Abstract
B10 cells are the most frequently investigated subset of Breg cells, capable of suppressing immunity through the expression of the immunosuppressive cytokine IL-10. B10 cells are enriched in phenotypically diverse B-cell subsets. Recently, CD9 was identified as a marker of B10 cells in mice (human B10 cells have a separate set of markers that do not overlap with murine B10 cells). Together with a combination of other B10 markers, CD9 can be used to distinguish both mature and immature B10 cells from nonregulatory B cells and support selective purification of B10 cells. Here we provide five methods for the characterization and activity evaluation of CD9+ B cells. The first method is used for the preparation of leukocytes, the second and third are used for the characterization of CD9+ B cells, while the last two methods serve to evaluate CD9+ B-cell activities. Finally, we detail the purification of RNA from B10 cells and the performance of transcriptomic assays.
Keywords: Regulatory B cells, B10 cell, CD9, IL-10, Transcriptome
1. Introduction
B cells play important roles in both promoting and suppressing immune responses [1]. B cells are capable of antigen presentation, antibody production, proinflammatory cytokine production, and acting as co-stimulators for immune promotion. Moreover, B cells can produce immune-suppressive cytokines such as IL-10, IL-35, and TGF-b1, induce regulatory T cells (Treg), and eliminate autoantigens for immune suppression [2]. Regulatory B cells (Breg) are B cells with immune suppressive functions. Bregs have been shown to be involved in various autoimmune- and inflammation-induced diseases [3], including systemic lupus erythematosus [4] and allergies [5], both in patients and in mouse models.
Bregs are found to be enriched in phenotypically diverse B-cell subsets [6]. Mice and humans possess distinct sets of Breg markers, and there is a scarcity of unique markers in either organism that can exclusively and exhaustively identify Breg cells. B10 cells constitute one of the most studied Breg subsets, capable of secreting IL-10 and thereby of suppressing immunity. In a search of unique markers that could allow B10 purification and biochemical characterization—important since the lack of unique surface markers has foiled detailed investigations into the origin, development, and immunological roles of B10 cells—B10 cell surface markers have been updated regularly from the original CD19+CD1dhiCD5+ phenotype [3, 7]. The identification of B10 cells requires stimulation with specific reagents such as LPS plus PMA, ionomycin, and monensin for cytokine secretion, with this stimulation possibly masking the real differences between B10 cells and other kinds of B cells, especially in terms of expression profiles.
We and others have identified CD9, a tetraspanin family transmembrane protein, as a marker of B10 cells in mice [6]. CD9 can distinguish both mature and immature B10 cells from nonregulatory B cells. Thus, it provides a system to study unperturbed gene expression profiles of Breg progenitors and Bregs without the stimulation required in the past to prompt the IL-10 secretion necessary for Breg identification. With the designation of CD9 as a marker of B10 cells, the identification of physiologically relevant transcription factors driving B10 differentiation becomes a real possibility. Furthermore, as CD9+ B cells possess immune suppressive function(s) and can be enriched easily, another application is their adoptive transfer into murine autoimmune disease models to study their immunoregulatory function in vivo. The elimination of immunosuppressive IL-10-expressing plasma cells could allow cytotoxic T-cell-dependent eradication of therapy-resistant tumors [8]. In this light, the possibility that CD9+ B-cell depletion could be an effective alternative to specific depletion of B10 cells for tumor immunotherapy should be investigated. Finally, transcriptomics of B10 cells can be performed on B10 cells that have been purified based on their positive expression of CD9. In this chapter we provide five methods for the characterization and functional evaluation of CD9+ B cells.
2. Materials
2.1. Equipment
5 mL (12 × 75 mm) and 14 mL (17 × 100 mm) polystyrene round-bottom tubes with snap caps.
50 mL (33 × 115 mm) conical centrifuge tubes.
35 mm dish or 6-well plate.
Cell culture plates: sterile 6-well, 24-well, or 48-well flat-bottom culture plates.
Pipettes, sterile pipette tips and disposable transfer pipettes.
Scissors, sterile cell strainers (40 μm) and sterile 1–10 mL syringes.
Device for immunomagnetic isolation of the cell type of interest (e.g., columns, magnets, and stand).
Refrigerated centrifuge with swinging-bucket rotor suitable for 5 mL, 14 mL, and 50 mL tubes.
BSL-2 cabinet/hood for cell culture, and cell culture supplies.
Vortexer.
CO2 incubator, 37 °C, 5% CO2.
