Abstract
In precision oncology, two major strategies are being pursued for predicting clinically relevant tumour behaviours, such as treatment response and emergence of drug resistance: inference based on genomic, transcriptomic, epigenomic and/or proteomic analysis of patient samples, and phenotypic assays in personalized cancer avatars. The latter approach has historically relied on in vivo mouse xenografts and in vitro organoids or 2D cell cultures. Recent progress in rapid combinatorial genetic modelling, the development of a genetically immunocompromised strain for xenotransplantation of human patient samples in adult zebrafish and the first clinical trial using xenotransplantation in zebrafish larvae for phenotypic testing of drug response bring this tiny vertebrate to the forefront of the precision medicine arena. In this Review , we discuss advances in transgenic and transplantation-based zebrafish cancer avatars, and how these models compare with and complement mouse xenografts and human organoids. We also outline the unique opportunities that these different models present for prediction studies and current challenges they face for future clinical deployment.
The biomedical community has been developing an ever-growing number of novel anticancer agents. After an era where chemotherapy was the only systemic treatment available, a broad range of new targeted therapies and, more recently immunotherapy agents, have entered clinical development or have received regulatory approval1,2. This expanding list of treatment options offers new hope for patients with cancer in need of systemic therapy, but also poses new challenges for oncologists. The deceptively simple question of which drug to prescribe to a specific patient is increasingly harder to address with certainty. Development of new agents is outpacing the long and complex trials required to identify which dosage, schedule and combinatorial strategy will confer the greatest clinical benefit to which defined patient population.
The field of precision oncology was born from the need to address these issues by creating a new prognostic and therapeutic paradigm focused on optimizing treatment for an individual patient, rather than a group. Precision medicine at large philosophically moves away from predictions based on comparison of group averages and focuses instead on a patient’s unique disease features. Specifically, in precision oncology this new paradigm has resulted in two major approaches. The first approach is the inference of disease behaviour based on either single or multiple omic (genomic, transcriptomic, epigenomic and proteomic) analyses of tumour biopsy samples3,4, which is relatively rapid and inexpensive as a result of improving technologies. The second approach is the functional testing of tumour behaviours and response to drugs using personalized cancer avatars obtained from in vitro cultures or in vivo xenotransplantation of tumour biopsy samples5–7. As a clinical prediction tool, the former approach is limited by our knowledge of the functional consequences of individual genetic mutations in genes outside the common cancer drivers, by the effect of combinations of events and by the complexity of clonal dynamics and tumour evolution that can contribute to drug response8. The latter approach overcomes this complexity by simply focusing on the tumour’s phenotypic behaviour and emulating the possible treatment settings; however, it is constrained by the biological fidelity of specific models as a proxy for patient response and by the logistical considerations of assay duration, cost and scalability and the challenge of testing multiple conditions5. Phenotypic testing of drug response has historically relied on growing patient-derived cancer cells in vitro as 2D cultures9 or 3D organoids6, or in vivo as mouse xenografts5,10,11. Recent technological advances in the generation of both transgenic12 and transplantation-based zebrafish cancer avatars13,14, combined with the intrinsic logistic advantages of scale, cost, time and multiplexing of conditions15, and the potential for automation16,17 bring zebrafish into the arena of phenotypic testing of drug response for precision cancer therapy.
In this Review, we highlight the history and evolution of tools that have made possible the rapid generation of combinatorial transgenic cancer models in zebrafish12,18, and the development of xenotransplantation of patient samples into larvae and adult immunodeficient zebrafish raised at 37 °C (REFS13,14). We discuss how zebrafish cancer avatars compare with human organoids and mouse xenografts for drug testing. Finally, we highlight some of the limitations and challenges for future clinical implementation. For detailed coverage of human cancer organoids and xenografts, we refer the reader to the work of colleagues5,6,10,11,19.
Advances in zebrafish cancer avatars
Stable transgenic cancer models
Due to the extensive conservation with humans of organ-specific genetic programmes and cancer-associated genes20, a closer resemblance to humans than mice in telomere biology21 and the relative ease of genetic manipulation of embryos by microinjection at the one-cell stage, zebrafish quickly emerged as a promising organism to model cancer in vivo22. In 2003, the first genetically engineered cancer model in zebrafish of T cell acute lymphoblastic leukaemia (T-ALL) was reported23 (FIG. 1). The mouse Myc open reading frame was placed under control of the zebrafish rag2 promoter, which restricts expression to lymphoid cells. When this was injected into wild-type zebrafish embryos, 5% of F0 zebrafish developed T-ALL between 1 month and 5 months of age. Germline transmission of the transgene increased the penetrance to 100% and reduced the latency of tumours. This model provided a remarkable proof of concept for cancer modelling in zebrafish and laid the foundation for new discoveries of mechanisms that drive leukaemogenesis. This study also paved the way for developing other tumour models in zebrafish, including pancreatic neuroendocrine tumours24 in 2004, rapidly followed by melanoma25 in 2005, and many others have been established since then, including conditional models that use Cre-mediated recombination, and chemically inducible models26–28. However, in most human cancers, particularly solid tumours, oncogenesis is thought to require multiple genetic drivers, including both the activation of an oncogene and the loss of a tumour suppressor29,30. Accordingly, zebrafish models based solely on the expression of an oncogene, with exception of RAS-driven models31, have generally shown poor penetrance, which has considerably restrained their use. Other limitations include the fact that many models were obtained serendipitously by expression of an oncogene that did not always reflect the early clonal genetic drivers of human tumours24,32. The degree of tissue specificity of the promoter driving oncogene expression is also critical for the faithful recapitulation of human tumorigenesis.
Fig. 1 |. Timeline of key developments in zebrafish cancer avatars.
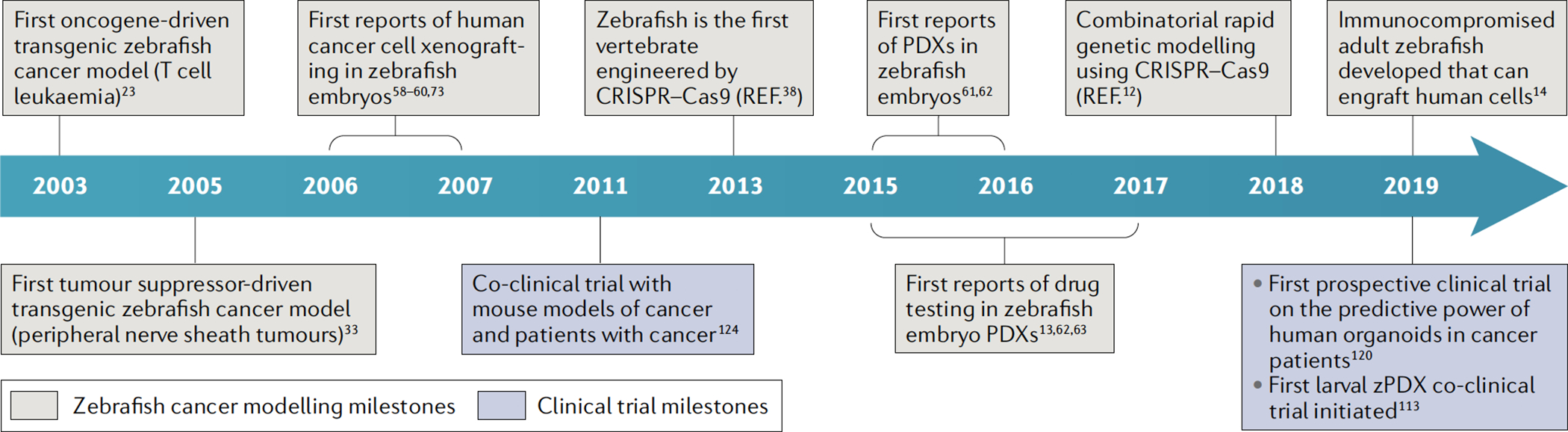
Timeline highlighting key milestones in the development of zebrafish cancer models towards personalized patient avatars (beige boxes). Salient milestones in clinical translation of cancer therapy response prediction using mouse, organoid and zebrafish patient avatars (purple boxes). PDX, patient-derived xenograft; zPDX, zebrafish patient-derived xenograft.
One-cell stage.
The zebrafish embryonic stage where the egg has been fertilized by sperm, but before the first cell division.
The first zebrafish model of melanoma used the most common driver found in human melanoma, BRAFV600E, placed under the control of the melanocyte-specific zebrafish mitfa promoter25. As in humans, BRAFV600E expression in zebrafish melanocytes generated only pigmented nevi. However, injection of the mitfa:BRAFV600E construct in zebrafish carrying an inactivating mutation in the tp53 tumour suppressor gene33, the human orthologue of which is mutated in approximately 15% of human melanomas, initiated the formation of malignant melanomas in 6% of zebrafish within 4 months25. These tumours were confirmed as melanomas by histologic and phenotypic analyses. Importantly, the penetrance of the disease reached nearly 100% in stable mitfa:BRAFV600E;tp53−/− F1 transgenic zebrafish. The mitfa:BRAFV600E;tp53−/− zebrafish melanoma model has subsequently served as a reliable basis for multiple genetic screens aiming at functionally probing human melanoma genetics in vivo, and has identified several new modulators of melanoma34. Although extremely valuable, this modelling approach posed some technical difficulties. First, like in other well-established transgenic zebrafish cancer models23,25,35,36, maintaining stable lines containing several transgenes is difficult. Second, the number of transgenes that can be combined remains limited, which makes it challenging to test complex genetic interactions. Third, unlike overexpression of human oncogenes, knockouts of endogenous zebrafish tumour suppressor genes are systemic, not tissue specific. Finally, most models are still based on transgenic expression of human oncogenes rather than knock-ins into endogenous or orthologue zebrafish loci and thus might not have physiologic levels of expression. Recent advances in genome editing technology have and will help overcome some of these limitations.
