Low-strength evidence suggests that, in low-risk pregnancies, risks of cesarean delivery, neonatal harms, and maternal harms were similar for outpatient and inpatient cervical ripening.
Abstract
OBJECTIVE:
To assess the comparative effectiveness and potential harms of cervical ripening in the outpatient compared with the inpatient setting, or different methods of ripening in the outpatient setting alone.
DATA SOURCES:
Searches for articles in English included MEDLINE, EMBASE, CINAHL, Cochrane Library, ClinicalTrials.gov, and reference lists (up to August 2020).
METHODS OF STUDY SELECTION:
Using predefined criteria and DistillerSR software, 10,853 citations were dual-reviewed for randomized controlled trials (RCTs) and cohort studies of outpatient cervical ripening using prostaglandins and mechanical methods in pregnant women at or beyond 37 weeks of gestation.
TABULATION, INTEGRATION, AND RESULTS:
Using prespecified criteria, study data abstraction and risk of bias assessment were conducted by two reviewers, random-effects meta-analyses were conducted and strength of evidence was assessed. We included 30 RCTs and 10 cohort studies (N=9,618) most generalizable to women aged 25–30 years with low-risk pregnancies. All findings were low or insufficient strength of evidence and not statistically significant. Incidence of cesarean delivery was not different for any comparison of inpatient and outpatient settings, or comparisons of different methods in the outpatient setting (most evidence available for single-balloon catheters and dinoprostone). Harms were inconsistently reported or inadequately defined. Differences were not found for neonatal infection (eg, sepsis) with outpatient compared with inpatient dinoprostone, birth trauma (eg, cephalohematoma) with outpatient compared with inpatient single-balloon catheter, shoulder dystocia with outpatient dinoprostone compared with placebo, maternal infection (eg, chorioamnionitis) with outpatient compared with inpatient single-balloon catheters or outpatient prostaglandins compared with placebo, and postpartum hemorrhage with outpatient catheter compared with inpatient dinoprostone. Evidence on misoprostol, hygroscopic dilators, and other outcomes (eg, perinatal mortality and time to vaginal birth) was insufficient.
CONCLUSION:
In women with low-risk pregnancies, outpatient cervical ripening with dinoprostone or single-balloon catheters did not increase cesarean deliveries. Although there were no clear differences in harms when comparing outpatient with inpatient cervical ripening, the certainty of evidence is low or insufficient to draw definitive conclusions.
SYSTEMATIC REVIEW REGISTRATION:
PROSPERO, CRD42020167406.
Induction of labor rates are rising in the United States, reaching 25.7 percent in 2017.1 Given the ARRIVE (A Randomized Trial of Induction Versus Expectant Management) trial findings that elective induction of labor was associated with lower cesarean delivery rate and no difference in serious perinatal harms compared with expectant management,2 it is anticipated that induction of labor rates will continue to rise.3,4 Approximately 84% of women who undergo induction of labor require cervical ripening.5,6 Traditionally, cervical ripening occurs inpatient using prostaglandins or mechanical methods (eg, balloon catheters).
Given that the cervical ripening process can be lengthy, inpatient cervical ripening requires numerous resources (eg, highly skilled labor and delivery staff), and some women prefer to be at home as long as possible before delivery, outpatient cervical ripening may be a reasonable alternative. However, risks and benefits of outpatient cervical ripening are not well-established. Its use remains controversial due to concerns about increased risk of harms combined with clinician and institutional risk-aversion driven by potential legal litigation.7,8 The 2009 American College of Obstetricians and Gynecologists3 Practice Bulletin on induction of labor was unable to reach a recommendation on outpatient cervical ripening. Because new evidence, not included in prior reviews,9–11 is available, an updated review of the evidence was requested by the American College of Obstetricians and Gynecologists to update their guidance. Therefore, we conducted a systematic review and meta-analysis comparing the effectiveness and potential harms of outpatient compared with inpatient cervical ripening, and comparing outcomes of different methods used in the outpatient setting. This article is a condensed version of the full report.12
SOURCES
A protocol was published a priori,13 and registered in the PROSPERO registry (CRD42020167406). Reporting of the review adheres to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement.14 A medical librarian conducted searches in Ovid MEDLINE, EMBASE, CINAHL, Cochrane Central Register of Controlled Trials, and Cochrane Database of Systematic Reviews from database inception to August 2020. The ClinicalTrials.gov registry was searched in December 2020 for both completed and on-going studies. References of included studies and prior systematic reviews were searched to locate additional studies. A Federal Register notice requesting “supplemental evidence and data for systematic review” did not result in the identification of new evidence. Experts were consulted before the design of the search strategies, and after a draft report was prepared. No study design restrictions were applied to the searches, but they were limited to English-language publications. Complete search strategies and inclusion criteria can be found in Appendices 1 and 2, available online at http://links.lww.com/AOG/C267.
STUDY SELECTION
To evaluate risks and benefits of outpatient cervical ripening, we included randomized controlled trials (RCTs) and observational (ie, cohort) studies with concurrent controls that enrolled women at or beyond 37 weeks of gestation undergoing cervical ripening in the outpatient setting (any method available in the United States), comparing either to an inpatient setting or another method in the outpatient setting.
Each citation identified through searches was screened for relevance by two reviewers. The full-text of articles with either reviewer indicating potential relevance was reviewed by two reviewers. Searches identified 10,853 references (Appendix 3, available online at http://links.lww.com/AOG/C267). After dual review of full-text of potentially eligible articles, 40 unique studies (in 43 publications)15–56 were included.
