI of the cranial nerves—more than meets the eye: technical considerations and advanced anatomy. Neuroimaging Clin N Am 2008; 18: 197–231. doi: 10.1016/j.nic.2008.02.002 [DOI] [PubMed] [Google Scholar]
- 7.Lee JH, Cheng K-L, Choi YJ, Baek JH. High-Resolution imaging of neural anatomy and pathology of the neck. Korean J Radiol 2017; 18: 180.Available from. doi: 10.3348/kjr.2017.18.1.180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Z, Meng Q, Chen Y, Li Z, Luo B, Yang Z, et al. 3-T imaging of the cranial nerves using three-dimensional reversed FISP with diffusion-weighted Mr sequence. J Magn Reson Imaging 2008; 27: 454–8. doi: 10.1002/jmri.21009 [DOI] [PubMed] [Google Scholar]
- 9.Chhabra A, Bajaj G, Wadhwa V, Quadri RS, White J, Myers LL, et al. Mr Neurographic evaluation of facial and neck pain: normal and abnormal craniospinal nerves below the skull base. RadioGraphics 2018; 38: 1498–513. doi: 10.1148/rg.2018170194 [DOI] [PubMed] [Google Scholar]
- 10.Klupp E, Cervantes B, Sollmann N, Treibel F, Weidlich D, Baum T, et al. Improved brachial plexus visualization using an adiabatic iMSDE-Prepared stir 3D TSE. Clin Neuroradiol 2019; 29: 631–8. doi: 10.1007/s00062-018-0706-0 [DOI] [PubMed] [Google Scholar]
- 11.Yoneyama M, Takahara T, Kwee TC, Nakamura M, Tabuchi T. Rapid high resolution Mr neurography with a diffusion-weighted pre-pulse. Magn Reson Med Sci 2013; 12: 111–9. doi: 10.2463/mrms.2012-0063 [DOI] [PubMed] [Google Scholar]
- 12.Saito S, Ozawa H, Fujioka M, Hikishima K, Hata J, Kurihara S, et al. Visualization of nerve fibers around the carotid bifurcation with use of a 9.4 tesla microscopic magnetic resonance diffusion tensor imaging with tractography. Head Neck 2018; 40: 2228–34. doi: 10.1002/hed.25318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacquesson T, Frindel C, Kocevar G, Berhouma M, Jouanneau E, Attyé A, et al. Overcoming challenges of cranial nerve tractography: a targeted review. Neurosurgery 2019; 84: 313–25. doi: 10.1093/neuros/nyy229 [DOI] [PubMed] [Google Scholar]
- 14.Burian E, Probst FA, Weidlich D, Cornelius C-P, Maier L, Robl T, et al. MRI of the inferior alveolar nerve and lingual nerve-anatomical variation and morphometric benchmark values of nerve diameters in healthy subjects. Clin Oral Investig 2020; 24: 2625–34. doi: 10.1007/s00784-019-03120-7 [DOI] [PubMed] [Google Scholar]
- 15.Dalrymple NC, Prasad SR, Freckleton MW, Chintapalli KN. Informatics in radiology (infoRAD): introduction to the language of three-dimensional imaging with multidetector CT. Radiographics 2005; 25: 1409–28. doi: 10.1148/rg.255055044 [DOI] [PubMed] [Google Scholar]
- 16.Napel S, Rubin GD, Jeffrey RB. STS-MIP: a new reconstruction technique for CT of the chest. J Comput Assist Tomogr 1993; 17: 832–8. [PubMed] [Google Scholar]
- 17.Joo W, Yoshioka F, Funaki T, Mizokami K, Rhoton AL. Microsurgical anatomy of the trigeminal nerve. Clin. Anat. 2014; 27: 61–88. doi: 10.1002/ca.22330 [DOI] [PubMed] [Google Scholar]
- 18.Woolfall P, Coulthard A. Pictorial review: trigeminal nerve: anatomy and pathology. Br J Radiol 2001; 74: 458–67. doi: 10.1259/bjr.74.881.740458 [DOI] [PubMed] [Google Scholar]
- 19.Bathla G, Hegde AN. The trigeminal nerve: an illustrated review of its imaging anatomy and pathology. Clin Radiol 2013; 68: 203–13. doi: 10.1016/j.crad.2012.05.019 [DOI] [PubMed] [Google Scholar]
