Abstract
Purpose
To assess the clinical effectiveness of cryoablation for palliation of painful bone metastases.
Materials and Methods
MOTION (Multicenter Study of Cryoablation for Palliation of Painful Bone Metastases) (ClinicalTrials.gov NCT 02511678) was a multicenter, prospective, single-arm study of adults with metastatic bone disease who were not candidates for or had not benefited from standard therapy, that took place from February 2016 to March 2018. At baseline, participants rated their pain using the Brief Pain Inventory-Short Form (reference range from 0 to 10 points); those with moderate to severe pain, who had at least one metastatic candidate tumor for ablation, were included. The primary effectiveness endpoint was change in pain score from baseline to week 8. Participants were followed for 24 weeks after treatment. Statistical analyses included descriptive statistics and logistic regression to evaluate changes in pain score over the postprocedure follow-up period.
Results
A total of 66 participants (mean age, 60.8 years ± 14.3 [standard deviation]; 35 [53.0%] men) were enrolled and received cryoablation; 65 completed follow-up. Mean change in pain score from baseline to week 8 was −2.61 points (95% CI: −3.45, −1.78). Mean pain scores improved by 2 points at week 1 and reached clinically meaningful levels (more than a 2-point decrease) after week 8; scores continued to improve throughout follow-up. Quality of life improved, opioid doses were stabilized, and functional status was maintained over 6 months. Serious adverse events occurred in three participants.
Conclusion
Cryoablation of metastatic bone tumors provided rapid and durable pain palliation, improved quality of life, and offered an alternative to opioids for pain control.
Keywords: Ablation Techniques, Metastases, Pain Management, Radiation Therapy/Oncology
Supplemental material is available for this article.
© RSNA, 2021
Keywords: Ablation Techniques, Metastases, Pain Management, Radiation Therapy/Oncology
Summary
Cryoablation is a safe and minimally invasive therapy option that provided rapid and durable pain relief, as well as an improved quality of life, in participants with bone metastases.
Key Points
■ Mean pain score improvement (from the Brief Pain Inventory-Short Form, scores 1–10) over the course of the first 8 weeks after cryoablation was −2.61 points (95% CI: −3.45, −1.78).
■ Quality of life improved over the course of the study period, with 27%–61% of participants rating their pain as “better than at the last visit” at follow-up visits (ranging from 1 to 24 weeks after treatment).
■ Palliation of painful metastatic bone lesions with cryoablation was rapid and durable for participants who were unresponsive to or were not candidates for standard pain therapy.
Introduction
Bone is a common site of metastasis in many types of cancer and can lead to devastating skeletal-related events such as hypercalcemia, pathologic bone fractures, metastatic epidural spinal cord compression, and bone pain (1,2). These events are associated with decreased physical function and quality of life (3–5). Objectives in treating patients with bone metastases include prevention of skeletal complications, palliation of pain, and maintaining quality of life. For patients with focal pain related to osseous metastatic disease, external beam radiation therapy is the standard of care, along with opioid and nonopioid analgesics (6–8). In patients who receive radiation therapy, pain can persist after treatment. The most commonly used dose (8 Gy) provided complete response in only 22% of patients and partial response in 38%; other doses fail to provide response in the majority of patients (9). Despite updated guidelines for managing metastatic bone pain, many patients still feel moderate to severe pain (10–12). For these patients, systemic analgesics are the only remaining option (5,13).
Over the past decade, minimally invasive or noninvasive image-guided percutaneous thermal ablation techniques, such as radiofrequency ablation, have become a well-established treatment modality and are now widely used as the primary or secondary treatment of bone tumor pain in patients who cannot undergo surgery or in whom radiation therapy has failed (14,15). Advantages of cryoablation over other thermal ablation techniques (radiofrequency or microwave ablation) include the ability to directly visualize the ablation margin, customize the size and shape of the ablation zones to match the target lesion, decrease intraprocedural and postprocedural pain, and shorten postprocedure hospital stays (13,16–18).
The primary objective of the Multicenter Study of Cryoablation for Palliation of Painful Bone Metastases (MOTION) was to assess the effectiveness of cryoablation for palliation of painful bone metastases in participants who were not candidates for traditional pain therapies or for whom traditional pain therapies had failed to provide adequate relief.
Materials and Methods
Study Design
MOTION (ClinicalTrials.gov NCT02511678) was a multicenter, international, single-arm 24-week prospective study examining the effectiveness and safety of a single cryoablation treatment for palliation in participants with painful bone metastases. The protocol was approved by each site’s institutional review board. Support for this study was provided by Galil Medical in the form of ablation probes, study coordinator support, and support for statistical services. None of the authors, some of whom were consultants for Galil Medical, had any access to the data other than acquisition until the study was closed and results presented.
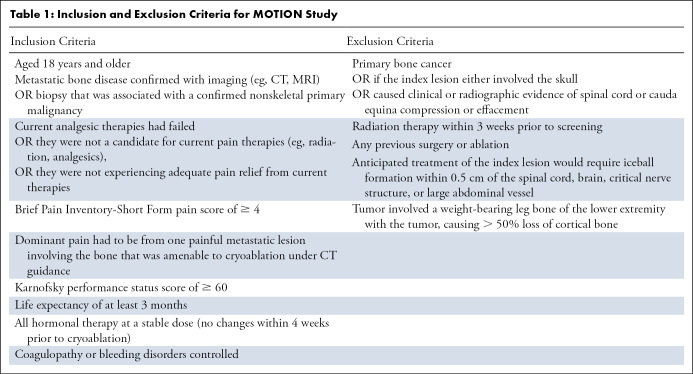
This study was an analysis of the prospective MOTION study. The study was conducted from February 2016 to March 2018 at 11 sites in the United States and France. Investigators at all sites were experienced in performing cryoablation specifically for the management of bone metastases–related pain. Participants aged 18 years or older were eligible for the study if: (a) they had metastatic bone confirmed with imaging (eg, CT or MRI) or biopsy that was associated with a confirmed nonskeletal primary malignancy; and (b) current analgesic therapies had failed, they were not a candidate for current pain therapies (eg, radiation, analgesics), or they were not experiencing adequate pain relief from current therapies. The pretreatment pain rating of “worst pain in the past 24 hours” had to be at least 4 on a scale of 0 (no pain) to 10 (pain as bad as the participant could imagine) on the Brief Pain Inventory-Short Form (BPI-SF). Participants were excluded if they had primary bone cancer or if treatment of the lesion would require iceball formation within 0.5 cm of the spinal cord, brain, or other critical nerve structure or large abdominal vessel. Additional inclusion and exclusion criteria are detailed in Table 1, and a flowchart is found in Figure 1.