Study characteristics and results were abstracted by one reviewer and checked for accuracy by a second. Primary outcomes were selected and defined a priori after consultation with an expert panel, according to Agency for Healthcare Research and Quality (AHRQ) methods. Primary outcomes assessed included birth-related outcomes (total time from admission to vaginal birth, total labor and delivery length of stay, and cesarean delivery rate overall), neonatal harms (perinatal mortality, hypoxic-ischemic encephalopathy, seizure, infection [confirmed sepsis or pneumonia], meconium aspiration syndrome, birth trauma [eg, bone fracture], and intracranial or subgaleal hemorrhage), and maternal harms (hemorrhage requiring transfusion, postpartum hemorrhage by mode of delivery [vaginal, cesarean], and uterine infection [ie, chorioamnionitis, endometritis]). The risk of bias of included studies was assessed by two reviewers, using preestablished criteria.57–59 Disagreements on inclusion decisions or risk of bias assessments were resolved through consensus. Profile-likelihood random effects models were used for meta-analysis of results from two or more studies, with heterogeneity assessed using both the χ2 test and the I-squared (I2) statistic. We reported relative risks (RRs) for dichotomous outcomes and mean differences for continuous outcomes, with 95% CIs. Prespecified subgroup analyses were planned for parity, maternal age, group B streptococcus status, diabetes (pregestational, gestational), hypertension (chronic, preeclampsia without severe features, gestational), fetal growth restriction, and gestational age at time of induction of labor (less than 39 weeks, 39–41 weeks, more than 41 weeks). The strength of evidence of primary outcome-intervention pairs were evaluated using the AHRQ methods.60 Based on input from clinical experts, we categorized the magnitude of effect as follows: a difference of less than 5%, little or no difference; 5–10%, small difference; 11–20%, moderate difference; greater than 20%, large difference.
RESULTS
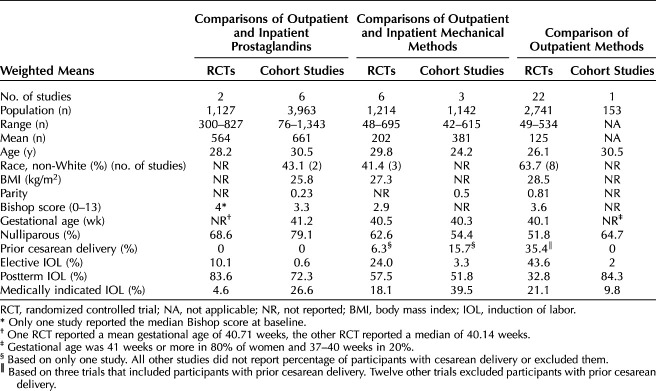
Thirty RCTs and 10 cohort studies were included, evaluating 9,618 women. The majority of the evidence pertained to comparisons of methods in the outpatient setting (22 RCTs, one cohort study). Four studies were rated good quality, 29 fair quality, and seven poor quality. A list of included studies and a list of excluded studies with reason for exclusion can be found in the full AHRQ report12 The characteristics of women enrolled in the included studies are summarized in Table 1, with detailed information on each study in the full AHRQ report. Participants' weighted mean age was 28.8 years and weighted body mass index (BMI, calculated as weight in kilograms divided by height in meters squared) was 26.7 in the six RCTs16,24,30,38,42,43 and one cohort study51 that reported it. Race was reported in 32.5% of studies, with most including majority White women (64–84%); however, three included majority Black women (61–88%), and one included majority Latina women (96%). Sixty-five percent of participants were nulliparous; only five studies reported on parity of participants (weighted mean parity 0.25). Data reported did not allow analysis of the percent nulliparous. Most studies (65%) excluded women with prior cesarean delivery; one RCT limited recruitment to women with prior vaginal birth,30 and another RCT recruited only women with prior cesarean delivery.44 Relatively few studies excluded women with preexisting comorbidities (pregestational diabetes 13%, gestational diabetes 10%, chronic hypertension 18%, gestational hypertension 20%). Across the studies, 5.6% of women enrolled had gestational diabetes mellitus, though one RCT reported that 69% of participants had gestational diabetes mellitus.35 Postterm pregnancy was the most frequently reported reason for cervical ripening (61.3%). Weighted mean Bishop score at baseline was 3.4 and mean gestational age was 40.6 weeks. Most studies were conducted in the United States (60%). Less than half (45%) reported a funding source; a nonprofit organization was the source in 50% of those that did report funding. Evidence tables of study and patient characteristics, study results, and risk of bias domain assessments for individual studies are available in the full AHRQ report.12
Table 1.
Study and Patient Characteristics
Tables 2 and 3 show the findings of studies and meta-analyses for primary outcomes for which there was sufficient evidence. There were multiple prespecified primary outcomes for which we did not find sufficient evidence, either due to the outcome not being reported or, more commonly, reported in ways that did not meet our criteria. For example, regarding the time to vaginal delivery primary outcome: most studies reported this outcome for any delivery mode, including cesarean, preventing disaggregation of vaginal birth observations. Other examples include neonatal infections, which were often “suspected” but without evidence of meeting diagnostic criteria, and meconium-related outcomes that failed to specify whether meconium aspiration syndrome was diagnosed. Given these areas of uncertainty, we relied on neonatal intensive care unit (NICU) admission as an indicator of true neonatal morbidity. If the neonatal morbidity event resulted in admission to a NICU, or similar unit, we included the outcome.
Table 2.
Primary Birth Outcomes With Sufficient Evidence, Cesarean Delivery
Table 3.
