Abstract
The Baltimore computed tomography (CT) prediction model for bleeding pelvic fractures is a multivariable decision tool that predicts angiopositivity from pelvic hematoma volume, active arterial bleeding, fracture patterns, and atherosclerosis. We hypothesized that quantitative markers of body composition and frailty could further improve model performance.
Methods:
This work is a retrospective secondary analysis of a single institution cohort used in the development of the Baltimore CT prediction model. The cohort includes 115 consecutive patients that underwent admission contrast-enhanced CT of the abdomen and pelvis for blunt trauma with pelvic ring disruption followed by conventional angiography. Major arterial injury requiring angioembolization served as the outcome variable. Angioembolization was required in 73/115 patients (63% of the cohort). Average age was 46.9 years (±SD 20.4). Body composition measurements were determined as 2-dimensional (2D) or 3-dimensional (3D) parameters and included mid-L3 trabecular bone attenuation, abdominal visceral fat area or volume, and percent muscle fat fraction (as a marker of sarcopenia) measured using segmentation and histogram analysis.
Results:
Models incorporating 2D (Model B) or 3D markers (model C) of body composition showed improvement over the original Baltimore model (model A) in all parameters of performance, quality, and fit (area under the receiver-operating curve [AUC], Akaike information criterion, Brier score, Hosmer-Lemeshow test, and adjusted-R2). Area under the receiver-operating curve increased from 0.83 (A), to 0.86 (B), and 0.88 (C). The greatest improvement was seen with 3D parameters.
Conclusion:
Once automated, quantitative visualization tools providing “free” 3D body composition information can be expected to improve personalized precision diagnostics, outcome prediction, and decision support in patients with bleeding pelvic fractures.
Keywords: body composition biomarkers, quantitative imaging, CT, pelvic fractures, multivariable modeling, personalized prediction, precision diagnostics
Résumé
Le modèle de prédiction par tomodensitométrie (TDM) de Baltimore pour les fractures hémorragiques du bassin est un outil de décision multifactoriel qui prédit la positivité angiographique du volume d’un hématome pelvien, d’un saignement artériel actif, des types de fractures et de l’athérosclérose. Nous avons émis l’hypothèse que des marqueurs quantitatifs de composition et de fragilité du corps pourraient améliorer la performance du modèle.
Méthodes :
Cette étude est une analyse secondaire rétrospective menée dans un établissement unique sur une cohorte utilisée pour l’élaboration du modèle de prédiction par TDM de Baltimore. La cohorte inclut 115 patients consécutifs ayant subi à l’admission une TDM avec agent de contraste de l’abdomen et du bassin pour traumatisme fermé avec rupture de la ceinture pelvienne, suivie d’une angiographie conventionnelle. Les lésions artérielles majeures nécessitant une embolisation vasculaire ont servi de variables de résultats. Une embolisation vasculaire a été nécessaire chez 73 des 115 patients (63 %) de la cohorte. L’âge moyen des patients était de 46,9 ans (ÉT : ± 20,4 ans). Les mesures de composition du corps ont été déterminées comme étant des paramètres bidimensionnels (2D) ou tridimensionnels (3D) et ont inclus une atténuation osseuse trabéculaire au milieu de L3, la surface ou le volume de la graisse viscérale abdominale et le pourcentage de la fraction muscle/tissu adipeux (marqueur de sarcopénie) mesuré au moyen de segmentations et d’une analyse des histogrammes.
Résultats :
Les modèles incorporant des marqueurs 2D (modèle B) ou 3D (modèle C) de la composition du corps ont présenté une amélioration par rapport au modèle initial de Baltimore (modèle A) sur tous les paramètres de performance, de qualité et d’adaptation (aire sous la courbe [ASC] d’efficacité du récepteur, critère d’information d’Akaike, score de Brier, test de Hosmer-Lemeshow et R2 ajusté). L’aire sous la courbe d’efficacité du récepteur a augmenté de 0,83 (A), à 0,86 (B), et 0,88 (C). La plus grande amélioration a été obtenue avec les paramètres 3D.
Conclusion :
Lorsqu’automatisés, les outils de visualisation quantitative fournissant « librement » des informations sur la composition corporelle en 3D mèneront à une amélioration de la précision de diagnostics personnalisés, de la prédiction de l’évolution et d’un soutien à la décision chez les patients atteints de fractures pelviennes hémorragiques.
Introduction
Pelvic fracture–related hemorrhage is a leading cause of mortality after blunt trauma.1–3 Venous bleeding is often amenable to a treatment pathway involving pelvic stabilization using provisional pelvic binders, external fixator devices, and definitive treatment with open reduction and internal fixation.4–6 Death is usually related to potentially reversible arterial bleeding, which is treated with angioembolization following initial computed tomography (CT). A recent multicenter study at 11 major level I trauma centers reported that 90% of patients with pelvic fractures presenting with shock will nevertheless undergo admission contrast enhanced CT in the trauma bay before intervention.7,8 Today with the speed and accessibility of trauma bay-adjacent CT scanners9,10 and shift toward prompt scanning without oral contrast,11,12 only a small fraction of patients in extremis will bypass initial CT until stabilized using damage control techniques.12–14 Computed tomography promotes early and rapid triage to conventional angiography for transcatheter embolization when arterial hemorrhage is identified or suspected4,9; however, predicting major arterial bleeding on angiography remains challenging even at CT.9,15–17 Intravenous contrast extravasation and segmented pelvic hematoma volumes are important CT-based predictors of arterial injury requiring angioembolization,18–22 although contrast extravasation can be intermittent due to tamponade, vasospasm, transient occlusion, and tenuous hemodynamics.23 Specific fracture patterns and the degree of pelvic ring instability are also predictive6,17,24; however, no single CT sign is deterministic. We have previously described multivariable model derived from a range of predictive CT features25—the Baltimore CT prediction model for major arterial injury after pelvic fractures. Independent predictors included in the final model are segmented hematoma volumes, measured using a semiautomated seeded region-growing method,22,5,26 intravenous contrast extravasation, displaced obturator ring fractures, rotational instability, and degree of atherosclerosis.
