Abstract
Cryoablation is a well-tolerated outpatient procedure that has been used to treat metastatic sites as well as small breast cancers in patients who are considered poor candidates for surgery. Recent studies suggest that cell disruption caused by cryoablation may increase the expression and immunogenicity of tumor neoantigens, which could enhance the ability of the immune system to recognize and attack cancer cells at both local and distant sites. Such an approach might broaden the role of immunotherapy for the treatment of breast cancer, which has previously demonstrated limited response to these agents, likely owing to the modest immunogenicity of most breast cancer subtypes. If cryoablation can induce a systemic tumor-specific response, it could enhance tumor susceptibility to immunotherapy agents. This review briefly summarizes the necessary components for generating an immune response against tumor cells, reviews the tumor microenvironment of breast cancer, describes the rationale for and limitations of immune checkpoint inhibition, highlights the potential for cryoablation to induce a systemic tumor-specific immune response, and describes the rationale for combining cryoablation and immune checkpoint inhibitors for the treatment of breast cancer.
Keywords: Ablation Techniques, Breast, Neoplasms-Primary, Percutaneous, Tumor Microenvironment, Tumor Response, Ultrasonography
© RSNA, 2021
Keywords: Ablation Techniques, Breast, Neoplasms-Primary, Percutaneous, Tumor Microenvironment, Tumor Response, Ultrasonography
Summary
Treating breast cancer with the combination of cryoablation and immune checkpoint inhibition is an appealing strategy owing to the synergistic mechanisms of these therapies. Cryoablation enhances tumor immunogenicity, which may facilitate response to immunotherapy, while immune checkpoint blockade may allow the body to mount a robust response to tumor-specific antigens released by cryoablation.
Essentials
■ Most breast cancers are not inherently immunogenic and thus, monotherapy with immune checkpoint inhibitors has had modest efficacy in selected subtypes of breast cancer.
■ Cancer cells destroyed by cryoablation release proteins that may lead to enhanced immune recognition and a sustained tumor-specific immune response that acts on both local and distant disease.
■ The combination of cryoablation and immunotherapy may provide better responses in breast cancer than either therapy alone.
Introduction
Cryoablation, the use of extreme cold to kill cancer cells, is a well-tolerated outpatient procedure that has been used to eradicate metastatic disease and treat small breast cancers in patients who are considered poor candidates for surgery (1–3). Recent studies demonstrate that, in addition to causing direct damage to neoplastic tissue, cryoablation induces a systemic tumor-specific immune response (4,5). These findings are of particular significance as agents targeting the immune system have rapidly become a mainstay in cancer treatment and are now the standard of care in the treatment of several malignancies, including melanoma, lung, and bladder cancer. The goal of using immunotherapy agents is to flag cancer cells as foreign so they are recognized by the immune system, while also modulating immune-regulating signals toward an inflammatory state directed against the tumor. Investigated strategies have included priming the immune system against tumor-associated antigens with cancer vaccines, injecting oncolytic viruses into tumors, delivering chimeric antigen receptor T-cell therapies, and administering antibodies against various immune system targets to exploit existing immunity (6). When antibodies are used to inhibit immune checkpoint molecules, this removes the physiologic brake on the activated immune system to allow a sustained antigen-specific immune response.
Studies examining the efficacy of immune checkpoint inhibition in breast cancer have primarily focused on antibodies targeting cytotoxic T-lymphocyte antigen-4 (CTLA-4), programmed cell death 1 protein (PD-1), and programmed cell death ligand 1 (PD-L1). However, while preclinical studies have been promising, early clinical studies of immune checkpoint inhibition for breast cancer have shown relatively modest responses (7,8). This has been primarily attributed to the modest immune response elicited by most breast cancers, which typically demonstrate lower mutational rates and lower expression of tumor-associated neoantigens (9). Emerging data suggest that this relative resistance of breast cancer to immunotherapy agents may be overcome by combining multiple strategies, particularly those that enhance tumor immunogenicity such as cryoablation. In this review, we describe how cryoablation and immune checkpoint inhibitors interact with the immune system, summarize recent data on their efficacy in the treatment of breast cancer, and outline the rationale for combined therapy.
