Abstract
Objective:
To study the effect of long-distance running on the morphological and T2* assessment of knee cartilage.
Methods:
3D-DESS and T2* mapping was performed in 12 amateur marathon runners (age: between 21 and 37 years) without obvious morphological cartilage damage. MRI was performed three times: within 24 h before the marathon, within 12 h after the marathon, and after a period of convalescence of two months. An automatic cartilage segmentation method was used to quantitatively assessed the morphological and T2* of knee cartilage pre- and post-marathon. The cartilage thickness, volume, and T2* values of 21 sub-regions were quantitatively assessed, respectively.
Results:
The femoral lateral central (FLC) cartilage thickness was increased when 12-h post-marathon compared with pre-marathon. The tibial medial anterior (TMA) cartilage thickness was decreased when 2 months post-marathon compared with pre-marathon. The tibial lateral posterior (TLP) cartilage volume was increased when 12-h post-marathon compared with pre-marathon. The cartilage T2* value in most sub-regions had the upward trend when 12-h post-marathon and restored trend when 2 months post-marathon, compared with pre-marathon. The femoral lateral anterior (FLA) and TMA cartilage volumes were decreased 2 months post-marathon compared with pre-marathon.
Conclusions:
The marathon had some effects on the thickness, volume, and T2* value of the knee cartilages. The thickness and volume of knee cartilage in most sub-regions were without significantly changes post-marathon compared with pre-marathon. T2* value of knee cartilage in most sub-regions was increased right after marathon and recovered 2 months later. The TLP and TMA subregions needed follow-up after marathon.
Advances in knowledge:
The morphological and T2* changes of knee cartilage after marathon were evaluated by MRI and automatic segmentation software. This study was the first to use cartilage automatic segmentation software to evaluate the effects of marathon on the morphology and biochemical components of articular cartilage, and to predict the most vulnerable articular cartilage subregions, for the convenience of future exercise adjustment and the avoidance of sports cartilage injury.
Introduction
The marathon has been widely used as a model of investigating the limits of physiology function. In 2018, the number of participants of marathon reached million.1 It is expected that the number of participants in 2020 will exceed 10 million. The continuous development and popularization of marathon sports has also brought some negative impacts while improving people’s physical fitness. For amateur marathon runners, they are more susceptible to damage to the musculoskeletal system due to lack of professional running knowledge and guidance than professional athlete. In longer ultra-marathons, 50–60% of the participants experience musculoskeletal problems.2 The early assessment of injuries for amateur marathon runners has become a hot topic of recent research and discussion.3,4
In marathon, the incidence of lower extremity running injuries is high due to repeated and huge loads acting on the lower extremity joints for a long time. The knee is the most frequently injured joint in runners.5 Common knee joint injuries include cartilage injury, sacroiliac tibial syndrome, patella pain syndrome, meniscus injury, bone marrow edema, patellar tendinitis, and ligament injury.6,7 Articular cartilage injury is one of the hot topics of research and discussion in recent years. Studies have shown that during a marathon, the average vertical force on the knee joint of a 70 KG athlete is 2800N, and articular cartilage plays an important role in the process of stress transmission to the sub-articular bone.8 As a biological shock absorber, articular cartilage can absorb and buffer stress to the greatest extent, and at the same time, it evenly transmits the force to the bone below the joint to avoid joint damage. However, articular cartilage is not easy to recover after injury. Many studies have shown that long-term load can cause degenerative changes of cartilage, leading to the occurrence of osteoarthritis.9–11
MRI was widely used to evaluate the morphological changes of knee joints before and after exercise, such as meniscus injury, joint effusion, bone marrow edema, peri-articular cysts, and ligament integrity changes.12–14 3-D US can evaluate the morphology of cartilage, and its measurements are reproducible and correlate strongly with MRI measurements.15 However, there are relatively few studies on knee ultrasound evaluating the cartilage, and its feasibility needs to be further verified. Those were morphological changes of the knee joint.
So far, MRI is the non-invasive and effective examination method that can observe and quantitatively evaluate joint cartilage in vivo. With the development of technology, a large number of quantitative MRI techniques have emerged in recent years, including T2-mapping, T2*-mapping, spin lattice relaxation in rotating frames (T1rho), and magnetic resonance delayed enhanced cartilage imaging (dGEMRIC), GAG chemical exchange saturation transfer imaging (MR gagCEST), et al. Among them, T2-mapping, T2*-mapping, and T1rho have been used in knee joint cartilage studies.12,16 The change in T2* value reflects a comprehensive change in lateral relaxation time and magnetic field heterogeneity, and is sensitive to the anisotropy of collagen fibers and moisture in articular cartilage.16 Therefore, this method is often used to study the changes in the composition of early chondrocyte matrix. In this study, T2 * mapping and high-resolution 3D MRI were used to assess the short-term and relatively long-term changes of knee cartilage in marathon runners.