Table 1:
Inclusion and Exclusion Criteria for MOTION Study
Figure 1:
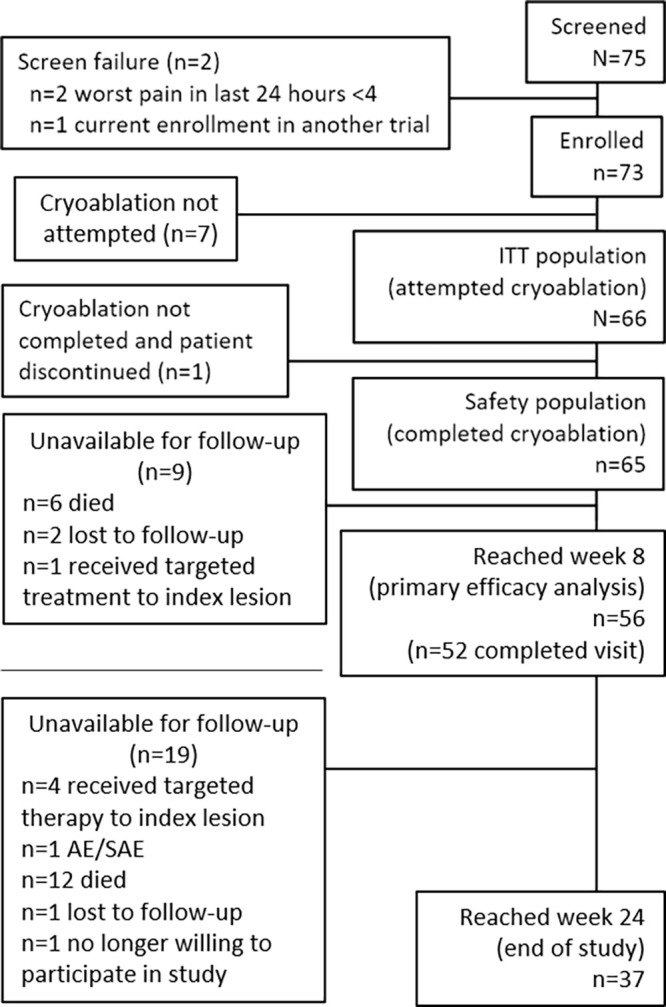
Participant disposition in the MOTION study. AE = adverse events, ITT = intention-to-treat, SA = serious adverse events. See Appendix E1 (supplement) for information on nine participants who did not undergo cryoablation.
Cryoablation Treatment
Cryoablation was performed with the Visual-IC Cryoablation System (Galil Medical, BTG International, and Boston Scientific) to a single metastatic bone lesion within 14 days of screening. In participants with multiple osseous lesions, the most painful lesion was selected as the index lesion. Clinical sites used a standard cryoablation protocol including two freeze-thaw cycles. If the operator felt that another cycle would improve coverage and local control, it was performed in select cases. CT images were obtained at intervals throughout the freeze cycles. Freeze duration varied to encompass the entire tumor or as much of the tumor as could be safely treated. Thermoprotective techniques for adjacent vital structures were recorded (ie, hydrodissection, CO2 dissection, balloon displacement, and skin warming). Technical success was defined as the ability to ablate the lesion successfully for pain palliation. Participants were not denied needed therapy for pain; however, those who received additional targeted therapies to the index tumor were excluded. Participants could receive concomitant pain medications and chemotherapy for treatment of recurrent or new tumor pain. Opioid medication doses were converted to a standardized morphine equivalent daily dose (MEDD).
Study Endpoints
Pain improvement was evaluated using a single item from the BPI-SF questionnaire completed by participants. This item asked participants to evaluate the level of the “worst pain in the last 24 hours.” On the BPI-SF, participants were asked to rate this pain on a scale of 1 to 10, with 1 being “no pain” and 10 being the “worst pain imaginable.” The primary effectiveness endpoint was the change from pretreatment baseline rating of worst pain in the last 24 hours to posttreatment week 8 rating. A clinically meaningful change for this item was defined as a reduction of at least 2 points. A responder analysis was conducted with response to cryoablation defined as a reduction of at least 2 points in worst pain score in the last 24 hours among participants with stable medication use, defined as less than or equal to 25% increase in MEDD.
Other endpoints included: (a) changes in worst pain scores and average pain scores from baseline; (b) change in analgesic use (both MEDD and nonsteroidal anti-inflammatory drugs); (c) use of additional therapies for persistent or recurrent pain associated with the index tumor or new metastases; (d) quality of life (as indicated by change from baseline in overall average BPI-SF); and (e) change in Karnofsky performance status as a measure of functional impairment. The safety endpoint was the incidence and severity of procedure or device-related adverse events.
Participants were followed for 6 months for pain response, change in quality of life, and analgesic use. Pain medications, Karnofsky performance status, participants’ opinions of overall treatment effect, and safety were assessed, and the BPI-SF questionnaire for the assessment of pain and quality of life were completed at screening, baseline (treatment day), and weeks 1, 4, 8, 12, 16, 20, and 24. Where possible, follow-up was completed during an in-person visit to their provider; when this was not possible, follow-up was completed by a telephone call. Adverse events were collected from baseline through 30 days after cryoablation and were characterized and graded using the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 4.03. Events ongoing after 30 days were followed to resolution or through 6 months from onset.
Statistical Analysis
Data were analyzed by a core statistical analytic support group outside of the participating centers, using SAS version 9.3 software. The intention-to-treat population comprised all participants for whom cryoablation was attempted or performed. The safety population comprised participants who completed cryoablation and for whom safety endpoints were successfully collected. For those participants for whom cryoablation was not successfully completed, participant data were excluded from subsequent analyses. For those for whom safety endpoint measurement could not be completed, data were included in analyses up until their last completed follow-up visit. Unless specified, data were analyzed using descriptive statistics (continuous variables) or frequency and percentage (categorical variables). Clinically meaningful change in the primary effectiveness endpoint required that the upper bound of the 95% CI for the mean change from baseline across all participants be less than −2 based on a one-sample t test. Responder analyses were conducted where a responder was defined as a participant having a greater than or equal to 2-point reduction in the worst pain score in the last 24 hours and no more than 25% increase in morphine equivalent dose from baseline at given time points.