Nevi.
Benign skin lesions of melanocytes, also known as moles, which are thought to be senescent.
Rapid combinatorial genetic modelling
The CRISPR–Cas9 system created new opportunities for in vivo cancer modelling37. It allowed the rapid mosaic inactivation of tumour suppressor genes, bypassing the need for stable transgenic lines. It also greatly increased the potential for combinatorial modelling through multiplexing of guide RNAs (gRNAs) targeting different genes, making possible both the recapitulation of complex human cancer genotypes and the in vivo evaluation of putative genetic interactions inferred from human cancer genomics. Finally, it facilitated large-scale screens with the goal of identifying new cancer drivers.
As in mice, CRISPR made possible the rapid establishment of knockout zebrafish lines38. Taking advantage of the Tol2 transposon technology39, which facilitates the insertion of DNA constructs into the zebrafish genome, CRISPR vectors have been developed for tissue-specific gene inactivation by assembling a zebrafish U6 promoter-driven gRNA cassette with a cas9 sequence placed under the control of tissue-restricted zebrafish promoters18. Efficient mosaic gene knockout was observed in embryonic tissues such as muscle and erythroid cells. By designing vectors to target zebrafish tumour suppressor genes, and to express human oncogenes in a melanocyte-specific manner, we were able to rapidly and robustly model all the major genotypes found in human melanoma by combining oncogenic mutations in BRAF, NRAS or KIT with loss-of-function mutations in cdkn2a, tp53, ptena or ptenb12 (FIG. 2a). Tumours were obtained as early as 3 weeks after injection (NRASQ61R expression and tp53 loss) for the most aggressive genotypes or within a few months (BRAFV600E expression and cdkn2a loss), consistent with observations in the human disease40,41.
Fig. 2 |. Generation of zebrafish avatars.
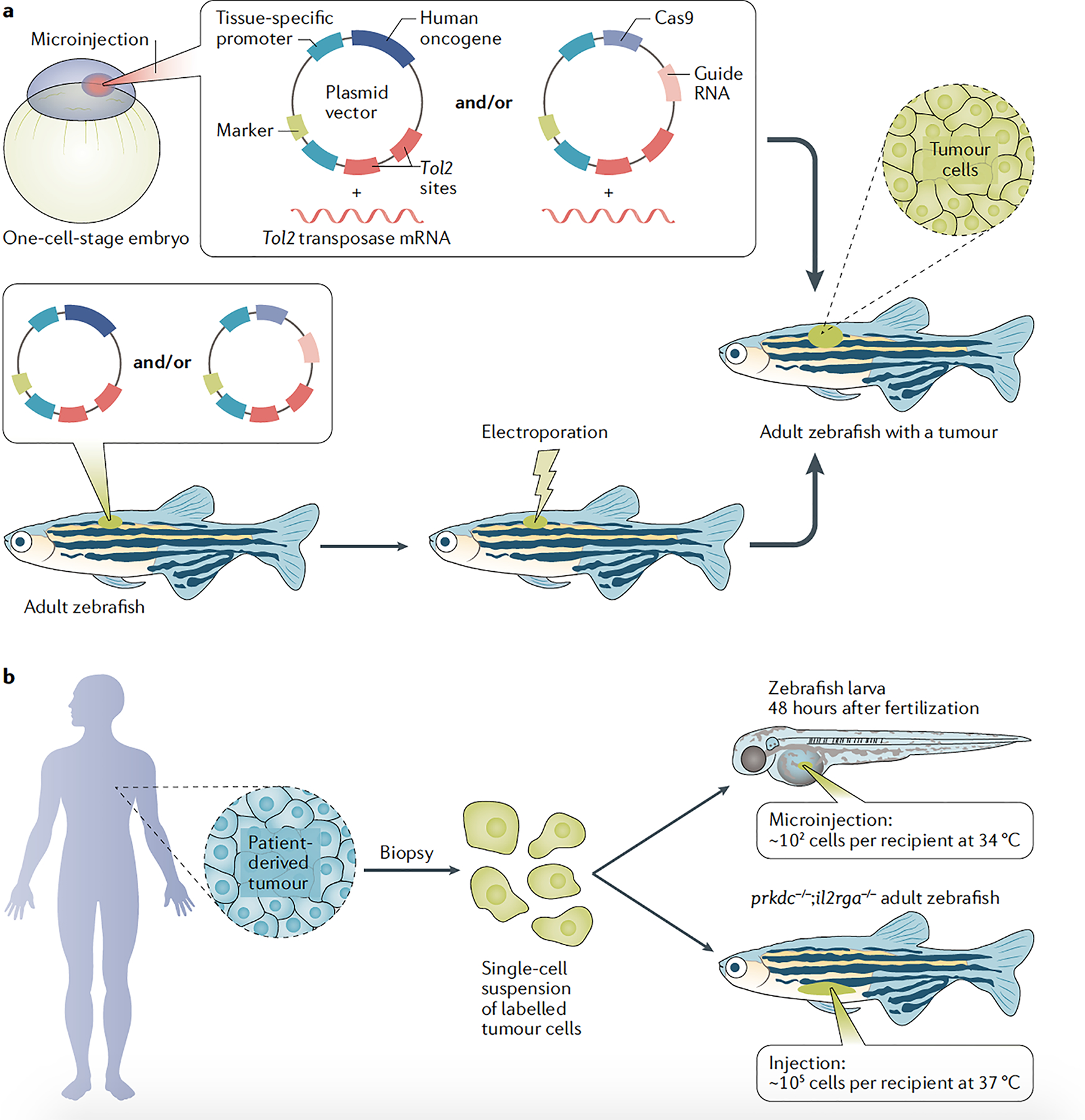
a | Mosaic transgenic zebrafish can be created using embryo microinjection or electroporation in adults. In embryos (top panel), Tol2 mRNA is co-injected with plasmid DNA vector(s) containing Tol2 sites, which are recognized by Tol2 protein and inserted in the host cell genome. For transgene electroporation in adult zebrafish (bottom panel), gene delivery is accomplished by co-injection of a DNA plasmid expressing Tol2 with transgenesis vector(s) before electroporation. Between Tol2 sites, transgenesis vectors typically include a (1) tissue-specific promoter driving a coding sequence, often a human oncogene12,23,34,36, or Cas9 for CRISPR knockout of tumour suppressors12,18, and (2) a transgenesis marker. The marker is often composed of a tissue-specific promoter and a fluorescent protein (for example, heart marker cmlc2:EGFP) or, in the case of melanoma, an mitfa minigene (promoter plus coding sequence to rescue the loss of mitfa), which allows melanocyte development itself to be used as a transgenesis marker in F0 zebrafish18,34. b | Zebrafish patient-derived xenografts can be created by transplantation of cancer cells (which are usually labelled) in 48-hour post-fertilization larvae raised at 34 °C or genetically immunocompromised adult zebrafish (prkdc−/−;il2rga−/−) raised at 37 °C. Zebrafish embryos and adults are kept at 26–28.5 °C, while human cells grow at 37 °C. To support human cell engraftment, embryos are raised at 34 °C (ref.13), which, while below the normal physiological temperature for human cells, is the upper limit tolerated by zebrafish embryos and larvae. Adult fish can tolerate living at 37 °C for months if the temperature is slowly raised from 28.5 to 37 °C by about 0.5–1 °C per day14.
Tol2 transposon
Tol2 is an active transposon derived from the medaka fish and is used as a gene delivery method.
U6 promoter
An rNA polymerase iii type 3 promoter commonly used to drive short hairpin rNAs or guide rNAs.
This technique thus allows rapid cancer modelling. It is also versatile and could be easily adapted for expression and inactivation of different oncogene and tumour suppressor combinations, respectively, in other organs, provided a robust tissue-restricted zebrafish promoter is available. For example, the Gutierrez group has shown that mutations in the gene patched 1 (ptch1) in the Hedgehog pathway increase T-ALL onset using a similar mosaic CRISPR approach42. Some genotypes reached 100% penetrance, indicating that mosaic expression of the vectors in the F0 generation was sufficient to generate tumours, which abolishes the need to establish stable transgenic lines for tissues where robust tissue-specific promoters are available. The fact that genetic driver alterations are introduced from birth constitutes a limitation of this approach, as developmental defects may be induced. One way of circumventing this issue is to deliver the vector combinations directly into somatic tissues of adult zebrafish, for example, using a recently reported electroporation method43. Finally, this modelling technique is scalable, as several vectors can be injected together to model more complex genotypes. We used this option to study the role of SPRED1 loss of function in mucosal melanoma, which was inferred from analysing human genomic data, and demonstrated the genetic cooperation between spred1 inactivation and KIT oncogenic mutations in a tp53-null or cdkn2a-null context12. Overexpression and CRISPR vectors can also be multiplexed further to test the relevance of putative cancer drivers in a high-throughput manner. The tools presented above make the zebrafish a prime model to recapitulate and functionally probe the genetics of human tumours in vivo at scale.
