Abstract
We review the use of telemedicine in glaucoma and its possible roles in the COVID-19 outbreak. We performed a literature search of published human studies on teleglaucoma on May 12, 2020, using search terms including “telemedicine” and “glaucoma” that were in English and published over the prior 10 years. This search strategy yielded a total of 14 relevant articles after manual curation. Of the 14 articles, 4 were from the same randomized control trial, 7 were prospective studies, 2 were retrospective studies, 1 was descriptive analysis, and 1 was cost-effective analysis. Seven discussed the common ophthalmologic measurements used in teleglaucoma. Four demonstrated the cost effectiveness of the use of teleglaucoma, and 3 articles investigated patient satisfaction with the use of teleglaucoma. Three articles investigated the correlation between teleglaucoma and face-to-face clinics. Five articles discussed the current use and opportunities of teleglaucoma.
When compared to in-person care, teleglaucoma is more time and cost-effective, shows high patient satisfaction and fair to good agreement with in-person care; however, there is great variation in the reported sensitivity of glaucoma screening, warranting further studies to establish its efficacy. For glaucoma management, both the sensitivity and specificity must be further improved before it could be put into extensive use. Nevertheless, it is worthwhile to explore the possible extensive application of teleglaucoma in monitoring “glaucoma suspects” and maintaining glaucoma follow-up during a pandemic outbreak to reduce the risk of transmission of infection.
Keywords: Telemedicine, Teleglaucoma, Glaucoma, COVID-19
1. Introduction
Glaucoma, a major cause of irreversible blindness (2,5,6,12,19,20,31,35), is estimated to affect more than 60 million people, accounting for about 3 million cases of blindness worldwide (3,23,25,29,30). Patients with glaucoma have optic neuropathy characterized by progressive degeneration (35). About half of the patients are unaware of their illness (3).
Teleglaucoma refers to the use of telemedicine for glaucoma (21). Teleglaucoma is generally conducted in a remote center where various ophthalmologic measurements are carried out by trained technicians or nurses to assess a patient's glaucoma status. The measurement results are then be sent to glaucoma specialists electronically to allow them to make a clinical decision (28). Teleglaucoma has 2 forms - glaucoma screening and glaucoma management. Teleglaucoma screening refers to the use of telemedicine to screen high risk individuals and assist ophthalmologists in diagnosing glaucoma. After the screening, newly diagnosed glaucoma patients could either be further evaluated with telemedicine, or they could be referred for in-person care. Early detection of glaucoma with adequate follow up can reduce the chance of vision loss (14); however, the use of teleglaucoma is still in its initial stages, although teleglaucoma has been used as a screening tool in countries such as Canada and the United States and has been shown to reduce the screening time and costs per patient (20,27). The second form of teleglaucoma is glaucoma management that enables frequent monitoring of glaucoma progression in existing patients. Teleglaucoma may be beneficial for ophthalmologists to assess in treatment compliance and to monitor clinical parameters such as intraocular pressure. Studies of teleglaucoma management conducted in the United Kingdom and Alberta, Canada, have shown teleglaucoma can potentially improve the efficacy of glaucoma follow up (3,9,27). It may also be able to enhance the patient-physician relationship through streamlining patients’ follow up experience and shortening visit time (18). We summarize the currentevidence for the use of teleglaucoma, mainly focusing on the cost effectiveness, safety, and patient satisfaction. We also discuss future opportunities and the possible role of teleglaucoma in maintaining regular patient follow-up during the time of a pandemic such as the COVID-19 outbreak.
2. Results
The search strategy yielded a total of 14 relevant articles after manual curation (Table 1 ). Out of the 14 articles, 4 were from the same randomized control trial, 6 were prospective studies, 2 were retrospective studies, 1 was descriptive analysis, and 1 was a cost-effectiveness analysis. Two articles focused on glaucoma screening, whereas 9 were related to glaucoma management. The remaining 3 studies covered both glaucoma screening and management. Seven articles discussed the common ophthalmologic measurements used in teleglaucoma (3,9,14,20,22,27,32). Four demonstrated the cost effectiveness of the use of teleglaucoma (3,15,27,33). Three investigated patient satisfaction after the use of teleglaucoma (3,9,14). Three investigated the agreement between teleglaucoma and face-to-face care (3,13,22). Five discussed current use and future opportunities of teleglaucoma (20,27,32,34,36).
Table 1.