Methods and materials
We recruited 12 amateur marathon runners (five males and seven females) for this study, who run marathons less than two times. The participants were between 21 and 37 years old and had a range of body mass index (BMI) of 17.6–27.2 kg m−2. Inclusion criteria were (1) age between 18 and 40 years old; (2) the BMI <28 kg m−2; (3) no history of knee joint trauma, surgery, or infections; (4) no history of chronic diseases requiring long-term drug therapy; and (5) no history of vigorous exercise after the marathon. The exclusion criteria were: (1) knee joint trauma occurring during the study period; (2) pre-marathon images showing morphological injury of the articular cartilage; (3) knee joint pain or other positive sign; and (4) MRI contraindications.
Magnetic resonance imaging protocols
MRI was performed on both knee joints of all subjects within 24 h before marathon, within 12 h after marathon, and after a period of convalescence of 2 months using a 3T MR scanner (MAGNETOM Verio, Siemens Healthcare, Erlangen, Germany), with a dedicated 8-channel knee coil. High-resolution morphological 3D knee images were obtained using a 3D-DESS sequence with selective water excitation. The imaging parameters were as following: voxel size 0.7 × 0.6 × 0.7 mm3, TR 14.45 ms, TE 5.17 ms, flip angle 25°, FOV: 160 × 160 mm2, slice thickness 0.68 mm, matrix: 256 × 256 × 240. Sagittal T2* maps were obtained using a gradient echo sequence, utilizing five echoes for the fit: TR = 1340 ms, TE = 4.36, 11.9, 19.44, 26.98, 34.52 ms, FOV = 160.0 × 160.0 mm2, matrix = 384 × 384, flip angle = 60°, slice thickness = 3.0 mm. All subjects rested for 1 h before the MRI examination and were examined supine with the lower edge of the patella as the scanning center, minimizing motion artifacts by using sandbags and sponges.
Cartilage segmentation
Knee cartilage was automatically segmented to 21 subregions using post-processing prototype software (Siemens Chondral Health, version 2.1, Siemens Healthcare, Erlangen, Germany). This software automatically divides the knee cartilage into three parts—femoral, patellar and tibia cartilage—consisting of 21 cartilage regions (listed in Table 1 and Figure 1). Cartilage volume, thickness and T2* relaxation time were acquired by automatic segmentation, respectively. Upon completion of automatic segmentation, manual adjustment was applied to avoid joint effusion and cartilage morphology resulting in automatic identification misalignment. Image analysis done by both observers with inter-observer agreement.
Table 1.
The 21 cartilage regions of knee were automatically divided by the software
| Three parts | Femoral cartilage | Patellar cartilage | Tibia cartilage |
|---|---|---|---|
| Subregions | FMP | PLI | TLP |
| FMC | PLC | TLC | |
| FMA | PLS | TLA | |
| FTM | PMI | TMP | |
| FTC | PMC | TMC | |
| FTL | PMS | TMA | |
| FLP | |||
| FLC | |||
| FLA |
FLA, Femoral lateral anterior; FLC, Femoral lateral central; FLP, Femoral lateral posterior; FMA, Femoral medial anterior; FMC, Femoral medial central; FMP, Femoral medial posterior; FTC, Femoral trochlea central; FTL, Femoral trochlea lateral; FTM, Femoral trochlea medial; PLC, Patellar lateral central; PLI, Patellar lateral inferior; PLS, Patellar lateral superior; PMC, Patellar medial central; PMI, Patellar medial inferior; PMS, Patellar medial superior; TLA, Tibial lateral anterior; TLC, Tibial lateral central; TLP, Tibial lateral posterior; TMA, Tibial medial anterior; TMC, Tibial medial central; TMP, Tibial medial posterior.
Figure 1.
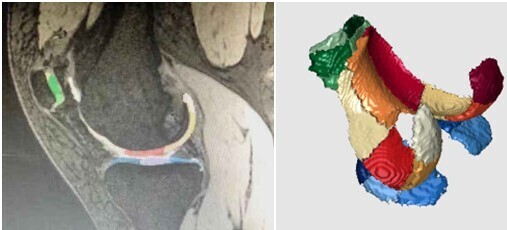
The sub-regions of knee cartilage automatically segmented by software with different colors. The green ones are patellar cartilage, the warm ones are femoral cartilage, and the blue ones are tibial cartilage.