Principles of an Antigen-specific Immune Response
The efficacy of the immune system in mediating tumor regression depends on the efficient induction and maintenance of tumor antigen–specific T-cell responses. The inherent genetic instability of most tumor cells results in the expression of aberrant antigenic proteins or the overexpression of normally repressed genes that ultimately provide a target for T-cell recognition.
The activation of effector T cells depends on two signals. The process is initiated once the T-cell receptor engages its cognate antigen through interaction with the major histocompatibility complex on antigen-presenting cells (APCs). The second signal is delivered when the B7 costimulatory molecule expressed on APCs binds to the CD28 ligand on the surface of T cells. This results in a proinflammatory state and T-cell expansion. If the costimulatory signal is not received, T cells presented with antigen become anergic.
After a T cell has been activated by these two signals, a physiologic negative feedback loop is initiated to prevent an overexuberant T-cell response and potential autoimmunity. This so-called coinhibitory signal is delivered when CTLA-4 on T cells binds to B7 on APCs. CTLA-4 is normally upregulated to promote regulatory T cell–associated immune suppression and dampen effector T-cell proliferation in the priming phase of the immune response, primarily acting in lymph nodes (10,11). A different coinhibitory molecule, PD-1, attenuates the proliferation of effector T cells in the effector phase, typically at the site of the tumor (10). PD-L1 is the ligand of PD-1 and is expressed on a variety of cells, including tumor cells, and is one mechanism by which tumors escape immune surveillance (12).
Initial attempts to exploit this immune system mechanism focused on enhancing the costimulatory signal required to activate T cells. Current investigations have shifted to blocking the coinhibitory signal delivered by CTLA-4 and other related targets, including the PD-1/PD-L1 axis, thereby removing the brake on the immune system.
Tumor-infiltrating Lymphocytes
Tumor-infiltrating lymphocytes (TILs) have been identified in some breast tumors, and when present, predict greater response to neoadjuvant chemotherapy and better overall survival rates (13–15). This advantage appears to be directly associated with the amount of lymphocytic infiltrate, with tumors demonstrating greater than 50% TILs (ie, lymphocyte-predominant breast cancers [LPBCs]) deriving greatest benefit. For example, a study of 2009 patients with node-positive breast cancer found that in estrogen receptor–negative/human epidermal growth factor receptor 2 (HER2)–negative breast cancers, every 10% increase in TILs was associated with a 15%–17% reduction in risk of recurrence and a 17%–27% reduction in risk of death (13). In another study of patients with locally advanced breast cancer treated with neoadjuvant chemotherapy, patients with LPBC had a 40% pathologic complete response as compared with a 7% pathologic complete response in patients with tumors that had no lymphocytic infiltrate (15). Unfortunately, while triple-negative and HER2-positive breast cancers have most consistently been associated with the presence of TILs and higher rates of LPBCs, lymphocyte-predominant infiltrates are relatively uncommon: across all subtypes, they are seen in less than 10% of breast cancers (16,17).
The composition of the lymphocytic infiltrate is important as well. Increased CD8+ effector T cells have been shown to predict improved clinical outcome, and higher numbers of intratumoral CD8+ effector T cells are associated with improved breast cancer–specific survival (18). Conversely, the presence of CD4+ regulatory T cells in the tumor has been associated with worse prognosis, including decreased disease-free and overall survival (19).
Checkpoint Inhibition
Immune checkpoint inhibitors represent a major disruptive breakthrough in cancer care. CTLA-4 blockade and its clinical success in melanoma therapy pioneered the field of checkpoint inhibition, which in turn led to the development of other targets, such as those of the PD-1/PD-L1 axis (20, 21). Response to these agents is likely affected by a number of both tumor- and host-related factors. For example, studies suggest that patient sex, age, gut microbiome, and human leukocyte antigen class I (HLA-I) genotype may have a role (22). In patients with melanoma, resistance to immune checkpoint inhibition has been associated with Wnt/β-catenin pathway activation, tumor loss of phosphatase and homolog expression, and mutations in interferon receptor–associated Janus kinase 1 (JAK1) or JAK2 (23). In breast cancer specifically, studies of immunotherapy response predictors have been largely limited to tumor PD-L1 expression, TIL density and composition, and tumor mutation load. A recent systematic review and meta-analysis demonstrated that PD-L1 positivity, higher TIL levels, and higher CD8+ T-cell levels predict better response to immunotherapy (24). Higher tumor mutational burden has also correlated with improved response, which has been discouraging given that most breast cancers demonstrate low tumor mutational burden (9,23,25).