Summary of selected relevant studies for teleglaucoma
| Study | Study design | Number of | Study aim | Uses of | Significant findings |
|---|---|---|---|---|---|
| patients | teleglaucoma | ||||
| Arora et al. (2014) (3) | prospective comparative study | 131 | To investigate the cycle time and access time between teleglaucoma and in person glaucoma care | Glaucoma management | Teleglaucoma is a more efficient way of managing patients with early stage glaucoma when compared with in person assessment |
| Clarke et al. (2017) (9) | prospective study | 204 | To investigate the agreement in determination of glaucoma status made in a virtual glaucoma clinic and face to face consultation | Glaucoma management | Virtual glaucoma clinic is a safe option and viable option for selective patients with glaucoma because of the low incidence of adverse misclassification and the slowly progressive nature of glaucoma |
| Gupta et al. (2013) (13) | Prospective study | 247 | To compare the use of teleophthalmology using indigenous equipment, compared to the clinical assessment in terms of the level of agreement and sensitivity and specificity of diagnosis and management different eye diseases | Glaucoma screening and management | Teleophthalmology was found to be effective in diagnosis and management decision of various eye diseases. |
| Hark et al. (2017) (15) | prospective, RCT | 906 | To discuss the screening results from the Philadelphia telemedicine glaucoma detection and follow up study | Glaucoma screening | Telemedicine screening intervention in primary care can be able to detect high rate of suspicious optic nerves, retinal diseases and ocular hypertension. |
| Hark et al. (2019) (16) | prospective study | 902 | To examine the use of IOP measurement, used in addition to nonmydriatic fundus photography in glaucoma telemedicine screening | Glaucoma screening | Telemedicine vision screening programs with IOP measurement in high-risk populations is applicable. |
| Hark, Acito et al. (2018) (14) | prospective, RCT | 184 | To examine the use of tele medicine for the detection of glaucoma and other eye diseases in primary care clinics | Glaucoma screening | Telemedicine was found to be useful in early detection of glaucoma and other ocular pathology |
| Hark Myers et al. (2018) (17) | prospective RCT | 906 | To discover determinants of unreadable fundus images in the Philadelphia Telemedicine Glaucoma Detection and Follow-up Study | Glaucoma screening | Understanding the causes of unreadable fundus images is likely to optimize the predictive accuracy, efficiency, and cost in ophthalmology in telemedicine |
| Kassam et al. (2013) (21) | Prospective study | 257 | To evaluate the use of teleglaucoma in the University of Alberta in 2011 | Glaucoma screening and management | The use of teleglaucoma is able to improve the diagnosis and management of glaucoma in industrialized and developing countries. |
| Kiage et al. (2013) (22) | prospective study | 309 | To compare a web-based teleglaucoma assessment with clinical slit lamp examination for the glaucoma screening among diabetics in a rural African district. | Glaucoma screening | Agreement between the ability to diagnose glaucoma using teleglaucoma when compared to clinical slit lamp examination was found to be moderate |
| Rathi et al. (2017) (27) | descriptive analysis | N/A | To describe the use of teleophthalmology in the hospital and outpatient settings | Glaucoma screening and management | Ophthalmic telemedicine in the United States is in its developing phase but may be able to improve compliance to evidence-based protocols. |
| Staffieri et al. (2011) (32) | prospective study | 133 | To evaluate the use of a telemedicine model in terms of decreasing glaucoma blindness through the early detection of undiagnosed glaucoma in high-risk individuals | Glaucoma screening | Telemedicine is an efficient way for screening, grading, and showing participants of examination results |
| Thomas et al. (2015) (33) | cost-effectiveness analysis | Population in rural Canada (exact number not specified) | To measure the cost effectiveness of teleglaucoma rural Canada | Glaucoma screening | Teleglaucoma allows better access to ophthalmic care and improves healthcare efficiency, specifically in rural areas. It also improve cost benefits. |
| Verma et al. (2014) (34) | Retrospective study | 247 | To evaluate the diagnostic outcomes and referral pathways of patients participated in a collaborative care patient centred teleglaucoma program | Glaucoma screening | Most patients did not require in-person consultation and can be managed by distance collaboration. Further investigations in the cost effectiveness for the program is needed |
| Wright et al. (2015) (36) | Retrospective study | 24257 | To determine the significance of specialist supervision in a new model of glaucoma service delivery | Glaucoma screening | Virtual review of glaucoma can reduce the chance of patients treated unnecessarily and reduce the demand for glaucoma appoints. |
3. Common ophthalmologic measurements used in teleglaucoma
A total of 7 studies discussed the types of ophthalmologic measurements they used in teleglaucoma (3,9,14,20,22,27,32). Common measurements included the examination of fundus images (3,9,14,20,22,32), automated visual field testing (3,9,20,22,27,32), intraocular pressure (IOP) (9,14,20,32), visual acuity (9,14,32), and questionnaires on patients’ symptoms (9,22). Slit lamp examination (3,9) and corneal thickness (20,27) were also used in either teleglaucoma screening or management in some of the studies. Optical coherence tomography (OCT) proved helpful in teleglaucoma screening and management (3,27). The study by Arora and coworkers also used the Pentacam for anterior segment imaging (3), whereas refractive status was included in the study by Staffieri and coworkers. (32) The above summary suggested that measurements that are more machine-dependent (i.e., visual field testing and IOP) are more commonly used in teleglaucoma. We believe that machines that are less operator dependent should give more objective results even when they are operated by less experienced personnel than ophthalmologists in a remote site.
The Philadelphia Telemedicine Glaucoma Detection Study emphasized the importance of intraocular pressure (IOP) measurement in teleglaucoma settings (16). During the first visit of the study, the IOP level was associated with both suspicious (P = 0.043) and nonsuspicious optic nerve head findings (P < 0.001). Furthermore, for patients with both suspicious optic nerve head findings and elevated IOP (>21 mm Hg) at the first visit, the odds ratio for being diagnosed with glaucoma at the second visit was 4.48 (95% CI, 1.50–13.93; P = 0.007), when compared to patients with neither suspicious nerve findings nor elevated IOP at screening. For patients with suspicious nerve findings, but normal IOP (<21 mm Hg), the odds of a glaucoma diagnosis at the second visit were only 2.04 (95% CI, 0.83–5.53; P = 0.152) (16). This suggested that abnormal optic disc findings on fundus images alone were not adequate to predict glaucoma occurrence. Inclusion of IOP measurement improved diagnostic accuracy.