Statistical analysis
Statistical analysis was done by the SPSS v.17.0 (Chicago, IL) and was expressed as mean ± standard deviation. p < 0.05 indicated that the difference was statistically significant. The paired rank sum test and Friedman M test were used to compare the volume, thickness and T2* relaxation parameter of cartilage and the rate of change in each parameter in the different regions.
Results
Thickness changes of subregions
The cartilage thicknesses of these subregions of FTM (Χ2 = 7.75, p < 0.05), FTL (Χ2 = 7.14, p < 0.05), TLP (Χ2 = 8.87, p < 0.05), and TMA (Χ2 = 5.87, p < 0.05) were negative significant differences, and the cartilage thickness of FLC subregion (Χ2 = 7.47, p < 0.05) (Table 2) was positive significant difference. Post-hoc test showed that the cartilage thickness of the FLC subregion was increased at 12 h post-marathon (p < 0.05; Figure 2), and the thickness of the TMA subregion was decreased at 2 months post-marathon (p < 0.05; Figure 2).
Table 2.
The thicknesses of 21 sub-regions of knee cartilage
| Sub regions | Pre-run | 12-h post-run | 2 months post-run | Χ2 | p value |
|---|---|---|---|---|---|
| FMP | 1.4460 ± 0.2165 | 1.4358 ± 0.1678 | 1.4470 ± 0.2003 | 1.000 | 0.607 |
| FMC | 1.4710 ± 0.16441 | 1.4608 ± 0.1718 | 1.4690 ± 0.2010 | 1.000 | 0.607 |
| FMA | 1.6960 ± 1.5196 | 1.6750 ± 0.1405 | 1.6050 ± 0.1697 | 1.750 | 0.417 |
| FTM | 1.6190 ± 0.1660 | 1.6133 ± 0.1601 | 1.5750 ± 0.1345 | 7.750 | 0.021 |
| FTC | 2.1540 ± 0.3617 | 2.1550 ± 0.3443 | 2.1290 ± 0.3388 | 2.480 | 0.289 |
| FTL | 1.8240 ± 0.2388 | 1.8408 ± 0.2242 | 1.7760 ± 0.2034 | 7.143 | 0.028 |
| FLP | 1.5490 ± 0.1460 | 1.5017 ± 0.1753 | 1.5000 ± 0.1425 | 1.724 | 0.422 |
| FLC | 1.7430 ± 0.2827 | 1.7783 ± 0.2837 | 1.7750 ± 0.2762 | 7.467 | 0.024 |
| FLA | 1.5620 ± 0.2480 | 1.5950 ± 0.2423 | 1.5640 ± 0.2457 | 4.323 | 0.115 |
| PLI | 1.6870 ± 0.3528 | 1.6958 ± 0.3175 | 1.6460 ± 0.2032 | 0.452 | 0.798 |
| PLC | 2.4290 ± 0.3801 | 2.4792 ± 0.3512 | 2.3810 ± 0.2666 | 3.250 | 0.197 |
| PLS | 1.7120 ± 0.3051 | 1.6867 ± 0.2265 | 1.7070 ± 0.2261 | 2.516 | 0.284 |
| PMI | 1.6920 ± 0.2138 | 1.7467 ± 0.1974 | 1.7730 ± 0.2155 | 0.452 | 0.798 |
| PMC | 2.8880 ± 0.3782 | 2.8800 ± 0.3235 | 2.9080 ± 0.2880 | 1.000 | 0.607 |
| PMS | 2.0230 ± 0.3629 | 1.9925 ± 0.2160 | 1.9300 ± 0.2385 | 0.250 | 0.882 |
| TLP | 1.8270 ± 0.3782 | 1.8708 ± 0.3168 | 1.8220 ± 0.2811 | 8.867 | 0.012 |
| TLC | 2.6370 ± 0.3935 | 2.6733 ± 0.3549 | 2.6490 ± 0.2901 | 4.323 | 0.115 |
| TLA | 1.6550 ± 0.2309 | 1.7033 ± 0.2573 | 1.6280 ± 0.1794 | 1.742 | 0.419 |
| TMP | 1.3670 ± 0.1430 | 1.3458 ± 0.1250 | 1.3210 ± 0.1187 | 2.000 | 0.368 |
| TMC | 1.7830 ± 0.1843 | 1.7458 ± 0.1908 | 1.6810 ± 0.1741 | 0.194 | 0.908 |
| TMA | 1.4090 ± 0.1543 | 1.3875 ± 0.1409 | 1.2790 ± 0.1534 | 5.871 | 0.053 |
Figure 2.

The thickness changes of each cartilage subregion in three times of MRI, asterisks indicate statistically significant difference.
