Abstract
COVID-19 has remained an uncontained, worldwide pandemic. While battling for the disease in China, six Traditional Chinese Medicine (TCM) recipes have been shown to be remarkably effective for treating patients with COVID-19. The present review discusses principles of TCM in curing infectious disease, and clinical evidence and mechanisms of the 6 most effective TCM recipes used in treating COVID-19 in 92% of all of the confirmed cases in China. Applications of TCM and specific recipes in the treatment of other viral infections, such as those caused by SARS-CoV, MERS-CoV, hepatitis B virus, hepatitis C virus, influenza A virus (including H1N1 and H7N9), influenza B, dengue virus as well as Ebola virus, are also discussed. Among the 6 TCM recipes, Jinhua Qinggan (JHQG) granules and Lianhua Qingwen (LHQW) capsules are recommended during medical observation; Lung Cleansing and Detoxifying Decoction (LCDD) is recommended for the treatment of both severe and non-severe patients; Xuanfeibaidu (XFBD) granules are recommended for treating moderate cases; while Huashibaidu (HSBD) and Xuebijing (XBJ) have been used in managing severe cases effectively. The common components and the active ingredients of the six TCM recipes have been summarized to reveal most promising drug candidates. The potential molecular mechanisms of the active ingredients in the six TCM recipes that target ACE2, 3CLpro and IL-6, revealed by molecular biological studies and/or network pharmacology prediction/molecular docking analysis/visualization analysis, are fully discussed. Therefore, further investigation of these TCM recipes may be of high translational value in enabling novel targeted therapies for COVID-19, potentially via purification and characterization of the active ingredients in the effective TCM recipes.
Keywords: Traditional Chinese Medicine (TCM), Viral infections, COVID-19, Jinhua Qinggan granules (JHQG), Lianhua Qingwen capsules (LHQW), Lung Cleansing and Detoxifying Decoction (LCDD), Xuanfeibaidu granules (XFBD), Huashibaidu granules (HSBD), Xuebijing (XBJ), Angiotensin converting enzyme 2 (ACE2), Coronavirus 3-chymotrypsin-like protease (3CLpro), Interleukin-6 (IL-6)
Abbreviations: 3CLpro, Coronavirus 3-chymotrypsin-like protease; ACE2, angiotensin converting enzyme 2; AGE-RAGE, Advanced glycation end products-Receptor for AGE; Akt, Protein kinase B; ARDS, Acute respiratory distress syndrome; BCL2, B-cell lymphoma 2; CASP3, Caspase 3; CCL2, C-C Motif Chemokine Ligand 2; COVID, Coronavirus disease; COVID-19, Coronavirus disease 2019; COX-2, Cyclooxygenase-2; CRP, C-reactive protein; CT, computerized tomography; CVA, cough variant asthma; DAVID, Database for Annotation, Visualization, and Integrated Discovery; EGFR, epidermal growth factor receptor; ESR, erythrocyte sedimentation rate; GO, gene ontology; HIF-1, Hypoxia-Inducible Factor-1; HNF4A, hepatocyte nuclear factor 4 alpha; HSBD, Huashibaidu granules; HSP90AA1, Heat shock protein HSP 90-alpha; HSP90AB1, Heat shock protein HSP 90-beta; HXZQ, Huoxiang Zhengqi dropping pills; IC50, 50% inhibitory concentration; ICU, intensive care unit; IFN-γ, Interferon gamma; IL-6, Interleukin-6; IP-10, Interferon gamma-induced protein 10; JHQG, Jinhua Qinggan granules; KEGG, Kyoto Encyclopedia of Genes and Genomes; LCDD, Lung Cleansing and Detoxifying Decoction; LHQW, Lianhua Qingwen capsules; MAPK, Mitogen-activated protein kinase; MCP-1, Monocyte Chemoattractant Protein-1; miRNAs, micro RNAs; NCOA2, Nuclear receptor coactivator 2; NFkB, Nuclear factor kappa B; NOS2, Nitric Oxide Synthase 2 (inducible Nitric Oxide Synthase/iNOS); Nrf2, nuclear factor erythroid 2-related factor 2; PCT, procalcitonin; PI3K, Phosphoinositide 3-kinase; PPARG, peroxisome proliferator-activated receptor gamma; PPI, protein-protein interaction; PTGS1, Prostaglandin-endoperoxide synthase 1; PTGS2, Prostaglandin-endoperoxide synthase 2; RCT, randomized controlled trial; ROS, reactive oxygen species; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; SIRS, systematic inflammatory response syndrome; SOCS1, suppressor of cytokine signaling 1; S protein, SARS-CoV-2 spike (S) glycoprotein; TCM, Traditional Chinese Medicine; TCMID, Traditional Chinese Medicine Integrated Database; TFs, transcription factors; TNF-α, Tumor Necrosis Factor Alpha; TP53, Tumor protein p53; UPLC-DAD-QTOF-MS, Ultra performance liquid chromatography coupled with diode-array detector and quadrupole time-of-flight mass spectrometry; WBC, white blood cell; WHO, World Health Organization; XBJ, Xuebijing; XFBD, Xuanfeibaidu granules
1. Introduction
COVID-19 has remained an uncontained, worldwide pandemic. During the fight against the disease in China, the National Health Commission of the People's Republic of China declared that 92% of the confirmed COVID-19 cases were treated with Traditional Chinese Medicine (TCM) in combination with Western Medicine, and the patients responded to the treatment to recover or much improve in more than 90% of the cases (ChinaDaily, 2020b). For patients with mild and moderate disease, early intervention with TCM has been shown to effectively prevent disease transition into severe and critical state (Ren, Zhang, & Wang, 2020). In the severe cases, TCM has helped to stabilize the patients for prolonged therapeutic window (Ren, Zhang, & Wang, 2020). Accumulating evidence has shown that early intervention of COVID-19 patients with TCM is important in improving cure rate, shortening the course of disease, delaying disease progression and reducing mortality rate (Ren, Zhang, & Wang, 2020). The current review discusses efficacies and mechanisms of TCM recipes that have proven to be robustly effective in treating patients with COVID-19 in China, milestones of TCM applications in treating infectious disease through the long history of China, as well as applications and efficacies of TCM in treating other types of viral infections including those caused by SARS-CoV, MERS-CoV, hepatitis B virus, hepatitis C virus, influenza A virus (including H1N1 and H7N9), influenza B, dengue virus as well as Ebola virus.
2. History of TCM in treating infectious disease
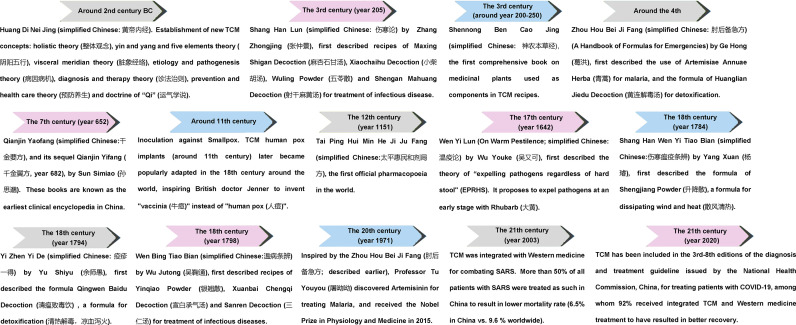
Historically, TCM has worked well in treating infectious disease (Fig. 1 ). During the past 3000 years of Chinese history, TCM has been used as the routine treatment regime for pandemic and endemic diseases. In dealing with these, a complete theoretical system of prevention and treatment of “pestilence” (refers to fatal epidemic disease, called “Wen Yi” (瘟疫) or “Wen Bing” (温病) in ancient China) has been developed. TCM was described to cure infectious diseases for the first time in Huangdi Neijing (The Yellow Emperor's Classic of Medicine, an ancient treatise on health and disease, simplified Chinese: 黄帝内经) over 2500 years ago, in which new TCM concepts were described including holistic theory (整体观念), yin and yang and five elements theory (阴阳五行), visceral meridian theory (脏象经络), etiology and pathogenesis theory (病因病机), diagnosis and therapy theory (诊法治则), prevention and health care theory (预防养生) and doctrine of “Qi” (运气学说) (Curran, 2008; Luo, Tang et al. 2020) (Fig. 1). The classical TCM book named Shanghan Lun (Treatise on Cold Damage Diseases as the translation, simplified Chinese: 伤寒论) describes a system of characterizing the disease by six main syndromes, with characteristic symptoms and pulses to guide diagnosis and appropriate individualized prescriptions for treatment; this book lays foundation for differential syndrome categorization and individualized treatment in TCM, especially for infectious disease. A book named Zhou hou bei ji fang, (“Emergency Prescriptions kept in one's Sleeve”, simplified Chinese: 肘后备急方) written by Ge Hong, describes TCM applications for treating malaria. The specific instructions of treatment in this book are the followings: ‘Immerse a handful of Qing Hao (青蒿) in two liters of water, wring out the juice and drink it all (青蒿一握, 以水二升渍, 绞取汁, 尽服之)’ (Tu, 2015). This has inspired Professor Youyou Tu (Chinese name: 屠呦呦) to isolate artemisinin from Qing Hao (青蒿) with ether, and discover its therapeutic effects on Malaria, which later claimed the 2015 Nobel Prize in Physiology and Medicine (Tu, 2015).
Fig. 1.
A brief history of TCM for pestilence prevention and treatment of infectious diseases. During the past 3,000 years of Chinese history, TCM has been used as the routine treatment regime for pandemic and endemic diseases. The milestone of TCM in pestilence prevention and treatment of infectious diseases are listed.
Formulated Chinese medicine has been used in managing previous pandemics, such as the two previous coronavirus outbreaks involving SARS-CoV in 2003 and MERS-CoV in 2012, and seasonal epidemics caused by influenza viruses and dengue virus (Li et al., 2020). During the 2002–2003 SARS (Severe Acute Respiratory Syndrome) epidemic in China, TCM was used in the prevention (Lau et al., 2005) and treatment of SARS (Chen & Nakamura, 2004), which had been shown to result in shorter hospitalization, reduced side effects from steroid treatments, and relief from dyspnoea and malaise (WHO, 2004). To date, dozens of Chinese herbs and hundreds of natural TCM ingredients have been reported to possess antiviral activities (Xian et al., 2020). TCM have been shown to possess antiviral activities against various viral strains including herpes simplex virus, influenza virus, human immunodeficiency virus, hepatitis B and C viruses, and SARS-CoV and MERS-CoV (Xian et al., 2020). When the human transmissible swine flu virus (H1N1) was reported in Mexico and the United States in April 2009, the Chinese government rapidly released three editions of documents entitled “Recommended Schemes for Pandemic Influenza A Diagnoses and Treatments” (Ge et al., 2010). Besides the two targeted anti-flu drugs, Oseltamivir and Zanamivir, four anti-flu TCM prescriptions were recommended for the treatment of H1N1 infection in the third edition (Ge et al., 2010). A meta-analysis of 30 studies including 3444 cases indicates that the mean time to defervescence in the TCM treatment group was significantly reduced, and that the duration of viral [Influenza A (H1N1)] shedding in the subgroup of patients receiving integrated treatment of Chinese and Western medicine, was also significantly shortened (Li, Wang, Guo, & Li, 2016).
TCM drugs are traditionally composed of many different herbs/components with known or unknown active ingredients that can target various pathways for a given class of medical indications, and are tunable to the symptoms of an individual (Ge et al., 2010). The evidence-based guideline on treating adult influenza patients with TCM has been developed (Wu et al., 2020). Earlier work by Wu et al. formulated six recommendations based on evidence synthesis and experts' consensus, namely Lianhua Qingwen capsule (LHQW, 连花清瘟胶囊), Jinhua Qinggan granule (JHQG, 金花清感颗粒), Banlangen granule (BLG, 板蓝根颗粒), Shufeng Jiedu capsule (SFJD, 疏风解毒胶囊), or Jinfang Baidu pill (JFBD, Jing Fang Bai Du San, 荆防败毒散) for treating mild influenza, depending on the manifestations (Wu, Chen, et al., 2020). LHQW in combination with antiviral medications and supportive therapy is recommended for treating severe influenza, or mild influenza in patients at higher risk of developing into severe disease (Wu, Chen, et al., 2020). TCM exerts its anti-influenza activity by regulating the immune response to interfere with both viral infection and host reactions, and might well be used as an alternative therapeutic option to treat influenza virus infection (Dai et al., 2020). Of note, clinical practice guidelines (CPG) have been developed by a guideline working group on TCM treatment of influenza according to the Institute of Medicine, the Appraisal of Guidelines for REsearch and Evaluation II (AGREE II instrument), and the World Health Organization (WHO) guideline handbook, to provide recommendations based on systematic reviews and evidence syntheses (Zhao et al., 2020). This CPG represents a first statement documenting the efficacies and practice guidelines of treating influenza with TCM (Zhao, Guo, et al., 2020).
3. The six TCM recipes proven most effective in treating COVID-19
During the fight against COVID-19 in China, TCM has been officially added to the 3rd-8th editions of the diagnosis and treatment guideline issued by the National Health Commission, China (Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7), 2020; NHC, 2020). According to the collective analyses from The State Council Information Office, China, three TCM decoctions and 3 formulated Chinese medicines (referred to as the “six most effective recipes” for the rest of the review) have been shown to be most effective in TCM treatments of COVID-19 patients in China (ChinaDaily 2020b; Luo, Gao et al., 2020).
