It has long been known that atherosclerotic disease is associated with the development of intimal calcification.1–3 Microcalcifications are a key feature of the early process of atherogenesis in humans.4, 5 Pathologic studies have shown that the degree of calcification progresses with plaque complexity as well as the degree of luminal narrowing.4–8 In humans, coronary artery calcifications (CAC) that appear in early adult life (prior to 50 years of age) predict myocardial infarction and cardiovascular death in the near term.9 The CAC score, a non-invasive measure of coronary plaque burden obtained through computed tomography (CT), extends the ability of traditional clinical risk scores, such as the Framingham Risk Score, to predict clinical cardiac events. Further, progression of CAC also independently predicts the risk of incident cardiac events.10–12 Intriguingly, statins, which are the mainstay of therapy for individuals at risk of atherosclerotic cardiovascular disease (ASCVD) and definitively reduce the risk of clinical cardiac events, are not associated with a reduction in calcium scores. This has been assessed in several randomized controlled clinical trials, each demonstrating that despite a significant LDL-lowering effect, CAC scores either did not change or trended toward higher levels in patients treated with statins.13–16 The explanation for this apparent paradox with statin treatment remains an area of debate and investigation.
In this issue of Arteriosclerosis, Thrombosis, and Vascular Biology, Xian et al. provide evidence that statin treatment promotes changes in the microarchitecture of vascular calcium, which suggests a possible mechanism by which statins may be associated with an increase in CAC and stabilize lesion remodeling. Using two different imaging modalities, the authors demonstrated that vascular calcification increased in Apoe−/− mice as they aged. Beginning at 56 weeks of age, the authors treated mice with pravastatin or vehicle in their drinking water. They assessed calcium deposits by micro-computed tomography (μCT) at baseline, after 10 weeks, and after 20 weeks of treatment, and found that aortic calcium content increased significantly in all mice over the study period. Analysis of 18F-NaF-micro-positron emission tomography (μPET) imaging demonstrated that after 10 weeks of treatment, the pravastatin group had less 18F uptake compared with controls, suggesting that the calcium deposits have a smaller surface area. The authors then extended their findings with complementary histopathological analysis demonstrating that, while mice treated with pravastatin had a similar amount of cross-sectional area of calcium deposits, there was an increase in the total number of deposits, with the majority of these being microdeposits (<50μm in diameter). The authors propose that this change in microarchitecture leads to a reduction in overall calcium deposit surface area that may influence biomechanical vulnerability and potentially decrease the risk of plaque rupture. Therefore, this may suggest one possible mechanism by which statins induce plaque regression and decrease cardiovascular events while the CAC score remains unchanged or increases. Indeed, Criqui and colleagues have shown that in humans, calcium density is inversely associated with event risk, suggesting that calcifications may contribute to lesion stability.17 Moreover, a study using serial CT angiographic assessments demonstrated more rapid calcification of lesions along with lower rate of atheroma progression