Volume changes of subregions
The cartilage volumes of these subregions of FLC (Χ2 = 12.25, p < 0.05), FLA (Χ2 = 6.28, p < 0.05), TLP (Χ2 = 8.97, p < 0.05), and TMA (Χ2 = 9.74, p < 0.05) were negative significant differences (Table 3). Post-hoc test showed that the cartilage volume of the TLP subregion was increased at 12-h post-marathon (p < 0.05; Figure 3), and the volumes of the FLA and TMA subregions were decreased at 2 months post-marathon (p < 0.05; Figure 3).
Table 3.
The volumes of 21 sub-regions of knee cartilage
| Sub regions | Pre-run | 12-h post-run | 2 months post-run | Χ2 | p-value |
|---|---|---|---|---|---|
| FMP | 0.6540 ± 0.1903 | 0.6283 ± 0.1358 | 0.6250 ± 0.1776 | 0.452 | 0.798 |
| FMC | 1.0750 ± 0.2431 | 1.0592 ± 0.1924 | 1.0810 ± 0.2392 | 0.839 | 0.657 |
| FMA | 0.9920 ± 0.1854 | 0.9692 ± 0.1768 | 0.9380 ± 0.1966 | 4.323 | 0.115 |
| FTM | 0.9170 ± 0.1889 | 0.9008 ± 0.1924 | 0.8780 ± 0.1614 | 5.250 | 0.072 |
| FTC | 1.7900 ± 0.4130 | 1.6900 ± 0.4274 | 1.7250 ± 0.3806 | 1.724 | 0.422 |
| FTL | 1.7710 ± 0.3767 | 1.7233 ± 0.3332 | 1.7080 ± 0.2971 | 4.710 | 0.095 |
| FLP | 0.6480 ± 0.1327 | 0.6333 ± 0.1328 | 0.6160 ± 0.1225 | 0.065 | 0.968 |
| FLC | 1.0550 ± 0.2309 | 1.0708 ± 0.2279 | 1.0600 ± 0.2184 | 12.250 | 0.002 |
| FLA | 1.0500 ± 0.2595 | 1.0533 ± 0.2666 | 1.0300 ± 0.2338 | 6.276 | 0.043 |
| PLI | 0.2440 ± 0.0698 | 0.2583 ± 0.0798 | 0.2500 ± 0.0572 | 1.462 | 0.482 |
| PLC | 0.6650 ± 0.1523 | 0.6442 ± 0.1664 | 0.6250 ± 0.1144 | 0.600 | 0.741 |
| PLS | 0.2050 ± 0.0871 | 0.1908 ± 0.0601 | 0.1750 ± 0.0344 | 1.750 | 0.417 |
| PMI | 0.4080 ± 0.1171 | 0.4242 ± 0.1084 | 0.4290 ± 0.1280 | 0.621 | 0.733 |
| PMC | 0.9360 ± 0.1702 | 0.9092 ± 0.1628 | 0.8990 ± 0.1678 | 0.581 | 0.748 |
| PMS | 0.4090 ± 0.1484 | 0.3925 ± 0.1017 | 0.3620 ± 0.0636 | 0.897 | 0.639 |
| TLP | 0.8580 ± 0.2754 | 0.8533 ± 0.2191 | 0.8060 ± 0.1970 | 8.968 | 0.011 |
| TLC | 1.0660 ± 0.2599 | 1.0433 ± 0.2338 | 1.0260 ± 0.2227 | 5.871 | 0.053 |
| TLA | 0.5350 ± 0.1681 | 0.5317 ± 0.1607 | 0.4960 ± 0.1405 | 0.452 | 0.798 |
| TMP | 0.5210 ± 0.0690 | 0.5042 ± 0.0948 | 0.5370 ± 0.1089 | 3.935 | 0.14 |
| TMC | 0.8550 ± 0.1702 | 0.8375 ± 0.1677 | 0.8190 ± 0.1856 | 3.071 | 0.215 |
| TMA | 0.5480 ± 0.1364 | 0.5325 ± 0.1280 | 0.4780 ± 0.1195 | 9.742 | 0.008 |
Figure 3.
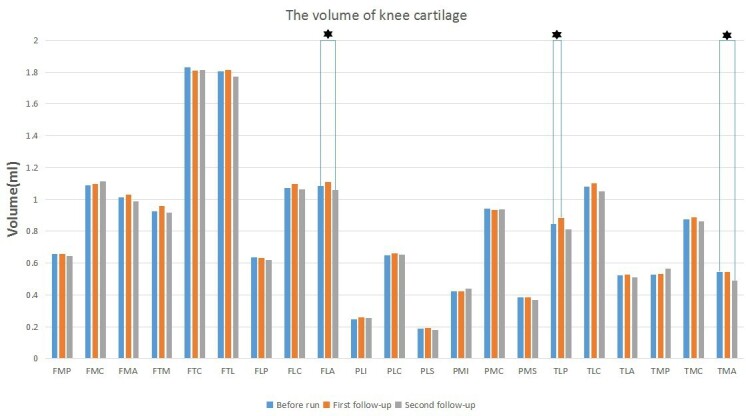
The volume changes of each cartilage subregion in three times of MRI, asterisks indicate statistically significant difference.
