Abstract
14-3-3 proteins are a family of proteins expressed throughout the body and implicated in many diseases from cancer to neurodegenerative disorders. While these proteins do not have direct enzymatic activity, they form a hub for many signaling pathways via protein-protein interactions. 14-3-3 interactions have proven difficult to target with traditional pharmacological methods due to the unique nature of their binding. However, recent advances in compound development utilizing a range of tools from thermodynamic binding site analysis to computational molecular modeling techniques have opened the door to targeting these interactions. Compounds are already being developed targeting 14-3-3 interactions with potential therapeutic implication for neurodegenerative disorders, but challenges still remain in optimizing specificity and target engagement in order to avoid unintended negative consequences arising from targeting 14-3-3 signaling networks.
Keywords: 14-3-3, Parkinson’s disease, Alzheimer’s disease, protein-protein interactions
14-3-3 Protein Discovery, Structure, and Function
14-3-3 proteins are a family of homologous proteins ubiquitously expressed throughout the body with particularly high expression in the brain, representing 1% of total soluble brain protein [1, 2]. The name 14-3-3 derives from the 14th fraction on the DEAE cellulose column and the 3.3 fractions in the subsequent starch gel electrophoresis containing the protein in the analysis of calf brain homogenates [3, 4]. These proteins show high conservation across evolution, from plants to higher order mammals [5]. In mammals, the 14-3-3 family contains seven isoforms including 14-3-3 β, γ, ε, η, ζ, σ, and τ/θ [6, 7]. Two isoforms designated as α and δ represent the phosphorylated forms of 14-3-3 β and ζ, respectively [8].
Each isoform contains nine alpha helices and dimerizes with the same or other 14-3-3 isoforms into homodimers or heterodimers. Upon dimerization, 14-3-3s form ‘W’-shaped amphipathic pockets that serve as the main binding site for binding partners. In 14-3-3 dimers, the first and second alpha helices of one monomer interact with the third and fourth alpha helices of the second monomer in an antiparallel fashion to form the bottom of the ‘W’ [9]. 14-3-3 proteins lack enzymatic activity and mediate their functionality through protein-protein interactions (PPIs) with several hundred binding partners or clients [10, 11]. 14-3-3s preferentially bind phosphorylated proteins containing phosphorylated serines or threonines via three distinct motifs: motif I R[S/F/Y/W]Xp(S/T)XP, motif II RX[S/Y/F/W/T/Q/A/D]Xp(S/T)X[P/L/M], or motif III (p(S/T)X1–2–COOH) [12]. Doubly phosphorylated proteins have higher affinity to 14-3-3s than singly phosphorylated proteins as long as they have the flexibility to reach both amphipathic grooves within 14-3-3 dimers [12]. Not all interactions with 14-3-3s require these phosphomotifs within the binding partner, and some 14-3-3 interactions do not depend on phosphorylation at all [13].
14-3-3s act as molecular adaptors through PPIs. Upon client binding, 14-3-3s bring together two separate proteins to interact, or 14-3-3s promote conformational change in the binding partner. These conformational changes alter the enzymatic activity of the binding partner or expose or hide localization motifs that affect the subcellular localization of the binding partner. One of the first enzymes shown to be regulated by 14-3-3s is tyrosine and tryptophan hydroxylase, from which the genetic moniker “YWHA” (corresponding to Tyrosine, Tryptophan Hydroxylase Activators) is derived [6]. Through a broad array of PPIs, 14-3-3s serve as a central hub for multiple signaling pathways and participate in many cellular functions, including apoptosis, cell trafficking, regulation of cytoskeletal dynamics, and neuronal plasticity. Consequently, 14-3-3s are implicated in many diseases including cancer, metabolic disorders, autoimmune diseases, and neurological disorders [14]. This review focuses on 14-3-3s and their role in neurodegenerative diseases and their potential as a target for therapeutic intervention.
14-3-3s in Neurological Disease
While several 14-3-3 isoform knockout (KO) mouse models are embryonic lethal [15], researchers have created several 14-3-3 mouse models that illustrate their role in neurologic function, with several KO mice demonstrating abnormalities during brain development. 14-3-3ζ KO mice show aberrant neuronal migration within the hippocampus and impaired learning and hyperactivity [16, 17]. A conditional 14-3-3ε/14-3-3ζ double KO mouse also reveals abnormal neuronal migration with seizures [15]. 14-3-3γ KO mice have migration defects and structural abnormalities in cortical neurons [18]. While complete 14-3-3ε KO usually results in embryonic death, 14-3-3ε +/− mice have working memory deficits and anxiety [19]. A functional KO mouse model created by expressing the pan 14-3-3 competitive antagonist dimeric fourteen-three-three peptide inhibitor (difopein) under the Thy1.2 neuron-specific promoter expressed postnatally demonstrates electrophysiological abnormalities, with impaired synaptic transmission and NMDAR-dependent long-term potentiation, along with structural changes marked by shortened dendrites and reduced spine density [20, 21]. Additionally, these mice demonstrate learning and memory deficits, hyperactivity, and impaired social interaction [20, 21]. These mouse models have pointed to a key role for 14-3-3s in brain function and are useful tools in exploring the roles of 14-3-3s in disease.
Parkinson’s Disease and Dementia with Lewy Bodies
Evidence for a key role of 14-3-3 proteins in Parkinson’s Disease (PD) and Dementia with Lewy Bodies (DLB) is growing. Patients with PD suffer from a variety of motor, cognitive, and psychiatric symptoms [22, 23]. DLB patients develop similar PD motor symptoms, yet cognitive decline including visual hallucinations and psychosis occurs much earlier compared to PD [24]. Both illnesses are marked by pathological inclusions termed Lewy bodies and Lewy neurites, predominantly composed of the aggregation-prone protein alpha-synuclein (αsyn) [25]. 14-3-3s colocalize with αsyn within Lewy Bodies of patients with PD and DLB [26, 27]. A reduction of 14-3-3s in soluble lysates from the temporal cortices of patients with DLB and an increase in 14-3-3 phosphorylation in insoluble brain lysates from PD and DLB patients have been observed [28]. Transcriptomic work shows dysregulation in 14-3-3 β, γ, and ζ in PD patients compared to controls [29].
Loss of 14-3-3 function may promote neurodegeneration in PD. The 14-3-3 inhibitor difopein promotes neuronal loss in neurotoxin models, such as rotenone and MPTP, while 14-3-3 overexpression, especially of the 14-3-3θ isoform, reduces toxicity [30–32]. 14-3-3s act as chaperones to regulate αsyn folding. 14-3-3η reduces αsyn fibrillization in vitro [33]. We recently found 14-3-3θ overexpression decreases while 14-3-3 inhibition accelerates αsyn oligomerization, internalization, and seeding in paracrine and fibrillar αsyn models [34]. In vivo difopein mice show an acceleration of αsyn aggregation, behavioral deficits, and neuronal loss with αsyn fibril injections, while 14-3-3θ overexpression delays αsyn aggregation and reduces behavioral deficits and neuron loss [35].
14-3-3s are major interacting proteins for leucine-rich repeat kinase 2 (LRRK2) [36–38]. LRRK2 mutations cause the most common familial form of PD and increase LRRK2 kinase activity which likely drives LRRK2 neurotoxicity [39–42]. Key 14-3-3 binding residues on LRRK2 include phosphorylated S910, S935, and S1444 [36, 43]. Several PD-causing LRRK2 mutations disrupt 14-3-3 binding to LRRK2 [36–38]. Difopein promotes LRRK2 kinase activity, while 14-3-3θ overexpression reduces mutant LRRK2 kinase activity [44]. 14-3-3θ’s effect on mutant LRRK2 kinase activity translates to a reduction in toxicity: 14-3-3θ overexpression in BAC transgenic G2019S-LRRK2 and R1441G-LRRK2 mice reversed neurite shortening observed in mutant LRRK2 neurons, while 14-3-3 inhibition exacerbated neurite shortening [44]. p21-activated protein kinase 6 (PAK6)-mediated phosphorylation of 14-3-3γ at S59 disrupts 14-3-3γ’s binding to LRRK2 [45] – pointing to modulators of this phosphorylation site as a potential therapeutic target in familial LRRK2-related PD. Alterations in the 14-3-3/LRRK2 interaction have been observed in animal PD models and in human idiopathic PD brains [46]. These findings point to modulation of 14-3-3/LRRK2 PPI as an area for further drug-target investigation.