Primary Harms Outcomes With Sufficient Evidence
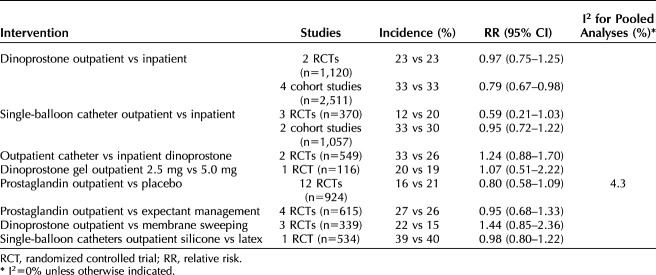
For birth outcomes, only cesarean delivery was adequately reported (Table 2). For all comparisons, findings were not statistically significantly different between groups. In terms of sample size, the body of evidence on dinoprostone outpatient compared with inpatient was the strongest, with 1,120 women in two RCTs and 2,511 in four cohort studies (Fig. 1). One of the cohort studies was poor quality; however, removal of the poor-quality study in sensitivity analysis did not alter the results. The incidence of cesarean delivery in cohort studies was greater than in RCTs, but the differences between groups were similar to the differences found in the RCTs. In a subgroup analysis in one cohort study20 the frequency of cesarean delivery with dinoprostone outpatient compared with inpatient in women with postterm pregnancies (adjusted odds ratio 0.74, 95% CI 0.54–1.01) was not significantly different from that of the full population (postterm and preterm rupture of membranes, adjusted odds ratio 0.71, 95% CI 0.54–0.95). The evidence on misoprostol was insufficient, limited to a single fair-quality cohort study (n=273).19 Cervical ripening using a single- or double-balloon catheter did not result in differences in cesarean delivery when used in the outpatient compared with inpatient settings (Fig. 2). Notably, the evidence on outpatient compared with inpatient for double-balloon catheter specifically was insufficient due to very small sample size, no corroborating evidence, and study limitations.55 In comparing catheters in the outpatient setting with dinoprostone in the inpatient setting, one study (n=217) conducted a subgroup analysis of women with modified Bishop score higher than 3 at the start of cervical ripening and found no difference in cesarean delivery (31% catheter vs 20% dinoprostone; RR 1.53, 95% CI 0.96–2.46).16
Fig. 1. Meta-analysis of cesarean delivery with prostaglandins for cervical ripening: outpatient (OP) vs inpatient (IP). *Risk ratio estimate calculated from author's adjusted odds ratio comparing inpatient with outpatient. RCT, randomized controlled trial.
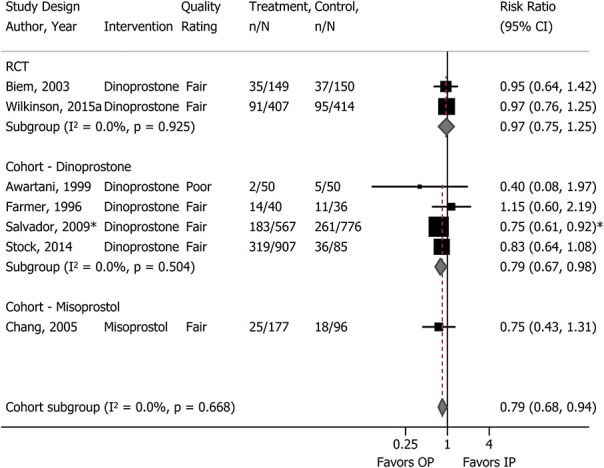
McDonagh. Outpatient Cervical Ripening. Obstet Gynecol 2021.
Fig. 2. Meta-analysis of cesarean delivery with catheters for cervical ripening: outpatient (OP) vs inpatient (IP). *Risk ratio estimate calculated from author's adjusted odds ratio comparing inpatient with outpatient. RCT, randomized controlled trial.
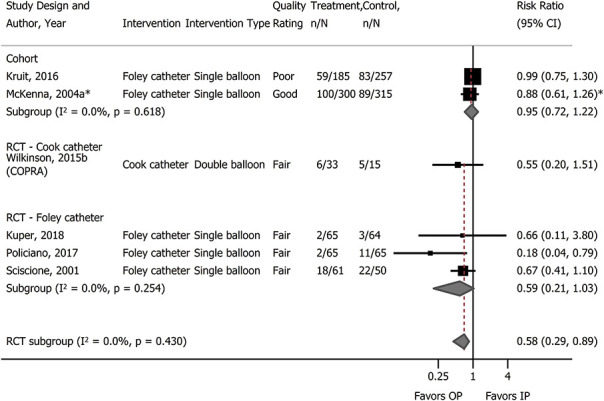
McDonagh. Outpatient Cervical Ripening. Obstet Gynecol 2021.
Figure 3 shows the meta-analysis of cesarean delivery comparing prostaglandin with placebo in the outpatient setting (seven dinoprostone [n=473] and five misoprostol [n=461]). Additional analyses did not identify publication bias (Appendix 4, available online at http://links.lww.com/AOG/C267) or variation in effects based on type of prostaglandin, gestational age (postterm pregnancies compared with mixed populations), or study quality. Two RCTs (one good-quality of misoprostol and one fair-quality of dinoprostone) conducted within-study subgroup analyses of cesarean delivery frequency according to parity.38,47 The direction of the effect in both studies varied according to parity; nulliparous women experienced more frequent cesarean delivery when outpatient cervical ripening involved a prostaglandin compared with placebo (misoprostol: 40% vs 37%; RR 1.09, 95% CI 0.49–2.41; dinoprostone: 43% vs 19%; RR 2.29, 95% CI 0.70–7.48). However, the studies were small (total n=118), and the difference did not reach statistical significance.
Fig. 3. Meta-analysis of cesarean delivery with prostaglandins vs placebo for cervical ripening in the outpatient setting.
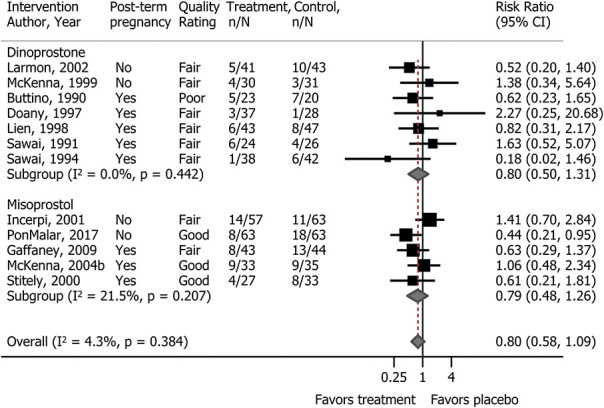
McDonagh. Outpatient Cervical Ripening. Obstet Gynecol 2021.
