Abstract
Background
The prognostic values of inflammation-based markers in well-differentiated pancreatic neuroendocrine neoplasms, diagnosed according to the new 2017 World Health Organization classification, have remained unclear. Therefore, we assessed the ability to predict the recurrence of such markers after curative resection in patients with these neoplasms.
Methods
Circulating/systemic neutrophil–lymphocyte, monocyte–lymphocyte, platelet–lymphocyte, and platelet–white cell ratios were evaluated in 120 patients who underwent curative resection for well-differentiated pancreatic neuroendocrine neoplasms without synchronous distant metastasis between 2001 and 2018. Recurrence-free-survival and overall survival were compared using Kaplan–Meier analysis and log-rank tests. Univariate or multivariate analyses, using a Cox proportional hazards model, were used to calculate hazard ratios with 95% confidence intervals.
Results
Univariate analysis demonstrated that preoperative neutrophil–lymphocyte ratio, tumor size, European Neuroendocrine Tumor Society TMN classification, 2017 World Health Organization classification, and venous invasion were associated with recurrence. The optimal preoperative neutrophil–lymphocyte ratio cut-off value was 2.62, based on receiver operating characteristic curve analysis. In multivariate analysis, a higher preoperative neutrophil–lymphocyte ratio (HR = 3.49 95% CI 1.05–11.7; P = 0.042) and 2017 World Health Organization classification (HR = 8.81, 95% CI 1.46–168.2; P = 0.015) were independent recurrence predictors.
Conclusions
The circulating/systemic neutrophil–lymphocyte ratio is a useful and convenient preoperative prognostic marker of recurrence in patients with well-differentiated pancreatic neuroendocrine neoplasm based on the 2017 World Health Organization classification.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12893-021-01178-3.
Keywords: Neutrophil–lymphocyte ratio (NLR), Pancreatic neuroendocrine neoplasm (PanNEN), Systemic immune-inflammatory marker
Background
Pancreatic neuroendocrine neoplasm (PanNEN) is a biologically heterogeneous and relatively rare malignancy, with an incidence rate of approximately 5 cases per 1 million person-years, which accounts for 1–2% of primary pancreatic neoplasms [1]. In recent years, the incidence of PanNEN detected clinically has significantly increased because of the advances in imaging modalities during the past few decades [2]. When the disease is clinically detected before it becomes symptomatic, the lesions are typically localized, increasing the possibility of curative resection and improving prognosis [3]. Although surgical resection is currently the only curative treatment for PanNEN [4], recurrence could occur at intervals, and therefore, reoperation for recurrent lesions may occasionally be required. Reoperation for distant metastases can lead to excellent long-term survival [5]. Even if unresectable metastases occur, novel targeted drugs, such as the multiple tyrosine kinase inhibitor sunitinib and the mTOR inhibitor everolimus, have been approved and registered for antiproliferative therapy for well-differentiated PanNEN [6, 7]. Therefore, it is essential to identify recurrence earlier. For this reason, indicators that could predict recurrence after surgery are required for the optimal management of PanNEN.
Several studies, however, have demonstrated that tumorigenesis and clinical manifestations of well-differentiated PanNEN are distinctively different from poorly differentiated PanNEN (neuroendocrine carcinoma; NEC), and thus, the determinants of treatment should be considered separately [8, 9]. The prognosis in NEC is poor [10], and the National Comprehensive Cancer Network (NCCN) guidelines recommend platinum-based systemic chemotherapy for patients with NEC [6]. For this reason, the 2017 World Health Organization (WHO) introduced significant changes to the classification of PanNEN. Of note, a new category of well-differentiated neoplasms, neuroendocrine tumors G3 (NET-G3), was introduced, and these are distinct from poorly differentiated NEC-G3 [11, 12].
Recently, systemic immune-inflammatory markers have been reported as factors that influence the outcomes of treatments, such as surgery, and the efficacy of chemotherapy in patients with various types of malignancies [13–18]. However, the ability of systemic immune-inflammatory markers to predict prognosis in patients with sole, well-differentiated PanNEN, based on the 2017 WHO classification [11], other than NEC or synchronous distant metastasis, has remained unknown.
In this study, we sought to evaluate whether systemic immune-inflammatory markers can be preoperative prognostic factors for predicting recurrence and overall survival after curative resection in patients with well-differentiated PanNEN based on the new 2017 WHO classification.
Materials and methods
Patients
We analyzed 132 consecutive cases who underwent surgery for primary, histologically confirmed PanNEN at the Department of Surgery, Tohoku University Hospital, between 2001 and 2018. Eight patients with synchronous hepatic metastasis during the surgery, two patients with NEC, one patient not suitable for curative resection, and one patient with an active infection at blood sampling were excluded from the study. Finally, 120 patients with well-differentiated PanNEN were enrolled in this study. Patient characteristics (age, sex), perioperative factors (serum albumin, hormonal secretion, tumor location, clinical stage), pathological findings (2017 WHO classification, tumor size, lymph node metastasis, surgical margin status, lymphovascular invasion), and prognosis were investigated retrospectively. Histopathological findings were assessed by experienced pathologists (FF, and HS). For all the patients, visual assessment ‘‘eyeballing calculation’’ was performed to assess Ki-67 index. TNM staging was adopted according to the European Neuroendocrine Tumor Society (ENETS) classification [19], and the new 2017 WHO classification of NET by the gastro-entero-pancreatic (GEP) system was used for histopathological classification [11]. Peripheral blood routine tests were performed within 14 days before surgery, according to our internal institutional policy. The serum neutrophil–lymphocyte ratio (NLR) was calculated as the number of neutrophils divided by the number of lymphocytes. The monocyte–lymphocyte ratio (MLR), platelet–lymphocyte ratio (PLR), and platelet–white cell ratio (PWR) were calculated in the same manner.