and appearance of high risk plaque features in statin-treated compared to statin-naïve patients over two years.18 While this raises some interesting parallels between the murine and human data, this must be interpreted with caution since the data in humans often define rapid progression as occurring over years and by definition are macroscopic advanced atheroma with large calcifications, whereas those described in mice are microscopic calcifications that develop and change over weeks. Interestingly, while not explored in the current study, human histological data has suggested that statins may also change the localization of calcium microdeposits, promoting their accumulation around the necrotic core in order to stabilize plaques and this is deserving of further study.7
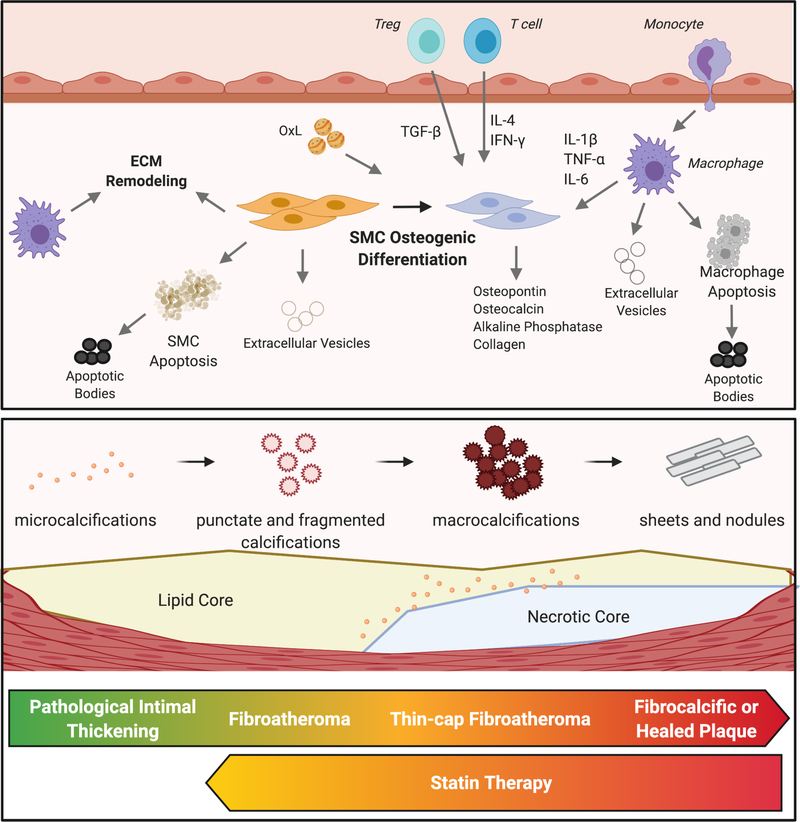
The authors of the present study explored possible mechanisms that might explain the increased microcalcifications seen in the pravastatin-treated group. Evolving data over the last 20 years has shown that vascular calcification is akin to osteogenesis.19 As part of this, vascular smooth muscle cells (VSMCs) differentiate into osteochondrogenic cells and may express high levels of typical markers for osteoblasts including collagen I, alkaline phosphatase, osteopontin, tissue factor, and osteocalcin (Figure 1).19 In this study, the authors did not find differences between the control and pravastatin-treated groups with regard to osteopontin expression, however, they did identify a larger number of alkaline phosphatase-positive cells in the pravastatin-treated group, suggesting a possible increase in these osteoblast-like cells. More recently, inflammation has also been shown to play an important role in driving the calcification of atherosclerotic plaques. An important role for both the innate and adaptive immune systems has emerged as it has now been shown that macrophages, neutrophils, and T cells can drive extracellular matrix remodeling as well as promote osteogenic differentiation of VSMCs.20 In addition, inflammation-driven apoptosis of VSMCs is also felt to be a driving force in the development of calcifications. While neither of these aspects were explored in the present report, these hypotheses will be important to address in subsequent studies.
Figure 1. Calcification within atherosclerotic plaques.
Osteogenic differentiation of smooth muscle cells (SMCs) is a central process to the calcification of lesions. These cells become phenotypic osteoblasts, able to calcify surrounding tissue through the production of a variety of stimuli. Inflammation, driven by both innate and adaptive immune cells, promotes this SMC phenotypic shift. Oxidized lipids (OxL) promote metabolic and phenotypic changes in SMCs and macrophages as well as by augmenting local inflammation. Apoptosis of macrophages and SMCs generate apoptotic bodies that may become calcified. Similarly, extracellular vesicles released by SMCs or macrophages can also become calcified and aggregate into microcalcifications. Macrophages and SMCs produce factors that remodel the extracellular matrix (ECM), providing a scaffolding on which microcalcifications can aggregate into macroscopic calcifications. As atherosclerotic lesions progress, microcalcifications and fragmented calcifications coalesce to form macrocalcifications. Late-stage plaques or those that have ruptured and healed are characterized by dense sheets of calcium. Statin therapy appears to promote a reversal of macrocalcifications to microcalcifications in mice.