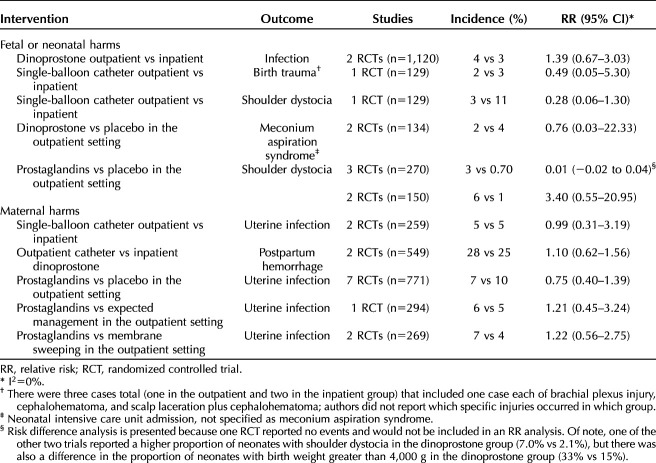
Evidence on harms associated with outpatient compared with inpatient cervical ripening or comparing two methods in the outpatient setting, is presented in Table 3; there were no statistically significant differences. Neonatal harm outcomes were rarely or inadequately reported. Neonatal infection (confirmed sepsis or pneumonia) was not different between groups comparing outpatient (4%) with inpatient dinoprostone (3%) (two RCTs, n=1,120). The incidences of birth trauma with outpatient compared with inpatient single-balloon catheter were similar.
Shoulder dystocia was also not different between groups (n=129, 3% outpatient vs 11% inpatient). When comparing outpatient cervical ripening regimens across three RCTs (n=270), shoulder dystocia occurred more frequently in the prostaglandin groups (3.1%) compared with placebo (0.70%), but was not statistically significant (risk difference 0.01, 95% CI −0.02 to 0.04). Closer examination of this outcome revealed the difference could be attributed to one small trial (n=90) in which no adjustment was made for differences in baseline clinical characteristics. For example, 33% of patients in dinoprostone group had fetal weight greater than 4 kg (a significant risk factor for shoulder dystocia) compared with 15% in placebo group.32 The other two studies had one or no events.27,50 Admission to NICU for meconium aspiration with dinoprostone compared with placebo in the outpatient setting was similar. Other primary neonatal harm outcomes were not reported, reported too infrequently to assess given the small samples sizes (ie, perinatal mortality, seizure), or were not reported as defined a priori (eg, hypoxic-ischemic encephalopathy, intracranial or subgaleal hemorrhage).
Table 3 shows maternal harms evidence. Uterine infection (chorioamnionitis or endometritis) was the most commonly reported outcome; there were no differences found between outpatient compared with inpatient single balloon catheters and outpatient prostaglandins compared with placebo, expectant management or membrane sweeping, and incidence was similar across groups. Postpartum hemorrhage was reported in two RCTs comparing outpatient catheters (single- and double-balloon) with inpatient dinoprostone, with the difference not statistically significant.16,23 Other primary maternal harm outcomes were either reported too infrequently to assess given the small samples sizes or not reported as prespecified (eg, postpartum hemorrhage by delivery mode, or requiring transfusion).
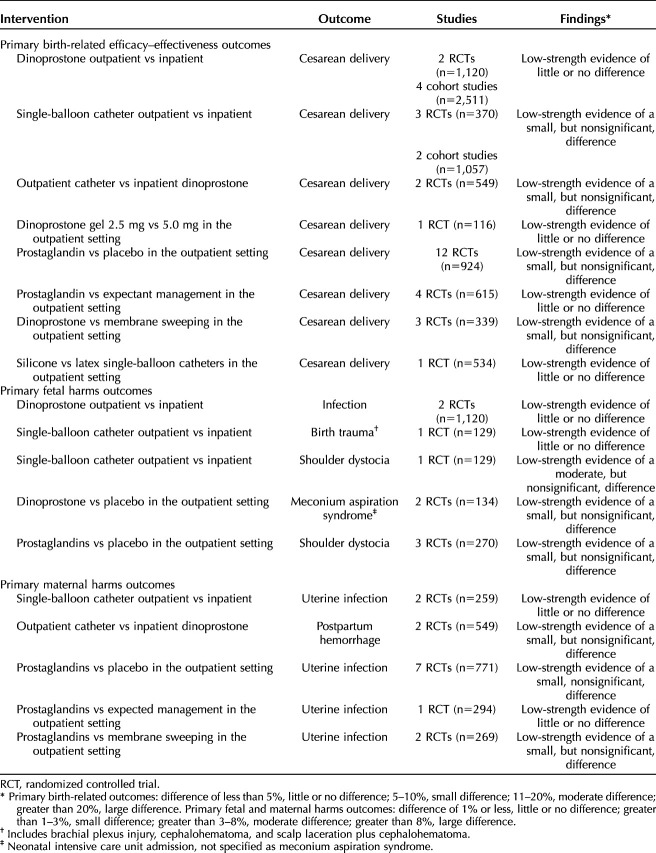
Table 4 shows all outcomes with sufficient evidence, the strength of the evidence, and the magnitude of effect category for the finding. No outcome was found to have better than low-strength evidence. The differences were not statistically significant for any outcome, and the magnitude of the difference was “little to none” or “small” in all but one. The magnitude of the difference between outpatient and inpatient single-balloon catheter was moderate (defined as greater than 3–8%) for shoulder dystocia, favoring the outpatient setting.
Table 4.