Zebrafish cancer xenografts
Zebrafish as transplant recipients.
The zebrafish has many inherent traits that make it an ideal transplant recipient. First, the engrafted normal or malignant cells can be easily tracked by fluorescent labelling of transplanted cells, and direct visualization of grafts is possible using the optically clear casper zebrafish strain as recipients44. Second, zebrafish are highly fecund, with matured females capable of producing hundreds of eggs per week, yet are small, so thousands of zebrafish can be kept in a single facility, and thus husbandry and maintenance costs are low relative to those for mice (while this might differ significantly between countries and institutions, it has been reported that the cost ratio is on the order of magnitude of approximately US$1.05 per mouse versus approximately US$0.01 per adult zebrafish)45. Most importantly, manual transplantation or microinjection can be performed in several hundred adult zebrafish or up to a few thousand zebrafish larvae, respectively, in a single day by a single operator (FIG. 2b), facilitating large-scale and high-throughput cell transplantation studies that are difficult to execute with immunocompromised mouse models46–53. Allogeneic transplantation of nonimmune matched zebrafish cells into partially immunocompromised adult zebrafish has now become routine. When paired with large-scale genetic screening and drug discovery platforms available in zebrafish models, such studies have provided valuable insights into intra-tumoural clonal evolution and heterogeneity, therapy resistance, invasion and metastasis, haematopoiesis and stem cell engraftment13,47,51,53–57.
Casper zebrafish strain.
An optically clear strain of zebrafish obtained by crossing the roy (mpv17−/−) and nacre (mitfa−/−) mutants, which results in the lack of iridophores and melanocytes, respectively.
Xenografts in zebrafish larvae.
The xenotransplantation of a human cancer cell line into zebrafish larvae was first described for melanoma in 2006–2007 (REFS58–60) (FIG. 1). Since then, a large number of studies have reported xenotransplantation of cancer cell lines into zebrafish larvae. However, especially earlier studies did not always include a rigorous assessment of xenograft survival and proliferation. Ten years later, three proof-of-concept studies were published that developed patient-derived xenografts (PDXs) in zebrafish larvae from both blood malignancies and solid tumours61–63, followed by others showing cooperation among tumour-cell subpopulations that can synergize to promote tumour progression64 and phenotypic testing of drug response13.
Adaptive and innate immune systems in zebrafish are highly conserved with mice and humans65. In zebrafish, innate immunity starts developing on the first day after fertilization, while adaptive immunity is morphologically and functionally mature only about 2–3 weeks after fertilization66–69. The short window of immune incompetency during early larval development has allowed transplantation and short-term survival of xenotransplanted human or mouse cells without the need to immunocompromise the zebrafish. With this approach, numerous cell types have been transplanted into zebrafish, which resulted in many important findings, including mechanisms governing cancer cell differentiation, proliferation and migration, as well as therapeutic response13,59,70,71. A particular advantage is the natural optical clarity of wild-type larval zebrafish before they develop pigmentation, which allows facile single-cell resolution imaging, analysis and quantification of transplanted cells, a trait superior to other transplant model organisms.
However, such assays have some inherent limitations. First, larval xenotransplantation experiments have a short window of about 7 days before engrafted cells are rejected by the acquired immune system13. Second, the small body size of zebrafish larvae restricts the number of transplanted cells to 100–200 per animal, a number that often fails to contain cancer-driving stem cells or accurately recapitulate the genetic heterogeneity and drug response behaviours driven by rare subclones found in human tumours13,72. Third, most xenotransplantation experiments using larval recipients are conducted below 37 °C (REF.13), and at those temperatures, transplanted human cells do not proliferate at the same rates or form tumour masses akin to those found in immunocompromised Nsg mice or in human patients13,14.
NSG mice.
genetically engineered immunocompromised strain of mice widely used as recipients for engraftment of human primary cells or tumours (for example, patient-derived xenografts).
Xenografts in adult zebrafish.
Immunodeficient zebrafish models were recently developed that engraft xenotransplanted patient-derived cancer cells into adult zebrafish recipients14 (FIG. 1). Previous attempts at facilitating long-term engraftment and survival of xenotransplanted cells in adult zebrafish involved transient ablation of the animal’s immune system using radiation or chemical treatment73,74. Gamma irradiation of wild-type or casper recipient fish allows robust engraftment of zebrafish tumour and haematopoietic cells for up to 20 days after transplantation75, since the host immune system recovers by 30 days after irradiation and rejects the graft, but has not been reported to make possible engraftment of human cells. Long-term chemically induced immunosuppression by constant drug dosing of the steroid drug dexamethasone permits engraftment of a subset of human tumours to time points exceeding 30 days73. As dexamethasone itself is a clinically used anticancer drug in leukaemias and lymphomas76, this approach would not be suitable for assessing blood and leukaemia cell engraftment. Furthermore, chemical immunosuppression introduces an additional layer of experimental variability and could result in drug interactions with agents tested that might confound experimental result interpretations, thus hindering standardization and scaling.
To overcome these limitations, the Langenau group generated an adult zebrafish xenotransplantation model that allows robust, long-term engraftment and proliferation of human and mouse cells by introducing homozygous inactivating mutations in prkdc (which encodes DNA-activated protein kinase catalytic subunit) and il2rga (which encodes interleukin-2 receptor-γa) into transparent casper zebrafish14. These immunodeficient zebrafish are optically clear, lack T cells, B cells and natural killer cells and can survive at 37 °C. Remarkably, no drawbacks were found in rearing zebrafish at 37 °C, other than additional work in husbandry. Viability is high and zebrafish well tolerate this temperature if acclimated over time. Moreover, acclimated zebrafish robustly engraft a large variety of both human cancer cell lines and PDXs in excess of 28 days14. Another major advantage of adult over larval transplant assays is that a significantly larger number of transplanted cells can be used per adult zebrafish (up to two million cells per zebrafish), in addition to the advantages of a longer experimental window and human physiological temperature. Growth kinetics, cell proliferation and apoptotic rates were identical when compared with those for the same tumours grown in NSG mice14. Building upon the experience in mouse models77,78, we expect that further engineering of the prkdc−/−il2rga−/− strain could overcome current limitations, due, for example, to limited conservation between fish and human cytokines, or residual adaptive immunity, and further improve the robustness and increase the range of human tumours engrafted.
Drug testing in zebrafish avatars
Engrafting a patient’s tumour or transgenic modelling with large cohorts of zebrafish permits testing of a wide array of clinically available drugs, and as such pairing responses in zebrafish avatars with clinical decision-making could help stratify patients for the most suitable treatment for their tumour (FIG. 3). The logistic advantages of zebrafish avatars significantly increases the scale of drug testing possible compared with other in vivo patient-specific avatars (TABLE 1).
Fig. 3 |. Drug administration in zebrafish.