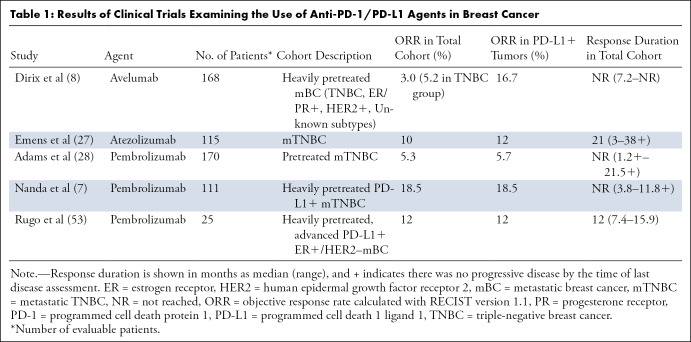
Recent clinical trials have investigated the efficacy of monotherapy with anti–PD-1/PD-L1 agents in breast cancer (7,8,24). The proportion of patients who respond to these agents has been modest, with objective response rates ranging from 6% to 19% in patients with PD-L1–positive tumors, and rates of 0%–4.7% in patients with PD-L1–negative tumors (7, 8,27,28) (Table 1). However, in patients who respond, the treatment effects are durable. PD-L1 expression is challenging to measure, and reporting has not been standardized, which poses an additional challenge to determining which patients might benefit from anti-PD-1/PD-L1 monotherapy (29). Regardless, the addition of agents that elicit an immune response and upregulate PD-L1 may enable those with PD-1–negative breast cancers to clinically benefit from PD-1 inhibitors (30).
Table 1:
Results of Clinical Trials Examining the Use of Anti-PD-1/PD-L1 Agents in Breast Cancer
There have been far fewer clinical trials investigating CTLA-4 antagonist monotherapy in breast cancer. In one phase 1 study of 26 patients with hormone receptor–positive metastatic breast cancer, tremelimumab (anti–CTLA-4) was combined with the aromatase inhibitor exemestane (26). Although disease stability was observed in 42% of patients, no patients showed partial or complete response. However, it is important to note that this subtype has historically low levels of TILs, and so may have been less likely to respond to immune modulation than those with higher levels of TILs.
Combination therapy with CTLA-4 and PD-1/PD-L1 antagonists is also currently being investigated. An ongoing phase I/II trial is comparing nivolumab (anti-PD-1) alone to combination therapy with ipilimumab (anti–CTLA-4) in solid cancers, including triple-negative breast cancer (ClinicalTrials.gov identifier NCT01928394). Two other trials are investigating the efficacy of durvalumab (anti–PD-L1) and tremelimumab (anti–CTLA-4) in patients with metastatic HER2-negative breast cancer (ClinicalTrials.gov identifier NCT02536794) and hormone receptor–positive, HER2-negative stage II or III breast cancer (ClinicalTrials.gov identifier NCT03132467).
Cryoablation
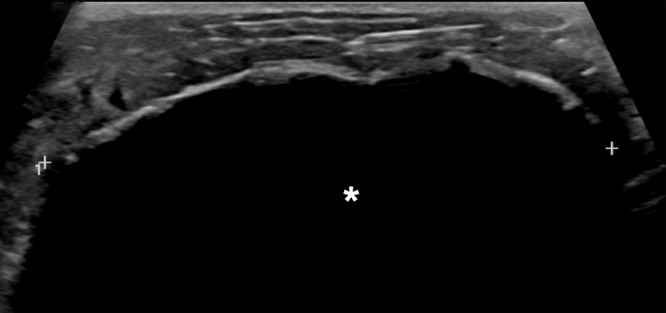
Breast cryoablation is an office-based US-guided percutaneous procedure that uses extremely cold temperatures to freeze tissue surrounding the tip of the ablation probe needle (31). It is well-tolerated by patients and achieves cosmetically satisfactory results (32). Cryoablation has been successfully used to target metastatic sites in the liver, kidney, lung, bone, and soft tissues, and clinical trials examining its efficacy in treating small, early-stage invasive ductal carcinoma have been promising (1–3). The Figure provides a representative example of cryoablation for the purpose of intraductal carcinoma treatment. Recently, clinical interest has emerged in using cryoablation to also elicit an immune response that acts on metastatic sites distant from the treated lesion.