Flow cytometer and sorter with multiparameter cytometry capability.
Bioanalyzer (Agilent Technologies).
Wet ice, to keep cells and reagents at 4 °C.
2.2. Mice
Female or male C57BL/6 J mice.
Female or male, 6–8 weeks old CD19 KO mice.
2.3. Cell Preparation, Culture, and Stimulation
Commercially available sterile Dulbecco’s phosphate-buffered saline (D-PBS) without Ca2+ and Mg2+. Store at 4 °C or on ice.
Ammonium-Chloride-Potassium (ACK) lysing buffer for the lysis of red blood cells.
MACS buffer: 0.5% bovine serum albumin (BSA) and 2 mM EDTA in D-PBS. Sterilize by filtration and keep sterile. Store at 4 °C or on ice.
Lymphocytes culture medium (LCM): 10% fetal bovine serum (FBS), 100 IU/mL penicillin, 100 μg/mL streptomycin, 2 mM L-Glutamine, 2 mM sodium pyruvate, 2 mM MEM NEAA, 4 mM HEPES buffer, and 55 μM 2-mercaptoethanol in sterile RPMI 1640.
Staining buffer: 2% FBS in D-PBS. Store at 4 °C.
Mouse CD19 beads for immunomagnetic isolation of B cells.
Purified anti-mouse CD40 antibody (Clone HM40–3).
Lipopolysaccharides (LPS) from Escherichia coli O111:B4: dissolve at 5 mg/mL in sterile D-PBS. Sterilize with a 0.2-μm pore size filter and store in 200 μL aliquots at −20 °C. The thawed aliquots should be kept at 4 °C.
Phorbol 12-myristate 13-acetate (PMA): dissolve at 100 ng/μ L in dimethyl sulfoxide (DMSO) and store in 20 μL aliquots at −20 °C.
Ionomycin: dissolve at 1 μg/μL in DMSO and store in 20 μL aliquots at −20 °C.
Monensin: the final working concentration is 2 μM. Store at 4 °C.
Dye for the discrimination of viable from nonviable cells in multicolor flow cytometric applications (e.g., LIVE/DEAD™ Fixable Near IR Dead Cell Stain Kit, LIVE/DEAD™ Fixable Blue Dead Cell Stain Kit, for UV excitation from Thermo Fisher). Reconstitute the dye according to the manufacturer’s instructions.
Violet fluorescent cell proliferation dye such as VPD450 (Becton Dickinson) or eFluor 450 (Thermo Fisher).
2.4. Cell Immunofluorescence Staining, Sorting, and Enrichment
Purified anti-mouse CD16/CD32 (Clone 2.4G2) mAb.
Fluorochrome-conjugated antibodies (see Table 1). All antibodies should be tittered to identify optimal concentration starting from specific manufacturer’s indications.
Cell fixation/permeabilization solution and permeabilization buffer.
1.5% formaldehyde fixing solution: dilute 36% formalin 24-fold in D-PBS, and store at room temperature for up to 1 month.
Mouse IL-10 secretion assay (Miltenyi Biotec).
Mouse CD90.2 beads.
FITC-microbeads.
DNase I: diluted at the final concentration of 20 mg/mL in buffer containing 20 mM Tris-HCl, 1 mM MgCl2, 50% (w/v) glycerol, pH 7.5. Store this solution at −15 to −25 °C for up to 18 months.
Table 1.
List of antibodies
| Staining | Target | Clone | Fluorophore |
|---|---|---|---|
| B cells | CD19 | 6D5 | APC |
| CD9 | MZ3 | FITC | |
| IL-10 | JES5-16E3 | PE | |
| T cells | CD4 | GK1.5 | PE/Cy7 |
| CD25 | PC61 | FITC | |
| Rat IgG2b | RTK4530 | PE | |
2.5. RNA Profiling of B10 Cells
Guanidine thiocyanate and phenol reagent for phenol–chloroform-based RNA extraction (e.g., Trizol by Thermo Fisher, Tri-Reagent by Sigma).
rRNA removal kit.
Illumina TruSeq and TruSeq Stranded.
Ingenuity Pathway Analysis (IPA) software program (Qiagen).
2.6. Ex Vivo Function of CD9+ B Cells
Transwell for 96-well plates, with a 0.4-μm membrane pore size.
96-well, cell culture-treated, U-shaped-bottom microplate.
Purified anti-mouse CD28 (Clone 37.51) antibody.
Purified anti-mouse CD3e (Clone 145–2C11) antibody.