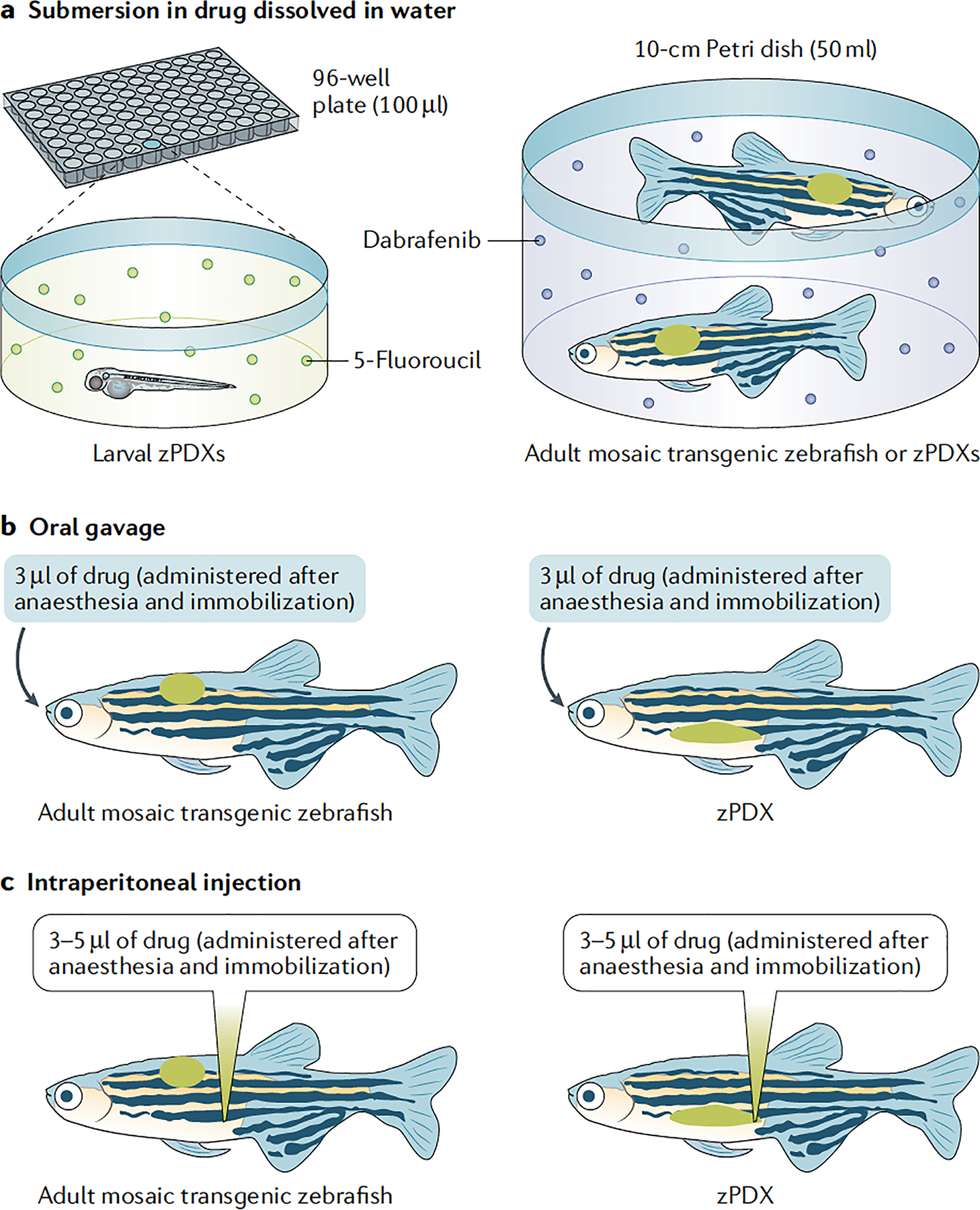
a | Submersion in a water and drug solution is used to treat larval zebrafish patient-derived xenografts (zPDXs) (10–20 24-hour post-fertilization embryos per well in a 24-well plate or one embryo per 96-well plate) or adult avatars (two adults in a 10-cm Petri dish). Example compounds shown were used by Fior et al.13 (5-fluorouracil treatment of larvae) and Ablain et al.12 (treatment of adults with the BRAF inhibitor dabrafenib). b | Oral gavage can also be used to treat adult avatars (mosaic transgenic zebrafish or zPDXs). Zebrafish are anaesthetized and immobilized using MS-222 alone or in combination with isoflurane. Oral gavage using a syringe with flexible tubing allows ~3 μl of drug solution to be dispensed (described by Dang et al.79). c | Intraperitoneal injection is another alternative to treat adult avatars (mosaic transgenic zebrafish or zPDXs). Zebrafish are anaesthetized and immobilized using MS-222 or MS-222 and isoflurane. Free-hand injections are performed with 10-μl capacity syringes to deliver up to 5 μl of drug solution. Approximately 3–5 μl is the tolerable range reported14,79.
Table 1 |.
Comparison of personalized cancer avatars
| Cancer avatar type | Assay duration | Costa | Logistic footprinta | Number of cells per recipient or condition | Drug screening throughputb | Cancer cell intrinsic target conservation | Patient TME conservation | Pharmacokinetic and dose optimization |
|---|---|---|---|---|---|---|---|---|
Mosaic transgenic zebrafish
|
Weeks to months | Low | Medium | NA (requires only patient tumour DNA sequencing) | Medium | 100% for transgenic drivers; variable for others | No | Yes |
| Zebrafish PDXs in larvae |
5–7 days | Low | Low | 102 | High | High | No | No |
Zebrafish PDXs in adu1tsc
|
Weeks to Months | Low | Medium | 105 | Medium | High | Low or no | Yes |
Human 3D organoids in vitro
|
1–2 weeks | Low | Low | 102–103 | Very high | High | No | No |
Mouse PDXs
|
Weeks to months | High | High | 105–106 | Low | High | Low or no | Yes |
NA , not applicable; PDX, patient-derived xenograft; TME, tumour microenvironment.
While cost and husbandry rules differ significantly by country and across institutions, previously reported cost and housing rules in North America and some European countries suggest that husbandry physical footprint space requirements (12 adult zebrafish versus 1 adult mouse per ~1.2 l)113 and cost per day (US cents per 3.5- l fish tank versus US dollars per mouse cage, approximately $1.05 per mouse versus approximately $0.01 per adult zebrafish)45 are at least an order of magnitude lower for zebrafish than for mice.
Drug screening throughput order of magnitude assuming a duration compatible with clinical decision-making (days to weeks): low (1–5 compounds), medium (10–50 compounds), high (~100 compounds), very high (~1000 compounds).
The data here are based on the proof-of-concept study by Yan et al.14. Further studies will be needed in adult zebrafish PDXs to generalize engraftment rates, assay duration and cell numbers across tumour types.
Drug administration routes in zebrafish
Zebrafish are treated by adding drugs directly to their water and submersing larvae and adults12,13. Furthermore, in adults, drugs can be administered by intraperitoneal injection79,80 or oral gavage14,79 (FIG. 3). Drug delivery in larvae via submersion therapy makes it impossible to accurately assess drug dosing, pharmacokinetics and pharmacodynamics (FIG. 3). In contrast, clinically relevant drug administration via oral gavage or intraperitoneal injection in adults allows more tightly controlled dosing and also assessment of pharmacokinetics by mass spectrometry using pooled blood plasma samples. Furthermore, unlike submersion, oral gavage and intraperitoneal injection can effectively deliver small molecules and antibodies that have low solubility in water. Pharmacokinetic differences between humans, mice and zebrafish are an important concern that the field has been confronted with, but from empirical testing have been used effectively to identify dose-conversion factors for oral gavage14,79 and for submersion therapy13 (also preliminarily shown in REF.81). Furthermore, oral gavage of both a chemotherapy agent (that is, temozolomide) and a targeted therapy (that is, olaparib) in adult zebrafish PDXs (zPDXs) led to a blood pharmacokinetic profile very similar to that in humans and mice14. Thus, while optimization will be required for drug dosing on a per case basis, there is robust proof of concept that pharmacokinetics per se is not a cross-species barrier for zebrafish avatars.
Clinical response determination
Patient clinical response to cancer treatment is determined by measuring maximum tumour diameter changes on imaging data from magnetic resonance imaging or computed tomography scans. On the basis of the size change, the patient’s response is classified according to the RECIST criteria82. In mouse xenograft tumours, calipers, computed tomography or positron emission tomography, or luciferase bioluminescence is commonly used for tumour response measurement. In zebrafish avatars, drug response has been measured in several ways: (1) direct imaging in transparent recipients of unlabelled tumour cells (particularly for naturally pigmented tumours as melanoma)79, (2) fluorescent imaging of tumour size (by either transducing the patient-derived cells with a fluorescent protein viral vector (for example, enhanced GFP), or with FUCCI4 to visualize cell cycle phases)14, (3) by exposing the cancer cells to viable fluorescent dyes13,14 or (4) non-invasively using ultra-sonography83. The number of cells (larval zPDX)13 and tumour surface area (both larval and adult zPDXs)14 (also preliminarily shown in REF.81) have been used as a tumour response measure using RECIST criteria. These methods, combined with the high throughput of in vivo screening offered by the zebrafish platform, provide faster imaging end points that capitalize on the ability to assess drug effects in real time down to single-cell resolution. The development of automated injector systems (reviewed previously17) and the emergence of automated imaging systems for zebrafish larvae16 further illustrate the high potential for automation and scaling of zPDXs, which would be important for standardization and potential clinical deployment.
RECIST criteria.
A set of published rules that define when patients with cancer improve (‘respond’), stay the same (‘stable’) or worsen (‘progression’) during treatments.
Types of agents that can be tested
The biological differences between the avatar models highlighted earlier and target conservation in different organisms have immediate implications for what type of therapeutic agents can be assessed effectively (TABLE 1). Many anticancer agents have been successfully used in zebrafish, including various types of chemotherapy, small-molecule inhibitors such as dasatinib and antibody-based therapies such as bevacizumab and cetuximab13,62,79,84–94. For example, the adult zPDX model was used to identify the combination of the poly(ADP-ribose) polymerase (PARP) inhibitor olaparib and the chemotherapy agent temozolomide as an effective therapy for rhabdomyosarcoma. The combination treatment efficacy was recapitulated in a mouse xenograft model, giving further proof of the equivalency of the two recipient model organisms for drug testing14. This drug combination is now entering clinical trials for paediatric rhabdomyosarcoma (NCT01858168)95 and represents the first clinical trial of a combination therapy initiated from xenograft preclinical studies using zebrafish. Chemotherapy has a high degree of conservation of targets across zebrafish and humans, while targeted therapies using small molecules or antibodies are highly dependent on the target’s structural conservation for its action. Thus, intrinsic targets in cancer cells can usually be assessed effectively only in xenograft cell transplant models62,79,84–92. On the other hand, targeted therapy directed to the tumour microenvironment (TME) will present a more variable degree of feasibility depending on exact target conservation. Similar considerations apply to transgenic models where we typically introduce human versions of driver oncogenes12,34,79, but the success of therapies targeting other genes or synthetic lethal approaches will differ depending on target conservation20,96,97. As in engraftment studies using NSG mice, the lack of a patient-specific immune tumour microenvironment and/or co-evolution in zPDX models, and lack of cross-species target conservation (for example, limited sequence conservation of immune checkpoints such as PD1, PDL1 and CTLA4) poses challenges to providing an effective proxy in zebrafish avatars to assess patient responses to agents that target the immune TME. Another confounding factor specifically in larvae is the potential toxic effects of many anticancer agents on the zebrafish recipient larva itself, as it goes through a rapid phase of development. Indeed an observed tumour shrinkage might also be the result of an indirect effect of the toxicity on the recipient itself that would compromise its ability to support cancer cell growth98–103.