Missing primary effectiveness data due to missing participant visits were incorporated using multiple imputation. Due to the arbitrary pattern of missingness and covariates or imputed variables being continuous, the multiple imputation method that was used was the Markov Chain Monte Carlo method. Separate sensitivity analyses were conducted where missing data were excluded (complete cases) or replaced with the last observation carried forward. Analyses of the primary efficacy endpoint by prior radiation therapy within 6 months were also performed. The safety endpoints of all adverse events assessed as “related or possibly related” to the study device or procedure through 30 days after cryoablation were summarized by count, incidence (95% CI), and severity.
Based on Galil Medical’s pilot trial results, the expected device performance was a mean 3.2 points (standard error of ± 2.7 points) reduction from baseline in worst pain score at 7 weeks after cryoablation. With these assumptions, the PASS 13 software (NCSS) was used to compute a sample size for a one-sample t test with superiority by a margin of −2.0, indicating a sample size of 42 would achieve 80% power to detect superiority at a significance level (α) of .025. Assuming an attrition rate at 8 weeks of 30%, at least 60 participants were required to be enrolled and treated.
Results
Participant Overview
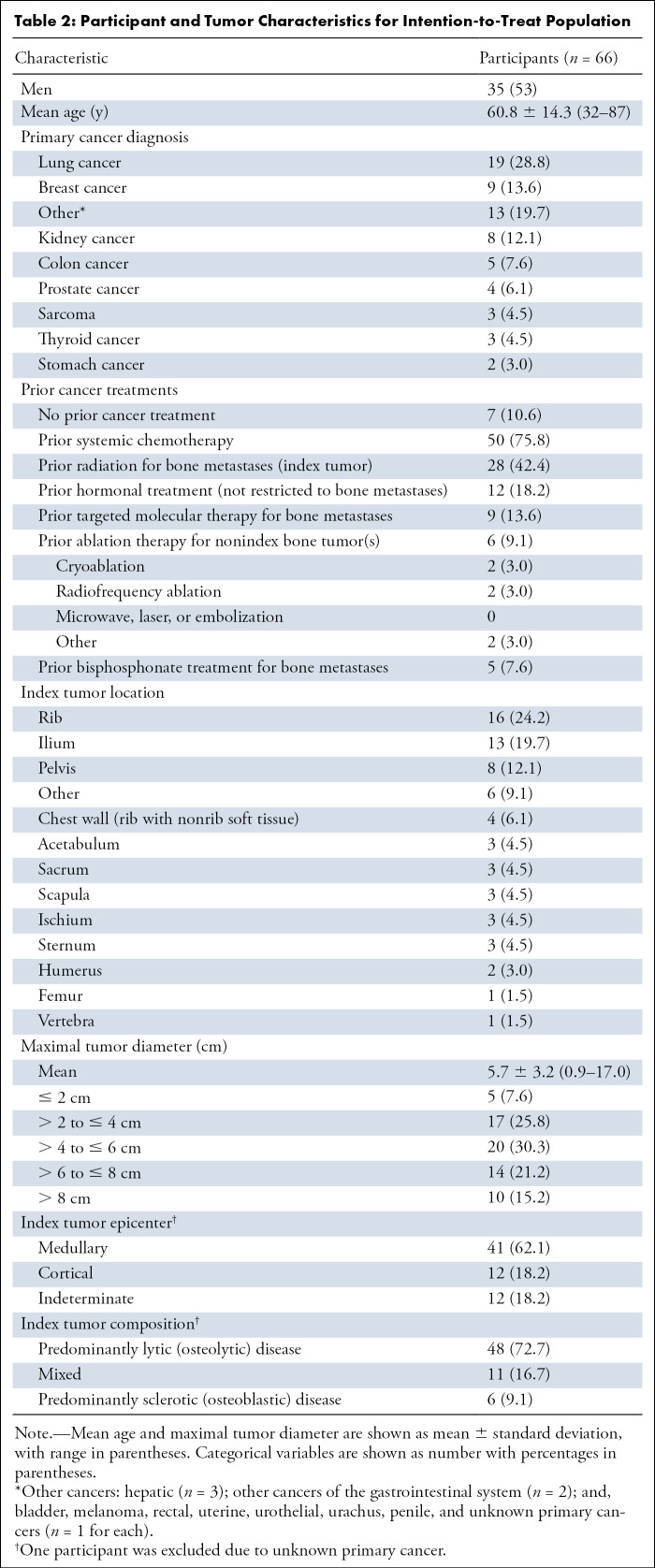
Of the 75 participants screened, two were excluded due to their worst pain in the last 24 hours being rated as less than a 4 in response to the BPI-SF pain item; one participant was also currently enrolled in another clinical trial (Fig 1 and Table 2). Of the 73 enrolled, the 66 for whom cryoablation was attempted were included in the intention-to-treat population; of these participants, cryoablation was not completed for one, and that participant was discontinued. This left the safety population, which included the 65 participants who completed cryoablation; of these patients, one did not complete follow-up, leaving 64 complete cases.