Alzheimer’s Disease
14-3-3s also interact with key proteins implicated in Alzheimer’s Disease (AD), the most common neurodegenerative disorder and the sixth-leading cause of death in the US [47]. Key pathological changes observed in AD include beta-amyloid (Aβ) plaques and neurofibrillary tangles (NFTs) comprised of the microtubule-associated protein tau aggregated into β sheets. NFTs disrupt neuronal functioning and likely cause neuronal death [48]. Mechanisms by which tau monomers aggregate are debated, but current evidence points to tau hyperphosphorylation as a main cause of aggregation, potentially due to reduced affinity of tau for microtubules [49].
Genetic variants in the 14-3-3 genes are associated with AD risk [50, 51]. 14-3-3s interact with Aβ and tau and are found in Aβ plaques and NFTs in human AD brains [52–55]. Samples from the temporal cortex of human AD patients show a 40% reduction in soluble 14-3-3s compared to age-matched controls and increased phosphorylation of 14-3-3θ at S232 [28]. These 14-3-3 alterations display an inverse relationship to cognitive function, and higher insoluble Aβ correlated with lower soluble 14-3-3 levels [28]. Interestingly, reduced 14-3-3θ levels in the hippocampus are correlated with reduced cognitive function in aged mice [56, 57], and difopein mice demonstrate impaired spine density and cognitive function [20, 21]. These findings suggest that low levels of 14-3-3s could promote spine loss and cognitive decline in aging and/or AD.
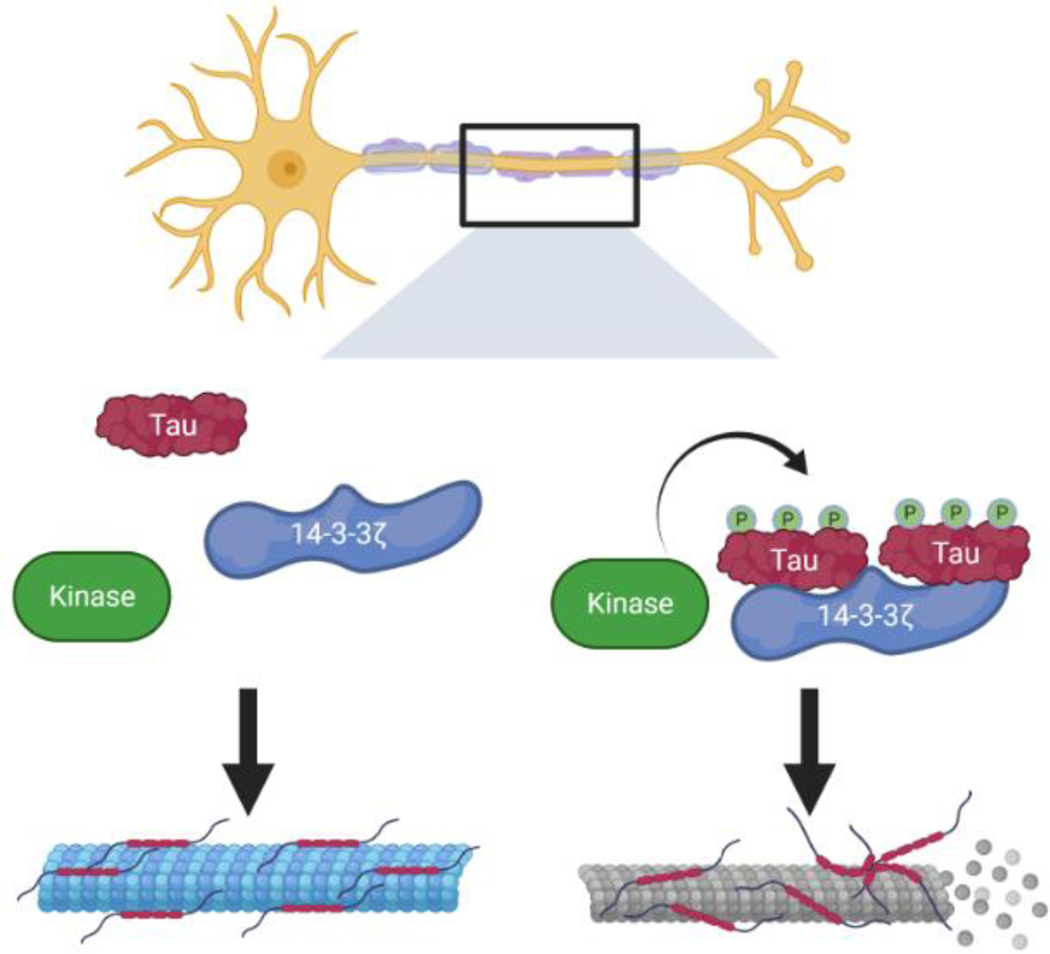
In contrast, 14-3-3s may promote toxicity mediated by tau aggregation (Figure 1). NFTs show increased levels of insoluble 14-3-3ζ, indicating this isoform may play a role in NFT formation [58]. In vitro assays demonstrate a dose-dependent increase in tau phosphorylation and aggregation with the addition of recombinant 14-3-3ζ [59–61]. 14-3-3ζ binding likely promotes tau as a substrate for kinases as opposed to 14-3-3ζ directly activating tau kinases, such as protein kinase A or neuronal Cdc2-like protein kinase [53]. Additionally, 14-3-3 overexpression disrupts tau’s binding to microtubules to decrease microtubule stability [62]. These results point to the interaction between tau and 14-3-3 as an excellent target for pharmacological research.
Figure 1. 14-3-3ζ may promote tau hyper-phosphorylation and aggregation seen in Alzheimer’s Disease.
Within the axons of cortical neurons, normal tau phosphorylation and activity leads to microtubule stabilization. However, 14-3-3ζ can bind tau and makes it a better substrate for various kinases. Excessive tau phosphorylation can then promote tau aggregation and consequent destabilization of microtubules.
14-3-3 Drug Discovery
14-3-3 dimer structure allows for many PPIs. The 14-3-3 dimer forms a ‘W’ shape, with each monomer imagined to be a ‘V’ that when combined form this ‘W’ [9]. The lateral lines on either side of each ‘V’ in the dimer ‘V V’ are the C-terminal ends of each monomer, and the medial two lines are the N-terminal ends. The amphipathic groove that serves as the main binding pockets for phosphoproteins is formed by positive residues Lys49, Arg56, Arg60, Arg127, and Tyr128 on the N-terminus (in human 14-3-3ζ numbering) and the hydrophobic residues of the C-terminus [63]. These amphipathic grooves within 14-3-3s allow for a variety of charged and uncharged conformations of binding partners. The grooves themselves are structurally rigid, but the proteins binding to 14-3-3 often contain disordered regions [64]. Key residues on binding partners interact with the amphipathic grooves of 14-3-3 dimers, and disordered regions change conformation as they approach. This bonding is marked by high specificity, low affinity, and thermodynamic favorability [65]. 14-3-3s can form multivalent interactions with their binding partners, which increases specificity and affinity compared to monovalent interactions [66, 67]. Consequently, 14-3-3s and other multivalent binders detect small changes in phosphorylation within signaling pathways and amplify that signal by binding activated molecules as well as downstream effectors [68].
