Abstract
To better explore the underlying mechanism of liver metastatic formation by placenta-specific protein 1 (PLAC1) in human colorectal cancer, we investigated the proliferation, invasion and angiogenic capabilities of human colorectal cancer cells with different liver metastatic potentials as well as the mechanism of action of PLAC1 in the metastatic process. The expression of PLAC1 was detected by reverse transcriptase PCR, western blot, and real-time PCR. The effect of PLAC1 on metastatic potential was determined by proliferation, invasion, and angiogenesis assays, including an in-vitro coculture system consisting of cancer cells and vascular endothelial cells that were used to detect the relationship between cancer cells and angiogenesis. In addition, we also determined PLAC1 downstream targets that preferentially contribute to the metastatic process. PLAC1 was expressed in HT-29, WiDr, and CaCo-2 colorectal cancer cells but not in Colo320 colorectal cancer cells. PLAC1 not only enhanced significantly the proliferation of CoLo320 and human umbilical vein endothelial cells (HUVECs) but also promoted the invasion of CoLo320 cells. The angiogenesis of HUVECs was enhanced by PLAC1 in a dose-dependent manner. In cocultured systems, angiogenesis was significantly increased by coculture with HT-29 cells. In addition, PLAC1 could promote angiogenesis in coculture with HT-29 cells. Furthermore, PLAC1-enhanced metastatic potential of colorectal cancer cells was dependent on the activation of the PI3K/Akt/NF-κB pathway. The activation of PI3K/Akt/NF-κB signaling by PLAC1 may be critical for metastasis of colorectal cancer cells. According to our results, we suggest that modification of PLAC1 function might be a promising new therapeutic approach to inhibit the aggressive spread of colorectal cancer.
Keywords: angiogenesis, colorectal cancer, placenta-specific 1, proliferation
Introduction
Colorectal cancer is one of the most common malignant tumors that seriously threaten human health. There are almost 1.36 million new colorectal cancer cases, and it causes more than a million patient deaths annually worldwide. It has the third highest incidence of malignant tumors in the world, ranking third in male patients and second in female patients, and it also ranks as the second leading cause of cancer-related death after lung cancer (Siegel RL et al., 2019). The present treatments for colorectal cancer are radical surgical operation, chemotherapy, radiotherapy, immunotherapy, and targeted therapy. However, many patients with colorectal cancer are diagnosed at an advanced stage, when surgical intervention is no longer effective to treat this disease. At least 40% of patients with colorectal cancer develop metastases (Ma et al., 2018), and there are no highly effective approaches against disseminated colorectal cancer. Therefore, new, nonsurgical therapeutic strategies are urgently needed for the treatment of advanced or metastatic colorectal cancer. There have not been highly effective approaches against metastasis of colorectal cancer so far. Recently, research on the microenvironment of solid tumors has shown that chemokines and their receptors have key functions in cancer metastatic processes, and chemokines play specific roles in the regulation of angiogenesis, the activation of a tumor-specific immune response and the induction of tumor cell proliferation in an autocrine or paracrine fashion (Ning et al., 2011; Ma et al., 2017a; Ma et al., 2017b).
Placenta-specific protein 1, encoded by PLAC1 gene, is a recently discovered placental antigen with limited normal tissue expression and fundamental roles in placental function and development (Fant et al., 2014). During embryo implantation, the invasion of trophocytes into the endometrium and the formation of blood vessels are very similar to the growth, invasion, and migration of tumors (Chang et al., 2014). Recently, increasing evidence has revealed that PLAC1 expression is activated in a variety of human cancers, including gastric, nonsmall-cell lung, liver and colorectal, and epithelial ovarian and breast cancer, as well as primary colorectal adenocarcinoma (Tchabo et al., 2009; Koslowski et al., 2009; Ghods et al., 2014; Liu et al., 2015). In addition, increased expression of PLAC1 was found to be positively correlated with the degree of tumor invasion, lymph node metastasis, and distant metastasis (Koslowski et al., 2007).
Recent studies showed that PLAC1 is expressed at high levels on the surface of trophoblast cells in the placenta and at low levels in the testis but is otherwise absent in normal somatic tissues. PLAC1 is expressed in human fetal tissues, and circulating PLAC1 mRNA increases during pregnancy (Concu et al., 2005; Devor et al., 2013) and as a result of pre-eclampsia, fetal injury and implantation failure (Matteo et al., 2013). Many genes normally expressed in the embryo become reactivated in cancer cells, and PLAC1 was the first such cancer/testis gene that related placentation to cancer. PLAC1 RNA was expressed in human cancer cell lines covering 17 different malignancies, including colorectal cancer (Silva et al., 2007). PLAC1 expression detected by immunohistonchemistry was upregulated in other cancers, such as serous endometrial adenocarcinoma and late-stage colon and liver cancer (Dong et al., 2008; Liu et al., 2014). There is a growing body of evidence showing that PLAC1 is frequently activated in a wide variety of cancer types and promotes cancer progression (Geiger et al., 2009). Overall, these data suggest that PLAC1 may have diagnostic value as a tumor-selective biomarker in colon cancer and other malignancies.
The aim of the present study was to investigate the effect of PLAC1 on metastatic potential and the underlying mechanism in colorectal cancer cells and elucidate the mechanism of PLAC1 in metastasis and in the interactions between colorectal cancer cells and stromal cells in the tumor microenvironment. Understanding the biological mechanisms responsible for the regulation of PLAC1 may enable better molecularly targeted therapies for the treatment of patients with metastatic colorectal cancer. PLAC1 may be regarded as a potential cancer-testis-placenta antigen therapeutic target for treating patients with metastatic colorectal cancer. Furthermore, our study will provide data strongly suggesting that the phosphatidylinositol PI3K/Akt/NF-κB signaling pathway plays an important role in PLAC1 simulation and that this process is involved in the development and metastasis of colorectal cancer.