Clinical follow-up
Postoperative follow-up evaluation included physical examinations, laboratory tests, and enhanced computed tomography (chest and abdominal cavity), once every 6 months. There were no patients who received neoadjuvant or adjuvant therapies in this cohort. Treatment after recurrence was determined by the available evidence at the time of surgery and based on the patient’s condition.
Statistical analysis and software
Recurrence-free-survival (RFS) and overall survival (OS) were calculated from the date of surgery to the date of recurrence, the date of death from any cause, or the date of last follow-up. To determine the appropriate cut-off values, we used receiver operating characteristic (ROC) curves and determined the area under the curve (AUC). Differences between groups were determined using t-tests in the case of normally distributed variables or by the Wilcoxon rank-sum test in the case of abnormally distributed variables for examining differences in continuous variable distributions, and Pearson’s chi-square tests for categorical variables. RFS probabilities were compared for various categories of interest using the Kaplan–Meier method with the log-rank test.
Prognostic factors were assessed with univariate and multivariate analyses, using Cox’s proportional hazards model. Hazard ratios (HR) with 95% confidence intervals (CIs) were calculated. P < 0.05 was considered to indicate statistical significance.
All statistical analyses were performed using the JMP Pro 14.2.0 statistical software (SAS Institute Inc., Cary, NC, USA) and GraphPad Prism Version 8.4.2 (GraphPad Software, San Diego, CA, USA).
Ethics approval
This study was approved by the ethics committee of the Tohoku University Hospital (Approval No. 2020-1-322). It was performed in adherence to the tenets of the Declaration of Helsinki and its later amendments. The need to obtain written informed consent was waived due to the retrospective nature of the study.
Results
Characteristics of patients with resected well-differentiated PanNEN
The demographic and clinicopathological features of the 120 patients who underwent curative resection of PanNEN are shown in Table 1. The median age was 60 years (range 12–88 years), and the median follow-up period in all patients was 64 months (range, 6–185 months). There were no perioperative deaths. The pathological findings (based on the 2017 WHO classification) were NET-G1 in 73 patients, NET-G2 in 45, and NET-G3 in 2. The median tumor size was 14.5 mm (range 4–168 mm). Pathology investigations confirmed lymph node metastasis in 18 patients (15.0%).
Table 1.
Clinicopathological characteristics of 120 patients with well-differentiated PanNEN
| Patient characteristics | n = 120 | % |
|---|---|---|
| Age, median(range) | 60 (12‒88) | |
| Sex | ||
| Male | 49 | 40.8 |
| Female | 71 | 59.2 |
| NLR, median (range) | 1.93 (0.44‒5.32) | |
| MLR, median (range) | 0.23 (0.11‒0.53) | |
| PLR, median (range) | 145.2 (42.5‒328.8) | |
| PWR, median (range) | 43.7 (11.9‒120) | |
| Albumin(g/L), median (range) | 41.0 (28‒49) | |
| Tumor size (mm), median (range) | 14.5 (4‒168) | |
| Operative procedures | ||
| PD | 38 | 31.7 |
| DP | 58 | 48.3 |
| TP | 2 | 1.7 |
| Partial resection | 22 | 18.3 |
| Surgical approach | ||
| Open | 78 | 65.0 |
| Laparoscopy | 42 | 35.0 |
| Surgical margin status | ||
| R0 | 116 | 96.7 |
| R1 | 4 | 3.3 |
| Tumor location | ||
| Head | 47 | 39.2 |
| Body/tail | 68 | 56.7 |
| Multiple | 5 | 4.2 |
| Ki-67 (%), median (range) | 1.83 (0.02‒28) | |
| Clinical stage | ||
| I | 74 | 61.7 |
| II | 27 | 23.3 |
| III | 19 | 15.0 |
| 2017 WHO classification | ||
| G1 | 73 | 60.8 |
| G2 | 45 | 37.5 |
| G3 | 2 | 1.7 |
| Hormonal function | ||
| No | 70 | 58.3 |
| Yes | 50 | 41.7 |
| Lymph node metastasis | ||
| No | 102 | 85.0 |
| Yes | 18 | 15.0 |
| Lymphatic invasion | ||
| No | 105 | 87.5 |
| Yes | 15 | 12.5 |
| Venous invasion | ||
| No | 91 | 75.8 |
| Yes | 29 | 24.2 |
Data are expressed as the median (range) or as absolute number
NLR neutrophil–lymphocyte, MLR monocyte–lymphocyte ratio, PLR platelet–lymphocyte, PWR platelet–white blood cell ratio, PD pancreaticoduodenectomy, DP distal pancreatectomy, TP total pancreatectomy
Clinicopathological features associated with recurrence and NLR