Purified anti-mouse CD9 (Clone MZ3) antibody and its isotype control. Customized without sodium azide.
2.7. Reconstitution of B-Cell-Deficient Mice and Contact Hypersensitivity (CHS) Assay
4-Ethoxymethylene-2-phenyl-2-oxazolin-5-one (oxazolone): reconstitute the powder at 100 mg/mL in acetone/olive oil (4:1 v/v).
Oxazolone solution: 10 mg/mL oxazolone in acetone/olive oil (4:1 v/v).
A constant force, calibrated digital thickness gauge.
Isoflurane and anesthesia ventilators (see Note 1).
0.5% proparacaine hydrochloride ophthalmic solution (see Note 1).
3. Methods
3.1. B-Cell Preparation and Culture
For cell culture or further application in vivo, all media, solutions, tips, and tubes should be sterile, and all operations should be performed in a biosafety cabinet (tissue culture hood).
3.1.1. Isolation of B Cells from the Spleen
Collect the spleens from C57BL/6 J mice and homogenize with the plunger of a 10-mL syringe on a 40-μm cell strainer in a 35-mm dish or 6-well plate containing 3 mL ice-cold D-PBS (see Note 2).
Transfer the homogenized solution to a 14-mL round-bottom tube and wash the 35-mm dish or 6-well plate with 2-mL D-PBS. Combine the suspensions and pipet several times for further disassociation of cells from the tissue, then spin at 500 × g for 8 min at 4 °C.
Discard the supernatant and resuspend the cell pellet in 5 mL of ACK lysing buffer by gentle brief vortex, and keep at room temperature (RT) for 5 min with occasional shaking, then spin at 300 × g for 5 min at 4 °C (see Note 3).
Discard the supernatant, resuspend the pellet in 5 mL of MACS buffer and then spin at 300 × g for 5 min to remove residual ACK lysis buffer. The obtained pellet consists of spleen mononuclear cells that can be either stained with fluorescence antibodies for analysis or cell sorting (see Subheading 3.4) or used to purify CD19+ B cells (step 5).
Purify CD19+ cells by immunomagnetic isolation. Use magnetic microbeads following the instructions outlined in the kit.
3.1.2. Isolation of Peritoneal B Cells
Isolate leukocytes from the peritoneal cavity by injecting 10 mL of ice-cold MACS buffer with a syringe into the peritoneal cavity, followed by careful aspiration of the fluid back into the syringe after a gentle palpitation of the abdomen. Use the appropriate number of mice which allows to obtain a leukocyte population sufficient to meet the needs of downstream analyses.
Wash collected cells once with MACS buffer and then proceed to perform the CD19+ B-cell isolation, employing the same protocol used in Step 5 of Subheading 3.1.1.
3.1.3. B-Cell Stimulation for B10 and B10pro Cell Analysis
The primary B cells enriched from splenocytes contain both B10 cells and B10pro cells which are capable of differentiating into B10 cells after culture with the anti-CD40 antibody for 48 h. For intracellular staining of IL-10, cells should be cultured for 5 h with 10 mg/mL LPS, 50 ng/mL PMA, 500 ng/mL ionomycin, and 2 mM monensin (LPI + M), which increase the accumulation of IL-10 by blocking the IL-10 transport process. For the cell sorting of alive IL-10+ B cells, cells should be stimulated with LPI rather than LPI + M for 4 h: taking advantage of the IL-10 secretion assay developed by Miltenyi Biotec, secreted IL-10 is captured by the catch reagent on the cell surface and cells can be stained for the cytokine without the need of permeabilization.
To induce B10pro cell differentiation into B10 cells, culture single-cell CD19+ B cells at 2 × 106 cells/mL in LCM containing 1 μg/mL purified anti-mouse CD40 antibody for 48 h at 37 °C in a tissue culture incubator with a 5% CO2 atmosphere.
For B10 cell analysis, culture single-cell primary CD19+ B cells at 2 × 106 cells/mL in LCM containing LPI + M at 37 °C in a tissue culture incubator with a 5% CO2 atmosphere for 5 h to induce IL-10 production (see Note 4).
For B10 and B10pro cell analysis, culture single-cell CD19+ B cells at 2 × 106 cells/mL in LCM containing 1 μg/mL purified anti-mouse CD40 antibody for 48 h plus LPI + M for the terminal 5 h at 37 °C in a tissue culture incubator with a 5% CO2 atmosphere.
