Abstract
Background
In premature infants, we investigated whether the duration of extra-uterine development influenced autonomic nervous system (ANS) maturation.
Methods
We performed a longitudinal cohort study of ANS maturation in preterm infants. Eligibility included birth gestational age (GA) <37 weeks, NICU admission, and expected survival. The cohort was divided into three birth GA groups: Group 1 (≤29 weeks), Group 2 (30–33 weeks), and Group 3 (≥34 weeks). ECG data were recorded weekly and analyzed for sympathetic and parasympathetic tone using heart rate variability (HRV). Quantile regression modeled the slope of ANS maturation among the groups by postnatal age to term equivalent age (TEA) (≥37weeks).
Results
100 infants, median (Q1-Q3) birth GA of 31.9 (28.7–33.9) weeks, were enrolled: Group 1 (n=35); Group 2 (n=40); and Group 3 (n=25). Earlier birth GA was associated with lower sympathetic and parasympathetic tone. However, the rate of autonomic maturation was similar, and at TEA there was no difference in HRV metrics across the three groups. The majority of infants (91%) did not experience significant neonatal morbidities.
Conclusion
Premature infants with low prematurity-related systemic morbidity have maturational trajectories of ANS development that are comparable across a wide range of ex-utero durations regardless of birth GA.
Introduction
At birth the autonomic nervous system (ANS) plays a major role in the successful transition from the fetal to the extra-uterine environment (1). The latter half of gestation and early neonatal periods are critical periods for maturation of the ANS (2). Consequently, premature birth has two important consequences for the developing ANS. First, autonomic maturation in the premature infant may be underdeveloped and ill-prepared to support the profound physiological changes at birth. Second, subsequent developmental changes occur in a vastly different and ‘unnatural’ extra-uterine milieu (1). Heart rate variability (HRV) has become a powerful tool for studying ANS tone and provides a measure of sympathetic and parasympathetic function and therefore ANS maturation (3–5).
A number of studies have suggested that ANS development may be altered in a premature extra-uterine environment (6,7). In addition, altered autonomic balance has been reported in infants with conditions including hypoxic-ischemic encephalopathy, intraventricular hemorrhage (IVH), sepsis, and necrotizing enterocolitis (NEC) (8–11). We have described an aberrant autonomic profile in prematurely born infants who experience apparent life-threatening events (or brief resolved unexplained events) after discharge from the neonatal intensive care unit (NICU) (12). Importantly, impaired early-life ANS development has been implicated in chronic cardiovascular and neuropsychiatric outcomes of later onset (13,14).
Earlier reports of autonomic dysmaturation in premature infants, including ours, have come from high-morbidity referral NICUs (6,15,16). In recent decades, there have been significant advances in neonatal critical care support with a decrease in the intensity of systemic morbidity (17). The primary objective of this study was to evaluate the trajectories of sympathetic and parasympathetic maturation (using HRV metrics) in premature infants over a wide range of birth gestational age (GA), cared for in a large, community NICU. We hypothesized that infants born at younger GA would have a slower ANS maturation and lower autonomic tone at term-equivalent age (TEA) compared to infants with older birth GA.
Methods
Participants
We performed a prospective longitudinal study of ANS maturation in preterm infants born at a large suburban community mother-baby hospital (Inova Women and Children’s Hospital, Fairfax, VA) from May 2017 to May 2019. The hospital delivers around 10,000 infants a year and has a 108-bed level IV NICU. Informed consent was obtained and the study was approved by the Children’s National Hospital and Inova Hospital Institutional Review Boards. Infants were inborn and either enrolled before birth or within 96 hours of birth. Eligibility criteria included infants with a birth GA of 23 1/7 to 36 6/7 weeks, requiring NICU admission, without a suspected genetic or syndromic condition, and expected to survive to NICU discharge. We recorded pregnancy findings of twin gestation, fetal growth restriction, pre-eclampsia, and pregnancy-induced hypertension and all significant postnatal medical complications, including grade III or IV IVH, NEC requiring surgery, patent ductus arteriosus (PDA) requiring surgical ligation, culture positive sepsis, or death.