Figure:

Images in a 73-year-old woman with grade II estrogen receptor/progesterone receptor–positive human epidermal growth factor receptor 2–negative intraductal carcinoma measuring 1.1 cm in size. US images acquired during cryoablation procedure are shown. (a) Long-axis view of tumor (arrow) prior to the procedure. (b) Long-axis view and (c) short-axis view after cryoprobe placement within the tumor (arrow). Arrowhead denotes edge of tumor, and caliper (+) denotes cryoprobe tip. (d) Long-axis view of the ice ball (*) enveloping the tumor. Calipers correspond to the margin of the ice ball.
Figure:

Images in a 73-year-old woman with grade II estrogen receptor/progesterone receptor–positive human epidermal growth factor receptor 2–negative intraductal carcinoma measuring 1.1 cm in size. US images acquired during cryoablation procedure are shown. (a) Long-axis view of tumor (arrow) prior to the procedure. (b) Long-axis view and (c) short-axis view after cryoprobe placement within the tumor (arrow). Arrowhead denotes edge of tumor, and caliper (+) denotes cryoprobe tip. (d) Long-axis view of the ice ball (*) enveloping the tumor. Calipers correspond to the margin of the ice ball.
Figure:

Images in a 73-year-old woman with grade II estrogen receptor/progesterone receptor–positive human epidermal growth factor receptor 2–negative intraductal carcinoma measuring 1.1 cm in size. US images acquired during cryoablation procedure are shown. (a) Long-axis view of tumor (arrow) prior to the procedure. (b) Long-axis view and (c) short-axis view after cryoprobe placement within the tumor (arrow). Arrowhead denotes edge of tumor, and caliper (+) denotes cryoprobe tip. (d) Long-axis view of the ice ball (*) enveloping the tumor. Calipers correspond to the margin of the ice ball.
Figure:
Images in a 73-year-old woman with grade II estrogen receptor/progesterone receptor–positive human epidermal growth factor receptor 2–negative intraductal carcinoma measuring 1.1 cm in size. US images acquired during cryoablation procedure are shown. (a) Long-axis view of tumor (arrow) prior to the procedure. (b) Long-axis view and (c) short-axis view after cryoprobe placement within the tumor (arrow). Arrowhead denotes edge of tumor, and caliper (+) denotes cryoprobe tip. (d) Long-axis view of the ice ball (*) enveloping the tumor. Calipers correspond to the margin of the ice ball.
The rationale for this interest can be explained by review of the mechanism of cryoablation. Two short freeze-thaw cycles at a high freeze rate result in coagulative necrosis of cells closest to the probe. In the freeze phase, water freezes in the extracellular space faster than it does in the intracellular space, which sets up an osmotic gradient that drives fluid out of cells, resulting in cellular dehydration. During the thaw phase, the osmotic gradient reverses so that water rapidly enters cells, causing cell rupture. Additional injury results from the formation and growth of ice crystals, as well as from endothelial cell dysfunction, which causes vascular stasis and ischemia of tumor cells (33,34).
The resultant coagulative necrosis of tumor cells elicits a local inflammatory response, and importantly, also causes the release of intact tumor antigens, cellular stress signals, and type 1 cytokines into circulation (35,36). These signals result in the recruitment of APCs to the tumor and enhanced presentation of tumor-specific antigens to T cells, thereby inducing a tumor-specific T-cell response (37,38). This has been demonstrated in animal models of breast cancer, specifically, in which high freeze rate cryoablation resulted in an increase in tumor-specific T cells in tumor-draining lymph nodes, a decrease in the number of regulatory T cells, and improved survival (4,5). Essentially, local ablative therapies convert the tumor into an in situ vaccine that induces a systemic antitumor immune response.