Prediction of response and resistance
The value of zebrafish avatars for precision cancer therapy boils down to (1) the predictive power, (2) the scale of experimentation and (3) the assay duration time to inform clinical decision-making. Thus, it is worth considering the benefit and limitations pertaining to the time and the effectiveness of zebrafish avatars in predicting not just drug sensitivity but also drug resistance. Drug resistance is often classified as primary or intrinsic resistance (when patients never respond to an agent) and secondary or acquired resistance (when a patient initially sensitive to the drug develops resistance). The former is often determined by the genetic make-up of the tumour’s dominant clones or the TME, while the latter has been proposed to be acquired as a result of Darwinian selection of minor genetically resistant pre-existing clones or transcriptional Lamarckian adaptation followed by genetic fixation of a trait104.
As an example, primary genetic resistance was probed in zebrafish by rapid combinatorial transgenic modelling of spred1 inactivation in the context of BRAF- or KIT-driven melanoma12. Treatment of adult transgenic zebrafish by submersion with targeted therapies for patients with mucosal melanoma showed this prevalent loss-of-function genetic event drives resistance to drugs inhibiting KIT tyrosine kinase activity12 (FIG. 3). Mechanistic studies further suggested the potential efficacy of combinations of MEK and KIT inhibitors12. Given the short duration of drug exposure required to identify primary resistance, both larval and adult zPDXs are well-suited models to address this question13. Larval zPDXs developed from tumour biopsy samples, much like tumour organoids105, might be particularly useful in the neoadjuvant setting before surgery to help rapidly pick an effective chemotherapy regimen.
On the other hand, such a short-term larval transplant assay with a limited number of cells per recipient is unlikely to be a good predictor of secondary resistance. This was evident in the work of Fior et al.13 mimicking a polyclonal tumour, where two isogenic cell lines (HCT116 cells with mutant KRAS and Hke3 cells with wild-type KRAS) were mixed 1:1 before transplantation, each labelled with a different fluorescent colour. While exposure of the polyclonal tumour to the folinic acid–5-fluorouracil–oxaliplatin (FOLFOX) chemotherapy regimen caused the tumour to shrink overall, the Hke3 resistant clone did not change in size13. Thus, without the differential colour labeling, an actual tumour would score overall as sensitive even if a very sizeable subclone failed to respond106. It is reasonable to speculate that the longer time window and the larger number of cells of adult zPDXs would better position these models to predict secondary resistance mechanisms based on expansion of a minor genetically resistant clone or allow the time required for transcriptional adaptation. Overall, the ability of these avatars to accurately model and predict resistance will be crucial for their translation to the clinic.
Comparison between cancer avatars
Zebrafish and mouse transgenic models
Different cancer avatars have different advantages and limitations, both biologically and logistically, that render their use more suitable to either preclinical drug development or personalized drug testing. In the case of transgenic cancer models in general, the key factors limiting their application in precision oncology are the time required to generate the avatar and the scale of combinatorial genetics to approximate genomic cancer complexity. For a comparison of zebrafish, mouse and organoid cancer avatar models, see TABLE 1.
Owing to recent advances in cloning and DNA synthesis methods, zebrafish transgenesis vectors can be generated in as little as 1–2 weeks. The time required to establish a transgenic mosaic tumour model in zebrafish ranges from 10 days to 8 weeks in early-onset models such as human KRASG12D-driven rhabdomyosarcoma36, mouse Myc-driven T-ALL107, NRASQ61K-driven melanoma12,31 or melanoma resulting from localized skin electroporation of a CRISPR vector targeting rb1 (which encodes retinoblastoma 1) in adult BRAFV600E;tp53−/− zebrafish43. Other transgenic models may take many months to develop, such as sarcoma models (for example, rhabdomyosarcoma driven by the PAX3–FOXO1 translocation under the control of the CMV promoter has 16% penetrance at 19 months)35 (FIG. 2). To model genomic complexity, combination of three to four genomic events has been successfully achieved, with a possibility to further scale up to ~10–20 alterations depending on limitations of pooled vector transgenesis approaches12,18. Strategies for expressing arrays of gRNAs from a single vector might further expand this limit for CRISPR knockout strategies108,109.
The speed and scalability of combinatorial genetics of zebrafish mosaic transgenic models far surpass those of most genetically engineered mouse models by avoiding the need to perform multigenerational crosses110. The small number of mouse models that leverage in vivo mosaic transgene delivery of plasmid DNA using hydrodynamic injection (liver cancer)111 or viral vectors (lung cancer)112 have similar capabilities to zebrafish models in terms of the time to tumour onset and combinatorial genetics. However, the challenges and cost of housing for mice, biosafety, vector production and handling pose a much greater barrier to the generation of a large enough number of animals for testing multiple therapeutic agents. In comparison, a single operator can perform a few thousand zebrafish embryo microinjections in a day to generate genetic patient avatars using only picograms to nanograms of plasmid DNA under biosafety level 1 conditions. Furthermore, the husbandry space requirements (12 adult zebrafish versus 1 adult mouse per ~1.2 l) and cost (of US cents per 3.5-l fish tank versus US dollars per mouse cage), while variable across countries and institutions, are at least an order of magnitude lower for zebrafish than for mice113.
Hydrodynamic injection.
rapid injection of a relatively large volume of DNA solution, which is used for gene delivery in mouse livers.
Mosaic transgenic zebrafish cancer models remain most useful today as tools for preclinical drug development of genotype-stratified targeted therapies, and for phenotypic dissection of genetic events identified in sequencing studies (for example, identification of genetic drivers of drug resistance, functional validation of a gene’s role or identification of novel combination treatments to overcome resistance). It is also important to note that both zebrafish and mouse transgenic models hae limited genetic heterogeneity, often do not recapitulate the sequential order of tumorigenic events seen in patients and have limited capabilities of modelling subclonal architecture12,34,43. Thus, modelling intra-tumour heterogeneity still presents major challenges and remains a barrier for these models to predict drug responses in patients.
PDXs and human organoids
Approaches using patient-derived tumour material for empirical drug testing are limited by several factors: (1) the ability to culture in vitro or successfully engraft in vivo patient-derived cancer cells in a recipient animal model; (2) the finite number of cells obtained from biopsy or surgical resection that can be used for in vitro culture or in vivo xenotransplantation; (3) the time between the biopsy and the readout of the assay; and (4) the degree of fidelity to which these models maintain representation of the tumour heterogeneity and clonal evolution within the patient, and also its microenvironment and co-evolution with the patient’s immune system.
With regard to the first point, xenotransplantation of patient-derived cancer cells into zebrafish has been reported to have a high degree of success in proof-of-concept studies, both in the successful establishment of zPDXs from a specific patient and in the proportion of recipient zebrafish showing engraftment from a specific donor. Fior et al.13 reported 100% donor engraftment in a small clinical study of patients with colorectal cancer (n = 5), with 47–89% of zebrafish larval recipients (n = 47–251) showing engraftment. Preliminary partial results of clinical study NCT03668418 (REF.114) conducted as a co-clinical trial involving 24 adult patients with pancreatic cancer (n = 12), colon cancer (n = 8) or gastric cancer (n = 4) similarly suggested engraftment of all donors, with comparable survival of recipient zebrafish larvae81. Other studies reported successful engraftment in larvae of PDXs from gastric cancer86, adenoid cystic carcinoma85 and pancreatic cancer87. Similarly, xenotransplantation in prkdc−/−,il2rga−/− recipient adult zebrafish at 37 °C of six PDXs from patients with melanoma (n = 2), breast cancer (n = 1), glioma (n = 1) or embryonal rhabdomyosarcoma (n = 2) resulted in engraftment of all six donors, with 35–80% of recipient animals showing engraftment (four to eight transplant recipients per donor patient)14.
Second, the number of cells used per recipient is both a limitation and an advantage. Patient material is a finite and often non-renewable resource, especially if it comes from a biopsy or the excision of a small tumour. Thus, organoids or zebrafish transplantation in larvae is advantageous since it requires only ~100 cells per well or recipient. Thus, even small quantities of patient-derived cells can be used effectively to test many conditions, drugs, dosing regimens or drug combinations. Xenotransplantation in adult zebrafish or mice, on the other hand, requires 105 or 105–106 cells, respectively5,14.
Third, organoid-based drug assays require 1–2 weeks105,115–117, zPDXs in larvae require 4–7 days13 and adult zebrafish and mouse PDXs require many weeks or even months to read out. On the other hand, the higher number of cells and the longer time window in adult zebrafish and mouse xenografts allows us to observe and perhaps model the effects of clonal evolution and adaptation, which has implications for drug resistance prediction discussed further below.