Quantitative markers of body composition at CT, including measurements of visceral, subcutaneous, and intermuscular adipose tissue; skeletal muscle mass; and osteopenia have been assessed in a variety of disease states. For example, the size of visceral and subcutaneous fat compartments are correlated with the risk of metabolic syndrome and diabetes mellitus.27 Changes in skeletal muscle mass over time are quantifiable in ICU patients with respiratory failure.28 Attenuation of vertebral trabecular bone (in Hounsfield units [HU]) can be employed for opportunistic screening of osteoporosis.29,30
Recently, markers of CT body composition have been used as surrogates of frailty in trauma patients. The degree of sarcopenia (loss of muscle mass) predicts disposition after hospitalization in elderly patients31; sarcopenia is independently associated with severe thoracic trauma and osteopenia with spine trauma after crash injury32; and both parameters also predict 1-year survival after trauma in elderly patients.33 Area measurements of psoas muscle mass correlate with decreased survival in elderly patients with hip fractures.34 In critically ill trauma patients, abdominal adiposity has been linked with the risk of acute kidney injury.35 Body composition markers can be incorporated into personalized explanatory models in acute blunt trauma for predicting the need for urgent intervention.
A variety of 2-dimensional (2D) surface area measurements and 3-dimensional (3D) volumetric measurements have been employed.27,28,32,35 In the present analysis, we hypothesized that discrimination, calibration, model quality, and overall model accuracy would improve with the addition of either 2D or 3D body composition measurements as predictor variables. These models were compared to one another and our originally published “conventional” multivariate Baltimore CT prediction model for angiopositivity after pelvic fracture from a development data set of 115 patients who underwent angiography following CT for blunt pelvic ring disruption.
Methods
Patient Cohort
The study was institutional review board–approved and Health Insurance Portability and Accountability Act–compliant. The previously described cohort consisted of 115 consecutive patients with blunt pelvic ring disruptions, age ≥18 years, who underwent admission contrast-enhanced multidetector CT (MDCT) of the abdomen and pelvis prior to digital subtraction angiography between January 2008 and October 2013. Major arterial injury requiring transarterial embolization was used as the dependent variable. Seventy-three of 115 patients (63% of the cohort) had positive angiograms requiring angioembolization. Sixty-seven percent of the cohort was male and 33% female. The average age was 46.9 years (SD ± 20.4). We refer readers to our previously published work for additional information regarding selection criteria, demographics, and patient characteristics.25
Image Analysis and Processing
Abdominopelvic CTs were routinely performed on 40- and 64-section MDCT scanners (Brilliance; Phillips Healthcare) in the arterial phase, typically as part of a single acquisition whole-body CT protocol, with the following scanner settings: 120 kVp, 250 mAs, 0.67 pitch; 0.5 second rotation time, and 0.625 collimation) using 100-mL Iohexol (350 mg/mL; Omnipaque; GE Healthcare), administered at a rate of 4 mL/sec with 50-mL saline flush. Computed tomography studies were archived at 3-mm section thickness.
Randomized de-identified studies were reviewed using a 3D software client (Aquarius iNtuition version 4.4; TeraRecon). All 2D and 3D body composition measurements were performed using the client by a single-blinded reader with 5 years of full-time clinical experience in trauma radiology. Single measurements of five 2D parameters and 5 corresponding 3D parameters were taken. The semiautomated volumetric measurement tools employed were previously validated and are approved for clinical use by the US Food and Drug Administration.
2-Dimensional parameters.
2-Dimensional measurements included: (1) mid-L3 vertebral body trabecular bone attenuation using a circular region of interest measuring 1 to 1.5 cm2 (note the existing precedent for use of L3-level measurements corresponding with sarcopenia and various trauma and non-trauma-related outcomes;32,36,37 (2) semi-automated measurements of visceral fat and (3) subcutaneous fat compartment areas (cm2) at the mid-L3 level using the client’s 2D fat analysis tool (see Figure 1); (4) manually traced total paraspinal muscle area (cm2) at the mid-L3 level; and (5) % fat fraction calculated using the client’s area histogram tool, with the fat attention range set to −300 to −10 HU, and muscle attenuation range set to −9 to −150 HU.
Figure 1.