Notably, when compared with heat-based modalities such as radiofrequency and microwave ablation, cryoablation induces a greater postablative immune response as demonstrated by markedly elevated levels of proinflammatory cytokines (39–42). Partly because of the analgesic effects of ice, it is also better tolerated than heat-based ablation, and so only local anesthesia is required for targets in the breast (43).
In preclinical studies of cryoablation in breast cancer, the elicited immune response has been associated with regression of tumors distant from the treated lesion, a phenomenon known as the abscopal effect (4,5,34). However, in practice, this is a rare phenomenon when using local ablation agents alone for the treatment of breast cancer and is limited to case reports (44,45).
Combined Cryoablation and Checkpoint Inhibition
Since cryoablation induces a systemic tumor-specific T-cell response and checkpoint inhibitors remove the breaks on the immune system, combining the two therapies may have synergistic effects. Cryoablation may enhance immune recognition of breast cancer, facilitating tumor response to immunotherapy. In turn, checkpoint inhibition may allow the body to mount a robust immune response to the tumor-specific antigens released by cryoablation, enabling destruction of tumor cells in not only the breast, but also in regional lymph nodes and occult metastatic sites (36). Essentially, combination therapy could allow the abscopal effect (36).
Studies of combination therapy in animal models of solid tumors have demonstrated efficacy. In a mouse model of prostate cancer, cryoablation of the primary tumor followed by anti–CTLA-4 therapy slowed growth or triggered rejection of injected second tumors modeling micrometastasis (46). Clinical trials studying combination therapy for the treatment of various cancers, including breast cancer, are ongoing (47) (Table 2). While the results of these larger trials are not yet available, a few pilot studies have published preliminary data that show promise. A study combining cryoablation with either ipilimumab (anti–CTLA-4) or pembrolizumab (anti–PD-1) for the treatment of metastatic melanoma is reporting responses in both local (cryoablated) and distant lesions (48).
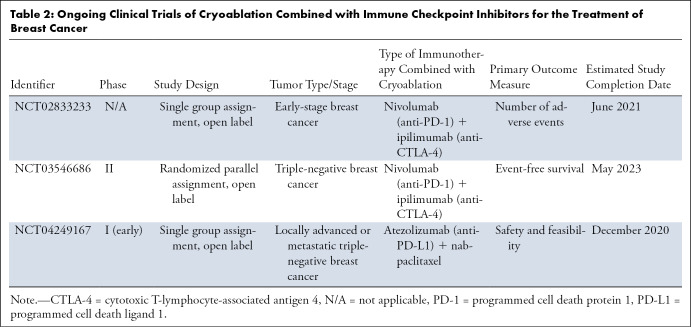
Table 2:
Ongoing Clinical Trials of Cryoablation Combined with Immune Checkpoint Inhibitors for the Treatment of Breast Cancer
In breast cancer specifically, a treat-and-resect pilot study has demonstrated that preoperative cryoablation and single-dose ipilimumab are safe alone and in combination (49). Furthermore, the combination therapy was associated with a potentially favorable intratumoral and systemic immunologic response as demonstrated by sustained elevations in type 1 cytokines, activated and proliferating CD8+ T cells, and posttreatment proliferative effector T cells relative to regulatory T cells within the tumor bed (49). In addition, this combination therapy was associated with upregulation of interferon gamma, which in turn has been shown to increase expression of PD-L1, and thus could benefit patients with low TILs and low PD-1/PD-L1 expression (49–51).
Given that breast cancer is generally less immunogenic than most cancers, it may require the combination of both anti–CTLA-4 and anti–PD-L1 agents with cryoablation to induce the abscopal effect. Accordingly, investigators are currently recruiting patients for clinical trials that use both nivolumab (anti–PD-1) and ipilimumab (anti–CTLA-4) with cryoablation for the treatment of breast cancer (ClinicalTrials.gov identifiers NCT02833233 and NCT03546686). The results of these studies will help determine if the theory behind combined therapy translates to improved clinical outcomes. Should any beneficial clinical immune response be noted, thorough analyses of multiple treatment parameters and additional adjuvants (eg, toll-like receptors) will be needed to further improve upon those responses. Finally, some checkpoint inhibitors are dose dependent, and a combined percutaneous approach may allow the administration of lower doses, which may help decrease adverse effects while maintaining effective clinical outcomes (52).