- 20.Dessouky R, Xi Y, Zuniga J, Chhabra A. Role of Mr neurography for the diagnosis of peripheral trigeminal nerve injuries in patients with prior molar tooth extraction. AJNR Am J Neuroradiol 2018; 39: 162–9. doi: 10.3174/ajnr.A5438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.21.Van der Cruyssen F, Peeters F, Croonenborghs T-M, Fransen J, Renton T, Politis C, et al. A systematic review on diagnostic test accuracy of magnetic resonance neurography versus clinical neurosensory assessment for post-traumatic trigeminal neuropathy in patients reporting neurosensory disturbance. Dentomaxillofac Radiol 2020;: 20200103. doi: 10.1259/dmfr.20200103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hwang L, Dessouky R, Xi Y, Amirlak B, Chhabra A. Mr neurography of greater occipital nerve neuropathy: initial experience in patients with migraine. AJNR Am J Neuroradiol 2017; 38: 2203–9. doi: 10.3174/ajnr.A5354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta S, Mends F, Hagiwara M, Fatterpekar G, Roehm PC. Imaging the facial nerve: a contemporary review. Radiol Res Pract 2013; 2013: 1–14. doi: 10.1155/2013/248039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Myckatyn TM, Mackinnon SE. A review of facial nerve anatomy. Semin Plast Surg 2004; 18: 5–11. doi: 10.1055/s-2004-823118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Casselman JW, Kuhweide R, Deimling M, Ampe W, Dehaene I, Meeus L. Constructive interference in steady state-3DFT MR imaging of the inner ear and cerebellopontine angle. AJNR Am J Neuroradiol 1993; 14: 47–57. [PMC free article] [PubMed] [Google Scholar]
- 26.Meng Q, Guan J, Rao L, Chu J, Li S, Zhou Z, et al. High-Resolution MRI of the intraparotid facial nerve based on a Microsurface coil and a 3D reversed fast imaging with steady-state precession DWI sequence at 3T. Am J Neuroradiol 2013; 34: 1643–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wen J, Desai NS, Jeffery D, Aygun N, Blitz A. High-Resolution isotropic three-dimensional MR imaging of the Extraforaminal segments of the cranial nerves. Magn Reson Imaging Clin N Am 2018; 26: 101–19Available from. doi: 10.1016/j.mric.2017.08.007 [DOI] [PubMed] [Google Scholar]
- 28.Fujii H, Fujita A, Yang A, Kanazawa H, Buch K, Sakai O, et al. Visualization of the peripheral branches of the mandibular division of the trigeminal nerve on 3D Double-Echo steady-state with water excitation sequence. AJNR Am J Neuroradiol 2015; 36: 1333–7. doi: 10.3174/ajnr.A4288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujii H, Fujita A, Kanazawa H, Sung E, Sakai O, Sugimoto H. Localization of parotid gland tumors in relation to the intraparotid facial nerve on 3D Double-Echo steady-state with water excitation sequence. AJNR Am J Neuroradiol 2019; 40: 1037–42. doi: 10.3174/ajnr.A6078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.García Santos JM, Sánchez Jiménez S, Tovar Pérez M, Moreno Cascales M, Lailhacar Marty J, Fernández-Villacañas Marín MA. Tracking the glossopharyngeal nerve pathway through anatomical references in cross-sectional imaging techniques: a pictorial review. Insights Imaging 2018; 9: 559–69. doi: 10.1007/s13244-018-0630-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakamoto Y. Morphological features of the branching pattern of the hypoglossal nerve. Anat Rec 2019; 302: 558–67. doi: 10.1002/ar.23819 [DOI] [PubMed] [Google Scholar]
- 32.Yuan H, Silberstein SD. Vagus nerve and vagus nerve stimulation, a comprehensive review: Part I. Headache 2016; 56: 71–8. doi: 10.1111/head.12647 [DOI] [PubMed] [Google Scholar]
- 33.Tubbs RS, Mortazavi MM, Loukas M, D'Antoni AV, Shoja MM, Chern JJ, et al. Anatomical study of the third occipital nerve and its potential role in occipital headache/neck pain following midline dissections of the craniocervical junction. J Neurosurg 2011; 15: 71–5. doi: 10.3171/2011.3.SPINE10854 [DOI] [PubMed] [Google Scholar]
- 34.Durazzo MD, Furlan JC, Teixeira GV, Friguglietti CUM, Kulcsar MAV, Magalhães RP, et al. Anatomic landmarks for localization of the spinal accessory nerve. Clin. Anat. 2009; 22: 471–5. doi: 10.1002/ca.20796 [DOI] [PubMed] [Google Scholar]