3.1. Cost effectiveness
The cost effectiveness of glaucoma consultation can be analyzed based on both time cost and money cost. Four of the articles commented on the cost effectiveness of teleglaucoma. The Philadelphia Telemedicine Glaucoma Detection mainly investigated the money cost of screening (15), whereas the cost-effectiveness analysis by Thomas and coworkers indicated that teleglaucoma is more cost effective as a screening tool for glaucoma (33). Arora and coworkers and Rathi and coworkers focused on time cost analysis and concluded that teleglaucoma was less time consuming than in-person visits. (3,27);
3.1.1. Teleglaucoma for screening
Cost analyses of the Philadelphia Telemedicine Glaucoma Detection Study have justified the cost effectiveness of teleglaucoma in glaucoma screening (15). The Philadelphia Study mainly conducted glaucoma screening with IOP measurement and funduscopic images on high risk individuals according to their ethnicities (mainly African Americans, Asians, Hispanics) or those with a positive family history of glaucoma. This screening method only incurred an average overall cost of USD$9.77 per participant, with a 23.7-minute mean visit time. More than 50% of overall costs were for the fundus photography, with an average cost of $4.96 and an average time of 12.2 minutes per patient. Despite the low screening cost, it yielded reasonably good results from the 906 patients, with 258 (25.8%) having a suspicious optic nerve appearance, 62 (6.8%) diagnosed with ocular hypertension, 102 (11.3%) with diabetic retinopathy and 68 (7.5%) with other retinal abnormalities.
Thomas and coworkers’ analysis further reaffirmed the cost-effectiveness of teleglaucoma for screening. (33) They calculated the cost effectiveness based on 3 major areas of expediture (human resources, information technology, and diagnostic equipment) and effectiveness based on quality of life improvement. Teleglaucoma was demonstrated to be more cost effective than in-person care for detecting glaucoma. When compared to no glaucoma screening, teleglaucoma had an incremental cost-effectiveness ratio (ICER) of $47.60/quality adjusted life year (QALY). In other words, spending an additional $47.60 in teleglaucoma for each patient will give an additional QALY. Its cost-effectiveness was higher when compared to $244.05/QALY in in-person glaucoma detection.
Thomas and coworkers also performed a Markov Probability Analysis demonstrating that the 30-year overall risk of glaucoma development and the risk of developing moderate glaucoma remain relatively the same in teleglaucoma when compared to in-person care, but teleglaucoma was able to prevent 24% more cases of glaucoma-related blindness after 30 years. For patients who were screened positive by teleglaucoma, the total reward was 15.7 QALYs, 1.1 less than rewards from in-person care. However, the per-patient cumulative cost for in-person care was significantly higher (by almost 3.5 times) than that of teleglaucoma after 30 years. Judging from the 30-year ICER, teleglaucoma was more cost-effective than in-person care with an ICER of-$27,460 per QALY per patient. (33) This suggested that the long-term implementation of teleglaucoma screening can save considerable cost whereas providing similar benefits to patients at risk of developing glaucoma. The 30-year cost-effectiveness analysis by Thomas and coworkers is, however, just a simulated model that may not accurately reflect drawbacks of teleglaucoma such as logistic difficulties and possible misdiagnosis.
3.1.2. Teleglaucoma for disease management
We are unable to identify articles that discussed the cost-effectiveness of glaucoma management; however, we identified an article which evaluated the time effectiveness of telegalucoma management.
In the study by Arora and coworkers, patients with early-stage glaucoma were either managed by teleglaucoma or in-person care. The mean access time (as defined as the duration from optometrists' date of referral to the date of diagnostic testing for glaucoma) was 45 ± 22 days in the teleglaucoma group, significantly lower than 88 ± 47 days in the in-person assessment group (P < 0.0001). (3) The same study also measured the time to the third next appointment (TNA). TNA was chosen as it was believed to better reflect system availability than the first or second appointment. In the teleglaucoma group the time to the TNA was significantly shorter (53 ± 12 days) when compared with that of the in-person pathway (192 ± 41 days) (P < 0.0001). Regarding the cycle time (as defined as the time used in consultation, from registration until departure), teleglaucoma group was also shorter (78 ± 20 minutes) than the in-person group (115 ± 44 minutes). Interestingly, only 19 ± 13% of the cycle time was waiting time for patients seen through teleglaucoma, when compared to 41 ± 24% for in-person assessments (P < 0.01). Therefore, it suggests that the patient's time can be better utilized when they are seen with teleglaucoma, and more timely management could be arranged for them.
4. Patient satisfaction
Patient satisfaction for teleglaucoma was addressed in 3 articles, and the overall comment was positive. (3,9,14) Aroroa and coworkers reported a mean satisfaction score of 4.67 ± 0.5 (out of 5) regarding the model and quality of teleglaucoma services they received (3). Similar positive responses were observed in the Philadelphia Study, with 82.2% and 85.4% of patients in 2 centers believing that the screening location for glaucoma to be very convenient (9). 97.8% and 94.9% of patients in these 2 centers claimed that they were very likely to return to the same location for future eye care visits. 100% of the patients were either satisfied or very satisfied with the eye screening, with 85% saying that they would recommend the eye-screening program to a friend or family member. (14)
5. Agreement between teleglaucoma and in-person care
Three studies have investigated the agreement between teleglaucoma and in-person care (9,13,22).