These binding properties promote a wide variety of 14-3-3 PPIs and make it an excellent target for pharmacological intervention. However, this multivalent binding forms a double-edged sword. When we find a potential target, how can we target that specific protein interaction? How do we know which combination of binding sites to target? In the following section we will walk through theoretical steps of binding site identification for an imaginary 14-3-3 binding pair. This section is meant to highlight interesting and unique methodologies of 14-3-3 drug discovery and not to be an all-encompassing guide on all experiments necessary for this process.
Imagine a 14-3-3 binding protein (“protein Z”) whose disruption of its 14-3-3 interaction promotes the development of PD but that little else is known about targeting this protein. The first step is to identify where 14-3-3 binds to protein Z. One useful tool is 14-3-3-Pred, a webserver developed to determine potential 14-3-3 binding phosphosites [69]. This tool was developed via a compilation of human proteome data from UniProt, annotated phosphoproteome from PhosphoSitePlus [70], and 300 confirmed 14-3-3 binding sites from the Annotation and Integrated Analysis of the 14-3-3 interactome (ANIA) database [71]. 14-3-3-Pred uses several models to predict 14-3-3 binding. A position-specific scoring matrix examines motifs of 4–12 amino acids to determine a score based on amino acid frequency derived from 14-3-3 binding and 14-3-3 non-binding datasets as well as a background dataset derived from peptides with annotated serine/threonine phosphorylation sites. Each motif receives a score based on the sum of the scores of each amino acid in the sequence. A second model used by 14-3-3-Pred is a support vector machine model (SVM) from PyML, a python library of various machine learning tools to assess motif binding affinity of the protein of interest based on training data of known 14-3-3 binding/non-binding motifs. Finally, an artificial neural network utilizing solvent accessibility, protein secondary structure, and protein region disorder also scores these motifs. Using 14-3-3-Pred, the authors found two novel high-scoring 14-3-3-binding peptides, FAM122A and FAM122B, and validated these hits in vitro using immunoprecipitation to show robust 14-3-3 binding affinity [69].
14-3-3-Pred identifies potential binding motifs for Protein Z using bioinformatic analysis. However, further characterization of potential sites in vivo is required to identify the crucial interaction sites. One potential next approach created by Stevers et al. utilizes both bioinformatics and thermodynamics [72]. This methodology was employed to examine the binding of 14-3-3β to nine phosphate sites on Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) and the binding of 14-3-3γ to six phosphate sites on LRRK2. Using fluorescence polarization assays, they determined 14-3-3’s binding affinity to singly or doubly phosphorylated peptide fragments of these proteins. Isothermal titration calorimetry provides binding enthalpy/entropy metrics within the different combinations of sites to estimate the overall effective molarity of the interaction. After thermodynamic properties of these binding interactions were determined, simulations were made of every combination of 14-3-3 multivalent binding to these phosphorylated sites on the target client. These simulations provide information about each binding site’s contribution to complex formation as well as how these binding sites can synergistically interact with each other. This thermodynamic modeling also allows for investigation into how modulation of different sites affects complex formation and points to new target sites in drug discovery.
Once we have identified promising binding sites using this method developed by Stevers et al. [72], the next stage targets these binding sites to stabilize the interaction between 14-3-3 and protein Z. Drug development targeting PPI interactions has proven difficult as these proteins usually have fewer well-defined, pocket-shaped binding sites [73, 74], thus making traditional methods for target identification such as high-throughput screening, fragment-based discovery, and bioinformatic ligand design difficult. An approach utilizing cysteine trapping to identify potential PPI stabilizers was used to identify stabilizers for the interaction between 14-3-3σ and Estrogen Receptor α (ERα) [75]. This method works by utilizing a cysteine residue on the 14-3-3 protein as well as a library of cysteine containing fragments. The cysteine fragments are incubated with 14-3-3 alone or 14-3-3 bound to a peptide fragment of the protein of interest (i.e. ERα) under mildly reducing conditions. Each cysteine fragment in the library is screened via intact protein mass spectrometry for binding ability. Once prime candidates are found, fluorescence anisotropy examines the fragment’s effect on binding affinity between the peptide (ERα) and 14-3-3σ. X-ray crystallography can determine the mechanism by which these fragments alter binding affinity. The main advantage of this methodology is the ability to rapidly screen through potential stabilizing fragments that can later be modified for future studies.
In summary, bioinformatic tools and thermodynamic approaches can identify key residues regulating 14-3-3/Protein Z interaction, as described above (Figure 2). These promising sites can then be targeted using cysteine trapping to screen for potential compounds to target our PPI of interest.
Figure 2. A theoretical framework for stabilizing the interaction between 14-3-3 and the imaginary Protein Z.
14-3-3-Pred can be used to find predicted binding sites. Once predicted sites are found, thermodynamic techniques such as fluorescence polarization and isothermal titration calorimetry can be used to determine which sites are critical to the 14-3-3/Protein Z interaction. Finally, cysteine trapping can be used to screen for compounds that stabilize the interaction between Protein Z and 14-3-3.
Current 14-3-3 PPI Modulators
Most compounds developed to target 14-3-3 PPIs are primarily developed to target signaling interactions implicated in cancers. However, recent work points to the potential therapeutic role for 14-3-3 PPI modulators in neurological disorders (Table 1).
Table 1.
Current 14-3-3 PPI modulators.
| Compound | Targeted PPI | Relevant disease | Reference |
|---|---|---|---|
| PPI inhibitors | |||
| BV01, BV101 | 14-3-3σ/c-abl | CML | [83] |
| BV02 | 14-3-3σ/c-abl | CML | [81] |
| BV02 derivatives | 14-3-3σ/c-abl | CML | [80] |
| Difopein | 14-3-3/Raf-1 | cancer | [77] |
| FOBISIN101 | 14-3-3/PRAS40 14-3-3/Raf-1 |
cancer | [115] |
| Fragment C3 and related compounds | 14-3-3ζ/SOS | cancer | [105] |
| Inosine phosphate | 14-3-3σ/c-abl | CML | [84] |
| Macrocyclic peptides | 14-3-3ζ/ExoS | Pseudomonal infection | [78] |
| pS214Tau peptides | 14-3-3ζ/tau | Alzheimer’s disease | [87] |
| Pyridoxal phosphate | 14-3-3σ/c-abl | CML | [84] |
| R18 | 14-3-3ζ/Raf-1 | --- | [76] |
| UTK01 | 14-3-3ε/ezrin-radixinmoesin | notochord development | [85] |
| PPI stabilizers | |||
| Cotylenin A | 14-3-3/PMA | leukemias | [90, 91] |
| Disulfide fragments | 14-3-3σ/ERα | breast cancer | [75] |
| Epibastatin | 14-3-3/PMA | --- | [108] |
| FC-A | 14-3-3/PMA | cancer spinal cord injury |
[88] [102] |
| FC derivatives | cancer spinal cord injury |
[93] [103] |
|
| DP-005 | 14-3-3/p65 | cancer, stroke | [112] |
| FC-NCPC | --- | spinal cord injury | [103] |
| FC-THF | 14-3-3ơ/TASK3 | cancer | [99] |
| ISIR-005 | 14-3-3ζ/Gab2 | CML | [98] |
| ISIR-042 | --- | cancer | [100] |
| ISIR-050 | --- | cancer | [101] |
| NV-1, NV-2, NV-3 | 14-3-3/TAZ | cancer | [110] |
| 6 new derivatives | 14-3-3σ/TASK | cancer | [104] |
| Pyrollidone 1 and derivatives | 14-3-3/PMA | --- | [107, 108] |
PPI Inhibitors
R18 was the first 14-3-3 PPI inhibitor discovered utilizing phage display [76]. X-ray crystallography shows R18 binds within 14-3-3ζ’s conserved amphipathic groove. R18 forms several bonds with three arginine and one lysine residue in 14-3-3ζ, and R18 significantly reduces the association of 14-3-3ζ and Raf-1 kinase [76]. A mutation in the hydrophobic groove of 14-3-3ζ/Raf1 interaction site decreases Raf-1 binding to 14-3-3ζ as well as R18 binding [76]. While R18 lacks specificity to 14-3-3 proteins, difopein, created as two molecules of R18 connected by a linker, is a high affinity competitive antagonist of 14-3-3s that fits within the amphipathic grooves [77].
