Abstract
Nickel nanoparticles (NiNPs) are increasingly used in nanotechnology applications, yet information on sex differences in NiNP-induced lung disease is lacking. The goal of this study was to explore mechanisms of susceptibility between male and female mice after acute or subchronic pulmonary exposure to NiNPs. For acute exposure, male and female mice received a single dose of NiNPs with or without LPS by oropharyngeal aspiration and necropsied 24 hours later. For subchronic exposure, mice received NiNPs with or without LPS six times over 3 weeks prior to necropsy. After acute exposure to NiNPs and LPS, male mice had elevated cytokines (CXCL1 and IL-6) and more neutrophils in bronchoalveolar lavage fluid (BALF), along with greater STAT3 phosphorylation in lung tissue. After subchronic exposure to NiNPs and LPS, male mice exhibited increased monocytes in BALF. Moreover, subchronic exposure of male mice to NiNP only induced higher CXCL1 and CCL2 in BALF along with increased alveolar infiltrates and CCL2 in lung tissue. STAT1 in lung tissue was induced by subchronic exposure to NiNPs in females but not males. Males had a greater induction of IL-6 mRNA in liver after acute exposure to NiNPs and LPS, and greater CCL2 mRNA in liver after subchronic NiNP exposure. These data indicate that susceptibility of males to acute lung inflammation involves enhanced neutrophilia with increased CXCL1 and IL-6/STAT3 signaling, whereas susceptibility to subchronic lung inflammation involves enhanced monocytic infiltration with increased CXCL1 and CCL2. STAT transcription factors appear to play a role in these sex differences. This study demonstrates sex differences in the lung inflammatory response of mice to NiNPs that has implications for human disease.
Keywords: nickel nanoparticles, inflammation, lung, sex, susceptibility
INTRODUCTION
With rapid development of nanotechnology, inhalation exposure to metal nanoparticles is inevitable, resulting in adverse health effects in the respiratory system (Bonner, 2010a; Lu et al., 2015). Exposure to metals in the workplace and in the environment is frequently accompanied by co-exposure to lipopolysaccharide (LPS), a ubiquitous agent derived from gram-negative bacteria that stimulates neutrophilic lung inflammation (Bonner et al., 1998; Duquenne and Marchand., 2013). Nickel nanoparticles (NiNPs) are used in various technological applications such as catalysis, battery manufacturing and textile applications due to their characteristics of high catalytic activity, high magnetism, low melting point and high burning point (Pumera, 2007; Zhao et al., 2008; Mai et al., 2012; He et al., 2013; Ishizaki, Yatsugi and Akedo, 2016; Sharma et al., 2018; Su et al., 2018). Moreover, NiNPs are gaining more attention as they have the potential to be used in biomedical devices or chemotherapeutic applications (Guo et al., 2009). Chronic inhalation exposure to nickel fumes in occupational settings has been linked to pulmonary diseases including fibrosis, asthma, and cancer (Nemery, 1990). Therefore, there is a concern that occupational exposure to NiNPs, either in the absence or presence of LPS, could further increase the incidence of these lung diseases. In particular, the nano-sized dimensions and high surface area of NiNPs make them uniquely toxic when inhaled since they have greater potential to be deposited in the lower respiratory tract and generate reactive oxygen species (ROS) (Unfried et al., 2007; Bonner, 2010a; Glista-Baker et al., 2014; Osman, Sexton and Saleem, 2019;). The generation of ROS induced by NiNPs in mesothelial cells and macrophages in vitro or in the lungs of mice in vivo leads to activation of hypoxia inducible factor- 1α (HIF-1α) transcription factor (Wan et al., 2011; Glista-Baker et al., 2012, Glista-Baker et al., 2014). HIF-1α activation is accompanied by the production of chemokines such as CCL2 and CXCL10 that mediate monocyte migration (Glista-Baker et al., 2012).
Previous studies have addressed mechanisms of nickel or NiNPs toxicity in vivo and in vitro, but no studies have investigated sex-specific pulmonary immune and inflammatory responses to nickel or NiNPs in experimental animal models. In epidemiology studies of human pulmonary diseases, sex has been a critical factor in determining the susceptibility towards a variety of agents that cause acute or chronic respiratory inflammation (Falagas et al., 2007; Gleeson et al., 2011; Casimir et al., 2013; Pinkerton et al., 2015; Klein and Flanagan, 2016). For example, males are more susceptible to acute lung inflammation caused by viral or bacterial infections and have a more severe prognosis with increased risk of respiratory tract infections, pulmonary fibrosis, and pneumonia (Jensen-Fangel et al., 2004; Gleeson et al., 2011; Kadioglu et al., 2011; Klein and Flanagan, 2016; Sathish and Prakash, 2016). On the other hand, females are more susceptible to chronic lung inflammation including asthma, chronic obstructive pulmonary disease, and autoimmune lung diseases (Postma, 2007; Pinkerton et al., 2015; Klein and Flanagan, 2016; Sathish and Prakash, 2016). Lung cancer, also known to be caused by nickel exposures, has been reported to be influenced by sex (Grimsrud et al., 2002; Egleston et al., 2009; Gasperino, 2011; Sathish and Prakash, 2016). One study suggested that estrogen signaling in women can affect the development of lung cancer regardless of smoking status (Baik and Eaton, 2012). Furthermore, a higher incidence was recorded globally in women developing lung cancer without any prior smoking experience compared to men (Thun et al., 2008). Yoshizaki et al. (2017) showed that male mice exposed to particulate matter less than 2.5 microns (PM2.5) had higher acute inflammatory makers such as IL-1β, IL-8Rα and COX-2 while female mice exposed to PM2.5 had higher chronic inflammatory maker such as IL-17. Since susceptibility to lung diseases is determined in part by sex, it is critical to better understand the basic mechanisms of susceptibility between male and female mice exposed to emerging nanomaterials in order to better predict the risk for human respiratory disease.
The generation of ROS by nickel is thought to be a major factor that initiates cytokine production, pulmonary inflammation and disease pathogenesis (Fu et al., 2014; Neubauer et al., 2015; Latvala et al., 2016; Cao et al., 2016; Mo et al., 2019). ROS activates NF-κB within human innate immune cells such as macrophages or human cancer cells to induce the expression of pro-inflammatory cytokines such as IL-8, which serves to recruit neutrophils, and IL-6, which mediates several biological functions including cell proliferation, differentiation and immune cell function (Morgan and Liu, 2011; Yoon et al., 2010). In experimental animal models, IL-6 has been reported to either induce or suppress CXCL1 (a murine homologue of IL-8) depending on the inflammatory stimulus and context of the exposure (Jones et al., 2006; Fielding et al., 2008). The biological effects of IL-6 are mediated in part through STAT3, which plays homeostatic roles in cell growth and differentiation. However, dysregulation of IL-6/STAT3 has been implicated in the pathogenesis of cancer, hepatic and pulmonary fibrosis, acute lung injury and allergic asthma (Gao et al., 2004; Wang et al., 2013; O’Donoghue et al., 2012; Zhao et al., 2016; Silva et al., 2017). In disease states, IL-6-induced STAT3 initiates a feed-back loop mechanism to amplify more IL-6 secretion to sustain cell proliferation through prolonging STAT3 activation (Hodge, Hurt and Farrar, 2005; Wang et al., 2013). Therefore, targeting STAT3 has been proposed for therapeutic intervention of multiple diseases. The biological effects of STAT3 in promoting cell proliferation are counteracted by the growth arrest activity of STAT1 (Thompson et al., 2015). We previously reported that STAT1 plays a protective role in lung fibrosis or exacerbation of allergic lung disease induced by multi-walled carbon nanotubes (MWCNTs) (Duke et al., 2017). Moreover, IL-6/STAT3 signaling serves to shift acute inflammation to chronic inflammation by promoting chronic monocytic inflammation through CCL2 production (Kaplanski et al., 2003; Gabay, 2006). Thus, IL-6/STAT3 signaling acts as a double-edged sword to antagonize STAT1 and intensify neutrophilic inflammation, but also facilitates the subsequent resolution of neutrophilic inflammation (Jones et al., 2006; Fielding et al., 2008). Differences in IL-6 production between males and females have been implicated in disease pathogenesis in both human and rodent models. Significantly higher hepatic IL-6 mRNA and serum IL-6 protein levels were reported in male mice compared to female mice during liver carcinogenesis induced by the chemical carcinogen diethylnitrosamine (DEN) (Naugler et al., 2007). Furthermore, higher serum IL-6 in men has been associated with a worse prognosis for non-small cell lung cancer (Silva et al., 2017). This phenomenon of differential IL-6 production between sexes could be partly due to the differences in levels of 17β-estradiol. Thus, differential IL-6 production and STAT3 signaling between sexes could play a role in susceptibility to lung diseases.
The goal of this study was to explore mechanisms of susceptibility to pulmonary inflammation between male and female mice after a single acute exposure or repeated subchronic exposures to NiNPs with or without LPS (endotoxin). Occupational and environmental exposure to NiNPs does not occur under pristine conditions, but rather is combined with low levels of LPS. Moreover, LPS can modify the biological impact of nanoparticles. For example, we previously reported that LPS enhances the pro-inflammatory and pro-fibrotic effects of MWCNTs in the lungs of male Sprague-Dawley rats (Cesta et al., 2010). Therefore, the LPS-nanoparticle co-exposure model is valuable towards determining susceptibility of individuals to real-world co-exposures. Based on epidemiology studies of respiratory diseases in human cohorts, we hypothesized that male mice would be more susceptible to NiNP-induced acute lung inflammation with or without LPS, while female mice would be more susceptible to NiNP-induced subchronic lung inflammation in the absence or presence of LPS. However, we discovered that male mice were more susceptible than female mice to both acute neutrophilic lung inflammation and subchronic monocytic lung inflammation in response to NiNPs and LPS. Moreover, the susceptibility of male mice to acute neutrophilic inflammation was correlated with changes with the induction of IL-6 and CXCL1 along with increased activation of STAT3, while susceptibility of the male mice to subchronic monocytic inflammation was hallmarked by increased CXCL1 and CCL2, with reduced STAT1 protein, in lung tissue.
METHODS
Nickel Nanoparticles:
NiNPs were purchased from Sun Innovations (Fremont, CA) and were characterized by the manufacturer as a sphere-shaped nanoparticle with a diameter of ~20 nm, metal purity of 99.9% and surface area of 40 to 60 m2/g. Moreover, these NiNPs have been previously described as insoluble in water and possess an oxidation state of zero (Glista-Baker et al., 2014). A more detailed independent characterization of NiNP size carried out in our laboratory using TEM showed an average mean (±SEM) particle diameter of 25.43 ±11.62 nm with agglomerates ranging from 250 – 600 nm when suspended in 0.1% pluronic F-68 (Sigma, St. Louis, MO) diluted in Gibco’s phosphate-buffered saline (DPBS) (Glista-Baker et al., 2012). For pulmonary exposure of mice, NiNPs suspended in 0.1% pluronic/DPBS were sonicated in a cuphorn sonicator (Qsonica, Newton, CT) for 2 minutes and vortexed immediately before dosing as described below. A representative TEM image of the NiNPs is shown in Fig. 1A.
Figure 1.
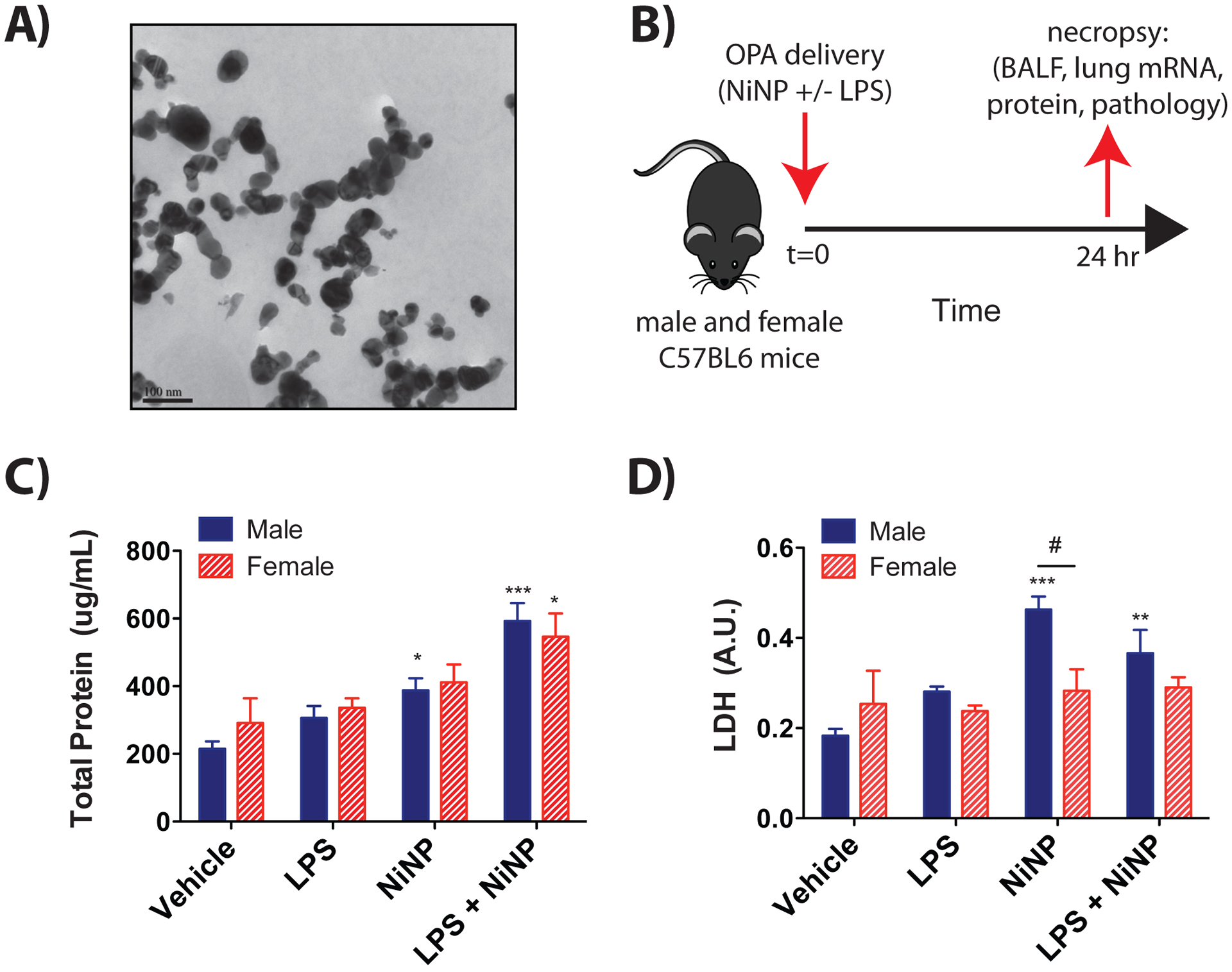
Acute lung exposure of male and female mice by oropharyngeal aspiration (OPA) to LPS, NiNPs, or LPS and NiNPs. A) Transmission electron microscopy (TEM) of nickel nanoparticles used in this study (bar equals 100 nm). B) Illustration of experimental design. C57BL/6J mice were exposed to vehicle (0.1% Pluronic in DPBS), LPS (5 μg/kg), NiNPs (4 mg/kg), or LPS (5 μg/kg) and NiNPs (4 mg/kg) co-exposure via OPA delivery. Mice were euthanized and necropsy performed 24 hrs after the exposure. C) Protein concentration in BALF determined by Pierce BCA Protein Assay. D) Lactate dehydrogenase (LDH) activity in BALF measured using Pierce LDH Cytotoxicity Assay. (n=6 mice per group, ***P<0.001, ** P<0.01 or *P<0.05 compared to vehicle determined by one-way ANOVA, #P<0.05 between sexes as determined by two-way ANOVA).