5.1. Teleglaucoma for screening
In Kiage and coworker's study, slit lamp examination was performed by comprehensive ophthalmologists, whereas teleglaucoma assessment was done by glaucoma specialists on 309 patients (22). The study investigated the Cohen's k score agreement of vertical cup disc ratio between teleglaucoma analysis and slit lamp examination. The agreement between the 2 examinations was moderate, 0.55. For focal glaucoma damage, the agreement between the 2 groups in terms of notching, peripapillary atrophy and disc hemorrhage was found to be fair (0.31, 0.24, and 0.25 respectively). The diagnostic precision of frequency doubling technology (FDT) to detect glaucoma in patients with glaucomatous disc damage was found to be in substantial agreement (0.84) (22). Another study performed by Gupta and coworkers demonstrated that the agreement between teleophthalmology and in clinic assessment was moderate for the diagnosis of glaucoma. The k value for glaucoma diagnosis was found to be 0.52 (13).
5.2. Teleglaucoma for management
Clarke and coworkers compared both interobserver and intraobserver agreement in clinical decisions of glaucoma status in face-to-face consultation versus teleglaucoma (9). Patients with poor mobility, poor visual field, or poor quality optic disc imaging were excluded. The k statistic for interobserver agreement was found to be 0.320 among both senior trainees and consultants when comparing face-to-face with virtual assessment, which is a fair agreement. For the interobserver agreement between consultants, the results showed a moderate agreement between face to face and virtual clinic (k statistic 0.406). For Intraobserver agreement, the agreement was found to be 0.274 for consultant and 0.264 for fellow trainees; however, 7 unstable patients were misclassified as stable by virtual review. This resulted in adverse disagreement, accounted for 3.4% (9). For the study performed by Gupta and coworkers, a moderate level of agreement in terms of management decisions was achieved, which a k value of 0.53 (13).
6. Specificity and sensitivity for teleglaucoma versus in-person clinic
6.1. Teleglaucoma for screening
Two studies demonstrated the sensitivity and specificity of the use of teleglaucoma for glaucoma detection. Gupta and coworkers showed the sensitivity and specificity of teleophthalmology for glaucoma diagnosis were 72.1 and 81.82, respectively (13). The study performed by Kiage and coworkers demonstrated the sensitivity and specificity for the diagnosis of glaucoma by teleglaucoma when compared to clinical examination, was 41.3% and 77.5%, respectively (22). It also showed that the positive predictive value and negative predicative value were 77.5% and 82.2% respectively. Both studies showed that the specificity is fair at around 80%, but the sensitivity for diagnosis of glaucoma is variable.
6.2. Teleglaucoma for management
Gupta and coworkers showed the sensitivity and specificity for teleophthalmology for glaucoma management decisions were 79.08 and 77, respectively. (13) Clarke and coworkers demonstrated that the sensitivity and specificity of virtual clinics for glaucoma remote virtual clinic decisions and clinical management decisions performed by consultants and senior trainees were 50% and 91.6%, respectively. (9) The sensitivity and specificity between virtual clinic decisions and clinical management decisions performed by consultants only were found to have a better result for sensitivity, 75% and 89.1%, respectively. A total of 3.4% of patients with glaucoma progression identified in an in-person clinic were misdiagnosed as stable in a virtual clinic. The 2 studies showed that teleglaucoma seems to have a fair specificity in assessing disease condition and progression, but the sensitivity was questionable.
Two patients with advanced visual field loss were found to have significant disagreement between face to face clinic and virtual clinic. (9) They were classified as significant misclassification events for virtual clinic, accounted for 1.9% of the cases. Trabeculectomy bleb leak, nonsight threatening anterior segment disorders could not be identified in the virtual clinic.
Clarke and coworkers also suggested that, with the low rate of significant misclassification, the slowly progressing nature of most glaucoma, and regular reassessment virtual clinics are a safe option for selected patients or glaucoma follow-up (9).
7. Current use and opportunities of teleglaucoma
Telemedicine in ophthalmology is now at its initial stage and not yet widely accepted as standard glaucoma management (27). Teleglaucoma has been used as a screening tool or for follow up in some parts of the world such as Alberta, Canada (34). The majority of telescreening of glaucoma includes using portable or handheld cameras for optic nerve photographs. Some other tools such as tonometers may be able to improve the accuracy of teleglaucoma diagnosis. The study by Kassam and coworkers described teleglaucoma use as a screening tool at Aga Khan University Hospital in Nairobi using mobile units equipped with a fundus camera and visual field machine. The study also suggested that teleglaucoma could improve the diagnosis and management of glaucoma in industrialized and developing countries (20). Staffieri and coworkers recommended that telemedicine can be used for screening for glaucoma in targeted patients with first degree relatives with primary open-angle glaucoma and increases the chances of identifing individuals with high risk of glaucoma or undiagnosed glaucoma. (32) It can also be used to reduce the chances of patients treated or followed up unnecessarily and decrease the demand for glaucoma appointments (36).
8. Challenges and limitations of teleglaucoma
First of all, from the ophthalmologist's perspective, the information provided during teleconsultation was highly variable (20). In conventional in-person consultation, this problem is less relevant as the glaucoma specialist can collect the information he/she wants by directly asking or examining the patients. With teleglaucoma, the diagnosis made by glaucoma specialists is entirely dependent on the information provided by the referrers. This is affected by the clinical skills of the referrers and the quality of diagnostic equipment in local centers. This problem was also mentioned by Shaw as 59% of ophthalmologists had “low confidence” in their ability to make clinical decisions solely based on ophthalmic images.(13)
In addition, the development of teleglaucoma may be limited by technical factors, including the bandwidth and storage of telecommunication devices. This makes the practice of teleglaucoma very difficult in developing countries. The high cost of ophthalmic imaging equipment and other hardware is another limiting factor as a retinal camera can cost over USD$10,000.