Materials and methods
Reagents and antibody
Recombinant human PLAC1 and anti-human PLAC1 antibodies were provided by R&D System Inc. (Minneapolis, Minnesota, USA). LY294002 (PI3K inhibitor) was ordered from Cell Signaling Technology (Beverly, Massachusetts, USA). The Akt inhibitor was purchased from BioVision (Mountain View, California, USA). The Akt, phospho-Akt (Ser473), PI3K p85, phospho-PI3K p85 (Tyr 458)/p55 (Tyr199), NF-κB p65, and phospho-NF-κB p65 (Ser536) antibodies were purchased from Cell Signaling Technology (Mountain View).
Cell culture
Human colorectal cancer cells (HT-29, WiDr, CaCo-2, and Colo320) were obtained from American Type Culture Collection (Rockville, Maryland, USA). HT-29 was incubated in McCoy’s medium containing 10% fetal bovine serum (FBS) (Gibco, Grand Island, New York, USA). WiDr and CaCo-2 cells were maintained in minimum essential medium Eagle with 10% FBS. Roswell Park Memorial Institute-1640 medium with 10% FBS was used to culture Colo320 cells. Human umbilical vein endothelial cells (HUVECs) were obtained from Kurabo Co., (Chuo-ku, Osaka, Japan) and incubated in HuMedia-EB2 medium containing 2% FBS, 5 ng/mL bFGF, 10 mg/mL heparin, 10 ng/mL epidermal growth factor, and 1 mg/mL hydrocortisone.
RT-PCR analysis
Total RNA from colorectal cancer cells was extracted by using an Isogen Kit, and the concentration of RNA was measured spectrophotometrically. Five microliters of total RNA was mixed with random hexamers and dNTP mix, incubated at 65°C for 5 min, chilled on ice, and then reverse-transcribed into cDNA using a cDNA Synthesis Mix, which included 10× RT buffer, 25 mM MgCl2, 0.1 MDTT, RNaseOUT, and 200 U SuperScript III RT (Invitrogen, San Diego, California, USA) at 50°C for 50 min. The reaction was discontinued at 85°C for 5 min. One microliter of each reaction mixture was used as a template for PCR. The following forward and reverse primers were used: PLAC1, 5′-TTCACCAGTGAGCACAA AGC-3′, and 5′-CCAGTCTATGG AGCACAGCA-3′. Amplification reactions were performed using a DNA Thermal Cycler.
Western blot analysis
Colorectal cancer cells and HUVECs (1 × 106/mL) were lysed in protein lysis buffer. Then, the protein concentrations were measured with a bicinchoninic acid protein assay kit (Pierce, Rockford, USA). The lysates (30 µg per lane) were separated by 10% SDS-PAGE and transferred to polyvinylidene membranes. The membranes were incubated in blocking buffer for 60 min at RT. The blocking buffer included 5% nonfat dry milk solubilized into Tris-buffered saline comprising 0.1% Tween 20 (TBS-T). After three 5 min washes with TBS-T, the cells were immunoblotted with each primary antibody, diluted 500- to 1000-fold by primary antibody dilution buffer, and incubated overnight at 4°C. Then, the membrane was washed with TBS-T three more times and subjected to incubation with the HRP-conjugated secondary antibody for 1 h at RT. The antibody complex was detected by using the ECL Western blotting observation and analysis system. The grayscale values of the strips were measured by Image J software. The relative expression level of the proteins was expressed as the ratio of the target protein to the internal reference protein.
Real-time quantitative RT-PCR
The qPCR was performed using a LightCycler apparatus. Freshly isolated RNA was converted to cDNA using the PrimeScriptTM TR Reagent kit (Takara Bio Inc., Shiga, Japan), and the PCR was performed using the TaqMan Gene Expression Assay Kit (Applied Biosystems, Foster City, California, USA). In brief, a mixture of 1 µL of total RNA and 1 µL of oligo dT primer was incubated at 37°C for 15 min and 85°C for 5 s for reverse transcription. The PCR was then performed in a 20 µL final volume containing the following: up to 20 µL of H2O, 10 µL of TaqMan Universal PCR Master Mix, No AmpErase UNG ordered separately, 1 µL of 20× TaqMan Gene Expression Assay Mix, and 9 µL of cDNA diluted in RNase-free water. After an initial incubation at 94°C for 15 s, temperature cycling was initiated with each cycle (a total of 45-50 cycles) as follows: denaturation at 95°C for 10 s, hybridization at 60°C for 30 s, and elongation at 72°C for 30 s. The fluorescence signal was acquired at the final step of hybridization. Melting curves were obtained with a temperature range of 65–95°C, read every 0.2°C, held for 5 s, and then incubated at 65°C for 1 min. Cycling conditions of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were consistent with the above steps. A standard curve for each run was generated from serial dilutions of cDNA of the HT-29 cell line. The mRNA expression level of PLAC1 was normalized to that of GAPDH and shown as the mean ± SD. Relative mRNA expression of PLAC1 was calculated using the following formula: A/G-A0/G0, in which A and G are the relative mRNA copy numbers of PLAC1 and GAPDH, while A0 and G0 are the relative mRNA levels of PLAC1 and GAPDH from the standard cDNA dilutions as control.
Proliferation assay
The colorectal cancer cells and HUVECs were planted at a density of 3 × 103 cells/100 µL into 96-well flat-bottomed plates and cultured overnight. The medium was exchanged, and the cells then cultured in the medium alone (control) or in the medium that included different concentrations of PLAC1 and anti-PLAC1 antibody. After 72 h of incubation, 10 µL of WST-1 reagent was added to each well, and the cells were incubated for another 4 h. Then, cell proliferation was measured by the WST-1 Cell Proliferation Assay System (Takara Bio Inc., Shiga, Japan). The absorbance was determined using a microplate reader at a test wavelength of 450 nm and a reference wavelength of 690 nm.