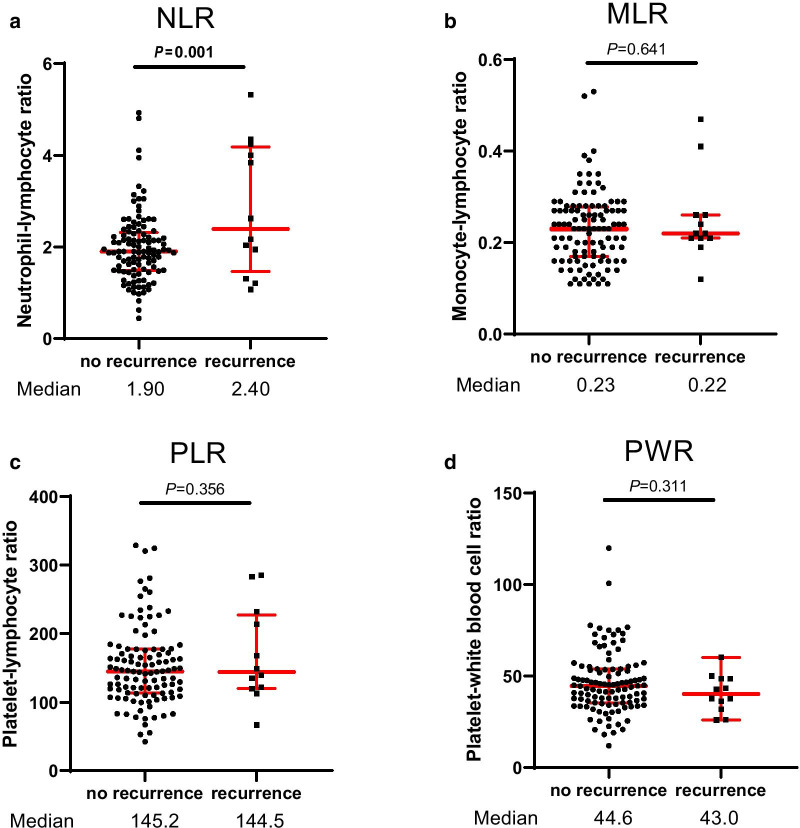
Postoperative recurrences were observed in 12 cases (10%). The sites of recurrence were in the liver in 10 patients, the para-aortic lymph node in 1, and the lung in 1. The 5- and 10-year RFS rates for the entire cohort were 92.0% and 78.7%, respectively. Three patients died due to PanNEN, 10 patients died due to other diseases, and the remaining 107 patients were alive at the end of the surveillance period. Thus, the 5- and 10-year disease-specific survival rates were 100% and 92.5%, respectively. The NLR was significantly higher in patients with recurrence than in those without recurrence (median NLR: 2.40 vs 1.90, P = 0.001), while the MLR, PLR, and PWR were not statistically significantly different between those with and those without recurrence (Fig. 1).
Fig. 1.
Distribution of the inflammation-based markers in PanNENs. The NLR was significantly higher in patients with recurrence than in those without recurrence, while the MLR, PLR, and PWR were not statistically different between those with and those without recurrence
An ROC curve was used to determine the cut-off value associated with postoperative recurrence. Each cut-off value of NLR and tumor size was defined as the highest log-rank statistic of any threshold. The optimal cut-off values for preoperative NLR and tumor size were 2.62 mm and 25 mm, respectively (Additional file 1: Fig. S1.). The Ki-67 index was statistically higher in patients with high NLR (≥ 2.62) than in patients with low NLR (< 2.62) (mean: 5.46 vs 3.14, P = 0.042). In contrast, age, sex, albumin, surgical margin status, clinical stage, 2017 WHO classification, tumor functionality, tumor size, tumor location, lymph node metastasis, and lymphovascular invasion were not associated with NLR status (Table 2). The recurrence rate was 33.3% and 31.0% in 18 patients with a high NLR (≥ 2.62) and 29 patients with larger tumors (≥ 25 mm), respectively.
Table 2.
Relationship between NLR and clinicopathological characteristics (n = 120)
| LNR < 2.62 (n = 102) | LNR ≥ 2.62 (n = 18) | P-value | |
|---|---|---|---|
| Age (years) | 57.7 ± 16.0 | 59.5 ± 14.7 | 0.763 |
| Sex | |||
| Female | 59 | 12 | 0.483 |
| Male | 43 | 6 | |
| Albumin (g/L) | 3.98 ± 0.40 | 4.01 ± 0.41 | 0.915 |
| Tumor size (mm) | 19.0 ± 19.4 | 22.6 ± 15.1 | 0.194 |
| Surgical margin status | |||
| R0 | 99 | 17 | 0.569 |
| R1 | 3 | 1 | |
| Tumor location | |||
| Head | 39 | 8 | 0.595 |
| Body/tail | 58 | 10 | |
| Multiple | 5 | 0 | |
| Ki-67 | 3.14 ± 3.83 | 5.46 ± 6.97 | 0.042 |
| Clinical stage | |||
| I | 64 | 10 | 0.714 |
| II | 23 | 4 | |
| III | 25 | 4 | |
| 2017 WHO classification | |||
| G1 | 64 | 9 | 0.361 |
| G2 | 37 | 8 | |
| G3 | 1 | 1 | |
| Hormonal function | |||
| No | 56 | 14 | 0.070 |
| Yes | 46 | 4 | |
| Lymph node metastasis | |||
| No | 88 | 14 | 0.352 |
| Yes | 14 | 4 | |
| Lymphatic invasion | |||
| No | 88 | 17 | 0.334 |
| Yes | 14 | 1 | |
| Venous invasion | |||
| No | 77 | 14 | 0.834 |
| Yes | 25 | 4 |
Results are expressed as mean ± SD or as absolute number
Comparison of clinical variables in relationship to RFS after curative resection
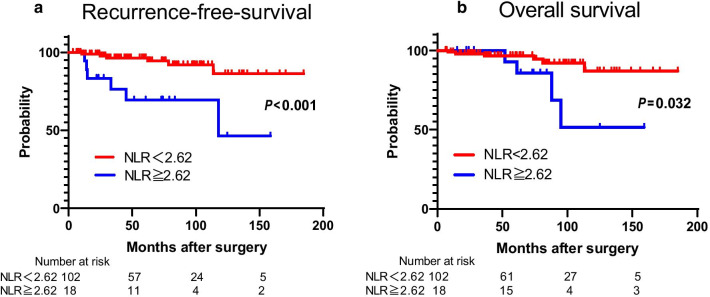
The results of the univariate and multivariate analyses for each of the clinicopathological variables are shown in Table 3. According to univariate analysis, the recurrence risk was about six times higher in patients with a high NLR than in those with a low NLR (95% CI 1.81–18.5, P = 0.004). Additionally, the TMN clinical-stage, 2017 WHO classification G2/3, tumor size, and venous invasion were also significantly predictive factors for recurrence (P < 0.05 for all). In contrast, age, sex, albumin, surgical margin status, hormonal function, tumor location, lymph node metastasis, and lymphatic invasion were not significant predictors of recurrence. Moreover, in multivariate analysis, higher NLR (HR = 3.49, 95% CI 1.05–11.7, P = 0.042) and 2017 WHO classification G2/3 (HR = 8.81, 95% CI 1.46–168.2, P = 0.015) were independent predictive factors for recurrence. A higher NLR showed a significant correlation with shorter RFS (median RFS duration, 117.8 months, P < 0.001) (Fig. 2a) and poor OS (median OS duration, 95.2 months, P = 0.032) after curative resection (Fig. 2b).