Moreover, practicing teleglaucoma in the community setting requires the primary care clinics to perform additional tasks to ensure patient compliance with recommendations from glaucoma specialists. (27) This may pose additional pressure to primary care units and affect treatment efficacy owing to less frequent follow-up to specialist clinics.
9. Discussion
As shown in various studies (3,15,27,33), teleglaucoma can significantly reduce the time and money costs of glaucoma consultation and requires less monetary cost for each QALY gained in both short-term and long-term analyses. Teleglaucoma management, however, was found to be variable, with suboptimal specificity and sensitivity. Clarke and coworkers found an unacceptably low sensitivity of only 50% between virtual clinic decisions and clinical management decisions performed by senior trainees and consultants. (9) When performed by consultants only there was a much better sensitivity of 75%, but similar specificity of 89.1%. Ideally the sensitivity for screening should be as high as possible, and a sensitivity of 41% would be too low for teleglaucoma to be implemented for screening. The sensitivity for the screening should be high even with relatively simple tests. Although expensive equipment may increase sensitivity and specificity, this would not be cost effective. Another way of improving sensitivity is only performing the screening test in high-risk populations, such as those with known family history of glaucoma, elderly patients, those with a history of pseudoexfoliation, chronic steroid use, and high IOP.
As glaucoma is a disease that results in damage to optic nerve and irreversible blindness, better specificity and sensitivity should be ensured when using teleglaucoma for disease management. Methods to improve the specificity and sensitivity of teleglaucoma for management should be explored to enhance safety. In order to apply teleglaucoma to disease management, measures may include examination of fundus images, visual field testing, intraocular pressure, visual acuity, corneal thickness, OCT, slit lamp examination and questionnaires on patient symptoms. It should be noted that tele-slit lamp examination may have a higher chance of error as skill is required for good quality examinations. The problem of low sensitivity might be mitigated by employing experienced ophthalmologists. Clarke and coworkers (9) demonstrated that experienced ophthalmologists have better agreement between teleglaucoma and inpatient clinics. Furthermore, the employment of devices with higher resolution to image the optic disc and OCT, or even using artificial intelligence to grade fundus photographs and OCT, might further improve sensitivity and specificity. (10,38) These, however, may increase the cost of teleglaucoma. The cost of improving sensitivity and specificity should be evaluated by more studies.
It is important to note that a significant proportion (17.1%) of patients who underwent teleglaucoma screening had unreadable fundus images. (17) This group of patients is advised to have ophthalmologic investigations as a high proportion was later diagnosed with different ocular pathologies such as cataract in the Philadelphia Telemedicine Glaucoma Detection Study. This also shows that the investigations used in teleglaucoma programs can also be used for screening and monitoring of other ocular conditions such as cataract and macular degeneration. Thus, the benefits of teleglaucoma are not just limited to better glaucoma outcome, but may also enhance the overall ocular health by diagnosing or monitoring concurrent eye diseases.
Teleglaucoma mainly serves 2 purposes, namely screening of high-risk underserved individuals and management of existing glaucoma patients. With regard to glaucoma screening, its role in monitoring ‘glaucoma suspects’ was discussed by Straffier and coworkers (32) In general, “glaucoma suspect” refers to patients with clinical signs consistent with, but not diagnostic of, glaucoma. These may include increased cup-disc ratio (CDR), elevated IOP (>21 mm Hg), thin corneas, visual field impairment, and/or ocular hypertension, but without no visual field or retinal nerve fiber layer thinning. This group of patients usually requires no active treatment, but regular monitoring is required. Face-to-face consultation may pose additional disease burden being time consuming and requiring higher consultation fees. The complicated in-person consultation may also discourage regular follow-up. Straffier and coworkers pointed out that applying teleglaucoma for screening of ‘glaucoma suspects’ is more cost effective and can yield more positive results (32). Using a glaucoma screening program, Mitchell and coworkers found that 3–5% of a population over 40 had glaucoma (26). Straffier and coworkers detected glaucoma in 11% of patients in a selected high-risk population (32). Our review showed that teleglaucoma is more cost effective, results in high patient satisfaction, and has moderate agreement with in-person care if it is conducted by senior ophthalmologists. It would be worthwhile to explore the possible application of teleglaucoma for the follow-up of ‘glaucoma suspects. After the initial screening, newly diagnosed cases of glaucoma could either undergo further evaluation with telemedicine or they could be referred to ophthalmologists for in-person care. The follow-up would be dependent on the availability of local resource and patient preference. Regarding glaucoma management, patients with advanced glaucoma may benefit from teleglaucoma as this may allow more frequent IOP and visual acuity monitoring. As a result, ophthalmologists gain a better understanding of their disease status and more accurately assess the effectiveness of their current glaucoma medication. Teleglaucoma may also help patients with early glaucoma as their disease management is often straightforward and does not require much input from glaucoma specialists. In most countries, the major expense associated with glaucoma management is the cost of experienced ophthalmologists. If technology could replace some of the tedious work of the glaucoma specialists in looking after glaucoma suspects or patients with early glaucoma, they could spend more time in managing difficult cases. Teleglaucoma may be important in more remote areas where in-person care by glaucoma specialists is not readily available.