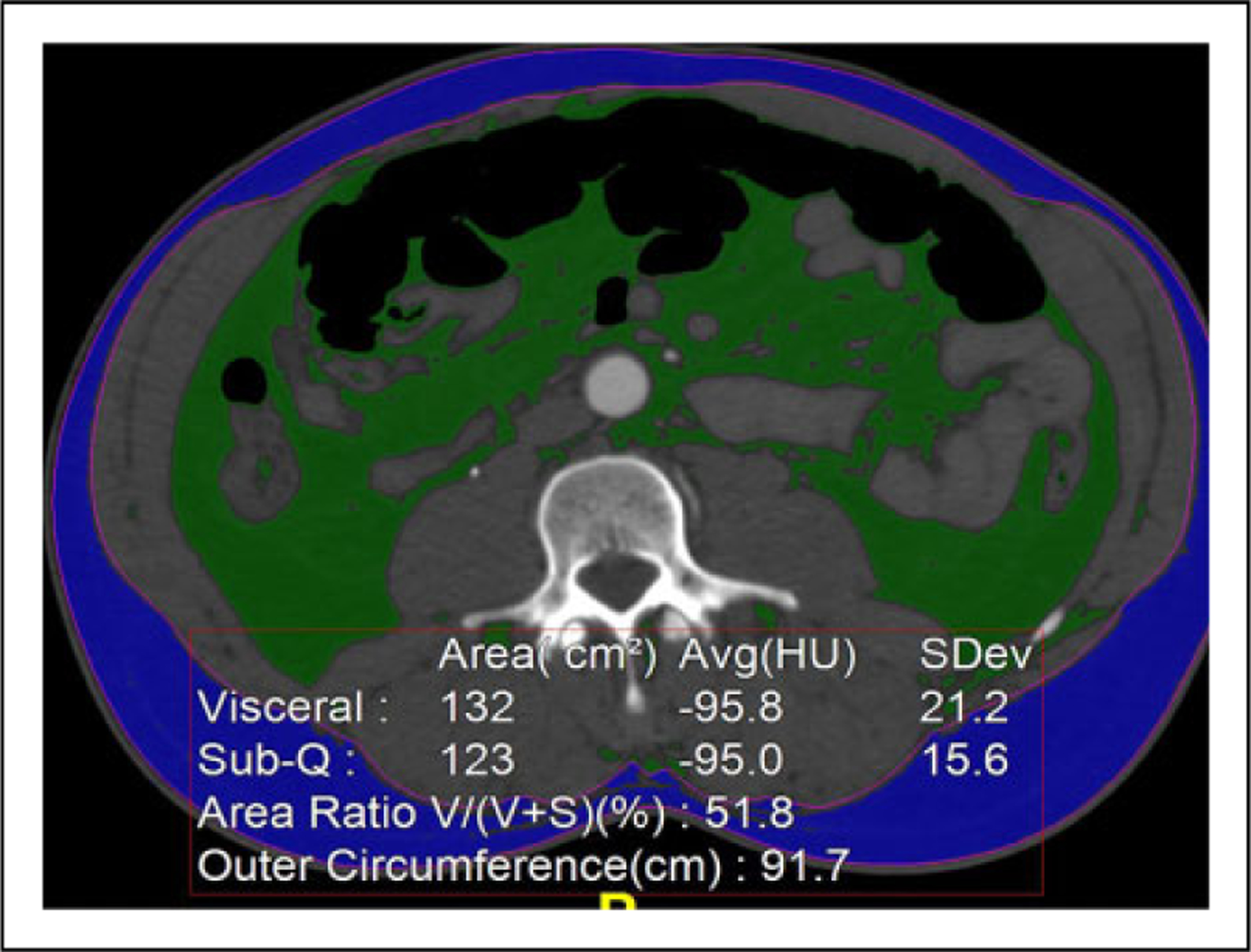
Fat analysis. Automated 2D fat analysis tool which distinguishes visceral from sub-q fat. The 3D tool performs the same function but provides visceral and sub-q fat volumes and volume ratios. 2D indicates 2-dimensional; 3D, 3-dimensional.
3-dimensional parameters.
Corresponding 3D parameters included: (1) spherical ROI HU measurements centered at the L3 mid-vertebral body level, with equator area of 1–1.5 cm2; (2) volumetric visceral fat; and (3) volumetric subcutaneous fat measurements (cm3) using the 3D fat analysis tool with range set using coronal images, from the diaphragmatic insertion at the body wall to the ischial spines; (4) semiautomated segmentation of the paraspinal muscles (cm3) using the region grow tool from the lower margins of the 12th ribs to the axial slice where the psoas muscle is no longer contiguous with the remaining paraspinal musculature; and (5) % fat fraction calculated using the volume histogram tool, with fat attenuation range set to −150 to −10 HU and muscle attenuation range set to −9 to −150 HU (see Figure 2).
Figure 2.
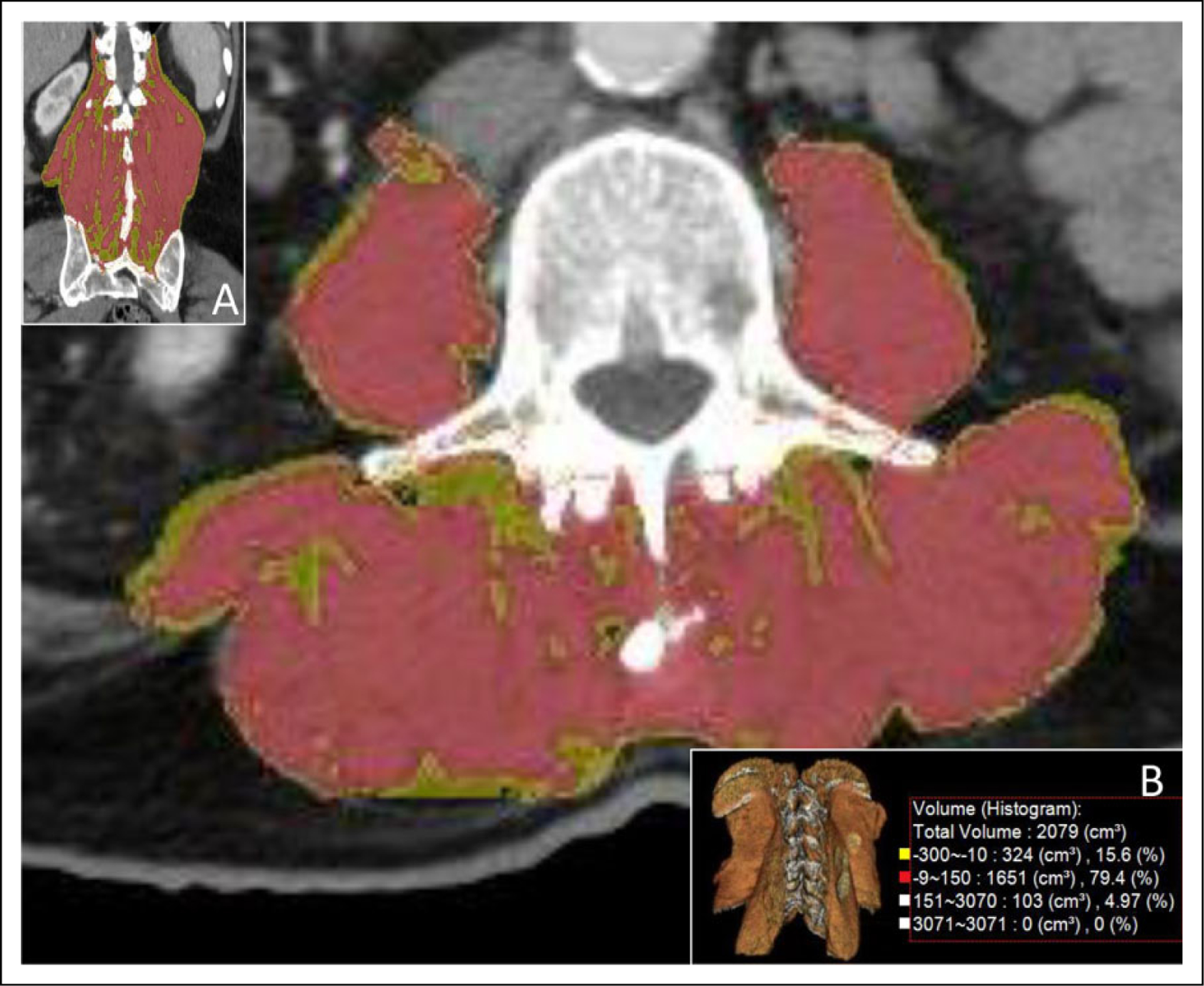
3D and histogram segmented paraspinal muscles. Semiautomated region-grow tool 3D segmentation of paraspinal muscles with histogram analysis (cm3) using the region grow tool. Parameters including % fat fraction are calculated with fat attenuation range set to −150 to −10 HU and muscle attenuation to −9 to −150 HU. 3D segmentation is shown in axial, coronal, and 3D planes (see insets A and B). Quantitative histogram analysis is shown in inset B. All Paraspinal muscles were segmented from the lower margins of the 12th ribs to the axial slice where the psoas muscle is no longer contiguous with the remaining paraspinal musculature, Segmented muscles included the psoas major anteriorly, quadratus lumborum laterally, the iliocostalis, longissimus, and spinalis posteriorly, and erector spinae posteriorly and inferiorly. 3D indicates 3-dimensional; HU, Hounsfield units.
Statistical Analysis