Lipopolysaccharide (LPS):
LPS from Escherichia coli (Serotype 026: B6) was purchased from Sigma-Aldrich (St. Louis, MO) and suspended in DPBS for the stock solution. LPS was diluted in DPBS solution to the desired concentration and vortexed before use.
Animal care:
Six to eight-week old male and female C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were dosed between 12–16 weeks as described below under ‘Experimental design’ after 2 weeks of acclimation. Mice were housed in pathogen-free, humidity, and temperature-controlled AAALAC (Association for Assessment and Accreditation of Laboratory Animal Care) accredited animal facility in the Toxicology Building. Mice were housed maximum of 3 per cage in a temperature and humidity-controlled facility and supplied with food and water ad libitum. All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at North Carolina State University.
Experimental design:
Both female and male wild type mice were randomly divided into 4 treatment groups (vehicle, LPS, NiNPs, or both LPS and NiNPs). 0.1% Pluronic solution in PBS will be used as a vehicle for the experiment. Each treatment group contained 6 mice per group per sex. For the acute exposure protocol, mice were exposed at 12 to 14 weeks of age by oropharyngeal aspiration (OPA) to 5 μg/kg of LPS, 4 mg/kg of NiNPs, both 5 μg/kg of LPS and 4 mg/kg of NiNPs, or equal volume of vehicle solution (100 μl) via oropharyngeal aspiration (OPA) under isoflurane anesthesia and euthanized 24 hrs post-exposure. We have previously shown that a single NiNP dose of 4 mg/kg delivered by OPA produces mild lung inflammation and fibrosis in mice without overt symptoms or mortality (Glista-Baker et al., 2014). The relatively low dose of LPS (5 μg/kg) was chosen since it induced only mild neutrophilic inflammation, but not lung injury, and was intended to model low dose occupational exposures rather than acute lung injury. For the subchronic exposure protocol, mice were exposed at 16 weeks of age by OPA on days 1, 3, 5, 15, 17, and 19 to 0.83 μg/kg of LPS, 0.67 mg/kg of NiNPs, both 0.83 μg/kg of LPS and 0.67 mg/kg NiNPs, or equal volume of vehicle solution and euthanized on day 24. The cumulative doses of NiNPs and LPS delivered over 6 repeated exposures in the subchronic study were chosen to be equivalent to the single delivered doses of NiNPs and LPS in the acute study, respectively. Acute study results were a combination of two separate experiments (n=3) whereas subchronic study was conducted all at once (n=6). All mice were 12–16 weeks old at the time when acute or subchronic exposures were initiated.
Necropsy and collection of samples:
Mice were euthanized via pentobarbital i.p. injection. At necropsy, the trachea was cannulated and two 0.5 mL aliquots of DPBS were instilled to collect BALF for analysis of cytokines, lactate dehydrogenase (LDH), total protein and inflammatory cell counts. A Thermo Scientific Cytospin 4 centrifuge (Thermo Fisher Scientific, Waltham, MA) was immediately used to spin cells from 100 μl of BALF onto glass slides. These cells were then fixed and stained with the Diff-Quik Stain Set (DadeBehring Inc., Newark, DE, USA) for cellular content analysis of BALF. The left lobe of the lung was inflated with neutral buffered formalin (Azer Scientific, Morgantown, PA) and fixed for 24 hrs, dehydrated in 70% ethanol for 3 days, and then embedded in paraffin for histology. Right lobes were divided equally into RNAlater (FisherScientific, Waltham, MA) or snap frozen in a cryo-tube for mRNA and protein analysis, respectively. Portions of spleen, kidney, heart, and liver were collected for histopathology, mRNA for RT-PCR, or protein for Western blot analysis. Whole blood was collected from the jugular veins and allowed to coagulate for 30 min in Serum Separator Tubes (BD Microtainer, Franklin Lakes, NJ), then centrifuged to obtain the serum.
Total protein and LDH analysis of BALF:
Lactate dehydrogenase (LDH) in BALF was assayed as an index of pulmonary cytotoxicity and was measured by the Pierce LDH Cytotoxicity Assay Kit (ThermoFisher, Waltham, MA) according to the manufacturer’s instructions. Absorbance values were measured at 450nm using the Multiskan EX microplate spectrophotometer (ThermoFisher, Waltham, MA). Total protein concentration in BALF was measured according to the manufacturer’s protocol with the Pierce BCA Protein Assay Kit (ThermoFisher, Waltham, MA). Absorbance was read at 450nm with a correction at 540nm using the Multiskan EX microplate spectrophotometer (ThermoFisher, Waltham, MA).
Cell counting and histopathology:
Average total cell counts per three microscopic fields were obtained from Diff-Quik stained Cytospin slides by counting cells in three frames per slide at 100x magnification and divided by three for the average of cell numbers as described previously (Shipkowski et al., 2015). Images were taken Olympus light microscope BX41 (Center Valley, PA) at 100x and every cell type including neutrophils, eosinophils, monocytes and macrophages were counted using ImageJ software with the Fiji expansion (Eliceiri/LOCI group, University of Wisconsin-Madison, Madison, WI) in a similar way by counting cells in three frames per slide and divided by three for the average of cell numbers. Monocytes were identified by a unilobular dumbbell-shaped nucleus and non-granulated cytoplasm whereas macrophages were identified by a rounded nucleus with granular cytoplasm. Paraffin embedded tissues were cut into three sections and stained with hematoxylin and eosin (H&E) for both acute and subchronic exposure protocol, or with Alcian blue/periodic acid- Schiff (AB/PAS) and/or Gomori’s trichrome stain for subchronic exposure protocol only. Semi-quantitative evaluation of the degree of inflammation was assessed on histopathology slides using an unbiased scoring protocol (See Supplementary File 1).
Cytokine quantification:
Cytokines (IL-6, CXCL1, TNF-α, IL-1β and CCL2) from BALF were measured by DuoSet enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Inc., Minneapolis, MN). Lung lysates were isolated with lysis buffer from snap-frozen mouse right lung lobes. The lung lysate protein concentration was determined using the Pierce BCA Protein Assay Kit (ThermoFisher Scientific, Waltham, MA). CCL2 in lung lysate was measured by DuoSet enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Inc., Minneapolis, MN). Cytokine concentrations in BALF or lung lysates were derived from the absorbance values and converted to concentration values based on the standards provided from the kit.
RT-PCR:
Applied Biosystems high capacity cDNA reverse transcription kit (ThermoFisher Scientific, Waltham, MA) was used to create cDNA from the mRNA isolated from the right lung lobes, spleen, liver, and heart using Quick-RNA™ MiniPrep (Zymo Research, Irvine, CA) according to the manufacturer’s instructions. The FastStart Universal Probe Master (Rox) (Roche, Basel, Switzerland) was then used to run Taqman qPCR on the Applied Biosystems QuantStudio3 Real-Time PCR System Thermal Cycling Block (ABI, Foster City, CA) to determine the comparative CT (ΔΔCT) fold change expression of IL-6, CXCL1, and CCL2 normalized to β-actin as the endogenous control.
Immunoblotting:
Whole lung protein lysates were isolated from snap-frozen mouse right lung lobes. The protein concentration was determined using the Pierce BCA Protein Assay Kit (ThermoFisher Scientific, Waltham, MA). Samples were loaded onto a Novex™ 4–12% SDS-PAGE gel (Invitrogen, Carlsbad, CA), and separated by electrophoresis with Pierce Pre-stained Protein MW Marker (ThermoFisher Scientific, Waltham, MA). Proteins were transferred to PVDF membranes, blocked with blocking buffer (1X TBS, 0.1% Tween-20 with 5% nonfat dry milk in water) for an hour, and incubated in primary antibody (1:1000 dilution) overnight at 4°C. pSTAT3 (Tyr 705), total STAT3, STAT1, and β-actin primary antibodies as well as anti-rabbit or anti-mouse secondary antibody (1:2000) were purchased from Cell Signaling Technology (Beverly, MA). Following primary antibody incubation, the membranes were washed with TBST (1X TBS and 0.1% Tween-20 in water) and then incubated with horseradish peroxidase-conjugated secondary antibody (1:2500 dilution) for an hour at the room temperature. The membrane was then washed in TBST once again for 30 minutes. Enhanced chemiluminescence (ECL) (ThermoFisher Scientific, Waltham, MA) was used to visualize immunoblot signals by using Amersham Imager 680 (GE Life Sciences, Marlborough, MA). Semi-quantitative densitometry was performed on Western blot protein bands using ImageQuant TL (GE LifeSciences). Volume intensity of each protein band was quantified from ImageQuantTL program according to the manufacturer’s protocol; phosphorylation protein bands were normalized to corresponding total protein bands, and total protein bands were normalized to the corresponding β-actin bands.
Statistical Analysis:
All statistical analyses were performed using Prism Software (GraphPad Inc., La Jolla, CA). Differences between treatment groups within the same sex were evaluated by one-way ANOVA with a Dunnett’s post-hoc test. A two-way ANOVA with a Bonferroni’s post- hoc test was used to test for significance between sexes among the treatment groups. Student’s T-test was also used to determine significant differences between males and females. Differences was determined to be significant at a P value < 0.05 or less. Specific information for each analysis performed is detailed in the figure legends.
RESULTS
Male mice are more susceptible than female mice to neutrophilic inflammation after a single acute exposure to NiNPs and LPS.
To determine whether there were sex differences in the acute lung inflammatory response to NiNPs with or without LPS, male and female C57BL/6J mice were treated with vehicle solution, 5 μg/kg LPS, 4 mg/kg NiNPs, or both 5 μg/kg LPS and 4 mg/kg NiNPs via OPA as illustrated in Figure 1B. Exposure to NiNPs and LPS caused a significant increase of total protein in the BALF of both male and female mice, indicating acute lung injury in both sexes (Fig. 1C). NiNPs or NiNPs and LPS caused a significant increase in cytotoxicity in male mice, but not in female mice, with a significant difference in LDH between sexes in the NiNP exposure group. (Fig. 1D). Representative images of BALF cell density on Diff-Quick stained Cytospin slides from male and female mice are shown in Fig. 2A. These data show that the average total cell density, counted on 3 microscopic fields per animal, is much higher in males and mostly composed of neutrophils along with macrophages containing cytoplasmic NiNP inclusions. Quantitative evaluation of inflammatory cells (neutrophils, macrophages, eosinophils, and lymphocytes) from the BALF Cytospin slides showed that male and female mice had a modest increase in numbers of total cells and neutrophils 24 hrs after exposure to LPS or NiNPs with no significant differences between sexes (Fig. 2A, 2B). However, co-exposure to NiNPs and LPS synergistically increased both the average total cell counts and the average number of neutrophils, with males having significantly higher numbers of cells compared to female mice. (Fig. 2A, 2B). No significant changes were detected in the number of macrophages after NiNPs or LPS and NiNPs (Fig. 2B). These results indicate that acute co-exposure to NiNPs and LPS induced significantly more neutrophilic inflammation in male mice compared to female mice. Qualitative evaluation of hematoxylin and eosin-stained lung sections showed that mice co-exposed to NiNPs and LPS had neutrophilic infiltration within the distal bronchoalveolar regions (Data not shown).
Figure 2.

Inflammatory cells in the BALF from male and female mice 24 hrs after exposure to LPS, NiNPs, or LPS and NiNPs using the protocol illustrated in Fig. 1. A) Representative microscopic images of BALF cells from male and female mice on Diff-Quick stained Cytospin slides from each exposure group (Magnification 200X; inset 1000X oil immersion). B) Average number of total cells, neutrophils or macrophage per three microscopic fields on BALF Cytospin slides after acute (24 hr) exposure to NiNPs with or without LPS (Average number of cells were counted as described in the methods). (n=5–6 mice per group, ***P<0.001 or **P< 0.01 or *P<0.05 compared to vehicle determined by one-way ANOVA, ###P<0.001 or #P<0.05 between sexes determined by two-way ANOVA).
Male mice produce a significantly higher amount of pro-inflammatory cytokines after a single acute exposure to NiNPs and LPS.