Almost all of the ocular investigations of the teleglaucoma programs in these studies were conducted by technicians in clinics; however, it is important to note that there are even more convenient forms of teleglaucoma with the emergence of home tonometry, and fundus photographs and perimetry on mobile phones. For instance, home tonometer was approved by FDA for clinical uses in 2017. This user-friendly device can yield extra information about IOP variability and allow more timely assessment of glaucoma progression. Moreover, home testing can significantly reduce the travelling and waiting time in face-to-face clinics. It is believed that further development and extensive uses of such devices can facilitate the monitoring and management of glaucoma patients. Hopefully in the future, home tonometry can be made available at an affordable cost, and play a similar role as continuous blood glucose monitoring does in diabetic patients
Disease burden is a major concern when using teleglaucoma in either screening or management. Teleglaucoma for screening may increase the disease burden to the society initially because more individuals with glaucoma would be picked up and require long-term follow up; however, early diagnosis of glaucoma may improve treatment outcome. With less impaired vision in glaucoma patients, this reduces the burden to society in the long run. Cost is one of the concerns, but we believe that a person should not be deprived of the chance to be diagnosed and managed early. We believe that, although universal screening might not be practical and beneficial, high risk individuals should be included. Screening should be targeted to maximize its cost effectiveness, Burr and coworkers suggest that targeted screening of high-risk groups such as those with positive family history or black ethnicity would be more appropriate than population screening. (7)
During the COVID-19 outbreak, many nonemergent hospital services are suspended to avoid disease transmission. (1,4,11,24,37). With regard to the possible chances of human to human transmission, the recent American Academye of Ophthalmology guidelines recommend outpatient centers should reduce the number of outpatient consultations days. (8) Telemedicine may be used to reduce the number of outpatients in clinics and decrease the chance of disease transmission. In remote regions, glaucoma tests can be performed by trained technicians in local clinics and sent electronically to glaucoma specialists.
Despite various advantages and uses of telemedicine, face-to-face consultation still is important in glaucoma monitoring. Management of glaucoma requires an integration of testing and patient preference. In-person care is essential especially for monitoring patients with poor drug compliance and side effects from glaucoma medications.
10. Conclusion
Teleglaucoma is more cost effective than in-person care, shows high patient satisfaction, and has fair to good agreement with in-person care. Teleglaucoma management may allow better utilization of resources and while not compromising outcomes. Further studies are required to evaluate the long term cost-effectiveness of teleglaucoma. The sensitivity and specificity of teleglaucoma screening and management needs to be further enhanced before it can be put into extensive use. Nevertheless, it is worthwhile to explore the application of teleglaucoma in monitoring “glaucoma suspects” and maintaining glaucoma follow-up in selected patients with mild and stable disease or for those who require frequent monitoring.
11. Method of literature search
11.1. Eligibility criteria
We searched for human studies published in 10 years, in English language, which discussed the use of telemedicine in glaucoma monitoring and compared the outcomes (in terms of cost-effectiveness, patient satisfaction, agreement of diagnosis, specificity and sensitivity) with traditional in-person care.
11.2. Information sources
The literature search was performed on PubMed by 2 of the authors (PYL and SCC) on 12 May 2020.
11.3. Search strategy
Search terms used were: (("telemedicine"[MeSH Terms] OR "telemedicine"[All Fields]) OR "telemedicine s"[All Fields]) AND ((("glaucoma"[MeSH Terms] OR "glaucoma"[All Fields]) OR "glaucomas"[All Fields])). The filters applied included human studies, 10 years, full text and English language.
11.4. Study selection
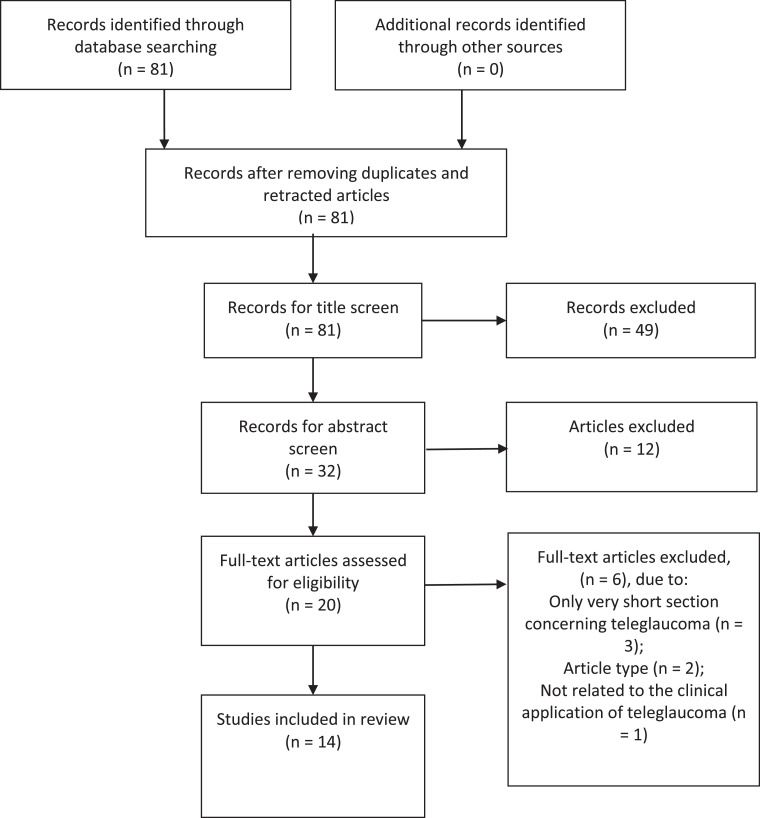
A total of 81 articles were yielded with the aforementioned search strategy. Two authors (PYL and SCC) then selected the papers independently. Papers were screened for eligibility by title and, if necessary, by examining the abstract. Only a few discrepancies in the selections of the 2 authors occurred. These were discussed and consensus was reached in line with our search criteria. Figure 1 describes the selection process for identified studies.