Table 3.
Prognostic factors for recurrence-free-survival in 120 patients with well-differentiated PanNEN
| Independent factor | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P‒value | Hazard ratio | 95% CI | P‒value | |
| Age (years) | 0.101 | |||||
| < 60 | Reference | |||||
| ≥ 60 | 0.36 | 0.08‒1.21 | ||||
| Sex | 0.09 | |||||
| Female | Reference | |||||
| Male | 2.66 | 0.84‒9.05 | ||||
| NLR | 0.004 | 0.042 | ||||
| < 2.62 | Reference | Reference | ||||
| ≥ 2.62 | 5.78 | 1.81‒18.5 | 3.49 | 1.05‒11.7 | ||
| Albumin (g/L) | 0.829 | |||||
| < 35 | Reference | |||||
| ≥ 35 | 0.79 | 0.15‒14.5 | ||||
| Tumor size (mm) | < 0.001 | 0.052 | ||||
| < 25 | Reference | Reference | ||||
| ≥ 25 | 10.2 | 3.05‒46.2 | 5.30 | 0.98‒81.5 | ||
| Surgical margin status | 0.337 | |||||
| R0 | Reference | |||||
| R1 | 2.74 | 0.35–21.5 | ||||
| Tumor location | 0.619 | |||||
| Head | Reference | |||||
| Body/tail | 0.95 | 0.28‒2.99 | ||||
| Multiple | NA | NA | ||||
| Clinical stage | 0.001 | 0.736 | ||||
| I | Reference | Reference | ||||
| II/III | 8.12 | 2.13‒52.9 | 1.19 | 0.06‒13.6 | ||
| 2017 WHO classification | < 0.001 | 0.015 | ||||
| G1 | Reference | Reference | ||||
| G2/G3 | 15.6 | 3.02‒285.6 | 8.81 | 1.46‒168.2 | ||
| Hormonal function | 0.151 | |||||
| No | Reference | |||||
| Yes | 2.46 | 0.73‒11.1 | ||||
| Lymph node metastasis | 0.063 | |||||
| No | Reference | |||||
| Yes | 3.49 | 0.93‒11.1 | ||||
| Lymphatic invasion | 0.150 | |||||
| No | Reference | |||||
| Yes | 2.89 | 0.64‒9.77 | ||||
| Venous invasion | 0.022 | 0.356 | ||||
| No | Reference | Reference | ||||
| Yes | 3.96 | 1.23‒12.7 | 1.17 | 0.29‒4.49 | ||
Variables associated with RFS according to the Cox proportional hazards regression model
RFS Recurrence-free-survival, NLR neutrophil–lymphocyte ratio, NA not available
P-value < 0.05 marked in bold font shows statistical significance
Fig. 2.
Recurrence-free-survival and overall survival for PanNENs stratified by NLR. A higher NLR showed a significant correlation with shorter RFS (median RFS duration, 117.8 months, P < 0.001) (a) and poor OS (median OS duration, 95.2 months, P = 0.032) after curative resection (b)
Subgroup analyses of the hormonal function associated with the NLR
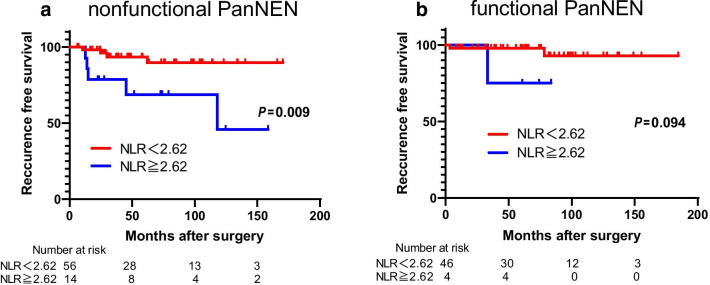
We then focused on the usefulness of the NLR for the classification of functional and nonfunctional PanNEN. We confirmed a strong association between NLR and RFS, especially in nonfunctional PanNEN (HR 4.95; 95% CI 1.430–20.1; P = 0.002) (Table 4). Additionally, a higher NLR was significantly associated with a shorter RFS in nonfunctional PanNEN (P = 0.009) (Fig. 3a). Contrary to nonfunctional PanNEN, NLR was not associated with RFS in functional PanNEN (P = 0.094) (Fig. 3b).