Physiologic Signal Collection
After birth, up to 96 hours of continuous electrocardiogram (ECG) was collected directly from the infants’ bedside monitor using custom software developed in MATLAB (Mathworks, Inc, Natick, MA, USA) and installed in a laptop. The hour of age of the infant at the beginning and end of the ECG data collection period varied based upon the time of infant arrival to the NICU and time of study consent. Following the infants first ECG data collection period, the laptop was used to collect bedside physiologic signals during the daytime for up to 7 hours per session at weekly intervals until NICU discharge. Simultaneous video was acquired during laptop sessions to identify patient care artifacts and movements.
Heart Rate Variability (HRV) Spectral Analysis
Autonomic function was measured by HRV analysis in time and frequency domains using the serial ECG data collection. From the ECG, beat-to-beat intervals between successive R-waves (RRi) were derived using a combination of Hilbert transform and adaptive threshold detection approach and RRi was calculated (4,18). Each weekly ECG recording session was partitioned into 10-minute epochs, for which the HRV metrics were measured and assigned the average HRV metrics for the session.
The ANS influences HRV at two frequencies; low frequency (LF) and high frequency (HF). The LF spectral division (0.04–0.15 Hz) reflects sympathetic and parasympathetic activity and is influenced by the baroreflex system, while the HF spectral division (0.4–1.5 Hz) reflects parasympathetic activity and is influenced by the respiratory system (4,19). LF (dB) and HF (dB) powers were calculated using the Welch periodogram approach with a frequency resolution of 0.016Hz. Next we determined the spectral powers in LF (dB) and HF (dB) as the median of the logarithm of the power in 0.05–0.25 Hz and 0.3–1 Hz frequency bands, respectively. We performed normalization of LF and HF frequencies to yield nHF and nLF, respectively. nLF power was calculated as the ratio of the sum of the powers in the 0.05–0.25 Hz band to the total power and nHF power as the ratio of the powers in 0.3–1 Hz band to the total power. We defined total power as the sum of the powers from 0.05–2 Hz. The normalization shows the balance in the LF and HF frequencies since together the normalized values are close to 1.
Time Domain Characterization
Detrended fluctuation analysis (DFA) is a modified root mean square (RMS) analysis approach that enables analysis of non-stationary signals (20,21), that can be caused by infant movement. Using the DFA fluctuation function we calculated RMS1 (sec) as the maximum value of the DFA fluctuation function for ‘s’ between 15–50 beats and RMS2 (sec) as the maximum of the DFA fluctuation function for ‘s’ between 100–150 beats (22). We also calculated the alpha (𝛼) exponent from the slope of the DFA fluctuation function versus ‘s’ in double logarithmic representation. Alpha 1 (𝛼1) was obtained from the region 15–30 beats (short term scale) and alpha 2 (𝛼2) was obtained from the region 35–150 beats (long-term scale/ ultralow frequency) (22). The RMS (sec) characterizes the variability in the RRi whereas the metrics characterize 𝛼 the autocorrelation in the RRi.
Statistical Analysis
The cohort was divided into three birth GA categories: Group 1 (≤29 weeks), Group 2 (30–33 weeks), and Group 3 (≥34 weeks). Descriptive statistics are presented, with categorical variables assessed using chi-square tests and continuous variables analyzed using either Kruskall-Wallis test or ANOVA depending on whether the data was normally distributed or not. Quantile regression was conducted to compare the post-natal age ANS metric trajectories of the three groups using the QREG2 procedure in STATA to account for repeated patient measures. An interaction term between group and post-natal age was included in the analysis to evaluate differences in ANS slopes over time. Non-significant interaction terms were removed from models prior to plotting trajectories. Correlations between spectral and time domain ANS metrics were assessed by Pearson correlation coefficient. Analysis was completed using SAS 9.4 and STATA SE version 16; a two-sided p-value of 0.05 was considered significant for all analyses.