Statistical analysis was performed using Stata (Stata) and R statistical software (R Foundation for Statistical Computing; https://www.r-project.org/). Logistic regression with backward elimination was used to generate new models, using predictors from the original model, together with either 2D or 3D body composition parameters. Individual predictors in the final models were assessed for significance (P < .05). Variables were interrogated for collinearity using the variance inflation factor (VIF). Discrimination and accuracy of the previously derived Baltimore CT prediction model (model A), the model including area measurements (model B), and the model including volumetric measurements (model C) were compared using the area under the receiver-operating curve (AUC) and Brier score. Overall model quality was compared using the Akaike information criterion (AIC). Higher AUC, lower Brier score, and lower AIC indicate improved performance. The ability of the models to explain variation in the population was compared using adjusted R2. Model calibration was evaluated using the Hosmer-Lemeshow statistic, with P values > .05 indicating goodness of fit.
In all 3 models, the VIF was < 4 for all variables, indicating absence of significant multicollinearity. K-fold cross-validation with 10 equal-sized subsamples was used to assess for model overfitting. Results of cross-validation were consistent across all folds with root mean square error of 0.18 (model A, standard error, 0.05); 0.18 (model B, standard error, 0.08), and 0.17 (model C, standard error, 0.06).
Results
The results of regression for model B and C are presented and compared with previously described results for model A (Tables 1–3). In both model B and C, all variables found to be important in the original model (hematoma volume, contrast extravasation, atherosclerosis, obturator ring fracture, and rotational instability), remained independently predictive of major arterial injury.
Table 1.
Model A (Baltimore CT Prediction Model).
| Predictor | β-coefficient | SE | Wald χ2 test | DF | P value | Exp(β) | 95% CI |
|---|---|---|---|---|---|---|---|
| Rotational instability | −1.237 | 0.545 | 5.163 | 1 | 0.023 | 0.290 | 0.1–0.844 |
| Obturator fracture | 1.134 | 0.534 | 4.507 | 1 | 0.034 | 3.107 | 1.091–8.848 |
| Atherosclerosis | 0.820 | 0.316 | 6.744 | 1 | 0.009 | 2.270 | 1.223–4.214 |
| Hematoma volume | 0.007 | 0.002 | 14.317 | 1 | 0.0002 | 1.007 | 1.003–1.010 |
| ICE > 6 mm | 1.099 | 0.479 | 5.270 | 1 | 0.022 | 3.002 | 1.174–7.674 |
Table 3.