In these six highly effective TCM recipes, Chinese formula medicines of Jinhua Qinggan granules (JHQG, 金花清感颗粒) and Lianhua Qingwen capsules (LHQW, 连花清瘟胶囊) are recommended for treating patients during medical observation/early stage of disease development, when fatigue and fever are presented as the primary clinical manifestations (Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7), 2020). Lung Cleansing and Detoxifying Decoction (LCDD) (also known as Qingfei Paidu, QFPD, 清肺排毒汤) is recommended for treating both severe and non-severe patients. Xuanfeibaidu granules (XFBD, 宣肺败毒颗粒) on the other hand, are recommended for treating moderate cases; while Huashibaidu granules (HSBD, 化湿败毒颗粒) and Xuebijing (XBJ, 血必净) have been used in severe cases (Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7), 2020).
Besides molecular biological studies, network pharmacology and high throughput molecular docking analyses, methods used to predict ligand-protein binding patterns/affinity by simulating ligand binding with receptor proteins, have been used to explore the potential molecular mechanisms of the six TCM recipes in treating COVID-19. The steps of the analyses are as the followings: at first, the active ingredients and their targets were identified using a TCM systems pharmacology database and analysis platform (TCMSP), which represents a database and platform of systems pharmacology for drug discovery from TCM recipes; a TCM Integrated Database (TCMID), in which records of TCM-related information are collected from different sources and through text-mining methods, and via links to common drug and disease databases including Drugbank, OMIM and PubChem, forming a network for integrative analyses of relationships between herbs/TCM components and their disease targets, as well as the relationships between the active ingredients of TCM and their targets; and a BATMAN-TCM (Bioinformatics Analysis Tool for Molecular mechANism of TCM (Jimilihan et al., 2020; Liu et al., 2016; Ru et al., 2014; Xue et al., 2013). Then, absorption, distribution, metabolism and excretion (ADME) parameters were used to screen the active ingredients (Jimilihan et al., 2020). Finally, PubChem, Chemical Book and ChemDraw softwares were used to determine the structures of the ingredients and construct the molecular docking ligands. In the network pharmacology and molecular docking analyses, 3CL hydrolase (3CLpro, also known as Mpro, main protease for SARS-CoV-2, essential for proteolytic maturation of the SARS-CoV) and ACE2 (angiotensin converting enzyme 2, receptor for SARS-CoV-2) have been used as receptors for molecular docking by Autodock software, which is a suite of automated docking tools designed to predict how small molecules, such as substrates or drug candidates, bind to a receptor of known 3D structure (Jimilihan et al., 2020). Further, the active ingredients and their targets that could potentially combine 3CLpro and/or ACE2 have been constructed using Cycloscape software (an open source software platform for visualizing molecular interaction networks and biological pathways, and for integration of these networks with annotations, gene expression profiles and other state data) to produce the TCM medicine-active ingredients-target network (Jimilihan et al., 2020). The protein-protein interaction (PPI) analysis of the targets was carried out by String (https://string-db.org/) database, and the potential targets of the active ingredients were identified (Jimilihan et al., 2020). The potential targets were subsequently analyzed for gene function by ClueGO: a Cytoscape plug-in to decipher functionally grouped gene ontology (GO) and pathway annotation networks (Bindea et al., 2009; Dennis Jr. et al., 2003; Jimilihan et al., 2020; Kanehisa & Goto, 2000). The targets can also be analyzed by DAVID (Database for Annotation, Visualization, and Integrated Discovery, a web-accessible program that integrates functional genomics annotations with intuitive graphical summaries) following identification of Kyoto Encyclopedia of Genes and Genomes (KEGG, a knowledge base for systematic analysis of gene functions, linking genomic information with higher order functional information) signaling pathways (Bindea et al., 2009; Dennis Jr. et al., 2003; Jimilihan et al., 2020; Kanehisa & Goto, 2000).
3.1. JHQG recipe
JHQG granules (金花清感颗粒) combine two classical recipes of Maxingshigan decoction (麻杏石甘汤) and Yinqiao San (银翘散), which are composed of Niubangzi (牛蒡子; Great Burdock Achene; Arctii Fructus), Qinghao (青蒿; Sweet Wormwood Herb; Artemisiae Annuae Herba), Bohe (薄荷; Wild Mint Herb; Menthae Haplocalycis Herba), Lianqiao (连翘; Forsythiae Fructus; Forsythia suspensa), Jinyinhua (金银花, Wild honeysuckle flower; Lonicerae Japonicae Flos), Kuxingren (苦杏仁; Bitter Almond; Armeniacae Semen Amarum), Shigao (石膏; Gypsum; Gypsum fibrosum), Gancao (甘草; Licorice; Glycyrrhizae Radix et Rhizoma), Huangqin (黄芩; Baikal Skullcap Root; Scutellariae Radix), Mahuang (麻黄; Ephedra; Ephedrae Herba), Zhebeimu (浙贝母; Thunberg Fritillary Bulb; Fritillariae Thunbergii Bulbus), and Zhimu (知母; Common Anemarrhena Rhizome; Anemarrhenae Rhizoma) (Liu et al., 2020).
3.1.1. JHQG recipe in the treatment of previous infectious diseases
JHQG granules (金花清感颗粒) was specifically formulated for treating influenza A (H1N1) by the expert group of TCM after the 2009 H1N1 influenza outbreak in Mexico (Qi, Qi, & Wang, 2016). JHQG recipe was found highly effective for treating H1N1 infection in animal models and clinical studies before the recipe became widely used (Yan, 2010). In one preclinical study, JHQG was found to markedly decrease mortality and improve survival, while reducing the severity of pulmonary lesions in mice infected with H1N1 virus (Yan, 2010). In another study, JHQG was able to reduce fever and inflammation in a rabbit model of H1N1 infection (Yan, 2010). In addition, a clinical study showed that JHQG markedly reduced duration of fever and alleviated symptoms (Yan, 2010). In a double-blinded, randomized controlled trial (RCT) of 136 influenza patients, JHQG (5 g each time, three times daily for 5 days) speeded up recovery of the patients (Li et al., 2013). In another double-blinded RCT of 174 influenza patients, the same dose of JHQG significantly reduced serum levels of CRP and IFN-γ (Qi et al., 2016).
3.1.2. JHQG recipe in the treatment of COVID-19
JHQG has proven to be an effective treatment for COVID-19, after being selected by joint efforts of Chinese and Western medical experts organized by Beijing Administration of Traditional Chinese Medicine, referring to more than 100 ancient prescriptions in TCM classics such as Treatise on Febrile Diseases (伤寒论), Wenyi Lun (瘟疫论) and Wenbing Tiaobian (温病条辨) (Liu et al., 2020). Accumulating evidence has demonstrated that JHQG significantly alleviated clinical symptoms of mild COVID-19 patients, such as fever, cough, fatigue, and expectoration, and can relieve anxiety of the patients (Duan et al., 2020). Liu et al. reported that 7-day viral clearance rate was significantly higher while pneumonia recovery time was significantly shorter in the JHQG treated group, in a study of 80 COVID-19 cases (Liu et al., 2020). Furthermore, in a case control study of 123 patients with COVID-19, JHQG significantly relieved symptoms of fever, cough, fatigue and anxiety in patients with mild COVID-19 (Duan et al., 2020). The active ingredients and potential molecular mechanisms of JHQG in treating COVID-19 have been explored (shared common components/ingredients with other TCM recipes and targeted pathways are discussed in more details in 2, 3). Shen et al. reported that the active ingredients of JHQG, 3-methoxy-glycerol, crude-glycerin and glycyrrhizin B, have strong binding activity for 3CLpro and ACE2 by network pharmacology and high throughput molecular docking analyses (Shen et al., 2020). The chemical constituents and molecular targets of the 12 components in JHQG have been identified using TCMSP (as described above). UniProt was used to search for the targets of the JHQG's ingredients, and Cytoscape 3.7.2 was used to construct the ingredients-target (gene) network. DAVID was used to perform enrichment analysis of GO functions and the KEGG pathways to predict the mechanisms of action. Cytoscape 3.7.2 based analyses indicate that the major active ingredients (including chlorogenic acid, forsythoside A, and ephedrine) of JHQG docked with 3CLpro, SARS-CoV-2 spike glycoprotein (S protein), ACE2, and suppressor of cytokine signaling 1 (SOCS1) (Ren et al., 2020). Visualization analysis with DAVID demonstrated that the core active ingredients of JHQG have strong affinity for 3CLpro, S protein, ACE2, and SOCS1, thereby inhibiting viral replication and binding to target cells, reducing host inflammation and activating antiviral immunity (Ren, Yin, et al., 2020). Network pharmacology and molecular docking analyses performed by Gong et al. indicate that the effective compounds in JHQG regulate multiple signaling pathways via binding to ACE2, involving Prostaglandin-endoperoxide synthase 1 (PTGS1), Prostaglandin-endoperoxide synthase 2 (PTGS2), Heat shock protein HSP 90-alpha (HSP90AA1), Heat shock protein HSP 90-beta (HSP90AB1), and Nuclear receptor coactivator 2 (NCOA2) (Gong, Guo, Li, Wang, & Gu, 2020). In addition, Simayi et al. elucidated that the active ingredients of JHQG, such as kaempferol, baicalein and oroxylin A, regulate multiple signaling pathways (such as PTGS2, B-cell lymphoma 2 (BCL2) and Caspase-3) by binding to ACE2 (Jimilihan et al., 2020). More detailed discussion of target pathways driven by specific active ingredients of JHQG and other TCM recipes is included in Section 3.
3.2. LHQW recipe
LHQW capsules (连花清瘟胶囊) is a TCM recipe composed of 13 herbs or components: Gancao (甘草; Licorice; Glycyrrhizae Radix et Rhizoma), Hongjingtian (红景天; Rhodiola; Rhodiolae Crenulatae Radix et Rhizoma), Bohenao (薄荷脑; Menthol; Menthol), Dahuang (大黄; Rhubarb Tangute Rhubarb; Rhei Radix Et Rhizoma), Mahuang (麻黄; Ephedra; Ephedrae Herba), Jinyinhua (金银花, Wild honeysuckle flower; Lonicerae Japonicae Flos), Guanghuoxiang (广藿香; Patchouli; Pogostemon patchouli), Lianqiao (连翘; Forsythiae Fructus; Forsythia suspensa), Shigao (石膏; Gypsum; Gypsum fibrosum), Kuxingren (苦杏仁; Bitter Almond; Armeniacae Semen Amarum), Yuxingcao (鱼腥草; Houttuyniae Herba; Houttuynia cordata Thunb), Banlangen (板蓝根; Indigowoad Root; Isatidis Radix), and Mianmaguanzhong (绵马贯众; Dryopteris Crassirhizoma; Dryopteridis Crassirhizomatis Rhizoma) (Ding et al., 2017; Tao et al., 2013).
3.2.1. LHQW recipe in the treatment of previous infectious diseases
LHQW capsules (连花清瘟胶囊) was patented in 2003 in China, and was approved for Phase II clinical trial by US FDA in 2015 (Ye et al., 2020). It has been used to treat influenza and shown to have broad-spectrum antiviral effects on a number of influenza viruses with immune regulatory effects (Ding et al., 2017; Tao et al., 2013). A meta-analysis of RCT revealed that LHQW treatment in patients infected with influenza A virus resulted in shorter duration of fever, cough, sore throat, and body ache, comparing to Oseltamivir (anti-viral drug) treated patients (Zhao, Yang, Lv, & Wei, 2014). Combinatory treatment of LHQW (200 mg/kg/day for 5 days) and Qseltamivir in a mouse model significantly diminished influenza B virus infection over the individual administration of either alone, indicating that LHQW could be used as an assistant medicine to enhance the effect of Qseltamivir in treating influenza B virus infection (Yang et al., 2020). Of note, it has been shown that fewer inflammatory cells were present in the lungs of influenza B virus infected mice treated with LHQW (Yang, Wang, et al., 2020). LHQW treated mice of influenza A virus (H1N1) infection had decreased viral replication, inflammation, and lung lesions (Gao et al., 2020). Notably, Cyclooxygenase-2 (COX-2) was validated as a viable pharmacological biomarker for the treatment efficacy of LHQW, since low- and high-doses of LHQW decreased COX-2 expression while the high dose of LHQW was more effective than the low dose in H1N1 influenza virus infected mice (Gao et al., 2020). Besides, LHQW displayed a significant inhibitory effect on COX-2 activity in vitro in a dose-dependent manner (Gao et al., 2020). In addition, LHQW inhibited proliferation of influenza viruses of various strains in vitro in Madin-Darby canine kidney cells (Ding et al., 2017). It also blocked the early stages (0−2h) of viral infection, suppressed virus-induced NF-kB activation and alleviated virus-induced gene expression of Interleukin-6 (IL-6), IL-8, tumor necrosis factor (TNF)-α, IP-10, and monocyte chemotactic protein 1 (MCP-1) in a dose-dependent manner (1.5-3 mg/mL) (Ding et al., 2017). Additionally, the viral titers and the levels of inflammatory cytokines were both decreased by LHQW administration (1,300 mg/kg/day for 5 days) in the lungs of the mice inoculated with variant of H1N1 influenza virus A/PR/8/34 (H1N1) (H274Y mutuant) (Ding et al., 2017).