Results
ANS tone and maturational trajectory was evaluated in 100 infants with median (Q1-Q3) birth GA of 31.9 (28.7–33.9) weeks over duration of 33.5 (18.5–60.0) NICU days. Thirty-five infants were in Group 1, 40 infants were in Group 2, and 25 infants were in Group 3. The clinical and demographic characteristics of the infants are reported in Table 1. All significant differences between the three groups were expected based on difference in birth GA (Table 1).
Table 1:
Clinical and Demographic Characteristics of the Preterm Infant Cohort
| Characteristic | Group 1 (n = 35) | Group 2 (n = 40) | Group 3 (n = 25) | Total cohort (n = 100) | P |
|---|---|---|---|---|---|
| Birth GA (week) | 28.0 (25.7–28.86) | 32.1 (31.5–33.4) | 34.7 (34.3–35.1) | 31.9 (28.7–33.9) | <0.0001 |
| Male gender | 17 (49) | 24 (60) | 12 (48) | 53 (53) | 0.52 |
| Maternal age (years) | 32.9 (5.5) | 33.7 (4.8) | 33.7 (5.8) | 33.4 (5.3) | 0.76 |
| Maternal parity ≥1 | 15 (44) | 10 (27) | 11 (55) | 36 (40) | 0.09 |
| Twin gestation | 6 (17) | 10 (25) | 8 (32) | 24 (24) | 0.41 |
| FGR | 2 (6) | 6 (15) | 4 (16) | 12 (12) | 0.36 |
| Pre-eclampsia | 7 (20) | 9 (23) | 2 (8) | 18 (18) | 0.31 |
| PIH | 2 (6) | 2 (5) | 2 (8) | 6 (6) | 0.88 |
| Antenatal betamethasone | 32 (91) | 32 (80) | 17 (68) | 81 (81) | 0.07 |
| Birth via CS | 33 (94) | 34 (85) | 19 (76) | 86 (86) | 0.13 |
| Birth weight (grams) | 1006.1 (283.9) | 1728.9 (325.9) | 2223.6 (315.7) | 1599.6 (568.6) | <0.0001 |
| Head circumference (cm) | 25.6 (3.1) | 29.9 (1.9) | 31.8 (1.6) | 28.9 (3.4) | <0.0001 |
| Apgar score (5-minute) | 8 (6–8) | 9 (8–9) | 9 (8–9) | 8 (7–9) | 0.0006 |
| Maximal respiratory support: | |||||
| mechanical | 19 (54) | 4 (10) | 1 (4) | 24 (24) | <0.0001 |
| ventilation | 16 (46) | 31 (78) | 11 (44) | 58 (58) | |
| CPAP | 0 (0) | 1 (3) | 0 (0) | 1 (1) | |
| nasal cannula room air | 0 (0) | 4 (10) | 13 (52) | 17 (17) | |
| cGA when on room air (weeks) | 39.6 (37.4–40.9) | 36.6 (35.7–37.3) | 36.6 (35.9–37.1) | 36.9 (36.0–38.1) | <0.0001 |
| IVH bundle | 32 (91) | 3 (8) | 0 (0) | 35 (35) | <0.0001 |
| Caffeine exposure | 35 (100) | 18 (45) | 0 (0) | 53 (53) | <0.0001 |
| Age of first recording (hours) | 47.5 (24.8) | 43.4 (28.5) | 40.9 (31.2) | 44.2 (27.8) | 0.66 |
| Number of ANS recordings | 10.1 (4.5) | 4.2 (1.9) | 1.8 (0.9) | 5.7 (4.5) | <0.0001 |
| NICU duration (days) | 86.6 (63.1–106.6) | 28.8 (22.7–44.8) | 11.9 (10.1–19.5) | 33.5 (18.5–60.0) | <0.0001 |
| cGA at discharge (weeks) | 39.4 (3.2) | 36.8 (1.3) | 37.0 (1.4) | 37.7 (2.4) | <0.0001 |