Invasion assay
The effect of PLAC1 on the invasive capability of human colorectal cancer cell lines and HUVECs was measured by using Matrigel-coated invasion chambers. The transwell chambers are separated by a polyethylene terephthalate membrane coated with Matrigel Matrix containing 8-μm pore-size polycarbonate membranes such that only invasive cells can migrate through the membrane to the reverse side. After rehydration f of the inserts at 37°C, cells were seeded at a density of 1 × 105 cells/well into the inner chambers of a cell culture insert and incubated at 37°C for 24 h with various concentrations of PLAC1. After 24 h of incubation, cells that did not pass through were removed from the upper surface of the membrane by scrubbing gently with cotton-tipped applicators. The cells that invaded the reverse side of the membrane were fixed with 70% ethanol, stained with Giemsa solution, and counted in five random fields of the low filter surface under a microscope at 200× magnification.
Angiogenesis assay
To explore the effect of PLAC1 on tubule formation by HUVECs, HUVECs and fibroblasts were co-incubated in basal medium using an angiogenesis kit according to the manufacturer’s protocols. First, HUVECs and fibroblasts were cocultured in 24-well plates with basal medium. The media were exchanged every two days with media containing various concentrations of PLAC1, with coincubation continuing for a total of 11 days. The coculturing system was stained with anti-CD31 antibody. The area of angiogenesis was measured quantitatively over 10 different microscope fields for each well using an image analyzer (Kurabo Co.).
Angiogenic activity during cocultivation with colorectal cancer cells
To further investigate the influence of different colon cancer cells (HT-29 secreting PLAC1 or Colo320 non-secreting PLAC1) on tubule formation by HUVEC. HT-29 or Colo320, HUVECs, and fibroblasts were cocultured adopting a double-chamber method. First, HUVEC/fibroblasts were cultured in a 24-well plate for 3 days, and then HT-29 or Colo320 cells (5 × 104 cells/mL) were incubated in trans-well chambers in which the bottom of chamber consisted of polycarbonate membrane with 0.45-μm pores, and then trans-well chambers were placed in HUVEC/fibroblast cultured 24-well plate. Approximately 100 ng/mL of PLAC1, or anti-PLAC1 Ab was added to the culture medium, and the medium was changed every two days. Cells were incubated for a total of 12 days, then the upper trans-wells were removed, and the 24-well plate chambers were washed with 100 mL of 1× PBS three times. Then the HUVEC tubular formation was stained with anti-CD31 antibody (R&D System Inc.) by the protocols of Manufacturer (Kurabo Co.), and the total area of tubular formation was measured as described above.
Statistical analysis
Using the t-test for paired observations or one-way ANOVA with a post hoc test (Dunnett’s multiple comparisons) for multiple group comparisons, comparisons between groups were made. Statistical significance was provided at P < 0.05. Data are presented as the mean ± SD. Each experiment was performed in triplicate.
Results
Expression of PLAC1 in colorectal cancer cell lines
Expression of PLAC1 mRNA was detected in HT-29, WiDr, and CaCo-2 cells but not in Colo320 colorectal cancer cells using RT-PCR (Fig. 1a). Similarly, by immunoblotting, HT-29, WiDr, and CaCo-2 colorectal cancer cell lines were analyzed for the expression of PLAC1 protein (Fig. 1b). We previously determined the liver-metastatic capability of human colorectal cancer cell lines by intrasplenic liver metastatic assay and classified them into either the high liver metastatic group (HT-29 and WiDr) or the low liver metastatic potential group (CaCo-2 and Colo320). The results showed that there was a positive relationship between the relative quantity of PLAC1 mRNA and metastatic potential. In other words, expression of PLAC1 mRNA in the high metastasis group (HT-29 and WiDr) was significantly higher than in the low metastasis group (CaCo-2 and Colo320, *P < 0.01, Fig. 1c).
Fig. 1.

Expression levels of PLAC1 in colorectal cancer cell lines. (a) PLAC1 mRNA was detected by RT-PCR in colorectal cancer cells. PCR-amplified products of reverse-transcribed mRNA (cDNA) from GenBank, using primers specific for PLAC1 PCR products, were separated through 2% agarose gels and stained with ethidium bromide. β-actin served as a loading control. (b) The protein expression of PLAC1 in colorectal cancer cell lines was determined in whole-cell lysates by western blotting analysis. The 30 µg/mL total cell lysate was subjected to 10% SDS-PAGE and transferred to a polyvinylidene difluoride membrane. The membrane was probed with antibodies to PLAC1. β-actin acted as a loading control. (c) Relative expression of PLAC1 mRNA in colorectal cancer cell lines compared to GAPDH was assessed using semiquantitative RT-PCR. The relative expression of PLAC1 mRNA was significantly higher in HT-29 and WiDr cells compared with CaCo-2 and Colo320 cells (*P < 0.01). GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Effect of PLAC1 on the proliferation of colorectal cancer cells and human umbilical vein endothelial cells
We next examined the proliferative effects of PLAC1 over a range of concentrations in colorectal cancer cells and HUVECs. The proliferative assay results showed that PLAC1 enhanced proliferation of Colo320 cells in a dose-dependent manner (compared with 0 ng/mL of PLAC1, **P < 0.05, *P < 0.01), and there was no significant promotion of proliferation in HT-29, WiDr and CaCo-2 cells (Fig. 2a). The growth of HUVECs was significantly enhanced by PLAC1 in a concentration-dependent manner when compared with controls (**P < 0.05, *P < 0.01), and this enhancement of proliferative capability was inhibited by the anti-PLAC1 antibody (compared with 100 ng/mL of PLAC1, *P < 0.01, Fig. 2b).