3.1.4. Differentiation of B10 cells from CD9+ B Cells
Stain the purified B cells obtained from Subheadings 3.1.1 and 3.1.2 with anti-CD19 and -CD9 antibodies, following the method of cell surface staining outlined in Subheading 3.2, steps 1–5). Resuspend the cells in D-PBS at 0.5–1 × 107 cells/mL and proceed to fluorescence-activated cell sorting (FACS) of viable CD19+CD9+ and CD19+CD9− cells (see Note 5).
Culture purified B cells at 2 × 106 cells/mL in 6-well plates with LCM containing LPI for 4 h to induce IL-10 secretion. Stain responding cells, in terms of IL-10 secretion, using the IL-10 secretion assay kit following manufacturer’s instructions and then stain the cells with CD19 and CD9 antibodies as previously described. Isolate viable CD9−IL10−, CD9+IL10−, and CD9+IL10+ B cells through FACS (see Fig. 1 and Note 5).
Culture the sorted CD9+, CD9−, CD9−IL10−, CD9+IL10−, and CD9+IL10+ B cells in 96-well plates with LCM containing 1 μg/mL purified anti-mouse CD40 antibody for 48 h at 37 °C plus LPI + M for the terminal 5 h.
At the end of the stimulation step, collect the cells for flow cytometry analysis of CD9+ Breg cells (see Subheading 3.2).
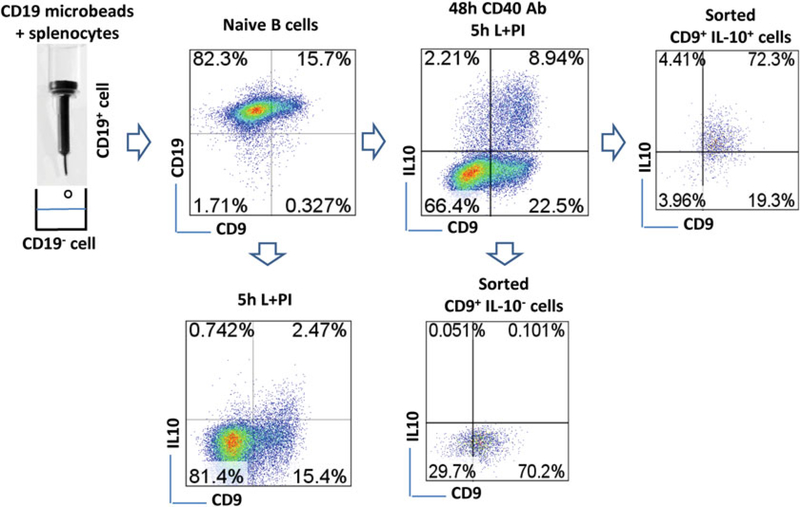
Fig. 1.
Cell sorting. Splenocytes are incubated with CD19 microbeads and then naïve CD19+ B cells are enriched through immunomagnetic separation. The frequency of CD19+CD9+ B cells is shown in the plot. Dot plots also show the expression of CD9 and IL-10 in CD19+ B cells which are treated either with LPI for 5 h or with an anti-CD40 antibody for 48 h plus LPI for the last 5 h, after staining with an IL-10 secretion assay kit as described in Subheading 3.1.4. The purity of CD9+IL-10+ and CD9+IL-10− B cells sorted from the cells cultured for 48 h is shown. After sorting, the fluorescence of the sorted cells demonstrates obvious attenuation
3.2. Flow Cytometry Analysis of CD9+ Breg Cells
The protocol outlined below allows the user to follow the expression of IL-10, CD9, and other reported Breg markers on B cells stimulated with either LPI +M for 5 h or with anti-CD40 antibody for 48 h plus LPI + M for the terminal 5 h. After staining, B cells should be analyzed by flow cytometry. For both cell surface and intracellular staining, cells should be kept on ice to prevent cell death and clogging. When cell surface staining is part of a FACS-based isolation protocol, then antibody staining should be performed in a biosafety cabinet. Conversely, when the procedure involves steps of intracellular staining, all the work can be performed on the bench without the use of a tissue culture hood.
Harvest the cultured cells to 5 mL tubes on ice, centrifuge at 300 × g for 5 min at 4 °C. Discard the supernatant and resuspend the pellet in 1 mL ice-cold D-PBS, centrifuge at 300 × g for 5 min at 4 °C.