We next evaluated cytokines or chemokines that play a role in acute neutrophilic inflammation (IL-6, CXCL1, TNF-α, IL-1β). Mouse CXCL1 mediates neutrophil migration via CXCR2, which is a receptor for human IL-8 (CXCL8), which also possesses neutrophil chemotactic properties (Fan et al., 2007). Both IL-6 and CXCL1 are known to play vital roles in neutrophilic lung inflammation as part of the innate immune response to pathogens or inhaled particles (Kaplanski et al., 2003; Gabay, 2006). We observed the increase in both IL-6 protein and mRNA levels after exposure to NiNPs or NiNPs and LPS, with males having significantly higher IL-6 levels than females (Fig. 3A,B). On the other hand, LPS alone did not induce a significant increase in IL-6 protein or mRNA in either male or female mice (Figure 3A,B). NiNPs, LPS or NiNPs and LPS caused a significant increase in CXCL1 protein levels in BALF in male mice, whereas only LPS treatment caused a significant increase in CXCL1 in BALF in female mice (Fig. 3C). Furthermore, CXCL1 protein levels in BALF induced by NiNPs or LPS and NiNPs treatments were significantly higher in male mice compared to the female mice (Fig. 3C). However, only NiNPs and LPS co-exposure caused a significant in CXCL1 mRNA levels in lung tissue, with no differences between males and females (Fig. 3D). No significant changes were observed in protein levels of TNF-α or IL-1β measured by ELISA (Data not shown).
Figure 3.
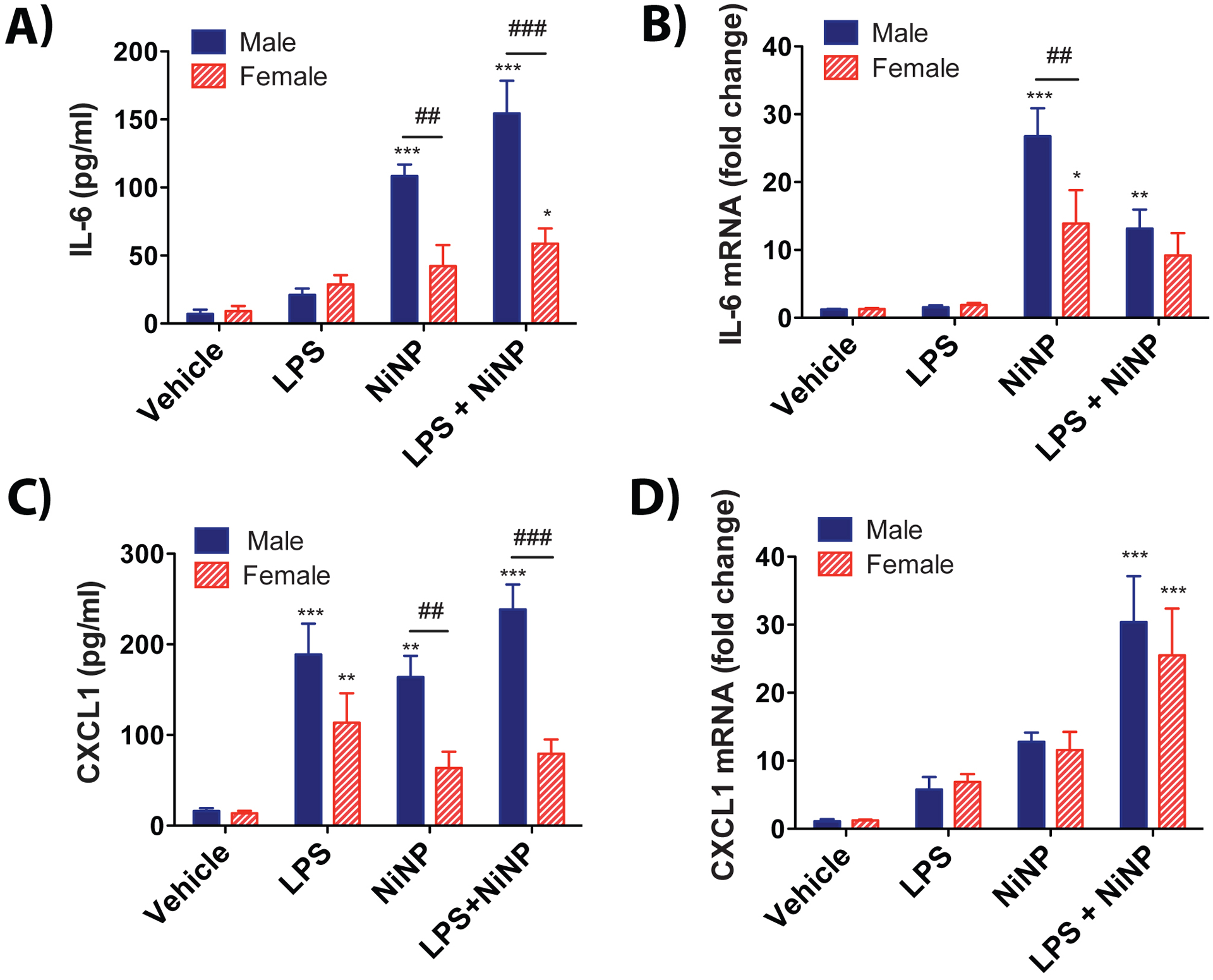
Levels of pro-inflammatory cytokines in BALF and lung tissue. A) IL-6 protein in BALF measured by ELISA. B) IL-6 mRNA in lung tissue measured by qRT-PCR. C) CXCL1 protein in BALF measured by ELISA. D) CXCL1 mRNA in lung tissue measured by qRT-PCR. (n=5–6 mice per group, ***P<0.001 or **P< 0.01 or *P<0.05 compared to vehicle determined by one-way ANOVA, ###P<0.001 or ##P<0.01 between sexes determined by two-way ANOVA).
STAT3 activation is greater in male mice after a single acute exposure to NiNPs.
As mentioned previously, STAT3 is pivotal transcription factor that plays a critical role in multiple pulmonary diseases including asthma, fibrosis and cancer (Wang et al., 2013). Moreover, IL-6 stimulates STAT3 activation resulting in amplification of IL-6 production (Hodge, Hurt and Farrar, 2005; Wang et al., 2013). Furthermore, STAT3 tyrosine 705 phosphorylation is necessary for IL-6 production (Wang et al., 2013). Densitometry of western blots showed that NiNP-treated male mice had a significantly higher STAT3 phosphorylation level (p-STAT3/STAT3 ratio) in lung tissue compared to vehicle and compared to NiNP treated female mice (Fig. 4A,B). LPS and NiNPs co-exposure also caused a significant increase in STAT3 phosphorylation in male mice, but no significant difference was detected between sexes (Fig. 4A,B). Total STAT3 levels were similar between male and female mice in all treatment groups. STAT1 phosphorylation was not detected by western blotting in lung tissue from male or female mice in the acute exposure. Lung protein levels of total STAT1 were increased by NiNPs, and LPS and NiNPS in male mice whereas only LPS increased total STAT1 in female mice in the acute exposure (Fig. 4C,D). However, no significant sex difference in total STAT1 level was found in different treatments (Fig. 4C,D).
Figure 4.
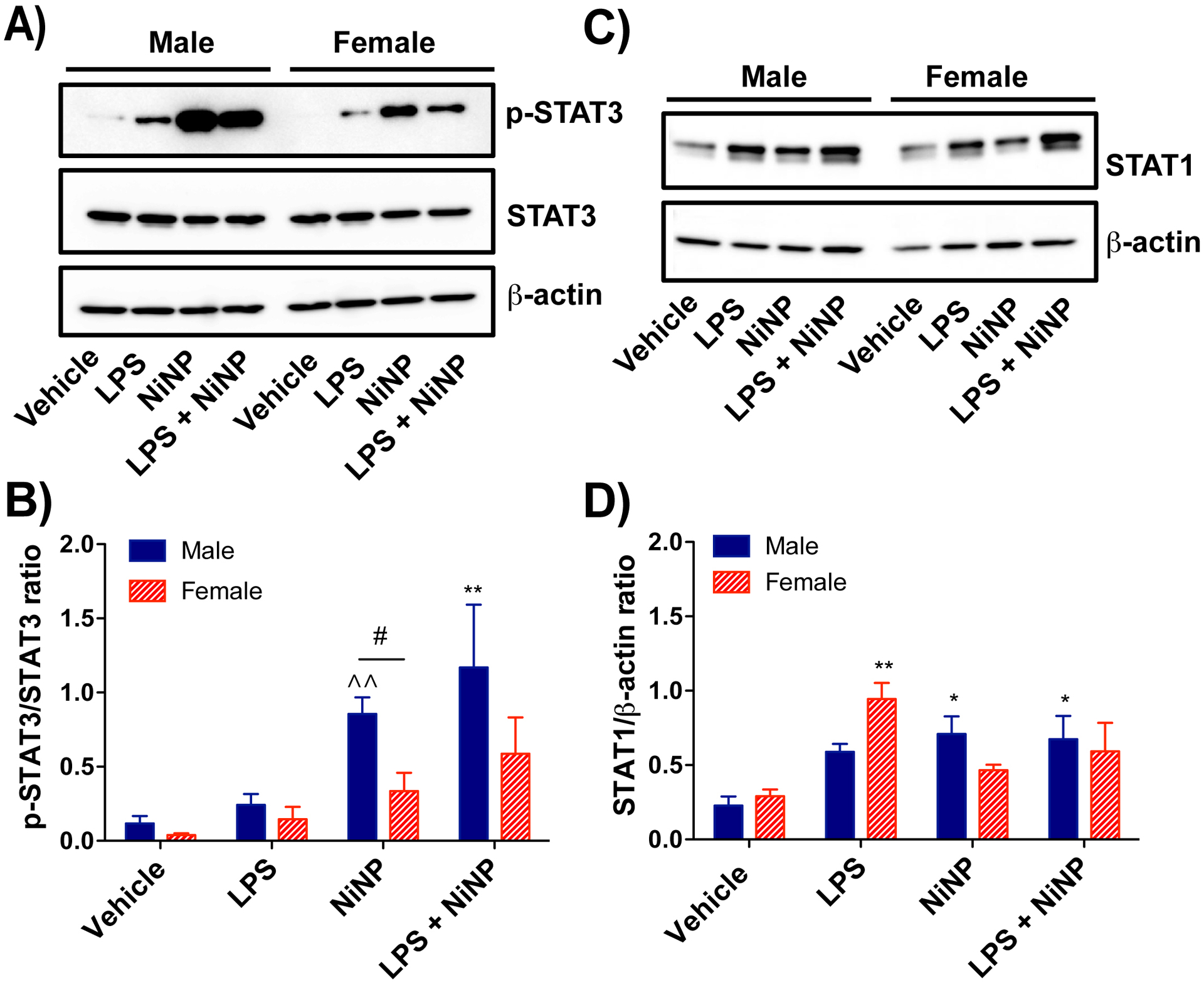
Western blot analysis of STAT proteins from the lung tissue of male or female mice after acute exposure to NiNPs in the absence or presence of LPS A) Representative western blots of phosphorylated STAT3 (p-STAT3), total STAT3, and β-actin in lung tissue from each treatment group in male and female mice. B) Quantitative densitometry of the average expression of pSTAT3 normalized for total STAT3. C) Representative western blots of total STAT1 and β-actin in lung tissue from each treatment group in male and female mice. D) Quantitative densitometry of the average expression of total STAT1 normalized for β-actin. (n=4–6, **P< 0.01 or *P<0.05 compared to vehicle determined by one-way ANOVA, ^^P<0.01 compared to vehicle by paired Student’s t-test, #P<0.05 between sexes using Student’s t-test).
Repeated subchronic exposure to NiNPs or NiNPs and LPS produces monocytic lung inflammation and the formation of crystals in BALF.
In order to model a subchronic, repeated exposure scenario, male and female C57BL/6J mice were treated via OPA with vehicle solution, LPS (0.83 μg/kg), NiNPs (0.67 mg/kg), or LPS and NiNPs six times over a period of 3 weeks as illustrated in Figure 5A. The total accumulated dose for the subchronic model was the same as that used for the acute experiment. Male mice had a significant increase in BALF total protein after NiNPs or NiNPs and LPS treatment, but there was no significant difference in total protein between sexes (Fig. 5B). Both male and female mice had a significant increase in LDH levels in BALF after exposure to NiNPs or NiNPs and LPS, with no significant differences between sexes (Fig. 5C). Evaluation of inflammatory cells in BALF after repeated subchronic exposure to NiNPs, LPS or NiNPs and LPS showed that most of the cells were monocytes, macrophages and lymphocytes. Monocytes were identified by a unilobular dumbbell-shaped nucleus and non-graulated cytoplasm whereas macrophages were identified by a rounded nucleus with granular cytoplasm, and often contained phagocytic inclusions in the NiNP treatment group. Example images of macrophages, monocytes, neutrophils and lymphocytes are shown in Fig. 6A. Representative images of BALF cell density on Diff-Quick stained Cytospin slides showed that male mice had a greater density of average total cell counts in the NiNPs and LPS co-exposure group (Fig. 6B). Differential cell counting showed that the increased cellularity in the BALF from male mice exposed to the combination of NiNPs and LPS was mainly due to monocytes and macrophages (Fig. 6C). LPS and NiNP treatment significantly induced a greater number of average total cell counts and monocytes in male mice compared to the female mice (Fig. 6C). Relatively low numbers of neutrophils were detected in BALF after subchronic exposure to NiNPs or the combination of LPS and NiNPs, but numbers of neutrophils were greater in males compared to females (Fig. 6C). No difference in lymphocytes were detected between sexes (Fig. 6C). These results indicate that repeated subchronic exposure to NiNPs or NiNPs and LPS produced monocytic lung inflammation that contrasted to the neutrophilic inflammation observed in the single acute exposure to NiNPs or NiNPs and LPS. Interestingly, repeated subchronic exposure to NiNPs caused a significant increase in the number of hexagonal crystals that were apparent in the BALF of both male and female mice and also seen in the lungs of male and female mice in situ (Fig. 7A,B,C). The number of NiNP-induced crystals were reduced in both sexes upon co-exposure with LPS.
Figure 5.

Subchronic exposure of male and female mice by repeated oropharyngeal aspiration (OPA) to LPS, NiNP, or LPS and NiNP over a period of three weeks. A) Illustration of experimental design. C57BL/6J mice were exposed to vehicle (0.1% Pluronic in DPBS), LPS (0.83 μg/kg), NiNPs (0.67 mg/kg), or combination of (0.83 μg/kg) LPS and (0.67 mg/kg) NiNPs on days 1, 3 and 5, then allowed to rest for week and exposed again on days 15, 17 and 19. Mice were euthanized and necropsy performed 24 days after the first exposure. B) Total protein concentration in BALF was determined by Pierce BCA Protein Assay. C) Lactate dehydrogenase (LDH) activity in BALF was measured using Pierce LDH Cytotoxicity Assay. (n=5–6 mice per group, ***P<0.001 or **P<0.01 or *P<0.05 compared to vehicle determined by one-way ANOVA).