Fig. 2.

PLAC1 promoted the proliferation of colorectal cancer cells. (a) HT-29, WiDr, CaCo-2, and Colo320 cells were cultured in the presence of different concentrations of PLAC1 for 72 h. Cell proliferation was determined by the Premix WST-1 Cell Assay System, and absorbance was read at 450 nm. The reference wavelength is 690 nm. (b) The effect of PLAC1 and anti-PLAC1 antibody on the proliferation of HUVECs. HUVECs were incubated in medium containing different concentrations of PLAC1 or anti-PLAC1 antibody for 72 h. **P < 0.05, *P < 0.01, compared with control (0 ng/mL).
The roles of PLAC1 in the invasive behavior of colorectal cancer cells and human umbilical vein endothelial cells
After pretreatment (or no treatment) with PLAC1, Colo320 cells were cultured for 24 h. At that point, the invasive capability was assessed. PLAC1 was found to enhance the invasiveness of Colo320 cells (Fig. 3a) and HUVECs (Fig. 3b) in a dose-dependent manner. The 100 ng/mL of PLAC1 was the most effective (*P < 0.01). On the other hand, the invasive ability was blocked by anti-PLAC1 antibody in Colo320 cells and HUVECs (compared with 100 ng/mL of PLAC1, *P < 0.01).
Fig. 3.

PLAC1 enhanced the invasiveness of colorectal cancers and HUVECs. (a) The invasion of colorectal cancer cells treated with PLAC1 was assessed by the BD Bio-Coat Matrigel invasion assay. Colo320 cells were incubated for 24 h; the invading cells were fixed and stained with Diff-Quick stain. The invading cells were counted in five random microscopic fields (×200). The invasive ability of Colo320 cells was significantly promoted by PLAC1 compared with the control (**P < 0.05, *P < 0.01). (a) 0 ng/mL of PLAC1; a-1: 1 ng/mL of PLAC1; a-2: 10 ng/mL of PLAC1; a-3: 100 ng/mL of PLAC1; a-4: 100 ng/mL of PLAC1 + 10 µg/mL of anti-PLAC1 antibody. *P < 0.01. (b) The invasive ability of HUVECs treated with PLAC1 was also measured by invasion assay. The invasiveness of HUVECs was significantly enhanced by PLAC1 compared with the control (*P < 0.01). B: 0 ng/mL of PLAC1; b-1: 1 ng/mL of PLAC1; b-2: 10 ng/mL of PLAC1; b-3: 100 ng/mL of PLAC1; b-4: 100 ng/mL of PLAC1 + 10 µg/mL of anti-PLAC1 antibody. *P < 0.01.
Effect of PLAC1 and colorectal cancer cells on human umbilical vein endothelial cell tube formation
To further investigate the role of PLAC1 in the microenvironment, we focused on the interaction between tumor cells and stromal cells by characterizing angiogenic activity in cells cocultured with fibroblasts and HUVECs and the effect of PLAC1 in this system. HUVEC tube formation was significantly enhanced by the presence of PLAC1 in a dose-dependent manner (compared with 0 ng/mL of PLAC1, *P < 0.01, Fig. 4a).
Fig. 4.
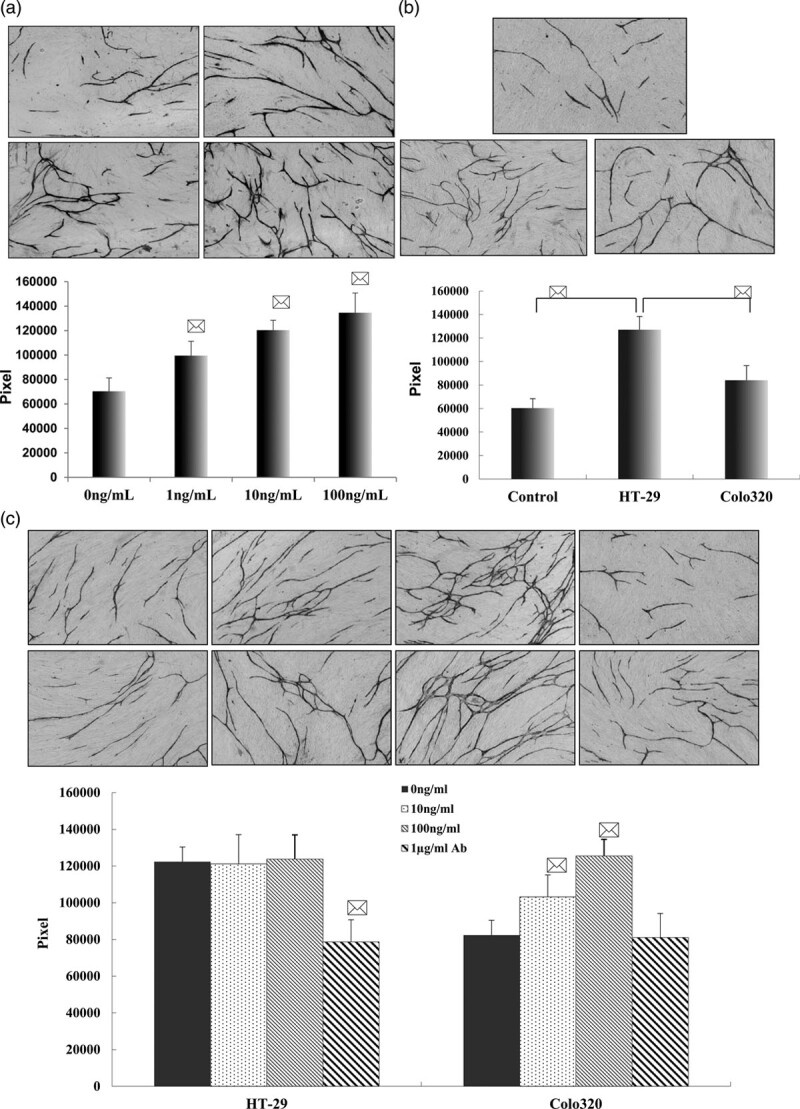
The effect of PLAC1 and colorectal cancer cells on angiogenesis. (a) Effect of PLAC1 pretreatment on tube formation. After incubation of HUVECs/fibroblasts in the presence of PLAC1 for 11 days, the tube formation was visualized with CD31 antibody staining. a: control; a-1: culture system treated with 1 ng/mL of PLAC1; a-2: culture system treated with 10 ng/mL PLAC1; a-3: culture system treated with 100 ng/mL PLAC1; GF (×200). The effect of PLAC1 on the tube formation area was measured quantitatively using an image analyzer. *P < 0.01 compared with control. (b) Effect of different metastatic potentials of colon cancer cells on angiogenesis. HT-29 cells with higher liver metastatic potential or Colo320 cells with lower liver metastatic potential on angiogenesis and HUVEC/fibroblasts consisted of a coculture system using a double chamber. Angiogenesis was assessed after 11 days of coculture. The angiogenesis was visualized with CD31 antibody staining (×200). The angiogenesis was significantly enhanced by HT-29 cells compared with Colo320 cells. b: control; b-1: coculture with HT-29 cells; b-2: coculture with Colo320 cells. *P < 0.01 compared with control (coculture without colorectal cancer cells) and coculture with Colo320 cells. (c) The different metastatic potentials of colorectal cancer cells treated with PLAC1 or anti-PLAC1 antibody affected angiogenesis in the coculture system. HT-29 or Colo320 cells were pretreated with PLAC1 or anti-PLAC1 antibody and cocultured with HUVECs/fibroblasts. All cells were coincubated for 11 days, and angiogenesis was visualized with CD31 antibody staining (×200). PLAC1 enhanced angiogenesis in a dose-dependent manner in the Colo320 coculture system. However, anti-PLAC1 Ab decreased angiogenesis in the HT-29 coculture system. c: coculture with HT-29 cells; c-1: coculture with HT-29 cells pretreated with 10 ng/mL PLAc1; c-2: coculture with HT-29 cells pretreated with 100 ng/mL PLAc1; c-3: coculture with HT-29 cells pretreated with 1 µg/mL anti-PLAC1 Ab; d: coculture with Colo320 cells; d-1: coculture with Colo320 cells pretreated with 10 ng/mL PLAC1; d-2: coculture with Colo320 cells pretreated with 100 ng/mL PLAC1; d-3: coculture with Colo320 cells pretreated with 1 µg/mL anti-PLAC1 Ab. *P < 0.01 compared with coculture colon cancer cells.
Effect of colorectal cancer cell coculture and PLAC1 on human umbilical vein endothelial cell tube formation
We next investigated the influence of colorectal cancer cell lines with different metastatic potentials on HUVEC tube formation using a double-chamber cell culture system. The counting of pixels was represented as the total length of tube formation. Tube formation was significantly enhanced by coculture with HT-29 cells compared with control (HUVECs and fibroblasts only) or coculture with Colo320 cells (*P < 0.01) (Fig. 4b). Moreover, the presence of PLAC1 significantly promoted tube formation in the Colo320 cell coculture system (*P < 0.01). In contrast, the enhanced tube formation of HUVECs was significantly inhibited by the addition of anti-PLAC1 antibody in the HT-29 cell coculture system (*P < 0.01) and was not inhibited by anti-PLAC1 antibody in the Colo320 cell coculture system (*P < 0.01) (Fig. 4c).
Activation of the PI3K, Akt and NF-κB signaling pathway after PLAC1 stimulation in human colorectal cancer cells and human umbilical vein endothelial cells
We used colorectal cancer cell lines and HUVECs to examine the activation of the PI3K/Akt/NF-κB signaling pathway, a potential downstream target of PLAC1. PLAC1 treatment increased PI3K phosphorylation in a dose-dependent manner in HT-29, Colo320 cells, and HUVECs (Fig. 5a). The relative expression level of phosphorylation of PI3K was measured by Image J software (compared with 0 ng/mL of PLAC1, **P < 0.05, *P < 0.01, Fig. 5b). The Akt kinase activity of colorectal cancer cells was remarkably enhanced by PLAC1 stimulation in a concentration-dependent manner (Fig. 5c). The relative expression level of phospho-Akt was measured by Image J software (compared with 0 ng/mL of PLAC1, **P < 0.05, *P < 0.01, Fig. 5d). Stronger activation of NF-κB phosphorylation activity was observed in HT-29, Colo320 cells and HUVECs stimulated by PLAC1 for 15 min in a dose-dependent manner (Fig. 5e). The relative expression level of phospho-NF-κB were measured by Image J software (compared with 0 ng/mL of PLAC1, **P < 0.05, *P < 0.01, Fig. 5f).
Fig. 5.

PLAC1-induced phosphorylation of PI3K, Akt, and NF-κB in human colorectal cancer cell lines and HUVECs HT-29, Colo320 cells, and HUVECs were treated with different concentrations of PLAC1 (1, 10, and 100 ng/mL) and cultured for 15 min. Cells were gathered and lysed by lysis buffer. Thirty micrograms of lysed protein were used for immunoblotting with a phospho-PI3K antibody (a, b), phospho-Akt antibody (c, d), and phospho-NF-κB antibody (e, f). Detection of total PI3K levels served as a loading control.