Several 14-3-3 PPI inhibitors have since been developed. Synthesized macrocyclic peptides were developed to disrupt the interaction between 14-3-3ζ and Exoenzyme S (ExoS), a virulence factor of Pseudomonas aeruginosa [78]. This compound strategy demonstrated the ability of researchers to target PPIs involving irregular, disordered peptide secondary structures as is common in PPI interactions and led to an interesting potential target for Pseudomonas aeruginosa infections [79].
Inhibitors can also target different cancers. For instance, BV02-derived inhibitors were developed to inhibit the interaction between c-Abl and 14-3-3σ in chronic myelogenous leukemia (CML) [80, 81]. When c-Abl binding to 14-3-3σ is disrupted, c-Abl will undergo nuclear translocation and activate apoptosis. In CML a translocation of the ABL and BCR genes leads to the formation of the constitutively active Bcr-Abl protein which fails to properly induce apoptosis [82]. Inhibition of its 14-3-3 interaction allows residual c-Abl not fused to Bcr to translocate to the nucleus and induce apoptosis. BV02 was identified out of 200,000 compounds screened in-silico for inhibition of the 14-3-3σ/c-Abl interaction [81]. BV01 and BV101 were similarly discovered [83]. Further BV02 modifications have led to more stable compounds that disrupt the 14-3-3σ/c-Abl interaction [80]. Other compounds targeting 14-3-3σ/c-Abl include inosine monophosphate and pyridoxal phosphate and may provide alternate treatments for CML [84].
PPI inhibitors have been used primarily as tools for investigating the role of 14-3-3s in neurological disease. For example, the compound UTKO1 was used to investigate the mechanisms underlying notochord tubulogenesis, by disrupting the interaction between ezrin/radixin/moesin and 14-3-3ε [85]. Difopein has been a critical tool to demonstrate the importance of 14-3-3s in regulating in α-syn and LRRK2 in PD, as noted above [34, 35, 44].
Inhibition of the 14-3-3ζ/tau interaction has been investigated as a potential therapeutic target for AD. As noted above, 14-3-3ζ binding to phosphorylated tau disrupts tau’s binding to microtubules and thus could contribute to microtubule instability in AD. Tau-derived compounds were recently developed to disrupt the 14-3-3ζ/tau interaction using a structure-guided approach. Based on X-ray crystallography that had previously identified pS214 and pS324 as the key 14-3-3 binding sites within tau [62], a pS214 tau peptide epitope was modified with extensions at the C-terminus with hydrophobic groups to better engage the fusicoccin hydrophobic pocket within the amphipathic 14-3-3 groove [86]. Fusicoccin is a fungal-derived compound first identified as a stabilizer of 14-3-3 PPIs (see below). The addition of a benzhydryl pyrrolidine moiety at the C-terminus particularly increased 14-3-3 binding [86]. 17 new compounds were created by further chemical modifications around the benzyhydryl pyrrolidine in order to further improve 14-3-3 binding at a deep-lying pocket near the fusicoccin pocket [87]. While their chemical modifications were aimed to target the deep-lying pocket, x-ray crystallography and thermodynamic analysis demonstrated that the seven analyzed compounds bound at the fusicoccin site instead. The three most potent peptide derivatives successfully disrupted the interaction between 14-3-3ζ and full-length tau [87]. While these compounds may not be ready for clinical studies, they provide new tools in studying the 14-3-3/tau interaction, and demonstrate how structure-based approaches could lead to potentially clinically useful therapies for AD.
PPI Stabilizers
PPI stabilizers have traditionally been neglected as a focus of drug development, but stabilization of 14-3-3 PPIs could have therapeutic value for PD and other disorders. Most PPI stabilizers have been discovered serendipitously, including the first discovered stabilizer of a 14-3-3 PPI, fusicoccin-A (FC). FC was identified as a natural compound produced by the fungus Phomopsis amygdali [88] that stabilizes the complex of 14-3-3 with a plasma membrane hydrogen ion pump found in plants (PMA) [89]. Cotylenin A is another compound of similar chemical structure from the fungus Cladosporium that stabilizes this same 14-3-3/PMA complex [90, 91]. FC primarily mediates its interaction with 14-3-3 clients via the C-terminal phosphorylation motif (motif III) [92]. After identifying specific structural constraints at the C-terminal residue of this binding motif, Sengupta et al. used bioinformatics to identify 119 candidate 14-3-3 PPIs that can be targeted by FC and found several novel 14-3-3 protein interactors [93]. Given the large number of 14-3-3 PPIs that could be targeted by FC, its use as a therapeutic agent is likely limited, although FC could still be an effective tool for understanding the role of 14-3-3 PPIs in disease.
FC-based compounds have been developed for cancer therapeutics, including the targeting of Grb-associated binder 2 (Gab2) and 14-3-3ζ. Increased Gab2 signaling is associated with several cancers, promotes Bcr-Abl inhibitor resistance in CML, and may promote cellular proliferation via increased MAPK signaling [94–96]. When bound to 14-3-3ζ, Gab2 cannot form the complex that promotes MAPK pro-proliferative signaling [97]. The FC-derived compound ISIR-005 stabilizes the 14-3-3ζ/Gab2 interaction by selectively binding the phosphorylated Gab2 at T391, which has increased binding affinity for 14-3-3ζ [98]. This FC compound demonstrates the feasibility of targeting this interaction to decrease pro-proliferative signaling. Further FC modifications have led to other compounds with anti-cancer properties, including ISIR-042, ISIR-050, and FC-THF [99–101]. Because of modifications promoting steric hindrance at motif I and II sites, FC-THF shows increased specificity towards the motif III sites and increased stabilization of the 14-3-3ơ/TASK3 interaction that plays a role in cancer [99].
FC-compounds may also treat spinal cord injury. FC promotes neurite outgrowth in neuronal culture and rescues axonal loss in spinal cord injury and optic nerve crush injury models [102]. Screening of FC derivatives identified nine compounds with a higher potency than FC, including FC-NCPC [103]. Proteomic analysis of GST-14-3-3ε pulldowns from cortical neurons treated with vehicle, FC, or FC-NCPC identified key 14-3-3 PPIs affected by these FC compounds. KEGG pathway analysis of 14-3-3 clients affected by FC or FC-NCPC treatment pointed to enrichment in the RAP1 signaling pathway, an important signaling pathway for cell adhesion, with some protein interactions being inhibited while others were stabilized. X-ray crystallography demonstrated that slight structural shifts in the 14-3-3 binding pocket by FC compounds could induce opposing effects to stabilize or destabilize specific 14-3-3 PPIs. This study shows how targeting PPIs at one site can affect a multitude of interactions within the same or different pathways.
Developing PPI stabilizers is more complex due to the need for three partners to interact compared to more traditional inhibition strategies. While strategic approaches to stabilizing 14-3-3 PPIs has been limited by limited structure-activity information, Andrei et al. demonstrate a successful rationally designed approach for developing new 14-3-3 PPI stabilizers by using molecular dynamic simulations based on the 14-3-3ơ/TASK/FC crystal structure [104]. By identifying key residues that add an additional hydrogen bond to interact with the 14-3-3 residue D215, they created six more compounds with increased potency compared to FC that stabilized several 14-3-3 PPIs and had functional effect in biological assays [104]. Such approaches could be used to develop more 14-3-3 PPI stabilizers.