Figure 6.

Inflammatory cells in the BALF of male and female mice 24 days after repeated subchronic exposure to LPS, NiNPs, or LPS and NiNPs. A) Microscopic images of representative cell types on BALF Cytospin slides used for identification (Magnification, 1000X oil immersion, taken from a male mouse exposed to NiNPs and LPS). B) Representative microscopic images of BALF cells from male and female mice on Diff-Quick stained Cytospin slides from each exposure group (Magnification 200X; inset 1000X oil immersion). C) Average number of total cells, monocytes, macrophages, neutrophils or lymphocytes per three microscopic fields on BALF Cytospin slides after repeated subchronic exposure to NiNPs with or without LPS. Average number of cells were counted as described in the methods. (n=5–6 mice per group, ***P<0.001 or **P< 0.01 or *P<0.05 compared to vehicle determined by one-way ANOVA, ###P<0.001, ##P<0.01 or #P<0.05 between sexes determined by two-way ANOVA).
Figure 7.
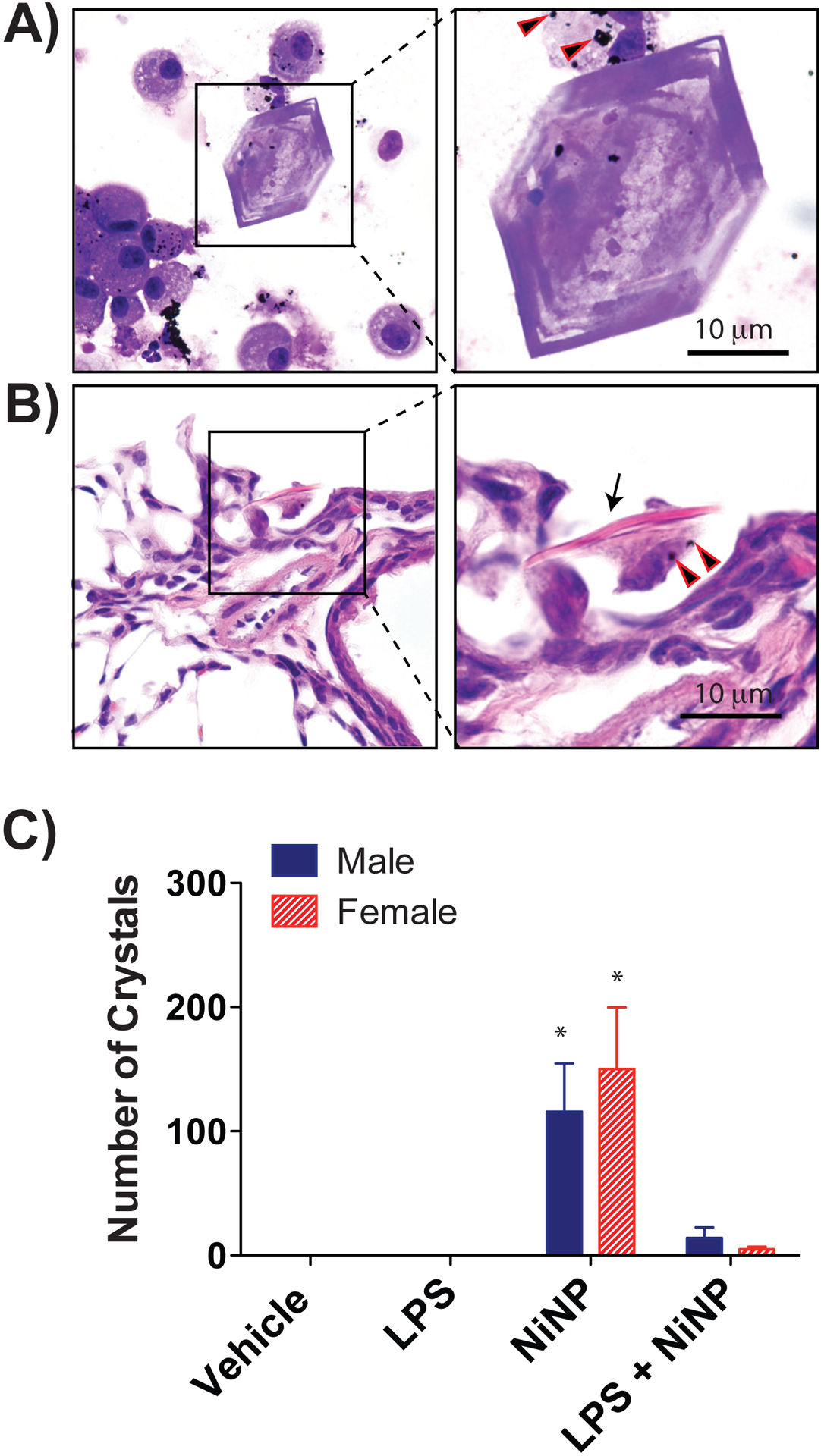
Hexagonal crystals in the lungs of male and female mice after repeated subchronic exposure to NiNPs. A) Representative microscopic image of Diff-Quick stained Cytospin slide from a male mouse 24 days after repeated exposure to NiNPs showing a crystal with BALF cells (Magnification 200X; inset 1000X oil immersion with arrowheads indicating NiNPs). B) Hematoxylin and eosin stained lung section from a male mouse after repeated subchronic exposure to NiNPs showing side view of a crystal (arrow) in the bronchoalveolar region associated with a macrophage containing NiNPs (arrowheads). C) Average number of crystals in the BALF of male and female mice after exposure to NiNPs in the absence or presence of LPS. The data represent the total numbers of crystals in Cytospin slides from each animal. (n=5–6 mice per group, *P<0.05 compared to vehicle determined by one-way ANOVA).
Male mice have greater alveolar inflammatory cell infiltration after repeated subchronic exposure to NiNPs and LPS.
Repeated subchronic exposure to NiNPs, LPS or NiNPs and LPS, using the protocol illustrated in Fig. 5, produced interstitial inflammatory lesions in the lungs of male and female mice (Fig. 8A). Three different inflammatory patterns were scored in a randomized and blinded fashion by a board-certified veterinary pathologist; 1) cellular infiltrates in alveolar lumina, 2) perivascular and peribronchiolar lymphocyte aggregates, and 3) dense epithelioid macrophages aggregates. The scoring system was: 0 = no inflammation, 1 = mild inflammation, 2 = moderate inflammation, and 3 = marked inflammation (See Supplementary File 1 for detailed protocol). NiNP-treated male mice had significantly higher scoring on alveolar lumen infiltrates compared to female NiNP-treated mice (Fig. 8B). Exposure to NiNPs or LPS and NiNPs significantly increased lymphocytic perivascular and peribronchiolar aggregates but there was no difference between sexes (Fig. 8B). Similarly, the score for lesions containing epithelioid macrophage aggregates was significantly increased in males and females by NiNPs or LPS and NiNPs, but there were no significant differences between males and females (Fig. 8B). NiNPs were still detectable in the lungs after 24 days, mainly within macrophages.
Figure 8.
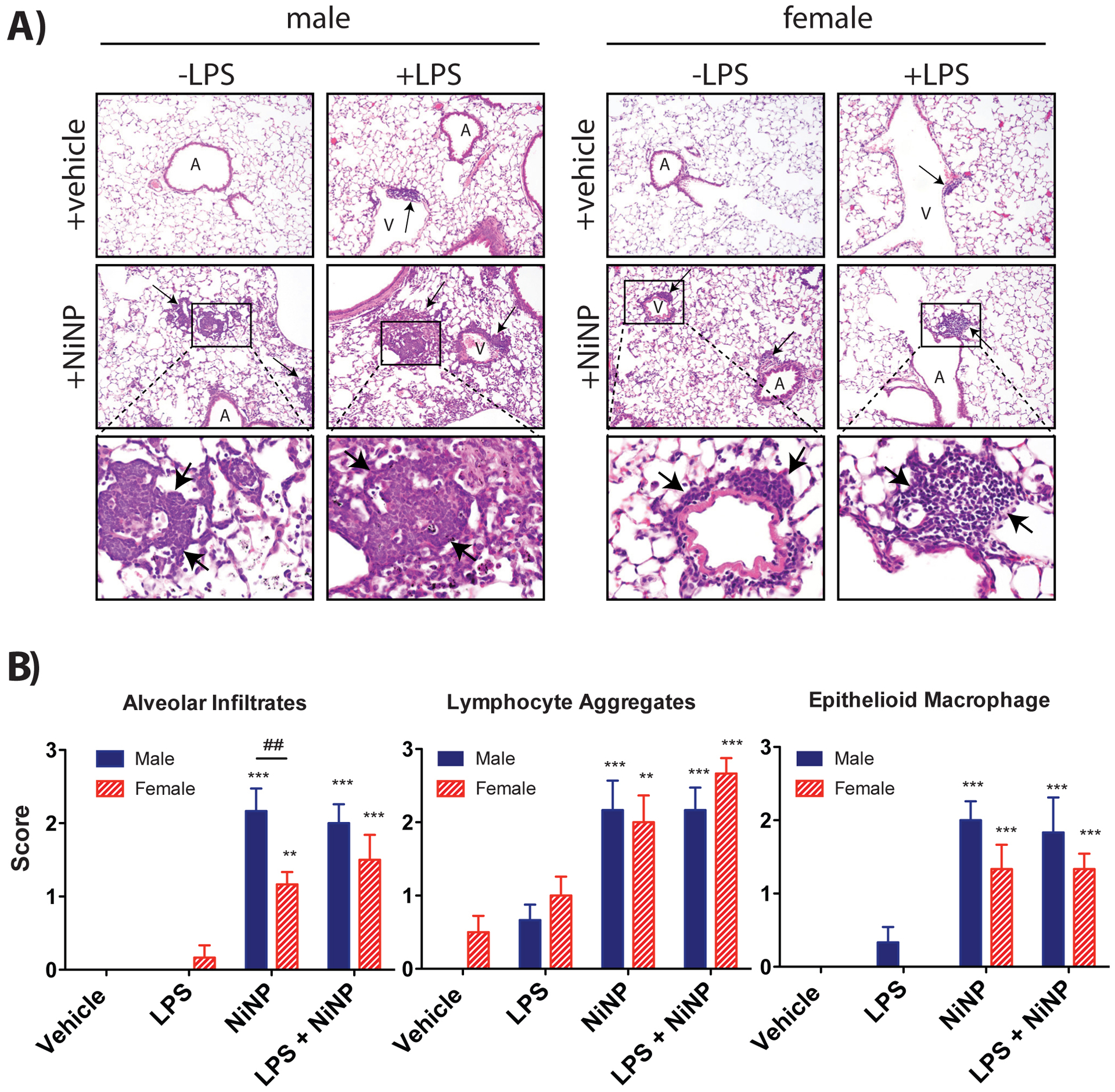
Lung pathology of male and female mice after repeated subchronic exposure to NiNPs in the absence or presence of LPS. A) Representative hematoxylin and eosin stained lung sections from mice treated with NiNPs or LPS and NiNPs. Inflammatory lesions are indicated by arrows. ‘A’ and ‘V’ indicate airways and vessels, respectively. B) Results of pathology scoring of inflammatory patterns showing relative scores for cellular infiltrates in alveolar lumina, perivascular and peribronchiolar lymphocyte aggregates and dense epithelioid macrophage aggregates. See methods and Supplementary File 1 for scoring system. (n=5–6 mice per group, ***P<0.001 or **P< 0.01 compared to vehicle determined by one-way ANOVA, ##P<0.01 between sexes determined by two-way ANOVA).
CXCL1 and CCL2 protein levels are increased in the BALF and lung tissue of male mice after repeated subchronic exposure to NiNPs.
CXCL1 protein in BALF was significantly increased by NiNPs in both male and female mice, and significantly increased by co-exposure to LPS and NiNP in male mice (Fig. 9A). Moreover, male mice had significantly higher levels of CXCL1 protein levels in BALF compared to female mice after repeated subchronic exposure to NiNPs or LPS and NiNPs (Fig. 9A). In contrast to the acute exposure protocol, where IL-6 levels in BALF were increased and greater in male mice (Fig. 3A), there were no changes in IL-6 protein in BALF, after subchronic exposure, except for a decrease in IL-6 in males exposed to LPS and NiNPs (Fig. 9B). CCL2 (MCP-1) is a major chemokine that regulates monocyte/macrophage migration and infiltration (Yang et al., 2020). Interestingly, CCL2 protein in BALF or lung tissue from male mice was significantly increased by NiNPs and higher than CCL2 levels in the BALF or lung tissue of female mice (Fig. 9C). CCL2 and CXCL1 mRNA induced by LPS and NiNPs did not correlate with cytokine protein levels measured by ELISA (Supplementary File 2). Moreover, lung mRNAs for CXCL1, IL-6, and CCL2 showed different expression patterns compared to BALF protein levels, with females having significantly higher cytokine mRNAs induced by NiNPs (Supplementary File 2). CXCL-10 also serves as a mononuclear cell chemokine (Zhao et al., 2017). No changes were detected in CXCL10 in BALF as measured by ELISA in any of the treatment groups in either male or female mice (Data not shown).
Figure 9.