Model C (3D Body Composition Parameters).a
| Predictor | β-coefficient | SE | Wald χ2 test | DF | P value | Exp(β) | 95% CI |
|---|---|---|---|---|---|---|---|
| Rotational instability | −1.726 | 0.626 | 7.618 | 1 | 0.006 | 0.178 | 0.0522–0.607 |
| Obturator fracture | 1.813 | 0.644 | 7.896 | 1 | 0.005 | 6.127 | 1.733–21.662 |
| Atherosclerosis | 1.616 | 0.799 | 4.080 | 1 | 0.043 | 5.030 | 1.051–24.08 |
| Hematoma volume | 0.007 | 0.002 | 12.603 | 1 | 0.000 | 1.007 | 1.003–1.011 |
| ICE > 6 mm | 1.627 | 0.562 | 8.352 | 1 | 0.004 | 5.090 | 1.691–15.321 |
| Sub-q fat volume | 0.000 | 0.000 | 4.368 | 1 | 0.037 | 1.000 | 1.000–1.000 |
| Paraspinal fat fraction (3D) | −0.099 | 0.041 | 5.954 | 1 | 0.015 | 0.905 | 0.836–0.981 |
| Constant | −2.503 | 0.970 | 6.656 | 1 | 0.010 | 0.082 | 0.0122–0.548 |
Abbreviations: CT, computed tomography; 2D, 2-dimensional; 3D, 3-dimensional.
All variables found to be important in the Baltimore CT prediction model (model A—hematoma volume, contrast extravasation, atherosclerosis, obturator ring fracture, and rotational instability), remained in model B and model C. In model B (2D), paraspinal fat fraction (cm2) was the only body composition measurement to reach significance (P = .03). In model C (3D), subcutaneous fat volume (cm3), and paraspinal muscle fat fraction (%) were both significant independent predictors of major arterial injury (P = .04 and .02, respectively).
In model B (2D parameters), paraspinal fat fraction (cm2) was the only body composition measurement to reach significance (P = .03). In model C, subcutaneous fat volume (cm3) and paraspinal muscle fat fraction (%) were both significant independent predictors of major arterial injury (P = .04 and .02, respectively). Paraspinal muscle volume remained in the final model incorporating volumetric body composition measurements and approached significance (P = .09). Surprisingly, age was not included in any of the final models; however, scatter matrices showed linear relationships with body composition markers. These are shown in Figure 3.
Figure 3.
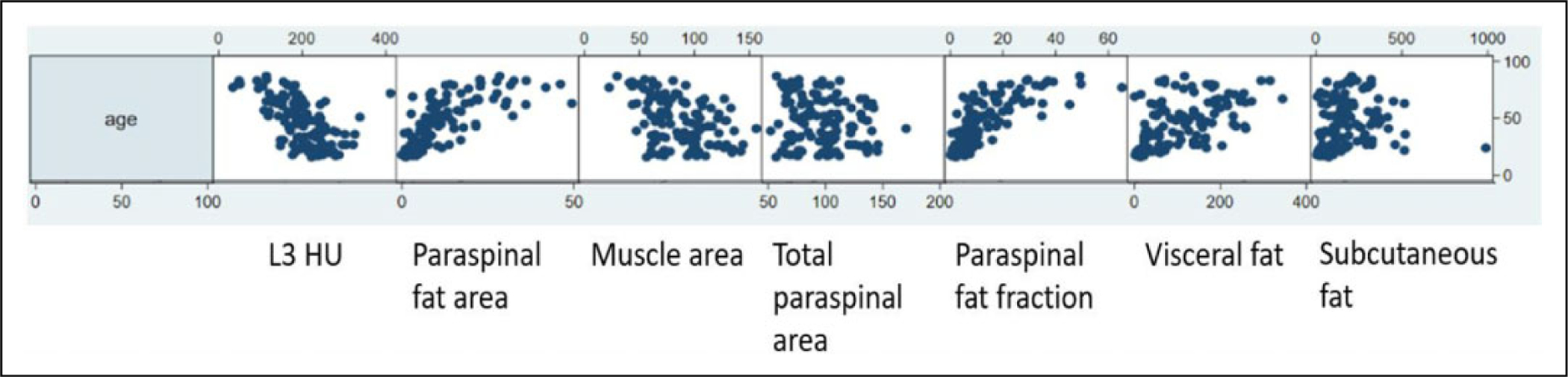
Scatter matrices for 2D measurements by age. Age (y-axis) is plotted against area measurements (x-axis) with units in cm2. Fat fraction is presented as a percentage. fat fraction indicates paraspinal fat area/total paraspinal area; L3 (third lumbar mid-vertebral level); parasp., paraspinal; sub-q, subcutaneous; 2D, 2-dimensional.
Regression diagnostics using the Hosmer-Lemeshow statistic revealed that all 3 models were well-calibrated to the actual data with P values of .53 (model A), .80 (model B), and .65 (model C). All measures of model performance showed successive incremental improvement from the originally published model (model A—incorporating bleeding features and pelvic fracture patterns but not markers of body composition). Improvement was greater using 3D (model C) than 2D (model B) body composition markers. Area under the receiver-operating curve increased from 0.83 (model A) to 0.86 (model B) to 0.88 (model C), and Brier score decreased from 0.16 to 0.14 to 0.13. Akaike information criterion decreased from 121 to 118 to 115. Adjusted R2 improved from 0.28 to 0.34 to 0.37. It should be noted that substantially lower R2 values are to be expected when using binary logistic regression, over the counterfactual example of linear regression with a continuous outcome.
Discussion
Quantitative CT biomarkers of body composition, such as those related to muscle mass, intermuscular fat, visceral and subcutaneous abdominal fat compartments, and bone density have been associated with a number of nontraumatic disease processes including metabolic syndrome and diabetes, wasting in the setting of respiratory failure, and osteoporosis.27–30 Recently, there has been a growing interest in the potential predictive utility of these biomarkers in the setting of trauma.31–33,35
Arterial hemorrhage after pelvic ring disruptions is a leading cause of death after trauma. Admission MDCT has become a widely accepted cornerstone of early triage; however, no single CT feature is deterministic. We have previously shown that a multivariate model incorporating (among other features) categorical grading of atherosclerosis severity as a marker of frailty has similar accuracy to radiologist prediction of major arterial injury requiring angiointervention. The importance of atherosclerosis as a predictor led us to explore the added value of body composition markers corresponding to sarcopenic obesity and osteopenia. These are additional markers of patient frailty that can potentially increase the risk of bleeding in pelvic trauma. Surprisingly, osteopenia was not found to be independently predictive of major bleeding. This may be due to the underrepresentation of elderly patients in the study cohort.