Table 4.
Subgroup analysis for recurrence-free-survival according to neutrophil–lymphocyte ratio
| NLR | n (%) | RFS | ||
|---|---|---|---|---|
| Hazard ratio | 95% CI | P‒value | ||
| Nonfunctional PanNEN | ||||
| < 2.62 | 56 (80) | Reference | 0.002 | |
| ≥ 2.62 | 14 (20) | 4.95 | 1.30‒20.1 | |
| Functional PanNEN | ||||
| < 2.62 | 47 (94) | Reference | 0.198 | |
| ≥ 2.62 | 3 (6) | 6.18 | 0.28‒66.4 | |
P-value < 0.05 marked in bold font shows statistical significance
Fig. 3.
Recurrence-free-survival for nonfunctional and functional PanNENs stratified by NLR. A higher NLR was significantly associated with a shorter RFS in nonfunctional PanNEN (a). Contrary to nonfunctional PanNEN, NLR was not associated with RFS in functional PanNEN (b)
Discussion
The current study demonstrated that elevated preoperative NLR and 2017 WHO classification independently predicted recurrence in patients with well-differentiated PanNEN after curative surgery. No previous studies have demonstrated that increased NLR serves as an independent prognostic factor in patients with nonmetastatic well-differentiated PanNEN, as defined by the 2017 WHO classification. This may be of potential clinical benefit in these patients. Furthermore, we observed that elevated preoperative NLR was predictive of a significantly shorter RFS in nonfunctional PanNEN patients.
Previously, PanNEN with lymph node metastasis, a higher Ki-67 index, and a higher 2010 WHO grade were reported to be associated with a significantly higher risk of recurrence [20, 21]. In contrast, a large international cohort study showed that the ENETs TNM classification was superior to the Union for International Cancer Control/American Joint Committee on Cancer/WHO staging system and could more accurately predict the clinical outcome of patients [22]. We revealed that the ENETs TNM classification was related to RFS in univariate analysis but not in multivariate analysis. A possible explanation for this finding is that we assessed the patients with curatively resected PanNEN and excluded metastatic stage IV patients in our present study. Clinically, the preoperative Ki-67 index obtained by fine-needle aspiration biopsy is less accurate due to intra-tumoral heterogeneity [23], which highlights the requirement of preoperative non-invasive prognostic indicators, such as inflammation-based markers. Preoperative precise assessment of recurrence risk of the patients allows clinically more relevant selection of an optimal surgical strategy, such as enucleation or further lymph node dissection.
In terms of systemic inflammation-based markers in PanNEN, preoperative NLR and PLR have been reported to be useful for predicting lymph node metastasis or recurrence [24–28]. However, these studies included a moderate number of patients with distant metastatic stage IV or poorly differentiated PanNEN (NEC), as defined as 2010 WHO NET-G3. Generally, poorly differentiated PanNEN has substantial distant metastases and a distinctly poor prognosis [29]. The 2010 WHO classification of NET-G3 included both well-differentiated and poorly differentiated PanNEN, resulting in a morphologically and biologically heterogeneous population [30]. Consequently, the 2017 WHO classification of NET-G3 was recategorized as only well-differentiated PanNEN, distinctively different from NEC. Indeed, the median RFS (6.7 months) and median OS (15.3 months) of surgically resected NEC were markedly shorter than in well-differentiated PanNEN in our institute (Additional file 1: Fig. S2.). Furthermore, the value of NLR for NEC was significantly higher than that in patients with well-differentiated PanNEN (Additional file 1: Fig. S3.). In our present study, we assessed the efficacy of NLR to predict recurrence in well-differentiated PanNEN, other than NEC or distant metastasis, based on the 2017 WHO classification. Hence, more prolonged RFS and OS were detected in our present study than in the previously reported ones [24–28].
NLR was recently reported to be associated with tumor progression in several human malignancies [14–18]. In addition, NLR could serve as a predictive marker in patients with not only PanNEN but also gastrointestinal NEN [27]. We previously reported that the NLR was a useful diagnostic marker for predicting intraductal papillary mucinous neoplasm with high-grade dysplasia/invasive carcinoma to differentiate low-grade dysplasia [31]. Previous studies reported that a high NLR was significantly consistent with accumulation of tumor infiltrating CD66b neutrophils or CD163+ macrophages in patients with PanNEN and pancreatic cancer, which results in poor RFS and OS [28, 32]. In general, neutrophils are markers of acute inflammation and could possibly promote tumor development and progression by providing an adequate tumor microenvironment via the production of cytokines and chemokines [33]. In addition, an increased number of lymphocytes play a crucial role in the host’s anticancer immune response; thus, lymphocytosis is generally associated with a better prognosis and a more favorable response to chemotherapy or radiation therapy in a variety of cancers [34]. Therefore, in cancer patients, peripheral blood neutrophilia and lymphopenia may reflect a weak anticancer reaction and worse clinical outcomes [35].
Regardless of the histological findings, there are hormonally functional and nonfunctional phenotypes in PanNEN. According to an epidemiological survey, the number of PanNEN patients has increased rapidly. In particular, hormonally nonfunctional PanNEN was most prevalent and increased significantly [36, 37]. To the best of our knowledge, no previous studies have demonstrated possible roles of NLR as a prognostic factor for RFS in distinct categories of nonfunctional and functional PanNEN. A high NLR has also been proven to be a risk factor of recurrence in nonfunctional PanNEN. In contrast, the NLR was statistically unrelated to RFS in functional PanNEN. One reason for this might be that we analyzed a relatively small number of the patients with hormonally functional PanNEN; only three patients in this subgroup had recurrence during the follow-up period.