Summary of Evidence Findings and Strength
DISCUSSION
This systematic review summaries 40 studies examining outpatient cervical ripening. We found no differences in cesarean delivery, neonatal or maternal outcomes by outpatient compared with inpatient cervical ripening. We also found no differences in outcomes when comparing different methods of outpatient ripening. Specifically, the incidence of cesarean delivery was similar in comparisons of outpatient with inpatient dinoprostone and single-balloon catheter, outpatient catheter with inpatient dinoprostone, and outpatient comparisons of dinoprostone 2.5 mg with 5 mg, silicone and latex single-balloon catheters, and prostaglandins with placebo, expectant management, or membrane sweeping. However, across the primary outcomes prioritized for this review, there was only low-strength evidence, with many scientific gaps where the evidence is insufficient to draw conclusions.
Compared with two prior systematic reviews, this review provides higher strength evidence, and direct comparisons of outpatient and inpatient cervical ripening outcomes.9,11 A prior 2017 Cochrane Review examined different methods of cervical ripening in the outpatient setting. The authors included 16 RCTs of prostaglandins compared with placebo, and concluded that there was insufficient evidence to detect differences in maternal or neonatal outcomes.11 Although other reviews included studies of outpatient cervical ripening, they were either nonsystematic reviews, or combined studies of outpatient and inpatient cervical ripening.9,10 Recently, an additional trial of cervical ripening with a single-balloon catheter in the outpatient compared with inpatient setting was published.61 The rates of cesarean delivery and maternal infection were not significantly different, which is consistent with the findings of this review.
The highest strength of evidence for outcomes of outpatient cervical ripening found in this review was low, with several important outcomes having insufficient evidence. A rating of low-strength evidence means that there is low certainty in the magnitude or direction of the findings, and that future studies could change the conclusions. Limitations of the evidence included 1) insufficient evidence for direct comparisons of different interventions in the outpatient setting, 2) inadequate data to determine differential benefit or harm for cervical ripening methods in specific maternal or fetal subgroups (ie, effect modification), and 3) evidence quantity and quality is low for specific interventions; these and others are discussed more fully in the full AHRQ report.12 Limitations of the review process included exclusion of observational studies without a concurrent control group (eg, pre–post studies), which may have provided some additional insights into harm outcomes, and studies published in languages other than English. Due to inadequate numbers of studies, we were unable to conduct publication bias assessments for most outcomes.
The finding that outpatient cervical ripening with dinoprostone and single-balloon catheters did not impose increased risk of cesarean delivery, with at least no strong signals of clinically important increased risk of harms, may be encouraging for women who are interested in outpatient cervical ripening. However, it is important to recognize that not all possible harms were adequately studied or reported, and that the findings apply most directly to women under age 30, with singleton fetuses in cephalic presentation, and no major comorbidities. The question of the characteristics of pregnant women and fetuses that will benefit most or have the lowest risk of harm is not addressed by this evidence. The best choice of agent for outpatient cervical ripening remains unknown. There is also little information to guide the use of double-balloon catheters, hygroscopic dilators, or misoprostol, or to compare doses and routes of administration of prostaglandins.
Based on our review, we suggest that additional RCTs are needed to corroborate these findings, particularly where there is only a single, small study available currently (eg, outpatient misoprostol, double-balloon catheters, dilators). These RCTs should be large enough to evaluate important harms and evaluate differential effectiveness and harms of outpatient cervical ripening in important subgroups, and additional factors not considered here (eg, augmentation of labor with synthetic oxytocin, epidural anesthesia).
Footnotes
This work was funded under Contract No 290-2015-00009-I from the Agency for Healthcare Research and Quality (AHRQ), U.S. Department of Health and Human Services (HHS). The Patient-Centered Outcomes Research Institute (PCORI) funded the report (PCORI Publication No. 2020-SR-03). The authors of this manuscript are responsible for its content. The content does not necessarily represent the official views of or imply endorsement by PCORI, AHRQ, or of the U.S. Department of Health and Human Services.
Financial Disclosure Amy Hermesch disclosed that money was paid to her from GenBioPro as a consultant. The other authors did not report any potential conflicts of interest.
The authors thank the following individuals for their contributions: Elaine Graham, MLS, for EPC program guidance; Leah Williams, BS, for editorial support; Shaun Ramirez and Frances Hsu for technical support; our Task Order Officer, Jill Huppert, MD, MPH, for guidance in review development; and EPC Associate Editor, Ian Saldanha, MBBS, MPH, PhD, for his review of this report.
Each author has confirmed compliance with the journal's requirements for authorship.
Published online ahead-of-print March 22, 2021.
Peer reviews and author correspondence are available at http://links.lww.com/AOG/C268.