- 35.Johal J, Iwanaga J, Tubbs K, Loukas M, Oskouian RJ, Tubbs RS. The accessory nerve: a comprehensive review of its anatomy, development, variations, landmarks and clinical considerations. Anat Rec 2019; 302: 620–9. doi: 10.1002/ar.23823 [DOI] [PubMed] [Google Scholar]
- 36.Overland J, Hodge JC, Breik O, Krishnan S. Surgical anatomy of the spinal accessory nerve: review of the literature and case report of a rare anatomical variant. J. Laryngol. Otol. 2016; 130: 969–72. doi: 10.1017/S0022215116008148 [DOI] [PubMed] [Google Scholar]
- 37.Kim DD, Caccamese JF, Ord RA. Variations in the course of the hypoglossal nerve: a case report and literature review. Int J Oral Maxillofac Surg 2003; 32: 568–70. doi: 10.1016/S0901-5027(02)90360-2 [DOI] [PubMed] [Google Scholar]
- 38.Lin H, Barkhaus P. Cranial nerve XII: the hypoglossal nerve. Semin Neurol 2009; 29: 045–52. doi: 10.1055/s-0028-1124022 [DOI] [PubMed] [Google Scholar]
- 39.Linn J, Peters F, Moriggl B, Naidich TP, Brückmann H, Yousry I. The jugular foramen: imaging strategy and detailed anatomy at 3T. AJNR Am J Neuroradiol 2009; 30: 34–41. doi: 10.3174/ajnr.A1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bovim G, Bonamico L, Fredriksen TA, Lindboe CF, Stolt-Nielsen A, Sjaastad O. Topographic variations in the peripheral course of the greater occipital nerve. Spine 1991; 16: 475–8. doi: 10.1097/00007632-199104000-00017 [DOI] [PubMed] [Google Scholar]
- 41.Mosser SW, Guyuron B, Janis JE, Rohrich RJ. The anatomy of the greater occipital nerve: implications for the etiology of migraine headaches. Plast Reconstr Surg 2004; 113: 693–7. doi: 10.1097/01.PRS.0000101502.22727.5D [DOI] [PubMed] [Google Scholar]
- 42.Kwon H-J, Kim H-S, O J, Kang HJ, Won JY, Yang H-M, et al. Anatomical analysis of the distribution patterns of occipital cutaneous nerves and the clinical implications for pain management. J Pain Res 2018; 11: 2023–31. doi: 10.2147/JPR.S175506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cohen-Gadol A, Kemp III W, Tubbs RShane, Rs T. The innervation of the scalp: a comprehensive review including anatomy, pathology, and neurosurgical correlates. Surg Neurol Int 2011; 2: 178. doi: 10.4103/2152-7806.90699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee M, Brown M, Chepla K, Okada H, Gatherwright J, Totonchi A, et al. An anatomical study of the lesser occipital nerve and its potential compression points: implications for surgical treatment of migraine headaches. Plast Reconstr Surg 2013; 132: 1551–6. doi: 10.1097/PRS.0b013e3182a80721 [DOI] [PubMed] [Google Scholar]
- 45.Pennisi E, Cruccu G, Manfredi M, Palladini G. Histometric study of myelinated fibers in the human trigeminal nerve. J Neurol Sci 1991; 105: 22–8. doi: 10.1016/0022-510X(91)90113-L [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y-xin, Zhang K-qi, Qin S-jia, Wang F. Autopsy study for maxillary nerve and its artery supply. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2012; 47: 836–40. [PubMed] [Google Scholar]
- 47.Soeira G, Abd el-Bary TH, Dujovny M, Slavin KV, Ausman JI. Microsurgical anatomy of the trigeminal nerve. Neurol Res 1994; 16: 273–83. doi: 10.1080/01616412.1994.11740240 [DOI] [PubMed] [Google Scholar]
- 48.Ikeda K, Ho KC, Nowicki BH, Haughton VM. Multiplanar Mr and anatomic study of the mandibular canal. AJNR Am J Neuroradiol 1996; 17: 579–84. [PMC free article] [PubMed] [Google Scholar]
- 49.Gershenson A, Nathan H, Luchansky E. Mental foramen and mental nerve: changes with age. Cells Tissues Organs 1986; 126: 21–8. doi: 10.1159/000146181 [DOI] [PubMed] [Google Scholar]