Activation of the PI3K/Akt/NF-κB signaling pathway after the stimulation of PLAC1 in colorectal cancer cells and human umbilical vein endothelial cells
To investigate the effect of PI3K inhibitor (LY294002) and Akt kinase inhibitor on the activation of NF-κB in HT-29, Colo320 cells, and HUVECs after stimulation by PLAC1, HT-29, Colo320, and HUVECs were pretreated with 50 µM PI3K inhibitor (LY294002) or 50 µM Akt kinase inhibitor for 5 min, then 100 ng/mL of PLAC1 was added to the culture medium and incubated for 15 min. The proteins were extracted and separated by SDS-PAGE and transferred to membranes, and the membranes were probed with an antibody directed against phospho-NF-κB. The results showed that PLAC1-mediated phospho-NF-κB was significantly blocked by 50 µM of LY294002 and Akt kinase inhibitor. These data indicate that PLAC1 enhanced the metastatic potential of colorectal cancer depending on the upregulation of the PLAC1/PI3K/Akt NF-κB signaling pathway (Fig. 6a and b).
Fig. 6.

Effect of Akt and PI3K inhibitor on PLAC1-induced phospho-NF-κB. HT-29, Colo320 cells, and HUVECs, after being pretreated with 50 μM Akt inhibitor and 50 μM LY294002 for 1 h, were incubated with 100 ng/mL PLAC1 for 30 min. The cells were gathered and lysed by lysis buffer. Thirty micrograms of lysed protein were used for immunoblotting with a phospho-NF-κB antibody. PLAC1-mediated phospho-NF-κB was significantly blocked by 50 µM of LY294002 and Akt kinase inhibitor. Detection of total NF-κB levels served as a loading control (a, b).
Discussion
Liver is one of the most common metastatic organs of colorectal cancer. Synchronous liver metastasis occurs in approximately 25% of patients, and advanced liver metastasis occurs in 30–40% of patients with colorectal cancer. Liver metastasis is also the leading cause of death in patients with colorectal cancer. The median survival time for untreated liver metastases was only 6.9 months, and the 5-year survival rate for unresectable patients was less than 5% (Morris et al., 2018; Mahmoudian et al., 2019). For this reason, the mechanism of colorectal cancer metastasis to the liver was explored; this mechanism is expected to be used for suppressing the invasion and metastasis of the tumor and to eventually provide a new approach to the prevention and treatment of colorectal cancer metastasis in clinical practice.
PLAC1 is a new member of the cancer-testis antigen family. It is characterized by its restricted expression in germ cells and placental trophoblastic tissues and its extensive expression in tumor tissues (Guo et al., 2017). The protein is closely related to the growth of the placenta and embryo and plays an important role in regulating the formation of the placenta and stabilizing the connection between the placenta and the mother, thus acting as a biological indicator to predict the prognosis of pathological pregnancy and embryo transfer. PLAC1 is extensively expressed in tumor tissue (Devor et al., 2016). CT antigen is overexpressed in various tumor types, while its expression is restricted to germ cells for normal tissues. Germ cells cannot be recognized by T cell receptor (TCR) gene-modified T cells due to the lack of expression of major histocompatibility complex molecules, which are responsible for antigen presentation. This feature makes these cells a potential target for cancer immunotherapy. PLAC1 is a membrane protein with immunogenicity. Although there is still no clear understanding of the effect of PLAC1 on tumor biological functions, its gene plays a very important role in tumor tissue expression and localization of cell surface proteins in placental tissue. Therefore, the CT antigen may act as an ideal target for tumor immunotherapy (Chang et al., 2014).
Placental growth is vital to embryo development. There are many similarities between the implantation and development of an embryo and the invasion and growth of the tumor. Similar to malignant cells, trophoblast cells migrate and invade into the uterus and its vessels to nourish the developing fetus. The expression of PLAC1 in colorectal cancer patients was proven to be associated with differentiation degree, depth of invasion, lymph node metastasis, distal metastasis, degree of malignancy and prognosis, suggesting that the abnormal expression of PLAC1 plays an important role in the development, progression and liver metastasis of colorectal cancer. PLAC1 may act as a tumor marker for assessing the malignancy, liver metastasis, and prognosis of colorectal cancer (Liu et al., 2008). To investigate the effects of PLAC1 on the metastatic potential of colorectal cancer, we assessed cell proliferation, invasion, and neovascularization. The results showed that exogenous PLAC1 could significantly increase the proliferation and invasion of the PLAC1-negative colorectal cancer cell line Colo320 in a dose-dependent manner, while there was no obvious enhancing effect on proliferation and invasion observed in PLAC1-positive colorectal cancer cells, such as HT-29, WiDr, and CaCo-2. The enhanced expression of PLAC1 was related to factors such as invasion depth, lymph node metastasis, and distal metastasis of colorectal cancer, suggesting that abnormal expression of PLAC1 may play an important role in the development of colorectal cancer and liver metastasis. PLAC1 had a potentiating effect on the proliferation and invasion of HUVECs, which was positively correlated with concentration. PLAC1 was expressed in up to 37.8% of primary tumor specimens, especially in 82.3 and 54.6% of primary breast cancer and tumor cell lines, respectively (Yuan et al., 2018). Silencing of the PLAC1 gene and anti-PLAC1 antibody can significantly inhibit the motility, proliferation, and invasiveness of non-small cell lung cancer