REFERENCES
- 1.Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Drake P. Births: final data for 2017. National vital statistics reports: from the centers for disease control and prevention, national center for health statistics, Natl Vital Stat Syst 2018;67:1–50 [Google Scholar]
- 2.Grobman WA, Rice MM, Reddy UM, Tita ATN, Silver RM, Mallett G, et al. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med 2018;379:513–23. doi: 10.1056/NEJMoa1800566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Induction of labor. ACOG Practice Bulletin No. 107. American College of Obstetricians and Gynecologists. Obstet Gynecol 2009;114:386–97. doi: 10.1097/AOG.0b013e3181b48ef5 [DOI] [PubMed] [Google Scholar]
- 4.Management of late-term and postterm pregnancies. Practice Bulletin No. 146. American College of Obstetricians Gynecologists. Obstet Gynecol 2014;124:390–6. doi: 10.1097/01.AOG.0000452744.06088.48 [DOI] [PubMed] [Google Scholar]
- 5.Bernardes TP, Broekhuijsen K, Koopmans CM, Boers KE, van Wyk L, Tajik P, et al. Caesarean section rates and adverse neonatal outcomes after induction of labour versus expectant management in women with an unripe cervix: a secondary analysis of the HYPITAT and DIGITAT trials. BJOG 2016;123:1501–8. doi: 10.1111/1471-0528.14028 [DOI] [PubMed] [Google Scholar]
- 6.Bartha JL, Romero-Carmona R, Martinez-Del-Fresno P, Comino-Delgado R. Bishop score and transvaginal ultrasound for preinduction cervical assessment: a randomized clinical trial. Ultrasound Obstet Gynecol 2005;25:155–9. doi: 10.1002/uog.1813 [DOI] [PubMed] [Google Scholar]
- 7.Carlson NS, Neal JL, Tilden EL, Smith DC, Breman RB, Lowe NK, et al. Influence of midwifery presence in United States centers on labor care and outcomes of low-risk parous women: a Consortium on Safe Labor study. Birth 2019;46:487–99. doi: 10.1111/birt.12405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neal JL, Carlson NS, Phillippi JC, Tilden EL, Smith DC, Breman RB, et al. Midwifery presence in United States medical centers and labor care and birth outcomes among low-risk nulliparous women: a Consortium on Safe Labor study. Birth 2019;46:475–48. doi: 10.1111/birt.12407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diederen M, Gommers J, Wilkinson C, Turnbull D, Mol B. Safety of the balloon catheter for cervical ripening in outpatient care: complications during the period from insertion to expulsion of a balloon catheter in the process of labour induction: a systematic review. BJOG 2018;125:1086–95. doi: 10.1111/1471-0528.15047 [DOI] [PubMed] [Google Scholar]
- 10.Smith LK. Outpatient induction of labour with prostaglandins: safety, effectiveness and women's views. Br J Midwifery 2017;25:774–82. doi: 10.12968/bjom.2017.25.12.774 [DOI] [Google Scholar]
- 11.Vogel JP, Osoti AO, Kelly AJ, Livio S, Norman JE, Alfirevic Z. Pharmacological and mechanical interventions for labour induction in outpatient settings. The Cochrane Database of Systematic Reviews 2017, Issue 9. Art. No.: CD007701. doi: 10.1002/14651858.CD007701.pub3 [DOI] [PMC free article] [PubMed]
- 12.McDonagh M, Skelly AC, Hermesch A, Tilden E, Brodt ED, Dana T, et al. Cervical ripening in the outpatient setting. Comparative Effectiveness Review No. 238. (Prepared by the Pacific Northwest Evidence-based Practice Center under Contract No. 290-2015-00009-I for the Agency for Healthcare Research and Quality and the Patient-Centered Outcomes Research Institute.) AHRQ Publication No. 21-EHC011. PCORI Publication No. 2020-SR-03. Agency for Healthcare Research and Quality; 2021. doi: 10.23970/AHRQEPCCER238. Posted final reports are located on the Effective Health Care Program search page. [DOI]
- 13.Agency for Healthcare Research and Quality. Cervical ripening in the outpatient setting: research protocol. Accessed February 19, 2021. https://effectivehealthcare.ahrq.gov/products/cervical-ripening/protocol [PubMed] [Google Scholar]
- 14.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. doi: 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Awartani KA, Turnell RW, Olatunbosun OA. A prospective study of induction of labor with prostaglandin vaginal gel: ambulatory versus in-patient administration. Clin Exp Obstet Gynecol 1999;26:162–5. [PubMed] [Google Scholar]
- 16.Beckmann M, Gibbons K, Flenady V, Kumar S. Induction of labour using prostaglandin E2 as an inpatient versus balloon catheter as an outpatient: a multicentre randomised controlled trial. BJOG 2020;127:571–9. doi: 10.1111/1471-0528.16030 [DOI] [PubMed] [Google Scholar]
- 17.Biem SR, Turnell RW, Olatunbosun O, Tauh M, Biem HJ. A randomized controlled trial of outpatient versus inpatient labour induction with vaginal controlled-release prostaglandin-E2: effectiveness and satisfaction. J Obstet Gynaecol Can 2003;25:23–31. doi: 10.1016/s1701-2163(16)31079-9 [DOI] [PubMed] [Google Scholar]
- 18.Buttino LT, Jr, Garite TJ. Intracervical prostaglandin in postdate pregnancy. A randomized trial. J Reprod Med 1990;35:155–8. [PubMed] [Google Scholar]
- 19.Chang DW, Velazquez MD, Colyer M, Klaus P, Mallipeddi SK, Rayburn WF. Vaginal misoprostol for cervical ripening at term: comparison of outpatient vs. inpatient administration. J Reprod Med 2005;50:735–9. [PubMed] [Google Scholar]