One limitation of the current study is that the authors did not see a significant increase in the overall amount of vascular calcification in pravastatin-treated mice over control, as has been previously shown in mice21 and humans.13–16 Further, the difference in 18F uptake seen after 10 weeks did not persist through the full 20 weeks of the study, although histologically, they did see differences in calcium at both 10 weeks and 20 weeks. The authors suggest that this may be because the pravastatin group may have been accumulating a higher number of microdeposits overall and that there are complex time-dependent changes in the character of these deposits. There are also several important limitations of studying statins in mice. While statins have been shown to lower cholesterol and lesion area in mice that are fed a high-fat ‘Western’ diet22, when used in Apoe−/− mice fed a standard ‘chow’ diet, the statins lead to increased cholesterol and increased plaque burden23, a finding which the authors have redemonstrated in their own study. This raises concerns about possible confounders in studying the mechanism of statin-induced calcification, since oxidated lipids are known to promote calcification themselves.19
In conclusion, the present report identifies a possible mechanism that may help explain the seemingly paradoxical effects of statins on vascular calcium and ASCVD risk. Further studies will be necessary to fully understand the impact of microcalcifications on vascular architecture.
Acknowledgements
The authors acknowledge Biorender.com for assistance in generation of the figure.
Sources of Funding
This work was supported by an American Heart Association Fellow-to-Faculty award (to ACD), and National Institutes of Health Grants: HL148137, HL146134, HL116263 (to MFL).
Disclosures
MFL: Consultant for Amgen, REGENXBIO, ESPERION Therapeutics; and Research Support from Merck, Genzyme, Sanofi Aventis, Ionis, Regeneron, FH Foundation.
References
- 1.Bolick LE, Blankenhorn DH. A quantitative study of coronary arterial calcification. Am J Pathol. 1961;39:511–519 [PMC free article] [PubMed] [Google Scholar]
- 2.Virchow R Cellular pathology as based upon physiological and pathological histology. New York, NY: Dover Publications; 1971, 1–554 [DOI] [PubMed] [Google Scholar]
- 3.Haust MD, Moore RH. Spontaneous lesions of the aorta in rabbit. In: Roberts JC, Straus R, eds. Comparative atherosclerosis: The morphology of spontaneous and induced atherosclerotic lesions in animals and its relation to human disease. New York, NY: Harper and Row; 1965:1–268 [Google Scholar]
- 4.Chatrou ML, Cleutjens JP, van der Vusse GJ, Roijers RB, Mutsaers PH, Schurgers LJ. Intra-section analysis of human coronary arteries reveals a potential role for microcalcifications in macrophage recruitment in the early stage of atherosclerosis. PLoS One. 2015;10:e0142335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stary HC. Natural history and histological classification of atherosclerotic lesions: An update. Arterioscler Thromb Vasc Biol. 2000;20:1177–1178 [DOI] [PubMed] [Google Scholar]
- 6.Stary HC. The development of calcium deposits in atherosclerotic lesions and their persistence after lipid regression. Am J Cardiol. 2001;88:16E–19E [DOI] [PubMed] [Google Scholar]
- 7.Mori H, Torii S, Kutyna M, Sakamoto A, Finn AV, Virmani R. Coronary artery calcification and its progression: What does it really mean? JACC Cardiovasc Imaging. 2018;11:127–142 [DOI] [PubMed] [Google Scholar]
- 8.Otsuka F, Sakakura K, Yahagi K, Joner M, Virmani R. Has our understanding of calcification in human coronary atherosclerosis progressed? Arterioscler Thromb Vasc Biol. 2014;34:724–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carr JJ, Jacobs DR Jr., Terry JG, Shay CM, Sidney S, Liu K, Schreiner PJ, Lewis CE, Shikany JM, Reis JP, Goff DC Jr. Association of coronary artery calcium in adults aged 32 to 46 years with incident coronary heart disease and death. JAMA Cardiol. 2017;2:391–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenland P, LaBree L, Azen SP, Doherty TM, Detrano RC. Coronary artery calcium score combined with framingham score for risk prediction in asymptomatic individuals. JAMA. 2004;291:210–215 [DOI] [PubMed] [Google Scholar]
