Abstract
Encephalopathy is one of the most frequent neurological complications of severe Coronavirus Disease 2019 (COVID-19) patients. Cytokine storm and sepsis, hypercatabolic states, the use of furosemide and dialytic therapy represent risk factors for thiamine deficiency and are also found in patients with severe COVID-19. In this retrospective case series, we report clinical and neurological findings of fifteen patients with COVID-19-associated Wernicke Encephalopathy (WE) and their response to treatment with intravenous thiamine. All patients had encephalopathy, with 67% displaying at least one additional sign of classic WE triad (ophthalmoparesis and ataxia). Two patients (13%) had the classic triad. All COVID-19 patients had significant improvement of the neurological manifestations between two to five days after intravenous thiamine administration. Eleven patients (73%) had good neurological outcome at hospital discharge and only two patients (13%) died. This case series suggests that thiamine deficiency may be an etiology of encephalopathy in severe COVID-19 patients and its treatment may represent a safety and low-cost response to reduce the neurological burden.
Keywords: Wernicke encephalopathy, COVID-19, Thiamine, Encephalopathy, Delirium, Acute respiratory distress syndrome (ARDS)
1. Introduction
Encephalopathy is one of the most frequent neurological complications in Coronavirus Disease (2019) (COVID-19) patients with Acute Respiratory Distress Syndrome (ARDS), having a prevalence varying between from 60 to 84% of the cases (Helms et al., 2020; Liotta et al., 2020). It is a clinical manifestation of acute brain dysfunction, characterized by delirium, torpor and, in more severe cases, coma (Helms et al., 2020). The occurrence of encephalopathy in COVID-19 patients is associated with higher morbidity, mortality, worse neurological outcomes and an estimated three-fold increase in the hospital length of stay (LOS) (Liotta et al., 2020). Recent studies with severe COVID-19 patients have revealed anatomopathological findings of hypoxic injury and microhemorrhage in central nervous system (CNS) without evidence of direct viral effect (Solomon et al., 2020). Evaluation of cerebrospinal fluid in this group of patients also failed to document viral infection (Edén et al., 2021). Although encephalopathy has a multifactorial etiology, in the majority of patients with COVID-19 its etiology remains unknown (Ghannan et al., 2020). Hypercatabolic states, such as cytokine storm and sepsis, in addition to the use of furosemide and dialytic therapy represent risk factors for thiamine deficiency (TD) (Moskowitz et al., 2020). Sepsis can be a heralding sign of TD (Fattal-Valevski A et al., 2005). Moreover, severe infection is commonly observed in the encephalopathic phase of TD (Wijnia JW et al., 2016). Wernicke encephalopathy (WE) is a condition associated with TD, characterized by encephalopathy, opthalmoparesis and ataxia. Due to the absence of the full clinical triad in majority of patients and the low sensibility of laboratory work-up of thiamine dosage, WE is an underrecognized disease (Sinha et al., 2019). Recently we have described the first three cases of WE in severe COVID-19 patients (Branco de Oliveira et al., 2021). Here, we provide a description of clinical and neurological characteristics of consecutive patients diagnosed with COVID-19-associated WE and their response to treatment with intravenous thiamine.
2. Methods
In this retrospective single center case series, a hospital-wide search was performed using the electronic medical records for all patients who underwent mechanical ventilation due to ARDS secondary to COVID-19 from April 1, 2020 to November 30, 2020 in a tertiary hospital of Sao Paulo State, Brazil. The inclusion criteria were as follows: 1) Reverse transcription-PCR (RT-PCR) positive for Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in oropharynx and nasopharynx samples prior to encephalopathy onset; 2) diagnosis of COVID-19-associated WE made after complete neurological examination and brain images studies; and 3) having undergone intravenous thiamine replacement for the treatment of encephalopathy. The dosage of thiamine used was 500 mg three times a day for five days. All patients were evaluated and followed by a team of neurologists with at least ten years of experience. The exclusion criteria were: 1) diagnosis of encephalopathy with a more likely etiology than thiamine deficiency, such as stroke, hypoxic-ischemic encephalopathy after prolonged cardiac arrest and hepatic encephalopathy; and 2) absence of evaluation and follow-up by a neurologist during hospitalization. The occurrence of comorbidities was searched in electronic medical records. The modified Rankin scale (mRS) was applied at hospital discharge for evaluation of the functional neurological outcomes.