- 50.von Arx T, Lozanoff S. Clinical oral anatomy. Switzerland: Springer International Publishing; 2017. pp. 561. [Google Scholar]
- 51.Komarnitki I, Andrzejczak-Sobocińska A, Tomczyk J, Deszczyńska K, Ciszek B. Clinical anatomy of the auriculotemporal nerve in the area of the infratemporal fossa. Folia Morphol 2012; 71: 187–93. [PubMed] [Google Scholar]
- 52.Vianna M, Adams M, Schachern P, Lazarini PR, Paparella MM, Cureoglu S. Bell’s palsy — A 3-dimensional temporal bone study. Otol Neurotol 2014; 35: 514–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.YL L, Fook-Chong S, Leoh TH, Dan YF, Lee MP, Gan HY, et al. High-resolution ultrasound in the evaluation and prognosis of Bell’s palsy. Eur J Neurol 2010; 17: 885–9. [DOI] [PubMed] [Google Scholar]
- 54.Tawfik EA. Sonographic characteristics of the facial nerve in healthy volunteers. Muscle Nerve 2015; 52: 767–71. doi: 10.1002/mus.24627 [DOI] [PubMed] [Google Scholar]
- 55.Martínez Pascual P, Maranillo E, Vázquez T, Simon de Blas C, Lasso JM, Sañudo JR. Extracranial course of the facial nerve revisited. Anat Rec 2019; 302: 599–608. doi: 10.1002/ar.23825 [DOI] [PubMed] [Google Scholar]
- 56.Hammer N, Löffler S, Cakmak YO, Ondruschka B, Planitzer U, Schultz M, et al. Cervical vagus nerve morphometry and vascularity in the context of nerve stimulation - A cadaveric study. Sci Rep 2018; 8: 1–9. doi: 10.1038/s41598-018-26135-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vacher C, Dauge M-C. Morphometric study of the cervical course of the hypoglossal nerve and its application to hypoglossal facial anastomosis. Surg Radiol Anat 2004; 26: 86–90. doi: 10.1007/s00276-003-0197-8 [DOI] [PubMed] [Google Scholar]
- 58.Ducic I, Moriarty M, Al-Attar A. Anatomical variations of the occipital nerves: implications for the treatment of chronic headaches. Plast Reconstr Surg 2009; 123: 859–63. doi: 10.1097/PRS.0b013e318199f080 [DOI] [PubMed] [Google Scholar]
- 59.Tubbs RS, Mortazavi MM, Loukas M, D'Antoni AV, Shoja MM, Chern JJ, et al. Anatomical study of the third occipital nerve and its potential role in occipital headache/neck pain following midline dissections of the craniocervical junction. J Neurosurg 2011; 15: 71–5. doi: 10.3171/2011.3.SPINE10854 [DOI] [PubMed] [Google Scholar]
- 60.Burian E, Probst FA, Weidlich D, Cornelius C-P, Maier L, Robl T, et al. Mri of the inferior alveolar nerve and lingual nerve-anatomical variation and morphometric benchmark values of nerve diameters in healthy subjects. Clin Oral Investig 2020; 24: 2625–34. doi: 10.1007/s00784-019-03120-7 [DOI] [PubMed] [Google Scholar]
- 61.Cox B, Zuniga JR, Panchal N, Cheng J, Chhabra A. Magnetic resonance neurography in the management of peripheral trigeminal neuropathy: experience in a tertiary care centre. Eur Radiol 2016; 26: 3392–400. doi: 10.1007/s00330-015-4182-5 [DOI] [PubMed] [Google Scholar]
- 62.Zuniga JR, Mistry C, Tikhonov I, Dessouky R, Chhabra A. Magnetic resonance neurography of traumatic and nontraumatic peripheral trigeminal neuropathies. J Oral Maxillofac Surg 2018; 76: 725–36. doi: 10.1016/j.joms.2017.11.007 [DOI] [PubMed] [Google Scholar]
- 63.Chhabra A JAP, Ph D, Flammang A, Gilson W, Carrino JA. Magnetic resonance neurography – techniques and interpretation. Magnetom flash-Clinical Neurol 2012; 2: 4–8. [Google Scholar]
- 64.Voskuilen L, de Heer P, van der Molen L, Balm AJM, van der Heijden F, Strijkers GJ, et al. A 12-channel flexible receiver coil for accelerated tongue imaging. MAGMA 2020; 33: 581–90. doi: 10.1007/s10334-019-00824-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wessig C, Bendszus M, Stoll G. In vivo visualization of focal demyelination in peripheral nerves by gadofluorine M-enhanced magnetic resonance imaging. Exp Neurol 2007; 204: 14–19. doi: 10.1016/j.expneurol.2006.09.022 [DOI] [PubMed] [Google Scholar]