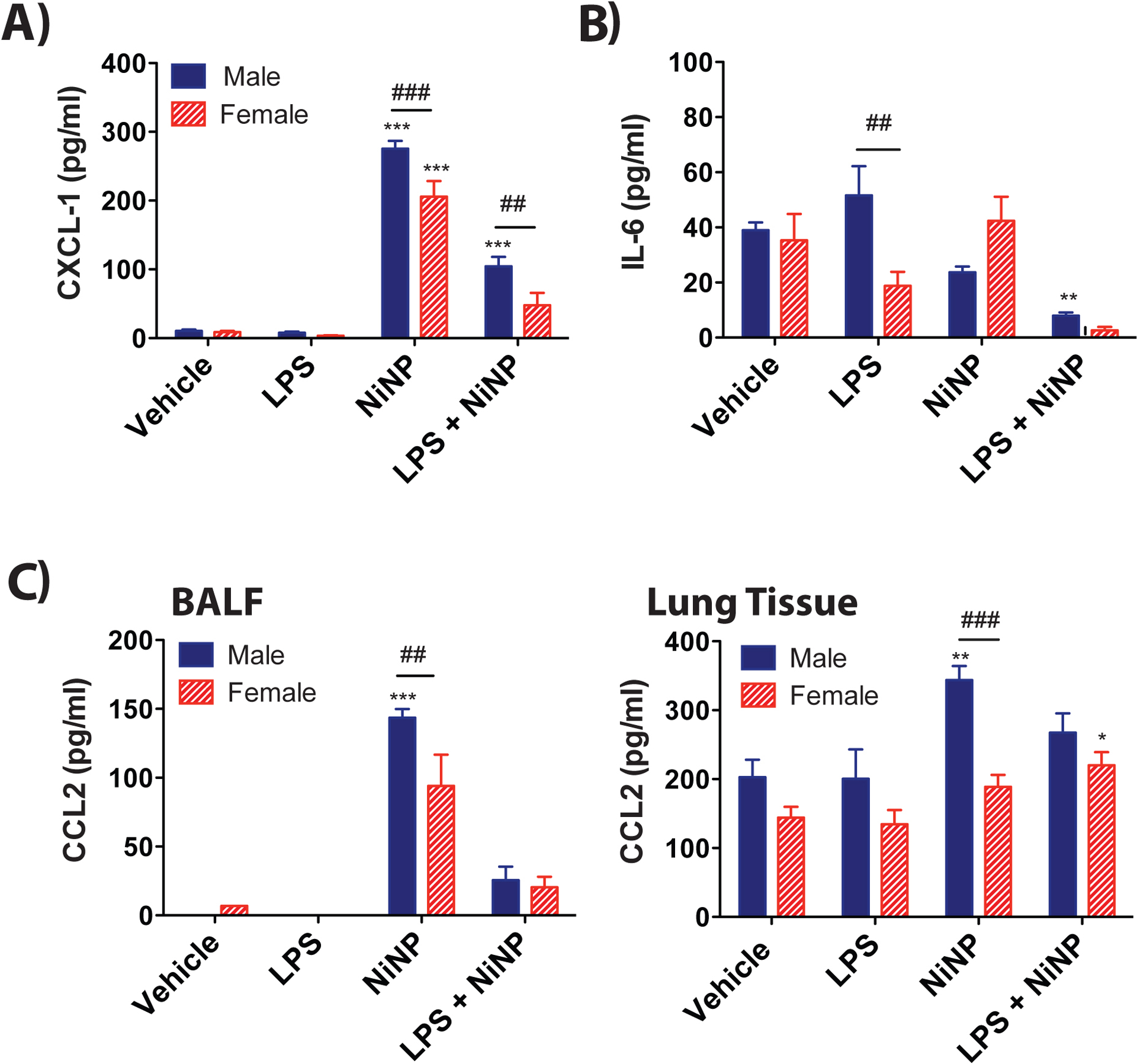
Protein levels of cytokines measured by ELISA in the lungs of male and female mice after repeated subchronic exposure to NiNPs in the absence or presence of LPS. A) CXCL1 protein in BALF. B) IL-6 protein in BALF. C) CCL2 protein in BALF and lung lysates. (n=5–6 mice per group, ***P<0.001 or **P< 0.01 or *P<0.05 compared to vehicle determined by one-way ANOVA, ##P<0.01 or ###P<0.001 between sexes determined by two-way ANOVA).
Female mice have significantly elevated protein levels of STAT1 after repeated exposure to NiNPs.
Unlike the acute exposure protocol that induced a high level of STAT3 phosphorylation in male mice exposed to NiNPs and LPS, subchronic exposure to NiNPs, in the absence or presence of LPS, did not induce STAT3 phosphorylation in the lungs of male mice (Fig. 10A,B). Subchronic exposure to NiNPs induced an increase in STAT3 phosphorylation in female mice only. There was a significantly lower level of basal phospho-STAT3 protein in lungs of female mice in the LPS treatment group. No differences in STAT3 phosphorylation and total STAT3 were observed between sexes. STAT1 is a protective transcription factor that suppresses allergen-induced lung inflammation in mice that is exacerbated nickel-containing multi-walled carbon nanotubes (Duke et al., 2017, Thompson et al., 2015). Interestingly, Western blotting of total lung protein for STAT1 showed that female mice had higher levels of total STAT1 compared to male mice after repeated, subchronic exposure to NiNPs (Fig. 10C,D). However, quantitative densitometry of STAT1 bands from Western blots of lung tissue showed a significant increase only in NiNP-exposed female mice, but not after repeated exposure to LPS and NiNPs.
Figure 10.
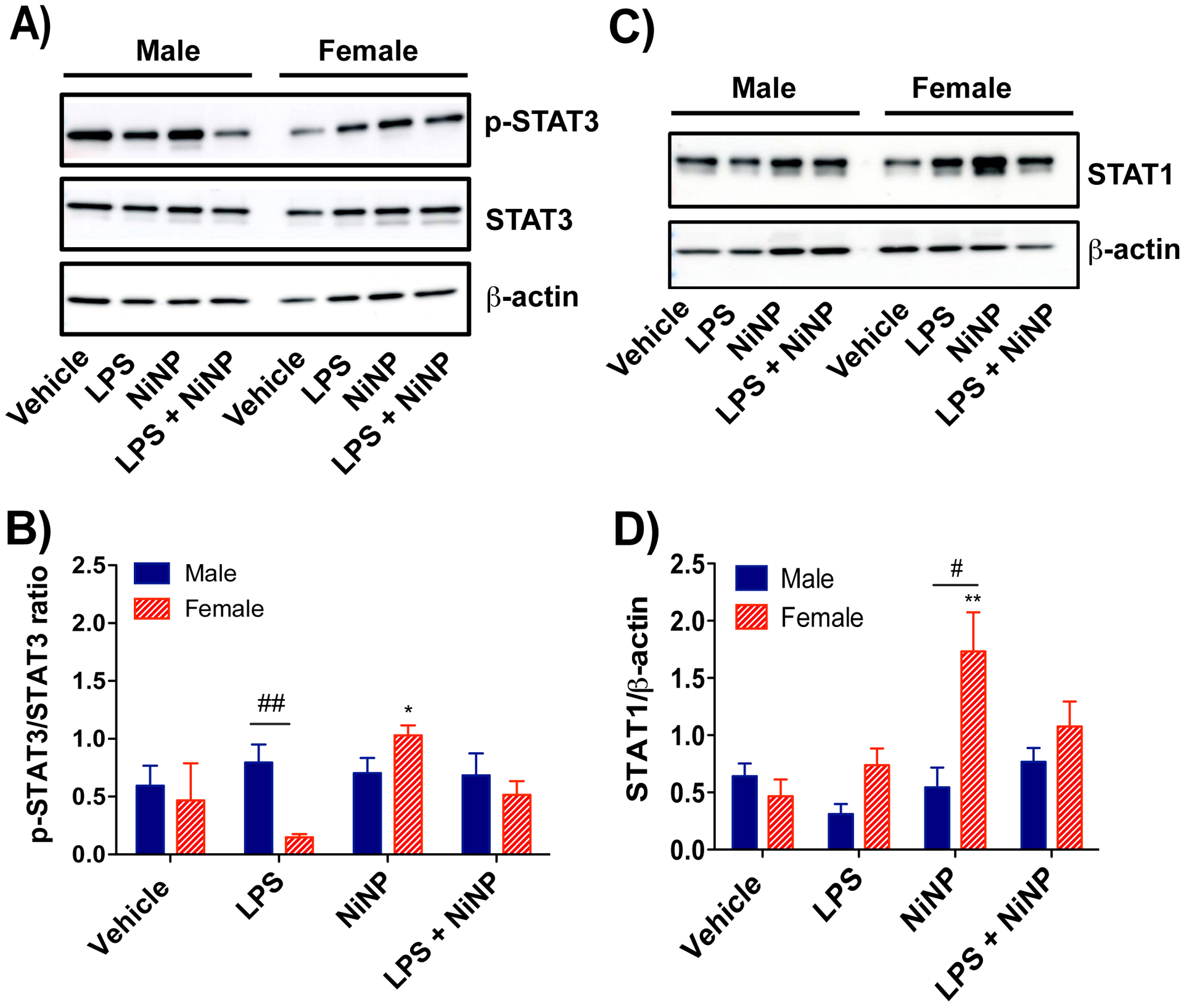
Western blot analysis of STAT proteins from the lung tissue of male or female mice after repeated subchronic exposure to NiNPs in the absence or presence of LPS. A) Representative western blots of p-STAT3, total STAT3, and β-actin in lung tissue from each treatment group in male and female mice. B) Quantitative densitometry of the average expression of p-STAT3 normalized for total STAT3. C) Representative western blot of total STAT1 and β-actin in lung tissue from each treatment group in male and female mice. D) Quantitative densitometry of the average expression of total STAT1 normalized for β-actin. (n=4–6 mice per group, **P< 0.01 or *P<0.05 compared to vehicle determined by one-way ANOVA, ##P<0.01 or #P<0.05 between sexes determined by using Student’s t-test).
Male mice have higher IL-6 mRNA in liver after acute NiNP exposure, but higher CCL2 mRNA in liver after repeated subchronic exposure to NiNPs.
In addition to lung, IL-6 and CCL2 mRNAs were also measured in liver, spleen, and heart, after acute or repeated, subchronic exposure to NiNPs in the absence or presence of LPS. In the acute exposure, IL-6 mRNA was significantly induced by LPS and NiNPs in the liver of male mice but not in female mice (Supplementary File 3). IL-6 mRNA levels were also higher in the heart from male mice compared to female mice after LPS or NiNP and LPS co-exposure (Supplementary File 3). LPS with or without NiNPs reduced IL-6 mRNA in heart tissue of female mice after 24 hrs (Supplementary File 3). IL-6 mRNA in spleen from female mice was statistically lower compared to the male mice in all treatments including vehicle (Supplementary File 3). After repeated subchronic exposure, no significant changes in IL-6 mRNA in the lung or other organs were observed (data not shown). However, CCL2 mRNA was significantly induced in the liver and heart from male mice compared to the female mice after repeated, subchronic exposure to NiNPs (Supplementary File 4).
DISCUSSION
The goal of this study was to explore sex differences in the lung inflammatory responses of mice after acute or repeated, subchronic exposure to NiNPs in the absence or presence of LPS and to investigate mechanisms of differential susceptibility between sexes. The LPS-nanoparticle co-exposure model is relevant to real-world occupational and environmental exposures to particles that are often contaminated with LPS (Bonner et al., 1998; Duquenne and Marchand., 2013; Cesta et al., 2010). To our knowledge, this is the first study to investigate sex differences in the lung inflammatory response to nickel or NiNPs in an animal model. Based on epidemiology studies of human cohorts showing that males are more susceptible to acute pulmonary diseases, whereas females are generally more susceptible to chronic pulmonary diseases, we hypothesized that male mice would be more susceptible to acute lung inflammation after a single acute exposure, while female mice would be more susceptible to lung inflammation caused by repeated, subchronic exposures. The acute exposure to NiNPs, in the absence or presence of LPS, showed that male mice had greater numbers of neutrophils in their BALF and produced higher levels of acute proinflammatory cytokines including IL-6 and CXCL1, demonstrating that in mice, males are more susceptible than females to acute inflammation. For the subchronic experiment, we observed higher numbers of monocytes in the BALF of male mice compared to female mice. Unbiased pathology scoring of subchronic lung lesions showed an increase in cellular infiltrates in alveolar lumina in male mice that were composed primarily of monocytes. The increase in cellular infiltrates in alveolar lumina and BALF monocytes in male mice after repeated subchronic exposure corresponded to higher CCL2 protein in lung tissue. Therefore, our data clearly demonstrate the susceptibility of male mice to acute neutrophilic inflammation after a single exposure to NiNPs, as well as susceptibility to chronic monocytic inflammation after repeated exposures to NiNPs. A hypothetical mechanism of sex-dependent susceptibility to acute and subchronic NiNP exposure is illustrated in Fig. 11.
Figure 11.

Hypothetical illustration of mechanisms underlying the susceptibility of male mice to a single acute or repeated subchronic exposure to NiNPs in the absence or presence of LPS. Red and green arrows indicate an increase or decrease in males compared to females.
We observed that a single acute exposure to NiNPs and LPS resulted in more severe inflammation in male mice within 24 hrs that involved increased neutrophilic infiltration with higher lung IL-6 mRNA and protein. IL-6 is a major inducer of STAT3 activation (Wang et al., 2013). The mechanism of male susceptibility to acute NiNP-induced inflammation could involve IL-6 acting through STAT3, since our data demonstrated that males had higher levels of STAT3 phosphorylation in lung tissue compared to females when exposed to NiNPs with or without LPS. STAT3 is an important transcription factor that is critical in generating inflammatory responses in many different pulmonary diseases including fibrosis and asthma as well as in other organ systems (Wang et al., 2013). IL-6 binding to its cognate cell-surface receptor (IL-6R) stimulates the phosphorylation of STAT3 and activated STAT3 works in a feedback loop mechanism to stimulate more IL-6 production (Tanaka et al, 2014). We suggest that higher CXCL1 and IL-6 production, with greater STAT3 phosphorylation, contributed to higher neutrophil counts and greater severity of acute lung inflammation, in male mice exposed to a single dose of NiNPs with or without LPS exposure. Moreover, numerically higher levels of IL-6 mRNA in male mice were also observed in other organs, including the liver, heart and spleen. It has previously been reported that the circulating IL-6 produced from acute kidney injury can induce CXCL1 production from endothelial cells and neutrophils to mediate lung inflammation in mice (Ahuja et al., 2012). This observation also suggests that the susceptibility of males to NiNP-induced inflammation is not limited to the lungs and could extend to increased susceptibility to inflammatory reactions in other organ systems. The higher level of NiNP-inducible IL-6 in lung and other organs of male mice in the present study could be due to differences in sex hormones. For example, a study by Kassem et al. (2010) showed that 17β-estradiol had no effect on the constitutive production of IL-6 by osteoblasts but had a profound inhibitory effect on inducible IL-6 production. Naugler et al. (2007) found that hepatocellular carcinoma (a classic inflammation-linked cancer) is more common in male mice due to sex specific IL-6 production. They found that injecting chemical carcinogen DEN significantly increased IL-6 serum level only in male mice. They also reported that, upon injection of DEN, ovariectomized female mice had a higher level of IL-6 mRNA, and estradiol supplement to the ovariectomized female mice significantly reduced IL-6 mRNA levels (Naugler et al., 2007). Furthermore, Sperry et al. (2008) found that persistent excessive IL-6 expression in men compared to women following traumatic injury and hemorrhagic shock is associated with higher number of multiple organ failure cases in men. Paimela et al. (2007) found that inhibitory effect of 17β-estradiol on IL-6 production is linked to decreased NF-κB DNA binding activity. Similarly, Galien and Garcia et al. (1997) found estrogen receptor signaling impairs IL-6 production by preventing c-rel and RelA proteins to bind to the NF-κB site of the IL-6 promoter. Therefore, we suggest that estradiol signaling in female mice exposed to NiNP, in the absence or presence of LPS, could reduce both IL-6 production and STAT3 activation resulting in less severe acute inflammation compared to male mice.