Add 100 μL of D-PBS containing the live/dead dye and anti-mouse CD16/CD32 mAb diluted from the stock according to manufacturer’s instructions. Gently vortex the tube briefly and incubate for 15 min on wet ice (~ 4 °C) in the dark (see Note 6).
Add 1 mL of ice-cold D-PBS to each tube to resuspend the cells and then centrifuge at 300 × g at 4 °C for 5 min to pellet the cells.
Proceed to cell surface staining. Add 100 μL of a mixture of anti-CD19 and anti-CD9 antibodies (see Table 1) diluted in staining buffer according to manufacturer’s instructions. A titration of antibody can be performed. Vortex briefly and then incubate for 20–30 min on wet ice in the dark (see Notes 6 and 7).
Add 1 mL of ice-cold D-PBS, centrifuge at 300 × g at 4 °C for 5 min to pellet the cells and discard the supernatant. Repeat once.
Thoroughly resuspend the cell pellet in fixation/permeabilization solution following manufacturer’s instructions. Incubate on wet ice in the dark for 20 min.
Add 1 mL of perm/wash buffer and centrifuge at 500 × g at 4 °C for 5 min. Discard the supernatant. Repeat once.
Thoroughly resuspend the permeabilized cells in 100 μL of ice-cold perm/wash buffer containing the PE anti-mouse IL-10 antibody, diluted according to manufacturer’s instructions. A titration of antibody can be performed. Incubate the cell mixture on wet ice for 30 min in the dark.
Wash the cells twice with ice-cold perm/wash buffer and resuspend the cell pellet in 250 μL of ice-cold 1.5% formaldehyde fixative. Mix the samples thoroughly by vortexing.
Keep the cells on ice or refrigerated at 4 °C before analyzing the stained cells by flow cytometry (see Fig. 1).
3.3. RNA Profiling of CD9+ B10 cells for the Analysis of the Transcriptome
3.3.1. Induction and Isolation of CD9+IL-10+ B Cells
Induce B10pro cell differentiation as in step 1 of Subheading 3.1.3. During the last 4 h of B-cell stimulation through the anti-CD40 mAb, add LPI to induce IL-10 secretion.
Stain and isolate the CD40-responding cells based on their secretion of IL-10 using the IL-10 secretion assay kit and following manufacturer’s instructions, as described previously [9]. Briefly, collect and wash the cells, incubate the cells with the IL-10 catch reagent, and culture the cells with LCM containing LPI for one additional hour to let the reagent on the cells catch the secreted IL-10. Stain the cells with the IL-10 detection antibody provided by the kit.
Collect the B cells and stain for dead cells and for the CD9 surface marker (see Subheading 3.2).
Isolate viable CD9+IL10− and CD9+IL10+ B cells through FACS. Collect cells in LCM (see Note 5).
3.3.2. RNA Preparation
Transfer the FACS-sorted CD9+IL10− and CD9+IL10+ populations to 50 mL tubes and add an equal volume of D-PBS. Centrifuge the cells at 500 × g at 4 °C for 10 min.
Discard the supernatant and resuspend the cell pellet in 1 mL of a guanidine thiocyanate and phenol reagent, such as Trizol™. Total RNA is prepared following manufacturer’s instructions. Resuspend the RNA in 30 μL of the indicated buffer.
Deplete the rRNA via a rRNA removal kit, following manufacturer’s instructions. Quantify the RNA amount by a device such as a Bioanalyzer (see Note 8).
Send the rRNA-depleted total RNA to an internal or external facility for RNA sequencing.
3.3.3. RNA Profiling
Libraries are prepared with Illumina TruSeq and TruSeq Stranded total RNA sample prep kits, and then sequenced with 50–60 million of 2 × 100 bp paired raw passing filter reads on an Illumina HiSeq 2000 V3 instrument.
All reads of total RNAs are first mapped to the mouse reference genome (mm9) with TopHat (v. 1.3.2). Cufflinks (v. 1.2.1) is subsequently applied to assemble the whole transcriptome and to identify all possible transcripts.
To obtain short RNAs, set the overlap radius as 1 and merge repeated samples. All resulting transcripts can be further annotated using a comprehensive collection of RNA databases including mRNA, snoRNA, long intergenic noncoding RNA (lincRNA), microRNA, and other annotated noncoding RNAs.
Once the transcriptome has been generated, the individual transcripts can be annotated. Specifically, all assembled transcripts can be overlapped with known RNAs in the collected database (information for source of coordinates of various RNAs can be accessed from [6]), and the transcripts can be annotated by their adjacent known RNAs. Biological replicates indicate that each RNA-seq data set should be generated from B cells isolated from separate mice.