While target compounds have yet to be identified, 14-3-3 PPI stabilizers could serve as potential treatments for PD by stabilization of 14-3-3 binding to LRRK2 and to αsyn. Interestingly, Pak6, a kinase whose 14-3-3 interaction is affected by FC compounds [103], regulates 14-3-3γ’s interaction with LRRK2 [45]. S910 and S935 have been identified as the key phosphosites to regulate LRRK2 binding to 14-3-3s [72], and development of compounds to target interactions at these phosphosites could lead to LRRK2-based therapies for PD.
Conclusions and Future Perspectives
In summary, we have reviewed the role of 14-3-3 PPIs in common neurodegenerative disorders and discussed the new approaches that can be used to develop better 14-3-3 PPI modulators. The field of 14-3-3 drug discovery is growing, with the identification of more and more PPI modulating compounds (Table 1). While 14-3-3 PPI modulator development has primarily focused on cancer, more studies are examining 14-3-3 PPI targeting for neurological disease. Most compounds developed to date disrupt 14-3-3 PPIs, yet pharmacological advances show several compounds can stabilize PPIs or even act as double agents to promote certain PPIs while simultaneously disrupting other PPIs [103]. Such double-edged compounds could be tailored to promote select 14-3-3 interactions while inhibiting others. PPI modulation continues to prove difficult compared to more traditional drug discovery methods, yet as technology examining 14-3-3 PPI interactions advances, the potential for targeting PPIs for therapeutics increases. Rational drug design employing structural information from X-ray crystallography along with computational molecular modeling techniques and even proteomics, as illustrated in several examples above, will lead to more potent and specific compounds [83, 87, 93, 103–106].
The key challenge and future direction for the field is developing specificity to target key PPIs without affecting other desirable 14-3-3 PPIs in order to avoid off-target toxicities (see Outstanding Questions). This is particularly important given the ubiquitous nature of 14-3-3s. Potential approaches include development of compounds that are highly specific for a particular 14-3-3 PPI important for the disease targeted and the use of drug-targeting strategies currently under development for other pharmaceutical agents. Some 14-3-3 PPI stabilizers show some degree of specificity. For example, the PPI stabilizer pyrrolidone 1 and its derivatives show specificity to 14-3-3/PMA and are inactive against other targets like Raf-1, p53, and Cdc25C [107, 108]. Different 14-3-3 isoforms demonstrate differential FC-stabilizing effects on binding clients, with 14-3-3σ PPIs being most affected by FC [109]. These isoform-dependent differences may be greater in vivo than revealed by this study in which peptide client proteins were used instead of full-length proteins. Development of newer FC-based compounds via chemical modifications of critical residues demonstrate the feasibility of developing more selective PPI compounds using rational structure-based and modeling approaches [104].
Outstanding Questions.
What are the key 14-3-3 isoforms most relevant to target in neurodegenerative diseases? Should different 14-3-3 isoforms be targeted for different disorders?
How can one develop very specific and selective 14-3-3 PPI modulators that target key PPIs without affecting other 14-3-3 PPIs?
What are the long-term consequences of targeting specific 14-3-3 PPIs for chronic diseases?
How can specific cell populations be targeted with 14-3-3 PPI therapeutics?
Targeting/screening for compounds that bind at less conserved, secondary sites outside of the central 14-3-3 amphipathic groove is one promising approach for developing more selective 14-3-3 PPIs. Sijbesma et al. recently identified small molecule peptide fragments that bound the 14-3-3/TAZ complex in secondary sites outside the conserved central groove [110]. Similarly, Ballone et al. used biophysical techniques and molecular docking simulations to develop specific inhibitors against the 14-3-3ζ/SOS1pS1161 complex by targeting sites outside the conserved groove [105]. Indeed, developing PPI stabilizers should theoretically be selective as these compounds would target select interfaces that are formed only about the multiprotein complex. Cooperativity analysis, in which evaluation of binding affinity of ternary complex vs. binary complex is elucidated, is another approach by which selective PPI stabilizers can be designed [111, 112]. PPI stabilization is driven not just by the intrinsic binding affinity of the compound for the individual binding partners (i.e. 14-3-3) but also by affinity for the cooperative complex (14-3-3/client) [111]. The more that a 14-3-3 PPI stabilizer promotes the cooperative binding between 14-3-3 and its client leads to improved complex stabilization and increased selectivity. Wolter et al. demonstrated this cooperativity phenomenon with the FC-derivative DP-005, which shows high cooperativity and selectivity for the 14-3-3 complex with the NFκB subunit p65 [112].
A related question is appropriate target engagement. The first step is crossing the blood-brain barrier for novel 14-3-3 PPI compounds – a concern for the development of any CNS-active therapeutic [113]. Drug specificity to the affected brain region and/or cell type is another important feature for successful therapeutics to avoid long-term toxicities in the chronic treatment of neurodegenerative disorders. This concern is not limited to 14-3-3 PPI modulators but an issue for the management of all chronic neurological disorders. While outside realm of this review, advances in drug-targeting approaches, including small molecules, aptamers, peptide or antibody-mediated strategies, and cell-targeting approaches [114], could be applied to novel 14-3-3 PPI modulators to achieve specific target engagement. Advancements in such drug targeting strategies have been successful for cancer-related disorders [114], and these technologies will likely be converted to treat neurological disorders.
In conclusion, 14-3-3 PPI targeting is an area of exciting new avenues for much needed therapeutics. 14-3-3 PPI modulation shows promise in a variety of neurological diseases. These PPI modulators not only have therapeutic potential but serve as critical tools to allow researchers to better understand the mechanisms by which 14-3-3s exert their biological effect.
Highlights.
Continuing evidence demonstrates the role of 14-3-3 proteins in common neurodegenerative disorders, including Parkinson’s Disease and Alzheimer’s Disease.
To date, targeting protein-protein interactions (PPIs), such as those used by 14-3-3 proteins, have proven difficult, but emerging methodologies may provide unique ways of targeting 14-3-3 PPIs.
Recent advances in targeting 14-3-3 interactions have identified several compounds that can act as double agents to promote certain PPIs while simultaneously disrupting other PPIs.
Rational drug design using X-ray crystallography along with computational molecular modeling techniques can lead to more potent and specific 14-3-3 PPI compounds.
Acknowledgements
This work was supported by grants from the NIH to TAY (R01 NS088533, R01 NS112203, R56 NS115767, P50 NS108675).