3.2.2. LHQW recipe in the treatment of COVID-19
LHQW has been recommended by the Evidence-Based Medicine Chapter of the China International Exchange and Promotive Association for Medical and Health Care (CPAM) and the Chinese Research Hospital Association (CRHA) to treat patients with mild or moderate COVID-19 in combination with conventional therapy, which refers to respiratory support, symptomatic treatment, antiviral treatment, and antibacterial treatment if needed (Jin et al., 2020). A retrospective analysis of clinical records showed that fever was better resolved and fever duration was shortened in patients treated with LHQW plus conventional therapy (21 confirmed COVID-19 patients), compared to conventional therapy alone (21 confirmed COVID-19 patients) (Yao, Liu, Li, Huang, & Cai, 2020). Results from a retrospective analysis of 54 COVID-19 patients indicate that LHQW is effective in significantly relieving symptoms of fever, cough and weakness, and in shortening duration of having these symptoms (Cheng & Li, 2020). Another RCT of mild COVID-19 patients showed that, compared with Arbidol (anti-influenza drug) treatment group of 148 patients, the total effective rate and the TCM syndrome scores (based on the TCM syndrome rating scale) were significantly improved after 7 day treatment with LHQW (6 g each time, three times daily) plus Arbidol in 147 patients (Yu, Li, Wan, & Wang, 2020). LHQW combined with Arbidol more effectively alleviated clinical symptoms and improved treatment efficacy in patients with mild COVID-19 (Yu et al., 2020). Besides, a study of 151 severe COVID-19 patients indicated that quadruple combination therapy of LHQW, Ribavirin, Lopinavir/ritonavir and Umifenovir may serve as a preferred protocol for treating severe COVID-19 patients (Li et al., 2020). This combination of LHQW with three drugs may result in maximal suppression of viral replication and infection through different mechanisms of action (Li, Yang, Liu, Yang, et al., 2020). Additionally, a study of 283 COVID-19 patients indicated that LHQW combined with Huoxiang Zhengqi dropping pills (HXZQ, 藿香正气滴丸) have advantages in the treatment of nausea, vomiting and limb soreness (Xiao et al., 2020). The combination of LHQW with HXZQ reduced the use of macrolides antibiotics and the number of diagnosed patients progressing into severe disease (Xiao et al., 2020). A prospective multicenter RCT of 284 COVID-19 patients indicate that clinical cure rate and the rate of recovery of chest CT manifestations were markedly higher in the patient group treated with LHQW (4 capsules thrice daily for 14 days) (Hu et al., 2020). Results from a recent meta-analysis of 154 COVID-19 patients indicate that the main clinical symptoms of fever, cough and fatigue disappeared faster in LHQW treated group; and that other symptoms of runny nose, sputum, nasal congestion, muscle pain, difficulty breathing, chest tightness, nausea and vomiting, and loss of appetite also disappeared faster while the duration of fever was significantly reduced by LHQW treatment (Zeng, Li, & Wu, 2020). In addition, Hu et al. evaluated the efficacy of LHQW in the treatment of common pneumonia and COVID-19 pneumonia by meta analysis of 42 studies involving 3793 subjects, in which LHQW treatment was found associated with improvements in flu-like symptoms and conversion of severe cases (Hu et al., 2020). Ultra performance liquid chromatography coupled with diode-array detector and quadrupole time-of-flight mass spectrometry (UPLC-DAD-QTOF-MS) was employed for qualitative and quantitative analyses of the major ingredients of LHQW, among which a total of 61 ingredients including flavonoids, phenylpropanoids, anthraquinones, triterpenoids, iridoids, and other types of compounds were unambiguously or tentatively identified by comparing the retention times and the accurate mass measurements with reference compounds and/or data in the literatures (Jia et al., 2015). Among them, twelve representative ingredients, including salidroside, chlorogenic acid, forsythoside E, cryptochlorogenic acid, amygdalin, sweroside, hyperin, rutin, forsythoside A, phillyrin, rhein, and glycyrrhizic acid were further quantified (Jia et al., 2015). In another study, the primary active ingredients of LHQW were identified by network pharmacology analysis, with a focus on 61 candidate ingredients to further identify their related targets (Wang et al., 2016). The main effective ingredient-target (MECT) network was constructed to reveal the main effective ingredients and their key targets for LHQW (Wang et al., 2016). It has been shown that the docking scores to 3CLpro of SARS-CoV-2 of three ingredients in LHQW, rutin, forsythoside E, and hyperoside, are better than that of Lopinavir (anti-viral drug) (Ye et al., 2020). In addition, at molecular levels, Li et al. observed that LHQW significantly inhibited SARS-CoV-2 replication in Vero E6 cells, and markedly reduced mRNA expression of pro-inflammatory cytokines of TNF-α, IL-6, CCL-2/MCP-1 and CXCL-10/IP-10 (Li et al., 2020). Recently, network pharmacology and molecular docking analyses performed by Xia et al. indicated that the six active ingredients (beta-carotene, kaempferol, luteolin, naringenin, quercetin and wogonin) in LHQW may play important roles in treating COVID-19 via binding to Akt1, which has been implicated in the pathogenesis of lung injury, lung fibrogenesis and SARS-CoV-2 infection (Xia et al., 2020).
3.3. XFBD recipe
XFBD granules (宣肺败毒颗粒) is composed of 13 TCM herbs or components, originated from 4 classic traditional recipes (Maxing Shigan Decoction, MXSG麻杏石甘汤; Maxing Yigan Decoction, MXYG, 麻杏薏甘汤; Qianjin Weijing Decoction, QJWJ, 千金苇茎汤 and Tingli Dazao Xiefei Decoction, TLDZXF, 葶苈大枣泻肺汤) (Wang et al., 2020). The individual components are Shegan (射干; Blackberrglily Rhizom; Belamcandae Rhizoma), Huajuhong (化橘红; Tomentose Pummelo Peel; Citri Grandis Exocarpium), Mabiancao (马鞭草; European Verbena Herb; Verbenae Herba), Mahuang (麻黄; Ephedra; Ephedrae Herba), Gancao (甘草; Licorice; Glycyrrhizae Radix et Rhizoma), Kuxingren (苦杏仁; Bitter Almond; Armeniacae Semen Amarum), Shigao (石膏; Gypsum; Gypsum fibrosum), Qinghao (青蒿; Sweet Wormwood Herb; Artemisiae Annuae Herba), Huzhang (虎杖; Giant Knotweed Rhizome; Polygoni Cuspidati Rhizoma et Radix), Tinglizi (葶苈子; Pepperweed Seed; Descurainiae Semen), Shengyiyiren (生薏苡仁; Coix Seed; Semen Coicis), Guanghuoxiang (广藿香; Patchouli; Pogostemon patchouli), Maocangshu (茅苍术; swordlike Atractylodes rhizome; Rhizoma Areactylodis lanceae), and Maogen (茅根; Lalang Grass Rhizome; Imperatae Rhizoma). As a new recipe specifically formulated for the treatment of COVID-19, it was not previously used to treat other viral infections.
3.3.1. XFBD recipe in the treatment of COVID-19
XFBD granules (宣肺败毒颗粒) was the first, newly formulated TCM recipe for COVD-19 treatment, developed employing some components that have been shown effective treating previous infections by corona viruses (Zhang, 2020a). Among the components of XFBD, Huzhang (虎杖) has the strongest anti-coronavirus effect while Mabiancao (马鞭草) has strong activity on lung damage, especially small airway damage and microthrombus caused by coronavirus (Zhang, 2020a). XFBD has been shown to significantly reduce fever, cough, fatigue and other symptoms in mild and moderate cases of COVID-19 patients, and prevent transition of mild/moderate cases into severe cases (Pan, Dong, Yang, Chen, & Peng, 2020). In a randomized trial of 42 COVID-19 patients, it has been shown that XFBD (1 bag, 2 times per day for 1 week), combined with conventional therapy, significantly improved disappearance rate of clinical symptoms (fever, cough, fatigue and loss of appetite), increased the number of WBC and lymphocytes, and reduced CRP and erythrocyte sedimentation rate (ESR) (Xiong, Wang, Du, & Ai, 2020). In a study of 280 patients with COVID-19 who were treated with XFBD, no case transformed into severe and critical conditions (ChinaDaily, 2020a). Approximately 326 out of the 1224 putative XFBD targets have been linked to the pathological mediators of COVID-19, among which 109 targets are enriched in the disease pathways of viral infection and lung injury (Wang et al., 2020). The primary biological pathways regulated by the key XFBD targets include those involved in viral infection, and parasites and bacterial infections (Wang, Li, et al., 2020). Network pharmacology and molecular docking analyses indicate that XFBD inhibits viral invasion and viral replication mainly by binding to ACE2 and 3CLp ro of SARS-CoV-2 through flavonoids and phytosterols (more discussion in Section 3), and may play a role in the treatment of COVID-19 by regulating key targets such as IL6, MAPK3, MAPK1, IL1β, CCL2, EGFR, and NOS2 (Wang, Song, et al., 2020).
3.4. HSBD recipe
Similar to XFBD above, HSBD (化湿败毒颗粒) was also specifically formulated for treating COVID-19 (Pan et al., 2020), and not previously used to treat other viral infections. It is derived with modifications from classic recipes of MXSG (麻杏石甘汤), Huoxiang Zhengqi San (藿香正气散), Xuanbai Chengqi Decoction (宣白承气汤), and TLDZXF (葶苈大枣泻肺汤) (Lai, Liang, He, Huang, & Wu, 2020). The components in HSBD include Caoguo (草果; Caoguo; Tsaoko Fructus), Fabanxia (法半夏; Pinellia Tuber; Rhizoma Pinelliae), Dahuang (大黄; Rhubarb Tangute Rhubarb; Rhei Radix Et Rhizoma), Shenghuangqi (生黄芪; Milkvetch Root; Astragali Radix), Chishao (赤芍; Red Paeoniae Trichocarpae; Paeoniae Radix Rubra), Kuxingren (苦杏仁; Bitter Almond; Armeniacae Semen Amarum), Fuling (茯苓; Poria; Indian Buead Tuckahoe), Huoxiang (藿香; Ageratum; Herba Agastachis), Mahuang (麻黄; Ephedra; Ephedrae Herba), Shigao (石膏; Gypsum; Gypsum fibrosum), Tinglizi (葶苈子; Pepperweed Seed; Descurainiae Semen), Gancao (甘草; Licorice; Glycyrrhizae Radix et Rhizoma), Houpo (厚朴; Officinal magnolia bark; Magnoliae Officinalis Cortex), and Cangshu (苍术; Atractylodes; Rhizoma Atractylodis) (Yang, 2020).
3.4.1. HSBD recipe in the treatment of COVID-19
Treatment of COVID-19 patients with HSBD (化湿败毒颗粒) have been optimized based on clinical practice at Wuhan Jinyintan Hospital, one of the major sites caring for the COVID-19 patients (Wu, 2020). It has been used for the treatment of mild, moderate and severe cases (Wu, 2020). Recently, HSBD was approved by the United Arab Emirates health authorities and listed as a registered emergency drug for treating COVID-19 (Wu, 2020). HSBD has been shown to be effective in significantly shortening the time to nucleic acid turning negative in patients with COVID-19 (Li et al., 2020). In addition, it can reduce the average length of hospitalization, and significantly improve clinical symptoms and pulmonary CT findings (Li, Wang, Li, Zheng, et al., 2020). Pan et al. have shown that HSBD can effectively relieve symptoms of severe COVID-19, and reduce the conversion rate of mild/moderate to moderate/severe cases, and decrease mortality rate of critical illness (Pan et al., 2020). In a study of 60 patients, HSBD (137 g, twice per day) strengthened the efficacy of Lopinavir-Ritonavir in the treatment of COVID-19 (Shi et al., 2020). Besides, HSBD was found to reduce viral load in lung tissues by 30% in a mouse model (Li, Wang, Li, Zheng, et al., 2020). It has been shown that HSBD has anti-inflammatory, anti-viral and immune-modulating effects (Lai et al., 2020). Xie et al. explored the potential mechanisms of HSBD in treating COVID-19 using network pharmacology and molecular docking analyses, from which 269 active ingredients and 2629 drug targets were retrieved (Xie et al., 2020). The GO functional enrichment analysis indicate that lipopolysaccharide, reactive oxygen species (ROS) and interferon-gamma-mediated signaling pathways are likely inhibited by HSBD in the treatment of COVID-19 (Xie et al., 2020). KEGG pathway enrichment analysis indicate that modulations of AGE-RAGE, TNF, NFkB, and RIG-I-like receptor signaling pathways might be involved in mediating therapeutic effects of HSBD on COVID-19 (Xie et al., 2020). Besides, network pharmacology and molecular docking analyses on molecular targets and mechanisms of HSBD in the treatment of COVID-19 indicate that compound–target network mainly involves 178 compounds and 272 corresponding targets, among which key targets include MAPK3, MAPK8, TP53, CASP3, IL-6, TNF, MAPK1, CCL2, and PTGS2 (Tao et al., 2020). A total of 522 GO items were identified by GO enrichment analysis, while 168 signaling pathways were identified by KEGG analysis, mainly involving TNF-α, PI3K/Akt, NOD-like receptor, MAPK, and HIF-1 signaling axis (Tao et al., 2020). Sun and colleagues have identified 138 active ingredients in HSBD by network pharmacology; and molecular docking analyses indicate that baicalein has the highest affinity for 3CLpro, whereas licorice phenol has the highest affinity for ACE2 (Sun, Tao, Xu, & Yuan, 2020). Likewise, Tao et al. showed that baicalein and quercetin are the top two ingredients of HSBD that have high affinity for ACE2, presumably leading to therapeutic efficacies via inhibition of ACE2 (Tao et al., 2020). In addition, network pharmacology and molecular docking technology performed by Xie et al. demonstrate that quercetin, luteolin and kaempferol have strong binding activities for 3CLpro, S protein and ACE2 (Xie et al., 2020).