T2* value changes of subregions
The cartilage T2* values of these subregions of FMC (Χ2 = 7.75, p < 0.05), FTM (Χ2 = 9.25, p < 0.05), FLP (Χ2 = 9.25, p < 0.05), PMC (Χ2 = 7.00, p < 0.05), and PMS (Χ2 = 6.25, p < 0.05) were positive significant differences, and the cartilage T2* value of TMA subregion (Χ2 = 7.00, p < 0.05) was negative difference (Table 4). Post-hoc test showed that the cartilage T2* values of these subregions of FMC, FMA, FLP, FLC, FLA, PLC, PMI, and PMC were increased at 12-h post-marathon, and the cartilage T2* value of TMC subregion was decreased at 12-h post-marathon (p < 0.05; Figure 4). The cartilage T2* values of these subregions of FMC, FTM, and FLP were increased at 2 months post-marathon, and the cartilage T2* value of TMA subregion was decreased at 2 months post-marathon (p < 0.05; Figure 4).
Table 4.
The T2* values of 21 sub-regions of knee cartilage
| Sub regions | Pre-run | 12 h post-run | 2 months post-run | Χ2 | p value |
|---|---|---|---|---|---|
| FMP | 26.8140 ± 2.9093 | 26.8117 ± 2.6064 | 26.0570 ± 3.5588 | 0.750 | 0.687 |
| FMC | 24.7960 ± 3.3544 | 28.2042 ± 3.9048 | 26.4010 ± 3.0143 | 7.750 | 0.021 |
| FMA | 23.7490 ± 3.4570 | 27.2967 ± 2.8618 | 24.7370 ± 4.8945 | 5.250 | 0.072 |
| FTM | 26.0240 ± 3.2980 | 29.5058 ± 7.0735 | 27.5550 ± 4.1877 | 9.250 | 0.01 |
| FTC | 31.7720 ± 5.7471 | 32.6842 ± 8.3347 | 31.3410 ± 5.2931 | 1.000 | 0.607 |
| FTL | 29.5850 ± 3.0265 | 29.8358 ± 4.4919 | 29.7330 ± 2.3493 | 0.250 | 0.882 |
| FLP | 23.4380 ± 2.8626 | 25.7108 ± 2.5188 | 25.0240 ± 3.0547 | 9.250 | 0.01 |
| FLC | 27.0000 ± 2.7988 | 30.3275 ± 4.7437 | 28.2640 ± 3.6302 | 5.250 | 0.072 |
| FLA | 24.5220 ± 6.3202 | 29.3975 ± 5.3554 | 25.2360 ± 3.8326 | 4.000 | 0.135 |
| PLI | 22.5560 ± 5.3755 | 25.3042 ± 4.7913 | 23.4290 ± 5.6574 | 2.250 | 0.325 |
| PLC | 22.4950 ± 5.5133 | 25.6225 ± 3.2824 | 23.4570 ± 3.7510 | 4.750 | 0.093 |
| PLS | 21.9810 ± 6.3075 | 24.1075 ± 2.6461 | 22.9980 ± 2.7201 | 4.750 | 0.093 |
| PMI | 25.1500 ± 2.8294 | 28.7158 ± 3.3098 | 27.4580 ± 6.7601 | 4.000 | 0.135 |
| PMC | 27.7510 ± 4.2947 | 31.7875 ± 3.0928 | 30.5200 ± 6.6304 | 7.000 | 0.03 |
| PMS | 23.8790 ± 4.3198 | 26.2150 ± 3.6092 | 26.3940 ± 6.3590 | 6.250 | 0.044 |
| TLP | 23.7470 ± 3.0178 | 23.0267 ± 2.5010 | 22.5250 ± 2.0028 | 2.250 | 0.325 |
| TLC | 21.1280 ± 3.0559 | 20.1642 ± 2.4046 | 21.1280 ± 3.5948 | 1.000 | 0.607 |
| TLA | 22.8000 ± 4.7100 | 22.5425 ± 2.8664 | 27.9630 ± 8.7169 | 2.250 | 0.325 |
| TMP | 18.8930 ± 2.5052 | 18.5575 ± 2.6489 | 18.4490 ± 1.9549 | 3.250 | 0.197 |
| TMC | 20.4810 ± 3.2483 | 17.9325 ± 1.7727 | 18.3240 ± 2.8053 | 5.250 | 0.072 |
| TMA | 23.7690 ± 5.6100 | 20.9917 ± 4.0921 | 20.9830 ± 6.2824 | 7.000 | 0.03 |
Figure 4.