Based on evidence from published epidemiology studies showing that women are generally more susceptible to chronic lung inflammation (Jensen-Fangel et al., 2004; Postma, 2007; Gleeson et al., 2011; Kadioglu et al., 2011; Casimir et al., 2013; Pinkerton et al., 2015; Klein and Flanagan, 2016), we initially postulated that female mice would be more susceptible to repeated subchronic exposures to NiNPs with or without LPS. However, we observed that male mice were more susceptible to subchronic lung inflammation after repeated exposures to NiNPs and LPS with greater numbers of infiltrating monocytes, whereas NiNPs or LPS alone did not produce a significant increase in monocytes isolated from BALF. This finding is, in part, consistent with a study by Yoshizaki and colleagues (2017) wherein greater numbers of infiltrating monocytes and macrophages were found in the lungs of male mice after repeated subchronic exposure to PM2.5. Furthermore, a retrospective cohort study demonstrated that an increase in monocyte counts in serum was found in patients that developed idiopathic pulmonary fibrosis and other fibrotic disorders (Scott et al., 2019). Several studies have shown that infiltrating monocytes require chemokine receptor CCR2 in order to migrate to inflammatory sites containing high levels of the chemokine ligand CCL2, and that the CCL2/CCR2 signaling pathway is involved in the pathogenesis of bleomycin-induced pulmonary fibrosis (Kurihara and Bravo, 1996; Okuma et al., 2004; O’Connor, Borsig and Heikenwalder, 2015). Furthermore, one of these reports suggested that the loss of CCR2 could improve the disease through regulating macrophage infiltration (Okuma et al., 2004). A study conducted by Groves et al. (2018) also showed that CCR2 knockout chimeric mouse exposed to radiation had reduced number of infiltrating monocytes and reduced pulmonary fibrosis compared to the wild type mice. Gillespie et al. (2010) found that both short term and long-term exposures to nickel hydroxide nanoparticles induced CCL2 in male mice and Morimoto et al. (2010) also reported that intratracheal instillation of nickel oxide nanoparticles induced CCL2/MCP-1 production in the male rats. Our findings show that NiNP-treated male mice had more CCL2 protein in BALF and whole lung lysates compared to females (Fig. 9). This suggests that the increase in lung monocytic inflammation in male mice after subchronic exposure to NiNPs in the present study could be mediated by CCL2/CCR2 signaling. However, a caveat is that we observed the greatest increase in CCL2 induced by NiNPs alone, whereas the greatest increase in monocytes occurred in the NiNP and LPS co-exposure group (Fig. 6). It is possible that CCL2 could have been increased earlier to signal monocyte influx and subsequently declined or other chemokines/mediators were contributory to monocyte influx. Also, lung mRNAs encoding CXCL1, IL-6, and CCL2 showed entirely different expression patterns compared to protein levels of these cytokines in BALF, with females having significant induction of all three cytokines after repeated, subchronic NiNP exposure (Supplementary File 2). These data reveal that mRNAs are not necessarily predictive of cytokine protein levels after repeated nanoparticle exposure.
Another interesting finding using the subchronic exposure protocol in the present study was that female mice showed higher total STAT1 protein in lung tissue, compared to the males, after repeated exposure to NiNPs. STAT1 mediates growth arrest and antagonizes the pro-inflammatory and proliferative actions of STAT3 (Ramana et al., 2001; Bonner, 2010b). Our laboratory previously reported that homozygous Stat1 knockout mice exposed multi-walled carbon nanotubes displayed increased mucous cell metaplasia and airway fibrosis compared to wild type mice, demonstrating that STAT1 plays a protective role in development of pulmonary fibrosis by inhibiting TGF-β1 production and collagen synthesis (Thompson et al., 2015; Duke et al., 2017). Although we did not detect phosphorylated STAT1 after the repeated subchronic exposure to NiNPs in the present study, we observed that female mice had a significantly higher total STAT1 levels when exposed to NiNPs compared to male mice. This observation suggests that the susceptibility of male mice to subchronic NiNP exposure may involve reduced levels of STAT1. STAT1 is phosphorylated on serine or tyrosine residues upon activation of cells with interferons (IFN-α,-β,-γ), growth factors (e.g., PDGF, EGF), or oxidative stress (Simon et al., 2002; Aaronson and Horvath, 2002; Citores et al., 2007; Meissl et al., 2017; Zibara et al., 2017). However, the activation of STAT1 or STAT3 leads to increased expression of unphosphorylated (U)-STAT1 or U-STAT3, respectively, that have important roles in constitutive transcription (Cheon et al., 2011). Therefore, increased total STAT1 (i.e., U-STAT1) in female mice treated with NiNPs (Fig. 10) represents a plausible mechanism for reduced lung inflammation that was observed in the subchronic exposure experiment. From a clinical perspective, men are more susceptible to diseases such as idiopathic pulmonary fibrosis and systemic sclerosis, and patients with both diseases have reduced levels of total STAT1 (Lindahl et al., 2013).
In contrast to the high numbers of neutrophils in the BALF of mice after an acute 24 hr exposure, very few neutrophils or IL-6 were observed in the BALF from male or female mice in the subchronic experiment suggesting that IL-6/STAT3 does not play a critical role in subchronic inflammation. However, as mentioned earlier in the introduction, work from others has shown that IL-6 is a key player for transitioning from acute neutrophilic inflammation to chronic monocytic inflammation. In acute inflammation, human endothelial cells produce IL-6 and IL-8 to recruit acute phase proteins and neutrophils to the inflammatory sites, respectively (Kaplanski et al., 2003; Gabay, 2006). As inflammation prolongs, recruited neutrophils induce IL-6Rα to shed soluble IL-6Rα (sIL-6Rα). sIL-6Rα then binds to IL-6 and gp-130 on endothelial cells to selectively produce CCL2, but not IL-8, for monocyte recruitment (Kaplanski et al., 2003; Gabay, 2006). The resulting increase in the production of CCL2 now favors monocytic inflammation over neutrophilic inflammation (Kaplanski et al., 2003; Gabay, 2006). Our findings follow this transition concept from acute to chronic inflammation, except that CXCL1 was still high in our subchronic experiment. However, since no increase in neutrophils was detected after repeated exposures to NiNPs, CXCL1 could play an alternative role, other than recruiting neutrophils, in subchronic inflammation. CXCL1-CXCR2 signaling has been shown to be critical for monocyte infiltration in the cardiac remodeling process in mice (Wang et al., 2018). Thus, IL-6 could play a role in the transitioning from acute to subchronic inflammation by promoting CCL2 production, which could act in coordination with CXCL1 to promote monocytic inflammation. Further study to elucidate the roles of IL-6 or CXCL1 in NiNP-induced lung inflammation should utilize transgenic mice that are deficient in these cytokines or their receptors.
We also observed crystal formations in the BALF of mice exposed to NiNPs in the subchronic exposure experiment. These crystals have been previously described as eosinophilic crystals in mice and resemble Charcot-Leyden crystals found in humans (Guo, Johnson and Schuh, 2000). The colorless crystals were termed ‘eosinophilic crystals’, because the positive charge of the crystalline surface attracts eosin from the hematoxylin and eosin staining protocol (Guo, Johnson and Schuh, 2000). It has been reported that eosinophilic crystals are formed in the BALF of mice with severe pulmonary injury (Guo, Johnson and Schuh, 2000). We have previously observed similar crystals in the lungs of p53 heterozygous mice after chronic exposure multi-walled carbon nanotubes (Duke et al., 2018). However, a much more frequent number of crystals were found in the present study and only observed in male or female mice exposed to NiNPs. Another study reported that eosinophilic crystals in the lungs of aged 129S4/SvJae mice were a cause of mortality (Hoenerhoff et al., 2006). Therefore, it is possible that repeated chronic exposures to NiNPs in humans could cause lung injury through crystal formation.
We have found that males are more susceptible to a single acute exposure to NiNPs or repeated subchronic exposure to NiNPs in the absence or presence of LPS. Susceptibility to acute NiNP exposure in male mice corresponded to enhanced IL-6/STAT-3 signaling, while susceptibility of male mice to subchronic NiNP exposure was associated with increased CCL2 and reduced levels of STAT-1. 17β-estradiol could play a role in regulating IL-6/STAT3 in acute inflammation or STAT-1 in subchronic inflammation induced by NiNPs with or without LPS. Future experiments should be undertaken using ovariectomized mice to confirm that this sex difference is regulated by a hormonal difference. Also, since hormone levels change throughout lifespan, age should be evaluated as a factor in the susceptibility to nanoparticles. In addition to biological factors, sex-dependent susceptibility could be dependent on the specific type of particle. For example, Ray and Holian et al. (2019) found that female mice were more susceptible than male mice to both acute and chronic MWCNT-induced inflammation, but male mice had worse alveolitis to chronic lung injury induced by repeated exposure to crystalline silica. Therefore, susceptibility to lung injury appears, at least in part, to depend on specific type of particle stimulus inciting the inflammatory response.
Our findings have important implications for susceptibility in occupational or environmental exposures to NiNPs in humans. NiNPs are widely used metal nanoparticles with many industrial applications, and have gained more attention in diverse fields including chemistry, physics, material science, and biology (Chen and Wu, 2000; Guo et al., 2009; Heuer-Jungemann et al., 2019). Therefore, investigating sex differences in acute and subchronic lung inflammatory responses of mice to NiNPs is an important advance towards assessing the toxicity and susceptibility in human populations. Chronic lung inflammation caused by repeated exposure to metals, including nickel, can cause pulmonary fibrosis (Nemery, 1990). Berge et al. (2003) found that soluble and sulfidic nickel are risk factors for pulmonary fibrosis in nickel refinery workers exposed occupationally. Siegesmund et al. (1974) reported that IPF patients had different types of metals in their lungs including nickel, cobalt, iron and chromium. Moreover, rodent studies show that nickel oxide nanoparticles can activate TGF-β1 to induce pulmonary fibrosis (Taskar and Coultas, 2006). With increasing evidence of nickel in promoting pulmonary fibrosis in humans, there is an urgent need to fully understand the risks associated with NiNPs that are increasingly used in industrial applications, and how susceptibility factors, including sex, play a role. Furthermore, with a higher incidence of men developing pulmonary fibrosis, the mechanisms of susceptibility to NiNPs in causing respiratory disease should be more carefully evaluated and compared with soluble nickel to determine the specificity of NiNP in causing greater lung inflammatory responses in male mice. In conclusion, we have found that the male mice are more susceptible than female mice to both acute neutrophilic lung inflammation and subchronic monocytic lung inflammation in response to exposure from NiNPs, in the absence or presence of LPS. Moreover, as illustrated in Fig. 11, susceptibility of male mice to acute NiNP-induced lung inflammation was correlated with elevated IL-6 and CXCL1 along with increased activation of STAT3, while susceptibility to chronic lung inflammation was correlated with increased CXCL1, CCL2 and suppressed STAT1. Our findings emphasize the importance of sex in risk assessment, therapeutic intervention, and treatment of nanoparticle-induced pulmonary inflammation.
Supplementary Material
Funding
This work was supported by NIEHS R01ES020897 (DJY, HYL, AJT, JCB), NIEHS Training Grant: T32 ES007046 (DJY, HYL), NIEHS P30 ES025128 (KEL, JCB).
LIST OF ABBREVIATIONS
- BALF
bronchoalveolar lavage fluid
- CCL2
chemokine (C-C motif) ligand 2
- CXCL1
C-X-C motif ligand chemokine 1
- DPBS
Dulbecco’s phosphate-buffered saline
- ELISA
enzyme linked immunosorbent assay
- IHC
immunohistochemistry
- IL
interleukin
- LPS
lipopolysaccharide
- MCP-1
monocyte chemoattractant protein-1 (CCL2)
- MWCNT
multi-walled carbon nanotube
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NiNPs
nickel nanoparticles
- OPA
oropharyngeal aspiration
- ROS
reactive oxygen species
- STAT
signal transducer and activator of transcription
- TGF-β1
transforming growth factor β1
- TEM
transmission electron microscope
Footnotes
Ethics approval and consent to participate:
Ethics approval for animal use was given by the North Carolina State University IACUC.
Consent for publication
Not applicable
Availability of data and material
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Competing interests
The authors declare that they have no competing interests.