Categorical data is reported as N(%) and continuous data is reported as median (Q1-Q3) or as mean (SD)
Abbreviations: cGA = corrected gestational age, CPAP = continuous positive airway pressure, FGR = fetal growth restriction, CS = cesarean section, GA = gestational age, Group 1 = birth GA ≤29 weeks, Group 2 = birth GA 30–33 weeks, Group 3 = birth GA ≥34 weeks, PIH = pregnancy-induced hypertension
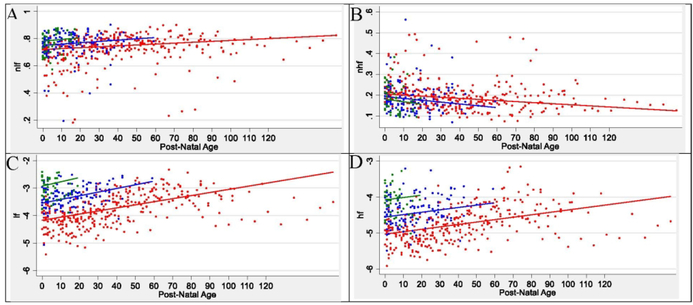
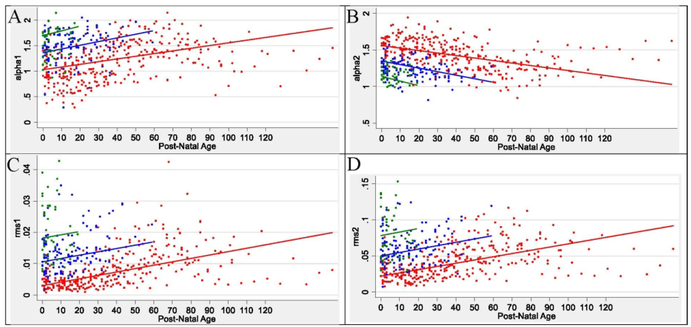
The first measurement of HRV metrics in the infants occurred at a mean (SD) of 44.2 (27.8) hours of age (Table 1). At the first recording, alpha 1, RMS1, RMS 2, nLF, LF, and HF were lowest in Group 1 and highest in Group 3 and alpha 2 and nHF were highest in Group 1 and lowest in Group 3. The maturational trajectories of the ANS metrics were assessed over time by postnatal age (Figure 1, Figure 2). Owing to a higher median (Q1-Q3) birth GA (34.7 [34.3–35.1] weeks), Group 3 had the shortest duration in the NICU (11.9 [10.1–19.5] days) during which to establish a clear trajectory/slope, compared to Groups 1 and 2 (Table 1). HRV metrics were assessed a mean (SD) of 10.1 (4.5) time-points per infant in Group 1, 4.2 (1.9) time-points per infant in Group 2, and 1.8 (0.9) time-points per infant in Group 3. The developmental trajectory/slope of the HRV metrics increased in all three groups over postnatal age, except alpha 2 and nHF which decreased over postnatal age (Figure 1, Figure 2). For each metric, there was no difference in slope over post-natal days for the three birth GA groups. At term equivalent age (TEA) (≥37 weeks), there was no significant difference in autonomic tone across the three birth GA groups. The ANS metrics of nLF, nHF, LF, HF, HR, alpha 1, RMS 1, RMS 2 showed significant correlation between each other (Table 2). Alpha 2 showed correlation with LF, HF, HR and alpha 1, but not with nLF and nHF.
Figure 1:
ANS Spectral Metrics Over Time in Preterm Newborn Cohort
Abbreviations: nLF = normalized low frequency; nHF = normalized high frequency; LF = low frequency; HF = high frequency
Infant ANS metrics of nLF (A), nHF (B), LF (C), and HF (D), at each measurement session are shown as a colored dot. Red dots are for infants in group 1 with birth GA ≤29 weeks, blue dots are for infants in group 2 with birth GA of 30–33 weeks, and green dots are for infants in group 3 with birth GA ≥34 weeks. The solid colored lines represent the modeled ANS metric trajectory over time in postnatal age in days for each birth GA group.