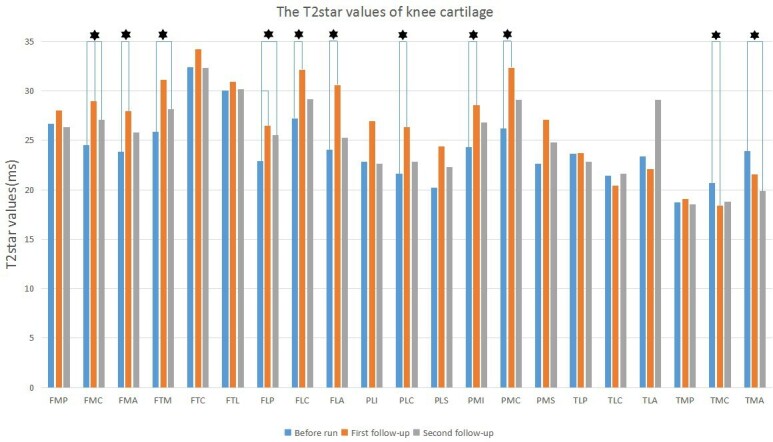
The T2* values changes of each cartilage subregion in three times of MRI, asterisks indicate statistically significant difference.
Discussion
Regarding the change of cartilage volume after long-distance running, scholars hold different views. Some scholars claimed that the mechanical load caused by long-distance running will not cause internal pressure on bone and cartilage because the developed anatomy of the knee joint, which prevents the cartilage volume from changing.8 On the contrary, Kessler founded that the cartilage volume of the patella and tibia were reduced by 7.0 and 5.1% after running 20 km, respectively. After 1 h rest, the cartilage volume is recovered.17
The above studies explored the short-term effects of running on cartilage deformation. In this study, the observation time windows were placed at before, 12 h after and 2 months after marathon. The results showed that the most sub-regions of cartilage without significant differences of volume and thickness among three MRI examinations. The significant differences were seen in FLC, TLP and TMA sub-regions both volume and thickness among three MRI examinations. We believed that the cartilage volume and thickness have changed to a certain extent after running. It can be inferred from this study, among the effects of marathon on cartilage morphology, the change of cartilage thickness was more sensitive than the change of cartilage volume in the evaluation of the impact of short time, because the subregions of cartilage thickness changes overlapped more with the subregions of cartilage T2* changes. However, in the evaluation of the effect of relatively long-time, the change of cartilage volume was more specific, and the effect of exercise on the subregion was more persistent where the cartilage volume was significantly reduced. After a recovery period of one hour, it was found that the reduction rate of cartilage volume was smaller than before, and it was no longer statistically significant, which is consistent with our research results.17 A study was focused on the short-term effects of marathon exercise on cartilage volume and thickness, and found that the volume and thickness of lateral femur cartilage decreased by a mean of 3.2 ± 3.0 and 1.7 ± 1.6%, respectively. No significant changes in cartilage volume and thickness were observed at other regions.4 The reduction of cartilage volume post marathon required a certain amount of time to recover, while the specific time for the cartilage volume to recover pre-marathon level after marathon remains to be studied and discussed.
In recent years, many scholars have used T2 mapping or T2 * mapping methods to explore the changes of cartilage during marathon, and the results obtained are not the same. T2-mapping technology is a multi-level multi-echo spin echo sequence. The T2 value is mainly affected by the water content in the cartilage and the fluidity of the water. The increase of T2 value often represents an increase in water content and loss of anisotropy of collagen fibers.3 Similar to T2-mapping, T2*-mapping is sensitive to the extracellular water content and the interaction between water molecules and collagen fibers. The high T2* value reflects high water content and mobility. The difference is that T2 mapping imaging is generally a spin echo sequence, and the image is susceptible to magnetic field inhomogeneity, while T2* mapping uses a multi echo gradient sequence, which has fast imaging speed, feasible three-dimensional acquisition, high spatial resolution, and comprehensive articular surface coverage.15
In this study, T2* map was used to evaluate the changes of biochemical composition of knee joint cartilage before and after the marathon. The results showed that T2* values of femoral cartilage, medial tibial cartilage, and medial patella area were significantly increased, except for the femoral trochlear. It was consistent with the results of Hesper’s.16 This was mainly related to the partial loss of glycosaminoglycan (GAG) in cartilage, which weakened the restriction of macromolecular substances on the mobility of free water, and the increased mobility of water lead to an increase of T2 * relaxation time. In addition, the high intensity of repeated loads made the skeletal collagen fiber changes resulting in the partial loss of anisotropy of collagen fibers. That was also an important factor for the increase of T2* relaxation time.