Table 2:
Participant and Tumor Characteristics for Intention-to-Treat Population
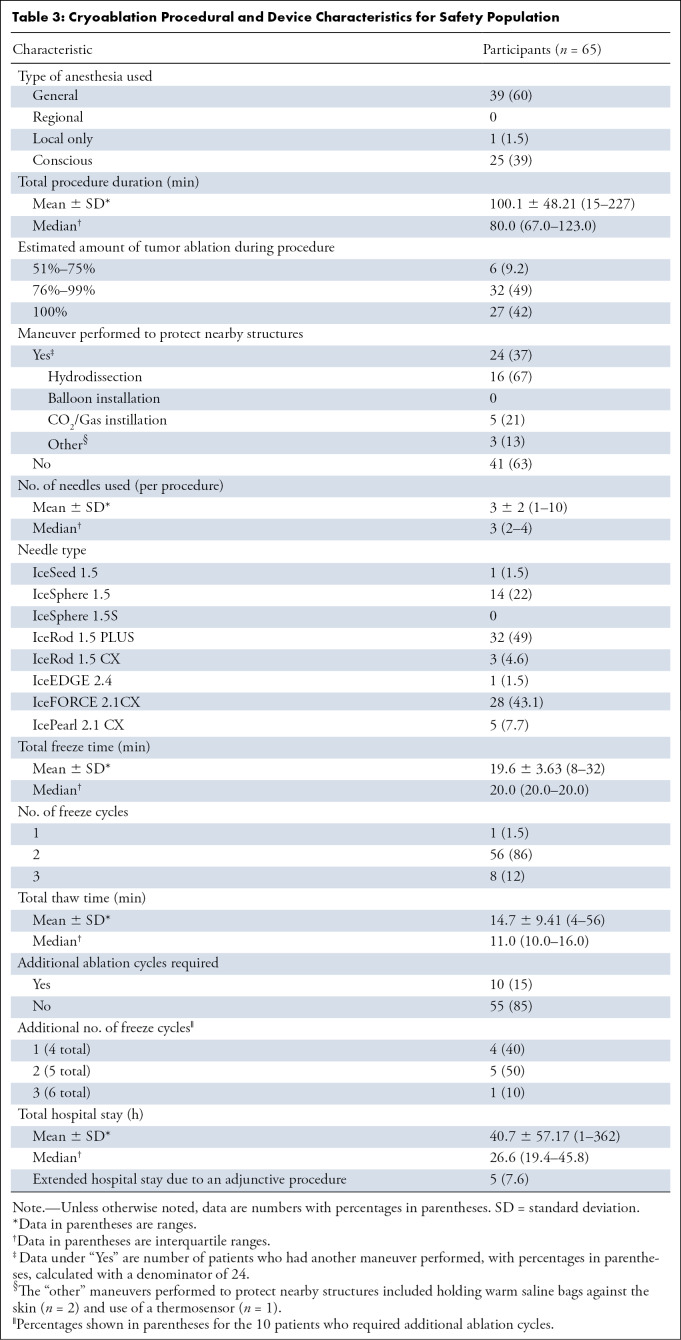
The number of participants for whom cryoablation was completed and for whom follow-up data were collected decreased over the course of the study due to loss to follow-up, missing week 8 visit, changes in care resulting in withdrawal from MOTION, or death: weeks 1 (98%; 64 of 65), 4 (83%; 54 of 65), 8 (76%; 50 of 65), 12 (62%; 40 of 65), 16 (57%; 37 of 65), 20 (52%; 34 of 65), and 24 (53%; 34 of 65) (Fig 1). Analyses using the intention-to-treat population utilized complete case analysis; this resulted in a decrease in the number of participants at each time point. Through the end of the study, 18 of the 65 participants (27.7%) in the safety population died (n = 15, progression of the underlying cancer; n = 1, cardiac arrest; n = 2, cause of death unknown). New metastatic disease occurred in 30 (46%) intention-to-treat participants and new painful bone tumors developed in 14 (21%). There were no device-, procedure-, or opioid-related deaths in the study. A thermoprotective maneuver was performed in 37% (24 of 65) of participants for adjacent vital structures. Procedural details (needle size, number of probes, etc) are summarized in Table 3.
Table 3:
Cryoablation Procedural and Device Characteristics for Safety Population
Effectiveness of Cryoablation for Reducing Pain
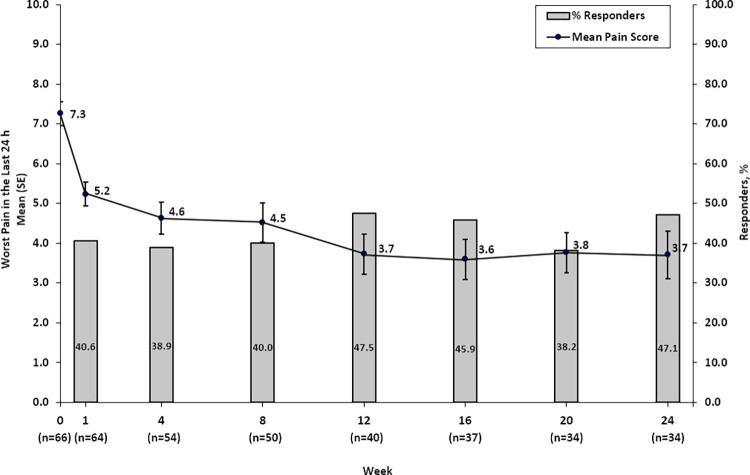
Clinically meaningful changes from baseline were observed at all time points after week 8 (Fig 2). The mean change in score for worst pain in the last 24 hours from baseline to week 8 was −2.61 ± 0.43 (95% CI: −3.45, −1.78). Mean scores improved by 2 points as early as week 1 in the complete-case sensitivity analysis and continued to improve, reaching clinically meaningful improvement starting after week 8; improvements were consistently sustained through week 24 (Fig 2). For mean pain scores in the intention-to-treat group, the 95% CI around the mean met the statistical test for clinically meaningful change at week 12 (95% CI: −4.4,−2.1), week 16 (95% CI: −4.7, −2.4), week 20 (95% CI: −4.6, −2.3), and week 24 (95% CI: −4.6, −2.3). Results for average pain followed the same pattern. Analyses were conducted to compare efficacy by tumor size (< 3 cm, 3–6 cm, > 6 cm); no difference was found in terms of the primary efficacy measure of change in worst pain the last 24 hours from baseline to week 8 of follow-up by tumor size (P = .06). Similar analyses were conducted to evaluate whether associations existed between efficacy and sex; again, no statistically significant differences were identified.
Figure 2:
After cryoablation, worst pain in the last 24 hours and percentage of responders with stable medication use from week 0 to week 24 for the intention-to-treat population. SE = standard error.