REFERENCES
- Aaronson DS and Horvath CM (2002) A road map for those who don’t know JAK-STAT. Science. 296, pp.1653–5. doi: 10.1126/science.1071545. [DOI] [PubMed] [Google Scholar]
- Ahuja N, Andres-Hernando A, Altmann C, Bhargava R, Bacalja J, Webb RG, He Z, Edelstein CL and Faubel S (2012) Circulating IL-6 mediates lung injury via CXCL1 production after acute kidney injury in mice. American Journal of Physiology - Renal Physiology. 303(6), pp. 864–72. doi: 10.1152/ajprenal.00025.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baik CS and Eaton KD (2012) Estrogen signaling in lung cancer: An opportunity for novel therapy. Cancers. 18(8), pp.1713. doi: 10.3390/cancers4040969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berge SR and Skyberg K (2003) Radiographic evidence of pulmonary fibrosis and possible etiologic factors at a nickel refinery in Norway. Journal of Environmental Monitoring. 5, pp. 681–8. doi: 10.1039/b209623b. [DOI] [PubMed] [Google Scholar]
- Bonner JC, Rice AB, Lindroos PM, O’Brien PO, Dreher KL, Rosas I, Alfaro-Moreno E and Osornio-Vargas AR (1998) Induction of the lung myofibroblast PDGF receptor system by urban ambient particles from Mexico City. American Journal of Respiratory Cell and Molecular Biology. 19(4), pp.672–80. doi: 10.1165/ajrcmb.19.4.3176. [DOI] [PubMed] [Google Scholar]
- Bonner JC (2010a) Nanoparticles as a Potential Cause of Pleural and Interstitial Lung Disease. Proceedings of the American Thoracic Society. 7(2), pp.138–41. doi: 10.1513/pats.200907-061rm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner JC (2010b) Mesenchymal cell survival in airway and interstitial pulmonary fibrosis. Fibrogenesis & Tissue Repair. 3(15). doi: 10.1186/1755-1536-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Fang Y, Lu Y, Qian F, Ma Q, He M, Pi H, Yu Z and Zhou Z (2016) Exposure to nickel oxide nanoparticles induces pulmonary inflammation through NLRP3 inflammasome activation in rats. International Journal of Nanomedicine. 11, pp.3331–46. doi: 10.2147/IJN.S106912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimir GJ, Lefèvre N, Corazza F and Duchateau J (2013) Sex and inflammation in respiratory diseases: a clinical viewpoint. Biology of Sex Differences. 4(16). doi: 10.1186/2042-6410-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesta MF, Ryman-Rasmussen JP, Wallace DG, Masinde T, Hurlburt G, Taylor AJ, Bonner JC (2010) Bacterial lipopolysaccharide enhances PDGF signaling and pulmonary fibrosis in rats exposed to carbon nanotubes. American Journal of Respiratory Cell and Molecular Biology. 43(2), pp.142–151. doi: 10.1165/rcmb.2009-0113OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citores L, Bai L, Sørensen V and Olsnes S (2007) Fibroblast growth factor receptor-induced phosphorylation of STAT1 at the Golgi apparatus without translocation to the nucleus. Journal of Cellular Physiology. 212(1), pp.148–56. doi: 10.1002/jcp.21014. [DOI] [PubMed] [Google Scholar]
- Chen DH and Wu SH (2000) Synthesis of nickel nanoparticles in water-in-oil microemulsions. Chemistry of Materials. 12(5), pp.1354–60. doi: 10.1021/cm991167y. [DOI] [Google Scholar]
- Cheon H, Yang J, Stark GR (2011) The functions of signal transducers and activators of transcriptions 1 and 3 as cytokine-inducible proteins. Journal of Interferon and Cytokine Research. 31(1), pp. 33–40. doi: 10.1089/jir.2010.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke KS, Taylor-Just AJ, Ihrie MD, Shipkowski KA, Thompson EA, Dandley EC, Parsons GN and Bonner JC (2017) STAT1-dependent and -independent pulmonary allergic and fibrogenic responses in mice after exposure to tangled versus rod-like multi-walled carbon nanotubes. Particle and Fibre Toxicology. 14(1), pp. 1–15. doi: 10.1186/s12989-017-0207-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke KS, Thompson EA, Ihrie MD, Taylor-Just AJ, Ash EA, Shipkowski KA, Hall JR, Tokarz DA, Cesta MF, Hubbs AF, Porter DW, Sargent LM and Bonner JC (2018) Role of p53 in the chronic pulmonary immune response to tangled or rod-like multi-walled carbon nanotubes. Nanotoxicology. 12(9), pp. 975–91. doi: 10.1080/17435390.2018.1502830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duquenne P and Marchand G (2013) Measurement of Endotoxins in Bioaerosols at Workplace: A Critical Review of Literature and a Standardization Issue. Annals of Work Exposure and Health. 57(2), pp.137–72. doi: 10.1093/annhyg/mes051. [DOI] [PubMed] [Google Scholar]
- Egleston BL, Meireles SI, Flieder DB and Clapper ML (2009) Population-Based Trends in Lung Cancer Incidence in Women. Seminars in Oncology, 36(6), pp. 506–515. doi: 10.1053/j.seminoncol.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falagas ME, Mourtzoukou EG and Vardakas KZ (2007) Sex differences in the incidence and severity of respiratory tract infections. Respiratory Medicine, 101(9), pp. 1845–1863. doi: 10.1016/j.rmed.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Fan X, Patera AC, Pong-Kennedy A, Deno G, Gonsiorek W, Manfra DJ, Vassileva G, Zeng M, Jackson C, Sullivan L, Sharif-Rodriguez W, Opdenakker G, Van Damme J, Hedrick JA, Lundell D, Lira SA and Hipkin RW (2007) Murine CXCR1 is a functional receptor for GCP-2/CXCL6 and interleukin-8/CXCL8. Journal of Biological Chemistry. 282(16), pp.11658–66. doi: 10.1074/jbc.M607705200. [DOI] [PubMed] [Google Scholar]
- Fielding CA, McLoughlin RM, McLeod L, Colmont CS, Najdovska M, Grail D, Ernst M, Jones SA, Topley N and Jenkins BJ (2008) IL-6 Regulates Neutrophil Trafficking during Acute Inflammation via STAT3. The Journal of Immunology. 181(3), pp.2189–95. doi: 10.4049/jimmunol.181.3.2189. [DOI] [PubMed] [Google Scholar]
- Fu PP, Xia Q, Hwang HM, Ray PC and Yu H (2014) Mechanisms of nanotoxicity: Generation of reactive oxygen species. Journal of Food and Drug Analysis. 22(1), pp.64–75. doi: 10.1016/j.jfda.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay C (2006) Interleukin-6 and chronic inflammation. Arthritis Research and Therapy. 8(Suppl 2), pp.53. doi: 10.1186/ar1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galien R and Garcia T (1997) Estrogen receptor impairs interleukin-6 expression by preventing protein binding on the NF-κB site. Nucleic Acids Research. 25(12), pp.2424–29. doi: 10.1093/nar/25.12.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Guo R-F, Speyer CL, Reuben J, Neff TA, Hoesel LM, Riedemann NC, McClintock SD, Sarma JV, Van Rooijen N, Zetoune FS and Ward PA (2004) Stat3 Activation in Acute Lung Injury. The Journal of Immunology. 172(12), pp.7703–12. doi: 10.4049/jimmunol.172.12.7703. [DOI] [PubMed] [Google Scholar]
- Gasperino J (2011) Gender is a risk factor for lung cancer. Medical Hypotheses. 76(3), pp.328–31. doi: 10.1016/j.mehy.2010.10.030. [DOI] [PubMed] [Google Scholar]
- Gillespie PA, Kang GS, Elder A, Gelein R, Chen L, Moreira AL, Koberstein J, Tchou-Wong KM, Gordon T and Chen LC (2010) Pulmonary response after exposure to inhaled nickel hydroxide nanoparticles: Short and long-term studies in mice. Nanotoxicology. 4(1), pp.106–19. doi: 10.3109/17435390903470101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson M, Bishop N, Oliveira M and Mccauley T (2011) Sex differences in immune variables and respiratory infection incidence in an athletic population. Exercise Immunology Review, 44(0), pp. 122–135. [PubMed] [Google Scholar]
- Glista-Baker EE, Taylor AJ, Sayers BC, Thompson EA and Bonner JC (2012) Nickel nanoparticles enhance platelet-derived growth factor-induced chemokine expression by mesothelial cells via prolonged mitogen-activated protein kinase activation. American Journal of Respiratory Cell and Molecular Biology, 47(4), pp. 552–561. doi: 10.1165/rcmb.2012-0023OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glista-Baker EE, Taylor AJ, Sayers BC, Thompson EA and Bonner JC (2014) Nickel Nanoparticles cause exaggerated lung and airway remodeling in mice lacking the T-box transcription factor, TBX21 (T-bet). Particle and Fibre Toxicology, 11(1). doi: 10.1186/1743-8977-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimsrud TK, Berge SR, Haldorsen T and Andersen A (2002) Exposure to different forms of nickel and risk of lung cancer. American Journal of Epidemiology. 156(12), pp.1123–32. doi: 10.1093/aje/kwf165. [DOI] [PubMed] [Google Scholar]
- Groves AM, Johnston CJ, Williams JP and Finkelstein JN (2018) Role of Infiltrating Monocytes in the Development of Radiation-Induced Pulmonary Fibrosis. Radiation Research. 189(3), pp.300–11. doi: 10.1667/RR14874.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Johnson RS and Schuh JACL (2000) Biochemical characterization of endogenously formed eosinophilic crystals in the lungs of mice. Journal of Biological Chemistry. 275, pp.8032–37. doi: 10.1074/jbc.275.11.8032. [DOI] [PubMed] [Google Scholar]
- Guo D, Wu C, Li J, Guo A, Li Q, Jiang H, Chen B and Wang X (2009) Synergistic effect of functionalized nickel nanoparticles and quercetin on inhibition of the SMMC-7721 cells proliferation. Nanoscale Research Letters. 4(12), pp.1395–402. doi: 10.1007/s11671-009-9411-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Zhong W, Au CT and Du Y (2013) Size dependence of the magnetic properties of Ni nanoparticles prepared by thermal decomposition method. Nanoscale Research Letters. 446(8). doi: 10.1186/1556-276X-8-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer-Jungemann A, Feliu N, Bakaimi I, Hamaly M, Alkilany A, Chakraborty I, Masood A, Casula MF, Kostopoulou A, Oh E, Susumu K, Stewart MH, Medintz IL, Stratakis E, Parak WJ and Kanaras AG (2019) The role of ligands in the chemical synthesis and applications of inorganic nanoparticles. Chemical Reviews, 119(8), pp. 4819–4880. doi: 10.1021/acs.chemrev.8b00733. [DOI] [PubMed] [Google Scholar]
- Hodge DR, Hurt EM and Farrar WL (2005) The role of IL-6 and STAT3 in inflammation and cancer. European Journal of Cancer. 4(16), pp.2502–12. doi: 10.1016/j.ejca.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Hoenerhoff MJ, Starost MF and Ward JM (2006) Eosinophilic crystalline pneumonia as a major cause of death in 129S4/SvJae mice. Veterinary Pathology. 43(5), pp.682–88. doi: 10.1354/vp.43-5-682. [DOI] [PubMed] [Google Scholar]
- Ishizaki T, Yatsugi K and Akedo K (2016) Effect of Particle Size on the Magnetic Properties of Ni Nanoparticles Synthesized with Trioctylphosphine as the Capping Agent. Nanomaterials. 6(9). doi: 10.3390/nano6090172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen-Fangel S, Mohey R, Johnsen SP, Andersen PL, Toft Sørensen H and Østergaard L (2004) Gender differences in hospitalization rates for respiratory tract infections in Danish youth. Scandinavian Journal of Infectious Diseases. 36(1), pp.31–6. doi: 10.1080/00365540310017618. [DOI] [PubMed] [Google Scholar]
- Jones MR, Quinton LJ, Simms BT, Lupa MM, Kogan MS and Mizgerd JP (2006) Roles of Interleukin‐6 in Activation of STAT Proteins and Recruitment of Neutrophils during Escherichia coli Pneumonia. The Journal of Infectious Diseases. 193(3), pp.360–9. doi: 10.1086/499312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadioglu A, Cuppone AM, Trappetti C, List T, Spreafico A, Pozzi G, Andrew PW and Oggioni MR (2011) Sex-Based Differences in Susceptibility to Respiratory and Systemic Pneumococcal Disease in Mice. The Journal of Infectious Diseases, 204(12), pp. 1971–1979. doi: 10.1093/infdis/jir657. [DOI] [PubMed] [Google Scholar]
- Kaplanski G, Marin V, Montero-Julian F, Mantovani A and Farnarier C (2003) IL-6: A regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends in Immunology. 24(1), pp.25–9. doi: 10.1016/S1471-4906(02)00013-3. [DOI] [PubMed] [Google Scholar]
- Kassem M, Harris SA, Spelsberg TC and Riggs BL (2010) Estrogen inhibits interleukin-6 production and gene expression in a human osteoblastic cell line with high levels of estrogen receptors. Journal of Bone and Mineral Research. 11(2), pp.193–9. doi: 10.1002/jbmr.5650110208. [DOI] [PubMed] [Google Scholar]
- Klein SL and Flanagan KL (2016) Sex differences in immune responses. Nat Rev Immunol, 16(10), pp. 626–38. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- Kurihara T and Bravo R (1996) Cloning and functional expression of mCCR2, a murine receptor for the C-C chemokines JE and FIC. Journal of Biological Chemistry. 271, pp.11603–6. doi: 10.1074/jbc.271.20.11603. [DOI] [PubMed] [Google Scholar]