There were some studies inconsistent with the results of this study. In the Mosher’s study, the T2 relaxation time of the superficial cartilage of the femur and tibia was decreased after the participants finished 30 min (approximately 5000 m) run.18 It was focus on the superficial cartilage of knee joint, while, this study was focus on the full layer of articular cartilage. Another aspect, there may be some relationship between the T2 or T2* relaxation time changes with the running distance and load bearing time. After short distance running, such as a half marathon, the values of T2 and T2 * maybe tend to decrease after running. The possible reason is that the fluid in the superficial cartilage transfer to the deep side with load bearing; after long distance running, T2 and T2 * values maybe tend to increase, such as full marathon, super marathon, due to partial degradation of proteoglycan in cartilage and changes in skeletal collagen fiber, resulting in partial loss of anisotropy of collagen fiber. Luke studied the changes of T2 value in the cartilage of the knee joint after the full marathon. The results showed that the T2 value of cartilage increased significantly within 48 h after running, which is the same as this study.
We found that the areas where the force concentrated have significant differences of T2* values after marathon compared with pre-marathon. The number of cartilage subregions of T2* value changed post-marathon was significantly higher than that of morphological changed. It indicated that T2* value was more sensitive to the biochemical and structural changes of cartilage than morphology. The T2* values of the femoral trochlear did not change significantly, while the T2* values of the medial cartilage area of the femur, tibia, and patella were obviously increase. It may be due to the large contact area of the medial tibiofemoral platform cartilage of the knee joint during exercise, and the stress will also act on the patellar platform cartilage. What’s more, the 60–80% of the knee pressure load is transmitted through the medial cartilage.
After comparing the T2* value changes between the pre-marathon and the 2 month recovery period, the T2* values of most areas were close to the pre-marathon level after a period of recovery, indicating that with the decrease of activity, the changes of the biochemical components would be eventually recovered. It was suggested that long-distance running would not cause continuous fluid changes. However, the T2* values of the medial femoral medial and lateral femoral posterior cartilage were still significantly different from pro-marathoon level after the recovery period, and further follow-up observation was essential.
After marathon, the cartilage thickness and volume were both changed in TLP and TMA subregions, and the TMA subregion also showed significant T2* changes. The results indicated that these two subregions were the high-frequency injury sites of knee joint post-marathon, and the changes of these two subregions should be followed up after marathon, so as to find the signs of sports injury early, to adjust the exercise intensity in time, and to avoid irreversible cartilage injury.
Limitations
This current study had several limitations. First, this study observed the changes of knee joint cartilage of amateur marathon athletes in the short- and relative long-time term after marathon. The results proved that the T2* value had changed to some extent, but the long-term impact of the marathon needed to be judged, so further follow-up and increasing the sample capacity were necessary. In addition, the time span of first measurement after marathon was large (12 h). Despite these limitations, this study still demonstrated statistically significant results indicated biochemical changes in knee cartilage after a marathon.
Conclusion
In conclusion, biochemical imaging was more sensitive than morphological examinations to study early changes in knee cartilage. The T2* values of knee cartilage in amateur marathon runners showed a “first rising and then decrease” trend, suggested that articular cartilage had a degree of reversible change during marathon running. The TLP and TMA subregions needed follow-up after marathon, so as to avoid irreversible cartilage injury due to high exercise intensity.
Footnotes
Funding: This research was supported by the projects from National Natural ScienceFoundation of China (No: 81701645).
Ethics approval and consent to participate: This paper was approved by all authors. Availability of data and material: The datasets used during the current study are available from the corresponding author upon request.
Contributor Information
Ping Zhang, Email: tj2012zp@163.com.
Baohai Yu, Email: 15533681084@163.com.
Ranxu Zhang, Email: heroine-zp321@163.com.
Xiaoshuai Chen, Email: 296545669@qq.com.
Shuying Shao, Email: 15533637121@163.com.
Yan Zeng, Email: 15633687121@163.com.
Jianling Cui, Email: jianlingcui@sina.com.
Jian Zhao, Email: zhaojiansohu@126.com.
REFERENCES
- 1.Marathon drives new wave of consumption in Chinese sports industry. 2019. Available from: http://www.china.org.cn/sports/2019-05/29/content_74833747.htm.