Figure 2:
ANS Time Domain Metrics Over Time in Preterm Newborn Cohort
Abbreviations: alpha 1= alpha short; alpha 2 = alpha long; RMS 1 = root mean square 1; RMS 2 = root mean square 2
Infant ANS metrics of alpha 1 (A), alpha 2 (B), RMS 1 (C), and RNS 2 (D), at each measurement session are shown as a colored dot. Red dots are for infants in group 1 with birth GA ≤29 weeks, blue dots are for infants in group 2 with birth GA of 30–33 weeks, and green dots are for infants in group 3 with birth GA ≥34 weeks. The solid colored lines represent the modeled ANS metric trajectory over time in postnatal age in days for each birth GA group.
Table 2:
Correlation of Spectral and Time Domain Metrics of ANS Tone
| nLF | nHF | LF | HF | HR | Alpha 1 | Alpha 2 | RMS 1 | RMS 2 | |
|---|---|---|---|---|---|---|---|---|---|
| nLF | |||||||||
| nHF | −0.95* | ||||||||
| LF | 0.37* | −0.25* | |||||||
| HF | −0.02 | 0.15* | 0.90* | ||||||
| HR | −0.15† | −0.03 | −0.50* | −0.52* | |||||
| Alpha 1 | 0.71* | −0.63* | 0.77* | 0.49* | −0.37* | ||||
| Alpha 2 | −0.05 | −0.05 | −0.79* | −0.80* | 0.44* | −0.54* | |||
| RMS 1 | 0.23* | −0.11‡ | 0.90* | 0.87* | −0.58* | 0.61* | −0.76* | ||
| RMS 2 | 0.39* | −0.29* | 0.91* | 0.82* | −0.56* | 0.70* | −0.62* | 0.94* |
P < 0.05
P < 0.01
P < 0.001
Abbreviations: nLF = normalized low frequency; nHF = normalized high frequency; LF = low frequency; HF = high frequency; HR = mean heart rate; alpha 1= alpha short; alpha 2 = alpha long; RMS 1 = root mean square 1; RMS 2 = root mean square 2
The majority of infants (91%) did not have a major medical complication of prematurity. Three infants had grade III or IV IVH. Four infants had a PDA requiring surgical ligation. No infants were transferred to another NICU for a higher level of care; one infant was transferred to another NICU due to parental preference. Ninety-eight infants survived to NICU discharge; one infant died due to NEC and sepsis and one due to severe sepsis.
Discussion
In our cohort of infants undergoing extra-uterine development after premature birth, we found that maturational trajectories in autonomic tone were comparable across a broad range of birth GA. We found that both sympathetic and parasympathetic tone were lowest among the most prematurely born infants, as expected. However, by the time of NICU discharge there were no significant differences in ANS tone among the different birth GA groups. Unlike previous reports (6,7,15), the findings in our cohort suggest that the duration of ANS maturation in an extra-uterine environment after premature birth may not by itself influence ANS development to term.
Maturation of sympathetic function starts earlier in gestation than that of the parasympathetic system. Normal parasympathetic maturation accelerates between 25–30 weeks’ gestation, when many premature newborns are undergoing transition (2,23). By 37–38 weeks there is an increase in vagal tone, as evidenced by increased high-frequency HRV (24). Although HRV metrics for both sympathetic tone (LF, nLF, alpha 1, RMS1, and RMS2) and parasympathetic tone (HF) increased with increasing postnatal age, the more rapid maturation of the sympathetic function translated into a negative slope for nHF.
We showed strong correlation between spectral and time domain metrics of ANS tone. Since nLF and nHF represent the relative power of the spectral frequencies, we see opposite direction of correlation between nLF and nHF, respectively for each of the ANS metrics, except with alpha 2. Alpha 2 is a measure of long-term fluctuations in HR. Alpha 2 correlates with the absolute value of HF and LF, and negatively correlates with increasing HRV, but does not correlate with the normalized metrics likely because normalized metrics do not represent absolute HRV. Alpha 1, a measure of short-term heart rate fluctuation influenced by sympathetic activity showed correlation with all metrics (25).