- 11.McEvoy JW, Blaha MJ, Defilippis AP, Budoff MJ, Nasir K, Blumenthal RS, Jones SR. Coronary artery calcium progression: An important clinical measurement? A review of published reports. J Am Coll Cardiol. 2010;56:1613–1622 [DOI] [PubMed] [Google Scholar]
- 12.Yeboah J, McClelland RL, Polonsky TS, Burke GL, Sibley CT, O’Leary D, Carr JJ, Goff DC, Greenland P, Herrington DM. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012;308:788–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Houslay ES, Cowell SJ, Prescott RJ, Reid J, Burton J, Northridge DB, Boon NA, Newby DE, Scottish Aortic S, Lipid Lowering Therapy IoRtI. Progressive coronary calcification despite intensive lipid-lowering treatment: A randomised controlled trial. Heart. 2006;92:1207–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raggi P, Davidson M, Callister TQ, Welty FK, Bachmann GA, Hecht H, Rumberger JA. Aggressive versus moderate lipid-lowering therapy in hypercholesterolemic postmenopausal women: Beyond endorsed lipid lowering with ebt scanning (belles). Circulation. 2005;112:563–571 [DOI] [PubMed] [Google Scholar]
- 15.Schmermund A, Achenbach S, Budde T, Buziashvili Y, Forster A, Friedrich G, Henein M, Kerkhoff G, Knollmann F, Kukharchuk V, Lahiri A, Leischik R, Moshage W, Schartl M, Siffert W, Steinhagen-Thiessen E, Sinitsyn V, Vogt A, Wiedeking B, Erbel R. Effect of intensive versus standard lipid-lowering treatment with atorvastatin on the progression of calcified coronary atherosclerosis over 12 months: A multicenter, randomized, double-blind trial. Circulation. 2006;113:427–437 [DOI] [PubMed] [Google Scholar]
- 16.Terry JG, Carr JJ, Kouba EO, Davis DH, Menon L, Bender K, Chandler ET, Morgan T, Crouse JR 3rd. Effect of simvastatin (80 mg) on coronary and abdominal aortic arterial calcium (from the coronary artery calcification treatment with zocor [catz] study). Am J Cardiol. 2007;99:1714–1717 [DOI] [PubMed] [Google Scholar]
- 17.Criqui MH, Denenberg JO, Ix JH, McClelland RL, Wassel CL, Rifkin DE, Carr JJ, Budoff MJ, Allison MA. Calcium density of coronary artery plaque and risk of incident cardiovascular events. JAMA. 2014;311:271–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee SE, Chang HJ, Sung JM, Park HB, Heo R, Rizvi A, Lin FY, Kumar A, Hadamitzky M, Kim YJ, Conte E, Andreini D, Pontone G, Budoff MJ, Gottlieb I, Lee BK, Chun EJ, Cademartiri F, Maffei E, Marques H, Leipsic JA, Shin S, Choi JH, Chinnaiyan K, Raff G, Virmani R, Samady H, Stone PH, Berman DS, Narula J, Shaw LJ, Bax JJ, Min JK. Effects of statins on coronary atherosclerotic plaques: The paradigm study. JACC Cardiovasc Imaging. 2018;11:1475–1484 [DOI] [PubMed] [Google Scholar]
- 19.Sage AP, Tintut Y, Demer LL. Regulatory mechanisms in vascular calcification. Nat Rev Cardiol. 2010;7:528–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Passos LSA, Lupieri A, Becker-Greene D, Aikawa E. Innate and adaptive immunity in cardiovascular calcification. Atherosclerosis. 2020;306:59–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X, Xiao S, Li Q. Pravastatin polarizes the phenotype of macrophages toward m2 and elevates serum cholesterol levels in apolipoprotein e knockout mice. J Int Med Res. 2018;46:3365–3373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gronros J, Wikstrom J, Brandt-Eliasson U, Forsberg GB, Behrendt M, Hansson GI, Gan LM. Effects of rosuvastatin on cardiovascular morphology and function in an apoe-knockout mouse model of atherosclerosis. Am J Physiol Heart Circ Physiol. 2008;295:H2046–2053 [DOI] [PubMed] [Google Scholar]
- 23.Wang YX, Martin-McNulty B, Huw LY, da Cunha V, Post J, Hinchman J, Vergona R, Sullivan ME, Dole W, Kauser K. Anti-atherosclerotic effect of simvastatin depends on the presence of apolipoprotein e. Atherosclerosis. 2002;162:23–31 [DOI] [PubMed] [Google Scholar]