3.5. XBJ recipe
XBJ injection (血必净) is a five-herb combination, which is composed of Danggui (当归; Angelica root; Angelica sinensis), Danshen (丹参; Salvia; Salviae Miltiorrhizae Radix et Rhizoma), Chishao (赤芍; Red Paeoniae Trichocarpae; Paeoniae Radix Rubra), Honghua (红花; Safflower; Carthami flos), and Chuanxiong (川芎; Szechuan Lovage Rhizome; Ligusticum chuanxiong Hort) (Song et al., 2020; Yin & Li, 2014).
3.5.1. XBJ recipe in the treatment of previous infectious diseases
After being approved in 2003 by The National Medical Products Administration (NMPA) (Chinese: 国家药品监督管理局) (formerly the China Food and Drug Administration, or CFDA), XBJ injection has been used to treat infections caused by H1N1, H7N9, MERS, Ebola virus and dengue virus (Tong, Wu, Ni, Shen, & Liu, 2020). XBJ has been frequently used as an add-on therapy to treat sepsis or septic shock, and has been shown to attenuate inflammation and bacteriostasis without side effects (Fan, Cheng, Fang, Chen, & Su, 2020; Gao, Chai, & Yao, 2013; Zheng et al., 2019). The combination of XBJ with Western Medicine Ulinastatin (UTI, an urinary trypsin inhibitor, commonly used as a drug to treat acute inflammatory disorders) was reported to be more effective than UTI alone in treating septic patients (Xiao et al., 2018). In addition, the combined therapy not only drastically reduced the 28-day mortality rate, mechanical ventilation time, length of ICU stay, occurrence rate of multiple organ dysfunction syndrome, and the acute physiology and chronic health evaluation score (APACHE II, a commonly used severity-of-disease classification system), but also decreased levels of procalcitonin (PCT), TNF-α, and IL-6 (Xiao et al., 2018). In a multi-center RCT of 710 critically-ill patients with severe community-acquired pneumonia, XBJ (100 mL, every12 hours, for at least 5 days up to a maximum of 7 days in adult patients) was found effective in improving survival rate, while shortening duration of mechanical ventilation and that of ICU stay (Song et al., 2019). Besides, a meta-analysis of 867 patients with sepsis demonstrated that XBJ treatment improved coagulopathy possibly by decreasing platelet counts, as well as by shortening of activated partial thromboplastin time, prothrombin time, and thrombin time (Hou, Feng, Lin, & Tan, 2015). In another meta-analysis of case–control studies on sepsis, XBJ was found to significantly reduce 28-day mortality and improve clinical parameters including the APACHE II score, white blood cell count, C-reactive protein (CRP) and procalcitonin levels, and body temperature (Shi, Hong, Qian, Cai, & Chen, 2017). In influenza A (H1N1) induced severe pneumonia in mice, XBJ reduced levels of inflammatory mediators of TNF-α and IL-6, and alleviated lung injury (Ma, Shan, Guo, & Wang, 2015).
3.5.2. XBJ recipe in the treatment of COVID-19
Based on the clinical evidence on XBJ treatment of sepsis, bacterial pneumonia and acute respiratory distress syndrome (ARDS), XBJ was recommended by China's National Health Commission to treat severe and critical cases of COVID-19, especially during systematic inflammatory response syndrome (SIRS) and/or multi-organ failure (Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7), 2020; Song et al., 2020). XBJ was used more frequently in severe/critical patients with significant benefits for earlier discharge (Huang et al., 2020). XBJ (100 mL, twice per day) combined with routine treatment resulted in significantly reduced IL-6 levels and body temperature in a group of 42 COVID-19 patients (Guo et al., 2020). In a retrospective study of 44 COVID-19 patients, XBJ (50 mL XBJ in 100 mL saline, i.v. for 7 days) treatment group (n = 22) showed reduced lung inflammation by CT imaging although nucleic acid yet turned negative (Zhang, Li, Zhang, Wang, & Jiang, 2020). In a randomized study of 60 severe COVID-19 patients, low dose (50 mL, twice per day for 7 days) and high dose (100 mL, twice per day for 7 days) of XBJ decreased levels of CRP and ESR compared to routine treatment group while only 100 mL XBJ increased white blood cell count (WBC) (Wen, Zhou, Jiang, & Huang, 2020). Besides, the APACHE II score after treatment was significantly lower in high dose XBJ group than those in low dose XBJ group and the group with routine treatment (Wen et al., 2020). By increasing Th1/Th2 ratio, XBJ injection has been shown to increase the number of Th1 cells in septic rats (Zhang, Sun, Wen, & Yin, 2006). Chen and colleagues reported that XBJ (18 mL/kg, i.p. twice at 0 h and 24 h.) improves survival in response to septic shock in a murine model of polymicrobial sepsis, partially through preventing cytokine storm, inhibiting inflammation and regulating the balance of Tregs and Th17 cells (Chen et al., 2018). In addition, XBJ has been shown to protect against SARS-CoV-2-induced cell death in virus infected Vero E6 cells, and to reduce the average size and the number of the plaque in a dose dependent fashion (12.5–50 mg/mL) (Ma et al., 2020). XBJ was also predicted to treat SARS-CoV-2 infection via modulation of arachidonic acid metabolic pathway (principally used to synthesize inflammatory cytokines, such as MCP-1, TNF, IL, IFN, etc.) by pharmacophore models (Ren et al., 2020). Kong et al. reported that quercetin, gallic acid, luteolin, rosmarinic acid, rutin, kaempferol hlorogenic acid, tanshinone II A, hydroxysafflor yellow A, and paeoniflorin are the primary active ingredients of XBJ, identified by network pharmacology to be responsible for the treatment effects of XBJ on COVID-19 (Kong et al., 2020). Hydroxysafflor yellow A, chlorogenic acid and salvianolic acid B were identified as major compositions in XBJ by molecular docking, through “multi-component, multi-target, multi-pathway” pattern to exert protective effects on inflammation and vascular endothelial cell injury (He, Duan, Li, & Zhang, 2020).
3.6. LCDD recipe
LCDD (清肺排毒汤) is composed of 4 classic TCM recipes: MSXG (麻杏石甘汤), She Gan Ma Huang decoction (SGMH, 射干麻黄汤), Xiao Chai Hu (XCH, 小柴胡), and Wu Ling San (WLS, 五苓散) that were all initially described in Treatise on Cold Damage Diseases (Shanghan Lun,伤寒论). Out of the 4 recipes, MXSG is the most important constitute shared among the 3 effective TCM decoctions found effective in treating COVID-19 as described here and above (LCDD, XFBD, HSBD) (Chen et al., 2020). MXSG (components: Mahuang, 麻黄; Gancao, 甘草; Kuxingren, 苦杏仁; Shigao, 石膏) has been used for the treatment of influenza by disrupting viral surface structure and inhibiting viral entry (Hsieh et al., 2012). Overall, LCDD is a recipe of 21 Chinese herbs/components, which include Xixin (细辛; Manchurian Wildginger Herb; Asari Radix Et Rhizoma), Shegan (射干; Blackberrglily Rhizome; Belamcandae Rhizoma), Shanyao (山药; Common Yam Rhizome; Dioscoreae Rhizoma), Kuandonghua (款冬花; Common Coltsfoot Flower; Farfarae Flos), Zhishi (枳实; Immature Bitter Orange; Aurantii Fructus Immaturus), Ziyuan (紫苑; Tatarian Aster Root; Asteris Radix et Rhizoma), Kuxingren (苦杏仁; Bitter Almond; Armeniacae Semen Amarum), Chenpi (陈皮; Tangerine Peel; Citri Reticulatae Pericarpium), Shengjiang (生姜; Fresh Ginger; Zingiberis Rhizoma Recens), Huangqin (黄芩; Baikal Skullcap Root; Scutellariae Radix), Guizhi (桂枝; Ramulus cinnamomi; Cinnamomi Ramulus), Mahuang (麻黄; Ephedra; Ephedrae Herba), Jiangbanxia (姜半夏; Pinelliaternata processed with ginger; Pinelliae Rhizoma), Fuling (茯苓; Poria; Indian Buead Tuckahoe), Huoxiang (藿香; Ageratum; Herba Agastachis), and Zhigancao (炙甘草; Prepared Liquorice Root; Glycyrrhizae Praeparata cum Melle Radix et Rhizoma) (Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7), 2020).
3.6.1. LCDD recipe in the treatment of previous infectious diseases
A prospective RCT of 410 patients with H1N1 influenza indicate that MXSG (麻杏石甘汤)–Yin Qiao San (银翘散) combination can serve as an alternative treatment for H1N1 infection (Wang et al., 2011). Time to fever resolution was reduced by 19% with Qseltamivir plus MXSG–Yin Qiao San treatment, compared to Oseltamivir treatment alone (Wang et al., 2011). In an experimental study, the transcriptomic analysis of lung tissues of LPS induced pneumonic rats revealed that MXSG regulates multiple complement and coagulation cascades as well as thrombin system to interfere with infection (Yang et al., 2020). Mechanistically, MXSG has been shown to attenuate host cell entry of influenza virus by regulating PI3K/AKT signaling pathway (Hsieh et al., 2012). The second recipe in LCDD, SGMH (射干麻黄汤), is composed of Mahuang (麻黄), Jiangbanxia (姜半夏), Shengjiang (生姜), Ziyuan (紫苑), Kuandonghua (款冬花), Shegan (射干), and Xixin (细辛); and is a classical prescription for the treatment of flu-like symptoms, tonsillitis, and asthma (Lin et al., 2020). It has been used to treat patients with cough variant asthma (CVA) (Wang et al., 2012), and shown to downregulate production of Th2/Th17 cells while upregulating CD4 + FoxP3+ Tregs cells in asthmatic airway hyperresponsiveness in mice (Lin et al., 2020). The third recipe in LCDD, XCH (小柴胡), is composed of Radix Gancao (甘草), Chai Hu (柴胡), Huangqin (黄芩), Banxia (半夏), Shengjiang (生姜), and has been shown to possess antiviral and various anticarcinogenic properties (Cheng, Ng, & Lin, 2006; Zheng et al., 2013). XCH has been used to treat chronic hepatitis B (Kong et al., 2019), and found to attenuate liver fibrosis by activating Nrf2 pathway (Li et al., 2017). The forth recipe in LCDD, WLS (五苓散), is composed of Guizhi (桂枝), Zexie (泽泻), Zhuling (猪苓), Cangshu (苍术), and Fuling (茯苓), and a well-known Chinese prescription for nephritic syndrome, which has been shown to improve kidney excretion function and inhibit inflammatory response (Yang et al., 2015). A recent study reported that many water-soluble polysaccharides in LCDD possess multiple bioactivities of immunomodulation (such as glycyrrhiza polysaccharide promoting the maturation, differentiation and reproduction of immune cells of lymphocytes and macrophages, as well as activating the reticuloendothelial system), anti-inflammation (such as ephedra polysaccharide regulating Factor-1/Smad2 signaling pathway and inhibiting the TLR4 signaling pathway), and anti-oxidation (such as bupleurum polysaccharide reducing the content of MDA in serum and bronchoalveolar lavage fluid (BALF) in models of acute lung injury), as well as antibacterial and antitussive activities (Cao et al., 2020).
3.6.2. LCDD recipe in the treatment of COVID-19
Among the 6 recipes found most effective in treating patients with COVID-19, LCDD (清肺排毒汤) is recommended as the primary recipe by the General Office of National Health Commission, China, and the Office of State TCM Administration (Notice, 2020). Recently, a retrospective study of 63 patients with confirmed COVID-19 showed that the combination of LCDD with Western Medicine exerted significantly better anti-inflammatory effects compared to treatment with Western Medicine alone, in patients with mild and moderate COVID-19 (Xin et al., 2020). Combined treatment not only diminished levels of CRP, creatine kinase, creatine kinase-myocardial band, lactate dehydrogenase, and blood urea nitrogen, but also mitigated the extent of multi-organ impairment (Xin et al., 2020). In a retrospective study of 98 patients with COVID-19, LCDD treatment relieved symptoms such as fever and cough, normalized laboratory indexes including LYMPH%, CPR, ESR, and reversed pulmonary CT imaging characteristics showing obvious absorption of inflammation, only 6 days after initiation of the treatment (Wang et al., 2020). Four provincial hospitals in China used LCDD to treat 214 patients with COVID-19, following a protocol of 3 days as a course of treatment, and the total effective rate was higher than 90% (Zhang, 2020b). Among them, symptoms and CT imaging data of 60% of the patients improved significantly, and 30% of patients had stable symptoms without exacerbation (Zhang, 2020b). Out of 1264 patients from 10 provinces in China who received LCDD treatment, 1214 patients recovered, according to the 7th version of the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (ChinaDaily, 2020b; Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7), 2020). The discharge criteria included were: 1) Body temperature back to normal for more than three days; 2) Respiratory symptoms improve obviously; 3) Pulmonary imaging shows obvious absorption of inflammation, 4) Nucleic acid tested negative twice consecutively from respiratory tract samples such as sputum and nasopharyngeal swabs (sampling interval being at least 24 h) (Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7), 2020). Of note, 96.1% (1214/1264, as above) of the patients treated with LCDD satisfied these criteria to be discharged, and no cases deteriorated during treatment (ChinaDaily, 2020b). More recently, in a retrospective multicenter study of 782 confirmed COVID-19 cases from 54 hospitals in 9 provinces of China, Shi et al. reported that early treatment with LCDD at less than 1 week, 1–2 weeks, or 2–3 weeks of onset of symptoms had a higher likelihood of recovery compared to treatment initiated 3 weeks after onset of symptoms (Shi et al., 2020). Early LCDD treatment was associated with favorable outcomes, including faster time to recovery, shorter time of viral shedding, and shorter time of hospital stay (Shi, Liu, et al., 2020).