Surveillance at shorter intervals might be required in patients with nonfunctional well-differentiated PanNEN with a high NLR and 2017 WHO G2/G3 classification to detect recurrence earlier after surgery. Furthermore, almost all well-differentiated PanNENs express somatostatin receptors; hence, somatostatin receptor scintigraphy should be considered in PanNEN patients with a high NLR to help identify distant metastases that could be missed by computed tomography or positron emission tomography before and after surgery [38–40]. Well-differentiated PanNEN with the risk factors described above may receive clinical benefits by adjuvant treatments such as somatostatin analogs after surgery. However, there is no current evidence or clinical indication for adjuvant therapy, and further studies that focus on these high-risk groups are required.
This study had some limitations. First, it was a retrospective review of a single, high-volume institution in the field of pancreatic tumors. Several studies reported the NLR cutoff values as a prognostic factor, but the cutoff values were different across these studies due to the variation in disease stage or heterogeneity of the patient population. Therefore, we need to perform the external validation study in a large population using a nationwide clinical database or multi-center trial to confirm our findings in the future. Second, although consecutive patients were enrolled, they were collected over a relatively long period, during which treatment strategies changed reasonably.
Conclusion
In summary, the results of our present study clearly demonstrated that the NLR could serve as a useful preoperative marker of clinical recurrence risks after the surgery. It is considered a convenient screening tool for the host immune response and should be incorporated into preoperative workups in the clinical management of well-differentiated PanNEN patients.
Supplementary Information
Additional file 1: Fig. S1. ROC curve for the NLR and tumor size in well-differentiated PanNENs. The ROC curve illustrated that NLR has an AUC of 0.664 (95% CI 0.464–0.864) and tumor size has an AUC of 0.801 (95% CI 0.675–0.945). Arrows indicate optimal cut-off values. Fig. S2. Recurrence-free-survival and overall survival for PanNENs stratified by the 2017 WHO classification. Recurrence-free-survival and overall survival of neuroendocrine carcinoma (NEC) were significantly shorter than those of well-differentiated PanNENs (NET G1-3). Fig. S3. Distribution of NLR in PanNENs stratified by the 2017 WHO classification. The value of NLR in patients with NEC was significantly higher than that in patients with well-differentiated PanNEN.
Acknowledgements
Not applicable.
Abbreviations
- PanNEN
Pancreatic neuroendocrine neoplasm
- NLR
Neutrophil–lymphocyte
- MLR
Monocyte–lymphocyte ratio
- PLR
Platelet–lymphocyte
- PWR
Platelet–white blood cell ratio
- RFS
Recurrence-free-survival
- OS
Overall survival
Authors' contributions
TM: protocol/project development, data collection and management, and manuscript writing. HO, TA, and SA: protocol/project development, data collection and management. TM, TK, and MU: protocol/project development, management, and manuscript editing. TH, TT, SM, and KA: data collection and management. KK and KM: data collection. MI and MM: data analysis and manuscript editing. FF and HS: histopathological analysis and assessment. All authors read and approved final manuscript.
Funding
No funding supported this work.
Availability of data and materials
The datasets used and analyzed during this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional Research Committee and with the 1964 Helsinki Declaration and its later amendments or with comparable ethical standards. This study was approved by the Ethics Committee of the Tohoku University Hospital (institutional review board approval No. 2019-1-303). The Ethics Committee of the Tohoku University Hospital waived the need for informed consent because of the of the study’s retrospective nature.
Consent for publication
Not applicable.
Competing interests
All authors have no conflicts of interest and no financial disclosures.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Oberg K, Eriksson B. Endocrine tumours of the pancreas. Best Pract Res Clin Gastroenterol. 2005;19(5):753–781. doi: 10.1016/j.bpg.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Cives M, Strosberg J. An update on gastroenteropancreatic neuroendocrine tumors. Oncology (Williston Park) 2014;28(9):749–756. [PubMed] [Google Scholar]
- 3.Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors: pancreatic endocrine tumors. Gastroenterology. 2008;135(5):1469–1492. doi: 10.1053/j.gastro.2008.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doi R. Determinants of surgical resection for pancreatic neuroendocrine tumors. J Hepatobiliary Pancreat Sci. 2015;22(8):610–617. doi: 10.1002/jhbp.224. [DOI] [PubMed] [Google Scholar]
- 5.Fendrich V, Langer P, Celik I, Bartsch DK, Zielke A, Ramaswamy A, et al. An aggressive surgical approach leads to long-term survival in patients with pancreatic endocrine tumors. Ann Surg. 2006;244(6):845–51; discussion 852–43. doi: 10.1097/01.sla.0000246951.21252.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah MH, Goldner WS, Halfdanarson TR, Bergsland E, Berlin JD, Halperin D, et al. NCCN Guidelines insights: neuroendocrine and adrenal tumors, Version 2.2018. J Natl Compr Canc Netw. 2018;16(6):693–702. doi: 10.6004/jnccn.2018.0056. [DOI] [PubMed] [Google Scholar]