and hepatocellular carcinoma (Yang et al., 2018; Wu et al., 2017). Moreover, silencing PLAC1 by siRNA and blocking PLAC1 by anti-PLAC1 antibody led to a decrease in phosphorylated Akt levels (Bufu et al., 2018). This result suggested that Akt kinase activation is involved in the execution of the downstream effect of PLAC1. Taken together, PLAC1 may be involved in tumor progression. To mimic the effect of PLAC1 on tumor angiogenesis in the microenvironment, we conducted tumor neovascularization in vitro by using a coculture system consisting of colon cancer cells and stromal cells and assessed the effect of colon cancer cells with different PLAC1 expression on neovascularization. The effect on neovascularization of HUVECs was significantly greater in the PLAC1-positive colorectal cancer cells cocultured than in the PLAC1-negative cells cocultured. In a culture system consisting of colorectal cancer cells HT-29 and HUVECs+fibroblasts, the enhanced neovascularization was inhibited by the addition of anti-PLAC1 antibody to the compartment of HT-29 cells. These results indicated that PLAC1 mainly enhanced the proliferation, invasion, neovascularization, and metastatic potential of colorectal cancer cells in PLAC1-positive colorectal cancer cells. Inhibition of PLAC1 expression can be a potential target for inhibiting colorectal cancer metastasis. The effect of PLAC1 on the microenvironment of colorectal cancer cells can be assessed in a more objective and realistic manner by using the constructed coculture system mimicking the microenvironment, which is of importance to understanding the specific mechanisms of growth, invasion, and metastasis of tumor cells. We also aimed at exploring the mechanism by which PLAC1 enhances proliferation, invasion, and neovascularization of colorectal cancer cells. Our results showed that PLAC1 promoted the proliferation, invasion, and neovascularization of colorectal cancer cells through the PI3K/Akt/NF-κB signaling pathway. The imbalance of the PI3K/Akt signaling pathway may trigger an array of procedures concerning cell growth, proliferation, apoptosis, exercise, invasion, cell cycle regulation, and telomerase activation, which may subsequently be involved in colorectal cancer development, progression and immune escape (Ren et al., 2016; Slattery et al., 2018). Activation of the PI3K/Akt signaling pathway may further activate the proliferation, differentiation, apoptosis, migration, and cell cycle regulation of its downstream target proteins and mediating cells. When cells are stimulated by specific cytokines, phosphorylation of the Akt protein in the PI3K/Akt signaling pathway induces a series of linked enzyme-catalyzed reactions that lead to the phosphorylation of inhibitory subunit alpha of NF-κB (IκB-α) downstream and dissociation from NF-κB. Currently, much attention has been paid to the PI3K/Akt signaling pathway, which may potentially be a highly specific target for anticancer therapy.
In summary, PLAC1 can promote proliferation, invasion, and neovascularization of colorectal cancer cells, enhancing their metastatic potential of colorectal cancer. The mechanism of this effect was related to the upregulation of cascade transmission among PI3K/Akt/NF-κB pathway members. Further research is still needed to identify the downstream target of the PI3K/Akt/NF-κB pathway for the enhanced metastatic potential of colorectal cancer mediated by PLAC. Subsequent topics of the research group include the inhibition of cascade transmission among members of the PI3K/Akt/NF-κB pathway, analysis of interactions between PLAC1 target genes and target pathways, as well as PLAC1 TCR gene-modified T cells as anti-tumor immunotherapy. It was speculated that PLAC1 may act as a potential target for the treatment of colorectal cancer, and elucidation of the above issues may facilitate the clinical treatment of colorectal cancer with positive expression of PLAC1. PLAC1/PI3K/Akt/NF-κB cascade may be critical for colorectal cancer cells to metastasize. Based on our results, we suggest that cancer-targeted therapies directed against PLAC1 may represent a promising new strategy against advanced colorectal cancer.
Acknowledgements
This work was supported by Grants from the National Natural and Science Foundation of China (81260325). Key Natural Science Research Projects in Universities of Anhui Province (KJ2019A0396).
Conflicts of interest
There are no conflicts of interest.
References
- Bufu T, Di X, Yilin Z, Gege L, Xi C, Ling W. (2018). Celastrol inhibits colorectal cancer cell proliferation and migration through suppression of MMP3 and MMP7 by the PI3K/AKT signaling pathway. Anticancer Drugs 29:530–538. [DOI] [PubMed] [Google Scholar]
- Chang WL, Yang Q, Zhang H, Lin HY, Zhou Z, Lu X, et al. (2014). Role of placenta-specific protein 1 in trophoblast invasion and migration. Reproduction 148:343–352. [DOI] [PubMed] [Google Scholar]
- Concu M, Banzola I, Farina A, Sekizawa A, Rizzo N, Marini M, et al. (2005). Rapid clearance of mRNA for PLAC1 gene in maternal blood after delivery. Fetal Diagn Ther 20:27–30. [DOI] [PubMed] [Google Scholar]
- Devor EJ. (2016). Placenta-specific protein 1 is conserved throughout the Placentalia under purifying selection. Scientificworldjournal 2014:537356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor EJ, Leslie KK. (2013). The oncoplacental gene placenta-specific protein 1 is highly expressed in endometrial tumors and cell lines. Obstet Gynecol Int 2013:807849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong XY, Peng JR, Ye YJ, Chen HS, Zhang LJ, Pang XW, et al. (2008). PLAC1 is a tumor-specific antigen capable of eliciting spontaneous antibody responses in human cancer patients. Int J Cancer 122:2038–2043. [DOI] [PubMed] [Google Scholar]