Fig. 1.
PRISMA flowchart illustrating selection process of articles
11.5. Data analysis
The following information was extracted from the selected articles independently by the 2 authors: year of publication, study type, study aim, sample size and overall findings.
Declaration of competing interests
(Not applicable)
Acknowledgments
Acknowledgements
Nil.
Declarations: Funding
(Not applicable)
Availability of data and material
(Not applicable)
Code availability
(Not applicable)
Author contributions
Idea for the article:
Bonnie Nga Kwan Choy
Literature search and data analysis:
Shing Chuen Chow, Pun Yuet Lam
Drafting and revision of work:
Shing Chuen Chow, Pun Yuet Lam, Bonnie Nga Kwan Choy, Jimmy Shiu Ming Lai
References
- 1.Ankita KA, Saxena S.K. In: Coronavirus Disease 2019 (COVID-19) Saxena S., editor. Medical Virology: From Pathogenesis to Disease Control [Internet]; 2020. An Ophthalmological Update. [Google Scholar]
- 2.Aref AA, Gedde SJ, Budenz DL. Glaucoma Drainage Implant Surgery. Dev Ophthalmol. 2017;59:43–52. doi: 10.1159/000458485. [DOI] [PubMed] [Google Scholar]
- 3.Arora S, Rudnisky CJ, Damji KF. Improved access and cycle time with an "in-house" patient-centered teleglaucoma program versus traditional in-person assessment. Telemed J E Health. 2014;20(5):439–445. doi: 10.1089/tmj.2013.0241. [DOI] [PubMed] [Google Scholar]
- 4.Awadasseid A, Wu Y, Tanaka Y, Zhang W. Initial success in the identification and management of the coronavirus disease 2019 (COVID-19) indicates human-to-human transmission in Wuhan, China. Int J Biol Sci. 2020;16(11):1846–1860. doi: 10.7150/ijbs.45018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bluwol E. [Glaucoma treatment] Rev Prat. 2016;66(5):508–513. [PubMed] [Google Scholar]
- 6.Bol P. [Glaucoma] Ned Tijdschr Tandheelkd. 2003;110(7):298–299. [PubMed] [Google Scholar]
- 7.Burr JM, Mowatt G, Hernández R, Siddiqui MA, Cook J, Lourenco T, et al. The clinical effectiveness and cost-effectiveness of screening for open angle glaucoma: a systematic review and economic evaluation. Health Technol Assess. 2007;11(41):1–190. doi: 10.3310/hta11410. iii-iv, ix-x. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention WHO. Important coronavirus updates for ophthalmologists 2020
- 9.Clarke J, Puertas R, Kotecha A, Foster PJ, Barton K. Virtual clinics in glaucoma care: face-to-face versus remote decision-making. Br J Ophthalmol. 2017;101(7):892–895. doi: 10.1136/bjophthalmol-2016-308993. [DOI] [PubMed] [Google Scholar]
- 10.Devalla SK, Liang Z, Pham TH, Boote C, Strouthidis NG, Thiery AH, et al. Glaucoma management in the era of artificial intelligence. Br J Ophthalmol. 2020;104(3):301–311. doi: 10.1136/bjophthalmol-2019-315016. [DOI] [PubMed] [Google Scholar]
- 11.Eslami H, Jalili M. The role of environmental factors to transmission of SARS-CoV-2 (COVID-19) AMB Express. 2020;10(1):92. doi: 10.1186/s13568-020-01028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flammer J, Orgül S, Costa VP, Orzalesi N, Krieglstein GK, Serra LM, et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 2002;21(4):359–393. doi: 10.1016/s1350-9462(02)00008-3. [DOI] [PubMed] [Google Scholar]
- 13.Gupta SC, Sinha SK, Dagar AB. Evaluation of the effectiveness of diagnostic & management decision by teleophthalmology using indigenous equipment in comparison with in-clinic assessment of patients. Indian J Med Res. 2013;138(4):531–535. [PMC free article] [PubMed] [Google Scholar]
- 14.Hark L, Acito M, Adeghate J, Henderer J, Okudolo J, Malik K, et al. Philadelphia telemedicine glaucoma detection and follow-up study: ocular findings at two health centers. J Health Care Poor Underserved. 2018;29(4):1400–1415. doi: 10.1353/hpu.2018.0103. [DOI] [PubMed] [Google Scholar]
- 15.Hark LA, Katz LJ, Myers JS, Waisbourd M, Johnson D, Pizzi LT, et al. Philadelphia telemedicine glaucoma detection and follow-up study: methods and screening results. Am J Ophthalmol. 2017;181:114–124. doi: 10.1016/j.ajo.2017.06.024. [DOI] [PubMed] [Google Scholar]
- 16.Hark LA, Myers JS, Pasquale LR, Razeghinejad MR, Maity A, Zhan T, et al. Philadelphia telemedicine glaucoma detection and follow-up study: intraocular pressure measurements found in a population at high risk for glaucoma. J Glaucoma. 2019;28(4):294–301. doi: 10.1097/IJG.0000000000001207. [DOI] [PubMed] [Google Scholar]
- 17.Hark LA, Myers JS, Rahmatnejad K, Wang Q, Zhan T, Hegarty SE, et al. Philadelphia telemedicine glaucoma detection and follow-up study: analysis of unreadable fundus images. J Glaucoma. 2018;27(11):999–1008. doi: 10.1097/IJG.0000000000001082. [DOI] [PubMed] [Google Scholar]