- 7.Frilling A, Modlin IM, Kidd M, Russell C, Breitenstein S, Salem R, et al. Recommendations for management of patients with neuroendocrine liver metastases. Lancet Oncol. 2014;15(1):e8–21. doi: 10.1016/S1470-2045(13)70362-0. [DOI] [PubMed] [Google Scholar]
- 8.Hijioka S, Hosoda W, Matsuo K, Ueno M, Furukawa M, Yoshitomi H, et al. Rb loss and KRAS mutation are predictors of the response to platinum-based chemotherapy in pancreatic neuroendocrine neoplasm with Grade 3: a Japanese multicenter pancreatic NEN-G3 study. Clin Cancer Res. 2017;23(16):4625–4632. doi: 10.1158/1078-0432.CCR-16-3135. [DOI] [PubMed] [Google Scholar]
- 9.Singhi AD, Klimstra DS. Well-differentiated pancreatic neuroendocrine tumours (PanNETs) and poorly differentiated pancreatic neuroendocrine carcinomas (PanNECs): concepts, issues and a practical diagnostic approach to high-grade (G3) cases. Histopathology. 2018;72(1):168–177. doi: 10.1111/his.13408. [DOI] [PubMed] [Google Scholar]
- 10.Sorbye H, Welin S, Langer SW, Vestermark LW, Holt N, Osterlund P, et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Ann Oncol. 2013;24(1):152–160. doi: 10.1093/annonc/mds276. [DOI] [PubMed] [Google Scholar]
- 11.Lloyd ROR, Kloppel G, et al. WHO classification of tumours of endocrine organs. 4. Lyon: International Agency for Research on Cancer Press; 2017. [Google Scholar]
- 12.Scoazec JY, Couvelard A. Classification of pancreatic neuroendocrine tumours: changes made in the 2017 WHO classification of tumours of endocrine organs and perspectives for the future. Ann Pathol. 2017;37(6):444–456. doi: 10.1016/j.annpat.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohira M, Yoshizumi T, Yugawa K, Kosai-Fujimoto Y, Inokuchi S, Motomura T, et al. Association of inflammatory biomarkers with long-term outcomes after curative surgery for mass-forming intrahepatic cholangiocarcinoma. Surg Today. 2020;50(4):379–388. doi: 10.1007/s00595-019-01905-7. [DOI] [PubMed] [Google Scholar]
- 15.Migita K, Matsumoto S, Wakatsuki K, Ito M, Kunishige T, Nakade H, et al. The prognostic significance of inflammation-based markers in patients with recurrent gastric cancer. Surg Today. 2018;48(3):282–291. doi: 10.1007/s00595-017-1582-y. [DOI] [PubMed] [Google Scholar]
- 16.Cananzi FCM, Ruspi L, Quagliuolo VL. Preoperative monocyte-to-lymphocyte ratio predicts recurrence in gastrointestinal stromal tumors. J Surg Oncol. 2019;119(7):1026. doi: 10.1002/jso.25412. [DOI] [PubMed] [Google Scholar]
- 17.Kawai M, Hirono S, Okada KI, Miyazawa M, Shimizu A, Kitahata Y, et al. Low lymphocyte monocyte ratio after neoadjuvant therapy predicts poor survival after pancreatectomy in patients with borderline resectable pancreatic cancer. Surgery. 2019;165(6):1151–1160. doi: 10.1016/j.surg.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 18.Chen F, Lin L, Yan L, Qiu Y, Cai L, He B. Preoperative neutrophil-to-lymphocyte ratio predicts the prognosis of oral squamous cell carcinoma: a large-sample prospective study. J Oral Maxillofac Surg. 2017;75(6):1275–1282. doi: 10.1016/j.joms.2016.11.022. [DOI] [PubMed] [Google Scholar]
- 19.Rindi G, Kloppel G, Alhman H, Caplin M, Couvelard A, de Herder WW, et al. TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2006;449(4):395–401. doi: 10.1007/s00428-006-0250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Genc CG, Falconi M, Partelli S, Muffatti F, van Eeden S, Doglioni C, et al. Recurrence of pancreatic neuroendocrine tumors and survival predicted by Ki67. Ann Surg Oncol. 2018;25(8):2467–2474. doi: 10.1245/s10434-018-6518-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song KB, Kim SC, Kim JH, Hong SM, Park KM, Hwang DW, et al. Prognostic factors in 151 patients with surgically resected non-functioning pancreatic neuroendocrine tumours. ANZ J Surg. 2016;86(7–8):563–567. doi: 10.1111/ans.12738. [DOI] [PubMed] [Google Scholar]
- 22.Rindi G, Falconi M, Klersy C, Albarello L, Boninsegna L, Buchler MW, et al. TNM staging of neoplasms of the endocrine pancreas: results from a large international cohort study. J Natl Cancer Inst. 2012;104(10):764–777. doi: 10.1093/jnci/djs208. [DOI] [PubMed] [Google Scholar]
- 23.Rebours V, Cordova J, Couvelard A, Fabre M, Palazzo L, Vullierme MP, et al. Can pancreatic neuroendocrine tumour biopsy accurately determine pathological characteristics? Dig Liver Dis. 2015;47(11):973–977. doi: 10.1016/j.dld.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Zou J, Li Q, Kou F, Zhu Y, Lu M, Li J, et al. Prognostic value of inflammation-based markers in advanced or metastatic neuroendocrine tumours. Curr Oncol. 2019;26(1):e30–e38. doi: 10.3747/co.26.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou B, Zhan C, Wu J, Liu J, Zhou J, Zheng S. Prognostic significance of preoperative neutrophil-to-lymphocyte ratio in surgically resectable pancreatic neuroendocrine tumors. Med Sci Monit. 2017;23:5574–5588. doi: 10.12659/MSM.907182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tong Z, Liu L, Zheng Y, Jiang W, Zhao P, Fang W, et al. Predictive value of preoperative peripheral blood neutrophil/lymphocyte ratio for lymph node metastasis in patients of resectable pancreatic neuroendocrine tumors: a nomogram-based study. World J Surg Oncol. 2017;15(1):108. doi: 10.1186/s12957-017-1169-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salman T, Kazaz SN, Varol U, Oflazoglu U, Unek IT, Kucukzeybek Y, et al. Prognostic value of the pretreatment neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio for patients with neuroendocrine tumors: an Izmir oncology group study. Chemotherapy. 2016;61(6):281–286. doi: 10.1159/000445045. [DOI] [PubMed] [Google Scholar]