Some limitations inherent to transplantation-based avatars are common between mouse and zebrafish PDXs. Mouse studies highlight how the engraftment bottlenecks and evolution in a recipient animal might alter patient representation or result in evolution patterns that are discordant between patient and mouse avatars118. Krivtsov and colleagues119 reported a 50% clonal discordance in mouse PDXs of leukaemia when analysing genetic driver allele frequency, and Golub and colleagues120 showed mouse-specific tumour evolution of a large number of PDXs across 24 cancer types. On the other hand, human organoids seem to maintain genetic representation of the patient source material during short-term in vitro culture115. Large-scale comparative studies will be needed to determine the degree to which genetic and transcriptional heterogeneity is preserved in adult zPDX avatars over time.
While clinical prediction studies have been limited in frequency and scale due to the young age of the field, the results of several proof-of-concept studies have been encouraging. Fior et al.13 tested the response to FOLFOX chemotherapy and cetuximab using five colorectal cancer PDXs in zebrafish larvae. Concordant results were obtained between zPDXs and actual patient clinical responses for FOLFOX (four of five patients) and cetuximab (three of three patients)13. While these numbers are obviously small, for comparison, the largest prospective clinical study to date on the predictive value of human organoids for treatment response enrolled 61 patients with colorectal cancer and reported a 63% success rate in establishing in vitro cultures from patient tissue, with only 29 of 61 patients (~47%) with an evaluable response and an 80% prediction rate121. This study thus highlights both the promise and also the challenges of phenotypic testing of drug response even in technologies more mature than zebrafish avatars. In addition to the intrinsic heterogeneity of cancer cells, an ever-growing body of evidence has shown the importance of other cell types present in the local TME in modulating therapy response122,123. Much like mouse PDXs, the contribution of local TME components in zebrafish xenotransplantation is limited, and host-systemic effects such as immune co-evolution and systemic endocrine conditioning are not captured by transplantation-based zebrafish avatars (TABLE 1). This structural constraint of all transplant-based but more broadly patient-derived tumour models limits the predictive power of all of these assays in predicting drug sensitivity.
Concluding remarks
Recent advances showing organoid fidelity in mimicking clinical tumour behaviours105,115–117 and the first report of a prospective clinical trial for organoids as predictors of patient outcome121 provided a proof of concept for the clinical value of phenotypic testing of drug response at large. Yet, the very same prospective study121 highlighted how narrow the predictive power of these in vitro tools still is. Similarly, since they were proposed in 2011, mouse co-clinical trials using PDXs have shown little progress beyond proof of concept due to logistical challenges5,124,125. Zebrafish cancer avatars offer unique advantages over current in vivo models in scale, cost and speed of model development and potential for automation.
In terms of personalized transgenic avatars, the time barriers (1–4 weeks for sequencing, data analysis and vector generation, plus weeks to months of tumour latency time) limit their benefit to inform clinical decision-making. Thus, they remain most useful as preclinical models to unravel cancer genetics and for drug discovery. Continued reductions in the cost and increases in the speed and adoption of both DNA sequencing and synthesis, together with improvements in genome engineering technologies, should allow the generation of ever-faster and more complex transgenic zebrafish models for profiling likely therapeutic responses of individual patients. These studies should benefit precision cancer medicine both by directly affecting the specific patients modelled and also by increasing our understanding of cancer genomics more generally. By functionally assessing the effect of specific mutations on drug response modulation, they should also increase our ability to formulate response predictions based on inferential models analysing the alterations observed in tumour biopsy samples from various types of omics profiling.
On the other hand, now that the technology for zebrafish xenograft cell transplantation has matured, and robust proof-of-concept studies have been provided for the generation of both larval and adult zPDXs, it is paramount for the field to move towards clinical deployment and to conduct clinical studies to assess their predictive power and value in informing clinical decisions. A first ongoing trial (NCT03668418)114 aiming to enrol 120 patients with cancer to assess the predictive power of larval zPDXs is a good example in this direction and will provide useful insights into the value of larval zPDXs overall. It is still unclear whether zPDXs in larvae can provide significant advantages compared with human organoids, and head-to-head studies will be needed to address this question. These larval models could be of value in clinical settings where speed and short-term responses are the goal, such as presurgery neoadjuvant chemotherapy. Beyond drug response prediction, assessment of metastatic behaviour has been proposed using zebrafish PDXs in larvae13, but further studies are needed to assess whether migration in the developing embryo is informative of metastatic risk or organ tropism of human cancer. Evidence of substantial biological and predictive equivalency between mouse PDXs and adult zPDXs, combined with the lower cost and husbandry logistic footprint, and higher throughput of drug screening of the zebrafish model would render it an attractive alternative for precision cancer therapy (TABLE 1). Ultimately cost–benefit analyses will guide which players in the phenotypic drug-testing field will move forward in the clinic. A high predictive power will be needed as motivation for the cost-intensive establishment and operation of dedicated facilities necessary for any avatar model. The potential clinical benefit to patients in terms of survival outcome and spared toxic effects, and the potential cost saving from avoiding wasteful and dangerous administration of ineffective treatments, are an unmet need that the further development of personalized cancer avatars strives to address.
Acknowledgements
L.I.Z. is supported by the Melanoma Research Alliance and US National Institutes of Health (grants P01CA163222 and R01 CA103846). M.F. was supported by Boehringer Ingelheim Fonds. D.M.L. is funded by the US National Institutes of Health (grants R01CA154923, R01CA211734, R01CA215118, R01CA226926 and R24OD016761) and the MGH Research Scholars Program. Y.C. is supported by the Alex Lemonade Stand Foundation.
Footnotes
Competing interests
L.I.Z. is a founder and stockholder of Fate Therapeutics Inc., Scholar Rock and Camp4 Therapeutics Inc., and is a scientific adviser for Stemgent. The other authors declare no competing interests.
Peer review information
Nature Reviews Cancer thanks M. G. Ferreira, Z. Gong, R. Stewart and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
References
- 1.National Cancer Institute. Targeted cancer therapies. NCI https://www.cancer.gov/about-cancer/treatment/types/targeted-therapies/targeted-therapies-fact-sheet (2020).
- 2.Morrison C Fresh from the biotech pipeline — 2018. Nat. Biotechnol 37, 118–123 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Friedman AA, Letai A, Fisher DE & Flaherty KT Precision medicine for cancer with next-generation functional diagnostics. Nat. Rev. Cancer 15, 747–756 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hyman DM, Taylor BS & Baselga J Implementing genome-driven oncology. Cell 168, 584–599 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clohessy JG & Pandolfi PP Mouse hospital and co-clinical trial project — from bench to bedside. Nat. Rev. Clin. Oncol 12, 491 (2015). [DOI] [PubMed] [Google Scholar]; This review discusses mouse co-clinical trials for precision cancer therapy.
- 6.Drost J & Clevers H Organoids in cancer research. Nat. Rev. Cancer 18, 407–418 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Tuveson D & Clevers H Cancer modeling meets human organoid technology. Science 364, 952–955 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Schwartzberg L, Kim ES, Liu D & Schrag D Precision oncology: who, how, what, when, and when not? Am. Soc. Clin. Oncol. Educ. Book 37, 160–169 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Strauss DG & Blinova K Clinical trials in a dish. Trends Pharmacol. Sci 38, 4–7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aparicio S, Hidalgo M & Kung AL Examining the utility of patient-derived xenograft mouse models. Nat. Rev. Cancer 15, 311 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Tentler JJ et al. Patient-derived tumour xenografts as models for oncology drug development. Nat. Rev. Clin. Oncol 9, 338 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ablain J et al. Human tumor genomics and zebrafish modeling identify SPRED1 loss as a driver of mucosal melanoma. Science 362, 1055–1060 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; This article shows rapid generation of genetic avatars in zebrafish using combinatorial mosaic transgenesis.
- 13.Fior R et al. Single-cell functional and chemosensitive profiling of combinatorial colorectal therapy in zebrafish xenografts. Proc. Natl Acad. Sci. USA 114, E8234–E8243 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study conducts phenotypic drug response testing and migration scoring of PDXs in zebrafish larvae.
- 14.Yan C et al. Visualizing engrafted human cancer and therapy responses in immunodeficient zebrafish. Cell 177, 1903–1914 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; This article demonstrates establishment of PDXs in adult zebrafish.
- 15.White R, Rose K & Zon L Zebrafish cancer: the state of the art and the path forward. Nat. Rev. Cancer 13, 624–636 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pulak R Tools for automating the imaging of zebrafish larvae. Methods 96, 118–126 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Zhao Y et al. A review of automated microinjection of zebrafish embryos. Micromachines 10, 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ablain J, Durand EM, Yang S, Zhou Y & Zon LI A CRISPR/Cas9 vector system for tissue-specific gene disruption in zebrafish. Dev. Cell 32, 756–764 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gengenbacher N, Singhal M & Augustin HG Preclinical mouse solid tumour models: status quo, challenges and perspectives. Nat. Rev. Cancer 17, 751 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Howe K et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 496, 498–503 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carneiro MC, de Castro IP & Ferreira MG Telomeres in aging and disease: lessons from zebrafish. Dis. Model. Mech 9, 737–748 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berghmans S et al. Making waves in cancer research: new models in the zebrafish. Biotechniques 39, 227–237 (2005). [DOI] [PubMed] [Google Scholar]
- 23.Langenau DM et al. Myc-induced T cell leukemia in transgenic zebrafish. Science 299, 887–890 (2003). [DOI] [PubMed] [Google Scholar]; This article reports the first transgenic cancer model in zebrafish.