Conclusion
Immunotherapy is a rapidly evolving field that seeks to harness the potential of the body’s immune system to recognize and eradicate neoplastic tissue. While the efficacy of these agents in breast cancer has previously been limited by the modest immune response mounted toward most tumors, cryoablation has the potential to enhance tumor immunogenicity, which may facilitate response to these agents. In turn, immune checkpoint inhibition removes the brakes on the activated immune system and by so doing may allow the body to mount a robust immune response to tumor-specific antigens released by cryoablation. Thus, combining cryoablation with immune checkpoint inhibition is a rational strategy aimed at improving immune recognition and activation, and may result in an augmented tumor-specific immune response that acts on both local and distant disease. The results of ongoing studies will determine the trajectory with which this combination approach may be used in the future to treat breast cancer.
Authors declared no funding for this work.
Disclosures of Conflicts of Interest: H.R.T. disclosed no relevant relationships. R.C.W. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed no relevant relationships. Other relationships: principal investigator in a clinical trial. W.M.S. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: received payment for lecturing from Lilly Oncology, Eisai, Daiichi-Sankyo, and AstraZeneca. Other relationships: disclosed no relevant relationships. P.J.L. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: consultant to Galil Medical and Endocare; issued patents with Endocare. Other relationships: disclosed no relevant relationships.
Abbreviations:
- APC
- antigen-presenting cell
- CTLA-4
- cytotoxic T-lymphocyte antigen-4
- HER2
- human epidermal growth factor receptor 2
- JAK
- Janus kinase
- LPBC
- lymphocyte-predominant breast cancer
- PD-1
- programmed cell death protein 1
- PD-L1
- programmed cell death ligand 1
- TIL
- tumor-infiltrating lymphocyte
References
- 1.Littrup PJ, Bang HJ, Currier BP, et al. Soft-tissue cryoablation in diffuse locations: feasibility and intermediate term outcomes. J Vasc Interv Radiol 2013;24(12):1817–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simmons RM, Ballman KV, Cox C, et al. A Phase II Trial Exploring the Success of Cryoablation Therapy in the Treatment of Invasive Breast Carcinoma: Results from ACOSOG (Alliance) Z1072. Ann Surg Oncol 2016;23(8):2438–2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pusceddu C, Paliogiannis P, Nigri G, Fancellu A. Cryoablation In The Management Of Breast Cancer: Evidence To Date. Breast Cancer (Dove Med Press; ) 2019;11:283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sabel MS, Nehs MA, Su G, Lowler KP, Ferrara JLM, Chang AE. Immunologic response to cryoablation of breast cancer. Breast Cancer Res Treat 2005;90(1):97–104. [DOI] [PubMed] [Google Scholar]
- 5.Sabel MS, Su G, Griffith KA, Chang AE. Rate of freeze alters the immunologic response after cryoablation of breast cancer. Ann Surg Oncol 2010;17(4):1187–1193. [DOI] [PubMed] [Google Scholar]
- 6.Sanchez K, Page D, McArthur HL. Immunotherapy in breast cancer: An overview of modern checkpoint blockade strategies and vaccines. Curr Probl Cancer 2016;40(2-4):151–162. [DOI] [PubMed] [Google Scholar]
- 7.Nanda R, Chow LQM, Dees EC, et al. Pembrolizumab in Patients With Advanced Triple-Negative Breast Cancer: Phase Ib KEYNOTE-012 Study. J Clin Oncol 2016;34(21):2460–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dirix LY, Takacs I, Jerusalem G, et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN Solid Tumor study. Breast Cancer Res Treat 2018;167(3):671–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol 2013;31:51–72. [DOI] [PubMed] [Google Scholar]
- 10.Ward RC, Kaufman HL. Targeting costimulatory pathways for tumor immunotherapy. Int Rev Immunol 2007;26(3-4):161–196. [DOI] [PubMed] [Google Scholar]