3. Results
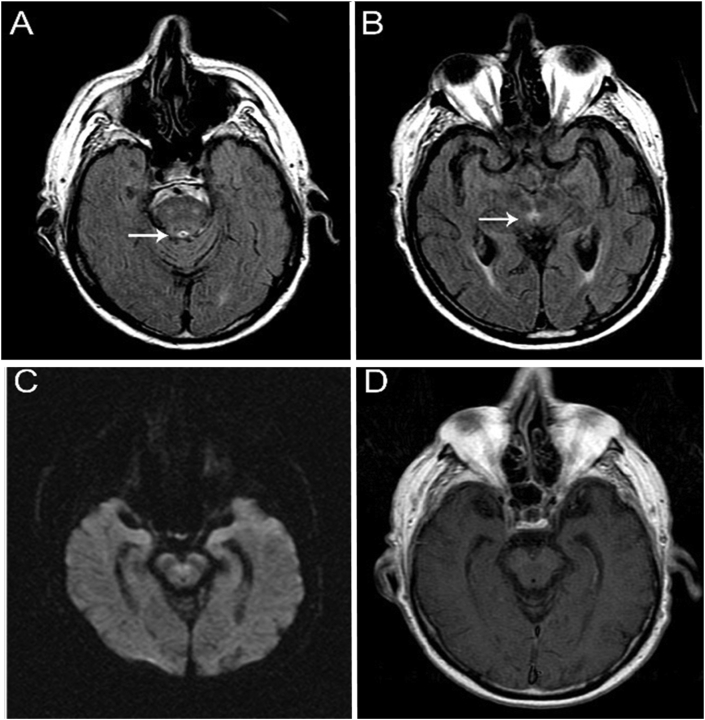
A total of 15 severe COVID-19 patients met the study inclusion criteria. All patients developed encephalopathy and other neurological and psychiatric findings, such as hallucination, confabulation, ophthalmoparesis, and ataxia, without spontaneous improvement for at least two days after removing of the sedatives. Table 1 depicts the patient’s clinical and neurological findings. The mean age was 60 years (46–86), with a 67% male predominance. The comorbidities found in the sample patients were systemic hypertension (47%), obesity (33%), coronary artery disease (33%), diabetes mellitus (33%), dyslipidemia (33%), hypothyroidism (13%), hypoparathyroidism (7%), obstructive sleep apnea (7%), bronchial asthma (7%), bariatric surgery (7%), myasthenia gravis (7%), anxiety disorder (7%) and insomnia disorder (7%). Although classically associated with TD, long-term alcohol use was not reported. The average length of hospitalization until WE diagnosis was 17 days. Furthermore, 86% of patients had acute renal injury, while 33% underwent hemodialysis. Sepsis and the use of furosemide were common risk factors in all 15 cases. Confabulation and visual hallucination were observed in 33% and 26% of patients, respectively. Six patients (40%) had ataxia, including truncal, appendicular and gait. Ophthalmoparesis, neuropathic pain and dysautonomia were found in 33%, 13% and 6.5% of patients, respectively. Two patients (13%) had the classic clinical triad of WE (encephalopathy, ophthalmoparesis and ataxia) and eight (54%) had encephalopathy associated with other classic symptom. Five (33%) had only encephalopathy. All patients were submitted to brain imaging, 12 of whom were assessed by computed tomography (CT) and 3 by magnetic resonance imaging (MRI) scans. Electroencephalogram (EEG) was performed in all patients. All CT scans and two of the three MRI depicted no abnormalities. One MRI revealed an abnormal high signal in FLAIR image in periaqueductal grey matter PAG (Fig. 1), a typical radiological finding of WE. In all patients, EEG showed diffuse slowing of rhythms in the theta-delta range and no epileptiform abnormalities. When performed, cerebrospinal fluid analysis was normal, without pleocytosis, abnormal protein level or elevated IgG index.
Table 1.