- 20.Cundiff GW, Simpson ML, Koenig N, Lee T. Observational study of neonatal safety for outpatient labour induction priming with dinoprostone vaginal insert. J Obstet Gynaecol Can 2017;39:354–60. doi: 10.1016/j.jogc.2017.01.003 [DOI] [PubMed] [Google Scholar]
- 21.Doany W, McCarty J. Outpatient management of the uncomplicated postdate pregnancy with intravaginal prostaglandin E2 gel and membrane stripping. J Matern Fetal Med 1997;6:71–8. doi: . [DOI] [PubMed] [Google Scholar]
- 22.Farmer KC, Schwartz WJ, III, Rayburn WF, Turnbull G. A cost-minimization analysis of intracervical prostaglandin E2 for cervical ripening in an outpatient versus inpatient setting. Clin Ther 1996;18:747–56. doi: 10.1016/s0149-2918(96)80224-4 [DOI] [PubMed] [Google Scholar]
- 23.Gaffaney CA, Saul LL, Rumney PJ, Morrison EH, Thomas S, Nageotte MP, et al. Outpatient oral misoprostol for prolonged pregnancies: a pilot investigation. Am J Perinatol 2009;26:673–7. doi: 10.1055/s-0029-1220790 [DOI] [PubMed] [Google Scholar]
- 24.Henry A, Madan A, Reid R, Tracy SK, Austin K, Welsh A, et al. Outpatient foley catheter versus inpatient prostaglandin E2 gel for induction of labour: a randomised trial. BMC Pregnancy Childbirth 2013;13:25. doi: 10.1186/1471-2393-13-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herabutya Y, Prasertsawat PO. A comparison of oral and intracervical prostaglandin E2 for ripening of the unfavourable cervix prior to induction of labour. J Med Assoc Thai 1988;71:269–73. [PubMed] [Google Scholar]
- 26.Howard K, Gerard K, Adelson P, Bryce R, Wilkinson C, Turnbull D. Women's preferences for inpatient and outpatient priming for labour induction: a discrete choice experiment. BMC Health Serv Res 2014;14:330. doi: 10.1186/1472-6963-14-330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Incerpi MH, Fassett MJ, Kjos SL, Tran SH, Wing DA. Vaginally administered misoprostol for outpatient cervical ripening in pregnancies complicated by diabetes mellitus. Am J Obstet Gynecol 2001;185:916–9. doi: 10.1067/mob.2001.117306 [DOI] [PubMed] [Google Scholar]
- 28.Kipikasa JH, Adair CD, Williamson J, Breen JM, Medford LK, Sanchez-Ramos L. Use of misoprostol on an outpatient basis for postdate pregnancy. Int J Gynaecol Obstet 2005;88:108–11. doi: 10.1016/j.ijgo.2004.10.006 [DOI] [PubMed] [Google Scholar]
- 29.Kruit H, Heikinheimo O, Ulander VM, Aitokallio-Tallberg A, Nupponen I, Paavonen J, et al. Foley catheter induction of labor as an outpatient procedure. J Perinatol 2016;36:618–22. doi: 10.1038/jp.2016.62 [DOI] [PubMed] [Google Scholar]
- 30.Kuper SG, Jauk VC, George DM, Edwards RK, Szychowski JM, Mazzoni SE, et al. Outpatient foley catheter for induction of labor in parous women: a randomized controlled trial. Obstet Gynecol 2018;132:94–101. doi: 10.1097/AOG.0000000000002678 [DOI] [PubMed] [Google Scholar]
- 31.Larmon JE, Magann EF, Dickerson GA, Morrison JC. Outpatient cervical ripening with prostaglandin E2 and estradiol. J Matern Fetal Neonatal Med 2002;11:113–7. doi: 10.1080/jmf.11.2.113.117 [DOI] [PubMed] [Google Scholar]
- 32.Lien JM, Morgan MA, Garite TJ, Kennedy KA, Sassoon DA, Freeman RK. Antepartum cervical ripening: applying prostaglandin E2 gel in conjunction with scheduled nonstress tests in postdate pregnancies. Am J Obstet Gynecol 1998;179:453–8. doi: 10.1016/s0002-9378(98)70378-3 [DOI] [PubMed] [Google Scholar]
- 33.Magann EF, Chauhan SP, McNamara MF, Bass JD, Estes CM, Morrison JC. Membrane sweeping versus dinoprostone vaginal insert in the management of pregnancies beyond 41 weeks with an unfavorable cervix. J Perinatol 1999;19:88–91. doi: 10.1038/sj.jp.7200133 [DOI] [PubMed] [Google Scholar]
- 34.Magann EF, Chauhan SP, Nevils BG, McNamara MF, Kinsella MJ, Morrison JC. Management of pregnancies beyond forty-one weeks' gestation with an unfavorable cervix. Am J Obstet Gynecol 1998;178:1279–87. doi: 10.1016/s0002-9378(98)70334-5 [DOI] [PubMed] [Google Scholar]
- 35.McGee TM, Gidaszewski B, Khajehei M, Tse T, Gibbs E. Foley catheter silicone versus latex for term outpatient induction of labour: a randomised trial. Aust N Z J Obstet Gynaecol 2019;59:235–42. doi: 10.1111/ajo.12828 [DOI] [PubMed] [Google Scholar]
- 36.McKenna DS, Costa SW, Samuels P. Prostaglandin E2 cervical ripening without subsequent induction of labor. Obstet Gynecol 1999;94:11–4. doi: 10.1016/s0029-7844(99)00244-6 [DOI] [PubMed] [Google Scholar]
- 37.McKenna DS, Duke JM. Effectiveness and infectious morbidity of outpatient cervical ripening with a Foley catheter. J Reprod Med 2004;49:28–32. [PubMed] [Google Scholar]
- 38.McKenna DS, Ester JB, Proffitt M, Waddell KR. Misoprostol outpatient cervical ripening without subsequent induction of labor: a randomized trial. Obstet Gynecol 2004b;104:579–84. doi: 10.1097/01.AOG.0000136479.72777.56 [DOI] [PubMed] [Google Scholar]
- 39.Meyer M, Pflum J, Howard D. Outpatient misoprostol compared with dinoprostone gel for preinduction cervical ripening: a randomized controlled trial. Obstet Gynecol 2005;105:466–72. doi: 10.1097/01.AOG.0000152341.31873.d9 [DOI] [PubMed] [Google Scholar]
- 40.Oboro VO, Tabowei TO. Outpatient misoprostol cervical ripening without subsequent induction of labor to prevent post-term pregnancy. Acta Obstet Gynecol Scand 2005;84:628–31. doi: 10.1111/j.0001-6349.2005.00655.x [DOI] [PubMed] [Google Scholar]