- 11.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med 2009;206(8):1717–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen LT, Ohashi PS. Clinical blockade of PD1 and LAG3--potential mechanisms of action. Nat Rev Immunol 2015;15(1):45–56. [DOI] [PubMed] [Google Scholar]
- 13.Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol 2013;31(7):860–867. [DOI] [PubMed] [Google Scholar]
- 14.Dushyanthen S, Beavis PA, Savas P, et al. Relevance of tumor-infiltrating lymphocytes in breast cancer. BMC Med 2015;13:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denkert C, Loibl S, Noske A, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol 2010;28(1):105–113. [Published correction appears in J Clin Oncol 2010;28(4):708. [DOI] [PubMed] [Google Scholar]
- 16.Stanton SE, Disis ML. Clinical significance of tumor-infiltrating lymphocytes in breast cancer. J Immunother Cancer 2016;4:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loi S, Drubay D, Adams S, et al. Tumor-Infiltrating Lymphocytes and Prognosis: A Pooled Individual Patient Analysis of Early-Stage Triple-Negative Breast Cancers. J Clin Oncol 2019;37(7):559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahmoud SMA, Paish EC, Powe DG, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol 2011;29(15):1949–1955. [DOI] [PubMed] [Google Scholar]
- 19.Bates GJ, Fox SB, Han C, et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol 2006;24(34):5373–5380. [DOI] [PubMed] [Google Scholar]
- 20.Schadendorf D, Hodi FS, Robert C, et al. Pooled Analysis of Long-Term Survival Data From Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. J Clin Oncol 2015;33(17):1889–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med 2015;372(26):2521–2532. [DOI] [PubMed] [Google Scholar]
- 22.Yan X, Zhang S, Deng Y, Wang P, Hou Q, Xu H. Prognostic Factors for Checkpoint Inhibitor Based Immunotherapy: An Update With New Evidences. Front Pharmacol 2018;9:1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rao SV, Moran AE, Graff JN. Predictors of response and resistance to checkpoint inhibitors in solid tumors. Ann Transl Med 2017;5(23):468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zou Y, Zou X, Zheng S, et al. Efficacy and predictive factors of immune checkpoint inhibitors in metastatic breast cancer: a systematic review and meta-analysis. Ther Adv Med Oncol 2020;12:1758835920940928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voutsadakis IA. High Tumor Mutation Burden and Other Immunotherapy Response Predictors in Breast Cancers: Associations and Therapeutic Opportunities. Target Oncol 2020;15(1):127–138. [DOI] [PubMed] [Google Scholar]
- 26.Vonderheide RH, LoRusso PM, Khalil M, et al. Tremelimumab in combination with exemestane in patients with advanced breast cancer and treatment-associated modulation of inducible costimulator expression on patient T cells. Clin Cancer Res 2010;16(13):3485–3494. [DOI] [PubMed] [Google Scholar]
- 27.Emens LA, Cruz C, Eder JP, et al. Long-term Clinical Outcomes and Biomarker Analyses of Atezolizumab Therapy for Patients With Metastatic Triple-Negative Breast Cancer: A Phase 1 Study. JAMA Oncol 2019;5(1):74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adams S, Schmid P, Rugo HS, et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann Oncol 2019;30(3):397–404. [DOI] [PubMed] [Google Scholar]
- 29.García-Teijido P, Cabal ML, Fernández IP, Pérez YF. Tumor-Infiltrating Lymphocytes in Triple Negative Breast Cancer: The Future of Immune Targeting. Clin Med Insights Oncol 2016;10(Suppl 1):31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burugu S, Asleh-Aburaya K, Nielsen TO. Immune infiltrates in the breast cancer microenvironment: detection, characterization and clinical implication. Breast Cancer 2017;24(1):3–15. [DOI] [PubMed] [Google Scholar]
- 31.Ward RC, Lourenco AP, Mainiero MB. Ultrasound-Guided Breast Cancer Cryoablation. AJR Am J Roentgenol 2019;213(3):716–722. [DOI] [PubMed] [Google Scholar]
- 32.Lanza E, Palussiere J, Buy X, et al. Percutaneous Image-Guided Cryoablation of Breast Cancer: A Systematic Review. J Vasc Interv Radiol 2015;26(11):1652–7.e1. [DOI] [PubMed] [Google Scholar]
- 33.Baust JG, Gage AA. The molecular basis of cryosurgery. BJU Int 2005;95(9):1187–1191. [DOI] [PubMed] [Google Scholar]