Footnotes
Disclaimer Statement
Talene Yacoubian has a U.S. Patent #7,919,262 on the use of 14-3-3s in neurodegeneration.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Martin H, et al. (1994) Subcellular localisation of 14-3-3 isoforms in rat brain using specific antibodies. J Neurochem 63, 2259–2265 [DOI] [PubMed] [Google Scholar]
- 2.Baxter HC, et al. (2002) Specific 14-3-3 isoform detection and immunolocalization in prion diseases. Biochem Soc Trans 30, 387–391 [DOI] [PubMed] [Google Scholar]
- 3.Moore BW (1967) Specific acidic proteins of the nervous system. Physiological and Biochemical Aspects of Nervous Integration, 343–359 [Google Scholar]
- 4.Moore BW, et al. (1968) ASSAY AND REGIONAL DISTRIBUTION OF A SOLUBLE PROTEIN CHARACTERISTIC OF THE NERVOUS SYSTEM*. Journal of Neurochemistry 15, 265–272 [DOI] [PubMed] [Google Scholar]
- 5.Zhu H, et al. (2016) Origin of a folded repeat protein from an intrinsically disordered ancestor. Elife 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ichimura T, et al. (1988) Molecular cloning of cDNA coding for brain-specific 14-3-3 protein, a protein kinase-dependent activator of tyrosine and tryptophan hydroxylases. Proc Natl Acad Sci U S A 85, 7084–7088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toker A, et al. (1992) Multiple isoforms of a protein kinase C inhibitor (KCIP-1/14-3-3) from sheep brain. Amino acid sequence of phosphorylated forms. Eur J Biochem 206, 453–461 [DOI] [PubMed] [Google Scholar]
- 8.Aitken A, et al. (1995) 14-3-3 alpha and delta are the phosphorylated forms of raf-activating 14-3-3 beta and zeta. In vivo stoichiometric phosphorylation in brain at a Ser-Pro-Glu-Lys MOTIF. J Biol Chem 270, 5706–5709 [DOI] [PubMed] [Google Scholar]
- 9.Sluchanko NN and Bustos DM (2019) Intrinsic disorder associated with 14-3-3 proteins and their partners. Prog Mol Biol Transl Sci 166, 19–61 [DOI] [PubMed] [Google Scholar]
- 10.Pennington KL, et al. (2018) The dynamic and stress-adaptive signaling hub of 14-3-3: emerging mechanisms of regulation and context-dependent protein-protein interactions. Oncogene 37, 5587–5604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stevers LM, et al. (2018) Modulators of 14-3-3 Protein-Protein Interactions. J Med Chem 61, 3755–3778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yaffe MB, et al. (1997) The structural basis for 14-3-3:phosphopeptide binding specificity. Cell 91, 961–971 [DOI] [PubMed] [Google Scholar]
- 13.Ottmann C, et al. (2007) Phosphorylation-independent interaction between 14-3-3 and exoenzyme S: from structure to pathogenesis. The EMBO journal 26, 902–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan X, et al. (2019) 14-3-3 Proteins Are on the Crossroads of Cancer, Aging, and Age-Related Neurodegenerative Disease. International journal of molecular sciences 20, 3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toyo-oka K, et al. (2014) 14-3-3epsilon and zeta regulate neurogenesis and differentiation of neuronal progenitor cells in the developing brain. J Neurosci 34, 12168–12181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheah PS, et al. (2012) Neurodevelopmental and neuropsychiatric behaviour defects arise from 14-3-3ζ deficiency. Mol Psychiatry 17, 451–466 [DOI] [PubMed] [Google Scholar]
- 17.Xu X, et al. (2015) 14-3-3ζ deficient mice in the BALB/c background display behavioural and anatomical defects associated with neurodevelopmental disorders. Sci Rep 5, 12434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wachi T, et al. (2016) Ablation of the 14-3-3gamma Protein Results in Neuronal Migration Delay and Morphological Defects in the Developing Cerebral Cortex. Dev Neurobiol 76, 600–614 [DOI] [PubMed] [Google Scholar]
- 19.Ikeda M, et al. (2008) Identification of YWHAE, a gene encoding 14-3-3epsilon, as a possible susceptibility gene for schizophrenia. Hum Mol Genet 17, 3212–3222 [DOI] [PubMed] [Google Scholar]
- 20.Foote M, et al. (2015) Inhibition of 14-3-3 Proteins Leads to Schizophrenia-Related Behavioral Phenotypes and Synaptic Defects in Mice. Biol Psychiatry 78, 386–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qiao H, et al. (2014) 14-3-3 proteins are required for hippocampal long-term potentiation and associative learning and memory. J Neurosci 34, 4801–4808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forno LS (1988) The Neuropathology Of Parkinson’S Disease. In Progress in Parkinson Research (Hefti F. and Weiner WJ, eds), pp. 11–21, Springer US [Google Scholar]
- 23.Jankovic J. (2008) Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry 79, 368–376 [DOI] [PubMed] [Google Scholar]
- 24.McKeith IG, et al. (2005) Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 65, 1863–1872 [DOI] [PubMed] [Google Scholar]
- 25.Spillantini MG, et al. (1997) Alpha-synuclein in Lewy bodies. Nature 388, 839–840 [DOI] [PubMed] [Google Scholar]
- 26.Berg D, et al. (2003) Specification of 14-3-3 proteins in Lewy bodies. Ann Neurol 54, 135. [DOI] [PubMed] [Google Scholar]
- 27.Kawamoto Y, et al. (2002) 14-3-3 proteins in Lewy bodies in Parkinson disease and diffuse Lewy body disease brains. J Neuropathol Exp Neurol 61, 245–253 [DOI] [PubMed] [Google Scholar]
- 28.McFerrin MB, et al. (2017) Dysregulation of 14-3-3 proteins in neurodegenerative diseases with Lewy body or Alzheimer pathology. Ann Clin Transl Neurol 4, 466–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ulitsky I, et al. (2010) DEGAS: de novo discovery of dysregulated pathways in human diseases. PLoS One 5, e13367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slone SR, et al. (2011) 14-3-3theta protects against neurotoxicity in a cellular Parkinson’s disease model through inhibition of the apoptotic factor Bax. PLoS One 6, e21720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yacoubian TA, et al. (2010) Differential neuroprotective effects of 14-3-3 proteins in models of Parkinson’s disease. Cell Death Dis 1, e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ding H, et al. (2015) 14-3-3 inhibition promotes dopaminergic neuron loss and 14-3-3θ overexpression promotes recovery in the MPTP mouse model of Parkinson’s disease. Neuroscience 307, 73–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plotegher N, et al. (2014) The chaperone-like protein 14-3-3η interacts with human α-synuclein aggregation intermediates rerouting the amyloidogenic pathway and reducing α-synuclein cellular toxicity. Hum Mol Genet 23, 5615–5629 [DOI] [PubMed] [Google Scholar]
- 34.Wang B, et al. (2018) 14-3-3 Proteins Reduce Cell-to-Cell Transfer and Propagation of Pathogenic alpha-Synuclein. J Neurosci 38, 8211–8232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Underwood R, et al. (2020) 14-3-3 mitigates alpha-synuclein aggregation and toxicity in the in vivo preformed fibril model. Acta Neuropathol Comm in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dzamko N, et al. (2010) Inhibition of LRRK2 kinase activity leads to dephosphorylation of Ser(910)/Ser(935), disruption of 14-3-3 binding and altered cytoplasmic localization. Biochem J 430, 405–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nichols RJ, et al. (2010) 14-3-3 binding to LRRK2 is disrupted by multiple Parkinson’s disease-associated mutations and regulates cytoplasmic localization. Biochem J 430, 393–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li X, et al. (2011) Phosphorylation-dependent 14-3-3 binding to LRRK2 is impaired by common mutations of familial Parkinson’s disease. PLoS One 6, e17153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greggio E, et al. (2006) Kinase activity is required for the toxic effects of mutant LRRK2/dardarin. Neurobiol Dis 23, 329–341 [DOI] [PubMed] [Google Scholar]