The generated list of differentially expressed factors can be analyzed through the “Core Analysis” option in the IPA software program [10]. Rank change (RC) and fold change (FC) levels of various noncoding RNAs, including long noncoding RNA (lnRNA), as well as mRNAs compared between B10+ and B10− cells can be analyzed.
3.4. Ex Vivo Activity of CD9+ B Cells
The protocol outlined here below allows the evaluation of the immune suppressive activity of CD9+ B cells through a T-cell proliferation assay. CD4+CD25− T cells are sorted from the CD19− non-B cell fraction, stained with a violet fluorescent cell proliferation dye such as VPD450, and then seeded in 96-well round-bottom plates coated with the anti-CD3e antibody at an appropriate density. CD9+ and CD9− B cells are sorted from the CD19+ B-cell fraction and then cocultured with VPD450-stained CD4+CD25− T cells. The coculture is treated with or without 10 μg/mL of anti-CD9 blocking antibody or of its isotype control to check whether CD9 is involved in the suppressive activity of CD9+ B cells.
3.4.1. T-Cell Proliferation Assay
Pretreat each well of a 96-well round-bottom plate with 100 μL of 5 μg/mL anti-CD3e antibody and then incubate overnight at 4 °C. Wash twice with 200 μL D-PBS per well before seeding cells for the T/B-cell coculture.
Isolate splenocytes and collect both the CD19+ B-cell and CD19− non-B-cell fractions using CD19 microbeads, following the instruction of the kit.
Prepare the T-cell fraction for the T/B-cell coculture. Stain unstimulated CD19− cells with anti-CD4 and anti-CD25 antibodies (see Table 1) for 30 min at 4 °C and then purify CD4+CD25− T cells through FACS. Finally, stain CD4+CD25− T cells with VPD450 following manufacturer’s instructions.
Prepare the CD19+CD9− and CD19+CD9+ B-cell fractions for the T/B-cell coculture. Subsets are sorted from CD19+ cells which were first stimulated for 48 h with anti-CD40 antibody and LPS for the last 5 h to prime the suppressive activity (see Note 9), and then stained with the anti-CD19 and anti-CD9 antibodies.
Seed both T and B cells on the 96-well round-bottom plate pretreated with the anti-CD3e antibody. Coculture 1–2 × 105 VPD450-stained CD4+CD25− T cells with different numbers of autologous CD19+CD9+ B cells or CD19+CD9− B cells, in the presence or absence of 10 μg/mL anti-CD9 blocking antibody or of its isotype control. Incubate the coculture for 72 h, but change the medium after 48 h to avoid nutrients exhaustion. If one of the final outcomes of the T/B-cell coculture is the detection of B10 cells by flow cytometry, then cells should be stimulated with PMA, ionomycin, and monensin for the terminal 5 h of culture.
At the end of the incubation, the cocultured cells should be collected, washed once with PBS, and then stained with the anti-CD9, -CD4, and -CD19 antibodies to check the final frequencies of the T-cell, B-cell, and B10-cell subsets by flow cytometry (see Note 10).
Finally, analyze the changes in T-cell proliferation by following the dilution of the VPD450 dye by flow cytometry.
3.4.2. Evaluation of Cell Contact Contribution on the T-Cell Proliferation Assay
A transwell system can be used to check whether cell–cell contact between Tcells and B cells is important for the suppressive function of CD9+ B cells on T cells.
Pretreat each well of specific plates with transwell with 5 μg/mL anti-CD3e and anti-CD28 antibody in a final volume of 200 μL (see Note 11). Incubate the plate overnight at 4 °C.
The day after, wash each well twice with 200 μL D-PBS before seeding cells (see Note 12).
Seed 1 × 105 VPD450-stained CD4+CD25− T cells in each well of both the receiver plate and the insert. The insert well is also loaded with different numbers of autologous CD19+CD9+ B cells or CD19+CD9− B cells.
Culture the cells for 72 h and then analyze T-cell proliferation by following the dilution of the VPD450 dye by flow cytometry.