Figure 3 shows CT images in a patient with successful cryoablation with no disease progression at 14 months of follow-up. Of the 64 patients for whom cryoablation was successfully completed and for whom complete follow-up data were available, the majority of participants achieved palliation (92% [59 of 64] of intention-to-treat participants), with median time to maximal pain relief (ie, lowest reported pain score) of 39.0 days (95% CI: 43.7, 72.4 days; n = 59). Most participants achieved their maximum palliation by week 1 (33.9%; 20 of 59), week 4 (25.4%; 15 of 59), or week 12 (15.3%; nine of 59). Several participants had continued improvements through the end of the study. Three of 59 participants (5.1%) had their lowest BPI-SF score at week 20, and four of 59 (6.8%) participants had their lowest BPI-SF score at week 24. Recurrence of worst pain at or above the baseline level at the treated site occurred in 36 of 64 (56.3%) intention-to-treat participants; median time to recurrence was 36.0 days (95% CI: 34.9, 65.5 days). Most participants who had a recurrence did so at week 1 (33.3%; 12 of 36), week 4 (27.8%; 10 of 36), or week 8 (19.4%; seven of 36).
Figure 3a:
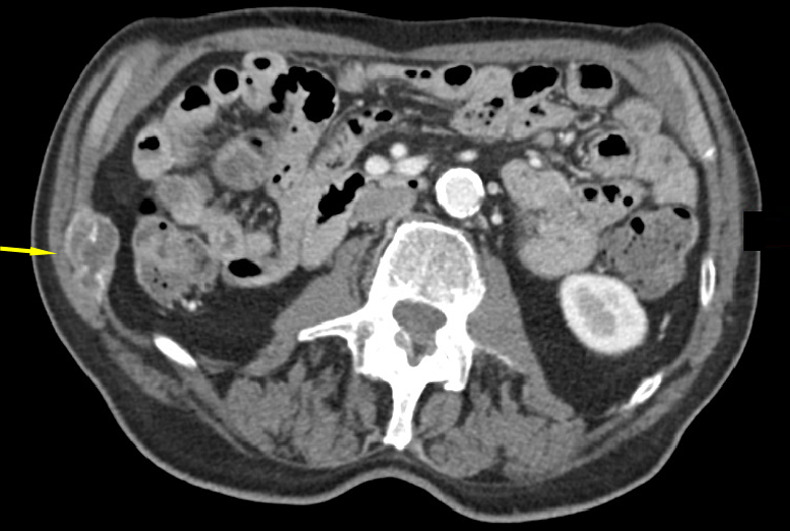
CT images in an 82-year-old man show tumor (a) before, (b) during, and (c) after cryoablation. (a) Yellow arrow shows metastatic leiomyosarcoma (right 11th rib lesion). (b) Yellow arrows around low-attenuation ice ball. Red arrow is CO2 displacement of the adjacent bowel. (c) Image from 14-month follow-up CT examination demonstrates no disease progression in tumor (yellow arrows).
Figure 3b:
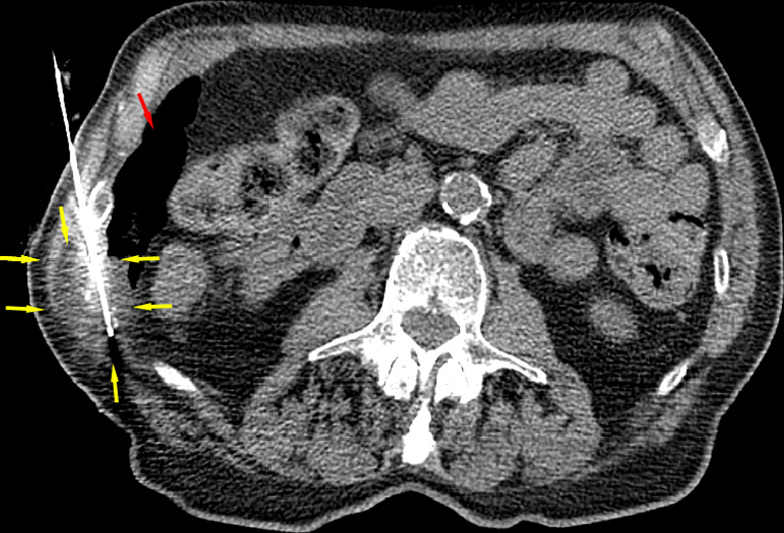
CT images in an 82-year-old man show tumor (a) before, (b) during, and (c) after cryoablation. (a) Yellow arrow shows metastatic leiomyosarcoma (right 11th rib lesion). (b) Yellow arrows around low-attenuation ice ball. Red arrow is CO2 displacement of the adjacent bowel. (c) Image from 14-month follow-up CT examination demonstrates no disease progression in tumor (yellow arrows).
Figure 3c:
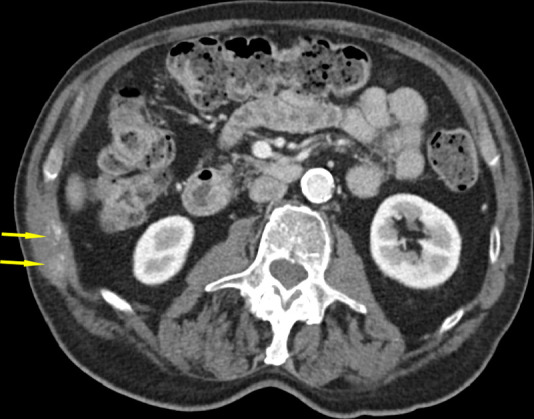
CT images in an 82-year-old man show tumor (a) before, (b) during, and (c) after cryoablation. (a) Yellow arrow shows metastatic leiomyosarcoma (right 11th rib lesion). (b) Yellow arrows around low-attenuation ice ball. Red arrow is CO2 displacement of the adjacent bowel. (c) Image from 14-month follow-up CT examination demonstrates no disease progression in tumor (yellow arrows).
In an analysis of the 64 patients for whom complete follow-up data were available, the percentage of responders over time ranged from 38% (13 of 34 at week 20, lowest percentage) to 48% (19 of 40 at week 12, highest percentage) over the 24-week follow-up period, weeks 1 through 24 (Fig 2). The percentages of participants with a reduction of at least 2 points in worst pain score, irrespective of medication use, was 58%–74% at week 4 through week 24. Additional analyses in the subgroups of participants with and without prior radiation therapy to the index tumor (Table 2) were consistent with the primary responder analyses. Among the patients with complete follow-up data, responder rates were similar between participants who did and did not have prior radiation at week 4 (42% [five of 12] vs 38% [16 of 42]) and week 8 (30% [three of 10] vs 43% [17 of 40]).