- 24.Yang HW et al. Targeted expression of human MYCN selectively causes pancreatic neuroendocrine tumors in transgenic zebrafish. Cancer Res. 64, 7256–7262 (2004). [DOI] [PubMed] [Google Scholar]
- 25.Patton EE et al. BRAF mutations are sufficient to promote nevi formation and cooperate with p53 in the genesis of melanoma. Curr. Biol 15, 249–254 (2005). [DOI] [PubMed] [Google Scholar]
- 26.Cagan RL, Zon LI & White RM Modeling cancer with flies and fish. Dev. Cell 49, 317–324 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirchberger S, Sturtzel C, Pascoal S & Distel M Quo natas, Danio? — Recent progress in modeling cancer in zebrafish. Front. Oncol 7, 186 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Letrado P, de Miguel I, Lamberto I, Diez-Martinez R & Oyarzabal J Zebrafish: speeding up the cancer drug discovery process. Cancer Res. 78, 6048–6058 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Vogelstein B et al. Cancer genome landscapes. Science 339, 1546–1558 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vogelstein B & Kinzler KW The path to cancer – three strikes and you’re out. N. Engl. J. Med 373, 1895–1898 (2015). [DOI] [PubMed] [Google Scholar]
- 31.McConnell AM et al. Neural crest state activation in NRAS driven melanoma, but not in NRAS-driven melanocyte expansion. Dev. Biol 449, 107–114 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pea A, Hruban RH & Wood LD Genetics of pancreatic neuroendocrine tumors: implications for the clinic. Expert Rev. Gastroenterol. Hepatol 9, 1407–1419 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berghmans S et al. tp53 mutant zebrafish develop malignant peripheral nerve sheath tumors. Proc. Natl Acad. Sci. USA 102, 407–412 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ceol CJ et al. The histone methyltransferase SETDB1 is recurrently amplified in melanoma and accelerates its onset. Nature 471, 513–517 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kendall GC et al. PAX3- FOXO1 transgenic zebrafish models identify HES3 as a mediator of rhabdomyosarcoma tumorigenesis. eLife 7, e33800 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Langenau DM et al. Effects of RAS on the genesis of embryonal rhabdomyosarcoma. Genes Dev. 21, 1382–1395 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanchez-Rivera FJ & Jacks T Applications of the CRISPR-Cas9 system in cancer biology. Nat. Rev. Cancer 15, 387–395 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hwang WY et al. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat. Biotechnol 31, 227–229 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reports the first use of CRISPR in zebrafish.
- 39.Kawakami K et al. A transposon-mediated gene trap approach identifies developmentally regulated genes in zebrafish. Dev. Cell 7, 133–144 (2004). [DOI] [PubMed] [Google Scholar]
- 40.Heppt MV et al. Prognostic significance of BRAF and NRAS mutations in melanoma: a German study from routine care. BMC Cancer 17, 536 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shain AH et al. The genetic evolution of melanoma from precursor lesions. N. Engl. J. Med 373, 1926–1936 (2015). [DOI] [PubMed] [Google Scholar]
- 42.Burns MA et al. Hedgehog pathway mutations drive oncogenic transformation in high-risk T-cell acute lymphoblastic leukemia. Leukemia 32, 2126–2137 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Callahan SJ et al. Cancer modeling by transgene electroporation in adult zebrafish (TEAZ). Dis. Model. Mech 11, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; This article shows mosaic transgenesis in adult zebrafish through electroporation of plasmid DNA.
- 44.White RM et al. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell 2, 183–189 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]; This article reports development of the optically clear casper zebrafish strain often used for transplantation studies.
- 45.Weintraub A All eyes on zebrafish. Lab. Anim 46, 323–326 (2017). [DOI] [PubMed] [Google Scholar]
- 46.Smith AC et al. High-throughput cell transplantation establishes that tumor-initiating cells are abundant in zebrafish T-cell acute lymphoblastic leukemia. Blood 115, 3296–3303 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blackburn JS et al. Clonal evolution enhances leukemia-propagating cell frequency in T cell acute lymphoblastic leukemia through Akt/mTORC1 pathway activation. Cancer Cell 25, 366–378 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang Q et al. Optimized cell transplantation using adult rag2 mutant zebrafish. Nat. Methods 11, 821–824 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; This article reports allotransplantation of zebrafish tumours in immunocompromised recipients.
- 49.Tang Q et al. Imaging tumour cell heterogeneity following cell transplantation into optically clear immune-deficient zebrafish. Nat. Commun 7, 10358 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hayes MN et al. Vangl2/RhoA signaling pathway regulates stem cell self-renewal programs and growth in rhabdomyosarcoma. Cell Stem Cell 22, 414–427.e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ignatius MS et al. The NOTCH1/SNAIL1/MEF2C pathway regulates growth and self-renewal in embryonal rhabdomyosarcoma. Cell Rep. 19, 2304–2318 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moore JC et al. Single-cell imaging of normal and malignant cell engraftment into optically clear prkdc-null SCID zebrafish. J. Exp. Med 213, 2575–2589 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tenente IM et al. Myogenic regulatory transcription factors regulate growth in rhabdomyosarcoma. eLife 6, e19214 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li P et al. Epoxyeicosatrienoic acids enhance embryonic haematopoiesis and adult marrow engraftment. Nature 523, 468–471 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tamplin OJ et al. Hematopoietic stem cell arrival triggers dynamic remodeling of the perivascular niche. Cell 160, 241–252 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heilmann S et al. A quantitative system for studying metastasis using transparent zebrafish. Cancer Res. 75, 4272–4282 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang M et al. Adipocyte-derived lipids mediate melanoma progression via FATP proteins. Cancer Discov. 8, 1006–1025 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nicoli S, Ribatti D, Cotelli F & Presta M Mammalian tumor xenografts induce neovascularization in zebrafish embryos. Cancer Res. 67, 2927–2931 (2007). [DOI] [PubMed] [Google Scholar]
- 59.Haldi M, Ton C, Seng WL & McGrath P Human melanoma cells transplanted into zebrafish proliferate, migrate, produce melanin, form masses and stimulate angiogenesis in zebrafish. Angiogenesis 9, 139–151 (2006). [DOI] [PubMed] [Google Scholar]
- 60.Topczewska JM et al. Embryonic and tumorigenic pathways converge via Nodal signaling: role in melanoma aggressiveness. Nat. Med 12, 925–932 (2006). [DOI] [PubMed] [Google Scholar]
- 61.Mercatali L et al. Development of a patient-derived xenograft (PDX) of breast cancer bone metastasis in a zebrafish model. Int. J. Mol. Sci 17, 1375 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bentley VL et al. Focused chemical genomics using zebrafish xenotransplantation as a pre-clinical therapeutic platform for T-cell acute lymphoblastic leukemia. Haematologica 100, 70–76 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lin J et al. A clinically relevant in vivo zebrafish model of human multiple myeloma to study preclinical therapeutic efficacy. Blood 128, 249–252 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chapman A et al. Heterogeneous tumor subpopulations cooperate to drive invasion. Cell Rep. 8, 688–695 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Renshaw SA & Trede NS A model 450 million years in the making: zebrafish and vertebrate immunity. Dis. Model. Mech 5, 38–47 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Langenau DM et al. In vivo tracking of T cell development, ablation, and engraftment in transgenic zebrafish. Proc. Natl Acad. Sci. USA 101, 7369–7374 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tang Q et al. Dissecting hematopoietic and renal cell heterogeneity in adult zebrafish at single-cell resolution using RNA sequencing. J. Exp. Med 214, 2875–2887 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Trede NS, Langenau DM, Traver D, Look AT & Zon LI The use of zebrafish to understand immunity. Immunity 20, 367–379 (2004). [DOI] [PubMed] [Google Scholar]
- 69.Willett CE, Cortes A, Zuasti A & Zapata AG Early hematopoiesis and developing lymphoid organs in the zebrafish. Dev. Dyn 214, 323–336 (1999). [DOI] [PubMed] [Google Scholar]
- 70.Konantz M et al. Zebrafish xenografts as a tool for in vivo studies on human cancer. Ann. NY Acad. Sci 1266, 124–137 (2012). [DOI] [PubMed] [Google Scholar]
- 71.He S et al. Neutrophil-mediated experimental metastasis is enhanced by VEGFR inhibition in a zebrafish xenograft model. J. Pathol 227, 431–445 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Visvader JE & Lindeman GJ Cancer stem cells: current status and evolving complexities. Cell Stem Cell 10, 717–728 (2012). [DOI] [PubMed] [Google Scholar]
- 73.Stoletov K, Montel V, Lester RD, Gonias SL & Klemke R High-resolution imaging of the dynamic tumor cell vascular interface in transparent zebrafish. Proc. Natl Acad. Sci. USA 104, 17406–17411 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Traver D et al. Transplantation and in vivo imaging of multilineage engraftment in zebrafish bloodless mutants. Nat. Immunol 4, 1238–1246 (2003). [DOI] [PubMed] [Google Scholar]
- 75.Traver D et al. Effects of lethal irradiation in zebrafish and rescue by hematopoietic cell transplantation. Blood 104, 1298–1305 (2004). [DOI] [PubMed] [Google Scholar]
- 76.Larsen EC et al. Dexamethasone and high-dose methotrexate improve outcome for children and young adults with high-risk B-acute lymphoblastic leukemia: a report from Children’s Oncology Group Study AALL0232. J. Clin. Oncol 34, 2380–2388 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Matsuda M et al. Human NK cell development in hIL-7 and hIL-15 knockin NOD/SCID/IL2rgKO mice. Life Sci. Alliance 2, 201800195 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Herndler-Brandstetter D et al. Humanized mouse model supports development, function, and tissue residency of human natural killer cells. Proc. Natl Acad. Sci. USA 114, E9626–E9634 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This article reports engineering of NSG mice towards humanized models and more robust PDX support.