Prematurity exposes infants to a broad spectrum of systemic morbidities, including cardiorespiratory, neurologic, and infectious complications (1). In an earlier study of 26 preterm infants born ≤28 weeks gestation, we found that the preterm infants had significantly lower ANS tone at TEA compared to low-risk term control infants (6). Different from the current study, our earlier study was performed in infants admitted to our regional referral NICU for high acuity prematurity-related complications. Furthermore, in the previous study (6), we compared autonomic tone at term between infants born ≤28 weeks GA and normal term-born infants. Our findings also differ from those of Patural et al., who found no significant longitudinal increase in the autonomic tone by TEA among 39 preterm infants (mean birth GA 28 weeks) (15). Infants in that study had a high degree of prematurity-related risk factors which may have impacted ANS development (15). Autonomic tone at birth was also not different by birth GA, which may have been due to prenatal factors (21). Another study of ex-premature infants described impaired autonomic maturation among those with abnormal neurodevelopmental outcomes (16). Infection-inflammation is another common complication of prematurity and is known to have an effect on the nucleus tractus solitarius, a primary sensory autonomic center (16). Conditions such as early neonatal sepsis and NEC have been associated with short-term depression of autonomic tone, although the long-term effects are less well-described (11,26). Other prematurity-related complications such as PDA can alter ANS tone in premature newborns (27). In our current study, the prevalence of these prematurity-related complications was relatively low (9% with a complication), which might explain the lack of an association between duration of premature extra-uterine life and autonomic maturation.
The infants in our cohort were cared for in a NICU in which many of the more recent advances toward a “kinder, gentler” practice of NICU care are routine, including quiet single-bed rooms, minimal early medical handling, avoidance of prolonged intubation and less invasive ventilation, and early skin-to-skin contact (17,28). In premature newborns, the prone body position may be more comforting as it is associated with higher parasympathetic tone (29). Early skin-to-skin maternal care in premature newborns has been shown to improve vagal tone and autonomic functioning into childhood (28,30). Based on the current findings, we speculate that this type of practice promotes autonomic maturation in the extra-uterine environment.
While spectral analysis is one of the commonly recommended analysis approaches to characterize HRV, DFA used in this study is a newer approach with advantages for analyzing infant HRV data (4). The principles behind DFA stem from statistical physics, and they allow us to address non-stationary components in the RRi and quantify HRV in a more reliable way (20). Kubios is open source software for HRV analysis and has an implementation of DFA in its package (31).
There are a number of strengths to our data, including the relatively large cohort, as well as the longitudinal HRV recordings. However, this study also has some limitations. The effects of birth GA and duration of extra-uterine development prior to NICU discharge are of course, inextricably (and inversely) linked. We reported the data by postnatal age as opposed to postmenstrual age to show ANS developmental trajectories for the infants originating at the same time point of birth. This enabled us to evaluate the impact of postnatal extra-uterine duration in the NICU on ANS development. Since we did not perform follow-up HRV studies after NICU discharge, we cannot comment on the enduring nature of these autonomic trajectories. A term newborn reference of HRV metrics to which to compare our preterm infant data at TEA was not available. HRV metrics change during the first hours after birth during fetal-neonatal transition complicating any comparison between HRV data in term newborns and preterm cohorts at TEA (32). Our current studies aim to develop a normative set of HRV metrics in uncomplicated term infants beyond the birth transitional period to serve as a reference dataset. Furthermore, most infants in our cohort were born by cesarean section; we cannot address the role of delivery mode on autonomic development (33). It is standard protocol at the NICU from which our cohort was recruited that infants born <29 weeks GA, spend the first 72 hours after birth with limited touch, bundled care, after which a cranial ultrasound is routinely performed. Infants above 28 weeks birth GA, only undergo cranial ultrasound if there is clinical suspicion of a brain lesion. It is therefore possible that some infants had unrecognized, sub-clinical brain injury which could have affected ANS maturation. Since pregnancy complications such as pre-eclampsia and fetal growth restriction were similar among the birth GA groups, we did not evaluate for a difference in HRV trajectory in those with these complications, however based on a study by Aye and colleagues these factors do not seem to affect HRV metrics at birth (34). Long-term follow-up of preterm infants born to pregnant women with these specific complications may warrant further study in a larger cohort.