Neurological manifestations, risk factors for thiamine deficiency and outcomes in the patients with Covid-19 and Wernicke Encephalopathy.
| Patient (sex,age)a | Neurological Manifestation | Hospitalization (days) | AKIb | Diuretics | Hemodialysis | Sepsis | mRSc |
|---|---|---|---|---|---|---|---|
| 1 (M,52) | Encephalopathyd, ophthalmoparesis | 33 | + | + | + | + | 2 |
| 2 (F,47) | Encephalopathy, ophthalmoparesis, hallucination, ataxia, neuropathic pain | 15 | + | + | – | + | 2 |
| 3 (M,51) | Encephalopathy,ataxia, dysautonomia | 27 | + | + | + | + | 2 |
| 4 (F,86) | Encephalopathy, ophthalmoparesis | 15 | + | + | + | + | 6 (died) |
| 5 (M,77) | Encephalopathy, ophthalmoparesis | 21 | + | + | + | + | 6 (died) |
| 6 (F,75) | Encephalopathy, delusions, confabulation | 14 | + | + | – | + | – |
| 7 (M,56) | Encephalopathy, confabulation, ataxia, neuropathic pain | 14 | + | + | – | + | 2 |
| 8 (M,46) | Encephalopathy, ataxia, confabulation, hallucination | 18 | + | + | – | + | 2 |
| 9 (M,42) | Encephalopathy | 18 | + | + | + | + | 1 |
| 10 (F,62) | Encephalopathy | 6 | – | + | – | + | 2 |
| 11 (M,50) | Encephalopathy, ophthalmoparesis, confabulation, delusions, hallucinations, ataxia | 10 | – | + | – | + | 2 |
| 12 (M,63) | Encephalopathy | 34 | + | + | – | + | 5 |
| 13 (F,63) | Encephalopathy | 11 | + | + | – | + | 2 |
| 14 (M,64) | Encephalopathy | 9 | + | + | – | + | 0 |
| 15 (M,66) | Encephalopathy, ataxia, confabulation, hallucination | 9 | + | + | – | + | 2 |
M:male; F:female; age: years.
AKI: acute kidney injury.
Modified Rankin Scale (0-no symptoms, 1-no significant disability, despite symptoms, able to perform all usual duties and activities, 2-slight disability, unable to perform all previous activities, but able to look after own affairs, without assistance, 3-moderate disability, requires some help, but able to walk without assistance, 4-moderately severe disability, unable to walk without assistance and unable to attend to own bodily needs without assistance, 5-severe disability, bedrriden, incontinent and requires constant nursing care and attention.
Encephalopathy: brain dysfunction associated with delirium, drowsiness, torpor or coma.
Fig. 1.
MRI scan showing FLAIR images with increased signal intensity in periaqueductal grey matter (A and B,white arrows). There was no restricted diffusion in DWI (C) and also no enhancement in T1 weighted image post-contrast (D).
All patients showed significant improvement of the neurological manifestations between two to five days after the intravenous thiamine replacement. In addition to promoting improvement of level and content of consciousness, treatment with thiamine led to a resolution of ophthalmoparesis, gait and appendicular ataxia and neuropathic pain. The mRS results revealed that eleven patients (73%) were independent for basic daily life activities (mRS 0–2) and two patients (13%) died (mRS 6) due to decompensation of their clinical status and comorbidities.
4. Discussion
Although encephalopathy is a frequent neurological complication of SARS-Cov-2 infection, its etiology remains misunderstood. Case series describing neuropathologic features and cerebrospinal fluid biomarkers in severe COVID-19 patients have failed to prove the direct viral effect or an immune-mediated encephalitis (Solomon et al., 2020; Edén et al., 2021). In the current case series, we report fifteen patients with severe COVID-19 and critical ill encephalopathy diagnosed after removing of the sedatives. Most patients had encephalopathy associated with another classical signal of WE, and only 13% had the full clinical triad, such as ophthalmoparesis, ataxia and encephalopathy, similarly to what has been reported in previous studies (Sinha et al., 2019). In one of the three brain MRI performed was observed a typical finding of WE. The presence of several risk factors for TD and the immediate neurological improvement with high dose intravenous thiamine, as observed in classic descriptions of WE treatment, reinforce the plausibility of the TD as a possible etiology for encephalopathy in COVID-19 patients. The thiamine-induced neurological improvement allowed the acceleration of weaning from ventilation, likely reducing the systemics complications as ventilator-associated pneumonia and recurrence of sepsis. Despite the severe cases reported, most of our patients were walking and independent to basic life activities at discharge. Moreover, the mortality rate was low (13%) than observed in severe compromised COVID-19 group of patients described in previous studies (Liotta el. 2020; Suleyman et al., 2020). Liotta et al. (2020) observed that only 32.1% of COVID-19 patients with encephalopathy (with or without ARDS) had good functional outcome (mRS 0–2) at hospital discharge. In this study, the mortality rate was 20% even with the inclusion of less severe patients than current case series. Another previous investigation with severe COVID-19 patients needing treatment in ICU reported 40% of mortality (Suleyman et al., 2020).