Studies have been carried out to examine inhibitory effects on COVID-19 of active ingredients in LCDD. Network pharmacology analysis conducted by Zhang et al. showed that MXSG in LCDD inhibits SARS-CoV-2 replication, as well as cytokine storm by promoting TH cell differentiation and T cell homeostatic proliferation (Zhang, Yang, He, G, & Li, 2020). Furthermore, through molecular docking prediction, it was found that patchouli alcohol, ergosterol and shionone in LCDD have strong anti-COVID-19 effect (Ren, Zhang, & Wang, 2020; Wu et al., 2020). Zhou et al. identified 87 major bioactive ingredients in LCDD using UHPLC-LTQ-Orbitrap-M, which include 43 flavonoids, 9 alkaloids, 4 triterpenoid saponins, 1 sesquiterpene, 2 coumarins, 10 phenolic acids and 18 other compounds (Zhou et al., 2020). Another study identified 58 flavonoids, 20 glycosides, 13 carboxylic acids, 7 saponins, 6 alkaloids, 4 terpenes, and other types of compounds by UPLC-Q-TOF/MS (Yang, Liu, et al., 2020). In this work, the Toll-like receptor signaling pathway was shown to play an important role in LCDD induced therapeutic effect on COVID-19 (Yang, Liu, et al., 2020). Zhao et al. elucidated that baicalin, glycyrrhizic acid, hesperidin and hyperoside are important ingredients and IL6, IL10, TNF-α, AKT1, TP53, PTGS2 and HMOX1 are important targets of LCDD to treat COVID-19 (Zhao et al., 2020). Su et al. found that baicalin and baicalein, active ingredients of Huangqin (黄芩), were the first identified non-covalent, non-peptidomimetic inhibitors of SARS-CoV-2 3CLpro (Su et al., 2020). Baicalin and baicalein were effective in decreasing the replication of coronaviruses in a cell-based system by interacting with two catalytic residues of 3CLpro (the crucial S1/S2 subsites and the oxyanion loop) to ensconce in the core of the substrate-binding pocket (Su et al., 2020). Liu and colleagues found that baicalein and ethanol extract of Huangqin (黄芩), inhibit the replication of SARS-CoV-2 in Vero cells (Liu et al., 2021). Using system biological analyses, Chen et al. reported that glycyrrhizic acid may be effective in regulating immune responses by acting on NOD-like and Toll-like signaling pathways to promote production of interferons, activate and balance T-cells, and to inhibit excessive inflammatory responses to prevent the onset of cytokine storm (Chen et al., 2020). Sekiou et al. found that quercetin, hispidulin, cirsimaritin, sulfasalazine, artemisin and curcumin exhibited potential inhibitory effects on 3CLpro and ACE2 using in-silico identification (Sekiou, Bouziane, Bouslama, & Djemel, 2020). Patchouli alcohol from Huoxiang (藿香), Shionone from Ziwan (紫菀, and Ergosterol from Zhuling (猪苓) showed affinity for 3CLpro and ACE2 by network pharmacology analyses (Wu, Wang, et al., 2020).
4. Common components, ingredients and targets of TCM recipes in treating COVID-19
4.1. Common components in the six TCM recipes effective for COVID-19 treatment
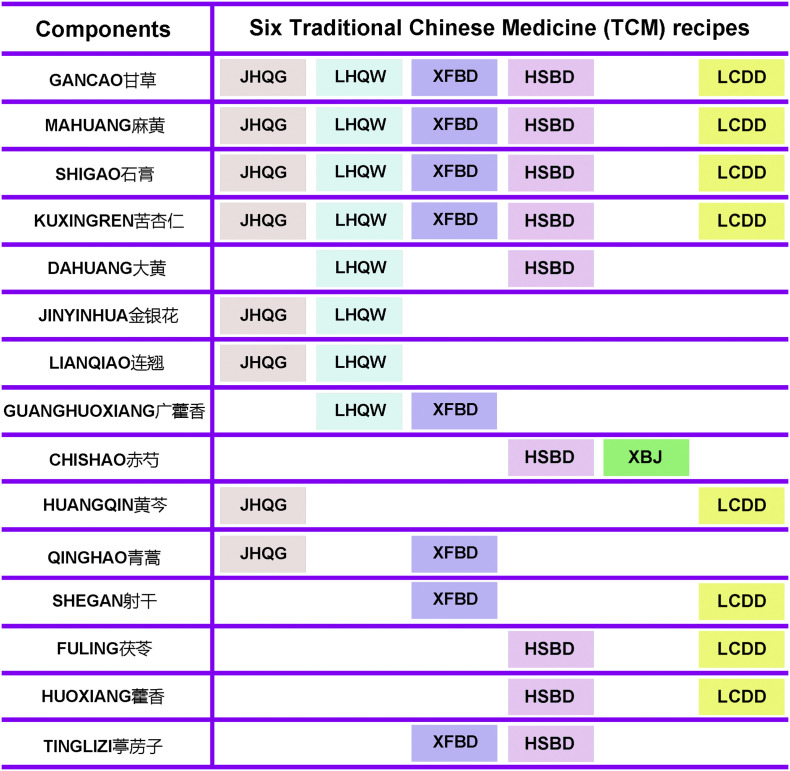
We compared the herbs/components cross the six most effective recipes of “three TCM decoctions and three formulated Chinese medicines” for COVID-19 treatment, and found some common herbs/components among them (Fig. 2 ). Gancao (甘草), Mahuang (麻黄), Shigao (石膏) and Kuxingren (苦杏仁) are the common components among LHQW, LCDD, XFBD, HSBD and JHQG. Jinyinhua (金银花) and Lianqiao (连翘) are the common herbs between LHQW and JHQG. Guanghuoxiang (广藿香) is the common herb between LHQW and XFBD. Chishao (赤芍) is the common herb between XBJ and HSBD. Huangqin (黄芩) is the common herbs of LCDD and JHQG. Fuling (茯苓) and Huoxiang (藿香) are the common herbs of LCDD and HSBD. Of note, Kuxingren (苦杏仁) and Mahuang (麻黄) are among the top 10 herbs/components that have been used most frequently in treating mild, moderate and severe cases of COVID-19 (Ang, Lee, Choi, Zhang, & Soo Lee, 2020). Shigao (石膏, the common component among LHQW, LCDD, XFBD, HSBD and JHQG), and Tinglizi (葶苈子), have been used for treating moderate and severe cases (Ang et al., 2020). In addition, Huoxiang (藿香), Lianqiao (连翘), Cangshu (苍术), and Huangqin (黄芩) (the common herbs between LCDD and JHQG) have been frequently used to treat patients of COVID-19 at mild and moderate stages (Ang et al., 2020). Notably, Gancao (甘草, the common herb of LHQW, LCDD, XFBD, HSBD and JHQG) is among the top 10 herbs/components used most frequently to treat patients at mild, moderate, severe and recovery stages (Ang et al., 2020). Network pharmacology analyses indicate that Lianqiao (连翘), Jinyinhua (金银花), Honghua (红花), and Mabiancao (马鞭草) have the most abundant active compounds known to have therapeutic effects on COVID-19, and they may therefore compose the best combination scheme of Chinese herbs/TCM components for COVID-19 treatment (Liang et al., 2020).
Fig. 2.
The common components of the six TCMs (JHQG, LHQW, XFBD, HSBD, XBJ and LCDD). All of the components in JHQG, LHQW, XFBD, HSBD, XBJ and LCDD were compared and the common components of the six TCM recipes are presented.
4.2. Common active ingredients in TCM recipes effective for the treatment of COVID-19
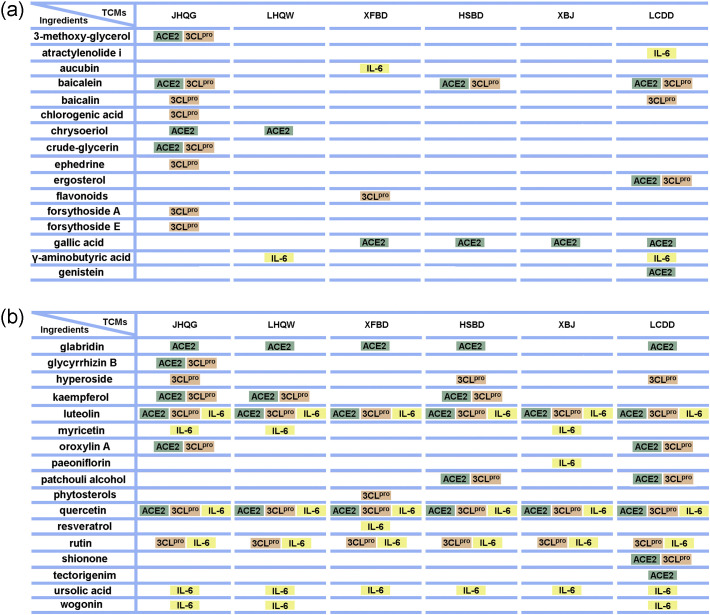
Network pharmacology analyses were employed to identify common active ingredients in TCM recipes proven effective for COVID-19 treatment (Fig. 3 ) (Liang et al., 2020; Niu et al., 2020). Quercetin, glabridin, and gallic acid, active ingredients of the six recipes discussed in Section 2, have been predicted to inhibit COVID-19 via ACE2 (Niu et al., 2020) (Fig. 3). Quercetin, ursolic acid, luteolin, and rutin were found to be the common active ingredients of the six recipes discussed in Section 2 that exert therapeutic effects on COVID-19 via modulation of IL-6 (Liang et al., 2020). Rutin is the unique ingredient shared by JHQG, LHQW, HSBD, XFBD, XBJ and LCDD that targets 3CLpro and IL-6 (Fig. 3). Ursolic acid is the unique ingredient shared by all of the six recipes that targets IL-6 alone (Fig. 3). Luteolin is one of the two ingredients shared by all of the six recipes that targets ACE2, 3CLpro and IL-6 (Fig. 3). Quercetin is the other ingredient shared by all of the six recipes that targets ACE2, 3CLpro and IL-6 to exert therapeutic effects on COVID-19 (Fig. 3). The results of molecular docking analyses indicate that quercetin is one of the top two ingredients (the other one is baicalein) of HSBD, which has high affinity for ACE2 (Tao et al., 2020). Therefore, quercetin in HSBD may regulate multiple signaling pathways through ACE2, which in turn exert therapeutic effects on COVID-19 (Tao et al., 2020). The results of molecular docking analyses indicate that quercetin has a high affinity for 3CLpro and ACE2. Sekiou et al. also reported that quercetin exhibits better inhibitory effects on 3CLpro and ACE2 than Hydroxy-Chloroquine using in-silico identification (Sekiou et al., 2020). Therefore, quercetin may represent one of the most important TCM ingredients that have played a major role in mechanistically inhibiting pathophysiological pathways of COVID-19.
Fig. 3.
The ingredients of the six TCM recipes (JHQG, LHQW, XFBD, HSBD, XBJ and LCDD) that target 3CLpro, ACE2 or/and IL-6. 3CLpro, ACE2 and IL-6 are three of the most important targets for treating COVID-19. The active ingredients in the six TCM recipes (JHQG, LHQW, XFBD, HSBD, XBJ and LCDD) that target/predicted to target 3CLpro, ACE2 and IL-6 are summarized.
4.3. TCM regulation of ACE2 for the treatment of COVID-19
Binding to ACE2 is a critical initial step for SARS-CoV-2 to enter into target cells (Hoffmann et al., 2020; Lan et al., 2020). Therefore, ACE2 is an important therapeutic target for COVID-19 (Monteil et al., 2020). Several active ingredients of TCM as discussed above, including quercetin, glabridin, and gallic acid, have been shown to downregulate ACE2 expression by regulating the aforementioned transcription factors (TFs) or miRNAs (Niu et al., 2020). The TFs and miRNAs include hepatocyte nuclear factor 4 alpha (HNF4A), peroxisome proliferator-activated receptor gamma (PPARG), hsa-miR-2113, and hsa-miR-421 (Niu et al., 2020). Network pharmacology analyses and high throughput molecular docking analyses showed that 3-methoxy-glycerol, crude-glycerin and glycyrrhizin B, the active ingredients of JHQG, have strong binding activity for ACE2 (Shen et al., 2020). Notably, these analyses revealed that the action mechanism of JHQG might be attributed to its active ingredients of kaempferol, baicalein and oroxylin A, which regulate multiple signaling pathways (such as PTGS1, PTGS2, BCL2, HSP90AB1, HSP90AA1, NCOA2 and CASP3) by binding to ACE2, thereby exerting therapeutic effects on COVID-19 (Gong et al., 2020; Jimilihan et al., 2020). Additional network pharmacology and molecular docking analyses indicate that XFBD inhibits viral invasion and viral replication mainly by binding to ACE2 through flavonoids and phytosterols (Wang, Song, et al., 2020). Baicalein and quercetin, the two major active ingredients of HSBD, were predicted by network pharmacology and molecular docking analyses to treat COVID-19 by binding ACE2 (Tao et al., 2020). The results of molecular docking predict that quercetin, luteolin, kaempferol, active ingredients from HSBD, physically bind to ACE2 (Lai et al., 2020). These findings demonstrate that HSBD exerts therapeutic effects on COVID-19 through ACE2 (Lai et al., 2020). In-silico identification results indicated that quercetin, hispidulin, cirsimaritin, sulfasalazine, artemisin and curcumin exhibit potential inhibitory effects on ACE2 (Sekiou et al., 2020). Besides, network pharmacology study showed that ACE2 was also targeted by patchouli alcohol from Huoxiang (藿香), shionone from Ziwan (紫菀), and ergosterol from Zhuling (猪苓) (Wu, Wang, et al., 2020). Therefore, ACE2 appears to be one of the major targets for TCM to exert therapeutic effects on COVID-19.