Conclusions
We report here the autonomic developmental trajectories as studied by HRV metrics in a cohort of premature infants born over a broad range of gestational ages. We found that the duration of preterm ex-utero development (i.e., between birth and TEA) did not significantly influence the rate of autonomic maturation. We speculate that the current findings differ from those in previous publications (6,7,15,16,30), because the source population of premature infants in the current study differs from populations previously reported. Specifically, the present study occurred in a large community-based inborn NICU, with a relatively low level of prematurity-related systemic morbidity rather than a high-morbidity premature population in a referral NICU. In addition, and in part because of the above, gentler, less intense transitional support (for example ventilatory support) was required. This question is currently under investigation.
Impact.
Heart rate variability can evaluate the maturation of the autonomic nervous system
Metrics of both the sympathetic and parasympathetic nervous system show maturation in the premature extrauterine milieu
The autonomic nervous system in preterm infants shows comparable maturation across a wide range of birth gestational ages
Preterm newborns with low medical morbidity have maturation of their autonomic nervous system while in the NICU
Modern NICU advances appear to support autonomic development in the preterm infant
Acknowledgements
We greatly appreciate the support of Inova Women and Children’s Hospital, Fairfax, VA and the families who allowed us to study their newborn infants.
Financial Support: This study was supported by the Children’s National Inova Collaborative (CNICA) Research Program, through institutional support from Children’s National Hospital, Washington, DC and the Inova Health System, Fairfax, VA. Dr. Mulkey received support by Award Numbers UL1TR001876 and KL2TR001877 from the NIH National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Footnotes
Disclosures: The authors have no disclosures to report.
Informed consent statement: Patient written informed consent was obtained for the study.
Category of Study: Clinical
References
- 1.Mulkey SB, Plessis AD. The critical role of the central autonomic nervous system in fetal-neonatal transition. Semin. Pediatr. Neurol. 28, 29–37 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Longin E, Gerstner T, Schaible T, Lenz T, Konig S Maturation of the autonomic nervous system: differences in heart rate variability in premature vs. term infants. J. Perinat. Med. 34, 303–308 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Fyfe KL, et al. The Effect of gestational age at birth on post-term maturation of heart rate variability. Sleep. 38,1635–1644 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Task Force of the European Society of Cardiology the North American Society of Pacing Electrophysiology. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur. Heart J. 93,1043–1065 (1996). [PubMed] [Google Scholar]
- 5.Malliani A, Lombardi F, Pagani M Power spectrum analysis of heart rate variability: a tool to explore neural regulatory mechanisms. Br. Heart J. 71, 1–2 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mulkey SB, et al. Autonomic nervous system depression at term in neurologically normal premature infants. Early Hum. Dev. 123, 11–16 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yiallourou SR, Witcombe NB, Sands SA, Walker AM, Horne RS The development of autonomic cardiovascular control is altered by preterm birth. Early Hum. Dev. 89,145–152 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Tuzcu V, Nas S, Ulusar U, Ugur A, Kaiser JR Altered heart rhythm dynamics in very low birth weight infants with impending intraventricular hemorrhage. Pediatrics. 123, 810815 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Massaro AN, et al. Heart rate variability in encephalopathic newborns during and after therapeutic hypothermia. J. Perinatol. 34, 836–841 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fairchild KD, O’Shea TM Heart rate characteristics: physiomarkers for detection of late-onset neonatal sepsis. Clin. Perinatol. 37, 581–598 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Shargabi T, et al. Changes in autonomic tone in premature infants developing necrotizing enterocolitis. Am. J. Perinatol. 35, 1079–1086 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Nino G, et al. Premature infants rehospitalized because of an apparent life-threatening event had distinctive autonomic developmental trajectories. Am. J. Respir. Crit. Care Med. 194, 379–381 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mulkey SB, du Plessis AJ Autonomic nervous system development and its impact on neuropsychiatric outcome. Pediatr. Res. 85, 120–126 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haraldsdottir K, et al. Heart rate recovery after maximal exercise is impaired in healthy young adults born preterm. Eur. J. Appl. Physiol. 119, 857–866 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patural H, et al. Autonomic cardiac control of very preterm newborns: a prolonged dysfunction. Early Hum. Dev. 84, 681–687 (2008). [DOI] [PubMed] [Google Scholar]