- 66.Takagi T, Nakamura M, Yamada M, Hikishima K, Momoshima S, Fujiyoshi K, et al. Visualization of peripheral nerve degeneration and regeneration: monitoring with diffusion tensor tractography. Neuroimage 2009; 44: 884–92. doi: 10.1016/j.neuroimage.2008.09.022 [DOI] [PubMed] [Google Scholar]
- 67.El Kininy W, Roddy D, Davy S, Roman E, O'Keane V, O'Hanlon E, El KW, O’Keefe V, O’Hanlon E, et al. Magnetic resonance diffusion weighted imaging using constrained spherical deconvolution-based tractography of the extracranial course of the facial nerve. Oral Surg Oral Med Oral Pathol Oral Radiol 2020; 130: 1–13. doi: 10.1016/j.oooo.2019.12.012 [DOI] [PubMed] [Google Scholar]
- 68.Rouchy R-C, Attyé A, Medici M, Renard F, Kastler A, Grand S, et al. Facial nerve tractography: a new tool for the detection of perineural spread in parotid cancers. Eur Radiol 2018; 28: 3861–71. doi: 10.1007/s00330-018-5318-1 [DOI] [PubMed] [Google Scholar]
- 69.Xie G, Zhang F, Leung L, Mooney MA, Epprecht L, Norton I, et al. Anatomical assessment of trigeminal nerve tractography using diffusion MRI: a comparison of acquisition b-values and single- and multi-fiber tracking strategies. Neuroimage Clin 2020; 25: 102160. doi: 10.1016/j.nicl.2019.102160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Terumitsu M, Matsuzawa H, Seo K, Watanabe M, Kurata S, Suda A, et al. High-Contrast high-resolution imaging of posttraumatic mandibular nerve by 3DAC-PROPELLER magnetic resonance imaging: correlation with the severity of sensory disturbance. Oral Surg Oral Med Oral Pathol Oral Radiol 2017; 124: 85–94. doi: 10.1016/j.oooo.2017.02.017 [DOI] [PubMed] [Google Scholar]
- 71.Shapey J, Vos SB, Vercauteren T, Bradford R, Saeed SR, Bisdas S, et al. Clinical applications for diffusion MRI and tractography of cranial nerves within the posterior fossa: a systematic review. Front Neurosci 2019; 13: 23. doi: 10.3389/fnins.2019.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cauley KA, Filippi CG. Diffusion-tensor imaging of small nerve bundles: cranial nerves, peripheral nerves, distal spinal cord, and lumbar nerve roots-clinical applications. AJR Am J Roentgenol 2013; 201: W326–35. doi: 10.2214/AJR.12.9230 [DOI] [PubMed] [Google Scholar]
- 73.Burian E, Sollmann N, Ritschl LM, Palla B, Maier L, Zimmer C, et al. High resolution MRI for quantitative assessment of inferior alveolar nerve impairment in course of mandible fractures: an imaging feasibility study. Sci Rep 2020; 10: 1–9. doi: 10.1038/s41598-020-68501-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chhabra A, Madhuranthakam AJ, Andreisek G. Magnetic resonance neurography: current perspectives and literature review. Eur Radiol 2018; 28: 698–707. doi: 10.1007/s00330-017-4976-8 [DOI] [PubMed] [Google Scholar]
- 75.Manoliu A, Ho M, Nanz D, Dappa E, Boss A, Grodzki DM, et al. Mr neurographic orthopantomogram: ultrashort echo-time imaging of mandibular bone and teeth complemented with high-resolution morphological and functional MR neurography. J Magn Reson Imaging 2016; 44: 393–400. doi: 10.1002/jmri.25178 [DOI] [PubMed] [Google Scholar]
- 76.Zhu B, Liu JZ, Cauley SF, Rosen BR, Rosen MS. Image reconstruction by domain-transform manifold learning. Nature 2018; 555: 487–92. doi: 10.1038/nature25988 [DOI] [PubMed] [Google Scholar]
- 77.Lundervold AS, Lundervold A. An overview of deep learning in medical imaging focusing on MRI. Zeitschrift für Medizinische Physik 2019; 29: 102–27Available from. doi: 10.1016/j.zemedi.2018.11.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.