- 41.Ohel G, Rahav D, Rothbart H, Ruach M. Randomised trial of outpatient induction of labor with vaginal PGE2 at 40-41 weeks of gestation versus expectant management. Arch Gynecol Obstet 1996;258:109–12. doi: 10.1007/s004040050110 [DOI] [PubMed] [Google Scholar]
- 42.Policiano C, Pimenta M, Martins D, Clode N. Outpatient versus inpatient cervix priming with foley catheter: a randomized trial. Eur J Obstet Gynecol Reprod Biol 2017;210:1–6. doi: 10.1016/j.ejogrb.2016.11.026 [DOI] [PubMed] [Google Scholar]
- 43.PonMalar J, Benjamin SJ, Abraham A, Rathore S, Jeyaseelan V, Mathews JE. Randomized double-blind placebo controlled study of preinduction cervical priming with 25 micro g of misoprostol in the outpatient setting to prevent formal induction of labour. Arch Gynecol Obstet 2017;295:33–8. doi: 10.1007/s00404-016-4173-z [DOI] [PubMed] [Google Scholar]
- 44.Rayburn WF, Gittens LN, Lucas MJ, Gall SA, Martin ME. Weekly administration of prostaglandin E2 gel compared with expectant management in women with previous cesareans. Prepidil gel study group. Obstet Gynecol 1999;94:250–4. doi: 10.1016/s0029-7844(99)00300-2 [DOI] [PubMed] [Google Scholar]
- 45.Salvador SC, Lynn Simpson M, Cundiff GW. Dinoprostone vaginal insert for labour induction: a comparison of outpatient and inpatient settings. J Obstet Gynaecol Can 2009;31:1028–34. doi: 10.1016/S1701-2163(16)34347-X [DOI] [PubMed] [Google Scholar]
- 46.Sawai SK, O'Brien WF, Mastrogiannis DS, Krammer J, Mastry MG, Porter GW. Patient-administered outpatient intravaginal prostaglandin E2 suppositories in post-date pregnancies: a double-blind, randomized, placebo-controlled study. Obstet Gynecol 1994;84:807–10. [PubMed] [Google Scholar]
- 47.Sawai SK, Williams MC, O'Brien WF, Angel JL, Mastrogiannis DS, Johnson L. Sequential outpatient application of intravaginal prostaglandin E2 gel in the management of postdates pregnancies. Obstet Gynecol 1991;78:19–23. [PubMed] [Google Scholar]
- 48.Sciscione AC, Muench M, Pollock M, Jenkins TM, Tildon-Burton J, Colmorgen GH. Transcervical foley catheter for preinduction cervical ripening in an outpatient versus inpatient setting. Obstet Gynecol 2001;98:751–6. doi: 10.1016/s0029-7844(01)01579-4 [DOI] [PubMed] [Google Scholar]
- 49.Smith CV, Miller A, Livezey GT. Double-blind comparison of 2.5 and 5.0 mg of prostaglandin E2 gel for preinduction cervical ripening. J Reprod Med 1996;41:745–8. [PubMed] [Google Scholar]
- 50.Stitely ML, Browning J, Fowler M, Gendron RT, Gherman RB. Outpatient cervical ripening with intravaginal misoprostol. Obstet Gynecol 2000;96:684–8. doi: 10.1016/s0029-7844(00)01034-6 [DOI] [PubMed] [Google Scholar]
- 51.Stock SJ, Taylor R, Mairs R, Azaghdani A, Hor K, Smith I, et al. Home cervical ripening with dinoprostone gel in nulliparous women with singleton pregnancies. Obstet Gynecol 2014;124:354–60. doi: 10.1097/AOG.0000000000000394 [DOI] [PubMed] [Google Scholar]
- 52.Turnbull D, Adelson P, Oster C, Bryce R, Fereday J, Wilkinson C. Psychosocial outcomes of a randomized controlled trial of outpatient cervical priming for induction of labor. Birth 2013;40:75–80. doi: 10.1111/birt.12035 [DOI] [PubMed] [Google Scholar]
- 53.Turnbull D, Adelson P, Oster C, Coffey J, Coomblas J, Bryce R, et al. The impact of outpatient priming for induction of labour on midwives' work demand, work autonomy and satisfaction. Women Birth 2013;26:207–12. doi: 10.1016/j.wombi.2013.03.001 [DOI] [PubMed] [Google Scholar]
- 54.Upadhyaya NB, Childs KD, Neiger R, Caudle MR. Ambulatory cervical ripening in term pregnancy. J Reprod Med 1999;44:363–6. [PubMed] [Google Scholar]
- 55.Wilkinson C, Adelson P, Turnbull D. A comparison of inpatient with outpatient balloon catheter cervical ripening: a pilot randomized controlled trial. BMC Pregnancy Childbirth 2015;15:126. doi: 10.1186/s12884-015-0550-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilkinson C, Bryce R, Adelson P, Turnbull D. A randomised controlled trial of outpatient compared with inpatient cervical ripening with prostaglandin E2 (OPRA study). BJOG 2015;122:94–104. doi: 10.1111/1471-0528.12846 [DOI] [PubMed] [Google Scholar]
- 57.Methods guide for effectiveness and comparative effectiveness reviews. AHRQ Publication No. 10(14)-EHC063-EF. Agency for Healthcare Research and Quality; 2014 [PubMed] [Google Scholar]
- 58.Viswanathan M, Patnode CD, Berkman ND, Bass EB, Chang S, Hartling L, et al. Assessing the risk of bias in systematic reviews of health care interventions. Methods guide for effectiveness and comparative effectiveness reviews. Agency for Healthcare Research and Quality; 2017. [PubMed] [Google Scholar]
- 59.Furlan AD, Malmivaara A, Chou R, Maher CG, Deyo RA, Schoene M, et al. 2015 updated method guideline for systematic reviews in the Cochrane Back and Neck Group. Spine 2015;40:1660–73. [DOI] [PubMed] [Google Scholar]
- 60.Berkman ND, Lohr KN, Ansari MT, Balk EM, Kane R, McDonagh M, et al. Grading the strength of a body of evidence when assessing health care interventions: an EPC update. J Clin Epidemiol 2015;68:1312–24. doi: 10.1016/j.jclinepi.2014.11.023 [DOI] [PubMed] [Google Scholar]
- 61.Ausbeck EB, Jauk VC, Xue Y, Files P, Kuper SG, Subramaniam A, et al. Outpatient foley catheter for induction of labor in nulliparous women: a randomized controlled trial. Obstet Gynecol 2020;136:597–606. doi: 10.1097/aog.0000000000004041 [DOI] [PubMed] [Google Scholar]