- 16.Thiriez G, et al. Altered autonomic control in preterm newborns with impaired neurological outcomes. Clin. Auton. Res. 25, 233–242 (2015). [DOI] [PubMed] [Google Scholar]
- 17.de Bijl-Marcus K, Brouwer AJ, De Vries LS, Groenendaal F, Wezel-Meijler GV Neonatal care bundles are associated with a reduction in the incidence of intraventricular haemorrhage in preterm infants: a multicentre cohort study. Arch. Dis. Child Fetal Neonatal Ed. November. 15 [Epub ahead of print] (2019). [DOI] [PubMed] [Google Scholar]
- 18.Ulusar UD, et al. Adaptive rule based fetal QRS complex detection using Hilbert transform. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2009, 4666–4669 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fyfe KL, Yiallourou SR, Wong FY, Horne RS The development of cardiovascular and cerebral vascular control in preterm infants. Sleep Med. Rev. 18, 299–310 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Peng CK, Havlin S, Stanley HE, Goldberger AL Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time series. Chaos. 5, 82–87 (1995). [DOI] [PubMed] [Google Scholar]
- 21.Govindan RB, Massaro AN, Niforatos N, du Plessis A Mitigating the effect of nonstationarity in spectral analysis-an application to neonate heart rate analysis. Comput. Biol. Med. 43, 2001–2006 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Govindan RB, Preissl H, Eswaran H, Campbell JQ, Lowery CL Detrended fluctuation analysis of short data sets: An application to fetal cardiac. Physica D: Nonlinera Phenomena. 226, 23–31 (2007). [Google Scholar]
- 23.Patural H, et al. Birth prematurity determines prolonged autonomic nervous system immaturity. Clin. Auton. Res. 14, 391–395 (2004). [DOI] [PubMed] [Google Scholar]
- 24.Clairambault J, Curzi-Dascalova L, Kauffmann F, Medigue C, Leffler C Heart rate variability in normal sleeping full-term and preterm neonates. Early Hum. Dev. 28, 169–183 (1992). [DOI] [PubMed] [Google Scholar]
- 25.Tulppo MP, et al. Physiological background of the loss of fractal heart rate dynamics. Circulation. 112, 314–319 (2005). [DOI] [PubMed] [Google Scholar]
- 26.Bohanon FJ, et al. Heart rate variability analysis is more sensitive at identifying neonatal sepsis than conventional vital signs. Am. J. Surg. 210, 661–667 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goudjil S, et al. Patent ductus arteriosus in preterm infants is associated with cardiac autonomic alteration and predominant parasympathetic stimulation. Early Hum. Dev. 89, 631634 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Sanders MR, Hall SL Trauma-informed care in the newborn intensive care unit: promoting safety, security and connectedness. J. Perinatol. 38, 3–10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gomes E, et al. Autonomic responses of premature newborns to body position and environmental noise in the neonatal intensive care unit. Rev. Bras. Ter. Intensiva. 31, 296–302 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feldman R, Rosenthal Z, Eidelman AI Maternal-preterm skin-to-skin contact enhances child physiologic organization and cognitive control across the first 10 years of life. Biol. Psychiatry. 75, 56–64 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Tarvainen MP, Niskanen JP, Lipponen JA, Ranta-Aho PO, Karjalainen PA Kubios HRV--heart rate variability analysis software. Comput. Methods Programs Biomed. 113, 210–220 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Shayani LA, da Cruz CJ, Porto LGG, Molina GE Cardiac Autonomic Function in the First Hours of Postnatal Life: An Observational Cross-Sectional Study in Term Neonates. Pediatr Cardiol. 40, 1703–1708 (2019). [DOI] [PubMed] [Google Scholar]
- 33.Mulkey SB, et al. The effect of labor and delivery mode on electrocortical and brainstem autonomic function during neonatal transition. Sci Rep. 9, 11020 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aye CYL, et al. Neonatal autonomic function after pregnancy complications and early cardiovascular development. Pediatr Res. 84:85–91 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]