- 40.Trinh J. and Farrer M. (2013) Advances in the genetics of Parkinson disease. Nat Rev Neurol 9, 445–454 [DOI] [PubMed] [Google Scholar]
- 41.Smith WW, et al. (2006) Kinase activity of mutant LRRK2 mediates neuronal toxicity. Nat Neurosci 9, 1231–1233 [DOI] [PubMed] [Google Scholar]
- 42.West AB, et al. (2007) Parkinson’s disease-associated mutations in LRRK2 link enhanced GTP-binding and kinase activities to neuronal toxicity. Hum Mol Genet 16, 223–232 [DOI] [PubMed] [Google Scholar]
- 43.Muda K, et al. (2014) Parkinson-related LRRK2 mutation R1441C/G/H impairs PKA phosphorylation of LRRK2 and disrupts its interaction with 14-3-3. Proc Natl Acad Sci U S A 111, E34–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lavalley NJ, et al. (2016) 14-3-3 Proteins regulate mutant LRRK2 kinase activity and neurite shortening. Hum Mol Genet 25, 109–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Civiero L, et al. (2017) PAK6 Phosphorylates 14-3-3γ to Regulate Steady State Phosphorylation of LRRK2. Front Mol Neurosci 10, 417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Di Maio R, et al. (2018) LRRK2 activation in idiopathic Parkinson’s disease. Sci Transl Med 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.(2020) 2020 Alzheimer’s disease facts and figures. Alzheimers Dement [DOI] [PubMed] [Google Scholar]
- 48.Ballard C, et al. (2011) Alzheimer’s disease. The Lancet 377, 1019–1031 [DOI] [PubMed] [Google Scholar]
- 49.Meraz‐Ríos MA, et al. (2010) Tau oligomers and aggregation in Alzheimer’s disease. Journal of neurochemistry 112, 1353–1367 [DOI] [PubMed] [Google Scholar]
- 50.Mateo I, et al. (2008) Gene-gene interaction between 14-3-3 zeta and butyrylcholinesterase modulates Alzheimer’s disease risk. European journal of neurology : the official journal of the European Federation of Neurological Societies 15, 219–222 [DOI] [PubMed] [Google Scholar]
- 51.Mateo I, et al. (2008) 14-3-3 zeta and tau genes interactively decrease Alzheimer’s disease risk. Dementia and geriatric cognitive disorders 25, 317–320 [DOI] [PubMed] [Google Scholar]
- 52.Liao L, et al. (2004) Proteomic characterization of postmortem amyloid plaques isolated by laser capture microdissection. J Biol Chem 279, 37061–37068 [DOI] [PubMed] [Google Scholar]
- 53.Hashiguchi M, et al. (2000) 14-3-3zeta is an effector of tau protein phosphorylation. J Biol Chem 275, 25247–25254 [DOI] [PubMed] [Google Scholar]
- 54.Sumioka A, et al. (2005) Role of 14-3-3gamma in FE65-dependent gene transactivation mediated by the amyloid beta-protein precursor cytoplasmic fragment. J Biol Chem 280, 42364–42374 [DOI] [PubMed] [Google Scholar]
- 55.Umahara T, et al. (2004) 14-3-3 proteins and zeta isoform containing neurofibrillary tangles in patients with Alzheimer’s disease. Acta Neuropathol 108, 279–286 [DOI] [PubMed] [Google Scholar]
- 56.VanGuilder HD, et al. (2011) Hippocampal dysregulation of synaptic plasticity-associated proteins with age-related cognitive decline. Neurobiol Dis 43, 201–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.VanGuilder HD, et al. (2010) Aging alters the expression of neurotransmission-regulating proteins in the hippocampal synaptoproteome. J Neurochem 113, 1577–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Umahara T, et al. (2004) 14-3-3 proteins and zeta isoform containing neurofibrillary tangles in patients with Alzheimer’s disease. Acta Neuropathologica 108, 279–286 [DOI] [PubMed] [Google Scholar]
- 59.Sadik G, et al. (2009) Phosphorylation of tau at Ser214 mediates its interaction with 14-3-3 protein: implications for the mechanism of tau aggregation. J Neurochem 108, 33–43 [DOI] [PubMed] [Google Scholar]
- 60.Hernández F, et al. (2004) Zeta 14-3-3 protein favours the formation of human tau fibrillar polymers. Neurosci Lett 357, 143–146 [DOI] [PubMed] [Google Scholar]
- 61.Qureshi HY, et al. (2013) Interaction of 14-3-3ζ with microtubule-associated protein tau within Alzheimer’s disease neurofibrillary tangles. Biochemistry 52, 6445–6455 [DOI] [PubMed] [Google Scholar]
- 62.Joo Y, et al. (2015) Involvement of 14-3-3 in tubulin instability and impaired axon development is mediated by Tau. Faseb j 29, 4133–4144 [DOI] [PubMed] [Google Scholar]
- 63.Liu D, et al. (1995) Crystal structure of the zeta isoform of the 14-3-3 protein. Nature 376, 191–194 [DOI] [PubMed] [Google Scholar]
- 64.Bustos DM and Iglesias AA (2006) Intrinsic disorder is a key characteristic in partners that bind 14-3-3 proteins. Proteins 63, 35–42 [DOI] [PubMed] [Google Scholar]
- 65.Wright PE and Dyson HJ (1999) Intrinsically unstructured proteins: re-assessing the protein structure-function paradigm. J Mol Biol 293, 321–331 [DOI] [PubMed] [Google Scholar]
- 66.Mammen M, et al. (1998) Polyvalent Interactions in Biological Systems: Implications for Design and Use of Multivalent Ligands and Inhibitors. Angew Chem Int Ed Engl 37, 2754–2794 [DOI] [PubMed] [Google Scholar]
- 67.Fasting C, et al. (2012) Multivalency as a chemical organization and action principle. Angew Chem Int Ed Engl 51, 10472–10498 [DOI] [PubMed] [Google Scholar]
- 68.Ferrell JE Jr. and Ha SH (2014) Ultrasensitivity part II: multisite phosphorylation, stoichiometric inhibitors, and positive feedback. Trends Biochem Sci 39, 556–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Madeira F, et al. (2015) 14-3-3-Pred: improved methods to predict 14-3-3-binding phosphopeptides. Bioinformatics 31, 2276–2283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hornbeck PV, et al. (2004) PhosphoSite: A bioinformatics resource dedicated to physiological protein phosphorylation. Proteomics 4, 1551–1561 [DOI] [PubMed] [Google Scholar]
- 71.Tinti M, et al. (2014) ANIA: ANnotation and Integrated Analysis of the 14-3-3 interactome. Database (Oxford) 2014, bat085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stevers LM, et al. (2018) A Thermodynamic Model for Multivalency in 14-3-3 Protein-Protein Interactions. J Am Chem Soc 140, 14498–14510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Edfeldt FN, et al. (2011) Fragment screening to predict druggability (ligandability) and lead discovery success. Drug Discov Today 16, 284–287 [DOI] [PubMed] [Google Scholar]
- 74.Surade S. and Blundell TL (2012) Structural biology and drug discovery of difficult targets: the limits of ligandability. Chem Biol 19, 42–50 [DOI] [PubMed] [Google Scholar]
- 75.Sijbesma E, et al. (2019) Site-Directed Fragment-Based Screening for the Discovery of Protein-Protein Interaction Stabilizers. J Am Chem Soc 141, 3524–3531 [DOI] [PubMed] [Google Scholar]
- 76.Wang B, et al. (1999) Isolation of high-affinity peptide antagonists of 14-3-3 proteins by phage display. Biochemistry 38, 12499–12504 [DOI] [PubMed] [Google Scholar]
- 77.Masters SC and Fu H. (2001) 14-3-3 proteins mediate an essential anti-apoptotic signal. J Biol Chem 276, 45193–45200 [DOI] [PubMed] [Google Scholar]
- 78.Glas A, et al. (2014) Constrained peptides with target-adapted cross-links as inhibitors of a pathogenic protein-protein interaction. Angew Chem Int Ed Engl 53, 2489–2493 [DOI] [PubMed] [Google Scholar]