At this time, methods for measuring these parameters are not well-established, and no standard has emerged. In published works, area measurements are primarily utilized.32–34 We compared models incorporating area and volumetric measurements corresponding with paraspinal muscle mass, intermuscular fat, visceral fat, and subcutaneous fat to our initially developed model. Our results show relatively modest but consistent improvement in every parameter of model quality, fit, and performance compared with our published Baltimore CT prediction model when markers of body composition are included. Improvement was more pronounced with volumetric measurements. Paraspinal fat fraction (P = .02) and subcutaneous fat (P = .04) were found to be independent volumetric predictors of major arterial bleeding after blunt pelvic ring disruptions.
The patient cohort used is retrospective, from a single institution, and restricted to patients who underwent angiography after CT, limiting the generalizability of our results. Reproducibility of the measurements will need to be assessed in future studies. Nevertheless, our findings build on our previous results showing the high explanatory value of volumetric hematoma segmentation for predicting major arterial bleeding, and incorporation of atherosclerosis as a single marker of frailty. The results suggest that future predictive models for major arterial bleeding after pelvic ring disruptions should include additional volumetric biomarkers, including those related to body composition. The limited ability of the models to explain variation in the population, demonstrated by low adjusted R2 values, indicate that additional predictors to those previously described in the literature should be identified, and existing predictors measured volumetrically wherever possible to derive more personalized precision diagnostic models and clinical prediction tools. The inclusion of volumetric parameters will preclude application of such predictive models at the point of care until such time that sufficiently rapid and robust auto-segmentation and classification algorithms emerge for each important variable. Another direction to consider is the possibility of assessing additional outcome measures such as length of hospital stay, length of ICU stay, and ventilator associated days to gain additional understanding of multivariable CT prediction for bleeding pelvic fractures.
Future Avenues of Investigation
Toward this end, we have since developed deep learning (DL) algorithms for the 3 major deterministic features in our Baltimore CT prediction model for bleeding pelvic fractures including segmentation and volumetric measurement of pelvic hematomas, active arterial bleeding, and automated pelvic fracture severity classification using the Tile system.38–41
Furthermore, recent work by Lee et al illustrates the feasibility of automated lean muscle mass measurements using a DL algorithm.42 Other authors have also shown that robust automated visceral muscle and subcutaneous adiposity quantitative visualization tools can be developed with DL.43 While our pilot single-center secondary analysis is exploratory and translational at the present time (our DL pelvic fracture and hemorrhage quantitative visualization tools are not yet combined with novel experimental DL body composition tools. At the time our study was conducted, only semiautomated methods were available). We show an important proof of concept; however, as mentioned, point of care use is currently impracticable due to the extensive time effort required to carry out all measurements. Future rapid, robust, and increasingly explanatory CT-volumetry-based computational models leveraging automated bleeding features (or other traumatic pathology) with body composition markers could serve important decision support functions, augmenting the radiologist’s ability to render objective and accurate point of care recommendations with no end-user burden. This has moved from fanciful pipe dream to software prototypes. Following containerization, these can be disseminated, initially as research tools through existing AI marketplaces, with future potential following multicenter clinical trials for widespread clinical adoption.
Table 2.
Model B (2D Body Composition Parameters).
| Predictor | β-coefficient | SE | Wald χ2 test | DF | P value | Exp(β) | 95% CI |
|---|---|---|---|---|---|---|---|
| Rotational instability | −1.687 | 0.616 | 7.500 | 1 | 0.006 | 0.185 | 0.055–0.62 |
| Obturator fracture | 1.649 | 0.629 | 6.880 | 1 | 0.009 | 5.200 | 1.52–17.8 |
| Atherosclerosis | 1.275 | 0.731 | 3.040 | 1 | 0.081 | 3.579 | 0.85–14.994 |
| Hematoma volume | 0.007 | 0.002 | 13.270 | 1 | 0.000 | 1.007 | 1.003–1.010 |
| ICE > 6 mm | 1.455 | 0.535 | 7.400 | 1 | 0.007 | 4.286 | 1.50–12.23 |
| Sub-q fat area | 0.003 | 0.002 | 2.770 | 1 | 0.096 | 1.003 | 0.999–1.007 |
| Paraspinal fat fraction (2D) | −0.075 | 0.035 | 4.560 | 1 | 0.033 | 0.928 | 0.866–0.993 |
| Constant | −2.738 | 0.934 | 8.590 | 1 | 0.003 | 0.065 | 0.010–0.404 |
Abbreviation: 2D, 2-dimensional.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by NIBIB NIH K08 EB027141-01A1 (PI: David Dreizin, MD); Accelerated Translational Incubator Pilot (ATIP) award, University of Maryland (PI: David Dreizin, MD); and RSNA Research Scholar Grant #1605.
Footnotes
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: David Dreizin is founder of TraumaVisual, LLC.
List of meetings at which the work was presented: ASER 2018.