WE diagnosis is challenging, with only 15% of cases diagnosed before death (Sinha et al., 2019). Classic clinical triad (encephalopathy, ophthalmoparesis and ataxia) occurs in only 10% of the patients (Sinha et al., 2019). Brain CT scan has only 10% of sensitivity for WE diagnosis and MRI, 50%, although 93% of specificity (Antunez et al., 1998). The typical MRI findings of WE are high signal in T2, Flair and DWI symmetrical images in mammillary bodies, medial thalamus, tectal plate and periaqueductal grey matter (Manzo et al., 2014). Atypical lesions may be observed in cerebral cortex, cerebellum and cranial nerve nuclei (Manzo et al., 2014). However, normal MRI do not exclude the WE diagnosis (Antunez et al., 1998). In one of our cases, the MRI evidenced an abnormal high signal in FLAIR image in periaqueductal grey matter (PAG). PAG has high concentration of a water channel protein, Aquaporine 4, expressed in the endfeet of astrocytes (Pittock et al., 2006).
Kanberg et al. (2020) proposed that astrocytic activation and injury are the first insult to central nervous system of moderate to severe COVID-19 patients, suggested by higher plasma concentration of glial fibrillary acidic protein (GFAp). Neuronal injury would occur later in the disease, evidenced by higher plasma concentration of neurofilament light chain (NfL) in patients with severe COVID-19 (Kanberg et al., 2020). Astrocytes seem to have a relevant role in the pathophysiology of TD (Afladal et al., 2014). Through impaired energy metabolism, the TD would promote abnormalities in the expression of key proteins of astrocytes, leading to the disruption of neuronal integrity. The measurement of plasma level of thiamine has been more often used in clinical practice. However, given that 80% of thiamine is in the erythrocytes, this measurement lacks sensitivity and specificity for WE diagnosis (Sinha et al., 2019). More accurate techniques as monophosphorylated and diphosphorylated thiamine dosage in red blood cells and measurement of erythrocyte transketolase activity are less available (Sinha et al., 2019). Thiamine has a key role as cofactor of pyruvato desidrogenase enzyme, contributing to the occurrence of mitochondrial phoporylative oxidation (Moskowitz el al., 2020). We hypothesized that depletion of thiamine would be promoted by high catabolic states associated with cytokine storm in critical COVID-19 patients. This condition could be aggravated by sepsis, furosemide and hemodialysis.
Our study has some limitations. The absence of a control group (not treated with thiamine) does not completely exclude the influence of others variables in addition to thiamine replacement in the improvement of neurological disturbances in the patients reported. This makes it essential to conduct a further clinical trial to better address the real impact of intravenous thiamine in the improvement of neurological outcomes and mortality in COVID-19 patients, as preliminarily suggested by our results. Another limitation is the absence of blood thiamine measurement in these patients. Finally, MRI was performed in only three patients due to the high risk of clinical decompensation in patient transport and long time spent in the examination.
The results reported in this case series suggest that thiamine replacement could have a beneficial effect in COVID-19-associated encephalopathy, improving functional outcomes and reducing mortality. Our findings also reinforce that the diagnosis of WE should be considered in patients with encephalopathy and risk factors for the depletion of thiamine, such as severe Covid-19 patients.
Funding
No targeted funding reported.
Author’s contribution
MVBO: conception of the manuscript, manuscript preoperation (writing of the first draw, review), including medical writing for content, clinical evaluation, diagnosis and treatment of the patient. DGB and MVMG: manuscript preparation, review and critique, including medical writing for content. SI and FHBL: manuscript preparation including review of manuscript and clinical evaluation of the patients. TI, RBI, TVCA and AMF manuscript preparation including review of manuscript. MS, VNO, VNS and DAGS: diagnosis and treatment of the patient.
Declaration of competing interest
None.
Acknowledgments
None.