Reduced Opioid Medication Use
Opioid medication use at baseline was reported by 48 of 66 (73%) participants, with a mean MEDD of 43.1 mg ± 79.0 (median, 12.6 mg; interquartile range, 6.3–42.0 mg). Opioid medication use was reported by 56%–69% of participants who attended visits at week 4 through week 24. The MEDD among complete-case participants decreased from week 4 to week 24 (Fig 4). Opioid pain medication use was stable (ie, increased ≤ 25% over baseline) over week 4 through week 24 in 57% (21 of 37) of participants.
Figure 4:
Mean total morphine equivalent daily dose (MEDD) among all participants (complete case). SE = standard error. (n = 65; complete case analysis).
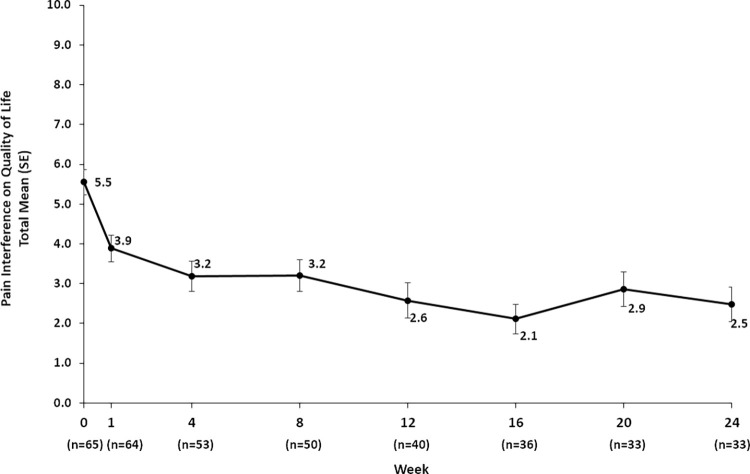
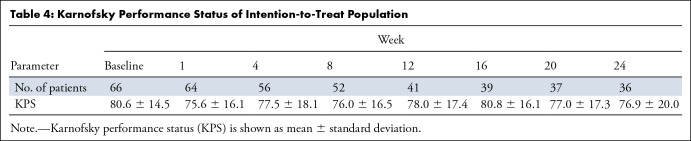
Improved Quality of Life
Quality of life consistently improved over 6 months (Fig 5). Functional status was maintained over 6 months (Table 4). The overall treatment effect was rated “better than at the last visit” by 60.9% (39 of 64) and 30% (11 of 37) of participants at weeks 1 and 24, respectively; treatment effect was rated “worse than at the last visit” by 13% (eight of 64) and 11% (four of 37) participants at weeks 1 and 24, respectively.
Figure 5:
Quality of life assessment from participant-reported interference of pain on quality of life from weeks 1 to 24 for the intention-to-treat population. For the Brief Pain Inventory-Short Form, patients recorded a score of 0 (does not interfere) to 10 (completely interferes) for the areas of general activity, mood, walking ability, relations, sleep, and enjoyment. For participants with responses of greater than 50% for the aforementioned areas, a total pain interference score, consisting of a mean of individual area scores, was calculated. SE = standard error.
Table 4:
Karnofsky Performance Status of Intention-to-Treat Population
Safety and Adverse Events
The safety population comprised 65 participants (Fig 1). Possibly related adverse events occurred in 22% (14 of 65) of participants. Of these, 3.1% (two of 65) were hematoma, 3.1% (two of 65) experienced nausea, and 3.1% (two of 65) experienced tumor pain; 7.7% (five of 65) were described as “other.” Each of the following was experienced by 1.5% (one of 65) participants: hypotension, pain at needle site, pleural effusion, skin burn and frostbite, and vomiting. Three of 65 participants (4.6%) each had one serious adverse event that was a grade 3 or 4 event (abdominal pain, hematoma, and skin burn or frostbite). One of these participants, a 49-year-old woman, had a grade 4 skin burn that led to withdrawal from the study and below-the-knee amputation. It was determined that the event was related to the study device and procedure. As the index lesion was below the knee, amputation resulted in removal of the index lesion; therefore, follow-up was not possible for the treated lesion. The other two serious adverse events resolved.
Discussion
Patients with bone metastasis generally have poor physical function, which has been shown to be directly correlated with pain; therefore, treatment is primarily aimed at alleviating pain and restoring function (3). In the MOTION study, mean scores for worst pain in the last 24 hours improved by 2 points as early as week 1 and reached the level of clinically meaningful improvement starting after week 8; improvements were sustained through week 24. Recurrence of worst pain in 56% of participants was recorded mostly in the first few weeks and was felt to be due to postprocedural pain and the associated immune response and inflammation with cryoablation. Quality of life parameters were improved, and functional status was preserved through the 6 months of follow-up. These results support the findings of prior retrospective and prospective studies that have demonstrated cryoablation provides rapid and durable nonopioid pain relief and opiate reduction in patients with painful metastatic disease to the bone (14,16,18–21). Notably, these findings are in line with the largest reported prospective study that included 69 metastatic bone tumors from 61 patients who were treated with cryoablation, which found that the mean score for worst pain in the past 24-hour period, scored using the BPI-SF, decreased from 7.1 prior to cyroablation to 5.1 1 week after treatment to 1.4 at 24 weeks after treatment (P < .0001) (19). In a systematic review of studies of cryoablation as treatment for cancer pain, cryoablation was highly effective in 496 patients with 580 treated lesions, 82.8% of which were bone lesions (21). Cryoablation provided a 62.5%, 70%, and 80.9% decrease in pain scores at 24 hours, 3 months, and 6 months after treatment, respectively (21). Cryoablation also decreased the need for opioid medications and substantially increased quality of life (21).
A substantial proportion of participants entered the MOTION study using opioid medications for palliation. At subsequent visits, fewer participants used opioid medications. Average opioid doses were stabilized throughout the 6 months of follow-up, demonstrating that cryoablation can provide a nonopioid alternative pain management strategy for patients. The observations of preserved functional status over the 6 months of follow-up, along with a trend of increasing quality of life over time, may in part be attributable to opioid dose stabilization, as long-term opioid use is associated with clinically significant impairment or distress (22).