References
- 1.Yoon W, Kim JK, Jeong YY, Seo JJ, Park JG, Kang HK. Pelvic arterial hemorrhage in patients with pelvic fractures: detection with contrast-enhanced CT. RadioGraphics 2004;24(6): 1591–1605. doi: 10.1148/rg.246045028 [DOI] [PubMed] [Google Scholar]
- 2.Demetriades D, Karaiskakis M, Toutouzas K, Alo K, Velmahos G, Chan L. Pelvic fractures: epidemiology and predictors of associated abdominal injuries and outcomes. J Am Coll Surg. 2002;195(1):1–10. doi: 10.1016/S1072-7515(02)01197-3 [DOI] [PubMed] [Google Scholar]
- 3.Sathy AK, Starr AJ, Smith WR, et al. The effect of pelvic fracture on mortality after trauma: an analysis of 63,000 trauma patients. J Bone Joint Surg. 2009;91(12):2803–2810. http://jbjs.org/content/91/12/2803.abstract [DOI] [PubMed] [Google Scholar]
- 4.Miller PR, Moore PS, Mansell E, Meredith JW, Chang MC. External fixation or arteriogram in bleeding pelvic fracture: initial therapy guided by markers of arterial hemorrhage. J Trau Acute Care Surg. 2003;54(3):437–443. [DOI] [PubMed] [Google Scholar]
- 5.Dreizin D, Bodanapally U, Mascarenhas D, et al. Quantitative MDCT assessment of binder effects after pelvic ring disruptions using segmented pelvic haematoma volumes and multiplanar caliper measurements. Europ Radiol. 2018;28(9):3953–3962. [DOI] [PubMed] [Google Scholar]
- 6.Dreizin D, LeBedis CA, Nascone JW. Imaging Acetabular Fractures. Radiol Clin. 2019;57(4):823–841. [DOI] [PubMed] [Google Scholar]
- 7.Costantini TW, Coimbra R, Holcomb JB, et al. Current management of hemorrhage from severe pelvic fractures: results of an American association for the surgery of trauma multi-institutional trial. J Trauma Acute Care Surg. 2016;80(5):717–725. [DOI] [PubMed] [Google Scholar]
- 8.Dreizin D Commentary on “multidetector CT in vascular injuries resulting from pelvic fractures”. RadioGraphics. 2019;39(7): 2130–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dreizin D, Munera F. Blunt polytrauma: evaluation with 64-section whole-body CT angiography. Radiographics. 2012; 32(3):609–631. [DOI] [PubMed] [Google Scholar]
- 10.Dreizin D, Munera F. Multidetector CT for penetrating torso trauma: state of the art. Radiology. 2015;277(2):338–355. [DOI] [PubMed] [Google Scholar]
- 11.Alabousi A, Patlas MN, Sne N, Katz DS. Is oral contrast necessary for multidetector computed tomography imaging of patients with acute abdominal pain? Can Assoc Radiol J. 2015;66(4): 318–322. [DOI] [PubMed] [Google Scholar]
- 12.Landry BA, Patlas MN, Faidi S, Coates A, Nicolaou S. Are we missing traumatic bowel and mesenteric injuries? Can Assoc Radiol J. 2016;67(4):420–425. [DOI] [PubMed] [Google Scholar]
- 13.Dreizin D, Bodanapally UK, Munera F. MDCT of complications and common postoperative findings following penetrating torso trauma. Emerg Radiol. 2015;22(5):553–563. [DOI] [PubMed] [Google Scholar]
- 14.Qamar SR, Evans D, Gibney B, et al. Emergent comprehensive imaging of the major trauma patient: a new paradigm for improved clinical decision-making. Can Assoc Radiol J. 2020. doi: 10.1177/0846537120914247 [DOI] [PubMed] [Google Scholar]
- 15.Anderson SW, Soto JA, Lucey BC, Burke PA, Hirsch EF, Rhea JT. Blunt trauma: feasibility and clinical utility of pelvic CT angiography performed with 64–detector row CT. Radiology 2008;246(2):410–419. doi: 10.1148/radiol.2462070082 [DOI] [PubMed] [Google Scholar]
- 16.Cullinane DC, Schiller HJ, Zielinski MD, et al. Eastern association for the surgery of trauma practice management guidelines for hemorrhage in pelvic fracture—update and systematic review. J Traum Acute Care Surg. 2011;71(6):1850–1868. doi: 10.1097/TA.0b013e31823dca9a [DOI] [PubMed] [Google Scholar]
- 17.Dreizin D, Nascone J, Davis DL, et al. Can MDCT unmask instability in binder-stabilized pelvic ring disruptions. American J Roentgenol. 2016;207(6):1244–1251. [DOI] [PubMed] [Google Scholar]
- 18.Blackmore C, Jurkovich GJ, Linnau KF, Cummings P, Hoffer EK, Rivara FP. Assessment of volume of hemorrhage and outcome from pelvic fracture. Archi Surg. 2003;138(5):504–509. doi: 10.1001/archsurg.138.5.504 [DOI] [PubMed] [Google Scholar]
- 19.Brasel KJ, Pham K, Yang H, Christensen R, Weigelt JA. Significance of contrast extravasation in patients with pelvic fracture. J Trauma Acute Care Surg. 2007;62(5):1149–1152. doi: 10.1097/TA.0b013e3180479827 [DOI] [PubMed] [Google Scholar]
- 20.Brown CVR, Kasotakis G, Wilcox A, Rhee P, Salim A, Demetriades D. Does pelvic hematoma on admission computed tomography predict active bleeding at angiography for pelvic fracture? American Surgeon. 2005;71(9):759–762. http://www.ingentaconnect.com/content/sesc/tas/2005/00000071/00000009/art00013 [PubMed] [Google Scholar]
- 21.Sheridan M, Blackmore C, Linnau K, Hoffer E, Lomoschitz F, Jurkovich G. Can CT predict the source of arterial hemorrhage in patients with pelvic fractures. Emerg Radiol. 2002;9(4):188–194. [DOI] [PubMed] [Google Scholar]
- 22.Dreizin D, Bodanapally UK, Neerchal N, Tirada N, Patlas M, Herskovits E. Volumetric analysis of pelvic hematomas after blunt trauma using semi-automated seeded region growing segmentation: a method validation study. Abdom Radiol. 2016; 41(11):2203–2208. [DOI] [PubMed] [Google Scholar]