3.5. In Vivo Activity of CD9+ B Cells
The protocol outlined in this section allows the evaluation of the in vivo immune suppressive activity of CD9+ B cells through the CHS assay. As FACS sorting takes a long time to obtain the desirable cell number and is not good for cell viability, we developed a method for immunomagnetic cell enrichment of CD9+ B cells. CD9+ and CD9− B cells are enriched with FITC-microbeads from CD90.2− cells (which themselves are enriched with CD90.2-microbeads) and then stained with anti-CD9 antibody conjugated to FITC. CD19 KO mice reconstituted with either CD9+ or CD9− B cells are first immunized with oxazolone in acetone/olive oil by application on the animal’s abdominal skin and subsequently challenged with oxazolone in acetone/olive oil by application to the animal’s ears. CHS is mediated by T cells and is reflected by the thickness of the swelling of the ears. Therefore, the suppressive activity of CD9+ B cells on T-cell-mediated immune response can be monitored through the ear thickness.
Enrich B cells from mouse splenocytes or peritoneal cavity cells by negative selection using CD90.2 microbeads and following the instructions of the kit. Culture the obtained CD90.2− cells with the anti-CD40 antibody for 48 h and LPS for the terminal 5 h (see Note 9 and 12).
Collect the cultured cells, wash and stain with the anti-CD9-FITC antibody as outlined in steps 1–5 in Subheading 3.2. CD9+ B cells are enriched using FITC-microbeads and following the instructions of the manufacturer. CD9− B cells represent the flow through fraction and are re-purified over the column to remove residual CD9+ B cells, while CD9+ cells are eluted from the column as a final step. Wash cells twice with D-PBS and finally resuspend both fractions in D-PBS at the final concentration of 2 × 107 B cells/mL (see Note 13).
Reconstitute CD19 KO mice by injecting intravenously 0.1 mL of 2 × 107 B cells/mL (CD9+ B cells or CD9− B cells) prepared as in step 2. Use a 29-G needle for the injection. As a control, inject 0.1 mL/mouse of D-PBS. For each condition, prepare groups of five CD19 KO mice of the same age and sex (see Note 14).
Two days later, induce skin inflammation in mice. Shave the hind flank hair of the mice with an electric or manual shaver. Mice are painted with a pipette and sensitized by a uniform topical application of 25 μL of 100 mg/mL oxazolone in acetone/olive oil (4:1 v/v) for two consecutive days.
On day 5, challenge sensitized mice by application of 10 μL of a 10 mg/mL oxazolone solution to the right ear (5 μL on the dorsal side and 5 μL on the ventral side). As a control, an identical amount of acetone/olive oil (4:1) is administered to the left ear.
Using a constant force, calibrated digital thickness gauge, perform three measurements of the thickness of the central portion of each ear lobe before challenge and 24, 48, 72, and 96 h after challenge (see Note 15).
Ear thickness is an indicator of T-cell-mediated immune response (i.e., smaller ear thickness shows greater immune suppressive activity). Therefore, by comparing ear thickness in CD19 KO mice reconstituted with either CD9+ or CD9− cells, it is possible to evaluate CD9+ B-cells suppressive activity.
Acknowledgments
This work is supported by Guangzhou Basic and Applied Basic Research Foundation (202002030127) to JS; the Fundamental Research Funds for the Central Universities (20ykzd08) to JS; Natural Science Foundation of Guangdong Province (2018A030313563) to JS; Program for Guangdong Introducing Innovative and Entrepreneurial Teams (2016ZT06S252) to JS; Guangdong Financial Fund for High-Caliber Hospital Construction to JS; NIAID (1R01AI099195 and R01AI134988) to U.B.
Footnotes
These reagents will be required if the operator opts for a retro-orbital injection, rather than an intravenous injection, for the reconstitution of B-cell-deficient mice.
The spleen should be mashed on the cell strainer without cutting it into smaller sections or digesting it with enzymes. Most of the cells prepared in this way are alive and grow well.
Vortex speed should be limited to the lowest rate at which the suspension can rise on the wall of the tube. A higher-than-necessary vortex rate may damage the cells.
When analyzing B10 cells obtained from the in vitro differentiation of B10pro cells, the LPI + M stimulus should be added during the last 5 h of the 48 h B-cell stimulation with anti-CD40 antibody.
From one spleen of an adult mouse, it should be possible to obtain 20–25 million CD19+ B cells using CD19 microbeads. After FACS, the number of obtained CD9+IL10+ B cells, CD9+IL10− B cells, and CD9− IL10− B cells will be approximately 0.2%, 0.4%, and 0.4–50%, respectively, of the total sorted B cells. The number of CD9+ B cells and CD9− B cells obtained is around 5% and 5–20%, respectively, of the total sorted B cells. When comparing the sorted subsets, it is better to set the yield of cell subsets with higher frequency comparable to the one with lowest frequency.