- 28.Harimoto N, Hoshino K, Muranushi R, Hagiwara K, Yamanaka T, Ishii N, et al. Prognostic significance of neutrophil-lymphocyte ratio in resectable pancreatic neuroendocrine tumors with special reference to tumor-associated macrophages. Pancreatology. 2019;19(6):897–902. doi: 10.1016/j.pan.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Strosberg JR, Coppola D, Klimstra DS, Phan AT, Kulke MH, Wiseman GA, et al. The NANETS consensus guidelines for the diagnosis and management of poorly differentiated (high-grade) extrapulmonary neuroendocrine carcinomas. Pancreas. 2010;39(6):799–800. doi: 10.1097/MPA.0b013e3181ebb56f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Basturk O, Yang Z, Tang LH, Hruban RH, Adsay V, McCall CM, et al. The high-grade (WHO G3) pancreatic neuroendocrine tumor category is morphologically and biologically heterogenous and includes both well differentiated and poorly differentiated neoplasms. Am J Surg Pathol. 2015;39(5):683–690. doi: 10.1097/PAS.0000000000000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hata T, Mizuma M, Motoi F, Ishida M, Morikawa T, Takadate T, et al. Diagnostic and prognostic impact of neutrophil-to-lymphocyte ratio for intraductal papillary mucinous neoplasms of the pancreas with high-grade dysplasia and associated invasive carcinoma. Pancreas. 2019;48(1):99–106. doi: 10.1097/MPA.0000000000001202. [DOI] [PubMed] [Google Scholar]
- 32.Takakura K, Ito Z, Suka M, Kanai T, Matsumoto Y, Odahara S, et al. Comprehensive assessment of the prognosis of pancreatic cancer: peripheral blood neutrophil–lymphocyte ratio and immunohistochemical analyses of the tumour site. Scand J Gastroenterol. 2016;51(5):610–617. doi: 10.3109/00365521.2015.1121515. [DOI] [PubMed] [Google Scholar]
- 33.Gregory AD, Houghton AM. Tumor-associated neutrophils: new targets for cancer therapy. Cancer Res. 2011;71(7):2411–2416. doi: 10.1158/0008-5472.CAN-10-2583. [DOI] [PubMed] [Google Scholar]
- 34.Barnes TA, Amir E. HYPE or HOPE: the prognostic value of infiltrating immune cells in cancer. Br J Cancer. 2017;117(4):451–460. doi: 10.1038/bjc.2017.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Halazun KJ, Hardy MA, Rana AA, Woodland DCT, Luyten EJ, Mahadev S, et al. Negative impact of neutrophil–lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Ann Surg. 2009;250(1):141–151. doi: 10.1097/SLA.0b013e3181a77e59. [DOI] [PubMed] [Google Scholar]
- 36.Ito T, Lee L, Hijioka M, Kawabe K, Kato M, Nakamura K, et al. The up-to-date review of epidemiological pancreatic neuroendocrine tumors in Japan. J Hepatobiliary Pancreat Sci. 2015;22(8):574–577. doi: 10.1002/jhbp.225. [DOI] [PubMed] [Google Scholar]
- 37.Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26(18):3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 38.Treglia G, Castaldi P, Rindi G, Giordano A, Rufini V. Diagnostic performance of Gallium-68 somatostatin receptor PET and PET/CT in patients with thoracic and gastroenteropancreatic neuroendocrine tumours: a meta-analysis. Endocrine. 2012;42(1):80–87. doi: 10.1007/s12020-012-9631-1. [DOI] [PubMed] [Google Scholar]
- 39.Sadowski SM, Neychev V, Millo C, Shih J, Nilubol N, Herscovitch P, et al. Prospective study of 68Ga-DOTATATE positron emission tomography/computed tomography for detecting gastro-entero-pancreatic neuroendocrine tumors and unknown primary sites. J Clin Oncol. 2016;34(6):588–596. doi: 10.1200/JCO.2015.64.0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsumoto T, Okabe H, Yamashita YI, Yusa T, Itoyama R, Nakao Y, et al. Clinical role of fludeoxyglucose (18F) positron emission tomography/computed tomography ((18)F-FDG PET/CT) in patients with pancreatic neuroendocrine tumors. Surg Today. 2019;49(1):21–26. doi: 10.1007/s00595-018-1703-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Fig. S1. ROC curve for the NLR and tumor size in well-differentiated PanNENs. The ROC curve illustrated that NLR has an AUC of 0.664 (95% CI 0.464–0.864) and tumor size has an AUC of 0.801 (95% CI 0.675–0.945). Arrows indicate optimal cut-off values. Fig. S2. Recurrence-free-survival and overall survival for PanNENs stratified by the 2017 WHO classification. Recurrence-free-survival and overall survival of neuroendocrine carcinoma (NEC) were significantly shorter than those of well-differentiated PanNENs (NET G1-3). Fig. S3. Distribution of NLR in PanNENs stratified by the 2017 WHO classification. The value of NLR in patients with NEC was significantly higher than that in patients with well-differentiated PanNEN.
Data Availability Statement
The datasets used and analyzed during this study are available from the corresponding author upon reasonable request.