- Fant M, Barerra-Saldana H, Dubinsky W, Poindexter B, Bick R. (2014). The PLAC1 protein localizes to membranous compartments in the apical region of the syncytiotrophoblast. Mol Reprod Dev 74:922–929. [DOI] [PubMed] [Google Scholar]
- Geiger TR, Peeper DS. (2009). Metastasis mechanisms. Biochim Biophys Acta 1796:293–308. [DOI] [PubMed] [Google Scholar]
- Ghods R, Ghahremani MH, Madjd Z, Asgari M, Abolhasani M, Tavasoli S, et al. (2014). High placenta-specific 1/low prostate-specific antigen expression pattern in high-grade prostate adenocarcinoma. Cancer Immunol Immunother 63:1319–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Xu D, Lu Y, Peng J, Jiang L. (2017). Detection of circulating tumor cells by reverse transcriptionquantitative polymerase chain reaction and magnetic activated cell sorting in the peripheral blood of patients with hepatocellular carcinoma. Mol Med Rep 16:5894–5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koslowski M, Sahin U, Mitnacht-Kraus R, Seitz G, Huber C, Türeci O. (2007). A placenta-specific gene ectopically activated in many human cancers is essentially involved in malignant cell processes. Cancer Res 67:9528–9534. [DOI] [PubMed] [Google Scholar]
- Koslowski M, Türeci O, Biesterfeld S, Seitz G, Huber C, Sahin U. (2009). Selective activation of trophoblast-specific PLAC1 in breast cancer by CCAAT/enhancer-binding protein beta (C/EBPbeta) isoform 2. J Biol Chem 284:28607–28615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Shen D, Kang X, Zhang C, Song Q. (2015). New tumour antigen PLAC1/CP1, a potentially useful prognostic marker and immunotherapy target for gastric adenocarcinoma. J Clin Pathol 68:913–916. [DOI] [PubMed] [Google Scholar]
- Liu F, Zhang H, Shen D, Wang S, Ye Y, Chen H, et al. (2014). Identification of two new HLA-A*0201-restricted cytotoxic T lymphocyte epitopes from colorectal carcinoma-associated antigen PLAC1/CP1. J Gastroenterol 49:419–426. [DOI] [PubMed] [Google Scholar]
- Liu FF, Dong XY, Pang XW, Xing Q, Wang HC, Zhang HG, et al. (2008). The specific immune response to tumor antigen CP1 and its correlation with improved survival in colon cancer patients. Gastroenterology 134:998–1006. [DOI] [PubMed] [Google Scholar]
- Ma J, Su H, Yu B, Guo T, Gong Z, Qi J, et al. (2018). CXCL12 gene silencing down-regulates metastatic potential via blockage of MAPK/PI3K/AP-1 signaling pathway in colon cancer. Clin Transl Oncol 20:1035–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Sun X, Guo T, Su H, Chen Q, Gong Z, et al. (2017a). Interleukin-1 receptor antagonist inhibits angiogenesis via blockage IL-1α/PI3K/NF-κβ pathway in human colon cancer cell. Cancer Manag Res 9:481–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JC, Sun XW, Su H, Chen Q, Guo TK, Li Y, et al. (2017b). Fibroblast-derived CXCL12/SDF-1α promotes CXCL6 secretion and co-operatively enhances metastatic potential through the PI3K/Akt/mTOR pathway in colon cancer. World J Gastroenterol 23:5167–5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudian J, Ghods R, Nazari M, Jeddi-Tehrani M, Ghahremani MH, Ghaffari-Tabrizi-Wizsy N, et al. (2019). PLAC1: biology and potential application in cancer immunotherapy. Cancer Immunol Immunother 68:1039–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteo M, Greco P, Levi Setti PE, Morenghi E, De Rosario F, Massenzio F, et al. (2013). Preliminary evidence for high anti-PLAC1 antibody levels in infertile patients with repeated unexplained implantation failure. Placenta 34:335–339. [DOI] [PubMed] [Google Scholar]
- Morris E, Treasure T. (2018). Surgical management and outcomes of colorectal cancer liver metastases. Cancer Epidemiol 52:160–161. [DOI] [PubMed] [Google Scholar]
- Ning Y, Manegold PC, Hong YK, Zhang W, Pohl A, Lurje G, et al. (2011). Interleukin-8 is associated with proliferation, migration, angiogenesis and chemosensitivity in vitro and in vivo in colon cancer cell line models. Int J Cancer 128:2038–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H, Wang Z, Zhang S, Ma H, Wang Y, Jia L, Li Y. (2016). IL-17A promotes the migration and invasiveness of colorectal cancer cells through NF-κB-mediated MMP expression. Oncol Res 23:249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, et al. (2020). Colorectal cancer statistics, 2020. CA Cancer J Clin 0:1–20. [DOI] [PubMed] [Google Scholar]
- Silva WA, Jr, Gnjatic S, Ritter E, Chua R, Cohen T, Hsu M, et al. (2007). PLAC1, a trophoblast-specific cell surface protein, is expressed in a range of human tumors and elicits spontaneous antibody responses. Cancer Immun 7:18. [PMC free article] [PubMed] [Google Scholar]
- Slattery ML, Mullany LE, Sakoda LC, Wolff RK, Stevens JR, Samowitz WS, Herrick JS. (2018). The PI3K/AKT signaling pathway: associations of miRNAs with dysregulated gene expression in colorectal cancer. Mol Carcinog 57:243–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchabo NE, Mhawech-Fauceglia P, Caballero OL, Villella J, Beck AF, Miliotto AJ, et al. (2009). Expression and serum immunoreactivity of developmentally restricted differentiation antigens in epithelial ovarian cancer. Cancer Immun 9;6. [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Lin X, Di X, Chen Y, Zhao H, Wang X. (2017). Oncogenic function of PLAC1 on the proliferation and metastasis in hepatocellular carcinoma cells. Oncol Rep 37:465–473. [DOI] [PubMed] [Google Scholar]
- Yuan H, Chen V, Boisvert M, Isaacs C, Glazer RI. (2018). PLAC1 as a serum biomarker for breast cancer. PLoS One 13:e0192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Zha TQ, He X, Chen L, Zhu Q, Wu WB, et al. (2018). Placenta-specific protein 1 promotes cell proliferation and invasion in non-small cell lung cancer. Oncol Rep 39:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]