Although the dilution ratio suggested by manufacturer’s instructions frequently works well, each lot of live/dead dye working solution and antibody staining solution must be titrated for optimal dilution prior to use in a critical experiment.
The cell surface staining mixture can include antibodies other than those directed against CD19 and CD9. More specifically, antibodies recognizing other Breg markers, such as CD1d and CD5, can be added.
Most of the facilities capable of providing RNA-seq services can also undertake rRNA depletion and rRNA-depleted total RNA quantification. It is recommended to choose such a service provider.
The incubation with anti-CD40 antibody and LPS is important to prime B10 cells. Differently from the stimulation protocol outlined in Subheading 3.1.4, PMA and ionomycin are not employed because such treatment would cause secretion of other cytokines, including proinflammatory cytokines, as well as IL-10 and force the B10 cells to transition into effector B10 cells, which does not reflect the physiological condition.
A coarser indicator of the suppressive effect of CD9+ B cells on T-cell proliferation is through the evaluation of the final ratio of T cells and B cells in the coculture.
In the transwell assay, the transwell plate is coated with an anti-CD28 antibody in addition to the anti-CD3e antibody. This because, differently from the normal T/B cell coculture, in the transwell assay T and B cells are physically separated (the receiver plate contains no B cells) and therefore the support to T-cell proliferation provided by B-cell molecules such as CD80 (which binds CD28) is missing.
The date of the experiment of B-cell enrichment should be properly scheduled in order to match the injection times of step 3 in the same section.
After enrichment with FITC-microbeads, a total of five million CD9+ B cells and 20 million CD9− B cells should be obtained starting from 100 million CD90.2− cells.
As an alternative to intravenous injection via the animal’s tail vein, cells can be retro-orbitally injected as described in detail by Yardeni et al. [11]. Consult your Institution’s Animal Care and Use Committee guidelines to determine if retro-orbital injection is permitted.
The reading of the gauge may keep changing as the thickness of the swelled ear is easy to deform under compression. Waiting an extended period of time to obtain a stable number is not advised as it would not accurately reflect the real ear thickness. It is recommended to record the number at a fixed time point post release of the gauge knob for all ears.
References
- 1.Zou F, Wang X, Han X et al. (2018) Expression and function of Tetraspanins and their interacting Partners in B Cells. Front Immunol 9:1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosser EC, Mauri C (2015) Regulatory B cells: origin, phenotype, and function. Immunity 42 (4):607–612 [DOI] [PubMed] [Google Scholar]
- 3.Kalampokis I, Yoshizaki A, Tedder TF (2013) IL-10-producing regulatory B cells (B10 cells) in autoimmune disease. Arthritis Res Ther 15: S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blair PA, Norena LY, Flores-Borja F et al. (2010) CD19(+)CD24(hi)CD38(hi) B cells 320 Jianbo Sun and Uttiya Basu exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. Immunity 32 (1):129–140 [DOI] [PubMed] [Google Scholar]
- 5.van de Veen W, Stanic B, Yaman G et al. (2013) IgG4 production is confined to human IL-10-producing regulatory B cells that suppress antigen-specific immune responses. J Allergy Clin Immunol 131(4):1204–1212 [DOI] [PubMed] [Google Scholar]
- 6.Sun J, Wang J, Pefanis E et al. (2015) Transcriptomics identify CD9 as a marker of murine IL-10-competent regulatory B cells. Cell Rep 13(6):1110–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yanaba K, Bouaziz JD, Haas KM et al. (2008) A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity 28(5):639–650 [DOI] [PubMed] [Google Scholar]
- 8.Lindner S, Dahlke K, Sontheimer K et al. (2013) Interleukin 21-induced granzyme B-expressing B cells infiltrate tumors and regulate T cells. Cancer Res 73(8):2468–2479 [DOI] [PubMed] [Google Scholar]
- 9.Matsushita T, Tedder TF (2011) Identifying regulatory B cells (B10 cells) that produce IL-10 in mice. Methods Mol Biol 677:99–111 [DOI] [PubMed] [Google Scholar]
- 10.Kramer A, Green J, Pollard J et al. (2014) Causal analysis approaches in ingenuity pathway analysis. Bioinformatics 30(4):523–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yardeni T, Eckhaus M, Morris HD et al. (2011) Retro-orbital injections in mice. Lab Anim 40 (5):155–160 [DOI] [PMC free article] [PubMed] [Google Scholar]