- 34.Mehta A, Oklu R, Sheth RA. Thermal Ablative Therapies and Immune Checkpoint Modulation: Can Locoregional Approaches Effect a Systemic Response? Gastroenterol Res Pract 2016;2016:9251375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer 2014;14(3):199–208. [DOI] [PubMed] [Google Scholar]
- 36.Abdo J, Cornell DL, Mittal SK, Agrawal DK. Immunotherapy Plus Cryotherapy: Potential Augmented Abscopal Effect for Advanced Cancers. Front Oncol 2018;8:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med 2006;203(5):1259–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Apetoh L, Ghiringhelli F, Tesniere A, et al. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol Rev 2007;220:47–59. [DOI] [PubMed] [Google Scholar]
- 39.Erinjeri JP, Thomas CT, Samoilia A, et al. Image-guided thermal ablation of tumors increases the plasma level of interleukin-6 and interleukin-10. J Vasc Interv Radiol 2013;24(8):1105–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahmad F, Gravante G, Bhardwaj N, et al. Changes in interleukin-1β and 6 after hepatic microwave tissue ablation compared with radiofrequency, cryotherapy and surgical resections. Am J Surg 2010;200(4):500–506. [DOI] [PubMed] [Google Scholar]
- 41.Jansen MC, van Hillegersberg R, Schoots IG, et al. Cryoablation induces greater inflammatory and coagulative responses than radiofrequency ablation or laser induced thermotherapy in a rat liver model. Surgery 2010;147(5):686–695. [DOI] [PubMed] [Google Scholar]
- 42.Chapman WC, Debelak JP, Wright Pinson C, et al. Hepatic cryoablation, but not radiofrequency ablation, results in lung inflammation. Ann Surg 2000;231(5):752–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Littrup PJ, Jallad B, Chandiwala-Mody P, D’Agostini M, Adam BA, Bouwman D. Cryotherapy for breast cancer: a feasibility study without excision. J Vasc Interv Radiol 2009;20(10):1329–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar AV, Patterson SG, Plaza MJ. Abscopal Effect following Cryoablation of Breast Cancer. J Vasc Interv Radiol 2019;30(3):466–469. [DOI] [PubMed] [Google Scholar]
- 45.Azami A, Suzuki N, Azami Y, et al. Abscopal effect following radiation monotherapy in breast cancer: A case report. Mol Clin Oncol 2018;9(3):283–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Waitz R, Solomon SB, Petre EN, et al. Potent induction of tumor immunity by combining tumor cryoablation with anti-CTLA-4 therapy. Cancer Res 2012;72(2):430–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aarts BM, Klompenhouwer EG, Rice SL, et al. Cryoablation and immunotherapy: an overview of evidence on its synergy. Insights Imaging 2019;10(1):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim DW, Haymaker C, McQuail N, et al. Pilot study of intratumoral (IT) cryoablation (cryo) in combination with systemic checkpoint blockade in patients with metastatic melanoma (MM). J Immunother Cancer 2015;3(Suppl 2):P137. [Google Scholar]
- 49.McArthur HL, Diab A, Page DB, et al. A Pilot Study of Preoperative Single-Dose Ipilimumab and/or Cryoablation in Women with Early-Stage Breast Cancer with Comprehensive Immune Profiling. Clin Cancer Res 2016;22(23):5729–5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tiainen S, Tumelius R, Rilla K, et al. High numbers of macrophages, especially M2-like (CD163-positive), correlate with hyaluronan accumulation and poor outcome in breast cancer. Histopathology 2015;66(6):873–883. [DOI] [PubMed] [Google Scholar]
- 51.Hollmén M, Roudnicky F, Karaman S, Detmar M. Characterization of macrophage--cancer cell crosstalk in estrogen receptor positive and triple-negative breast cancer. Sci Rep 2015;5:9188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer 2017;5(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rugo HS, Delord JP, Im SA, et al. Safety and antitumor activity of pembrolizumab in patients with estrogen receptor–positive/human epidermal growth factor receptor 2–negative advanced breast cancer. Clin Cancer Res 2018;24(12):2804–2811. [DOI] [PubMed] [Google Scholar]