4.4. TCM regulation of 3CLpro for the treatment of COVID-19
Coronavirus 3-chymotrypsin-like protease (known as Mpro, 3CL hydrolase or 3CLpro) is a three-domain (domains I to III) cysteine protease that is highly conserved among coronaviruses and required for proteolytic maturation of the coronaviruses (Dai et al., 2020; Elmezayen, Al-Obaidi, Sahin, & Yelekci, 2020; Zhang et al., 2020). Besides, 3CLpro has no human homolog (Dai et al., 2020). Therefore, 3CLpro is an attractive drug target for coronaviruses including SARS-CoV-2 (Dai et al., 2020; Elmezayen et al., 2020; Zhang, Lin, et al., 2020). Su et al. found that baicalin and baicalein, active ingredients of Huangqin (黄芩), were the first identified non-covalent, non-peptidomimetic inhibitors of SARS-CoV-2 3CLpro, effective in decreasing the replication of coronaviruses in a cell-based system, by interacting with two catalytic residues (the crucial S1/S2 subsites and the oxyanion loop) of the 3CLpro to ensconce in the core of the substrate-binding pocket (Su et al., 2020). 3CLpro was predicted to be the target of the active ingredients of JHQG (3-methoxy-glycerol, crude-glycerin and glycyrrhizin B,3-methoxy-glycerol, crude-glycerin and glycyrrhizin B) by network pharmacology prediction and high throughput molecular docking analyses (Shen et al., 2020). Chlorogenic acid, forsythoside A, and ephedrine were found to be the core active ingredients of JHQG that have strong affinity for 3CLpro by visualization analysis, thereby inhibiting viral replication (Ren, Yin, et al., 2020). Network pharmacology and molecular docking analyses indicated that flavonoids and phytosterols are the two ingredients in XFBD that inhibit SARS-CoV-2 invasion and replication mainly by binding to 3CLpro, thereby exerting therapeutic effects on COVID-19 (Wang, Song, et al., 2020). Quercetin, luteolin, kaempferol as the active ingredients of HSBD, were predicted to have high affinity for 3CLpro, indicating effects of HSBD on viral replication and maturation. Liu and colleagues found that baicalein and ethanol extract of Huangqin (黄芩), inhibit 3CLpro activity in vitro with activity assay using a peptide substrate (Thr-Ser-Ala-Val-Leu-Gln-pNA) according to the published procedure of SARS-CoV 3CLpro assay (Liu et al., 2021). Besides, the docking model verified that baicalein binds well to the substrate binding site of 3CLpro (Liu et al., 2021). The carbonyl group of baicalein was hydrogen bonded with the E166 backbone amide group of 3CLpro, while catalytic residues H41 and C145 of 3CLpro were well covered by baicalein, accounting for baicalein's inhibitory effect on SARS-CoV-2 via 3CLpro (Liu et al., 2021). In addition, patchouli alcohol, shionone, and ergosterol from LCDD were all predicted by network pharmacology to treat COVID-19 via targeting of 3CLpro (Wu, Wang, et al., 2020). Therefore, 3CLpro may have served as an important target for TCM in preventing the replication and maturation of SARS-CoV-2 to treat COVID-19, and that detailed molecular mechanisms of TCM interacting with 3CLpro for the treatment of COVID-19 warrant further investigation.
4.5. TCM regulation of IL-6 for the treatment of COVID-19
Cytokine storm is a hyperactive immune response characterized by release of interferons, interleukins, tumor-necrosis factors, chemokines, and several other mediators, which represents one of the major pathological features of COVID-19 (Sinha, Matthay, & Calfee, 2020). IL-6, a proinflammatory cytokine, is a key mediator of the acute inflammatory response and the cytokine storm (Sinha et al., 2020; Turnquist, Ryan, Horikawa, Harris, & Harris, 2020; Zhang et al., 2020). Elevation in serum IL-6 levels correlates with ARDS, multi-organ failure, and other adverse clinical outcomes in COVID-19 (Moore & June, 2020). IL-6 has been shown to be a good biomarker for earlier detection of COVID-19 progression (Wang, Fei, Li, Zhao, & Yu, 2020), and a promising target for the treatment of COVID-19 (Montesarchio et al., 2020; Sinha et al., 2020). In addition to targeting ACE2 and 3CLpro, TCM has also been shown to modulate IL-6 to relieve COVID-19 symptoms. Network pharmacology was used by Niu et al. to identify the phytochemicals in TCM for the treatment of COVID-19 that regulates IL-6 (Liang et al., 2020). Quercetin, ursolic acid, luteolin, and rutin, active ingredients of the six recipes discussed in Section 2, have been shown to decrease IL-6 levels (Liang et al., 2020). Network pharmacology and molecular docking analyses indicate that XFBD may play a role in the treatment of COVID-19 by regulating IL-6 after viral infection of host cells, exerting anti-cytokine storm and anti-oxidation effects (Wang, Song, et al., 2020). Analyses applied to identification of molecular targets and mechanisms of HSBD in the treatment of COVID-19 indicate that compound–target network mainly contains 178 compounds and 272 corresponding targets, among which IL-6 is a key target (Tao et al., 2020).
5. Conclusion
TCM has a well-documented history for treating infectious disease. During the fight against COVID-19 in China, six TCM recipes of JHQG, LHQW, XFBD, HSBD, XBJ and LCDD have been shown to be most effective in treating patients with COVID-19. We have systematically reviewed the components, active ingredients, and the potential molecular mechanisms of the 6 TCM recipes in treating COVID-19 and infections by other viruses. The common components/active ingredients among the 6 recipes were also identified to represent most promising drug candidates, characterization of which may result in rapid development of novel therapeutics for the treatment of COVID-19. These ingredients may exert therapeutic effects on COVID-19 via targeting of ACE2, 3CLpro and IL-6. Therefore, the key active ingredients of TCM recipes and their molecular mechanisms driving therapeutic efficacies on COVID-19, warrant in-depth and immediate investigation to help better manage the devastating disease of COVID-19.
Declaration of Competing Interest
The authors declare that there are no conflicts of interest.
References
- Ang L., Lee H.W., Choi J.Y., Zhang J., Soo Lee M. Herbal medicine and pattern identification for treating COVID-19: A rapid review of guidelines. Integrative Medicine Research. 2020;9:100407. doi: 10.1016/j.imr.2020.100407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindea G., Mlecnik B., Hackl H., Charoentong P., Tosolini M., Kirilovsky A.…Galon J. ClueGO: A Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics. 2009;25:1091–1093. doi: 10.1093/bioinformatics/btp101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao P., Wu S., Wu T., Deng Y., Zhang Q., Wang K., Zhang Y. The important role of polysaccharides from a traditional Chinese medicine-Lung Cleansing and Detoxifying Decoction against the COVID-19 pandemic. Carbohydrate Polymers. 2020;240:116346. doi: 10.1016/j.carbpol.2020.116346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Wang Y.K., Gao Y., Hu L.S., Yang J.W., Wang J.R.…Cao Y.B. Protection against COVID-19 injury by qingfei paidu decoction via anti-viral, anti-inflammatory activity and metabolic programming. Biomedicine & Pharmacotherapy. 2020;129:110281. doi: 10.1016/j.biopha.2020.110281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Hu C., Hood M., Zhang X., Zhang L., Kan J., Du J. A novel combination of vitamin C, Curcumin and Glycyrrhizic acid potentially regulates immune and inflammatory response associated with coronavirus infections: A perspective from system biology analysis. Nutrients. 2020:12. doi: 10.3390/nu12041193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Feng Y., Shen X., Pan G., Fan G., Gao X.…Zhu Y. Anti-sepsis protection of Xuebijing injection is mediated by differential regulation of pro- and anti-inflammatory Th17 and T regulatory cells in a murine model of polymicrobial sepsis. Journal of Ethnopharmacology. 2018;211:358–365. doi: 10.1016/j.jep.2017.10.001. [DOI] [PubMed] [Google Scholar]
- Chen Z., Nakamura T. Statistical evidence for the usefulness of Chinese medicine in the treatment of SARS. Phytotherapy Research. 2004;18:592–594. doi: 10.1002/ptr.1485. [DOI] [PubMed] [Google Scholar]
- Cheng D., Li Y. Clinical effectiveness and case analysis in 54 NCP patients treated with Lanhuaqingwen granules. World Chinese Medicine. 2020;15:150–154. [Google Scholar]
- Cheng P.W., Ng L.T., Lin C.C. Xiao chai hu tang inhibits CVB1 virus infection of CCFS-1 cells through the induction of Type I interferon expression. International Immunopharmacology. 2006;6:1003–1012. doi: 10.1016/j.intimp.2006.01.011. [DOI] [PubMed] [Google Scholar]
- ChinaDaily 2020. https://cn.Chinadaily.Com.Cn/a/202003/15/ws5e6ddca7a3107bb6b57a6998.html
- ChinaDaily 2020. https://covid-19.chinadaily.com.cn/a/202003/24/WS5e795bb6a3101282172816c2.html
- Curran J. The yellow emperor’s classic of internal medicine. BMJ. 2008;336(7647):777. [Google Scholar]
- Dai W., Zhang B., Jiang X.M., Su H., Li J., Zhao Y.…Liu H. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science. 2020;368:1331–1335. doi: 10.1126/science.abb4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis G., Jr., Sherman B.T., Hosack D.A., Yang J., Gao W., Lane H.C., Lempicki R.A. DAVID: Database for annotation, visualization, and integrated discovery. Genome Biology. 2003;4:P3. [PubMed] [Google Scholar]
- Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7) Chinese Medical Journal. 2020;133:1087–1095. doi: 10.1097/CM9.0000000000000819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y., Zeng L., Li R., Chen Q., Zhou B., Chen Q.…Zhang F. The Chinese prescription lianhuaqingwen capsule exerts anti-influenza activity through the inhibition of viral propagation and impacts immune function. BMC Complementary and Alternative Medicine. 2017;17:130. doi: 10.1186/s12906-017-1585-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan C., Xia W., Zheng C., Sun G., Li Z., Li Q.…Liu Q. Clinical observation of Jinhua Qinggan granule in treating pneumonia infected by novel coronavirus. Journal of Traditional Chinese Medicine. 2020;61:1473–1477. [Google Scholar]
- Elmezayen A.D., Al-Obaidi A., Sahin A.T., Yelekci K. Drug repurposing for coronavirus (COVID-19): In silico screening of known drugs against coronavirus 3CL hydrolase and protease enzymes. Journal of Biomolecular Structure & Dynamics. 2020:1–13. doi: 10.1080/07391102.2020.1758791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan T.T., Cheng B.L., Fang X.M., Chen Y.C., Su F. Application of Chinese medicine in the management of critical conditions: A review on sepsis. The American Journal of Chinese Medicine. 2020;48:1315–1330. doi: 10.1142/S0192415X20500640. [DOI] [PubMed] [Google Scholar]
- Gao D., Niu M., Wei S.Z., Zhang C.E., Zhou Y.F., Yang Z.W.…Xiao X.H. Identification of a pharmacological biomarker for the bioassay-based quality control of a thirteen-component TCM formula (Lianhua Qingwen) used in treating influenza A virus (H1N1) infection. Frontiers in Pharmacology. 2020;11:746. doi: 10.3389/fphar.2020.00746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Chai Y., Yao Y. Advancement in the research of mechanism of immune dysfunction in sepsis and the regulatory effects of Xuebijing injection. Zhonghua Shao Shang Za Zhi. 2013;29:162–165. [PubMed] [Google Scholar]
- Ge H., Wang Y.F., Xu J., Gu Q., Liu H.B., Xiao P.G.…Su H. Anti-influenza agents from traditional Chinese medicine. Natural Product Reports. 2010;27:1758–1780. doi: 10.1039/c0np00005a. [DOI] [PubMed] [Google Scholar]
- Gong P., Guo Y., Li X., Wang N., Gu J. Exploring active compounds of Jinhua qinggan granules for prevention of covid-19 based on network pharmacology and molecular docking. Chinese Traditional and Herbal Drugs. 2020;51:1685–1692. [Google Scholar]
- Guo H., Zheng J., Huang G., Xiang Y., Lang C., Li B.…Wan S. Xuebijing injection in the treatment of COVID-19: A retrospective case-control study. Annals of Palliative Medicine. 2020;9:3235–3248. doi: 10.21037/apm-20-1478. [DOI] [PubMed] [Google Scholar]
- He T., Duan C., Li X., Zhang J. Potential mechanism of xuebijing injection in treatment of coronavirus pneumonia based on network pharmacology and molecular docking. The Chinese Journal of Modern Applied Pharmacy. 2020;37:398–405. [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S.…Pohlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou S.Y., Feng X.H., Lin C.L., Tan Y.F. Efficacy of Xuebijing for coagulopathy in patients with sepsis. Saudi Medical Journal. 2015;36:164–169. doi: 10.15537/smj.2015.2.9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh C.F., Lo C.W., Liu C.H., Lin S., Yen H.R., Lin T.Y., Horng J.T. Mechanism by which Ma-Xing-Shi-Gan-Tang inhibits the entry of influenza virus. Journal of Ethnopharmacology. 2012;143:57–67. doi: 10.1016/j.jep.2012.05.061. [DOI] [PubMed] [Google Scholar]