- 79.Karlberg T, et al. (2018) 14-3-3 proteins activate Pseudomonas exotoxins-S and -T by chaperoning a hydrophobic surface. Nat Commun 9, 3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Iralde-Lorente L, et al. (2019) Chemically stable inhibitors of 14-3-3 protein-protein interactions derived from BV02. J Enzyme Inhib Med Chem 34, 657–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Corradi V, et al. (2010) Identification of the first non-peptidic small molecule inhibitor of the c-Abl/14-3-3 protein-protein interactions able to drive sensitive and Imatinib-resistant leukemia cells to apoptosis. Bioorg Med Chem Lett 20, 6133–6137 [DOI] [PubMed] [Google Scholar]
- 82.Bedi A, et al. (1994) Inhibition of apoptosis by BCR-ABL in chronic myeloid leukemia. Blood 83, 2038–2044 [PubMed] [Google Scholar]
- 83.Corradi V, et al. (2011) Computational techniques are valuable tools for the discovery of protein-protein interaction inhibitors: the 14-3-3sigma case. Bioorg Med Chem Lett 21, 6867–6871 [DOI] [PubMed] [Google Scholar]
- 84.Iralde-Lorente L, et al. (2020) Identification of Phosphate-Containing Compounds as New Inhibitors of 14-3-3/c-Abl Protein-Protein Interaction. ACS Chem Biol 15, 1026–1035 [DOI] [PubMed] [Google Scholar]
- 85.Mizotani Y, et al. (2018) 14-3-3εa directs the pulsatile transport of basal factors toward the apical domain for lumen growth in tubulogenesis. Proc Natl Acad Sci U S A 115, E8873–e8881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Milroy LG, et al. (2015) Stabilizer-Guided Inhibition of Protein-Protein Interactions. Angew Chem Int Ed Engl 54, 15720–15724 [DOI] [PubMed] [Google Scholar]
- 87.Andrei SA, et al. (2018) Inhibition of 14-3-3/Tau by Hybrid Small-Molecule Peptides Operating via Two Different Binding Modes. ACS Chem Neurosci 9, 2639–2654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pitman MG, et al. (1975) Relation between permeability to potassium and sodium ions and fusicoccin-stimulated hydrogen-ion efflux in barley roots. Planta 126, 61–73 [DOI] [PubMed] [Google Scholar]
- 89.Oecking C, et al. (1994) The fusicoccin receptor of plants is a member of the 14-3-3 superfamily of eukaryotic regulatory proteins. FEBS Lett 352, 163–166 [DOI] [PubMed] [Google Scholar]
- 90.Ottmann C, et al. (2009) A structural rationale for selective stabilization of anti-tumor interactions of 14-3-3 proteins by cotylenin A. J Mol Biol 386, 913–919 [DOI] [PubMed] [Google Scholar]
- 91.Asahi K, et al. (1997) Cotylenin A, a plant-growth regulator, induces the differentiation in murine and human myeloid leukemia cells. Biochem Biophys Res Commun 238, 758–763 [DOI] [PubMed] [Google Scholar]
- 92.Paiardini A, et al. (2014) The phytotoxin fusicoccin differently regulates 14-3-3 proteins association to mode III targets. IUBMB Life 66, 52–62 [DOI] [PubMed] [Google Scholar]
- 93.Sengupta A, et al. (2020) Analysis of Interactions Stabilized by Fusicoccin A Reveals an Expanded Suite of Potential 14-3-3 Binding Partners. ACS Chem Biol 15, 305–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brummer T, et al. (2006) Increased proliferation and altered growth factor dependence of human mammary epithelial cells overexpressing the Gab2 docking protein. J Biol Chem 281, 626–637 [DOI] [PubMed] [Google Scholar]
- 95.Wöhrle FU, et al. (2013) Gab2 signaling in chronic myeloid leukemia cells confers resistance to multiple Bcr-Abl inhibitors. Leukemia 27, 118–129 [DOI] [PubMed] [Google Scholar]
- 96.Nishida K, et al. (1999) Gab-family adapter proteins act downstream of cytokine and growth factor receptors and T- and B-cell antigen receptors. Blood 93, 1809–1816 [PubMed] [Google Scholar]
- 97.Brummer T, et al. (2008) Phosphorylation-dependent binding of 14-3-3 terminates signalling by the Gab2 docking protein. Embo j 27, 2305–2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bier D, et al. (2016) Small-Molecule Stabilization of the 14-3-3/Gab2 Protein-Protein Interaction (PPI) Interface. ChemMedChem 11, 911–918 [DOI] [PubMed] [Google Scholar]
- 99.Anders C, et al. (2013) A semisynthetic fusicoccane stabilizes a protein-protein interaction and enhances the expression of K+ channels at the cell surface. Chem Biol 20, 583–593 [DOI] [PubMed] [Google Scholar]
- 100.Kawakami K, et al. (2012) A novel fusicoccin derivative preferentially targets hypoxic tumor cells and inhibits tumor growth in xenografts. Anticancer Agents Med Chem 12, 791–800 [DOI] [PubMed] [Google Scholar]
- 101.Inoue T, et al. (2018) Semisynthesis and biological evaluation of a cotylenin A mimic derived from fusicoccin A. Bioorg Med Chem Lett 28, 646–650 [DOI] [PubMed] [Google Scholar]
- 102.Kaplan A, et al. (2017) Small-Molecule Stabilization of 14-3-3 Protein-Protein Interactions Stimulates Axon Regeneration. Neuron 93, 1082–1093 e1085 [DOI] [PubMed] [Google Scholar]
- 103.Kaplan A, et al. (2020) Polypharmacological Perturbation of the 14-3-3 Adaptor Protein Interactome Stimulates Neurite Outgrowth. Cell Chem Biol 27(6):657–667 e6 [DOI] [PubMed] [Google Scholar]
- 104.Andrei SA, et al. (2018) Rationally Designed Semisynthetic Natural Product Analogues for Stabilization of 14-3-3 Protein-Protein Interactions. Angew Chem Int Ed Engl 57, 13470–13474 [DOI] [PubMed] [Google Scholar]
- 105.Ballone A, et al. (2020) Experimental and Computational Druggability Exploration of the 14-3-3zeta/SOS1pS(1161) PPI Interface. J Chem Inf Model doi: 10.1021/acs.jcim.0c00722 [DOI] [PubMed] [Google Scholar]
- 106.Mori M, et al. (2013) Small molecules modulation of 14-3-3 protein-protein interactions. Drug Discov Today Technol 10, e541–547 [DOI] [PubMed] [Google Scholar]
- 107.Richter A, et al. (2012) An optimised small-molecule stabiliser of the 14-3-3-PMA2 protein-protein interaction. Chemistry 18, 6520–6527 [DOI] [PubMed] [Google Scholar]
- 108.Rose R, et al. (2010) Identification and structure of small-molecule stabilizers of 14-3-3 protein-protein interactions. Angew Chem Int Ed Engl 49, 4129–4132 [DOI] [PubMed] [Google Scholar]
- 109.Sengupta A, et al. (2020) Probing the 14-3-3 Isoform-Specificity Profile of Protein-Protein Interactions Stabilized by Fusicoccin A. ACS Omega 5, 25029–25035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sijbesma E, et al. (2017) Identification of Two Secondary Ligand Binding Sites in 14-3-3 Proteins Using Fragment Screening. Biochemistry 56, 3972–3982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.de Vink PJ, et al. (2019) Cooperativity basis for small-molecule stabilization of protein-protein interactions. Chem Sci 10, 2869–2874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wolter M, et al. (2020) Selectivity via Cooperativity: Preferential Stabilization of the p65/14-3-3 Interaction with Semisynthetic Natural Products. J Am Chem Soc 142, 11772–11783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Banks WA (2016) From blood-brain barrier to blood-brain interface: new opportunities for CNS drug delivery. Nat Rev Drug Discov 15, 275–292 [DOI] [PubMed] [Google Scholar]
- 114.Zhao Z, et al. (2020) Targeting Strategies for Tissue-Specific Drug Delivery. Cell 181, 151–167 [DOI] [PubMed] [Google Scholar]
- 115.Zhao J, et al. (2011) Discovery and structural characterization of a small molecule 14-3-3 protein-protein interaction inhibitor. Proc Natl Acad Sci U S A 108, 16212–16216 [DOI] [PMC free article] [PubMed] [Google Scholar]