References
- Afadlal S., Labetoulle R., Hazell A.S. Role of astrocytes in thiamine deficiency. Metab. Brain Dis. 2014 Dec;29(4):1061–1068. doi: 10.1007/s11011-014-9571-y. Epub 2014 Jun 15. PMID: 24929329. [DOI] [PubMed] [Google Scholar]
- Antunez E., Estruch R., Cardenal C., Nicolas J.M., Fernandez-Sola J., Urbano-Marquez A. Usefulness of CT and MR imaging in the diagnosis of acute Wernicke’s encephalopathy. AJR Am. J. Roentgenol. 1998;171(4):1131–1137. doi: 10.2214/ajr.171.4.9763009. [DOI] [PubMed] [Google Scholar]
- Branco de Oliveira M.V., Galera Bernabé D. Wernicke encephalopathy in COVID-19 patients: report of three cases. Front. Neurol. 2021;12 doi: 10.3389/fneur.2021.629.273. https://www.frontiersin.org/article/10.3389/fneur.2021.629273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edén A., Kanberg N., Gostner J., Fuchs D., Hagberg L., Andersson L.M., Lindh M., Price R.W., Zetterberg H., Gisslén M. CSF biomarkers in patients with COVID-19 and neurologic symptoms: a case series. Neurology. 2021;96(2):e294–e300. doi: 10.1212/WNL.0000000000010977. Epub 2020 Oct 1. PMID: 33004602. [DOI] [PubMed] [Google Scholar]
- Fattal-Valevski A. Out-break of life-threatening thiamine deficiency in infants in Israel caused by a defective soy-based formula. Pediatrics. 2005;115:e233–238. doi: 10.1542/peds.2004-1255. [DOI] [PubMed] [Google Scholar]
- Ghannam M., Alshaer Q., Al-Chalabi M., Zakarna L., Robertson J., Manousakis G. Neurological involvement of coronavirus disease 2019: a systematic review. J. Neurol. 2020;267(11):3135–3153. doi: 10.1007/s00415-020-09990-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms J., Kremer S., Merdji H. Neurologic features in severe SARS-CoV-2 infection. N. Engl. J. Med. 2020;382(23):2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanberg N., Ashton N.J., Andersson L.M., Yilmaz A., Lindh M., Nilsson S., Price R.W., Blennow K., Zetterberg H., Gisslén M. Neurochemical evidence of astrocytic and neuronal injury commonly found in COVID-19. Neurology. 2020;95(12):e1754–e1759. doi: 10.1212/WNL.0000000000010111. Epub 2020 Jun 16. PMID: 32546655. [DOI] [PubMed] [Google Scholar]
- Liotta E.M., Barra A., Clark J.R. Frequent neurologic manifestations and encephalopathy-associated morbidity in Covid-19 patients. Ann Clin Transl Neurol. 2020;7(11):2221–2230. doi: 10.1002/acn3.51210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzo G., De Gennaro A., Cozzolino A., Serino A., Fenza G., Manto A. MR imaging findings in alcoholic and nonalcoholic acute Wernicke’s encephalopathy: a review. BioMed Res. Int. 2014:503596. doi: 10.1155/2014/503596. Epub 2014 Jun 24. PMID: 25050351; PMCID: PMC4094710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz A., Donnino M.W. Thiamine (vitamin B1) in septic shock: a targeted therapy. J. Thorac. Dis. 2020;12(1):S78–S83. doi: 10.21037/jtd.2019.12.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittock S.J., Weinshenker B.G., Lucchinetti C.F., Wingerchuk D.M., Corboy J.R., Lennon V.A. Neuromyelitis optica brain lesions localized at sites of high aquaporin 4 expression. Arch. Neurol. 2006;63(7):964–968. doi: 10.1001/archneur.63.7.964. [DOI] [PubMed] [Google Scholar]
- Sinha S., Kataria A., Kolla B.P., Thusius N., Loukianova L.L. Wernicke encephalopathy-clinical pearls. Mayo Clin. Proc. 2019;94(6):1065–1072. doi: 10.1016/j.mayocp.2019.02.018. [DOI] [PubMed] [Google Scholar]
- Solomon I.H., Normandin E., Bhattacharyya S. Neuropathological features of covid-19. N. Engl. J. Med. 2020;383(10):989–992. doi: 10.1056/NEJMc2019373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suleyman G., Fadel R.A., Malette K.M. Clinical characteristics and morbidity associated with coronavirus disease 2019 in a series of patients in metropolitan detroit. JAMA Netw Open. 2020;3(6) doi: 10.1001/jamanetworkopen.2020.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijnia J.W. Severe infections are common in thiamine deficiency and may be related to cognitive outcomes: a cohort study of 68 patients with Wernicke-Korsakoff syndrome. Psychosomatics. 2016;57(6):624–633. doi: 10.1016/j.psym.2016.06.004. [DOI] [PubMed] [Google Scholar]