- Latvala S, Hedberg J, Di Bucchianico S, Möller L, Wallinder IO, Elihn K and Karlsson HL (2016) Nickel release, ROS generation and toxicity of Ni and NiO micro- and nanoparticle. PLoS ONE. 11(7). doi: 10.1371/journal.pone.0159684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl GE, Stock CJW, Shi-Wen X, Leoni P, Sestini P, Howat SL, Bou-Gharios G, Nicholson AG, Denton CP, Grutters JC, Maher TM, Wells AU, Abraham DJ and Renzoni EA (2013) Microarray profiling reveals suppressed interferon stimulated gene program in fibroblasts from scleroderma-associated interstitial lung disease. Respiratory Research. 14(80). doi: 10.1186/1465-9921-14-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S, Zhang W, Zhang R, Liu P, Wang, Qiangxiang, Shang Y, Wu M, Donaldson K and Wang, Qingyue (2015) Comparison of cellular toxicity caused by ambient ultrafine particles and engineered metal oxide nanoparticles. Particle and Fibre Toxicology. 12(5). doi: 10.1186/s12989-015-0082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai YJ, Tu JP, Gu CD and Wang XL (2012) Graphene anchored with nickel nanoparticles as a high-performance anode material for lithium ion batteries. Journal of Power Sources. 209, pp.1–6. doi: 10.1016/j.jpowsour.2012.02.073. [DOI] [Google Scholar]
- Meissl K, Macho-Maschler S, Müller M and Strobl B (2017) ‘The good and the bad faces of STAT1 in solid tumours’, Cytokine. 89, pp.12–20. doi: 10.1016/j.cyto.2015.11.011. [DOI] [PubMed] [Google Scholar]
- Mo Y, Jiang M, Zhang Y, Wan R, Li J, Zhong CJ, Li H, Tang S and Zhang Q (2019) Comparative mouse lung injury by nickel nanoparticles with differential surface modification. Journal of Nanobiotechnology. 17(2). doi: 10.1186/s12951-018-0436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MJ and Liu ZG (2011) Crosstalk of reactive oxygen species and NF-κB signaling. Cell Research. 21(1), pp.103–15. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto Y, Ogami A, Todoroki M, Yamamoto M, Murakami M, Hirohashi M, Oyabu T, Myojo T, Nishi KI, Kadoya C, Yamasaki S, Nagatomo H, Fujita K, Endoh S, Uchida K, Yamamoto K, Kobayashi N, Nakanishi J and Tanaka I (2010) Expression of inflammation-related cytokines following intratracheal instillation of nickel oxide nanoparticles. Nanotoxicology. 4(2), pp.161–76. doi: 10.1667/rr14874.1. [DOI] [PubMed] [Google Scholar]
- Naugler WE, Sakurai T, Kim S, Maeda S, Kim KH, Elsharkawy AM and Karin M (2007) Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science, 317(5834), pp. 121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- Nemery B (1990) Metal toxicity and the respiratory tract. European Respiratory Journal. 3(2), pp.202–19. doi: 10.1097/00043764-199012000-00003. [DOI] [PubMed] [Google Scholar]
- Neubauer N, Palomaeki J, Karisola P, Alenius H and Kasper G (2015) Size-dependent ROS production by palladium and nickel nanoparticles in cellular and acellular environments - An indication for the catalytic nature of their interactions. Nanotoxicology. 9(8), pp.1059–66. doi: 10.3109/17435390.2015.1019585. [DOI] [PubMed] [Google Scholar]
- O’Connor T, Borsig L and Heikenwalder M (2015) CCL2-CCR2 Signaling in Disease Pathogenesis. Endocrine, Metabolic & Immune Disorders-Drug Targets. 15(2), pp.105–18. doi: 10.2174/1871530315666150316120920. [DOI] [PubMed] [Google Scholar]
- O’Donoghue RJJ, Knight DA, Richards CD, Prêle CM, Lau HL, Jarnicki AG, Jones J, Bozinovski S, Vlahos R, Thiem S, McKenzie BS, Wang B, Stumbles P, Laurent GJ, McAnulty RJ, Rose-John S, Zhu HJ, Anderson GP, Ernst MR and Mutsaers SE (2012) Genetic partitioning of interleukin-6 signalling in mice dissociates Stat3 from Smad3-mediated lung fibrosis. EMBO Molecular Medicine. 4(9), pp.939–51. doi: 10.1002/emmm.201100604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuma T, Terasaki Y, Kaikita K, Kobayashi H, Kuziel WA, Kawasuji M and Takeya M (2004) C-C chemokine receptor 2 (CCR2) deficiency improves bleomycin-induced pulmonary fibrosis by attenuation of both macrophage infiltration and production of macrophage-derived matrix metalloproteinases. Journal of Pathology. 204(5), pp.594–604. doi: 10.1002/path.1667. [DOI] [PubMed] [Google Scholar]
- Osman NM, Sexton DW and Saleem IY (2019) Toxicological assessment of nanoparticle interactions with the pulmonary system. Nanotoxicology. 14(1), pp.21–58. doi: 10.1080/17435390.2019.1661043. [DOI] [PubMed] [Google Scholar]
- Paimela T, Ryhänen T, Mannermaa E, Ojala J, Kalesnykas G, Salminen A and Kaarniranta K (2007) The effect of 17β-estradiol on IL-6 secretion and NF-κB DNA-binding activity in human retinal pigment epithelial cells. Immunology Letters. 110(2), pp.139–44. doi: 10.1016/j.imlet.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Pinkerton KE, Harbaugh M, Han MLK, Le Saux CJ, Van Winkle LS, Martin WJ, Kosgei RJ, Carter EJ, Sitkin N, Smiley-Jewell SM and George M (2015) Women and lung disease: Sex differences and global health disparities. American Journal of Respiratory and Critical Care Medicine. 192(1), pp.11–6. doi: 10.1164/rccm.201409-1740PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma DS (2007) Gender differences in asthma development and progression. Gender medicine. 4(Suppl B), pp.S133–46. doi: 10.1016/s1550-8579(07)80054-4. [DOI] [PubMed] [Google Scholar]
- Pumera M (2007) Carbon nanotubes contain residual metal catalyst nanoparticles even after washing with nitric acid at elevated temperature because these metal nanoparticles are sheathed by several graphene sheets. Langmuir. 23(11), pp.6453–58. doi: 10.1021/la070088v. [DOI] [PubMed] [Google Scholar]
- Ramana CV, Gil MP, Han Y, Ransohoff RM, Schreiber RD and Stark GR (2001) Stat1-independent regulation of gene expression in response to IFN-γ. Proceedings of the National Academy of Sciences of the United States of America. 98, pp.6674–9. doi: 10.1073/pnas.111164198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray JL and Holian A (2019) Sex differences in the inflammatory immune response to multi-walled carbon nanotubes and crystalline silica. Inhalation Toxicology, 31(7), pp. 285–97. doi: 10.1080/08958378.2019.1669743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathish V and Prakash YS (2016) Sex Differences in Pulmonary Anatomy and Physiology: Implications for Health and Disease. Sex Differences In Physiology. pp.89–103. doi: 10.1016/B978-0-12-802388-4.00006-9. [DOI] [Google Scholar]
- Scott MKD, Quinn K, Li Q, Carroll R, Warsinske H, Vallania F, Chen S, Carns MA, Aren K, Sun J, Koloms K, Lee J, Baral J, Kropski J, Zhao H, Herzog E, Martinez FJ, Moore BB, Hinchcliff M, Denny J, Kaminski N, Herazo-Maya JD, Shah NH and Khatri P (2019) Increased monocyte count as a cellular biomarker for poor outcomes in fibrotic diseases: a retrospective, multicentre cohort study. The Lancet Respiratory Medicine. 7(6), pp. 497–508. doi: 10.1016/S2213-2600(18)30508-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Hickman J, Gazit N, Rabkin E and Mishin Y (2018) Nickel nanoparticles set a new record of strength. Nature Communications. 9(1). doi: 10.1038/s41467-018-06575-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipkowski KA, Taylor AJ, Thompson EA, Glista-Baker EE, Sayers BC, Messenger ZJ, Bauer RN, Jaspers I, Bonner JC (2015) An allergic lung microenvironment suppresses carbon nanotube-induced inflammasome activation via STAT6-dependent inhibition of caspase-1. PLoS One. 10(6): e0128888. doi: 10.1371/journal.pone.0128888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegesmund KA, Funahashi A, Pintar K, Funahashi A and Pintar K (1974) Identification of Metals in Lung From a Patient With Interstitial Pneumonia. Archives of Environmental Health. 28(6), pp. 345–9. doi: 10.1080/00039896.1974.10666506. [DOI] [PubMed] [Google Scholar]
- Silva EM, Mariano VS, Aguiar Pastrez PR, Pinto MC, Castro AG, Syrjanen KJ and Longatto-Filho A (2017) High systemic IL-6 is associated with worse prognosis in patients with non-small cell lung cancer. PLoS ONE. 12(7). doi: 10.1371/journal.pone.0181125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon AR, Takahashi S, Severgnini M, Fanburg BL and Cochran BH (2002) Role of the JAK-STAT pathway in PDGF-stimulated proliferation of human airway smooth muscle cells. American Journal of Physiology - Lung Cellular and Molecular Physiology. 282:L1296–304. doi: 10.1152/ajplung.00315.2001. [DOI] [PubMed] [Google Scholar]
- Sperry JL, Friese RS, Frankel HL, West MA, Cuschieri J, Moore EE, Harbrecht BG, Peitzman AB, Billiar TR, Maier RV, Remick DG and Minei JP (2008) Male gender is associated with excessive IL-6 expression following severe injury. Journal of Trauma - Injury, Infection and Critical Care. 64(3), pp.572–8. doi: 10.1097/TA.0b013e3181650fdf. [DOI] [PubMed] [Google Scholar]
- Su F, Qiu X, Liang F, Tanaka M, Qu T, Yao Y, Ma W, Yang B, Dai Y, Hayashi K and Watanabe T (2018) Preparation of nickel nanoparticles by direct current arc discharge method and their catalytic application in hybrid Na-air battery. Nanomaterials. 8(9), pp.684. doi: 10.3390/nano8090684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Narazaki M and Kishimoto T (2014) IL-6 in inflammation, Immunity, And disease. Cold Spring Harbor Perspectives in Biology. 6(10). doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taskar VS and Coultas DB (2006) Is idiopathic pulmonary fibrosis an environmental disease?. Proceedings of the American Thoracic Society. 3(4), pp.293–8. doi: 10.1513/pats.200512-131TK. [DOI] [PubMed] [Google Scholar]
- Thompson EA, Sayers BC, Glista-Baker EE, Shipkowski KA, Ihrie MD, Duke KS, Taylor AJ and Bonner JC (2015) Role of signal transducer and activator of transcription 1 in murine allergen-induced airway remodeling and exacerbation by carbon nanotubes. American Journal of Respiratory Cell and Molecular Biology. 53(5), pp.625–36. doi: 10.1165/rcmb.2014-0221OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thun MJ, Hannan LM, Adams-Campbell LL, Boffetta P, Buring JE, Feskanich D, Flanders WD, Sun HJ, Katanoda K, Kolonel LN, Lee IM, Marugame T, Palmer JR, Riboli E, Sobue T, Avila-Tang E, Wilkens LR and Samet JM (2008) Lung cancer occurrence in never-smokers: An analysis of 13 cohorts and 22 cancer registry studies. PLoS Medicine. doi: 10.1371/journal.pmed.0050185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unfried K, Albrecht C, Klotz LO, Von Mikecz A, Grether-Beck S and Schins RPF (2007) Cellular responses to nanoparticles: Target structures and mechanisms. Nanotoxicology. 1(1), pp.52–71. doi: 10.1080/00222930701314932 [DOI] [Google Scholar]
- Wan R, Mo Y, Chien S, Li Yihua, Li Yixin, Tollerud DJ and Zhang Q (2011) The role of hypoxia inducible factor-1α in the increased MMP-2 and MMP-9 production by human monocytes exposed to nickel nanoparticles. Nanotoxicology. 5(4), pp.568–82. doi: 10.3109/17435390.2010.537791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, van Boxel-Dezaire AHH, Cheon H, Yang J and Stark GR (2013) STAT3 activation in response to IL-6 is prolonged by the binding of IL-6 receptor to EGF receptor. Proceedings of the National Academy of Sciences. 110(42), pp.16975–80. doi: 10.1073/pnas.1315862110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhang YL, Lin QY, Liu Yu, Guan XM, Ma XL, Cao HJ, Liu Ying, Bai J, Xia YL, Du J and Li HH (2018) CXCL1-CXCR2 axis mediates angiotensin II-induced cardiac hypertrophy and remodeling through regulation of monocyte infiltration. European Heart Journal. 39(20), pp.1818–31. doi: 10.1093/eurheartj/ehy085. [DOI] [PubMed] [Google Scholar]
- Yang J, Agarwal M, Ling S, Teitz-Tennenbaum S, Zemans RL, Osterholzer JJ, Sisson TH and Kim KK (2020) Diverse Injury Pathways Induce Alveolar Epithelial Cell CCL2/12 Which Promotes Lung Fibrosis. American Journal of Respiratory Cell and Molecular Biology, (734), pp. 1–44. doi: 10.1165/rcmb.2019-0297oc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon S, Woo SU, Kang JH, Kim K, Kwon MH, Park S, Shin HJ, Gwak HS and Chwae YJ (2010) STAT3 transcriptional factor activated by reactive oxygen species induces IL6 in starvation-induced autophagy of cancer cells. Autophagy. 6(8), pp.1125–38. doi: 10.4161/auto.6.8.13547. [DOI] [PubMed] [Google Scholar]
- Yoshizaki K, Brito JM, Silva LF, Lino-dos-Santos-Franco A, Frias DP, e Silva RCR, Amato-Lourenço LF, Saldiva PHN, de Fátima Lopes Calvo Tibério I, Mauad T and Macchione M (2017) The effects of particulate matter on inflammation of respiratory system: Differences between male and female. Science of the Total Environment. 586, pp.284–95. doi: 10.1016/j.scitotenv.2017.01.221. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Topping T, Bingert JF, Thornton JJ, Dangelewicz AM, Li Y, Liu W, Zhu Y, Zhou Y and Lavernia EJ (2008) High tensile ductility and strength in bulk nanostructured nickel. Advanced Materials. 20, pp.3028–33. doi: 10.1002/adma.200800214. [DOI] [Google Scholar]
- Zhao Q, Kim T, Pang J, Sun W, Yang X, Wang J, Song Y, Zhang H, Sun H, Rangan V, Deshpande S, Tang H, Cvijic ME, Westhouse R, Olah T, Xie J, Struthers M and Salter-Cid L (2017) A novel function of CXCL10 in mediating monocyte production of proinflammatory cytokines. Journal of Leukocyte Biology. 102(5), pp.1271–80. doi: 10.1189/jlb.5a0717-302. [DOI] [PubMed] [Google Scholar]
- Zibara K, Zeidan A, Bjeije H, Kassem N, Badran B and El-Zein N (2017) ROS mediates interferon gamma induced phosphorylation of Src, through the Raf/ERK pathway, in MCF-7 human breast cancer cell line. Journal of Cell Communication and Signaling. 11(1), pp.57–67. doi: 10.1007/s12079-016-0362-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