While radiation therapy is the standard of care for bone metastasis, it typically takes 2 weeks after treatment for patients to feel relief, and pain relief is unlikely to improve further until after the first 6 weeks of therapy (14). In the MOTION study, 42% (28 of 66) of participants entered having had prior radiation therapy to the index lesion. The response among these participants was similar to that in participants who had no prior radiation therapy, which demonstrates that the palliative effect of the cryoablation procedure is rapid and durable across subgroups, including for those with no other viable local treatment option. Furthermore, failure to respond to radiation treatment should be an indication, rather than an exclusionary factor, for cryoablation.
Percutaneous cryoablation is safe, with a low major complication rate (23). In four large studies of cryoablation in bone, the major complication rate was 3.2% across 250 lesions (23). In the largest retrospective case series of percutaneous cryoablation, Auloge et al reviewed 320 primary or metastatic bone tumors that were treated with cryoablation from 2008 to 2017 and found a total complication rate of 9.1% (95% CI: 6%, 12.2%) and a major complication rate of 2.5% (24). Among the major complications, 50% were fractures, while cryoablation site infection, tumor seeding, bleeding, and severe hypotension each occurred at a rate of 0.3% (24). For all complications, statistically significant risk factors included having an Eastern Cooperative Oncology Group Performance Score greater than 2, long-bone cryoablation, and use of more than three cryoprobes (24). For major complications, risk factors included age older than 70 years and use of more than three cryoprobes (24). In the MOTION study, cryoablation was safe. Serious adverse events that were either related or possibly related to the procedure or device occurred in three (4.6%) participants, which is within the range of adverse events previously reported (14,24).
Limitations of MOTION include selection of 8 weeks for the primary endpoint. While the mean change in score for worst pain in the last 24 hours from baseline to week 8 was −2.61 and met the definition of clinically meaningful change, the upper bound of the 95% CI around this mean was −1.78. As the protocol specified that the upper bound be lower than −2, the protocol-defined statistical success criterion was not met at week 8. A larger sample size may have generated the power to achieve statistical success at week 8. Alternatively, the selection of a time point after week 8 would have garnered statistical success for the primary endpoint. Week 8 was selected based on historical data regarding patient attrition rates from prospective and retrospective studies of bone ablation. The rate of attrition in this terminally ill population was expected to be approximately 30%. Locations of the treated lesions were representative of those typical in the study population; however, it should be noted that spine lesions constituted a minority of the sites treated. Cryoablative technology is not frequently used in the spine in practice; this could have resulted in selection bias against treatment of painful spinal metastases. An additional limitation was the single-arm design of the study, thus impacting the ability to directly compare these results with other interventions.
Pain reporting is subjective and may be conflated by the phenomenon of pain unmasking, in which treatment of patients with multiple metastases produces a good response in the treated site but unmasks other painful sites (25). Over the course of the MOTION study, new metastatic disease occurred in 46.2% (30 of 65) of participants with complete follow-up, and new painful bone tumors occurred in 21.5% (14 of 65). Despite the progression of cancer over 6 months, the percentage of participants who responded to palliation with cryoablation was high. A study design element to possibly include in future studies would be a query to patients regarding interest in opioid dose reduction. Although high attrition rates in previous studies made the selection of a later time point for the primary efficacy endpoint analysis in the MOTION trial seem impractical, participants in the MOTION study actually showed clinically meaningful improvements starting at all time points after week 8, which were maintained through every visit until the end of the 6-month study.
The efficacy and safety results of the MOTION study consistently demonstrate that in patients who are unresponsive to or are not candidates for standard pain therapy, palliation of painful metastatic bone lesions with cryoablation was rapid and durable and improved quality of life. Cryoablation may provide a nonopioid alternative in the armamentarium of palliative treatment.
APPENDIX
Study supported by BTG and Boston Scientific.
Disclosures of Conflicts of Interest: J.W.J. Activities related to the present article: disclosed grant to author’s institution from Washington University for study support for data collection and cryoprobes for ablation; author received consultancy fees from Galil Medical and BTG regarding development of a microwave device, not related to cryoablation; Galil Medical and BTG provided cryoprobes for the study and assistance with manuscript, including graphs and tables. Activities not related to the present article: author received consultancy fees from Bard, Merit Medical, and Stryker. Other relationships: disclosed no relevant relationships. J.D.P. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: author received consultancy fees from Galil Medical and BTG; author’s institution is a study grant recipient from Galil Medical and BTG for an investigator-initiated study. Other relationships: author’s institution has patents pending from Focused Cryo involving a new cryoablation device. J.G. Activities related to the present article: author received fees from BTG and Galil for participation in review activities. Activities not related to the present article: author received payment for lectures including service on speakers bureaus from Canon, J&J, Medtronic, and BTG. Other relationships: disclosed no relevant relationships. A.G. disclosed no relevant relationships. X.B. disclosed no relevant relationships. J.P. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed consultancy fees paid to author’s institution from Boston Scientific; disclosed money paid to author from Philips for development of educational presentations. Other relationships: disclosed no relevant relationships. A.N.K. Activities related to the present article: disclosed grant to author’s institution from Galil Medical as sponsor for this trial. Activities not related to the present article: disclosed grants/grants pending to author’s institution from Philips and EDDA Technology; author received royalties from UpToDate. Other relationships: disclosed no relevant relationships. M.C. Activities related to the present article: author’s institution received grant from Galil Medical; author received consultancy fees or honorarium from Medtronic and Varian. Activities not related to the present article: disclosed no relevant relationships. Other relationships: disclosed no relevant relationships. S.G. disclosed no relevant relationships. F.A. Activities related to the present article: author’s institution participated in MOTION clinical trial and received expenses from Galil as per study protocol. Activities not related to the present article: disclosed no relevant relationships. Other relationships: disclosed no relevant relationships. A.J.H. disclosed no relevant relationships. J.I. disclosed no relevant relationships. F.P. disclosed no relevant relationships. C.M. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: author received consultancy fees from Deeplink Medical. Other relationships: disclosed no relevant relationships. P.J.L. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: author received honoraria and support for travel to meetings from Endocare and Galil Medical for invited talks on cryoablation, not related to the trial or associated data. Other relationships: disclosed no relevant relationships. T.d.B. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: author received consultancy fees from Terumo and Guerbet; author’s institution has grants/grants pending from Terumo; author received payment for lectures including service on speakers bureaus from Guerbet, Boston Scientific, Terumo, AstraZeneca, GE Healthcare, and Nanobiotix. Other relationships: disclosed no relevant relationships. F.D. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: author received consultancy fees from Medtronics, Ablatech, GE, Galil Medical, and Terumo. Other relationships: disclosed no relevant relationships.