- 18.Holekamp NM. Moving from clinic to home: what the future holds for ophthalmic telemedicine. Am J Ophthalmol. 2018;187:xxviii–xxxxxv. doi: 10.1016/j.ajo.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Infeld DA, O'Shea JG. Glaucoma: diagnosis and management. Postgrad Med J. 1998;74(878):709–715. doi: 10.1136/pgmj.74.878.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kassam F, Amin S, Sogbesan E, Damji KF. The use of teleglaucoma at the University of Alberta. J Telemed Telecare. 2012;18(7):367–373. doi: 10.1258/jtt.2012.120313. [DOI] [PubMed] [Google Scholar]
- 21.Kassam F, Yogesan K, Sogbesan E, Pasquale LR, Damji KF. Teleglaucoma: improving access and efficiency for glaucoma care. Middle East Afr J Ophthalmol. 2013;20(2):142–149. doi: 10.4103/0974-9233.110619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiage D, Kherani IN, Gichuhi S, Damji KF, Nyenze M. The Muranga Teleophthalmology Study: comparison of virtual (teleglaucoma) with in-person clinical assessment to diagnose glaucoma. Middle East Afr J Ophthalmol. 2013;20(2):150–157. doi: 10.4103/0974-9233.110604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kyari F, Abdull MM, Bastawrous A, Gilbert CE, Faal H. Epidemiology of glaucoma in sub-saharan Africa: prevalence, incidence and risk factors. Middle East Afr J Ophthalmol. 2013;20(2):111–125. doi: 10.4103/0974-9233.110605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int J Antimicrob Agents. 2020;55(3) doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maggio F. Glaucoma. Top Companion Anim Med. 2015;30(3):86–96. doi: 10.1053/j.tcam.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell P, Smith W, Attebo K, Healey PR. Prevalence of open-angle glaucoma in Australia. The Blue Mountains Eye Study. Ophthalmology. 1996;103(10):1661–1669. doi: 10.1016/s0161-6420(96)30449-1. [DOI] [PubMed] [Google Scholar]
- 27.Rathi S, Tsui E, Mehta N, Zahid S, Schuman JS. The current state of teleophthalmology in the United States. Ophthalmology. 2017;124(12):1729–1734. doi: 10.1016/j.ophtha.2017.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharafeldin N, Kawaguchi A, Sundaram A, Campbell S, Rudnisky C, Weis E, et al. Review of economic evaluations of teleophthalmology as a screening strategy for chronic eye disease in adults. Br J Ophthalmol. 2018;102(11):1485–1491. doi: 10.1136/bjophthalmol-2017-311452. [DOI] [PubMed] [Google Scholar]
- 29.Shaw J. Teleophthalmology: Ready for Prime Time? EyeNet Magazine. 2016.
- 30.Sim DH. Glaucoma update–what physicians and the public need to know. Singapore Med J. 1999;40(4):317–320. [PubMed] [Google Scholar]
- 31.Sivalingam E. Glaucoma: an overview. J Ophthalmic Nurs Technol. 1996;15(1):15–18. [PubMed] [Google Scholar]
- 32.Staffieri SE, Ruddle JB, Kearns LS, Barbour JM, Edwards TL, Paul P, et al. Telemedicine model to prevent blindness from familial glaucoma. Clin Exp Ophthalmol. 2011;39(8):760–765. doi: 10.1111/j.1442-9071.2011.02556.x. [DOI] [PubMed] [Google Scholar]
- 33.Thomas S, Hodge W, Malvankar-Mehta M. The cost-effectiveness analysis of teleglaucoma screening device. PLoS One. 2015;10(9) doi: 10.1371/journal.pone.0137913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verma S, Arora S, Kassam F, Edwards MC, Damji KF. Northern Alberta remote teleglaucoma program: clinical outcomes and patient disposition. Can J Ophthalmol. 2014;49(2):135–140. doi: 10.1016/j.jcjo.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 35.Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311(18):1901–1911. doi: 10.1001/jama.2014.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wright HR, Diamond JP. Service innovation in glaucoma management: using a web-based electronic patient record to facilitate virtual specialist supervision of a shared care glaucoma programme. Br J Ophthalmol. 2015;99(3):313–317. doi: 10.1136/bjophthalmol-2014-305588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xia J, Tong J, Liu M, Shen Y, Guo D. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J Med Virol. 2020;92(6):589–594. doi: 10.1002/jmv.25725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng C, Johnson TV, Garg A, Boland MV. Artificial intelligence in glaucoma. Curr Opin Ophthalmol. 2019;30(2):97–103. doi: 10.1097/ICU.0000000000000552. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
(Not applicable)