- 79.Dang M, Henderson RE, Garraway LA & Zon LI Long-term drug administration in the adult zebrafish using oral gavage for cancer preclinical studies. Dis. Model. Mech 9, 811–820 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study administers a drug in adult zebrafish through intraperitoneal injection and oral gavage.
- 80.Samaee SM, Seyedin S & Varga ZM An affordable intraperitoneal injection setup for juvenile and adult zebrafish. Zebrafish 14, 77–79 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Usai A et al. A model of zebrafish avatar for co-clinical trials. Cancers 12, 677 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; This article reports preliminary results of zebrafish co-clinical trial NCT03668418.
- 82.Eisenhauer EA et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009). [DOI] [PubMed] [Google Scholar]
- 83.Goessling W, North TE & Zon LI Ultrasound biomicroscopy permits in vivo characterization of zebrafish liver tumors. Nat. Methods 4, 551–553 (2007). [DOI] [PubMed] [Google Scholar]
- 84.Jin Y et al. Comparison of efficacy and toxicity of bevacizumab, endostar and apatinib in transgenic and human lung cancer xenograft zebrafish model. Sci. Rep 8, 15837 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen C et al. A multiplex preclinical model for adenoid cystic carcinoma of the salivary gland identifies regorafenib as a potential therapeutic drug. Sci. Rep 7, 11410 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wu JQ et al. Patient-derived xenograft in zebrafish embryos: a new platform for translational research in gastric cancer. J. Exp. Clin. Cancer Res 36, 160 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang L et al. Patient-derived heterogeneous xenograft model of pancreatic cancer using zebrafish larvae as hosts for comparative drug assessment. J. Vis. Exp 146, e59507 (2019). [DOI] [PubMed] [Google Scholar]; This is a method guide for the drug testing of zPDXs in larvae.
- 88.Ikonomopoulou MP et al. Gomesin inhibits melanoma growth by manipulating key signaling cascades that control cell death and proliferation. Sci. Rep 8, 11519 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang G et al. The novel autophagy inhibitor elaiophylin exerts antitumor activity against multiple myeloma with mutant TP53 in part through endoplasmic reticulum stress-induced apoptosis. Cancer Biol. Ther 18, 584–595 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.von Massenhausen A et al. Targeting DDR2 in head and neck squamous cell carcinoma with dasatinib. Int. J. Cancer 139, 2359–2369 (2016). [DOI] [PubMed] [Google Scholar]
- 91.Ochoa-Alvarez JA et al. Antibody and lectin target podoplanin to inhibit oral squamous carcinoma cell migration and viability by distinct mechanisms. Oncotarget 6, 9045–9060 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ghotra VP et al. SYK is a candidate kinase target for the treatment of advanced prostate cancer. Cancer Res. 75, 230–240 (2015). [DOI] [PubMed] [Google Scholar]
- 93.van der Ent W et al. Ewing sarcoma inhibition by disruption of EWSR1-FLI1 transcriptional activity and reactivation of p53. J. Pathol 233, 415–424 (2014). [DOI] [PubMed] [Google Scholar]
- 94.Tan DS et al. Bosutinib inhibits migration and invasion via ACK1 in KRAS mutant non-small cell lung cancer. Mol. Cancer 13, 13 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01858168 (2013). [DOI] [PubMed]
- 96.Liu PH et al. An IRAK1-PIN1 signalling axis drives intrinsic tumour resistance to radiation therapy. Nat. Cell Biol 21, 203–213 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Smith MP et al. Effect of SMURF2 targeting on susceptibility to MEK inhibitors in melanoma. J. Natl Cancer Inst 105, 33–46 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yoganantharjah P & Gibert Y The use of the zebrafish model to aid in drug discovery and target validation. Curr. Top. Med. Chem 17, 2041–2055 (2017). [DOI] [PubMed] [Google Scholar]
- 99.Sarmah S & Marrs JA Zebrafish as a vertebrate model system to evaluate effects of environmental toxicants on cardiac development and function. Int. J. Mol. Sci 17, 2123 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chakraborty C, Sharma AR, Sharma G & Lee SS Zebrafish: a complete animal model to enumerate the nanoparticle toxicity. J. Nanobiotech 14, 65 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kanungo J, Cuevas E, Ali SF & Paule MG Zebrafish model in drug safety assessment. Curr. Pharm. Des 20, 5416–5429 (2014). [DOI] [PubMed] [Google Scholar]
- 102.Chakravarthy S, Sadagopan S, Nair A & Sukumaran SK Zebrafish as an in vivo high-throughput model for genotoxicity. Zebrafish 11, 154–166 (2014). [DOI] [PubMed] [Google Scholar]
- 103.Peterson RT & Macrae CA Systematic approaches to toxicology in the zebrafish. Annu. Rev. Pharmacol. Toxicol 52, 433–453 (2012). [DOI] [PubMed] [Google Scholar]
- 104.Boumahdi S & de Sauvage FJ The great escape: tumour cell plasticity in resistance to targeted therapy. Nat. Rev. Drug Discov 19, 39–56 (2020). [DOI] [PubMed] [Google Scholar]
- 105.Yao Y et al. Patient-derived organoids predict chemoradiation responses of locally advanced rectal cancer. Cell Stem Cell 26, 17–26.e6 (2020). [DOI] [PubMed] [Google Scholar]; This article reports the use of organoids to assess drug response in the neoadjuvant setting.
- 106.Fazio M & Zon LI Fishing for answers in precision cancer medicine. Proc. Natl Acad. Sci. USA 114, 10306–10308 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Baeten JT & de Jong JLO Genetic models of leukemia in zebrafish. Front. Cell Dev. Biol 6, 115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kurata M et al. Highly multiplexed genome engineering using CRISPR/Cas9 gRNA arrays. PLoS one 13, e0198714 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang Y et al. A gRNA-tRNA array for CRISPR-Cas9 based rapid multiplexed genome editing in Saccharomyces cerevisiae. Nat. Commun 10, 1053 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kuzu OF, Nguyen FD, Noory MA & Sharma A Current state of animal (mouse) modeling in melanoma research. Cancer Growth Metastasis 8 (Suppl. 1), 81–94 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xue W et al. CRISPR-mediated direct mutation of cancer genes in the mouse liver. Nature 514, 380–384 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sanchez-Rivera FJ et al. Rapid modelling of cooperating genetic events in cancer through somatic genome editing. Nature 516, 428–431 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; This article reports mosaic transgenesis in mouse cancer models.
- 113.National Research Council. Guide for the Care and Use of Laboratory Animals 8th edn (National Academies Press, 2011) [PubMed] [Google Scholar]
- 114.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03668418 (2018). [DOI] [PubMed] [Google Scholar]
- 115.Vlachogiannis G et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science 359, 920–926 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tiriac H et al. Organoid profiling identifies common responders to chemotherapy in pancreatic cancer. Cancer Discov. 8, 1112–1129 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ganesh K et al. A rectal cancer organoid platform to study individual responses to chemoradiation. Nat. Med 25, 1607–1614 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ben-David U, Beroukhim R & Golub TR Genomic evolution of cancer models: perils and opportunities. Nat. Rev. Cancer 19, 97–109 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang K et al. Patient-derived xenotransplants can recapitulate the genetic driver landscape of acute leukemias. Leukemia 31, 151–158 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ben-David U et al. Patient-derived xenografts undergo mouse-specific tumor evolution. Nat. Genet 49, 1567–1575 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This article shows the evolution of mouse PDXs compared with donor patients.
- 121.Ooft SN et al. Patient-derived organoids can predict response to chemotherapy in metastatic colorectal cancer patients. Sci. Transl Med 11, eaay2574 (2019). [DOI] [PubMed] [Google Scholar]
- 122.Hirata E & Sahai E Tumor microenvironment and differential responses to therapy. Cold Spring Harb. Perspect. Med 10.1101/cshperspect.a026781 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Binnewies M et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med 24, 541–550 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vargas R et al. Case study: patient-derived clear cell adenocarcinoma xenograft model longitudinally predicts treatment response. NPJ Precis. Oncol 2, 14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nardella C, Lunardi A, Patnaik A, Cantley LC & Pandolfi PP The APL paradigm and the ‘co-clinical trial’ project. Cancer Discov. 1, 108–116 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]