- Hu C., Liang M., Gong F., He B., Zhao D., Zhang G. Efficacy of Lianhua Qingwen compared with conventional drugs in the treatment of common pneumonia and COVID-19 pneumonia: A meta-analysis. Evidence-based Complementary and Alternative Medicine. 2020;2020:5157089. doi: 10.1155/2020/5157089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K., Guan W.J., Bi Y., Zhang W., Li L., Zhang B.…Zhong N.S. Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: A multicenter, prospective, randomized controlled trial. Phytomedicine. 2020;2020 doi: 10.1016/j.phymed.2020.153242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Cai C., Zang J., Xie J., Xu D., Zheng F.…Xie Y. Treatment strategies of hospitalized patients with coronavirus disease-19. Aging (Albany NY) 2020;12:11224–11237. doi: 10.18632/aging.103370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia W., Wang C., Wang Y., Pan G., Jiang M., Li Z., Zhu Y. Qualitative and quantitative analysis of the major constituents in Chinese medical preparation Lianhua-Qingwen capsule by UPLC-DAD-QTOF-MS. The Scientific World Journal. 2015;2015:731765. doi: 10.1155/2015/731765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimilihan S., Maimaitiming N., Ainiwaer W., Maierdan Y., Muhadaisi N., Nulibiya M., Zhou W.T. Study on the active components in the adjuvant treatment of novel coronavirus pneumonia (covid-19) with Jinhua Qinggan granules based on network pharmacology and molecular docking. Journal of Chinese Medicinal Materials. 2020;43:1275–1283. [Google Scholar]
- Jin Y.H., Zhan Q.Y., Peng Z.Y., Ren X.Q., Yin X.T., Cai L.…Wang X.H. Chemoprophylaxis, diagnosis, treatments, and discharge management of COVID-19: An evidence-based clinical practice guideline (updated version) Military Medical Research. 2020;7:1–33. doi: 10.1186/s40779-020-00270-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M., Goto S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Research. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Y., Lin L., Chen Y., Lai S., Wu W., Chen J. Mechanism of XueBiJing injection on treatment of coronavirus disease 2019 based on network pharmacology. Modernization of Traditional Chinese Medicine and Materia Materia-World Science and Technology. 2020;22:552–560. [Google Scholar]
- Kong Z., Liang N., Yang G.L., Zhang Z., Liu Y., Li J.…Liu J.P. Xiao Chai Hu Tang, a herbal medicine, for chronic hepatitis B. Cochrane Database of Systematic Reviews. 2019;2019 doi: 10.1002/14651858.CD013090.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Q., Liang A., He M., Huang X., Wu W. Pharmacological mechanism and network pharmacology research of Huashibaidu formula in treating COVID-19. Natural Product Research and Development. 2020;32:909–919. [Google Scholar]
- Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S.…Wang X. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- Lau T.F., Leung P.C., Wong E.L., Fong C., Cheng K.F., Zhang S.C.…Ko W.M. Using herbal medicine as a means of prevention experience during the SARS crisis. The American Journal of Chinese Medicine. 2005;33:345–356. doi: 10.1142/S0192415X05002965. [DOI] [PubMed] [Google Scholar]
- Li G.Q., Zhao J., Tu Z.T., Li J.B., Liu Q.Q., Shi L.Q.…Hu J.Q. Treating influenza patients of wind-heat affecting Fei syndrome by Jinhua Qinggan granule: A double-blinded randomized control trial. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2013;33:1631–1635. [PubMed] [Google Scholar]
- Li H., Yang L., Liu F.F., Ma X.N., He P.L., Tang W.…Zuo J.P. Overview of therapeutic drug research for COVID-19 in China. Acta Pharmacologica Sinica. 2020;41:1133–1140. doi: 10.1038/s41401-020-0438-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Hu R., Xu S., Li Y., Qin Y., Wu Q., Xiao Z. Xiaochaihutang attenuates liver fibrosis by activation of Nrf2 pathway in rats. Biomedicine & Pharmacotherapy. 2017;96:847–853. doi: 10.1016/j.biopha.2017.10.065. [DOI] [PubMed] [Google Scholar]
- Li J.H., Wang R.Q., Guo W.J., Li J.S. Efficacy and safety of traditional Chinese medicine for the treatment of influenza A (H1N1): A meta-analysis. Journal of the Chinese Medical Association. 2016;79:281–291. doi: 10.1016/j.jcma.2015.10.009. [DOI] [PubMed] [Google Scholar]
- Li Q., Wang H., Li X., Zheng Y., Wei Y., Zhang P.…Tong X. The role played by traditional Chinese medicine in preventing and treating COVID-19 in China. Frontiers in Medicine. 2020;14:681–688. doi: 10.1007/s11684-020-0801-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Hou Y., Huang J., Pan W., Ma Q., Shi Y.…Yang Z. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2) Pharmacological Research. 2020;156:104761. doi: 10.1016/j.phrs.2020.104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Yang Y., Liu L., Yang X., Zhao X., Li Y.…Huang S. Effect of combination antiviral therapy on hematological profiles in 151 adults hospitalized with severe coronavirus disease 2019. Pharmacological Research. 2020;160:105036. doi: 10.1016/j.phrs.2020.105036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C., Niu W., Wu F., Cao W., Wu Z., Chao Y.C., Peng F. Network pharmacology for the identification of phytochemicals in traditional Chinese medicine for COVID-19 that may regulate interleukin-6. Bioscience Reports. 2020;41 doi: 10.1042/BSR20202583. (BSR20202583) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.C., Wang Y.Y., Chen S.M., Liu Y.T., Li J.Q., Li F.…Zhang Z.D. Shegan-Mahuang decoction ameliorates asthmatic airway hyperresponsiveness by downregulating Th2/Th17 cells but upregulating CD4+FoxP3+ Tregs. Journal of Ethnopharmacology. 2020;253:112656. doi: 10.1016/j.jep.2020.112656. [DOI] [PubMed] [Google Scholar]
- Liu H., Ye F., Sun Q., Liang H., Li C., Li S.…Lai L. Scutellaria baicalensis extract and baicalein inhibit replication of SARS-CoV-2 and its 3C-like proteasein vitro. Journal of Enzyme Inhibition and Medicinal Chemistry. 2021;36:497–503. doi: 10.1080/14756366.2021.1873977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Guo F., Wang Y., Li C., Zhang X., Li H.…He F. BATMAN-TCM: A bioinformatics analysis tool for molecular mechANism of traditional Chinese medicine. Scientific Reports. 2016;6:21146. doi: 10.1038/srep21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Li X., Gou C., Li L., Luo X., Zhang C.…Wang X. Effect of Jinhua Qinggan granules on novel coronavirus pneumonia in patients. Journal of Traditional Chinese Medicine. 2020;40:467–472. doi: 10.19852/j.cnki.jtcm.2020.03.016. [DOI] [PubMed] [Google Scholar]
- Luo H., Gao Y., Zou J., Zhang S., Chen H., Liu Q.…Wang S. Reflections on treatment of COVID-19 with traditional Chinese medicine. Chinese Medicine. 2020;15:94. doi: 10.1186/s13020-020-00375-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H., Tang Q.L., Shang Y.X., Liang S.B., Yang M., Robinson N., Liu J.P. Can Chinese medicine be used for prevention of Corona virus disease 2019 (COVID-19)? A review of historical classics, research evidence and current prevention programs. Chinese Journal of Integrative Medicine. 2020;26:243–250. doi: 10.1007/s11655-020-3192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q., Qiu M., Zhou H., Chen J., Yang X., Deng Z.…Yang Z. The study on the treatment of Xuebijing injection (XBJ) in adults with severe or critical Corona Virus Disease 2019 and the inhibitory effect of XBJ against SARS-CoV-2. Pharmacological Research. 2020;160:105073. doi: 10.1016/j.phrs.2020.105073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Shan M., Guo Y., Wang Y. Study on treatment of influenza A H1N1 induced severe pneumonia by Xuebijing injection. World Chinese Medicine. 2015;10:243–246. [Google Scholar]
- Monteil V., Kwon H., Prado P., Hagelkruys A., Wimmer R.A., Stahl M.…Penninger J.M. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181:905–913. doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesarchio V., Parrela R., Iommelli C., Bianco A., Manzillo E., Fraganza F.…Ascierto P.A. Outcomes and biomarker analyses among patients with COVID-19 treated with interleukin 6 (IL-6) receptor antagonist sarilumab at a single institution in Italy. Journal for Immunotherapy of Cancer. 2020;8 doi: 10.1136/jitc-2020-001089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020;368:473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- NHC Full text: Diagnosis and treatment protocol for COVID-19 patients (tentative 8th edition) 2020. http://en.nhc.gov.cn/2020-09/07/c_81565.htm [DOI] [PMC free article] [PubMed]
- Niu W., Wu F., Cui H., Cao W., Chao Y., Wu Z.…Liang C. Network pharmacology analysis to identify phytochemicals in traditional Chinese medicines that may regulate ACE2 for the treatment of COVID-19. Evidence-based Complementary and Alternative Medicine. 2020;2020:7493281. doi: 10.1155/2020/7493281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notice Recommendation of combination "Qingfei Paidu Decoction" with Western medicine on treating COVID-19. 2020. http://yzs.satcm.gov.cn/zhengcewenjian/2020-02-07/12876.html
- Pan X., Dong L., Yang L., Chen D., Peng C. Potential drugs for the treatment of the novel coronavirus pneumonia (COVID-19) in China. Virus Research. 2020;286:198057. doi: 10.1016/j.virusres.2020.198057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J., Qi X., Wang X. Clinical efficacy of different doses of Jinhuaqinggan granule on influenza and serum levels of cytokines. Modern Medical Journal. 2016;44:1664–1669. [Google Scholar]
- Ren J.L., Zhang A.H., Wang X.J. Traditional Chinese medicine for COVID-19 treatment. Pharmacological Research. 2020;155:104743. doi: 10.1016/j.phrs.2020.104743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y., Yao M.C., Huo X.Q., Gu Y., Zhu W.X., Qiao Y.J., Zhang Y.L. Study on treatment of “cytokine storm” by anti-2019-nCoV prescriptions based on arachidonic acid metabolic pathway. Zhongguo Zhong Yao Za Zhi. 2020;45:1225–1231. doi: 10.19540/j.cnki.cjcmm.20200224.405. [DOI] [PubMed] [Google Scholar]
- Ren Y., Yin Z., Dai J., Yang Z., Ye B., Ma Y.…Shi Y. Evidence-based complementary and alternative medicine exploring active components and mechanism of Jinhua Qinggan granules in treatment of covid-19 based on virus-host interaction. Natural Product Communications. 2020;15:1–11. [Google Scholar]
- Ru J., Li P., Wang J., Zhou W., Li B., Huang C.…Yang L. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. Journal of Cheminformatics. 2014;6:13. doi: 10.1186/1758-2946-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiou O., Bouziane I., Bouslama Z., Djemel A. In-silico identification of potent inhibitors of covid-19 main protease (mpro) and angiotensin converting enzyme 2 (ace2) from natural products: Quercetin, hispidulin, and cirsimaritin exhibited better potential inhibition than hydroxy-chloroquine against covid-19 main protease active site and ace2. Chemrxiv. 2020 (Preprint) [Google Scholar]
- Shen F., Fu Z., Wu Y., Li L., Zhao Y., Xia Y., Kuang G. The potential molecular mechanism of active compounds binding SARS-CoV-2 specific target proteins in Huaqing granules treat COVID-19 based on network pharmacology and high-throughput molecular docking fellowship. Modernization of Traditional Chinese Medicine and Materia Materia-World Science and Technology. 2020;22:622–631. [Google Scholar]
- Shi H., Hong Y., Qian J., Cai X., Chen S. Xuebijing in the treatment of patients with sepsis. The American Journal of Emergency Medicine. 2017;35:285–291. doi: 10.1016/j.ajem.2016.11.007. [DOI] [PubMed] [Google Scholar]
- Shi N., Guo L., Liu B., Bian Y., Chen R., Chen S.…Huang L. Efficacy and safety of Chinese herbal medicine versus Lopinavir-Ritonavir in adult patients with coronavirus disease 2019: A non-randomized controlled trial. Phytomedicine. 2020;81:153367. doi: 10.1016/j.phymed.2020.153367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi N., Liu B., Liang N., Ma Y., Ge Y., Yi H.…Wang Y. Association between early treatment with Qingfei Paidu decoction and favorable clinical outcomes in patients with COVID-19: A retrospective multicenter cohort study. Pharmacological Research. 2020;161:105290. doi: 10.1016/j.phrs.2020.105290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha P., Matthay M.A., Calfee C.S. Is a “cytokine storm” relevant to COVID-19? JAMA Internal Medicine. 2020;180:1152–1154. doi: 10.1001/jamainternmed.2020.3313. [DOI] [PubMed] [Google Scholar]
- Song Y., Yao C., Yao Y., Han H., Zhao X., Yu K.…Bai C. XueBiJing injection versus placebo for critically ill patients with severe community-acquired pneumonia: A randomized controlled trial. Critical Care Medicine. 2019;47:e735–e743. doi: 10.1097/CCM.0000000000003842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y., Zhang M., Yin L., Wang K., Zhou Y., Zhou M., Lu Y. COVID-19 treatment: Close to a cure? A rapid review of pharmacotherapies for the novel coronavirus (SARS-CoV-2) International Journal of Antimicrobial Agents. 2020;56:106080. doi: 10.1016/j.ijantimicag.2020.106080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H., Yao S., Zhao W., Li M., Liu J., Shang W.…Xu Y. Discovery of baicalin and baicalein as novel, natural product inhibitors of SARS-CoV-2 3CL protease in vitro. bioRxiv. 2020 [Google Scholar]