- 2.Knechtle B, Nikolaidis PT. Physiology and pathophysiology in Ultra-Marathon running. Front Physiol 2018; 9: 634. doi: 10.3389/fphys.2018.00634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qiu L, Perez J, Emerson C, Barrera CM, Zhong J, Nham F, et al. Biochemical changes in knee articular cartilage of novice half-marathon runners. J Int Med Res 2019; 47: 5671–9. doi: 10.1177/0300060519874140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hinterwimmer S, Feucht MJ, Steinbrech C, Graichen H, von Eisenhart-Rothe R. The effect of a six-month training program followed by a marathon run on knee joint cartilage volume and thickness in marathon beginners. Knee Surg Sports Traumatol Arthrosc 2014; 22: 1353–9. doi: 10.1007/s00167-013-2686-6 [DOI] [PubMed] [Google Scholar]
- 5.Tschopp M, Brunner F. Diseases and overuse injuries of the lower extremities in long distance runners. Z Rheumatol 2017; 76: 443–50. doi: 10.1007/s00393-017-0276-6 [DOI] [PubMed] [Google Scholar]
- 6.Fields KB. Running injuries - changing trends and demographics. Curr Sports Med Rep 2011; 10: 299–303. doi: 10.1249/JSR.0b013e31822d403f [DOI] [PubMed] [Google Scholar]
- 7.Jin J. JAMA patient page. running injuries. JAMA 2014; 312: 202. doi: 10.1001/jama.2013.283011 [DOI] [PubMed] [Google Scholar]
- 8.Hohmann E, Wörtler K, Imhoff AB. Mr imaging of the hip and knee before and after marathon running. Am J Sports Med 2004; 32: 55–9. doi: 10.1177/0363546503258904 [DOI] [PubMed] [Google Scholar]
- 9.Roseti L, Desando G, Cavallo C, Petretta M, Grigolo B. Articular cartilage regeneration in osteoarthritis. Cells 2019; 8: 1305: E130523 10 2019. doi: 10.3390/cells8111305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willick SE, Hansen PA. Running and osteoarthritis. Clin Sports Med 2010; 29: 417–28. doi: 10.1016/j.csm.2010.03.006 [DOI] [PubMed] [Google Scholar]
- 11.Cymet TC, Sinkov V. Does long-distance running cause osteoarthritis? J Am Osteopath Assoc 2006; 106: 342–5. [PubMed] [Google Scholar]
- 12.Chen M, Qiu L, Shen S, Wang F, Zhang J, Zhang C, et al. The influences of walking, running and stair activity on knee articular cartilage: quantitative MRI using T1 Rho and T2 mapping. PLoS One 2017; 12: e0187008. doi: 10.1371/journal.pone.0187008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoessly ML, Wildi LM. Magnetic resonance imaging findings in the knee before and after long-distance Running-Documentation of irreversible structural damage? A systematic review. Am J Sports Med 2017; 45: 1206–17. doi: 10.1177/0363546516656180 [DOI] [PubMed] [Google Scholar]
- 14.Esculier J-F, Jarrett M, Krowchuk NM, Rauscher A, Wiggermann V, Taunton JE, et al. Cartilage recovery in runners with and without knee osteoarthritis: a pilot study. Knee 2019; 26: 1049–57. doi: 10.1016/j.knee.2019.07.011 [DOI] [PubMed] [Google Scholar]
- 15.Ohashi S, Ohnishi I, Matsumoto T, Bessho M, Matsuyama J, Tobita K, et al. Measurement of articular cartilage thickness using a three-dimensional image reconstructed from B-mode ultrasonography mechanical scans feasibility study by comparison with MRI-derived data. Ultrasound Med Biol 2012; 38: 402–11. doi: 10.1016/j.ultrasmedbio.2011.11.019 [DOI] [PubMed] [Google Scholar]
- 16.Hesper T, Miese FR, Hosalkar HS, Behringer M, Zilkens C, Antoch G, et al. Quantitative T2(*) assessment of knee joint cartilage after running a marathon. Eur J Radiol 2015; 84: 284–9. doi: 10.1016/j.ejrad.2014.11.021 [DOI] [PubMed] [Google Scholar]
- 17.Kessler MA, Glaser C, Tittel S, Reiser M, Imhoff AB. Recovery of the menisci and articular cartilage of runners after cessation of exercise: additional aspects of in vivo investigation based on 3-dimensional magnetic resonance imaging. Am J Sports Med 2008; 36: 966–70. doi: 10.1177/0363546507313093 [DOI] [PubMed] [Google Scholar]
- 18.Mosher TJ, Liu Y, Torok CM. Functional cartilage MRI T2 mapping: evaluating the effect of age and training on knee cartilage response to running. Osteoarthritis Cartilage 2010; 18: 358–64. doi: 10.1016/j.joca.2009.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]