Abbreviations:
- BPI-SF
- Brief Pain Inventory-Short Form
- MEDD
- morphine equivalent daily dose
- MOTION
- Multicenter Study of Cryoablation for Palliation of Painful Bone Metastases
References
- 1.Li S, Peng Y, Weinhandl ED, et al. Estimated number of prevalent cases of metastatic bone disease in the US adult population. Clin Epidemiol 2012;4:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hernandez RK, Wade SW, Reich A, Pirolli M, Liede A, Lyman GH. Incidence of bone metastases in patients with solid tumors: analysis of oncology electronic medical records in the United States. BMC Cancer 2018;18(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janssen SJ, Pereira NRP, Thio QCBS, et al. Physical function and pain intensity in patients with metastatic bone disease. J Surg Oncol 2019;120(3):376–381. [DOI] [PubMed] [Google Scholar]
- 4.Tsuzuki S, Park SH, Eber MR, Peters CM, Shiozawa Y. Skeletal complications in cancer patients with bone metastases. Int J Urol 2016;23(10):825–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.von Moos R, Costa L, Ripamonti CI, Niepel D, Santini D. Improving quality of life in patients with advanced cancer: Targeting metastatic bone pain. Eur J Cancer 2017;71:80–94. [DOI] [PubMed] [Google Scholar]
- 6.Paice JA, Portenoy R, Lacchetti C, et al. Management of Chronic Pain in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2016;34(27):3325–3345. [DOI] [PubMed] [Google Scholar]
- 7.Lutz S, Balboni T, Jones J, et al. Palliative radiation therapy for bone metastases: Update of an ASTRO Evidence-Based Guideline. Pract Radiat Oncol 2017;7(1):4–12. [DOI] [PubMed] [Google Scholar]
- 8.Fallon M, Giusti R, Aielli F, et al. Management of cancer pain in adult patients: ESMO Clinical Practice Guidelines. Ann Oncol 2018;29(Suppl 4):iv166–iv191. [DOI] [PubMed] [Google Scholar]
- 9.Chow R, Hoskin P, Hollenberg D, et al. Efficacy of single fraction conventional radiation therapy for painful uncomplicated bone metastases: a systematic review and meta-analysis. Ann Palliat Med 2017;6(2):125–142. [DOI] [PubMed] [Google Scholar]
- 10.Kwon JH. Overcoming barriers in cancer pain management. J Clin Oncol 2014;32(16):1727–1733 [Published correction appears in J Clin Oncol 2014;32(16):2117.]. [DOI] [PubMed] [Google Scholar]
- 11.Vieira C, Fragoso M, Pereira D, Medeiros R. Pain prevalence and treatment in patients with metastatic bone disease. Oncol Lett 2019;17(3):3362–3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van den Beuken-van Everdingen MH, Hochstenbach LM, Joosten EA, Tjan-Heijnen VC, Janssen DJ. Update on Prevalence of Pain in Patients With Cancer: Systematic Review and Meta-Analysis. J Pain Symptom Manage 2016;51(6):1070–1090.e9. [DOI] [PubMed] [Google Scholar]
- 13.Callstrom MR, Kurup AN. Percutaneous ablation for bone and soft tissue metastases--why cryoablation? Skeletal Radiol 2009;38(9):835–839. [DOI] [PubMed] [Google Scholar]
- 14.Gennaro N, Sconfienza LM, Ambrogi F, Boveri S, Lanza E. Thermal ablation to relieve pain from metastatic bone disease: a systematic review. Skeletal Radiol 2019;48(8):1161–1169. [DOI] [PubMed] [Google Scholar]
- 15.Kurup AN, Callstrom MR. Expanding role of percutaneous ablative and consolidative treatments for musculoskeletal tumours. Clin Radiol 2017;72(8):645–656. [DOI] [PubMed] [Google Scholar]
- 16.Prologo JD, Passalacqua M, Patel I, Bohnert N, Corn DJ. Image-guided cryoablation for the treatment of painful musculoskeletal metastatic disease: a single-center experience. Skeletal Radiol 2014;43(11):1551–1559. [DOI] [PubMed] [Google Scholar]
- 17.Truesdale CM, Soulen MC, Clark TW, et al. Percutaneous computed tomography-guided renal mass radiofrequency ablation versus cryoablation: doses of sedation medication used. J Vasc Interv Radiol 2013;24(3):347–350. [DOI] [PubMed] [Google Scholar]
- 18.Thacker PG, Callstrom MR, Curry TB, et al. Palliation of painful metastatic disease involving bone with imaging-guided treatment: comparison of patients’ immediate response to radiofrequency ablation and cryoablation. AJR Am J Roentgenol 2011;197(2):510–515. [DOI] [PubMed] [Google Scholar]
- 19.Callstrom MR, Dupuy DE, Solomon SB, et al. Percutaneous image-guided cryoablation of painful metastases involving bone: multicenter trial. Cancer 2013;119(5):1033–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wallace AN, McWilliams SR, Connolly SE, et al. Percutaneous Image-Guided Cryoablation of Musculoskeletal Metastases: Pain Palliation and Local Tumor Control. J Vasc Interv Radiol 2016;27(12):1788–1796. [DOI] [PubMed] [Google Scholar]
- 21.Ferrer-Mileo L, Luque Blanco AI, González-Barboteo J. Efficacy of Cryoablation to Control Cancer Pain: A Systematic Review. Pain Pract 2018;18(8):1083–1098. [DOI] [PubMed] [Google Scholar]
- 22.Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain--United States, 2016. JAMA 2016;315(15):1624–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jennings JW. Is Percutaneous Bone Cryoablation Safe? Radiology 2019;291(2):529–530. [DOI] [PubMed] [Google Scholar]
- 24.Auloge P, Cazzato RL, Rousseau C, et al. Complications of Percutaneous Bone Tumor Cryoablation: A 10-year Experience. Radiology 2019;291(2):521–528. [DOI] [PubMed] [Google Scholar]
- 25.Chow E, Wu JS, Hoskin P, Coia LR, Bentzen SM, Blitzer PH. International consensus on palliative radiotherapy endpoints for future clinical trials in bone metastases. Radiother Oncol 2002;64(3):275–280. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.