- Sun X., Tao J., Xu S., Yuan B. The molecular mechanism of treating COVID-19 with Huashi Baidu formula based on network pharmacology. Journal of Chinese Medicinal Materials. 2020;43:2050–2055. [Google Scholar]
- Tao Q., Du J., Li X., Zeng J., Tan B., Xu J.…Chen X.L. Network pharmacology and molecular docking analysis on molecular targets and mechanisms of Huashi Baidu formula in the treatment of COVID-19. Drug Development and Industrial Pharmacy. 2020;46:1345–1353. doi: 10.1080/03639045.2020.1788070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Z., Yang Y., Shi W., Xue M., Yang W., Song Z.…Yuan Y. Complementary and alternative medicine is expected to make greater contribution in controlling the prevalence of influenza. Bioscience Trends. 2013;7:253–256. [PubMed] [Google Scholar]
- Tong T., Wu Y.Q., Ni W.J., Shen A.Z., Liu S. The potential insights of Traditional Chinese Medicine on treatment of COVID-19. Chinese Medicine. 2020;15:51. doi: 10.1186/s13020-020-00326-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Y. Tu Youyou biographical. 2015. https://www.nobelprize.org/prizes/medicine/2015/tu/biographical/
- Turnquist C., Ryan B.M., Horikawa I., Harris B.T., Harris C.C. Cytokine storms in cancer and COVID-19. Cancer Cell. 2020;38:598–601. doi: 10.1016/j.ccell.2020.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Cao B., Liu Q.Q., Zou Z.Q., Liang Z.A., Gu L.…Jiang L.D. Oseltamivir compared with the Chinese traditional therapy maxingshigan-yinqiaosan in the treatment of H1N1 influenza: A randomized trial. Annals of Internal Medicine. 2011;155:217–225. doi: 10.7326/0003-4819-155-4-201108160-00005. [DOI] [PubMed] [Google Scholar]
- Wang C., Fei D., Li X., Zhao M., Yu K. IL-6 may be a good biomarker for earlier detection of COVID-19 progression. Intensive Care Medicine. 2020;46:1475–1476. doi: 10.1007/s00134-020-06065-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.H., Zhong Y., Zhang Y., Liu J.P., Wang Y.F., Jia W.N.…Gao X.M. A network analysis of the Chinese medicine Lianhua-Qingwen formula to identify its main effective components. Molecular BioSystems. 2016;12:606–613. doi: 10.1039/c5mb00448a. [DOI] [PubMed] [Google Scholar]
- Wang H., Li G., Cao Y., Lin M., Deng L., Guan A. Montelukast combined with Shegan Mahuang Tang for treating of 86 patients with cough variant asthma. Chinese Journal of Experimental Traditional Medical Formulae. 2012;18:273–275. [Google Scholar]
- Wang H., Song H., Wang D., Ma X., Zou D., Miao J., Yang W. The molecular mechanism of Xuanfei Baidu formula in the treatment of COVID-19 antiviral effect based on network pharmacology and molecular docking. Journal of Hainan Medical University. 2020 (czh-360) [Google Scholar]
- Wang R., Yang S., Xie C., Shen Q., Li M., Lei X.…Huang M. Clinical observation of qingfeipaidu decoction in the treatment of covid-19. Pharmacology and Clinics of Chinese Materia Medica. 2020;36(1):13–18. [Google Scholar]
- Wang Y., Li X., Zhang J.H., Xue R., Qian J.Y., Zhang X.H.…Zhang B.L. Mechanism of Xuanfei Baidu Tang in treatment of COVID-19 based on network pharmacology. Zhongguo Zhong Yao Za Zhi. 2020;45:2249–2256. doi: 10.19540/j.cnki.cjcmm.20200325.401. [DOI] [PubMed] [Google Scholar]
- Wen L., Zhou Z., Jiang D., Huang K. Effect of Xuebijing injection on inflammatory markers and disease outcome of coronavirus disease 2019. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2020;32:426–429. doi: 10.3760/cma.j.cn121430-20200406-00386. [DOI] [PubMed] [Google Scholar]
- WHO Sars: Clinical trials on treatment using a combination of traditional chinese medicine and western medicine. 2004. https://apps.who.int/iris/handle/10665/43029
- Wu H., Wang J., Yang Y., Li T., Cao Y., Qu Y.…Sun Y. Preliminary exploration of the mechanism of Qingfei Paidu decoction against novel coronavirus pneumonia based on network pharmacology and molecular docking technology. Acta Pharmaceutica Sinica. 2020;55:374–383. [Google Scholar]
- Wu L., Chen Y., Ma Y., Yang Z., Yang N., Deng W.…Lin L. Clinical practice guideline on treating influenza in adult patients with Chinese patent medicines. Pharmacological Research. 2020;160:105101. doi: 10.1016/j.phrs.2020.105101. [DOI] [PubMed] [Google Scholar]
- Wu Y. 2020. https://www.cacms.ac.cn/zykxy/dtyw/202010/c1a215f451174a9a90e3e8a74ab832d2.shtml
- Xia Q.D., Xun Y., Lu J.L., Lu Y.C., Yang Y.Y., Zhou P.…Wang S.G. Network pharmacology and molecular docking analyses on Lianhua Qingwen capsule indicate Akt1 is a potential target to treat and prevent COVID-19. Cell Proliferation. 2020;53 doi: 10.1111/cpr.12949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian Y., Zhang J., Bian Z., Zhou H., Zhang Z., Lin Z., Xu H. Bioactive natural compounds against human coronaviruses: A review and perspective. Acta Pharmaceutica Sinica B. 2020;10:1163–1174. doi: 10.1016/j.apsb.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao M., Tian J., Zhou Y., Xu X., Min X., Lv Y.…Tong X. Efficacy of Huoxiang Zhengqi dropping pills and Lianhua Qingwen granules in treatment of COVID-19: A randomized controlled trial. Pharmacological Research. 2020;161:105126. doi: 10.1016/j.phrs.2020.105126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S.H., Luo L., Liu X.H., Zhou Y.M., Liu H.M., Huang Z.F. Curative efficacy and safety of traditional Chinese medicine xuebijing injections combined with ulinastatin for treating sepsis in the Chinese population: A meta-analysis. Medicine (Baltimore) 2018;97 doi: 10.1097/MD.0000000000010971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y., Zhong C., Ji S., Huang B., Huang H., Zhan S., Liu X. Study of molecular mechanism of Huashibaidu decoction on COVID-19 based on network pharmacology and molecular docking technology. Pharmacology and Clinics of Chinese Materia Medica. 2020;36(3):28–35. [Google Scholar]
- Xin S., Cheng X., Zhu B., Liao X., Yang F., Song L.…Yu R. Clinical retrospective study on the efficacy of Qingfei Paidu decoction combined with Western medicine for COVID-19 treatment. Biomedicine & Pharmacotherapy. 2020;129:110500. doi: 10.1016/j.biopha.2020.110500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W.Z., Wang G., Du J., Ai W. Efficacy of herbal medicine (Xuanfei Baidu decoction) combined with conventional drug in treating COVID-19: A pilot randomized clinical trial. Integrative Medicine Research. 2020;9:100489. doi: 10.1016/j.imr.2020.100489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue R., Fang Z., Zhang M., Yi Z., Wen C., Shi T. TCMID: Traditional Chinese Medicine integrative database for herb molecular mechanism analysis. Nucleic Acids Research. 2013;41:D1089–D1095. doi: 10.1093/nar/gks1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan D. Traditional Chinese Medicine in treating influenza A (H1N1)—Jinhua Qinggan granules. Family Chinese Medicine. 2010:26–28. [Google Scholar]
- Yang C., Wang Y., He J., Yan W., Jiang H., Chen Q.…Yang Z. Lianhua-Qingwen displays antiviral and anti-inflammatory activity and synergistic effects with Oseltamivir against influenza B virus infection in the mouse model. Evidence-based Complementary and Alternative Medicine. 2020;2020:3196375. doi: 10.1155/2020/3196375. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yang R., Liu H., Bai C., Wang Y., Zhang X., Guo R.…Wang Y. Chemical composition and pharmacological mechanism of Qingfei Paidu Decoction and Ma Xing Shi Gan Decoction against Coronavirus Disease 2019 (COVID-19): In silico and experimental study. Pharmacological Research. 2020;157:104820. doi: 10.1016/j.phrs.2020.104820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X. Material basis research of anti-COVID-19 Huashibaidu granule gormula. Modern Chinese Medicine. 2020;22:672–689. [Google Scholar]
- Yang Y., Zhang D.M., Liu J.H., Hu L.S., Xue Q.C., Ding X.Q., Kong L.D. Wuling San protects kidney dysfunction by inhibiting renal TLR4/MyD88 signaling and NLRP3 inflammasome activation in high fructose-induced hyperuricemic mice. Journal of Ethnopharmacology. 2015;169:49–59. doi: 10.1016/j.jep.2015.04.011. [DOI] [PubMed] [Google Scholar]
- Yao K., Liu M., Li X., Huang J., Cai H. Retrospective clinical analysis on treatment of coronavirus disease 2019 with traditional chinese medicine lianhua qingwen. Chinese Journal of Experimental Traditional Medical Formulae. 2020;26:8–12. [Google Scholar]
- Ye C., Gao M., Lin W., Yu K., Li P., Chen G. Theoretical study of the anti-NCP molecular mechanism of Traditional Chinese Medicine Lianhua-Qingwen Formula (LQF) ChemRxiv. 2020 doi: 10.26434/chemrxiv.12016236.v1. Preprint. [DOI] [Google Scholar]
- Yin Q., Li C. Treatment effects of xuebijing injection in severe septic patients with disseminated intravascular coagulation. Evidence-based Complementary and Alternative Medicine. 2014;2014:949254. doi: 10.1155/2014/949254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P., Li Y., Wan S., Wang Y. Effects of lianhua qingwen granules plus arbidol on treatment of mild corona virus disease-19. Chinese Pharmaceutical Journal. 2020;55:1042–1045. [Google Scholar]
- Zeng M., Li L., Wu Z. Traditional Chinese medicine Lianhua Qingwen treating corona virus disease 2019(COVID-19): Meta-analysis of randomized controlled trials. PLoS One. 2020;15 doi: 10.1371/journal.pone.0238828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B. Xuanfeibaidu Granules is a product of "Chinese Medicine +Technology". 2020. http://www.xinhuanet.com/politics/2020-03/17/c_1125724808.htm
- Zhang C., Li Z., Zhang S., Wang W., Jiang X. Clinical observation of Xuebijing in the treatment of COVID-19. Chinese Journal of Hospital Pharmacy. 2020;40:964–967. [Google Scholar]
- Zhang K. Is traditional Chinese medicine useful in the treatment of COVID-19? The American Journal of Emergency Medicine. 2020;38:2238. doi: 10.1016/j.ajem.2020.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Lin D., Sun X., Curth U., Drosten C., Sauerhering L.…Hilgenfeld R. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved alpha-ketoamide inhibitors. Science. 2020;368:409–412. doi: 10.1126/science.abb3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Sun C., Wen Y., Yin C. Effect of treatment with xuebijing injection on serum inflammatory mediators and th1/2 of spleen in rats with sepsis. Chinese Journal of Critical Care Medicine. 2006;18:673–676. [PubMed] [Google Scholar]
- Zhang X., Tan Y., Ling Y., Lu G., Liu F., Yi Z.…Lu H. Viral and host factors related to the clinical outcome of COVID-19. Nature. 2020;583:437–440. doi: 10.1038/s41586-020-2355-0. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Yang H., He X., G W., Li J. The curative mechanism of Maxingshigan decoction on cytokine storm of COVID-19 pneumonia based on network pharmacology. World Chinese Medicine. 2020;15:1908–1913. [Google Scholar]
- Zhao G., Guo Y., Chen W., Li B., Chen T., Lu Y.…Liu Q. Protocol for developing clinical practice guidelines on traditional Chinese medicine therapy for influenza. Annals of Palliative Medicine. 2020 doi: 10.21037/apm-20-862. (apm-20-862) [DOI] [PubMed] [Google Scholar]
- Zhao J., Tian S., Lu D., Yang J., Zeng H., Zhang F.…Zhang W. Systems pharmacological study illustrates the immune regulation, anti-infection, anti-inflammation, and multi-organ protection mechanism of Qing-Fei-Pai-Du decoction in the treatment of COVID-19. Phytomedicine. 2020 doi: 10.1016/j.phymed.2020.153315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P., Yang H.Z., Lv H.Y., Wei Z.M. Efficacy of Lianhuaqingwen capsule compared with oseltamivir for influenza A virus infection: A meta-analysis of randomized, controlled trials. Alternative Therapies in Health and Medicine. 2014;20:25–30. [PubMed] [Google Scholar]
- Zheng N., Dai J., Cao H., Sun S., Fang J., Li Q.…Huang S. Current understanding on antihepatocarcinoma effects of xiao chai hu tang and its constituents. Evidence-based Complementary and Alternative Medicine. 2013;2013:529458. doi: 10.1155/2013/529458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng R., Wang H., Liu Z., Wang X., Li J., Lei X.…Shang H. A real-world study on adverse drug reactions to Xuebijing injection: Hospital intensive monitoring based on 93 hospitals (31,913 cases) Annals of Translational Medicine. 2019;7:117. doi: 10.21037/atm.2018.09.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y.Y., Gao W.Y., Gu X.R., Chen Z.Q., Zhao H.Y., Bian B.L.…Tan Y. Identification and attribution of chemical constituents of Qingfei Paidu Decoction based on UHPLC-LTQ-Orbitrap-MS technology. Zhongguo Zhong Yao Za Zhi. 2020;45:3035–3044. doi: 10.19540/j.cnki.cjcmm.20200423.202. [DOI] [PubMed] [Google Scholar]