- 23.Dreizin D, Liang Y, Dent J, Akhter N, Mascarenhas D, Scalea TM. Diagnostic value of CT contrast extravasation for major arterial injury after pelvic fracture: a meta-analysis. Ameri J Emerg Med. 2019. doi: 10.1016/j.ajem.2019.11.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eastridge BJ, Starr A, Minei JP, O’Keefe GE. The importance of fracture pattern in guiding therapeutic decision-making in patients with hemorrhagic shock and pelvic ring disruptions. J Trauma Acute Care Surg. 2002;53(3):446–451. http://journals.lww.com/jtrauma/Fulltext/2002/09000/The_Importance_of_Fracture_Pattern_in_Guiding.9.aspx [DOI] [PubMed] [Google Scholar]
- 25.Dreizin D, Bodanapally U, Boscak A, et al. CT prediction model for major arterial injury after blunt pelvic ring disruption. Radiology. 2018;287(3):1061–1069. [DOI] [PubMed] [Google Scholar]
- 26.Battey TW, Dreizin D, Bodanapally UK, et al. A comparison of segmented abdominopelvic fluid volumes with conventional CT signs of abdominal compartment syndrome in a trauma population. Abdom Radiol (NY). 2019;44(7):2648–2655. [DOI] [PubMed] [Google Scholar]
- 27.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments. Circulation 2007; 116(1):39–48. [DOI] [PubMed] [Google Scholar]
- 28.Braunschweig CA, Sheean PM, Peterson SJ, et al. Exploitation of diagnostic computed tomography scans to assess the impact of nutrition support on body composition changes in respiratory failure patients. JPEN J Parenter Enteral Nutr. 2014;38(7):880–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pickhardt PJ, Pooler BD, Lauder T, del Rio AM, Bruce RJ, Binkley N. Opportunistic screening for osteoporosis using abdominal computed tomography scans obtained for other indications. Ann Intern Med. 2013;158(8):588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pickhardt P, Lauder T, Pooler B, et al. Effect of IV contrast on lumbar trabecular attenuation at routine abdominal CT: correlation with DXA and implications for opportunistic osteoporosis screening. Osteoporosis Int. 2016;27(1):147–152. [DOI] [PubMed] [Google Scholar]
- 31.Fairchild B, Webb TP, Xiang Q, Tarima S, Brasel KJ. Sarcopenia and frailty in elderly trauma patients. World J Surg. 2015;39(2):373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oskutis MQ, Lauerman MH, Kufera JA, et al. Are frailty markers associated with serious thoracic and spinal injuries among motor vehicle crash occupants? J Traum Acute Care Surge. 2016;81(1): 156–161. [DOI] [PubMed] [Google Scholar]
- 33.Kaplan SJ, Pham TN, Arbabi S, et al. Association of radiologic indicators of frailty with 1-year mortality in older trauma patients: opportunistic screening for sarcopenia and osteopenia. JAMA Surg. 2017;152(2):e164604–e164604. [DOI] [PubMed] [Google Scholar]
- 34.Boutin RD, Bamrungchart S, Bateni CP, et al. CT of patients with hip fracture: muscle size and attenuation help predict mortality. AJR Am J Roentgenol. 2017;208(6):W208–W215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shashaty MG, Kalkan E, Bellamy SL, et al. Computed tomography-defined abdominal adiposity is associated with acute kidney injury in critically ill trauma patients. Crit Care Med. 2014;42(7):1619–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ufuk F, Herek D, Yüksel D. Diagnosis of sarcopenia in head and neck computed tomography: cervical muscle mass as a strong indicator of sarcopenia. Clin Exp Otorhinolaryngol. 2019;12(3): 317–324. doi: 10.21053/ceo.2018.01613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lauerman MH, Raithel M, Kufera J, et al. Comparison of individual and composite radiographic markers of frailty in trauma. Injury 2019;50(1):149–155. [DOI] [PubMed] [Google Scholar]
- 38.Dreizin D, Zhou Y, Zhang Y, Tirada N, Yuille AL. Performance of a deep learning algorithm for automated segmentation and quantification of traumatic pelvic hematomas on CT. J Digit Imag. 2020;33(1):243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou Y, Dreizin D, Li Y, Zhang Z, Wang Y, Yuille A. Multi-scale attentional network for multi-focal segmentation of active bleed after pelvic fractures. In: Suk HI, Liu M, Yan P, Lian C, eds. Machine learning in medical imaging. MLMI 2019. Lecture notes in computer science, vol. 11861. Cham: Springer; 2019. https://link.springer.com/chapter/10.1007/978-3-030-32692-0_53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dreizin D, Zhou Y, Chen T, et al. Deep learning-based quantitative visualization and measurement of extraperitoneal hematoma volumes in patients with pelvic fractures: potential role in personalized forecasting and decision support. J Trau Acute Care Surge. 2020;88(3):425–433. doi: 10.1097/ta.0000000000002566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dreizin D, Goldmann F, Chen T, Unberath M.Automated CT pelvic fracture severity grading with deep learning: association with clinical outcomes. Conference on Machine Intelligence in Medical Imaging (C-MIMI). Austin, TX, September 22–23, 2019. SIIM. [Google Scholar]
- 42.Lee H, Troschel FM, Tajmir S, et al. Pixel-level deep segmentation: artificial intelligence quantifies muscle on computed tomography for body morphometric analysis. J Digit Imaging. 2017; 30(4):487–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weston AD, Korfiatis P, Kline TL, et al. Automated abdominal segmentation of CT scans for body composition analysis using deep learning. Radiology. 2019;290(3):669–679. [DOI] [PubMed] [Google Scholar]


